Abstract
The mechanism through which developmental programming of offspring overweight/obesity following in utero exposure to maternal overweight/obesity operates is unknown but may operate through biologic pathways involving offspring anthropometry at birth. Thus, we sought to examine to what extent the association between in utero exposure to maternal overweight/obesity and childhood overweight/obesity is mediated by birth anthropometry. Analyses were conducted on a retrospective cohort with data obtained from one hospital system. A natural effects model framework was used to estimate the natural direct effect and natural indirect effect of birth anthropometry (weight, length, head circumference, ponderal index, and small-for-gestational-age [SGA] or large-for-gestational-age [LGA]) for the association between pre-pregnancy maternal BMI category (overweight/obese vs normal weight) and offspring overweight/obesity in childhood. Models were adjusted for maternal and child sociodemographics. 3,950 mother-child dyads were included in analyses (1,467 [57.8%] of mothers and 913 [34.4%] of children were overweight/obese). Results suggest that a small percent of the effect of maternal pre-pregnancy BMI overweight/obesity on offspring overweight/obesity operated through offspring anthropometry at birth (weight: 15.5%, length: 5.2%, head circumference: 8.5%, ponderal index: 2.2%, SGA: 2.9%, and LGA: 4.2%). There was a small increase in the percent mediated when gestational diabetes or hypertensive disorders were added to the models. Our study suggests that some measures of birth anthropometry mediate the association between maternal pre-pregnancy overweight/obesity and offspring overweight/obesity in childhood, and that the size of this mediated effect is small.
Keywords: Childhood obesity, maternal obesity, pregnancy, developmental programming, mediation, indirect effects, direct effects
INTRODUCTION
In the United States, rates of maternal obesity are climbing, especially among minority groups, with over half of non-Hispanic Black females of childbearing age being categorized as obese1. Compared to mothers categorized as normal weight, mothers with obesity have at least 3-times the odds of gestational diabetes and pregnancy-induced hypertensive disorders, and differential rates of gestational weight gain1-3. In turn, infants born to these mothers are more likely to be preterm, large-for-gestational-age, and have a higher fat mass as compared to infants born to unaffected mothers1,4-8. Into childhood and adolescence, these offspring are typically at an increased risk of excess adiposity and developing cardiometabolic disorders6,9,10.
Though researchers have provided compelling hypotheses, the mechanism through which developmental programming of offspring disease operates is largely unknown11-15. Within the context of the association between maternal pre-pregnancy overweight/obesity and offspring overweight/obesity, birthweight and other measures of birth anthropometry may play a mediating role. Very few studies have performed a formal mediation analysis of this association with results differing across these studies, potentially due to inconsistencies in measurements of birth anthropometry and study populations16-18. Large-for-gestational-age (LGA) status and ponderal index have been previously identified as significant mediators of the association between maternal metabolic disorders and offspring childhood obesity16,17. However, a recent study found that birthweight was not a significant mediator of the association between maternal pre-pregnancy BMI and offspring BMI percentile in childhood18. As far as we are aware, no prior studies have examined multiple measures of infant birth anthropometry to assess what specific measures might be mediators of this association. Infant birthweight and its classifications (e.g. LGA) capture only one dimension of fetal growth. Infant length and head circumference may provide additional information regarding the mechanism underlying the association between maternal overweight/obesity and offspring overweight/obesity in childhood . Furthermore, the role of factors related to maternal overweight/obesity and offspring birth and childhood anthropometry (e.g. gestational diabetes, hypertensive disorders of pregnancy, gestational weight gain) has not been elucidated. Understanding the role that birth anthropometry and other early life factors play in mediating or moderating this association may help scientists to understand the biologic mechanisms contributing to developmental programming.
Thus, our primary study aim was to assess to what extent the association between intrauterine exposure to maternal pre-pregnancy overweight/obesity and childhood overweight/obesity was mediated by birth anthropometry. Our secondary study aim was to examine whether maternal gestational cardiometabolic disorders moderated or mediated our associations of interest.
METHODS
Cohort Selection
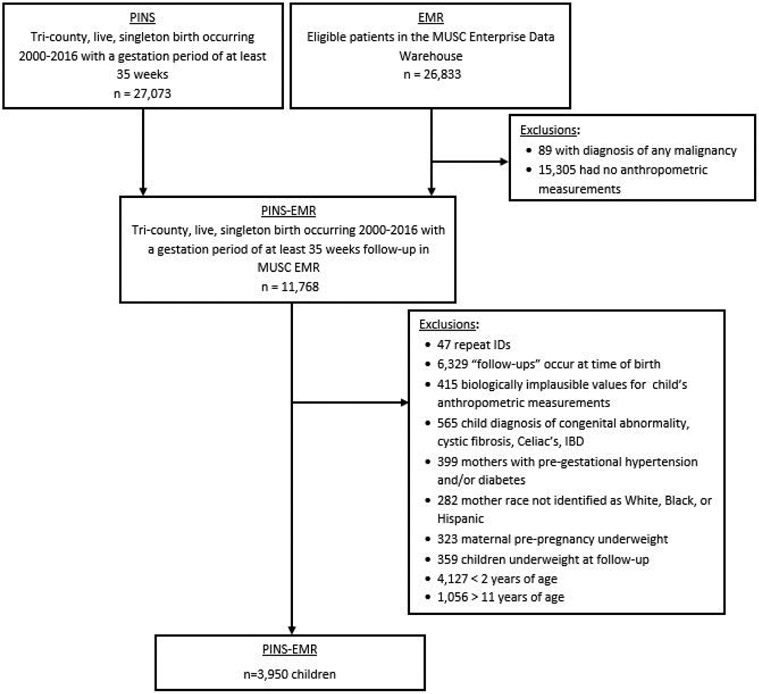
A retrospective hospital-based cohort was designed by linking the Perinatal Information Systems (PINS) and the electronic medical record from the Medical University of South Carolina (MUSC). The PINS is a database repository for abstracted medical records of: all mothers who deliver at MUSC, all babies who were delivered at MUSC, and all babies who were delivered at community hospitals and subsequently transferred to MUSC. PINS data is captured on a data abstraction form by three specially trained data abstractors with the opportunity to record over a thousand maternal and newborn data elements. Pregnancies from PINS were linked to the electronic medical record for: 1) confirmation of maternal diabetes diagnosis, and 2) offspring height, weight, and/or body mass index (BMI) until 18 years of age. See Figure 1 for the flow chart defining our study population and exclusions. The Institutional Review Board at the Medical University of South Carolina reviewed and approved this study.
Figure 1:
Flow-chart of Study Sample Size
Exposure
The primary exposure of interest was maternal pre-pregnancy BMI dichotomized as normal weight versus overweight/obese. Maternal pre-pregnancy BMI from PINS was abstracted from the electronic medical record and based on nurse or physician assessments. Mothers were classified into the following BMI categories: normal weight (18.5 kg/m2 < BMI < 25.0 kg/m2) or overweight/obese (BMI > 25.0 kg/m2). Underweight mothers (n=323) were excluded from analyses.
Secondary analyses compared mothers with obesity (BMI ≥ 30.0 kg/m2) to normal weight mothers.
Outcome
The primary study outcome was offspring BMI percentile dichotomized as overweight/obese or normal weight at follow-up in childhood. The electronic medical record provided assessments of offspring height (meters), weight (kgs), and/or BMI measured by nurses or physicians. Age- and sex-adjusted BMI percentiles were calculated using the Centers for Disease Control (CDC) National Health and Nutrition Examination Survey (NHANES) criteria19. Any values flagged as likely to be biologically implausible by the CDC program were set to missing (n=415). Offspring with BMI < 5th percentile were classified as underweight, BMI ≥ 5th and less than the 85th percentile as normal weight, and BMI ≥ 85th percentile as overweight/obese. Underweight offspring (n=359) were excluded from analyses.
Secondary analyses compared offspring with obesity (BMI ≥ 95th percentile) to offspring categorized as normal weight at follow-up.
Mediators
We had four continuous mediating variables that we examined in our analyses: birthweight (g), birth length (cm), head circumference at birth (cm), ponderal index (kg/m3). These mediators were obtained from the PINS dataset and measured by nurses or physicians at birth. Ponderal index was calculated as a measure of mass per length at birth cubed (kg/m3)20. Twenty-eight measures of length and head circumference, and thirty-seven measures of ponderal index were set to missing based on having biologically implausible outlying values.
Birthweight-for-gestational-age percentiles were calculated based on estimates from Talge et al and infants were categorized as large-for-gestational age (>90th birthweight-for-gestational-age percentile) or small-for-gestational age (<10th birthweight-for-gestational-age percentile)21. These two variables were assessed as potential mediators, with reference categories being infants born appropriate-for-gestational-age (between the 10th and 90th birthweight-for-gestational-age percentiles).
In secondary analyses, we sought to assess mediation and moderation by maternal gestational cardiometabolic disorders. These included gestational diabetes, hypertensive disorders of pregnancy, or gestational weight gain (lbs). We defined gestational diabetes based on its diagnosis either in the PINS dataset or electronic medical record. We defined hypertensive disorders of pregnancy based on a diagnosis of either gestational hypertension, pre-eclampsia, or eclampsia in the PINS dataset.
Covariates
Covariates adjusted for in all models included maternal and child sociodemographics. Continuous study covariates included: maternal age (years), maternal education (number of years), and year of birth (2000-2016). Categorical study covariates include: maternal smoking (yes/no), maternal insurance (Private/Self-pay, Medicaid), maternal race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic), child’s sex (male/female), child’s age (2-5 years, 5-7 years, 8-11 years), and first birth (yes/no).
Statistical Analyses
SAS System (version 9.4; SAS institute; Cary, NC) and RStudio (Version 3.5.2) were used to run all analyses. A p-value of 0.05 was used for assessing statistically significant moderated mediation. Prior to running analyses, normality assumptions were assessed using histograms and outliers were set to missing as detailed above.
Mediation analyses were run using the medflex package in R, which enabled us to flexibly estimate direct and indirect effects for non-parametric data within a natural effect model framework22. We ran all models first using an imputation-based approach, which operates by fitting a working model for the outcome mean. This approach with bootstrap standard errors allowed us to accommodate missing outcomes in our dataset through computation of nested counterfactuals. The natural indirect, natural direct, and total effects were estimated as odds ratios (ORs) and 95% confidence intervals (95% CI).
Figure 2 presents a directed acyclic graph (DAG) of the hypothesized association between maternal pre-pregnancy BMI and child’s BMI percentile. The indirect effect represents the amount of the total effect of maternal pre-pregnancy BMI on child’s BMI percentile that operates via the mediator of interest. In Figure 2, this indirect effect is represented as the arrow pathways a and c. Within a counterfactual framework, the natural indirect effect is defined as the change in the outcome (odds of child overweight/obesity) that would be observed if we could change the mediator (birth anthropometry) to what would be observed if the exposure was changed but without actually changing the exposure (maternal overweight/obesity). The direct effect represents the amount of the total effect of maternal pre-pregnancy BMI on child’s BMI percentile that does not operate via the mediator of interest. In Figure 2, this direct effect is represented as the arrow pathway b. Within a counterfactual framework, the natural direct effect is defined as the change in the outcome (odds of child overweight/obesity) that would be observed if we changed the exposure (maternal overweight/obesity) while leaving the mediator at its natural value for the unchanged exposure. The total effect is the sum of these effects and represents the total effect of the exposure on the outcome. In Figure 2, this total effect is the sum of the a, b, and c pathways.
Figure 2.
Directed Acyclic Graph showing the hypothesized relationship between maternal pre-pregnancy body mass index and child’s body mass index. Bolded terms are primary exposure and outcome. Italicized terms are model covariates. Bolded and italicized terms are hypothesized mediators. GWG, GDM, and HTN were examined to assess whether they mediated or moderated the primary association of interest. Solid lines represent our natural direct (b), indirect (a + c), and total (a + b + c) effects of interest. For secondary analyses, the natural direct (b), indirect (d + e + c), and total (b + c + d + e) effects of interest were examined. Abbreviations: gestational weight gain (GWG), gestational diabetes (GDM), hypertensive disorders of pregnancy (HDP), body mass index (BMI)
For each of the measures of birth anthropometry, we ran natural effects models examining the association between maternal overweight/obesity and child’s overweight/obesity with adjustment for maternal and child sociodemographics (maternal age, education, insurance, smoking status, first birth, year of birth, and child’s age and sex). The percent of the association mediated was estimated using .
In secondary analyses, we examined moderation and mediation of these effects by maternal gestational cardiometabolic disorders. For moderation analyses, we sought to assess whether the direct or indirect effects generalized across different population strata by examining effect modification of these effects by levels of maternal gestational cardiometabolic disorders. We assessed these moderators for association with weight, length, head circumference, and ponderal index at birth as mediators; sample sizes were insufficient to assess moderation in models with small- or large-for-gestational-age as mediators. Moderated mediation (moderation of the indirect effects) occurs when the effect the independent variable (maternal pre-pregnancy overweight/obesity) on the dependent variable (offspring overweight/obesity) via a mediator variable (weight, length, head circumference, ponderal index at birth) differs depending on levels of the moderating variable (gestational diabetes, hypertensive disorders of pregnancy, gestational weight gain). For mediation analyses including these factors, we examined the natural direct and indirect effects for models including birth anthropometry plus gestational weight gain, gestational diabetes, or hypertensive disorders of pregnancy; additional combinations of mediators (e.g. examining a pathway operating through gestational weight gain, gestational diabetes, birth weight) could not be fit due to insufficient sample size. In figure 2, this secondary mediation analyses assesses the direct effect (b), indirect effect (c + d + e) and total effect (b + c + d + e) of maternal pre-pregnancy BMI on child’s BMI percentile.
We ran several sensitivity analyses. First, we limited our population only to full-term infants (≥ 37 weeks gestation). Second, we used maternal pre-pregnancy obesity (versus normal weight) as our exposure and child obesity (versus normal weight) as our outcome. Overweight children were excluded in this analysis. Third, as non-Hispanic Blacks made up about 60% of our total sample, we limited our analyses to this race/ethnic group; other subgroup sample sizes were too small to examine in sensitivity analyses.
RESULTS
Of the 3950 mother-child dyads were included in analyses, 2537 were not missing exposure data and 2656 were not missing outcome data. Of those without missing data, 1467 (57.8%) of mothers and 913 (34.4%) of children were overweight/obese, and 774 (30.5%) of mothers and 476 (17.9%) of children were obese. Table 1 presents maternal and child sample characteristics by maternal pre-pregnancy BMI categories.
Table 1.
Maternal and Child Sample Characteristics by Maternal Pre-Pregnancy BMI Categories
| Normal Weight (N=1,467) |
Overweight/Obese (N=1,070) |
Total (N=2,537) |
|
|---|---|---|---|
| Maternal Sociodemographics | |||
| Maternal Age at Child’s Birth (Mean ± SD) | 24.9 ± 6.1 | 25.4 ± 5.7 | 25.2 ± 5.9 |
| Maternal Education (years) (Mean ± SD) | 12.0 ± 3.1 | 11.7 ± 2.3 | 11.8 ± 2.7 |
| Maternal Medicaid (N (%)) | 637 (59.6%) | 983 (67.1%) | 1620 (63.9%) |
| Maternal Smoking (N (%)) | 102 (9.5%) | 117 (8.0%) | 219 (8.6%) |
| First Birth (N (%)) | 481 (45.0%) | 497 (33.9%) | 978 (38.5%) |
| Maternal Race/Ethnicity (N (%)) | |||
| Non-Hispanic White | 274 (25.6%) | 230 (15.7%) | 504 (19.9%) |
| Non-Hispanic Black | 560 (52.3%) | 962 (65.6%) | 1522 (60.0%) |
| Hispanic | 236 (22.1%) | 275 (18.7%) | 511 (20.1%) |
| Maternal Gestational Cardiometabolic Disorders | |||
| Gestational Diabetes (N (%)) | 75 (7.0%) | 168 (11.5%) | 243 (9.6%) |
| Maternal Hypertension (N (%)) | 73 (6.8%) | 192 (13.1%) | 265 (10.4%) |
| Maternal Gestational Weight Gain (lbs) (Mean ± SD) | 31.5 ± 14.5 | 27.5 ± 16.2 | 29.2 ± 15.6 |
| Birth Anthropometry | |||
| Birthweight (g) (Mean ± SD) | 3,163 ± 482 | 3,256 ± 501 | 3,217 ± 496 |
| Birth length (cm) (Mean ± SD) | 499.6 ± 27.3 | 501.1 ± 27.2 | 500.5 ± 27.3 |
| Birth Head Circumference (cm) (Mean ± SD) | 33.5 ± 1.7 | 33.7 ± 1.7 | 33.7 ± 1.7 |
| Ponderal Index (kg/m3) (Mean ± SD) | 25.4 ± 3.3 | 25.9 ± 3.4 | 25.7 ± 3.4 |
| Large-for-Gestational-Age (N (%)) | 37 (3.6%) | 107 (7.6%) | 144 (5.9%) |
| Small-for-Gestational-Age (N (%)) | 163 (15.8%) | 175 (12.4%) | 338 (13.8%) |
| Child’s Sociodemographics | |||
| Male (N (%)) | 567 (53.0%) | 764 (52.1%) | 1331 (52.5%) |
| Child Age (years) (Mean ± SD) | 6.0 ± 2.8 | 5.8 ± 2.7 | 5.9 ± 2.7 |
The natural direct, natural indirect, and total effects of maternal pre-pregnancy BMI category on child’s odds of overweight/obesity by birth anthropometry are presented in Table 2. Across all models, the total effect of maternal pre-pregnancy overweight/obesity on offspring overweight/obesity in childhood was elevated. The natural direct effect of maternal pre-pregnancy BMI category on child’s odds of overweight/obesity was similarly elevated in all models, though the magnitude of this effect differed depending on the mediator of interest. The natural direct effect for birthweight may be interpreted as: altering the level of maternal pre-pregnancy BMI from normal weight to overweight/obese while controlling for birthweight (i.e. setting birthweight to levels naturally observed given maternal pre-pregnancy BMI category) increases the odds of child’s overweight/obesity by 1.69 (95% CI: 1.32, 2.12). The natural indirect effect was elevated for all potential mediators, but 95% confidence intervals did not bisect 1.00 for birthweight and head circumference, suggesting these factors may mediate the exposure-outcome association. Of note, across all potential mediators, the natural indirect effects were relatively small when compared to the natural direct effects. The natural indirect effect for birthweight may be interpreted as: altering the level of birthweight as observed for normal weight mothers to levels that would have been observed for overweight/obese mothers, while controlling for maternal pre-pregnancy BMI category at any given level, increased the odds of child’s overweight/obesity by 1.07 (95% CI: 1.03, 1.11).
Table 2.
Natural Direct, Natural Indirect, and Total Effects of Maternal Pre-Pregnancy Overweight/Obesity on Child’s Overweight/Obesity for Measures of Birth Anthropometry and in the Presence of Multiple Mediatorsa,b
| Birth anthropometry | Birth anthropometry + gestational weight gain |
Birth anthropometry + gestational diabetes |
Birth anthropometry + hypertensive disorders of pregnancy |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Percent Mediated |
OR (95% CI) | Percent Mediated |
OR (95% CI) | Percent Mediated |
OR (95% CI) | Percent Mediated |
|
| Birthweight | ||||||||
| Natural direct effect | 1.68 (1.33, 2.15) | 1.69 (1.34, 2.13) | 1.67 (1.31, 2.10) | 1.66 (1.31, 2.09) | ||||
| Natural indirect effect | 1.07 (1.03, 1.11) | 15.50% | 1.07 (1.02, 1.12) | 15.05% | 1.08 (1.04, 1.12) | 16.29% | 1.09 (1.03, 1.14) | 17.60% |
| Total effect | 1.81 (1.43, 2.31) | 1.82 (1.43, 2.28) | 1.81 (1.41, 2.26) | 1.81 (1.42, 2.26) | ||||
| Birth Length | ||||||||
| Natural direct effect | 1.76 (1.39, 2.22) | 1.78 (1.41, 2.23) | 1.75 (1.38, 2.21) | 1.75 (1.37, 2.18) | ||||
| Natural indirect effect | 1.02 (1.00, 1.05) | 5.16% | 1.02 (0.98, 1.05) | 3.67% | 1.03 (1.00, 1.06) | 6.31% | 1.03 (0.99, 1.07) | 7.01% |
| Total effect | 1.81 (1.42, 2.27) | 1.81 (1.44, 2.27) | 1.80 (1.42, 2.27) | 1.80 (1.41, 2.25) | ||||
| Birth Head Circumference | ||||||||
| Natural direct effect | 1.71 (1.34, 2.15) | 1.73 (1.35, 2.18) | 1.69 (1.33, 2.12) | 1.70 (1.33, 2.14) | ||||
| Natural indirect effect | 1.04 (1.01, 1.07)* | 8.48% | 1.03 (0.99, 1.07) | 6.98% | 1.04 (1.01, 1.08) | 9.81% | 1.04 (1.00, 1.09) | 9.81% |
| Total effect | 1.78 (1.39, 2.23) | 1.78 (1.39, 2.25) | 1.76 (1.39, 2.21) | 1.78 (1.39, 2.23) | ||||
| Birth Ponderal Index | ||||||||
| Natural direct effect | 1.79 (1.43, 2.26) | 1.81 (1.42, 2.27) | 1.78 (1.41, 2.23) | 1.77 (1.39, 2.24) | ||||
| Natural indirect effect | 1.01 (0.99, 1.03) | 2.18% | 1.00 (0.98, 1.03) | 0.78% | 1.02 (0.99, 1.04) | 3.32% | 1.02 (0.99, 1.06) | 4.56% |
| Total effect | 1.81 (1.44, 2.29) | 1.81 (1.43, 2.28) | 1.81 (1.43, 2.26) | 1.81 (1.42, 2.28) | ||||
| Small-for-Gestational-Age | ||||||||
| Natural direct effect | 1.74 (1.36, 2.21) | 1.77 (1.36, 2.26) | 1.73 (1.35, 2.20) | 1.74 (1.35, 2.20) | ||||
| Natural indirect effect | 1.01 (1.00, 1.03) | 2.91% | 1.00 (0.97, 1.04) | 1.09% | 1.02 (0.99, 1.05) | 4.64% | 1.02 (0.98, 1.06) | 4.56% |
| Total effect | 1.77 (1.37, 2.23) | 1.78 (1.37, 2.27) | 1.76 (1.38, 2.24) | 1.77 (1.38, 2.24) | ||||
| Large-for-Gestational-Age | ||||||||
| Natural direct effect | 1.86 (1.43, 2.38) | 1.89 (1.45, 2.43) | 1.84 (1.42, 2.36) | -- | ||||
| Natural indirect effect | 1.02 (0.99, 1.05) | 4.23% | 1.01 (0.97, 1.05) | 2.20% | 1.03 (0.99, 1.07) | 5.87% | -- | -- |
| Total effect | 1.90 (1.46, 2.43) | 1.91 (1.47, 2.45) | 1.90 (1.46, 2.42) | -- | ||||
Model adjusted for maternal & child sociodemographics (maternal age, maternal education, maternal insurance, maternal smoking, first birth, child’s sex, and child’s age)
n=3950 mother-child dyads examined using imputation-based methods, which fit a working model for the outcome mean
p=0.04 test for moderation by gestational weight gain
In analyses examining whether maternal gestational cardiometabolic disorders moderated the association between maternal pre-pregnancy overweight/obesity and child’s overweight/obesity, gestational weight gain presented itself as a statistically significant moderator of the natural indirect effect operating via offspring’s head circumference (p=0.04). This suggests that the impact of maternal overweight/obesity on offspring overweight/obesity operating via head circumference is significantly different by amount of maternal gestational weight gain. This association was not observed in sensitivity analyses.
In analyses examining whether maternal gestational cardiometabolic disorders were secondary mediators of the association between maternal pre-pregnancy overweight/obesity and child’s overweight/obesity, hypertensive disorders of pregnancy, and gestational diabetes were additional mediators of this association though their addition to models did not increase the mediated effect by much (Table 2). Models including hypertensive disorders of pregnancy and large-for-gestational-age would not converge.
Results of sensitivity analyses for our mediation analyses did not substantially differ from those in our primary analyses (Supplementary Materials). Head circumference at birth no longer appeared to be a mediator of interest within the context of the association between maternal pre-pregnancy obesity and child obesity, and for analyses limited to non-Hispanic Blacks. Effect sizes were much larger for natural direct and total effects in analyses examining maternal pre-pregnancy obesity and child obesity. None of our moderated mediation analyses suggested significant moderation by our variables of interest. Results of multiple mediator analyses for each of our sensitivity analyses were similar to our primary analyses though effect sizes were larger in analyses examining maternal pre-pregnancy obesity and child obesity.
DISCUSSION
The results of this study demonstrate that maternal pre-pregnancy overweight/obesity has a consistent association with offspring overweight/obesity in childhood, with a small portion of this association mediated by birthweight and head circumference. Pathways involving birth anthropometry plus gestational diabetes or hypertensive disorders of pregnancy as secondary mediators had only a slightly larger mediated effect. Across all models, there was little consistent evidence of maternal gestational cardiometabolic disorders serving as moderators of the association of interest.
Multiple previous studies have identified maternal overweight/obesity pre-pregnancy as a risk factor for offspring overweight/obesity across the lifespan9,23. Similarly, we demonstrated in the current study that children 2-11 years of age exposed in utero to maternal overweight/obesity were more likely to be overweight/obese as opposed to unexposed children. Though the true mechanisms underlying the association between maternal overweight/obesity and offspring overweight/obesity are unknown, most authors hypothesize that offspring adaptations to maternal metabolic dysfunction occur at both the biologic and epigenetic level, and may also operate through modifications to the offspring microbiome24,25. Biologically, maternal overweight/obesity pre-pregnancy may result in an increase in the transfer of fuels across the placenta and induce fetal hyperglycemia or hyperinsulinemia24,26. This in turn, may cause increased fetal production of anabolic hormones and growth factors, resulting in increased adiposity that is sustained throughout the offspring’s lifetime24-26. Offspring birthweight and other measures of anthropometry at birth may act along the causal pathway between these exposures and long-term health outcomes. This is the proposed causal pathway we sought to examine in our study.
Three formal mediation analyses have been previously performed examining mediation between in utero exposure to maternal metabolic disorders and anthropometric outcomes in childhood or adolescence16-18,27. Lamb et al performed mediation analyses of early life predictors of offspring BMI in childhood on 1,178 subjects at increased genetic risk for Type 1 diabetes16. This study identified large-for-gestational-age as a significant mediator of the association between maternal diabetes and offspring childhood obesity16. A path analysis by Morgen et al reported that ponderal index as a mediator of the association between maternal or paternal BMI and offspring BMI at age 7 and 11 years17. More recently, Adane et al published a study showing that birthweight was not a significant mediator of the association between maternal pre-pregnancy BMI and offspring BMI percentile in childhood18. This contrasts with our study, which reported birthweight as a mediator of this association. Pooling the results of these studies suggests that the effects of in utero exposure to maternal overweight/obesity on offspring overweight/obesity may be minimally mediated by offspring birth anthropometry.
As far as we are aware, no prior studies have examined or found head circumference at birth to be a mediator of the association between in utero exposure to maternal metabolic disorders and offspring overweight/obesity in childhood. One prior study reported a relationship between early life head circumference and an earlier age at adiposity rebound28. These authors suggest that constraints on head circumference in utero may lead to rapid postnatal growth in an effort to increase infant fat stores.
The association between maternal pre-pregnancy BMI on offspring anthropometry at birth and throughout the lifecourse has been previously established25. Maternal pre-pregnancy BMI has also been associated with excess gestational weight gain, gestational diabetes, and hypertensive disorders of pregnancy1,25. Excess gestational weight gain, gestational diabetes, and hypertensive disorders of pregnancy have been associated with each other as well as with offspring anthropometric outcomes25. These complex relationships have not been appropriately accounted for in prior mediation analyses, which have either ignored the role that gestational cardiometabolic disorders may play in their association of interest or simply adjusted for these disorders in analyses17,29. Our results suggest that gestational diabetes and hypertensive disorders of pregnancy may play roles as additional mediators of the association between maternal pre-pregnancy overweight/obesity and offspring overweight/obesity.
Our study has several notable strengths. First, our study is novel in that we were able to control for many confounding factors and to examine the role that gestational cardiometabolic disorders may play within the context of our association. Second, our study is comprised of predominantly non-Hispanic Blacks, a relatively understudied race/ethnic group, despite being disproportionately impacted by the obesity epidemic1,30. Third, we examined the natural indirect effects of several potential mediators of the association of interest including weight, length, head circumference, ponderal index, and being small-for-gestational-age or large-for-gestational-age at birth. These are measures traditionally obtained at birth and may provide clinicians with information concerning short- and long-term outcomes, including offspring overweight/obesity.
Our study also had several notable limitations. Administrative datasets were used to form our cohort, which may have led to poor data quality. Diagnoses in electronic medical records tend to underestimate the true prevalence of disease, which may result in bias of our results towards the null31. Fortunately, evidence suggests that childhood anthropometrics collected during routine practice are fairly accurate, with one study reporting an accuracy of 97.3%32. However, PINS is a research-quality database rigorously assessed for accuracy and was supplemented with the electronic medical record when there was overlap to ensure accurate diagnoses were captured. Missing data was common in these administrative datasets. Using an imputation-based approach in Medflex allowed us to estimate our associations even in the presence of missing outcomes.
However, we still had substantial missingness; thus, results should be generalized with caution. One major assumption of causal mediation analyses is adequate control for confounding, which may not have been met in our dataset. Thus, though the term “effect” is often used in mediation analyses, we are not proposing a causal relationship in the current study. Further studies may help to better control for potential confounders and be able to better estimate the causal effects for this association. Finally, the impact of the postnatal environment on modifying or mediating the effect of prenatal exposures on offspring health is largely unknown, and we were unable to examine these types of effects within our analyses. Future research should focus on identifying whether postnatal factors can reduce the negative effect of harmful prenatal exposures on offspring health.
CONCLUSION
As the prevalence of maternal obesity pre- and during pregnancy increases, it is paramount that additional research into the impact of this exposure on offspring short- and long-term wellbeing be conducted, and that the potential pathways whereby this exposure may be impacting offspring are examined. Our study suggests that birth anthropometry may mediate the association between maternal pre-pregnancy overweight/obesity and offspring overweight/obesity in childhood, though the magnitude of this mediated effect is small. Future studies should attempt to replicate these results and assess other pathways of interest to identify additional mediators and moderators of this association.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT
This work was supported by the Medical University of South Carolina’s Clinical and Translational Research Institute through the National Institute of Health [UL1 TR001450 and TL1 TR001451], and the Office Of The Director of the National Institutes of Health [UG3OD023316].
Footnotes
CONFLICTS OF INTEREST
The authors declare they have no actual or potential conflicts of interests. Study sponsor(s) had no role in study design, collection, and analysis, interpretation of data, report writing, or decision to submit the paper for publication.
ETHICS STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant United States guidelines on human subjects research and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional review board at the Medical University of South Carolina.
REFERENCES
- 1.Poston L, Caleyachetty R, Cnattingius S, et al. Maternal obesity 1 Preconceptional and maternal obesity : epidemiology and health consequences LANCET Diabetes Endocrinol [Internet], Elsevier Ltd; 2016;8587(16). Available from: 10.1016/S2213-8587(16)30217-0 [DOI] [PubMed] [Google Scholar]
- 2.Feresu SA, Wang Y, Dickinson S. Relationship between maternal obesity and prenatal, metabolic syndrome, obstetrical and perinatal complications of pregnancy in Indiana, 2008-2010 BMC Pregnancy Childbirth [Internet]. BioMed Central; 2015. October 16 [cited 2019 Jan 15];15:266 Available from: http://www.ncbi.nlm.nih.gov/pubmed/26475596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaillard R, Steegers EAP, Franco OH, Hofman A, Jaddoe VWV. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study Int J Obes (Lond) [Internet]. Nature Publishing Group; 2015;39(4):677–85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25287752 [DOI] [PubMed] [Google Scholar]
- 4.Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101(2):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan KM, Gale C, Hyde MJ, Santhakumaran S, Modi N. Diabetes in pregnancy and infant adiposity: systematic review and meta-analysis. Arch Dis Child - Fetal Neonatal Ed [Internet]. 2017;102(1):F65–F72. Available from: http://fn.bmj.com/lookup/doi/10.1136/archdischild-2015-309750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojha S, Robinson L, Symonds ME, Budge H. Suboptimal maternal nutrition affects offspring health in adult life Early Hum Dev [Internet]. Elsevier Ltd; 2013;89(11):909–913. Available from: 10.1016/j.earlhumdev.2013.08.022 [DOI] [PubMed] [Google Scholar]
- 7.Stang J, Huffman LG. Position of the Academy of Nutrition and Dietetics: Obesity, Reproduction, and Pregnancy Outcomes. J Acad Nutr Diet. 2016;116(4):677–691. [DOI] [PubMed] [Google Scholar]
- 8.Berglind D, Willmer M, Näslund E, Tynelius P, Sørensen TIA, Rasmussen F. Differences in gestational weight gain between pregnancies before and after maternal bariatric surgery correlate with differences in birth weight but not with scores on the body mass index in early childhood. Pediatr Obes. 2014;9(6):427–434. [DOI] [PubMed] [Google Scholar]
- 9.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-Pregnancy Body Mass Index in Relation to Infant Birth Weight and Offspring Overweight/Obesity: A Systematic Review and Meta-Analysis. PLoS One. 2013;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philipps LH, Santhakumaran S, Gale C, et al. The diabetic pregnancy and offspring BMI in childhood: A systematic review and meta-analysis. Diabetologia. 2011;54(8):1957–1966. [DOI] [PubMed] [Google Scholar]
- 11.Gillman MW, Rich-Edwards JW. The fetal origins of adult disease: from sceptic to convert. Paediatr Perinat Epidemiol. 2000;14:192–193. [DOI] [PubMed] [Google Scholar]
- 12.Hales CN, Barker DJP. The thrifty phenotype hypothesis: Type 2 diabetes. Br Med Bull [Internet]. 2001;60(1):5–20. Available from: http://bmb.oxfordjournals.org/cgi/content/abstract/60/1/5 [DOI] [PubMed] [Google Scholar]
- 13.Bateson P, Barker D, Clutton-Brock T, et al. Developmental plasticity and human health. Nature. 2004;430(6998):419–421. [DOI] [PubMed] [Google Scholar]
- 14.Wells JCK. Environmental quality, developmental plasticity and the thirfty phenotype: a review of evolutionary models. Evol Bioinforma. 2007;3:109–120. [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson M, Gluckman P. Developmental origins of noncommunicable disease: population and public health implications. Am J Clin Nutr [Internet]. 2011;94(6):1754–1758. Available from: http://ajcn.nutrition.org/content/94/6_Suppl/1754S.full.pdf+html [DOI] [PubMed] [Google Scholar]
- 16.Lamb MM, Dabelea D, Yin X, et al. Early-life predictors of higher body mass index in healthy children. Ann Nutr Metab. 2010;56(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgen CS, Ängquist L, Baker JL, Andersen AMN, Michaelsen KF, Sørensen TIA. Prenatal risk factors influencing childhood BMI and overweight independent of birth weight and infancy BMI: A path analysis within the Danish National Birth Cohort Int J Obes [Internet]. Nature Publishing Group; 2018;42(4):594–602. Available from: 10.1038/ijo.2017.217 [DOI] [PubMed] [Google Scholar]
- 18.Adane AA, Tooth LR, Mishra GD. The role of offspring’s birthweight on the association between pre-pregnancy obesity and offspring’s childhood anthropometrics: a mediation analysis. 2019; Available from: 10.1017/S2040174418001137 [DOI] [PubMed] [Google Scholar]
- 19.Division of Nutrition Physical Activity and Obesity National Center for Chronic Disease Prevention and Health Promotion. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) [Internet]. 2016. [cited 2018 Mar 10]. Available from: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm [Google Scholar]
- 20.Crusell M, Damm P, Hansen T, et al. Ponderal index at birth associates with later risk of gestational diabetes mellitus Arch Gynecol Obstet [Internet]. Springer Berlin Heidelberg; 2017. August 17 [cited 2019 Jan 23];296(2):249–256. Available from: http://link.springer.com/10.1007/s00404-017-4427-4 [DOI] [PubMed] [Google Scholar]
- 21.Talge NM, Mudd LM, Sikorskii A, Basso O. United States Birth Weight Reference Corrected For Implausible Gestational Age Estimates. Pediatrics [Internet]. 2014;133(5):844–853. Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2013-3285 [DOI] [PubMed] [Google Scholar]
- 22.Steen J, Loeys T. medflex : An R Package for Flexible Mediation Analysis using Natural Effect Models. 2017;76(11). [Google Scholar]
- 23.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review Am J Prev Med [Internet], Elsevier; 2016;50(6):761–779. Available from: http://dx.doi.Org/10.1016/j.amepre.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 24.American College of Obstetricians and Gynecologists. Weight gain during pregnancy. Comm. Opin 2013. [Google Scholar]
- 25.Catalano PM, Shankar K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356(m). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawlor DA, Lichtenstein P, Långström N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation [Internet], 2011;123(3):258–65. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4440894&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Olsen SF, Mendola P, et al. Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: A prospective cohort study. Am J Clin Nutr. 2016;103(3):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eriksson JG, Kajantie E, Lampl M, Osmond C, Barker DJP. Small head circumference at birth and early age at adiposity rebound. Acta Physiol. 2014. January;210(l):154–160. [DOI] [PubMed] [Google Scholar]
- 29.Adane AA, Tooth LR, Mishra GD. The role of offspring’s birthweight on the association between pre-pregnancy obesity and offspring’s childhood anthropometrics: a mediation analysis. 2019; [DOI] [PubMed] [Google Scholar]
- 30.Fryar CD, Carroll MD, Ogden CL. Prevalence of Overweight and Obesity Among Children and Adolescents: United States, 1963–1965 Through 2011–2012. Natl Cent Heal Stat [Internet]. 2014;(September):1963–1965. Available from: http://www.cdc.gov/nchs/data/hestat/obesity_child_11_12/obesity_child_11_12.pdf [Google Scholar]
- 31.Spratt SE, Pereira K, Granger BB, et al. Assessing electronic health record phenotypes against gold-standard diagnostic criteria for diabetes mellitus J Am Med Informatics Assoc. Oxford University Press; 2017. April 1;24(e1):e121–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carsley S, Birken CS, Parkin PC, Pullenayegum E, Tu K. Completeness and accuracy of anthropometric measurements in electronic medical records for children attending primary care J Innov Heal Informatics. British Computer Society; 2018;25(1):19–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.