Abstract
Aims & Background:
Non-alcoholic fatty liver disease (NAFLD) is rising in young adults, with potential implications for reproductive-aged women. Whether NAFLD during pregnancy confers more serious risks for maternal or perinatal health is unclear.
Methods:
Using weighted discharge data from the United States National Inpatient Sample, we evaluated temporal trends of NAFLD in pregnancies after 20 weeks gestation, and compared outcomes to pregnancies with other chronic liver diseases (CLD) or no CLD. Study outcomes included pre-term birth, postpartum hemorrhage, hypertensive complications (pre-eclampsia, eclampsia, and/or hemolysis, elevated liver enzymes, and low platelets syndrome), and maternal or fetal death. NAFLD prevalence was estimated by calendar year and temporal trends tested by linear regression. Outcomes were analyzed by logistic regression adjusted for age, race, multiple gestation, and pre-pregnancy diabetes, obesity, dyslipidemia and hypertension.
Results:
Among 18,574,225 pregnancies, 5,640 had NAFLD and 115,210 had other, non-NAFLD CLD. Pregnancies with NAFLD nearly tripled from 10.5/100,000 pregnancies in 2007 to 28.9/100,000 in 2015 (p<.001). NAFLD versus other groups had more gestational diabetes (23% vs 7–8%), hypertensive complications (16% vs 4%), postpartum hemorrhage (6 vs 3–5%), and pre-term birth (9% vs 5–7%), p values < 0.01. On adjusted analysis, compared to no CLD, NAFLD was associated with hypertensive complications (OR 3.1, 95% CI 2.6–3.8, p<.001), pre-term birth (OR 1.6, 95% CI 1.3–2.0, p<.001), postpartum hemorrhage (OR 1.7, 95% CI 1.3–2.2) and possibly maternal (OR 17.8, 95% CI 2.1–149 p=.01), but not fetal death (p=.90).
Conclusion:
NAFLD in pregnancy has nearly tripled in the last decade and is independently associated with hypertensive complications, postpartum hemorrhage and pre-term birth. NAFLD should be considered a high-risk obstetric condition, with clinical implications for pre-conception counseling and pregnancy care.
Keywords: nonalcoholic steatohepatitis, reproductive health, complications, chronic liver disease
Graphical Abstract
Lay summary:
The prevalence of non-alcoholic fatty liver disease (NAFLD) in pregnancy has almost tripled over the past 10 years. Having NAFLD during pregnancy increases risks for both the mother and the baby, including hypertensive complications of pregnancy, bleeding after delivery, and pre-term birth. Thus, women with NAFLD warrant pre-conception counseling regarding these risks, and management by a high-risk obstetrician during pregnancy.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease (CLD) in the United States (U.S.), and includes simple steatosis as well as manifestations of hepatocellular injury and fibrosis, known as non-alcoholic steatohepatitis (NASH).(1) Emerging U.S. data highlight the largest rise in NAFLD incidence among adults under age 40 years,(2) and NASH is now the leading indication for liver transplantation in young adults, as well as in women.(3, 4) The public health implications of NAFLD/NASH in young adults, including reproductive-aged women, is therefore vast.
NAFLD is considered the hepatic manifestation of the metabolic syndrome, and is tightly linked with obesity and diabetes (DM).(5) The obesity epidemic has affected reproductive-aged women, with obesity present in over one third of U.S. women ages 20–39 years.(6) Pregnancy itself is a relative insulin resistant state and concurrent maternal obesity further increases the risk for gestational diabetes.(7) The adverse risks of obesity and gestational diabetes on perinatal outcomes are well established, although whether NAFLD is independently associated with more serious pregnancy-related complications is unknown.
In the current study, we leverage discharge records from the National Inpatient Sample (NIS) database to evaluate temporal trends in NAFLD prevalence during pregnancy and to determine whether NAFLD in pregnancy is associated with adverse maternal and perinatal outcomes. Understanding the role of NAFLD in pregnancy outcomes has implications for pre-conception counseling in women with NAFLD, and in optimizing the management of pregnant women with NAFLD to enhance immediate and long-term maternal and perinatal health.
METHODS
Study population:
Using the United States 2007–2016 National Inpatient Sample (NIS) database, we retrospectively evaluated hospital discharge records identifying pregnancies in women 18 years or older with a diagnosis or procedure indicating a delivery event including live and stillbirths after 20 weeks of gestation. Extra-uterine pregnancies were excluded. To avoid double counting records for the same pregnancy, only codes for a final pregnancy event were included. Non-natural terminations, miscarriages, spontaneous and missed abortions were excluded. Pregnancies from each delivery discharge were classified as having NAFLD, non-NAFLD chronic liver disease (CLD), or no CLD using corresponding International Classification of Diseases (ICD) 9 &10 codes (see Supplemental Table 1 for comprehensive list). Other CLDs included alcoholic liver disease, chronic viral hepatitis, autoimmune or disorders of copper or iron metabolism, and any unspecified cirrhosis. Acute liver diseases including diagnoses such as acute viral hepatitis or acute liver failure were excluded, as well pregnancies with discharge codes for dual diagnoses of NAFLD plus another CLD, or NAFLD plus an alcohol use disorder (Supplemental Table 1).
Data source:
The NIS is the largest all-payer U.S. inpatient care database, sampling approximately 8 million hospital stays annually.(8) Data elements include primary and secondary diagnoses and procedures, discharge status, demographic and clinical variables. The NIS is designed to yield nationally representative, weighted estimates of hospital discharges. In 2012, the NIS was redesigned to improve national estimates; it now approximates a 20-percent stratified sample of all discharges from U.S. community hospitals while years prior to 2012 sampled hospitals from which all discharges were retained. The NIS is sampled from the State Inpatient Databases, which includes all inpatient data and contributes to the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project (HCUP). In 2016, data came from 46 states, plus the District of Columbia, representing more than 97 percent of the U.S. population.
Study outcomes:
Maternal pregnancy outcomes included hypertensive complications (defined as a pre-eclampsia, eclampsia, or hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, postpartum hemorrhage, or death during the delivery admission. HELLP was included in the hypertensive complications category as it reflects a more severe variant of pre-eclampsia.(9) Perinatal outcomes included pre-term birth (<37 weeks), fetal growth restriction (FGR), large for gestational age (LGA), and fetal death (stillbirth or intrauterine fetal demise). With the exception of maternal death, identified using the variable “DIED”, each outcome used the ICD Ninth Revision, Clinical Modification (ICD-9-CM) and Tenth Revision (ICD-10-CM) beginning in October 2015 (Supplemental Table 1). Outcomes were not mutually exclusive. ICD-9-CM codes primarily came from Chapter 11 of the ICD manual on Complications of Pregnancy, Childbirth, and the Puerperium (codes 630–679), as well as from non-pregnancy-related chapters. ICD-10-CM codes primarily came from Chapter 15 Pregnancy, childbirth and the puerperium (codes O00-O9A) and Chapter 16 Certain conditions originating in the perinatal period (codes P00-P96).
Covariates of interest:
Covariates included demographics (age, race/ethnicity, rural-based hospital defined as population <50,0000), multiple gestation, and pre-existing comorbidities including obesity, diabetes, dyslipidemia, hypertension (HTN), and cirrhosis. Additional pregnancy characteristics included gestational diabetes (GDM), gestational hypertension, and cesarean section. Demographic data were collected directly from the NIS database; remaining variables were based on discharge diagnosis and procedural codes (see Supplemental Table 1 for complete list of ICD-9 and ICD-10 codes). Codes were chosen based on review of pregnancy-related HCUP publications(10, 11), HCUP comorbidity software, and ICD manuals.(8)
Statistical analysis:
NAFLD prevalence and 95% confidence intervals (CI) were estimated per 100,000 pregnancies by calendar year, and temporal trend in NAFLD prevalence in pregnancy was tested by linear regression. We used the supplemental NIS trend weight files(12) to allow for a continuous study of national trends spanning the 2012 database redesign.(13) The trend analysis was limited to 2007 through the third quarter of 2015 due changes in NAFLD coding with the release of ICD10. Specifically, a code for nonalcoholic steatohepatitis (NASH) was introduced, potentially inflating NAFLD rates after the coding update.
Using the most recent 5 years of data (2012 to 2016), the association of NAFLD with maternal and perinatal outcomes was assessed by logistic regression adjusting for baseline pregnancy factors with plausible associations with NAFLD or outcomes of interest. These included age, race, multiple gestation, pre-pregnancy diabetes, obesity, dyslipidemia and hypertension. The analysis was restricted to 2012–2016 due to change in hospital sampling in 2012. Though gestational diabetes mellitus (GDM) does not reflect a baseline pregnancy characteristic, it is associated with NAFLD(14) as well as adverse obstetric outcomes(15), thus efforts were specifically taken to ensure observed findings were not driven by GDM, including additional adjustment for GDM, as well as testing for interactions between GDM and liver disease categories. Additional sensitivity analyses were also performed to evaluate the consistency of findings. These included: 1) restricting analyses to pregnancies without pre-existing diabetes 2) restricting analyses of hypertensive complications to pregnancies without pre-existing hypertension 3) restricting analyses to the ICD-9 time period to ensure that findings were not driven by the introduction of NASH coding with ICD-10, and 4) excluding pregnancies with hypertensive complications (which includes HELLP), from the analyses of postpartum hemorrhage to explore whether observed differences could relate to low platelet counts with HELLP syndrome. Finally, we acknowledged that NAFLD is likely underdiagnosed, and women with metabolic risk factors may have had undiagnosed NAFLD, and misclassified in the no CLD group. Thus, we additionally performed sensitivity analyses after excluding pregnancies with obesity, diabetes, or dyslipidemia from the no CLD group, and compared those study outcomes to pregnancies with NAFLD.
Adjusted odds ratios (AORs) and CIs were computed for each study outcome using logistic regression, accounting for the complex survey design via Taylor series linearization for variance estimation. Descriptive statistics were evaluated via t-test for age and via Rao-Scott (sample-design adjusted) chi-square goodness-of-fit tests for categorical variables. All statistical tests were two-sided at significance level of 0.05, including significance levels for interaction terms. Computations were completed in SAS version 9.4 (SAS Institute; Cary, North Carolina). The analyzed NIS dataset was purchased by the University of California, San Francisco, and permission obtained for analysis after completion of a signed Data Use Agreement form.
RESULTS:
Rising Prevalence of NAFLD in Pregnancy
During study years 2007–2016, there were 37,775,491 eligible pregnancies; 8,523 with NAFLD, 196,701 with other CLD, and 37,570,267 with no CLD. The prevalence of NAFLD in pregnancy increased over time, with rates nearly tripling from 10.5/100,000 pregnancies in 2007, to 28.9/100,000 pregnancies in 2015 (p<.001) (Figure 1).
Figure 1.
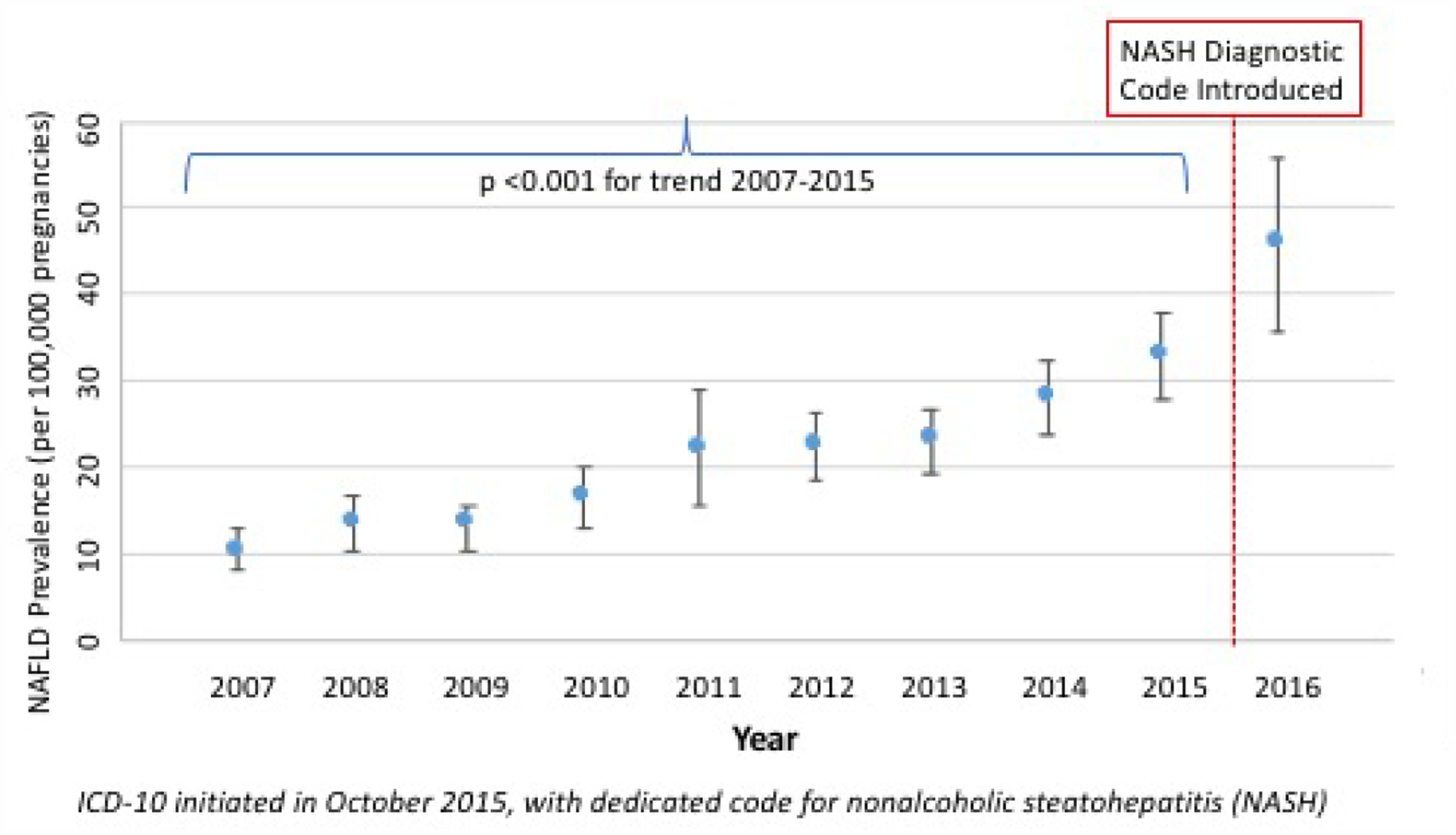
Temporal Trends in NAFLD Prevalence in Pregnancy (2007–2016). P-value < 0.001 (test of trend).
In the most recent 5 years of data (2012–2016), there were 18,574,225 eligible pregnancies for evaluating the association of NAFLD with maternal and perinatal outcomes; 5,640 with NAFLD (0.1% with cirrhosis), 115,210 with other CLD (0.7% with cirrhosis), and 18,453,375 with no CLD. The mean age of women in the NAFLD, other CLD, and no CLD groups were 31, 30, and 29 years, respectively (Table 1). Notable racial/ethnic differences included a higher proportion of Hispanics with NAFLD and higher proportion of Asian-Pacific Islanders with other CLD. Pre-existing metabolic comorbidities were more common in pregnancies with NAFLD including diabetes, obesity, dyslipidemia, and hypertension (all p values <.001) (Table 1). Most notably, obesity was present in nearly 40% of pregnancies with NAFLD (vs 6–7% in other groups), pre-pregnancy hypertension was thrice as common with NAFLD (15.5% vs <5% in other groups), and dyslipidemia present in 7.4% of the NAFLD group as compared to <0.5% of the other pregnancy groups. A slightly higher proportion of pregnancies with NAFLD had multiple gestation (3% vs 1.8%, p values <.001).
Table 1.
Cohort Characteristics by Liver Disease Status
| Characteristic | NAFLD n=5,640 |
Other CLD n=115,210 |
No CLD n= 18,453,375 |
NAFLD vs Other CLD p-value | NAFLD vs no CLD p-value |
|---|---|---|---|---|---|
| Age, mean (SE), years | 30.5 (0.17) | 29.5 (0.05) | 28.5 (0.02) | <.001 | <.001 |
| Race/ethnicity, n (%)*: | |||||
| White | 2,255 (42.1) | 61,490 (57.2) | 9,266,372 (53.6) | ||
| Black | 395 (7.4) | 12,475 (11.6) | 2,504,590 (14.5) | ||
| Hispanic | 2,045 (38.2) | 7,750 (7.2) | 3,561,489 (20.6) | <.001 | <.001 |
| Asian/Pacific Islander | 385 (7.2) | 20,125 (18.7) | 998,985 (5.8) | ||
| Other | 270 (5.0) | 5,730 (5.3) | 951,815 (5.5) | ||
| Urban or rural-based hospital, n (%)**: | |||||
| Rural | 500 (8.9) | 19,140 (16.6) | 2,558,737 (13.9) | <.001 | <.001 |
| Urban | 5,130 (91.1) | 95,840 (83.4) | 15,843,742 (86.1) | ||
| Multiple gestation, n (%) | 165 (2.9) | 2,120 (1.8) | 327,995 (1.8) | <.001 | <.001 |
| Diabetes, n (%) | 635 (11.3) | 1,610 (1.4) | 202,345 (1.1) | <.001 | <.001 |
| Obesity, n (%) | 2,235 (39.6) | 7,315 (6.3) | 1,336,310 (7.2) | <.001 | <.001 |
| Dyslipidemia, n (%) | 415 (7.4) | 240 (0.2) | 33,340 (0.2) | <.001 | <.001 |
| Hypertension, n (%) | 875 (15.5) | 4,630 (4.0) | 565,680 (3.1) | <.001 | <.001 |
| Cirrhosis, n (%) | 5 (0.09) | 830 (0.7) | ----- | .01 | ----- |
Abbreviations: nonalcoholic fatty liver disease (NAFLD), chronic liver disease (CLD);
missing in n=290 NAFLD (5.1%), 7,640 other CLD (6.7%), and n=1,170,125 other CLD (6.3%);
missing in n=10 NAFLD (0.2), 230 other CLD (0.2%), and n=50,895 other CLD (0.3%). T-test and chi-squared tests were used to compare continuous and dichotomized measures, respectively, with p <0.05 considered statistically significant.
Maternal Complications in Pregnancies With NAFLD versus non-NAFLD CLD and no CLD
Gestational DM, gestational hypertension, hypertensive complications (pre-eclampsia, eclampsia, and/or HELLP), caesarean section, and postpartum hemorrhage were significantly more common in pregnancies with NAFLD, as compared to pregnancies with other CLD or no CLD (p values <.001) (Table 2). GDM was present in 23% of NAFLD pregnancies vs 7–8% in the other two groups. Hypertensive complications occurred in 16% of NAFLD vs 4% of other groups, and 52% of NAFLD pregnancies resulted in caesarean section as compared to 33% and 36% of other CLD and no CLD groups, respectively (p values <.001). Maternal mortality was also more common with NAFLD as compared to no CLD in pregnancy (0.1% vs 0.005%, p<.001).
Table 2.
Prevalence of Maternal and Perinatal Outcomes by Liver Disease Status in Pregnancy
| Prevalence, n (%) | p-values (vs NAFLD) | ||||
|---|---|---|---|---|---|
| NAFLD n=5,640 |
OtherCLD n=115,210 |
No CLD n=18,453,375 | Other CLD | No CLD | |
| Maternal Outcomes, n (%) | |||||
| Gestational DM | 1,290 (22.9) | 9,225 (8.0) | 1,289,640 (7.0) | <.001 | <.001 |
| Gestational HTN | 335 (5.9) | 3,920 (3.4) | 717,800 (3.9) | <.001 | <.001 |
| Hypertensive complications (preeclampsia, eclampsia, and/or HELLP syndrome) | 905 (16.0) | 4,380 (3.8) | 713,045 (3.9) | <.001 | <.001 |
| Cesarean section | 2,905 (51.5) | 41,935 (36.4) | 6,076,293 (32.9) | <.001 | <.001 |
| Postpartum hemorrhage | 355 (6.3) | 5,235 (4.5) | 589,670 (3.2) | .007 | <.001 |
| Maternal death | 5(0.1) | 35 (0.03) | 920 (0.005) | .29 | <.001 |
| Perinatal Outcomes, n (%) | |||||
| Pre-term birth (<37 weeks) | 500 (8.9) | 7,825 (6.8) | 846,805 (4.6) | .008 | <.001 |
| Fetal growth restriction | 75 (1.3) | 4,115 (3.6) | 372,910 (2.0) | <.001 | .10 |
| Large for gestational age | 300 (5.3) | 1,925 (1.7) | 489,770 (2.7) | <.001 | <.001 |
| Fetal death | 45 (0.8) | 1,085 (0.9) | 127,730 (0.7) | .62 | .67 |
Abbreviations: nonalcoholic fatty liver disease (NAFLD), chronic liver disease (CLD), diabetes mellitus (DM), hypertension (HTN), hemolysis, elevated liver tests, low platelets (HELLP). Chi-squared tests were used to compare proportions, with p <0.05 considered statistically significant.
After adjusting for age, race, multiple gestation and all pre-existing metabolic disease codes (Table 3), NAFLD as compared to no CLD was associated with hypertensive complications (AOR 3.1, 95% CI 2.6–3.8, p<.001) as well as postpartum hemorrhage (AOR 1.7, 95% CI 1.3–2.1, p<.001). NAFLD also conferred a higher odds of maternal mortality (AOR 17.9, 95% CI 2.1–149, p=.01), although this estimate reflects only 5 deaths in the NAFLD group. Compared to pregnancies with other CLD, NAFLD was also associated with a three-fold higher odds of hypertensive complications (AOR 3.1, 95% CI 2.5–3.8, p<.001). The odds of postpartum hemorrhage and maternal death were similar between NAFLD and other CLD groups (Table 3).
Table 3.
NAFLD and Adverse Maternal and Perinatal Outcomes: Adjusted Analyses
| NAFLD vs Other CLD | NAFLD vs no CLD | |||
|---|---|---|---|---|
| AOR*, 95% CI | p value | AOR*, 95% CI | p value | |
| Maternal Outcomes | ||||
| Hypertensive complications (preeclampsia, eclampsia, and/or HELLP syndrome) | 3.09 (2.54–3.76) | <.001 | 3.13 (2.61–3.75) | <.001 |
| Postpartum hemorrhage | 1.19 (0.91–1.55) | .20 | 1.67 (1.28–2.16) | <.001 |
| Maternal deatht | 3.80 (0.37–39) | .26 | 17.82 (2.13–149) | .008 |
| Perinatal Outcomes | ||||
| Pre-term birth (<37 weeks) | 1.03 (0.81–1.31) | .84 | 1.60 (1.27–2.02) | <.001 |
| Fetal growth restriction | 0.42 (0.25–0.71) | .001 | 0.73 (0.44–1.21) | 0.22 |
| Large for gestational age | 1.84 (1.36–2.48) | <.001 | 1.14 (0.86–1.5) | 0.36 |
| Fetal death | 0.69 (0.35–1.35) | .28 | 0.96 (0.5–1.86) | 0.90 |
Abbreviations: nonalcoholic fatty liver disease (NAFLD), chronic liver disease (CLD), diabetes mellitus (DM), hypertension (HTN), hemolysis, elevated liver tests, low platelets (HELLP)
Adjusted odds ratios (AORs) and confidence intervals (CIs) were computed for each study outcome using logistic regression and adjusted for age, race, multiple gestation, pre-existing diabetes, hypertension, dyslipidemia, and obesity, with p <0.05 considered statistically significant; N= 17,263,795 for all models except maternal death (17,260,870 for maternal death model) of available 18,574,225;
Reflects 5 deaths in NAFLD group, 35 deaths in other CLD group and 920 deaths in no CLD group.
Perinatal Complications in Pregnancies With NAFLD versus non-NAFLD CLD and no CLD
Pre-term birth was more common with NAFLD compared to other CLD and no CLD groups (9% vs 7% and 5%, respectively), as was large for gestational age (5% vs < 3% in other groups), p values <.001 (Table 2). Conversely, fetal growth restriction (FGR) was less common in pregnancies with NAFLD at 1.3% vs 3.6% in the other CLD group (p <.001), and vs 2.0% in the no CLD group (p=.10) (Table 2). On multivariate analysis, adjusting for age, race, multiple gestation and all pre-existing metabolic diseases, NAFLD was associated with pre-term birth compared to pregnancies without CLD (AOR 1.6, 95% CI 1.3–2.0 p<.001) (Table 3). Compared to other CLD in pregnancy, NAFLD was associated with a lower odds of FGR (AOR 0.4, 95% CI 0.2–0.7, p<.001), and conversely a higher odds of having large for gestational age infants (AOR 1.8, 95% CI 1.4–2.5, p<.001). No differences in fetal growth or size were observed on adjusted analyses comparing pregnancies with NAFLD versus no CLD (Table 3).
Sensitivity Analyses
All statistically significant estimates remained stable with inclusion of GDM to our fully adjusted model (Supplemental Table 2). No significant interactions between NAFLD and GDM were identified for the observed comparisons between NAFLD and no CLD groups (all interaction p values > 0.1). When NAFLD was compared to other CLD, a statistically significant interaction was observed for hypertensive complications, with lower odds of hypertensive complications identified in pregnancies with NAFLD and GDM (AOR 1.97, 95% CI 1.3–3.0, p=.001) as compared to pregnancies with NAFLD without GDM (AOR 3.29, 95% CI 2.6–4.1, p<.001), p=.03 for the comparison. Observed estimates remained stable after exclusion of pre-exising diabetes (Supplemental Table 3). We also evaluated the association of NAFLD with hypertensive complications after excluding pre-existing hypertension, and estimates remained stable, including adjusted models for NAFLD compared to other CLD (AOR 3.36, 95% CI 2.74–4.13, p<0.001) and compared to no CLD (AOR 3.37, 95% CI 2.78–4.08, p<0.001). Estimates also remained stable for study outcomes restricted to the ICD-9 time period, with the exception of large for gestational age, for which NAFLD also became significantly associated with LGA when compared to the no CLD group (Supplemental Table 4). In a sensitivity analysis of postpartum hemorrhage excluding hypertensive complications (pre-eclampsia, eclampsia, and/or HELLP) the association of NAFLD was slightly attenuated (AOR 1.4, 95% CI 1.0–1.8) but remained significant as compared to pregnancies with no CLD. Moreover, when we excluded women with risk factors for NAFLD from the no CLD group, the observed estimates for NAFLD associated risks further increased, indicating that our primary models reflect conservative estimates (Supplemental Table 5).
DISCUSSION:
In this large nationally representative U.S. database, we identified a more than tripling of NAFLD prevalence in pregnancies over the past 10 years. Pregnant women with NAFLD had significantly higher odds of serious maternal and perinatal complications, highlighting the importance of this emerging “high risk pregnancy” group. All metabolic co-morbidities, as well as caesarean deliveries, were more common in pregnancies affected by NAFLD. Moreover, after adjusting for metabolic risk factors, NAFLD remained associated with hypertensive complications, postpartum hemorrhage and preterm birth. There was a higher odds of maternal mortality as compared to pregnancies without CLD, though maternal death was overall quite rare, thus findings must be interpreted with caution. Overall these data do support the need for increased awareness of the risks of NAFLD in pregnant women and their need for linkage with high-risk obstetric care.
Recent U.S. data show a five-fold rise in NAFLD incidence over the past 20 years, with the largest rise in adults under the age of 40 years.(2) Our data highlight the impact of this national epidemic on reproductive-aged women in particular, with NAFLD rates in pregnancy tripling since 2007. These findings align with trends in obesity and diabetes in young adults(16), including a rise in gestational diabetes in U.S. women, thought to relate to the growing number of pregnancies in overweight and obese women.(17) Taken together these data support the need for more routine consideration of NAFLD in pregnancy, particularly in women with existing metabolic comorbidities.
The most striking finding from this study was the more than three-fold higher risk of hypertensive complications, including pre-eclampsia, eclampsia, or HELLP in pregnancies affected by NAFLD. Moreoever, this increased risk was apparent when compared to pregnancies affected by other chronic liver diseases, supporting its more specific association with NAFLD versus liver disease in general. While obesity and pre-existing HTN are established risk factors for pre-eclampsia(18), the current study highlights the independent association of NAFLD with the spectrum of hypertensive complications, which persisted despite adjustment for baseline metabolic co-morbidities. This finding has direct implications for pregnancy counseling in women with NAFLD, and supports the need for future studies to evaluate the potential role of prophylactic measures in pregnant women with NAFLD, such as aspirin use to help mitigate this risk.
The mechanisms by which NAFLD may promote adverse maternal and perinatal events are not well defined, although observed estimates remained stable despite extensive efforts to evaluate for potential confounding factors, including sensitivity and stratified analyses evaluating the contribution of pre-existing metabolic disease and gestational diabetes. Insulin resistance in the setting of NAFLD is associated with hepatic production of inflammatory mediators such as tumor necrosis factor-alpha and interleukin −1ß.(19) Insulin resistance outside of pregnancy is also known to promote activation of the renin-angiotensin-aldosterone pathway, leading to elevated blood pressure. Activation of the RAA system in women with NAFLD may contribute to their increased risk of hypertensive complications in pregnancy. It is less clear why NAFLD was so strongly associated with postpartum hemorrhage. Underreporting of advanced liver disease is possible, though perhaps less likely in a young cohort of women who were able to conceive. In a sensitivity analysis excluding pre-eclampsia, eclampsia, and HELLP, estimates for postpartum hemorrhage were attenuated though remained significant, indicating that bleeding risk was not entirely explained by these conditions. An enhanced awareness of maternal and perinatal risks in women with NAFLD should promote prospective mechanistic studies to understand these pathways.
A smaller study from Sweden capturing 110 pregnant women with NAFLD also identified increased risk of pre-eclampsia and pre-term birth.(20) In that study NAFLD was associated with low birthweight infants which contrasts with the association of NAFLD with large for gestational age infants observed by us and others.(21) Differences may be due to variability in study populations as well as comorbid exposures; the Swedish study did not capture alcohol history, thus alcoholic steatosis could have been misclassified as NAFLD. A smaller cross sectional study from Sri Lanka also identified NAFLD as a risk factor for having either pre-eclampsia or gestational hypertension(22), although due to modest sample size, more serious outcomes were not evaluated, such as HELLP, eclampsia, postpartum hemorrhage, or maternal and fetal mortality.
The current study did focus on serious adverse maternal and perinatal outcomes rather than the more established association of NAFLD with gestational diabetes. Pregnancy is an insulin resistant state, which is a normal physiologic adaptation to the growing fetus to ensure their adequate carbohydrate supply.(23) Concurrent NAFLD in pregnancy appears to compound this insulin resistant state, leading to increased risk of GDM. A prospective Korean study assessed for NAFLD in early pregnancy and found presence and severity of steatosis on ultrasound to be associated with the development of GDM.(24) In a similar study from Canada, hepatic steatosis in early pregnancy was associated with a composite outcome of impaired fasting glucose, impaired glucose tolerance, or GDM at 24–28 weeks of gestation.(25) In our study cohort GDM was nearly three times as common in pregnancies with NAFLD, though additional adjustment for GDM did not explain their higher risk for maternal and perinatal complications.
In addition to more immediate peripartum risks, NAFLD in pregnant mothers may adversely affect the long-term health of their children. Parental history of NAFLD is associated with risk of NAFLD in children(26) although whether having NAFLD during pregnancy influences this risk is unknown. Other forms of metabolic disease during pregnancy do appear to contribute to childhood NAFLD, including baseline and trajectory of weight gain in pregnancy.(27) Higher maternal BMI is also predictive of greater hepatic lipid and fat content in infants, as measured by magnetic resonance imaging in early neonatal life.(28–30) Stillborn babies of mothers with GDM also have increased prevalence of histologically-confirmed steatosis.(31) At the time of pediatric NAFLD diagnosis, between 25–50% of children have been shown to have NASH, up to 25% of whom have advanced fibrosis.(32) Thus, the rising rates of NAFLD in pregnancy may have much farther reaching implications for liver disease in youth.
The current study has some notable limitations and important strengths. The NIS is a large, nationally representative dataset, although our study was dependent upon discharge diagnoses to ensure that we only captured study outcomes from individual pregnancies. Although we do not know the timing of NAFLD diagnosis, this chronic liver condition is expected to have been present throughout pregnancy. We were careful to analyze our data with attention to the nuances of NIS discharge records, as well as changes in sampling schemes over time.(33) The rising prevalence of NAFLD in pregnancy likely reflects both the growing awareness of NAFLD, in addition to its rising incidence. Excluding ICD10 data, which introduced a dedicated NASH code, did not affect our results. Our findings also mirror other studies that show increased NAFLD incidence across study settings, including in women of child-bearing age.(2, 34) The prevalence of NAFLD in our study was also low, and thus likely underestimates disease, although we remained adequately powered to detect clinically relevant risks in pregnancies with NAFLD compared to non-NAFLD groups. It is also likely that some pregnancies in the no CLD group included undiagnosed NAFLD, particularly those with metabolic risk factors. However, this misclassification would bias results towards the null, which we confirmed to be the case in sensitivity analyses excluding pregnancies with metabolic conditions from the no CLD group. The NIS does lack patient identifiers, thus the same participant may have been captured during multiple pregnancies. However, prevalence estimates reflect person-year within a calendar period, which is less likely to result in double-counting. Furthermore, each pregnancy provides unique information on individual perinatal outcomes. Pre-pregnancy metabolic comorbidities and GDM were treated as potential confounders in our analysis, and while we performed extensive sensitivity analyses demonstrating stability of results, residual confounding cannot be excluded. It is also possible that pregnancies with adverse outcomes were more likely to have testing for liver disease (i.e. confounding by indication), leading to increased NAFLD detection. However, for our three main study outcomes (pre-term birth, postpartum hemorrhage, and hypertensive complications), confounding by indication was not felt to be likely for the following reasons. A prior study also identified an association of NAFLD with pre-term birth when NAFLD was assessed earlier in pregnancy, and thus prior to the occurrence of pre-term birth.(20) The standard of care evaluation and management of postpartum hemorrhage does not include liver enzymes or abdominal imaging, and when imaging is indicated would typically entail only pelvic ultrasonography.(35, 36) Finally, the observed risk of hypertensive complications in NAFLD pregnancies was also noted when compared to pregnancies with other chronic liver diseases, for which prior liver tests and/or imaging would also have been performed.
In summary, we identified a near tripling of NAFLD prevalence in pregnancies over the past ten years. While pregnancies with NAFLD were more likely to have co-existing metabolic disease, NAFLD was associated with adverse maternal and perinatal outcomes independent of metabolic comorbidities. These data support a critical need to recognize the public health implications of NAFLD in reproductive-aged women, and ensure that women with NAFLD receive adequate pre-conception counseling, including efforts to optimize metabolic health. Moreover, women with NAFLD in pregnancy should be managed by high-risk obstetrics, with a goal of improving outcomes in this growing population of mothers and infants.
Supplementary Material
Supplemental Figure 1. Temporal Trends in non-NAFLD Chronic Liver Disease Prevalence in Pregnancy (2007–2016). P-value < 0.001 (test of trend).
Highlights:
The prevalence of non-alcoholic fatty liver disease (NAFLD) has nearly tripled over the past decade.
Maternal and perinatal complications are more common with NAFLD as compared to pregnancies without liver disease and pregnancies with other causes of liver disease.
NAFLD in pregnancy is independently associated with pre-eclampsia, eclampsia, or hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, post-partum hemorrhage, and pre-term birth.
Women with NAFLD warrant pre-conception counseling and management by high-risk obstetricians during pregnancy.
Acknowledgments:
We thank the UCSF Liver Center (P30 DK026743) for their support.
Financial support: MS is supported by a K23 award from National Institutes of Health to (DK111944).
Abbreviations:
- NAFLD
Nonalcoholic Fatty Liver Disease
- CLD
Chronic Liver Disease
- U.S.
United States
- NASH
Nonalcoholic Steatohepatitis
- DM
Diabetes Mellitus
- NIS
National Inpatient Sample
- ICD
International Classification of Diseases
- HCUP
Healthcare Cost and Utilization Project
- HELLP
Hemolysis Elevated Liver Enzymes Low Platelets
- FGR
Fetal Growth Restriction
- LGA
Large For Gestational Age
- CI
Confidence Interval
- GDM
Gestational Diabetes Mellitus
- AOR
Adjusted Odds Ratio
- BMI
Body Mass Index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: MS is the site principal investigator for a NAFLD clinical trial funded by Zydus pharmaceuticals. NT is on the advisory board at Intercept Pharmaceuticals and receives grant support Gilead Sciences. No other authors have relevant conflicts of interest.
References:
- 1.Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology. 2018;67(5):1726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic Steatohepatitis is the Most Rapidly Increasing Indication for Liver Transplantation in Young Adults in the United States. J Clin Gastroenterol. 2018;52(4):339–46. [DOI] [PubMed] [Google Scholar]
- 4.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. The American journal of gastroenterology. 2018;113(11):1649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. Jama. 2012;307(5):491–7. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health. 2010;100(6):1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HCUP Databases. Healthcare Cost and Utilization Project (HCUP): Agency for Healthcare Research and Quality, Rockville, MD; 2018. [Available from: www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 9.Aloizos S, Seretis C, Liakos N, Aravosita P, Mystakelli C, Kanna E, et al. HELLP syndrome: understanding and management of a pregnancy-specific disease. J Obstet Gynaecol. 2013;33(4):331–7. [DOI] [PubMed] [Google Scholar]
- 10.Wier LM, Witt E, Burgess J, Elixhauser A. Hospitalizations Related to Diabetes in Pregnancy, 2008: Statistical Brief #102. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Rockville (MD) 2006. [PubMed] [Google Scholar]
- 11.Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, Wolff T, Steiner CA, Elixhauser A. Delivery Hospitalizations Involving Preeclampsia and Eclampsia, 2005–2014: Statistical Brief #222. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Rockville (MD) 2006. [PubMed] [Google Scholar]
- 12.[Available from: https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp.
- 13.Houchens R, Ross D, Elixhauser A. Using the HCUP National Inpatient Sample to Estimate Trends. U.S. Agency for Healthcare Research and Quality; 2016. [Google Scholar]
- 14.Ajmera VH, Gunderson EP, VanWagner LB, Lewis CE, Carr JJ, Terrault NA. Gestational Diabetes Mellitus Is Strongly Associated With Non-Alcoholic Fatty Liver Disease. The American journal of gastroenterology. 2016;111(5):658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. [DOI] [PubMed] [Google Scholar]
- 16.Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15(4):230–40. [DOI] [PubMed] [Google Scholar]
- 17.Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124(5):804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15(5):275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7(6):496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagstrom H, Hoijer J, Ludvigsson JF, Bottai M, Ekbom A, Hultcrantz R, et al. Adverse outcomes of pregnancy in women with non-alcoholic fatty liver disease. Liver international : official journal of the International Association for the Study of the Liver. 2016;36(2):268–74. [DOI] [PubMed] [Google Scholar]
- 21.Lee SM, Kim BJ, Koo JN, Norwitz ER, Oh IH, Kim SM, et al. Nonalcoholic fatty liver disease is a risk factor for large-for-gestational-age birthweight. PloS one. 2019;14(8):e0221400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herath RP, Siriwardana SR, Ekanayake CD, Abeysekara V, Kodithuwakku SUA, Herath HP. Non-alcoholic fatty liver disease and pregnancy complications among Sri Lankan women: A cross sectional analytical study. PloS one. 2019;14(4):e0215326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivan E, Homko CJ, Chen X, Reece EA, Boden G. Effect of insulin on fat metabolism during and after normal pregnancy. Diabetes. 1999;48(4):834–8. [DOI] [PubMed] [Google Scholar]
- 24.Lee SM, Kwak SH, Koo JN, Oh IH, Kwon JE, Kim BJ, et al. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia. 2019;62(2):238–48. [DOI] [PubMed] [Google Scholar]
- 25.De Souza LR, Berger H, Retnakaran R, Vlachou PA, Maguire JL, Nathens AB, et al. Non-Alcoholic Fatty Liver Disease in Early Pregnancy Predicts Dysglycemia in Mid-Pregnancy: Prospective Study. The American journal of gastroenterology. 2016;111(5):665–70. [DOI] [PubMed] [Google Scholar]
- 26.Long MT, Gurary EB, Massaro JM, Ma J, Hoffmann U, Chung RT, et al. Parental non-alcoholic fatty liver disease increases risk of non-alcoholic fatty liver disease in offspring. Liver international : official journal of the International Association for the Study of the Liver. 2019;39(4):740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayonrinde OT, Adams LA, Mori TA, Beilin LJ, de Klerk N, Pennell CE, et al. Sex differences between parental pregnancy characteristics and nonalcoholic fatty liver disease in adolescents. Hepatology. 2018;67(1):108–22. [DOI] [PubMed] [Google Scholar]
- 28.Modi N, Murgasova D, Ruager-Martin R, Thomas EL, Hyde MJ, Gale C, et al. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res. 2011;70(3):287–91. [DOI] [PubMed] [Google Scholar]
- 29.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr. 2013;162(5):930–6 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gale C, Thomas EL, Jeffries S, Durighel G, Logan KM, Parkinson JR, et al. Adiposity and hepatic lipid in healthy full-term, breastfed, and formula-fed human infants: a prospective short-term longitudinal cohort study. Am J Clin Nutr. 2014;99(5):1034–40. [DOI] [PubMed] [Google Scholar]
- 31.Patel KR, White FV, Deutsch GH. Hepatic steatosis is prevalent in stillborns delivered to women with diabetes mellitus. J Pediatr Gastroenterol Nutr. 2015;60(2):152–8. [DOI] [PubMed] [Google Scholar]
- 32.Goyal NP, Schwimmer JB. The Progression and Natural History of Pediatric Nonalcoholic Fatty Liver Disease. Clinics in liver disease. 2016;20(2):325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, et al. Adherence to Methodological Standards in Research Using the National Inpatient Sample. Jama. 2017;318(20):2011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology. 2018;15(1):11–20. [DOI] [PubMed] [Google Scholar]
- 35.Committee on Practice B-O. Practice Bulletin No. 183: Postpartum Hemorrhage. Obstetrics and gynecology. 2017;130(4):e168–e86. [DOI] [PubMed] [Google Scholar]
- 36.Prevention and Management of Postpartum Haemorrhage: Green-top Guideline No. 52. BJOG. 2017;124(5):e106–e49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Temporal Trends in non-NAFLD Chronic Liver Disease Prevalence in Pregnancy (2007–2016). P-value < 0.001 (test of trend).


