Abstract
Objectives
As schools plan for re-opening, understanding the potential role children play in the coronavirus infectious disease 2019 (COVID-19) pandemic and the factors that drive severe illness in children is critical.
Study design
Children ages 0-22 years with suspected severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection presenting to urgent care clinics or being hospitalized for confirmed/suspected SARS-CoV-2 infection or multisystem inflammatory syndrome in children (MIS-C) at Massachusetts General Hospital were offered enrollment in the Massachusetts General Hospital Pediatric COVID-19 Biorepository. Enrolled children provided nasopharyngeal, oropharyngeal, and/or blood specimens. SARS-CoV-2 viral load, ACE2 RNA levels, and serology for SARS-CoV-2 were quantified.
Results
A total of 192 children (mean age, 10.2 ± 7.0 years) were enrolled. Forty-nine children (26%) were diagnosed with acute SARS-CoV-2 infection; an additional 18 children (9%) met the criteria for MIS-C. Only 25 children (51%) with acute SARS-CoV-2 infection presented with fever; symptoms of SARS-CoV-2 infection, if present, were nonspecific. Nasopharyngeal viral load was highest in children in the first 2 days of symptoms, significantly higher than hospitalized adults with severe disease (P = .002). Age did not impact viral load, but younger children had lower angiotensin-converting enzyme 2 expression (P = .004). Immunoglobulin M (IgM) and Immunoglobulin G (IgG) to the receptor binding domain of the SARS-CoV-2 spike protein were increased in severe MIS-C (P < .001), with dysregulated humoral responses observed.
Conclusions
This study reveals that children may be a potential source of contagion in the SARS-CoV-2 pandemic despite having milder disease or a lack of symptoms; immune dysregulation is implicated in severe postinfectious MIS-C.
Abbreviations: ACE2, Angiotensin-converting enzyme; COVID-19, Coronavirus disease-19; IPO8, Importin-8; IRB, Institutional Review Board; MGH, Massachusetts General Hospital; MIS-C, Multisystem inflammatory syndrome in children; NT-proBNB, N-terminal pro b-type natriuretic peptide; RBD, Receptor binding domain; RSV, Respiratory syncytial virus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
See related article, p 31
As schools plan for reopening, debates around the role children play in the coronavirus disease-19 (COVID-19) pandemic persist. Concerns have been raised as to whether allowing children to congregate in the classroom will fuel the spread of the pandemic. On an individual level, families are worried how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection could affect their children and family. Particular concern is elevated for families belonging to low socioeconomic classes, where the prevalence of SARS-CoV-2 infection is higher, and where multigenerational cohabitation is the norm, increasing the risk of transmitting the infection to vulnerable grandparents and older adults.1
The manner in which children contribute to the spread of SARS-CoV-2 is unclear. Children are less likely to become seriously ill from SARS-CoV-2; however, asymptomatic carriers, including children, can spread infection and carry virus into their household.2 , 3 Children infected with SARS-CoV-2 tend to have milder symptoms with significantly lower mortality than is seen in adult infection.4 It has been hypothesized that children have reduced incidence of COVID-19 because angiotensin-converting enzyme 2 (ACE2) expression in the nasopharynx increases with age; however, ACE2 expression has not been studied in the upper airways of children infected with SARS-CoV-2.5 Understanding infectious burden and the potential for transmissibility within the pediatric population is critical for developing both short- and long-term responses, including public health policies, to the current pandemic.
Although an acute SARS-CoV-2 infection tends to be mild or symptom free in most pediatric cases, some children develop a multisystem inflammatory syndrome in children (MIS-C) several weeks after possible SARS-CoV-2 infection or exposure, with severe cardiac complications, including hypotension, shock, and acute heart failure.6, 7, 8 Understanding postinfectious immune responses in pediatric SARS-CoV-2 infection, especially MIS-C, is critical for designing treatment and prevention strategies.9
Here, we describe the pediatric impact of COVID-19, specifically focusing on viral burden, susceptibility to disease, and immune responses.
Methods
Patient Selection
Pediatric patients ≤22 years of age presenting to Massachusetts General Hospital Respiratory Infection Control clinics for medical evaluation of symptoms concerning for COVID-19 or admitted for acute symptoms related to COVID-19 or MIS-C were offered enrollment in the Institutional Review Board (IRB)-approved Massachusetts General Hospital (MGH) Pediatric COVID-19 Biorepository (#2020P000955). For the ACE2 gene expression analysis, children presenting for well visits and newborns born during the COVID-19 pandemic were enrolled in the MGH Pediatric COVID-19 biorepository. For the virology and antibody studies, adult patients being evaluated for COVID-19 in the outpatient or inpatient setting were enrolled through the IRB-approved MGH COVID-19 biorepository (#2020P000804) (Table I; available at www.jpeds.com).
Once informed consent, and if appropriate, assent, were verbally obtained by the patients or parent/guardian in accordance with IRB guidelines, nasopharyngeal and oropharyngeal swabs were obtained and placed in phosphate buffered saline. The samples were immediately aliquoted and stored at −80°C. Venipuncture was performed; plasma and serum were collected and immediately stored at −80°C.
Study Definitions
Individuals with SARS-CoV-2 (+) had a nasopharyngeal swab sample positive for SARS-CoV-2 by clinical quantitative PCR (qPCR) testing. Individuals without SARS-CoV-2 (−) had negative nasopharyngeal qPCR testing. MIS-C was defined per the Centers for Disease Control and Prevention criteria: fever >38°C for >24 hours, laboratory evidence of inflammation, ≥2 organs involved, and no alternative plausible diagnoses and a positive SARS-CoV-2 test by RT-PCR, serology or antigen test, or exposure to an individual with COVID-19 within 4 weeks before the onset of symptoms.
Data Collection
Medical records were reviewed to assess demographic and clinical factors, including age, medical history, presenting features and clinical testing, household contacts, and other possible risk factors at presentation. Data were stored in a REDcap database.
SARS-CoV-2 Viral Load Quantification
SARS-CoV-2 RNA levels were quantified with a quantitative viral load assay using the US Centers for Disease Control and Prevention 2019-nCoV_N1 primers and probe set as previously described.10 Plasma and respiratory samples were centrifuged at approximately 21 000 × g for 2 hours at 4°C. RNA was extracted from serum and respiratory specimens using the TRIzol-LS (Thermo Fisher Scientific Inc, Waltham, Massachusetts)-based method, followed by RNA purification, and quantification with the 1 × TaqPath 1-Step RT-qPCR Master Mix, CG (Thermo Fisher). Quantification of the Importin-8 (IPO8) housekeeping gene RNA level was performed to determine the quality of the respiratory sample collection.11, 12, 13 An internal virion control (RCAS) was spiked into each sample and quantified to determine the efficiency of RNA extraction and qPCR amplification.14 SARS-CoV-2 pseudoviral reference standards (SeraCare, Milford, Massachusetts) were used as positive controls for each run. SARS-CoV-2 viral loads of <40 RNA copies/mL were categorized as undetectable and set at 1.0 log10 RNA copies/mL.
ACE2 Expression in the Upper Airway
The cDNA was transcribed from RNA extracted from nasopharyngeal and oropharyngeal swabs using TRIzol-LS reagent (Thermo Fisher) and then purified by isopropanol extraction. qPCR standards were created using a hACE2 plasmid and MEGAscript T7 transcription kit (Thermo Fisher), purified with the RNeasy MinElute spin column kit (Qiagen, Venlo, the Netherlands), and quantified by nanodrop. ACE2 and IPO8 Gene expression was assessed by qPCR using iTaq Universal SYBR Green mix (Bio-Rad Laboratories, Hercules, California) with ACE2 primers (forward: AAACATACTGTGACCCCGCAT; reverse: CCAAGCCTCAGCATATTGAACA) as previously used and IPO8 primers (Bio-Rad Laboratories, Hercules, California).15 ACE2 and IPO8 RNA were used to generate standard curve to quantitate copy numbers per sample and ACE2 expression relative to IPO8 was calculated as previous.16
IgG and IgM Titers Measured by ELISA
SARS2-CoV2-receptor binding domain (RBD) (in-house, HEK293 cells provided by Aaron Schmidt, Ragon Institute) and SARS2-CoV2-NC (Aalto Bio Reagents Ltd., Dublin, Ireland) specific plasma antibodies were quantified by ELISA. The average plus 5 × or 3 × standard deviation of included negative adult plasma controls were defined as negative cutoff for Immunoglobulin G (IgG) or Immunoglobulin M (IgM), respectively. SARS-CoV-2-RBD specific monoclonal human IgG1 or IgM antibody (clone: CR3022) was added in a 2-fold dilution curve starting at 2.5 μg/mL to each plate and specific IgG or IgM concentrations were calculated.
IgG1 and IgM Titers Measured by Luminex
SARS2-CoV2-RBD, SARS2-CoV2-NC, SARS2-CoV2-S (provided by Eric Fischer, Dana Farber), and RBD domains of the coronavirus strains NL-63, HKU1, 229E and OC43 (in-house, provided by Aaron Schmidt) specific antibody isotypes were analyzed by Luminex multiplexing.17 Antigens were carboxy-coupled to Luminex microspheres (Luminex Corp, Austin, Texas) and incubated with polyclonal plasma samples containing IgM and IgG1. Isotypes were probed with fluorophore tagged secondary antibody and relative concentrations analyzed by flow cytometry.
Statistical Analyses
The Mann-Whitney U test assessed statistical significance between 2 outcomes; the Kruskal-Wallis test assessed comparisons of continuous variables. For all categorical comparisons, the Fisher exact test was used. The Spearman rank correlation tested relationships between 2 variables. Prism software (Prism 8, Graphpad Software, San Diego, California) was used to analyze and graph data.
Results
The MGH Pediatric COVID-19 biorepository enrolled 192 patients (mean age, 10.2 ± 7.0 years), whose demographics are summarized in Table II (available at www.jpeds.com). Of all enrolled, 49 (26%) were SARS-CoV-2 (+), 18 (9%) had MIS-C, and 125 (65%) were SARS-CoV-2 (−).
Patient Demographics
Children ages 0-22 years participated in this study, with children ages 11-16 years most highly represented in the SARS-CoV-2 (+) cohort (16 [34%]) and children ages 1-4 years most highly represented in the MIS-C cohort (7 [39%]). Only 2 (4%) of the SARS-CoV-2 (+) cohort were <1 year of age, although this age group was previously reported as being at higher risk.18 Sex was equally distributed between children with and without acute SARS-CoV-2 infection, although there was a male predominance in the MIS-C group (14 [78%]). Latino/Hispanic children were most highly represented in both the SARS-CoV-2 (−) and SARS-CoV-2 (+) groups. Twenty-five children (51%) infected acutely with SARS-CoV-2 came from low-income communities, as compared with 1 (2%) from a high-income community (Fisher exact test, P < .001).
All children enrolled in the pediatric COVID-19 biorepository had the option of providing nasopharyngeal, oropharyngeal, and blood specimens for research. Eighty-three children provided a nasopharyngeal specimen, 105 provided an oropharyngeal specimen, and 100 provided a blood sample.
Presenting Symptoms
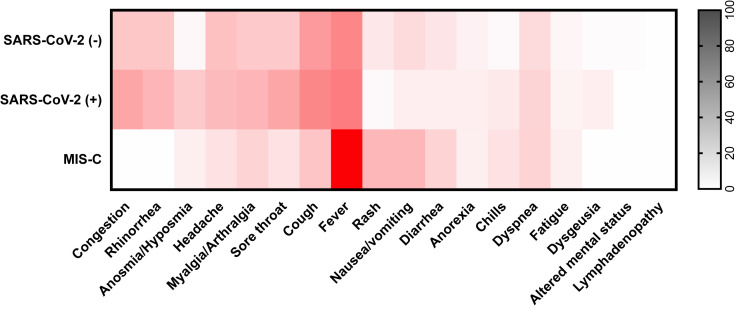
SARS-CoV-2 infection and non-COVID-19-related illnesses presented similarly. Both SARS-CoV-2 (−) and SARS-CoV-2 (+) children commonly reported fever (62 [40%] vs 25 [51%], respectively), cough (55 [36%] vs 23 [47%]), congestion (29 [19%] vs 17 [35%]), rhinorrhea (29 [19%] vs 14 [29%]), and headache (33 [21%] vs 13 [27%]), none of which were significantly different between the 2 groups. Anosmia was more common in the SARS-CoV-2 (+) group (3 [2%] vs 10 [20%]; P ≤ .001), as was sore throat (26 [28%] vs 17 [35%]; P = .04). In addition to fever, MIS-C presented more often with nausea/vomiting (5 [29%]; P < .001) and rash (5 [28%]; P < .001) and less often with symptoms of an upper respiratory tract infection. Temperatures documented on examination did not differ among the three cohorts (Figure 1 and Table III [Table III available at www.jpeds.com]).
Figure 1.
Presenting symptoms of enrolled patients. Darker color intensity depicts increased prevalence of a symptom within each cohort. Patients were grouped by SARS-CoV-2 qPCR results (positive or negative) or diagnosis of MIS-C.
Comorbidities
None of the SARS-CoV-2 (+) or MIS-C children had heart disease, hypertension, or diabetes, which are risk factors for infection in the adult population; however, 13 (27%) of SARS-CoV-2 (+) children were obese, as compared with 2 (11%) of the MIS-C cohort.19 Asthma was a common feature in SARS-CoV-2 (−) patients (29 [19%]) whereas SARS-CoV-2 (+) and MIS-C patient groups displayed typical population rates of asthma (6 [12%] and 2 [11%], respectively).20 Other pulmonary diseases, immune/autoimmune diseases, and neuro/neurodevelopmental diseases were assessed and were not seen in high levels in any cohort.
Nine children (18%) infected with SARS-CoV-2 and 10 children (56%) with MIS-C did not have a known infected household contact. Of the children acutely infected with SARS-CoV-2, 26 (53%) attended grade school. None of the 7 preschool/kindergarteners tested positive for SARS-CoV-2 or developed MIS-C.
SARS-CoV-2 Viral Load
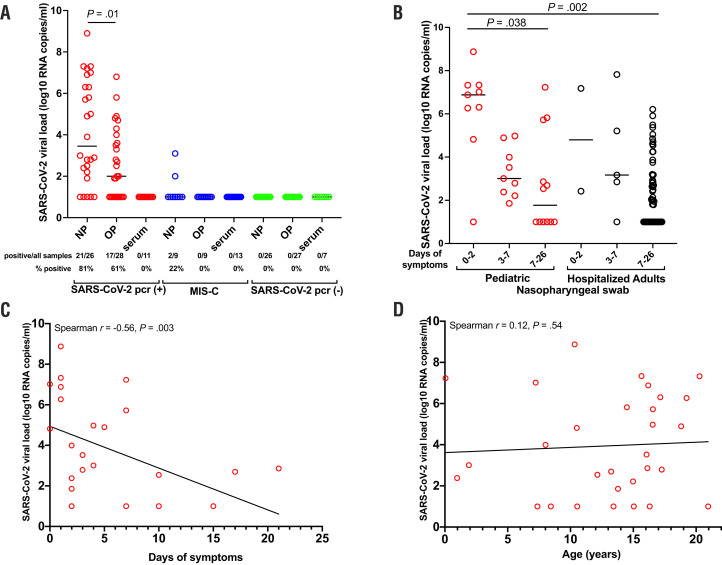
Nasopharyngeal and oropharyngeal swabs and serum were tested to quantify SARS-CoV-2 viral load. Higher levels of viral load were detected in nasopharyngeal swabs compared with oropharyngeal swabs (unpaired t test, P = .01; Figure 2, A). Only 2 children (11%) with MIS-C had a detectable viral load from nasopharyngeal swabs (Figure 2, A). The viral load in respiratory secretions of children was high, despite mild or absent symptoms, at 6.2 log10 RNA copies/mL (range, 1.0-8.9 log10 RNA copies/mL) during days 0-2 of symptoms. Of the 11 asymptomatic children presenting for SARS-CoV-2 testing based on exposure to an infected individual rather than symptoms, 3 (27%) tested positive for SARS-CoV-2 infection. Pediatric patients displayed no apparent difference in viral load compared with adults requiring intubation for severe SARS-CoV-2 infection when stratified by time. The viral load in children in the asymptomatic/early infection phase was significantly higher than in hospitalized adults with severe disease with over 7 days of symptoms (P = .002) (Figure 2, B). Nasopharyngeal viral load decreased over time (Spearman r = −0.56, P = .003) (Figure 2, C). Age did not impact the ability to carry a high viral load (Figure 2, D). Of note, our cohort included a limited number of infants, and children <6 years of age were less likely to provide a nasopharyngeal swab for research. No SARS-CoV-2 RNA was detected in the serum of any children (Figure 2, A).
Figure 2.
Infective SARS-CoV-2 viral load in children. A, Viral loads from nasopharyngeal, oropharyngeal, and blood were quantified within SARS-CoV-2 (+), MIS-C, and SARS-CoV-2 (−) cohorts. Viral load in nasopharyngeal and oropharyngeal specimens from SARS-CoV-2 (+) children were compared with the Mann-Whitney U test, median presented. B, SARS-CoV-2 viral loads were categorized by symptom duration, including asymptomatic period to day 2 of symptoms, days 3-7 of symptoms, and days 7-26 of symptoms. The median is presented and comparisons are by the Kruskal-Wallis test. Nasopharyngeal viral load was correlated with, C, days of symptoms and, D, age; Spearman correlation. NP, nasopharyngeal; OP, oropharyngeal.
SARS-CoV-2 Viral Binding Sites
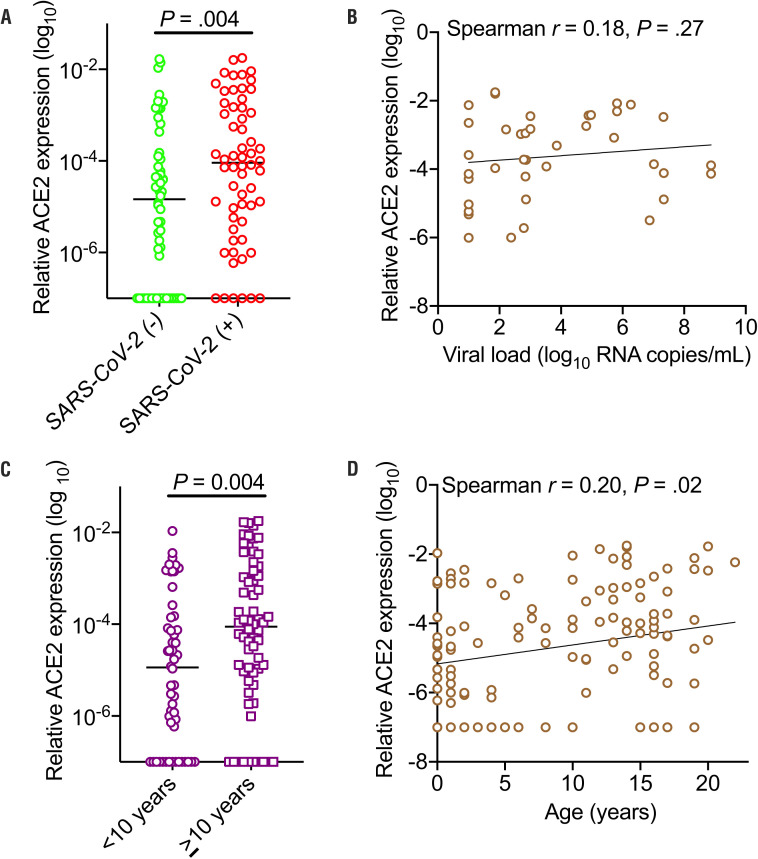
ACE2 gene expression was quantified from nasopharyngeal and oropharyngeal swabs of SARS-CoV-2 (+) and SARS-CoV-2 (−) children, plus from swabs from asymptomatic children presenting for well-visits and from newborns who were also enrolled in the MGH pediatric COVID-19 biorepository. ACE2 expression was higher in SARS-CoV-2-infected children (including MIS-C) as compared with noninfected children (P = .004) (Figure 3, A). Within the SARS-CoV-2-infected cohort, ACE2 expression did not correlate with viral load, suggesting that although increased ACE2 expression increased susceptibility for infection, once infected, children could carry high viral loads regardless of the level of ACE2 expression (Figure 3, B). Children <10 years had lower ACE2 expression as compared with older children (P = .004) (Figure 3, C). Within the pediatric cohort, ACE2 expression increased with age (Spearman r = 0.20, P = .02) (Figure 3, D).
Figure 3.
ACE2 expression in the upper airways of children. A, Relative expression of ACE2 (log10) categorized by SARS-CoV-2 infection, median presented, and significance tested by Mann-Whitney U test. B, Correlation of relative ACE2 expression and viral load (log10 RNA copies/mL); Spearman correlation. C, Relative expression of ACE2 (log10) categorized by age <10 years or ≥10 years, median presented, and significance tested by Mann-Whitney U test. D, Correlation of ACE2 expression with age; Spearman correlation.
SARS-CoV-2 Antibody Response
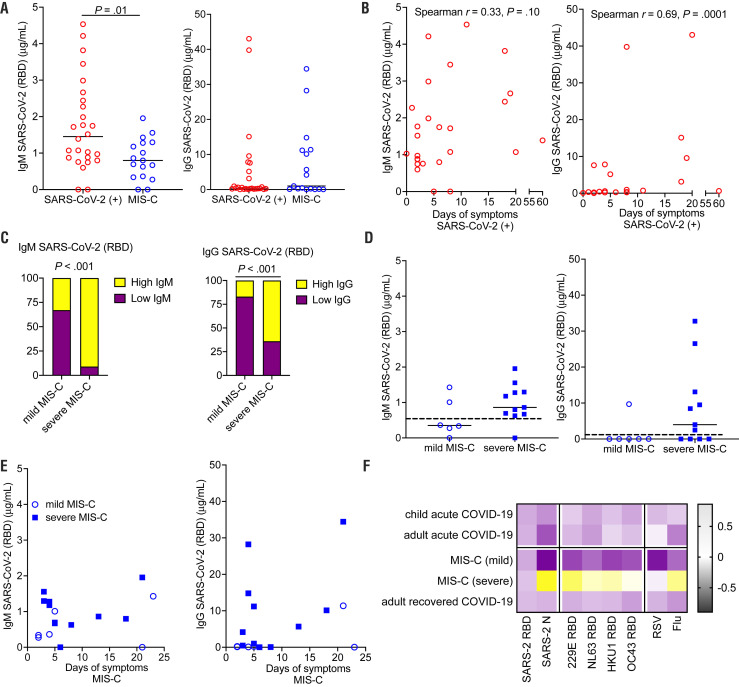
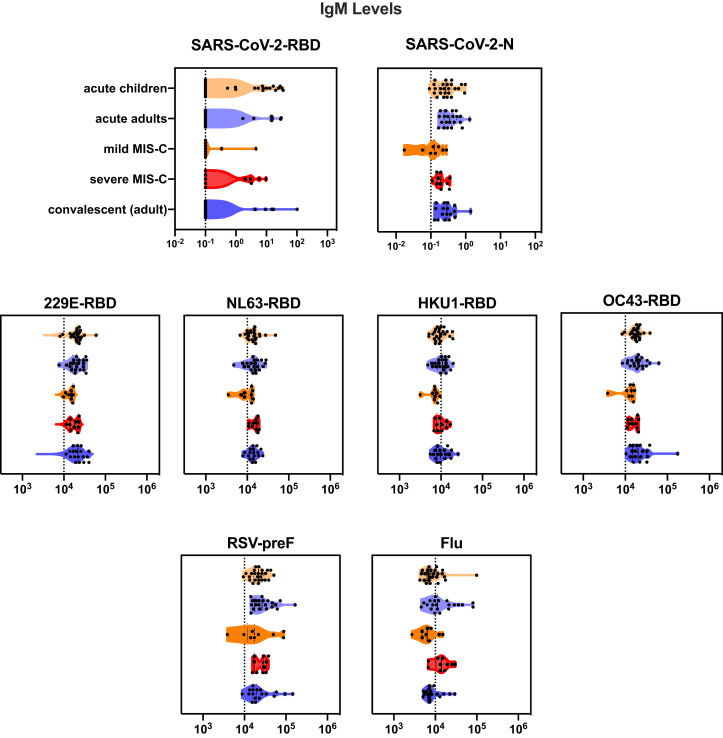
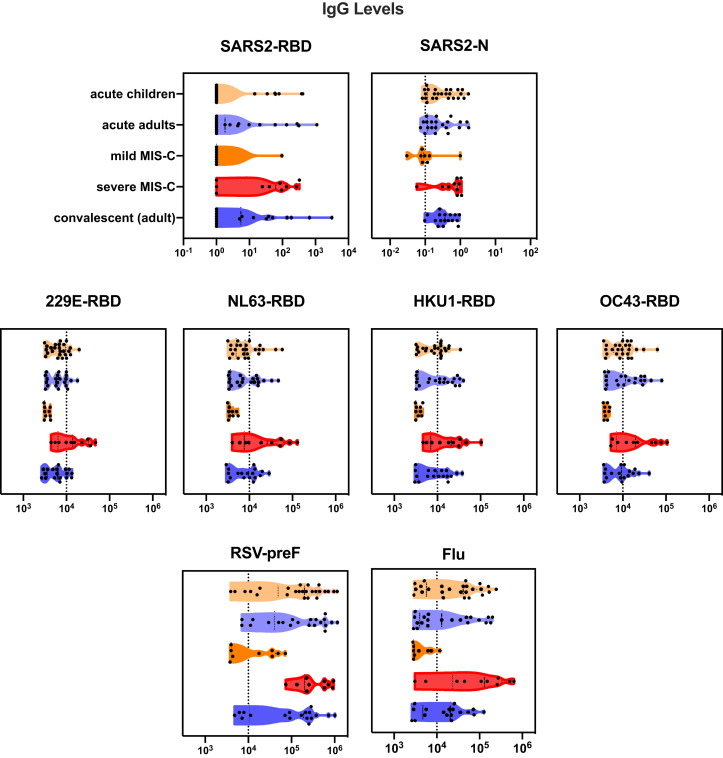
To determine immune responses to SARS-CoV-2 infection, antibodies to the RBD component of the spike protein of SARS-CoV-2 were quantified. Children with acute SARS-CoV-2 infection were more likely than those with MIS-C to have an elevated IgM to RDB (P = .01), consistent with the resolution of acute SARS-CoV-2 infection in children with MIS-C (Figure 4, A). IgG levels increased in acute SARS-CoV-2 infection with increased duration of symptoms (Spearman correlation, r = 0.44; P = .02) (Figure 4, B). Children with severe MIS-C (defined as children with MIS-C with hypotension or cardiac abnormalities requiring therapeutic intervention such as steroids, intravenous immunoglobulin, and/or anakinra) were more likely to have elevated IgM and IgG SARS-CoV-2 responses compared with mild MIS-C (Fisher exact test, each P < .001) (Figure 4, C). Both IgM and IgG SARS-CoV-2 levels in mild MIS-C were below a threshold of 0.5 mg/mL (Figure 4, D), consistent with waning immune responses seen in adults after acute infection.21 There was no correlation of IgG level with duration of symptoms seen in MIS-C (Figure 4, E). Children presenting with severe MIS-C tended to have broadly elevated IgG responses to a multitude of respiratory viruses, including other coronaviruses, 229E, NL63, HKU1, and OC43, respiratory syncytial virus (RSV), and influenza. This was not seen in milder cases of MIS-C, acute SARS-CoV-2 infection in children, adults hospitalized for SARS-CoV-2 infection, or recovered adults, pointing to a generalized enhancement of humoral immune responses as a marker of severe MIS-C (Figure 4, F, Figure 5 [available at www.jpeds.com], and Figure 6 [available at www.jpeds.com]).
Figure 4.
SARS-CoV-2 antibody response in children infected with SARS-CoV-2. A, Peak IgM and IgG to the RBD component of SARS-CoV-2 were quantified for children acutely infected with SARS-CoV-2 and children presenting with MIS-C. Comparison by Mann-Whitney U tests; median presented. B, IgM and IgG responses in acute SARS-CoV-2 infection were correlated with days of symptoms; Spearman correlation. C, Percent of children mild vs severe MIS-C with elevated IgM or IgG (above a threshold of 0.5 μg/mL) were compared by Fisher exact test. D, Peak IgM and IgG levels were compared between mild and severe MIS-C, Mann-Whitney U tests; median presented. Dotted line represents 0.5 μg/mL threshold for defining high or low antibody response, E, IgM and IgG responses in acute SARS-CoV-2 infection were correlated with days of symptoms; Spearman correlation. F, Heat map depicts relative IgG responses to SARS-CoV-2 RBD and SARS-CoV-2 N capsid protein, other coronaviruses (strains 229E, NL63, HKU1, and OC43), RSV, and influenza (flu). In addition to showing antibody response for children with acute SARS-CoV-2 infection and mild and severe MIS-C, antibody levels from adults with acute SARS-CoV-2 and adults recovered from SARS-CoV-2 infection are displayed.
Figure 5.
Violin plot of IgM antibodies to SARS-CoV-2 RBD and SARS-CoV-2 N capsid protein, other coronaviruses (strains 229E, NL63, HKU1, and OC43), and RSV and influenza.
Figure 6.
Violin plot of IgG antibodies to SARS-CoV-2 RBD and SARS-CoV-2 N capsid protein, other coronaviruses (strains 229E, NL63, HKU1, and OC4), RSV, and influenza.
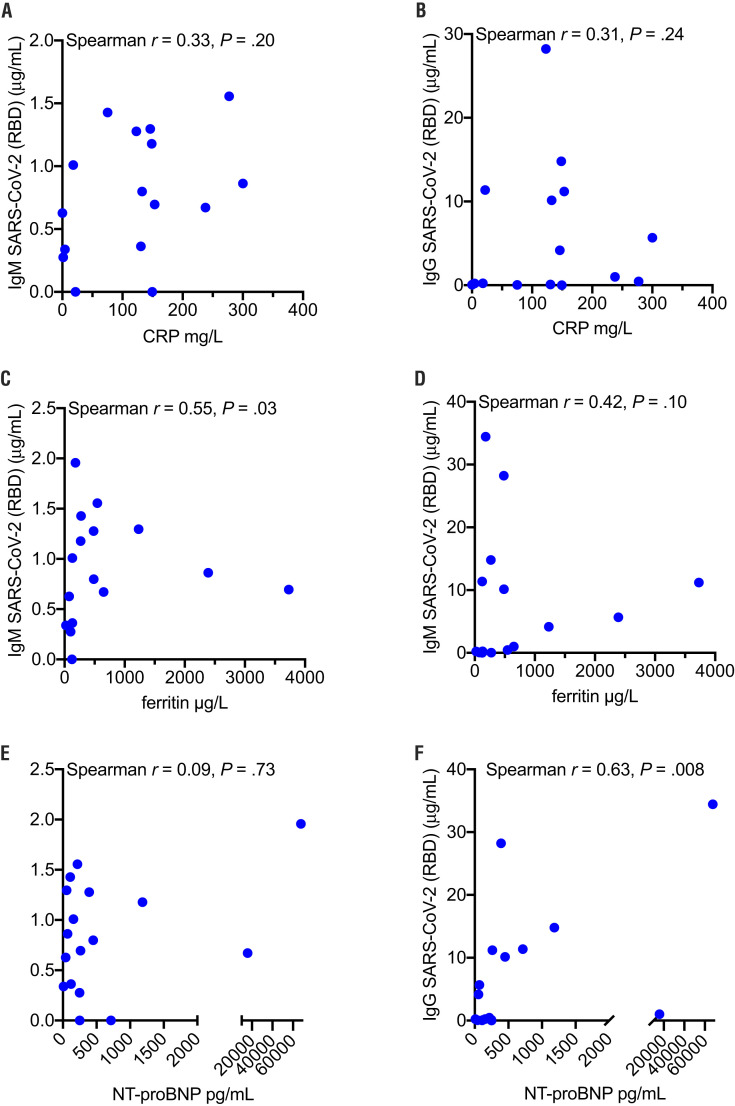
To further characterize the inflammatory response in MIS-C, correlations between SARS-CoV-2 antibodies and inflammatory markers were analyzed. These included C-reactive protein, a generalized inflammatory marker; ferritin, a marker of macrophage activation; and N-terminal pro b-type natriuretic peptide (NT-proBNP), a peptide secreted by cardiomyocytes during heart failure (Figure 7; available at www.jpeds.com). A positive correlation was found between ferritin and both IgM SARS-CoV-2 (Spearman correlation r = 0.55; P = .03) and IgG SARS-CoV-2 (Spearman correlation r = 0.42; P = .10), suggesting an interplay between monocytes/macrophages and SARS-CoV-2 antibodies in MIS-C. A significant correlation was noted with NT-proBNP and IgG SARS-CoV-2 (Spearman correlation r = 0.63; P = .008), although there was no correlation between NT-proBNP and IgM SARS-CoV-2.
Figure 7.
Correlation of inflammatory markers and SARS-CoV-2 antibody responses in MIS-C. IgM and IgG SARS-CoV-2 (RBD component) were correlated with A and B, C-reactive protein (CRP), respectively; C and D, ferritin, respectively; and E and F, NT-proBNP, respectively. Spearman correlation.
Discussion
We present findings from the largest pediatric COVID-19 biospecimen repository, describing viral load, ACE2 expression, and antibody responses as they relate to children with acute SARS-CoV-2 infection and MIS-C. We found that children can carry high levels of virus in their upper airways, particularly early in an acute SARS-CoV-2 infection, yet they display relatively mild or no symptoms. However, there was no age correlation with viral load, indicating that infants through young adults can carry equally high levels of virus. However, SARS-CoV-2-infected children have higher levels of ACE2 expression, which may predispose certain children to infection. Children with MIS-C do not have high levels of viral load on nasopharyngeal or oropharyngeal viral testing, nor do they have detectable viremia; however, they do have hyperactive antibody responses.
From an infection control perspective, it is critical to identify infected children early for quarantine purposes. One-third of school-aged children presenting with illness during the height of the local pandemic were found to have SARS-CoV-2 infection. However, children display relatively mild or no symptoms. Although ACE2 expression was increased in SARS-CoV-2-infected children, ACE2 expression did not impact viral load within the upper airway. Similarly, although younger children had a decreased ACE2 expression, age also did not impact viral load. This finding suggests that, regardless of disease susceptibility, children can carry high viral loads, which is a key consideration when opening up schools and daycare centers.
Moreover, when present, the symptoms of SARS-CoV-2 are nonspecific and overlap considerably with non-COVID-related illnesses. Identifying SARS-CoV-2 infection in children will become even more challenging during pollen allergy season and influenza season this fall. Further, some children carry very high viral loads even before symptoms develop. In contrast, children with severe symptoms, such as MIS-C, do not have high levels of viral load on nasopharyngeal or oropharyngeal viral testing, nor do they have detectable viremia. Overall, the lack of correlations between viral load and symptoms will complicate infection control strategies for children.
Children with severe MIS-C have elevated SARS-CoV-2 IgM and IgG levels; IgG levels are not only elevated in SARS-CoV-2, but also in the other coronaviruses, influenza, and RSV. The broad, nonspecific antibody response points to T- and B-cell over-reactivity, or to auto-antibodies that may be driving an inflammatory process causing MIS-C.22 Elevated ferritin levels in MIS-C, which positively correlate with SARS-CoV-2 serology, also suggest an interplay with macrophage activation. Further, SARS-CoV-2 IgG are positively correlated with NT-proBNP, a marker of heart failure, which could indicate mechanism of disease or provide a correlation with disease severity.
Limiting the spread of SARS-CoV-2 infections in children is of particular concern as schools plan for reopening. Our findings suggest that it would be ineffective to rely on symptoms or temperature monitoring to identify SARS-CoV-2 infection. Instead, infection control measures should minimize the possibility of viral spread, with focus on strategies including social distancing precautions, mask use, and/or remote learning. Moreover, schools could screen all students for SARS-CoV-2 infection and establish routine screening protocols. Without infection control measures such as these, there is significant risk that the pandemic will persist, and children could carry the virus into the home, exposing adults who are at greater risk of developing severe disease. This risk is particularly high in lower income communities, where household size may be larger with multigenerational cohabitation and greater housing density. These recommendations contradict previous reports from the initial phase of the pandemic, which found children to be less likely to be the index case for viral transmission within a household.23 However, in our cohort, nearly 20% of acute SARS-CoV-2 infections and more than one-half of the MIS-C cases did not have a known household exposure to SARS-CoV-2. Although transmissibility was not assessed in this study, children with high viral loads and nonspecific symptoms including rhinorrhea and cough can likely transmit SARS-CoV-2 as easily as other viral infections spread by respiratory particles. If schools were to reopen fully without necessary precautions, it is likely that children will play a larger role in this pandemic.
Our initial findings show that although a low expression of ACE2 in younger children (<10 years of age) likely corresponds to reduced infection rates, children of all ages, once infected, can carry high SARS-CoV-2 viral loads. Symptom monitoring is an ineffective strategy for identifying infected children. Children can develop severe illness during the postinfectious stage with a hyperinflammatory antibody response. Potential transmission of SARS-CoV-2 between children and families should be considered when designing strategies to mitigate the COVID-19 pandemic.
Data Statement
Data sharing statement available at www.jpeds.com.
Footnotes
Supported by the National Heart, Lung, and Blood Institute (5K08HL143183 to L.Y.), the Cystic Fibrosis Foundation (YONKER18Q0 to L.Y.), the National Institute of Child Health and Human Development (K08 HD094638 [to A.N.] and R01HD100022 [to A.E.]), Mark and Lisa Schwartz (to J.L.), the National Institute of Diabetes and Digestive and Kidney Diseases (DK039773, DK072381 [to J.B.] and DK104344 [to A.F.]), the National Institute of Allergy and Infectious Disease (K24AI141036 to I.B.), the Centers for Disease Control and Prevention (U01CK000490 to E.R.), and the Department of Pediatrics and the Department of Obstetrics/Gynecology at Massachusetts General Hospital (to L.Y. and A.E.). The authors declare no conflicts of interest.
Appendix
Table I.
Adult samples included for comparative purposes in virology and antibody assays
| Characteristics | Adult patients, hospitalized for COVID-19 |
|---|---|
| Virology assays (n = 162) | |
| Age, years | 58 ± 16 |
| Male sex | 107 (66.05) |
| BMI | 29.3 ± 6.6 |
| Past medical history | |
| Hypertension | 97 (60) |
| Active cancer | 3 (2) |
| Chronic lung disease | 32 (20) |
| Diabetes | 75 (46) |
| Intubated | 100 (62) |
| Deaths | 22 (14) |
| Antibody assays (n = 39) | |
| Age years, mean (SD) | 39 ± 16 |
| Urgent care, total | 21 (54) |
| SARS-CoV-2 (+) | 12 (57) |
| Recovered | 18 (46) |
Values are mean ± SD or number (%).
Table II.
Patient characteristics (n = 192) of children not infected with SARS-CoV-2, children with SARS-CoV-2 infection, and children diagnosed with MIS-C
| Patient characteristics | SARS-CoV-2 (−) (n = 125) | SARS-CoV-2 (+) (n = 49) | MIS-C (n = 18) |
|---|---|---|---|
| Age, years | 9.6 ± 7.1 | 12.7 ± 6.3 | 7.7 ± 7.0 |
| Age group | |||
| <1 year | 11 (8.8) | 2 (4.3) | 2 (11.1) |
| 1-4 years | 32 (25.6) | 5 (10.6) | 7 (38.9) |
| 5-10 years | 29 (23.2) | 11 (23.4) | 4 (22.2) |
| 11-16 years | 26 (20.8) | 16 (34.0) | 2 (11.1) |
| 17-22 years | 27 (21.6) | 13 (27.7) | 3 (16.7) |
| Male sex | 67 (53.6) | 23 (46.9) | 14 (77.8) |
| Race | |||
| American Indian/Alaska Native | 0 (0) | 0 (0) | 0 (0) |
| Asian | 7 (5.6) | 1 (2.0) | 1 (5.6) |
| Black or African American | 5 (4.0) | 4 (8.2) | 2 (11.1) |
| Native Hawaiian/Pacific Islander | 0 (0) | 0 (0) | 0 (0) |
| White | 43 (34.4) | 7 (14.3) | 9 (50.0) |
| Unknown | 26 (20.8) | 10 (20.4) | 2 (11.1) |
| Ethnicity | |||
| Latino/Hispanic | 63 (50.4) | 29 (59.2) | 6 (33.3) |
| Non-Latino/Non-Hispanic | 43 (34.4) | 11 (22.4) | 10 (55.6) |
| Past medical history | |||
| History of cardiac or metabolic disease | |||
| Congenital heart disease | 4 (3.2) | 0 (0) | 0 (0) |
| Hypertension | 3 (2.4) | 0 (0) | 0 (0) |
| Diabetes type 1 | 1 (0.8) | 0 (0) | 0 (0) |
| Diabetes type 2 | 0 (0) | 0 (0) | 0 (0) |
| Dyslipidemia | 0 (0) | 2 (4.1) | 0 (0) |
| Obesity | 12 (9.6) | 13 (26.5) | 2 (11.1) |
| History of pulmonary disease | |||
| Asthma | 26 (20.8) | 6 (12.2) | 2 (11.1) |
| Pneumonia | 5 (5.6) | 3 (6.1) | 0 (0) |
| History of preterm delivery | 11 (8.8) | 2 (4.1) | 1 (5.6) |
| Cystic fibrosis | 0 (0) | 0 (0) | 0 (0) |
| History of immune/autoimmune disease | |||
| Rheumatologic disease | 1 (0.8) | 0 (0) | 0 (0) |
| Inflammatory bowel disease | 0 (0) | 1 (2.0) | 1 (5.6) |
| Immunodeficiency | 0 (0) | 0 (0) | 0 (0) |
| History of neuro/neurodevelopmental disorders | |||
| Seizure | 5 (4.0) | 7 (14.3) | 0 (0) |
| Attention deficit hyperactivity disorder | 12 (9.6) | 5 (10.2) | 1 (5.6) |
| Autism | 2 (1.6) | 1 (2.0) | 1 (5.6) |
| Cerebral palsy | 0 (0) | 2 (4.1) | 0 (0) |
| Down syndrome | 1 (0.8) | 0 (0) | 0 (0) |
| Vaccinations up to date | 101 (80.8) | 41 (83.7) | 14 (77.8) |
| Household exposures | |||
| Mother | 21 (16.8) | 20 (40.8) | 4 (22.2) |
| Father | 11 (8.8) | 13 (26.5) | 2 (11.1) |
| Sibling | 8 (6.4) | 9 (18.4) | 1 (5.6) |
| Other | 19 (15.2) | 9 (18.4) | 5 (27.8) |
| No household exposure | 70 (56.0) | 9 (18.4) | 10 (55.6) |
| Daycare/school | |||
| Nanny/home daycare | 27 (21.6) | 6 (12.2) | 7 (38.9) |
| Group daycare | 7 (5.6) | 1 (2.0) | 1 (5.6) |
| Preschool/kindergarten | 7 (5.6) | 0 (0) | 0 (0) |
| Grade school | 48 (38.4) | 26 (53.1) | 6 (33.3) |
| College | 4 (3.2) | 2 (4.1) | 0 (0) |
| Unknown | 32 (25.6) | 14 (28.6) | 4 (22.2) |
Values are average ± SD or number (%).
Table III.
Presenting symptoms of enrolled patients
| Symptoms reported | Comparison of acute SARS-CoV-2 (+) and MIS-C |
||||
|---|---|---|---|---|---|
| Comparison of SARS-CoV-2 (−) and (+) |
MIS-C | P value | |||
| SARS-CoV-2 (−) | SARS-CoV-2 (+) | P value | |||
| Congestion | 27 (21.6) | 17 (34.7) | .08 | 0 (0) | .002 |
| Rhinorrhea | 27 (21.6) | 14 (28.6) | .33 | 0 (0) | <.001 |
| Anosmia/hyposmia | 3 (2.4) | 10 (20.4) | <.001 | 1 (5.6) | .005 |
| Headache | 30 (24.0) | 13 (26.5) | .75 | 2 (11.1) | .006 |
| Myalgia/arthralgia | 26 (20.8) | 14 (28.6) | .25 | 3 (16.7) | .06 |
| Sore throat | 26 (20.8) | 17 (34.7) | .04 | 2 (11.1) | <.001 |
| Cough | 49 (39.2) | 23 (46.9) | .32 | 4 (22.2) | .003 |
| Fever | 59 (47.2) | 25 (51.0) | .67 | 18 (100.0) | <.001 |
| Rash | 11 (8.8) | 1 (2.0) | .06 | 5 (27.8) | <.001 |
| Nausea/vomiting | 17 (13.6) | 3 (6.1) | .10 | 5 (27.8) | <.001 |
| Diarrhea | 12 (9.6) | 3 (6.1) | .44 | 3 (16.7) | .02 |
| Anorexia | 6 (4.8) | 3 (6.1) | >.99 | 1 (5.6) | >.99 |
| Chills | 2 (1.6) | 4 (8.2) | .10 | 2 (11.1) | .63 |
| Dyspnea | 17 (13.6) | 8 (16.3) | .84 | 3 (16.7) | >.99 |
| Fatigue | 4 (3.2) | 2 (4.1) | >.99 | 1 (5.6) | .75 |
| Dysgeusia | 1 (0.8) | 3 (6.1) | .12 | 0 (0) | .03 |
| Altered mental status | 1 (0.8) | 0 (0) | >.99 | 0 (0) | >.99 |
| Lymphadenopathy | 0 (0) | 0 (0) | N/A | 0 (0) | N/A |
N/A, not applicable.
Comparisons between symptoms reported in acute SARS-CoV-2 infection and non-SARS-CoV-2 illnesses, and SARS-CoV-2 (+) and MIS-C are compared by Fisher exact test. Values are number (%).
Supplementary Data
References
- 1.Clark E., Fredricks K., Woc-Colburn L., Bottazzi M.E., Weatherhead J. Disproportionate impact of the COVID-19 pandemic on immigrant communities in the United States. PLoS Negl Trop Dis. 2020;14:e0008484. doi: 10.1371/journal.pntd.0008484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Team C.C.-R. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furukawa N.W., Brooks J.T., Sobel J. Evidence supporting transmission of severe acute respiratory syndrome oronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26:e201595. doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S., et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belhadjer Z., Meot M., Bajolle F., Khraiche D., Legendre A., Abakka S., et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048360. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Fialkowski A., Gernez Y., Arya P., Weinacht K.G., Bernard Kinane T., Yonker L.M. Insight into the pediatric and adult dichotomy of COVID-19: age-related differences in the immune response to SARS-CoV-2 infection. Pediatr Pulmonol. 2020 doi: 10.1002/ppul.24981. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Fajnzylber J.M., Regan J., Coxen K., Corry H., Wong C., Rosenthal A., et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. medRxiv. 2020;2020 doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguewa P.A., Agorreta J., Blanco D., Lozano M.D., Gomez-Roman J., Sanchez B.A., et al. Identification of importin 8 (IPO8) as the most accurate reference gene for the clinicopathological analysis of lung specimens. BMC Mol Biol. 2008;9:103. doi: 10.1186/1471-2199-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riemer A.B., Keskin D.B., Reinherz E.L. Identification and validation of reference genes for expression studies in human keratinocyte cell lines treated with and without interferon-gamma - a method for qRT-PCR reference gene determination. Exp Dermatol. 2012;21:625–629. doi: 10.1111/j.1600-0625.2012.01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizzolla A., Wang Z., Groom J.R., Kedzierska K., Brooks A.G., Reading P.C., et al. Nasal-associated lymphoid tissues (NALTs) support the recall but not priming of influenza virus-specific cytotoxic T cells. Proc Natl Acad Sci U S A. 2017;114:5225–5230. doi: 10.1073/pnas.1620194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer S., Wiegand A.P., Maldarelli F., Bazmi H., Mican J.M., Polis M., et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma D., Chen C.B., Jhanji V., Xu C., Yuan X.L., Liang J.J., et al. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye (Lond) 2020;34:1212–1219. doi: 10.1038/s41433-020-0939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yahya M., Rulli M., Toivonen L., Waris M., Peltola V. Detection of host response to viral respiratory infection by measurement of messenger RNA for MxA, TRIM21, and viperin in nasal swabs. J Infect Dis. 2017;216:1099–1103. doi: 10.1093/infdis/jix458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown E.P., Licht A.F., Dugast A.S., Choi I., Bailey-Kellogg C., Alter G., et al. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J Immunol Methods. 2012;386:117–123. doi: 10.1016/j.jim.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 19.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knorr R.S., Condon S.K., Dwyer F.M., Hoffman D.F. Tracking pediatric asthma: the Massachusetts experience using school health records. Environ Health Perspect. 2004;112:1424–1427. doi: 10.1289/ehp.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu A., Li Y., Peng J., Huang Y., Xu D. Antibody responses against SARS-CoV-2 in COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.26241. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng M.H., Zhang S., Porritt R.A., Arditi M., Bahar I. An insertion unique to SARS-CoV-2 exhibits superantigenic character strengthened by recent mutations. bioRxiv. 2020 [Google Scholar]
- 23.Posfay-Barbe K.M., Wagner N., Gauthey M., Moussaoui D., Loevy N., Diana A., et al. COVID-19 in children and the dynamics of infection in families. Pediatrics. 2020;146:e20201576. doi: 10.1542/peds.2020-1576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.