Abstract
Despite significant efforts to improve glioblastoma multiforme (GBM) treatment, GBM remains one of the most lethal cancers. Effective GBM treatments require sensitive intraoperative tumor visualization and effective postoperative chemotherapeutic delivery. Unfortunately, the diffusive and infiltrating nature of GBM limit the detection of GBM tumors, and current intraoperative visualization methods limit complete tumor resection. In addition, although chemotherapy is often used to eliminate any cancerous tissue remaining after surgery, most chemotherapeutic drugs do not effectively cross the brain-blood barrier (BBB) or enter GBM tumors. As a result, GBM has limited treatment options with high recurrence rates, and methods that improve its complete visualization during surgery and treatment are needed. Herein, we report a fluorescent nanoparticle platform for the near-infrared fluorescence (NIRF)-based tumor boundary visualization and image-guided drug delivery into GBM tumors. Our nanoplatform is based on ferumoxytol (FMX), an FDA-approved MRI-sensitive superparamagnetic iron oxide nanoparticle, which is conjugated with hepthamethine cyanine (HMC), a NIRF ligand that specifically targets the organic anion transporter polypeptides (OATPs) that are overexpressed in GBM. We have shown that HMC-FMX nanoparticles cross the BBB and selectively accumulate in the tumor using orthotopic GBM mouse models, enabling NIRF-based visualization of infiltrating tumor tissue. In addition, HMC-FMX can encapsulate chemotherapeutic drugs, such as paclitaxel or cisplatin, and deliver these agents into GBM tumors, reducing tumor size and increasing survival. Taken together, these observations indicate that HMC-FMX is a promising nanoprobe for GBM surgical visualization and drug delivery.
Keywords: glioblastoma, iron oxide nanoparticles, ferumoxytol, drug delivery, fluorescence imaging
Graphical Abstract
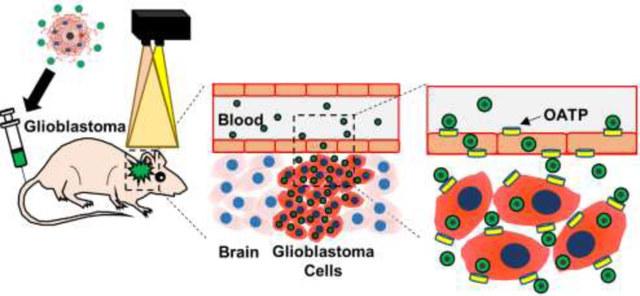
Despite advances in the diagnosis and treatment of glioblastoma multiforme (GBM), patients with GBM have a median survival of only 15 months and a five-year survival rate of 10%.1–4 The standard of care for GBM is surgery, to remove as much tumor mass from the patient as possible, followed by a combination of radiation and chemotherapy, to treat residual tumor tissue. Despite this broad combination of treatment methods, GBM recurrence remains a significant issue in patients. As a result, many patients with GBM undergo treatments that are palliative rather than curative. For these reasons, developing techniques to locate GBM tumors, visualize their boundaries during surgery and deliver anticancer drugs selectively into GBM tumors could improve therapeutic outcomes and reduce disease recurrence.
Effective GBM imaging methods are required to identify both the location and boundaries of GBM tumors prior to and during surgery. Magnetic resonance imaging (MRI) is a non-invasive imaging method that has been used clinically to detect both primary and metastatic brain tumors.5–7 MRI requires clinical instruments that are cumbersome to use during surgery, and the poor sensitivity of MRI limits its ability to detect infiltrating brain tumor cells. Meanwhile, intraoperative near-infrared fluorescence (NIRF) imaging at excitation wavelengths between 700–800 nm could identify GBM tumor boundaries more precisely during surgery and enable neurosurgeons to resect tumors with high sensitivity and precision. Unfortunately, the lack of highly fluorescent agents that localize to GBM tumors, particularly to their infiltrating regions, hampers the use of this technology.
Current intraoperative NIRF imaging methods for GBM and other high grade gliomas have demonstrated limited success at treating and extending the life of these patients, partially because they are unable to visualize infiltrating tumors and do not carry a chemotherapeutic to treat the remaining GBM cells. For example, 5-aminolevulinic acid (5-ALA) is administered orally to GBM patients prior to surgery.8–10 5-ALA is then converted into fluorescent protoporphyrin IX (pPIX) within the GBM cells mitochondria and pPIX can be detected by fluorescence with emission at 635 nm following excitation at 410 nm to visualize GBM tumor margins. More recently, a fluorescent indocyanine green-chlorotoxin (ICG-CTX) peptide conjugate has been shown to cross the BBB, label GBM tumors and facilitate tumor border visualization during surgery.11–14 Unfortunately, both 5-ALA and ICG-CTX imaging methods may not identify single migratory cancer cells or small colonies of infiltrating GBM cells. Since neither 5-ALA nor ICG-CTX can directly treat these remaining cancer cells, these cells remain in a patient and may lead to disease recurrence. In a clinical trial comparing imaging methods, 5-ALA-guided NIRF imaging failed to increase progression-free survival at 6 months postsurgery compared to intraoperative MRI.15
Successful GBM treatments also require effective chemotherapeutics that can cross the blood-brain barrier (BBB) and enter brain tumors, but clinically available chemotherapeutic drugs have limited success at treating GBM. Temozolomide (TMZ) is the only currently approved first-line chemotherapeutic drug for GBM, and it is administered to patients after surgery in combination with radiotherapy.16, 17 Although TMZ crosses the BBB and accumulates within GBM tumors, TMZ treatments in combination with radiotherapy have only demonstrated modest increases in median survival compared to radiotherapy alone. Although taxanes and platinum-based compounds, such as paclitaxel and cisplatin, have demonstrated efficacy against GBM cells in vitro and delayed the progression of breast, prostate and lung cancers in patients, they have not been successfully used to treat GBM. Therefore, we hypothesized that a tumor-targeted, near infrared fluorescent nanoparticle-based system that can selectively cross the BBB and identify GBM tumors non-invasively during surgery, while also functioning as GBM-targeting drug delivery system, would improve GBM treatment significantly.
Herein, we report the development of an image-guided approach for intraoperative tumor boundary assessment and chemotherapeutic treatment of GBM using near infrared fluorescent nanoprobes (Figure 1). These nanoprobes are derived from carboxymethyl dextran-coated superparamagnetic iron oxide nanoparticles (SPIONs) (Figure 1a). Ferumoxytol (FMX) is a 15–30 nm diameter carboxymethyl dextran-coated superparamagnetic iron oxide nanoparticle that is currently used to treat iron-deficiency anemia.18 In biodistribution studies, FMX was completely metabolized within 6–8 weeks of administration and stored in the liver as iron.19, 20 In addition to its therapeutic applications, FMX is increasingly used off-label for MR angiography and liver imaging at lower doses than those approved for anemia treatment.21–23 In toxicity studies, large doses of FMX (12-fold greater than the maximum clinical dose) presented no significant toxicity and minimal side effects.20, 24 In addition, a recent multicenter study showed that the use of FMX in MRI was well-tolerated in patients, indicating a positive safety profile.25 Various SPION formulations including FMX have been shown to encapsulate hydrophobic drugs within their polymer coatings and deliver them to tumors in mouse models.26 To create these fluorescent nanoprobes, we conjugated an OATP-targeting heptamethine carbocyanine (HMC) fluorescent ligand onto the surface of FMX (Figure 1b). Conjugation of HMC to FMX endows the resulting HMC-labelled FMX-based nanoprobe (HMC-FMX) with both magnetic and fluorescent properties, as well as BBB-crossing and GBM-targeting capabilities (Figure 1c). We found that HMC-FMX crosses the BBB and accumulates within GBM tumors, allowing GBM tumor boundaries to be identified. In addition, upon encapsulation of anticancer drugs, the resulting therapeutic nanoprobe delivered these drugs into GBM tumors. Taken together, these results show that HMC-FMX nanoprobes hold promise to improve GBM therapy.
Figure 1. HMC-FMX facilitates GBM tumor detection and drug delivery across the BBB.
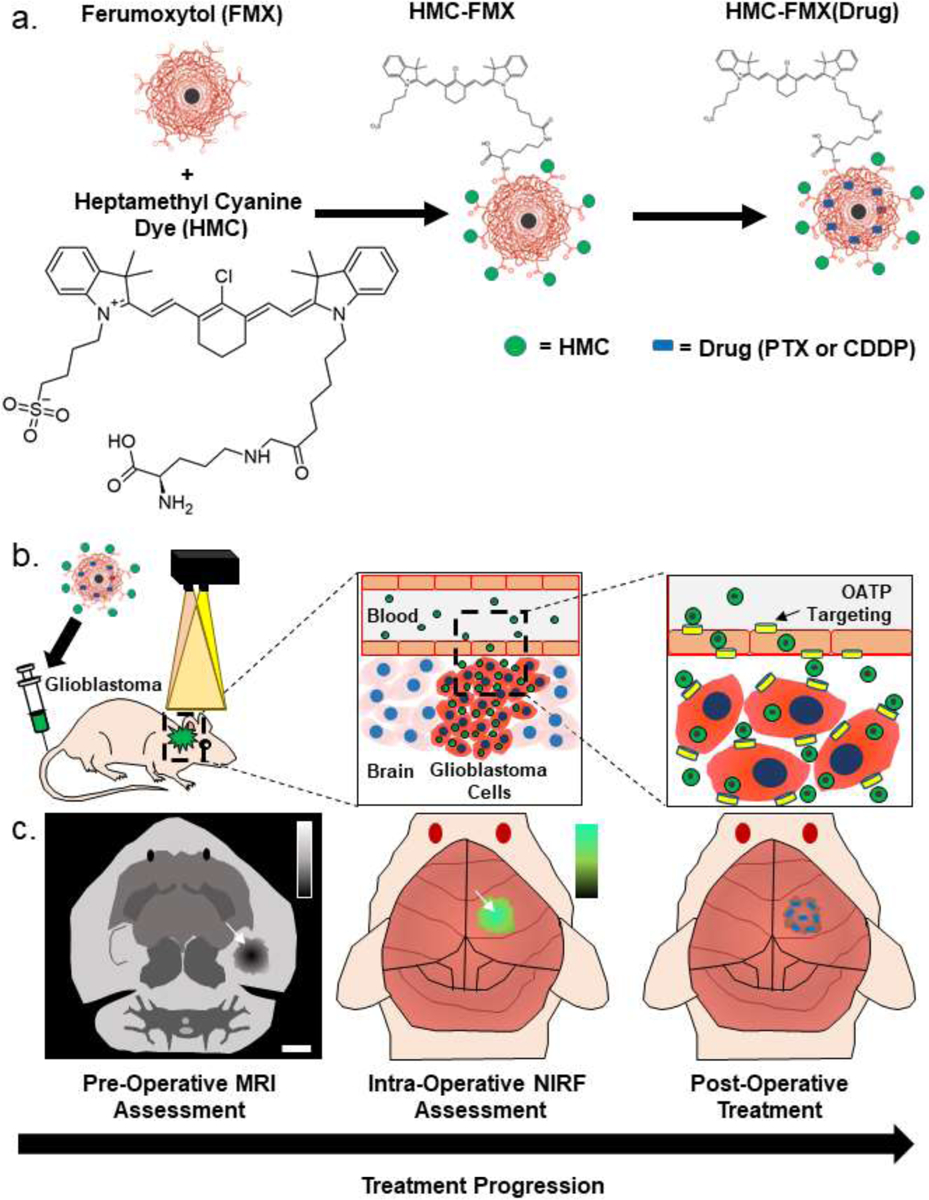
(a). HMC-FMX accumulates in GBM tumors via OATP transport following intravenous administration, and the accumulated HMC-FMX can be detected by a fluorescence imaging camera (b). As FMX can also encapsulate chemotherapeutic drugs, drug-loaded HMC-FMX nanoprobes can identify tumors and then deliver chemotherapeutic drugs across the BBB into any remaining GBM tumor tissue following surgery (c).
RESULTS AND DISCUSSION
HMC-FMX is a Sensitive Near Infrared Magnetofluorescent Nanoprobe
To develop a BBB-crossing and glioma-targeting nanoprobe, we conjugated HMC ligands to ferumoxytol (FMX) (Figure 1a). Because HMC is insoluble in water and not reactive with carboxylic acid groups of FMX, we synthesized a lysine-modified, water-soluble HMC analog (HMC-Lys) based on protocols reported previously in the literature (Figure S1).27 Following this synthesis, HMC-Lys could be directly conjugated to FMX using simple EDC/NHS coupling chemistry. Our synthesis method was similar to previous reports.28 Conjugation of HMC to superparamagnetic FMX will allow these HMC-conjugated FMX-based nanoprobes (HMC-FMX) to be imaged by both MRI and NIRF.
Both the NIRF and OATP-targeting properties of HMC are beneficial for nanoprobe targeting of GBM. HMC exhibits near-infrared fluorescence (NIRF), with emission at 800 nm following excitation at 750 nm, similar to the clinically available dye indocyanine green (ICG) (Figure S2).29 HMC has been previously reported to target overexpressed OATPs in animal models of solid tumors. To assess the feasibility of HMC-FMX to target human GBM tumors, we performed a screening of OATP RNA levels in GBM tumors and normal brain tissue using published web portals.30 GBM tumors were identified to overexpress OATP2A1 and OATP2B1 compared to normal brain tissue, while having similar or reduced expression of other OATP transporters (Figures S3 and S4). Since these transporters are also overexpressed on the BBB,27 we speculated that HMC targeting would allow for GBM-selective accumulation of HMC-FMX nanoprobes.
To image HMC-FMX fluorescence, we used a Synchronized Near InfraRed Imaging System (SIRIS) camera, which has been designed for NIRF image-guided surgery using ICG and ICG-based fluorescent agents.11 The SIRIS produces high definition quality brightfield images, which are superimposed with maps of fluorescent dye distribution. Surgeons can use these images to direct the intraoperative detection and resection of tumors in real time. Because HMC-Lys has similar optical properties to ICG, we expect the SIRIS to image HMC and HMC-FMX as well.11 Upon excitation of HMC-FMX at 785 nm using the SIRIS camera, a bright NIRF signal is observed from HMC-FMX (Figure 2a). Conjugation of HMC-Lys to FMX slightly decreased its absorbance intensity and increased its fluorescence intensity, but the peak absorbance and fluorescence wavelengths were minimally affected (HMC-Lys vs. HMC-FMX; absorbance, 776 nm each; fluorescence, 796.5 vs. 799 nm) (Figure 2b). Bright and stable NIR fluorescence is observed from HMC-FMX even after illumination for 3 hours under surgical conditions (Figure 2c). However, it should be noted that while the fluorescence remained stable under surgical conditions, signal quantification in lower concentration samples showed that the fluorescence intensity decreased by about 50% over 3 h under constant exposure conditions (Figure S5). If reduced signal intensity were observed during long surgeries, the surgeon could increase the signal by increasing the SIRIS camera exposure time or by using intermittent exposure conditions. The fluorescence of HMC-FMX remained strong even at low HMC concentrations (Figure 2d). We quantified HMC-FMX fluorescence from 0.3 to 5554 nM HMC (Figure 2e) and used the linear range of detection between 0.3 and 22 nM HMC to calculate detection limits (Figure 2e, insert). Based on the slope of the graph, we calculated an HMC-FMX detection limit of 0.2 nM HMC, but a detection limit to 1.4 nM HMC was observed by sight (Figure 2d,e).
Figure 2. Fluorescence and MRI characterization of HMC-FMX.
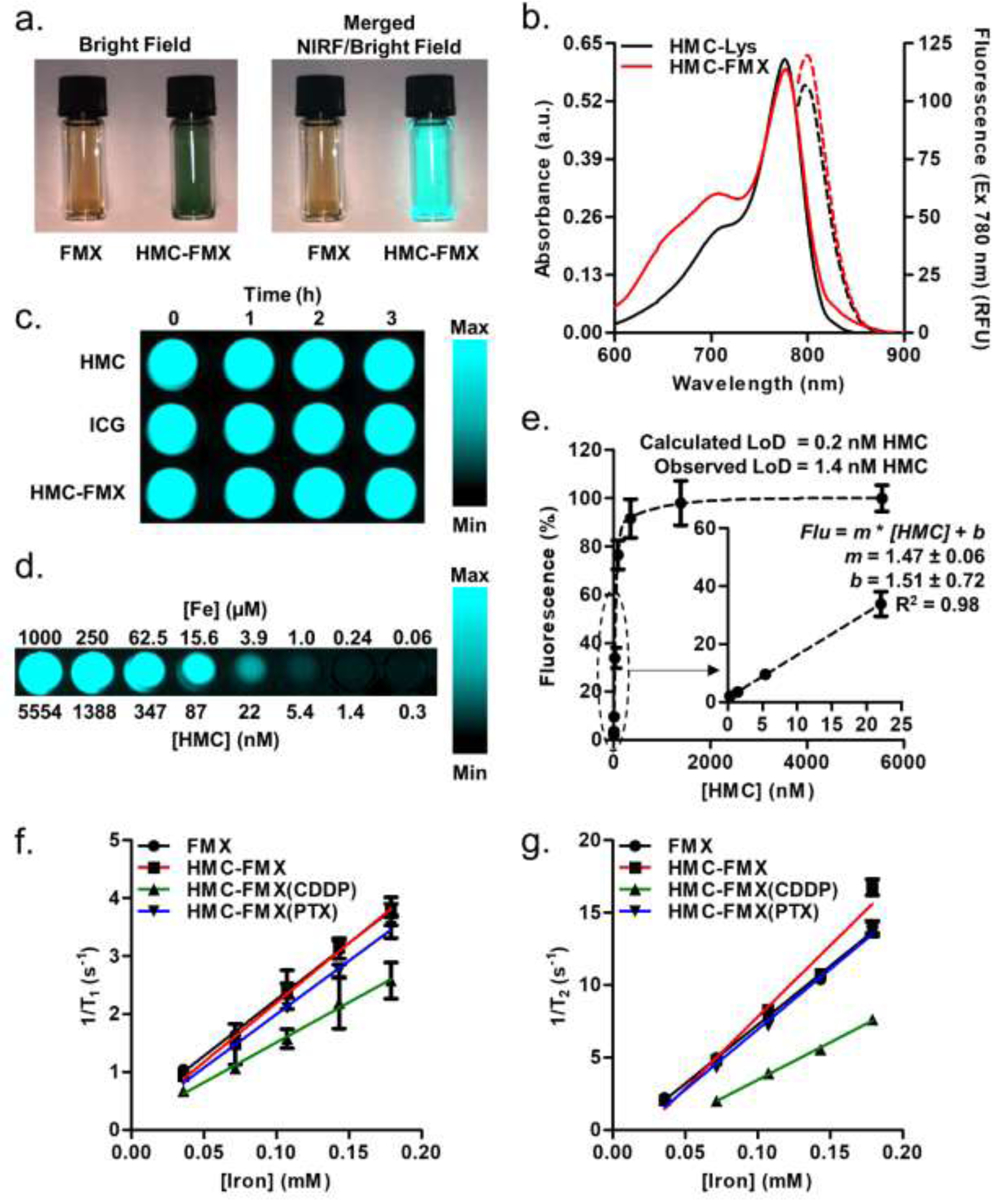
Brightfield and merged NIRF/brightfield images of FMX and HMC-FMX (a). Absorbance (solid lines) and fluorescence (dashed lines) spectra of HMC-Lys (black) and HMC-FMX (red) (b). Photostability of HMC, ICG, and HMC-FMX under intraoperative imaging conditions. (c). Fluorescence measurement of HMC-FMX at high exposure time (d). Fluorescence quantification and detection limit of HMC-FMX (e, insert). Measurement of r1 and r2 relaxivity of nanoprobes (f, g).
The R1 and R2 relaxivity of HMC-FMX nanoprobes were also measured (Figures 2f and 2g) from linear regressions of the relaxation data using equation 1 below.
| 1) |
In equation 1, T is the relaxation time, r is the relaxivity, [Iron] is the iron concentration in the sample and b is the intercept. Both FMX and HMC-FMX had similar r1 and r2 values, indicating that dye conjugation does not hinder the superparamagnetic properties of FMX (Table 1). The loading of CDDP into nanoprobes significantly decreased the r1 and r2 values of the nanoprobes, but PTX loading had no effect. This observation suggests that CDDP could be interacting stronger with FMX than PTX.
Table 1.
Relaxivity properties of FMX, HMC-FMX, HMC-FMX(CDDP) and HMC-FMX(PTX).
| Sample | r1 (mM−1 s−1) | b1 (s−1) | R2 | r2 (mM−1 s−1) | b2 (s−1) | R2 | r2/r1 |
|---|---|---|---|---|---|---|---|
| FMX | 19.5 ± 0.4 | 0.3 ± 0.1 | 0.99 | 81.1 ± 2.8 | −0.1 ± 0.1 | 0.99 | 4.2 ± 0.2 |
| HMC-FMX | 20.5 ± 0.9 | 0.1 ± 0.1 | 0.99 | 98.7 ± 9.5 | −2.1 ± 1.1 | 0.97 | 4.8 ± 0.5 |
| HMC-FMX(CDDP) | 13.8 ± 0.6** | 0.1 ± 0.1 | 0.99 | 51.6 ± 1.7* | −1.7 ± 0.2 | 0.99 | 3.7 ± 0.2* |
| HMC-FMX(PTX) | 18.4 ± 0.5 | 0.2 ± 0.1 | 0.99 | 83.2 ± 3.6 | −1.4 ± 0.4 | 0.99 | 4.5 ± 0.2 |
The physical-chemical properties of HMC-FMX were also measured and compared to FMX. Because FMX is used clinically and approved by the FDA, its physical-chemical properties have been previously characterized in detail.31, 32 HMC conjugation increased the diameter of FMX and reduced its surface charge (Table 2), likely due to the conversion of carboxylic acid groups into amide bonds. We measured HMC conjugation to HMC-FMX using a previously described method,33 and calculated that an average of 40 HMC molecules were attached to each FMX nanoparticle. During storage in 20% serum at 37°C for 72 h, the diameter of HMC-FMX increased (28 nm to 38 nm), but the surface charge remained unchanged (Figure S6).
Table 2.
Physical-chemical properties of HMC-FMX compared to FMX as measured by dynamic light scattering (DLS).
| Sample | Diameter (nm) | Zeta Potential (mV) | [HMC] (mM) | HMC per NP (mol HMC/mol FMX) |
|---|---|---|---|---|
| FMX | 23.2 ± 0.4 | −20.6 ± 1.2 | - | - |
| HMC-FMX | 37.0 ± 3.0*** | −11.8 ± 0.3 | 0.20 ± 0.02 | 40.2 ± 0.3 |
HMC-FMX Targets GBM Cells Selectively via OATP
Next, we tested the ability of HMC-FMX to selectively target and internalize into various GBM cell lines. Five GBM cell lines (A-172, LN-18, LN-229, T-98G and U-87) and one non-cancerous neuronal cell line (HT-22) were incubated with HMC-FMX (300 μM iron) for 1 h and imaged using fluorescence microscopy. NIR fluorescence was observed within all GBM cell lines, but minimal fluorescence was observed in the neuronal cell line (Figure S7a). This observation was verified in flow cytometry studies (Figure S7b). The observed cancer cell-selective uptake of HMC is not shared with other cyanine dyes, despite their structural resemblance.29 A quantification of iron uptake in FMX- and HMC-FMX-treated cells indicated that HMC targeting of nanoprobes increased iron uptake into cancer cells (Figure S7c). This result suggests that HMC targeting enables the uptake of intact nanoprobes. In addition, when U-87 cells were pre-incubated with various OATP inhibitors selected from literature,34 HMC-FMX uptake was reduced, verifying that HMC-FMX uptake is mediated by OATP (Figure S8).
Because the BBB acts as an additional barrier to GBM tumor targeting compared to targeting other tumor types, OATP targeting of GBM tumors requires transporters that are expressed on both cancer cells and on the tumor-associated BBB. OATPs are a family of 11 transmembrane glycoproteins that transport many substances into cells, including drugs and hormones that cannot freely diffuse through cellular membranes.35, 36 Because they are overexpressed in tumors of various biological origins, OATP targeting of other solid tumors has gathered interest for tumor-selective delivery of drugs and imaging agents.35, 37 OATP1A2 and OATP2B1 are highly expressed in both the luminal and basal membranes of the endothelial cells forming the BBB in humans, and OATP2B1 overexpression has been noted on many types of cancer cells.35, 36, 38 Previous studies have reported reduced blood-to-brain transport of several agents in mice lacking OATP1A2, suggesting a role for this particular OATP in facilitating HMC transport across the BBB.39, 40 However, our genetic analysis in human tissue samples demonstrated that while OATP2A1 and OATP2B1 are overexpressed in primary GBM tumor samples, OATP1A2 expression is unchanged compared to normal brain tissue. This observation suggests that the overexpression of OATP2B1 in the tumor-associated vascular endothelium and glioma cells could contribute to the HMC-FMX’s BBB crossing and eventual accumulation in GBM cells. Further studies are needed in order to elucidate the specific contributions of OATP on the vascular endothelial and GBM cells to HMC-FMX uptake in brain tumors.
HMC-FMX Specifically Localizes to and Fluorescently Labels GBM Tumors in an Intracranial GBM Mouse Model
To demonstrate the tumor localization and retention of HMC-FMX in U-87 tumors in an orthotopic GBM mouse model, HMC-FMX (1 mg HMC and 4 mg iron/kg mice) was injected intravenously into mice, and then mice were euthanized at 3, 24, 48 and 168 h post-injection. Following euthanasia, major organs were collected, and the fluorescence in each organ was quantified (Figure 3a). For fluorescence quantification, fluorescent brain tumors were separated from non-fluorescent normal brain. As soon as 3 h after injection of HMC-FMX, fluorescence can be seen in the tumor within the mouse brain, as well as in other major organs (Figure 3b). In general, the fluorescence in most organs decreased following injection, except for fluorescence in lungs which increased up to 24 h and then decreased. Within a week, fluorescence is significantly reduced in most major organs. In contrast, tumor fluorescence increased up to 168 h post-injection, indicating not only GBM targeting but also retention of HMC-FMX in tumors from 3 to 168 h post-injection. Brain tumors could be distinguished from normal brain tissue in ex vivo imaging (Figure 3c). The tumor-to-brain fluorescent intensity ratio increased at 48 h post-injection and remained stable up to 168 h post-injection (Figure 3d), indicating that tumors can be easily observed within this broad time window. Surprisingly, we were able to observe fluorescence from brain tumors through the skin and skull before tumor excision (Figure S9). Finally, preliminary pharmacokinetic studies show that HMC fluorescence in the blood decreases after injection, with the lowest values being observed after 48 and 168 h (Figure 3e). Taken together, the successful association and corresponding NIRF labeling of GBM tumors by HMC-FMX suggest that HMC-FMX can visualize these tumors during surgery. Furthermore, HMC-FMX provides a broad window (1–7 days post injection) where one could measure NIRF within GBM tumors during surgery.
Figure 3. HMC-FMX accumulates in human orthotopic GBM tumors in mice.
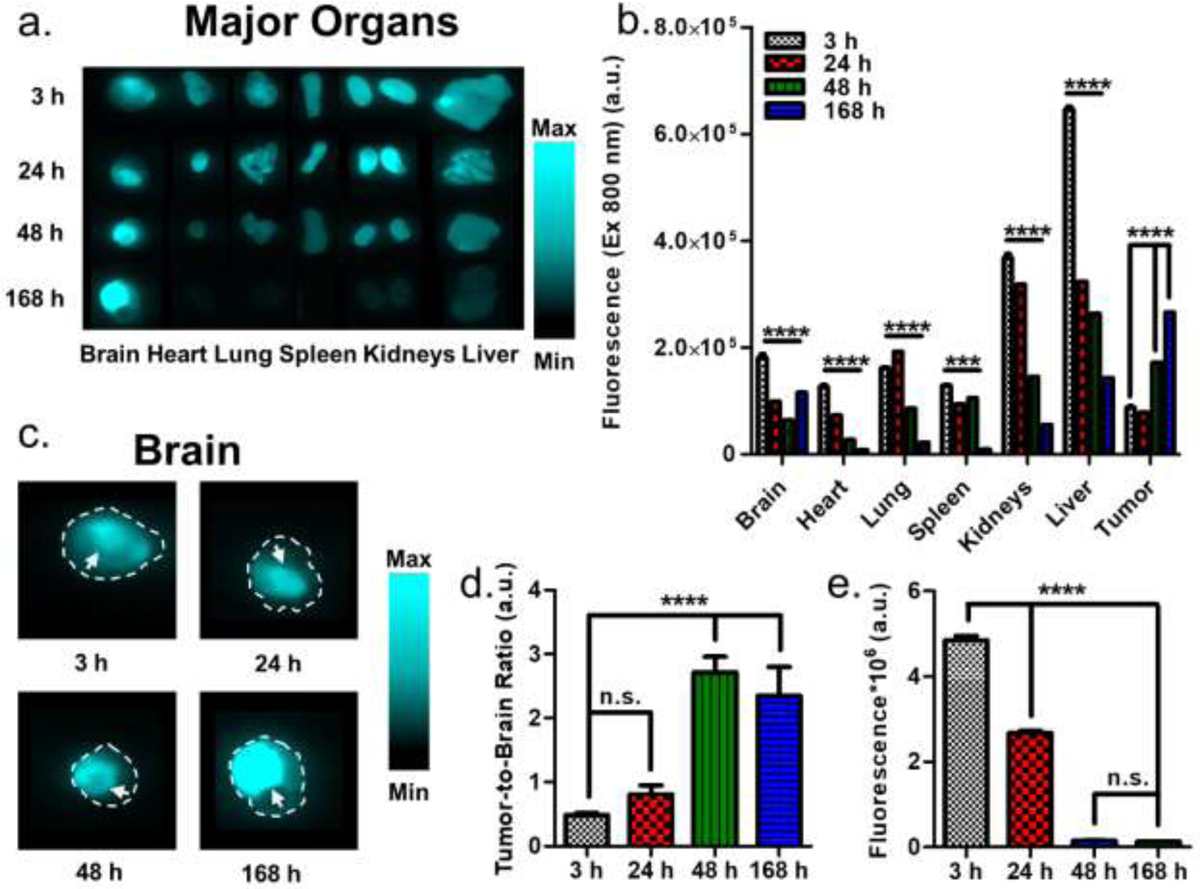
HMC-FMX fluorescence in organs at 3, 24, 48 and 168 h post-injection (a). HMC-FMX fluorescence quantification (b). Larger image of brains from ex vivo imaging. Arrows indicate brain tumor, while the dotted lines indicate brain outlines from corresponding brightfield images (c). Tumor-to-normal brain fluorescence ratio (d) and blood fluorescence quantification (e).
HMC-FMX Facilitates GBM Tumor Resection via Fluorescence Image-Guided Surgery
Next, GBM tumor-bearing mice were injected with either HMC-FMX, IR820-FMX, HMC or ICG to observe the tumor-selective accumulation of these nanoprobes and dyes. IR820-FMX is a non-targeting fluorescent nanoprobe that was prepared by conjugating the NIRF dye IR820 to FMX. Mice were euthanized 24 h after injection of the corresponding nanoprobes or dyes, and their brains were extracted to observe probe accumulation by fluorescence imaging. We chose a 24 h timepoint for imaging because this is the earliest time point at which we observed tumor-associated fluorescence from HMC-FMX, and it maximizes any potential signals from other treatments. Strong GBM tumor-associated fluorescence was observed in isolated brains from mice injected with HMC-FMX, with minimal fluorescence in the rest of the normal brain (Figure 4). The GBM tumor was clearly visualized by the SIRIS camera.
Figure 4. HMC-FMX specifically targets GBM tumors in mice.
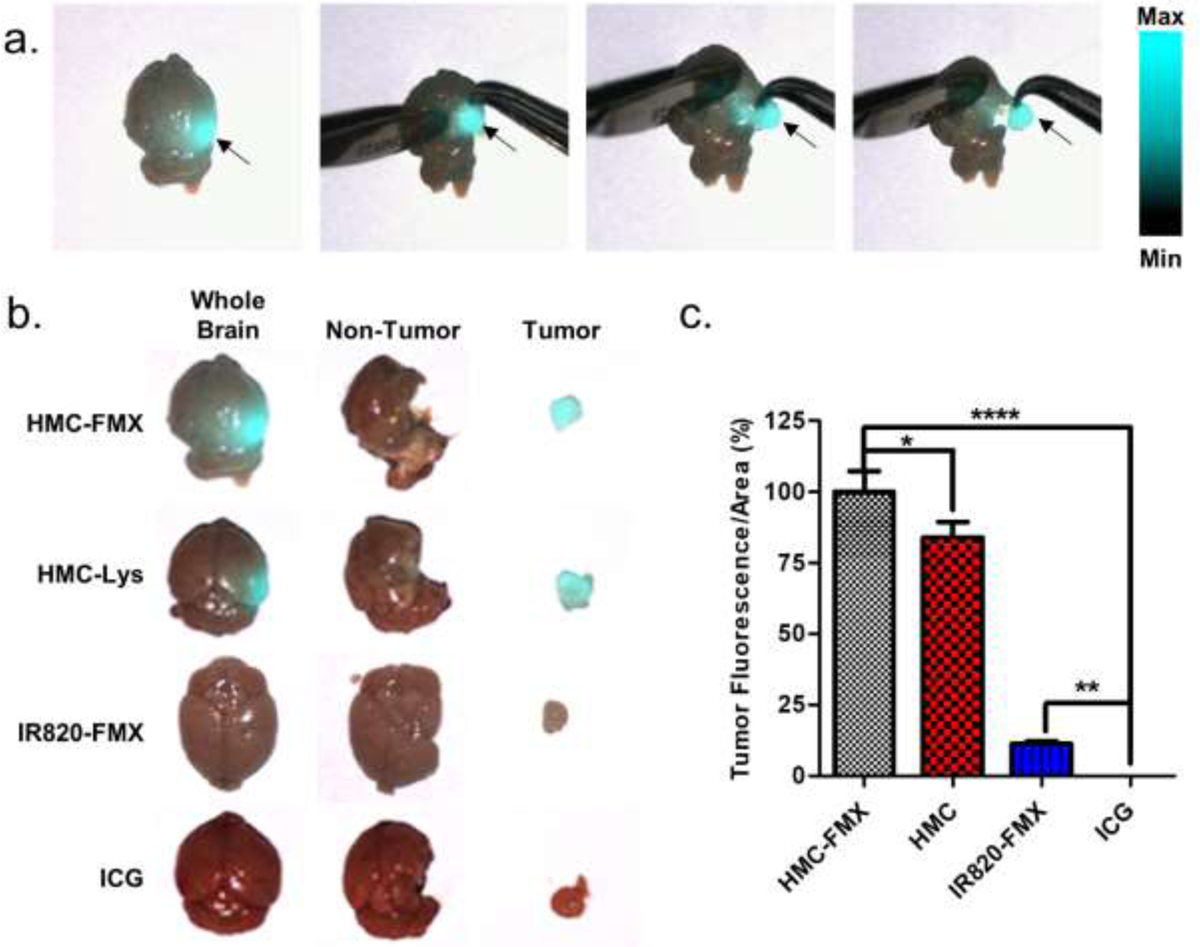
Mock fluorescence image-guided surgery to remove GBM tumors (a). Mice were injected with HMC-FMX, HMC-Lys, IR820-FMX or ICG, and then images of either mouse brains with GBM tumors, non-tumor brain or excised tumors were taken 24 h post-injection either before (whole brain) or after (non-tumor, tumor) mock surgery (b). Quantification of fluorescence signals from tumors (c). Fluorescence signals from GBM tumors that were treated with ICG were very small.
We extracted tumors from the mouse brain after removing the brain from the skull, rather than performing the extraction procedure in situ, in order to clearly image the entire brain. Figure 4a show snapshots of a movie recording this procedure. A clear delineation of the tumor borders was observed in mice injected with either HMC-FMX or HMC, and GBM tumors were successfully extracted from these brains (Figure 4b). These results show that the tumor targeting ability of HMC is not compromised upon conjugation to FMX. In contrast, neither IR820-FMX nor ICG show tumor localization as the extracted tumor is not fluorescently labeled. The fluorescence signal from extracted tumors was quantified and normalized to tumor area for each sample (Figure 4c). HMC-FMX yielded the strongest signal, followed by HMC-Lys. Both IR820-FMX and ICG treatments yielded weak fluorescence signals. These results suggest that not only does HMC conjugation to nanoparticles allow nanoprobes to cross the BBB and enter GBM tumors, but also that nanoparticle conjugation enhances the fluorescence signals of GBM tumors compared to HMC-Lys alone.
HMC-FMX Accurately Identifies Brain Tumors in Intracranial Mouse Models of GBM
To assess the accuracy of GBM tumor identification by HMC-FMX, HMC-FMX was injected into mice with large, infiltrating GBM tumors, and their brains were isolated after 24 h for fluorescence imaging and tumor resection using the SIRIS. Strong tumor-associated NIRF was observed in isolated mice brains. Using this fluorescence, primary GBM tumors were extracted from the normal brain (Figure 5a,b). Some fluorescence remained in the surgical bed of the brain (yellow arrows, Figure 5c), suggesting the presence of infiltrating GBM tissue within the surgical cavity. Without intraoperative NIRF imaging, the infiltrating GBM tumor tissue could not be identified, and if left behind in the surgical cavity, it could cause recurrence of the tumor.
Figure 5. HMC-FMX crosses the BBB and targets orthotopic GBM tumors in mice.
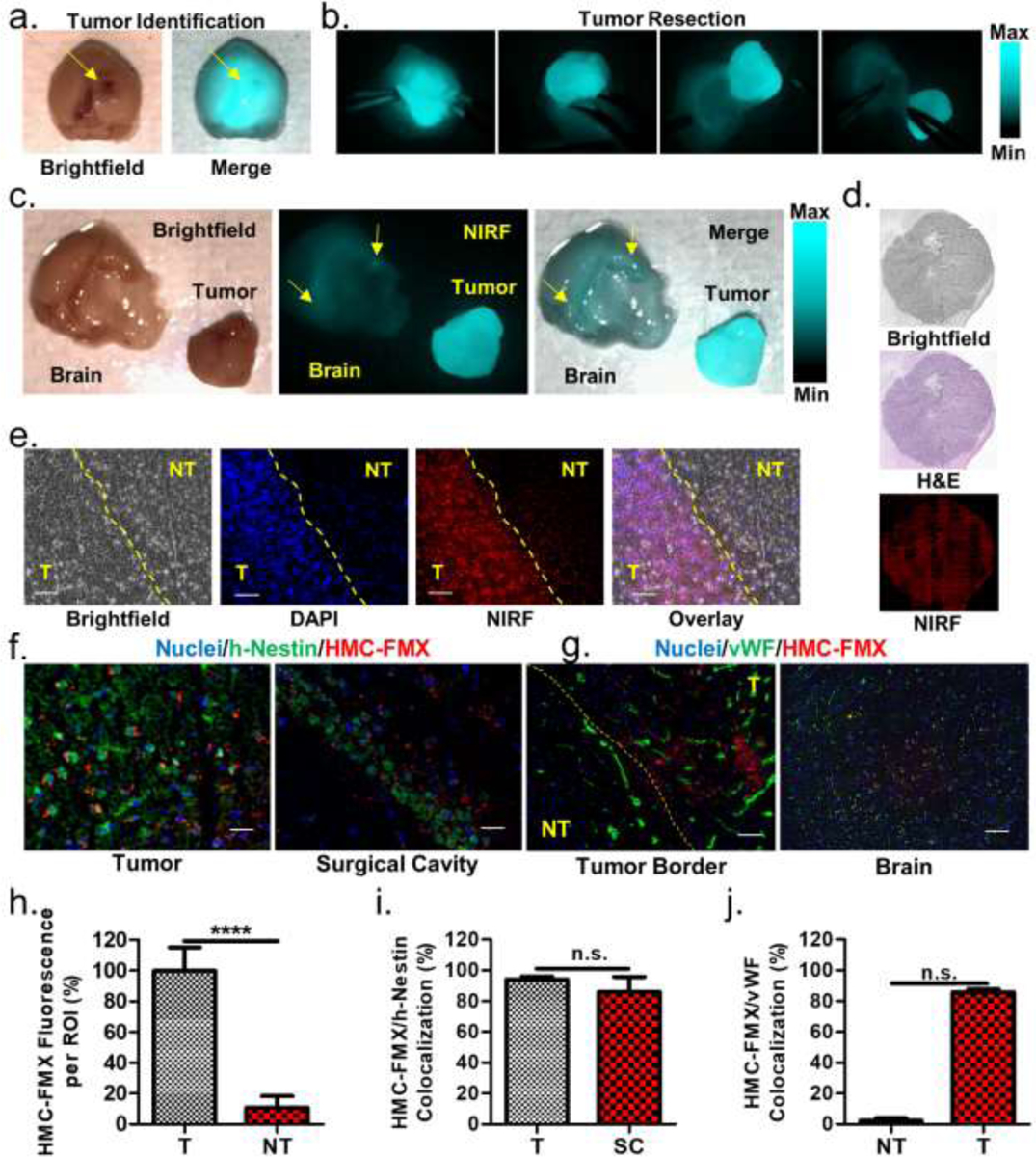
Brightfield and merged brightfield/NIRF image of a mouse brain with a large GBM tumor (a). SIRIS-recorded snapshots of a tumor resection procedure (b). Brightfield, NIRF, and merged images of the brain and extracted primary GBM tumor (c). Fluorescence is observed both in the primary tumor and remaining in the surgical bed (yellow arrows). Microscopic images of brain tissue slices from HMC-FMX-treated GBM tumor-bearing mice. Significant correspondence is observed between H&E and HMC-FMX NIRF tumor identification methods (d). Magnified fluorescence images of the tumor border show correspondence between nuclear density and HMC-FMX NIRF tumor identification methods (e). The yellow line is a superimposed border based on brightfield images. Immunohistopathology of tumor and surgical bed indicate that HMC-FMX (red) associates with h-nestin (green) on U-87 cells (f). No association was observed between HMC-FMX (red) and von Willebrand factor (vWF) signal (green) on blood vessels in tumors, but significant association was observed in the normal brain (g). Quantification of HMC-FMX fluorescence in tumor (T) and non-tumor (NT) regions of part e (h), HMC-FMX/h-nestin co-localization in tumor (T) and surgical cavity (SC) of part f (i), and HMC-FMX/vWF co-localization in non-tumor (NT) and tumor (T) of part g (j). In each fluorescence microscope image, scale bars are 100 μm.
To further verify the ability of HMC-FMX to label GBM tumors, delineate tumor borders and identify residual infiltrating tissue, tissue sections of mouse brains with GBM tumors, isolated GBM tumors and the surgical cavity were collected and analyzed by fluorescence microscopy and histological staining. Both H&E staining and NIRF identified similar tumor boundaries, corroborating the association of HMC-FMX with tumor tissue (Figure 5d). The tumor boundary was analyzed further by fluorescence microscopy. In order to identify tumor tissue by fluorescence microscopy, the nuclear stain DAPI was used. DAPI staining was expected to identify the size, shape and density of nuclei on the tumor boundary, and regions of tissue with brighter, denser, elongated nuclei were defined as tumor tissue. NIRF signals were highly co-localized within the tumor tissue region of images, further indicating that HMC-FMX localizes within GBM tumors (Figure 5e). To further assess the co-localization of HMC-FMX with tumor tissue, both tumor and surgical cavity tissue samples were labelled with an anti-human-nestin (h-nestin) antibody identifying human U-87 cells (Figure 5f).41, 42 We also labelled tumor border and normal brain samples with an anti-von Willebrand factor (vWF) antibody identifying vasculature (Figure 5g). Bright NIRF signals were observed in cancerous regions in the tumor borders, while only weak NIRF signals were observed in non-cancerous regions (Figure 5h). It should be noted that the tumor border was identified by visual inspection of the tissue sample, rather than by fluorescence, and that fluorescence signals may identify the tumor border more accurately than visual inspection. Next, HMC-FMX NIRF signals co-localize with h-nestin signal in both the tumor and the surgical cavity, showing that the nanoprobe signal corresponds with U-87 cells in both primary tumors and infiltrating tissue (Figure 5i). HMC-FMX NIRF signal does not co-localize with vasculature within tumors, but it does co-localize with vasculature within the normal brain (Figure 5j). This result further verifies that HMC-FMX crosses the BBB within tumors and enters GBM cells, and it also suggests that weak HMC-FMX fluorescence signals from healthy tissues are due to the small amount of circulating nanoparticles remaining in the blood. As a result, HMC-FMX is expected to identify both primary GBM tumors and infiltrating tumor tissue around the tumor cavity and facilitate intraoperative NIRF image-guided tumor extraction.
HMC-FMX Identifies Patient-Derived GBM Cancer Stem Cell-Based Tumors in a Mouse Model
Because of the complex genetic variability of GBM, reliable animal models are needed to better recapitulate the biology of human GBM and predict therapeutic outcomes for individual patients.43, 44 Toward this goal, we have developed orthotopic GBM xenograft mouse models using patient-derived GBM cancer stem cells (CSCs).45, 46 These CSC-derived tumors resemble both the infiltrating and migratory nature of human GBM while also maintaining its genetic characteristics. We reasoned that the use of CSC-derived GBM mouse models to study the targeting and labelling of GBM tumors by HMC-FMX would be more predictive of its future clinical applicability than traditional cell line-based models. First, we tested the uptake of HMC-FMX by patient-derived GBM CSCs. Following treatment with HMC-FMX for 24 h, GBM CSC spheroids demonstrated bright NIRF (Figure 6a). When orthotopic GBM xenograft mouse models were generated using these cells, migratory and infiltrating brain tumors were clearly visualized by HMC-FMX NIRF imaging (Figure 6b). GBM CSC-derived tumors were located further from the cell injection site and were more infiltrating that those observed in a U-87 tumors (Figure 6c). The NIRF signal in SIRIS images correlates with the tumor area identified by H&E staining (Figure 6d,e). Because HMC-FMX localizes to primary tumors, migrating tumors and infiltrating tumor tissue, HMC-FMX nanoprobes have significant potential for GBM tumor identification during NIRF image-guided surgery.
Figure 6. HMC-FMX targets patient-derived GBM CSCs and CSC-derived orthotopic GBM tumors in mice.
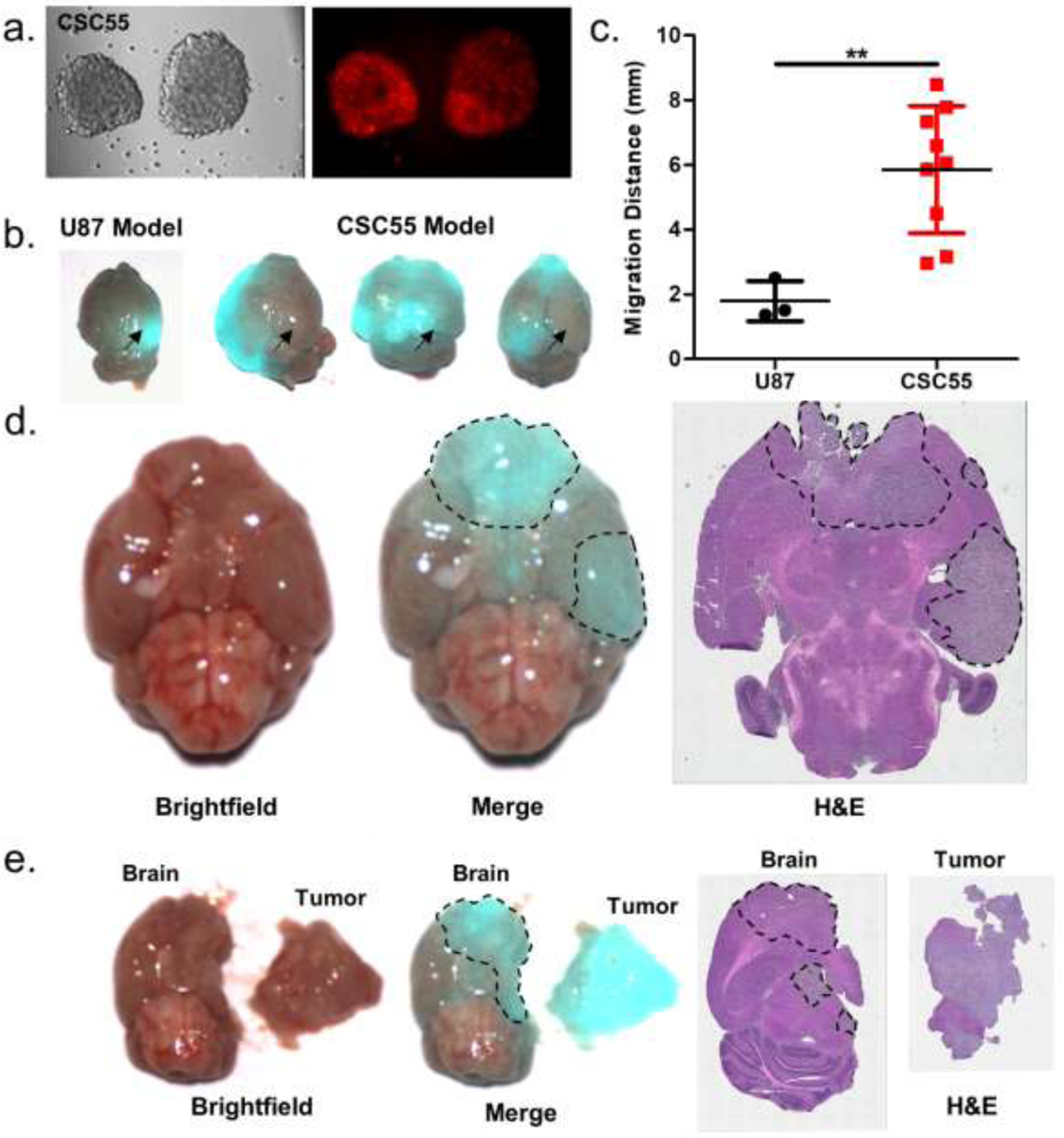
GBM CSC spheroids fluorescently labeled with HMC-FMX (a). Accumulation of HMC-FMX in orthotopic GBM tumor xenografts from U-87 and GBM CSCs (b). Arrow indicates cell injection site. Migration distance for U-87 and GBM CSC-derived tumors from injection site (c). Brightfield, merged brightfield/NIRF and H&E staining of GBM CSC-derived tumors before (d) and after fluorescence image-guided resection (e).
The potential effects of intracranial injections on BBB disruption and tumor detection in GBM mouse models should be considered. In this study, orthotopic GBM mouse models were established using two cell lines: U-87 primary GBM cells and CSC55 GBM CSCs. Brain tumors are recognized as regions within the brain that have a compromised BBB,47 in addition, the injection procedure by itself may also compromised the surrounding BBB, creating a more permemeable brain tumor. We expect the injection site within the brain to heal within one week following injection, and imaging experiments were performed three to four weeks post-injection to minimize any transitory effects of injection site damage on BBB integrity. In contrast to U-87 cells that grow within the injection site, CSC55 cells can migrate to distant sites within the brain, developing tumors in regions away from the injection point and therefore less affected by the injection procedure. HMC-FMX detected both U-87- and CSC55-derived tumors within mice, regardless of their proximity to the injection site. Therefore, one could reason that the migratory and infiltrating GBM tumors would have a less compromised or intact BBB than those developed at the injection site. Further investigation of HMC-FMX tumor detection in non-xenograft-based GBM models, such as carcinogen-induced or genetically engineered mouse models, may allow potential injection site effects on GBM BBB disruption to be more thoroughly investigated.
HMC-FMX Can Deliver Anticancer Drugs to GBM Cells and GBM Stem Cell Spheroids
Previously, SPIONs like FMX were shown to encapsulate drugs at physiological pH within the hydrophobic pockets of their polymeric coatings via hydrophobic and electrostatic interactions.48 At pH 6.5 or below, drugs released from FMX, leading to cancer cell death. As a result, FMX-encapsulated chemotherapeutic drugs have been shown to more efficiently reduce tumor growth than free drugs.26 Therefore, we reasoned that HMC-FMX can encapsulate chemotherapeutic drugs and deliver them across the BBB and into GBM tumors, reducing tumor growth and increasing overall survival. Paclitaxel (PTX) and cisplatin (CDDP) were selected as model drugs for HMC-FMX encapsulation. PTX was selected because it is effective against a variety of cancer types such as ovarian, breast, and lung.49, 50 However, its use in the treatment of gliomas has been limited, presumably due to poor BBB penetration. Although CDDP is used to treat recurrent GBM, high CDDP doses are needed for effective treatment because its BBB penetration is limited. Encapsulation of both PTX and CDDP into HMC-FMX was hypothesized to improve drug delivery to GBM tumors, while also minimizing their systemic toxicity.
We encapsulated PTX and CDDP into HMC-FMX, obtaining HMC-FMX(PTX) and HMC-FMX(CDDP) nanoprobes, respectively. These drug-loaded nanoprobes demonstrated similar diameters and zeta potentials to unloaded HMC-FMX, and they were able to encapsulate drugs at high concentrations (Table S1). HMC-FMX can encapsulate hydrophobic drugs for several weeks at 4°C in PBS (Figure S10). Drug-loaded HMC-FMX nanoprobes released all their encapsulated drug within 48 h in PBS at 37°C (PTX, t1/2 = 7.6 h; CDDP, t1/2 = 2.2 h) (Figure S11). The presence of serum significantly decreased the release rates of PTX but not CDDP (PTX, t1/2 = 74.2 h; CDDP, t1/2 = 1.8 h).
When various GBM cell lines were incubated with HMC-FMX(PTX) for 72 h, significant changes in cell morphology were observed corresponding to nanoparticle uptake (Figure S12). Based on this observation, cells were treated with various concentrations of either free drug, untargeted drug-loaded nanoprobes or HMC-targeted drug-loaded nanoparticles, and the IC50 values of the treated cells were measured and compared (Figure S13a). IC50 values were tabulated (Table S2). FMX(PTX)- and HMC-FMX(PTX)-treated cells demonstrated higher IC50 values than PTX-treated cells (Figure S13b). However, FMX(CDDP)- and HMC-FMX(CDDP)-treated cells demonstrated lower IC50 values than CDDP-treated cells. To further investigate the cause of GBM cell death following nanoprobe treatment, we performed a flow cytometry analysis of HMC-FMX(PTX)-treated U-87 cells (5 nM PTX) with 7AAD/annexin V staining (Figure S13c). PBS-treated control cells demonstrated high cell viability (90.2% viable cells) and minimal amounts of early apoptotic (2.9%), late apoptotic (5.5%) and necrotic cells (1.4%). In contrast, HMC-FMX(PTX)-treated cells demonstrated reduced cell viability (56.4%) corresponding to increases in both early (28.0%) and late apoptotic cells (12.1%). The number of necrotic cells (3.5%) in HMC-FMX(PTX)-treated cells remained low.
As GBM CSCs are involved in tumor initiation, migration and therapeutic resistance, we tested the ability of HMC-FMX(PTX) and HMC-FMX(CDDP) to reduce the viability of patient-derived GBM CSCs. Following 5 μM drug treatment for 7 days using nanoprobes, the spheroid formation ability of GBM CSCs was greatly inhibited (Figure S14a). While PBS treatment had minimal effect on spheroid formation, both HMC-FMX(PTX) and HMC-FMX(CDDP) decreased the percent of viable cells to 41.3% and 13.5%, respectively (Figure S14b,c).
HMC-FMX Delivers Anticancer Drugs to GBM Tumors, Decreases Tumor Growth and Increases Overall Survival
We next treated U-87 orthotopic GBM tumor-bearing mice with HMC-FMX(PTX) and measured tumor growth by MRI (Figure 7a). Nanoprobe treatment (1 mg HMC, 3 mg PTX and 4 mg iron/kg mice twice a week for 3 weeks) resulted in a dramatic reduction of tumor growth, with no visible tumors by MRI for up to 42 days after tumor inoculation (Figure 7b). Tumor size differences were statistically significant after 35 days, and the full statistical comparison of each treatment is in Table S3. After ceasing treatment with HMC-FMX(PTX), mice developed tumors that were visible by MRI (Figure S15), suggesting that GBM tumors were established in these mice and treatment suppressed tumor growth. Histopathological studies of the brain corroborate the absence of brain tumors in HMC-FMX(PTX)-treated mice (Figure 7c). These studies also demonstrated no visible signs of toxicity in major organs from HMC-FMX(PTX)-treated mice.
Figure 7. HMC-FMX(PTX) reduces the growth of U-87 GBM tumors in mice.
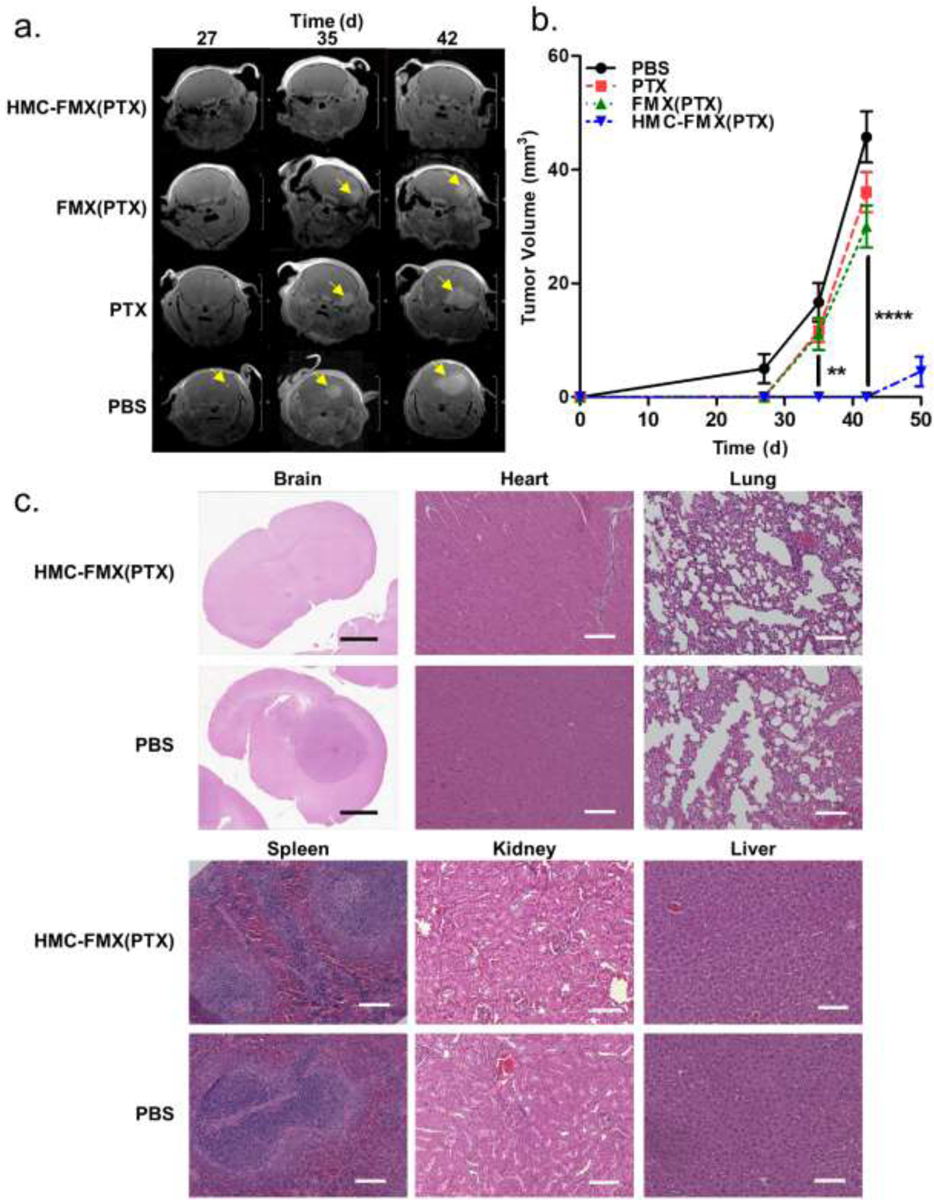
Representative brain MRI images of treated mice by gadolinium-enhanced MRI (a). Yellow arrows indicate tumors. Tumor volume measurements of treated mice by MRI (n=5 per group) (b). Histopathological analysis of brain and other organs with and without nanoprobe treatment (c). No tumor is visible in the HMC-FMX(PTX)-treated mice brains during the treatment period, but tumors are observed in PBS-treated mice. No visible signs of toxicity are observed in HMC-FMX(PTX)-treated mice. Scale bars are 100 μm.
Finally, the effects of HMC-FMX(PTX) and HMC-FMX(CDDP) on the median survival of mice with GBM tumors were investigated. Either PBS (negative control), free drug, non-targeted nanoprobe or targeted nanoprobe were injected into mice eight times over three weeks in order to mimic a potential clinical GBM treatment regimen (Figure 8a). In these experiments, the amount of drug, dye and iron were kept constant between treatment groups. HMC-FMX(PTX) treatments increased the median survival of mice from 32 days (PBS treatment) to 41 days (Figure 8b). A 9-day increase in median survival represents a 28% increase in median survival compared to PBS treatment. Additionally, there was no statistically significant differences among the median survivals of PBS-, PTX- and FMX(PTX)-treated mice. In contrast, HMC-FMX(CDDP) treatments improved the median survival of mice to 55 days (Figure 8c). Compared to PBS treatments, the 23 day increase in median survival from HMC-FMX(CDDP) treatments represents a 72% increase in median survival. Similar to the HMC-FMX(PTX) experiments, there were no statistically significant differences among the median survivals of PBS-, CDDP- and FMX(CDDP)-treated mice. Notably, HMC-FMX(CDDP) nanoprobes were more effective than HMC-FMX(PTX) nanoprobes at increasing the survival of mice with GBM tumors. We were able to detect the delivery of platinum into brain tumors indicating GBM-selective uptake of CDDP, but we were unable to distinguish iron uptake from endogenous iron in the normal brain (Figure S16). It is possible; however, that some of the CDDP is released while in circulation from HMC-FMX(CDDP) and accumulates in GBM tumors, therefore having an effect. If this was true, then one would see no difference between the efficacy of HMC-FMX(CDDP) and FMX(CDDP) in the survival of mice with orthotopic GBM tumors (Figure 8C). Therefore, we attribute the observed platinum accumulation within GBM tumors to nanoparticle-mediated delivery of drug across the BBB. Taken together, these survival experiments indicate that HMC targeting is necessary for the drug-loaded nanoprobes to cross the BBB and increase GBM survival in mice.
Figure 8. Kaplan-Meier survival graphs of mice with orthotopic GBM tumors.
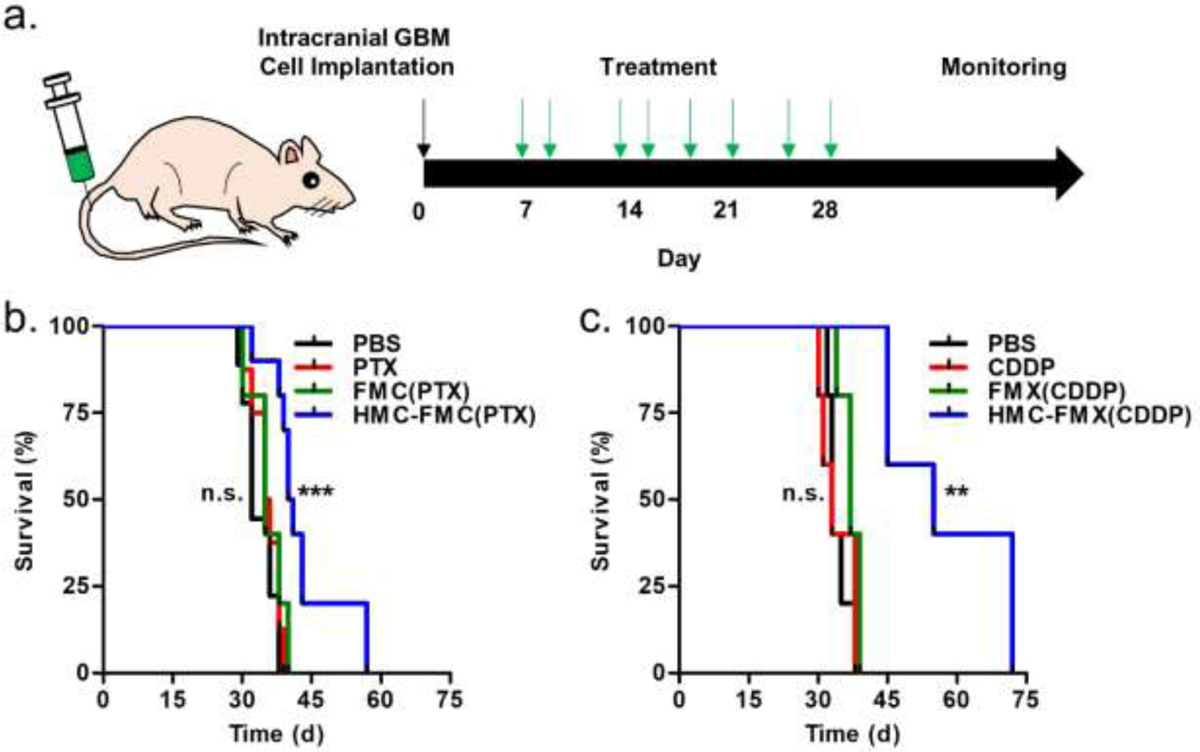
Mice were treated with eight doses of either PBS (negative control), drug, drug-loaded non-targeted nanoprobes, or drug-loaded HMC-targeted nanoprobes, and then monitored until they became symptomatic and were euthanized (a). Either PTX-loaded (b) or CDDP-loaded HMC-FMX nanoprobes (c) were used. Each injection contained 3 mg drug or 4 mg iron/kg or both.
Due to the physical and developmental differences between GBM tumors in mouse models and human GBM tumors, the effects of both the tumor-to-brain volume ratio and absolute tumor size on GBM treatment in mice must be carefully considered. Because mice have smaller brains than humans, mice cannot develop brain tumors that mimic the size of human brain tumors by the time they present clinically (about 5000 mm3).51 Based on an initial tumor volume of 2 mm3 from a 2 ul injection of cells and a mouse brain mass of 0.450 g, we estimate an initial tumor-to-brain ratio of 0.0044 mm3 tumor/g brain tissue. Similarly, a human with a 5000 mm3 tumor at presentation with a 1350 g brain would have an estimated tumor-to-brain ratio of 0.0037 mm3 tumor/g brain tissue. Because these values are in agreement, we speculate that our orthotopic GBM tumors maintain a similar tumor-to-size ratio as human brain tumors when they are early in their development. In our orthotopic GBM mouse model, all mice had to be euthanized when their brain tumors were less than 60 mm3, and we were unable to consider any effects of a larger tumor volume on drug delivery into GBM tumors. Based on our survival experiments, HMC-FMX nanoprobes were able to increase the median survival of mice with GBM tumors that are of a similar tumor-to-brain size ratio as human GBM tumors. We expect that these observations in mice will be applicable to larger human tumors and cancer cells remaining in the surgical bed following resection, but additional GBM animal models are needed to test this hypothesis. Toward this end, we will develop a large animal model that can more accurately mimic both the size and growth of human GBM tumors.
CONCLUSIONS
In summary, our results showed that HMC-FMX can label GBM tumors with near infrared fluorescence in orthotopic GBM mouse models. HMC-FMX labels both primary and infiltrating GBM tumors with bright NIRF signals, facilitating GBM resection by identifying infiltrating tumor tissue that can evade visualization by the surgeon. In addition, HMC-FMX was able to label migratory GBM CSCs derived from patient cell lines. We also showed that HMC-FMX can cross the BBB and accumulate within GBM cells, delivering chemotherapeutic drugs into GBM tumors, reducing tumor volume and increasing survival. In contrast to other imaging and drug delivery methods for GBM treatment, HMC-FMX could simultaneously improve the visualization of infiltrating GBM tumors during resection and deliver either clinically available or experimental non-BBB-crossing drugs to residual GBM cells.
MATERIALS AND METHODS
Materials
Ferumoxytol (FMX) was from AMAG Pharmaceuticals (Waltham, MA). Paclitaxel (PTX), cisplatin (CDDP), DAPI (4’,6-diamidino-2-phyenylindole), Alexa Fluor 568-labeled donkey anti-sheep, Alexa Fluor 488-labeled donkey anti-mouse H+L cross-absorbed secondary antibodies, antibiotic-Antimycotic solution (AA), Prolonged Gold antifade reagent with DAPI and CTS Neurobasal-A Medium were from Thermo Fischer Scientific (Waltham, MA). 2,3,3-trimethylindolenine, 1,4-butanesulfone, 6-bromohexanoic acid, nitric acid, phosphorous oxychloride, cyclohexanone, aniline, tert-butyl methyl ether, dianaline hydrochloride, sodium acetate, N,N’-dicyclohexylcarbodiimide (DCC), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS) and N-Boc-lysine were from Sigma Aldrich (St. Louis, MO). Dimethyl sulfoxide (DMSO), acetonitrile (ACN), methanol, diethyl ether, hexane, dimethyl formamide (DMF), ethanol, acetone, trifluoroacetic acid (TFA), 2-ethanesulfonic acid (MES), phosphate buffered saline (PBS), sodium borate, Triton X-100, crystal violet, OCT tissue freezing medium, Dulbecco’s Modified Eagle’s Medium (DMEM), Eagle’s Minimum Essential Medium (EMEM), dialysis membrane with molecular weight cutoff (MWCO) of 6–8 kD and 500 μl dialysis cups were from Fischer Scientific (Pittsburgh, PA). Fetal Bovine Serum (FBS) was purchased from Atlanta Biologicals (Flowery Branch, GA). Deionized water was prepared using a Milli-Q reverse osmosis system (Millipore Sigma, Burlington, MA). Sodium borate buffer (0.1 M, pH 8.5) was prepared on site. Human glioblastoma cell lines U-87, A-172, LN-18, and T98-G were obtained from American Type Culture Collection (ATCC, Manassas, VA). Organic anion transporter polypeptide (OATP) inhibitors (Telmisartan, Rifamycin, and Atazanavir), paraformaldehyde (PFA), and donkey serum were purchased from Sigma-Aldrich (St. Louis, MO). Sodium dodecyl sulfate (SDS) was purchased from Bio-Rad Laboratories (Hercules, CA). Sheep anti-Von Willebrand Factor (ab11713) and mouse anti-Nestin (ab6320) antibodies were purchased from Abcam (Cambridge, United Kingdom). Alexa Fluor 568 Donkey anti-sheep IgG secondary antibody (A-21099) and Alexa Fluor 488 Donkey-anti mouse IgG secondary antibody (A-21202) were purchased from Invitrogen (Carlsbad, CA). PE Annexin V Apoptosis Detection Kit I was from BD Pharmingen (San Jose, CA). A microliter-scale syringe and needle for animal surgeries was from Hamilton (Reno, NV). PFA (4% w/v in PBS) and SDS solutions (2% w/v in deionized water) were prepared for cell culture experiments. B-27 supplement (50x) serum-free and Penicillin-Streptomycin (Pen-Strep) solution were from Life Technologies (Carlsbad, CA). Recombinant human EGF protein and recombinant human FGF protein were purchased from R&D systems (Minneapolis, MN). Heparin was purchased from Stemcell Technologies (Vancouver, Canada).
HMC-FMX Nanoprobe Preparation
Magnetofluorescent nanoprobes were prepared by conjugating the near-infrared dye heptamethine carbocyanine (HMC) to the surface of ferumoxytol (FMX). To accomplish this, a lysine-modified derivative of heptamethyl carbocyanine (HMC-Lys) was prepared as described previously52, 53 and conjugated to carboxylic acid groups on FMX. A synthesis protocol for HMC-Lys is included in the supplemental information. To prepare HMC-FMX nanoprobes, FMX (1 ml, 30 mg iron/ml), NHS (24 mg) and EDC (35 mg) were dissolved in 2000 ul MES buffer (pH 6.0, 100 mM) and allow to stand at room temperature for 30 minutes. Then HMC-Lys (10 mg) in 1000 ul DMSO was added and incubated overnight at room temperature in the dark. The reaction product was purified by dialysis in PBS for 3 days. The purified HMC-FMX product was collected from the dialysis bag, diluted with PBS to an iron concentration of 30 mM, and then stored at 4°C in the dark until future use. The diameter and surface charge of HMC-FMX was determined via dynamic light scattering (DLS, Zetasizer Nano ZS, Malvern, UK) by diluting the nanoprobe in PBS to a 1 mM iron concentration, and light scattering was measured using 173° backscattered light with a 4 mW laser at 633 nm. Dye conjugation to HMC-FMX was quantified by measuring the absorbance of iron-precipitated HMC-FMX solution using a plater reader (SpectaMax M5, Molecular Devices, San Jose, CA). Briefly, HMC-FMX was diluted 1:1 with acetonitrile and incubated for 2 h at room temperature. The sample was then centrifuged to remove the precipitated iron, and the supernatant was collected. The absorbance of the supernatant at 780 nm was compared to a calibration curve of known concentration HMC standards. Using this value, the number of HMC molecules attached to each per FMX nanoparticle in HMC-FMX was determined by following published procedures.33
Optical Characterization of ICG, HMC and HMC-FMX
HMC-Lys, ICG and HMC-FMX were dissolved at a 10 μM dye concentration in PBS, and their absorbance spectra were measured using a plate reader as described in the previous section. For fluorescence measurements, HMC-Lys, ICG and HMC-FMX were dissolved at 1 μM dye concentration in PBS, and their absorbance spectra were measured using a PerkinElmer LS-55 fluorescence spectrometer (Waltham, MA). In addition, both FMX and HMC-FMX were dissolved at 1.67 mg/ml iron in HPLC vials, and the fluorescence was measured using a SIRIS camera.
MR Characterization of Nanoprobes
Both drug-loaded and empty nanoprobes [FMX, HMC-FMX, HMC-FMX(PTX) and HMC-FMX(CDDP)] were diluted to 2, 4, 6, 8 and 10 μg/ml iron concentrations. The samples were placed into a clinical scale MRI instrument (Magnetom Vida, Siemens Healthineers, 3.0 T), and T1 and T2 relaxation times were measured. The relaxation times were graphed against the sample concentrations, and relaxivity values were calculated as the slope of best fit line for each sample using Graphpad Prism.
Fluorescence Stability of Nanoprobes
HMC-FMX was diluted in PBS to a 1 mM iron concentration and stored under the SIRIS camera mimicking intraoperative light conditions. Sample fluorescence was measured with the SIRIS camera at 0, 1, 2 or 3 h. The fluorescence was quantified for each time point by measuring total fluorescence signal in each image and subtracting background signal from a blank image using ImageJ (Bethesda, MD). The fluorescence for each sample was normalized to the initial sample fluorescence. In addition, HMC-FMX dilutions of 1000, 250, 62.5, 15.6, 3.9, 1.0, 0.24 and 0.06 nM iron concentration were prepared, and the fluorescence of each sample was measured and quantified as described above. The fluorescence for each sample was normalized to the highest concentration sample fluorescence. The limit of detection was calculated as three times the standard deviation of the calibration curve divided by the slope of the calibration curve.
Physical Stability of Nanoprobes
HMC-FMX was diluted in PBS to a 1 mM iron concentration, and DLS measurements were performed every 24 hours as described earlier. Between measurements, HMC-FMX was stored at 4°C in the dark. In addition, HMC-FMX was diluted in 20% FBS to a 1 mM iron concentration, and DLS measurements were performed as described earlier. The sample was then stored at 37°C in the dark. After 72 h, the samples were removed, and DLS measurements were repeated.
Drug Loading into HMC-FMX Nanoprobes
PTX and CDDP were loaded into FMX or HMC-FMX by a solvent diffusion method.54 Briefly, stock solutions of FMX and HMC-FMX (30 mM iron in PBS) were prepared. Stock solutions of PTX and CDDP (10 mg/ml in DMSO) were also prepared. The PTX or CDDP solution (200 μl) was added dropwise to either FMX or HMC-FMX solutions (1800 μl) while vortexing, and then each sample was mixed overnight at room temperature to prepare the corresponding drug loaded FMX or HMC-FMX. The resulting FMX(PTX), FMX(CDDP), HMC-FMX(PTX) or HMC-FMX(CDDP) samples were purified by centrifugal filtration, diluted to a 30 mM iron concentration and stored at 4°C until future use. Drug loading in PTX nanoparticles were then quantified by HPLC. Briefly, 100 μl aliquots of the PTX-loaded FMX or HMC-FMX were each diluted with 100 μl of PBS (pH 4.0) and 200 μl of acetonitrile. Diluted nanoprobe samples were incubated for 2 h at room temperature to release drug from nanoprobes and precipitate iron. The samples were then centrifuged, and the supernatants from each sample were collected for HPLC analysis (Agilent 1100, Hichrom Apollo C18 column, 150×4.6 mm, 5 μm pore size, 1 ml/min flow rate, 10 μl injection). Absorbance peaks from each sample were compared to a calibration curve of known concentration PTX standards (60% ACN/40% water containing 0.1% TFA, room temperature, 230 nm, 4.92 min retention time). CDDP-loaded nanoparticles were quantified by inductively coupled plasma optical emission spectroscopy (ICP-OES) (Shimadzu Scientific Instrument, ICPE-9000). Briefly, 200 ul of sample were diluted to 10 ml, and platinum concentrations were calculated in each sample. Based on platinum concentrations, the amount of CDDP loaded into nanoparticles could be calculated. The mass-based drug concentration in each sample and the molar drug loading (mol of drug/mol iron in nanoprobe) were then calculated.
Drug Release from HMC-FMX Nanoprobes
Drug release was measured from HMC-FMX nanoprobes using a dialysis method. Briefly, HMC-FMX(CDDP) or HMC-FMX(PTX) were dissolved at 1.67 mg/ml iron in PBS, or at 1.34 mg/ml iron in 20% FBS. Each solution (1.5 ml) was added to dialysis cups, and dialysis cassettes were placed into 15 ml of dialysis solution. The nanoprobe solutions were dialyzed for 72 h at 37°C. Aliquots of nanoprobe solution (200 ul) were removed at 0, 1, 3, 6, 24, 48 and 72 h. Drug concentrations were quantified in each sample as described earlier. To maintain a sink condition, the dialysis solution was changed each time the nanoprobe solution was sampled. For each sample, the amount of drug remaining at each timepoint was calculated as percent of drug remaining in the nanoprobe solution divided by the amount of drug at the initial timepoint. The drug released was calculated as 100 minus the percent drug remaining. The percent of drug remaining was graphed against time, and the curve was fitted to a first order exponential association graph using GraphPad Prism 5 (San Diego, CA).
Drug Loading Stability of HMC-FMX Nanoprobes
HMC-FMX(PTX) was stored at 4°C in the dark. Aliquots of nanoprobe solution (200 ul) were removed after 0, 7, 14, 21 and 28 d. Drug concentrations were quantified in each sample as described earlier.
GBM Cell Culture
U-87, A-172 and HT-22 cell lines were grown in DMEM supplemented with 10% FBS and 1% AA. LN-18 and LN-229 cell lines were grown in DMEM supplemented with 5% FBS and 1% AA. T-98G was grown in EMEM supplemented with 10% FBS and 1% AA. Cells were grown according to ATCC recommendations. GBM cancer stem cell line CSC55 was generated from a GBM patient at Cedars Sinai Medical Center, following approved Institutional Review Board (IRB) guidelines and stored in a Biobank Core Facility.55–58 GBM stem cells were grown in CTS Neurobasal-A Medium supplemented with 2% B-27, 1% Pen-Strep, 50 ng/ml EGF, 20 ng/ml FGF, and 160 ng/ml Heparin. Cells were maintained at 37 °C in a humidified incubator with 5% CO2. All cell lines used in this study were used within ten passages of their initial reconstitution.
HMC-FMX Nanoprobe Uptake in Cultured Cells
HT-22, U-87, A-172, LN-18, LN-229, T98-G, and CSC55 cells were seeded in 6 well plates at a density of 100,000 cells per well. The cells were then treated with HMC-FMX (0.17 nM HMC, 30 μM iron). After 1 h of treatment, the GBM cell lines were washed with PBS, fixed with PFA solution, counterstained with DAPI (300 nM in PBS), and imaged on a fluorescence microscope (Keyence BZ-X710, Keyence, Osaka, Japan). Because CSC55 cells were not adherent, these cells were not fixed or counterstained before imaging. Fluorescence images of nuclei (DAPI filter) and HMC (Cy7 filter) were taken at a constant brightness and exposure time. Imaging experiments were also repeated with HMC-FMX(PTX). To determine the effects of OATP inhibitors on HMC dye uptake, U-87 cells were seeded in 24 well plates at a density of 2.5 × 104 cells/well. Cells were then pre-incubated with OATP inhibitors telmisartan (25 μM), rifamycin (20 μM) or atazanavir (20 μM) for 5 h prior to the treatment with HMC-FMX (0.87 nM HMC, 150 μM iron for each). Three hours after the treatment, cells were washed twice with PBS, fixed with PFA solution, and counterstained with DAPI. The uptake and accumulation of HMC-FMX with or without inhibitors were observed under a fluorescence microscope (Keyence BZ-X710, Keyence, Osaka, Japan). Fluorescence was quantified in images using ImageJ. In addition, HMC-FMX-treated cells were resuspended and fixed in 2% PFA for flow cytometry analysis using a BD LSR Fortessa flow cytometer (BD Biosciences, San Jose, CA).
HMC-FMX Iron Uptake Quantification
U-87 cells were seeded in 10 cm dishes at a density of 2,200,000 cells per dish. The cells were then treated with HMC-FMX (1.7 nM HMC, 300 μM iron). After 24 h of treatment, the cell lines were washed with PBS, resuspended and pelleted. Cell pellets were resuspended in 500 μl of RIPA buffer and allowed to lyse for 30 minutes. Lysed pellets were analyzed by ICP-OES to determine iron content.
Cell Viability Assay
Cell viability was assessed initially using a cell adhesion assay. Briefly, U-87, A-172, LN-18, LN-229 and T-98G cells were seeded in 96-well plates at a density of 5 × 103 cells/well. After 24 h, cells were treated with increasing concentrations of HMC-FMX(PTX), FMX(PTX), PTX, HMC-FMX(CDDP), FMX(CDDP) or CDDP. After 72 h, the cells were washed twice with PBS, fixed with PFA solution, and stained with crystal violet (5 mg/ml in deionized water) for 10 min. The cells were then washed twice with DI water, dried at room temperature and lysed with SDS solution for 30 min at room temperature. Following lysis, the absorbance of crystal violet was measured at 595 nm. A blank measurement was subtracted from each measurement, and measurements were normalized to untreated samples. IC50 values were calculated for each cell line and treatment using GraphPad Prism 5. In addition, a flow cytometry-based apoptosis assay (Annexin/7-AAD) was used to further investigate cell death. In these experiments, cells were seeded in 6-well plates at a density of 1 × 105 cells/well. Cells were treated with increasing concentrations of HMC-FMX(PTX), HMC-FMX(CDDP) or corresponding treatment, whereas PBS treatment was done as a negative control. Cells were pelleted, washed twice with ice-cold PBS, and then suspended in binding buffer at a concentration of 1 × 106 cells/ml. 100 μl of the solution was transferred to a 5 ml culture tube respectively. Five μl of PE Annexin V and 5 μl of 7-AAD were added to the samples, and then the samples were gently vortexed and incubated for 15 min at room temperature in the dark. To quantify cell viability, 400 μl of binding buffer was added to each tube and the samples were analyzed using a BD LSR Fortessa flow cytometer within 1 h of staining. Data and graphs were analyzed using FlowJo 10 (Ashland, OR).
Orthotopic U-87 GBM Model Development and Nanoprobe Administration
Mouse experiments were conducted in strict compliance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Cedars Sinai Medical Center. Nu/Nu female mice (3–4 weeks old) were obtained from Charles River (Wilmington, MA). Animals were housed in a pathogen-free animal house and were given sterilized water and fed with rodent chow ad libitum. Orthotopic brain tumors were established in mice by stereotactically injecting 105 U-87 cells or CSC55 GBM CSCs into the mouse brain right basal ganglia field under anesthesia. Mice were monitored daily and allowed to recover for one week post-operation before any further experiments.
HMC-FMX Biodistribution and GBM Targeting Studies
Brain tumor-bearing mice were intravenously injected with HMC-FMX (1 mg HMC and 4 mg iron/kg). At 3, 24, 48 and 168 h post-injection, mice were euthanized and then imaged using the SIRIS. Following whole body imaging, mice brains, hearts, lungs, livers, kidneys and spleens were harvested and imaged to visualized fluorescence distribution using SIRIS. In addition, fluorescence images of these organs were taken using an Odyssey fluorescence imager (Li-COR, Lincoln, NE) to quantify the total fluorescence in each organ for each timepoint. In these images, the fluorescence of brain tumors was quantified separately from the normal brain. A 50 μl blood sample was also collected from the euthanized mice. To quantify the amount of HMC-FMX in circulation at each time point, blood samples were diluted 20-fold with PBS and imaged on the Odyssey imager.
Ex Vivo GBM Tumor Extraction Using Near Infrared Fluorescence Imaging
To evaluate the potential for near-infrared fluorescence (NIRF) image-guided surgical removal of GBM tumors using magnetofluorescent nanoprobes, we performed a mock ex vivo procedure to remove brain tumors from a mouse brain. Briefly, tumor-bearing mice (n=5) were intravenously injected with HMC-FMX (1 mg HMC in 4 mg iron/kg). After 24 h, mice were euthanized and their brains were removed. Mice brains were placed under the SIRIS camera to visualize the fluorescent GBM tumor after HMC-FMX uptake. Once a fluorescent brain tumor was visualized, it was separated from the non-fluorescent normal brain tissue. Procedures were recorded using a Hauppauge High Definition Personal Video Recorder (Hauppauge, NY). Additionally, still images of the procedure were taken using built-in capabilities of the SIRIS camera system software.
Immunohistochemistry
After euthanasia, brains from mice treated with HMC-FMX for 24 h were harvested, embedded in OCT tissue freezing medium, and stored at −80°C. Frozen brains were sectioned at a 10 μm thickness using a Leica CM3050S cryostat (Leica Biosystems, Wetzlar, Germany). Prior to staining, tissue sections were air-dried at room temperature for 1 h, fixed in 4% PFA for 10 min, and rinsed in PBS. Brain sections were then incubated in a humidity chamber with a blocking solution (5% donkey serum and 0.1% Triton X-100 in PBS) at room temperature for 1 h. Blocked samples were then incubated with either sheep anti-von Willebrand factor or mouse anti-Nestin primary antibodies (1:100 in blocking solution) overnight in a humidity chamber at 4°C in the dark. Following primary antibody incubation, tissue sections were rinsed 3 times with PBS and then stained with either anti-sheep or anti mouse secondary antibodies (1:250 in PBS), respectively, at room temperature for 1 h. Stained tissue sections were rinsed 3 times with PBS and then mounted with Prolonged Gold antifade reagent containing DAPI. Tissue section images were acquired using the Keyence BZ X-700 fluorescence microscope.
Hematoxylin and Eosin (H&E) Staining
Mice were treated with either 8 doses of HMC-FMX(PTX) or PBS (see efficacy studies below). Brain, heart, lung, liver, spleen, and kidneys were harvested from these mice upon euthanasia and stored in 4% PFA for 48 h, followed by 70% ethanol. Within one week of ethanol storage, the organs were sent to the Biobank and Translational Research Core at Cedars Sinai Medical Center for tissue sectioning and H&E staining. To examine nanoprobe toxicity, brightfield images of H&E-stained tissue sections from nanoprobe-treated mice were taken using the Keyence BZ X-700 fluorescence microscope and compared to H&E-stained tissue sections from PBS-treated mice.
HMC-FMX GBM Tumor Growth Monitoring and GBM Survival Studies
Brain tumor-bearing mice (n=5) were administered with HMC-FMX(PTX) or HMC-FMX(CDDP) via intravenous tail vein injection (2.5 mg PTX/kg or 2.5 mg CDDP/kg) twice a week for a total of 8 tail vein injections. As controls, mice were also injected with FMX(PTX), FMX(CDDP), PTX or CDDP at the same drug concentration (n=5 for each treatment). In addition, mice were injected with equal volumes of PBS as a negative control (n=5). All mice were monitored daily for signs of pain or distress, and their body weights were measured every 3 days. Tumor growth was monitored using an MRI 9.4 Tesla microMR scanner (Bruker BioSpec, Billerica MA, USA) using a volume transmit coil for excitation and a four-channel array surface coil for detection. Briefly, mice were injected with gadolinium (75 mg/kg) and then imaged immediately. A T2*-weighted 2D fast gradient echo pulse sequence with a flip angle of 20°, a repetition time of 70 ms, multiple echo times of 1.5–12.6 ms (8 echoes with echo spacing of 1.6 ms), a matrix of 128×128 pixels, a field of view of 4.5×2.7 cm, one excitation and a slice thickness of 0.6 mm (1 mm for 3T scanner) was used to acquire the MR images at 27, 35, and 42 days after tumor inoculation. All mice with MRI-visible tumors were euthanized at day 42 and their brain were harvested for H&E analysis. To assure that treated mice without visible tumors had been successfully inoculated with GBM cells, HMC-FMX(PTX)-treated mice were allowed to grow tumors following the end of treatment and were euthanized as soon as visible tumors were observed by MRI. For survival studies, the mice were then administered twice a week with nanoprobes and treatments at the same dose as described above. Mice were monitored daily for signs of pain or neurological symptoms indicative of GBM such as lethargy, lack of movement or difficulty eating. Mice that displayed advanced neurological symptoms of GBM were euthanized.
HMC-FMX Drug Delivery Studies
Brain tumor-bearing mice (n=5) were administered with a single dose of HMC-FMX(CDDP) (2.5 mg CDDP/kg). After 48 h, mice were euthanized, and their brains were removed. Using the SIRIS camera, fluorescent brain tumors were separated from the non-fluorescent normal brain. Brain tumors and normal brain were digested in nitric acid for 1 h at 90°C. Both iron and platinum in digested tissue samples were quantified by ICP-OES.
Statistics
Data are presented as mean ± standard deviation from at least three independent experiments. For pairs of measurements, the statistical significance was analyzed by Student’s t test. For groups of measurements, the data were compared by a one-way ANOVA with Bonferroni’s post-test correction. P values less than 0.05 were considered as statistically significant. Statistical significance was denoted by n.s. (p > 0.05), * (p < 0.05), ** (p < 0.01), *** (p < 0.001) or **** (p < 0.0001). Statistics and fitting of experimental data were performed with Prism 5 (GraphPad, San Diego, CA).
Supplementary Material
ACKNOWLEDGEMENT
This work was supported in part by NIH/NIBIB grant R01EB019288 and internal funding from Cedars Sinai Medical Center awarded to JMP. The authors thank the Imaging Core of the Cedars-Sinai Biomedical Research Institute for the use of the MRI facilities and technical advice.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests
SUPPORTING INFORMATION
This material is available free of charge via the Internet at http://pubs.acs.org. Supporting information contains the following information:
1. Synthesis protocol for HMC-Lys
2. Absorbance and fluorescence spectra of HMC-Lys and ICG
3. RNA expression of OATPs in GBM and normal brain tissue
4. Photostability of HMC-Lys, HMC-FMX and ICG under intraoperative imaging conditions
5. Physical stability of HMC-FMX in PBS and 20% FBS
6. Cellular uptake of HMC-FMX in neuronal and GBM cell lines
7. OATP inhibition of HMC-FMX uptake
8. NIRF imaging of whole mice after HMC-FMX treatment
9. Physical characterization of drug-loaded HMC-FMX nanoprobes
10. Drug release profiles of HMC-FMX(PTX) and HMC-FMX(CDDP)
11. Cellular uptake of drug-loaded HMC-FMX
12. Cytotoxicity of HMC-FMX(PTX) and HMC-FMX(CDDP) in GBM cell lines and GBM CSCs
13. MRI of brain tumors in HMC-FMX(PTX)-treated mice after treatment cessation
14. Quantification of iron and CDDP in brain tumors after 48 h following HMC-FMX(CDDP) treatment
REFERENCES:
- 1.Gittleman HR; Ostrom QT; Rouse CD; Dowling JA; de Blank PM; Kruchko CA; Elder JB; Rosenfeld SS; Selman WR; Sloan AE; Barnholtz-Sloan JS Trends in Central Nervous System Tumor Incidence Relative to Other Common Cancers in Adults, Adolescents, and Children in the United States, 2000 to 2010. Cancer 2015, 121, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT; Gittleman H; Fulop J; Liu M; Blanda R; Kromer C; Wolinsky Y; Kruchko C; Barnholtz-Sloan JS CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol 2015, 17 Suppl 4, iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouse C; Gittleman H; Ostrom QT; Kruchko C; Barnholtz-Sloan JS Years of Potential Life Lost for Brain and CNS Tumors Relative to Other Cancers in Adults in the United States, 2010. Neuro Oncol 2016, 18, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young RM; Jamshidi A; Davis G; Sherman JH Current Trends in the Surgical Management and Treatment of Adult Glioblastoma. Ann Transl Med 2015, 3, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyder F; Manjura Hoque S Brain Tumor Diagnostics and Therapeutics with Superparamagnetic Ferrite Nanoparticles. Contrast Media Mol Imaging 2017, 2017, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalpathy-Cramer J; Gerstner ER; Emblem KE; Andronesi O; Rosen B Advanced Magnetic Resonance Imaging of the Physical Processes in Human Glioblastoma. Cancer Res 2014, 74, 4622–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao J; Hsu CH; Li Z; Kim TS; Hwang LP; Lin YC; Lin YY Magnetic Resonance Nano-Theranostics for Glioblastoma Multiforme. Curr Pharm Des 2015, 21, 5256–5266. [DOI] [PubMed] [Google Scholar]
- 8.Ferraro N; Barbarite E; Albert TR; Berchmans E; Shah AH; Bregy A; Ivan ME; Brown T; Komotar RJ The Role of 5-Aminolevulinic Acid in Brain Tumor Surgery: A Systematic Review. Neurosurg Rev 2016, 39, 545–555. [DOI] [PubMed] [Google Scholar]
- 9.Stummer W; Pichlmeier U; Meinel T; Wiestler OD; Zanella F; Reulen HJ Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol 2006, 7, 392–401. [DOI] [PubMed] [Google Scholar]
- 10.Tonn JC; Stummer W Fluorescence-Guided Resection of Malignant Gliomas Using 5-Aminolevulinic Acid: Practical Use, Risks, and Pitfalls. Clin Neurosurg 2008, 55, 20–26. [PubMed] [Google Scholar]
- 11.Butte PV; Mamelak A; Parrish-Novak J; Drazin D; Shweikeh F; Gangalum PR; Chesnokova A; Ljubimova JY; Black K Near-Infrared Imaging of Brain Tumors Using the Tumor Paint BLZ-100 to Achieve Near-Complete Resection of Brain Tumors. Neurosurg Focus 2014, 36, 1–8. [DOI] [PubMed] [Google Scholar]
- 12.Lyons SA; O’Neal J; Sontheimer H Chlorotoxin, a Scorpion-Derived Peptide, Specifically Binds to Gliomas and Tumors of Neuroectodermal Origin. Glia 2002, 39, 162–173. [DOI] [PubMed] [Google Scholar]
- 13.Mamelak AN; Jacoby DB Targeted Delivery of Antitumoral Therapy to Glioma and Other Malignancies with Synthetic Chlorotoxin (TM-601). Expert Opin Drug Deliv 2007, 4, 175–186. [DOI] [PubMed] [Google Scholar]
- 14.Yu XF; Sun Z; Li M; Xiang Y; Wang QQ; Tang F; Wu Y; Cao Z; Li W Neurotoxin-Conjugated Upconversion Nanoprobes for Direct Visualization of Tumors under Near-Infrared Irradiation. Biomaterials 2010, 31, 8724–8731. [DOI] [PubMed] [Google Scholar]
- 15.Roder C; Bisdas S; Ebner FH; Honegger J; Naegele T; Ernemann U; Tatagiba M Maximizing the Extent of Resection and Survival Benefit of Patients in Glioblastoma Surgery: High-Field iMRI versus Conventional and 5-ALA-Assisted Surgery. European Journal of Surgical Oncology (EJSO) 2014, 40, 297–304. [DOI] [PubMed] [Google Scholar]
- 16.Stupp R; Mason WP; van den Bent MJ; Weller M; Fisher B; Taphoorn MJ; Belanger K; Brandes AA; Marosi C; Bogdahn U; Curschmann J; Janzer RC; Ludwin SK; Gorlia T; Allgeier A; Lacombe D; Cairncross JG; Eisenhauer E; Mirimanoff RO Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med 2005, 352, 987–996. [DOI] [PubMed] [Google Scholar]
- 17.Stupp R; Taillibert S; Kanner AA; Kesari S; Steinberg DM; Toms SA; Taylor LP; Lieberman F; Silvani A; Fink KL; Barnett GH; Zhu JJ; Henson JW; Engelhard HH; Chen TC; Tran DD; Sroubek J; Tran ND; Hottinger AF; Landolfi J, et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [DOI] [PubMed] [Google Scholar]
- 18.Auerbach M; Strauss W; Auerbach S; Rineer S; Bahrain H Safety and Efficacy of Total Dose Infusion of 1,020 Mg of Ferumoxytol Administered over 15 Min. Am J Hematol 2013, 88, 944–947. [DOI] [PubMed] [Google Scholar]
- 19.Cheng K; Shen D; Hensley MT; Middleton R; Sun B; Liu W; De Couto G; Marban E Magnetic Antibody-Linked Nanomatchmakers for Therapeutic Cell Targeting. Nat Commun 2014, 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu M; Cohen MH; Rieves D; Pazdur R FDA Report: Ferumoxytol for Intravenous Iron Therapy in Adult Patients with Chronic Kidney Disease. Am J Hematol 2010, 85, 315–319. [DOI] [PubMed] [Google Scholar]
- 21.Bashir MR; Bhatti L; Marin D; Nelson RC Emerging Applications for Ferumoxytol as a Contrast Agent in MRI. J Magn Reson Imaging 2015, 41, 884–898. [DOI] [PubMed] [Google Scholar]
- 22.Hope MD; Hope TA; Zhu C; Faraji F; Haraldsson H; Ordovas KG; Saloner D Vascular Imaging with Ferumoxytol as a Contrast Agent. AJR Am J Roentgenol 2015, 205, W366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toth GB; Varallyay CG; Horvath A; Bashir MR; Choyke PL; Daldrup-Link HE; Dosa E; Finn JP; Gahramanov S; Harisinghani M; Macdougall I; Neuwelt A; Vasanawala SS; Ambady P; Barajas R; Cetas JS; Ciporen J; DeLoughery TJ; Doolittle ND; Fu R, et al. Current and Potential Imaging Applications of Ferumoxytol for Magnetic Resonance Imaging. Kidney Int 2017, 92, 47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwenk MH Ferumoxytol: A New Intravenous Iron Preparation for the Treatment of Iron Deficiency Anemia in Patients with Chronic Kidney Disease. Pharmacotherapy 2010, 30, 70–79. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen K-L; Yoshida T; Kathuria-Prakash N; Zaki IH; Varallyay CG; Semple SI; Saouaf R; Rigsby CK; Stoumpos S; Whitehead KK; Griffin LM; Saloner D; Hope MD; Prince MR; Fogel MA; Schiebler ML; Roditi GH; Radjenovic A; Newby DE; Neuwelt EA, et al. Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI. Radiology 2019, 293, 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaittanis C; Shaffer TM; Ogirala A; Santra S; Perez JM; Chiosis G; Li Y; Josephson L; Grimm J Environment-Responsive Nanophores for Therapy and Treatment Monitoring via Molecular MRI Quenching. Nat Commun 2014, 5, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu JB; Shi C; Chu GC-Y; Xu Q; Zhang Y; Li Q; Yu JS; Zhau HE; Chung LWK Near-Infrared Fluorescence Heptamethine Carbocyanine Dyes Mediate Imaging and Targeted Drug Delivery for Human Brain Tumor. Biomaterials 2015, 67, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S; George Thomas R; Ju Moon M; Ju Park H; Park I-K; Lee B-I; Yeon Jeong Y Near-Infrared Heptamethine Cyanine Based Iron Oxide Nanoparticles for Tumor Targeted Multimodal Imaging and Photothermal Therapy. Sci. Rep 2017, 7, 2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X; Shi C; Tong R; Qian W; Zhau HE; Wang R; Zhu G; Cheng J; Yang VW; Cheng T; Henary M; Strekowski L; Chung LW Near IR Heptamethine Cyanine Dye-Mediated Cancer Imaging. Clin Cancer Res 2010, 16, 2833–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandrashekar DS; Bashel B; Balasubramanya SAH; Creighton CJ; Ponce-Rodriguez I; Chakravarthi B; Varambally S UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia (New York, N.Y.) 2017, 19, 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bryant LH; Kim SJ; Hobson M; Milo B; Kovacs ZI; Jikaria N; Lewis BK; Aronova MA; Sousa AA; Zhang G; Leapman RD; Frank JA Physicochemical Characterization of Ferumoxytol, Heparin and Protamine Nanocomplexes for Improved Magnetic Labeling of Stem Cells. Nanomed. Nanotechnol. Biol. Med 2017, 13, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balakrishnan VS; Rao M; Kausz AT; Brenner L; Pereira BJG; Frigo TB; Lewis JM Physicochemical Properties of Ferumoxytol, A New Intravenous Iron Preparation. Eur. J. Clin. Invest 2009, 39, 489–496. [DOI] [PubMed] [Google Scholar]
- 33.Josephson L; Tung CH; Moore A; Weissleder R High-Efficiency Intracellular Magnetic Labeling with Novel Superparamagnetic-TAT Peptide Conjugates. Bioconjug Chem 1999, 10, 186–191. [DOI] [PubMed] [Google Scholar]
- 34.Karlgren M; Vildhede A; Norinder U; Wisniewski JR; Kimoto E; Lai Y; Haglund U; Artursson P Classification of Inhibitors of Hepatic Organic Anion Transporting Polypeptides (OATPs): Influence of Protein Expression on Drug-Drug Interactions. J Med Chem 2012, 55, 4740–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obaidat A; Roth M; Hagenbuch B The Expression and Function of Organic Anion Transporting Polypeptides in Normal Tissues and in Cancer. Annu Rev Pharmacol Toxicol 2012, 52, 135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth M; Obaidat A; Hagenbuch B OATPs, OATs and OCTs: The Organic Anion and Cation Transporters of the SLCO and SLC22A Gene Superfamilies. Br J Pharmacol 2012, 165, 1260–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi C; Wu JB; Pan D Review on Near-Infrared Heptamethine Cyanine Dyes as Theranostic Agents for Tumor Imaging, Targeting, and Photodynamic Therapy. J Biomed Opt 2016, 21, 1–11. [DOI] [PubMed] [Google Scholar]
- 38.Buxhofer-Ausch V; Secky L; Wlcek K; Svoboda M; Kounnis V; Briasoulis E; Tzakos AG; Jaeger W; Thalhammer T Tumor-Specific Expression of Organic Anion-Transporting Polypeptides: Transporters as Novel Targets for Cancer Therapy. J Drug Deliv 2013, 2013, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bronger H; Konig J; Kopplow K; Steiner HH; Ahmadi R; Herold-Mende C; Keppler D; Nies AT ABCC Drug Efflux Pumps and Organic Anion Uptake Transporters in Human Gliomas and the Blood-Tumor Barrier. Cancer Res 2005, 65, 11419–11428. [DOI] [PubMed] [Google Scholar]
- 40.Ose A; Kusuhara H; Endo C; Tohyama K; Miyajima M; Kitamura S; Sugiyama Y Functional Characterization of Mouse Organic Anion Transporting Peptide 1A4 in the Uptake and Efflux of Drugs across the Blood-Brain Barrier. Drug Metab Dispos 2010, 38, 168–176. [DOI] [PubMed] [Google Scholar]
- 41.Miconi G; Palumbo P; Dehcordi SR; La Torre C; Lombardi F; Evtoski Z; Cimini AM; Galzio R; Cifone MG; Cinque B Immunophenotypic Characterization of Human Glioblastoma Stem Cells: Correlation with Clinical Outcome. J Cell Biochem 2015, 116, 864–876. [DOI] [PubMed] [Google Scholar]
- 42.Tamborini M; Locatelli E; Rasile M; Monaco I; Rodighiero S; Corradini I; Franchini MC; Passoni L; Matteoli M A Combined Approach Employing Chlorotoxin-Nanovectors and Low Dose Radiation To Reach Infiltrating Tumor Niches in Glioblastoma. ACS Nano 2016, 10, 2509–2520. [DOI] [PubMed] [Google Scholar]
- 43.Irtenkauf SM; Sobiechowski S; Hasselbach LA; Nelson KK; Transou AD; Carlton ET; Mikkelsen T; deCarvalho AC Optimization of Glioblastoma Mouse Orthotopic Xenograft Models for Translational Research. Comp Med 2017, 67, 300–314. [PMC free article] [PubMed] [Google Scholar]
- 44.William D; Mullins CS; Schneider B; Orthmann A; Lamp N; Krohn M; Hoffmann A; Classen CF; Linnebacher M Optimized Creation of Glioblastoma Patient Derived Xenografts for Use in Preclinical Studies. J Transl Med 2017, 15, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beier D; Hau P; Proescholdt M; Lohmeier A; Wischhusen J; Oefner PJ; Aigner L; Brawanski A; Bogdahn U; Beier CP CD133(+) and CD133(−) Glioblastoma-Derived Cancer Stem Cells Show Differential Growth Characteristics and Molecular Profiles. Cancer Res 2007, 67, 4010–4015. [DOI] [PubMed] [Google Scholar]
- 46.Goffart N; Kroonen J; Rogister B Glioblastoma-Initiating Cells: Relationship with Neural Stem Cells and the Micro-Environment. Cancers (Basel) 2013, 5, 1049–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deeken JF; Löscher W The Blood-Brain Barrier and Cancer: Transporters, Treatment, and Trojan Horses. Clinical Cancer Research 2007, 13, 1663–1674. [DOI] [PubMed] [Google Scholar]
- 48.Santra S; Jativa SD; Kaittanis C; Normand G; Grimm J; Perez JM Gadolinium-Encapsulating Iron Oxide Nanoprobe as Activatable NMR/MRI Contrast Agent. ACS Nano 2012, 6, 7281–7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardin C; Shum E; Singh AP; Perez-Soler R; Cheng H Emerging Treatment Using Tubulin Inhibitors in Advanced Non-Small Cell Lung Cancer. Expert Opin Pharmacother 2017, 18, 701–716. [DOI] [PubMed] [Google Scholar]
- 50.Oruc Z; Kaplan MA; Arslan C An Update on the Currently Available and Future Chemotherapy for Treating Bone Metastases in Breast Cancer Patients. Expert Opin Pharmacother 2018, 19, 1305–1316. [DOI] [PubMed] [Google Scholar]
- 51.Hendrix P; Hans E; Griessenauer CJ; Simgen A; Oertel J; Karbach J Neurocognitive Status in Patients with Newly-Diagnosed Brain Tumors in Good Neurological Condition: The Impact of Tumor Type, Volume, and Location. Clinical Neurology and Neurosurgery 2017, 156, 55–62. [DOI] [PubMed] [Google Scholar]
- 52.Xiao L; Zhang Y; Yue W; Xie XZ; Wang JP; Chordia MD; Chung LWK; Pan DF Heptamethine Cyanine Based Cu-64-PET Probe PC-1001 for Cancer Imaging: Synthesis and In Vivo Evaluation. Nucl Med Biol 2013, 40, 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y; Xiao L; Popovic K; Xie XZ; Chordia MD; Chung LWK; Williams MB; Yue W; Pan DF Novel Cancer-Targeting SPECT/NIRF Dual-Modality Imaging Probe Tc-99m-PC-1007: Synthesis and Biological Evaluation. Bioorg Med Chem Lett 2013, 23, 6350–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santra S; Kaittanis C; Grimm J; Perez JM Drug/Dye-Loaded, Multifunctional Iron Oxide Nanoparticles for Combined Targeted Cancer Therapy and Dual Optical/Magnetic Resonance Imaging. Small 2009, 5, 1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edwards LA; Li A; Berel D; Madany M; Kim NH; Liu M; Hymowitz M; Uy B; Jung R; Xu M; Black KL; Rentsendorj A; Fan X; Zhang W; Yu JS ZEB1 Regulates Glioma Stemness through LIF Repression. Sci Rep 2017, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu G; Yuan X; Zeng Z; Tunici P; Ng H; Abdulkadir IR; Lu L; Irvin D; Black KL; Yu JS Analysis of Gene Expression and Chemoresistance of CD133+ Cancer Stem Cells in Glioblastoma. Mol Cancer 2006, 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu Q; Yuan X; Liu G; Black KL; Yu JS Hedgehog Signaling Regulates Brain Tumor-Initiating Cell Proliferation and Portends Shorter Survival for Patients with PTEN-Coexpressing Glioblastomas. Stem Cells 2008, 26, 3018–3026. [DOI] [PubMed] [Google Scholar]
- 58.Yuan XP; Curtin J; Xiong YZ; Liu GT; Waschsmann-Hogiu S; Farkas DL; Black KL; Yu JS Isolation of Cancer Stem Cells from Adult Glioblastoma Multiforme. Oncogene 2004, 23, 9392–9400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


