Abstract
The immune system has evolved multiple mechanisms to restrict microbial infections and regulate inflammatory responses. Without appropriate regulation, infection-induced inflammatory pathology can be deadly. The innate immune system recognizes the microbial molecules conserved in many pathogens and engages a rapid response by producing inflammatory mediators and activating programmed cell death pathways, including pyroptosis, apoptosis, and necroptosis. Activation of pattern recognition receptors, in combination with inflammatory cytokine-induced signaling through death domain-containing receptors, initiates a highly interconnected cell death process called PANoptosis (pyroptosis, apoptosis, necroptosis). Broadly speaking, PANoptosis is critical for restricting a wide range of pathogens (including bacteria, viruses, fungi, and parasites), which we describe in this review. We propose that re-examining the role of cell death and inflammatory cytokines through the lens of PANoptosis will advance our understanding of host–pathogen evolution and may reveal new treatment strategies for controlling a wide range of infectious diseases.
Current Opinion in Microbiology 2021, 59:42–49
This review comes from a themed issue on Host–microbe interactions: bacteria and viruses
Edited by Thirumala-Devi Kanneganti and Wolf-Dietrich Hardt
For a complete overview see the Issue and the Editorial
Available online 20th August 2020
https://doi.org/10.1016/j.mib.2020.07.012
1369-5274/© 2020 Elsevier Ltd. All rights reserved.
Introduction
Programmed cell death plays an important role in limiting the severity of infection by multiple pathogens, but until recently, studies examining the contribution of each cell death pathway, using genetic mouse models, were largely lacking. In this review, we focus on the role of pyroptosis, apoptosis, and necroptosis during microbial infection, as they are interconnected by shared regulatory proteins and signaling pathways. Pyroptosis and necroptosis are two programmed cell death pathways that are similar in that they are lytic forms of cell death driven by activation of the pore-forming proteins gasdermin D (GSDMD) and mixed lineage kinase domain-like (MLKL), respectively. During pyroptosis, proximal sensors (including NOD-like receptor [NLR] pyrin-containing 1 [NLRP1], NLRP3, NLR family apoptosis inhibitory protein [NAIP]-NLR caspase recruitment domain [CARD]-containing 4 [NLRC4], Pyrin, and absent in melanoma 2 [AIM2]) are activated by direct ligand binding (AIM2: dsDNA; NAIP-NLRC4: type 3 secretion system [T3SS] needle, T3SS rod, or flagellin) or by indirect sensing of changes in cellular state (NLRP1: anthrax lethal toxin protease activity; Pyrin: Rho GTPase-inactivating toxins; NLRP3: broad range of cellular stressors including bacterial pore-forming toxins and viral infection, reviewed [1, 2, 3]). These proximal sensors may then interact with an adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC), which facilitates binding to and activation of caspase-1. Caspase-1 then proteolytically activates GSDMD, releasing its N-terminal pore-forming domain from the auto-inhibitory C-terminal domain, and cleaves the inactive pro–IL-1β and pro–IL-18 into their active forms [4]. The pore-forming activity of GSDMD then mediates pyroptotic cell lysis and release of intracellular cytokines and damage-associated molecular patterns (DAMPs). Similarly, necroptosis occurs following receptor-interacting serine/threonine-protein kinase (RIPK) 3-dependent phosphorylation of MLKL, promoting MLKL translocation to the plasma membrane and disruption of membrane integrity [5]. Unlike these lytic cell death pathways, apoptosis is often described as an ordered process characterized by cell shrinking and the blebbing off of membrane and intracellular components rather than rapid membrane disruption; this blebbing allows for inflammatory cell clearance by phagocytosis, limiting the release of intracellular cytokines and inflammatory DAMPs. Apoptosis is instigated by initiator caspase (caspase-8/9) cleavage of executioner caspases (caspases-3/6/7) which, through cleavage of a broad range of substrate proteins, drives apoptotic cell death processes [6].
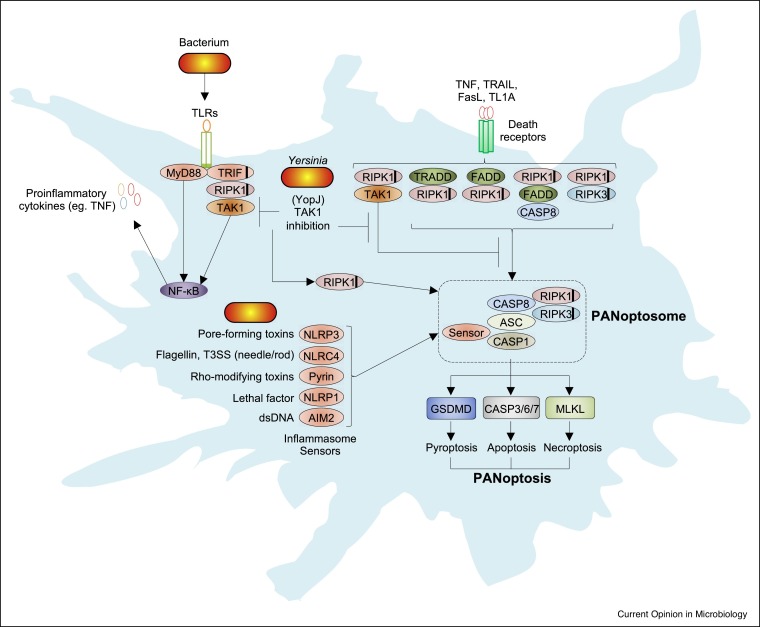
In this review we will focus on PANoptosis, a unique inflammatory programmed cell death regulated by the PANoptosome (Figure 1, Figure 2 ) [7, 8, 9]. Early studies identified that pyroptosis, via caspase-1, could cleave apoptotic caspases and poly (ADP-ribose) polymerase 1 (PARP1), that the apoptotic caspase-8 was important for the NLRP3-dependent inflammasome, and that overlap between pyroptosis, apoptosis, and necroptosis was important for different disease models, establishing a link between pyroptosis, apoptosis, and necroptosis regulators [10, 11, 12, 13, 14]. Depending on the proximal sensor of different microbial infections, the sensor molecule(s) can initiate the formation of a protein complex termed the PANoptosome, which provides a molecular scaffold that allows for interactions and activation of the machinery required for the inflammasome/pyroptosis (such as an inflammasome sensor, ASC, and caspase-1), apoptosis (caspase-8), and necroptosis (RIPK3, RIPK1) [8,9,15••,16••,17•]. One such proximal sensor, Z-DNA-binding protein 1 (ZBP1), binds influenza A virus (IAV) viral ribonucleoproteins (vRNPs) and forms a complex called the ZBP1-dependent PANoptosome consisting of RIPK3, RIPK1, caspase-6, caspase-8, ASC, NLRP3, and caspase-1 [17•,18]. Similarly, microbial pathogen-associated molecular patterns (PAMPs), through Toll-like receptors (TLRs) or death receptor signaling (including tumor necrosis factor [TNF] receptor 1 [TNFR1], Fas, TRAIL-R, and DR3), can promote RIPK1-dependent PANoptosome formation when regulatory proteins like transforming growth factor β-activated kinase 1 (TAK1) are inhibited [16••]. Similar to inflammasome complexes, which share core proteins but are also defined by their distinct proximal sensors (i.e. NLRP3 inflammasome versus NLRC4 inflammasome), distinct PANoptosomes may be named by their proximal sensors (i.e. ZBP1 PANoptosome). Together, these PANoptosome complexes promote activation of downstream cell death effectors representing pyroptosis (caspase-1 and GSDMD), apoptosis (caspase-3/7), and necroptosis (RIPK3 and MLKL). Deletion of all the PANoptosome components was required to protect macrophages from cell death during infection by multiple pathogens, suggesting it plays an important role in control of infections [15••,62]. This conceptual reframing of programmed cell death is important for understanding how the host immune system restricts pathogens in the face of adversarial microbial virulence factor manipulation of these same inflammatory signaling and cell death pathways.
Figure 1.
Activation of PANoptosis during bacterial infection.
Bacterial infection is sensed by TLRs and other innate receptors to produce NFκB-dependent inflammatory cytokines, including those of the TNF superfamily, which promote further inflammatory signaling through death receptors (including TNFR1, Fas, TRAIL-R, and DR3). Together, TLRs and death receptors signal through adaptor proteins (TRADD, FADD, RIPK1) that can engage downstream signaling pathways including TAK1 and NF-κB. In the absence of TAK1 activity, signaling through these receptors can promote cell death via RIPK1. TAK1-mediated suppression of PANoptosis is disrupted by the Yersinia T3SS effector YopJ, resulting in formation of a PANoptosome and activation of downstream pyroptosis, apoptosis, and necroptosis executioners as indicated. Inflammasome sensors detect their respective bacterial PAMPs and, through the adaptor ASC, can recruit and activate caspase-1/8. Black lines within proteins represent a RHIM interaction domain.
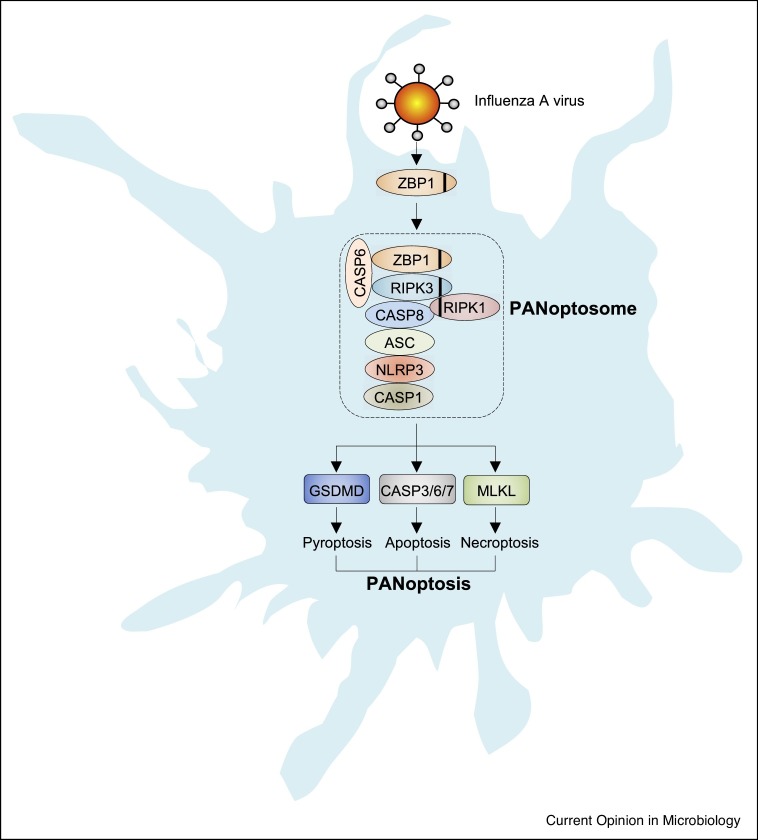
Figure 2.
ZBP1 as a master regulator of PANoptosis during IAV infection.
ZBP1 is an innate sensor of IAV that triggers PANoptosis and inflammation. ZBP1 activation leads to its interaction with RIPK3, caspase-6, RIPK1, and caspase-8 to assemble the PANoptosome. The ZBP1-dependent PANoptosome engages NLRP3 inflammasome activation and GSDMD-dependent pyroptosis. The activation of caspase-8 leads to caspase-3, caspase-6, caspase-7 activation and apoptosis and can also cleave GSDMD, while inactivation of caspase-8 leads to phosphorylation of MLKL and necroptosis. Black lines within proteins represent the RHIM interaction domain.
Here we will explore the physiological roles that PANoptosis plays during microbial infection and reflect on how it may be subverted by pathogens. In addition to bacterial infection, we will explore how viral pathogens also engage and manipulate multiple cell death regulators as a part of their virulence strategy and how fungal and parasitic infection may also initiate PANoptosis. Throughout, we will describe the cell death components that regulate PANoptosis during different infections, including those from pyroptosis (NLRP3, NLRC4, AIM2, ASC, caspase-1, and GSDMD), extrinsic apoptosis (TNFR1, caspase-8, caspase-3, and caspase-7), and necroptosis (TNFR1, caspase-8, RIPK1, RIPK3, and MLKL), and discuss how they play a role in PANoptosome formation. Critical regulators of these pathways, specifically molecules that regulate more than one of these defined pathways (including ZBP1, TAK1, RIPK1, and Fas-associated death domain [FADD]) will also be explored for their role in pathogen control. Re-evaluating the molecular determinants of cell death during infection with our updated understanding of the key molecular players is informative, as many previous studies examined cell death in immortalized cell lines, which inherently have dysregulated cell survival and cell death pathways.
PANoptosis in bacterial infection
Conceptually, cell death appears to protect against most acute bacterial pathogens that infect hosts and, in many cases, even more successfully restricts non-pathogenic or opportunistic bacteria [19,20]. Earlier studies on cell death, before the identification of many of the key regulatory genes, suggested multiple forms of cell death are induced following infection with different bacterial pathogens [20]. Armed with the current understanding of the key molecules involved in different cell death pathways and how they are extensively interconnected, future research must carefully re-examine cell death during infections. A generalized model for how bacteria may engage PANoptosis is presented in Figure 1. Signaling through TLRs and the death receptor family members and their adaptors (including Toll/interleukin-1 receptor domain-containing adaptor-inducing interferon [IFN]-β [TRIF], MyD88, FADD, and RIPK1) coordinate the activation and inhibition of cell death through changes in gene expression, protein localization, and activation status of cell death initiator proteins. Here, we present examples of how recent research utilizing different bacterial infection models has helped to illuminate the role of cell death in pathogen control.
Yersinia as a model for PANoptosis in host defense
Research into the modes of cell death activated during Yersinia infection has provided a useful model for understanding how PANoptosis can be activated by bacterial infection. Yersinia possess a T3SS effector protein, YopJ, which inhibits the host protein TAK1 [Sweet et al., Cell Microbiol 2007; Paquette et al., PNAS 2012].We discovered the critical role of TAK1 in cell death, showing that bone marrow-derived macrophages (BMDMs) lacking TAK1 undergo spontaneous cell death and that inhibition of TAK1 is sufficient to drive PANoptotic cell death involving caspase-8, RIPK1, RIPK3, MLKL, NLRP3, caspase-1, and GSDMD [23••,63,64]. TAK1-deficient cells form a complex consisting of RIPK1, caspase-8, ASC, and NLRP3 that activates downstream caspases-3/7, phosphorylates MLKL, and cleaves GSDMD, causing RIPK1-dependent PANoptosis [16••,63,64]. In the context of Yersinia, YopJ is secreted into macrophages and inhibits TAK1 and inhibitor of nuclear factor-κB kinase (IKK) to engage a RIPK1– and caspase-8–dependent cleavage of GSDMD and GSDME, resulting in cell death [21•,22•]. Together, the combined engagement of pyroptotic, apoptotic, and necroptotic machinery promotes intracellular clearance of Yersinia and release of inflammatory cytokines including IL-1β and IL-18. Depending on the cell type and the degree of inflammatory stimulation, differential expression and activation states of cell death signaling proteins are likely to dictate the relative degree to which each single cell death pathway is activated. The activation of PANoptosis during Yersinia infection is critical for the destruction of the intracellular niche and bacterial clearance.
Complex interplay of bacterial effectors and PANoptosis
As with Yersinia, Salmonella enterica serovar Typhimurium has been shown to both activate and regulate PANoptosis [15••]. Canonical pyroptotic ligands including the T3SS needle and rod proteins and flagellin each activate the NAIP-NLRC4 inflammasomes [24, 25, 26]. Similar to Yersinia, Salmonella T3SS effector proteins (AvrA, SspH1, SseL, GtgA, SpvC, SopB, SseK1/4) extensively inhibit TLR-mediated and TNF-mediated inflammatory signaling. Similar to Yersinia’s YopJ, Salmonella effector proteins may balance the production of inflammatory mediators and, in some cells, may trigger a PANoptotic cell death response as a consequence [27]. Despite the activity of many of the immunomodulatory effectors secreted by Salmonella, NLRC4 inflammasome activation readily occurs and can recruit NLRP3 to a combined inflammasome complex [28], which could potentially recruit other PANoptosome components to drive cell death. Indeed, this complex has also been shown to recruit caspase-8, and FADD/caspase-8 have both been shown to regulate the priming and activation of NLRP3-dependent inflammasome activation during infection [12,29]. In addition, Salmonella lipopolysaccharide (LPS) can be exposed from the Salmonella-containing vesicle by IFN-mediated expression of guanylate binding proteins (GBPs), which lyse the intracellular vacuole and allow caspase-11 sensing of LPS and activation of GSDMD, promoting additional NLRP3-dependent inflammasome activation [30,31]. Activation of TLR/TNF signaling during infection, in combination with variable expression of apoptosis-regulatory proteins, may also promote necroptosis, which has been suggested by data showing that MLKL phosphorylation occurs during BMDM infection [15••,32]. Interestingly, caspase-8–mediated suppression of necroptosis during intestinal Salmonella infection appears to be important for limiting excessive inflammatory damage in the host, suggesting cell-specific and tissue-specific responses should be carefully examined depending on the particular microbe [33].
Similar to Salmonella, enteropathogenic Escherichia coli (EPEC) possess T3SS effectors that block various arms of regulated cell death. NleB and NleF inhibit death receptor-mediated apoptosis and necroptosis by negatively regulating death domain (DD) interactions between FADD, TRADD, RIPK1, and caspase-8 or by directly inhibiting caspase activity, while EspL restricts necroptosis and pyroptosis via proteolytic cleavage of the receptor-interacting protein homotypic interaction motif (RHIM) domains of RIPK1, RIPK3, TRIF, and ZBP1. Loss of these effectors consequently may allow for increased activation of PANoptosis [34, 35, 36]. Other bacteria, including Listeria monocytogenes and Francisella novicida, also have the potential to activate multiple cell death processes [15••,37,38]. Francisella infection activates the AIM2 inflammasome, and recent studies have identified that AIM2, through ASC, can also promote apoptosis via caspase-8 [39]. Francisella-induced IFNs also promote expression of TRAIL, which signals through death receptors to promote apoptosis [38]. Highly virulent species of Francisella have also been described to induce necroptosis, but the molecular mechanisms of this are poorly studied [40]. Together, these overlapping modes of cell death activation provide another example of how PANoptosis can restrict infection by a wide range of bacteria possessing a wide range of PAMPs and virulence regulators.
PANoptosis in viral infection
PANoptosis plays a key role in viral infection and functions as an antiviral strategy restricting a variety of viruses. Here, we present examples of recent research utilizing different viral infection models that have helped to illuminate the role of multiple cell death pathways in pathogen control.
Influenza A virus (IAV) as a model for PANoptosis in host defense
Recently, ZBP1 has been identified as a sensor of IAV vRNPs that triggers PANoptosis (Figure 2) [18]. During infection, ZBP1 interacts with RIPK3 via the RHIM domain and recruits caspase-8 and the NLRP3 inflammasome sensor to promote assembly of a PANoptosome complex that drives PANoptosis [18,41, 42, 43]. The ZBP1-dependent PANoptosome is required for NLRP3– and capsase-1–dependent inflammasome activation, pyroptosis, and maturation of proinflammatory cytokines including IL-1β and IL-18 during IAV infection. IAV infection also results in activation of apoptotic caspases-8, caspases-3, and caspases-7. Inhibition of caspase activity during IAV infection, with the pan-caspase inhibitor z-VAD, promotes necroptosis [18]. More recently, caspase-6 was identified as a PANoptosome component that facilitates RHIM-dependent binding of RIPK3 with ZBP1, promoting ZBP1-mediated PANoptosis [17•].
Similar to how bacteria engage PANoptotic regulators through activation of TLR/TNF signaling and regulation of RIPK1 activation states, ZBP1 is regulated by upstream innate immune sensors. IAV RNA sensing by RIG-I or TLR3 results in expression of type I IFNs and signaling through the type I IFN receptor (IFNAR), upregulation of IFN regulatory factor 1 (IRF1), and IRF1-dependent ZBP1 expression [41,44]. Structurally, ZBP1 contains two nucleic acid Zα binding domains which were initially described to bind DNA in the Z-conformation and have been proposed to sense other nucleic acids (including vRNPs) in a conformation-dependent manner [41,45]. Once expressed, ZBP1 functions as a sensor of IAV infection by interacting with vRNP complexes (consisting of viral polymerases [PA, PB1, PB2], NP, and viral RNA) in the cytoplasm and forms a ZBP1-dependent PANoptosome [17•,18,43]. Cytoplasmic ZBP1 can translocate to the nucleus where viral genome replication occurs, indicating that sensing of IAV can occur in either the nucleus or the cytoplasm. Indeed, a recent study found that replicating IAV Z-RNAs are recognized by the Zα2 domain of ZBP1 in the nucleus of infected cells as viral nucleic acid ligands, which activate RIPK3 and MLKL, resulting in MLKL-dependent nuclear envelope rupture and necroptosis [42,46]. It remains unclear whether the Z-RNAs sensed by ZBP1 in the nucleus can assemble a PANoptosome in the cytoplasm. Taken together, these studies demonstrate ZBP1 interactions with various components of IAV to induce PANoptosis.
ZBP1-mediated PANoptosis in response to other viral pathogens
Although ZBP1 regulates PANoptosis in response to both mouse-adapted and human IAV strains, other RNA viruses including vesicular stomatitis virus (VSV), Sendai virus (SeV), and respiratory syncytial virus (RSV) do not appear to activate ZBP1-dependent PANoptosis [18,47]. While ZBP1 has not been shown to be required for cell death during infection with VSV, loss of key PANoptotic components including caspase-1/11, RIPK3, and caspase-8 reduces the amount of cell death observed during VSV infection [15••]. Additionally, cells require caspase-8/RIPK3 to induce PANoptosis during infection with the coronavirus murine hepatits virus (MHV), a model for studying coronavirus infections [62], though the role of ZBP1 remains unclear in this context. These data suggest that, unlike ZBP1-dependent activation of PANoptosis through direct sensing of IAV infection, innate receptor signaling through TLRs, TNFR1, and/or other unidentified sensors may initiate PANoptosis in other viral infections.
PANoptosis in viral pathogenesis
PANoptosis can be beneficial to the host as it triggers the infiltration of immune cells to remove infectious viral agents. However, excessive PANoptosis may also drive detrimental inflammation and tissue damage without an apparent benefit to the host. For example, ZBP1-dependent PANoptosis restricts replication of IAV but also contributes to virus-induced lung immunopathology [44,47,48]. ZBP1 is also important for the restriction of West Nile virus and Zika virus infection [49,50], but the role of PANoptosis is poorly understood. The balance of ZBP1-dependent PANoptosis and immunopathology is also highlighted by recent work showing that caspase-6, which promotes PANoptosis, is important for limiting IAV-induced lung immunopathology, presumably by promoting ZBP1-dependent PANoptosis [17•]. Together, these findings highlight how virus-induced PANoptosis must be balanced to reduce excessive inflammation while retaining antiviral functions. These studies indicate that activation or suppression of PANoptosis may be therapeutically targeted to improve viral infection outcomes in the future.
Complex interplay of viral modulators of PANoptosis
Because of the importance of PANoptotic cell death in limiting viral replication and promoting clearance, viruses have undergone selective pressure to limit PANoptosis and permit their intracellular replication. Several viruses encode proteins that interfere with PANoptotic signaling [51]. MCMV-encoded viral inhibitor of RIP activation (vIRA), the product of the M45 gene, is a potent RHIM-containing cell death suppressor that targets ZBP1, RIPK3, and RIPK1 [47], potentially restricting PANoptosome formation to evade PANoptosis. The HSV-1 protein ICP6 has a key role in restricting ZBP1-dependent cell death in human cells; however, this is not the case in murine cells, suggesting that host–pathogen adaptations may be driven by viral-mediated inhibition of PANoptosis [52]. These studies suggest that viruses have undergone selection to evade host cell PANoptosis, and these adaptations potentially help define the host range of different viruses. Future research may reveal inhibitors of these viral PANoptosis regulators that have therapeutic applications for treating viral infection.
PANoptosis in fungal and parasitic infections
While the majority of studies have focused on bacterial and viral pathogens which activate PANoptosis during infection, conceptually fungal and parasitic infections could result in similar cell death outcomes. Inflammatory signaling mediated by TLRs and death receptor family members is conserved during these infections. In addition, fungal pathogens such as Aspergillus fumigatus and Candida albicans activate the innate C-type lectin receptors (CLRs). Through these CLRs, fungi engage the molecule SYK, which drives formation of a molecular complex consisting of CARD9, BCL10, MALT1, ASC, caspase-8, NLRP3, AIM2, and caspase-1, which together likely promote PANoptosis [53, 54, 55, 56, 57]. The apicomplexan pathogen Toxoplasma gondii activates pyroptosis via NLRP1b and, similar to fungal infection, activates the SYK-CARD9 signaling axis to promote NLRP3/ASC/caspase-1 inflammasome activation and GSDMD-independent cell death, suggesting other gasdermin family members or other PANoptosis signaling components converge to restrict this parasitic infection [58,59]. These findings suggest that PANoptosis plays important roles in the host response to a wide variety of microbial agents.
Summary and future directions
The concept of PANoptosis can be leveraged to improve our understanding of selection pressures and protein–protein interactions among different pathogen–host pairs, emphasizing the importance of this programmed cell death process. While here we largely explored examples of acute infectious agents, the same framework can be used to understand the strategies of chronic infectious agents as well as commensal, zoonotic, and opportunistic infections. This concept is also applicable to both extracellular and intracellular pathogens, as many microbes considered to be largely extracellular have been shown to activate the NLRP3 inflammasome [4], potentially through activation of a hypothetical PANoptosome not requiring direct microbial ligand sensing. Recent work utilizing Casp1/11 −/− Casp8 −/− Ripk3 −/− mice also provides genetic evidence for PANoptosome components regulating cell death during infections of BMDMs with Salmonella, Listeria, IAV, and VSV [15••]. Additionally, in a murine colorectal cancer model, IRF1-dependent activation of PANoptosis prevents AOM/DSS-induced colorectal tumorigenesis, suggesting a broader role for PANoptosis in cancer [60]. Adaptive immune responses may also promote clearance of pathogens through cooperation with PANoptosis, as CD8+ T cells produce death receptor cytokines and lyse infected cells via secretion of perforin, which promotes NLRP3-dependent inflammasome activation [61]. Characterization of the interactions between microbial virulence factors and the many regulatory proteins known to modulate inflammatory signaling are likely to reveal unique mechanisms of PANoptosis activation. Considering the critical role PANoptosis plays in controlling multiple microbial infections, future studies should reveal new treatment strategies to both control infection and minimize inflammatory pathology with benefits to the host.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We apologize to our colleagues whose work was not cited due to space limitations. Research studies from our laboratory are supported by the U.S. National Institutes of Health (AR056296, CA253095, AI124346, and AI101935 to T.-D.K.), the American Lebanese Syrian Associated Charities (to T.-D.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the members of the Kanneganti lab for their comments and discussion throughout the development of this manuscript.
Footnotes
Given her role as Guest Editor, Thirumala-Devi Kanneganti had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Wolf-Dietrich Hardt.
References
- 1.Swanson K.V., Deng M., Ting J.P.-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tartey S., Kanneganti T.-D. Differential role of the NLRP3 inflammasome in infection and tumorigenesis. Immunology. 2019;156:329–338. doi: 10.1111/imm.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Place D.E., Kanneganti T.-D. Recent advances in inflammasome biology. Curr Opin Immunol. 2018;50:32–38. doi: 10.1016/j.coi.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Man S.M., Karki R., Kanneganti T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dondelinger Y., Declercq W., Montessuit S., Roelandt R., Goncalves A., Bruggeman I., Hulpiau P., Weber K., Sehon C.A., Marquis R.W., et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malireddi R.K.S., Kesavardhana S., Kanneganti T.-D. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis) Front Cell Infect Microbiol. 2019;9 doi: 10.3389/fcimb.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samir P., Malireddi R.K.S., Kanneganti T.-D. The PANoptosome: a deadly protein complex driving pyroptosis, apoptosis, and necroptosis (PANoptosis) Front Cell Infect Microbiol. 2020;10:238. doi: 10.3389/fcimb.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malireddi R.K.S., Tweedell R.E., Kanneganti T.-D. PANoptosis components, regulation, and implications. Aging (Albany NY) 2020;12:11163–11164. doi: 10.18632/aging.103528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamkanfi M., Kanneganti T.-D., Van Damme P., Vanden Berghe T., Vanoverberghe I., Vandekerckhove J., Vandenabeele P., Gevaert K., Núñez G. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malireddi R.K.S., Ippagunta S., Lamkanfi M., Kanneganti T.-D. Cutting edge: proteolytic inactivation of poly(ADP-Ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J Immunol. 2010;185:3127–3130. doi: 10.4049/jimmunol.1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurung P., Anand P.K., Malireddi R.K.S., Walle L.V., Opdenbosch N.V., Dillon C.P., Weinlich R., Green D.R., Lamkanfi M., Kanneganti T.-D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukens J.R., Gross J.M., Calabrese C., Iwakura Y., Lamkanfi M., Vogel P., Kanneganti T.-D. Critical role for inflammasome-independent IL-1β production in osteomyelitis. Proc Natl Acad Sci USA. 2014;111:1066–1071. doi: 10.1073/pnas.1318688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurung P., Burton A., Kanneganti T.-D. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1β–mediated osteomyelitis. PNAS. 2016;113:4452–4457. doi: 10.1073/pnas.1601636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Christgen S., Zheng M., Kesavardhana S., Karki R., Malireddi R.K.S., Banoth B., Place D.E., Briard B., Sharma B.R., Tuladhar S., et al. Identification of the PANoptosome: a molecular platform triggering cell death. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors here show how cells lacking key components of the PANoptosome are dramatically protected from cell death, while cells lacking each of the individual cell death pathways undergo extensive cell death. Together, these findings suggest that the host activates multiple overlapping cell death pathways to control microbes.
- 16••.Malireddi R.K.S., Gurung P., Kesavardhana S., Samir P., Burton A., Mummareddy H., Vogel P., Pelletier S., Burgula S., Kanneganti T.-D. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity–independent pyroptosis, apoptosis, necroptosis, and inflammatory diseaseRIPK1 kinase activity–independent cell death and inflammasome activation. J Exp Med. 2020;217 doi: 10.1084/jem.20191644. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows the role of TAK1 inhibition in PANoptosis and identifies, for the first time, a key role for RIPK1 in promoting formation of a PANoptosome through its scaffolding function, independent from its kinase function. This function is unique for TLR-mediated signaling, as TNF-dependent RIPK1-driven PANoptosis requires RIPK1 kinase function. This study has major implications for complex inflammatory signaling-mediated cell death.
- 17•.Zheng M., Karki R., Vogel P., Kanneganti T.-D. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell. 2020;181:674–687.e13. doi: 10.1016/j.cell.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show a novel role for caspase-6 in promoting ZBP1-dependent PANoptosome formation. Before this study, the role for caspase-6 in cell death was poorly understood.
- 18.Kuriakose T., Man S.M., Malireddi R.K.S., Karki R., Kesavardhana S., Place D.E., Neale G., Vogel P., Kanneganti T.-D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorgensen I., Rayamajhi M., Miao E.A. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacey C.A., Miao E.A. Programmed cell death in the evolutionary race against bacterial virulence factors. Cold Spring Harb Perspect Biol. 2020;12 doi: 10.1101/cshperspect.a036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Orning P., Weng D., Starheim K., Ratner D., Best Z., Lee B., Brooks A., Xia S., Wu H., Kelliher M.A., et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies a pathogen-encoded virulence factor, YopJ, which inhibits TAK1 and IKK signaling during infection and leads to RIPK1-dependent and caspase-8-dependent cleavage of GSDMD, activation of the NLRP3 inflammasome, and cell death.
- 22•.Sarhan J., Liu B.C., Muendlein H.I., Li P., Nilson R., Tang A.Y., Rongvaux A., Bunnell S.C., Shao F., Green D.R., et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. PNAS. 2018;115:E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies that during pathogen infection, the apoptotic caspase-8 can cleave the pore-forming protein GSDMD, the pyroptosis executioner molecule downstream of caspase-1. This provides evidence that the pyroptotic and apoptotic pathways are highly interconnected during infection with bacteria.
- 23••.Malireddi R.K.S., Gurung P., Mavuluri J., Dasari T.K., Klco J.M., Chi H., Kanneganti T.-D. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med. 2018;215:1023–1034. doi: 10.1084/jem.20171922. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows for the first time that inhibition of TAK1 causes spontaneous cell death and identifies TAK1 as a master regulator of PANoptosis. The authors go on to show that TAK1 is crucial for limiting spontaneous myeloid cell death in vivo, with implications for myeloproliferative diseases including cancer.
- 24.Franchi L., Amer A., Body-Malapel M., Kanneganti T.-D., Ozören N., Jagirdar R., Inohara N., Vandenabeele P., Bertin J., Coyle A., et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 25.Miao E.A., Ernst R.K., Dors M., Mao D.P., Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci U S A. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broz P., Newton K., Lamkanfi M., Mariathasan S., Dixit V.M., Monack D.M. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wemyss M.A., Pearson J.S. host cell death responses to non-typhoidal Salmonella infection. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Man S.M., Hopkins L.J., Nugent E., Cox S., Glück I.M., Tourlomousis P., Wright J.A., Cicuta P., Monie T.P., Bryant C.E. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. PNAS. 2014;111:7403–7408. doi: 10.1073/pnas.1402911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Man S.M., Tourlomousis P., Hopkins L., Monie T.P., Fitzgerald K.A., Bryant C.E. Salmonella infection induces recruitment of caspase-8 to the inflammasome to modulate IL-1β production. J Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Man S.M., Karki R., Sasai M., Place D.E., Kesavardhana S., Temirov J., Frase S., Zhu Q., Malireddi R.K.S., Kuriakose T., et al. IRGB10 liberates bacterial ligands for sensing by the AIM2 and caspase-11-NLRP3 inflammasomes. Cell. 2016;167:382–396.e17. doi: 10.1016/j.cell.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meunier E., Dick M.S., Dreier R.F., Schürmann N., Broz D.K., Warming S., Roose-Girma M., Bumann D., Kayagaki N., Takeda K., et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509 doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 32.Hos N.J., Ganesan R., Gutiérrez S., Hos D., Klimek J., Abdullah Z., Krönke M., Robinson N. Type I interferon enhances necroptosis of Salmonella Typhimurium-infected macrophages by impairing antioxidative stress responses. J Cell Biol. 2017;216:4107–4121. doi: 10.1083/jcb.201701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hefele M., Stolzer I., Ruder B., He G.-W., Mahapatro M., Wirtz S., Neurath M.F., Günther C. Intestinal epithelial caspase-8 signaling is essential to prevent necroptosis during Salmonella Typhimurium induced enteritis. Mucosal Immunol. 2018;11:1191–1202. doi: 10.1038/s41385-018-0011-x. [DOI] [PubMed] [Google Scholar]
- 34.Pearson J.S., Giogha C., Ong S.Y., Kennedy C.L., Kelly M., Robinson K.S., Lung T.W.F., Mansell A., Riedmaier P., Oates C.V.L., et al. A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature. 2013;501:247–251. doi: 10.1038/nature12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson J.S., Giogha C., Mühlen S., Nachbur U., Pham C.L.L., Zhang Y., Hildebrand J.M., Oates C.V., Lung T.W.F., Ingle D., et al. EspL is a bacterial cysteine protease effector that cleaves RHIM proteins to block necroptosis and inflammation. Nat Microbiol. 2017;2:16258. doi: 10.1038/nmicrobiol.2016.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S., Zhang L., Yao Q., Li L., Dong N., Rong J., Gao W., Ding X., Sun L., Chen X., et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature. 2013;501:242–246. doi: 10.1038/nature12436. [DOI] [PubMed] [Google Scholar]
- 37.McDougal C.E., Sauer J.-D. Listeria monocytogenes: the impact of cell death on infection and immunity. Pathogens. 2018;7 doi: 10.3390/pathogens7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Q., Man S.M., Karki R., Malireddi R.K.S., Kanneganti T.-D. Detrimental type I interferon signaling dominates protective AIM2 inflammasome responses during Francisella novicida infection. Cell Rep. 2018;22:3168–3174. doi: 10.1016/j.celrep.2018.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierini R., Juruj C., Perret M., Jones C.L., Mangeot P., Weiss D.S., Henry T. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19:1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh A., Periasamy S., Malik M., Bakshi C.S., Stephen L., Ault J.G., Mannella C.A., Sellati T.J. Necroptotic debris including damaged mitochondria elicits sepsis-like syndrome during late-phase tularemia. Cell Death Discov. 2017;3:1–12. doi: 10.1038/cddiscovery.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kesavardhana S., Kuriakose T., Guy C.S., Samir P., Malireddi R.K.S., Mishra A., Kanneganti T.-D. ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J Exp Med. 2017;214:2217–2229. doi: 10.1084/jem.20170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kesavardhana S., Malireddi R.K.S., Burton A.R., Porter S.N., Vogel P., Pruett-Miller S.M., Kanneganti T.-D. The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J Biol Chem. 2020;295:8325–8330. doi: 10.1074/jbc.RA120.013752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kesavardhana S., Kanneganti T.-D. ZBP1: a STARGᐰTE to decode the biology of Z-nucleic acids in disease. J Exp Med. 2020;217 doi: 10.1084/jem.20200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuriakose T., Zheng M., Neale G., Kanneganti T.-D. IRF1 is a transcriptional regulator of ZBP1 promoting NLRP3 inflammasome activation and cell death during influenza virus infection. J Immunol. 2018;200:1489–1495. doi: 10.4049/jimmunol.1701538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz T., Behlke J., Lowenhaupt K., Heinemann U., Rich A. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat Struct Biol. 2001;8:761–765. doi: 10.1038/nsb0901-761. [DOI] [PubMed] [Google Scholar]
- 46.Zhang T., Yin C., Boyd D.F., Quarato G., Ingram J.P., Shubina M., Ragan K.B., Ishizuka T., Crawford J.C., Tummers B., et al. Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell. 2020;180:1115–1129.e13. doi: 10.1016/j.cell.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Upton J.W., Kaiser W.J., Mocarski E.S. DAI/ ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thapa R.J., Ingram J.P., Ragan K.B., Nogusa S., Boyd D.F., Benitez A.A., Sridharan H., Kosoff R., Shubina M., Landsteiner V.J., et al. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothan H.A., Arora K., Natekar J.P., Strate P.G., Brinton M.A., Kumar M. Z-DNA-binding protein 1 is critical for controlling virus replication and survival in west nile virus encephalitis. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daniels B.P., Kofman S.B., Smith J.R., Norris G.T., Snyder A.G., Kolb J.P., Gao X., Locasale J.W., Martinez J., Gale M., et al. The nucleotide sensor ZBP1 and kinase RIPK3 induce the enzyme IRG1 to promote an antiviral metabolic state in neurons. Immunity. 2019;50:64–76.e4. doi: 10.1016/j.immuni.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuriakose T., Kanneganti T.-D. Pyroptosis in antiviral immunity. Curr Top Microbiol Immunol. 2019 doi: 10.1007/82_2019_189. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo H., Gilley R.P., Fisher A., Lane R., Landsteiner V.J., Ragan K.B., Dovey C.M., Carette J.E., Upton J.W., Mocarski E.S., et al. Species-independent contribution of ZBP1/DAI/DLM-1-triggered necroptosis in host defense against HSV1. Cell Death Dis. 2018;9:816. doi: 10.1038/s41419-018-0868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saïd-Sadier N., Padilla E., Langsley G., Ojcius D.M. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the syk tyrosine kinase. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gringhuis S.I., Kaptein T.M., Wevers B.A., Theelen B., van der Vlist M., Boekhout T., Geijtenbeek T.B.H. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 55.Ganesan S., Rathinam V.A.K., Bossaller L., Army K., Kaiser W.J., Mocarski E.S., Dillon C.P., Green D.R., Mayadas T.N., Levitz S.M., et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1β production in response to β-glucans and the fungal pathogen, Candida albicans. J Immunol. 2014;193:2519–2530. doi: 10.4049/jimmunol.1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karki R., Man S.M., Malireddi R.K.S., Gurung P., Vogel P., Lamkanfi M., Kanneganti T.-D. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe. 2015;17:357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Briard B., Karki R., Malireddi R.K.S., Bhattacharya A., Place D.E., Mavuluri J., Peters J.L., Vogel P., Yamamoto M., Kanneganti T.-D. Fungal ligands released by innate immune effectors promote inflammasome activation during Aspergillus fumigatus infection. Nat Microbiol. 2018;4:316–327. doi: 10.1038/s41564-018-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pandori W.J., Lima T.S., Mallya S., Kao T.H., Gov L., Lodoen M.B. Toxoplasma gondii activates a Syk-CARD9-NF-κB signaling axis and gasdermin D-independent release of IL-1β during infection of primary human monocytes. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ewald S.E., Chavarria-Smith J., Boothroyd J.C. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect Immun. 2014;82:460–468. doi: 10.1128/IAI.01170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karki R., Sharma B.R., Lee E., Banoth B., Malireddi R.K.S., Samir P., Tuladhar S., Mummareddy H., Burton A.R., Vogel P., et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight. 2020;5 doi: 10.1172/jci.insight.136720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao Y., Chen S., Cao M., Fan X., Yang T., Huang Y., Song X., Li Y., Ye L., Shen N., et al. Antigen-specific CD8 + T cell feedback activates NLRP3 inflammasome in antigen-presenting cells through perforin. Nat Commun. 2017;8:1–17. doi: 10.1038/ncomms15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng M., Williams E.P., Malireddi R.K.S., et al. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection [published online ahead of print, 2020 Aug 6] J Biol Chem. 2020 doi: 10.1074/jbc.RA120.015036. jbc.RA120.015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sweet C.R., Conlon J., Golenbock D.T., Goguen J., Silverman N. YopJ targets TRAF proteins to inhibit TLR-mediated NF-kappaB, MAPK and IRF3 signal transduction. Cell Microbiol. 2007;9:2700–2715. doi: 10.1111/j.1462-5822.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 64.Paquette N., Conlon J., Sweet C., et al. Serine/threonine acetylation of TGF#xps1#-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc Natl Acad Sci U S A. 2012;109:12710–12715. doi: 10.1073/pnas.1008203109. [DOI] [PMC free article] [PubMed] [Google Scholar]