Highlights
-
•
Educational mobile text messages were effective to control the severity of ECC.
-
•
They influenced changes in parental reports about children’s sugar consumption.
-
•
Also, they seemed beneficial to improve parental electronic health literacy levels.
Keywords: eHealth, Early childhood caries, Dental caries, Randomized controlled trial, mHealth
Abstract
Objectives
To evaluate the effectiveness of educational messages as an aid in the control of early childhood caries (ECC) in low socioeconomic children.
Methods
A single-blinded, randomized, and parallel-group study was conducted with 104 dyads of parents and children aged between 36–60 months, recruited in preschools from Bauru, Brazil. The participants were randomly allocated into control and intervention groups (1:1), stratified by parental eHealth literacy scores (eHEALS) and children's caries experience. Every 2 weeks, text messages were sent to parents of intervention group via WhatsApp. Visible plaque index (VPI) and the International Caries Detection and Assessment System (ICDAS) were assessed at baseline, 3- and 6-month follow-ups, while eHEALS and dietary habits were determined at baseline and 6-month follow-up. Statistical analysis was performed to intra and intergroup comparisons through Fischer’s exact and McNemar tests, and Mann-Whitney U and Friedman tests, respectively (P < 0.05).
Results
Despite similarities between groups, intervention increased parental eHEALS scores, influencing the reports about the children’s consumption of sugar-free sweets and controlling the severity of ECC.
Conclusion
Therefore, mobile text messages were effective to control the severity of ECC in low socioeconomic preschoolers, improving parental eHealth literacy and changing children’s dietary patterns.
Clinical significance
These findings demonstrate that parental-oriented WhatsApp messages can contribute to oral health education of socioeconomic vulnerable parents towards risk behavior changes to control ECC.
1. Introduction
Early childhood caries (ECC) is defined as the presence of one or more decayed (non-cavitated or cavitated lesions), missing (due to caries), or filled surfaces, in any primary tooth of a child under age six [1]. It has a complex etiology, being a biofilm- and sugar-dependent disease, influenced by environmental, socioeconomic, biological, and behavioral factors [2]. ECC is the tenth most prevalent non-communicable disease worldwide [3], impacting the quality of life of preschoolers and their families [4], because its physical, social, and psychological damages [5].
The prevalence of ECC is higher among low socioeconomic populations, due to several aspects, such as negative health beliefs, external locus of control, and limited levels of self-efficacy, oral health knowledge, and health literacy [[5], [6], [7]], which hamper the adherence and engagement of parents with strategies focused on behavior changes. The influence of these drawbacks on oral health outcomes may be overcame by parental education [8], in a context of family- and child-centered care [9]. Thus, personal approaches as motivational interviews, home visits, and telephone contacts have proven effective in the management of the disease [10,11].
However, barriers found in developing countries, such as low income, limited health infrastructure and high costs of services, prevent the access of families and children to health education [[12], [13], [14]]. In Brazil, 29 % of adults are functional illiterates, with 71 % presenting inadequate oral health literacy [15,16], i.e., people more prone to underuse preventive services and misinterpret health information [17].
The dissemination of information and communication technologies (ICTs) empowered individuals to make decisions about their own health conditions [18,19]. For instance, 101 million of Brazilian people access the Internet using their smartphones [20]. It is well-known that mobile health (mHealth) interventions based on text messages (reminder, alert, motivation, and prevention) have a significant potential to improve the self-management of non-communicable diseases [[21], [22], [23]], such as diabetes, hypertension, and obesity [24,25]. These strategies are also being considered promising to improve oral hygiene, plaque removal, gingivitis, and oral health literacy [26,27].
Taking into account the lack of clinical trials testing specific interventions to the management of ECC and its risk factors [5], the aim of this study was to evaluate the effectiveness of oral health educational text messages to aid in the control of early childhood caries in low socioeconomic children, considering caries experience, dental biofilm, and dietary habits. The hypotheses were that educational mobile text messages would have beneficial effects on the control of visible dental plaque, dental caries, and sugar consumption in children, and on the improvement of eHealth literacy of parents or caregivers after 6-month follow-up.
2. Methods
2.1. Trial design
This study was designed as a single-blind, 2-parallel arm, and randomized controlled trial (RCT) with 6-month follow-up [28]. The research protocol was reviewed and approved by the Council on Ethics in Human Research from the Bauru School of Dentistry, in accordance with the ethical standards of the Declaration of Helsinki, registered in the Brazilian Registry of Clinical Trials, and assigned with the universal trial number U111-1216-1393. This article was written in accordance with the checklist and guidelines of the Consolidated Standards of Reporting Trials (CONSORT) [29].
2.2. Participants
Dyads of parents and children were recruited during visits to preschools from Bauru, Brazil, if satisfying the following inclusion criteria: (i) children aged between 36 and 60 months; (ii) children with low socioeconomic levels; (iii) children with an ICDAS score < 4 [30]; (iv) parents or caregivers with a mobile phone with Internet access; (v) parents or caregivers who accepted to participate in all stages of research by signing a written informed consent form; and (vi) parents or caregivers who already had WhatsApp Messenger app installed on their smartphones, or those who agreed to install it for participating in the study.
The risk of dental caries of participants was determined based on the criteria of caries risk assessment of the American Academy of Pediatric Dentistry [31], through information collected from a questionnaire containing sociodemographic and child-related health information, such as age, gender, race, and education level, and from a dental examination performed by two trained and calibrated dentists (ML and APS).
2.3. Intervention
Every 2 weeks, the parents or caregivers of the intervention group received educational mobile text messages related to the management of ECC via WhatsApp Messenger. The electronic messages were conceptualized using simple and understandable language in accordance with the findings reported by Lotto et al. [32], and based on the principles of the Health Belief Model [33], to increase the parental awareness about the risk factors and consequences of ECC towards the adoption of healthy behaviors [34]. The main characteristics of the messages are summarized in Table 1 .
Table 1.
Characteristics of educational text messages.
| Messages | Topics and Aims | Word counts |
|---|---|---|
| #1 | Concept of early childhood caries | 71 |
| Aim: to explain that preschoolers are also affected by dental caries, presenting its negative impact on children's quality of life | ||
| #2 | Toothbrushing | 142 |
| Aim: to present and reinforce important aspects about toothbrushes and toothpastes | ||
| #3 | Frequency of oral hygiene | 128 |
| Aim: to define the appropriate frequency of toothbrushing and flossing | ||
| #4 | Antibiotics and dental caries | 125 |
| Aim: to elucidate the antibiotics usage is not the cause of ECC against a common negative health belief | ||
| #5 | ECC as a controllable disease | 98 |
| Aim: to clarify that dental caries is a controllable disease independently of systemic factors | ||
| #6 | Sugar consumption | 85 |
| Aim: to emphasize the low consumption of fermentable sugars for children, to prevent ECC | ||
| #7 | Fluoride usage | 143 |
| Aim: to explain the importance of fluoride to control ECC | ||
| #8 | Parental responsibility for children’s oral hygiene | 110 |
| Aim: to show the need of parental engagement in all aspects related to children’s oral hygiene | ||
| #9 | Dental demineralization | 128 |
| Aim: to educate parents or caregivers about dental demineralization | ||
| #10 | Dental caries consequences | 94 |
| Aim: to inform parents or cargivers about negative consequences of ECC | ||
| #11 | Frequency of dental visits | 80 |
| Aim: to advise parents or caregivers about the importance of periodic dental visits | ||
| #12 | Mouthwashes | 147 |
| Aim: to present the recommendation against the use of mouthwashes for preschoolers | ||
| #13 | Diet | 112 |
| Aim: to demonstrate the relationship between the high consumption of sugary-rich foods and ECC |
Simultaneously, audio narration readings were also sent to parents and caregivers, to improve their access to the educational contents. The participants were instructed to activate the function read receipts to confirm their adherence and engagement with the intervention; however, some parents or caregivers seemed disinterested in receiving the messages consistently over time (non-engaged participants). It was interpreted as a perception of self-sufficiency of parents in dealing adequately with their children’s oral health care.
2.4. Measurements and outcomes
All children were examined in the preschools by 2 trained and calibrated dentists (ML and APS) (kappa intraexaminer = 0.72 and 0.76; kappa interxaminer = 0.71) at baseline, 3- and 6-month follow-ups.
The training and calibration of the International Caries Detection and Assessment System (ICDAS) [30] were performed by an official calibrator (DR), including (i) 4 h of didactic sessions with presentation of images, and discussions about ICDAS codes and examination protocols, (ii) 2 days of training with teeth coded with ICDAS between 0 and 6, through the examination of patients and extracted teeth in the laboratory, and (iii) the review of findings to identify differences in the interpretation of exams until reaching the consensus between the investigators.
Children were examined positioned on school desks with the aid of artificial lighting. Initially, the visible plaque index (VPI) [35] was determined by the assessment of buccal surfaces of 6 deciduous teeth (#55, #53, #51, #71, #73 and #75) according to the absence (0) or presence (1) of dental plaque. The indices were calculated by the ratio of the sum of all codes per the number of assessed teeth, with values ranging between 0 and 1.
Subsequently, dental surfaces were cleaned with an electric toothbrush (Vitality Precision Clean, Oral B, P&G, Cincinnati, United States) to evaluate dental caries. The proportions of caries-free participants (ICDAS = 0), and children who increased their maximum ICDAS code (≥ 1) or diagnosed with dentin caries lesions (ICDAS ≥ 4) were considered to the analysis of individual-based changes in dental caries severity over time. Also, the mean ICDAS/teeth was calculated by the ratio of the sum of all codes by the number of teeth, with values varying from 0 to 6, to the analysis of group-based changes in dental caries progression for 6 months.
Additionally, parents or caregivers were asked to respond a validated Brazilian version of the eHealth Literacy Scale (eHEALS) [36] and a questionnaire about dietary habits developed by Llena and Forner [37], at baseline and 6-month follow-up. The eHEALS was applied by a trained professional who provided the participants with a sheet containing 8 items related to skills needed for the adequate consumption of eHealth information. The answers of each item were arranged into a 5-point Likert scale, with options ranging from completely agree to strongly disagree. The participants were instructed to classify each item according to their own perception, achieving a total score varying from 8 to 40, with higher scores representing higher self-perceived eHealth literacy [36].
The dietary habits were determined according to the frequency of consumption of 9 categories of foods: (1) foods containing sticky sugars: dried fruit, candies containing sugar, jellies, jams, and sauces; (2) food containing starch and sugar: cookies, cereals, and industrialized cakes; (3) candy without sugars; (4) milk and dairy products containing sugar: chocolate, yogurt, creams, ice creams and flans; (5) milk and dairy products without sugar: pure milk, sugar-free yogurt, and cheese; (6) sugary beverages: juices and soft drinks; (7) fruits: fruits and juices; (8) semi hydrolyzed starch-rich foods: potato chips, French fries, industrialized bread, and rolls; and (9) sugar-free foods: nuts, bread, pasta, and noodles. The frequencies of consumption were classified as high (every day or every week) or low (once a month or never) levels [37].
2.5. Sample size
The calculation of sample size was performed using the Open Source Epidemiologic Statistics for Public Health, following the criteria and outcomes described by Zotti et al. [38]. It was based on the caries incidence rates of control (31.4 %, 11 out of 35) and intervention (8.3 %, 3 out of 36) groups over 12 months, considering 5% level of significance, 80 % power, and an attrition of 10 %. The final sample size was 104 dyads of parents and children (52 in each group).
2.6. Randomization and blinding
The eligible dyads were randomly allocated (1:1) to control or intervention groups, stratified by dichotomized eHEALS scores (parents) and ICDAS (children), based on median values. The randomization was performed via a computer-generated list [39] through random block sizes for the allocation of participants. The balance between groups was also checked to mean age and gender distribution. The allocation sequence was concealed. The blinding of allocations was guaranteed by using closed and opaque envelopes, which were maintained confidentially by an independent researcher (TC).
This was a single-blind study. During the intervention, the messages were sent out to the participants by a specific investigator (PEAA). All investigators and participants were unaware of the assignment group during data collection; however, the parents or caregivers of intervention group were obviously conscious about the educational messages that they received over time.
2.7. Data analysis
Statistical analysis was performed using SPSS Statistics software 21.0 (IBM SPSS Statistics). The data was presented with descriptive statistics, being examined for lost values, outliers, normality, and homogeneity. To investigate the quality of randomization, potential differences between the characteristics of participants of the control and intervention groups were determined systematically, applying Student t tests for continuous variables and Chi-square test for categorical variables.
These analyses were performed in accordance with the principle of intention-to-treat (ITT), taking into account all participants randomized for the trial groups. Missing data were handled by multiple imputation (MI) to create 25 imputed datasets, considering specific groups, age, gender, and measures collected at baseline. The incomplete response variables were VPI and ICDAS at 3- and 6-month follow-ups, and eHEALS and dietary habits at 6-month follow-up.
All quantitative variables were submitted to Shapiro-Wilk and Levene tests to check normality and homogeneity of variances, respectively. As these assumptions were not reached, statistical comparisons were conducted using nonparametric tests.
The results of VPI, ICDAS/teeth and eHEALS were compared intergroup and intragroup by Mann-Whitney U and Friedman tests, respectively. Fischer’s exact and McNemar tests were employed to detect differences in the distribution of the frequency of caries-free participants, children who increased their maximum ICDAS code, children with the diagnosis of dentin caries lesions, and the pattern of children’s food consumption inter- and intragroup over time, respectively. P values < 0.05 was considered significant.
Although non-significant, missing data appeared to be more common in the intervention group for VPI and ICDAS [5.8 % (control) vs. 9.2 % (intervention) of complete cases, P = 0.715] and eHEALS [30.8 % (control) vs. 40.4 % (intervention) of complete cases, P = 0.413]. Also, children with missing values presented higher means of VPI (0.44 vs. 0.40) and ICDAS (0.44 vs. 0.37) at baseline than their counterparts. In this sense, it was postulated that (i) children who failed to attend in follow-ups were likely to have been in relatively poorer oral health conditions, and (ii) parents who failed to complete the questionnaires were likely to have had lower eHEALS scores.
Then, the missing at random (MAR) and missing not at random (MNAR) sensitivity analyses were performed to determine difference means and 95 % confidence intervals to normalized values of continuous variables at 6-month follow-up (> 5% missing outcome data), using a pattern-mixture approach following MI. We assumed that children’s VPI and ICDAS could be up to 10 % higher (c = 1.1), whereas parental eHEALS scores could be up to 10 % lower (c = 0.9) compared to the MAR setting (c = 1). This sensitivity parameter c was allowed to differ in up to 5% between control and intervention groups. It resulted in 13 different scenarios, with c = 1.10, 1.05, 1.0, 0.95, or 0.9.
3. Results
3.1. Participants
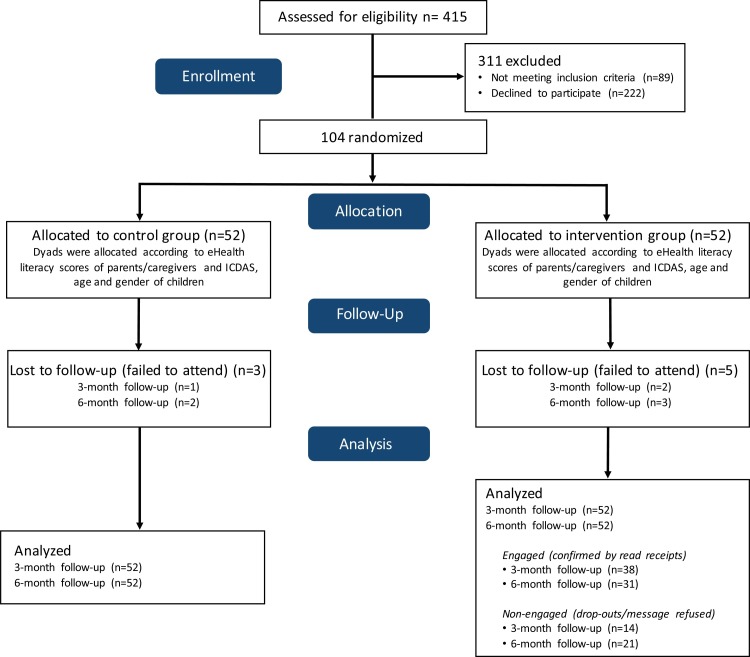
A total of 415 children were screened during the recruitment (March 2019 to July 2019), being excluded 89 (21.45 %) for not meeting the inclusion criteria, and 222 (53.49 %) for declining to participate. A hundred and four dyads of parents and children were enrolled into the study. They were randomly allocated in control and intervention groups (n = 52 each). Throughout the study (August 2019 to February 2020), 3 children (5.76 %) of the control group and 5 children (9.61 %) of the intervention group failed to attend in dental appointments (attrition of 7.69 %). During the follow-up period, 21 (40.3 %) parents of the intervention group refused to receive educational mobile text messages, being 14 (26.9 %) along the first 3 months. The recruitment, randomization, allocation, and follow-up of participants are outlined in the CONSORT flow diagram (Fig. 1 ).
Fig. 1.
Participant flow diagram.
3.2. Characterization of groups
The sociodemographic and clinical characteristics of groups are depicted in Tables 2 ,3 ,4 and 6 . At baseline, both groups were statistically similar in relation to mean age, gender distribution, parental eHEALS scores, VPI, mean ICDAS/teeth, proportion of caries-free children, and dietary habits. All children were recruited in preschools located in low socioeconomic suburbs of the city.
Table 2.
Sociodemographic characteristics of participants at baseline.
| Control | Intervention | Total | P | |
|---|---|---|---|---|
| (n = 52) | (n = 52) | (n = 104) | ||
| Age (children) | ||||
| Mean ± SD | 3.6 ± 0.6 | 3.4 ± 0.6 | 3.5 ± 0.6 | |
| Median | 4.0 | 3.0 | 3.0 | 0.195 |
| Gender (children) | ||||
| Female | 29 (55.7 %) | 28 (53.8 %) | 57 (100 %) | |
| Male | 23 (44.3 %) | 24 (46.2 %) | 47 (100 %) | 0.845 |
| eHEALS (parents) | ||||
| Mean ± SD | 24.5 ± 8.5 | 24.0 ± 8.1 | 24.3 ± 8.3 | |
| Median | 25.5 | 25.0 | 25.0 | 0.943 |
3.3. Primary outcomes
The clinical outcomes of control and intervention groups were statistically similar over time (Tables 3, 4 and 6).
Table 3.
The comparison of proportions of children without caries lesions (ICDAS = 0), who increased maximum ICDAS (at least ≥ 1), and diagnosed with dentin caries lesions (ICDAS ≥ 4) between groups over time. P values represent the significance level between groups regarding the same time (independent measures, Fischer’s exact test), while P’ values represent the significance level between times regarding the same group (repeated measures, McNemar’s test, P < 0.05). The asterisks indicate significant statistical differences.
| Baseline |
3-month follow-up |
6-month follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 52) | Intervention (n = 52) | P | Control (n = 52) | Intervention (n = 52) | P | Control (n = 52) | Intervention (n = 52) | P | ||
| Caries free (ICDAS = 0) | Yes | 10 (19.23 %) | 5 (9.62 %) | 0.264 | 6 (11.54 %) | 5 (9.62 %) | 1.000 | 5 (9.62 %) | 5 (9.62 %) | 1.000 |
| No | 42 (80.77 %) | 47 (90.38 %) | 46 (88.46 %) | 47 (90.38 %) | 47 (90.38 %) | 47 (90.38 %) | ||||
| P’ | 0.125 | 1.000 | 0.063 | 1.000 | ||||||
| Increased ICDAS (at least ≥ 1) | Yes | – | – | – | 11 (21.15 %) | 8 (15.38 %) | 0.613 | 19 (36.54 %) | 12 (23.08 %) | 0.198 |
| No | – | – | 41 (76.92 %) | 44 (84.62 %) | 33 (63.46 %) | 40 (76.92 %) | ||||
| P’ | – | – | – | – | 0.008** | 0.125 | ||||
| Dentin caries (ICDAS ≥ 4) | Yes | 0 (0%) | 0 (0%) | 1.000 | 3 (5.77 %) | 2 (3.85 %) | 1.000 | 6 (11.54 %) | 6 (11.54 %) | 1.000 |
| No | 52 (100 %) | 52 (100 %) | 49 (94.23 %) | 50 (96.15 %) | 46 (88.46 %) | 46 (88.46 %) | ||||
| P’ | 0.250 | 0.500 | 0.031** | 0.031** | ||||||
Although the percentages of children with dentin caries lesions were exactly the same in both groups after 6 months, the proportion of participants with the increment of maximum ICDAS did not increase significantly in the intervention group (15.4%–23.1%, P = 0.125), differently of that observed in the control group (21.2%–36.5%, P = 0.008) between 3- and 6-month follow-ups. Also, it is noteworthy the stability of caries-free children in the intervention group (n=5), instead of a considerable reduction found in the control group (50 %, from n=10 to 5) (Table 3).
The significant increment of VPI observed in the intervention group at 3-month follow-up was not detected over time, whereas mean ICDAS/teeth increased consistently in both groups (Table 4).
Table 4.
Mean (±SD) and median (IQR) of VPI, ICDAS/teeth and eHEALS according to groups over time. P values represent the significance level of intergroup differences (Mann-Whitney, control vs. intervention). P’ values represent the significance level of intragroup differences (Friedman, follow-ups vs. baseline). The asterisks indicate significant statistical differences.
| Baseline |
3-month follow-up |
6-month follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 52) | Intervention (n = 52) | P | Control (n = 52) | Intervention (n = 52) | P | Control (n = 52) | Intervention (n = 52) | P | ||
| VPI | Mean ± SD | 0.39 ± 0.34 | 0.43 ± 0.32 | 0.503 | 0.39 ± 0.32 | 0.47 ± 0.33 | 0.212 | 0.31 ± 0.33 | 0.38 ± 0.31 | 0.204 |
| Median (IIQ) | 0.33 (0.67) | 0.33 (0.50) | 0.33 (0.63) | 0.50 (0.50) | 0.26 (0.63) | 0.33 (0.50) | ||||
| P’ | 0.869 | 0.040** | 0.139 | 0.398 | ||||||
| ICDAS/ teeth | Mean ± SD | 0.35 ± 0.32 | 0.40 ± 0.41 | 0.717 | 0.43 ± 0.37 | 0.54 ± 0.49 | 0.195 | 0.52 ± 0.42 | 0.59 ± 0.52 | 0.737 |
| Median (IIQ) | 0.28 (0.54) | 0.25 (0.49) | 0.33 (0.64) | 0.48 (0.59) | 0.38 (0.64) | 0.55 (0.63) | ||||
| P’ | <0.001** | <0.001** | <0.001** | <0.001** | ||||||
| eHEALS | Mean ± SD | 24.53 ± 8.50 | 24.02 ± 8.14 | 0.943 | 23.73 ± 9.79 | 26.39 ± 10.10 | 0.091 | |||
| Median (IIQ) | 25.50 (12.0) | 25.00 (11.0) | 24.00 (11.0) | 28.02 (13.0) | ||||||
| P’ | 0.376 | 0.001** | ||||||||
According to the results of the sensitivity analysis, data imputation-related changes in model output values varied until 5.41 % for VPI and 8.82 % for ICDAS/teeth at 6-month follow-up, considering the ratios between MAR and all MNAR scenarios (assumption i) (Table 5 ). The similarities of groups for VPI and ICDAS/teeth are clearly detected by the analysis of 95 % confidence intervals.
Table 5.
Different MNAR assumptions for missing VPI, ICDAS and eHEALS data.
| Scenario number (assumption) | MNAR rescaling parametersa |
VPI mean difference [95 % CI] | ICDAS mean difference [95 % CI] | eHEALS mean difference [95 % CI] | |
|---|---|---|---|---|---|
| ccontrol | cintervention | ||||
| 1 (MAR) | 1 | 1 | 0.074 [-0.037 to 0.184] | 0.034 [-0.143 to 0.212] | 2.340 [-1.300 to 5.980] |
| 2 (i) | 1.10 | 1.10 | 0.077 [-0.036 to 0.191] | 0.036 [-0.145 to 0.217] | 2.793 [-1.102 to 6.687] |
| 3 (i) | 1.10 | 1.05 | 0.075 [-0.037 to 0.187] | 0.034 [-0.146 to 0.214] | 2.158 [-1.644 to 5.961] |
| 4 (i) | 1.05 | 1.10 | 0.078 [-0.035 to 0.192] | 0.037 [-0.144 to 0.218] | 3.158 [-0.711 to 7.027] |
| 5 (i) | 1.05 | 1.05 | 0.074 [-0.038 to 0.186] | 0.035 [-0.145 to 0.214] | 2.541 [-1.247 to 6.329] |
| 6 (i) | 1.05 | 1 | 0.073 [-0.038 to 0.184] | 0.033 [-0.145 to 0.211] | 1.869 [-1.697 to 5.641] |
| 7 (i) | 1 | 1.05 | 0.076 [-0.036 to 0.187] | 0.036 [-0.144 to 0.216] | 2.905 [-0.863 to 6.673] |
| 8 (ii) | 1 | 0.95 | 0.072 [-0.039 to 0.182] | 0.032 [-0.145 to 0.210] | 1.853 [-1.803 to 5.509] |
| 9 (ii) | 0.95 | 1 | 0.075 [-0.036 to 0.185] | 0.036 [-0.142 to 0.213] | 2.728 [-0.907 to 6.362] |
| 10 (ii) | 0.95 | 0.95 | 0.073 [-0.037 to 0.183] | 0.033 [-0.143 to 0.210] | 2.240 [-1.400 to 5.880] |
| 11 (ii) | 0.95 | 0.90 | 0.071 [-0.039 to 0.181] | 0.031 [-0.144 to 0.207] | 1.694 [-1.965 to 5.353] |
| 12 (ii) | 0.90 | 0.95 | 0.074 [-0.036 to 0.184] | 0.034 [-0.142 to 0.211] | 2.596 [-1.060 to 6.253] |
| 13 (ii) | 0.90 | 0.90 | 0.072 [-0.037 to 0.182] | 0.032 [-0.143 to 0.208] | 2.057 [-1.614 to 5.727] |
All results are based on imputed data comparing the control and intervention groups at 6-month follow-up (n = 104). For participants with complete clinical (n = 96; 92.3 %) and questionnaire-based data (n = 67; 64.4 %), the observed VPI mean difference was 0.067 [95 % CI: -0.050 to 0.184], the ICDAS mean difference was -0.029 [-0.207 to 0.148], and the eHEALS mean difference was 2.351 [-1.951 to 6.654].
CI confidence interval, MAR missing at random, MNAR missing not at random, mean difference (intervention – control).
How missing data are assumed to differ from the MAR-imputed values. ccontrol = 0.9 means that all imputed values in the control group have been reduced in 10 %.
Moreover, parents of the intervention group significantly reported a higher consumption of sugar-free sweets and a lower consumption of sugar-free foods by their children (Table 6 ).
Table 6.
Proportion of children with high frequency of consumption of different types of foods. Significant statistical differences were not detected between groups at baseline and 6-month follow-up (independent measures, Fischer’s exact test). The asterisks indicate significant statistical differences of the consumption of sugar-free sweets and foods in the intervention group, regarding distinct times (repeated measures, McNemar’s test, P < 0.05).
| Foods | Baseline |
6-month follow-up |
||
|---|---|---|---|---|
| Control | Intervention | Control | Intervention | |
| Sticky sugar-rich foods | 67.3 | 67.3 | 78.8 | 73.1 |
| Foods containing starch and sugar | 55.8 | 55.8 | 55.8 | 55.8 |
| Sugar-free sweets | 21.2 | 9.6** | 23.1 | 36.5** |
| Sugared milk and dairy products | 73.1 | 67.3 | 71.2 | 63.5 |
| Non-sugary milk and dairy products | 44.2 | 30.8 | 44.2 | 44.2 |
| Sugary liquids | 69.2 | 76.9 | 61.5 | 69.2 |
| Fruit | 69.2 | 65.4 | 69.2 | 61.5 |
| Foods rich in semi-hydrolyzed starch | 59.6 | 67.3 | 55.8 | 53.8 |
| Sugar-free foods | 75.0 | 69.2** | 59.6 | 50.0** |
3.4. Secondary outcomes
Although parental eHealth literacy scores were statistically similar between groups at 6-month follow-up, eHEALS increased significantly in the intervention group (+10.32 %, P = 0.001), in contrast with a non-significant decrease observed in the control group (-2.65 %, P = 0.376) (Table 4). Interestingly, scores increased even more among engaged parents in comparison to non-engaged ones (14.11 % vs. 3.85 %).
The sensitivity analysis demonstrated that data imputation-related changes model output values varied until 27.61 % for eHEALS at 6-month follow-up, considering the ratios between MAR and all MNAR scenarios (assumption ii). In addition, the analysis of 95 % confidence intervals demonstrates a trend of the mean difference being even higher between intervention and control groups (Table 5).
3.5. Harms
There is a likelihood that parents of the intervention group have been exhausted in receiving periodic educational messages over time. Additionally, although this research protocol was considered to be applied during 12 months, in April 2020, this study needed to be discontinued early because of the pandemic coronavirus (COVID-19).
4. Discussion
These findings indicate the effectiveness of mobile text messages to aid in the control of ECC in low socioeconomic children. Although observed clinical outcomes were similar between groups, this educational strategy increased eHealth literacy of parents, influencing reports of changes in the patterns of sugar consumption of preschoolers. In this context, the intervention seemed to contribute with better children’s oral health status, controlling the severity of the disease, demonstrated by higher proportions of participants without variation of their maximum ICDAS, and by the maintenance of caries-free participants over time. These results are consistent with the positive influence of educational messages on the engagement and attitudes against ECC in vulnerable groups as described by Borelli et al. [40]. Other mobile educational strategies were also able to improve oral health aspects, although they were usually applied in samples of different ages from developed countries [27,38,41,42].
Indeed, this intervention was effective even considering the low levels of eHealth literacy of participants at baseline and its short follow-up, which could make difficult the engagement of parents in accessing WhatsApp messages [43,44], and the observation of significant effects of educational mobile messages on clinical measures [45], respectively. In these circumstances, we suppose the observation of intergroup differences after 9 months, in relation to caries-free and increment of maximum ICDAS outcomes. This hypothesis emerged from the analysis of trends through the observation of indicators in 3 data points (data not shown), being also corroborated by the reference used to determine this sample size [38]. Definitely, these arguments should be taken into account with caution, since they were based on a simple mathematical approach compared with an evidence obtained from adolescents.
Moreover, the significant increases of children with dentin caries and the mean ICDAS/teeth deserve attention in relation to the progression of ECC in both groups. In this sense, the polarization of ECC even within a homogenous socioeconomic vulnerable population must be considered, indicating that other factors apart from income may contribute to disease susceptibility [46]. For the highest caries risk group, it is expected that the association of educational text messages with other preventive strategies (e.g. regular dental visits to fluoride varnish applications) would achieve better control of the progression of dental caries [30]. Also, it is important to note that central tendency measures as the mean ICDAS/teeth are excellent summary indices for groups; however, they may not be useful to evaluate the results of oral health-based interventions, because it reflects a disease measure separated from those affected individuals [47].
Regarding sugar consumption, although it is discouraged during the first years of life, it exceeds that recommended for the management of ECC in most developing countries, due to cultural, social, and economic issues [48]. Notwithstanding, this intervention seemed effective to concern parents about the role of dietary habits on dental caries, since they reported an increase of free-sugar sweets intake and a decrease of sugar-free foods by their children. Although these outcomes seem controversial, the list of sugar-free foods that was made available for participants included meals rich in carbohydrates (pasta and bread) and salt (dehydrated noodles), both considered inadequate for the development of other conditions as obesity and hypertension, i.e., educational contents may influence parents towards a healthy diet.
The relative stability of the presence of visible plaque in both groups, with a negligible trend of reduction over 6 months, can be explained by the practical difficulties of parents in following professional recommendations about oral hygiene, mainly because children’s negative behaviors [32]. In addition, health behaviors of participants enrolled in clinical trials can be improved by the Hawthorne effect, independently of the group [49,50]; however, VPI seems more adequate to be a complementary information about caries risk assessment of preschoolers than to be a considerable clinical indicator to the control of ECC. In this sense, the main efforts for the primary prevention of the disease are focused on the delivery of educational information to promote the reduction of sugar consumption and the use of topical fluoride [5].
This model seemed feasible to aid in parental behavior changes, to develop personalized messages for child and family-centered care, aiming to educate parents or caregivers for adequate decision-making related to their children's health. Also, text messages can be important to gather oral health-related information towards the elucidation of doubts and beliefs that may arise from inadvertent consumption of mis- or disinformation [51]. In this context, dentists must offer support to all personal demands, making constant improvements in the process of professional-person communication, using alternative ways to present the information, such as figures, videos or illustrations [52]. It is emphasized that those options were not applied in this study to improve the chance that all participants could visualize the contents independently their Internet data package. Hence, the development of policies for inclusive digital practices, such as universal access to free Internet and gadgets, would be desirable to amplify the delivery of mHealth solutions in developing countries, mainly to socioeconomic vulnerable populations [53], empowering people to self-care.
This study presents some limitations. First, not all parents or caregivers returned completing questionnaires at 6-month follow-up, which could influence the interpretation of eHEALS and dietary habits outcomes. Then, we performed the sensitivity analysis considering the influence of different scenarios of missing data multiple imputation on these results, which demonstrated a trend of enlargement in the difference of eHEALS scores between groups. Second, parents of the intervention group may get exhausted from consuming periodic educational text messages about a health issue that they were not used to discuss out of dental office. To minimize this discomfort, the messages were sent them only every two weeks. Third, the educational messages were formulated from doubts and beliefs of other parental groups [32], limiting the availability of contents for these participants. In contrast, the educational messages seemed to address the management of ECC-related risk behaviors. Fourth, this study needed to be discontinued due to COVID-19, preventing the participants' feedback on their general impressions and satisfaction about this novel approach for health promotion.
In conclusion, parental-oriented mobile text messages were effective to control the severity of ECC in low socioeconomic preschoolers, improving parental eHealth literacy and changing children’s dietary habits. Further studies should be performed to elucidate the role of this promising educational strategy for the management of ECC in distinct populations, considering longer follow-ups.
CRediT authorship contribution statement
Matheus Lotto: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Visualization. Anna Paola Strieder: Investigation, Writing - review & editing. Patricia Estefania Ayala Aguirre: Investigation, Writing - review & editing. Thais Marchini Oliveira: Formal analysis, Writing - review & editing. Maria Aparecida Andrade Moreira Machado: Formal analysis, Writing - review & editing. Daniela Rios: Resources, Writing - review & editing. Thiago Cruvinel: Conceptualization, Methodology, Formal analysis, Investigation, Writing - review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
This work was supported by the Sao Paulo Research Foundation (grant #2017/25899-7).
References
- 1.Drury T.F., Horowitz A.M., Ismail A.I., Maertens M.P., Rozier R.G., Selwitz R.H. Diagnosing and reporting early childhood caries for research proposes. A report of a workshop sponsored by the National Institute of Dental and Craniofacial Research, the health resources and services administration, and the health care financing administration. J. Public Health Dent. 1999;59(3):192–197. doi: 10.1111/j.1752-7325.1999.tb03268.x. PMID: 10649591. [DOI] [PubMed] [Google Scholar]
- 2.Fontana M. The clinical, environmental, and behavior factors that foster early childhood caries: evidence for caries risk assessment. Pediatr. Dent. 2015;37(2):217–225. PMID:26063551. [PubMed] [Google Scholar]
- 3.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J.L., Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J. Den. Res. 2015;94(5):650–658. doi: 10.1177/0022034515573272. PMID:25740856. [DOI] [PubMed] [Google Scholar]
- 4.Phantumvanit P., Makino Y., Ogawa H., Rugg-Gunn A., Moynihan P., Petersen P.E. WHO global consultation on public health intervention against early childhood caries. Community Dent. Oral Epidemiol. 2018;46(3):280–287. doi: 10.1111/cdoe.12362. PMID:29380407. [DOI] [PubMed] [Google Scholar]
- 5.Tinanoff N., Baez R.J., Guillory C.D., Donly K.J., Feldens C.A., McGrath C. Early childhood caries epidemiology, aetiology, risk assessment, societal burden, management, education, and policy: global perspective. Int. J. Paediatr. Dent. 2019;29(3):238–248. doi: 10.1111/ipd.12484. PMID:31099128. [DOI] [PubMed] [Google Scholar]
- 6.Rai N.K., Tiwari T. Parental factors influencing the development of early childhood caries in developing nations: a systematic review. Front. Public Health. 2018;6:64. doi: 10.3389/fpubh.2018.00064. PMID:29616206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finnegan D.A., Rainchuso L., Jenkins S., Kierce E., Rothman A. Immigrant caregivers of young children: oral health beliegs, attitudes, and eatly childhood caries knowledge. J. Community Health. 2016;41(2):250–257. doi: 10.1007/s10900-015-0090-5. PMID:26370378. [DOI] [PubMed] [Google Scholar]
- 8.Thwin K.M., Zatsu T., Ueno M., Kawagushi Y. Effects of oral health education in Myanmar prescholl children and guardians, J. Invesig. Clin. Dent. 2018;9(3):e12346. doi: 10.1111/jicd.12346. PMID:29873195. [DOI] [PubMed] [Google Scholar]
- 9.Smith J.D., George S.M.S., Prado G. Family-centered positive behavior support interventions in early childhood to prevent obesity. Child Dev. 2017;88(2):427–435. doi: 10.1111/cdev.12738. PMID:28195411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plonka K.A., Pukallus M.L., Barnett A., Holcombre T.F., Walsh L.J., Seow W.K. A controlled, longitudinal study of home visits compared to telephone contects to prevent early chilhood caries. Int. J. Paediatr. Dent. 2013;23(1) doi: 10.1111/j.1365-263X.2011.01219.x. 23-21 PMID:22251427. [DOI] [PubMed] [Google Scholar]
- 11.Jiang S., McGrath C., Lo E.C., Ho S.M., Gao X. Motivational interviewing to prevent early childhood caries: a randomized controlled trial. J. Dent. 2020 doi: 10.1016/j.jdent.2020.103349. Online ahead of print PMID:32330548. [DOI] [PubMed] [Google Scholar]
- 12.Henshaw M.M., Borrelli B., Gregorich S.E., Heaton B., Tooley E.M., Santo W. Randomized trial of motivational interviewing to prevent early childhood caries in public housing. JDR Clin. Trans. Res. 2018;3(4):353–365. doi: 10.1177/2380084418794377. PMID:30238060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Donnell O. Access to health care in developing countries: breaking down demand side barriers. Cad. Saude Publica. 2007;23(12):2820–2834. doi: 10.1590/s0102-311x2007001200003. PMID:18157324. [DOI] [PubMed] [Google Scholar]
- 14.Bhandari N., Shi Y., Jung K. Seeking health information online: does limited healthcare access matter? J. Am. Med. Inform. Assoc. 2014;21(6):1113–1117. doi: 10.1136/amiajnl-2013-002350. PMID:24948558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Instituto Paulo Montenegro . 2018. Indicador De Alfabetismo Funcional.https://drive.google.com/file/d/1ez-6jrlrRRUm9JJ3MkwxEUffltjCTEI6/view (Accessed 01 June 2020) [Google Scholar]
- 16.Batista M.J., Lawrence H.P., Sousa M.L.R. Oral health literacy and oral health outcomes in an adult population in Brazil. BMC Public Health. 2017;18(1):60. doi: 10.1186/s12889-017-4443-0. PMID:28747157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berkman N.D., Sheridan S.L., Donahue K.E., Halpern D.J., Vieira A., Crotty K. Health litercy interventions and outcomes: an updated systematic review. Evid. Rep. Assess. Summ. (Summ) 2011;199:1–941. PMID:23126607. [PMC free article] [PubMed] [Google Scholar]
- 18.Cruvinel T., Aguirre P.E.A., Lotto M., Oliveira T.M., Rios D., Cruvinel A.F.P. Digital behavior surveillance: monitoring dental caries and toothache interests of Google users from developing countries. Oral Dis. 2019;25(1):339–347. doi: 10.1111/odi.12986. PMID:30270556. [DOI] [PubMed] [Google Scholar]
- 19.Lotto M., Aguirre P.E.A., Strieder A.P., Cruvinel A.F.P., Cruvinel T. Levels of toothache-related interests of Google and YouTube users from developed and developing countries over time. PeerJ. 2019;7:e7706. doi: 10.7717/peerj.7706. PMID:31616582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statista . 2020. Brazil: Mobile Phone Internet Users in Brazil 2017-2023.https://www.statista.com/statistics/259749/mobile-phone-internet-users-in-brazil/ (accessed 01 June 2020) [Google Scholar]
- 21.Choi W., Zheng H., Franklin P., Tulu B. mHealth technologies for osteoarthritis self-management and treatment: a systematic review. Health Informatics J. 2019;25(3):984–1003. doi: 10.1177/1460458217735676. PMID:29090628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otu A., Ebenso B., Okuzu O., Osifo-Dawodu E. Using a mHealth tutorial application to change knowledge and attitude of frontline health workers to Ebola virus disease in Nigeria: a before-and-after study. Hum. Resour. Health. 2016;14:5. doi: 10.1186/s12960-016-0100-4. PMID:26872824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcolino M.S., Oliveira J.A.Q., D’Agostino M., Ribeiro A.L., Alkmim M.B.M., Novillo-Ortiz D. The impact of mHealth interventions: systematic review of systematic reviews. JMIR Mhealth Uhealth. 2018;6(1):e23. doi: 10.2196/mhealth.8873. PMID:29343463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong S., Berkhouse H., Schooler M., Pu W., Sun A., Gong E. Effectiveness of mHealth interventions in improving medication adherence among people with hypertension: a systematic review. Curr. Hypertens. Rep. 2018;20(10):86. doi: 10.1007/s11906-018-0886-7. PMID:30088110. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Xue H., Huang Y., Huang L., Zhang D. A systematic review of application and effectiveness of mHealth interventions for obesity and diabetes treatment and self-management. Adv. Nutr. 2017;8(3):449–462. doi: 10.3945/an.116.014100. PMID:28507010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkilzy M., Midani R., Höfer M., Splieth C. Improving toothbrushing with a smartphone app: results of a randomized controlled trial. Caries Res. 2019;53(6):628–635. doi: 10.1159/000499868. PMID:31132765. [DOI] [PubMed] [Google Scholar]
- 27.Toniazzo M.P., Nodari D., Muniz F.W.M.G., Weidlich P. Effect of mHealth in improving oral hygiene: a systematic review with meta-analysis. J. Clin. Periodontol. 2019;46(3):297–309. doi: 10.1111/jcpe.13083. PMID:30761580. [DOI] [PubMed] [Google Scholar]
- 28.Aguirre P.E.A., Lotto M., Strieder A.P., Cruvinel A.F.P., Cruvinel T. The effectiveness of educational mobile messages for assisting in the prevention of early childhood caries: protocol for a randomized controlled trial. JMIR Res. Protoc. 2019;8(9) doi: 10.2196/13656. PMID:31482856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 statement: Update guidelines for reporting parallel group randomized trials. BMC Med. 2010;24:8–18. doi: 10.1186/1741-7015-8-18. PMID:20334633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitts N.B., Ekstrand K.R., Foundation I.C.D.A.S. International caries detection and assessment system (ICDAS) and its International caries classification and management system (ICCMS) – methods for staging of the caries process and enabling dentists to manage caries, Community Dent. Oral. Epidemiol. 2013;41(1):e41–52. doi: 10.1111/cdoe.12025. PMID:24916677. [DOI] [PubMed] [Google Scholar]
- 31.American Academy of Pediatric Dentistry . first ed. American Academy of Pediatric Dentistry; Chicago: 2019. The Reference Manual of Pediatric Dentistry. [Google Scholar]
- 32.Lotto M., Strieder A.P., Aguirre P.E.A., Machado M.A.A.M., Rios D., Cruvinel A. Parental perspectives on early childhood caries: a qualitative study. Int. J. Paediatr. Dent. 2020;30(4):451–458. doi: 10.1111/ipd.12622. PMID:32011057. [DOI] [PubMed] [Google Scholar]
- 33.Rosenstock I.M. The Health Belief Model and preventive health behavior. Health Educ. Behav. 1974;2:354–386. doi: 10.1177/109019817400200405. [DOI] [Google Scholar]
- 34.Parandeh L., Shafaie F.S., Mlajouti J., Mirghafourvand M., Asghari-Jafarabadi M. The effect of educational text message based on health belief model on osteoporosis preventive behaviors in women: a randomized controlled clinical trial. Women Health. 2019;59(10) doi: 10.1080/03630242.2019.1590495. https://doi.org/1128-1140.10.1080/03630242.2019.1590495. PMID:30955478. [DOI] [PubMed] [Google Scholar]
- 35.Ainamo J., Bay I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975;25(4):29–35. PMID:1058834. [PubMed] [Google Scholar]
- 36.Norman C.D., Skinner H.A. eHEALS: the eHealth literacy scale. J. Med. Internet Res. 2006;8(4):e27. doi: 10.2196/jmir.8.4.e27. PMID:17213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llena C., Forner L. Dietary habits in a child population in relation to caries experience. Caries Res. 2008;42(5):387–393. doi: 10.1159/000154784. PMID:18781067. [DOI] [PubMed] [Google Scholar]
- 38.Zotti F., Dalessandri D., Salgarello S., Piancino M., Bonetti S., Visconti L. Usefulness of an app in improving oral hygiene compliance in adolescent orthodontic patients. Angle Orthod. 2016;86(1):101–107. doi: 10.2319/010915-19.1. PMID:25799001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dallal’s Jerry. 2020. Randomization Plans: Randomizing Subjects to a Single Treatment.http://www.jerrydallal.com/random/assign.htm (accessed 01 June 2020) [Google Scholar]
- 40.Borrelli B., Henshaw M., Endrighi R., Adams W.G., Heeren T., Rosen R.K. An interactive parent-targeted text messaging intervention to improve oral health in children attending urban pediatric clinics: feasibility randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(11) doi: 10.2196/14247. PMID:31710306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross M.C., Campbell P.M., Tadlock L.P., Taylor R.W., Buschang P.H. Effect of automated messaging on oral hygiene in adolescent orthodontic patients: a randomized controlled trial. Angle Orthod. 2019;89(2):262–267. doi: 10.2319/040618-260.1. PMID:30516416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheerman J.F.M., van Meijel B., van Empelen P., Verrips G.H.W., van Loveren C., Twisk J.W.R. The effect of using a mobile application (“WhiteTeeth”) on improving oral hygiene: a randomized controlled trial. Int. J. Dent. Hyg. 2020;18(1):79–83. doi: 10.1111/idh.12415. PMID:31291683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho J., Park D., Lee H.E. Cognitive factors of using health apps: systematic analysis of relationships among health consciousness, health information orientation, eHealth literacy, and health app use efficacy. J. Med. Internet Res. 2014;16(5):e125. doi: 10.2196/jmir.3283. PMID:24824062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richtering S.S., Hyun K., Neubeck L., Coorey G., Chalmers J., Usherwood T. eHealth literacy: predictors in a population with moderate-to-high cardiovascular risk. JMIR Hum. Factors. 2017;4(1):e4. doi: 10.2196/humanfactors.6217. PMID:28130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ernsting C., Stühmann L.M., Dombrowski S.U., Voigt-Antons J., Kuhlmey A., Gellert P. Associations of health app use and perceived effectiveness in people with cardiovascular diseases and diabetes: population-based survey. JMIR Mhealth Uhealth. 2019;7(3):e12179. doi: 10.2196/10.2196/12179. PMID:30920383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nunes A.M.M., Silva A.A.M., Alves C.M.C., Hugo F.N., Ribeiro C.C. Factors underlying the polarization of early childhood caries within a high-risk population. BMC Public Health. 2014;14:988. doi: 10.2196/10.1186/1471-2458-14-988. PMID:25245978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ElSalhy M., Ali U., Lai H., Flores-Mir C., Amim M. Caries reporting in studies that used the international caries detection and assessment system: a scoping review. Community Dent. Oral Epidemiol. 2019;47(1):92–102. doi: 10.1111/cdoe.12430. PMID: 30334280. [DOI] [PubMed] [Google Scholar]
- 48.Fisberg M., Kovalskys I., Gómez G., Rigotti A., Sanabria L.Y.C., Garcia M.C.Y., Torres R.G.P. Total and added sugar intake: assessment in eight Latin American countries. Nutrients. 2018;10(4):389. doi: 10.3390/nu10040389. PMID:29565308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sedgwick P., Greenwood N. Understanding the hawthorne effect. BMJ. 2015;315:h4672. doi: 10.1136/bmj.h4672. PMID:26341898. [DOI] [PubMed] [Google Scholar]
- 50.McCarney R., Werner J., Iliffe S., van Haselen R., Griffin M., Fisher P. The Hawthorne effect: a randomized, controlled trial. BMC Med. Res. Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. PMID:17608932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strieder A.P., Aguirre P.E.A., Lotto M., Cruvinel A.F.P., Cruvinel T. Digital behavior surveillance for monitoring the interests of Google users in amber necklace in different countries. Int. J. Paediatr. Dent. 2019;29(5):603–614. doi: 10.1111/ipd.12500. PMID:30920686. [DOI] [PubMed] [Google Scholar]
- 52.Hyde S., Gansky S.A., Gonzalez-Vargas M.J., Husting S.R., Cheng N.F., Millstein S.G. Developing an acceptability assessment of preventive dental treatments. J. Public Health Dent. 2009;69(1):18–23. doi: 10.1111/j.1752-7325.2008.00088.x. PMID:18662256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vidyasagar D. Digital divide and digital dividend in the age of information technology. J. Perinatol. 2006;26(5):313–315. doi: 10.1038/sj.jp.7211494. PMID:16598298. [DOI] [PubMed] [Google Scholar]