Poor maternal environments, such as under- or over-nutrition and obesity, can increase the risk for the development of obesity, type 2 diabetes, and cardiovascular disease in offspring1–9. Recent studies in animal models have shown that maternal exercise before and during pregnancy abolishes the age-related development of impaired glucose metabolism10–15, decreased cardiovascular function16, and increased adiposity11,15; however, the underlying mechanisms for maternal exercise to improve offspring health have not been identified. Here, we identify an exercise-induced increase in the oligosaccharide 3’siallylactose (3’SL) in milk in humans and mice, and show that the beneficial effects of maternal exercise on mice offspring metabolic health and cardiac function are mediated by 3’SL. In global 3’SL knockout mice (3’SL−/−), maternal exercise training failed to improve offspring metabolic health or cardiac function in mice. There was no beneficial effect of maternal exercise on wild-type (WT) offspring who consumed milk from exercise-trained 3’SL−/− dams, whereas supplementing 3’SL during lactation to WT mice improved metabolic health and cardiac function in offspring during adulthood. Importantly, supplementation of 3’SL negated the detrimental effects of a high-fat diet on body composition and metabolism. This study reveals a critical role for the oligosaccharide 3’SL in milk to mediate the effects of maternal exercise on offspring health. 3’SL supplementation is a potential therapeutic approach to combat the development of obesity, type 2 diabetes, and cardiovascular disease.
Six-week-old C57BL/6 female mice were subdivided into singular housing in standard cages (SED) or in cages containing an exercise wheel (TRAIN). The exercise training paradigm consisted of voluntary wheel-running two weeks prior to conception and throughout the three weeks of gestation. All mothers were provided a high-fat diet for two weeks prior to conception and throughout gestation as previous studies have indicated a more pronounced effect of maternal exercise on the metabolic health of female offspring in the presence of a maternal high-fat diet11,15. To isolate the effects of exercise-trained milk on metabolic health and cardiac function in adult offspring, a cross-fostering model was utilized. Offspring are referred to by the treatment of the mothers during gestation/birth followed by treatment of mothers during lactation (i.e. BIRTH MOM - FOSTER MOM). Offspring from sedentary dams were cross-fostered immediately after birth with exercise-trained dams (SED-TRAIN), and offspring from exercise-trained dams were cross-fostered with sedentary dams (TRAIN-SED). At 52 weeks of age, SED-TRAIN male offspring had reduced body weight and % fat mass (Figure 1a–c), improved glucose tolerance (Figure 1d), decreased fasting insulin (Figure 1e), improved insulin tolerance (Figure 1f), and enhanced ejection fraction (Figure 1g; Supplementary Table 1) compared to TRAIN-SED offspring. Thus, fostering of male offspring from sedentary dams with dams that were exercise-trained results in improved metabolic and cardiac health, highlighting the importance of exercise-induced adaptations to breastmilk as a critical mediator to confer the beneficial effects of maternal exercise.
Figure 1. Exercise-induced adaptations to milk improve metabolic health and cardiac function in offspring.

(a) Body weight over lifespan and (b) at 52 wks, (c)% fat mass, (d) glucose tolerance test (GTT) area under the curve (AUC), (e) fasting insulin, (f) insulin tolerance (ITT), and (g) ejection fraction in male cross-fostered offspring from high-fat fed Sedentary dams raised by high-fat fed Sedentary dams (SED-SED), high-fat fed Trained dams raised by high-fat fed Trained dams (TRAIN-TRAIN), high-fat fed Trained dams raised by high-fat fed Sedentary dams (TRAIN-SED), or high-fat fed Sedentary dams raised by high-fat fed Trained dams (SED-TRAIN). (h) Body weight over lifespan and (i) at 52 wks, (j) % fat mass, (k) GTT AUC, (l) fasting insulin, (m) ITT, and (n) ejection fraction in female cross-fostered offspring. Data are expressed as the mean ± SEM (for males, SED-SED n=12; TRAIN-TRAIN n=9; TRAIN-SED n=8; SED-TRAIN n=9 for Figures a-f; SED-SED n=8; TRAIN-TRAIN n=10; TRAIN-SED n=8; SED-TRAIN n=8 for Figure g. For females, SED-SED n=12; TRAIN-TRAIN n=8; TRAIN-SED n=12; SED-TRAIN n=10 for Figures h-m; SED-SED n=8; TRAIN-TRAIN n=9; TRAIN-SED n=8; SED-TRAIN n=9 for Figure n). Two-way ANOVA was used for a, d, f, h, k, and m with Tukey’s multiple comparisons tests; one-way ANOVA was used for b, c, e, g, i, j, l, and n with Tukey’s multiple comparisons tests. Asterisks represent differences between SED-SED and all other groups (*P < 0.05; **P < 0.01; ***P < 0.001); # represents differences between TRAIN-SED and SED-TRAIN; $ represents differences between TRAIN-TRAIN and SED-TRAIN ($P < 0.05).
To confirm that the mechanism for improved offspring health was due to exercise-induced adaptations to the milk and not the act of cross-fostering itself, offspring from sedentary dams were cross-fostered with sedentary dams (SED-SED), and offspring from trained dams were cross-fostered with other trained dams (TRAIN- TRAIN). Male TRAIN-TRAIN offspring had decreased body weight and % fat mass (Figure 1a–c), improved glucose tolerance, decreased fasting insulin, improved insulin tolerance, and enhanced ejection fraction (Figure 1d–g; Supplementary Table 1) compared to SED-SED offspring. Maternal exercise improved glucose tolerance in TRAIN-TRAIN, TRAIN-SED, and SED-TRAIN offspring, but the greatest improvement in glucose tolerance was in the SED-TRAIN offspring, suggesting that some of the beneficial effects of maternal exercise are conferred via breastmilk. Cardiac function was improved in both the TRAIN-TRAIN and SED-TRAIN offspring, indicating the importance of exercise-induced adaptations to breastmilk to improve cardiac function in offspring.
Interestingly, and similar to previous studies11,15, we find that the effects of maternal exercise on offspring metabolic health are more pronounced in male vs. female offspring. Female SED-TRAIN offspring had reduced body weight and % fat mass (Figure 1h–j) and improved glucose tolerance at 52 weeks of age (Figure 1k) compared to TRAIN- SED offspring. Fasting insulin and insulin tolerance were not altered in TRAIN-SED or SED-TRAIN offspring (Figure 1l–m). Female SED-TRAIN offspring had better cardiac function than TRAIN-SED at 52 weeks of age (Figure 1n; Supplementary Table 2). As with the males, we determined if the improved health of female offspring was due to the cross-fostering itself or exercise-induced adaptations to milk. There was no difference between SED-SED or TRAIN-TRAIN female offspring in body weight or % fat mass (Figure 1h–j). TRAIN-TRAIN female offspring had improved glucose tolerance and decreased fasting insulin compared to SED-SED female offspring (Figure 1k–l), but there was no effect on insulin tolerance (Figure 1m) or ejection fraction (Figure 1n; Supplementary Table 1). These cross-foster experiments demonstrate that the exercise-induced adaptations to milk are important and may confer the beneficial effects of maternal exercise on body composition, glucose tolerance, and cardiac function in offspring.
We next investigated which components in breastmilk mediate the observed effects. Human milk oligosaccharides (HMOs) are present at a concentration of 5–15% in humans and rodents, but comprise less than 0.5% of bovine milk, which is the basis for most infant formula17. Milk oligosaccharides are unique complex glycans present in the milk of all mammals. All milk oligosaccharides carry lactose at the reducing end, which is synthesized only in the mammary gland and only during lactation and late pregnancy. Lactose synthesis requires the expression of alpha-lactalbumin which serves as a co-factor to a galactosyltransferase in the Golgi that shifts its substrate specificity in the presence of alpha-lactalbumin to use glucose as an acceptor. Glucose and galactose together form lactose, which is then extended and modified to generate milk oligosaccharides. Because lactose synthesis is a requirement, milk oligosaccharides are only synthesized in the mammary gland and only during lactation18. Human milk contains more than 150 different oligosaccharides, while bovine milk contains ~40. In contrast, mouse milk contains only two oligosaccharides, primarily 3’-sialyllactose (3’SL) and to a lesser extent 6’-sialyllactose – both of which are also present in human and bovine milk17. 3’SL is the dominant sialylated milk oligosaccharide in human milk, and is the predominant oligosaccharide in mouse milk, accounting for 90–95% of the HMO content, while its structural isomer, 6’SL, accounts for the other ~5–10%. Little is known about how factors such as maternal diet, lifestyle, or activity influence HMO composition, and the role that these oligosaccharides play in metabolic health or cardiac function of offspring has not been identified.
To determine if maternal physical activity correlated with the concentration of specific HMOs in human milk, average activity and steps per day were recorded during pregnancy in 139 adult women and correlated to HMO levels at 2 months postpartum (Supplementary Table 3). 3’SL content in the milk was positively correlated to average activity and steps per day, and negatively correlated to BMI (Figure 2a–c). Since 3’SL was positively correlated with activity and steps per day, and negatively correlated with BMI, we performed multiple regression analyses to determine if the increase in 3’SL was confounded by BMI. These analyses revealed that even when BMI is accounted for, 3’SL is still significantly correlated with steps per day and activity (Supplementary Table 4). There was no effect of average activity per day or BMI to affect 6’SL, and average steps per day were negatively correlated with 6’SL in the milk (Extended Data Figure 1a–c). These data indicate a positive correlation among physical activity and 3’SL content in milk in humans, regardless of BMI. To determine if exercise affected HMO composition in a mouse, we performed high performance liquid chromatography (HPLC) on mouse milk from dams fed a chow or high-fat diet and determined the effects of both diet and exercise on 3’SL and 6’SL. Similar to what was observed in humans, a maternal high-fat diet reduced 3’SL while maternal exercise increased 3’SL in milk, regardless of diet (Figure 2d). The concentration of 6’SL was not changed in response to diet or exercise (Extended Data Figure 1d).
Figure 2. Maternal exercise increases 3’SL in milk in humans and rodents; 3’SL is required for the beneficial effects of maternal exercise to mediate improvements in offspring metabolic health and cardiac function.
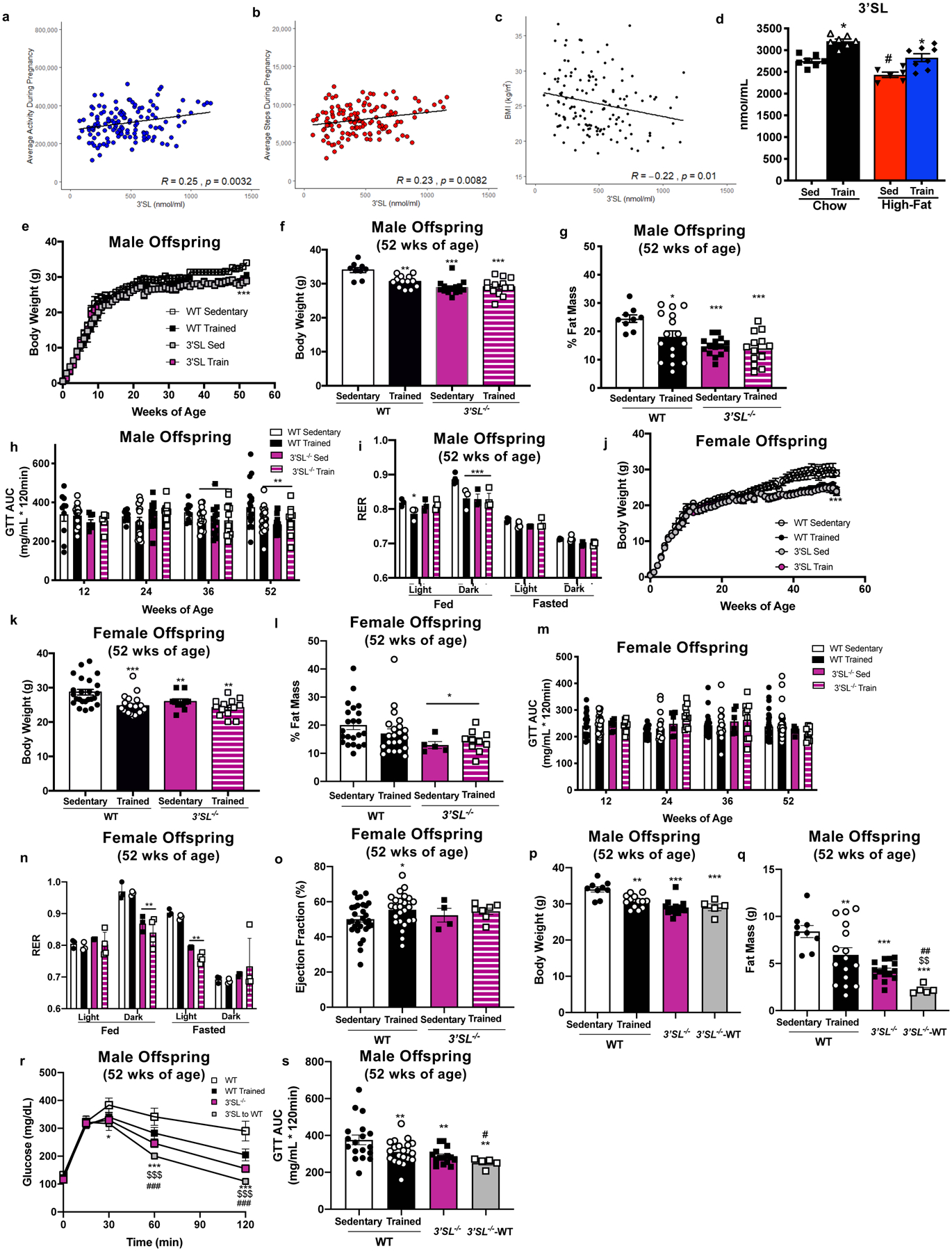
3’SL in human participants correlated to (a) average activity during pregnancy, (b) average steps per day during pregnancy, and (c) BMI; N=139, data are analyzed by Pearson’s correlation. (d) 3’SL in breastmilk from sedentary and exercise-trained chow-fed and high-fat fed dams. Data are expressed as the mean ± SEM (Sed Chow n=7; Train Chow n=7; Sed High-Fat n=6; Train High-Fat n=8). Asterisks represent differences compared with Sedentary Chow vs. Exercise-trained Chow (*P < 0.05); Sedentary High-Fat vs. Exercise-trained High-fat (#P < 0.05). (e) Body weight over lifespan and (f) at 52 wks, (g) % fat mass, (h) GTT AUC, and (i) respiratory exchange ratio (RER) in wild-type or 3’SL−/− male offspring. Data are expressed as the mean ± SEM (WT Sed n=9; WT Train n=16; 3’SL−/− Sed n=13; 3’SL−/− Train n=13 for Figures e-h; n=3 per group for Figure i). (j) Body weight over lifespan and (k) at 52 wks, (l) % fat mass, (m) GTT AUC, (n) respiratory exchange ratio (RER) and (o) ejection fraction in wild-type or 3’SL−/− female offspring. Data are expressed as the mean ± SEM (WT Sed n=24; WT Train n=22; 3’SL−/− Sed n=5; 3’SL−/− Train n=10 for Figures j-m; n=3 per group for Figure n; WT Sed n=30; WT Train n=25; 3’SL−/− Sed n=5; 3’SL−/− Train n=7 for Figure o.) Asterisks represent differences compared to WT Sedentary offspring (*P < 0.05; **P < 0.01; ***P < 0.001). (p) Body weight, (q) fat mass, (r) glucose tolerance excursion curve, and (s) GTT AUC in male offspring. Data are expressed as the mean ± SEM (WT Sed n=9; WT Train n=13; 3’SL−/− n=15; 3’SL−/− raised by WT (3’SL−/− -WT) n=5 for Figures p-s). Asterisks represent differences compared to WT Sedentary offspring (*P < 0.05; **P < 0.01; ***P < 0.001); $ represents differences compared to wild-type trained offspring ($ $P<0.01); and # represents differences compared to 3’SL−/− offspring (##P<0.01). Two-way ANOVA was used for e, h, i, j, m, n and r with Tukey’s multiple comparisons tests; one-way ANOVA was used for d, f, g, k, l, o, p, q, and s with Tukey’s multiple comparisons tests.
In order to determine if the increase in 3’SL was the predominant component responsible for the improved metabolic and cardiac phenotype in offspring from exercise-trained dams, we utilized St3gal4 knockout mice that lack 3’SL in their milk (3’SL−/− mice)19. When given ad libitum access to wheel cages the 3’SL−/− mice did not perform voluntary exercise, so a forced treadmill exercise paradigm was used. Both wild-type (WT) and 3’SL−/− mice were fed a chow diet and separated into two groups; sedentary or exercise-trained. Mice were trained for 60 min/day, 5 days/week at 0.8mph and a 10% incline for 2 weeks, followed by housing with male mice of the same genotype. Pregnant mice continued with the same treadmill paradigm for the 3 weeks of gestation. WT and 3’SL−/− mice had similar adaptations to exercise-training, determined by Citrate Synthase (CS) and hexokinase II (HKII) expression in tibialis anterior skeletal muscle (Extended Data Figure 1e,f). Similar to previous studies investigating the effects of voluntary maternal exercise on offspring10–15, forced maternal treadmill exercise of WT dams had significant effects on male offspring including decreased body weight and % fat mass (Figure 2e–g) and improved glucose tolerance (Figure 2h). In fact, maternal treadmill exercise resulted in similar improvements to metabolic health of male offspring as maternal wheel cage running10–15. Interestingly, comparison of offspring from sedentary WT vs sedentary 3’SL−/− dams revealed that there were pronounced effects of the lack of 3’SL, as these offspring had lower body weights, % fat mass, and GTT area under the curve (Figure 2e–h), suggesting that the absence of systemic 3’SL causes an altered metabolic phenotype. There was no further effect of maternal exercise to alter body weight, % fat mass, or glucose tolerance in male offspring from 3’SL−/− mothers (Figure 2e–h). There was also no effect of maternal exercise on insulin tolerance among male offspring from WT or 3’SL−/− dams (Extended Data Figure 1g). To determine if there were changes in fuel utilization, respiratory exchange ratio (RER) was measured. During the light-fed phase, RER was decreased in WT exercise-trained offspring and 3’SL−/− offspring (Figure 2i), suggesting that these mice had greater utilization of fat. There was no additional effect of maternal exercise to decrease RER in male offspring from 3’SL−/− mice. There was no effect of maternal exercise on cardiac function among male offspring from wild-type or 3’SL−/− dams (Extended Data Figure 1h; Supplementary Table 5).
Female offspring from WT, exercise-trained dams had decreased body weight (Figure 2j,k), but there was no effect of maternal exercise on % fat mass (Figure 2l), glucose tolerance (Figure 2m), insulin tolerance (Extended Data Figure 1i), or RER (Figure 2n). Similar to the males, female offspring from 3’SL−/− dams had decreased % body fat (Figure 2l), glucose tolerance (Figure 2m) and RER (Figure 2n) compared to offspring from WT dams. Maternal exercise in WT dams enhanced cardiac function in female offspring and protected offspring from the normal aging-induced changes in heart structure (Figure 2o; Supplementary Table 6). Maternal exercise in 3’SL−/− dams did not result in improved metabolic health or cardiac function in female offspring (Figure 2j–o). These data indicate that, similar to previous studies in chow-fed dams, there is a minimal effect of maternal exercise on metabolic health of female offspring15 and there was no effect of maternal exercise on metabolic health in 3’SL−/− mice. Importantly, these data are the first to demonstrate enhanced cardiac function in female offspring from WT, chow-fed, exercise-trained dams, but this effect was absent in female offspring from 3’SL−/− dams.
Together these data indicate that in the absence of 3’SL (3’SL−/−), there were no additional effects of maternal exercise to affect metabolic or cardiac health of offspring. However, the absence of 3’SL resulted in an altered metabolic phenotype including decreased body weight, % fat mass, and reduced GTT. To determine the effects of an absence of 3’SL on whole-body metabolic health, we performed exercise capacity tests and investigated the effects of 3’SL−/− mice on a high-fat diet. An exercise exhaustion test in male mice revealed significantly impaired exercise capacity in 3’SL−/− mice compared to WT mice (Extended Data Figure 1j). 3’SL−/− mice had an improved GTT and reduced fat mass compared to WT mice. To determine if the improved GTT was a result of the reduced body fat or a direct effect of glucose metabolism, a GTT was performed in male and female mice at 24 weeks of age based on lean mass. Surprisingly, both male and female 3’SL−/− mice had impaired glucose tolerance compared to WT mice, indicating that the altered metabolic phenotype was a result of the reduction in fat mass (Extended Data Figure 1k). To determine if the absence of 3’SL affects susceptibility to diet-induced obesity, male WT or 3’SL−/− mice were placed on a high-fat diet for 6 weeks. In contrast to chow-fed mice, after 6 weeks of a high-fat diet there was no difference between wild-type or 3’SL−/− mice in body weight, fat mass, or lean mass (Extended Data Figure 1l–n). In fact, in high-fat fed mice, glucose metabolism was impaired in 3’SL−/− mice compared to WT mice (Extended Data Figure 1o,p). These data suggest that the systemic absence of 3’SL reduces exercise capacity, impairs metabolic health, and increases susceptibility to the detrimental effects of a high-fat diet.
Since the systemic absence of 3’SL altered metabolic health, we investigated whether increasing 3’SL in a 3’SL−/− mice could improve metabolic health of offspring. To do this, offspring from 3’SL−/− were cross-fostered to wild-type dams (3’SL-WT). Interestingly, male 3’SL-WT mice had an additional decrease in body weight, fat mass, and improved glucose tolerance compared to 3’SL−/− mice (Figure 2p–s). There was no additional effect of increasing 3’SL in 3’SL−/− (3’SL-WT) on metabolic health of female mice (Extended Data Figure 1q–t), but ejection fraction was increased compared to 3’SL−/− mice (Supplementary Table 7). These data indicate that physiological consumption of 3’SL in 3’SL−/− mice at least partially restores the metabolic benefits in 3’SL−/− male mice and the improved cardiac function in 3’SL−/− female mice.
Expression of metabolic genes were measured to determine if they were associated with the altered metabolic phenotype in 3’SL−/− mice. Previous studies have shown that voluntary maternal exercise alters expression of hepatic genes in offspring15, so the effect of forced maternal exercise on hepatic gene expression was investigated by measuring genes involved in gluconeogenesis, Krebs cycle activity, and oxidative phosphorylation in offspring at 52 weeks of age. In WT mice, maternal exercise increased several genes involved in gluconeogenesis (Extended Data Figure 2a,b), Krebs cycle activity (Extended Data Figure 2c,d), and oxidative phosphorylation (Extended Data Figure 2e,f). Maternal exercise did not affect hepatic gene expression in offspring from 3’SL−/− mice (Extended Data Figure 2a–f). Strikingly, expression of hepatic genes involved in gluconeogenesis, Krebs cycle activity, and oxidative phosphorylation (Extended Data Figure 2a–f) were increased in male and female 3SL−/− mice compared to WT mice, indicating that the systemic absence of 3’SL may affect hepatic metabolism and could contribute to the altered metabolic phenotype in 3SL−/− mice.
Expression of cardiac genes involved in mitochondrial activity (Extended Data Figure 3a,b), fibrosis (Extended Data Figure 3c,d), and fetal reprogramming (Extended Data Figure 3e,f) were also measured. There was no effect of maternal exercise on gene expression in offspring from 3’SL−/− mice, but expression of several genes involved in cardiac metabolism and function were enhanced, even in the absence of maternal exercise. It is possible that this increase is a compensatory adaptation to the systemic absence of 3’SL.
To determine if there were exercise-induced adaptations to milk independent of 3’SL that improved metabolic and cardiac health of offspring, we investigated the effects of cross-fostering WT mice to sedentary and exercise-trained 3’SL−/− dams. WT offspring from sedentary dams were cross-fostered to 3’SL−/− sedentary (WT-3’SL SED) or 3’SL−/− exercise-trained (WT-3’SL TRAIN) dams. There was no difference between WT-3’SL SED or WT-3’SL TRAIN male or female offspring on body weight (Figure 3a), % fat mass (Figure 3b,c), glucose tolerance (Figure 3d,e), RER (Figure 3f,g), or ejection fraction (Figure 3h,i; Supplementary Tables 8, 9). These data indicate that in contrast to milk from exercise-trained WT dams (Figure 1), milk from exercise-trained, 3’SL−/− dams does not confer any beneficial effects to WT offspring, demonstrating the importance of 3’SL in milk to improve metabolic health and cardiac function in offspring.
Figure 3. Milk from exercise-trained 3’SL−/− dams did not improve metabolic health or cardiac function of wild-type (WT) mice.
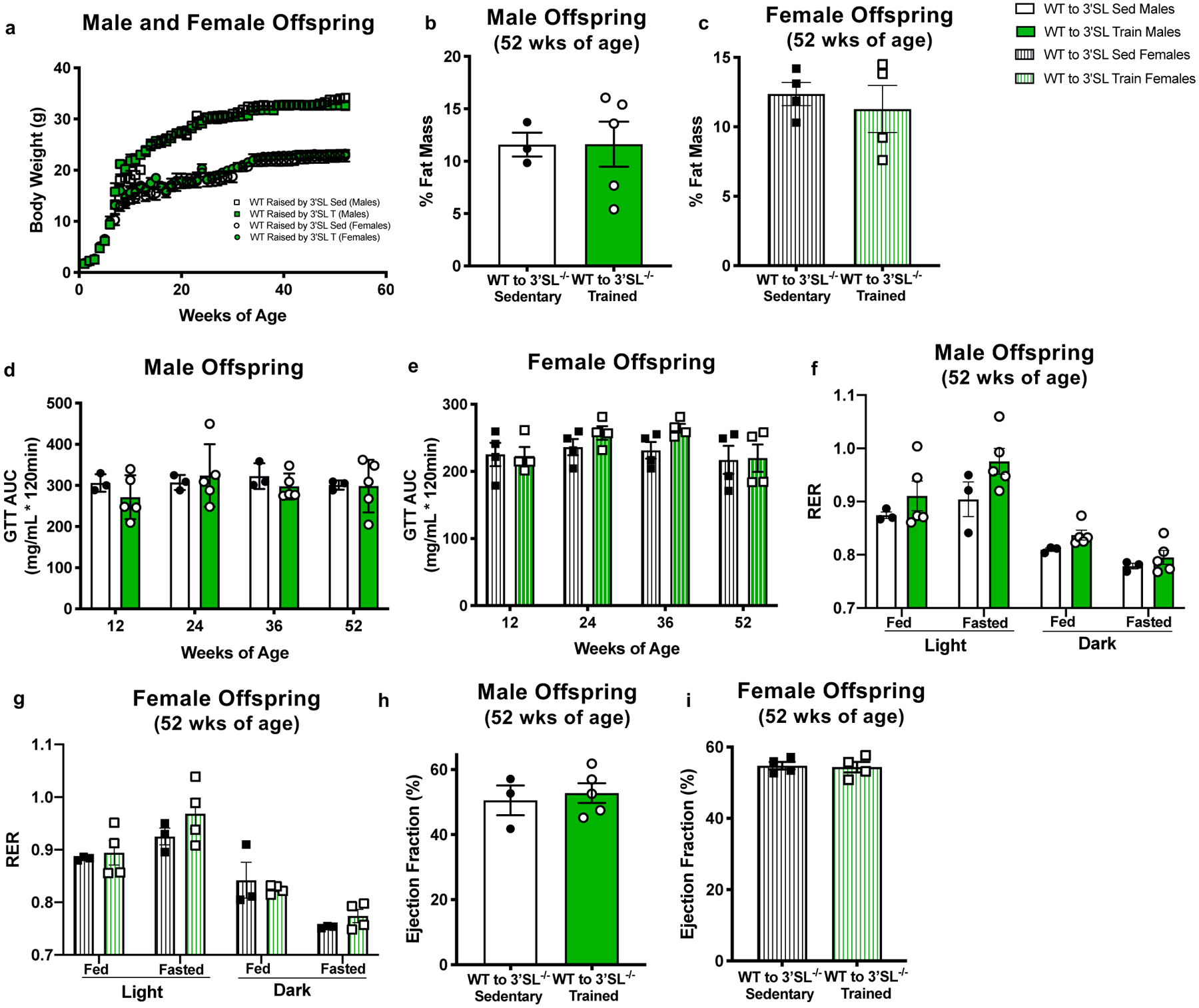
Offspring from WT mice were cross-fostered to 3’SL−/− Sedentary (WT-3’SL SED) or 3’SL−/− exercise-trained (WT-3’SL TRAIN) dams. (a) body weight; (b,c) % fat mass; (d,e) GTT AUC; (f,g) RER; and (h,i) ejection fraction. Data are expressed as the mean ± SEM (for males, WT-3’SL Sed n=3; WT-3’SL Train n=5. For females, WT-3’SL Sed n=4; WT-3’SL Train n=4). Two-way ANOVA was used for a, d, e, f, and g; unpaired two-tailed Student’s t-test was used for b, c, h, and i.
Finally, to determine if solely increasing 3’SL can improve metabolic health in male offspring and cardiac function in female offspring, we supplemented a cohort of offspring from sedentary, high-fat fed mice with exogenous 3’SL (600nmol) or a vehicle (PBS) through a pipette tip daily during their nursing period. This dose was selected based on the exercise-induced increase in 3’SL measured in mouse milk; this dose lowered body weight at 8 weeks of age (Extended Data Figure 4a–h) without preventing the pups from consuming other nutrients, which may contribute to metabolic and cardiac development. Importantly, this effect was specific to 3’SL, as supplementation of 6’SL did not alter body weight, body composition, or glucose tolerance in male or female mice at 8 weeks of age (Extended Data Figure 4i–p). Supplementation of 3’SL in pups increased circulating levels of 3’SL to an amount similar to offspring from exercise-trained dams at 14 days of age (Extended Data Figure 4q). Similar to offspring from exercise-trained dams (Fig 1,2), male offspring who were supplemented with 3’SL had decreased body weights (Figure 4a,b), decreased % fat mass (Figure 4c), improved glucose tolerance (Figure 4d), and decreased fasting insulin (Figure 4e) at 52 weeks of age. Supplementation of 3’SL did not affect insulin tolerance (Figure 4f) and tended to increase ejection fraction and increased left ventricular mass and wall thickness (Figure 4g; Supplementary Table 10) in male offspring. Female offspring supplemented with 3’SL had decreased body weights (Figure 4a,h) and reduced fasting insulin (Figure 4k), but % fat mass (Figure 4i), glucose tolerance (Figure 4j), and insulin tolerance (Figure 4l) were not affected. However, similar to the effects of maternal exercise, supplementation of 3’SL enhanced ejection fraction and increased left ventricular mass and wall thickness in female offspring at 24 weeks of age (Figure 4m; Supplementary Table 11). Expression of hepatic genes involved in glucose and fatty acid metabolism were significantly increased in male offspring that were supplemented with 3’SL during the nursing period (Extended Data Figure 5a–d), but 3’SL supplementation had minimal effect on cardiac gene expression (Extended Data Figure 5e–g). These data indicate that supplementation of a physiological concentration of 3’SL during the nursing period is sufficient to mediate beneficial effects similar to those resulting from maternal exercise.
Figure 4. Supplementation of 3’SL during the lactation period improves metabolic health and cardiac function of adult offspring.
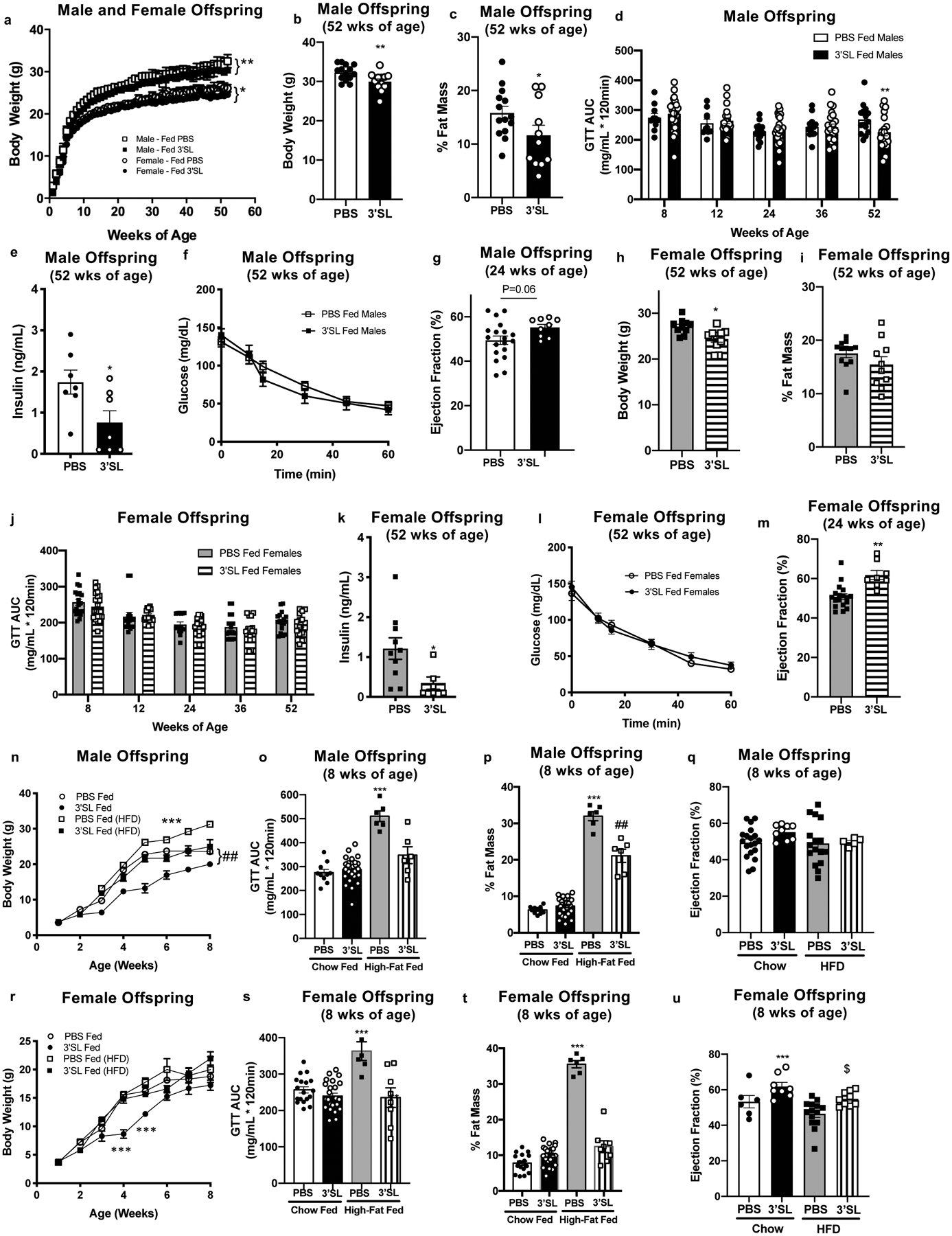
(a) Body weight, (b) body weight at 52 weeks; (c) % fat mass; (d) GTT AUC; (e) fasting insulin; (f) ITT and (g) ejection fraction in male offspring. (h) Body weight at 52 weeks; (i) % fat mass; (j) GTT AUC; (k) fasting insulin; (l) ITT and (m) ejection fraction in female offspring. Data are expressed as the mean ± SEM (for males, PBS Fed n=14, 3’SL Fed n=11 for Figures a, b, c, and f; PBS Fed n=15, 3’SL Fed n=20 for Figure d; PBS Fed n=7, 3’SL Fed n=7 for Figure e; PBS Fed n=19, 3’SL Fed n=9 for Figure g. For Females, PBS Fed n=12, 3’SL Fed n=11 for Figures a, h, i, l; PBS Fed n=17, 3’SL Fed n=17 for Figure j; PBS Fed n=10, 3’SL Fed n=6 for Figure k; PBS Fed n=18, 3’SL Fed n=8 for Figure m). Asterisks represent differences compared to PBS fed offspring of the same gender (*P < 0.05; **P < 0.01). (n) Body weight; (o) GTT AUC, (p) % fat mass, and (q) ejection fraction in male offspring supplemented with PBS or 600nmol 3’SL fed and placed on a chow or high-fat diet after weaning. Data are expressed as the mean ± SEM (PBS fed chow n=10; 3’SL fed chow n=26; PBS fed HFD n=6; 3’SL fed HFD n=6). Asterisks represent differences compared to all other groups (***P < 0.001) or compared to PBS and 3’SL chow fed (##P<0.01). (r) Body weight; (s) GTT AUC, (t) % fat mass, and (u) ejection fraction in female offspring supplemented with PBS or 600nmol 3’SL fed and placed on a chow diet or a high-fat diet after weaning. Data are expressed as the mean ± SEM (PBS fed chow n=18; 3’SL fed chow n=25; PBS fed HFD n=6; 3’SL fed HFD n=8). Asterisks represent differences compared to all other groups (***P < 0.001) or compared to PBS high-fat diet fed ($P<0.05). Two-way ANOVA was used for a, d, f, j, l, n and r with Tukey’s multiple comparisons tests; one-way ANOVA was used for o, p, q, s, t, and u with Tukey’s multiple comparisons tests; unpaired two-tailed Student’s t-test was used for b, c, e, g, h, i, and m.
To determine if supplementation of 3’SL could protect against the development of obesity or metabolic disease, mice supplemented with 3’SL or PBS were placed on a high-fat diet immediately after weaning. Male mice supplemented with PBS and placed on a high-fat diet had an increase in body weight, % fat mass, and an impairment in GTT compared to chow fed mice at 8 weeks of age (Figure 4n–p). Strikingly, supplementation with 3’SL completely abolished the effects of a high-fat diet on body weight and glucose tolerance in male mice (Figure 4n–p). There was no effect of 3’SL on ejection fraction in male offspring (Figure 4q). There was no difference in body weight in PBS or 3’SL fed female mice placed on a high-fat diet (Figure 4r), but female mice supplemented with 3’SL and fed a high-fat diet did not have the impairment in % fat mass or glucose tolerance observed in PBS supplemented, high-fat diet fed mice (Figure 4s,t). Supplementation of 3’SL protected the negative effects of a high-fat diet on ejection fraction in female mice (Figure 4u). Together these data suggest that supplementation of 3’SL improves metabolic health in male offspring, and cardiac function in female offspring, similar to effects seen with the consumption of exercise-trained milk (Figure 1), and importantly, supplementation of 3’SL protects against the detrimental effect of a high-fat diet on body composition and glucose tolerance.
In conclusion, these data demonstrate that an exercise-induced adaptation to milk is essential to improve the metabolic health in adult male offspring and cardiac function in adult female offspring, and that the human milk oligosaccharide (HMO) 3’SL is a critical component to mediate these beneficial effects. Increasing 3’SL during the lactation period could have a dramatic therapeutic potential for obesity, type 2 diabetes, and cardiovascular disease.
Methods
Additional information is available in the Nature Research Reporting Summary linked to this article.
Human Studies
Participants (N=139) were mothers with body mass index (BMI, kg/m2) between 18 and 35 kg/m2 enrolled in the longitudinal Glowing study (www.clinicaltrials.gov, ID# NCT01131117). Participants were healthy, without preexisting or ongoing medical conditions (e.g., diabetes mellitus, hypertension), use of medications during pregnancy known to influence fetal growth, or use of tobacco or alcohol products. Anthropometric measures were obtained at enrollment (≤ gestation week 10) using standardized methods. Body weight was measured to the nearest 0.1 kg on a tared scale (Perspective Enterprises, Portage, MI, USA) and standing height was measured using a standard wall-mounted stadiometer to the nearest 0.1 cm (Tanita Corp., Tokyo, Japan). BMI was computed as kg/m2. Physical activity (PA) was assessed using accelerometry (Actical, Philips Respironics Co. Inc., Bend, Oregon, USA) at gestation weeks 12, 24 and 36 to yield average prenatal daily total activity counts (Activity) and average prenatal daily steps (Steps). Participants collected a human milk sample at the second feeding of the day (around 9 am, whichever came first) by expressing fully one breast at 2 months postnatal. Demographics of human subjects are presented in Supplementary Table 1. The samples were stored at −70°C and shipped to the University of California, San Diego for human milk oligosaccharides analyses. The study protocols were approved by the Institutional Review Board of the University of Arkansas for Medical Sciences.
Mice and Training Paradigms
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of The Ohio State University (# 2019A00000119-R1) and were in accordance with NIH guidelines.
Wheel-cage training:
Six-week-old C57BL/6 virgin female mice were fed a chow (21% kcal from fat; 9F5020; Teklad) or high-fat diet (60% kcal from fat; Research Diets Inc.) for two weeks preconception, during gestation, and until pup weaning. Mice were additionally divided into two subgroups: trained (mice housed with running wheels preconception and during gestation); or sedentary (housed in static cages). All male breeders were 10-week-old C57BL/6 mice maintained on a chow diet and were sedentary. To control for potential differences in sires, breeding was done as harems. Litters were culled to five mice, and offspring were chow-fed and housed in static cages (sedentary) from birth onwards.
Treadmill training:
Six-week-old C57BL/6 (Charles Rivers Laboratories) or St3gal4 knockout (B6.129-St3gal4tm1.Jxm/J; Jackson Laboratories) (3’SL−/−) virgin female mice were fed a chow (21% kcal from fat; 9F5020; Teklad) for two weeks preconception, during gestation, and until pup weaning. Mice were additionally divided into two subgroups: trained (mice undergoing forced exercise on a treadmill preconception and during gestation); or sedentary (housed in static cages). The treadmill training program consisted of 60 min/day, 5 days/week at 0.8mph (21 m/min) and a 10% incline. All mice successfully completed the training program. All male breeders were 10-week-old C57BL/6 or 3’SL−/− mice maintained on a chow diet and were sedentary. To control for potential differences in sires, breeding was done as harems. Litters were culled to five mice, and offspring were chow-fed and housed in static cages (sedentary) from birth onwards.
Exhaustion Test:
Twelve-week-old wild-type (C57BL/6) (Charles River Laboratories) or 3’SL−/− male mice underwent an exercise test to exhaustion. Briefly, mice were subjected to a graded ramp treadmill running protocol. The treadmill speed began at 0.2 miles/h and increased 0.2 miles/h every 5 min until the speed reached 0.8 miles/h. The incline was increased by 5% every 5 min, to a maximum of 25%. The speed and incline were then kept constant (0.8 miles/h and 25%) until the mice reached exhaustion20.
Cross-fostering:
Twenty-four hours after birth, litters were “cross-fostered” from their birth mother to a foster mother21. Litters born to sedentary mothers were fostered with trained mothers, and vice versa; litters born to trained mothers were fostered with sedentary mothers. Litters born to sedentary mothers were fostered with other sedentary mothers, and litters born to trained mothers were fostered to other trained mothers. Litters born to wild-type (C57BL/6) mothers were fostered with 3’SL−/− sedentary or trained mothers, and vice versa; litters born to 3’SL−/− sedentary mothers were fostered with wild-type sedentary mothers. These litters were left nursing with their foster mother from the second day after birth until weaning, and after weaning were kept sedentary and on a chow diet.
Isolation of Milk
For isolation of milk, six-week-old female C57BL/6 (Charles Rivers Laboratories) mice were exercise-trained starting two weeks before pregnancy in running wheel cages, with four groups of sedentary chow fed, sedentary high fat fed, trained chow fed, and trained high fat fed. Pups were removed from the dams 24h prior to milk isolation, and the milk was isolated 7 days after birth22. To isolate the milk, the mammary gland was excised and placed on a dish on ice for 4h, and the excreted milk was collected.
3’sialyllactose (3’SL) Feeding
Six-week-old female C57BL/6 (Charles Rivers Laboratories) mice were kept sedentary and placed on high fat diet starting two weeks prior to conception, then bred with chow fed sedentary 8-week-old male C57BL/6 (Charles Rivers Laboratories) mice. The dams were split into six groups, PBS (phosphate-buffered saline) fed or 3’SL fed (300, 600, 900, or 1200nmol), or 6’SL fed (300nmol) and litters culled to the same size for each dam23. Feeding was done by oral gavage. 3’SL fed pups were given 3’SL in PBS solution. Mice were fed varying concentrations of 3’SL throughout their nursing period to mimic the physiological increase in 3’SL throughout the nursing period; mice were fed 150 nmol of 3’SL on days one through three; 300–600 nmol on days four through six; and 600–1200 nmol on days seven to twenty-one19. The 6’SL fed mice were fed 150 nmol of 6’SL on days one through three; 300nmol on days four through twenty-one. The PBS fed controls were fed the same volume solution as the 3’SL supplemented mice.
High Performance Liquid Chromatography (HPLC)
Concentrations of 3’-sialyllactose (3’SL) and 6’-siallyllactose (6’SL), the two milk oligosaccharides that are conserved between mice and humans, were measured by High Performance Liquid Chromatography (HPLC)24.
Body Composition, Metabolic, and Cardiac Testing
Body weight was measured weekly through 52 weeks using an OHAUS NV212 balance. Body composition was determined using an EchoMRI instrument (EchoMRI, LLC) with canola oil calibration25. Glucose Tolerance testing (GTT) was performed on a 12 hour fast (20:00h – 8:00h) with drinking water ad lib available. Blood glucose was assessed at baseline by a tail vein prick11,15,26 using a commercial glucose monitor (OneTouch Ultra2). Glucose was administered by intraperitoneal injection (2g glucose/kg body weight or per kg lean mass) at 0 min, and the tail vein prick was used to measure blood glucose levels at 0, 15, 30, 60, and 120 minutes post injection. Insulin Tolerance testing (ITT) was performed on a 2 hour fast (10:00h – 12:00h) with drinking water ad lib. Baseline blood glucose levels were measured using a tail vein prick. Insulin was administered intraperitoneal injection (1 unit per kg body weight) at 0 minutes. Blood glucose levels were measured at 0, 10, 15, 30, 45, and 60 minutes post injection. If mice dropped below 40 mg/mL glucose they were given an injection of 200 μL of 10% glucose (0.1g/mL) to prevent seizures. Ejection fraction was measured using a Visual Sonics Vevo 2100 with a MX550D 32–55 MHz transducer. Mice were anesthetized with 1–2% isoflurane and echocardiography conducted. Images were measured at baseline and ejection fraction measured27. Male mice who were WT (C57BL/6) or 3’SL−/− who underwent high-fat feeding were placed on a high-fat diet ad lib (60% kcal from fat; Research Diets Inc.) for 6 wks.
Comprehensive Lab Animal Monitoring System
The Comprehensive Lab Animal Monitoring System (Oxymax Opto-M3; Columbus Instruments) was used to measure activity level, volume of O2 consumption, volume of CO2 production, and heat production. Total energy expenditure of mice was calculated as described previously28.
Biochemical Methods.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) was performed on tissue after mice were sacrificed at 52 weeks. At the time of sacrifice, tissue was flash frozen and stored at −80°C until processing. mRNA levels in the tissue were measured by qRT-PCR (Roche LightCycler 480II) using SYBR Green detection (QuantaBio). The primers used are included in Supplementary Tables 12 and 1315. Tissue processing and immunoblotting were performed as previously described29. Briefly, tibialis anterior muscle lysates were solubilized in Laemmli buffer, separated by SDS-PAGE, and transferred to nitrocellulose membranes. The membranes were incubated with antibodies specific for Hexokinase II (HKII) and citrate synthase (CS), obtained from a commercial source (Abcam). The immunoreactive proteins were detected with enhanced chemiluminescence and quantified by densitometry. Circulating plasma insulin concentration was measured using a standard ELISA kit (Millipore).
Statistical Analysis.
The data are presented as means ± SEM. Statistical significance was defined as P < 0.05 and determined by one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis, or unpaired two-tailed Student’s t-test. For experiments with human subjects, Pearson’s correlations were used for analysis. For experiments that were carried out at various ages (12, 24, 36, and 52 weeks of age), statistical analyses were determined based on the control group at that specific time point, and comparisons among ages were not analyzed.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Correspondence and requests for materials should be addressed to K.I.S.
Extended Data
Extended Data 1. Effects of maternal exercise in dams.
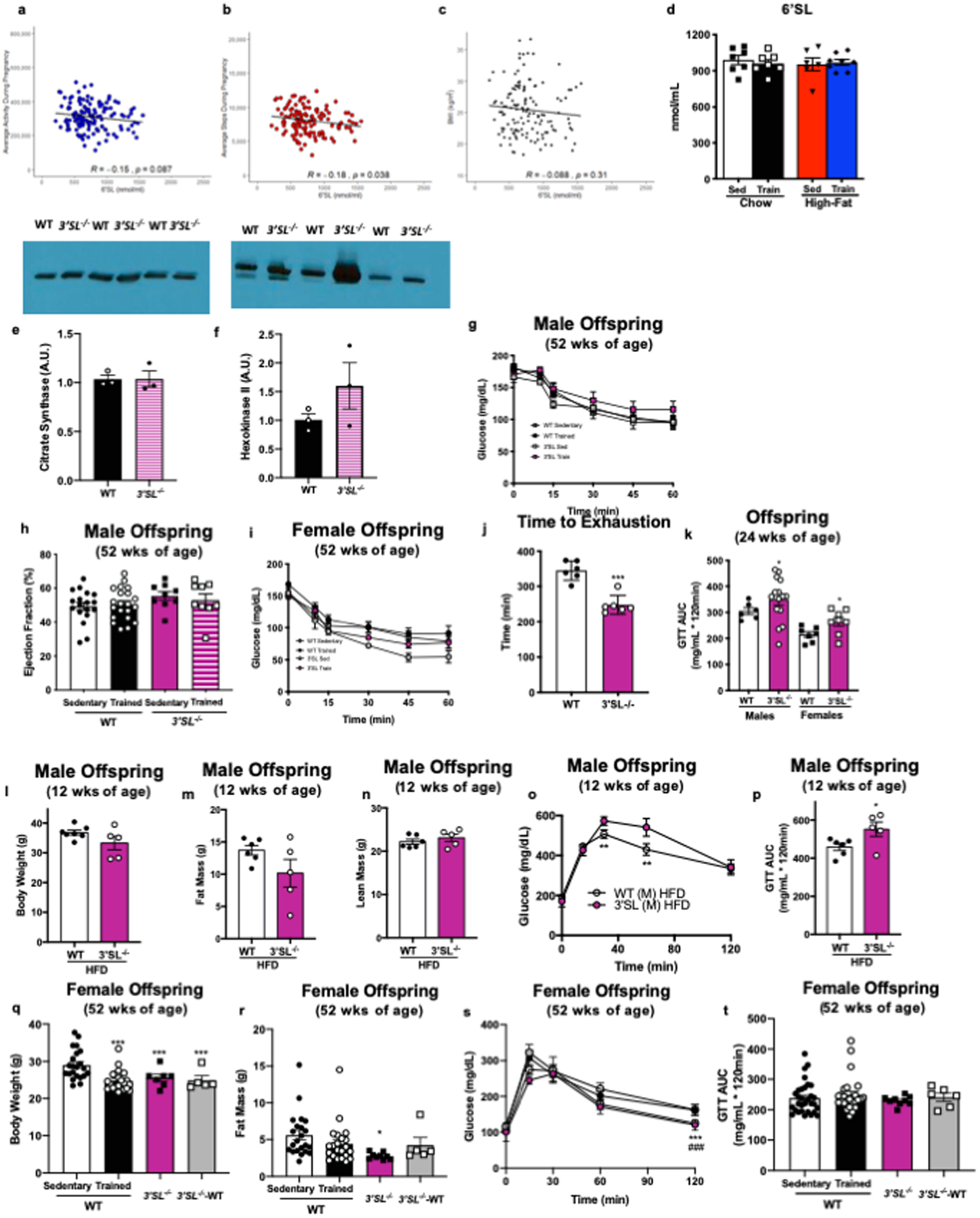
6’SL in human participants (a) average activity during pregnancy, (b) average steps per day during pregnancy, and (c) BMI; N=139, data analyzed by Pearson’s correlation. (d) 6’SL in breastmilk from sedentary and exercise-trained chow-fed and high-fat fed dams. Data are expressed as the mean ± SEM (Sed Chow n=7; Train Chow n=7; Sed High-Fat n=6; Train High-Fat n=8). There was no difference in (e) citrate synthase of (f) hexokinase 2 (HK2) protein expression in tibialis anterior skeletal muscles of wild-type (WT) and 3’SL−/− exercise-trained dams (n=3/group). (g) ITT, and (g) ejection fraction in male offspring from sedentary or exercise-trained wild-type or 3’SL−/− dams, and (i) ITT in female offspring from sedentary or exercise-trained wild-type or 3’SL−/− dams. Data are expressed as the mean ± SEM (for males, WT Sed n=18, WT Train n=21, 3’SL−/− Sed n=9, 3’SL−/− Train n=9. For females, WT Sed n=24; WT Train n=22; 3’SL−/− Sed n=5; 3’SL−/− Train n=10). (j) Exercise capacity test in wild-type or 3’SL−/− male mice (n=6/group). Asterisks represent differences compared to WT (***P < 0.001). (k) GTT by lean mass and wild-type or 3’SL−/− male or female mice at 24 wks of age. Data are expressed as the mean ± SEM (n=6 WT males; n=7 WT females; n=15; 3’SL−/− males; n=8; 3’SL−/− females). Asterisks represent differences compared to WT of same gender (*P < 0.05). (l) Body weight; (m) fat mass; (n) lean mass; (o) glucose excursion curve; and (p) GTT AUC in 12-week-old male WT (n=13) or 3’SL−/− (n=5) mice placed on a high-fat diet for 6 weeks. Data are expressed as the mean ± SEM. Asterisks represent differences compared to 3’SL−/− (*P < 0.05; **P<0.01). (q) Body weight, (r) fat mass, (s) glucose tolerance excursion curve, and (t) GTT AUC in female offspring from wild-type sedentary (n=22) or exercise-trained dams (n=22), 3’SL−/− sedentary dams (n=12), or 3’SL−/− sedentary dams cross-fostered to sedentary wild-type dams (3’SL-WT) (n=6). Data are expressed as the mean ± SEM. Asterisks represent differences compared to WT Sedentary offspring (*P < 0.05; **P < 0.01; ***P < 0.001); and # represents differences compared to WT Trained offspring (###P<0.01). Two-way ANOVA was used for g, i, o, and s with Tukey’s multiple comparisons tests; one-way ANOVA was used for d, h, q, r, and t, with Tukey’s multiple comparisons tests; unpaired two-tailed Student’s t-test was used for e, f, j, k, l, m, n, and p.
Extended Data 2. Maternal exercise alters expression of hepatic genes.
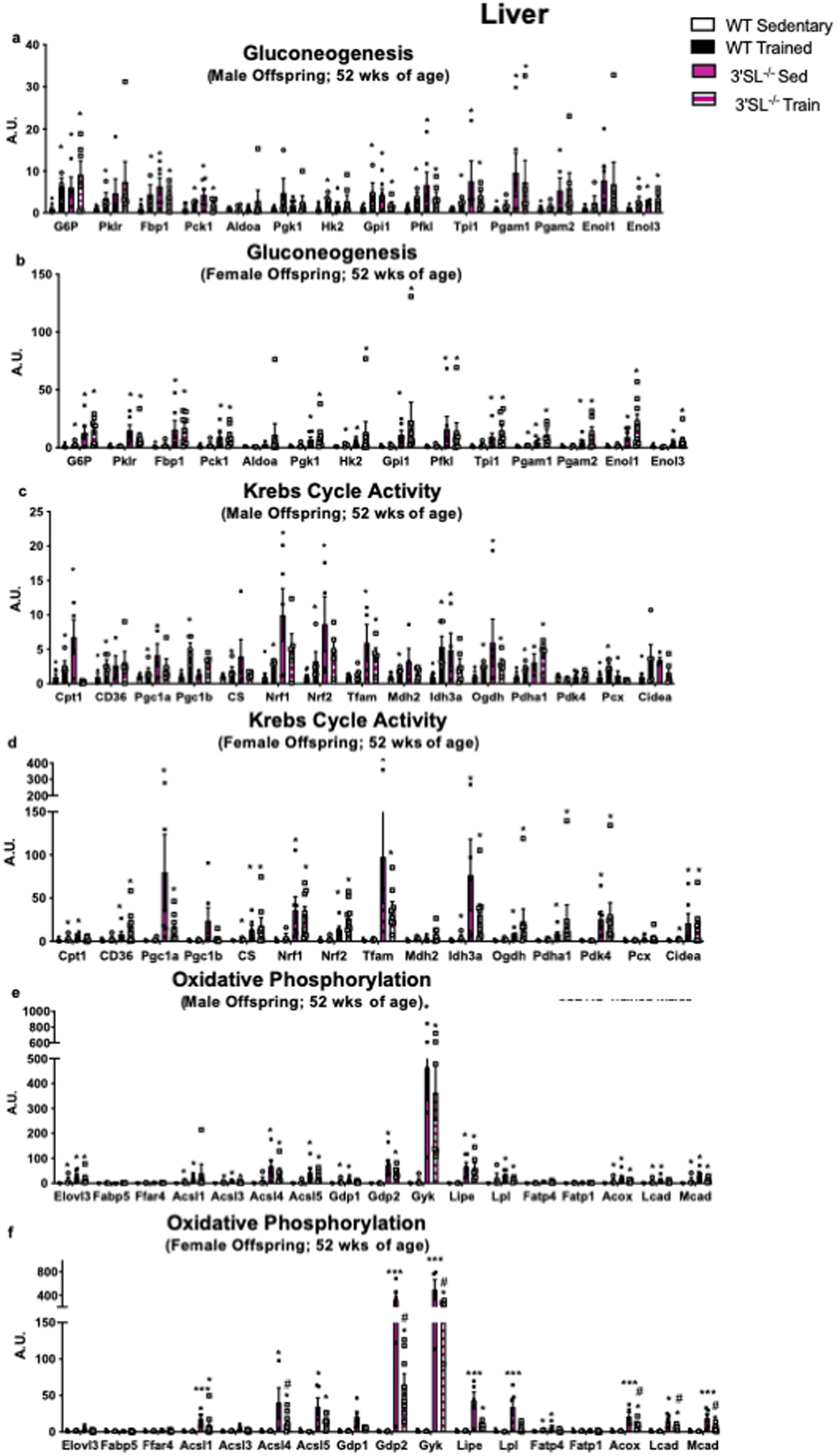
Expression of hepatic genes involved in (a,b) gluconeogenesis and glucose metabolism, (c,d) mitochondrial activity and Krebs Cycle activity, and (e,f) fatty acid transport and oxidation and male and female offspring from wild-type (WT) and 3’SL−/− mice. Data are expressed as the mean ± SEM (for males, WT Sed n=8, WT Train n=5, 3’SL−/− Sed n=6, 3’SL−/− Train n=6. For females, WT Sed n=9, WT Train n=4, 3’SL−/− Sed n=6, 3’SL−/− Train n=8). Asterisks represent differences compared to WT Sedentary offspring (*P < 0.05; ***P<0.001) or compared to 3’SL−/− Sedentary offspring (#P<0.05). One-way ANOVA was used for a-f with Tukey’s multiple comparisons tests.
Extended Data 3. Maternal exercise alters expression of cardiac genes.
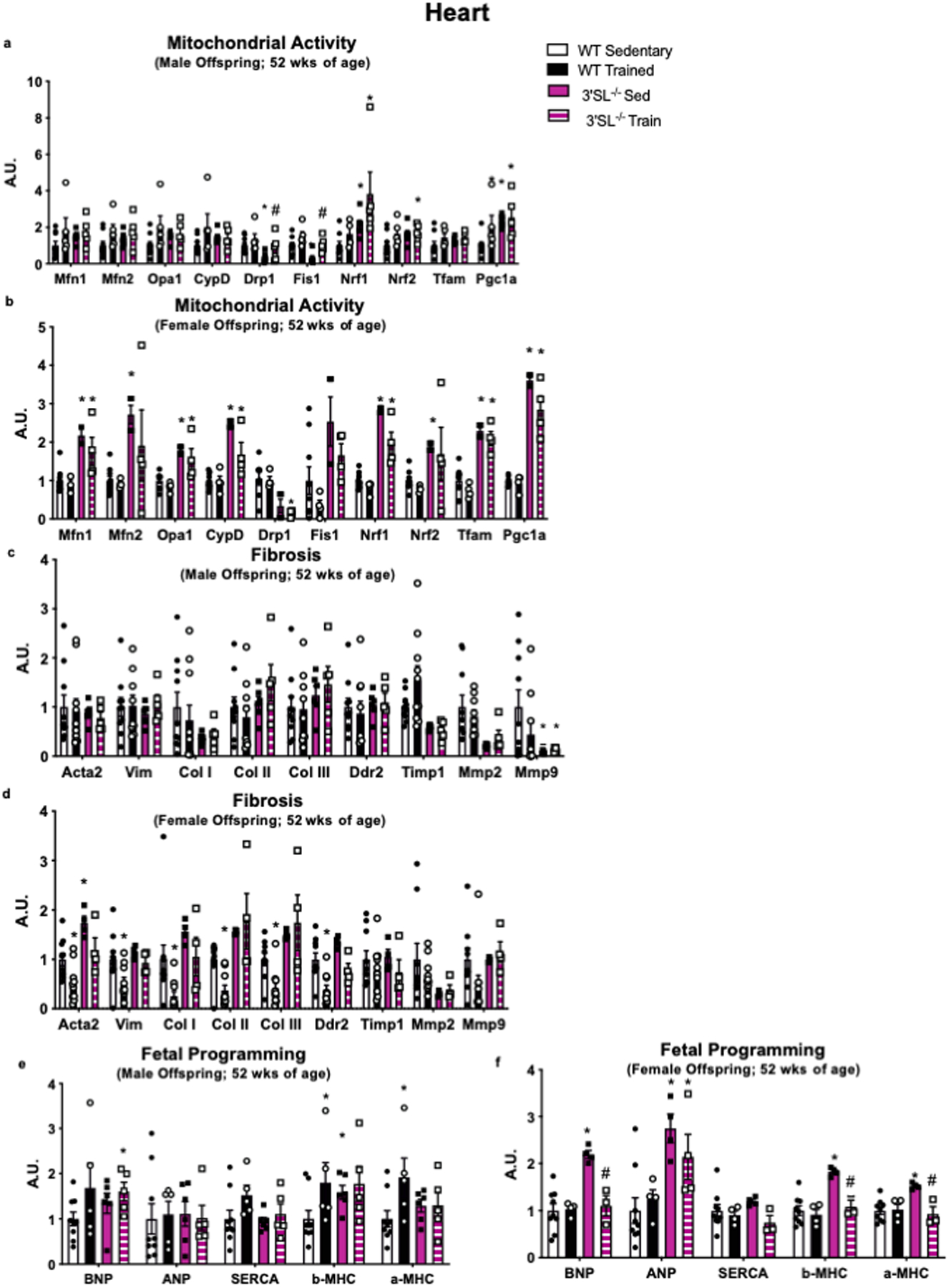
Expression of cardiac genes involved in (a,b) mitochondrial activity, (c,d) fibrosis, and (e,f) fetal reprogramming and male and female offspring from WT and 3’SL−/− mice. Data are expressed as the mean ± SEM (for males, WT Sed n=9, WT Train n=9, 3’SL−/− Sed n=6, 3’SL−/− Train n=5 for Figure a; WT Sed n=9, WT Train n=5, 3’SL−/− Sed n=6, 3’SL−/− Train n=5 for Figures c, e. For females, WT Sed n=9, WT Train n=10, 3’SL−/− Sed n=4, 3’SL−/− Train n=4 for Figures b, d; WT Sed n=9, WT Train n=4, 3’SL−/− Sed n=4, 3’SL−/− Train n=4 for Figure f). Asterisks represent differences compared to Sedentary offspring of the same gender (*P < 0.05; **P < 0.01) or compared to 3’SL−/− Sedentary offspring (#P<0.05). One-way ANOVA was used for a-f with Tukey’s multiple comparisons tests.
Extended Data 4. Supplementation with 3’SL improves metabolic health of offspring.
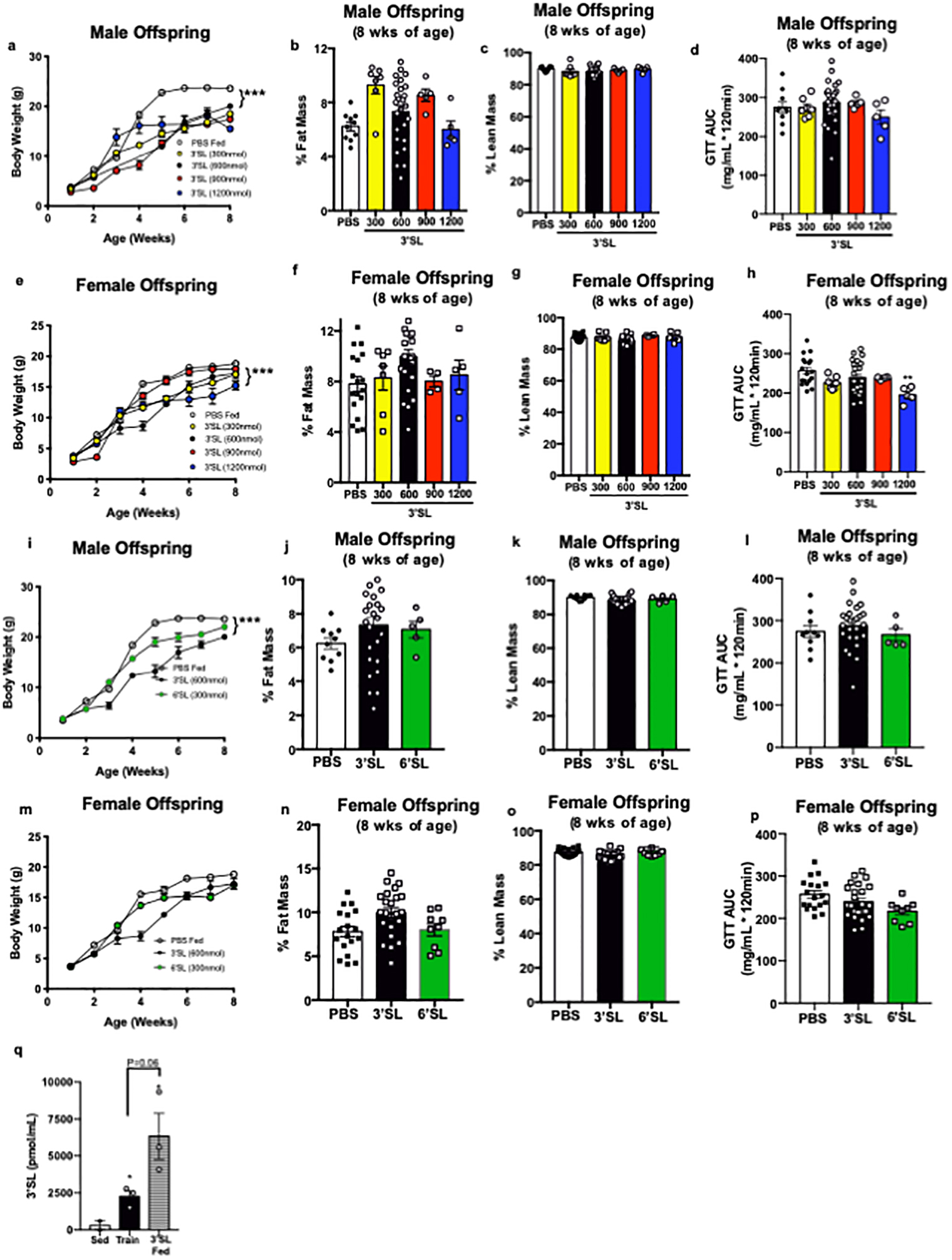
(a) Body weight, (b) % fat mass, (c) % lean mass, and (d) GTT AUC in male mice supplemented with 300 (n=7), 600 (n=26), 900 (n=5), or 1200 (n=5) nmol/day of 3’SL, or PBS fed (n=10). Data are expressed as the mean ± SEM. Asterisks represent differences compared to PBS fed (***P<0.001). (e) Body weight, (f) % fat mass, (g) % lean mass, and (h) GTT AUC in female mice supplemented with 300 (n=7), 600 (n=24), 900 (n=4), or 1200 (n=5) nmol/day of 3’SL, or PBS fed (n=18). Data are expressed as the mean ± SEM. Asterisks represent differences compared to PBS fed (**P<0.01). (i) Body weight, (j) % fat mass, (k) % lean mass, and (l) GTT AUC in male mice supplemented with 600 nmol/day 3’SL (n=26), 300 nmol/day 6’SL (n=5), or PBS fed (n=10). Data are expressed as the mean ± SEM. Asterisks represent differences compared to PBS fed (***P<0.001). (m) Body weight, (n) % fat mass, (o) % lean mass, and (p) GTT AUC in female mice supplemented with 600 nmol/day 3’SL (n=24), 300 nmol/day 6’SL (n=9), or PBS fed (n=18). Data are expressed as the mean ± SEM. (q) 3’SL in circulation of male offspring from sedentary dams (n=2), exercise-trained dams (n=3), or pups fed 3’SL (n=3). Data are expressed as the mean ± SEM. Asterisks represent differences compared to sedentary (*P<0.05). Two-way ANOVA was used for a, e, i, and m with Tukey’s multiple comparisons tests; one-way ANOVA was used for b, c, d, f, g, h, j, k, l, n, o, p and q with Tukey’s multiple comparisons tests.
Extended Data 5. Supplementation of 3’SL alters expression of hepatic and cardiac genes.
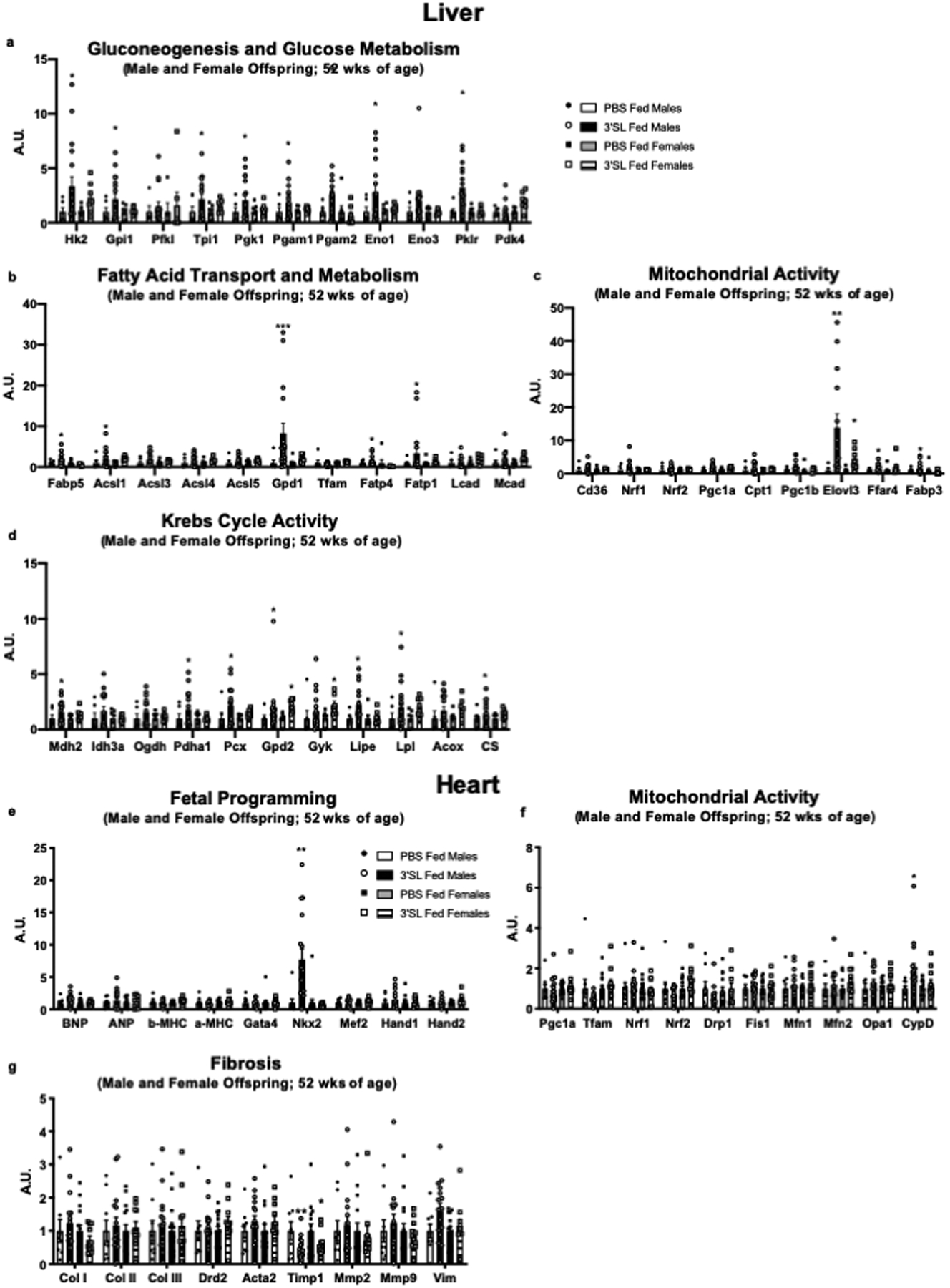
Expression of hepatic genes involved in (a) gluconeogenesis and glucose metabolism, (b) fatty acid transport and metabolism, (c) mitochondrial activity, and (d) Krebs Cycle activity in male and female from PBS and 3’SL fed offspring. Expression of cardiac genes involved in (e) fetal programming genes, (f) mitochondrial activity, and (g) fibrosis in male and female offspring from PBS and 3’SL fed offspring. Data are expressed as the mean ± SEM (for males, PBS Fed n=6, 3’SL Fed n=17. For females, PBS Fed n=7, 3’SL Fed n=7 for Figures a-d; PBS Fed n=15, 3’SL Fed n=11 for Figures e-g). Asterisks represent differences compared to PBS fed offspring of the same gender (*P < 0.05; **P < 0.01; ***P < 0.001). One-way ANOVA was used for a-g with Tukey’s multiple comparisons tests.
Supplementary Material
Acknowledgements
This work was supported by NIH grant R01HL138738 to K.I.S. and R01AG060542 to K.I.S. and M.T.Z., R01-DK101043 (to L.J.G.), and the Joslin Diabetes Center DRC (P30 DK36836). K.M.P. was supported by T32HL134616. The authors thank Drs. Peter J. Mohler and E. Douglas Lewandowski for critical discussions.
Footnotes
Competing Interests
The authors have declared that no conflict of interest exists.
References
- 1.Isganaitis E et al. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes 58, 1192–1200, doi: 10.2337/db08-1266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isganaitis E et al. Developmental programming by maternal insulin resistance: hyperinsulinemia, glucose intolerance, and dysregulated lipid metabolism in male offspring of insulin-resistant mice. Diabetes 63, 688–700, doi: 10.2337/db13-0558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo M et al. Early life nutrition modulates muscle stem cell number: implications for muscle mass and repair. Stem cells and development 20, 1763–1769, doi: 10.1089/scd.2010.0349 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCurdy CE et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. The Journal of clinical investigation 119, 323–335, doi: 10.1172/jci32661 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hales CN et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ (Clinical research ed.) 303, 1019–1022 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phipps K et al. Fetal growth and impaired glucose tolerance in men and women. Diabetologia 36, 225–228 (1993). [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ In utero programming of chronic disease. Clinical science (London, England : 1979) 95, 115–128 (1998). [PubMed] [Google Scholar]
- 8.Barker DJ In utero programming of cardiovascular disease. Theriogenology 53, 555–574 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Ravelli GP, Stein ZA & Susser MW Obesity in young men after famine exposure in utero and early infancy. The New England journal of medicine 295, 349–353, doi: 10.1056/nejm197608122950701 (1976). [DOI] [PubMed] [Google Scholar]
- 10.Harris JE, Baer LA & Stanford KI Maternal Exercise Improves the Metabolic Health of Adult Offspring. Trends in endocrinology and metabolism: TEM 29, 164–177, doi: 10.1016/j.tem.2018.01.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanford KI et al. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 64, 427–433, doi: 10.2337/db13-1848 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter LG et al. Perinatal exercise improves glucose homeostasis in adult offspring. American journal of physiology. Endocrinology and metabolism 303, E1061–1068, doi: 10.1152/ajpendo.00213.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter LG, Qi NR, De Cabo R & Pearson KJ Maternal exercise improves insulin sensitivity in mature rat offspring. Medicine and science in sports and exercise 45, 832–840, doi: 10.1249/MSS.0b013e31827de953 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laker RC et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1alpha gene and age-dependent metabolic dysfunction in the offspring. Diabetes 63, 1605–1611, doi: 10.2337/db13-1614 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanford KI et al. Maternal exercise improves glucose tolerance in female offspring. Diabetes 66, doi: 10.2337/db17-0098 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beeson JH et al. Maternal exercise intervention in obese pregnancy improves the cardiovascular health of the adult male offspring. Molecular metabolism 16, 35–44, doi: 10.1016/j.molmet.2018.06.009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bode L Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 22, 1147–1162, doi: 10.1093/glycob/cws074 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akers RM, Bauman DE, Capuco AV, Goodman GT & Tucker HA Prolactin regulation of milk secretion and biochemical differentiation of mammary epithelial cells in periparturient cows. Endocrinology 109, 23–30, doi: 10.1210/endo-109-1-23 (1981). [DOI] [PubMed] [Google Scholar]
- 19.Fuhrer A et al. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. The Journal of experimental medicine 207, 2843–2854, doi: 10.1084/jem.20101098 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An D et al. Overexpression of TRB3 in muscle alters muscle fiber type and improves exercise capacity in mice. American journal of physiology. Regulatory, integrative and comparative physiology 306, R925–933, doi: 10.1152/ajpregu.00027.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarty R Cross-fostering: Elucidating the effects of gene x environment interactions on phenotypic development. Neuroscience and Biobehavioral Reviews 73, 219–254, doi: 10.1016/j.neubiorev.2016.12.025 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Manthey CF, Autran CA, Eckmann L & Bode L Human Milk Oligosaccharides Protect Against Enteropathogenic Escherichia coli Attachment In Vitro and EPEC Colonization in Suckling Mice. Journal of Pediatric Gastroenterology and Nutrition 58, 165–168, doi: 10.1097/mpg.0000000000000172 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monaco MH et al. Evaluation of Sialyllactose Supplementation of a Prebiotic-Containing Formula on Growth, Intestinal Development, and Bacterial Colonization in the Neonatal Piglet. Current Developments in Nutrition 2, doi: 10.1093/cdn/nzy067 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thongaram T, Hoeflinger JL, Chow J & Miller MJ Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. Journal of Dairy Science 100, 7825–7833, doi: 10.3168/jds.2017-12753 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Lee DH et al. Comparison of the association of predicted fat mass, body mass index, and other obesity indicators with type 2 diabetes risk: two large prospective studies in US men and women. European Journal of Epidemiology 33, 1113–1123, doi: 10.1007/s10654-018-0433-5 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Stanford KI et al. Paternal Exercise Improves Glucose Metabolism in Adult Offspring. Diabetes 67, 2530–2540, doi: 10.2337/db18-0667 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roof SR et al. Obligatory role of neuronal nitric oxide synthase in the heart’s antioxidant adaptation with exercise. Journal of molecular and cellular cardiology 81, 54–61, doi: 10.1016/j.yjmcc.2015.01.003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albarado DC et al. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology 145, 243–252, doi: 10.1210/en.2003-0452 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Lessard SJ et al. Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes 62, 2717–2727, doi: 10.2337/db13-0062 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Correspondence and requests for materials should be addressed to K.I.S.


