Abstract
BACKGROUND
Vedolizumab (VDZ), a humanised monoclonal antibody that selectively inhibits alpha4-beta7 integrins is approved for use in adult moderate to severe ulcerative colitis (UC) patients.
AIM
To assess the efficacy and safety of VDZ in the real-world management of UC in a large multicenter cohort involving two countries and to identify predictors of achieving remission.
METHODS
A retrospective review of Australian and Oxford, United Kingdom data for UC patients. Clinical response at 3 mo, endoscopic remission at 6 mo and clinical remission at 3, 6 and 12 mo were assessed. Cox regression models and Kaplan Meier curves were performed to assess the time to remission, time to failure and the covariates influencing them. Safety outcomes were recorded.
RESULTS
Three hundred and three UC patients from 14 centres in Australia and United Kingdom, [60% n = 182, anti-TNF naïve] were included. The clinical response was 79% at 3 mo with more Australian patients achieving clinical response compared to Oxford (83% vs 70% P = 0.01). Clinical remission for all patients was 56%, 62% and 60% at 3, 6 and 12 mo respectively. Anti-TNF naive patients were more likely to achieve remission than exposed patients at all the time points (3 mo 66% vs 40% P < 0.001, 6 mo 73% vs 46% P < 0.001, 12 mo 66% vs 51% P = 0.03). More Australian patients achieved endoscopic remission at 6 mo compared to Oxford (69% vs 43% P = 0.01). On multi-variate analysis, anti-TNF naïve patients were 1.8 (95%CI: 1.3-2.3) times more likely to achieve remission than anti-TNF exposed (P < 0.001). 32 patients (11%) had colectomy by 12 mo.
CONCLUSION
VDZ was safe and effective with 60% of UC patients achieving clinical remission at 12 mo and prior anti-TNF exposure influenced this outcome.
Keywords: Vedolizumab, Ulcerative colitis, Outcomes
Core tip: Vedolizumab (VDZ) is a gut selective anti-integrin used for treatment of Ulcerative colitis (UC). Evidence is needed to support its use in real life setting involving multiple centers and two countries to reduce physician, site and country biases. This is a retrospective review of prospectively collected database involving 303 UC patients from Australia and Oxford, United Kingdom treated with VDZ. Clinical response was observed in 79% of patients at 3 mo and clinical remission in 56%, 62% and 60% at 3 mo, 6 mo and 12 mo respectively. Anti-tumor necrosis factor (anti-TNF) naïve patients were 1.8 times more likely to achieve remission than anti-TNF exposed and 11% of patients required colectomy by 12 mo. We concluded that VDZ is a safe and effective biologic medication used for treatment of UC.
INTRODUCTION
The aim of treatment in ulcerative colitis (UC) is to achieve sustained clinical, mucosal and histological healing[1,2]. The choice of treatment depends on several factors including induction, or maintenance, of disease remission, severity of disease, extent and location of bowel involvement, disease phenotype and individual characteristics of the drug and patient. The use of conventional medications may be limited either by a lack of efficacy (5-aminosalicylates) or side effects [steroids/azathioprine (AZA)/6 mercaptopurine (6MP)/methotrexate (MTX)][3]. Its’ use, however, is not without potential side effects including, development of opportunistic infections, reactivation of tuberculosis and an increased risk of melanoma[4].
Vedolizumab (VDZ), a humanised monoclonal antibody, selectively inhibits the migration of alpha4-beta7 inflammatory cells to the gastrointestinal tract, making it a biological agent without systemic immunosuppression and thus potentially reducing side-effects. In GEMINI 1, the randomised, double-blind placebo-controlled trial of VDZ in UC, the response rate for induction at week six was 47.1% with a response rate of 41.8% at week fifty two after eight-weekly VDZ treatments[5]. Patients enrolled in clinical trials, however, do not entirely represent the patients seen in routine clinical practice as demonstrated by a retrospective study where only 31% of 206 patients with moderate-to-severe inflammatory bowel disease (IBD) were eligible to participate in such a clinical trial[6].
Our aim was to assess the response and remission rates to VDZ in the real world, the time taken to achieve this, mucosal healing rates, adverse/serious events, the rates of colectomy and the predictors influencing remission in the first 12 mo of VDZ therapy through a multicenter consortium in a real-world setting.
MATERIALS AND METHODS
Study design
This was a multicenter retrospective review of prospectively collected data involving 14 IBD centers in Australia New Zealand inflammatory bowel disease consortium and data was also collected at a major IBD center in United Kingdom, thus reducing physician, site and country bias. All the centers involved in the study had a dedicated IBD team. In Australia, patients with UC refractory to conventional treatment, which was defined as failure of three, or more, mo of a 5-aminosalycylate and failure of three or more mo of an immunomodulator (AZA, 6MP or MTX) and 6 wk weaning dose of prednisolone that commenced at 40mg per day or more, were able to access VDZ from 2015 through the government funded pharmaceutical benefit scheme (PBS). In the United Kingdom, VDZ was given to patients at the physicians’ discretion if the conventional treatment and/or anti-tumor necrosis factor (anti-TNF) medications had failed to control the disease.
Consecutive patients with UC diagnosed as per the standard criteria[7] who received at least induction VDZ therapy were considered for the study. All patients who finished VDZ induction therapy were included in the study for analysis. VDZ was given as standard intravenous (IV) induction dosing of 300mg at 0, 2 and 6 wk followed by maintenance therapy of 8 weekly IV infusions. Patients continued to take, or wean off, steroids, 5-aminosalicylates (oral and rectal therapy) as deemed appropriate by the treating physician. Patients taking immunosuppressant medications, including AZA, 6MP, MTX orally, or rectal tacrolimus, continued on these medications under the treating physicians’ preference as guided by the disease control. There were no mandated changes to a patient’s regular IBD medications. The use of steroids and/or immunomodulators and their time of cessation was recorded for analysis.
A retrospective review of the IBD databases that contained prospectively-entered data included baseline patient demographics and disease characteristics classified by the Montreal classification[8], concomitant use of steroids and immunomodulator medications, prior exposure to anti-TNF medications, adverse events and colectomy rates.
Assessments tools and criteria
The Montreal classification was used to classify UC[8]. The Partial Mayo clinical score was used to assess disease control and is composed of 3 items, which includes stool frequency, rectal bleeding and the physician global assessment which were each scored individually from 0 to 3 at baseline, 3, 6 and 12 mo. The higher the score, more severe the disease and maximum score was 9. The Mayo endoscopic score (MES) is classified into four levels of severity from 0-4 based on mucosal friability, vascular pattern, friability and erosions. Mayo 0-1 was inactive disease while Mayo 2 and Mayo 3 were mild-moderate and moderate-severe disease respectively. Ulcerative colitis endoscopic index of severity (UCEIS) is composed of 3 items, which includes vascular pattern, bleeding and erosions/ulcers with score ranging from 0-2 for vascular pattern and scores 0-3 for bleeding and erosions/ulcers with higher scores indicating severe disease and the maximum score was 9. The response and remission to VDZ was assessed clinically using partial Mayo clinical score in both Australia and the United Kingdom sites. MES was used for assessing endoscopic appearance in Australia, and the UCEIS was used in the United Kingdom.
Evaluation of clinical efficiency at 3, 6 and 12 mo
Induction: Clinical efficiency of VDZ induction therapy was assessed as either clinical response or clinical remission at 3 mo. A response to VDZ was defined as a decrease in the Partial Mayo score of ≥ 3 from baseline, while clinical remission was defined as Partial Mayo score of < 2.
Maintenance
Clinical efficacy of VDZ was also assessed at 6 mo and 12 mo of VDZ therapy. The Partial Mayo score again was used to assess clinical response and clinical remission. Data was collected if VDZ was ceased due to side-effects, or loss of response (LOR) that resulted in a switch of the therapy away from VDZ, or surgery if it was required.
Endoscopic assessment of disease control was undertaken after approximately 6 mo of VDZ. An MES of 0 or 1 was defined as an endoscopic remission with a score > 1 indicating active disease. An UCEIS score of 0-1 was also defined as endoscopic remission with a score > 1 indicating active disease.
Corticosteroid and Immunomodulator therapy
Corticosteroid therapy was defined as the use of any oral steroid including prednisolone and budesonide. Immunomodulator therapy includes any oral or rectal immunomodulator which includes AZA, 6MP, MTX (oral or parenteral), tacrolimus (oral or rectal), ciclosporin and mycophenylate. Corticosteroid and immunomodulator use was assessed at baseline, 3, 6 and 12 mo.
Safety
The development of infusion reactions, adverse events and serious adverse events were all recorded. Infusion reactions were defined as any adverse event that occurred on the day, or the day after, the infusion. Adverse events were defined as any untoward medical occurrence not resulting in discontinuation of the VDZ or hospitalization. Adverse events were graded as serious if they resulted in discontinuation of VDZ, hospitalization of the patient, or patient death.
Statistical analysis
Data for each patient from their first dose of VDZ to either last infusion within the study period or cessation of the VDZ were included for analysis. All statistical analysis was conducted using SPSS version 24 (IBM Corp, released 2016). Statistical significance was set at P < 0.05. Patient demographic and disease profile information was described using frequency and percent for categorically classified variables, with mean and standard deviation and median and range used to describe scale variables. VDZ treatment outcomes are described at baseline, 3, 6 and 12 mo. Sample size varied across measures and was reported accordingly.
Two Cox Regression models and Kaplan Meier curves were performed separately for each site. The first model examined time (weeks) to remission. Time to censor was calculated as the difference between the date of remission (censor event) and the date the patient started the study. The second model examined time (weeks) to failure. Failure was defined as Partial Mayo clinical score ≥ 2 or MES ≥ 2, or the need for a change in the biologic agent, or requiring colectomy. Time to censor was calculated as the difference between this date of failure (censor event) and the date the patient started the study, truncated to 60 wk. Both models examined the covariates gender, disease duration (year), smoking (non–smoker/current smoker or ex smoker), disease location, age at which VDZ was given (year), and previous immunomodulator exposure. For the failure model, a remission covariate was also included (median split ≤ 13/> 13 wk of time to remission). For both models where remission or failure occurred at the start of the study, a small constant (0.01) was added to the time to event variable. Cox model proportional hazard assumption was tested using Schoenfeld residuals with no violations. A chi square analysis was used to investigate the remission and failure censor variables with anti-TNF, and site differences across categorical variables. Disease duration, age vedolizumab started, remission time and failure time were examined for normality using Shapiro Wilk and violations were noted. Therefore site differences for these continuous variables were examined using the non-parametric alternative Mann Whitney U Test. Further Cox Regression models and Kaplan Meier curves were performed using the combined data set with site included as a variable.
RESULTS
Patient characteristics
Three hundred and thress UC patients (Australia n = 210 and Oxford, United Kingdom, n = 93) from 15 centers (Australia n = 14) were included in the study. Of the 303 patients, patient data was available in 278 at 3 mo, 250 patients at 6 mo and 209 patients at 12 mo. Of the 303 patients, 15 patients were in remission at the start of VDZ and VDZ was commenced due to side effects to the anti-TNF agents, these patients were analysed separately at each time point (Figure 1).
Figure 1.
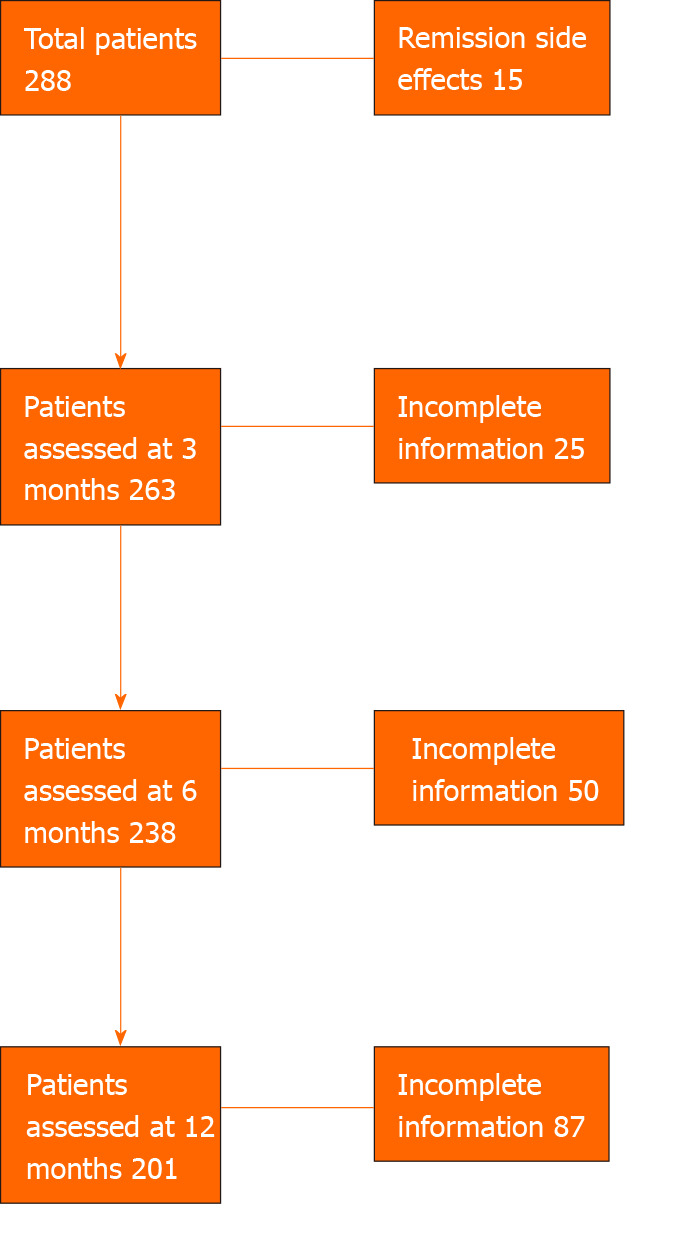
Flowchart.
Patient demographics and clinical characteristics
Of the total 303 patients, 60% (n = 182) were anti-TNF naïve and VDZ was their first biologic agent, while 40% (n = 121) had prior anti-TNF exposure with a secondary LOR in 20% (n = 61) and primary LOR in 15% (n = 45). VDZ was commenced in 5% (n = 15) of patients due to side-effects from anti-TNF therapy and these patients were in clinical remission at VDZ induction, so were analyzed separately. A total of 47% (n = 143) were female (Table 1).
Table 1.
Clinical characteristics of study population
| Characteristic | Total (n = 303) | Australia (n = 210) | Oxford (n = 93) | |
| Gender | ||||
| Female, n (%) | 143 (47) | 95 (45) | 48 (52) | |
| Median age VDZ given (range, yr) | 35 (16-84) | 36 (19-78) | 35 (16-84) | |
| Median disease duration (range, yr) | 6 (0.2-48) | 7 (1-48) | 5.4 (0.2-39.2) | |
| Montreal classification, n (%) | ||||
| Age | ||||
| A1 | 34 (11) | 33 (16) | 1 (1) | |
| A2 | 170 (56) | 120 (57) | 50 (54) | |
| A3 | 99 (33) | 57 (27) | 42 (45) | |
| Location | ||||
| E1 | 18 (6) | 15 (7) | 3 (3) | |
| E2 | 114 (38) | 72 (34) | 42 (45) | |
| E3 | 170 (56) | 122 (58) | 48 (52) | |
| Missing | 1 | 1 | 0 | |
| Family History, n (%) | 29 (12) | 22 (15) | 7 (7) | |
| First degree | 19 | 12 | 7 | |
| Second degree | 10 | 10 | 0 | |
| None | 212 | 126 | 86 | |
| Smoking, n | ||||
| Never | 226 | 140 | 86 | |
| Current | 9 | 6 | 3 | |
| Ex smoker | 45 | 41 | 4 | |
| Anti-TNF naïve, n (%) | 182 (60) | 122 (58.1) | 60 (65) | |
| Anti-TNF exposed, n (%) | 121 (40) | 88 (41.9) | 33 (35) | |
| Primary LOR | 45 (15) | 29 (13.8) | 16 (17) | |
| Secondary LOR | 61 (20) | 47 (22.4) | 14 (15) | |
| Side-effects | 15 (5) | 12 (5.7) | 3 (3.2) | |
| Steroids at VDZ initiation, n (%) | 191 (63) | 134 (64) | 57 (61.2) | |
| Prednisone | 162 (53) | 108 (51) | 54 (58) | |
| Budesonide | 29 (10) | 26 (12) | 3 (3) | |
| Immunomodulation at VDZ initiation, n (%) | 175 (58) | 135 (64) | 40 (43) | |
| AZA/6MP | 136 (45) | 108 (51) | 28 (30) | |
| Methotrexate | 19 (6) | 11 (5) | 8 (9) | |
| Tacrolimus | 17 (6) | 16 (8) | 1 (1) | |
| Others (Cyclo&Myco) | 3 (1) | 0 | 3 (3) | |
| Mean Partial Mayo before VDZ initiation | 5 (2-9) | 6 (2-9) | 5 (2-9) | |
VDZ: Vedolizumab; min: Minimum; max: Maximum; TNF: Tumor necrosis factor; Primary LOR: Primary loss of response; Secondary LOR: Secondary loss of response; AZA: Azathioprine; 6MP: 6-mercaptopurine; Cyclo: Ciclosporine; Myco: Mycophenolate; Init, Initiation.
The median age at which VDZ was started was 35 years (range 16-84 years), while the median disease duration was 6 years (range 0.2-48 years) prior to the commencement of VDZ. A family history was present in 12% (n = 29) and 81% (n = 226) were non-smokers at the time of commencing VDZ. All patients were classified by the Montreal classification[8] and 56% were diagnosed with UC between the ages of 17-40 years (n = 170, A2) compared to younger than 17 years in 11% (n = 34, A1) and 33% older than 40 years (n = 99, A3). Disease extent was extensive in 56% of patients (n = 170, E3) with 38% with suffering left-sided colitis (n = 114, E2) and 6% patients with proctitis (n = 18, E1). Of all patients, 63% (n = 191) were on steroids at the commencement of VDZ, 53% (n = 162) were taking prednisone and 10% (n = 29) budesonide. 58% (n = 175) of the patients were on an immunomodulator, with the thiopurines (AZA/6MP) being the most commonly used in 45% (n = 136), followed by MTX, tacrolimus, ciclosporine and mycophenolate. The mean partial Mayo score was 5 (range 2-9) at commencement of VDZ.
No significant differences were observed between the Australian and Oxford patients for prior anti-TNF exposure (P = 0.36), sex (P = 0.3), family history (P = 0.43), and age at which VDZ was started (P = 0.35). Significant differences between the Australian and Oxford patients, however, were observed for smoking with more Oxford patients having never smoked (P < 0.001) but there was no difference in the current smokers at time of VDZ commencement. Immunomodulator usage at the commencement of VDZ was greater in Australian than Oxford patients (P < 0.001), and the disease duration in Australian patients was longer prior to VDZ commencement (P < 0.048).
Assessment at 3 mo, 6 mo and 12 mo
A total of 263 patients were not in remission at commencement of VDZ, and of these 79% (n = 208) achieved a clinical response and 56% (n = 148) achieved clinical remission by 3 mo. Three mo data was missing for 25 patients (9 Australia, 16 Oxford), and thus these were not included in the induction analysis but there was data for the clinical status of these patients at 6 and 12 mo.
At 3 mo, Australian patients were more likely to achieve response (P = 0.01), but were not more likely to achieve remission than Oxford patients (P = 0.58) (Table 2). Anti-TNF naïve patients were more likely to achieve both response (P = 0.03) and remission (P < 0.001) at 3 mo compared to patients who had prior anti-TNF exposure. Within the anti-TNF exposed group, there was no significant difference between the patients who had a primary or secondary LOR to an anti-TNF agent in achieving clinical response (P = 0.9) or remission (P = 0.8) to VDZ at 3 mo.
Table 2.
Response and remission at 3 mo of vedolizumab therapy
| Total | Australia | Oxford | P value | |
| Response, n (%) | 208 (79) | 157 (83) | 51 (70) | 0.01 |
| Remission, n (%) | 148 (56) | 104 (55) | 44 (59) | 0.58 |
| Total | Anti-TNF naive | Anti-TNF exposed | P value | |
| Response, n (%) | 208 (79) | 138 (83) | 70 (72) | 0.03 |
| Remission, n (%) | 148 (56) | 109 (66) | 39 (40) | < 0.001 |
| Anti-TNF exposed | Primary LOR | Secondary LOR | P value | |
| Response, n (%) | 70 (72) | 30 (73) | 40 (71) | 0.85 |
| Remission, n (%) | 39 (40) | 16 (39) | 23 (41) | 0.83 |
VDZ: Vedolizumab; TNF: Tumor necrosis factor; Primary LOR: Primary loss of response to anti-TNF agent; Secondary LOR: Secondary loss of response to anti-TNF agent.
Of the total 238 patients assessed at 6 mo, 62% (n = 147) were in remission and of the 201 patients assessed at 12 mo, 60% (n = 120) were in remission (Table 3). No significant difference was found between Australia and Oxford in the number of patients in remission at 6 mo (P = 0.3) or at 12 mo (P = 0.09). Anti-TNF naïve patients were more likely to achieve remission both at 6 mo (P < 0.001) and 12 mo (P = 0.03) than those previously exposed. Within the anti-TNF exposed group, there was no significant difference between the patients who had primary or secondary LOR to anti-TNF agents in clinical remission at 6 mo (P = 0.2) or 12 mo (P = 0.3).
Table 3.
Remission at 6 mo and 12 mo of vedolizumab therapy
| Total | Australia | Oxford | P value | |
| Remission at 6 mo, n (%) | 147 (62) | 110/173(64) | 37/65 (57) | 0.34 |
| Remission at 12 mo, n (%) | 120/201 (60) | 87/138 (63) | 33/65 (52) | 0.09 |
| Total | Anti-TNF naive | Anti-TNF exposed | ||
| Remission at 6 mo, n (%) | 147/238 (62) | 103/142 (73) | 44/96 (46) | < 0.001 |
| Remission at 12 mo, n (%) | 120/201 (60) | 76/115 (66) | 44/86 (51) | 0.03 |
| Anti-TNF exposed | Primary LOR | Secondary LOR | ||
| Remission at 6 mo, n (%) | 44/96 (46) | 16/42 (38) | 28/54 (52) | 0.18 |
| Remission at 12 mo, n (%) | 44/86 (51) | 17/38 (44) | 27/48 (56) | 0.28 |
VDZ: Vedolizumab; TNF: Tumor necrosis factor; Primary LOR: Primary loss of response to anti-TNF agent; Secondary LOR: Secondary loss of response to anti-TNF agent.
Of the 15 patients who were in remission at the start of VDZ, where the indication was side effects to anti-TNF agents, 66% (n = 10/15) at 3 mo, 58% (n = 7/12) at 6 mo, 50% (n = 4/8) at 1 year were still in clinical remission.
Endoscopy
A total of 108 patients had endoscopy at 6 mo, 78 in Australia and 30 in Oxford. Of the Oxford patients, 43% (13/30) had an UCEIS of ≤ 1 indicating endoscopic remission. Of the Australian patients, 69% (54/78) had an MES of 0-1 indicating endoscopic remission. A significantly greater percentage of patients achieved endoscopic remission in Australia compared to Oxford (P = 0.01). A MES score of 0 was achieved in 31% (24/78) of the Australian cohort. A MES ≥ 2 was reported in 31% (n = 24) of Australian patients and a UCEIS ≥ 2 was reported in 57% (n = 17) of Oxford patients.
Time to remission
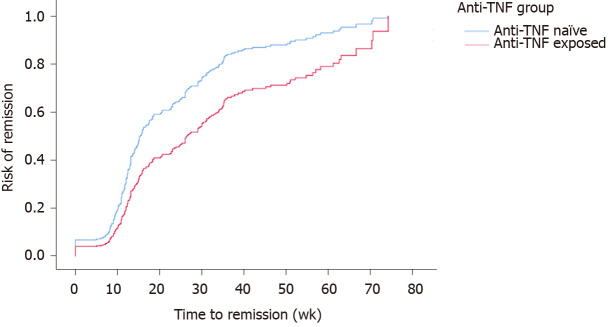
A total of 224 (73.9%) patients were censored as being in “remission”. While controlling for the site, univariate cox regression models for time to remission found no significant associations for gender (P = 0.3), disease duration (P = 0.6), smoking status (P = 0.9), age at which VDZ was given (P = 0.7), immunomodulator exposure (P = 0.8) and these were also not significant when considered with anti-TNF exposure. The final model included anti-TNF exposure and the site with the Log Rank (Mantel-Cox) reporting a significant difference (P < 0.001) between time-to-remission for anti-TNF exposure, with anti-TNF naïve patients 1.8 times more likely to achieve clinical remission (95%CI: 1.3-2.3) (Figure 2).
Figure 2.
Cumulative remission rate of anti-tumor necrosis factor naïve patients to vedolizumab therapy (vs anti-tumor necrosis factor exposed patients, P < 0.001).
Time to failure
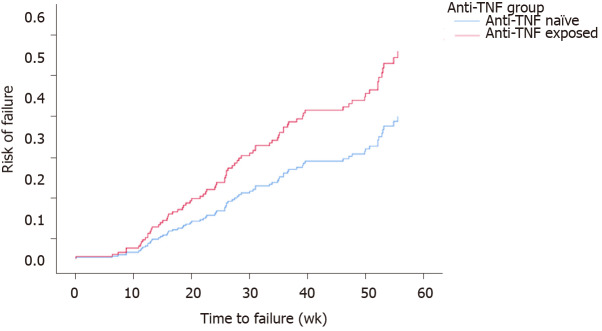
A total of 84 (27.7%) patients were censored as ‘failure’. Controlling for site, univariate cox regression models for time-to-failure found no significant associations for gender (P = 0.4), smoking status (P = 0.3), age at which VDZ was given (P = 0.9), immunomodulator exposure (P = 0.2). These factors remained not significant when considered with anti-TNF exposure. Disease duration was significant (OR = 0.95, 95%CI: 0.93-1.00 P = 0.048), however, was no longer significant when considered with anti-TNF exposure. The final model included anti-TNF exposure and site with the Log Rank (Mantel-Cox) reporting significant difference (P = 0.011) between time-to-failure for anti-TNF groups with anti-TNF exposure patients 1.8 times more likely to lose response (95%CI: 1.16-2.75) (Figure 3).
Figure 3.
Cumulative loss of response of anti-tumor necrosis factor exposed patients to Vedolizumab therapy (vs anti-tumor necrosis factor naïve patients, P = 0.011).
Safety
The tolerability of VDZ was high with only 8% (n = 25) of all patients reporting an adverse event. Infections (7%, n = 21) were by far the most common adverse event. Two patients reported a serious infection, one was Haemophagocytic syndrome due to Cytomegalovirus and another was from Klebsiella sepsis, both from Australia and both patients were on dual immunosuppression thorough out the study period. A total of 9 patients who received VDZ reported respiratory complications of whom 4 patients reported sinusitis, 2 patients an upper respiratory tract infection, one patient each of nasopharyngitis, pharyngitis and pneumonia. Gastrointestinal infections were reported in 8 patients. Clostridium difficile was the most common gastrointestinal infection (4 patients) followed by Strongyloides (one patient), Campylobacter (one patient), and Salmonella (one patient). A buttock abscess was reported in one patient. Oral thrush was reported in an Oxford patient was attributed to VDZ use. The other complications due to VDZ use reported in our cohort during the study period include rash in one patient, delayed hypersensitivity in one patient and arthralgia and headaches in another patient (Table 4). No deaths were attributed to VDZ use.
Table 4.
Complications of vedolizumab therapy
| Complication | Australia (n = 20) | Oxford (n = 5) |
| Respiratory infections | URTI (2) | Pneumonia (1) |
| Sinusitis (4) | Pharyngitis (1) | |
| Nasopharyngitis (1) | ||
| Gastrointestinal Infections | Strongyloidis (1) | Gastroenteritis (1) |
| Clostridium difficile (4) | Buttock abscess (1) | |
| Campylobacter (1) | Oral Thrush (1) | |
| Salmonella (1) | ||
| Serious infections | Haemophagocytic syndrome due to CMV (1) | NA |
| Klebsiella (1) | ||
| Others | Rash (1) | NA |
| Delayed hypersensitivity reaction (1) | ||
| Arthralgia and Headaches (1) |
VDZ: Vedolizumab; URTI: Upper respiratory tract infection; NA: Not applicable.
A colectomy was undertaken in 11% (n = 32) patients by 12 mo. No significant difference (P = 0.25) in the number of patients requiring colectomy in Australia (n = 19/210) and Oxford (n = 13/93) was observed. Anti-TNF exposed patients (23/121) were more likely to require colectomy compared to anti-TNF naïve (9/182) by 12 mo (P = 0.0005) but when patients with primary and secondary LOR to anti-TNF agents were compared, no significant difference was noted (Table 5).
Table 5.
Colectomy at 12 mo of vedolizumab therapy
| Colectomy, n | Australia | Oxford | P value |
| 19/210 | 13/93 | 0.25 | |
| Colectomy, n | Anti-TNF naive | Anti-TNF exposed | P value |
| 9/182 | 23/121 | 0.0005 | |
| Colectomy, n | Primary LOS | Secondary LOS | P value |
| 10/45 | 23/61 | 0.795 |
VDZ: Vedolizumab; TNF: Tumor necrosis factor; Primary LOR: Primary loss of response; Secondary LOR: Secondary loss of response.
DISCUSSION
In this study of 303 UC patients treated with VDZ from 15 specialist IBD centers in two countries, VDZ was noted to be both safe and effective. This study, and the GETAID studies[9], are the only studies where VDZ data is collected from multiple centers encompassing two countries, thus effectively reducing physician, site and country biases.
The key findings in this cohort were that the week 12 response in UC was 79% while remission rates were 56%, 62% and 60% at 3, 6 and 12 mo respectively. No differences were observed between the two countries in achieving remission at all time points. Anti-TNF-exposed patients, however, were almost twice as likely to lose response to VDZ compared to anti-TNF naïve patients but no difference in VDZ outcomes were observed between patients who had a primary and secondary LOR to anti-TNF agents. Adverse events were observed in 8% of patients and 11% patients required colectomy by 12 mo.
Our results were comparable to prior work from other real world studies. A Swedish real world study observed that 64% of UC patients achieved clinical remission at the end of the follow-up period[10], while a French study demonstrated that 40.5% were in steroid-free clinic remission at week 54[11]. The discrepancy in the clinical response rates could be explained by the difference in clinical characteristics of the patients entering the study and also different clinical criteria (steroid-free remission in French study) used to assess the patients. Similarly, other real world data have shown that prior exposure to anti-TNF agents reduces VDZ effectiveness, but no difference in VDZ outcomes when the patients had either primary or secondary LOR to anti-TNF agents is in line with our findings[12]. The adverse event profile with VDZ treatment was also similar to what has been previously reported[13].
Mucosal healing in UC is associated with improved long-term clinical outcomes and STRIDE guidelines identified it as a therapeutic goal[1]. With MES as an endpoint, we report mucosal healing rates of 69% in Australian Cohort at 6 mo compared to 50% in ACT1, 46% in ACT2 at week 30 with Infliximab[14], 59% in PURSUIT at week 30 with Golimumab[15] , 25% in ULTRA2 at week 52 with adalimumab[16], 51% in GEMINI1 with VDZ at week 52[5].The high mucosal healing rates observed in our study could be due to high concomitant immunomodulator use in Australia(64%), however further prospective studies are needed to prove the role of immunomodulator use with VDZ.
We chose week 12 to assess the response and remission, in contrast to GEMINI 1 time of assessment for response at week 6[5]. This was done in accordance with Australian PBS criteria, which stipulates that patients must be in remission after induction at clinic review before applying for maintenance VDZ. It appears, however, that the full effect of VDZ may take longer than 12 wk as a longer duration of treatment is associated with a higher rate remission at 6 (62%) than at 3 mo (56%). This study thus suggests that the PBS funding criteria may need to be re-attended to benefit the patients. Compared to its predecessor Natalizumab, an alpha4 antagonist, VDZ is a more selective integrin antagonist blocking only alpha4beta7 and thus does not effect lymphocyte trafficking to central nervous system, thereby theoretically eliminating the risk of progressive multifocal leukoencephalopathy (PML), a catastrophic side effect of Natalizumab[17]. No case of PML occurred in our study or any other previous studies with VDZ[18,19].
More Australian patients compared to Oxford patients achieved clinical response at 3 mo (83% vs 70% P = 0.01) and endoscopic remission at 6 mo (69% vs 43% P = 0.01). This may be due to more patients on concomitant immunomodulation in Australia compared to Oxford (64% vs 43% at VDZ initiation). Further analysis of our data and more prospective studies need to be done to define the role of concomitant immunomodulation with VDZ.
Our observed rate of VDZ efficacy at 12 mo in UC (60%) was comparable to the rates reported with anti-TNF agents[20,21]. While there are no head to head randomized control trials comparing VDZ and infliximab in UC, VDZ showed a significantly better durable clinical response (OR = 3.18, 95%CI: 1.14-9.20) and clinical remission (OR = 2.93, 95%CI: 1.03-8.28) when compared to infliximab in a network meta-analysis[22]. With no major safety concerns[23], in the treatment algorithm ladder of UC, we argue that VDZ should be considered as the first biologic when conventional treatments fail due to its gut selectivity. This is most relevant in patients at high risk of serious infections such as the elderly, those with chronic obstructive airway disease or cardiac disease. If cost allows, it should even be considered before conventional treatment such as AZA due to the same feature and with more and more biosimilars reducing the cost of biologics we may see this in future.
VDZ is also an attractive option in patients who have failed prior anti-TNF agent. Anti-TNF therapy is effective for the treatment of moderate to severe UC, however a significant proportion of patients either fail to respond to anti-TNF therapy termed or lose response with time[24]. Second and third anti-TNF agents can be used in such patients, however, it is a game of diminishing returns, as Golimumab efficacy data has shown that clinical response diminishes with each subsequent anti-TNF agent[25]. Rather than giving another anti-TNF agent, VDZ provides a unique mechanism of action with gut selectivity and less side effects. VDZ does work in anti-TNF refractory IBD patients[26] and our study supports this with 51% of TNF exposed patients achieving remission at 12 mo with VDZ therapy.
There are several limitations to our study, the most significant of which is retrospective review of the data (although the data in Australia pertaining to each VDZ application to PBS was collected prospectively). Different endoscopic assessment scores were used in Australia and United Kingdom, although a significant correlation was found between the two scores in a recent study[27]. There was consistency, however, in the clinical and endoscopic outcomes across the institutions. One another limitation is we did not report the number of patients who were steroid free and on immunomodulators at different time points in our study although our aim is to look at that in future by obtaining further information from the centers.
As more and more biologic agents become available for the treatment of IBD, the role of VDZ needs to be defined. Real-world data is important in developing treatment algorithms, which will ultimately help physicians make important treatment decisions in complex IBD patients. This study has shown that VDZ is safe and effective in achieving clinical and endoscopic remission in UC patients.
ARTICLE HIGHLIGHTS
Research background
Vedolizumab (VDZ) is a gut selective anti-integrin used for treatment of ulcerative colitis (UC). Evidence needed to assess it efficacy and safety in a real world setting.
Research motivation
Efficacy and safety of VDZ needs to be assessed, involving multiple inflammatory bowel disease (IBD) centers from two countries to reduce physician, site and country biases.
Research objectives
In this real world study, we aim to assess the clinical response, clinical, endoscopic remission and the factors influencing them in UC patients from Australia and Oxford in United Kingdom treated with VDZ.
Research methods
Retrospective review of prospectively entered patient database, treated with VDZ. Three hundred and three UC patients from 14 Australian centers and Oxford (United Kingdom) were included. Clinical response and remission was assessed at 3, 6 and 12 mo using Mayo score across all centers. Endoscopic remission was assessed at 6 mo using mayo endoscopic score in Australia and ulcerative colitis endoscopic score of severity score in Oxford. Cox regression models and Kaplan Meier curves were performed to assess the time to remission, time to failure and the covariates influencing them. Safety was assessed through adverse event reporting.
Research results
Clinical response for all patients was 79% at 3 mo and number of patients achieving clinical remission increased from 3 mo (56%) to 6 mo (62%) and remained almost stable at 12 mo (60%). No significant difference was observed between the two countries in achieving clinical remission at all points and a significantly greater proportion of Australian patients achieved mucosal healing compared to Oxford, which could be due to more patients using concomitant immunomodulation in Australia. Anti-tumor necrosis factor (anti-TNF) exposed patients were almost twice more likely to lose response to VDZ compared to anti-TNF naïve patients but no difference in outcomes were observed between patients who had a primary and secondary loss of response to anti-TNF agents. The role of concomitant immunomodulation in achieving above outcomes need to be elucidated in future prospective studies.
Research conclusions
VDZ can be safely and effectively used to treat UC patients in a real world setting. However patients who had prior anti-TNF therapy were more likely to fail compared to anti-TNF naïve patients.
Research perspective
VDZ use was reviewed in real world setting involving multiple IBD centers from two countries. This study helps physicians find VDZ its place in the treatment algorithm of complex IBD patient management. Future prospective studies are needed to evaluate the benefit of using concomitant immunomodulation with VDZ.
ACKNOWLEDGEMENTS
Australia New Zealand inflammatory bowel disease consortium Australian centers involved in the study include St John of God Hospital Subiaco (Perth), Mater Hospital (Brisbane), Eastern Health (Melbourne), Fiona Stanley Hospital (Perth), Austin Health (Melbourne), QIMR Berghofer Medical Research Institute (Brisbane), St Vincent’s Hospital (Melbourne), Northern Health (Melbourne), The Alfred Hospital, (Melbourne), St Vincent’s Hospital (Sydney), Flinders Medical Centre (Adelaide), Liverpool Hospital (Sydney), Royal Adelaide Hospital & University of Adelaide (Adelaide), Townsville Hospital (Townsville). John Radcliffe, Oxford (United Kingdom) also contributed for the study.
Footnotes
Institutional review board statement: This study comes under the investigation into natural history of inflammatory bowel disease approved by South Metropolitan area health service.
Informed consent statement: Patients were not required to give informed consent for the study because the data obtained and used for analysis was anonymous.
Conflict-of-interest statement: All authors declare no conflict of interest related to this article.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: April 4, 2020
First decision: April 26, 2020
Article in press: July 30, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zaltman C S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
Contributor Information
Samba Siva Reddy Pulusu, Centre for Inflammatory Bowel Diseases, St John of God Hospital, Subiaco 6008, Western Australia, Australia.
Ashish Srinivasan, Translational Gastroenterology Unit, NIHR Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Oxford OX3 9DU, United Kingdom.
Krupa Krishnaprasad, Inflammatory Bowel Disease Research Group, Queensland institute of Medical Research, Herston 4006, Queensland, Australia.
Daniel Cheng, Department of Gastroenterology, Mater Hospital, Brisbane 4101, Queensland, Australia.
Jakob Begun, Department of Gastroenterology, Mater Hospital, South Brisbane 4101, Queensland, Australia.
Charlotte Keung, Department of Gastroenterology, Eastern Health, Box Hill 3128, Victoria, Australia.
Daniel Van Langenberg, Department of Gastroenterology, Eastern Health, Box Hill 3128, Victoria, Australia.
Lena Thin, Department of Gastroenterology, Fiona Stanley Hospital, Murdoch 6150, Western Australia, Australia.
Tamara Mogilevski, Department of Gastroenterology, Austin Health, Heidelberg 3084, Victoria, Australia.
Peter De Cruz, Department of Gastroenterology, Austin Health, Heidelberg 3084, Victoria, Australia.
Graham Radford-Smith, Department of Gastroenterology, Mater Hospital, South Brisbane 4101, Queensland, Australia.
Emma Flanagan, Department of Gastroenterology, St Vincent’s Hospital, Fitzroy 3065, Victoria, Australia.
Sally Bell, Department of Gastroenterology, St Vincent’s Hospital, Fitzroy 3065, Victoria, Australia.
Soleiman Kashkooli, Department of Gastroenterology, Northern Health, Epping 3076, Victoria, Australia.
Miles Sparrow, Department of Gastroenterology, The Alfred Hospital, Melbourne 3004, Victoria, Australia.
Simon Ghaly, Department of Gastroenterology, St Vincent’s Hospital, Darlinghurst 2010, New South Wales, Australia.
Peter Bampton, Department of Gastroenterology, Flinders Medical Centre, Bedford Park 5042, South Australia, Australia.
Elise Sawyer, Department of Gastroenterology, Liverpool Hospital, Sydney 2170, New South Wales, Australia.
Susan Connor, Department of Gastroenterology, Liverpool Hospital, Sydney 2170, New South Wales, Australia.
Quart-ul-ain Rizvi, Department of Gastroenterology, Royal Adelaide Hospital & University of Adelaide, Adelaide 5000, South Australia, Australia.
Jane M Andrews, Department of Gastroenterology, Royal Adelaide Hospital & University of Adelaide, Adelaide 5000, South Australia, Australia.
Gillian Mahy, Department of Gastroenterology, Townsville Hospital, Douglas 4814, Queensland, Australia.
Paola Chivers, Institute for Health Research, University of Notre Dame, Fremantle 6160, Western Australia, Australia.
Simon Travis, Translational Gastroenterology Unit, NIHR Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Oxford OX3 9DU, United Kingdom.
Ian Craig Lawrance, Centre for Inflammatory Bowel Diseases, St John of God Hospital, Subiaco 6008, Western Australia, Australia; School of Medicine and Pharmacology, University of Western Australia, Crawley 6009, Western Australia, Australia. ian.lawrance@uwa.edu.au.
Data sharing statement
No additional data are available.
References
- 1.Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV, Jr, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 2.Bryant RV, Burger DC, Delo J, Walsh AJ, Thomas S, von Herbay A, Buchel OC, White L, Brain O, Keshav S, Warren BF, Travis SP. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2016;65:408–414. doi: 10.1136/gutjnl-2015-309598. [DOI] [PubMed] [Google Scholar]
- 3.Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008;14:354–377. doi: 10.3748/wjg.14.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targownik LE, Bernstein CN. Infectious and malignant complications of TNF inhibitor therapy in IBD. Am J Gastroenterol. 2013;108:1835–1842, quiz 1843. doi: 10.1038/ajg.2013.294. [DOI] [PubMed] [Google Scholar]
- 5.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 6.Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002–7; quiz e78. doi: 10.1016/j.cgh.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J, European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR] ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 8.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amiot A, Serrero M, Peyrin-Biroulet L, Filippi J, Pariente B, Roblin X, Buisson A, Stefanescu C, Trang-Poisson C, Altwegg R, Marteau P, Vaysse T, Bourrier A, Nancey S, Laharie D, Allez M, Savoye G, Moreau J, Vuitton L, Viennot S, Bouguen G, Abitbol V, Fumery M, Gagniere C, Bouhnik Y OBSERV-IBD study group, the GETAID. Three-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multi-centre cohort study. Aliment Pharmacol Ther. 2019;50:40–53. doi: 10.1111/apt.15294. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson C, Marsal J, Bergemalm D, Vigren L, Björk J, Eberhardson M, Karling P, Söderman C SWIBREG Vedolizumab Study Group, Myrelid P, Cao Y, Sjöberg D, Thörn M, Karlén P, Hertervig E, Strid H, Ludvigsson JF, Almer S, Halfvarson J. Long-term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish National Quality Registry for Inflammatory Bowel Disease (SWIBREG) Scand J Gastroenterol. 2017;52:722–729. doi: 10.1080/00365521.2017.1304987. [DOI] [PubMed] [Google Scholar]
- 11.Amiot A, Serrero M, Peyrin-Biroulet L, Filippi J, Pariente B, Roblin X, Buisson A, Stefanescu C, Trang-Poisson C, Altwegg R, Marteau P, Vaysse T, Bourrier A, Nancey S, Laharie D, Allez M, Savoye G, Moreau J, Vuitton L, Viennot S, Aubourg A, Pelletier AL, Bouguen G, Abitbol V, Gagniere C, Bouhnik Y, OBSERV-IBD study group and the GETAID. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther. 2017;46:310–321. doi: 10.1111/apt.14167. [DOI] [PubMed] [Google Scholar]
- 12.Shmidt E, Kochhar G, Hartke J, Chilukuri P, Meserve J, Chaudrey K, Koliani-Pace JL, Hirten R, Faleck D, Barocas M, Luo M, Lasch K, Boland BS, Singh S, Vande Casteele N, Sagi SV, Fischer M, Chang S, Bohm M, Lukin D, Sultan K, Swaminath A, Hudesman D, Gupta N, Kane S, Loftus EV, Jr, Sandborn WJ, Siegel CA, Sands BE, Colombel JF, Shen B, Dulai PS. Predictors and Management of Loss of Response to Vedolizumab in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24:2461–2467. doi: 10.1093/ibd/izy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loftus EV, Jr, Colombel JF, Feagan BG, Vermeire S, Sandborn WJ, Sands BE, Danese S, D'Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I, McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Ulcerative Colitis. J Crohns Colitis. 2017;11:400–411. doi: 10.1093/ecco-jcc/jjw177. [DOI] [PubMed] [Google Scholar]
- 14.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W, Gibson PR, Collins J, Järnerot G, Rutgeerts P PURSUIT-Maintenance Study Group. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96–109.e1. doi: 10.1053/j.gastro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–65.e1-3. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 18.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Dubé R, Cohen A, Steinhart AH, Landau S, Aguzzi RA, Fox IH, Vandervoort MK. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 19.Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, Ponich T, Fox I, Feagan BG. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18:1470–1479. doi: 10.1002/ibd.21896. [DOI] [PubMed] [Google Scholar]
- 20.Bálint A, Farkas K, Palatka K, Lakner L, Miheller P, Rácz I, Hegede G, Vincze Á, Horváth G, Szabó A, Nagy F, Szepes Z, Gábor Z, Zsigmond F, Zsóri Á, Juhász M, Csontos Á, Szűcs M, Bor R, Milassin Á, Rutka M, Molnár T. Efficacy and Safety of Adalimumab in Ulcerative Colitis Refractory to Conventional Therapy in Routine Clinical Practice. J Crohns Colitis. 2016;10:26–30. doi: 10.1093/ecco-jcc/jjv169. [DOI] [PubMed] [Google Scholar]
- 21.Oussalah A, Evesque L, Laharie D, Roblin X, Boschetti G, Nancey S, Filippi J, Flourié B, Hebuterne X, Bigard MA, Peyrin-Biroulet L. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol. 2010;105:2617–2625. doi: 10.1038/ajg.2010.345. [DOI] [PubMed] [Google Scholar]
- 22.Vickers AD, Ainsworth C, Mody R, Bergman A, Ling CS, Medjedovic J, Smyth M. Systematic Review with Network Meta-Analysis: Comparative Efficacy of Biologics in the Treatment of Moderately to Severely Active Ulcerative Colitis. PLoS One. 2016;11:e0165435. doi: 10.1371/journal.pone.0165435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3–15. doi: 10.1111/apt.14075. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Horin S. Loss of response to anti-tumor necrosis factors: what is the next step? Dig Dis. 2014;32:384–388. doi: 10.1159/000358142. [DOI] [PubMed] [Google Scholar]
- 25.Taxonera C, Rodríguez C, Bertoletti F, Menchén L, Arribas J, Sierra M, Arias L, Martínez-Montiel P, Juan A, Iglesias E, Algaba A, Manceñido N, Rivero M, Barreiro-de Acosta M, López-Serrano P, Argüelles-Arias F, Gutierrez A, Busquets D, Gisbert JP, Olivares D, Calvo M, Alba C Collaborators. Clinical Outcomes of Golimumab as First, Second or Third Anti-TNF Agent in Patients with Moderate-to-Severe Ulcerative Colitis. Inflamm Bowel Dis. 2017;23:1394–1402. doi: 10.1097/MIB.0000000000001144. [DOI] [PubMed] [Google Scholar]
- 26.Shelton E, Allegretti JR, Stevens B, Lucci M, Khalili H, Nguyen DD, Sauk J, Giallourakis C, Garber J, Hamilton MJ, Tomczak M, Makrauer F, Burakoff RB, Levine J, de Silva P, Friedman S, Ananthakrishnan A, Korzenik JR, Yajnik V. Efficacy of Vedolizumab as Induction Therapy in Refractory IBD Patients: A Multicenter Cohort. Inflamm Bowel Dis. 2015;21:2879–2885. doi: 10.1097/MIB.0000000000000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie T, Zhang T, Ding C, Dai X, Li Y, Guo Z, Wei Y, Gong J, Zhu W, Li J. Ulcerative Colitis Endoscopic Index of Severity (UCEIS) versus Mayo Endoscopic Score (MES) in guiding the need for colectomy in patients with acute severe colitis. Gastroenterol Rep (Oxf) 2018;6:38–44. doi: 10.1093/gastro/gox016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.