Co-morbidity scores have improved our ability to predict outcome after T-cell replete allogeneic hematopoietic stem cell transplantation (HCT).1 Prediction tools such as the Sorror Hematopoietic Cell Transplantation-Specific Comorbidity Index (HCT-CI) have been developed and validated in conventional T-replete HCT. 2–4 Ex vivo T cell depletion (TCD) of the graft improves the tolerability of HCT and may be impacted differently by factors determined to affect T-cell replete HCT outcome.5, 6 The European Group for Blood and Marrow Transplantation (EBMT) risk scores may be used to assign risks of conventional T-replete HCT to an individual patient.7 Lodewyck et al evaluated the utility of the EBMT score in T-cell depleted unrelated donor transplantation for acute leukemia and myelodysplasia. 8 Peripheral blood absolute lymphocyte/monocyte ratio (LMR) has also been shown to predict clinical outcomes in patients with lymphoma receiving autologous HCT.9–11 We therefore evaluated published comorbidity measures and other standard clinical biomarkers of outcome in a series of patients with hematologic malignancies undergoing myeloablative TCD transplants. Pre-transplant factors measured were: HCT-CI, EBMT risk scores, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS), serum CRP, albumin (ALB), pre-albumin (PAB), ferritin, ALC, absolute monocyte count (AMC), and LMR. LMR was calculated from the pre-transplant (week −2) complete blood cell count (CBC).
This is a single institute retrospective study of 79 consecutive patients with hematologic malignancies undergoing myeloablative TCD HCT between October 2006 and March 2012. All patients received a myeloablative preparative regimen. Granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood (PBSC) from their HLA-identical sibling donors underwent CD34+ selection. Overall survival probabilities were estimated by the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariate Cox proportional hazards regression models were used to identify factors associated with OS and NRM. Nonlinear continuous covariates were optimally categorized by the classification and regression tree (CART) method based on the Gini index and cross-validation.12 In the final multivariate Cox model, backwards variable selection was used to eliminate the unimportant factors. To compare the competing models and evaluate the added predictive ability of new risk factors beyond traditional risk indicators, the increment in the C-statistic (area under the receiver operating characteristic [ROC] curve) and a continuous category-free form of the net reclassification improvement were calculated.13 Statistical analyses were performed using the R statistical software, version 3.1.2.
We studied a cohort of 79 patients with hematological malignancies receiving an HLA-identical sibling T-Cell depleted HCT. Univariate Cox regression was used to examine all potential factors associated with mortality. Kaplan-Meier estimate of OS by HCT-CI ≥ 5 was significantly different compared to HCI-CI≤4 (unadjusted HR 2.09, 95% CI 1.10–3.96, P=0.02, Figure 1A). Of note, when LMR was dichotomized around median of 3, the OS estimates for LMR< 3 and LMR≥3 were statistically significant (unadjusted HR 1.91, 95% CI 1.05–3.46, P=0.03, Figure 1B). However, further analysis revealed that a cut-off value of 25 divided the patients with LMR≥3 into two different groups with estimated OS 10% for LMR>25 and OS 66.7% for LMR 3–25 (P<0.001, Figure 1C), and unadjusted HR 2.89 (95% CI, 1.41–5.92, P=0.004) for LMR < 3 and 3.87 (95% CI 1.59–9.42, P=0.003) for LMR> 25 compared with LMR 3–25, respectively. In addition, we compared the ROC curves and C-statistics (area under the curve [AUC]) of using either a dichotomous LMR or a three-category LMR to predict 5-year OS. The three-category (<3, >25 vs. 3–25) LMR was more predictive of OS compared to the dichotomous (<3 vs. ≥3) LMR (ΔC-statistic was 0.12 (P=0.018). Moreover, LMR low-risk (3–25) and high risk (<3 or >25) levels further significantly stratified the mortality risk in the survival estimates for HCT-CI≤4 and HCT-CI≥5 subgroups (Figure 1D).
Figure 1. Kaplan-Meier overall survival curves for important pre-transplant biomarkers (A, B, C, D).
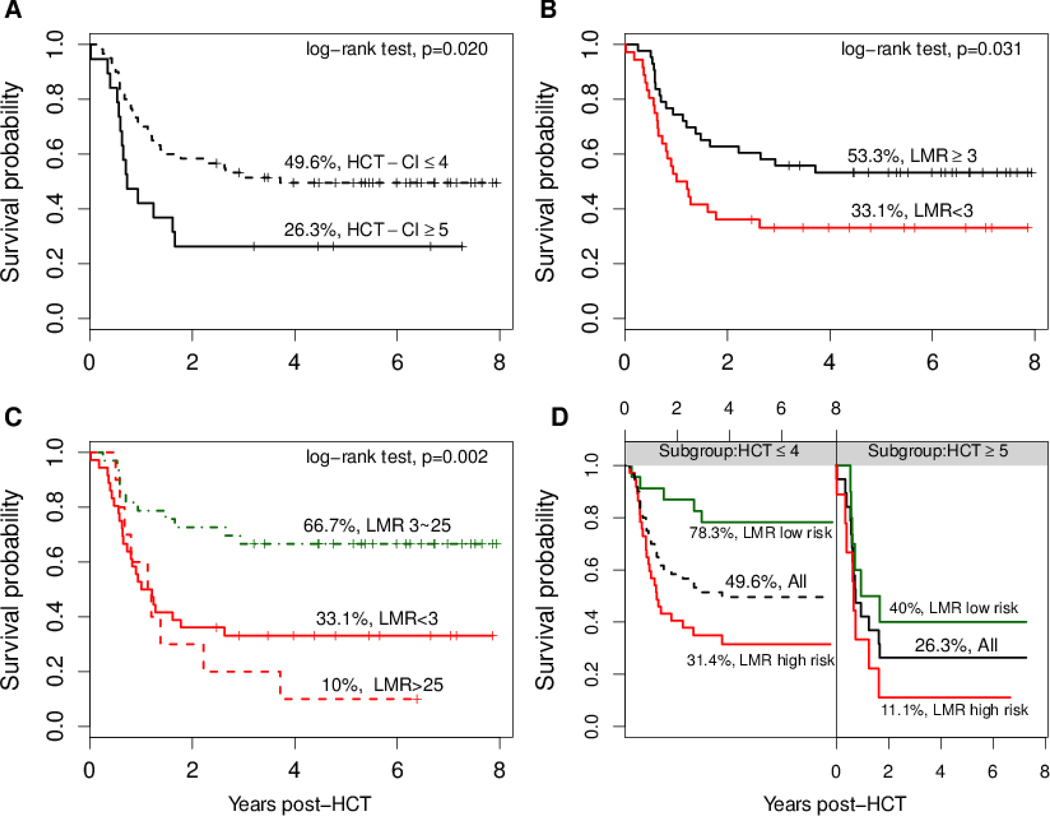
In Figure 1D, LMR high risk group contains LMR <3 or >25 and LMR low risk group is 3 to 25.
Multivariate Cox regression was used to adjust for potential confounding factors such as age, protocol and to examine the association of HCT-CI and EBMT risk scores and pre-transplant biomarkers with mortality. Only HCT-CI and LMR categorical factors were selected in the final model using a backwards variable selection procedure. Table 1 presented and compared three multivariate models. Model 1 contains age, protocol and EBMT score. In Model 2, we added HCT-CI score to Model 1; in Model 3, we added LMR to Model 2. The C-statistics for Model 1–3 were 0.55, 0.63 and 0.79, respectively, with significant improvement between Model 1 and 3 (ΔC-statistic = 0.24, P =0.002) and between Model 2 and 3 (ΔC-statistic = 0.16, P =0.02). Both the incremental C-statistic and net reclassification improvement (NRI) analysis suggested that the addition of HCT-CI score, and HCT-CI score plus LMR together improved the model discrimination and mortality risk prediction compared to the model with only EBMT. Only HCT-CI (adjusted HR 3.18, 95% CI 1.56–6.49 for HCI-CI ≥5) and LMR (adjusted HR 3.31, 95% CI 1.60–6.86 for LMR<3; adjusted HR 6.20, 95% CI 2.28–16.9 for LMR> 25) were highly significant in the finally selected Model 3 (Table 1). Similar analysis was performed to identify risk factors with NRM (Table 1). The multivariate analysis, controlling for age and protocol, showed neither the Model 4 with EBMT score or Model 5 after adding HCT-CI score was predictive of NRM with a low C-statistics of 0.56 and 0.58, respectively (Table 1). Of note, HCT-CI score was also found to be a significant predictor of NRM after introducing LMR to the multivariate model (Model 6 in Table 1). This association was also suggested in the nonparametric estimate of cumulative incidence of NRM by HCT-CI and LMR.
Table 1.
Multivariate analysis of predictors for mortality and non-relapse mortality
| Mortalitya | ||||||
|---|---|---|---|---|---|---|
| Model 1: EBMT score b | Model 2: EBMT and HCT-CI scores c | Model 3: EBMT, HCT-CI scores and LMRd | ||||
| Factors | HR | P | HR | P | HR | P |
| (95% CI) | (95% CI) | (95% CI) | ||||
| EBMT score | 1.06 | 0.63 | 1.03 | 0.84 | 0.997 | 0.98 |
| (0.85–1.32) | (0.81–1.29) | (0.81–1.23) | ||||
| HCT-CI scores (≥ 5 vs. ≤4) | -- | -- | 2.12 | 0.029 | 3.18 | 0.0015 |
| (1.08– 4.17) | (1.56–6.49) | |||||
| LMR | -- | -- | -- | -- | ||
| <3 vs. 3–25 | 3.31 | 0.0013 | ||||
| (1.60–6.86) | ||||||
| 620 | ||||||
| >25 vs. 3–25 | (2.28–16.9) | <0.001 | ||||
| Non-relapse mortalitya | ||||||
| Model 4: EBMT score e | Model 5: EBMT and HCT-CI scores f | Model 6: EBMT, HCT-CI scores and LMRg | ||||
| Factors | HR | P | HR | P | HR | P |
| (95% CI) | (95% CI) | (95% CI) | ||||
| EBMT score | 1.08 | 0.61 | 1.05 | 0.75 | 0.996 | 0.98 |
| (0.80–1.45) | (0.77–1.43) | (0.75–1.33) | ||||
| HCT-CI scores (≥ 5 vs. ≤4) | -- | -- | 2.11 | 0.12 | 3.58 | 0.011 |
| (0.83– 5.39) | (1.34– 9.54) | |||||
| LMR | -- | -- | -- | -- | ||
| <3 vs. 3–25 | 6.18 | <0.001 | ||||
| (2.15– 17.7) | ||||||
| >25 vs. 3–25 | 8.47 | 0.002 | ||||
| (2.22–32.3) | ||||||
Note:
Age, protocol (06-H-0248 vs. 07-H-0136), gender, disease-risk, CRP, ferritin, albumin, pre-albumin, ALC, AMC and ECOG performance score were examined in the multivariate model and were not significant. There were no missing values.
For Model 1 with age, protocol and EBMT score, the C-statistic (AUC) was 0.55 with respect to 5-year mortality.
Model 2 contains Model 1 variables plus HCT-CI scores; the C-statistic was 0.63. The net reclassification improvement (NRI) was 48% and the ΔC-statistic (change in AUC) was 0.08 (P =0.11) compared to Model 1.
Model 3 contains Model 2 variables plus LMR, the C-statistic was 0.79. Compared to Model 1, NRI = 78% and ΔC-statistic = 0.24 (P =0.002). Compared to Model 2, NRI=77% and ΔC-statistic = 0.16 (P =0.016).
For Model 4 with age, protocol and EBMT score, the C-statistic was 0.56 with respect to 5-year non-relapse mortality.
Model 5 contains Model 4 variables plus HCT-CI scores; the C-statistic was 0.58. NRI= 33% and the ΔC-statistic =0.017 (P =0.6) compared to Model 4.
Model 6 contains Model 5 variables plus LMR, the C-statistic was 0.74. Compared to Model 4, NRI = 102% and ΔC-statistic = 0.18 (P =0.014). Compared to Model 5, NRI=103% and ΔC-statistic = 0.16 (P =0.020).
Abbreviations: AUC, area under the receiver operating characteristic curve (ROC) curve; CI, confidence intervals; EBMT, European Group for Blood and Marrow Transplantation; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; HR, hazard ratio; LMR, absolute lymphocyte/monocyte ratio.
Based on the multivariate analysis results (Table 1), we identified the HCT-CI score and LMR are two significant predictive factors. A simple predictive score for CD34+ selected myeloablative transplantation may be calculated by summation of scores derived from the HCT-CI [≤4 =Score 0, ≥5=Score 1] and LMR [3–25= Score 0, <3=Score 1, >25=Score 2]. A cumulative score of 0 is low risk score; 1 is intermediate risk score; and 2–3 is the highest risk score. The predictive scores [range, 0 to 3] may differentiate three risk groups for hematopoietic cell transplantation OS and NRM. The survival decreased (OS estimates were 78.3%, 40.4% and 10.5% for three risk groups, respectively, P<0.001) and the non-relapse mortality increased with increasing risk score (NRM cumulative incidence were 17.4%, 27.3% and 63.2% for three risk groups, respectively, P=0.003).
This is the first study to explore the potential of biomarkers to refine clinically-based comorbidity scores in the prediction of outcome after CD34+ selected (TCD) HCT. In multivariate analysis of our TCD HCT cohort, HCT-CI ≥5 strongly predicted for higher overall mortality (HR=3.18, P=0.001) and higher NRM (HR=3.58, P=0.01) compared with a lower HCT-CI (Table 1). Of the biological risk factors examined, LMR emerged as the most discriminative biological risk factor. An extreme LMR of <3 or >25 were associated with increased overall mortality (HR of 3.31, P= 0.001 and 6.2, P <0.001, respectively) as well as higher NRM (HR of 6.18, P <0.001 and 8.47, P =0.002, respectively). While the utility of HCT-CI is already well established in allogeneic transplantation, the LMR has not been explored in any allogeneic HCT setting, although there are reports associating LMR with outcomes in autologous HCT.11 Among biomarkers the LMR has the advantage of being routinely performed in all patients prior to HCT. Our interpretation of the LMR is that the groups with highest (>25) and lowest (<3) LMR represent patients with very low monocyte counts and poor marrow function or very low lymphocyte counts associated with profound immunosuppression, respectively. Both extremes represent surrogates for poorer lympho-hematopoietic reserve or tissue damage from treatment. As the three-way segregation of the LMR gave the greatest discrimination between high and low risk (33%, 67% and 10% OS), we tried to combine this with the independent variable HCT-CI to calculate a simple predictive outcome score which may discriminate most between success or failure of the transplant. This score may differentiate three risk groups for HCT with decreasing OS and increasing NRM as the score increases. The LMR appears to reflect host immunity and stem cell reserve. Our LMR data are derived solely from the routine clinical laboratory complete blood cell counts. Efforts to better understand mechanisms are ongoing, including lymphocyte subset analysis.
In conclusion, this is the first study exploring comorbidity scores and biomarkers after CD34+ selected HCT. Our results suggest that HCT-CI score and LMR are important independent clinical predictors of OS and NRM in TCD HCT. The three-category (<3, >25 vs. 3–25) LMR was more predictive of OS and NRM compared to the dichotomous (<3 vs. ≥3) LMR. The utility of these predictive factors will need to be validated on other larger cohorts of patients and in different HCT settings. If confirmed, these measures may be useful for stratifying clinical trials in ex vivo TCD-HCT and counseling patients prior to HCT.
ACKNOWLEDGMENT
This work was supported by the intramural research program of the NHLBI, NIH.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Artz AS, Pollyea DA, Kocherginsky M, Stock W, Rich E, Odenike O et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2006; 12(9): 954–964. [DOI] [PubMed] [Google Scholar]
- 2.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106(8): 2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorror ML, Storer B, Storb RF. Validation of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in single and multiple institutions: limitations and inferences. Biol Blood Marrow Transplant 2009; 15(6): 757–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raimondi R, Tosetto A, Oneto R, Cavazzina R, Rodeghiero F, Bacigalupo A et al. Validation of the Hematopoietic Cell Transplantation-Specific Comorbidity Index: a prospective, multicenter GITMO study. Blood 2012; 120(6): 1327–1333. [DOI] [PubMed] [Google Scholar]
- 5.Le RQ, Bevans M, Savani BN, Mitchell SA, Stringaris K, Koklanaris E et al. Favorable outcomes in patients surviving 5 or more years after allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant 2010; 16(8): 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le RQ, Melenhorst JJ, Battiwalla M, Hill B, Memon S, Savani BN et al. Evolution of the donor T-cell repertoire in recipients in the second decade after allogeneic stem cell transplantation. Blood 2011; 117(19): 5250–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gratwohl A The EBMT risk score. Bone Marrow Transplant 2012; 47(6): 749–756. doi: 10.1038/bmt.2011.110 [DOI] [PubMed] [Google Scholar]
- 8.Lodewyck T, Oudshoorn M, van der Holt B, Petersen E, Spierings E, von dem Borne PA et al. Predictive impact of allele-matching and EBMT risk score for outcome after T-cell depleted unrelated donor transplantation in poor-risk acute leukemia and myelodysplasia. Leukemia 2011; 25(10): 1548–1554. [DOI] [PubMed] [Google Scholar]
- 9.Porrata LF, Ristow K, Colgan JP, Habermann TM, Witzig TE, Inwards DJ et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphoma. Haematologica 2012; 97(2): 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thoma MD, Huneke TJ, DeCook LJ, Johnson ND, Wiegand RA, Litzow MR et al. Peripheral blood lymphocyte and monocyte recovery and survival in acute leukemia postmyeloablative allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant 2012; 18(4): 600–607. [DOI] [PubMed] [Google Scholar]
- 11.Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Hogan WJ et al. Day 100 Peripheral Blood Absolute Lymphocyte/Monocyte Ratio and Survival in Classical Hodgkin’s Lymphoma Postautologous Peripheral Blood Hematopoietic Stem Cell Transplantation. Bone Marrow Res 2013; 2013: 658371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breiman LJ, Friedman H, Olshen RA, Stone CG. Classification and Regression Trees. In: Wadsworth International Group; Belmont, CA, 1984. [Google Scholar]
- 13.Pencina MJ, D’Agostino RB Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011; 30(1): 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


