Abstract
The human coronavirus disease (COVID-19) is now a global pandemic. Social distancing, hand hygiene and the use of personal protective equipment dominate the current fight against COVID-19. In developing countries, the need for clean water provision, sanitation and hygiene has only received limited attention. The current perspective examines the latest evidence on the occurrence, persistence and faecal-oral transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the etiological agent causing COVID-19. Evidence shows that SARS-CoV-2 proliferate in the human gastrointestinal system, and is shed via faeces. SARS-CoV-2 can survive and remain viable for up to 6 to 9 days on surfaces. Recent wastewater-based epidemiological studies from several countries also detected SARS-CoV-2 RNA in raw wastewaters. Shell disorder analysis shows that SARS-CoV-2 has a rigid outer shell conferring resilience, and a low shell disorder conferring moderate potential for faecal-oral transmission. Taken together, these findings point to potential faecal-oral transmission of SARS-CoV-2, which may partly explain its rapid transmission. Three potential mechanisms may account for SARS-CoV-2 faecal-oral transmission: (1) untreated contaminated drinking water, (2) raw and poorly cooked marine and aquatic foods from contaminated sources, (3) raw wastewater-based vegetatble production systems (e.g., salads) and aquaculture, and (4) vector-mediated transmission from faecal sources to foods, particularly those from open markets and street vending. SARS-CoV-2 faecal-oral transmission could be particularly high in developing countries due to several risk factors, including; (1) poor drinking water, wastewater and sanitation infrastructure, (2) poor hygiene and food handling practices, (3) unhygienic and rudimentary funeral practices, including home burials close to drinking water sources, and (4) poor social security and health care systems with low capacity to cope with disease outbreaks. Hence, clean drinking water provision, proper sanitation, food safety and hygiene could be critical in the current fight against COVID-19. Future research directions on COVID-19 faecal-oral transmission are highlighted.
Keywords: Coronavirus disease, Drinking water contamination, Exposure risk factors, Human gastrointestinal tract, On-site sanitation, SARS-CoV-2
Graphical abstract
1. Introduction
The coronavirus disease (COVID-19) was first reported in Wuhan, China towards the end of 2019, and has since been declared a global pandemic. According to recent World Health Organization (2020b) updates (14 August 2020), 20,730,456 confirmed COVID-19 cases and 751,154 deaths have been reported globally (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). Current efforts to stop further spread of the COVID-19 include social distancing via self-isolation and national quarantines or ‘lockdowns’, hand hygiene and the use of personal protective equipment (PPE) (WHO, 2020a). The current control measures are premised on the understanding that COVID-19 is principally transmitted from infected persons via respiratory droplets released during coughing and sneezing, and through direct contact routes with infected persons and surfaces (WHO, 2020a).
Recent evidence shows that, SARS-CoV-2, the etiological agent causing COVID-19 proliferate in the human gastrointestinal system (Wu et al., 2020a), and is shed via faeces of symptomatic or asymptomatic infected persons (Lodder and de Roda Husman, 2020; Xu et al., 2020). Subsequently, SARS-CoV-2 can be released into wastewater and on-site sanitation systems, and even via open defaecation. Once in faeces, wastewater or on-site sanitation systems, SARS-CoV-2 transmission may possibly occur via inhalation of contaminated aerosols and droplets from wastewater plumbing systems especially in densely populated residential areas (Gormley et al., 2020; Meng et al., 2020). Potential faecal-oral transmission along the human gut-wastewater-food continuum has also been suggested (Ding and Liang, 2020; Goh et al., 2020b; Gu et al., 2020; Heller et al., 2020; Hindson, 2020). The detection of SARS-CoV-2 RNA in the human gastrointestinal system, faeces and untreated wastewater points to potential COVID-19 faecal-oral transmission (Ahmed et al., 2020; Randazzo et al., 2020; Wu et al., 2020a; Lodder and de Roda Husman, 2020; Xiao et al., 2020; Yeo et al., 2020). A second line of recent evidence is drawn from a series of studies based on shell disorder analysis, showing that SARS-CoV-2 has a rigid shell and low shell disorder, hence is susceptible to faecal-oral transmission (Goh et al., 2019a, Goh et al., 2019b, Goh et al., 2020a, Goh et al., 2020b). Accordingly, the faecal-oral transmission route has recently attracted significant research attention as a potential second transmission mechanism (Heller et al., 2020; Hindson, 2020; Yeo et al., 2020), but epidemiological evidence confirming this hypothesis is still lacking. The existence of multiple COVID-19 transmission mechanisms may account for the rapid spread of the pandemic.
Earlier reviews, including scoping and overview articles on COVID-19 focused on the following: (1) occurrence and persistence of SARS-CoV-2 RNA in wastewaters (Ahmed et al., 2020; Carducci et al., 2018; La Rosa et al., 2020a; Naddeo and Liu, 2020), (2) faecal-oral hypothesis (Amirian, 2020; Ding and Liang, 2020; Heller et al., 2020; Hindson, 2020), and (3) generic water, sanitation and hygiene (WASH) issues (Odih et al., 2020; WHO, 2020a). This evidence is scattered in several recent articles, but comprehensive perspectives on the current evidence and its implication on the transmission and control of COVID-19 in developing countries are still limited. The few exceptions include: (1) one perspective paper discussing the opportunities and constraints associated with the application of wastewater-based epidemiology for COVID-19 surveillance in Africa (Street et al., 2020), (2) a recent communication highlighting the factors promoting the spread of COVID-19 in low-income countries, and proposed mitigation measures (Adelodun et al., 2020), and (3) a field study presenting SARS-CoV-2 RNA and human adenovirus in river water impacted by raw sewage wastewater in Ecuador (Guerrero-Latorre et al., 2020).
The lack of effective barriers for preventing the transmission of COVID-19 via wastewater, poor sanitation and hygiene practices motivates the need for an in-depth analysis of the potential for faecal-oral transmission of COVID-19 in developing countries. The faecal-oral transmission mode changes the human exposure scenario, particularly in developing countries due to several risk factors, including lack of clean drinking water, coupled with poor sanitation and hygiene. However, currently missing in literature is a comprehensive review of the faecal-oral hypothesis, and the potential implications of water, sanitation and hygiene (WASH) in the context of developing countries. Moreover, limited reviews exist discussing the potential faecal-oral pathways, and risk factors controlling human exposure to COVID-19 in developing countries. Similar to the spread of COVID-19, the scientific evidence on the characteristics, occurrence, and transmission of SARS-CoV-2 is rapidly evolving. Thus, updates and mitigation measures recommended by national and international COVID-19 response agencies may lag behind the scientific advances. In view of this, there is urgent need for a rapid synthesis and communication of perspectives on the recent scientific evidence with potential implications on practice in the fight against COVID-19. Therefore, the current perspective is timely, because the COVID-19 epicentre has now shifted to developing countries, including those in Africa, South America and the Caribbeans.
The current perspective presents a comprehensive analysis of recent evidence on the occurrence, persistence and faecal-oral transmission of SARS-CoV-2, and implications on the spread and control of COVID-19 in developing countries. This perspective posits that; (1) an increasing body of evidence exists pointing to the potential faecal-oral COVID-19 transmission route, and (2) potential human exposure risks via faecal-oral transmission could be particularly high in developing countries, hence clean water provision, and proper sanitation and waste management could be critical in the fight against COVID-19. This perspective does not intend to downplay the role of current control measures based on social distancing, hand hygiene and the use of PPE. Instead, it proposes that; (1) the potential for faecal-oral transmission warrants particular public and research attention in developing countries, and (2) lacking empirical evidence confirming the faecal-oral transmission, clean water provision, proper sanitation and waste management may need to be considered as precautionary measures to complement current efforts in the fight against COVID-19. The current perspective paper discusses the basis for these proposals particularly in developing countries.
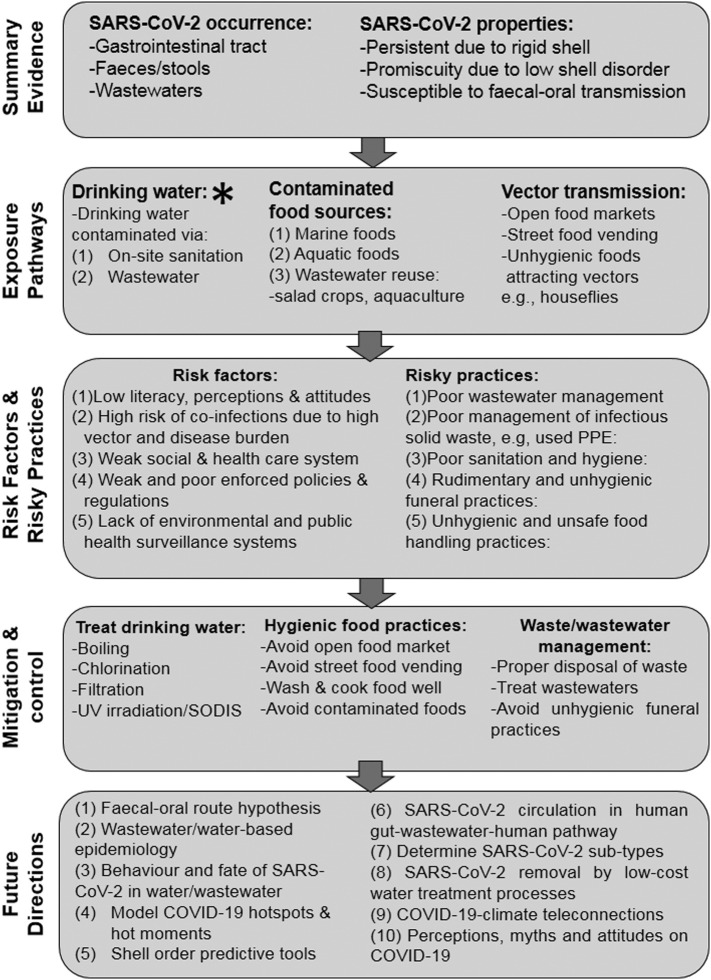
Fig. 1 presents a summary of the focus of the current perspective. First, the various lines of evidence pointing to potential faecal-oral transmission of COVID-19 are presented. Then, the potential human exposure pathways via the faecal-oral route are discussed. Risk factors and risky practices potentially predisposing human health to COVID-19 via the faecal-oral route are then highlighted. The potential critical role of clean water provision, proper sanitation, food safety and hygiene, and solid waste management systems in the fight against COVID-19 and future pandemics is discussed. Finally, key knowledge gaps pertaining to COVID-19 faecal-oral transmission in developing countries are highlighted.
Fig. 1.
Summary evidence, potential human exposure pathways, risk factors and risky practices, mitigation and research needs on faecal-oral transmission of COVID-19 in developing regions. * Transmission via contaminated drinking water is potentially more significant than the other routes.
2. Overview of methodology
To address the study objectives three sequential steps were followed: (1) searching and retrieval of articles, (2) verification of articles for relevance, and (3) review of articles and synthesis of results. Literature searches and retrieval were conducted using Boolean search techniques, which are regarded as efficient for searching and retrieving literature from a large pool in scholarly scientific databases. A detailed description of the generic search procedures is presented in recent reviews by the current author (Gwenzi, 2020a, Gwenzi, 2020c). Online scholarly databases included in the literature search were: (1) ScienceDirect®, (2) Clarivate's Web of Science®, (3) Researchgate®, (4) Scopus®, and (5) Google Scholar®, among others. The Boolean search techniques were used to search for a combination of keywords/words using, among others; ‘AND’, ‘OR’, ‘NEAR’, ‘AND NOT’, ‘NOT’ as well as truncation. Keywords representing ‘COVID-19’, ‘SARS-CoV-2’, and their variants such as ‘coronavirus 2019’ were used. These were combined with keywords corresponding to various thematic topics/objectives of the current study. For example, for ‘faecal-oral’ transmission, the following search terms and their variants were used: ‘novel’ transmission, ‘faecal-oral’, ‘drinking water’, ‘vector(s)’, ‘sanitation’ and ‘hygiene’, among others. Given the limited number of articles on faecal-oral transmission of SARS-CoV-2, the search was extended to include other human coronaviruses such as SARS and Middle East respiratory syndrome (MERS), bacteriophages and their surrogates. Note that, some non-reviewed literature, including pre-prints exists on COVID-19, but their citations were kept to a minimum in the current study. The only exceptions were five pre-prints (i.e., Danchin et al., 2020; Fears et al., 2020; Ma et al., 2020; Pastorino et al., 2020; Wikramaratna et al., 2020), which were cited and referred to as anecdotal evidence.
The retrieved articles were manually checked or verified for relevance to the study objectives. Additional literature was retrieved by manually searching the reference lists of the relevant articles. The relevant articles were then reviewed, and the key findings summarized. Given that the current study is a perspective, a qualitative analysis was conducted. A quantitative analysis based on meta-analytic and/or bibliometric methods was not feasible because no sufficient experimental data exist for such analysis.
3. COVID-19 faecal-oral hypothesis: a summary of the inferential evidence
3.1. Human gastrointestinal tract as a SARS-CoV-2 reservoir
Recent studies show that SARS-CoV-2 colonize and proliferate in the human gastrointestinal system, and persistent faecal viral shedding occur from symptomatic and asymptomatic infected persons (Fig. 1). SARS-CoV-2 RNA has been detected in faecal samples from COVID-19 patients and asymptomatic carriers (He et al., 2020; Pan et al., 2020; Woelfel et al., 2020; Xu et al., 2020; Young et al., 2020; Zhang et al., 2020b). For example, Xiao et al. (2020) showed that SARS-CoV-2 infected the rectal, duodenal and gastric epithelial cells, which in turn, release infectious SARS-CoV-2 virions into the human gastrointestinal tract.
A coronavirus virion is a complete virus particle comprising of the following: (1) several structural proteins or glycoproteins, (2) a phospholipid membrane, and (3) a viral RNA (Ashour et al., 2020; Race et al., 2020). A detailed description of the structure and functions of the various components of SARS-CoV-2 is presented in earlier papers (Ashour et al., 2020; Race et al., 2020). Briefly, the structural proteins consist of the following: (1) the spike (S), (2) membrane (M), and (3) envelope (E), while the nucleocapsid (N) protein occurs inside the virus particle, and interacts with the virus RNA (Race et al., 2020). The E protein is critical in the virus production or replication. The S glycoprotein is responsible for the formation of the spikes on the surface of viral particle, which mediate viral entry into the host cells (Race et al., 2020). The binding capacity of the coronavirus S proteins to cellular receptors is thought to be promoted by: (1) angiotensin-converting enzyme 2 (ACE2), which facilitates virus entry into host cell, and (2) serine protease enzymes (e.g., TMPRSS2 and TMPRSS4), responsible for S protein priming (Race et al., 2020; Hoffmann et al., 2020; Zang et al., 2020). Collectively, the outer structural proteins (shell) confer protein binding specificity or promiscuity, while the RNA confers infectivity or virulence (Goh et al., 2020b). As discussed later, the structural proteins, also referred to as the shell, also confer environmental stability or resilience (Goh et al., 2020a, Goh et al., 2020b).
A COVID-19 infected person sheds SARS-CoV-2 RNA for a mean period of approximately 14 to 21 days, and the magnitude of shedding ranges between 102 and 108 RNA copies per gram, but these vary among patients (Lescure et al., 2020; Pan et al., 2020; Woelfel et al., 2020). A high shedding period of 33 days was reported in one patient after respiratory samples tested negative, while faecal samples from another patient tested positive for 47 days following the first onset of symptoms (Wu et al., 2020a). Wu et al. (2020a) also report the longer shedding of SARS-CoV-2 RNA in faecal samples than in respiratory samples. Wu et al. (2020a) further report that faecal samples in their study tested positive for SARS-CoV-2 RNA for a mean of 11.2 days after respiratory tract samples became negative for SARS-CoV-2 RNA. Two inferences can be made from Wu et al.'s (2020a) findings: (1) SARS-CoV-2 could have been actively replicating in the patient's gastrointestinal tract, and/or, (2) SARS-CoV-2 persisted for a longer period in the gastrointestinal tract than in the respiratory tract. Faecal shedding of SARS-CoV-2 occurs during incubation period before manifestation of symptoms, during illness and after recovery, independent of diarrhoea and intestinal infections (Holshue et al., 2020; Woelfel et al., 2020; Wu et al., 2020a; Xiao et al., 2020; Zhang et al., 2020b). Therefore, depending on the nature of sanitation, faecal shedding may release SARS-CoV-2 RNA: (1) via open defaecation, (2) into on-site sanitation systems (i.e., pit latrines, septic tanks), and (3) into municipal wastewater systems.
SARS-CoV-2, and other coronaviruses may survive for up to 6 to 9 days on surfaces (Van Doremalen et al., 2020; Kampf et al., 2020). In one study, SARS-CoV-2 was incubated at various temperatures for up to 14 days in a virus transport medium, and then tested for infectivity (Chin et al., 2020). The results showed that the virus was still infective even on day 14 when incubated at 4 oC, but was inactivated in 5 min when incubated at 70 °C. The study also investigated the stability of the virus on various surfaces by dropping the cultured virus onto the surfaces under room temperature of 22 °C and relative humidity of 65%. The infective virus persisted longer on treated or artificial smooth surfaces specifically plastic and steel than on rough surfaces such as wood, cloth and tissue paper (Chin et al., 2020). However, the mechanisms account for the differences in stability between smooth and rough surface are unclear. Goh et al. (2020b) also showed that, unlike other coronaviruses, SARS-CoV-2 has a strange structural feature – a hard or rigid shell that confers high persistence or stability outside the human body and body fluids (Section 2). The survival times depend on viral strain and environmental conditions, including nature of surface, air humidity and temperature (Kampf et al., 2020). Survival times could be potentially longer in contaminated aquatic systems, including wastewaters due to the conducive environment and occurrence of organisms that may serve as putative alternative hosts for SARS-CoV-2. However, further research is required to determine survival times in wastewaters, possible intermediate host organisms in aquatic systems, drinking water sources and potential vectors such as houseflies.
3.2. Wastewater-based epidemiological evidence
Wastewater and on-site sanitation systems receive, and act as reservoirs of SARS-CoV-2 from multiple point and non-point sources in a catchment (Ahmed et al., 2020; Zhang et al., 2020a). These sources include; (1) faeces released via the toilet system, (2) household wastewaters from bathing of infected persons, (3) laundry wastewater from washing of infectious materials such as contaminated clothes and personal protective equipment, and (4) wastewaters from health care, autopsy and thanatopraxy/embalming facilities, including funeral homes (Gwenzi, 2020a, Gwenzi, 2020c; Zhang et al., 2020a). The occurrence of SARS-CoV-2 and other human pathogens in wastewater forms the basis for wastewater-based epidemiology (WBE). WBE is emerging field entailing surveillance of wastewater systems for the presence of pathogens including SARS-CoV-2 to gain clues on the occurrence of human infections such as COVID-19 within a catchment or community (Choi et al., 2018; Lorenzo and Picó, 2019; Ahmed et al., 2020; Daughton, 2020; Mallapaty, 2020).
An increasing body of literature drawn from developed countries including Australia, Spain, France, Netherlands, the USA, and Italy, among others, has detected SARS-CoV-2 RNA in raw wastewater systems (Ahmed et al., 2020; Lodder and de Roda Husman, 2020; Medema et al., 2020). Lodder and de Roda Husman (2020) reported the presence of SARS-CoV-2 RNA in raw wastewater samples. Moreover, the potential to use WBE for COVID-19 surveillance in Africa has been discussed in an earlier perspective (Street et al., 2020). However, unlike in developed countries, the use of diverse sanitation systems in Africa, including centralised sewer systems, and on-site sanitation systems such as pit latrines, bucket latrines and septic tanks could present significant challenges to WBE.
One WBE study conducted in Australia using reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) showed two positive detections of SARS-CoV-2 RNA in untreated wastewater within a 6-day sampling period (Ahmed et al., 2020). Using Monte Carlo simulation modelling, the same authors estimated that a median range of between 171 and 1090 infected persons occurred in the catchment. An independent study of six wastewater treatment plants in a low COVID-19 prevalence area in Murcia (Spain) detected 5.29 log genomic copies/L of SARS-CoV-2 RNA in untreated wastewaters (Randazzo et al., 2020). The same authors showed that, SARS-CoV-2 circulation among the population occurred before the first official cases were reported. This is because, the mean incubation period of SARS-CoV-2, which is the time between infection and manifestation of symptoms is about 5 days (Lauer et al., 2020). Secondary and tertiary effluents from the wastewater treatment plants using a combination of advanced water treatment processes (UV irradiation, disinfection) all tested negative for SARS-CoV-2 RNA, indicating the effectiveness of the disinfection processes used (Randazzo et al., 2020). Contrary, in Wuchang Fangcang Hospital, China, SARS-CoV-2 RNA was detected in medical wastewater from septic tanks after disinfection with 800 g/m3 sodium hypochlorite (Zhang et al., 2020a). The detection of SARS-CoV-2 RNA after chlorination of raw wastewater in septic tanks of a hospital could be attributed to the fact that free chlorine was not detected in the effluent. Thus, one may expect even higher concentrations and persistence period in raw wastewaters in on-site sanitation systems in developing countries, where no chlorination is practised. Further evidence on the occurrence of SARS-CoV-2 and other coronaviruses in the wastewater system has been reported in Paris (France) and Hong Kong, among others (Gormley et al., 2020; Lodder and de Roda Husman, 2020). Due to the interconnectedness of the wastewater system, air-borne transmission of coronaviruses via the wastewater system, including the widely reported 2003 super-spreading SARS event in Hong Kong has been reported (WHO, 2003; Gormley et al., 2020). Further studies are required to evaluate the global validity of WBE as a tool for estimating COVID-19 prevalence, especially in developing countries where severe shortages of diagnostic equipment constrain comprehensive clinical testing.
The findings of WBE are significant in our understanding of the occurrence, transmission and control of COVID-19 especially in developing countries. First, this evidence point to the fact that SARS-CoV-2 RNA occurs and persists in the wastewater system and on-site sanitation systems, which could in turn act as potential reservoirs of SARS-CoV-2. Subsequently, hydrological processes, including direct discharges, spillages, runoff/erosion, infiltration, groundwater recharge, and surface water-groundwater exchanges may disseminate SARS-CoV-2 from various faecal sources into other environmental compartments, including drinking water sources. In this regard, the untreated wastewater could play a key role in the faecal-oral transmission of SARS-CoV-2, yet this aspect has not been fully investigated. In turn, effective wastewater treatment, entailing advanced treatment processes may act as a barrier for preventing faecal-oral transmission of COVID-19. Second, the fact that SARS-CoV-2 RNA has been detected in wastewater systems ahead of official reports of COVID-19 cases indicates that WBE may act as an early warning system (Randazzo et al., 2020). Hence, WBE constitutes an under-utilized critical tool for COVID-19 surveillance especially in developing countries lacking comprehensive diagnostic systems for SARS-CoV-2 testing. In such regions, surveillance data from WBE can be used to trigger emergency preparedness and response systems.
Besides literature reporting SARS-CoV-2 RNA in wastewater, to date, only one study has documented SARS-CoV-2 in river water impacted by raw sewage wastewater in a low-income country (Guerrero-Latorre et al., 2020). Guerrero-Latorre et al. (2020) investigated the occurrence of SARS-CoV-2 at three locations along the urban rivers in Quito (Ecuador) during the peak of COVID-19 cases. In the same study, the SARS-CoV-2 and the human adenovirus, a human viral indicator were concentrated using the skimmed milk flocculation method, and subsequently assayed using the N1 and N2 target regions. SARS-CoV-2 RNA was detected in all water samples, ranging from 2,91E+05 to 3,19E+06 genomic copies (GC)/L for N1, and from 2,07E+05 to 2,22E+06 GC/L for N2. The human adenovirus was detected in high concentrations ranging between 1,13E+04 and 2,60E+05 GC/L (Guerrero-Latorre et al., 2020). The high concentration of human adenovirus is indicative of a high microbial contamination of aquatic systems via human excreta (Rusiñol et al., 2014; Guerrero-Latorre et al., 2020). The authors concluded that the presence of SARS-CoV-2 as well as water-borne pathogens (human adenovirus) river water might pose a risk of infection for downstream communities. Note that, although the title of Guerrero-Latorre et al.'s (2020) paper, and the results reported therein refer to ‘SARS-CoV-2’, the authors tested SARS-CoV-2 RNA rather than SARS-CoV-2. Distinguishing between SARS-CoV-2 RNA and SARS-CoV-2 in literature on COVID-19 is critical to avoid misleading interpretation of the research results. This is because, SARS-CoV-2 RNA is not synonymous with viable and infective SARS-CoV-2 (Section 3.3). Although SARS-CoV-2 RNA is not indicative of viable and infective SARS-CoV-2, studies detecting SARS-CoV-2 RNA in wastewater and surface water impacted by raw sewage seem to contradict and supersede World Health Organization statement that, ‘Currently, there are no studies on the survival of the COVID-19 virus in drinking-water or sewage’ (WHO, 2020a, WHO, 2020b, WHO, 2020c). Understandably, WHO updates, and even national COVID-19 mitigation responses may lag behind the rapidly evolving scientific knowledge.
Literature on the occurrence and persistence of SARS-CoV-2 and bacteriophages used as surrogates or models of coronaviruses in water and wastewater matrices has been reviewed elsewhere (Barcelo, 2020; Nghiem et al., 2020: Race et al., 2020). Recent reviews also exist on the occurrence and persistence of SARS-CoV-2 in faces, urine, raw wastewater and sewage sludge based on studies drawn from various countries (Collivignarelli et al., 2020; Kitajima et al., 2020). In summary, some studies reported that enveloped coronaviruses in wastewater are relatively short-lived, as evidenced by 3-log10 reduction in virus titre occurring within 2–3 days (Gundy et al., 2009). Other studies suggest longer survival periods in water and wastewater matrices. For example, SARS-CoV-1 survived in sewage for 14 days at 4 °C, and for 2 days at 20 °C, while its RNA was detected for 8 days, although the virus was inactive (Gundy et al., 2009; Wang et al., 2005). Studies using transmissible gastroenteritis (TGEV) and mouse hepatitis in water as surrogate coronaviruses showed that the viruses remained infectious in water and sewage for days to weeks (Rutalab et al., 2009). Specifically, at 25 oC, the time required for 99% reduction in TGEV in reagent-grade water was 22 days (Rutalab et al., 2009). Evidently, significant variations exist among the results and conclusions drawn therefrom. This partly reflects the fact that, the evidence was obtained under different environmental conditions using different viruses or their surrogates rather than SARS-CoV-2. Consequently, scientific opinion remains divided on whether or not to consider the faecal-oral transmission. On the one hand, due to this perceived rapid degradation in water, some authors suggest that human infections from water-borne coronaviruses, including SARS-CoV-2 are unlikely (e.g., Farkas et al., 2020). On the other hand, based on data suggesting longer survival periods, coupled with the positive detection of SARS-CoV-2 in stool samples, some authors suggest that faecal transmission routes should be considered (e.g., Barcelo, 2020).
Analysis of literature reporting SARS-CoV-2 in wastewaters (e.g., Ahmed et al., 2020; Medema et al., 2020; Naddeo and Liu, 2020; Wu et al., 2020a, Wu et al., 2020b) further revealed the following: (1) none of the available studies investigating the survival of coronaviruses in water and wastewater matrices were conducted in developing countries, (2) no data is available on the survival of coronaviruses in sewage and wastewaters from on-site sanitation systems common used in developing countries, and (3) evidence on the fate of SARS-CoV-2 from the time of discharge into the wastewaters to its final degradation are not available. Therefore, the survival times of SARS-CoV-2 in water and wastewater matrices in developing countries under environmentally relevant conditions is poorly understood. The current perspective posits that, the survival times reported in literature could be sufficient to allow the contamination of drinking water sources with SARS-CoV-2 and pose human health risks in developing countries especially where such drinking water sources are closely juxtaposed with on-site sanitation. Thus, the occurrence and persistence of SARS-CoV-2 in water and wastewater in developing countries require further investigation.
3.3. Shell rigidity and disorder as proxies of persistence and transmission mode
Goh and colleagues pioneered shell disorder analysis of viruses using artificial intelligence and molecular techniques to gain insights into the persistence, transmission mode and virulence of viruses and coronaviruses (Goh, 2017; Goh et al., 2015a, Goh et al., 2015b, Goh et al., 2016, Goh et al., 2019a, Goh et al., 2019b, Goh et al., 2020a, Goh et al., 2020b). Using shell disorder analysis, Goh et al. (2020b) showed that, SARS-CoV-2 is very strange and unique relative to other coronaviruses, because it has the hardest or most rigid outer shell among the coronaviruses. A hard or rigid outer shell is indicative of a resilient or persistent coronavirus outside and within the body and body fluids, while a soft shell reflects low resilience or persistence (Goh et al., 2020a, Goh et al., 2020b). SARS-CoV-2 also has the lowest shell disorder measured using percentage intrinsic disorder (PID) among the coronaviruses (Goh et al., 2020b). The degree of shell disorder is related to the mode of infection and virulence (Goh et al., 2015a, Goh et al., 2020b, Goh et al., 2016, Goh et al., 2019a, Goh et al., 2019b, Goh et al., 2020a). Low PID values typical of SARS-CoV-2 are indicative of both moderate respiratory and faecal-oral transmission potentials (Goh et al., 2020b). Higher PID values are often associated with higher infectivity via respiratory transmission, because high disorders point to greater promiscuity of the virus with respect to binding to host proteins (Goh et al., 2013; Goh, 2017). Therefore, the potential high persistence or stability of SARS-CoV-2, coupled with its low shell disorder appears to give further credence to the faecal-oral transmission hypothesis. The link between shell disorder analysis and persistence of viruses is interesting, as it points to a possibility to develop predictive tools for estimating the stability of virus in environmental media. However, further work is required to develop and validate such predictive tools.
Further evidence shows that the versatility of SARS-CoV-2 is not only limited to its persistence and transmission modes, but also the point of infection (i.e., lung versus gut). Coronaviruses are also versatile with respect to their capacity to preferentially infect the gastrointestinal (gut tropism) or the respiratory system (lung tropism) (Song et al., 2005). Some studies suggest that, the lung tropism causes severe adverse health effects, while the gut version is relatively less harmful (Holshue et al., 2020). Anecdotal evidence reported in a pre-print based on a modelling study of the COVID-19 outbreak in Wuhan City pointed to the existence of a second transmission route (Danchin et al., 2020). Although the work is not peer-reviewed, Danchin et al. (2020) showed that a model that did not account for a second propagation route based on environment-human transmission vial faecal-oral route cannot explain the Wuhan COVID-19 outbreak compared to a similar model accounting for the second transmission route. The same authors also concluded that sub-groups of SARS-CoV-2 existed, an observation which appears consistent with the existence of the lung and gut tropisms. This further suggests that coronaviruses, including SARS-CoV-2 could be amenable to multiple transmission via respiratory droplets, direct contact with contaminated surfaces and faecal-oral route. Yet several opinions may exist on the mechanisms accounting for such behaviours, thus this is subject to further research.
In summary, to this point, it is evident that: (1) SARS-CoV-2 colonizes and proliferates in the human gastrointestinal tract, (2) symptomatic and asymptomatic infected persons shed SARS-CoV-2 via faeces, and (3) SARS-CoV-2 RNA has been detected in wastewaters, and could potentially occur in on-site sanitation systems (pit latrines, septic tanks). Finally, the hard outer shell of SARS-CoV-2 and low shell disorder, point to potential persistence and moderate potential for faecal-oral transmission. Taken together, these findings suggest that faecal-oral transmission of SARS-CoV-2 cannot be totally discounted especially in developing countries due to several risk factors (Section 4.2).
3.4. A summary critique of the current evidence
3.4.1. Weak scientific evidence based on quantitative microbial risk assessment (QMRA)
The bulk of literature reporting SARS-CoV-2 in environmental and human media, including wastewaters, saliva, sputum, faeces and human gut samples rely on the detection of SARS-CoV-2 RNA (Ahmed et al., 2020; Kitajima et al., 2020). Yet consensus exists that SARS-CoV-2 RNA and even intact virions and their detection do not equate to viable and infectious SARS-CoV-2 virus. For example, WHO, 2020a, WHO, 2020b, WHO, 2020c cautions that, ‘…the detection of RNA in environmental samples based on PCR-based assays is not indicative of viable virus that could be transmissible.’ By comparison, limited studies have successfully cultured SARS-CoV-2 from such media. The lack of such studies possibly indicates the challenges associated with the culturing viruses outside their host cells. To the author's knowledge, only one study has cultured SARS-CoV-2 from human stools (Zang et al., 2020). The study showed that serine protease enzymes (TMPRSS2 and TMPRSS4) enhance the SARS-CoV-2 infection of enterocytes in the human small intestines. However, rapid inactivation of SARS-CoV-2 was observed in simulated colonic fluid. Barring the study by Zang et al. (2020), it remains unclear whether the shedding, and subsequent detection of SARS-CoV-2 RNA in both environmental (e.g., wastewaters) and human media (e.g., stools) is a true indicator of viable and infective viruses. In addition, currently, no established technique exists for estimating infectious virus particles from data on the occurrence and persistence of SARS-CoV-2 RNA in environmental media. Due to person-to-person variations, coupled with knowledge gaps in the virulence and pathogenicity of SARS-CoV-2, the quantitative estimation of the human health risks such as transmission and fatality rates from SARS-CoV-2 RNA data is even more complicated. Therefore, drawing accurate inferences of potential human health risks based on the detection of SARS-CoV-2 RNA in environmental media still presents significant challenges.
The possibility of novel transmission of SARS-CoV-2 via multiple faecal-oral route pathways further complicates the estimation of human health risks. Moreover, the potential for human exposure to viruses, including SARS-CoV-2 via bioaerosols and wastewater aerosols has been highlighted (Carducci et al., 2018; Fears et al., 2020; Kitajima et al., 2020; Meng et al., 2020). For example, anecdotal evidence based on a laboratory study investigating the persistence of SARS-CoV-2 in aerosols showed that the virus retains its viability and infectivity in aerosols for up to 16 h (Fears et al., 2020). This raises the possibility for human exposure to SARS-CoV-2 through bioaerosols and wastewater aerosols. Human exposure to SARS-CoV-2 via bioaerosols could be particularly important in crowded environments. Similarly, human exposure to SARS-CoV-2 via wastewater aerosols could be significant in shared sanitation systems especially in crowded informal settlements in developing countries (Gwenzi, 2020b). However, as Kitajima et al. (2020) pointed out, studies investigating human exposure to SARS-CoV-2 via bioaerosols and wastewater aerosols are scarce. Therefore, further research is required to better understand the human exposure and health risks associated with wastewater aerosols. This calls for further research on the development, validation and application of tools and models based on quantitative microbial risk assessment (QMRA).
QMRA relies on quantitative dose-response models or relationships between human exposure, and probability of occurrence of human health outcomes or endpoints such as infection or illness (Kitajima et al., 2020). QMRA requires data on the nature, concentrations, behaviour and fate of a biohazard (i.e., SARS-CoV-2) in environmental media as well as the multiple human exposure pathways. Yet quantitative dose-response models are not yet available for SARS-CoV-2 (Kitajima et al., 2020), while the environmental behaviour and fate of SARS-CoV-2 are still poorly understood. For example, studies investigating the environmental factors determining the persistence and fate of SARS-CoV-2 in real wastewaters and sewage sludges are still limited (Collivignarelli et al., 2020). Therefore, the development and validation of accurate predictive tools based on QMRA constitute one of the research frontiers in COVID-19. Such future research should consider both conventional and novel SARS-CoV-2 transmission pathways to gain a comprehensive understanding of the human exposure and health risks of SARS-CoV-2. QMRA tools can also be applied to address ‘what if’ mitigation scenarios, hence, could provide critical information for the development of effective COVID-19 control measures.
3.4.2. Some inconsistencies and uncertainties in current evidence
The evidence pointing to faecal-oral transmission of COVID-19 is still characterized by high uncertainties. These uncertainties are associated with knowledge gaps, untested assumptions, analytical techniques, and data inconsistencies. To some extent, such a high uncertainty is understandable, given that COVID-19 is an emerging human infectious disease characterized by several unknowns. Indeed, the high uncertainty associated with estimating human health risks arising from novel transmission has been highlighted by the UK Food Safety Agency in the case of COVID-19 transmission via cross-contaminated food (Oakenfull and Wilson, 2020). As discussed later (Section 4.2), several knowledge gaps still exist on the faecal-oral hypothesis and the associated novel transmission pathways, including, among others: (1) the validity and significance of novel transmission pathways via drinking water, shared sanitation systems, and vector and food-mediated transmission, and (2) the socio-economic and environmental settings under which such novel transmission mechanisms are valid and significant.
Uncertainties also arise from the sampling and analytical techniques (i.e., RT-qPCR) and proxy indicators (i.e., SARS-CoV-2 RNA) currently used to evaluate the occurrence and persistence of SARS-CoV-2 in environmental media. The limitations associated with current extraction and analytical methods have been discussed in a few recent studies (Kitajima et al., 2020). These limitations include: (1) low capacity of current techniques to extract viral RNA from enveloped coronaviruses such as SARS-CoV-2, and (2) the possibility of false-positive and negative results. Anecdotal evidence shows that false-negative results are more common than false-positive ones (Ma et al., 2020; Wikramaratna et al., 2020). False-negative results under-estimate the COVID-19 cases, which in turn, have significant adverse implications for the subsequent diagnosis, transmission and control of COVID-19.
Some contradictory results also exist: on the one hand, some studies suggest that SARS-CoV-2 is unstable in the environment because it has a delicate envelope (Kitajima et al., 2020; Nghiem et al., 2020). On the other hand, work by Goh et al., 2020a, Goh et al., 2020b based on shell disorder analysis suggested that SARS-CoV-2 has a very rigid shell conferring persistence. Moreover, technical updates and briefs by WHO, 2020a, WHO, 2020c suggest that ‘no evidence’ exists on the occurrence and persistence of SARS-CoV-2 in sewage, wastewater or drinking water. Yet some studies conducted in France, the USA, Italy, Netherlands, Australia and China have detected SARS-CoV-2 RNA in wastewaters (Ahmed et al., 2020; La Rosa et al., 2020b; Lodder and de Roda Husman, 2020; Medema et al., 2020; Wu et al., 2020a), even after disinfection (Zhang et al., 2020a). Moreover, while some authors point to the potential faecal-oral transmission of SARS-CoV-2 (He et al., 2020; Hindson, 2020; Wang et al., 2020; Yeo et al., 2020, Goh et al., 2020a, Goh et al., 2020b), WHO, 2020b, WHO, 2020c is of the opinion that the human health risks are low. One also wonders, if data confirmed that SARS-CoV-2 may persist on surfaces for up to 9 days (Van Doremalen et al., 2020; Kampf et al., 2020), then; (1) what prevents it from persisting in wastewaters after faecal shedding?, and (2) how long will it take for SARS-CoV-2 to undergo inactivation once released into wastewater, and what environmental factors control such inactivation? This calls for further research to understanding the persistence of SARS-CoV-2 in wastewaters and drinking water systems under environmentally relevant conditions. The current evidence is largely limited to developed countries, while corresponding data from developing nations are unavailable. This then raises the question whether or not existing evidence is universal, and representative of the situation in developing countries, where several risk factors exist (Section 3.2). Until these, and several questions are addressed, the faecal-oral transmission mechanism will remain a hypothesis that is yet to be validated. To this end, all the plausible routes that could potentially contribute to faecal-oral transmission are discussed. Subsequent in-depth research is expected to provide the evidence on their potential relative contribution to COVID-19 transmission. In view of these inconsistencies and uncertainties, it remains problematic to draw strong conclusions and recommendations. Notwithstanding these limitations, the significance and implications of the current evidence, and the need to consider precautionary measures are discussed.
4. Significance of the COVID-19 faecal-oral hypothesis in developing countries
4.1. Potential faecal-oral transmission pathways
4.1.1. Contaminated drinking water
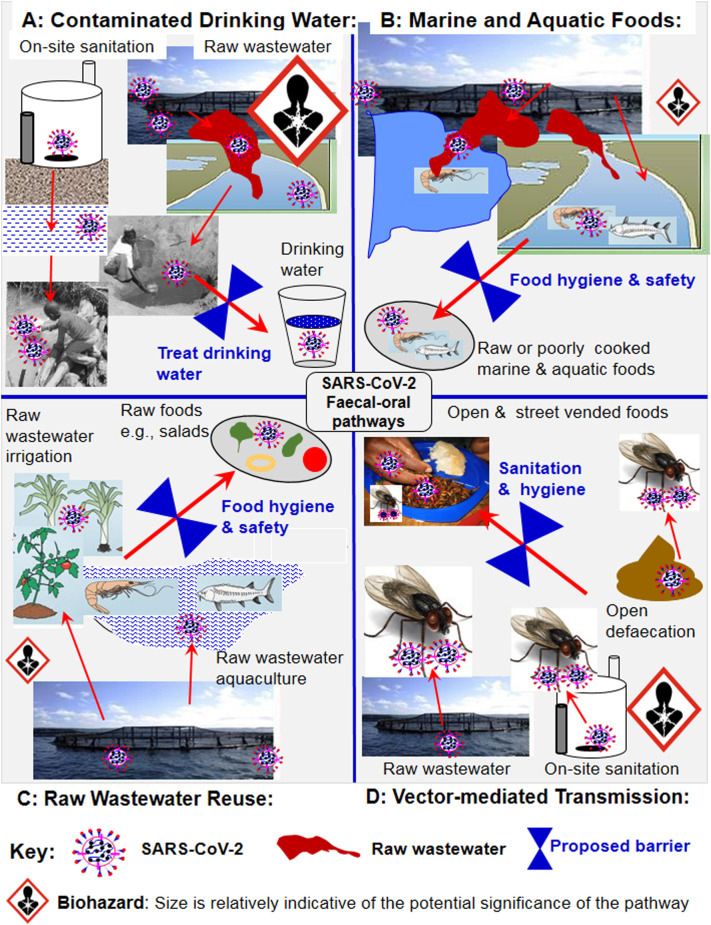
The faecal-oral route has three potential transmission sub-pathways; (1) contaminated drinking water, (2) raw and poorly cooked contaminated aquatic, marine, aquacultural and wastewater-irrigated foods (e.g., salads), and (3) vector-mediated transmission from faecal sources to foods (Heller et al., 2020; Fig. 2 ). Contamination of drinking water sources may occur via seepages from on-site sanitation systems such as pit latrines and septic tanks into surface and shallow groundwater systems serving as groundwater sources (Fig. 2). A study conducted on other coronaviruses reported that a 99.9% die-off occurred after 10 days in tap water at 23 °C and over 100 days at 4 °C (Gundy et al., 2009). This finding also suggests a longer survival time of coronaviruses in tap water than in wastewater. If these results are also valid for SARS-CoV-2, then this relatively long survival time in drinking water may increase the risk of human exposure via ingestion of contaminated drinking water.
Fig. 2.
Potential COVID-19 faecal-oral transmission pathways and proposed water, sanitation and hygiene (WASH) barriers to minimize human exposure and health risks.
The risk of contamination of drinking water via this route could be high in cases where drinking water sources are closely juxtaposed with wastewater and on-site sanitation systems. Communities with potential high risks of COVID-19 transmission include: (1) densely populated informal settlements such as squatter camps, slums and refugee camps without access to centralised drinking water systems (Corburn et al., 2020; WHO, 2020a, WHO, 2020b, WHO, 2020c), and (2) urban and peri-urban areas without reliable sources of drinking water, forcing communities to depend on unsafe surface and groundwater sources (Oswald et al., 2007). Evidence exists linking drinking water contamination via wastewater and on-site sanitation to outbreaks of human infections such as cholera and typhoid in developing countries, including those in Africa (Ahmed et al., 2011; Gwenzi and Sanganyado, 2019). However, data relating to SARS-CoV-2 are scarce, and it is currently unclear whether or not indicator and pathogenic microorganisms often detected in contaminated aquatic systems are intermediate hosts of SARS-CoV-2. Ironically, developing countries face a dilemma in this regard: (1) on the one hand, they face the highest risk of drinking water contamination via wastewater and on-site sanitation systems, but lack capacity for regular surveillance for SARS-CoV-2, and (2) on the other hand, existing wastewater and drinking water treatment systems are often dilapidated and overloaded, resulting in low treatment efficiencies. Advanced water treatment processes have high initial and operating costs, hence are rarely used in water and wastewater treatment in low-income countries. Consequently, the discharge of raw and partially treated wastewaters into aquatic systems serving as drinking water sources is common (Angassa et al., 2020).
Vulnerable and low-income communities in rural, urban, and peri-urban areas often rely on shared community water points such as wells, boreholes and community water taps (Amin et al., 2016). In such situations, social distancing is problematic due to over-crowding. For example, in Zimbabwe, informal observations showed excessive over-crowding and queues of women and children at community water points such as boreholes. Moreover, shared water sources may transmit SARS-CoV-2 via fomite or contact with contaminated surfaces such as handles of water abstraction devices. This is because, frequent and regular cleaning of water extraction devices using sanitizers and disinfectants is not feasible on community water sources. Sanitizers and PPE such as disposal gloves are often expensive and beyond the reach of low-income communities.
4.1.2. Contaminated foods
Raw and partially treated wastewater discharges may contaminate marine and surface aquatic systems serving as human food sources (Angassa et al., 2020). However, studies investigating SARS-CoV-2 contamination in the food value chain from farm to fork are still lacking. Similar to drinking water, the occurrence and persistence of SARS-CoV-2 on food warrant further investigation. Such studies should entail mimicking food contamination along the food value-chain by spiking the cultured virus or its surrogates onto the foods under environmentally relevant conditions. This will then be followed by determining the persistence and degradation kinetics of SARS-CoV-2 or its surrougates over time.
Currently, no conclusive evidence exists on whether or not aquatic organisms, including microorganisms such as faecal bacteria occurring in typical contaminated aquatic systems could act as intermediate hosts, reservoirs and vectors of SARS-CoV-2 and other coronaviruses. The only evidence available drawn from south east Asia, based on genomic and phylogenetic analysis has pointed to wild animals such as insectivorous horseshoe bats, palm civets and rodents as potential reservoirs and intermediate hosts of coronaviruses and coronavirus-like viruses (Guan et al., 2003; Lau et al., 2005; Li et al., 2005; Bennett, 2006). The potential existence of intermediate hosts for SARS-CoV-2 is plausible, but uncertainties still exist on the exact species involved, and whether such species or related ones occur in other developing regions such as Africa.
Lacking evidence, one may speculate that SARS-CoV-2 originating from raw wastewater may contaminate marine and aquatic foods such as fish and crustaceans (Fig. 2). Areas with potential risk of food transmission may include; (1) marine foods from coastal areas receiving untreated wastewater, (2) aquatic foods obtained from surface aquatic systems receiving raw or partially treated wastewaters, and (3) raw wastewater-irrigated salad crops. Other practices promoting food contamination include, (1) raw wastewater-aquacultural systems, and (2) raw wastewater irrigation of crops consumed uncooked such as salads. However, further research is required to determine the occurrence and persistence of wastewater-derived SARS-CoV-2 in marine and surface aquatic systems, and food from such sources. Such studies should also investigate the effects of various food pre-treatment and culinary methods on the persistence of SARV-CoV-2. Studies based on genomic and phylogenetic analysis are required to determine whether or not a possibility exists for SARS-CoV-2 to jump from aquatic systems to humans via aquatic and terrestrial animals and vice versa. This is particularly important given the intricate interactions between humans and wildlife, including the widespread consumption of wild aquatic and terrestrial animals (Sichewo et al., 2020). Data on environmental reservoirs and intermediate hosts of SARS-CoV-2 and other coronaviruses will provide insights into how such viruses persist and circulate in the environment between outbreaks.
4.1.3. Vector-mediated transmission
A number of vectors such as houseflies, cockroaches and rodents that frequent households and environments with faeces such as pit latrines, bucket latrines and septic tanks are well-known reservoirs and vectors of human pathogens (Akter et al., 2020; Al-Khalifa et al., 2020). Vectors may harbour viruses in their intestinal tracts and on their bodies, and contaminate human food and surfaces (Dehghani and Kassiri, 2020; Heller et al., 2020). Open defaecation, and wastewater and on-site sanitation systems such as pit latrines and septic tanks produce odours, and attract disease vectors including rodents and insects that can transmit pathogens, including SARS-CoV-2 (Fig. 2). However, no strong evidence exists on vector-transmitted SARS-CoV-2, except one review pointing to the possibility of vector-transmission of SARS-CoV-2 (Dehghani and Kassiri, 2020). Socio-economic settings and practices that could potentially promote vector transmission of COVID-19 may include; (1) communities living close to sewage, and wastewater treatment and conveyance utilities that attract rodents and insects, (2) communities relying on open defaecation, and unimproved, rudimentary and poorly designed on-site sanitation systems that allow rodents and insects to come into contact with faecal matter, and (3) open and street vending of foods, which is prevalent in developing countries. As reported for other pathogens (Songe et al., 2017), vectors may come into direct contact with faecal SARS-CoV-2 in the case of open defaecation, and in wastewater and on-site sanitation systems, and subsequently transfer SARS-CoV-2 to food (Fig. 2). However, the occurrence and transmission of COVID-19 via the vector-food-human pathway warrant further investigation focusing on households, open food markets and street vended foods in developing countries.
To date, empirical evidence validating the faecal-oral hypothesis and its associated novel transmission pathways remains scarce. In developing countries, these novel transmission mechanisms could be negligible due to effective control barriers including proper waste, wastewater and water treatment, coupled with effective food quality and safety procedures. In contrast, given the several risk factors, and lack of effective control barriers, these transmission mechanisms cannot be totally discounted in developing countries. While these novel transmission mechanisms could be currently regarded as insignificant relative to conventional transmission routes, only further research could confirm this notion. This perspective serves to stimulate further discussion and research on the subject, paying particular attention to developing countries. Such research may also provide insights into the transmission and control of future SARS.
4.2. Potential risk factors promoting COVID-19 transmission
Several potential risk factors and risky practices predisposing human health to risks in developing countries are discussed in reviews (Gwenzi, 2020a, Gwenzi, 2020c). Fig. 1 summarizes the factors and practices potentially promoting the COVID-19 faecal-oral transmission:
-
(1)
Poor wastewater management: Overloaded and dilapidated conventional wastewater treatment systems are inefficient. Moreover, infectious raw wastewaters from health care systems and the funeral industry are directly discharged into the sewer system without any pre-treatment (Gwenzi, 2020a, Gwenzi, 2020c). This contaminates marine and aquatic systems serving as drinking water and food sources.
-
(2)
Poor management of infectious solid waste: Properly designed sanitary landfills and incinerators for the disposal of hazardous materials such infectious solid waste from the health care system and funeral industry are still lacking. Instead, infectious wastes are co-mixed with general waste and co-disposed of in non-sanitary waste dumps (Patwary et al., 2011; Ali et al., 2017), thereby creating the risk of aquatic pollution via leachates and runoff.
-
(3)
Poor sanitation and hygiene: Unhygienic practices such as open defaecation, and the use of unimproved and poorly designed on-site sanitation systems such as pit latrines, bucket latrines and septic tanks are common. In most informal settlements such as slums, squatter camps and refugee camps, on-site sanitation systems may be closely juxtaposed with vulnerable drinking water sources such as wetlands, and surface and shallow groundwater systems (Potgieter et al., 2020). This is because, in such settings, the landholding per household is very small, leading to overcrowding. Thus, adherence to the following WHO recommendations is not achievable: (1) a minimum of 1.5 m depth difference between the bottom of the on-site sanitation system and the groundwater table, and (2) a minimum of 30 m horizontal distance between on-site sanitation systems and drinking water sources (Tilley et al., 2014; WHO, 2020a, WHO, 2020b, WHO, 2020c). Due to overcrowding, on-site sanitation systems are often shared among a large number of households (Heijnen et al., 2014; Simiyu et al., 2017), thereby increasing the risk of community transmission via fomites and aerosols (Caruso and Freeman, 2020). Overloading of on-site sanitation systems, leading to overflowing and spillages, and subsequent contamination of water sources may also occur.
-
(4)
Rudimentary and unhygienic funeral practices: Risky funeral practices are prevalent in poor communities, socio-cultural and religious settings, including: (i) home burials, where graves are located within homesteads close to drinking water sources (Ringane et al., 2019; Zume, 2011), (ii) cemeteries and graves located in wetlands and areas with shallow groundwater systems (Abia et al., 2018), and (iii) rudimentary earth burials of human cadavers wrapped in a blanket or cloth without a coffin (Gwenzi, 2020a, Gwenzi, 2020c). COVID-19 mass fatalities and burials may further promote such practices, thereby increasing the risk of groundwater contamination.
-
(5)
Unhygienic food handling practices: Food safety and hygiene regulations and practices are often weak and poorly enforced, hence food vending in open food markets and streets is common especially in Africa (Songe et al., 2017). For example, in Zambia, houseflies harbouring pathogenic antibiotic resistant strains of Escherichia coli and Salmonella infested and contaminated fish in food markets (Songe et al., 2017). Hence, vending of food in streets and open markets may potentially increase the risk of transmission of SARS-CoV-2 via vector-mediated food contamination.
-
(6)
Low literacy levels, attitudes and perceptions: Literacy levels are often low, resulting in limited understanding of the occurrence, transmission and health risks of human infections, including COVID-19. This is particularly true for emerging infections, because even local health care workers may have very limited understanding of the disease. Anecdotal evidence also shows that strong attitudes and perceptions may exist in some regions – for example, in Zimbabwe and possibly several African countries, a widely held notion exists that, COVID-19 only affects people of specific racial descents and regions, while native black Africans in Africa are immune to COVID-19. This belief may stem from the currently low infection cases reported in Africa compared to other regions, and also political leaders and government officials propagating the same misconception. Such attitudes and perceptions may promote COVID-19 transmission.
-
(7)
High risk of co-infections: The predominantly tropical environments in most developing regions such as sub-Saharan Africa may favour the proliferation and persistence of human disease vectors and pathogens (Barclay, 2008). High vector and disease burdens, including co-infections such as cholera, typhoid, malaria and even HIV/AIDS may predispose humans to the adverse health effects of COVID-19. Co-infections may synergistically interact with COVID-19, resulting in adverse human health outcomes (Lin et al., 2020; Park et al., 2020).
-
(8)
Weak social security and health care systems: Social security and health care systems are weak and poorly funded, thus have limited capacity to cope with large-scale outbreaks of infectious diseases including COVID-19 (Ji et al., 2020). The high infection and mortality rates associated with outbreaks of cholera and typhoid in developing countries (e.g., Zimbabwe) (Ahmed et al., 2011) clearly demonstrate that such social security and health care systems even fail to cope with outbreaks of treatable conventional or medieval diseases. In such settings, compulsory social distancing measures such as national quarantines or lockdowns may threaten, and even have adverse effects on livelihoods and food security of vulnerable communities. This may have long-lasting impacts, which may in turn, prolong or even constrain post-COVID-19 recovery.
-
(9)
Lack of surveillance systems: Systematic environmental, occupational and public health surveillance systems are either weak or non-existent. Yet such surveillance systems provide critical information for the development of comprehensive early detection systems and mitigation of COVID-19. Lacking surveillance data, responses to COVID-19 in developing countries are likely to be slow and poorly coordinated, thereby creating ideal conditions for the rapid spread of highly infectious diseases, including COVID-19.
-
(10)
Weak and poorly enforced policies and regulations: Environmental, occupational and public health policies and regulations are often weak, fragmented and poorly enforced (Gwenzi, 2020a, Gwenzi, 2020c). In addition, public health and environmental regulatory agencies, and those responsible for disaster response are often poorly funded and equipped, and lack critical expertise in emerging infectious diseases such as COVID-19. Hence, developing countries often strongly depend on the donor and international community for both resources and expertise, a scenario which may promote COVID-19 transmission and delay its control.
4.3. On the lack of research testing the faecal-oral hypothesis
The need for in-depth research to confirm whether or not the faecal-oral route contributes to the transmission of COVID-19 has been discussed in a few earlier papers (Heller et al., 2020; Hindson, 2020). However, until now, what has been missing is a comprehensive perspective discussing the hypothesis in the context of developing countries. Therefore, the current perspective addresses this gap, and adds to the growing voice calling for research to develop the scientific and empirical evidence base to confirm or dispel the faecal-oral hypothesis and its associated novel transmission pathways. Such empirical evidence will bring closure to the current speculation about the faecal-oral hypothesis. In addition, confirming or dispelling the hypothesis will provide key information on whether or not additional barriers are need to prevent novel transmission of COVID-19.
It is quite surprising and worrying that, nearly half a year after the first outbreak of COVID-19 in Wuhan in December 2019, field and laboratory studies directly investigating this hypothesis are still scarce. This is despite the fact that, the COVID-19 epicentre, as evidenced by confirmed cases and deaths, seem to have shifted to developing countries including those in Africa, South America and the Caribbeans. Thus, one may raise the question, ‘Why is the global research community, including those directly residing in developing countries in south east Asia, South America, Africa and the Caribbeans taking so long to test the faecal-oral hypothesis?’. The reasons accounting for this trend remain unclear to the author. However, one may speculate that: (1) outside China, the bulk of research on COVID-19 is drawn from developed countries in Europe and north America, where effective barriers based on advanced wastewater and water treatment make the hypothesis less relevant, and (2) developing countries, where the hypothesis could be most relevant, have weak research systems, characterized by severe lack of funding, research expertise and infrastructure such as analytical equipment. Finally, it appears the global research community, policy makers and practitioners particularly those in developing countries were caught by surprise and highly unprepared for the COVID-19 outbreak. Hence, the limited available resources, including funding, diagnostic equipment and personnel seem to focus on and prioritize the control of further transmission based on well-established transmission modes rather than investigating novel ones proposed here. Due to lack of evidence, such novel transmission mechanisms could be regarded as less significant compared to conventional transmission modes via respiratory droplets and direct contact. Although this notion could be valid for policy makers and practitioners, the current perspectiveargues that, the global research community, regional and national research institutions, think-tanks in public health, and universities should play their role by providing evidence to inform policy and practice.
In its simplest form, validating the faecal-oral hypothesis entails testing for SARS-CoV-2 RNA in drinking water along the source, storage and distribution systems, including at the point of human consumption. Currently, no compelling data exist to enable the evaluation of the relative significance of the various potential faecal-oral pathways to COVID-19 transmission. However, based on experiential and inferential evidence, priority should be given to transmission via contaminated drinking water (Fig. 1). This is because, compared to vector and food-mediated transmission, faecal contamination of drinking water sources is well-documented in developing countries (Ahmed et al., 2011). Moreover, a significant population in developing countries also relies on untreated water from unsafe sources, creating opportunities for potential human exposure. Although not directly related to COVID-19, evidence also exists linking faecal contamination of drinking water to human health risks including recurrent outbreaks of diarrhoeal infections, cholera and typhoid (Ahmed et al., 2011; Gwenzi and Sanganyado, 2019). Such research should also target and prioritize areas associated with putative faecal contamination such as informal settlements including slums, squatter camps and refugee camps. Communities in such informal settlements often rely on untreated raw water abstracted from shared and unsafe drinking water sources, which are often closely juxtaposed with on-site sanitation systems (Pujari et al., 2012: Potgieter et al., 2020). Developing countries with recurrent outbreaks of human infections transmitted via the faecal-oral route such as cholera and typhoid could constitute the ideal settings or ‘worst-case scenario’ to investigate the faecal-oral hypothesis.
5. Clean water, sanitation and waste management as potential critical control points
The summary evidence on the occurrence of SARS-CoV-2 in human gastrointestinal system, faeces and wastewater systems, and the potential faecal-oral transmission of COVID-19 have potential implications on the control of COVID-19 especially in developing countries. Clearly, the faecal-oral transmission route, is not a single pathway, but a set of multiple sub-pathways, including, drinking water, contaminated food and vector-mediated processes (Fig. 2). The current perspective investigated whether or not clean water provision, sanitation and waste management are critical control points in the fight of COVID-19 in developing countries. Fig. 1 and the following discussion partly address this question. However, evidence on the faecal-oral hypothesis remains weak and inconclusive, hence making strong recommendations based on weak evidence and uncertainty is problematic. Therefore, lacking strong evidence, the control measures premised on the faecal-oral transmission hypothesis summarized here should be considered as precautionary measures. These measures seek to complement, rather than replace social distancing, hand hygiene and the use of PPE, which are premised on COVID-19 transmission via respiratory droplets and direct contact routes.
5.1. Proper wastewater and sanitation systems
The fact that wastewater and on-sanitation systems are potential reservoirs of SARS-CoV-2, and the concept of wastewater-based epidemiology were discussed. Moreover, the dissemination of SARS-CoV-2 from faeces (in the case of open defaecation), wastewaters and on-site sanitation systems via hydrological processes may contaminate marine and aquatic systems serving as drinking water and food sources. Therefore, wastewater and sanitation-based barriers could be critical precautionary measures in preventing faecal-oral transmission arising from poor wastewater and sanitation (Fig. 2). This include, proper sanitation entailing; (1) stopping open defaecation, and (2) safe and hygienic disposal of human excreta in properly sited and designed on-site sanitation or sewer systems. Proper wastewater management practices include: (1) stopping the discharge of raw or partially treated wastewaters into marine and aquatic systems, and (2) advanced wastewater treatment using ultra-violet irradiation and disinfection to effectively inactivate SARS-CoV-2 (Randazzo et al., 2020). These control measures demonstrate the criticality of improved sanitation and wastewater treatment and disposal as potential barriers against faecal-oral transmission of COVID-19.
5.2. Clean drinking water provision
The risk factors and risky practices contributing to contamination of drinking water sources, and communities most likely to be at risk were highlighted. Based on this understanding, potential precautionary barriers to prevent transmission via contaminated drinking include, reliable supply of clean and regularly tested drinking water via: (1) centralised drinking water systems, where available, and/or (2) mobile systems such as water bowsers in the case of unconnected communities close to centralised drinking water systems. In the absence of centralised drinking water treatment systems, drinking water disinfection is recommended in communities with potential risk of transmission, where contamination is either suspected or confirmed (Fig. 2).
A number of generic guidelines exist on drinking water treatment in the case of disease outbreaks, and recent updates are also available (WHO, 2020a). Specifically, the World Health Organization recommends: (1) boiling, (2) high-performing ultrafiltration, and (3) nanomembrane filtration. Solar disinfection (SODIS), ultra-violet (UV) irradiation and chlorination using an appropriate dosage of free chlorine are recommended for non-turbid waters (WHO, 2019). However, one study detecting SARS-CoV-2 RNA in wastewater following chlorination with 800 g/m3 sodium hypochlorite (Zhang et al., 2020a) raises concerns about the effectiveness of conventional dosages of chlorination. Notably, current recommendations are generic and largely based on inferential evidence for other viruses and coronaviruses. This is because data on the occurrence and removal of SARS-CoV-2 in drinking water using the stated methods are scarce. Several low-cost water treatment methods also exist, including metallic iron filters, biosand filters, and ceramic filters (with or without silver nanoparticles), but their capacity to SARS-CoV-2 is also currently unknown. An exception is boiling, where some data exist. Coronaviruses such as SARS are highly sensitive to heat, and can be inactivated by heating at 56–60 °C for 30 min (Pastorino et al., 2020; Wu et al., 2020a). Anecdotal evidence from a pre-print shows that, heating at 92 °C for 15 min was more effective in achieving a 6 log reduction in SARS-CoV-2 than lower temperatures and longer heating times of 56 °C for 30 min and 60 °C for 60 min (Pastorino et al., 2020). Thus, boiling for at least 15 min may effectively disinfect contaminated drinking water. However, barring boiling, and probably solar disinfection, the technology (e.g., filters, chlorinating agents) and know-how to install and effectively operate ultrafiltration, nanomembrane filtration, UV irradiation and chlorination may not be readily available for most vulnerable communities in developing regions. In addition, unless such technologies are provided by the donor community or government, their appropriateness in developing countries could be questioned.
5.3. Behavioural changes in hygiene, food handling and funeral practices
Several high risky practices in hygiene, food handling and preparation, and funeral practices promoting SARS-CoV-2 faecal-oral transmission were highlighted. This calls for behavioural changes, and a long-term shift in such risk practices. Specifically, this should entail: (1) safe and hygienic disposal of human cadavers, and solid waste and wastewaters from health care systems and funeral industry, (2) avoiding selling and consumption of food from open markets and street vending sources, and (3) improving food hygiene, handling and preparation practices including thorough washing and proper cooking, and (4) removing breeding grounds and conditions promoting vectors such as rodents and houseflies through regular spraying using insecticides.
5.4. Strengthening policies, regulations and institutions
Safeguarding human health against COVID-19 and future outbreaks of infectious diseases requires effective regulatory frameworks, coupled with well-funded and equipped institutions, and social security and health care systems with relevant expertise. Effective and well-coordinated policies, regulation and institutions are critical in developing resilience, and early warning and response systems in developing countries. This aspect is cross-cutting, thus extends beyond infectious diseases to include environmental pollution and the associated health risks. Earlier reviews present a detailed discussion of the need to develop better regulations, policies and institutions to safeguard human health in developing countries (Gwenzi, 2020a, Gwenzi, 2020c).
6. Looking ahead: decision scenarios and future directions
6.1. Decision scenarios under imperfect knowledge and uncertainties
The world is currently in the middle of the COVID-19 crisis, and inferential evidence summarized here and elsewhere (e.g., Heller et al., 2020; Hindson, 2020; Yeo et al., 2020) further highlights the possibility of a COVID-19 faecal-oral transmission hypothesis. This emerging evidence may require additional precautionary measures complementary to existing ones. Yet admittedly, the evidence is still fraught with several unknowns and uncertainties pertaining to COVID-19 transmission and infectivity mechanisms. Against this background, a practical question then arises: ‘Should clean water provision, and improved sanitation, hygiene, and solid waste and wastewater management programmes (hereafter referred to as WASH for brevity) be accorded similar prominence and priority as current generic mitigation measures or is it too early to call?’ This question is likely to divide public, policy and scientific opinions, but potential dominant opinions are highlighted here to stimulate further debate.
-
(1)
Scenario 1 (‘business-as-usual’)
This 'busines-as-usual' scenario entails that WASH programmes can wait, while special attention is paid to social distancing, hand hygiene and the use of PPE. Proponents of this opinion may argue that: (i) evidence on faecal-oral transmission of COVID-19 is limited and inconclusive relative to transmission via respiratory droplets and the direct contact route, and (ii) integrating WASH programmes in current mitigation strategies will stretch the limited resources, and compromise the effectiveness of the mitigation strategy. Others may also argue that, changing a mitigation strategy in the middle of a global health crisis creates confusion and causes loss of confidence, resulting in disastrous health outcomes.
-
(2)
Scenario 2 (WASH is a critical pillar in fighting COVID-19)
In this scenario, clean water provision, hygiene and sanitation (WASH) are perceived as critical pillars of the public health systems. Hence, WASH programmes should be accorded equal importance, and integrated into existing mitigation programmes. This decision scenario is consistent with the recommendations of an earlier communication targeting low-income countries (Adelodun et al., 2020). Adelodun et al. (2020) recommended the following mitigation measures: (1) decentralised wastewater treatment, (2) community-wide monitoring of SARS-CoV-2 in wastewater, (3) improved sanitation, and (4) developing point-of-use wastewater treatment devices. Those in support of this viewpoint may argue that, the health risks and impacts of COVID-19 are very high and far-reaching. Hence, in the face of imperfect knowledge, developing countries should assume the ‘worst-case scenario’ and take precautionary measures by integrating WASH to complement existing mitigation measures. Such proponents may also argue that WASH programmes have the potential to bring long-lasting and far-reaching health benefits, not only for COVID-19, but for several other human infections, including future outbreaks of similar pandemics (Hyde, 2020). In addition, strengthening the WASH pillar will complement the current control methods based on social distancing, hand hygiene and the use of PPE by reducing human interactions particularly among women and children due to their critical role in household water provision, including water fetching. Moreover, reliable clean water supplies are critical for the recommended frequent and regular hand washing (WHO, 2020c). Hence, those in support of this viewpoint may propose that additional resources need to be mobilized to support both WASH and existing social distancing, hand hygiene and the use of PPE.
-
(3)
Scenario 3 (new evidence warrants a ‘lighter’ version of WASH)
This scenario entails that more attention and resources should be paid to social distancing, hand hygiene and the use of PPE, while a ‘lighter’ version of WASH is implemented to complement existing control measures in light of the new evidence. Here, the term ‘lighter’ WASH is used relative to scenario 1, and implies promoting basic WASH practices such as proper disposal of human excreta, washing and proper cooking of food and low-cost drinking water treatment without diverting a significant portion of time, effort and resources from current mitigation efforts. Such a viewpoint will reflect the current weight of evidence, uncertainties and constraints posed by limited resources to effectively and simultaneously implement both WASH programmes and social distancing, hand hygiene and the use of PPE. Reliable water supply is also critical to achieve frequent hand washing recommended under the current generic mitigation measures. A survey of recent updates from WHO (2020a) seem to subscribe to this viewpoint. For example, WHO, 2020a, WHO, 2020b, WHO, 2020c does not explicitly recommend the implementation of dedicated WASH programmes as a COVID-19 mitigation strategy. This is probably because WHO does not currently recognize faecal-oral route as a key COVID-19 transmission, as evidenced by its conclusion that ‘no evidence’ exists on the risk of faecal-oral transmission, while the risk of contamination of drinking water supplies is ‘low’ (WHO, 2019, 2020a). Notably, these WHO updates were published before the bulk of the recent evidence presented here, including results in pre-prints were available. Thus, some may argue that, in view of the risk factors and risky practices highlighted, the validity of such statements and WHO updates in developed countries could be questionable.
The scenarios highlighted here suggest that making decisions and specific recommendations in the middle of a crisis is a non-trivial task. The fact that the scientific evidence is rapidly changing, and still characterized by several unknowns and uncertainties further complicates the decision-making process. For similar reasons, the current perspective will not attempt to provide a ‘yes’ or ‘no’ answer to the question raised, nor make specific recommendations. Rather, the decision scenarios and their merits were highlighted to stimulate further debate on, and draw attention to the topic. Experts in the epidemiology of infectious viral diseases may provide a more authoritative evaluation of the weight of evidence, and make decisive recommendations. However, whether such decisions and recommendations will be made and implemented under the current COVID-19 pandemic or future similar outbreaks remains unknown. The need to further validate the evidence, and address gaps and uncertainties is apparent.
6.2. Future research directions
Future research in developing countries should address these knowledge gaps (Fig. 1):
-
(1)
Validating the faecal-oral transmission hypothesis
Several lines of evidences pointing to the possibility of COVID-19 transmission via the faecal-oral route were highlighted, but evidence remains weak and inconclusive. Systematic epidemiological evidence are required to test this hypothesis in developing countries, where faecal contamination of water sources is well-pronounced due several risk factors. Such studies should also determine the relative contribution and significance of the various pathways (i.e., drinking water, vectors, food contamination) to faecal-oral transmission of COVID-19.
-
(2)
Wastewater and drinking water-based epidemiology
WBE may provide cues and early insights on the occurrence of COVID-19 in developing countries where analytical equipment for comprehensive clinical screening of humans is scarce. Given that wastewaters are closely coupled to drinking water systems through cross-connections and contamination, WBE can also be extended to drinking water sources. Such WBE studies can be coupled to spatial tools such as geographical information systems to map potential 'hotpots' and 'hot moments'.
-
(3)
Shell order analysis predictive tools for estimating SARS-CoV-2 persistence
Goh et al., 2020a, Goh et al., 2020b have pioneered a potentially interesting and promising tool based on shell order analysis for predicting the transmission and environmental stability of coronaviruses such as SARS-CoV-2 and other viruses. However, the predictive capacity of shell order analysis for SARS-CoV-2 and other viruses has not been fully explored. Thus, further work should investigate the following: (1) potential interactive effects between inherent shell structure of viruses and environmental conditions on the stability of viruses, and (2) validate the predictive capacity of shell order analysis for several other viruses of human health concern, including Ebola virus, Zika virus, Nipah, and HIV, among others. Once the predictive capacity of shell order analysis has been confirmed, this can act as a potential tool to estimate the transmission and environmental stability of future coronaviruses.
-
(4)
Occurrence and behaviour of SARS-CoV-2 in the tropics
The bulk of evidence on the occurrence and stability of SARS-CoV-2 is limited to developed countries, which experience predominantly temperate climates. Research is needed to investigate the occurrence, persistence and fate of SARS-CoV-2 in various environmental media in tropical environments in developing regions such as Africa. Such media should include, aquatic and marine foods, wastewater, drinking water sources, and solid wastes from potential sources such as health care facilities, funeral homes, and isolation and quarantine centres.
-
(5)
Tracking COVID-19 along the human gut-wastewater-human exposure pathway
Systematic studies are required to track the circulation of SARS-CoV-2 along the human gut-wastewater-human exposure pathways. Emerging tools such as genomics, coupled with in silico techniques and network analysis are ideal for analysis of complex networks. Such information is critical for the development of barriers to stop the transmission along the faecal-oral route.
-
(6)
Do SARS-CoV-2 in gut and lung systems represent different sub-types?
SARS-CoV-2 may preferentially colonize either the respiratory system (lung tropism) or the human gastrointestinal tract (gut tropism), resulting in different human health outcomes (Holshue et al., 2020). This raises the question, ‘Do the two tropisms reflect contrasting sources and human exposure mechanisms, and if not, what controls the differential distribution within the human body?’. Currently, it is unclear whether the lung and gut tropisms represent different SARS-CoV-2 sub-types, because RT-qPCR currently used for SARS-CoV-2 determination lack capacity to differentiate between SARS-CoV-2 sub-types. Further studies based on whole genome analysis are needed to address these knowledge gaps.
-
(7)
Removal of SARS-CoV-2 in water and wastewater treatment systems
Data on the occurrence, persistence and removal of SARS-CoV-2 in conventional and often overloaded wastewater and drinking water treatment plants in developing countries are scarce. Moreover, barring evidence on effect of heat inactivation/boiling, limited data exist on the capacity of solar disinfection, biosand filtration and ceramic filters to remove SARS-CoV-2 in contaminated drinking water. Low-cost drinking water treatment methods are commonly used by vulnerable communities in developing countries and humanitarian disaster situations. These aspects warrant further research.
-
(8)
Predicting potential COVID-19 ‘hotspots’ and ‘hot moments’
Modelling studies predominantly conducted in Asia have predicted COVID-19 outbreaks using climatic/weather data, and showed that the number of infections are negatively correlated with temperature (Shi et al., 2020; Wu et al., 2020b). The bulk of these modelling studies were conducted after outbreaks (i.e., posterior). Therefore, it will be interesting to conduct similar anterior modelling studies using historical data or proxies from remote sensing in regions currently experiencing low infection outbreaks (e.g., Africa) to test the validity and universality of such modelling approaches. Such modelling efforts should focus on predicting areas (‘hotspots’) and periods (‘hot moments’) with exceptionally high outbreaks of COVID-19. Such modelling studies may entail using a combination of tools including internet, mobile technologies, spatial tools (GIS), and emerging tools such as genomics and big data analytics (e.g., artificial intelligence).
-
(9)
Climatic teleconnections and coronavirus outbreaks
Earth system dynamics and climatic phenomena (e.g., ENSO, La Niña) are often linked to vector and viral disease outbreaks (e.g., Chikungunya, dengue) in tropical Africa, Asia and South America through teleconnections (Anyamba et al., 2019). Teleconnections occur when an earth system phenomenon triggers the outbreak of a disease at long distance away. Thus, understanding the earth system-disease inter-relationships and teleconnections with coronavirus such as COVID-19 is critical for better understanding, forecasting, prevention and control of future viral infections.
-
(10)
Understanding the human factor in COVID-19 transmission and control
Similar to other pandemics such as HIV/AIDS, perceptions, myths and attitudes among policy-makers, community and religious leaders, practitioners, and the public may play a role in the transmission and control of COVID-19. These perceptions, myths and attitudes, collectively referred to here as the ‘the human factor’, could be significant in developing countries, including those in Africa. This is due to strong socio-cultural and religious beliefs, and gender norms, which are not as strong in developed countries. Yet an in-depth understanding of the perceptions, myths and attitudes towards the transmission and control of COVID-19 is still lacking.
6.3. Testing the faecal-oral transmission hypothesis in developing regions
Epidemiological evidence is required to validate the COVID-19 faecal-oral transmission hypothesis, and address related knowledge gaps highlighted here. The formal hypothesis to guide such research can be formulated thus, ‘The faecal-oral route significantly contribute to COVID-19 transmission, and the contribution of this transmission mechanism is more pronounced in developing countries with poor water/wastewater, sanitation and health care systems and infrastructure than their developed counterparts.’ One may speculate that, if the hypothesis is true, then global hotspots of water-borne diseases particularly cholera and typhoid such as Zimbabwe could be the next epicentres of COVID-19. Conversely, if the null hypothesis is true, no peculiar patterns in COVID-19 outbreaks will be evident in global hotspots of water-borne diseases relative to other regions.
The physical location of such research presents a challenge, because conditions promoting COVID-19 faecal-oral transmission in developing regions in Africa, south-east Asia and South America are not juxtaposed with the availability of advanced research systems, which are found in developed countries. The current author argues that, research to validate the faecal-oral hypothesis, and address the several knowledge gaps highlighted here is best conducted in developing regions due to the several risk factors and practices discussed. In view of these risk factors and practices, coupled with predominantly tropical environments favouring a high vector and disease burden, developing regions can be conceptually viewed as a ‘human-dominated large-scale laboratory’ ideal for testing various hypotheses on human epidemiology. Here, the notion of viewing developing regions as ‘a human-dominated large-scale laboratory’ is not meant in any way to downplay the need to consider and address ethical, transparency, fairness, consent and cultural considerations in conducting such research. Rather, this notion is solely meant to demonstrate the appropriateness of such settings to investigate the highlighted hypotheses. This proposition resonates well with earlier suggestions by the current and other authors (Barclay, 2008; Gwenzi and Chaukura, 2018). For example, Barclay (2008) pointed out that, two regions are ideal for conducting large-scale epidemiology research on infectious disease outbreaks: (1) south-east Asia, because of the intimate co-existence and interactions between humans and animals, and (2) sub-Saharan Africa, due to the prevalence of several risk factors including the greatest burden of infectious diseases. Moreover, both regions have some of the worst social security and health care systems, weak or non-existent environmental and public health surveillance systems, and endemic poverty.
Proposals that research on the epidemiology of certain human infectious diseases is best conducted in certain geographical regions (south-east Asia and Africa in this case) are often met with strong revulsions based on long-standing mistrust and historical racial connotations. Yet in some instances, including the current one on faecal-oral transmission of COVID-19, developing regions could provide the ideal biophysical and socio-economic settings for generating the epidemiological evidence to address this hypothesis. This then creates a dilemma: on the one hand, any research on the subject initiated and coordinated by foreign researchers may face strong resistance from governments in developing countries and rights activists. On the other hand, on their own, low income countries have limited capacity to implement comprehensive research to address the knowledge gaps highlighted. This is due to severe lack of funding, research infrastructure such as analytical equipment, and expertise. In addition, research involving infective SARS-CoV-2 may require strict biosafety controls exceeding level 2, which are often lacking in developing countries. Hence, the best compromise could be collaborative projects bringing together foreign scientists, local scientists and international agencies such as WHO. In such joint projects, international scientists, developed countries and international agencies may provide the bulk of resources including funding and expertise. To ensure transparency, and build trust among the various partners, the actual research should be designed and implemented jointly by local and foreign scientists with the participation of relevant institutions and regulatory agencies in developing countries. The research outputs from such joint projects should be shared fairly, and widely disseminated. Mechanisms are also need to be developed on how any intellectual property rights, including vaccines, arising from such projects will be shared among the partners.
7. Concluding remarks
The current perspective examined recent evidence to show that SARS-CoV-2 colonize and proliferate in the human gastrointestinal system, which acts as a reservoir of SARS-CoV-2. The SARS-CoV-2 is then shed into the environmenta via open defaecation, on-site sanitation facilities, and wastewater or sewer systems via faeces. Wastewater-based epidemiological evidence confirms the occurrence and the persistence of SARS-CoV-2 RNA in wastewaters. The circulation of SARS-CoV-2 among the human population may even precede the manifestation of symptoms, and the first official reports of COVID-19 cases. This recent evidence seems to support the COVID-19 faecal-oral transmission hypothesis particularly in developing countries. The notion of faecal-oral transmission seems to be further corroborated by results of shell disorder analysis and modelling, which indicate persistence and the potential for faecal-route transmission. The faecal-oral route is not a single pathway, but rather, consists of multiple potential sub-pathways; (1) contaminated drinking water, (2) contaminated marine and aquatic foods, and (3) vector-mediated transmission from faecal sources to food. Collectively, these findings could be significant in the following respects: (1) provide new insights on the potential transmission of COVID-19, and (2) highlight the potential need for precautionary control measures in developing countries. Due to several risk factors and risky practices, the possibility for COVID-19 faecal-oral transmission and the corresponding adverse human health outcomes could be putatively higher in developing than developed countries. Several precautionary barriers to prevent faecal-oral transmission were highlighted. Specifically, clean water provision, coupled with proper sanitation, hygiene, and waste and wastewater management practices could be potential critical control points, which can complement current efforts in the fight against COVID-19. Finally, decision scenarios and future directions were highlighted.
Declaration of competing interest
The author declares no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Acknowledgements
The research received no external funding, and was solely funded by the author's personal resources. I acknowledge with thanks the comments from the two anonymous reviewers that greatly improved the manuscript and its presentation. I am also indebted to two anonymous reviwers who provided comments on an earlier version of the manuscript.
Editor: Damia Barcelo
References
- Abia A.L.K., Ubomba-Jaswa E., Schmidt C., Dippenaar M.A. Where did they come from—multi-drug resistant pathogenic Escherichia coli in a cemetery environment? Antibiotics. 2018;7(3):73. doi: 10.3390/antibiotics7030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelodun B., Ajibade F.O., Ibrahim R.G., Bakare H.O., Choi K.-S. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: any sustainable preventive measures to curtail the scourge in low-income countries? Sci. Total Environ. 2020;742:108334. doi: 10.1016/j.scitotenv.2020.140680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Bardhan P.K., Iqbal A., Mazumder R.N., Khan A.I., Islam M.S., Siddique A.K., Cravioto A. The 2008 cholera epidemic in Zimbabwe: experience of the icddr, b team in the field. J. Health Popul. Nutr. 2011;29(5):541. doi: 10.3329/jhpn.v29i5.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter S., Sabuj A.A.M., Haque Z.F., Rahman M.T., Kafi M.A., Saha S. Detection of antibiotic-resistant bacteria and their resistance genes from houseflies. Veterinary World. 2020;13(2):266. doi: 10.14202/vetworld.2020.266-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Wang W., Chaudhry N., Geng Y. Hospital waste management in developing countries: a mini review. Waste Manag. Res. 2017;35(6):581–592. doi: 10.1177/0734242X17691344. [DOI] [PubMed] [Google Scholar]
- Al-Khalifa M.S., Mashaly A.M., Al-Qahtni A.H. Insect species colonized indoor and outdoor human corpses in Riyadh, Saudi Arabia. Journal of King Saud University-Science. 2020;32(3):A1812–1817. doi: 10.1016/j.jksus.2020.01.034. [DOI] [Google Scholar]
- Amin N., Crider Y.S., Unicomb L., Das K.K., Gope P.S., Mahmud Z.H., Islam M.S., Davis J., Luby S.P., Pickering A.J. Field trial of an automated batch chlorinator system at shared water points in an urban community of Dhaka, Bangladesh. Journal of Water, Sanitation and Hygiene for Development. 2016;6(1):32–41. [Google Scholar]
- Amirian E.S. Potential fecal transmission of SARS-CoV-2: current evidence and implications for public health. Int. J. Infect. Dis. 2020;95:363–370. doi: 10.1016/j.ijid.2020.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angassa K., Leta S., Mulat W., Kloos H. Seasonal characterization of municipal wastewater and performance evaluation of a constructed wetland system in Addis Ababa, Ethiopia. International Journal of Energy and Water Resources. 2020:1–12. [Google Scholar]
- Anyamba A., Chretien J.P., Britch S.C., Soebiyanto R.P., Small J.L., Jepsen R., Forshey B.M., Sanchez J.L., Smith R.D., Harris R., Tucker C.J. Global disease outbreaks associated with the 2015–2016 El Nino event. Sci. Rep. 2019;9(1):1–14. doi: 10.1038/s41598-018-38034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour H.M., Elkhatib W.F., Rahman M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo M. An environmental and health perspective for COVID-19 outbreak: meteorology and air quality influence, sewage epidemiology indicator, hospitals disinfection, drug therapies and recommendations. Journal of Environmental Chemical Engineering. 2020;8:104006. doi: 10.1016/j.jece.2020.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay E. Predicting the next pandemic. Lancet. 2008;372(9643):1025–1026. doi: 10.1016/S0140-6736(08)61425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. Bats and human emerging diseases. Epidemiol. Infect. 2006;134:905–907. doi: 10.1017/S0950268806006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A., Donzelli G., Cioni L., Federigi I., Lombardi R., Verani M. Quantitative microbial risk assessment for workers exposed to bioaerosol in wastewater treatment plants aimed at the choice and setup of safety measures. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso B.A., Freeman M.C. Shared sanitation and the spread of COVID-19: risks and next steps. The Lancet Planetary Health. 2020;4(5):e173. doi: 10.1016/S2542-5196(20)30086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., Peiris M., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020:414. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P.M., Tscharke B.J., Donner E., O’Brien J.W., Grant S.C., Kaserzon S.L., Mackie R., O’Malley E., Crosbie N.D., Thomas K.V., Mueller J.F. Wastewater-based epidemiology biomarkers: past, present and future. TrAC - Trends Anal Chem. 2018:317. [Google Scholar]
- Collivignarelli M.C., Collivignarelli C., Miino M.C., Abbà A., Pedrazzani R., Bertanza G. SARS-CoV-2 in sewer systems and connected facilities. Process Saf. Environ. Prot. 2020;143:196–203. doi: 10.1016/j.psep.2020.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corburn J., Vlahov D., Mberu B., Riley L., Caiaffa W.T., Rashid S.F., Ko A., Patel S., Jukur S., Martínez-Herrera E., Jayasinghe S., Agarwal S., Nguendo-Yongsi B., Weru J., Ouma S., Edmundo K., Oni T., Ayad H. Slum health: arresting COVID-19 and improving well-being in urban informal settlements. J. Urban Health. 2020;97:348–357. doi: 10.1007/s11524-020-00438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin A., Ng T.W.P., Turinici G. 2020. A New Transmission Route for the Propagation of the SARS-CoV-2 Coronavirus. MedRxiv Preprint. (Posted on February 18, 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. The international imperative to rapidly and inexpensively monitor community-wide Covid-19 infection status and trends. Sci. Total Environ. 2020;365:138149. doi: 10.1016/j.scitotenv.2020.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani R., Kassiri H. A brief review on the possible role of houseflies and cockroaches in the mechanical transmission of coronavirus disease 2019 (COVID-19) Arch. Clin. Infect. Dis. 2020;15:e102863. [Google Scholar]
- Ding S., Liang T.J. Is SARS-CoV-2 also an enteric pathogen with potential fecal-oral transmission: a COVID-19 virological and clinical review. Gastroenterology. 2020;159(1):53–61. doi: 10.1053/j.gastro.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas K., Walker D.I., Adriaenssens E.M., McDonald J.E., Hillary L.S., Malham S.K., Jones D.L. Viral indicators for tracking domestic wastewater contamination in the aquatic environment. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears A.C., Klimstra W.B., Duprex P., Hartman A., Weaver S.C., Plante K.C., Mirchandani D., Plante J.A., Aguilar P.V., Fernández D., Nalca A., Totura A., Dyer D., Kearney B., Lackemeyer M., Bohannon J.K., Johnson R., Garry R.F., Reed D.S., Roy C.J. 2020. Comparative Dynamic Aerosol Efficiencies of Three Emergent Coronaviruses and the Unusual Persistence of SARS-CoV-2 in Aerosol Suspensions. medRxiv. [DOI] [Google Scholar]
- Goh G.K. Simplicity Research Institute; Singapore: 2017. Viral Shapeshifters: Strange Behavoirs of HiV and Other Viruses. [Google Scholar]
- Goh G.K., Dunker A.K., Uversky V.N. Prediction of intrinsic disorder in MERS-CoV/ HCoV- EMC supports a high oral-fecal transmission. PLoS Curr. 2013;13:5. doi: 10.1371/currents.outbreaks.22254b58675cdebc256dbe3c5aa6498b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K., Dunker A.K., Uversky V.N. Detection of links between Ebola nucleocapsid and virulence using disorder analysis. Mol. BioSyst. 2015;11:2337–2344. doi: 10.1039/c5mb00240k. [DOI] [PubMed] [Google Scholar]
- Goh G.K., Dunker A.K., Uversky V.N. Shell disorder, immune evasion and transmission behaviors among human and animal retroviruses. Mol. BioSyst. 2015;11:2312–2323. doi: 10.1039/c5mb00277j. [DOI] [PubMed] [Google Scholar]
- Goh G.K., Dunker A.K., Uversky V.N. Correlating flavivirus virulence and levels of intrinsic disorder in shell proteins: protective roles vs. immune evasion. Mol. BioSyst. 2016;12:1881–1891. doi: 10.1039/c6mb00228e. [DOI] [PubMed] [Google Scholar]
- Goh G.K., Dunker A.K., Foster J.A., Uversky V.N. Zika and flavivirus disorder: virulence and fetal morbidity. Biomolecules. 2019;9:e710. doi: 10.3390/biom9110710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K., Dunker A.K., Foster J.A., Uversky V.N. HIV vaccine mystery and viral shell disorder. Biomolecules. 2019;9:178. doi: 10.3390/biom9050178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K., Dunker A.K., Foster J.A., Uversky V.N. Nipah shell disorder, mode of transmission and virulence. Microb. Pathog. 2020;141:103976. doi: 10.1016/j.micpath.2020.103976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G.K.-M., Dunke A.K., Foster J.A., Uversky V.N. Shell disorder analysis predicts greater resilience of the SARS-CoV-2 (COVID-19) outside the body and in body fluids. Microb. Pathog. 2020;144:104177. doi: 10.1016/j.micpath.2020.104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Health. 2020;8:e643. doi: 10.1016/S2214-109X(20)30112-1. Doi:10.1016/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Granda M.G., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food and Environmental Virology. 2009;1:10. [Google Scholar]
- Gwenzi W. Autopsy, thanatopraxy, cemeteries and crematoria as hotspots of toxic organic contaminants in the funeral industry continuum. Sci. Total Environ. 2020:141819. doi: 10.1016/j.scitotenv.2020.141819. (in Press) [DOI] [PubMed] [Google Scholar]
- Gwenzi W. Dangerous liaisons?: as the COVID-19 wave hits Africa with potential for novel transmission dynamics. J. Public Health. 2020 doi: 10.1007/s10389-020-01467-w. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwenzi W. The ‘thanato-resistome’ - the funeral industry as a potential reservoir of antibiotic resistance: early insights and perspectives. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.141120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwenzi W., Chaukura N. Organic contaminants in African aquatic systems: Current knowledge, health risks, and future research directions. Sci. Total Environ. 2018;619-620:1493–1514. doi: 10.1016/j.scitotenv.2017.11.121. [DOI] [PubMed] [Google Scholar]
- Gwenzi W., Sanganyado E. Recurrent cholera outbreaks in sub-Saharan Africa: moving beyond epidemiology to understand the environmental reservoirs and drivers. Challenges. 2019;10(1) doi: 10.3390/challe10010001. [DOI] [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., Mo X., Chen Y., Liao B., Chen W., Hu F., Zhang Q., Zhong M., Wu Y., Zhao L., Zhang F., Cowling B.J., Li F., Leung G.M. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Heijnen M., Cumming O., Peletz R., Chan G.K.S., Brown J., Baker K., Clasen T. Shared sanitation versus individual household latrines: a systematic review of health outcomes. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: are we asking the right questions? Sci. Total Environ. 2020:138919. doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson J. COVID-19: faecal–oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020;17(5):259. doi: 10.1038/s41575-020-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 cell entrydepends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde K. Residential water quality and the spread of COVID-19 in the United States (April 9, 2020) 2020. https://ssrn.com/abstract=3572341 Available at SSRN:
- Ji Y., Ma Z., Peppelenbosch M.P., Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob. Health. 2020;8(4) doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. Science of The Total Environment; 2020. SARS-CoV-2 in Wastewater: State of the Knowledge and Research Needs; p. 139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods-a scoping review. Water Res. 2020;115899:28. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Tot. Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K.P., et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. PNAS USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F.X., Bouadma L., Nguyen D., Parisey M., Wicky P.H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., Enouf V. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. The Lancet Infectious Diseases. 2020;2:1–10. doi: 10.1016/s1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., et al. Bats are natural reservoirs of SARS-like coronaviruses. Science (Washington) 2005;310:676–683. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- Lin D., Liu L., Zhang M., Hu Y., Yang Q., Guo J., Guo Y., Dai Y., Xu Y., Cai Y., Chen X. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci. China Life Sci. 2020:1–4. doi: 10.1007/s11427-020-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo M., Picó Y. Wastewater-based epidemiology: current status and future prospects. Curr Opin Environ Sci Heal. 2019;9:77–84. doi: 10.1016/j.coesh.2019.05.007. [DOI] [Google Scholar]
- Ma L., Cao G., Tang S., Yang D., Shi W., Wang X., et al. 2020. One Nosocomial Cluster Following With a Familial Cluster of COVID-19 Cases: The Potential Transmission Risk in Patients With Negative Swab Tests. [DOI] [Google Scholar]
- Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580(7802):176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Meng X., Huang X., Zhou P., Li C., Wu A. Alert for SARS-CoV-2 infection caused by fecal aerosols in rural areas in China. Infection Control & Hospital Epidemiology. 2020:1–4. doi: 10.1017/ice.2020.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddeo V., Liu H. Editorial perspectives: 2019 novel coronavirus (SARS-369 CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ Sci Water Res Technol. 2020;6(5):1213–1216. doi: 10.1039/D0EW90015J. [DOI] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies in Chemical and Environmental Engineering. 2020;1 doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakenfull R.J., Wilson A.J. Food Safety Agency (FSA)-UK; 2020. Qualitative Risk Assessment: What is the risk of food or food contact materials being a source or transmission route of SARS-CoV-2 for UK consumers? https://www.food.gov.uk/sites/default/files/media/document/web-version-qualitative-risk-assessment-risk-of-food-or-food-contact-materials-as-transmission-route-of-sars-cov-2-002.pdf Accessed 10 June 2020.
- Odih E.E., Afolayan A.O., Akintayo I., Okeke I.N. Could water and sanitation shortfalls exacerbate SARS-CoV-2 transmission risks? The American Journal of Tropical Medicine and Hygiene. 2020;103(2):554–557. doi: 10.4269/ajtmh.20-0462. (tpmd200462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald W.E., Lescano A.G., Bern C., Calderon M.M., Cabrera L., Gilman R.H. Fecal contamination of drinking water within peri-urban households, Lima, Peru. The American journal of tropical medicine and hygiene. 2007;77(4):699–704. [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 270 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Thwaites R.S., Openshaw P.J. COVID-19: lessons from SARS and MERS. Eur. J. Immunol. 2020;50(3):308. [Google Scholar]
- Pastorino B., Touret F., lles M., de Lamballerie X., Charrel R.N. 2020. Evaluation of Heating and Chemical Protocols for Inactivating SARS-CoV-2. BioRxiv Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwary M.A., O’Hare W.T., Sarker M.H. Assessment of occupational and environmental safety associated with medical waste disposal in developing countries: a qualitative approach. Saf. Sci. 2011;49(8–9):1200–1207. [Google Scholar]
- Potgieter N., Karambwe S., Mudau L.S., Barnard T., Traore A. Human enteric pathogens in eight rivers used as rural household drinking water sources in the northern region of South Africa. Int. J. Environ. Res. Public Health. 2020;17(6):2079. doi: 10.3390/ijerph17062079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujari P.R., Padmakar C., Labhasetwar P.K., Mahore P., Ganguly A.K. Assessment of the impact of on-site sanitation systems on groundwater pollution in two diverse geological settings—a case study from India. Environ. Monit. Assess. 2012;184(1):251–263. doi: 10.1007/s10661-011-1965-2. [DOI] [PubMed] [Google Scholar]
- Race M., Ferraro A., Galdieroc E., Guida M., Nunez-Delgado A., Pirozzi F., Siciliano A., Fabbricino M. Current emerging SARS-CoV-2 pandemic: potential direct/indirect negative impacts of virus persistence and related therapeutic drugs on the aquatic compartments. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringane A., Milovanovic Μ., Makete F., Omar Τ., Martinson Ν., Lebina L. An observational study of safe and risky practices in funeral homes in South Africa. SAMJ: South African Medical Journal. 2019;109(8):587–591. doi: 10.7196/SAMJ.2019.v109i8.13523. [DOI] [PubMed] [Google Scholar]
- Rusiñol M., Fernandez-Cassi X., Hundesa A., Vieira C., Kern A., Eriksson I., et al. Application of human and animal viral microbial source tracking tools in fresh and marine waters from five different geographical areas. Water Res. 2014;59:119–129. doi: 10.1016/j.watres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Rutalab W.A., Weberb D.J., Sobseya M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P., Dong Y., Yan H., Zhao C., Li X., Liu W., He M., Tang S., Xi S. Science of The Total Environment; 2020. Impact of Temperature on the Dynamics of the COVID-19 Outbreak in China; p. 138890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichewo P.R., Vander Kelen C., Thys S., Michel A.L. Risk practices for bovine tuberculosis transmission to cattle and livestock farming communities living at wildlife-livestock-human interface in northern KwaZulu Natal, South Africa. PLoS Negl. Trop. Dis. 2020;14(3) doi: 10.1371/journal.pntd.0007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiyu S., Swilling M., Cairncross S., Rheingans R. Determinants of quality of shared sanitation facilities in informal settlements: case study of Kisumu, Kenya. BMC Public Health. 2017;17(1):68. doi: 10.1186/s12889-016-4009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.-D., Tu C.-C., Zhang G.-W., et al. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. U. S. A. 2005;102(7):2430. doi: 10.1073/pnas.0409608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songe M.M., Hang’ombe B.M., Knight-Jones T.J., Grace D. Antimicrobial resistant enteropathogenic Escherichia coli and Salmonella spp. in houseflies infesting fish in food markets in Zambia. International Journal of Environmental Research and Public Health. 2017;14(1):21. doi: 10.3390/ijerph14010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street R., Malema S., Mahlangeni N., Mathee A. Wastewater surveillance for Covid-19: an African perspective. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley E., Ulrich L., Luthi C., Reymond P., Zurbrügg C. 2nd revised edition. Swiss Federal Institute of Aquatic Science and Technology (Eawag); Dübendorf, Switzerland: 2014. Compendium of Sanitation Systems and Technologies.https://www.eawag.ch/en/department/sandec/publications/compendium/ Available at: (Accessed 30 April 2020) [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-W., Li J.-S., Guo T.-K., Zhen B., Kong Q.-X., Yi B., Li Z., Song N., Jin M., Xiao W.-J., Zhu X.-M., Gu C.-Q., Yin J., Wei W., Yao W., Liu C., Li J.-F., Ou G.-R., Wang M.-N., Fang T.-Y., Wang G.-J., Qiu Y.-H., Wu H.-H., Chao F.-H., Li J.-W. Concentration and detection of SARS coronavirus in sewage from Xiaotang Shan hospital and the 309th hospital. J. Virol. Methods. 2005;128:156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO World Health Organization; 2003. Consensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS) https://apps.who.int/iris/handle/10665/70863
- WHO Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. 2020. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precautionrecommendations Accessed on 5 May 2020.
- WHO Novel coronavirus – nCoV-2019. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Accessed on 14 August 2020.
- WHO . World Health Organization; 2020. Water, Sanitation, Hygiene and Waste Management for COVID-19: Technical Brief, 03 March 2020. [Google Scholar]
- Wikramaratna P., Paton R.S., Ghafari M., Lourenço J. 2020. Estimating False-Negative Detection Rate of SARS-CoV-2 by RT-PCR. MedRxiv pre-print. Posted April 7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelfel R., Corman V.M., Guggemos W., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Jing W., Liu J., Ma Q., Yuan J., Wang Y., Du M., Liu M. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci. Total Environ. 2020:139051. doi: 10.1016/j.scitotenv.2020.139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet. 2020;313(5):335–337. doi: 10.1016/S2468-1253(20)30048-0. Gastroenterol Hepatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. Jama. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang R., Castro M.F., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., Liu Z., Brulois K.F., Wang X., Greenberg H.B., Diamond M.S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Science Immunology. 2020;13:5(47). doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J., et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2(8):123–124. [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang hospital. Sci. Total Environ. 2020;741:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zume J.T. Risks of burial practices on groundwater quality in rural north-central Nigeria. J. Water Health. 2011;9(3):609–616. doi: 10.2166/wh.2011.193. [DOI] [PubMed] [Google Scholar]