Abstract
Purpose:
Accurate life expectancy estimates are required to inform prostate cancer treatment decisions. However, few models are specific to the population served or easily implemented in a clinical setting. We sought to create life expectancy estimates specific to Veterans diagnosed with prostate cancer.
Materials and Methods:
Using national Veterans Health Administration electronic health records, we identified Veterans diagnosed with prostate cancer between 2000 and 2015. We abstracted demographics, comorbidities, oncologic staging, and treatment information. We fit Cox Proportional Hazards models to determine the impact of age, comorbidity, cancer risk, and race on survival. We stratified life expectancy estimates by age, comorbidity and cancer stage.
Results:
Our analytic cohort included 145,678 patients. Survival modeling demonstrated the importance of age and comorbidity across all cancer risk categories. Life expectancy estimates generated from age and comorbidity data were predictive of overall survival (C-index 0.676, 95%CI 0.674 to 0.679) and visualized using Kaplan-Meier plots and heatmaps stratified by age and comorbidity. Separate life expectancy estimates were generated for patients with localized or advanced disease. These life expectancy estimates calibrate well across prostate cancer risk categories.
Conclusions:
Life expectancy estimates are essential to providing patient-centered prostate cancer care. We developed accessible life expectancy estimation tools for Veterans diagnosed with prostate cancer that can be used in routine clinical practice to inform medical-decision making.
Keywords: Prostatic neoplasms, Life expectancy, Veterans Health, Comorbidity
1. Introduction:
Due to the indolent nature of most prostate cancers, treatment decisions require careful balancing of life expectancy and cancer prognosis. As the population ages, it is critical to incorporate life expectancy estimates into treatment planning to avoid unnecessary treatment and its potential life-altering side effects and financial costs. These trade-offs are especially relevant to Veterans receiving care in the Veterans Health Administration (VHA). Veterans diagnosed with cancer within the VHA are known to have lower performance status and higher rates of severe comorbidity[1]. Despite the comorbidity profile of patients treated in the VHA and the knowledge that prostate cancer is often clinically insignificant, Veterans remain at risk of overtreatment[2].
Major prostate cancer guidelines recommend incorporating life expectancy estimates in treatment decisions. However, both clinicians and patients have been shown to be poor raters of life expectancy[3–5]. Consequently, models have been proposed to better estimate general and cancer-specific life expectancy in prostate cancer patients. However, few life expectancy tools are implemented into routine clinical practice[6]. These tools are often overly complicated, requiring inaccessible inputs that are unrealistic for use in the clinic setting[7]. Additionally, existing life expectancy estimates may not be used if they are not transportable to a specific population. The poor survival of participants in the PIVOT trial (primarily drawn from the VHA) underscores the need for tools to generate VHA-specific life expectancy estimates[8, 9].
We set out to develop life expectancy estimates for Veterans with prostate cancer treated within the VHA, the largest national integrated health care system in the US. We hypothesized that even in a population of older men with heavy comorbidity burden, age and comorbidity will be strongly associated with survival. Furthermore, our goal was to develop age- andcomorbidity-adjusted life expectancy estimates to facilitate implementation into routine practice.
2. Materials and Methods:
2.1. Analytic Cohort
We obtained IRB approval and research permissions for VHA research data access. We identified all patients diagnosed with prostate cancer between 2000–2015 using the VHA electronic health record, which includes all VHA inpatient and outpatient services. We identified incident prostate cancer diagnoses using the Corporate Data Warehouse Oncology file, a national dataset designed to capture all patients diagnosed with or treated for cancer in the VHA[10]. We limited our analysis to patients from 50 to 90 years of age (more than 98% of the total cohort) as life expectancy estimates would be most relevant to patients in this age range.
2.2. Covariates
We abstracted patient comorbidities, demographics, tumor staging, and Gleason score from the Corporate Data Warehouse. PSA data were obtained directly from electronic health record laboratory results when available[11, 12]. Patients without sufficient information to allow cancer risk classification were excluded. We ascertained comorbidities included in the Deyo-Romano adaption of the Charlson Comorbidity Index (Supplemental Table I) [13]. The primary treatment was defined using mutually exclusive categories: surgery, radiotherapy, androgen deprivation therapy (ADT), or conservative management, as reported previously[9].
2.3. Survival Outcomes
We evaluated survival using the US Department of Veterans Affairs Vital Status File, which aggregates mortality data from the VHA, the Centers for Medicare and Medicaid, the Social Security Administration, and the National Cemetery Association (as Veterans are eligible for death and burial benefits). The Vital Status File combines these sources to provide a high-quality source of mortality information[14].
2.4. Stratification
We calculated each patient’s comorbidity burden in the two years prior to diagnosis and grouped them by the number of comorbidities present (0, 1, 2, 3, 4, 5, or 6 or more comorbidities). We stratified patients by prostate cancer risk category using the D’Amico risk classifications for localized prostate cancer[15], plus categories for locally advanced and metastatic disease. Low risk prostate cancer was defined as Gleason score ≤ 6, PSA < 10, and Stage ≤ T2a. Intermediate risk constituted patients with Gleason score=7, or PSA = 10 to 20, or Stage = T2b. High risk patients had Gleason score ≥ 8, or PSA > 20, or Stage = T2c-T3. We defined locally advanced disease as clinical stage T4 or N1, and metastatic disease as clinical stage M > 0. We also stratified patients that received prostate cancer treatment with surgery or radiation.
2.5. Statistical Analysis
We summarized key baseline characteristics of patients by providing the mean (standard deviation) for continuous variables and a frequency distribution for categorical variables. We calculated survival time from the date of diagnosis to the date of death. For patients alive on or after December 31, 2017, we censored the follow up time after this point. We visualized unadjusted survival using Kaplan-Meier analysis, both for the entire cohort and for each risk category. We calculated the median overall survival estimate using the product-limit method for each patient age and comorbidity group, generating a summary figure and heat map colorized to identify 5-year and 10-year life expectancy thresholds. In cases where an age/comorbidity category had not reached a median overall survival estimate, the category was assigned 15 years for the purpose of visualizing survival. If there were insufficient patients in an age/comorbidity category, the value from the result from the last available comorbidity category for that age group was carried forward.
We fit Cox proportional hazards regression analysis using covariates of age (as a continuous variable) and the number of comorbidities. This same analysis was performed for subsets of patients with localized or metastatic disease. We then fit models for the entire cohort that added prostate cancer risk category at diagnosis. We additionally tested differences by adding race/ethnicity as a covariate. Finally, to test the performance of our life-expectancy model and heat map visual aid, we used Kaplan-Meier analyses to evaluate if the survival estimates stratified observed survival across each risk category. Then, we fit a Cox model using the median life expectancy estimate, derived from age and comorbidity data, as the sole variable. We evaluated the goodness of fit of each model using the concordance index (C-index).
We performed all statistical analysis within the VA Informatics and Computing Infrastructure (VINCI) platform using SAS Enterprise Guide v7 (Cary, NC) and figures were generated using JMP Pro v14 (Cary, NC).
3. Results:
Our analytic cohort included 145,678 patients with comprehensive oncologic data available, from a total of 181,009 patients diagnosed with prostate cancer between 2000–2015. The mean age of the cohort was 69.0 years (+/− 8.4). 69.4% of patients identified as white, 26.2% as Black and 1.1% were of other race/ethnicity. The mean number of comorbidities was 1.3 (+/− 1.2) (Table I). The mean follow-up was 7.5 years (+/− 4.4), and the median follow-up was 7.0 years (interquartile range 3.8 to 10.7).
Table I:
Demographics and clinical characteristics of men with prostate cancer receiving care in the VHA between 2000–2015.
| Patient Characteristics | N=145,678 |
|---|---|
| Age (mean +/− SD) | 69.0 +/− 8.4 |
| No. Comorbidities (mean +/− SD) | 1.3 +/− 1.2 |
| Race/Ethnicity (%) | |
| White | 69.4 |
| Black | 26.2 |
| Other | 1.1 |
| Missing | 3.3 |
| Prostate Cancer Risk Category (%) | |
| Low Risk | 45.7 |
| Intermediate Risk | 16.6 |
| High Risk | 29.3 |
| Locally Advanced | 1.3 |
| Metastatic | |
| Gleason Score (%) | |
| <=6 | 61.7 |
| 7 | 14.3 |
| >=8 | 22.2 |
| Missing | 1.8 |
| PSA (%) | |
| < 10 | 59.8 |
| 10–20 | 17.7 |
| >20 | 9.3 |
| Missing | 13.2 |
| Clinical T Stage (%) | |
| T1 | 58.2 |
| T2 | 32.6 |
| T3 | 3.2 |
| T4 | 1.4 |
| Unknown | 4.7 |
| Clinical N Stage (%) | |
| N0 | 87.9 |
| N1 | 2.5 |
| NX | 9.7 |
| Clinical M Stage (%) | |
| M0 | 86.8 |
| M1 | 7.1 |
| MX | 6.1 |
| Initial Treatment (%) | |
| Surgery | 18.0 |
| Radiotherapy | 31.8 |
| Conservative | 24.1 |
| Androgen Deprivation | 16.1 |
Increasing comorbidity and age were strongly associated with worse overall survival. Figure I illustrates the worsening survival with increasing age and comorbidity burden. Overall survival decreased with increasing prostate cancer risk category. The percentage of patients with more than 10-year survival was 68.7% for low risk disease, 56.4% for intermediate risk patients, 51.9% for high risk patients, 32.3% for patients with locally advanced disease and 9.9% for patients with metastatic disease. Importantly, within each risk and staging category, patient age and number of comorbidities remained associated with overall survival (Figure II).
Figure I:
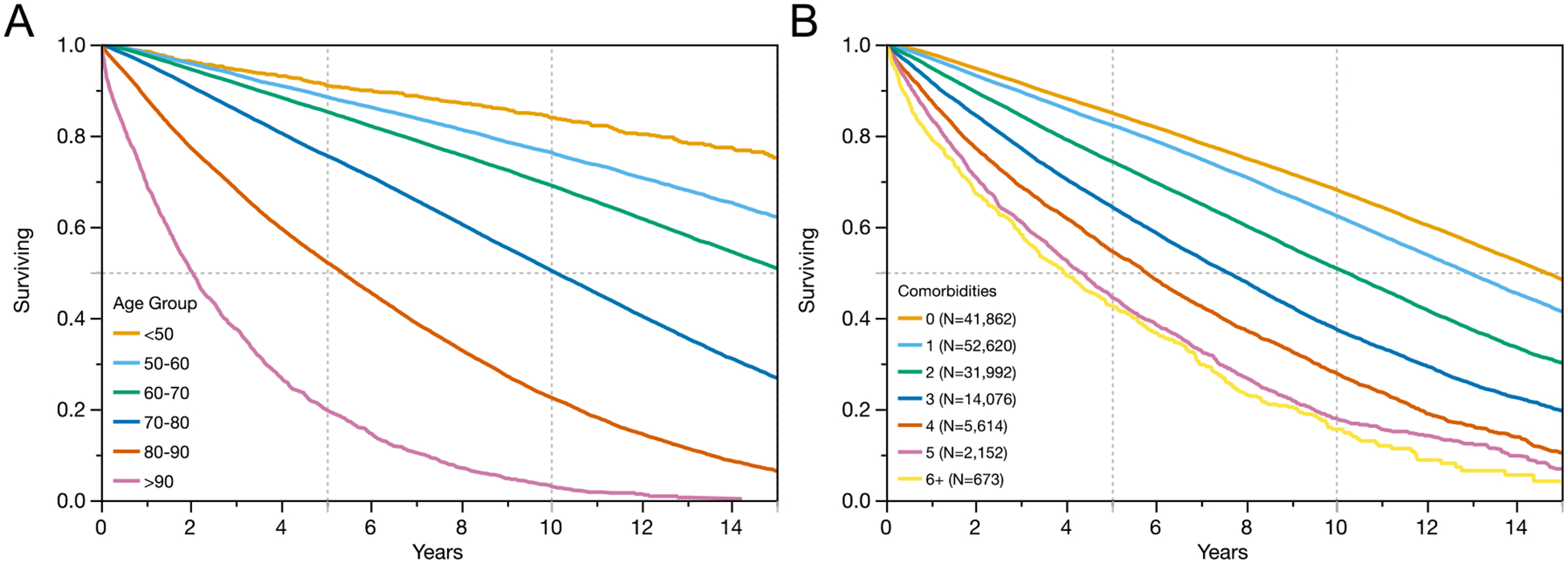
The importance of age (A) and number of comorbid conditions (B) at the time of prostate cancer diagnosis on overall survival among 145,678 Veterans receiving care in the VHA.
Figure II:
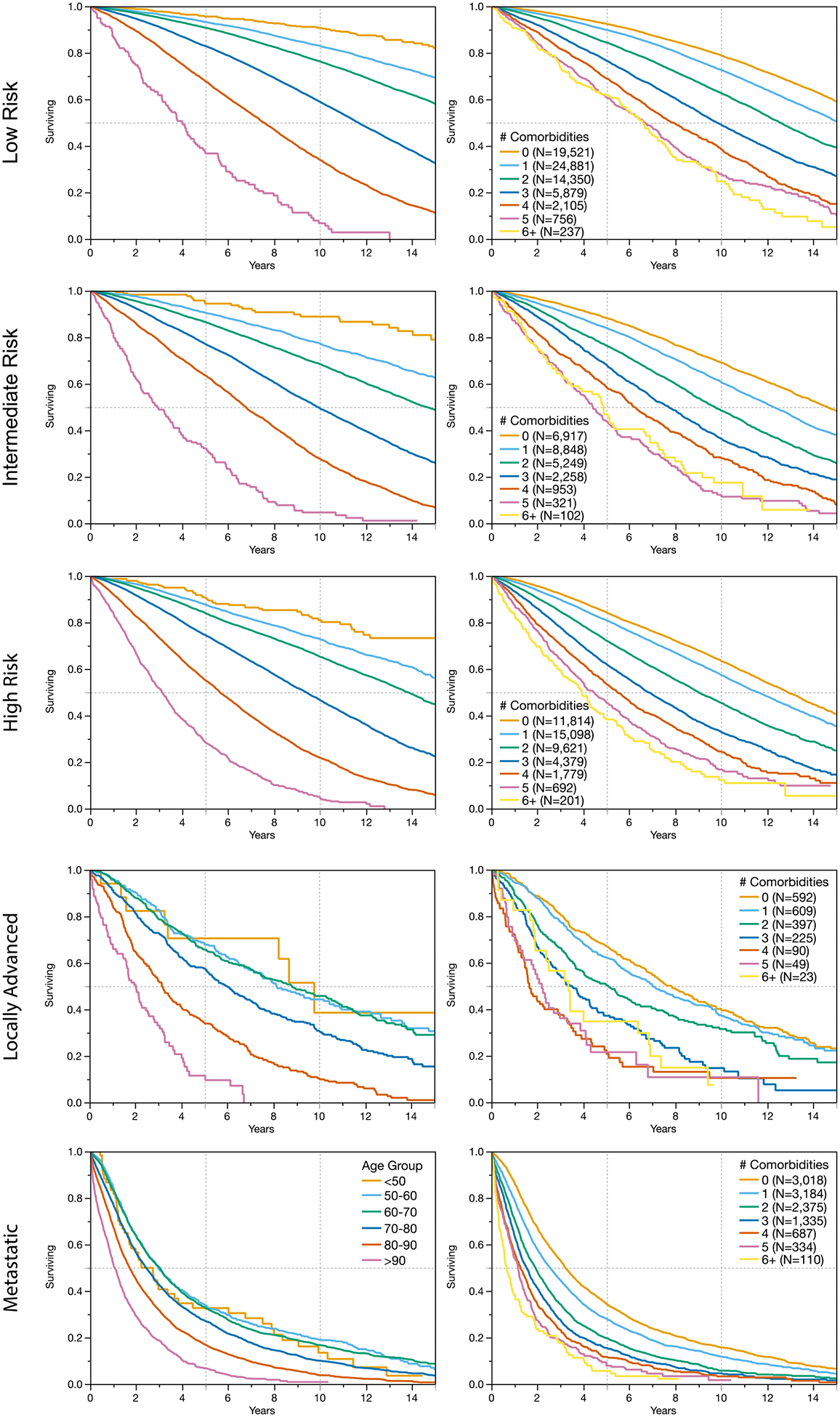
Increasing age and number of comorbidities is associated with worsening overall survival in patients with prostate cancer across D’Amico risk groups, as well as for patients with locally advanced disease (clinical staging N>0 or T4) and metastatic disease.
Increasing age (HR 1.39 per 5-year increase, 95% Confidence Interval [95%CI] 1.39 to 1.40) and comorbidity were shown to be independently associated with worse overall survival in multivariable proportional hazards models. The overall fit of this model including patient age and comorbidity was good (C-index 0.68, 95%CI 0.676 to 0.681) (Table II).
Table II:
Multivariable proportional hazard models demonstrating the association between clinical factors and overall survival. Model I includes patient age and the number of comorbidities. Model II adds the prostate cancer risk group / stage information. Finally, model III incorporates race/ethnicity. The fit of each model is included as the C-index.
| Model I Age and Comorbidity | Model II Age, Comorbidity, and Risk Group | Model III Age, Comorbidity, Risk Group, and Race/ethnicity | |
|---|---|---|---|
| HR (CI) | HR (CI) | HR (CI) | |
| Age (per 5 year increase) Number of Comorbidities | 1.39 (1.39–1.40) | 1.32 (1.31–1.33) | 1.40 (1.39–1.40) |
| 0 | Ref | Ref | Ref |
| 1 | 1.15 (1.12–1.17) | 1.20 (1.17–1.22) | 1.20 (1.17–1.22) |
| 2 | 1.53 (1.50–1.57) | 1.60 (1.57–1.64) | 1.60 (1.56–1.64) |
| 3 | 2.05 (1.99–2.10) | 2.12 (2.07–2.18) | 2.12 (2.06–2.18) |
| 4 | 2.67 (2.57–2.77) | 2.69 (2.59–2.79) | 2.68 (2.59–2.78) |
| 5 | 3.38 (3.20–3.57) | 3.38 (3.20–3.57) | 3.37 (3.19–3.56) |
| 6 | 3.64 (3.32–3.99) | 3.55 (3.24–3.89) | 3.54 (3.23–3.88) |
| 7+ | 6.01 (5.27–6.85) | 5.46 (4.79–6.23) | 5.45 (4.78–6.22) |
| Risk Group | |||
| Low | Ref | Ref | |
| Intermediate | 1.31 (1.28–1.34) | 1.31 (1.28–1.34) | |
| High | 1.49 (1.46–1.52) | 1.48 (1.46–1.51) | |
| Locally Advanced | 2.72 (2.56–2.88) | 2.72 (2.56–2.88) | |
| Metastatic | 5.94 (5.79–6.10) | ||
| Race/ethnicity | Ref | ||
| White | 5.92 (5.77–6.08) | ||
| Black | 1.03 (1.01–1.05) | ||
| Other | 0.88 (0.81–0.96) | ||
| Missing | 0.95 (0.91–1.00) | ||
| C-index | 0.678 | 0.729 | 0.738 |
We generated visualizations of the median overall survival by patient age and number of comorbidities at diagnosis for patients diagnosed with localized disease (Figures III) and metastatic disease (Figure IV), as well as for patients treated with either surgery or radiation (Supplemental Figure I). The heat map’s green coloring indicates a greater than 10-year median life expectancy while red coloring indicates patient categories with less than 5-year median life expectancy. In multivariable proportional hazards models for patients presenting with metastatic disease, we found that age (HR 1.11, 95%CI 1.10 to 1.12) and number of comorbidities remained predictive of survival (C-Index 0.6788, 95%CI 0.6764 to 0.6812).
Figure III:
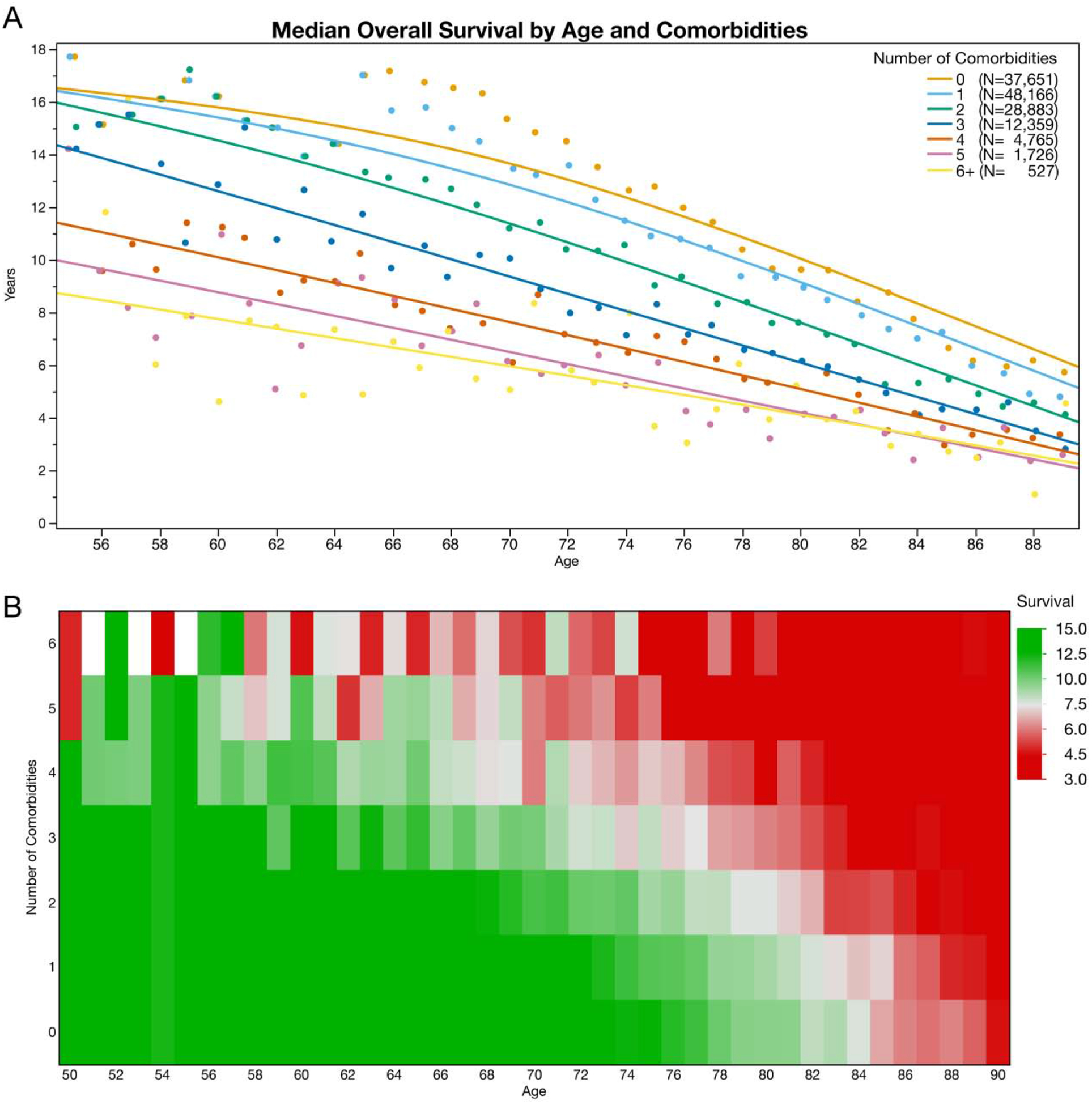
Median overall survival for Veterans diagnosed with localized prostate cancer in the VHA. A) Median estimated overall survival is stratified by patient age and number of comorbidities. B) Median overall survival is represented in a heat map with green coloring illustrating estimated survival of greater than 10 years, while red coloring illustrates estimated median survival of less than 5 years.
Figure IV:
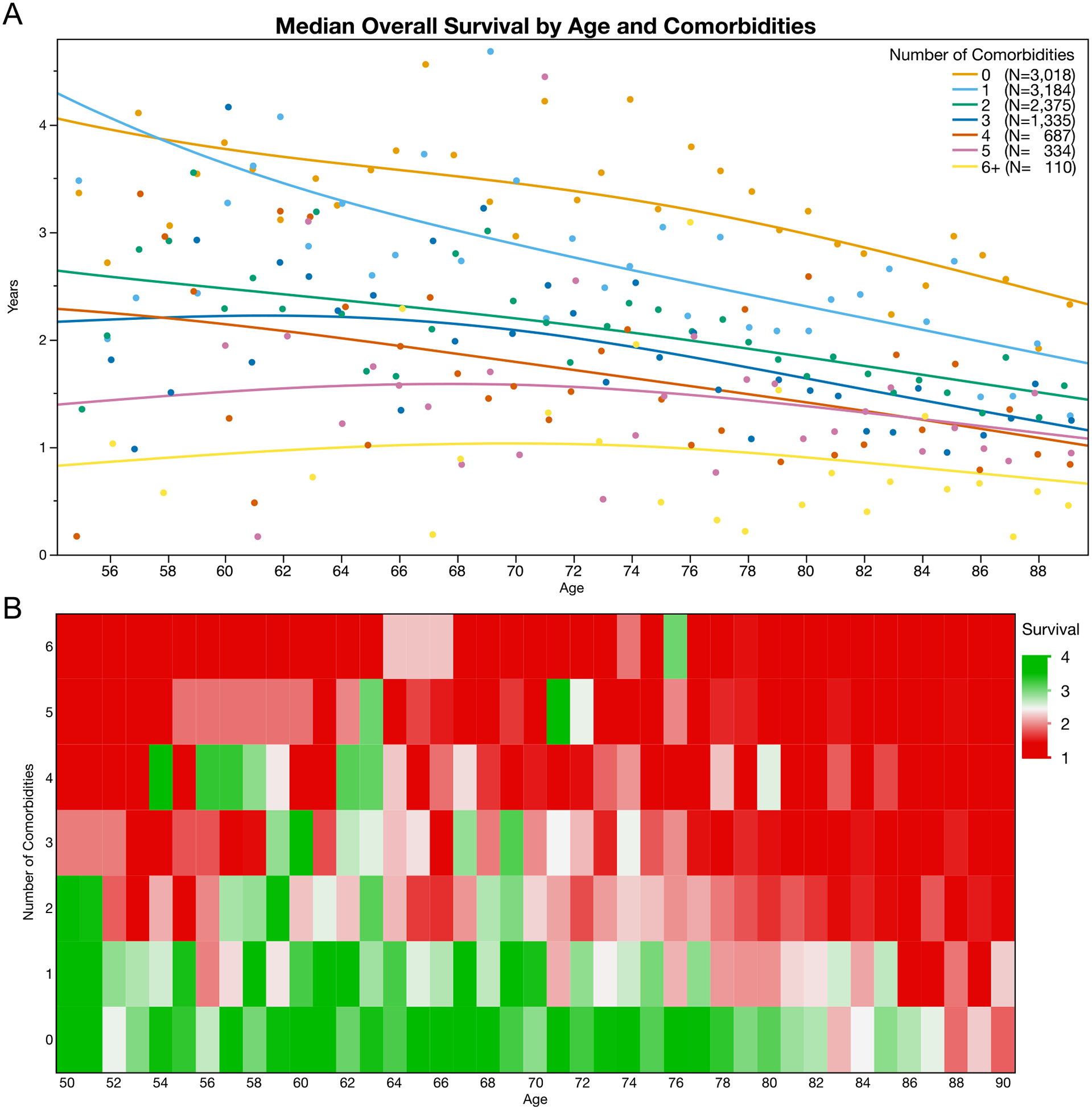
Median overall survival is poor among Veterans with metastatic prostate cancer at the time of diagnosis. Even among patients with metastatic disease, median estimated life expectancy decreases with increasing age and increasing number of comorbid conditions. (A) The median overall survival is shown by age and number of comorbidities. (B) A heatmap illustrates median life expectancy with red coloring representing less than 2 years and green coloring representing four or more years.
The median overall survival estimates were significantly associated with overall survival for patients across all risk categories. In Figure V, the calibration of these life expectancy estimates is shown by comparing the observed survival for patients stratified by their life expectancy estimate. The performance of the life expectancy estimates alone (C-index of 0.676, 95%CI 0.674 to 0.679) was similar to that of models that included each patient’s age and number of comorbidities.
Figure V:
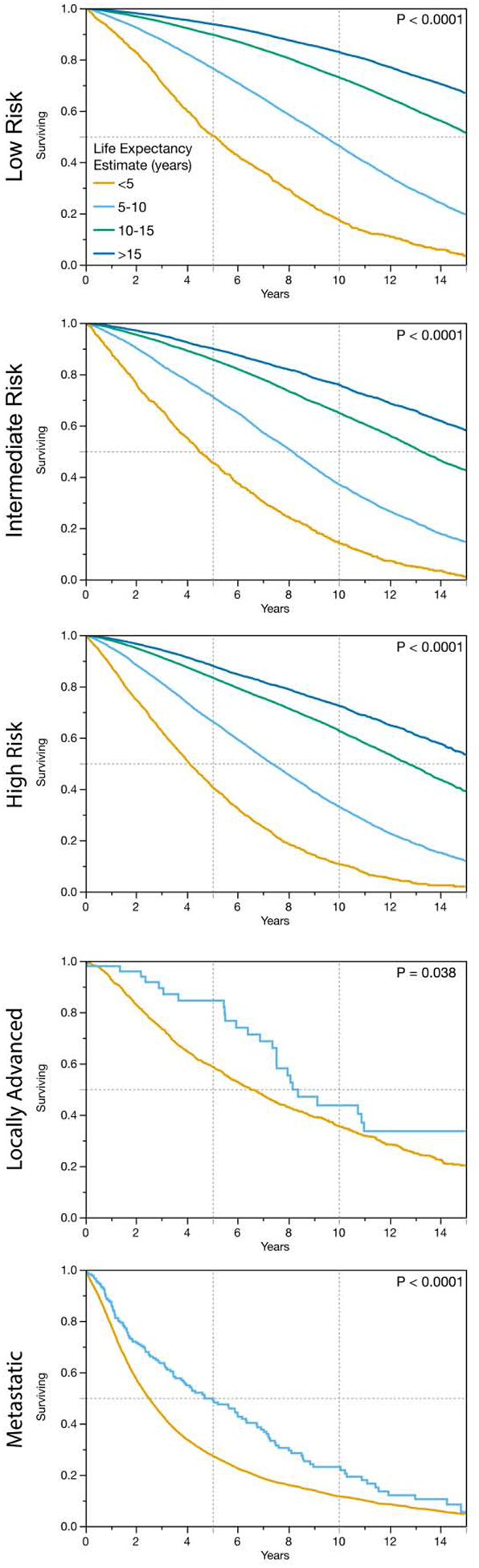
The performance of the life expectancy estimate is compared with observed survival data demonstrating good calibration of these estimates across all prostate cancer risk categories.
The median life expectancy by age and number of comorbidities for the entire cohort is further visualized in Supplemental Figure II. Given that patients of Black race/ethnicity represented more than one quarter of the cohort, we generated separate survival estimates for this cohort (Supplemental Figure III). Black race/ethnicity was associated with slightly worse survival (HR 1.03, 95%CI 1.01 to 1.05) after adjusting for age, comorbidity, and prostate cancer risk group. However, after further adjusting for the initial treatment received, Black race/ethnicity was no longer significantly associated with overall survival (HR 1.05 Black compared to white, 95%CI 0.99 to 1.12).
4. Discussion:
In this study, we created estimates of life expectancy among nearly 150,000 Veterans newly diagnosed with prostate cancer using real-world survival data from the VHA. Despite clinical guidelines supporting the use of life expectancy estimates, it has been consistently demonstrated that few clinicians take advantage of these tools to calculate life expectancy in routine practice. Kim et al. found that only one fourth of US Urologists and Radiation Oncologists use life expectancy prediction tools[16], which is consistent with surveys of European clinicians[17].To encourage use in a clinic setting, we avoided laborious inputs, requiring only the patient’s age, the number of comorbid diagnoses (without assigning disease specific weights), and limited stage information.
These data underscore the importance of patient age and comorbidity when estimating life expectancy among patients with prostate cancer. Veterans with prostate cancer have been found to have unique sociodemographic and oncologic risk factors that may be associated with worse survival outcomes[18]. Poor overall survival outcomes were demonstrated in the PIVOT trial (which primarily enrolled Veterans receiving treatment in the VHA) when compared with other prospective prostate cancer trials and cohorts[8, 9]. Therefore, we present a tool to estimate life expectancy and guide initial treatment decisions in this patient population with a high burden of comorbidity and competing risks of mortality, where other tools for estimating life expectancy might not apply. This tool complements the Prostate Cancer Comorbidity Index (PCCI) created by Daskivich et al[19], which was also derived using data from Veterans receiving care in the VHA. The PCCI score incorporates administrative claims data and assigns weights to each comorbid condition and is ideally suited for health services research. Here we show that patient age and an unweighted count of comorbid conditions can be used to provide life expectancy estimates in a busy clinic environment.
These estimates also support prior reports that many patients with limited life expectancy receive aggressive therapy[2, 20]. Loeb et al. have demonstrated that the adoption of conservative management of patients with low-risk prostate cancer has continued to increase throughout the VHA[21]. However, additional work needs to be done to limit overtreatment of patients – particularly that of patients with multimorbidity. This tool can help initiate a conversation with patients to balance treatment options in patients with limited life expectancy.
We also calculate life expectancy estimates in a large population of patients of Black race/ethnicity. The modest effect of race/ethnicity on survival did not meet our statistical significance threshold after adjusting for initial treatment type. This supports previous work demonstrating that race/ethnicity may not be a prognostic factor for mortality, specifically in an integrated health care system[22, 23]. These data also provide additional information to support treatment decisions among Black men, who may be less likely to select active surveillance for low-risk prostate cancer[24].
Additionally, our data provides “real world” survival outcomes among a large cohort of Veterans with locally-advanced and metastatic prostate cancer at the time of diagnosis. Most existing life expectancy estimates for patients with metastatic prostate cancer have been derived from clinical trial outcomes which are known to enroll patients with fewer comorbidities and better performance status, and may not reflect those seen in clinics in the VHA[25]. Equally important, we demonstrate that even among patients with clinically significant prostate cancer, age and comorbidity remain important components of survival estimates. This information can be used to better understand the potential benefits in reducing morbidity in men with aggressive prostate cancer. For Veterans with locally advanced disease, only one in three patients survive 10 years, while only one in four Veterans with metastatic disease at presentation survive beyond 5 years.
Our study has notable limitations. This life expectancy tool is intended for Veterans with prostate cancer, and the generalizability of these life expectancy estimates to men outside of the VHA is unknown. Notably, previous studies have demonstrated that after adjusting for known comorbidity burden, cancer patients treated in the VHA have been shown to have equal or better cancer-specific survival and all-cause mortality than patients treated in the community[1, 26]. Despite our focus on the Veteran population, the methods used provide a practical framework for incorporating real world data from an integrated health care system to generate life expectancy tools specific to other relevant populations. Additionally, we opted to limit the included comorbidities to diseases in the Charlson Comorbidity Index, a validated and common comorbidity instrument. However, this approach does not include several common comorbidities and may underestimate the total comorbidity burden for each patient. This tool was designed for use in the clinic and does not assign weights for each comorbidity. For researchers interested in more granular estimates that incorporate disease severity, we have also developed and validated the PCCI to assign disease-specific weights when estimating life expectancy using administrative claims data[27]. Finally, any life expectancy estimate model may not “calibrate” to individual patients and necessitates caution when used in clinical settings[28]. As such, we believe that life expectancy estimates can be a starting point during treatment discussion but may not capture all variables and patient preferences relevant to treatment choices.
Despite these limitations, our study has important strengths. This simple life expectancy tool can be implemented in clinical practice and a useful reference for clinicians and patients receiving prostate cancer care in the VHA. This approach has the potential to better inform patients making prostate cancer treatment decisions, and to reduce overtreatment of prostate cancer among US Veterans receiving care in the VHA. Clinicians can use these comorbidity-based life expectancy estimates to encourage positive health behaviors that may provide a long-term benefit to patients. In the future, researchers can apply these tools to better understand the comparative effectiveness of treatments in cohorts with multimorbidity or limited life expectancy. For example, these tools could place long-term survival for patients included in the PIVOT trial in context. Finally, this method can then be applied to other malignancies to improve life expectancy estimates across the VHA system, and similar approaches can be applied to other large population-based cohorts.
5. Conclusions:
Life expectancy estimates are essential to providing individualized prostate cancer treatment. We demonstrate life expectancy estimates using patient age and comorbidity specific to Veterans receiving care in the VHA. We provide these estimates for clinicians caring for Veterans as a reference for use in routine clinical care.
Supplementary Material
Supplement Figure I: Median overall survival following surgery (A) and radiation (B) represented as a heat map with green coloring illustration estimated survival of greater than 10 years, while red coloring illustrates estimated median survival of less than 5 years.
Supplemental Figure II: Median overall survival for all Veterans diagnosed with prostate cancer in the VHA. A) Median estimated overall survival is stratified by patient age and number of comorbidities. B) Median overall survival is represented in a heat map with green coloring illustrating estimated survival of greater than 10 years, while red coloring illustrates estimated median survival of less than 5 years.
Supplement Figure III: Median overall survival for Veterans of Black race/ethnicity diagnosed with prostate cancer in the VHA. (A) Median estimated overall survival is stratified by patient age and number of comorbidities. (B) Median overall survival is represented in a heat map with green coloring illustrating estimated survival of greater than 10 years, while red coloring illustrates estimated median survival of less than 5 years.
Highlights:
The authors have generated life expectancy estimates using patient age and comorbidity burden for Veterans diagnosed with prostate cancer
These life expectancy estimates can be quickly calculated in a busy clinic environment
These life expectancy estimates, specific to Veterans, calibrate well with observed survival across prostate cancer risk categories
Life expectancy estimates can be used to inform treatment decisions, and may minimize the risk of under- and over-treatment of prostate cancer
Acknowledgements / Funding:
This work was supported in part by VA Merit Review (I01 HX0021261 to JL), National Cancer Institute (R37 CA222885 to TAS) from the United States (U.S.). This work was also supported by Career Development Award (K08 CA230155 to TJD) from the National Cancer Institute. Department of Veterans Affairs Health Services Research and Development Service work was supported using resources and facilities at the VA Informatics and Computing Infrastructure (VINCI), VA HSR RES 13–457. The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Landrum MB, Keating NL, Lamont EB, Bozeman SR, Krasnow SH, Shulman L, et al. Survival of older patients with cancer in the Veterans Health Administration versus fee-for-service Medicare. J Clin Oncol. 2012;30:1072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Daskivich TJ, Chamie K, Kwan L, Labo J, Palvolgyi R, Dash A, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117:2058–66. [DOI] [PubMed] [Google Scholar]
- [3].Walz J, Gallina A, Perrotte P, Jeldres C, Trinh QD, Hutterer GC, et al. Clinicians are poor raters of life-expectancy before radical prostatectomy or definitive radiotherapy for localized prostate cancer. BJU Int. 2007;100:1254–8. [DOI] [PubMed] [Google Scholar]
- [4].Wilson JR, Clarke MG, Ewings P, Graham JD, MacDonagh R. The assessment of patient life-expectancy: how accurate are urologists and oncologists? BJU Int. 2005;95:794–8. [DOI] [PubMed] [Google Scholar]
- [5].Allen LA, Yager JE, Funk MJ, Levy WC, Tulsky JA, Bowers MT, et al. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. JAMA. 2008;299:2533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kent M, Vickers AJ. A systematic literature review of life expectancy prediction tools for patients with localized prostate cancer. J Urol. 2015;193:1938–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fontanella P, Benecchi L, Grasso A, Patel V, Albala D, Abbou C, et al. Decision-making tools in prostate cancer: from risk grouping to nomograms. Minerva Urol Nefrol. 2017;69:556–66. [DOI] [PubMed] [Google Scholar]
- [8].Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barbosa PV, Thomas IC, Srinivas S, Buyyounouski MK, Chung BI, Chertow GM, et al. Overall Survival in Patients with Localized Prostate Cancer in the US Veterans Health Administration: Is PIVOT Generalizable? Eur Urol. 2016;70:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fihn SD, Francis J, Clancy C, Nielson C, Nelson K, Rumsfeld J, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33:1203–11. [DOI] [PubMed] [Google Scholar]
- [11].Mittakanti HR, Thomas IC, Shelton JB, Makarov DV, Skolarus TA, Cooperberg MR, et al. Accuracy of Prostate-Specific Antigen Values in Prostate Cancer Registries. J Clin Oncol. 2016;34:3586–7. [DOI] [PubMed] [Google Scholar]
- [12].Guo DP, Thomas IC, Mittakanti HR, Shelton JB, Makarov DV, Skolarus TA, et al. The Research Implications of Prostate Specific Antigen Registry Errors: Data from the Veterans Health Administration. J Urol. 2018;200:541–8. [DOI] [PubMed] [Google Scholar]
- [13].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- [14].Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. [DOI] [PubMed] [Google Scholar]
- [16].Kim SP, Karnes RJ, Nguyen PL, Ziegenfuss JY, Han LC, Thompson RH, et al. Clinical implementation of quality of life instruments and prediction tools for localized prostate cancer: results from a national survey of radiation oncologists and urologists. J Urol. 2013;189:2092–8. [DOI] [PubMed] [Google Scholar]
- [17].Bhatt NR, Davis NF, Breen K, Flood HD, Giri SK. Life expectancy calculation in urology: Are we equitably treating older patients? Cent European J Urol. 2017;70:368–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cooperberg M, Lubeck DP, Penson D, Mehta SS, Carroll PR, Kane CJ. Sociodemographic and clinical risk characteristics of patients with prostate cancer within the Veterans Affairs health care system: data from CaPSURE. J Urol. 2003;170:905–8. [DOI] [PubMed] [Google Scholar]
- [19].Daskivich TJ, Kwan L, Dash A, Saigal C, Litwin MS. An Age Adjusted Comorbidity Index to Predict Long-Term, Other Cause Mortality in Men with Prostate Cancer. J Urol. 2015;194:73–8. [DOI] [PubMed] [Google Scholar]
- [20].Daskivich TJ, Lai J, Dick AW, Setodji CM, Hanley JM, Litwin MS, et al. Variation in treatment associated with life expectancy in a population-based cohort of men with early-stage prostate cancer. Cancer. 2014;120:3642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Loeb S, Byrne N, Makarov DV, Lepor H, Walter D. Use of Conservative Management for Low-Risk Prostate Cancer in the Veterans Affairs Integrated Health Care System From 2005–2015. JAMA. 2018;319:2231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Graham-Steed T, Uchio E, Wells CK, Aslan M, Ko J, Concato J. ‘Race’ and prostate cancer mortality in equal-access healthcare systems. The American journal of medicine. 2013;126:1084–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dess RT, Hartman HE, Mahal BA, Soni PD, Jackson WC, Cooperberg MR, et al. Association of Black Race With Prostate Cancer-Specific and Other-Cause Mortality. JAMA Oncol. 2019;5:975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Butler S, Muralidhar V, Chavez J, Fullerton Z, Mahal A, Nezolosky M, et al. Active Surveillance for Low-Risk Prostate Cancer in Black Patients. N Engl J Med. 2019;380:2070–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Al-Refaie WB, Vickers SM, Zhong W, Parsons H, Rothenberger D, Habermann EB. Cancer trials versus the real world in the United States. Ann Surg. 2011;254:438–42; discussion 42–3. [DOI] [PubMed] [Google Scholar]
- [26].Keating NL, Landrum MB, Lamont EB, Bozeman SR, Krasnow SH, Shulman LN, et al. Quality of care for older patients with cancer in the Veterans Health Administration versus the private sector: a cohort study. Ann Intern Med. 2011;154:727–36. [DOI] [PubMed] [Google Scholar]
- [27].Daskivich TJ, Thomas IC, Luu M, Shelton JB, Makarov DV, Skolarus TA, et al. External Validation of the Prostate Cancer Specific Comorbidity Index, A Claims Based Tool for the Prediction of Life Expectancy in Men with Prostate Cancer. J Urol. 2019:101097JU0000000000000287. [DOI] [PubMed] [Google Scholar]
- [28].Leppert JT, Asch SM, Bergman J. Ethical Pitfalls When Estimating Life Expectancy for Patients with Prostate Cancer. J Urol. 2018;200:709–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure I: Median overall survival following surgery (A) and radiation (B) represented as a heat map with green coloring illustration estimated survival of greater than 10 years, while red coloring illustrates estimated median survival of less than 5 years.
Supplemental Figure II: Median overall survival for all Veterans diagnosed with prostate cancer in the VHA. A) Median estimated overall survival is stratified by patient age and number of comorbidities. B) Median overall survival is represented in a heat map with green coloring illustrating estimated survival of greater than 10 years, while red coloring illustrates estimated median survival of less than 5 years.
Supplement Figure III: Median overall survival for Veterans of Black race/ethnicity diagnosed with prostate cancer in the VHA. (A) Median estimated overall survival is stratified by patient age and number of comorbidities. (B) Median overall survival is represented in a heat map with green coloring illustrating estimated survival of greater than 10 years, while red coloring illustrates estimated median survival of less than 5 years.


