Abstract
Background:
GlycA, a nuclear magnetic resonance composite marker of systemic inflammation, reflects serum concentration and glycosylation state of main acute phase reactants. Prior studies have shown plasma GlycA levels were associated with cardiovascular disease (CVD) even after adjusting for other inflammatory markers. However, little is known about the association of GlycA with the heart failure (HF) subtypes: preserved (HFpEF) or reduced (HFrEF) ejection fraction. We examined the association of GlycA with incident HF and its subtypes in a multi-ethnic cohort.
Methods:
We studied 6,507 MESA participants aged 45–84 without baseline CVD or HF who had data on GlycA and incident hospitalized HF. We used multivariable-adjusted Cox hazards models to evaluate the association of GlycA with incident total HF, HFpEF and HFrEF. Models were adjusted for sociodemographics, CVD risk factors and inflammatory biomarkers.
Results:
The mean (SD) for age was 62 (10) years and for GlycA was 375 (82) μmol/L; 53% women. Over a median follow-up of 14.0 years, participants in the highest quartile of GlycA, compared to lowest, experienced increased risk of developing any HF [Hazard Ratio 1.48 (95% CI 1.01–2.18)] in fully-adjusted models. However, this increased risk was only seen for HFpEF [2.18 (1.15–4.13)] and not HFrEF [1.06 (0.63–1.79)]. There was no significant interaction by sex, age or race/ethnicity.
Conclusions:
GlycA was associated with an increased risk of any HF, and in particular, HFpEF. Future studies should examine mechanisms that might explain differential association of GlycA with HF subtypes, and whether therapeutic lowering of GlycA can prevent HFpEF development.
Registration:
URL: https://clinicaltrials.gov; Unique Identifier: NCT00005487
The global burden of heart failure (HF) is tremendous, affecting over 23 million people worldwide.1 The estimated prevalence of HF is expected to increase by 46% from 2012 to 2030, which translates to more than 8 million people in the U.S. alone, with a corresponding increase in total healthcare cost from $21 billion in to $53 billion.2 Thus, prevention and early detection of HF is imperative to improve clinical outcomes and reduce healthcare costs. The American College of Cardiology (ACC)/American Heart Association (AHA) guidelines have classified HF into two broad categories: HF with reduced ejection fraction (HFrEF), and HF with preserved ejection fraction (HFpEF).3
Blood biomarkers may serve as prognostic and diagnostic tools to evaluate the risk of HF development, detect the presence of HF, and guide therapy. To date, numerous biomarkers have been studied and found associated with HF. Some examples are high-sensitivity C-Reactive Protein (hsCRP), interleukin-6 (IL-6), and tumor necrosis factor (TNF)-α (e.g., inflammatory markers), procollagen type III aminopropeptide, and interleukin 33/ST2 (extracellular matrix remodeling marker), natriuretic peptides (wall strain markers), copeptin (neurohormonal activation marker) and cardiac troponins (cardiomyocyte injury marker).4 The different types of biomarkers show the complexity of HF pathogenesis and progression.
Inflammatory activation has been shown to play a key role in HF pathogenesis.5, 6 Inflammatory cytokines levels are not only increased in HF patients, but also correlated with the severity of the disease.7 Cytokines like TNF-α can trigger electrolyte imbalance which contributes to adverse cardiac remodeling leading to HF.8 Notably, inflammatory biomarkers are elevated relatively early in the disease progress which makes them potentially useful in predicting risk of HF development unlike other biomarkers representing neurohormonal activation or wall strain which are elevated in more advanced disease states.9 Although inflammation can contribute to the whole spectrum of HF phenotypes, inflammation appears to be more strongly associated with HFpEF than with HFrEF.6, 10, 11 While several studies have shown the prognostic value of the inflammatory biomarkers in HF,12–15 their associations with incident HF have been inconsistent.16 Thus, there is interest in finding a more reliable marker which may better predict inflammatory-mediated HF risk.
GlycA has the potential to be a more superior biomarker for measuring inflammation and predicting cardiovascular disease (CVD) outcomes. GlycA is a novel biomarker for systemic inflammation that reflects the integrated concentrations and glycosylation states of several abundant acute-phase inflammatory proteins, including α1-acid glycoprotein, haptoglobin, α1-antitrypsin, α1-antichymotrypsin and transferrin.17 Measured by nuclear magnetic resonance (NMR) spectroscopy, GlycA can serve as a reliable biomarker compared to other common inflammatory markers, due to its low intra-individual variability and greater analytic precision.17 Prior studies from the Multi-Ethnic Study of Atherosclerosis (MESA) and other cohorts have shown higher plasma GlycA levels were associated with increased risk of CVD events, peripheral arterial disease, and mortality, even after adjusting for other inflammatory markers.14, 18–22 GlycA is also closely linked to obesity and other metabolic risk factors that predispose to cardiac remodeling.23 However, the association of GlycA with HF subtypes has not been previously evaluated. Since HFpEF and HFrEF have different etiologic profiles, comparing associations of GlycA with the different HF subtypes may lead to a discovery of a better prediction marker or therapeutic target for HF.
Therefore, we examined the association of GlycA with HF and its subtypes in a multi-ethnic cohort free of baseline CVD. Given the stronger association of other inflammatory markers with HFpEF than with HFrEF,6, 10, 11 we hypothesized that higher plasma GlycA levels will similarly have a stronger association with HFpEF than HFrEF, and that the association of GlycA with HFpEF will be independent of traditional CVD risk factors and other inflammatory markers.
Material and Methods
Transparency and Openness
Requests for access to MESA data can be made through the NIH BioLincc Open program at: https://biolincc.nhlbi.nih.gov/studies/mesa/
Study Sample
A detailed description of the MESA study design has been previously published.24 MESA is a concurrent cohort study which enrolled ethnically diverse men and women free of known CVD, including HF, at baseline from 6 different field centers in the United States to follow the progression of CVD, study its risk factors and assess its characteristics. At study entry, MESA recruited a total of 6,814 individuals from ages 45 to 84 years old, whose racial/ethnic distribution was 38% White, 28% African, 22% Hispanic, and 12% Asian. Since the initial visit between 2000 and 2002, there have been 5 subsequent visits, at which participant demographics, medical history, and physical examination results were collected, and continuous follow-up for clinical CVD events. Participants excluded from this study were those missing incident HF follow-up data, GlycA measurement at baseline, or covariate data. The primary analysis was comprised of 6,507 participants (Figure 1). The MESA study received approval from the institutional review boards at each participating field center and obtained informed consent from each study participant.
Figure 1.
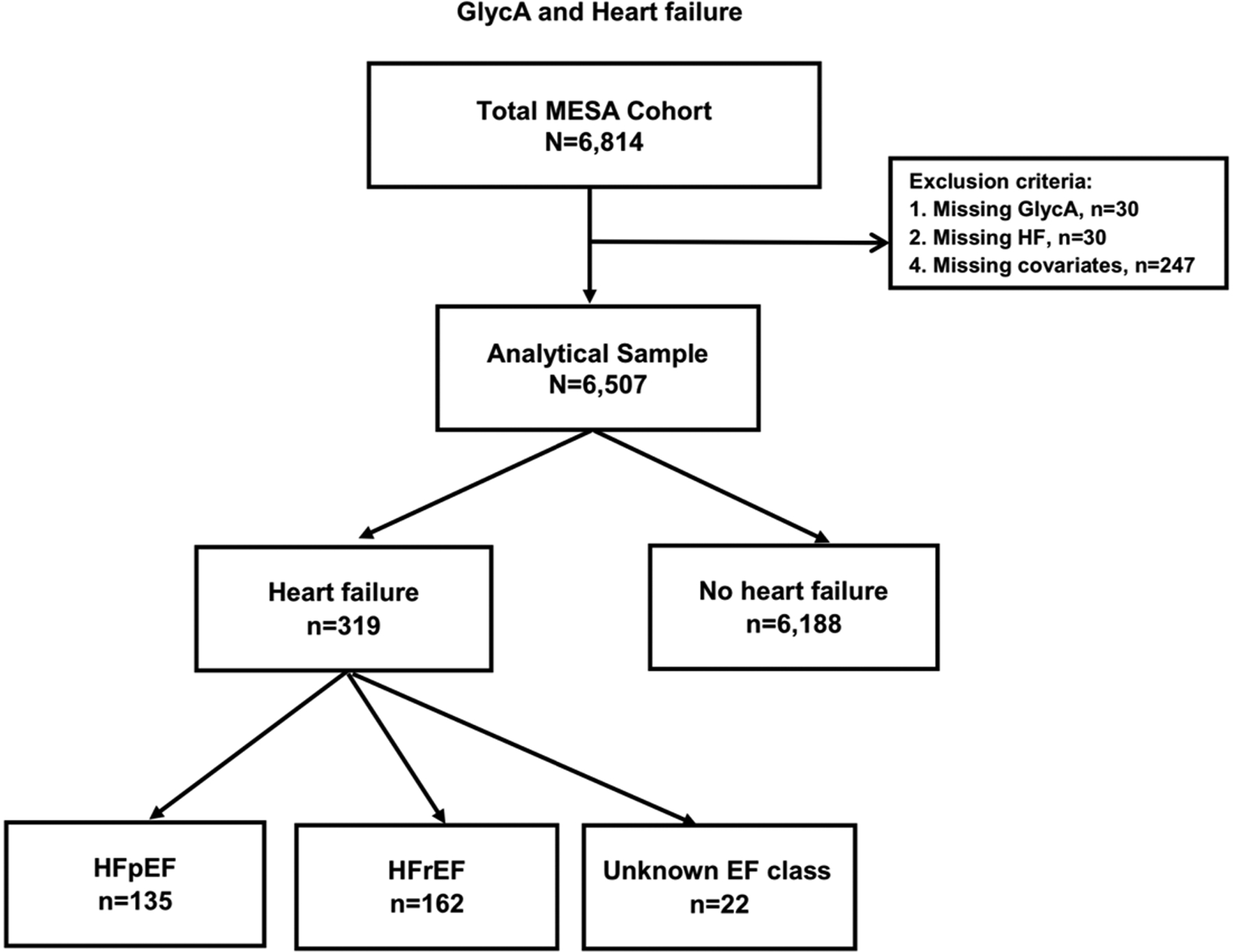
Flow diagram illustrating study sample inclusion and exclusion criteria.
GlycA Assessment
GlycA was measured from EDTA plasma samples drawn after a 12-hour fast during the MESA baseline visit (2000–2002) and stored at −70°C. The GlycA NMR signal was quantified by NMR LipoProfile® testing as previously described.14, 25 This assay detects the level of N-acetyl methyl group protons on the N-acetylglucosamine residues of the glycan portion of several abundant acute phase serum proteins, which are elevated during inflammation.17 The intra-assay and inter-assay coefficients of variation for the GlycA assay were 1.9% and 2.6%, respectively. Prior work from MESA found that the intraindividual variability of GlycA, assessed weekly for 5 weeks in 23 healthy volunteers, was 4.3%, lower than for hsCRP which was 29.2%.17 GlycA levels have previously been shown to be similar regardless of the length of storage, type of samples, and fasting state.17
Covariates
Covariates of interest for this analysis were obtained at visit 1 and included demographics (age, sex, race/ethnicity, and MESA site), behavioral factors (smoking status, pack-years of smoking, and physical activity), socioeconomic factors (education, health insurance), Body Mass Index (BMI), traditional CVD risk factors [systolic blood pressure (SBP), use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medication, diabetes and estimated glomerular filtration rate (eGFR)], and laboratory markers [N-terminal pro-B-type natriuretic peptide (NT-proBNP) and other inflammatory markers (hsCRP, IL-6, fibrinogen)].
Level of education was categorized into 9 levels: no schooling, grades 1–8, grades 9–11, completed high school/GED, some college but no degree, technical school certificate, associate degree, bachelor’s degree, graduate or professional school. Height and weight were measured according to the standard protocol, and BMI was calculated as weight divided by squared height (kg/m2). Total amount of moderate and vigorous physical activity was estimated in metabolic equivalent minutes per week using a 28-item Typical Week Physical Activity Questionnaire.26 Baseline systolic and diastolic blood pressure was measured in the seated position with the Dinamap automated blood pressure device, and the average of the last 2 out of 3 measurements were used in analyses. The chronic kidney disease epidemiology collaboration formula was used to calculate the eGFR.27 A positive diabetes status was determined by a fasting blood glucose level ≥126 mg/dL, self-reported diagnosis of diabetes, or use of diabetes medication, which was assess through a medication inventory approach. Other inflammatory biomarkers, including hsCRP, IL-6, and fibrinogen were measured from the stored plasma samples obtained at the baseline examination by methods previously reported.28, 29 NT-proBNP was measured using the Elecsys proBNP immunoassay (Roche Diagnostics Corporation, Indianapolis, IN).30
Outcomes Assessment
MESA study participants or their next of kin were contacted every 9 to 12 months after enrollment and asked about interim hospitalizations. Hospitalized HF events were adjudicated by a physician committee on the basis of the medical records using standardized criteria.31–33 We evaluated probable or definite HF events, with probable HF defined as a physician diagnosis and medical treatment for HF, and definite HF requiring at least one additional objective findings such as evidence of pulmonary congestion on chest X-ray, reduced left ventricular EF by echocardiography or ventriculography, or evidence of left ventricular diastolic dysfunction.31 HF events were censored in 2015, and dichotomized by EF reported in the hospital record and defined as HFpEF if EF ≥50% and HFrEF if EF <50%. There were not enough events to consider three categories of HF subtypes including HF with mid-range EF (40–49%). HF events with missing EF in the medical records were omitted from the analysis (Figure 1).
Statistical Analysis
We modeled plasma GlycA level in quartiles as well as continuously per one standard deviation (SD) increment. Descriptive statistics were used to present baseline characteristics by presence of HF or its subtypes, HFpEF or HFrEF. We presented continuous variables as means (SD) or median (interquartile interval, IQI), and categorical variables as frequency (percent). Participants were followed from the first visit until the development of a study endpoint, death, drop-out, or until December 31, 2015. Unadjusted HF incident rates were calculated per 1000-person years. We tested and satisfied the proportional hazards assumption using Schoenfeld residuals. Multivariable-adjusted Cox proportional hazard regression models were used to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) for the association of GlycA with incident total HF events and the subtypes of HFpEF or HFrEF.
We evaluated 4 progressively adjusted models. For model 1, we adjusted for demographics (age, sex, race/ethnicity, MESA site). In model 2, we adjusted for all model 1 covariates as well as socioeconomic, behavioral, and adiposity measures (education, health insurance, BMI, smoking status, pack-years of smoking, and physical activity). For model 3, we adjusted for CVD risk factors (SBP, use of antihypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medication, diabetes and eGFR) in addition to all covariates adjusted in model 2. In model 4 (our primary model), we further adjusted for other commonly studied inflammatory markers: hsCRP, IL-6, and fibrinogen, which were log-transformed for analysis. Finally, we performed a supplemental model (model 5) where we adjusted for the CVD risk factors in model 3 plus NT-proBNP, a biomarker of wall strain which may reflect subclinical HF.
We performed several additional analyses. First, we used restricted cubic splines adjusted for the variables in Model 4 with knots placed at the 5th, 25th, 65th, 95th percentiles to allow a more flexible distribution in characterizing the nonlinear association between GlycA (continuous) levels with incident HF, HFpEF, and HFrEF, Second, we tested for multiplicative effect modification of the association of GlycA with each HF subtype by age, sex, and race/ethnicity, also using Model 4. Third, we performed a sensitivity analysis examining the association of GlycA with HF and its subtypes after excluding individuals who had interim ASCVD (fatal and non-fatal coronary heart disease and stroke events; atherosclerotic CVD), using the same 4 primary models.
The analyses were performed using STATA version 15.0 (StataCorp LP, College Station, TX). P values were two-sided, with significance level set at 0.05.
Results
Baseline characteristics
The baseline characteristics of the 6,507 participants included in the analyses are shown in Table 1 by HF status. Among the sample, the mean (SD) for age was 62 (10) years with 53% being women, 39% White, 27% Black, 22% Hispanic, and 12% Chinese. The mean (SD) for plasma GlycA level was 375 (82) μmol/L. Over a median (IQI) follow-up time of 14.0 (11.5–14.7) years, a total of 319 participants (5%) experienced HF. Among those who experienced HF with available data on systolic function, we did not have data on the EF of 22 (7%) participants [i.e. HF with unknown subtype]. Of the remaining 297 participants, 135 (42%) people experienced HFpEF and 162 (51%) experienced HFrEF (Figure 1).
Table 1.
Baseline Characteristics of Participants by Heart Failure, Multi-Ethnic Study of Atherosclerosis (2002–2015)
| Total | HF | No HF | P value | HFpEF | HFrEF | P value | |
|---|---|---|---|---|---|---|---|
| N | 6,507 | 319 (5%) | 6,188 (95%) | 135 (42%)* | 162 (51%)* | ||
| † Age, years | 62 (10) | 68 (9) | 62 (10) | <0.001 | 69 (9) | 67 (9) | 0.03 |
| < 65 years | 3,667 (56%) | 95 (30%) | 3,572 (58%) | <0.001 | 34 (25%) | 57 (35%) | 0.06 |
| ≥ 65 years | 2,840 (44%) | 224 (70%) | 2,616 (42%) | 101 (75%) | 105 (65%) | ||
| Sex | |||||||
| Men | 3,065 (47%) | 191 (60%) | 2,874 (46%) | <0.001 | 66 (49%) | 112 (69%) | <0.001 |
| Women | 3,442 (53%) | 128 (40%) | 3,314 (54%) | 69 (51%) | 50 (31%) | ||
| Race/ethnicity | |||||||
| White | 2,532 (39%) | 130 (41%) | 2,402 (39%) | 0.04 | 59 (44%) | 62 (38%) | 0.007 |
| Chinese American | 787 (12%) | 23 (7%) | 764 (12%) | 14 (10%) | 4 (2%) | ||
| Black | 1,757 (27%) | 96 (30%) | 1,661 (27%) | 33 (24%) | 61 (38%) | ||
| Hispanic | 1,431 (22%) | 70 (22%) | 1,361 (22%) | 29 (21%) | 35 (22%) | ||
| Education | |||||||
| ≥ bachelor’s degree | 2,304 (35%) | 96 (30%) | 2,208 (36%) | 0.04 | 42 (31%) | 47 (29%) | 0.69 |
| < bachelor’s degree | 4,203 (65%) | 223 (70%) | 3,980 (64%) | 93 (69%) | 115 (71%) | ||
| † BMI, kg/m2 | 28 (5) | 30 (6) | 28 (5) | <0.001 | 30 (6) | 29 (5) | 0.14 |
| Current smoker | 832 (13%) | 45 (14%) | 787 (13%) | 0.007 | 16 (12%) | 27 (17%) | 0.50 |
| Former smoker | 2,390 (37%) | 140(44%) | 2,250 (36%) | 62 (46%) | 70 (43%) | ||
| Never smoker | 3,285 (50%) | 134 (42%) | 3,151 (51%) | 57 (42%) | 65 (40%) | ||
| ‡ Pack-years of smoking if >0, median (IQI) | 17 (6–33) | 20 (7–38) | 16 (6–32) | 0.01 | 21 (7–46) | 18 (7–31) | 0.03 |
| ‡ Physical activity MET-minutes/week | 4,028 (1,985–7,530) | 3,540 (1,680–6,480) | 4,050 (1,995–7576) | 0.17 | 3,255 (2100–5,330) | 3,966 (1,680–7,943) | 0.23 |
| † Systolic blood pressure, mmHg | 126 (22) | 138 (23) | 126 (21) | <0.001 | 139 (23) | 137 (23) | 0.54 |
| † eGFR, ml/min per 1.73m2 | 78 (16) | 72 (19) | 78 (16) | <0.001 | 73 (18) | 71 (19) | 0.48 |
| † Total cholesterol, mg/dL | 194 (35) | 190 (35) | 194 (35) | 0.05 | 189 (35) | 190 (37) | 0.88 |
| † HDL-C, mg/dL | 51 (15) | 48 (14) | 51 (15) | 0.001 | 50 (14) | 47 (13) | 0.04 |
| Diabetes mellitus | 810 (12%) | 91 (29%) | 719 (12%) | <0.001 | 36 (27%) | 45 (28%) | 0.97 |
| Antihypertensive medication | 2,405 (37%) | 188 (59%) | 2,217 (36%) | <0.001 | 80 (59%) | 93 (57%) | 0.75 |
| Lipid-lowering medication | 1,065 (16%) | 61 (19%) | 1,004 (16%) | 0.17 | 23 (17%) | 34 (21%) | 0.39 |
| ‡ NT-proBNP | 53 (24, 107) | 117 (59, 251) | 51 (23, 102) | <0.001 | 117 (61, 232) | 125 (59, 269) | 0.28 |
| ‡ GlycA, μmol/L | 375 (337–419) | 389 (352–438) | 374 (337–419) | <0.001 | 393 (361–447) | 381 (345–427) | 0.01 |
| ‡ hsCRP, mg/L | 1.9 (0.8–4.2) | 2.5 (1.1–5.0) | 1.9 (0.8–4.1) | 0.003 | 2.5 (1.1–5.6) | 2.4 (1.2–4.6) | 0.13 |
| ‡ IL-6 pg/mL | 1.2 (0.8–1.9) | 1.5 (1.1–2.5) | 1.2 (0.8–1.9) | <0.001 | 1.6 (1.1–2.7) | 1.5 (1.0–2.3) | 0.15 |
| ‡ Fibrinogen, mg/dL | 337 (295–388) | 352 (314–406) | 337 (294–387) | <0.001 | 349 (319–408) | 352 (303–402) | 0.38 |
Abbreviations: HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; BMI, body mass index; MET, metabolic equivalent of task; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6.
n=22 (7%) of HF cases were unknown subtype.
Data are presented as mean (standard deviation) for continuous variables and as count (percentages) for categorical variables, unless otherwise specified
Data are presented as median (IQI)
Those who developed incident total HF tended to be older, White, men, and have a higher BMI, median pack-years of smoking, SBP, prevalence of diabetes mellitus, higher median NT-proBNP, and higher median level of inflammatory biomarkers, such as GlycA, hsCRP, IL-6, and fibrinogen. In addition, they had lower average eGFR, total cholesterol, and HDL-C. Participants with any HF were also more likely to take medications for hypertension. Among people who developed any HF, those with HFpEF tended to be older, women, white, and have a higher median pack-years of smoking, and HDL-C than those with HFrEF. Moreover, patients with HFpEF showed elevated levels of GlycA compared to those with HFrEF. There was no significant difference in NT-proBNP levels between those with incident HFpEF and HFrEF.
Associations of GlycA with HF subtypes
The incidence rates and HRs for the association of GlycA with HF subtypes are shown in Table 2. Among our study participants, incidence rate (95% CI) per 1000 person-yrs for any HF, HFpEF and HFrEF were 4.0 (3.6–4.5), 1.7 (1.4–2.0) and 2.0 (1.7–2.4) respectively.
Table 2:
The Association of GlycA with Total HF and its subtypes of HFpEF and HFrEF: The Multi-Ethnic Study of Atherosclerosis (2000 to 2015)*
| GlycA in μmol/L [Median (IQI)] |
Quartile 1 [314 (294–327)] |
Quartile 2 [358 (348–367)] |
Quartile 3 [396 (386–406)] |
Quartile 4 [451 (434–477)] |
Per 1 SD (61 μmol/L) increment |
|---|---|---|---|---|---|
| Incidence rates (95% CI) and Hazard ratios (95% CI) of total HF events (n=319) by GlycA: N=6,507 § | |||||
| N | 1,647 | 1,608 | 1,631 | 1,621 | 6,507 |
| Cases | 58 | 74 | 78 | 109 | 319 |
| Incidence rate† | 2.8 (2.2–3.6) | 3.7 (2.9–4.6) | 3.9 (3.1–4.9) | 5.7 (4.7–6.9) | 4.0 (3.6–4.5) |
| Hazard Ratio‡ | |||||
| Model 1 | 1 (reference) | 1.30 (0.92–1.83) | 1.46 (1.03–2.06)* | 2.46 (1.76–3.43)* | 1.43 (1.29–1.59)* |
| Model 2 | 1 (reference) | 1.21 (0.86–1.71) | 1.26 (0.89–1.79) | 2.03 (1.44–2.86)* | 1.36 (1.21–1.52)* |
| Model 3 | 1 (reference) | 1.12 (0.79–1.59) | 1.13 (0.79–1.61) | 1.69 (1.19–2.40)* | 1.26 (1.13–1.42)* |
| Model 4 | 1 (reference) | 1.09 (0.77–1.55) | 1.06 (0.74–1.53) | 1.48 (1.01–2.18)* | 1.22 (1.07–1.39)* |
| Model 5 | 1 (reference) | 1.17 (0.82,1.66) | 1.13 (0.79,1.61) | 1.60 (1.12, 2.28)* | 1.20 (1.07,1.35)* |
| Incidence rates (95% CI) and Hazard ratios (95% CI) of HFpEF (n=135) by GlycA: N=6,507 § | |||||
| N | 1,647 | 1,608 | 1,631 | 1,621 | 6,507 |
| Cases | 18 | 30 | 36 | 51 | 135 |
| Incidence rate† | 0.9 (0.5–1.4) | 1.5 (1.0–2.1) | 1.8 (1.3–2.5) | 2.7 (2.0–3.5) | 1.7 (1.4–2.0) |
| Hazard Ratio‡ | |||||
| Model 1 | 1 (reference) | 1.67 (0.93–3.00) | 2.03 (1.14–3.60)* | 3.30 (1.88–5.79)* | 1.56 (1.33–1.82)* |
| Model 2 | 1 (reference) | 1.50 (0.83–2.71) | 1.65 (0.92–2.96) | 2.57 (1.44–4.59)* | 1.47 (1.24–1.74)* |
| Model 3 | 1 (reference) | 1.42 (0.78–2.57) | 1.60 (0.88–2.88) | 2.38 (1.32–4.29)* | 1.41 (1.19–1.67)* |
| Model 4 | 1 (reference) | 1.40 (0.77–2.54) | 1.55 (0.85–2.84) | 2.18 (1.15–4.13)* | 1.41 (1.15–1.71)* |
| Model 5 | 1 (reference) | 1.47 (0.81,2.67) | 1.61 (0.89,2.91) | 2.41 (1.33, 4.37)* | 1.38 (1.17, 1.64)* |
| Incidence rates (95% CI) and Hazard ratios (95% CI) of HFrEF (n=162) by GlycA: N=6,507 § | |||||
| N | 1,647 | 1,608 | 1,631 | 1,621 | 6,507 |
| Cases | 38 | 38 | 37 | 49 | 162 |
| Incidence rate† | 1.8 (1.3–2.5) | 1.9 (1.4–2.6) | 1.9 (1.3–2.6) | 2.6 (1.9–3.4) | 2.0 (1.7–2.4) |
| Hazard Ratio‡ | |||||
| Model 1 | 1 (reference) | 1.02 (0.65–1.60) | 1.09 (0.69–1.73) | 1.81 (1.16–2.82)* | 1.28 (1.10–1.50)* |
| Model 2 | 1 (reference) | 0.98 (0.62–1.55) | 0.98 (0.61–1.56) | 1.57 (0.99–2.48) | 1.22 (1.04–1.45)* |
| Model 3 | 1 (reference) | 0.91 (0.58–1.44) | 0.84 (0.53–1.35) | 1.24 (0.78–1.98) | 1.12 (0.94–1.33) |
| Model 4 | 1 (reference) | 0.88 (0.55–1.39) | 0.78 (0.48–1.27) | 1.06 (0.63–1.79) | 1.05 (0.86–1.28) |
| Model 5 | 1 (reference) | 0.94 (0.59,1.48) | 0.84 (0.52,1.35) | 1.08 (0.66,1.74) | 1.03 (0.87,1.22) |
Abbreviations: HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; CI, confidence interval
Statistically significant results at P <0.05
Incidence rates reported are per 1000 person-years.
Hazard ratios are adjusted as follows:
Model 1: adjusted for age, sex, and race/ethnicity, MESA site
Model 2: model 1 plus education, health insurance, BMI, smoking status, pack-years of smoking, and physical activity.
Model 3: model 2 plus systolic blood pressure, use of antihypertensive medication, total cholesterol, HDL- cholesterol, use of lipid-lowering medications, diabetes and eGFR
Model 4: model 3 plus ln(CRP), ln(IL-6) and ln(Fibrinogen)
Model 5: model 3 plus ln(NT-ProBNP)
Using GlycA quartiles, P = 0.47, 0.54 and 0.77 for interaction by sex for HF, HFpEF and HFrEF, respectively; P= 0.82, 0.60 and 0.86 for interaction by age for HF, HFpEF and HFrEF, respectively; P = 0.71, 0.18 and 0.60 for interaction by race for HF, HFpEF and HFrEF, respectively.
After adjusting for demographics, such as age, sex, race/ethnicity, and MESA site (model 1), compared to the lowest GlycA quartile, the adjusted HRs (95% CI) for incident total HF were 1.30 (0.92–1.83), 1.46 (1.03–2.06), and 2.46 (1.76–3.43) for the 2nd, 3rd and 4th quartiles, respectively, showing statistically significant associations between any incident HF and the top two quartiles of GlycA. The highest GlycA quartile remained significantly positively associated with any HF after further adjustment for socioeconomic and behavioral factors (model 2), CVD risk factors (model 3), and other biomarkers for inflammation (model 4), but the association was attenuated.
The adjusted HRs (95% CI) for HFpEF using model 1 were 1.67 (0.93–3.00), 2.03 (1.14–3.60), and 3.30 (1.88–5.79) for the 2nd, 3rd and 4th quartiles of GlycA, compared to the 1st quartile. Having a similar pattern with the overall HF analysis, the top two quartiles of GlycA showed statistically significant association with HFpEF, and the highest quartile remained positively associated with HFpEF in all models. Notably in the primary model 4 which was adjusted for CVD risk factors and other inflammatory markers, there was a 2-fold increased risk of incident HFpEF for the highest quartile of GlycA compared to the lowest [2.18 (1.15–4.13)].
The adjusted HRs (95% CI) for HFrEF using model 1 were 1.02 (0.65–1.60), 1.09 (0.69–1.73), and 1.81 (1.16–2.82). Unlike for HFpEF, HFrEF was only significantly positively associated with the highest GlycA quartile, and was no longer statistical significant upon additional adjustments in models 2, 3, and 4.
Of note, in our primary model 4, which adjusted for CVD risk factors and all of the inflammatory markers in the same model, while GlycA was associated with any HF and HFpEF risk, there was no independent association of hsCRP, IL-6, or fibrinogen with either HF outcome in this mutually-adjusted model. Additionally in our exploratory model adjusting for CVD risk factors plus NT-proBNP (model 5), GlycA remained still significantly associated with any HF and with HFpEF
There were no significant interactions of GlycA with either HF subtypes by sex, age, or race/ethnicity (p-values shown in footnote to Table 2). In restricted cubic spline models, the association of GlycA with risk of any HF and its subtypes was generally linear for all outcomes, but was stronger for HFpEF than HFrEF (Figure 2).
Figure 2.
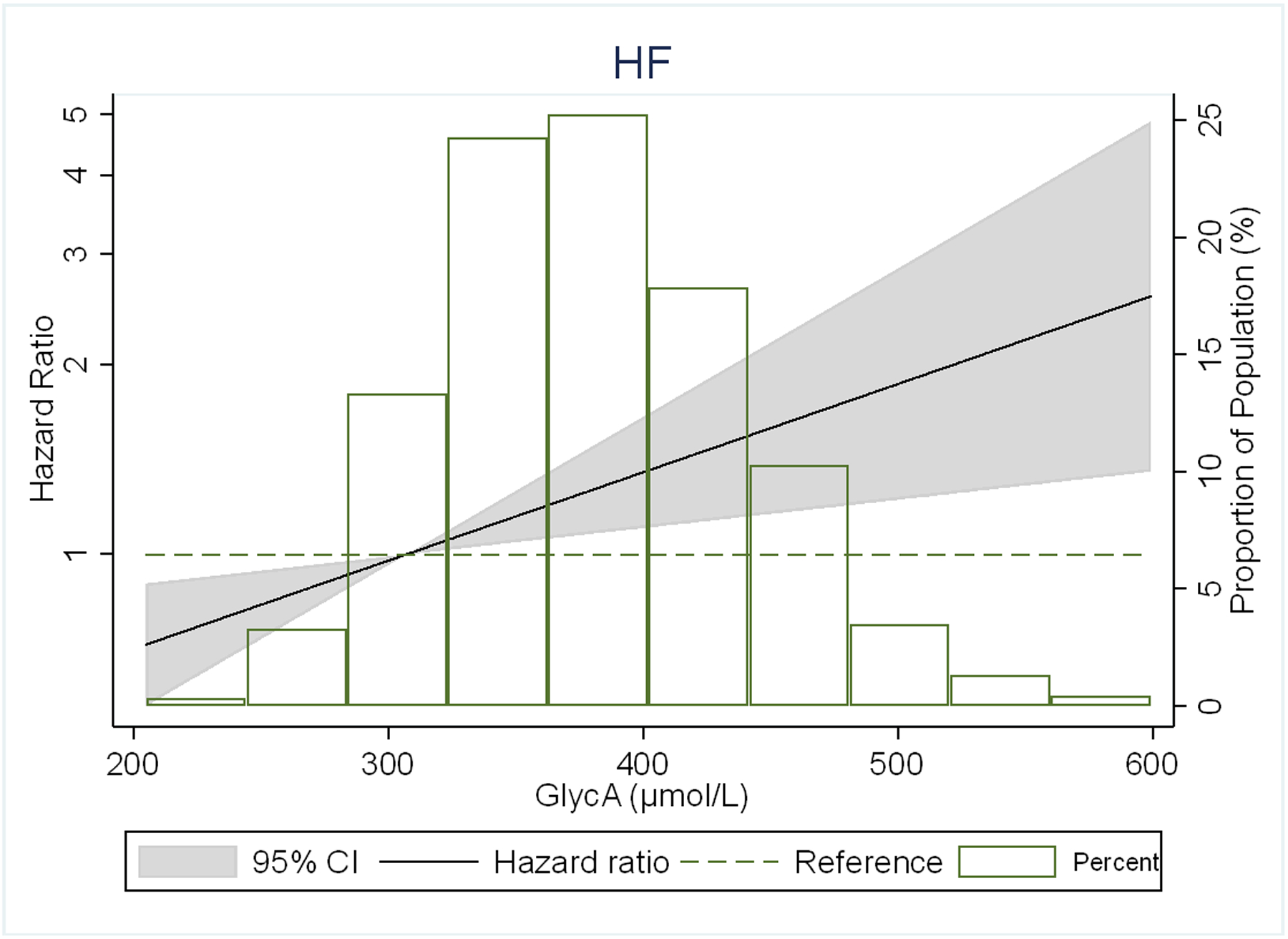
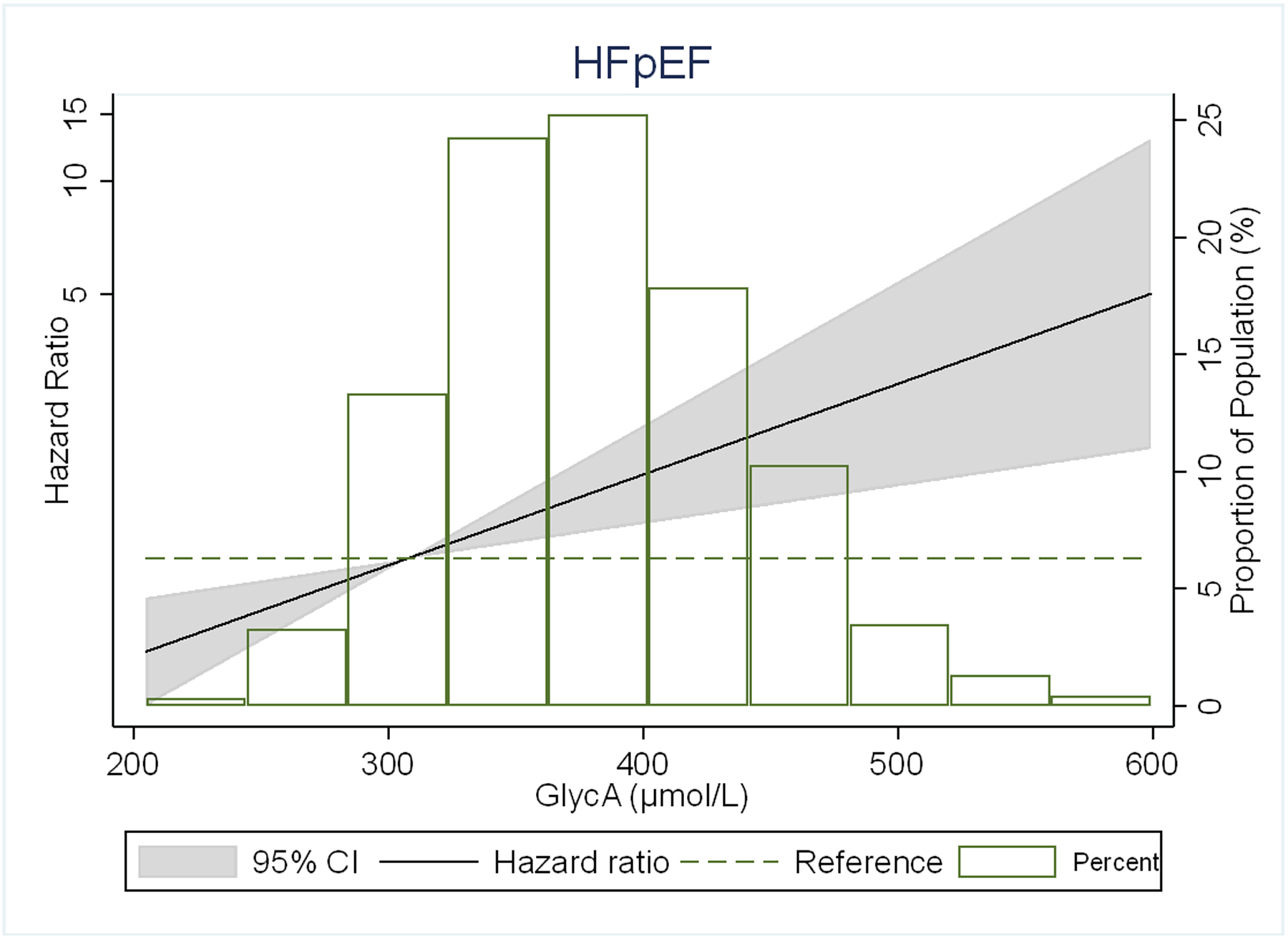
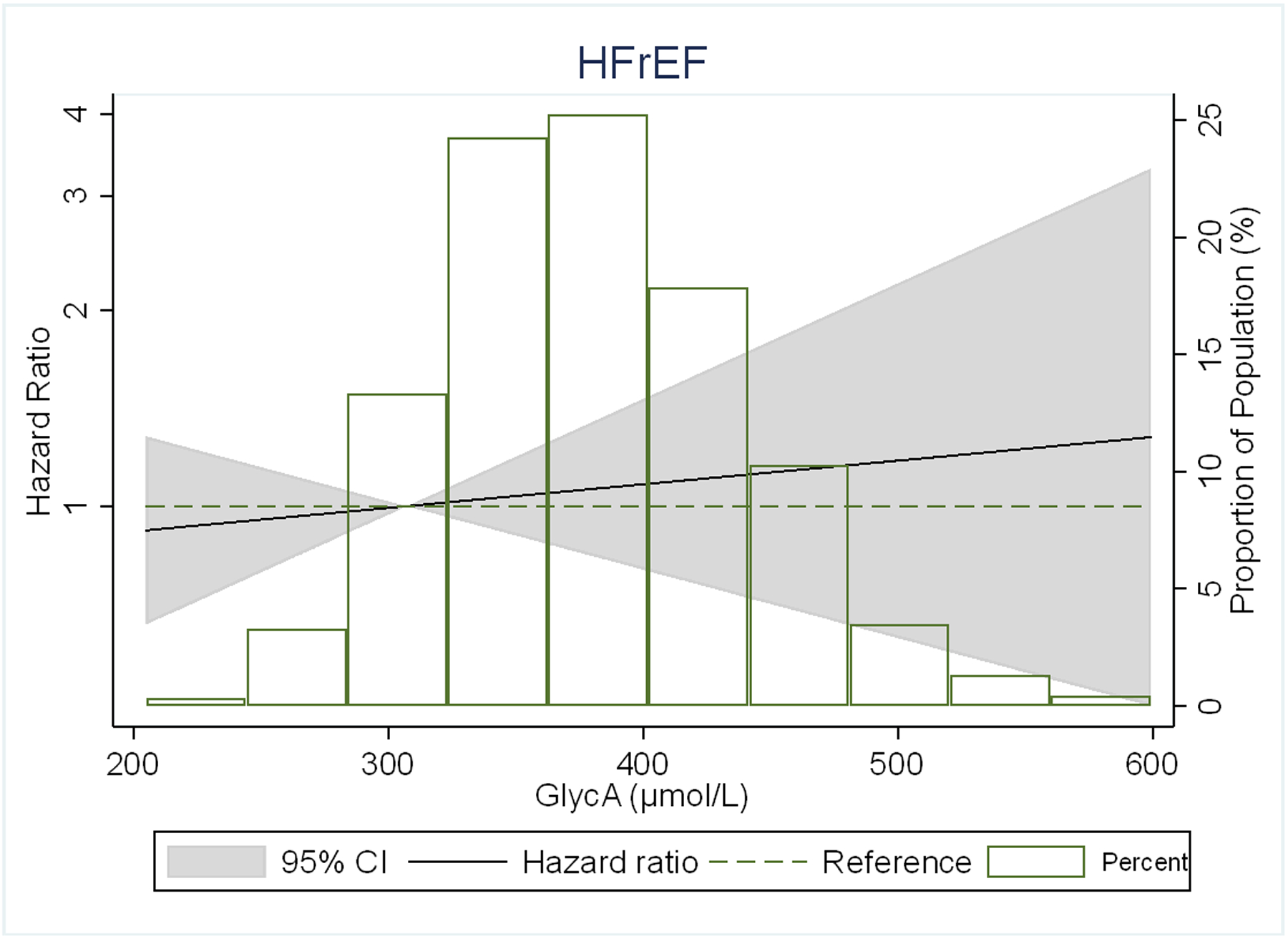
Adjusted* restricted cubic spline models showing the association of GlycA levels with hazard ratio of (A) HF, (B) HFpEF, (C) HFrEF.
*Spline models adjusted for age, sex, and race/ethnicity, MESA site, education, health insurance, BMI, smoking status, pack-years of smoking, physical activity, systolic blood pressure, use of antihypertensive medication, total cholesterol, HDL- cholesterol, use of lipid-lowering medications, diabetes, eGFR, ln(CRP), ln(IL-6) and ln(Fibrinogen).
The 4 knots are at 5th, 25th, 65th and 95th percentiles. Black curves represent the HR for the type of HF by proportion of population with the respective GlycA concentration. The 95% CI is represented by the gray shadow.
Given that GlycA has already been demonstrated to be associated with incident CVD events, we performed a sensitivity analysis to determine whether GlycA was still associated with any HF and its subtypes among those who had not had an interim CVD event (Supplemental Table 1). With exclusion of participants with interim CVD events (n=893) after the baseline visit, the model 1-adjusted HR (95% CI) per 1 SD higher GlycA was 1.45 (1.21–1.73), 1.41 (1.10–1.82), and 1.39 (1.06–1.82) for any HF, HFpEF, and HFrEF, respectively. After fully adjusting for the respective covariates (in primary model 4), the highest GlycA quartile were statistically significantly associated with any HF from model 1 to model 3, and the top two GlycA quartiles with HFpEF in the first model. However, no association was observed for the highest quartile of GlycA, compared to the lowest, for HFrEF in the initial model, and for either HF subtypes in the fully adjusted model, although there were 202 fewer incident HF events than in primary analysis.
Discussion
In this ethnically diverse community-based cohort free of clinical CVD at baseline, we have found that a higher plasma level of the novel composite inflammatory biomarker GlycA was independently associated with total incident hospitalized HF events and more particularly with the subtype of HFpEF, over 14 years of follow-up. Notably, there was greater than a 2-fold adjusted risk of HFpEF for the highest GlycA quartile compared to the lowest. These findings were consistent even after adjusting for NT-proBNP, a biomarker of wall strain which might reflect subclinical HF. Upon excluding individuals who developed ASCVD after the baseline visit in the sensitivity analysis, we observed some attenuation of the association in the context of fewer interim HF events; however, findings were generally similar to the primary analysis. To our knowledge, this is the first study evaluating the association of GlycA with HFpEF and HFrEF. Our findings suggest that GlycA may be an important inflammatory biomarker of risk of HF, especially HFpEF, even after taking into account other more commonly measured inflammatory biomarkers such as hsCRP.
Inflammatory activation plays a key role in the progression of HF,5 by impacting the pathogenesis of HF, and its underlying comorbidities including atherosclerosis, diabetes, and obesity.34–36 Increased concentrations of inflammatory cytokines like IL-6, TNF-α, and IL-1 have been reported in HF patients.7 The levels of inflammatory biomarkers have been shown to provide prognostic information in HF patients.12, 37 Previous studies have found a stronger association of other inflammatory markers with HFpEF than with HFrEF,6, 10, 11 and we now confirm a similar relationship with GlycA.
Prior work in MESA has found that GlycA is modestly to moderately correlated with other markers of inflammation [with Pearson correlation coefficient for GlycA with d-dimer (0.09), IL-6 (0.29), hsCRP (0.47), and fibrinogen (0.49)].23 Nevertheless, previous studies have shown that plasma GlycA levels were associated with CVD events and all-cause mortality even after adjusting for other inflammatory markers such as hsCRP, d-dimer, and IL-6.14, 18–22 In our analysis when all of the inflammatory markers were mutually adjusted for each other, we found only GlycA to be associated with any HF and HFpEF, whereas the other inflammatory markers were not independently associated with HF (any HF, HFpEF or HFrEF) outcomes. GlycA is also linked to subclinical atherosclerosis and its progression38–40 and with reduced cardiovascular health by the AHA’s Life Simple 7 metrics.23 Among patients with established HFpEF (n=248) who underwent cardiac catheterization at a single center, higher GlycA levels were associated with increased risk of death or major adverse cardiovascular events, suggesting GlycA might help identify subgroups of HFpEF patients at greatest risk for adverse events.41
However, whether GlycA levels can predict incident HFpEF among a community-based cohort free of HF at baseline had not been well-established before this study. In a prior analysis from MESA,14 Duprez, et al. examined the associations between GlycA and CVD events, coronary heart disease, stroke, and overall HF and showed that GlycA was predictive of any CVD, including HF. However, that analysis did not examine the association of GlycA with the specific HF subtypes of HFpEF and HFrEF, which we newly demonstrate here.
Differences in clinical characteristics and patients outcomes for HFpEF and HFrEF have been well established.42, 43 Patients with HFrEF have a younger median age with greater prevalence of previous myocardial infarction, contributing to ischemic cardiomyopathy.44 Patients with HFpEF have an older median age and show a higher likelihood of with non-cardiac comorbidities such as diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and obesity.44 All these comorbidities have the potential to induce systemic inflammation.45 The high prevalence of comorbidities in patients with HFpEF can be translated to differential association of inflammation with HFpEF compared to HFrEF, as suggested by our analyses
So far, attempts to utilize therapies that have been proven to work in HFrEF patients (such as angiotensin receptor blockers, ACE inhibitors, angiotensin receptor-neprilysin inhibitors) to reduce mortality and hospitalizations have so far been largely unsuccessful in patients with HFpEF.46–49 The lack of effective therapy for patients with HFpEF delineates the importance of preventing the development of HFpEF, which could be aided by the identification of a stable inflammatory biomarker. Besides providing biological insights, our findings showing differing associations of GlycA with new onset HFpEF and HFrEF implies that prevention strategies might need to be different for HFpEF and HFrEF.
GlycA is currently already a commercially available lab test in the United States through LabCorp. However, for successful use of GlycA as a biomarker for HF risk predictor or therapeutic target, we must next study whether therapeutic lowering of GlycA either by lifestyle adjustments or pharmacotherapy can slow or prevent HFpEF. Prior study has shown that while statin therapy decreased CVD events, statins only minimally decreased GlycA levels.19 Interestingly, immunomodulatory treatments targeting TNF in patients with psoriasis have been shown to reduce GlycA levels and vascular inflammation.40 Such therapies could be potentially used clinically to lower GlycA levels if confirmed in other studies of patients at risk of inflammatory disease.
There were several limitations in our study that should be noted. First, our study was observational, and we cannot infer causality about GlycA and HF risk. Despite our attempt to include all covariates that likely are to influence the development of HF in the statistical models, residual confounding remains a possibility. Second, only baseline GlycA levels were available, and thus we were unable to account for changes in GlycA levels over time. Previous work has shown that a single baseline measure could accurately capture the short-term inflammatory status for a 6 month period.50 However, whether the same accuracy holds for longer periods of time is not clear. Third, there were few individuals with HF who had a mid-range LVEF of 40-<50% (n=31), so due to reduced statistical power we were unable to use the 3 classification system proposed by the European Society of Cardiology and therefore focused our analysis on HFpEF and HFrEF, dichotomized at LVEF of 50%.
Strengths of this study include a large sample size, long duration of follow-up of 14.0 years, and ethnic and racial diversity. To our knowledge, our study was the first to examine the association of GlycA with both HF subtypes, HFpEF and HFrEF, separately; however, further external validation of our findings in other cohorts is warranted.
Conclusions
In sum, we found that higher plasma GlycA levels were associated with total incident HF, and in particular the subtype of HFpEF but not HFrEF, independently of traditional CVD risk factors and other inflammatory markers. Our findings lend support to the current understanding of the different pathophysiology of HFpEF and HFrEF, and suggest that inflammatory markers may offer different prognostic information based on HF subtype. Future study is warranted to examine mechanisms that might explain differential association of GlycA with HFpEF vs HFrEF, and whether therapeutic lowering of GlycA can prevent HFpEF development.
Supplementary Material
Clinical Perspective.
What is new?
Higher GlycA has been shown to be associated with cardiovascular diseases and may be a more reliable prognostic marker of HF; however its relation with HF subtypes, specifically, was unknown.
We found in a multi-ethnic cohort followed for a median of 14 years, higher plasma GlycA levels were associated with HF with preserved (HFpEF) but not reduced (HFrEF) ejection fraction, independently of traditional CVD risk factors and other inflammatory markers
Whereas the other inflammatory markers (high sensitivity C-reactive protein, interleukin-6, and fibrinogen) were not independently associated with either HF type in mutually-adjusted models including GlycA.
What are the Clinical Implications?
Our findings lend support to the current understanding of the different pathophysiology of HFpEF and HFrEF, and suggest that inflammatory markers may offer different prognostic information based on HF subtype.
Our findings provides rationale for further study of GlycA as a biomarker to improve HF risk prediction or to direct therapy.
Future studies should examine mechanisms that might explain differential association of GlycA with HFpEF vs HFrEF, and whether therapeutic lowering of GlycA can prevent HFpEF development.
Acknowledgments
The authors thank the other investigators, the staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding:
The MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. Drs. Michos and Zhao are additionally funded by the Blumenthal Scholars Award in Preventive Cardiology at Johns Hopkins University. Sunyoung Jang was supported by the Dean’s Research Fund at Johns Hopkins University Medical School.
Abbreviations
- ACC
American College of Cardiology
- AHA
American Heart Association
- BMI
Body Mass Index
- CVD
Cardiovascular Disease
- CI
Confidence Intervals
- eGFR
Estimated Glomerular Filtration Rate
- HR
Hazard Ratios
- hsCRP
High Sensitivity C-Reactive Protein
- HF
Heart Failure
- HFrEF
Heart Failure with Reduced Ejection Fraction
- HFpEF
Heart Failure with Preserved Ejection Fraction
- IL-6
Interleukin 6
- ILI
Interquartile Interval
- MESA
Multi-Ethnic Study of Atherosclerosis
- NT-proBNP
N-terminal pro-B-type Natriuretic Peptide
- NMR
Nuclear Magnetic Resonance
- SD
Standard Deviation
- SBP
Systolic Blood Pressure
- TNF
Tumor Necrosis Factor
Footnotes
Disclosures:
Dr. Otvos is employed by LabCorp. None of the other authors report any disclosures
References
- 1.Ziaeian B and Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention and Stroke C. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology F and American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 4.Liquori ME, Christenson RH, Collinson PO and Defilippi CR. Cardiac biomarkers in heart failure. Clin Biochem. 2014;47:327–37. [DOI] [PubMed] [Google Scholar]
- 5.Shirazi LF, Bissett J, Romeo F and Mehta JL. Role of Inflammation in Heart Failure. Curr Atheroscler Rep. 2017;19:27. [DOI] [PubMed] [Google Scholar]
- 6.Murphy SP, Kakkar R, McCarthy CP and Januzzi JL, Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1324–1340. [DOI] [PubMed] [Google Scholar]
- 7.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB and Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 1996;27:1201–6. [DOI] [PubMed] [Google Scholar]
- 8.Tschope C and Lam CS. Diastolic heart failure: What we still don’t know. Looking for new concepts, diagnostic approaches, and the role of comorbidities. Herz. 2012;37:875–9. [DOI] [PubMed] [Google Scholar]
- 9.Diwan A, Tran T, Misra A and Mann DL. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med. 2003;3:161–82. [DOI] [PubMed] [Google Scholar]
- 10.Tromp J, Khan MA, Klip IT, Meyer S, de Boer RA, Jaarsma T, Hillege H, van Veldhuisen DJ, van der Meer P and Voors AA. Biomarker Profiles in Heart Failure Patients With Preserved and Reduced Ejection Fraction. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P and Voors AA. Identifying Pathophysiological Mechanisms in Heart Failure With Reduced Versus Preserved Ejection Fraction. J Am Coll Cardiol. 2018;72:1081–1090. [DOI] [PubMed] [Google Scholar]
- 12.Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr., Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR, American Heart Association Clinical Pharmacology Committee of the Council on Clinical C, Council on Basic Cardiovascular S, Council on Cardiovascular Disease in the Y, Council on C, Stroke N, Council on Cardiopulmonary CCP, Resuscitation, Council on E, Prevention, Council on Functional G, Translational B, Council on Quality of C and Outcomes R. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2017;135:e1054–e1091. [DOI] [PubMed] [Google Scholar]
- 13.de Boer RA, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, Bahrami H, van der Harst P, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL Jr., Shah SJ, Levy D, Herrington DM, Larson MG, van Gilst WH, Gottdiener JS, Bertoni AG and Ho JE. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol. 2018;3:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R and Jacobs DR Jr. Comparison of the Predictive Value of GlycA and Other Biomarkers of Inflammation for Total Death, Incident Cardiovascular Events, Noncardiovascular and Noncancer Inflammatory-Related Events, and Total Cancer Events. Clin Chem. 2016;62:1020–31. [DOI] [PubMed] [Google Scholar]
- 15.Sani CM, Pogue EPL, Hrabia JB, Zayachkowski AG, Zawadka MM, Poniatowski AG, Dlugosz D, Lesniak W, Kruszelnicka O, Chyrchel B and Surdacki A. Association between low-grade chronic inflammation and depressed left atrial compliance in heart failure with preserved ejection fraction: A retrospective analysis. Folia Med Cracov. 2018;58:45–55. [DOI] [PubMed] [Google Scholar]
- 16.Ohkuma T, Jun M, Woodward M, Zoungas S, Cooper ME, Grobbee DE, Hamet P, Mancia G, Williams B, Welsh P, Sattar N, Shaw JE, Rahimi K, Chalmers J and Group AC. Cardiac Stress and Inflammatory Markers as Predictors of Heart Failure in Patients With Type 2 Diabetes: The ADVANCE Trial. Diabetes Care. 2017;40:1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH and Tracy RP. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin Chem. 2015;61:714–23. [DOI] [PubMed] [Google Scholar]
- 18.Akinkuolie AO, Buring JE, Ridker PM and Mora S. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc. 2014;3:e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akinkuolie AO, Glynn RJ, Padmanabhan L, Ridker PM and Mora S. Circulating N-Linked Glycoprotein Side-Chain Biomarker, Rosuvastatin Therapy, and Incident Cardiovascular Disease: An Analysis From the JUPITER Trial. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fashanu OE, Oyenuga AO, Zhao D, Tibuakuu M, Mora S, Otvos JD, Stein JH and Michos ED. GlycA, a Novel Inflammatory Marker and Its Association With Peripheral Arterial Disease and Carotid Plaque: The Multi-Ethnic Study of Atherosclerosis. Angiology. 2019;70:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muhlestein JB, May HT, Galenko O, Knowlton KU, Otvos JD, Connelly MA, Lappe DL and Anderson JL. GlycA and hsCRP are independent and additive predictors of future cardiovascular events among patients undergoing angiography: The intermountain heart collaborative study. Am Heart J. 2018;202:27–32. [DOI] [PubMed] [Google Scholar]
- 22.Gruppen EG, Riphagen IJ, Connelly MA, Otvos JD, Bakker SJ and Dullaart RP. GlycA, a Pro-Inflammatory Glycoprotein Biomarker, and Incident Cardiovascular Disease: Relationship with C-Reactive Protein and Renal Function. PloS one. 2015;10:e0139057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson EA, Tibuakuu M, Zhao D, Akinkuolie AO, Otvos JD, Duprez DA, Jacobs DR Jr., Mora S and Michos ED. Associations of ideal cardiovascular health with GlycA, a novel inflammatory marker: The Multi-Ethnic Study of Atherosclerosis. Clin Cardiol. 2018;41:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 25.Duprez DA, Otvos J, Tracy RP, Feingold KR, Greenland P, Gross MD, Lima JA, Mackey RH, Neaton JD, Sanchez OA and Jacobs DR. High-Density Lipoprotein Subclasses and Noncardiovascular, Noncancer Chronic Inflammatory-Related Events Versus Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4:e002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vella CA, Allison MA, Cushman M, Jenny NS, Miles MP, Larsen B, Lakoski SG, Michos ED and Blaha MJ. Physical Activity and Adiposity-related Inflammation: The MESA. Med Sci Sports Exerc. 2017;49:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS and Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelton SP, Narla V, Blaha MJ, Nasir K, Blumenthal RS, Jenny NS, Al-Mallah MH and Michos ED. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2014;113:644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osibogun O, Ogunmoroti O, Tibuakuu M, Benson EM and Michos ED. Sex differences in the association between ideal cardiovascular health and biomarkers of cardiovascular disease among adults in the United States: a cross-sectional analysis from the multiethnic study of atherosclerosis. BMJ Open. 2019;9:e031414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying W, Zhao D, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Sharma K, Shah SJ, Heckbert SR, Lima JA, deFilippi CR, Budoff MJ, Post WS and Michos ED. Sex Hormones and Change in N-Terminal Pro-B-Type Natriuretic Peptide Levels: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2018;103:4304–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chahal H, Bluemke DA, Wu CO, McClelland R, Liu K, Shea SJ, Burke G, Balfour P, Herrington D, Shi P, Post W, Olson J, Watson KE, Folsom AR and Lima JAC. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart. 2015;101:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS and Michos ED. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart Failure. 2018;6:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fliotsos M, Zhao D, Rao VN, Ndumele CE, Guallar E, Burke GL, Vaidya D, Delaney JCA and Michos ED. Body Mass Index From Early-, Mid-, and Older-Adulthood and Risk of Heart Failure and Atherosclerotic Cardiovascular Disease: MESA. J Am Heart Assoc. 2018;7:e009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby P and Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116:307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lumeng CN and Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tall AR and Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Bauersachs J and Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail. 2017;19:1379–1389. [DOI] [PubMed] [Google Scholar]
- 38.Ezeigwe A, Fashanu OE, Zhao D, Budoff MJ, Otvos JD, Thomas IC, Mora S, Tibuakuu M and Michos ED. The novel inflammatory marker GlycA and the prevalence and progression of valvular and thoracic aortic calcification: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019;282:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tibuakuu M, Fashanu OE, Zhao D, Otvos JD, Brown TT, Haberlen SA, Guallar E, Budoff MJ, Palella FJ Jr., Martinson JJ, Akinkuolie AO, Mora S, Post WS and Michos ED. GlycA, a novel inflammatory marker, is associated with subclinical coronary disease. AIDS. 2019;33:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, Krishnamoorthy P, Ng Q, Aridi TZ, Salahuddin T, Natarajan B, Lockshin BN, Ahlman MA, Chen MY, Rader DJ, Reilly MP, Remaley AT, Bluemke DA, Playford MP, Gelfand JM and Mehta NN. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res. 2016;119:1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly JP, Hunter WG, McGarrah RW, Craig D, Haynes C, Velazquez EJ, Felker GM, Hernandez AF, Newgard CB, Shah SH and Kraus WE. Novel Protein Glycan Inflammatory Biomarkers Predict Adverse Events in Heart Failure with Preserved Ejection Fraction. J Cardiac Fail. 2015;21 [abstract]. [Google Scholar]
- 42.Abebe TB, Gebreyohannes EA, Tefera YG and Abegaz TM. Patients with HFpEF and HFrEF have different clinical characteristics but similar prognosis: a retrospective cohort study. BMC Cardiovasc Disord. 2016;16:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ and van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. [DOI] [PubMed] [Google Scholar]
- 44.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA and Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. [DOI] [PubMed] [Google Scholar]
- 45.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J and Health ABCSI. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanwar M, Walter C, Clarke M and Patarroyo-Aponte M. Targeting heart failure with preserved ejection fraction: current status and future prospects. Vasc Health Risk Manag. 2016;12:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A and Investigators IP. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. [DOI] [PubMed] [Google Scholar]
- 48.Zakeri R and Cowie MR. Heart failure with preserved ejection fraction: controversies, challenges and future directions. Heart. 2018;104:377–384. [DOI] [PubMed] [Google Scholar]
- 49.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Dungen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, Investigators P-H and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 50.Navarro SL, Brasky TM, Schwarz Y, Song X, Wang CY, Kristal AR, Kratz M, White E and Lampe JW. Reliability of serum biomarkers of inflammation from repeated measures in healthy individuals. Cancer Epidemiol Biomarkers Prev. 2012;21:1167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


