Abstract
Objective
In Berlin, the first public severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing site started 1 day after the first case in the city occurred. We describe epidemiological and clinical characteristics and aim at identifying risk factors for SARS-CoV-2 detection during the first 6 weeks of operation.
Methods
Testing followed national recommendations, but was also based on the physician's discretion. We related patient characteristics to SARS-CoV-2 test positivity for exploratory analyses using a cross-sectional, observational study design.
Results
Between 3 March and 13 April 2020, 5179 individuals attended the site (median age 34 years; interquartile range 26–47 years). The median time since disease onset was 4 days (interquartile range 2–7 days). Among 4333 persons tested, 333 (7.7%) were positive. Test positivity increased up to 10.3% (96/929) during the first 3 weeks and then declined, paralleling Germany's lock-down and the course of the epidemic in Berlin. Strict adherence to testing guidelines resulted in 10.4% (262/2530) test positivity, compared with 3.9% (71/1803) among individuals tested for other indications. A nightclub was a transmission hotspot; 27.7% (26/94) of one night's visitors were found positive. Smell and/or taste dysfunction indicated coronavirus disease 2019 (COVID-19) with 85.6% specificity (95% CI 82.1%–88.1%). Four per cent (14/333) of those infected were asymptomatic. Risk factors for detection of SARS-CoV-2 infection were recent contact with a positive case (second week after contact, OR 3.42; 95% CI 2.48–4.71), travel to regions of high pandemic activity (e.g. Austria, OR 4.16; 95% CI 2.48–6.99), recent onset of symptoms (second week, OR 3.61; 95% CI 1.87–6.98) and an impaired sense of smell/taste (4.08; 95% CI 2.36–7.03).
Conclusions
In this young population, early-onset presentation of COVID-19 resembled flu-like symptoms, except for smell and/or taste dysfunction. Risk factors for SARS-CoV-2 detection were return from regions with high incidence and contact with confirmed SARS-CoV-2 cases, particularly when tests were administered within the first 2 weeks after contact and/or onset of symptoms.
Keywords: Asymptomatic, Coronavirus, Coronavirus disease 2019, Olfaction disorders, Screening, Severe acute respiratory syndrome coronavirus 2
Introduction
Since its emergence in China [1], severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread at an extraordinary pace. In Germany, the first case of SARS-CoV-2 infection was detected on 27 January 2020 [2]. Germany rapidly implemented testing capacities for SARS-CoV-2, and the national Public Health institute (Robert Koch-Institute, RKI), provided recommendations for testing [3]. Charité—Universitätsmedizin Berlin opened the SARS-CoV-2 test site on Campus Virchow-Klinikum located in the northwest of Berlin 1 day after the first coronavirus disease 2019 (COVID-19) patient was identified in the city. The site aimed to provide testing for the general population, and to reduce workload for the emergency departments. Because clinical manifestations range from absent or unspecific signs to severe acute respiratory distress [4], individuals with COVID-19 may visit different medical departments, and so pose a risk of transmission among medical staff across the hospital [5].
Most clinical descriptions have focused on COVID-19 among hospitalized patients, with fever, fatigue, dry cough and shortness of breath as common symptoms [[6], [7], [8]]. Individuals with mild symptoms have not been investigated extensively, although this group may contribute to community transmission, thereby impeding containment efforts [9,10].
The Charité test site offered the possibility to consult individuals with suspicion of SARS-CoV-2 infection and to assess the proportion of infected individuals among outpatients during the early epidemic. The present analysis of >5000 individuals aims to describe the epidemiological characteristics and clinical manifestation of SARS-CoV-2 infection to identify factors associated with SARS-CoV-2 detection in an outpatient setting.
Methods
The first COVID-19 case in Berlin was identified on 2 March 2020. The test site commenced operations on 3 March 2020, with opening hours from 08:00 to 16:00.
Upon presentation, physicians interviewed patients via an intercom with visual contact through window screens. Taking of medical history and assessment of symptoms were guided by a questionnaire also available as a web application CovApp (https://covapp.charite.de/). If indicated, a combined oro- and nasopharyngeal swab was obtained and tested for SARS-CoV-2 using RT-PCR in the central hospital laboratory [11]. Other respiratory pathogens were not tested, and patients were not examined physically.
The decision on testing largely followed RKI recommendations, which changed over time. Until 23 March 2020, they comprised mainly symptomatic individuals either with contact to SARS-CoV-2 cases, or with return from an area of risk, as determined and continuously redefined by the RKI, within 14 days before disease. As of 24 March, only individuals with acute respiratory symptoms and with contact to confirmed SARS-CoV-2 cases, or those without contact depending on risk factors including occupation, age and underlying clinical conditions, remained (see Supplementary material, Table S1). If test capacity was sufficient, acute respiratory symptoms alone justified testing; ultimately, testing was based on the attending physician's discretion. Use of the test site was also influenced by the implementation of video consultations and an ordinance on contact restrictions effective 23 March 2020, stating that persons ‘shall stay in their home at all times’.
For the present analysis, ethical approval was obtained from Charité’s institutional review board (EA4/083/20). Data width and granularity differed partially between patients, because the designated risk areas were modified over time and impaired sense of smell/taste was added as a symptom. Data were continuously entered into the hospital information system. After extraction, data were pseudonymized for analysis.
Descriptive analyses comprised clinical and epidemiological factors among individuals with positive and negative test results (undetermined test results were labelled as negative), and among individuals with and without a SARS-CoV-2 test. Wilcoxon rank sum test was used to compare continuous data and χ2 test was used for categorical data. The primary analysis examined potential risk factors for SARS-CoV-2 infection. We calculated crude and adjusted odds ratios with 95% CI (Wald) with the outcome SARS-CoV-2-infected patient using logistic regression models. Independent variables available that made clinical sense for this analysis were age, sex, underlying diseases, referral types, travel destinations, duration since return from travel (no travel/return ≤7 days/return 8–14 days/return >14 days/return date missing), contact with known SARS-CoV-2 case, duration since last contact (no contact/contact ≤7 days/contact 8–14 days/contact >14 days/contact date missing), symptom types and duration since onset of symptoms (no symptoms/symptoms ≤7 days/symptoms 8–14 days/symptoms >14 days/symptom date missing). For parameters of duration, missing data were included as an own category. Sensitivity analyses among all individuals tested included (a) an analysis of factors potentially on the causal pathway of SARS-CoV-2 (excluding symptoms and times), (b) separate models for the first and last 4 weeks with different screening recommendations and initiation of contact/travel restrictions and (c) separate models with undetermined test results labelled positive. For all multivariable regression models, p values were calculated using type III test, which examines the significance of each partial effect in consideration of interaction with other effects in the model. Variable selection was stepwise forward with p < 0.05 for entering factors into the model, p < 0.06 for removing factors, and p < 0.05 was considered significant. No interactions were tested in the models. Age and sex were included in all models, and individuals for whom the data were not collected were included as ‘not applicable’ in the models. For each model, the c-statistic was calculated. Analyses were exploratory in nature. All analyses were performed using R (software), SPSS (IBM SPSS statistics, Somer, NY, USA) and SAS (SAS Institute, Cary, NC, USA).
Results
Patient attendance, positivity rate and associated factors
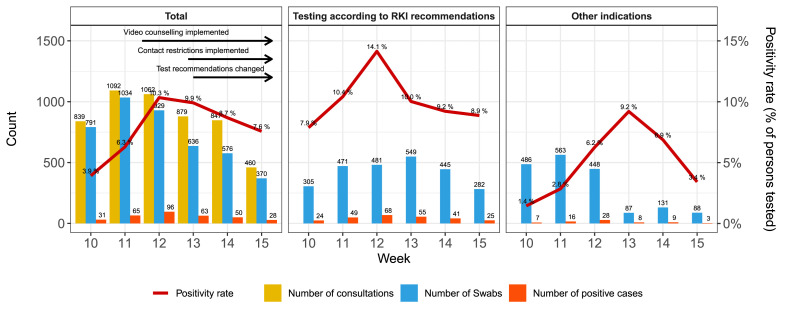
Between 3 March and 13 April 2020, 5179 patients consulted the test site (Fig. 1 ).
Fig. 1.
Patient attendance and SARS-CoV-2 positivity. (a) Number of consultations, tests and positive cases by calendar week (which are shifted by 1 day and start on Tuesday). (b) Number of patients tested and tested positive if decision on testing strictly followed RKI recommendations. (c) Number of tested patients missed by RKI recommendations.
Of the individuals attending, 83.7% (n = 4333) were tested for the presence of SARS-CoV-2, with 333 positive and 38 undetermined test results (7.7% and 0.9%, respectively). None of the individuals with an undetermined test result was tested again. Table 1 shows the basic patient characteristics of people tested, and Table S1 (see Supplementary material) shows a comparison of the individuals tested and not tested for SARS-CoV-2. The proportion of individuals tested among all individuals consulting declined over the course of the 6 weeks.
Table 1.
Basic characteristics of patients with SARS-CoV-2 among all patients tested (n = 4333)
| Total | Negative | Positive | Positive % among patients tested (total) | Crude OR (95% CI) | P valuea | |
|---|---|---|---|---|---|---|
| Patients | 4333 | 4000 | 333 | 7.69 | ||
| Age (years), median (IQR) | 34 (26–47) | 34 (26–47) | 34 (28–47) | 1.00 (1–1.01) | 0.269 | |
| Male sex, n (%) | 2127 (49.1%) | 1938 (48.5%) | 189 (56.8%) | 8.89 | 1.40 (1.12–1.75) | 0.004 |
| Chronic lung disease | 418 (9.6%) | 388 (9.7%) | 30 (9%) | 7.18 | 0.92 (0.62–1.36) | 0.682 |
| Chronic heart disease | 259 (6%) | 244 (6.1%) | 15 (4.5%) | 5.79 | 0.73 (0.43–1.24) | 0.241 |
| Diabetes | 108 (2.5%) | 101 (2.5%) | 7 (2.1%) | 6.48 | 0.83 (0.38–1.8) | 0.635 |
| Obesity | 135 (3.1%) | 126 (3.2%) | 9 (2.7%) | 6.67 | 0.85 (0.43–1.7) | 0.652 |
| Referral | ||||||
| Charité employee | 433 (10%) | 414 (10.4%) | 19 (5.7%) | 4.39 | 0.52 (0.33–0.84) | 0.008 |
| Without referral | 1843 (42.5%) | 1675 (41.9%) | 168 (50.5%) | 9.12 | 1.43 (1.14–1.79) | 0.002 |
| Referral from doctor | 558 (12.9%) | 529 (13.2%) | 29 (8.7%) | 5.20 | 0.61 (0.41–0.9) | 0.013 |
| Otherb | 644 (14.9%) | 597 (14.9%) | 47 (14.1%) | 7.30 | 0.88 (0.65–1.18) | 0.387 |
| No data | 855 (19.7%) | 785 (19.6%) | 70 (21%) | 8.19 | 1.09 (0.83–1.44) | 0.539 |
| Travel | ||||||
| No travel | 3309 (76.4%) | 3066 (76.7%) | 243 (73%) | 7.34 | 0.82 (0.64–1.06) | 0.130 |
| Austria | 119 (2.7%) | 96 (2.4%) | 23 (6.9%) | 19.33 | 3.02 (1.89–4.83) | <0.0001 |
| USA | 21 (0.5%) | 17 (0.4%) | 4 (1.2%) | 19.05 | 2.85 (0.96–8.52) | 0.061 |
| Switzerland | 31 (0.7%) | 28 (0.7%) | 3 (0.9%) | 9.68 | 1.29 (0.39–4.27) | 0.677 |
| Spain | 44 (1%) | 40 (1%) | 4 (1.2%) | 9.09 | 1.2 (0.43–3.39) | 0.725 |
| France | 33 (0.8%) | 30 (0.8%) | 3 (0.9%) | 9.09 | 1.2 (0.37–3.96) | 0.761 |
| Within Germany | 228 (5.3%) | 209 (5.2%) | 19 (5.7%) | 8.33 | 1.1 (0.68–1.78) | 0.706 |
| Italy | 244 (5.6%) | 235 (5.9%) | 9 (2.7%) | 3.69 | 0.45 (0.23–0.87) | 0.019 |
| Otherc | 260 (6%) | 241 (6%) | 19 (5.7%) | 7.31 | 0.94 (0.58–1.53) | 0.814 |
| Destination missing | 44 (1%) | 38 (1%) | 6 (1.8%) | 13.64 | 1.91 (0.8–4.56) | 0.143 |
| Time since return (days), median (IQR) | 7 (3–1) | 7 (3–14) | 9 (3–15) | 0.635 | ||
| No travel | 3309 (76.4%) | 3066 (76.7%) | 243 (73%) | 7.34 | 1 = reference | 0.245d |
| ≤7 days | 403 (9.3%) | 367 (9.2%) | 36 (10.8%) | 8.93 | 1.24 (0.86–1.79) | 0.254 |
| 8–14 days | 264 (6.1%) | 245 (6.1%) | 19 (5.7%) | 7.20 | 0.98 (0.6–1.59) | 0.930 |
| >14 days | 233 (5.4%) | 207 (5.2%) | 26 (7.8%) | 11.16 | 1.59 (1.03–2.43) | 0.035 |
| Return date missing | 124 (2.9%) | 115 (2.9%) | 9 (2.7%) | 7.26 | 0.99 (0.5–1.97) | 0.971 |
| Contact | ||||||
| Contact to confirmed COVID-19 case | 1645 (38%) | 1456 (36.4%) | 189 (56.8%) | 11.49 | 2.29 (1.83–2.88) | <0.0001 |
| Time since last contact (days), median (IQR) | 6 (3–9) | 6 (3–9) | 6 (3–8) | 0.707 | ||
| No contact | 2688 (62%) | 2544 (63.6%) | 144 (43.2%) | 5.36 | 1 = reference | <0.0001d |
| ≤7 days | 815 (18.8%) | 723 (18.1%) | 92 (27.6%) | 11.29 | 2.25 (1.71–2.96) | <0.0001 |
| 8–14 days | 503 (11.6%) | 433 (10.8%) | 70 (21%) | 13.92 | 2.86 (2.11–3.87) | <0.0001 |
| >14 days | 134 (3.1%) | 125 (3.1%) | 9 (2.7%) | 6.72 | 1.27 (0.63–2.55) | 0.499 |
| Contact date missing | 193 (4.5%) | 175 (4.4%) | 18 (5.4%) | 9.33 | 1.82 (1.09–3.04) | 0.023 |
| Symptoms | ||||||
| Presence of any symptoms | 3890 (89.8%) | 3569 (89.2%) | 321 (96.4%) | 8.25 | 3.23 (1.80–6.37) | <0.0001 |
| Time since symptom onset (days), median (IQR) | 4 (2–7) | 4 (2–7) | 4 (2–7) | 0.440 | ||
| No symptoms | 443 (10.2%) | 431 (10.8%) | 12 (3.6%) | 2.71 | 1 = reference | <0.0001d |
| ≤7 days | 2060 (47.5%) | 1877 (46.9%) | 183 (55%) | 8.88 | 3.50 (1.94–6.34) | <0.0001 |
| 8–14 days | 700 (16.2%) | 629 (15.7%) | 71 (21.3%) | 10.14 | 4.05 (2.17–7.57) | <0.0001 |
| >14 days | 330 (7.6%) | 316 (7.9%) | 14 (4.2%) | 4.24 | 1.59 (0.73–3.49) | 0.246 |
| Symptom date missing | 800 (18.5%) | 747 (18.7%) | 53 (15.9%) | 6.63 | 2.55 (1.35–4.82) | 0.004 |
| Recommended for testing by RKI | 2530 (58.4%) | 2268 (56.7%) | 262 (78.7%) | 10.36 | 2.82 (2.15–3.69) | <0.0001 |
IQR, interquartile range; RKI, Robert Koch-Institute.
Data are n (column %) unless indicated otherwise.
χ2 test.
Other referrals included referral via hotline, from local public health authorities or from employer.
Other countries included China, Iran and other countries with n < 10 travellers.
Type III test.
As outlined above, RKI test recommendations broadened over time. As of 24 March, suspected cases without epidemiological link were included. RKI recommendations were followed in 58.4% (2530/4333) of individuals. In this subgroup, test positivity was more than twice as high compared with tests without recommendation (10.4%, 262/2530 versus 3.9%, 71/1803; Fig. 1). Among all individuals that tested positive, 21.3% were identified outside the RKI recommendations (71/333).
In addition, we analysed several subgroups. On 6 March, local health authorities informed the public of an individual with COVID-19 who had visited a nightclub on 29 February. Between 6 March and 16 March, 94 persons who had visited the club the same evening presented at the test site. Of those, 27.7% (26/94) tested positive, representing 74.2% (23/31) of all confirmed cases at the test site during the first week. RKI would not have recommended 23.4% (22/94) of club visitors for testing, of whom 27.3% (6/22) had a positive test result.
Charité employees comprised 8.6% of the attendees (447/5179), and among those tested (433/4333), 4.4% (19/433) were positive. Of all Charité employees tested, 28.9% (125/433) did not report any symptoms, compared with 8.2% (318/3900) of other patients. RKI recommendations would have classified 45.5% of Charité employees for testing (197/433), and 26.3% (5/19) of those tested positive would have been missed. Previous contact with a confirmed case was reported by 56.3% (232/433) of Charité employees and 36.3% (1413/3900) of other patients; test results of Charité employees with contact were less often positive (2.4%, 8/232 versus 12.8%, 181/1413).
Clinical presentation of SARS-CoV-2 infection
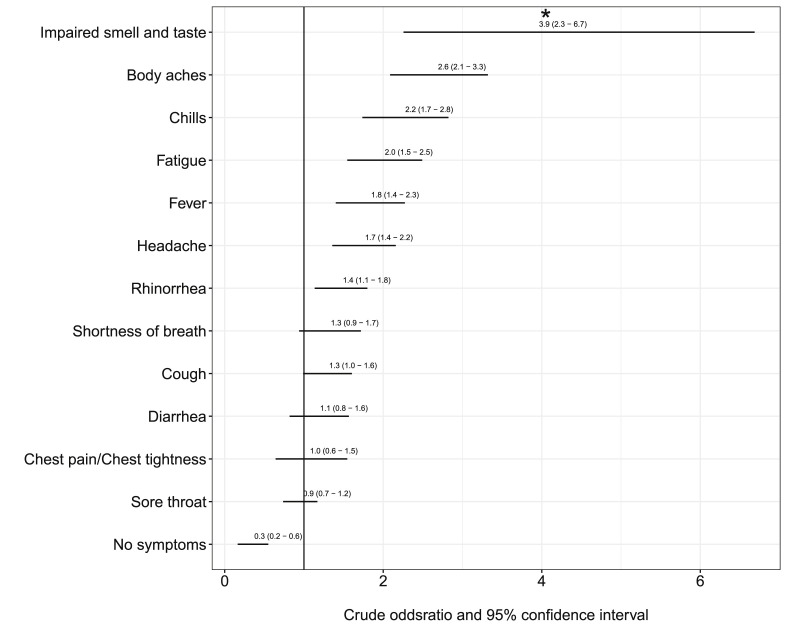
The clinical picture of individuals who tested positive was, although still mild, relatively more severe, with higher proportions of body aches, chills, fatigue, fever, headache and rhinorrhoea (Table 2 , Fig. 2 ). SARS-CoV-2-positive patients showed an increased proportion of affected sense of smell/taste compared with individuals who tested negative, but 72.4% (21/29) also showed symptoms of rhinorrhoea. Chemosensory dysfunction was the only symptom with some use to differentiate between SARS-CoV-2 infection and other respiratory illness with 85.6% specificity (95% CI 82.1%–88.1%); its sensitivity in detecting SARS-CoV-2 infection was 39.7% (95% CI 28.5%–51.0%); positive and negative predictive values were 20.6% (95% CI 13.9%–27.2%) and 93.8% (95% CI 91.0%–95.6%), respectively.
Table 2.
Symptoms at presentation among individuals tested positive or negative for SARS-CoV-2
| Symptom | Negative (n = 4000) |
Positive (n = 333) |
p value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| No symptoms | 431 | 10.8 | 12 | 3.6 | <0.001 |
| Fever | 968 | 24.2 | 121 | 36.3 | <0.001 |
| Shortness of breath | 597 | 14.9 | 61 | 18.3 | 0.098 |
| Chest tightness/pain | 308 | 7.7 | 26 | 7.8 | 0.943 |
| Chills | 827 | 20.7 | 122 | 36.6 | <0.001 |
| Fatigue | 1888 | 47.2 | 212 | 63.7 | <0.001 |
| Body aches | 1145 | 28.6 | 171 | 51.4 | <0.001 |
| Cough | 2405 | 60.1 | 218 | 65.5 | 0.056 |
| Rhinorrhoea | 1594 | 39.9 | 162 | 48.6 | 0.002 |
| Diarrhoea | 547 | 13.7 | 51 | 15.3 | 0.405 |
| Sore throat | 1984 | 49.6 | 159 | 47.7 | 0.516 |
| Headache | 1713 | 42.8 | 187 | 56.2 | <0.001 |
| Impaired smell/taste (n = 850) | 112/777 | 14.4 | 29/73 | 39.7 | <0.001 |
Fig. 2.
Symptoms of patients tested positive and negative for SARS-CoV-2; crude, unadjusted odds ratios. Asterisk represents findings for a subset of n = 850 patients.
Table 3 presents the multivariate analysis of factors associated with the detection of SARS-CoV-2 infection.
Table 3.
Multivariate analysis of factors associated with of SARS-CoV-2 infection
| Adjusted odds ratio | 95% CI | |
|---|---|---|
| Age (years) | 1.011 | (1.002–1.019) |
| Male sex | 1.397 | (1.103–1.769) |
| Chronic heart disease | 0.546 | (0.307–0.971) |
| Without referral | 1.42 | (1.122–1.798) |
| Contact to known SARS-CoV-2 case | ||
| No contact | 1 = reference | |
| ≤7 days | 3.177 | (2.361–4.274) |
| 8–14 days | 3.415 | (2.475–4.711) |
| >14 days | 1.283 | (0.619–2.663) |
| Last date of contact missing | 2.527 | (1.462–4.367) |
| Travel | ||
| Travel to USA | 3.893 | (1.241–12.207) |
| Travel to Austri | 4.163 | (2.48–6.989) |
| Time since onset of symptoms. | ||
| Reference no symptom | 1 = reference | |
| ≤7 days | 2.674 | (1.426–5.016) |
| 8–14 days | 3.613 | (1.871–6.977) |
| >14 days | 1.498 | (0.661–3.396) |
| Date symptom onset missing | 2.355 | (1.208–4.592) |
| Symptom type | ||
| Fever | 1.412 | (1.081–1.843) |
| Chills | 1.496 | (1.136–1.972) |
| Body aches | 2.155 | (1.664–2.792) |
| Sore throat | 0.613 | (0.479–0.783) |
| Impaired sense of smell/tastea | ||
| No impaired senste of smell/taste | 1 = reference | |
| No data | 1.028 | (0.723–1.462) |
| Impaired senste of smell/taste | 4.076 | (2.365–7.025) |
Parameters included in the model were age, sex, underlying diseases, referral types, duration since return from travel, travel destinations, duration since last contact, duration since onset of symptoms and symptom types.
Data collected in a subset of patients (n = 850); c-statistic (area unter the curve) = 0.741.
The probability of detecting SARS-CoV-2 was influenced not only by patient factors, symptomatology and travel to high-prevalence countries, but also by time since disease onset or last contact with confirmed cases.
Sensitivity analyses for the first 4 weeks and last 4 weeks confirmed the main findings from primary analysis (see Supplementary material, Table S2). Separate models for factors supposedly on the causal pathway, for the entire 6 weeks and also broken down to the first and last 4 weeks identified the same risk factors, except for a protective effect for Charité employees (see Supplementary material, Table S3). Risk factors associated with exposure were more pronounced in the first 4 weeks, before the test recommendations changed and contact restrictions were imposed (see Supplementary material, Tables S2 and S3). Sensitivity analysis with undetermined laboratory results labelled positive instead of negative confirmed all previous findings (see Supplementary material, Tables S4 and S5).
Discussion
In this description of SARS-CoV-2-infected individuals from the early epidemic in Berlin, Germany, most patients were young and showed mild symptoms of a respiratory tract infection. Important risk factors for detection of SARS-CoV-2 infection were recent contact with a positive case, travel to designated areas of risk, recent onset of symptoms, symptoms including impaired sense of smell/taste and unspecific symptoms such as fever, chills and body aches.
As of 14 April, Berlin counted 4668 cases [12], of whom 333 had been identified at the Charité test site (7%). If we had tested individuals strictly according to RKI recommendations, more than 20% of SARS-CoV-2-infected individuals attending the site would have been missed and could have contributed to additional silent community transmission. This illustrates the delicate balance between efficient use of limited resources and broadening test indications to identify otherwise undetected carriers.
Travelling abroad was a risk factor for SARS-CoV-2 infection, particularly in the weeks following winter holidays in February. Among risk areas as determined by RKI, returnees from Austria, a popular destination for winter sports, had a four times increased chance to have a positive test result. Anecdotally, a single bar in an Austrian ski resort was a transmission hotspot, which only became apparent with increasing numbers of sick returnees [13,14]. In contrast, extensive media coverage on the COVID-19 tragedy in Italy might have alerted the public and caused returnees from non-affected Italian regions to attend the test site. This could explain why returnees from a country with a high case number had reduced odds of infection.
Unsurprisingly, exposure to individuals with COVID-19 was associated with SARS-CoV-2 infection. However, a considerable number of exposed individuals tested negative, including household members. As contact persons are usually tested only once and tests could be performed either too early or too late to detect viral RNA, negative test, the results should be interpreted with caution. Secondary-attack rates for household members have been estimated to be around 16% [15]. The extent of individual variation in the number of secondary cases (dispersion) may be substantial, but is undetermined [16]. The transmission events within one crowded nightclub support temporary lock-down strategies and stress the importance of rapid contact tracing in potential superspreading events and isolation of cases without waiting for screening results to contain the outbreak.
The clinical suspicion of SARS-CoV-2 infection based on symptoms proved virtually impossible, as the pandemic reached Berlin during the peak of influenza and cold season [17]. Almost two-thirds of patients presented with cough, but respiratory symptoms did not predict positive test results. Only an impaired sense of smell/taste was clearly increased among SARS-CoV-2-infected patients, confirming olfactory disorders as potential early symptoms of COVID-19 [18,19]. We may have found symptoms more specific of SARS-CoV-2 if we had compared with other diseases, which was beyond the scope of the test site.
In our study, the proportion of asymptomatic cases was relatively small, contrasting with higher numbers reported elsewhere [20]. One obvious explanation is the largely symptom-based screening. However, more than one in four among hospital staff was asymptomatic upon presentation, indicating the risk of undetected nosocomial transmission.
Symptom/risk-based screening is widely used to contain the epidemic [21] and may yield more diagnoses compared with a less restricted strategy, but detection of SARS-CoV-2 clearly depended on the time since the symptoms began. Some individuals may have been tested beyond the detection period, as viral RNA concentrations appear to decline rapidly after infection, and could fall below detection thresholds after day 5 of disease onset [22]. Considering the threat posed by silent transmission through asymptomatic carriage, less restricted screening approaches may be crucial to interrupt transmission chains with potentially dramatic consequences if health-care staff or potential superspreaders are affected. Because screening without repeated sampling in persons tested negative may lead to a false sense of security, especially in settings with a high pre-test probability, targeted screening programmes at potential transmission hubs, such as personnel in long-term care facilities or schools, should encompass repeated sampling. Sentinel surveillance based on symptoms and/or absence from work, school or kindergartens could complement laboratory-based test strategies, speed up detection of clusters and trigger public health action, for example partial school closures, contact tracing and targeted testing.
There are limitations to our findings' validity and representativeness. The patient population may have changed over time, as did testing recommendations. Initial media reports on long waiting times and contact restrictions might have prevented individuals from consulting the test site. Some individuals could have exaggerated sympoms to obtain a test, while others may have been denied testing before they developed symptoms.
Conclusions
These data from the first operational SARS-CoV-2 test site in Berlin illustrate the predominantly mild disease resembling common cold or influenza among outpatients. Impaired sense of smell/taste may have value as a clinical symptom to guide the decision on whether or not to test individuals, but warrants further evaluation. The absence of severe cases might result from the outpatient setting, the early phase of the epidemic, access to testing and the young patient population.
Typical risk factors such as travel to high-incidence regions, contact with positive individuals and gatherings were identified for the majority of the patients, whereas the source of infection remained unsolved for others. The impact of a single spreading event (nightclub) on the early case count in Berlin cautions against using premature data for extrapolating figures. In light of potential transmission from mildly symptomatic and asymptomatic infected individuals, potential superspreading events should entail rapid contact tracing and subsequent isolation regardless of testing, which may be a slow and insecure tool if the chance for contacts to be infected is high.
All screening strategies depend on public acceptance, access to and usage of testing capacities, and testing recommendations, among other factors. Broad-scale testing targeted at high-risk populations such as travel returnees from high-incidence regions and contact with confirmed cases should be scheduled ideally within the first 2 weeks after contact and/or onset of symptoms.
Transparency declaration
The authors declare that they have no conflicts of interest.
No specific funding was received for this work.
Contributions
FM prepared the manuscript, and collated and analysed data. MG contributed to the manuscript, and implemented and coordinated testing and data collection. JH edited the manuscript and coordinated data collection. WvL collated and analysed data, and prepared the figures. FS analysed data and BP analysed data, edited the manuscricpt and oversaw testing. SR and MB extracted data and FH and AKL collected data and edited the manuscript. FK collected and collated data, and edited the manuscript. SB collected and analysed data. HR coordinated the testing site and CH, VK and AT coordinated digital data collection (covapp.de). PG oversaw testing and edited the manuscript; FPM oversaw testing, data collection and analysis, and edited the manuscript; and JS directed roll-out and edited the manuscript.
Acknowledgements
We thank all medical and non-medical Charité staff who contributed to the rapid and effective implementation of patient management, testing and data collection at the SARS-CoV-2-testing site Charité—Universitätsmedizin Berlin, Campus Virchow-Klinikum.
Editor: L. Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.08.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV Infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert Koch Institute COVID-19-Verdacht: Maßnahmen und Testkriterien - Orientierungshilfe für Ärzte 2020. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Massnahmen_Verdachtsfall_Infografik_Tab.html Available from:
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H. Early lessons from the frontline of the 2019-nCoV outbreak. Lancet. 2020;395:687. doi: 10.1016/S0140-6736(20)30356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Xu H., Rebaza A., Sharma L., Dela Cruz C.S. Protecting health-care workers from subclinical coronavirus infection. Lancet Resp Med. 2020;8 doi: 10.1016/S2213-2600(20)30066-7. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:1–8. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert Koch Institute . Robert Koch Institute; Berlin, Germany: 2020. Coronavirus disease 2019 (COVID-19) daily situation report of the Robert Koch Institute. 14.04.2020. [Google Scholar]
- 13.Felbermayr G., Hinz J., Chowdhry S. Apres-ski: the spread of coronavirus from Ischgl through Germany. Covid Econ Vetted Real-Time Pap. 2020;22:177–204. [Google Scholar]
- 14.Rudan I. A cascade of causes that led to the COVID-19 tragedy in Italy and in other European Union countries. J Glob Health. 2020;10:1–10. doi: 10.7189/jogh-10-010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W., Zhang B., Lu J., Liu S., Chang Z., Cao P. The characteristics of household transmission of COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa450. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020;25:2000058. doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Activity of acute respiratory infections in Germany. 2020. https://influenza.rki.de/MapArchive.aspx [Internet]. (cited 16 April 2020). Available from:
- 18.Hopkins C., Surda P., Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020;58(3):295–298. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 19.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan. Eurosurveillance. 2020;25:1–5. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gostic Katelyn. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. eLife. 2020;9:1–18. doi: 10.7554/eLife.55570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.