Graphical abstract
Abstract
As the incidence rate of invasive fungal infections has increased with the use of modern medical interventions, so too has the occurrence of fungi invading the brain. Fungi such as Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus often infect immunocompromised individuals, and can use several strategies to invade the central nervous system (CNS) by penetrating the blood–brain barrier. Once in the brain parenchyma the specialized resident immune cells need to effectively recognize the fungus and mount an appropriate immune response to clear the infection, without causing debilitating immune-mediated toxicity and neuronal damage. Here we review the current knowledge pertaining to the antifungal response of the CNS and highlight areas where future research is required.
Current Opinion in Microbiology 2020, 58:41–46
This review comes from a themed issue on Host-microbe interactions: fungi
Edited by Agostinho Carvalho and Frank L van de Veerdonk
For a complete overview see the Issue and the Editorial
Available online 20th August 2020
https://doi.org/10.1016/j.mib.2020.07.011
1369-5274/Published by Elsevier Ltd.
Introduction
In recent decades, invasive fungal infections (IFIs) have increased due to expanded immunosuppressed populations and invasive medical interventions [1]. Candida albicans infects critically ill and immunocompromised patients and is the most common cause of hospital-acquired fungemia [2]. Cryptococcus neoformans causes invasive central nervous system (CNS) infections in HIV/AIDS patients, while the related Cryptococcus gattii typically infects the lungs of putatively immunocompetent individuals [3]. Aspergillus fumigatus is responsible for invasive infections in patients with neutropenia or allogeneic hematopoietic stem cell transplantation (HSCT). Current treatments for IFIs remain insufficient, with mortalities >50% [4].
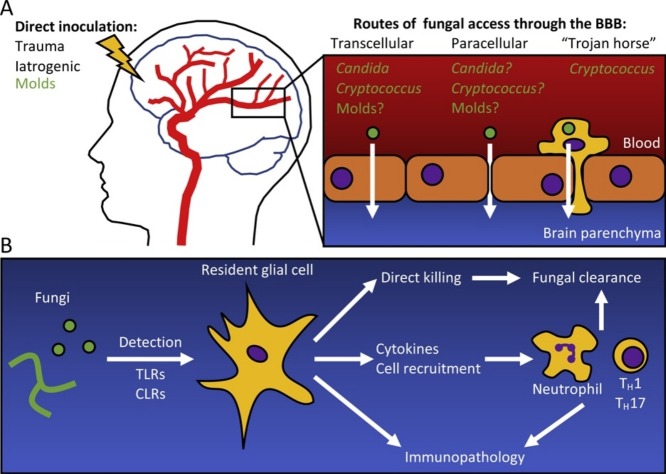
As IFIs disseminate, a common target tissue is the CNS. Additionally, fungi can gain direct access to the CNS following traumatic inoculation or neurosurgical procedures [5]. Within the CNS, fungi come in contact with the brain’s specialized resident immune system, comprising barrier-associated macrophages, microglia and astrocytes [6]. This review summarizes our current understanding of the most common CNS-associated IFIs in terms of at-risk populations, mechanisms of fungal CNS entry, and host immune responses (Figure 1 ). Gaps in our knowledge and opportunities for future research are highlighted.
Figure 1.
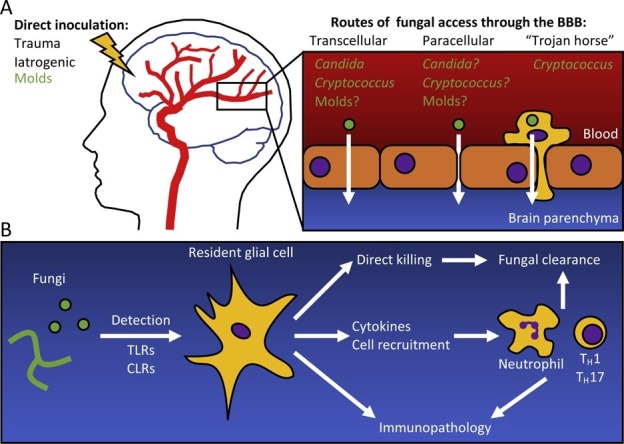
(a) Fungi enter the brain through either direct inoculation, or by crossing the blood–brain barrier (BBB). Direct inoculation can be the result of a traumatic event, or iatrogenically through neurosurgery. Fungi can cross the BBB by being taken up by endothelial cells (transcellular), degrading the tight junctions between the cells (paracellular), or carried across the BBB in a phagocyte (‘Trojan horse’). Brain Image adapted from https://www.health.harvard.edu/heart-health/the-crucial-controversial-carotid-artery-part-i-the-artery-in-health-and-disease. (b) Fungi are detected in the brain by resident glial cells (microglia, astrocytes) through Toll-like receptors (TLR) and C-type lectin receptors (CLR). Glial cells can then directly engage the fungi, clearing them from the brain, or recruit immune cells through the release of cytokines and chemoattractants. Innate immune cells such as neutrophils, and adaptive immune cells such as TH1 and TH17 lymphocytes are effective at clearing the infection. However, excessive inflammation and immunotoxicity can lead to irreversible neurological damage.
CNS candidiasis
Candida is the most common human fungal pathogen. Mucosal infections and candidemia are more common [7], whereas CNS infections are relatively rare [8]. C. albicans enters the CNS through bloodstream dissemination, causing encephalitis as a result of an ineffective antifungal immune response. Populations at-risk for Candida encephalitis include premature infants and severely immunocompromised patients with prolonged neutropenia, HSCT, and specific genetic deficiencies [8].
The mechanism by which C. albicans crosses the blood–brain barrier (BBB) and invades the CNS remains poorly understood. Evidence exists of C. albicans crossing between endothelial cells (paracellular route) and being taken up by endothelial cells and exiting the basal surface (transcellular route) [9]. The importance of the paracellular route for accessing the BBB remains unclear. C. albicans can degrade the tight junction protein E-cadherin through secreted aspartyl proteases [10] and E-cadherin is expressed by bovine brain endothelial cells [11]. However, expression of E-cadherin by human or mouse brain endothelial cells has not been reported. In contrast, greater evidence exists implicating the transcellular route for C. albicans accessing the brain. C. albicans interacts with the heat-shock protein gp96 on brain microvascular endothelial cells via its invasin Als3 [12]. Gp96 is uniquely expressed on brain endothelial cells, and its interaction with Als3 induces fungal endocytosis. Less is known about how the fungus exits the endothelial cell to gain access into the CNS; whether it is through active exocytosis, or a fungus-induced lytic process causing endothelial cell death remains unknown. Magnetic resonance imaging suggested the latter scenario, as C. albicans-infected mice display loss of BBB integrity [13]. However, it is unclear whether loss of BBB integrity is due to endothelial cell lysis as a result of the fungus exiting them, or due to damage from the ensuing CNS infection. While C. albicans deep-tissue invasion is often assumed to require hyphal differentiation, it is important to note that this is not necessary for CNS invasion, as heterologous expression of Als3 in Saccharomyces cerevisiae resulted in their uptake by brain endothelial cells [12], and a yeast-locked strain of C. albicans establishes CNS invasion in mice [14•]. Intravital microscopy studies are required to further clarify how C. albicans establishes CNS infection.
Microglia play a central role in anti-Candida CNS host responses. In a low-dose candidemia model, self-limited fungal granulomas develop in infected brains surrounded by microglia and astrocytes [15•]. While limited cellular recruitment was noted, amyloid-β peptides bound to C. albicans and enhanced microglial phagocytosis and killing [15•]. Accordingly, amyloid-deficient mice had larger fungal lesions and impaired fungal-clearing ability [15•].
Candida encephalitis stemming from high-dose candidemia requires neutrophil recruitment to avoid uncontrolled CNS invasion [16]. Human and animal studies have identified a role for Caspase Recruitment Domain 9 (CARD9), encoding a signal adaptor downstream of fungal-sensing C-type lectin receptors (CLRs), in recruiting neutrophils to the Candida-infected CNS [14•,16]. Brain-resident microglia are pivotal in orchestrating the Card9-dependent release of neutrophil-recruiting CXCL1 [14•]. Microglia respond to the fungal toxin candidalysin, upregulating c-Fos in a Card9-dependent manner [14•,17] and releasing interleukin (IL)-1β into their surroundings via Card9-dependent effects on Il1b transcription and inflammasome activation [14•]. IL-1β is required for the subsequent production of microglial CXCL1, with contribution from astrocytes acting in trans [14•]. The molecular basis of microglial-actrocyte cross-talk however, remains incompletely defined.
Cryptococcosis
The two Cryptococcus human pathogens are C. neoformans and C. gattii [18]. C. neoformans causes life-threatening pulmonary and CNS infections in immunocompromised individuals like those living with HIV/AIDS or undergoing immunosuppressive therapy [19]. C. gattii mostly causes lung infections, and can affect putatively immunocompetent individuals [3]. A recently described risk factor for C. gattii CNS infections is the presence of neutralizing anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibodies, which were detected in 7 of 9 putatively immunocompetent patients with C. gattii CNS infections [20]. Anti-GM-CSF autoantibodies may also contribute to susceptibility to non-gattii CNS cryptococcosis, as recently shown in a patient with C. neoformans var. grubii CNS vasculitis [21]. Both fungi are initially inhaled as dessicated yeasts from their environmental niches (i.e. trees, bird guano) [18]. Inhalation establishes a pulmonary infection, before dissemination to the blood and CNS. Cryptococci possess an array of infection-enabling virulence factors including a carbohydrate-rich capsule shielding pathogen-associated molecular patterns (PAMPs) from immune detection, and melanin production that protects from reactive oxygen species (ROS) [19].
Cryptococcus traverses the BBB and gains CNS entry through multiple mechanisms. In vitro experiments demonstrated the ability of phagocytes containing engulfed viable C. neoformans to cross a brain endothelial cell layer, suggesting that Cryptococcus can utilize a ‘Trojan horse’ strategy for brain invasion, albeit with a lesser efficiency than free yeast cells [22]. This observation is supported by the demonstration that mouse intravenous administration of monocytes containing phagocytosed C. neoformans develop cryptococcal encephalitis with greater fungal burden than mice inoculated with free yeast cells [23]. Intravital imaging observed Ly6Clow monocytes being recruited to the CNS microvasculature in a TNFR-dependent manner, engulfing cryptococcal cells and carrying them across the endothelium into the CNS [24•]. Phagocyte depletion of mice using chlodronate liposomes reduced fungal burden in the spleen, lungs and brains [23], further underscoring the importance of this entry mechanism. These data were partially corroborated using inflammatory-monocyte-depleted CCR2-DTR mice, which were more resistant to cryptococcosis with lower lung and lymph node fungal burden, yet similar brain fungal burden [25]. This discrepancy may suggest that CCR2-negative phagocytes are sufficient to deliver Cryptococcus to the CNS, or that phagocyte-independent mechanisms are operational. Indeed, as mentioned above, Cryptococcus can traverse the BBB in the absence of a ‘Trojan horse’ cell. Intravital microscopy experiments visualizing C. neoformans within the brain vasculature observed the abrupt arrest of yeasts as they reached capillaries of similar diameter, followed by their transmigration across the microvasculature into the brain [26]. While fungal arrest in the microcapillaries was independent of endothelial adherence, expression of urease by C. neoformans was required for BBB crossing, in keeping with a urease-deficient strain being impaired at establishing CNS infection [26]. While it remains unclear how urease enhances cryptococcal brain invasion, it is speculated that urease-dependent localized production of toxic ammonia levels might increase BBB permeability [26]. C. neoformans hyaluronic acid interacts with CD44 on brain endothelial cells in vitro, inducing yeast phagocytosis and initiating transcellular migration [27,28•]. This method of transcellular migration occurs via CD44-dependent phosphorylation of the tyrosine kinase receptor EphA2, which orchestrates the GTPase-mediated cytoskeletal actin reorganization necessary for transcytosis [28•]. Accordingly, CD44-deficient mice are resistant to cryptococcosis, exhibiting prolonged survival and reduced brain fungal burden [29]. Additionally, prolonged cytoskeletal rearrangement through EphA2 engagement causes degradation of the tight junctions of brain endothelial cells that are critical for maintaining BBB integrity [28•]. Supporting this notion, extended exposure of endothelial cells to C. neoformans in vitro results in degradation of the tight junction-stabilizing protein occludin [30].
Following CNS entry, the two cryptococcal species exhibit different pathologies, likely reflective of the patient populations they infect and their differential interactions with the immune system. While C. gattii primarily features large cryptococcomas in the mouse brain, these were rare in C. neoformans-infected mice [31]. C. neoformans elicits a relatively weak immune response, driven by the combination of PAMP concealement via its capsule and infection of immunocompromised hosts. Conversely, C. gattii infection manifests a robust inflammatory response. When stimulated in vitro with several strains from both Cryptococcus species, human monocytes produced greater quantities of TNFα, IL-1β, IL-6, IL-17 and IL-22 in response to C. gattii strains than C. neoformans [32]. However, a mouse model of pulmonary infection found reduced inflammation in response to C. gattii strain JP02 compared to C. neoformans strain H99, and in vitro stimulation of the mouse dendritic cell (DC) line JAWSII with the two strains exhibited IL-6 release in response to H99, but not JP02 [33]. The PAMP responsible for this pro-inflammatory response is acetylated glucuronoxylomannan, which resides in the capsule of C. neoformans, but not C. gattii [33]. Consequently, C. gattii induced attenuated adaptive TH1 and TH17 responses compared to C. neoformans, both via DC-mediated stimulation in vitro, and during pulmonary infection in vivo [34]. Further studies are required to determine whether this discrepancy of the immune activation potential of C. gattii is due to fundamental differences between humans and mice, or other factors.
Interestingly, while CARD9-deficiency renders patients susceptible to cerebral candidiasis, it appears dispensable for CNS cryptococcosis. In agreement, a Card9-deficient mouse model of pulmonary cryptococcosis found no increased brain fungal burden [35•]. Instead, Card9 drives lung macrophage killing of C. neoformans by promoting a protective M1-like phenotype [35•]. A possible explanation for the limited role of Card9 in defense against cerebral cryptococcosis is that cryptococcal recognition is less dependent on Card9-mediated CLR signaling due to masking of CLR ligands by the cryptococcal capsule [36]. Instead, microglia rely on Toll-like receptors for Cryptococcus recognition and phagocytosis, and pro-inflammatory cytokine production (e.g. TNFα, IL-1β, IL-6) [37,38]. The resulting inflammatory milieu attracts neutrophils and initiates an effective adaptive immune response [39]. During cryptococcosis, microglia upregulate MHCII [40], enhancing their ability for antigen presentation.
As shown by the susceptibility of HIV/AIDS patients to cryptococcosis, CD4+ T-cells are essential for fungal clearance. CD4+ T-cells are recruited to the brain in experimental cryptococcosis via increased TNFα, CCL2 and CCL5 [40]. Interestingly, treating C. neoformans-infected mice with poly-ICLC resulted in heightened TH1/TH17 responses and reduced mortality and lowered lung and brain fungal burden in a IFNαR1-dependent and MDA5-dependent, but TLR3-independent manner [41]. While poly-ICLC increased survival and decreased pulmonary fungal burden in C. gattii-infected mice, it had no effect of brain fungal burden [41]. While the T-cell response is effective at fungal clearance, the ensuing inflammation can cause irreversible and fatal CNS damage [40], a phenomenon mirrored clinically by immune reconstitution inflammatory syndrome and post-infectious inflammatory response syndrome. Better understanding of the pathogenic T-cell mechanisms in these conditions could help developing targeted immunotherapies.
Mold infections
Aspergillus, Mucorales, Scedosporium and Cladophialophora molds can infrequently cause CNS infections in susceptible hosts, associated with mortality rates between 50–100% [5,8]. These infections occur secondary to dissemination of pulmonary disease in immunocompromised patients (Aspergillus), direct extension from sinoorbital disease in diabetic hosts (mucormycosis) or inoculation during trauma or neurosurgical procedures, independent of the host immune status [5]. Notably, Scedosporium causes CNS infections following near-drownings [5], and Cladophialophora infects putatively immunocompetent individuals in tropical areas [42].
Little is known about how molds cross the BBB and invade the CNS. It is thought that Aspergillus invades through hyphal expansion that causes endothelial destruction [9]. Additionally, Aspergillus toxins were shown to affect the barrier integrity of human brain microvascular endothelial cells in vitro. Indeed, aflatoxin caused endothelial cell death [43], while gliotoxin reduced barrier integrity without disrupting tight junctions [44]. Rhizopus oryzae, the most common cause of mucormycosis, disseminates throughout the body by angioinvasion via the interaction between endothelial cell GRP78 and fungal CotH3 [45,46]. GRP78 is expressed by brain endothelial cells [45], and blocking CotH3 resulted in reduced brain fungal burden [46], suggesting this protein–protein interaction is involved in R. oryzae CNS invasion.
Similar to Candida, CARD9 is critical in mounting effective anti-Aspergillus CNS responses [47]. A CARD9-deficient patient was described with CNS aspergillosis, associated with impaired neutrophil accumulation [47]. Pulmonary aspergillosis was not observed in that patient [47], despite mouse studies showing a partial role for Card9 mediating pulmonary neutrophil recruitment [48]. CNS aspergillosis also develops in hematological malignancy patients receiving the BTK inhibitor Ibrutinib, with up to 40–50% of cases involving the CNS [49]. While the underlying mechanisms remain unclear, Ibrutinib affects TLR-dependent macrophage activation, and thus could have a similar effect on microglia [49].
Invasive pulmonary aspergillosis has recently been associated with severe influenza infection, and on at least one occasion a patient had developed fungal brain abscesses [50]. Recently, invasive pulmonary aspergillosis has also been observed in association with SARS-CoV-2 infection [51], and thus it will be important to determine whether cerebral aspergillosis may also manifest under these circumstances in patients receiving corticosteroids.
Little is known about host-Cladophialophora interactions in the CNS. A key feature of Cladophialophora is that in addition to conidia, its hyphae are also melanized, which may promote resistance from ROS [42]. The ability of molds to resist the CNS immune response leads to granuloma formation as seen with Cladophialophora [52], Aspergillus [53] and Scedosporium [54], which may contain the fungus from further spreading, but is not permissive for disease eradication.
Conclusions
CNS fungal infections typically affect patients with iatrogenic or inherited immunosuppressive conditions with high mortality despite treatment. Recent work has began outlining the mechanisms by which different fungi invade into the CNS. The host immune responses that promote protection or immunopathology in the fungal-infected CNS are also now emerging (Figure 1). A better understanding of the cellular and molecular basis of host–fungal interactions in the CNS could help devise strategies to enhance immunity and neuroprotection, block fungal CNS entry and improve patient outcomes.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
B.D.S. and M.S.L. are supported by the Division of Intramural Research, National Institute of Allergy and Infectious Disease, National Institutes of Health. R.A.D. is supported by the Medical Research Council (MR/S024611) and (SBF004/1008).
References
- 1.Lionakis M.S., Levitz S.M. Host control of fungal infections: lessons from basic studies and human cohorts. Annu Rev Immunol. 2018;36:157–191. doi: 10.1146/annurev-immunol-042617-053318. [DOI] [PubMed] [Google Scholar]
- 2.Suleyman G., Alangaden G.J. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect Dis Clin North Am. 2016;30:1023–1052. doi: 10.1016/j.idc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Bielska E., May R.C. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res. 2016;16:fov106. doi: 10.1093/femsyr/fov106. [DOI] [PubMed] [Google Scholar]
- 4.Brown G.D., et al. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 5.Miceli M.H. Central nervous system infections due to Aspergillus and other hyaline molds. J Fungi (Basel) 2019;5 doi: 10.3390/jof5030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rua R., McGavern D.B. Advances in meningeal immunity. Trends Mol Med. 2018;24:542–559. doi: 10.1016/j.molmed.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer F.L., Wilson D., Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goralska K., Blaszkowska J., Dzikowiec M. Neuroinfections caused by fungi. Infection. 2018;46:443–459. doi: 10.1007/s15010-018-1152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheppard D.C., Filler S.G. Host cell invasion by medically important fungi. Cold Spring Harb Perspect Med. 2014;5:a019687. doi: 10.1101/cshperspect.a019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villar C.C., et al. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun. 2007;75:2126–2135. doi: 10.1128/IAI.00054-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbruscato T.J., Davis T.P. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–286. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y., et al. Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog. 2011;7:e1002305. doi: 10.1371/journal.ppat.1002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarathna D.H., et al. MRI confirms loss of blood-brain barrier integrity in a mouse model of disseminated candidiasis. NMR Biomed. 2013;26:1125–1134. doi: 10.1002/nbm.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Drummond R.A., et al. CARD9(+) microglia promote antifungal immunity via IL-1beta- and CXCL1-mediated neutrophil recruitment. Nat Immunol. 2019;20:559–570. doi: 10.1038/s41590-019-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Establishes the Card9-dependent role of microglia in recruiting neutrophils to the brain during Candida encephalitis.
- 15•.Wu Y., et al. Microglia and amyloid precursor protein coordinate control of transient Candida cerebritis with memory deficits. Nat Commun. 2019;10:58. doi: 10.1038/s41467-018-07991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Observed transient encephalitis associated with low-grade systemic infection, controlled by antimicrobial effects of amyloid-β.
- 16.Drummond R.A., et al. CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLoS Pathog. 2015;11:e1005293. doi: 10.1371/journal.ppat.1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyes D.L., et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon-Chung K.J., et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med. 2014;4:a019760. doi: 10.1101/cshperspect.a019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skolnik K., Houston S., Mody C.H. Cryptococcal lung infections. Clin Chest Med. 2017;38:451–464. doi: 10.1016/j.ccm.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Saijo T., et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. 2014;5:e00912–e00914. doi: 10.1128/mBio.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrineau S., et al. Anti-GM-CSF autoantibodies and Cryptococcus neoformans var. grubii CNS vasculitis. J Clin Immunol. 2020;40:767–769. doi: 10.1007/s10875-020-00796-5. [DOI] [PubMed] [Google Scholar]
- 22.Santiago-Tirado F.H., et al. Trojan horse transit contributes to blood-brain barrier crossing of a eukaryotic pathogen. mBio. 2017;8 doi: 10.1128/mBio.02183-16. p. e02183-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlier C., et al. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Sun D., et al. VCAM1/VLA4 interaction mediates Ly6Clow monocyte recruitment to the brain in a TNFR signaling dependent manner during fungal infection. PLoS Pathog. 2020;16:e1008361. doi: 10.1371/journal.ppat.1008361. [DOI] [PMC free article] [PubMed] [Google Scholar]; Used intravital microscopy to discover a role for Ly6Clow monocytes in transporting C. neoformans across the BBB, and defined important leukocyte and endothelial factors required for this to occur.
- 25.Heung L.J., Hohl T.M. Inflammatory monocytes are detrimental to the host immune response during acute infection with Cryptococcus neoformans. PLoS Pathog. 2019;15:e1007627. doi: 10.1371/journal.ppat.1007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi M., et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 2010;120:1683–1693. doi: 10.1172/JCI41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jong A., et al. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell Microbiol. 2008;10:1313–1326. doi: 10.1111/j.1462-5822.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 28•.Aaron P.A., et al. The blood-brain barrier internalises Cryptococcus neoformans via the EphA2-tyrosine kinase receptor. Cell Microbiol. 2018;20 doi: 10.1111/cmi.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using transcriptomic data of human brain endothelial cells, described how C. neoformans co-opts the EPH tryrosine kinase pathway via CD44 to gain entry to the cells for transcellular transmigration.
- 29.Jong A., et al. Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J Biol Chem. 2012;287:15298–15306. doi: 10.1074/jbc.M112.353375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S.H., et al. Cryptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J Med Microbiol. 2003;52:961–970. doi: 10.1099/jmm.0.05230-0. [DOI] [PubMed] [Google Scholar]
- 31.Ngamskulrungroj P., et al. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio. 2012;3 doi: 10.1128/mBio.00103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoffelen T., et al. Cryptococcus gattii induces a cytokine pattern that is distinct from other cryptococcal species. PLoS One. 2013;8:e55579. doi: 10.1371/journal.pone.0055579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urai M., et al. Evasion of innate immune responses by the highly virulent Cryptococcus gattii by altering capsule glucuronoxylomannan structure. Front Cell Infect Microbiol. 2015;5:101. doi: 10.3389/fcimb.2015.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angkasekwinai P., et al. Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect Immun. 2014;82:3880–3890. doi: 10.1128/IAI.01773-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Campuzano A., et al. CARD9 is required for classical macrophage activation and the induction of protective immunity against pulmonary cryptococcosis. mBio. 2020;11:e03005–e03019. doi: 10.1128/mBio.03005-19. [DOI] [PMC free article] [PubMed] [Google Scholar]; In contrast to C. albicans and mold infections, this study did not find a role for Card9 in C. neoformans CNS infection, showing that it is not a pan-fungal encephalitis defense mechanism. Card9 instead played a role in the pulmonary phase of the disease.
- 36.Snarr B.D., Qureshi S.T., Sheppard D.C. Immune recognition of fungal polysaccharides. J Fungi (Basel) 2017;3 doi: 10.3390/jof3030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barluzzi R., et al. Role of the capsule in microglial cell–Cryptococcus neoformans interaction: impairment of antifungal activity but not of secretory functions. Med Mycol. 1998;36:189–197. [PubMed] [Google Scholar]
- 38.Redlich S., et al. Toll-like receptor stimulation increases phagocytosis of Cryptococcus neoformans by microglial cells. J Neuroinflammation. 2013;10:71. doi: 10.1186/1742-2094-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun D., et al. Intravascular clearance of disseminating Cryptococcus neoformans in the brain can be improved by enhancing neutrophil recruitment in mice. Eur J Immunol. 2016;46:1704–1714. doi: 10.1002/eji.201546239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neal L.M., et al. CD4(+) T cells orchestrate lethal immune pathology despite fungal clearance during Cryptococcus neoformans meningoencephalitis. mBio. 2017;8 doi: 10.1128/mBio.01415-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sionov E., et al. Type I IFN induction via Poly-ICLC protects mice against cryptococcosis. PLoS Pathog. 2015;11:e1005040. doi: 10.1371/journal.ppat.1005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kantarcioglu A.S., et al. An updated comprehensive systematic review of Cladophialophora bantiana and analysis of epidemiology, clinical characteristics, and outcome of cerebral cases. Med Mycol. 2017;55:579–604. doi: 10.1093/mmy/myw124. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi H., et al. Cytotoxic effects of aflatoxin B1 on human brain microvascular endothelial cells of the blood-brain barrier. Med Mycol. 2015;53:409–416. doi: 10.1093/mmy/myv010. [DOI] [PubMed] [Google Scholar]
- 44.Patel R., et al. Gliotoxin penetrates and impairs the integrity of the blood-brain barrier in vitro. Mycotoxin Res. 2018;34:257–268. doi: 10.1007/s12550-018-0320-7. [DOI] [PubMed] [Google Scholar]
- 45.Liu M., et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120:1914–1924. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gebremariam T., et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J Clin Invest. 2014;124:237–250. doi: 10.1172/JCI71349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rieber N., et al. Extrapulmonary Aspergillus infection in patients with CARD9 deficiency. JCI Insight. 2016;1:e89890. doi: 10.1172/jci.insight.89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jhingran A., et al. Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection. PLoS Pathog. 2015;11:e1004589. doi: 10.1371/journal.ppat.1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lionakis M.S., et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31:833–843. doi: 10.1016/j.ccell.2017.04.012. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim M.J., et al. A case of acute cerebral aspergillosis complicating influenza A/H1N1pdm 2009. Infect Chemother. 2013;45:225–229. doi: 10.3947/ic.2013.45.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arastehfar A., et al. COVID-19 associated pulmonary aspergillosis (CAPA)-from immunology to treatment. J Fungi (Basel) 2020;6 doi: 10.3390/jof6020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakrabarti A., et al. Brain abscess due to Cladophialophora bantiana: a review of 124 cases. Med Mycol. 2016;54:111–119. doi: 10.1093/mmy/myv091. [DOI] [PubMed] [Google Scholar]
- 53.Jariwal R., et al. Granulomatous invasive Aspergillus flavus infection involving the nasal sinuses and brain. J Investig Med High Impact Case Rep. 2018;6:1–4. doi: 10.1177/2324709618770473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ampawong S., Luplertlop N. Experimental scedosporiosis induces cerebral oedema associated with abscess regarding aquaporin-4 and Nrf-2 depletions. Biomed Res Int. 2019;2019:6076571. doi: 10.1155/2019/6076571. [DOI] [PMC free article] [PubMed] [Google Scholar]