Abstract
Background and Purpose:
Atrial fibrillation (AF) is a leading cause of cardioembolic stroke, but the relations between AF and non-cardioembolic stroke subtypes are unclear. Since AF may be unrecognized, and because AF has a substantial genetic basis, we assessed for predisposition to AF across ischemic stroke subtypes.
Methods:
We examined associations between AF genetic risk and Trial of Org 10172 in Acute Stroke Treatment stroke subtypes in 2,374 ambulatory individuals with ischemic stroke and 5,175 without from the Wellcome Trust Case-Control Consortium 2 using logistic regression. We calculated AF genetic risk scores using single nucleotide polymorphisms (SNPs) associated with AF in a prior independent analysis across a range of preselected significance thresholds.
Results:
There were 460 (19.4%) individuals with cardioembolic, 498 (21.0%) with large vessel, 474 (20.0%) with small vessel, and 814 (32.3%) with strokes of undetermined cause. Most AF genetic risk scores were associated with stroke, with the strongest association (P=6×10−4) attributed to scores of 944 SNPs (each associated with AF at P<1×10−3 in a prior analysis). Associations between AF genetic risk and stroke were enriched in the cardioembolic stroke subset (strongest P=1.2×10−9, 944 SNP score). In contrast, AF genetic risk was not significantly associated with non-cardioembolic stroke subtypes.
Conclusions:
Comprehensive AF genetic risk scores were specific for cardioembolic stroke. Incomplete workups and subtype misclassification may have limited the power to detect associations with strokes of undetermined etiology. Future studies are warranted to determine whether AF genetic risk is a useful biomarker to enhance clinical discrimination of stroke etiologies.
MeSH keywords: atrial fibrillation, stroke, genetics, risk factors
Journal subject terms: Atrial fibrillation, Ischemic stroke, genetics, risk factors, epidemiology
Whereas one in five ischemic strokes can be attributed to cardioembolism in the setting of atrial fibrillation (AF),1, 2 a prevalent and heritable3–8 arrhythmia, a substantial proportion of additional strokes may arise in association with AF. For example, a cause for stroke is not identified in up to one-third of patients,9–11 and unrecognized AF has been identified in about thirty percent of such individuals during long-term follow-up.12–16 However, AF may be challenging to identify given the occasionally paroxysmal and asymptomatic nature of the arrhythmia. Moreover, AF confers a markedly increased risk of recurrent stroke,17 and treatment with anticoagulation reduces the risk of stroke in patients with identified AF.18 As such, there is a critical need to understand the extent to which AF contributes to strokes which may be attributed to other etiologies, and strokes in which no obvious etiology is identified.
Ischemic stroke is heritable,19 and recent discoveries indicate that genetic variants associated with AF are also associated with stroke.20 The two most significant susceptibility loci for cardioembolism are also the two loci most strongly associated with AF.21–26 We recently observed that comprehensive genome-wide genetic risk scores for AF have greater power for predicting AF than more limited scores, and were significantly associated with cardioembolic stroke.27 Therefore, in order to assess the contribution of AF to different clinically-determined stroke mechanisms, we examined the relations between genome-wide measures of AF genetic risk and ischemic stroke subtypes.
METHODS
Study participants
Participants in the study included 2,374 ambulatory individuals with ischemic strokes and 5,175 population-based controls (1958 Birth Cohort and National Blood Service Donors) from the Wellcome Trust Case-Control Consortium 2 (WTCCC2) ischemic stroke study.24 Descriptions of the individual studies comprising the WTCCC2 sample are provided in the online supplement. All individuals were of self-reported European ancestry. Participating studies were approved by relevant institutional review boards, and all participants gave written or oral consent for study participation, including genetic research, as approved by the local institutional body.
Stroke ascertainment
Ischemic stroke cases were ascertained from 3 sites across the UK – Edinburgh, Oxford and St. George’s (SGUL). The University of Edinburgh collection comprised 727 ischemic stroke cases, consecutively collected as part of the Edinburgh Stroke Study. The University of Oxford collection comprised 896 ischemic stroke cases, consecutively collected as part of the Oxford vascular study (OXVASC). The SGUL collection comprised 1224 ischemic stroke samples from a hospital based setting. All patients underwent recording with continuous telemetry as inpatients and had electrocardiograms. Ischemic stroke subtypes were determined according to Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria based on relevant clinical imaging.28 Strokes of other determined etiology, using the TOAST criteria, were excluded from the case-control populations prior to genotyping. Full details regarding stroke ascertainment, neuroimaging, and other clinical work-up are provided in the online supplement.
Genotyping and imputation
All WTCCC2 cases were genotyped as part of the WTCCC2 Ischemic Stroke study using the Illumina Human660W-Quad array. Controls were genotyped using the Illumina Human1.2M-Duo. Quality control procedures involved excluding single nucleotide polymorphisms (SNPs) not genotyped on all case and control collections and SNPs with Fisher information measure <0.98, genotype call rate <0.95, minor allele frequency <0.01 or Hardy-Weinberg P <1×10−20 in either the case or control collections. Samples were excluded if identified as outliers on call rate, heterozygosity, ancestry, and average probe intensity based on a Bayesian clustering algorithm. Samples were also removed if they exhibited discrepancies between inferred and recorded sex or cryptic relatedness with other WTCCC2 samples (pairwise identity-by-descent >0.05). Imputation was performed to the 1000 Genomes Phase 1 Integrated variant set. Phasing was perform using SHAPEIT29 v2.778 and imputation was performed using IMPUTE v2.3.0.30
AF genetic risk
We selected SNPs in approximate linkage equilibrium by pruning31 2.2 million HapMap variants included in a prior independent meta-analysis of genome-wide association studies for AF from the AFGen consortium (6,707 individuals with and 53,436 without AF).32 Specifically, we recursively extracted SNPs within a sliding 250 kilobase window that were uncorrelated on the basis of an r2 value of 0.3 using PLINK v1.90b3.32.28
For each individual in the WTCCC2 sample, we calculated AF genetic risk scores by summing the dosage of each AF risk allele (which can range from zero to two), weighted by the natural logarithm of the relative risk for each SNP determined from the prior independent AFGen meta-analysis.32 For example, if a score is comprised of three SNPs, and the log-relative risks of the three SNPs are 0.3, 0.4, and 0.5, respectively, then if the individual was heterozygous for each of the three SNPs the score would be ((0.3 * 1) + (0.4 * 1) + (0.5 * 1) = 1.2). If the individual was homozygous for risk alleles at all three SNPs, the score would be ((0.3 * 2) + (0.4 * 2) + (0.5 * 2) = 2.4). Thus, genetic risk scores for each individual were single linear predictors which we treated as continuous variables.
Since inclusion of SNPs liberally associated with a trait in genetic risk scores may increase the proportion of variance in the trait explained by the score,33–36 we created scores based on SNPs associated with AF in the prior analysis32 at nine different significance thresholds, which we selected a priori: (P<0.0001, <0.001, <0.01, <0.05, <0.1, <0.2, <0.3, <0.4, and <0.5).
Statistical analysis
We examined the associations between AF genetic risk scores and stroke subtypes using multivariable logistic regression with adjustment for two ancestry-informative principal components. AF genetic risk was entered into models as a continuous variable. We specifically examined the associations between scores and all ischemic, cardioembolic, large vessel, small vessel, and unknown stroke subtypes. We used the same set of referents for all analyses.
The a priori significance threshold for all analyses was P<0.05 using two-sided tests. Analyses were conducted using PLINK v1.90b3.3231 and R 3.2.2.37
RESULTS
Participant characteristics are summarized in Table 1. Among the 2,374 ischemic stroke cases, there were 460 (19.4%) individuals with cardioembolic, 498 (21.0%) with large vessel, 474 (20.0%) with small vessel stroke subtypes, and 814 (34.2%) with strokes of undetermined cause. A further 128 cases (5.4%) had stroke of tandem etiology and were not considered in the subtype analyses.
Table 1.
Characteristics of WTCCC2 participants included in the analysis.
| N | Age, years | Men (%) | History of AF N (%) |
History of IHD N (%) |
MRI N (%) |
Echocardiogram N (%) |
Extracranial Imaging N (%) |
|
|---|---|---|---|---|---|---|---|---|
| All ischemic stroke | 2,374 | 72.2 ± 12.5 | 53.8 | 479 (20.1%) | 552 (23.3%) | 881 (37.1%) | 847 (35.7%) | 2176 (91.7%) |
| Cardioembolic | 460 (19.4%) | 75.4 ± 12.5 | 62.1 | 362 (78.7%) | 141 (30.7%) | 113 (24.6%) | 259 (56.3%) | 393 (85.4%) |
| Large vessel disease | 498 (20.1%) | 68.2 ± 10.8 | 66.2 | 2 (0.4%) | 136 (27.3%) | 196 (39.4%) | 133 (26.7%) | 487 (98.2%) |
| Small vessel disease | 474 (20.0%) | 69.6 ± 11.7 | 52.3 | 10 (2.1%) | 76 (16.0%) | 285 (60.1%) | 139 (29.1%) | 455 (96.0%) |
| Unknown | 814 (34.3%) | 70.8 ± 13.8 | 45.7 | 26 (3.2%) | 153 (18.8%) | 248 (30.5%) | 248 (30.5%) | 726 (89.2%) |
| Referents | 5,175 | – | 49.5 | - | - | - | - | - |
Data presented as mean ± standard deviation or N (%) unless otherwise specified. A further 128 (5.4%) of individuals had stroke of tandem etiology and were not included in any subgroup analyses. N/A, information not available. IHD, ischemic heart disease. AF, atrial fibrillation. All patients underwent CT imaging and an ECG. Extracranial cerebral arterial imaging includes carotid and vertebral artery ultrasound, or computed tomographic angiography, or magnetic resonance angiography.
AF genetic risk scores were comprised of between 172 and 162,456 SNPs across the nine different preselected SNP significance thresholds (Table 2). AF genetic risk scores were associated with all ischemic stroke, with p-values ranging from 6.0×10−4 (944 SNP score) to 0.18 (44,127 SNP score) (Figure).
Table 2.
Number of SNPs included in each atrial fibrillation genetic risk score.
| Discovery sample P value for association with AF | <1×10−4 | <1×10−3 | <0.01 | <0.05 | <0.1 | <0.2 | <0.3 | <0.4 | <0.5 |
| N SNPs in genetic risk score | 172 | 944 | 6,182 | 24,599 | 44,127 | 78,329 | 109,124 | 137,122 | 162,456 |
AF=atrial fibrillation; SNP=single nucleotide polymorphism
Figure.
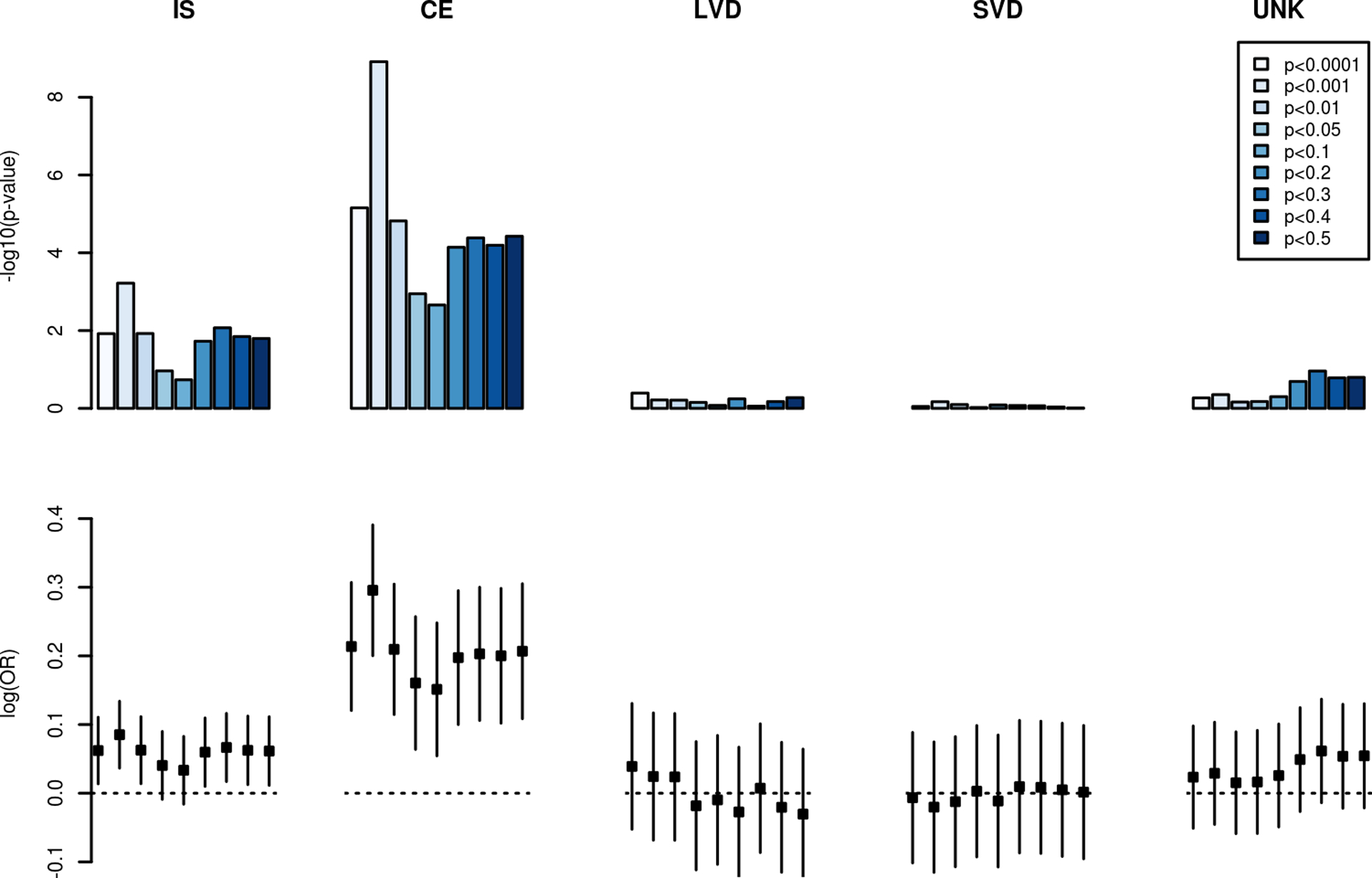
Association between atrial fibrillation genetic risk and ischemic stroke subtypes.
The strength of association between genetic risk scores comprised of atrial fibrillation genetic markers and ischemic stroke subtypes are displayed in the top panel. Separate scores were calculated corresponding to differences in the strength of association between each variant and atrial fibrillation in a prior independent analysis.32 The magnitude of association per 1-unit change in each genetic risk score is displayed in the bottom panel.
IS, all ischemic stroke; CE, Cardioembolic stroke; LVD, large vessel disease stroke; SVD, small vessel disease stroke; UNK, stroke of unknown etiology.
Among the stroke subtypes, AF genetic risk was enriched among the subset of individuals with cardioembolic stroke. We observed significant associations between AF genetic risk and cardioembolic stroke across all SNP scores, with the strongest association (P=1.2×10−9) accounted for by a score comprised of SNPs associated with AF at P<1×10−3 in the prior AFGen analysis.32 AF genetic risk scores were not significantly associated with large vessel disease, small vessel disease, or unknown stroke subtypes.
DISCUSSION
In our analysis of 2,374 individuals with ischemic stroke and 5,175 population-based referents of European ancestry, we observed that comprehensive AF genetic risk scores were significantly associated with stroke. The association was almost entirely explained by an association with cardioembolic stroke, whereas scores were not significantly associated with non-cardioembolic stroke etiologies. In aggregate, these findings indicate that the associations between AF genetic risk and ischemic stroke are specific for cardioembolic stroke.
Our findings support and extend prior analyses examining the relations between AF and ischemic stroke. AF is a well-recognized risk factor for stroke.13 Genome-wide association studies of ischemic stroke, in which a subset of individuals from the present analysis were included, identified variants at the top two AF susceptibility loci on chromosomes 4q25 and 16q22 among the subset with cardioembolic strokes.23–25 A genetic risk score comprised of top genome-wide significant variants for clinical traits known to be associated with ischemic stroke, including 14 variants associated with AF, was associated with ischemic stroke in a previous analysis.38 Our study extends prior literature by relating comprehensive genome-wide estimates of AF genetic risk to specific stroke subtypes in a large and well-characterized sample.
Our findings have three major implications. First, by testing scores comprised of SNPs associated with AF at different significance thresholds in a prior independent analysis, our findings indicate that common genetic variants associated with AF at more liberal thresholds than the stringent genome-wide significance threshold typically employed are associated with ischemic stroke. We observed similar findings in a separate analysis in which we assessed associations between AF genetic risk and incident AF.27 Our analysis identified an informative subset of variants that may be relevant for assessing AF genetic risk in future studies. Discovery efforts in larger samples and using improved imputation reference panels may improve our understanding of genetic risk associated with both AF and stroke.
Second, our observation that AF genetic risk is associated nearly exclusively with cardioembolic stroke highlights the possibility that AF genetic risk may serve as a discriminating marker for strokes caused by thromboembolism rather than other mechanisms. The fact that the AF genetic risk score most significantly associated with cardioembolic stroke had no discernible association with other stroke classifications highlights the relative effectiveness of TOAST classification in distinguishing cardioembolic from other stroke etiologies. Identification of quantitative thresholds of AF genetic risk that maximize discrimination between cardioembolic and non-cardioembolic stroke subtypes may inform prospective efforts to test the clinical utility of AF genetic risk. Future consideration of both the clinical utility, as well as cost-effectiveness, of genetic risk stratification to distinguish stroke mechanisms may be warranted.
Third, we did not observe a significant association between AF genetic risk and stroke of unknown etiology. However, we only had sufficient power to identify an association if AF and stroke of unknown etiology were moderately highly genetically correlated (r2>0.5), meaning we cannot rule out a moderate association between the two traits, particularly if only a fraction of the strokes of unknown etiology were due to AF. Moreover, the WTCCC2 dataset is a heavily curated research tool, which may not mirror the imperfect standard community practice of stroke subtyping. Future larger studies utilizing genetic risk of AF and other stroke risk factors may yield insights into the contribution of heritable risk factors to cryptogenic stroke.
Our study should be interpreted in the context of the observational study design. Individuals included in the analysis were of European ancestry, and therefore the results may not be generalizable to other ancestral groups. We had limited power to assess the relations between AF genetic risk and non-cardioembolic stroke subtypes. We were unable to adjust for clinical risk factors associated with ischemic stroke and AF, and therefore cannot exclude confounding between genetic risk and ischemic stroke. Nevertheless, we have separately observed significant associations between genetic markers and incident AF independent of clinical AF risk factors, which are highly correlated with stroke risk factors.39 Individuals with strokes of unknown etiology had clinical workups at the discretion of their treating providers, some of which may have been incomplete. Incomplete workups may have resulted in misclassification since further workup may have attributed strokes to an etiologic subgroup, and thereby biased our analyses examining associations between AF genetic risk scores and stroke of undetermined etiology towards the null if strokes were caused by other non-cardioembolic etiologies. Moreover, the absence of systematic long-term cardiac rhythm monitoring results does not enable assessment of incident AF in patients with ischemic strokes. Future analyses with more complete phenotyping in the undetermined subset, and with a larger number of individuals with undetermined stroke, are warranted. It is also possible that more precise scores of AF genetic risk may be more specific for cardioembolic stroke subtypes and yield associations with strokes of undetermined etiology. Large genome-wide association studies of AF are ongoing, and are expected to yield more precise estimates of AF risk associated with each SNP marker.
Conclusions
In our analysis of 2,374 individuals with stroke and 5,175 population-based referents, we observed that AF genetic risk was strongly associated with ischemic stroke. Genetic risk scores comprised of variants liberally associated with AF were associated specifically with the cardioembolic stroke subtype, indicating that the TOAST classification system effectively distinguishes cardioembolic from noncardioembolic stroke subtypes. Our observations suggest that polygenic AF risk is an important determinant of stroke risk. Future analyses are warranted to determine whether utilizing information AF genetic risk can help distinguish between stroke subtypes.
Supplementary Material
SOURCES OF FUNDING
Dr. Lubitz is supported by NIH grants K23HL114724 and a Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105. Dr. Traylor is supported by a British Heart Foundation programme grant (RG/16/4/32218). Dr. Ellinor and Benjamin are supported by 1RO1HL092577, R01HL128914. Dr. Ellinor is supported by grants from the National Institutes of Health K24HL105780 and an Established Investigator Award from the American Heart Association (13EIA14220013) and by the Fondation Leducq (14CVD01). Dr. Dichgans and Dr. Malik were supported by grants from the Deutsche Forschungsgemeinschaft (CRC 1123 [B3] and Munich Cluster for Systems Neurology [SyNergy]), the German Federal Ministry of Education and Research (BMBF, e:Med programme e:AtheroSysMed), the FP7/2007-2103 European Union project CVgenes@target (grant agreement No Health-F2-2013-601456), the European Union Horizon2020 projects SVDs@target (grant agreement No 66688) and CoSTREAM (grant agreement No 667375), the Fondation Leducq (Transatlantic Network of Excellence on the Pathogenesis of Small Vessel Disease of the Brain), the Vascular Dementia Research Foundation, and the Jackstaedt Foundation.
Footnotes
DISCLOSURES
Dr. Lubitz has received consulting support from St. Jude Medical for the use of implantable atrial fibrillation detection technologies, and grant funding from Boehringer Ingelheim to test physician electronic notification methods to improve adherence to guideline-directed anticoagulation. Dr. Ellinor is a principal investigator on a Bayer HealthCare grant to the Broad Institute focused on the mechanisms and therapeutics for atrial fibrillation.
References
- 1.Hart RG, Halperin JL. Atrial fibrillation and stroke : Concepts and controversies. Stroke. 2001;32:803–808 [DOI] [PubMed] [Google Scholar]
- 2.Marini C, De Santis F, Sacco S, Russo T, Olivieri L, Totaro R, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: Results from a population-based study. Stroke. 2005;36:1115–1119 [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Parise H, D’Agostino RB Sr., Lloyd-Jones DM, Vasan RS, Wang TJ, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855 [DOI] [PubMed] [Google Scholar]
- 4.Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184 [DOI] [PubMed] [Google Scholar]
- 5.Arnar DO, Thorvaldsson S, Manolio TA, Thorgeirsson G, Kristjansson K, Hakonarson H, et al. Familial aggregation of atrial fibrillation in iceland. Eur Heart J. 2006;27:708–712 [DOI] [PubMed] [Google Scholar]
- 6.Christophersen IE, Ravn LS, Budtz-Joergensen E, Skytthe A, Haunsoe S, Svendsen JH, et al. Familial aggregation of atrial fibrillation: A study in danish twins. Circ Arrhythm Electrophysiol. 2009;2:378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus GM, Smith LM, Vittinghoff E, Tseng ZH, Badhwar N, Lee BK, et al. A first-degree family history in lone atrial fibrillation patients. Heart Rhythm. 2008;5:826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petty GW, Brown RD Jr., Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: A population-based study of incidence and risk factors. Stroke. 1999;30:2513–2516 [DOI] [PubMed] [Google Scholar]
- 10.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to toast criteria: Incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke. 2001;32:2735–2740 [DOI] [PubMed] [Google Scholar]
- 11.Hajat C, Heuschmann PU, Coshall C, Padayachee S, Chambers J, Rudd AG, et al. Incidence of aetiological subtypes of stroke in a multi-ethnic population based study: The south london stroke register. J Neurol Neurosurg Psychiatry. 2011;82:527–533 [DOI] [PubMed] [Google Scholar]
- 12.Seet RC, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation. 2011;124:477–486 [DOI] [PubMed] [Google Scholar]
- 13.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. The New England journal of medicine. 2012;366:120–129 [DOI] [PubMed] [Google Scholar]
- 14.Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: A systematic review and meta-analysis. Stroke. 2014 [DOI] [PubMed] [Google Scholar]
- 15.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486 [DOI] [PubMed] [Google Scholar]
- 16.Gladstone DJ, Spring M, Dorian P, Panzov V, Thorpe KE, Hall J, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477 [DOI] [PubMed] [Google Scholar]
- 17.Penado S, Cano M, Acha O, Hernandez JL, Riancho JA. Atrial fibrillation as a risk factor for stroke recurrence. Am J Med. 2003;114:206–210 [DOI] [PubMed] [Google Scholar]
- 18.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867 [DOI] [PubMed] [Google Scholar]
- 19.Seshadri S, Beiser A, Pikula A, Himali JJ, Kelly-Hayes M, Debette S, et al. Parental occurrence of stroke and risk of stroke in their children: The framingham study. Circulation. 2010;121:1304–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tada H, Shiffman D, Smith JG, Sjogren M, Lubitz SA, Ellinor PT, et al. Twelve-single nucleotide polymorphism genetic risk score identifies individuals at increased risk for future atrial fibrillation and stroke. Stroke. 2014;45:2856–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gretarsdottir S, Thorleifsson G, Manolescu A, Styrkarsdottir U, Helgadottir A, Gschwendtner A, et al. Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Ann Neurol. 2008;64:402–409 [DOI] [PubMed] [Google Scholar]
- 22.Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, Thorgeirsson G, et al. A sequence variant in zfhx3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemmens R, Buysschaert I, Geelen V, Fernandez-Cadenas I, Montaner J, Schmidt H, et al. The association of the 4q25 susceptibility variant for atrial fibrillation with stroke is limited to stroke of cardioembolic etiology. Stroke; a journal of cerebral circulation. 2010;41:1850–1857 [DOI] [PubMed] [Google Scholar]
- 24.International Stroke Genetics C, Wellcome Trust Case Control C, Bellenguez C, Bevan S, Gschwendtner A, Spencer CC, et al. Genome-wide association study identifies a variant in hdac9 associated with large vessel ischemic stroke. Nat Genet. 2012;44:328–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, et al. Genetic risk factors for ischaemic stroke and its subtypes (the metastroke collaboration): A meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neurology Working Group of the Cohorts for H, Aging Research in Genomic Epidemiology C, Stroke Genetics N, International Stroke Genetics C. Identification of additional risk loci for stroke and small vessel disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubitz SA, Yin X, Lin H, Kolek M, Smith JG, Trompet S, et al. Genetic risk prediction of atrial fibrillation. Circulation. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 29.O’Connell J, Gurdasani D, Delaneau O, Pirastu N, Ulivi S, Cocca M, et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet. 2014;10:e1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. Plink: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans DM, Visscher PM, Wray NR. Harnessing the information contained within genome-wide association studies to improve individual prediction of complex disease risk. Human molecular genetics. 2009;18:3525–3531 [DOI] [PubMed] [Google Scholar]
- 34.Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehret GB, Lamparter D, Hoggart CJ, Whittaker JC, Beckmann JS, Kutalik Z. A multi-snp locus-association method reveals a substantial fraction of the missing heritability. American journal of human genetics. 2012;91:863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R core team. R: A language and environment for statistical computing. (https://www.r-project.org/). Last accessed March 22, 2017.
- 38.Malik R, Bevan S, Nalls MA, Holliday EG, Devan WJ, Cheng YC, et al. Multilocus genetic risk score associates with ischemic stroke in case-control and prospective cohort studies. Stroke. 2014;45:394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christophersen IE, Yin X, Larson MG, Lubitz SA, Magnani JW, McManus DD, et al. A comparison of the charge-af and the cha2ds2-vasc risk scores for prediction of atrial fibrillation in the framingham heart study. Am Heart J. 2016;178:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


