Abstract
Recent studies indicate that neuroimmune factors, including the cytokine interleukin-6 (IL-6), play a role in the CNS actions of alcohol. The cerebellum is a sensitive target of alcohol, but few studies have examined a potential role for neuroimmune factors in the actions of alcohol on this brain region. A number of studies have shown that synaptic transmission, and in particular inhibitory synaptic transmission, is an important cerebellar target of alcohol. IL-6 also alters synaptic transmission, although it is unknown if IL-6 targets are also targets of alcohol. This is an important issue because alcohol induces glial production of IL-6, which could then covertly influence the actions of alcohol. The persistent cerebellar effects of both IL-6 and alcohol typically involve chronic exposure and, presumably, altered gene and protein expression. Thus, in the current studies we tested the possibility that proteins involved in inhibitory and excitatory synaptic transmission in the cerebellum are common targets of alcohol and IL-6. We used transgenic mice that express elevated levels of astrocyte produced IL-6 to model persistently elevated expression of IL-6, as would occur in alcohol use disorders, and a chronic intermittent alcohol exposure/withdrawal paradigm (CIE/withdrawal) that is known to produce alcohol dependence. Multiple cerebellar synaptic proteins were assessed by Western blot. Results show that IL-6 and CIE/withdrawal have both unique and common actions that affect synaptic protein expression. These common targets could provide sites for IL-6/alcohol exposure/withdrawal interactions and play an important role in cerebellar symptoms of alcohol use such as ataxia.
Keywords: GABAA receptor subunits, glutamate receptor subunits, glia, neuroimmune, cytokine, vesicular transporters
Graphical Abstract
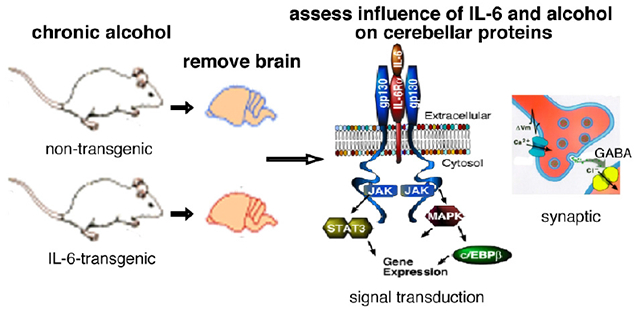
Overview.
Comparison of effects of IL-6 and alcohol, alone and together, on cerebella from non-transgenic (littermate controls) and IL-6 transgenic mice revealed that proteins involved in inhibitory GABAergic synaptic transmission are important targets of both IL-6 and alcohol.
Introduction
The cerebellum is a CNS region that shows high sensitivity to alcohol both during development and in the adult (Alfonso-Loeches and Guerri, 2011;Kumar et al., 2013;Oscar-Berman and Marinkovic, 2007). Both cerebellar neurons and glial cells are targets of alcohol actions, and show altered cellular or synaptic function following acute or chronic alcohol exposure, effects that underlie alcohol-induced changes in cerebellar function (Dar, 2015;Luo, 2015;Riikonen et al., 2002;Stowell and Majewska, 2020;Valenzuela and Jotty, 2015;Worst and Vrana, 2005;Zhang et al., 2015). Well-known behavioral consequences of excessive alcohol use include ataxia and motor dysfunction, among other cerebellar symptoms (Dar, 2015;Luo, 2015). In addition to the well-known role of the cerebellum in motor coordination and learning, recent studies indicate that the cerebellum participates in cognitive and emotional processes, and psychiatric disorders (Carta et al., 2019;Fitzpatrick and Crowe, 2013;Phillips et al., 2015;Rossi and Richardson, 2018). For example, connections occur between the cerebellum and CNS regions involved in fear and anxiety, and studies indicate a role of the cerebellum in these higher-order processes (Lange et al., 2015;Moreno-Rius, 2018;Timmann et al., 2010). The cerebellum also communicates with CNS regions involved with alcohol reward and alcohol drinking (Rossi and Richardson, 2018). Thus, effects of alcohol on the cerebellum could impact the function of several CNS regions and a variety of behaviors.
The actions of alcohol on the cerebellum and other CNS regions depend on a variety of factors such as alcohol dose, route of alcohol administration, age of the subjects, and local environmental factors. Recent studies indicate that alcohol induces the production of neuroimmune factors, including the cytokine IL-6, in the cerebellum and other CNS regions, thus affecting the local cellular environment (Kane et al., 2014;Lippai et al., 2013). IL-6 is produced primarily by glial cells of the CNS, the astrocytes, and microglia, and is an important signaling molecule in the CNS. IL-6 has been shown to alter the physiology of cerebellar cells (Gruol, 2015;Gruol et al., 2014;Peng et al., 2005). Thus, indirect effects alcohol on the cerebellum could occur as a consequence of alcohol-induced production of IL-6.
Given that little is known about the actions of IL-6 and interactions between IL-6 and alcohol in the CNS, and that emerging research indicates that neuroimmune activation is an important aspect of the effects of alcohol on the CNS (Crews et al., 2017;Robinson et al., 2014), our studies have focused on the issue of alcohol/IL-6 interactions. In recent studies we assessed potential interactions between effects of IL-6 and chronic alcohol on CNS function using transgenic mice that express elevated levels of IL-6 in the CNS through increased astrocyte expression (Gruol et al., 2018). The IL-6 tg mice were used as a model for subjects that have experienced tonically elevated levels of IL-6 in the CNS, such as occurs with long-term alcohol abuse. This model can inform on structural and functional consequences of CNS produced IL-6 and how these consequences affect the actions of alcohol, information that is important to an understanding of the mechanisms underlying the actions of alcohol on the CNS. Results showed that IL-6 produces neuroadaptive changes in the CNS and that interactions occurred between the neuroadaptive changes and actions of chronic intermittent alcohol exposure/withdrawal (ClE/withdrawal). These changes included altered brain activity as measured in EEG recordings and altered expression/activation of proteins involved in IL-6 signal transduction and inhibitory GABAergic synaptic transmission in the hippocampus. Results also showed that IL-6 and alcohol have similar cellular and synaptic targets in the hippocampus. Actions of IL-6 and alcohol at these targets could underlie the IL-6/alcohol interactions affecting EEG activity in these studies and could be a contributing factor to the effects of alcohol on the CNS under conditions of alcohol-induced IL-6 production.
In the current studies we have extended our investigation into the cellular and synaptic actions of IL-6 and IL-6/alcohol interactions in the CNS of IL-6 tg mice to the cerebellum, which is the brain region that expresses the highest level of IL-6 mRNA in the CNS of the IL-6 tg mice (Campbell et al., 1993). Interestingly, the IL-6 tg mice express ataxia, a process that is altered by alcohol, suggesting a potential role for IL-6 in cerebellar control of movement. Both acute and chronic alcohol have been reported to produce elevated levels of IL-6 mRNA in the cerebellum. For example, in adult rats an acute high dose of alcohol (4 g/kg, i.p.) increased cerebellar levels of IL-6 mRNA, but reduced cerebellar levels of IL-1 and TNFa mRNA (Doremus-Fitzwater et al., 2014). Chronic exposure to alcohol (6 g/kg by gavage for 10 days) also increased expression of IL-6 mRNA in the cerebellum with no effect on TNFα mRNA levels (Kane, et al., 2014).
Little is known about the consequences of the alcohol-induced IL-6 production in the cerebellum. To address this issue, we have analyzed effects of IL-6 and alcohol on synaptic proteins in cerebella from alcohol naïve and CIE/withdrawn IL-6 tg and non-tg littermate control mice. Based on results from our studies of the hippocampus, we focused on proteins involved in inhibitory synaptic transmission mediated by the GABAA subtype of GABA receptors (GABAAR), which are an important and highly sensitive target of alcohol in cerebellar circuits and have been implicated in cerebellar dysfunction associated with excessive alcohol use (Belmeguenai et al., 2008;Botta et al., 2007;Carta et al., 2004;Carta et al., 2006;He et al., 2013;Hirono et al., 2009;Kaplan et al., 2016;Li et al., 2018;Mapelli et al., 2009;Rossi and Richardson, 2018;Saeed Dar, 2006;Simonyi et al., 1996;Su et al., 2010;Valenzuela and Jotty, 2015;Wadleigh and Valenzuela, 2012;Zamudio-Bulcock et al., 2018). Alcohol-induced effects on GABAAR function in the cerebellum impacts both motor and non-motor processes associated with cerebellar circuitry (e.g., cognitive/emotive/reward) (Blednov et al., 2017;Rossi and Richardson, 2018;Valenzuela and Jotty, 2015;Wu et al., 2014). The GABAA subtype of GABA receptors (GABAAR) are multi-subunit (pentameric) ligand-gated ionotropic channels. The pentamers are comprised of different subunits (e.g., α1-α6, β1-β3, γ1-γ3, δ, ε, π, θ, and ρ1-ρ3) that form channels permeable to chloride ions. Depending on the subunit composition, the GABAARs mediate phasic (i.e., synaptic) or tonic (extrasynaptic) inhibition. In the cerebellum, receptors mediating phasic inhibition typically contain the a1 subunit, whereas receptors mediating tonic inhibition typically contain the α5 or α6 subunit. For example, in cerebellar granule neurons from adult rodents, tonic inhibition is mediated by GABAARs containing the α6 subunit (Santhakumar et al., 2006). Tonic inhibition is the primary form of synaptic inhibition in the granule neurons and is enhanced by low doses of acute alcohol (5-30 mM), although the exact mechanism mediating this action of alcohol is controversial (Botta, et al., 2007;Hamann et al., 2002;Valenzuela and Jotty, 2015). The increased tonic inhibition produced by acute alcohol has been proposed to play a role in alcohol-induced motor effects (Hanchar et al., 2005).
In the current studies a number of synaptic proteins were examined for effects of IL-6, alcohol and IL-6/alcohol interactions included the GABAAR subunits alpha-1, alpha-5 and alpha-6, the postsynaptic scaffolding protein gephyrin, the GABA synthesizing enzymes GAD65/67, and the GABA vesicular transporter VGAT. In addition, three proteins involved in excitatory synaptic transmission were examined, the AMPA receptor subunit GluR1, the glutamate vesicular transporter VGLUT1, and the astrocyte transporter GLAST. Glutamine synthetase, the astrocyte protein involved in both GABA and glutamate cycles, was also examined. Results show that prolonged exposure to IL-6 in the IL-6 tg cerebellum alters the expression of proteins involved in both inhibitory and excitatory synaptic function. Both increases and decreases in protein levels were observed. CIE/withdrawal reversed effects of IL-6 for some proteins but enhanced effects of IL-6 for others. Taken together, these results provide support for the hypotheses that alcohol-induced production of IL-6 can influence the effects of alcohol on synaptic pathways. Presumably behaviors mediated by those pathways would also be affected.
Experimental procedures
Transgenic mice
Heterozygous IL-6-tg mice (n = 21) with elevated expression of astrocyte produced IL-6 in the CNS and littermate controls (n = 23) were used in these experiments. Male and female mice of both genotypes (similar numbers) were used, although sex differences were not a focus of the studies. Construction of the transgenic mice was described previously (Campbell, et al., 1993). The elevated expression of IL-6 was targeted to astrocytes using an expression vector derived from the murine glial fibrillary acidic protein (GFAP) gene. The mice were derived from the low expressor 167 IL-6 tg mouse line. The low expressor line expresses higher levels of IL-6 than wildtype mice but lower levels than the high expressor line (Campbell, et al., 1993). The mice used in the current studies were obtained by breeding heterozygous IL-6 tg mice from the 167 line with wild-type C57BL/6J. Genotyping was carried out by PCR analysis of tail DNA using standard methods. The mice were subjected to a CIE/withdrawal treatment paradigm at 4-5 months of age and were 6-7 months of age at the completion of the treatment, at which time the animals were sacrificed and tissue collected for protein assays. All animal procedures were performed in accordance with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
Elevated levels of IL-6 mRNA in the cerebellum of the IL-6 tg mice is evident at 1 week postnatal but does not become prominent until about 1 month postnatal, suggesting that IL-6 production is not prominent until about 1 month postnatal (Chiang et al., 1994). Thus, with respect to alcohol-induced production of IL-6 this model is likely to be most relevant to alcohol use that starts in the juvenile or adolescent stage of life, a pattern of alcohol use that has significant risk for developing alcohol dependence and is currently an important societal issue (Foltran et al., 2011;Hingson et al., 2006). Studies in animal models have shown that ethanol exposure increases IL-6 levels in the CNS of adolescents as well as adults (Doremus-Fitzwater et al., 2015). The IL-6-tg mice exhibit progressive neurological symptoms including ataxia. However, structural features and neurological symptoms in the low expressor line used for the current studies are relatively minor until about 12 months of age (Brett et al., 1995;Campbell, et al., 1993;Gyengesi et al., 2019;Heyser et al., 1997).
Chronic intermittent alcohol (ethanol) treatment
The cerebellum was obtained from brains of animals that were used for studies of EEG activity during alcohol withdrawal. Protein activation/expression in the hippocampus and cerebellum were also investigated in parallel studies after completion of the EEG studies. Results from the EEG studies and studies of protein activation/expression in the hippocampus have been published (Gruol, et al., 2018). The current studies report results on synaptic proteins in the cerebellum.
A well-established paradigm for alcohol exposure that is a model for alcohol dependence, CIE/withdrawal in vapor chambers (La Jolla Alcohol Research, La Jolla, CA), was used for these studies (Becker and Lopez, 2004;Griffin et al., 2009;Lopez and Becker, 2014), as described previously (Gruol, et al., 2018). Male and female alcohol naïve IL-6-tg and non-tg mice in the CIE group were injected with 1.75 g/kg alcohol plus 68.1 mg/kg pyrazole (an alcohol dehydrogenase inhibitor; used to stabilize blood alcohol levels) prior to alcohol exposure and placed in chambers used for alcohol vapor exposure. The mice were exposed to three cycles of CIE/withdrawal. For each cycle, the animals were exposed to 3 days of alcohol vapor/withdrawal (16 h vapor on/ 8 h off each day) and then left undisturbed for 72 hrs. At the end of the 72 hrs, the next CIE/withdrawal cycle was started. Control (alcohol naïve) mice were injected with 68.1 mg/kg pyrazole in saline and placed in air chambers for the same period as the alcohol exposed animals. Target blood alcohol levels were 150-225 mg%. Blood alcohol levels (BAL) were measured at the end of the 16 hr alcohol exposure period on the 2nd day of alcohol exposure for each of the 3 CIE/withdrawal cycles. Blood alcohol levels (BALs) were measured on the 2nd day of alcohol exposure rather than the 3rd day to avoid potential stress that could affect EEG recordings that were made during withdrawal from the 3rd day of alcohol exposure. Mean BAL values (averaged for 3 alcohol exposure periods) were 150 mg/dl for non-tg mice and 136 mg/dl for IL-6 tg mice and were not significantly different (Gruol, et al., 2018). Relative to doses of alcohol commonly used for experimental studies, these BALs are considered to reflect moderately high levels of alcohol.
Protein assays
Animals were sacrificed for biochemical studies at the termination of the 3rd CIE/withdrawal cycle, immediately after the 24 hour withdrawal period. Preparation of protein samples and protein assays were carried as previously described for hippocampus (Gruol, et al., 2018). Briefly, the cerebellum was removed from the brain of the mice and snap frozen in liquid nitrogen. For each animal, proteins were extracted from the whole cerebellum by sonication in cold lysis buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.5% NP-40, a Complete Protease Inhibitor Cocktail Tablet (Roche Diagnostics, Mannheim, Germany), and a cocktail of phosphatase inhibitors (Na+ pyrophosphate, β-glycerophosphate, NaF, Na+ orthovanadate; all from Sigma-Aldrich). The samples were incubated on ice for 30 minutes, centrifuged at 13,860 x g for 30 minutes at 4°C, and the supernatants were collected. Protein concentration in the supernatants was determined using the Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA). Aliquots were stored at −80°C.
Expression levels of cerebellar synaptic and cellular proteins were determined in a representative number of animals (male and female, randomly selected) from each genotype by Western blot following previously published protocols (Gruol, et al., 2014;Nelson et al., 2012). Briefly, cerebellar protein samples from IL-6 tg and non-tg mice were subjected to SDS-PAGE using 4-12% Novex NuPAGE Bis-Tris gels (Invitrogen Life Technologies, Grand Island, NY). Each gel contained protein samples from both genotypes and treatment groups. Proteins were transferred to Immobilon-P membranes (Millipore, Billerica, MA). Uniform transfer was assessed by Ponceau S staining (Pierce, Rockford, IL). Membranes were washed and blocked (5% casein solution; Pierce), incubated in primary antibody overnight (4°C), washed, and then incubated (room temperature) in secondary antibody coupled to horseradish peroxidase (HRP). Protein bands were visualized by chemiluminescence and quantified by densitometry measurements using NIH Image software (http://rsb.info.nih.gov/nih-image/). Membranes were stripped and reprobed for beta-actin. To standardize results, for each Western blot the density of the band of interest was normalized to the density of the band for beta-actin in the same lane. Normalized data were then normalized to the average normalized value for cerebellum from alcohol naïve non-tg mice run on the same gel. For each protein, several Western blots containing samples from different animal were performed, so that multiple measurements were made on the cerebellum of each animal. Mean values were calculated for each protein and animal and the mean values were grouped according to genotype and treatment for statistical analyses.
The following antibodies were used for Western blot studies: a purified rabbit polyclonal antibody produced by immunizing rabbits with a synthetic peptide derived from human GAD65/67 (glutamic acid decarboxylase 65/67)(#PA5-38102, 1-2000, Invitrogen Life Technologies); a purified mouse monoclonal antibody to the alpha-1 subunit of GABAAR (GABAAR alpha-1) produced by immunizing mice with fusion protein amino acids 355-394 of GABAAR alpha-1 (375-136, 1-500, UC Davis/NIH NeuroMab Facility); a purified mouse monoclonal antibody to the alpha-5 subunit of GABAAR (GABAAR alpha-5) produced by immunizing mice with a fusion protein containing a sequence from the cytoplasmic domain of human GABAAR alpha-5 subunit (375-401, 1-500, UC Davis/NIH NeuroMab Facility); a purified mouse monoclonal antibody to the alpha-6 subunit of GABAAR (GABAAR alpha-6) produced by immunizing mice with a fusion protein containing a sequence from the cytoplasmic domain (near the C-terminus) of mouse GABAAR alpha-6 (75-388, 1-500, UC Davis/NIH NeuroMab Facility); supernatant containing a mouse monoclonal antibody to vesicular GABA transporter 1 (VGAT) produced by immunizing mice with a fusion protein containing a sequence from the cytoplasmic N-terminus domain of mouse VGAT (73-457, 1-4, UC Davis/NIH NeuroMab Facility); an antibody raised against a fusion protein from the N-terminus of human gephyrin (anti-gephyrin clone L106/83, UC Davis/NIH NeuroMab Facility); a purified mouse monoclonal antibody to subunit 1 of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtype of glutamate receptor (GluR) produced by immunizing mice with a fusion protein containing a sequence from the extracellular N-terminus region of subunit 1 of rat GluR (GluR1) (75-327, 1-1000, UC Davis/NIH NeuroMab Facility); a purified mouse monoclonal antibody to the vesicular glutamate transporter 1 (VGLUT) produced by immunizing mice with a fusion protein containing a sequence from the cytoplasmic C-terminus region of rat VGLUT (75-066, 1-500, UC Davis/NIH NeuroMab Facility); a purified rabbit antibody to a synthetic peptide corresponding to a sequnce within human glutamine synthetase (Glu Syn)(PA5-28940, 1-4000, ThermoFisher Scientific, Rockford, IL); a purified rabbit antibody to a synthetic peptide corresponding to a sequnce within human EAAT1 (i.e., Na+-dependent glutamate-aspartate transporter, also known as GLAST)(PA5-19709, 1-4000, ThermoFisher scientific), a monoclonal antibody to beta-actin produced by immunizing mice with a synthetic peptide corresponding to a sequences in the amino-terminal of human beta-actin (AB#3700, 1:10,000; Cell Signaling Technology); a monoclonal antibody to beta-actin produced by immunizing rabbits with a synthetic peptide corresponding to a sequences in the amino-terminal of human beta-actin (AB#4970, 1:5,000; Cell Signaling Technology).
Statistics
ANOVA (for parametric data) or the Mann-Whitney test (for non-parametric data) was used for statistical analysis of data. Normality was determined by the Kolmogorov-Smirnov (K-S) test and variance by the F-test. For presentation, data were graphed as box plots and show median, maximum, and minimum. Statistical significance was set at p ≤ 0.05. n = number of animals used. StatView 5.0 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. There was no significant difference between results from females and males, and data have been combined for presentation purposes.
Results
GABARs
Postsynaptic GABAARs containing the alpha-1 subunit are widely expressed in the cerebellum, with abundance in the granule cell layer and mainly localized to the synapse where they participate in phasic synaptic inhibition (Hortnagl et al., 2013;Pirker et al., 2000;Poltl et al., 2003). In alcohol naïve mice, GABAAR alpha-1 levels were significantly lower in cerebellum from IL-6 tg mice compared to cerebellum from non-tg mice (U = 4, n1 = 9, n2 = 7, p = 0.004, Mann-Whitney) (Fig. 1A). No significant effect of CIE/withdrawal on GABAAR alpha-1 levels was observed in cerebellum from non-tg mice (F(1,11) = 3.59 p = 0.08) or IL-6 tg mice (U = 21, n1 = 7, n2 = 9, p = 0.06, Mann-Whitney) compared to cerebellum from the respective alcohol naïve non-tg and IL-6 tg mice control mice (Fig. 1A).
Fig. 1.
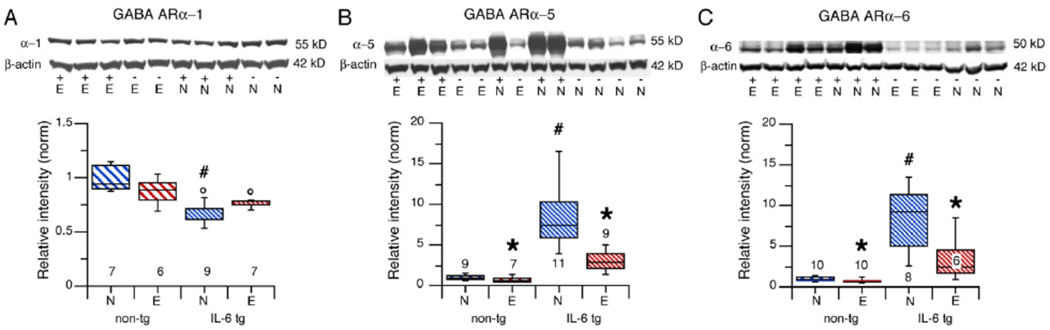
Effects of IL-6 and CIE/withdrawal on GABAAR subunit levels in cerebellum from IL-6 tg and non-tg mice. A, B, C. Graphs showing levels of GABAAR alpha-1 (A), GABAAR alpha-5 (B) and GABAAR alpha-6 (C) in cerebella from alcohol naïve (N) and CIE/withdrawn (E) non-tg and IL-6 tg mice. Representative Western blots are shown above the graphs. # = significantly different from non-tg of the same treatment group. * = significantly different from naïve of the same genotype. In this and other figures the numbers of animals measured are shown next to, within or below the graph. N = naïve, E = CIE/withdrawn. In Western blots − = non-tg, + = IL-6 tg.
In the cerebellum, GABAARs containing the alpha-5 subunit are primarily expressed in Purkinje neurons, are localized extra-synaptically, and are involved in tonic inhibition (Hortnagl, et al., 2013;Pirker, et al., 2000). Levels of GABAAR alpha-5 in cerebellum from alcohol naive IL-6 tg mice were significantly higher than levels in cerebellum from alcohol naive non-tg mice (U = 0, n = 11, n2 = 9, p = 0.0002, Mann-Whitney) (Fig. 1B). There was significant decrease in the level of GABAAR alpha-5 in cerebellum from CIE/withdrawn non-tg mice (F(1,14) = 5.42, p = 0.04) and IL-6 tg mice (U = 4, n1 = 11, n2 = 9, p = 0.0005, Mann-Whitney) compared to cerebellum from the respective alcohol naive non-tg and IL-6 tg mice (Fig. 1B).
GABAARs containing the alpha-6 subunit are prominently expressed in the granule neurons of the cerebellum (Kato, 1990;Laurie et al., 1992;Pirker, et al., 2000;Thompson et al., 1992). They are located both extrasynaptically and synaptically and primarily contribute to tonic inhibition but can also participate in phasic inhibition (Santhakumar, et al., 2006). Levels of GABAAR alpha-6 in cerebellum from alcohol naive IL-6 tg mice were significantly higher than in cerebellum from alcohol naive non-tg mice (U = 0, n1 = 8, n2 = 10, p = 0.0004, Mann-Whitney) (Fig. 1C). CIE/withdrawal significantly decrease the level of GABAaR alpha-6 in cerebellum from both non-tg (F(1,18) = 5.95 p = 0.03) and IL-6 tg mice (F(1,12) = 7.00, p = 0.02) when compared to cerebellum from the respective alcohol naive IL-6 tg and non-tg mice (Fig.1C).
Gephyrin
Gephyrin is a scaffolding protein that plays a central role in the distribution and clustering of GABAARs at postsynaptic sites on neurons (Tyagarajan and Fritschy, 2014). Gephyrin is widely distributed at synaptic sites and co-localized with GABAARs in the cerebellum (Sassoe-Pognetto et al., 2000). For example, immunostaining revealed that GABAaR alpha-1 subunit staining on dendrites in the molecular and granule cell layers was extensively co-localized with staining for gephyrin (Essrich et al., 1998;Sassoe-Pognetto, et al., 2000).
Gephyrin levels in cerebellum of alcohol naïve IL-6 tg mice were significantly reduced compared to gephyrin levels in cerebellum of alcohol naïve non-tg mice (U = 0, n1 = 11, n2 = 10, p = 0.0001, Mann-Whitney)(Fig. 2A, left panel), consistent with the reduced levels of GABAAR alpha-1. Cerebellum of CIE/withdrawn non-tg mice showed a significant decrease in gephyrin levels compared to cerebellum of alcohol naïve non-tg mice (F(1,18) = 8.37, p = 0.01). There was no significant effect of CIE/withdrawal on gephyrin levels in cerebellum from IL-6 tg mice (F(1,18) = 0.68, p = 0.36) (Fig. 2A, right panel). Thus, gephyrin levels in the cerebellum of IL-6 tg mice were resistant to the effects of CIE/withdrawal observed in the cerebellum of non-tg mice, perhaps because the levels of gephyrin were already down regulated by IL-6 in the cerebellum of the alcohol naïve IL-6 tg mice.
Fig. 2.
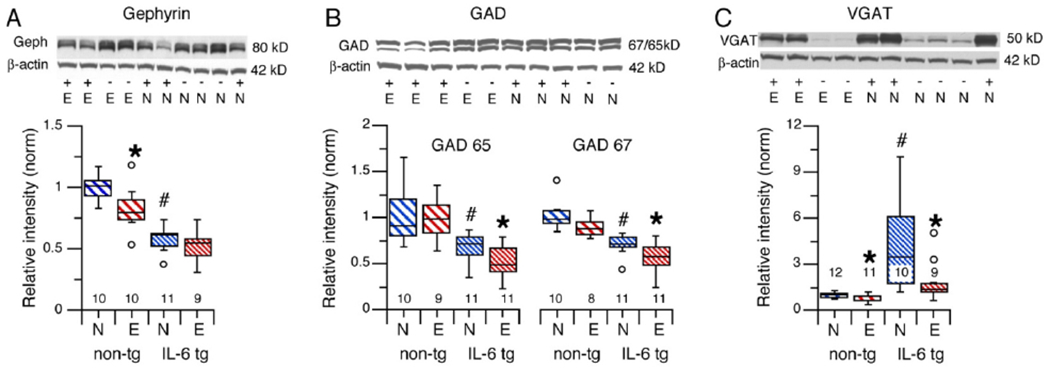
Effects of IL-6 and CIE/withdrawal on levels of cerebellar proteins related to GABAergic synaptic transmission in cerebellum of IL-6 tg and non-tg mice. A, B, C. Graphs show gephyrin (A), GAD 65/67 (B) and VGAT (C) levels in cerebellum from alcohol naïve and CIE/withdrawn IL-6 tg and non-tg mice. Representative Western blots are shown above the graphs. # = significantly different from non-tg of the same treatment group. * = significantly different from control of the same genotype. The open circles represent outliers in the data.
GAD65/67
The inhibitory transmitter GABA is synthesized through α-decarboxylation of glutamic acid by GAD 65/67, two isozymes encoded by different genes and identified by their molecular weight. Both isozymes are found in GABAergic neurons of the cerebellum, with GAD67 predominately localized to cell bodies and GAD65 predominately localized to axon terminals (Esclapez et al., 1994).
GAD 65 and GAD 67 levels in cerebellum of alcohol naïve IL-6 tg mice were significantly reduced compared to GAD 65 and GAD 67 levels, respectively, in cerebellum of alcohol naïve non-tg mice (GAD65: U = 16, = 10, n2 = 11, p = 0.006; GAD67: U =0, = 10, n2 = 10, p =0.002, Mann-Whitney)(Fig. 2B).
There was no significant difference in GAD 65 or GAD 67 levels between cerebellum from CIE/withdrawn non-tg mice compared to cerebellum of alcohol naïve non-tg mice (GAD 65, F(1,21) = 0.02, p = 0.88); GAD 67, (F(1,19) = 3.88, p = 0.06) (Fig. 2B). A significant decrease was observed in GAD 65 and 67 levels in cerebellum of CIE/withdrawn IL-6 tg mice compared to cerebellum of alcohol naïve IL-6 tg mice (GAD 65, F(1,17) = 8.06, p = 0.01; GAD 67, F(1,16) = 8.07, p = 0.01) (Fig. 2B). Thus, GAD 65/67 levels in cerebellum from IL-6 tg mice were sensitive to effects of CIE/withdrawal, whereas GAD 65/67 levels in cerebellum from non-tg mice were resistant.
VGAT
VGAT (subtype 1) is a vesicular transporter that mediates GABA uptake into synaptic vesicles. VGAT is highly expressed in the cerebellum, in presynaptic terminals, axons, and glial cells (Chiu et al., 2005). Mice with cerebellar Purkinje neuron specific VGAT knock out (L7-VGAT) retain cerebellar structure but show reduced VGAT protein and exhibit motor discoordination including ataxia, consistent with an important role for VGAT and inhibitory GABAergic transmission in cerebellar control of motor function (Kayakabe et al., 2013).
VGAT levels in cerebellum of alcohol naïve mice were significantly higher in cerebellum from IL-6 tg mice than in cerebellum of non-tg mice (U = 2, n1 = 12, n2 = 12, p <0.001, Mann-Whitney)(Fig. 2C). VGAT levels were significantly decreased in cerebellum from both CIE/withdrawn IL-6 tg and non-tg mice compared to cerebellum from the respective alcohol naïve mice (IL-6 tg, U = 17.00, n1 = 9, n2 = 10, p = 0.02; non-tg, F(1,21) = 5.89, p = 0.02)(Fig. 2C).
GluR1
GluR1, a subunit of the AMPA subtype of glutamate receptor, is expressed in the cerebellum in inhibitory cerebellar neurons, at synapses associated with excitatory glutamatergic parallel fiber and climbing synapses, at inhibitory basket cell axons and in Bergman glial cells (Baude et al., 1994;Castejon and Dailey, 2009;Martin et al., 1993). GluR1 levels in cerebellum of alcohol naïve IL-6 tg mice were significantly reduced compared to GluR1 levels in cerebellum of alcohol naïve non-tg mice (U = 4, n1 = 11, n2 = 12, p = 0.0001, Mann-Whitney)(Fig. 3A1). CIE/withdrawal significantly decreased GluR1 levels in cerebellum of CIE/withdrawn non-tg mice compared to cerebellum of alcohol naïve non-tg mice (U = 5 n1 = 12, n2 = 9, p = 0.0005, Mann-Whitney) (Fig. 3A2), but did not significantly effect GluR1 levels in cerebellum from IL-6 tg mice (F(1,17) = 1.12, p = 0.74) (Fig. 3A3). Thus, GluR1 levels in cerebellum from non-tg mice were sensitive to effects of CIE/withdrawal, whereas GluR1 levels in cerebellum from IL-6 tg mice were resistant.
Fig. 3.
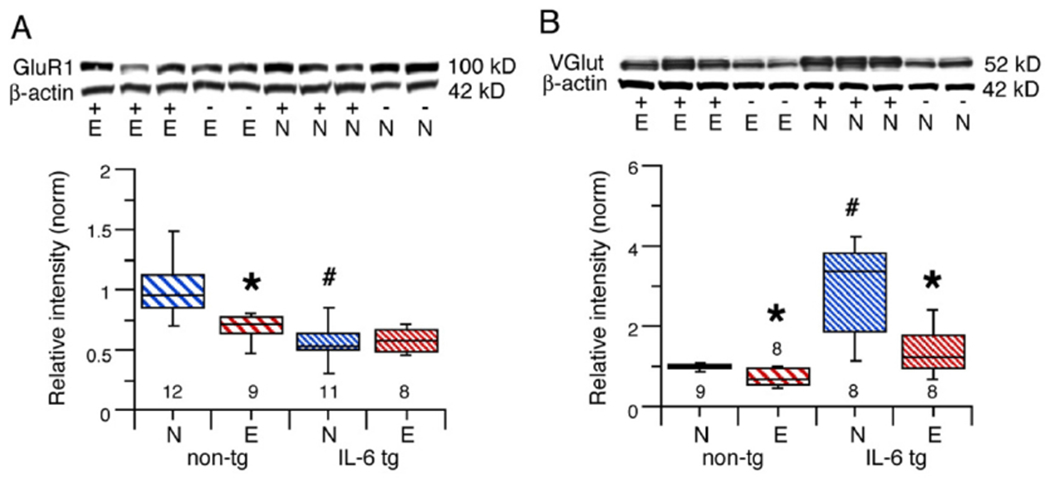
Effects of IL-6 and CIE/withdrawal on levels of cerebellar proteins related to glutamatergic synaptic transmission in cerebellum of IL-6 tg and non-tg mice. A,B. Graphs show GluR1 (A) and VGLUT (B) levels in cerebellum from alcohol naïve and CIE/withdrawn IL-6 tg and non-tg mice. Representative Western blots are shown above the graphs. # = significantly different from non-tg of the same treatment group. * = significantly different from control of the same genotype.
VGLUT1
VGLUT1 is a vesicular transporter that mediates glutamate uptake into synaptic vesicles. VGLUT1 is expressed in neurons of two excitatory synaptic pathways in the cerebellum, parallel fibers of cerebellar granule neurons, and mossy fibers originating from extracerebellar CNS regions (Hioki et al., 2003). VGLUT1 levels in cerebellum of alcohol naïve mice were significantly higher in cerebellum from IL-6 tg mice than in cerebellum of non-tg mice (U = 0, n1 = 8, n2 = 9, p = 0.0005, Mann-Whitney) (Fig. 3B). CIE/withdrawal produced a significant decrease in VGLUT1 levels in cerebellum of both CIE/withdrawn non-tg and IL-6 tg mice compared to cerebellum from their respective alcohol naïve mice (non-tg, U = 11, n1 = 8, n2 = 9, p = 0.02; IL-6 tg, U = 9, n1 = 8, n2 = 8, p = 0.02, Mann-Whitney test) (Fig. 3B).
Glial Proteins
Two proteins expressed by cerebellar astrocytes and Bergman glia were also studied, Glu Syn and GLAST.
Glu Syn
Glu Syn is a glutamate catabolizing enzyme exclusively located in astrocytes of the CNS where it catalyzes the synthesis of glutamine from glutamate and ammonia (glutamine-glutamate cycle) (Jayakumar and Norenberg, 2016). Glu Syn, in conjunction with high affinity glutamate transporters, which sequester glutamate into astrocytes, controls the concentration of glutamate at the synapse. Glutamine produced by astrocytes contributes to glutamate levels in both excitatory and inhibitory neurons. In excitatory neurons, glutamine is converted to the transmitter glutamate (glutamine-glutamate cycle), whereas in inhibitory neurons, glutamine is converted to glutamate, which is the precursor to the transmitter GABA (glutamine-GABA cycle) (Bak et al., 2006).
Glu Syn levels in cerebellum from alcohol naïve IL-6 tg mice were significantly higher level than in cerebellum from alcohol naïve non-tg mice (U = 8.0, n1 = 7, n2 = 7, p = 0.04, Mann-Whitney test; Fig. 4A). CIE/withdrawal significantly increased the level of Glu Syn in cerebellum from non-tg mice (U = 7.0, n1 = 7, n2 = 7, p = 0.03, Mann-Whitney test), while significantly decreasing the level of Glu Syn in cerebellum from IL-6 tg mice (U = 1.0, n1 = 7, n2 = 7, p = 0.003, Mann-Whitney test) compared to the respective alcohol naïve control (Fig. 4A).
Fig. 4.
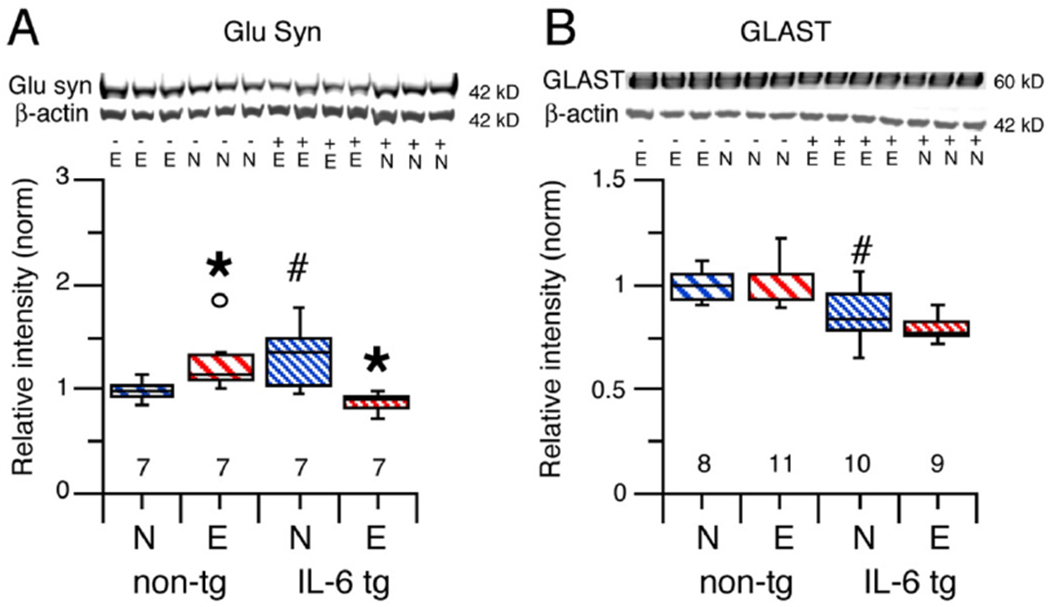
Effects of IL-6 and CIE/withdrawal on levels of cell specific proteins in cerebellum of IL-6 tg and non-tg mice. A,B. Graphs show Glu Syn (A) and GLAST (B) levels in cerebellum from alcohol naïve and CIE/withdrawn IL-6 tg and non-tg mice. Representative Western blots are shown above the graphs. # = significantly different from non-tg of the same treatment group. * = significantly different from control of the same genotype. The open circle represents an outlier in the data.
GLAST
Glutamate transporters, including GLAST, play a major role in control of the concentration of glutamate at the synapse. GLAST is located primarily on astrocytes and is critical to the removal of glutamate from the synapse. GLAST is prominently expressed in Bergman glia of the cerebellum and plays a prominent role in glutamate uptake in the cerebellum (Watase et al., 1998).
GLAST levels in cerebellum from alcohol naïve IL-6 tg mice were significantly lower than in cerebellum from alcohol naïve non-tg mice (F (1,16) = 6.96, p = 0.02; Fig. 4B). There was no effect of CIE/withdrawal on cerebellar GLAST levels for either genotype (IL-6 tg, F(1,17) = 2.37, p = 0.14; non-tg, F(1,17) = 0.35, p = 0.56)(Fig. 4B).
Discussion
In the current studies, we identify neuroadaptive changes in the expression of synaptic associated proteins in the cerebellum as a consequence of persistently expressed elevated levels of IL-6, a condition that occurs with excessive alcohol consumption. The proteins examined included neuronal and glial proteins associated with inhibitory (i.e., GABAAR alpha-1, alpha-5 and alpha-6, gephyrin, GAD65/67, VGAT, Glu Syn), and excitatory (i.e., GluR1, VGLUT1, Glu Syn and GLAST) synaptic transmission. In addition, interactions between the effects of IL-6 and the effects of CIE/withdrawal were identified, suggesting similar targets of IL-6 and CIE/withdrawal action. These interactions reversed or enhanced the effects of IL-6. Results are summarized in Table 1. In addition, complementary data from studies of hippocampal proteins are included for comparison purposes. Both similarities (e.g., effects of IL-6 and CIE/withdrawal on GABAAR alpha-5) and differences (e.g., effects of IL-6 on GABAAR alpha-1) were observed between results from the cerebellum and hippocampus. Similarities could reflect similar underlying mechanisms, whereas differences could reflect differences in cell types, IL-6 levels, or other factors between the two CNS regions. Although effects of alcohol on the levels of some of the synaptic proteins examined in this study have been shown previously by others to be altered by alcohol, few studies have examined the cerebellum.
Table 1.
Effect of alcohol exposure/withdrawal on protein expression
| Protein | Cerebellum | Hippocampus@ | ||||
|---|---|---|---|---|---|---|
| Naïve | CIE/withdrawn | Naïve | CIE/withdrawn | |||
| IL-6 +/− |
IL-6 +/− |
IL-6 −/− |
IL-6 +/− |
IL-6 +/− |
IL-6 −/− |
|
| 1. Synaptic proteins: GABA related | ||||||
| GABAAR α-1 | ↓ | ns | ns | ns | ns | ns |
| GABAAR α-5 | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ |
| GABAAR α-6 | ↑ | ↓ | ↓ | - | - | - |
| gephyrin | ↓ | ns | ↓ | ns | ns | ns |
| GAD 65 | ↓ | ↓ | ns | ns | ns | ns |
| GAD 67 | ↓ | ↓ | ns | ns | ns | ns |
| VGAT | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ |
| 2. Synaptic proteins: Glutamate related | ||||||
| GluR1 | ↓ | ns | ↓ | - | - | - |
| VGLUT1 | ↑ | ↓ | ↓ | - | - | - |
| 3. Glial proteins | ||||||
| GFAP | ↑$ | ↓$ | ns$ | ↑ | ↑ | ↑ |
| Glu Syn | ↑ | ↓ | ↑ | - | - | - |
| GLAST | ↓ | ns | ns | - | - | - |
↓ = decrease; ↑= increase; ns = no significant difference; − = not determined; naïve IL-6 tg (IL-6 +/−) results are relative to naïve non-tg (IL-6 −/−); CIE/withdrawn results are relative to alcohol naïve of the same genotype.
@ = Data from (Gruol, et al., 2018);
$ =Data from (Gruol et al., 2020)
The whole cerebellum was used for these studies so that sufficient tissue was available for analysis of multiple proteins. Thus, cerebellar regions or cell types involved in these changes could not be determined. However, the primary cellular region of the cerebellum, the cortical region, contains two major neuronal types, the granule neurons and Purkinje neurons. Granule neurons are small excitatory glutamatergic neurons and are the most populous neurons in the cerebellum. Purkinje neurons are large inhibitory neurons that have an expansive dendritic structure that receives extensive synaptic input from the granule neurons. Thus, granule neurons and Purkinje neurons are likely to be the primary targets of IL-6 and CIE/withdrawal detected in our studies, although axon terminals from excitatory neurons that innervate the cerebellum (e.g., climbing fibers and mossy fibers) and the inhibitory interneurons (e.g., Golgi cell, basket cell, and stellate cells) could also contribute. Both granule neurons and Purkinje neurons are sensitive to alcohol and IL-6, although studies on effects of chronic alcohol exposure/withdrawal on these neuronal types are limited (Gruol, 2013;Luo, 2015;Qiu et al., 1995;Valenzuela and Jotty, 2015).
Presynaptic effects
Both presynaptic and postsynaptic proteins associated with excitatory and inhibitory synaptic transmission were altered in the cerebellum of alcohol naïve IL-6 tg mice, indicative of neuroadaptive effects of IL-6. Some of these changes could reflect compensatory mechanisms. Presynaptic effects included a decrease in the level of the synthetic enzymes for GABA (GAD 65/67), which would predict lower levels of presynaptic GABA, and an increase in the level of vesicular transporter (VGAT) involved in sequestration of GABA into the synaptic vesicles. The combined increase in VGAT and decrease in GAD 65/67 levels could reflect compensatory presynaptic adjustments to normalize activity at inhibitory synapses in the cerebellum of the IL-6 tg mice. However, these types of presynaptic changes, if they resulted in reduced presynaptic GABA, could contribute to the increased seizure sensitivity of the IL-6 tg mice (Samland et al., 2003). Involvement of the cerebellum in seizure activity has been noted for several pathological conditions (e.g., (Kros et al., 2017;Marcian et al., 2016;Yu and Krook-Magnuson, 2015)).
CIE/withdrawal reduced GAD65/67 levels, and presumably presynaptic GABA levels, in the cerebellum from IL-6 tg mice, thereby increasing the reduction in GAD65/65 observed in the alcohol naïve IL-6 tg mice. CIE/withdrawal also reduced VGAT levels, but in this case both genotypes were affected. However, the effect was more prominent in the cerebellum of the IL-6 tg mice, which started from a higher level of VGAT under alcohol naïve conditions than the non-tg mice. The added effect of CIE/withdrawal to reduce GAD65/67 and VGAT in the IL-6 tg mice would predict lower presynaptic GABA and a greater reduction in inhibitory influences, a situation that could contribute to more prominent alcohol withdrawal symptoms such as the withdrawal hyperexcitability observed in the IL-6 tg mice (Gruol, et al., 2018;Jung, 2015;Keir and Morrow, 1994;Rewal et al., 2005). The lower levels of VGAT in the non-tg mice could also contribute to hyperexcitability during withdrawal in the non-tg mice. Reduced levels of GAD 65 could affect alcohol consumption if an IL-6-induced reduction in GAD65 was also expressed in brain areas that control drinking. Studies in GAD 65 knock out mice show that reduced GAD 65 levels increase alcohol intake compared to wildtype mice (Blednov et al., 2010).
The level of vesicular transporter (VGLUT) involved in sequestration of the excitatory transmitter glutamate into the synaptic vesicles of axon terminals of excitatory neurons was also increased in the cerebellum of the alcohol naïve IL-6 tg mice. VGLUT has been shown to regulate the glutamate content of the synaptic vesicles, the quanta released from the terminals and, consequently, synaptic strength at glutamatergic synapses (Wojcik et al., 2004). Overexpression of VGLUT1, the primary VGLUT isoform expressed in the cerebellum, has been shown to increase the amount of glutamate released from synaptic vesicles and enhance synaptic transmission (Wilson et al., 2005). Thus, the IL-6 induced increase in VGLUT1 levels would predict an enhancement of excitatory synaptic responses in the cerebellum of IL-6 tg mice, which could contribute to the increased seizure sensitivity of the IL-6 tg mice (Samland, et al., 2003). CIE/withdrawal reduced the level of VGLUT1 in cerebellum from both IL-6 tg and non-tg mice. In the cerebellum of the IL-6 tg mice, this action could counter the effects of increased levels of VGLUT1 observed in the cerebellum of alcohol naïve IL-6 tg mice.
Postsynaptic effects
Postsynaptic effects in the cerebellum of alcohol naïve IL-6 tg mice include a reduction in receptor subunits involved in phasic inhibitory (i.e., GABAAR alpha-1) and excitatory (i.e., GluRI) synaptic transmission, as well as the level of the scaffolding protein gephyrin, which is involved in receptor clustering at GABAergic synapses (Tyagarajan and Fritschy, 2014). In addition, levels of GABAAR alpha-5 and GABAAR alpha-6, which are primarily associated with tonic inhibition of Purkinje neurons and cerebellar granule neurons, respectively (Hortnagl, et al., 2013;Pirker, et al., 2000;Santhakumar, et al., 2006) were increased in cerebellum of alcohol naïve IL-6 tg mice. The increased levels of GABAAR subunits mediating tonic inhibition, the lower levels of GABAAR alpha-1, which mediates phasic inhibition, and lower levels of GAD65/67, which predicts reduced GABA levels, could shift the primary inhibitory influence from phasic to tonic in the cerebellum of the alcohol naïve IL-6 tg mice. Such changes could contribute to the reduced firing rate observed in Purkinje neurons in the cerebellum of IL-6 tg mice (Nelson et al., 1999) and in Purkinje neurons in cerebellar cultures chronically treated with IL-6 (Nelson et al., 2002)..
Like IL-6, CIE/withdrawal altered the level of several proteins involved in the GABAergic inhibitory influences, with some changes occurring in both genotypes. GABAAR alpha-5 and GABAAR alpha-6 were reduced by CIE/withdrawal in cerebellum from both genotypes, whereas the level of GABAAR alpha-1 was not altered by CIE/withdrawal in either genotype. These results suggest that the CIE/withdrawal could shift the primary GABAergic inhibitory mechanism in the cerebellum from tonic to phasic, thereby acting in opposition to the effects of IL-6. Gephyrin was also reduced in the cerebellum from non-tg mice, which could negatively impact both phasic and tonic inhibition due to disruption of receptor clustering, which is regulated by gephyrin. Compared to non-tg mice, cerebellar levels of GABAAR alpha-1 (reduced) and GABAAR alpha-5 (increased) and GABAAR alpha 6 (increased) in the IL-6 tg mice reflect differences similar that induced by chronic alcohol exposure in the CNS of mice (Mhatre et al., 1993;Mhatre and Ticku, 1992;Wu et al., 1995). This similarity support the use of the IL-6 tg mice as a model for the consequences of alcohol-induced elevated levels of IL-6 on the CNS.
Effects of IL-6 on levels of GABAAR alpha-1 and GABAAR alpha-5 containing receptors could have important implications relative to a potential impact of alcohol-induced production of IL-6 in the CNS on the behavioral actions of alcohol. Several lines of evidence from studies of rodents indicate that both GABAAR alpha-1 and GABAAR alpha-5 containing receptors play an important role in the behavioral and abuse-related effects of alcohol (McKay et al., 2004;Pickering et al., 2007;Stephens et al., 2017). For example, studies of a5-receptor subunit knock-out mice show reduced withdrawal hyperexcitability after an acute alcohol challenge and lower alcohol consumption, indicating a role for GABAAR alpha-5 containing receptors in these behaviors (Boehm et al., 2004). Pharmacological studies also indicate a role for GABAAR alpha-5 containing receptors in alcohol drinking (June et al., 2001). GABAAR alpha-1 knockout mice show decreased alcohol consumption and other alcohol associated behaviors (Blednov et al., 2003;Stephens, et al., 2017). Studies on alcohol drinking in the IL-6 tg mice are currently underway in the laboratory. Studies of IL-6 knockout mice have implicated IL-6 in alcohol consumption (Blednov et al., 2012).
The effects of CIE/withdrawal on levels of receptor subunits involved in inhibitory GABAergic synaptic transmission observed in our studies differ from results reported for studies of cerebellum of wildtype rats. In rats, chronic alcohol administration decreased GABAAR alpha-1 mRNA and protein levels in the cerebellum and increased GABAAR alpha-5 and -6 levels (Mehta and Ticku, 1999;Mhatre and Ticku, 1992;Morrow et al., 1992;Petrie et al., 2001), which would enhance tonic inhibition and reduce phasic inhibition. In our studies, GABAAR alpha-5 and -6 levels were decreased in both genotypes, which would reduce tonic inhibition, and there was no effect of CIE/withdrawal on GABAAR alpha-1 in either genotype, indicating that this GABAAR subunit was not a target of alcohol in our experiment. Several experimental differences could explain the discrepancies between these two studies, including different methods of alcohol administration, different endpoints measured (e.g., mRNA vs. protein), higher BALs used in previous studies (e.g., BALs of 223± 21 mg/dl compared to ~ 150 mg/dl in our studies), and the time of animal sacrifice relative to alcohol exposure (e.g., during alcohol exposure vs. after withdrawal).
CIE/withdrawal did not alter GluR1 levels in the cerebellum of IL-6 tg mice, whereas GluR1 levels were reduced in cerebellum from non-tg mice. GluR1 is an important subunit of the AMPA subtype of glutamate receptor and is expressed in cerebellar neurons (e.g., granule neurons and Purkinje neurons) and Bergman glia (Baude, et al., 1994;Yamazaki et al., 2010). GluR1 has been shown to play a role in synaptic functions important for fine motor control (Saab et al., 2012). Thus, the CIE/withdrawal induced reduction of GluR1 in cerebellum of the non-tg mice could involve both neurons and Bergman glia. GFAP is also expressed in Bergman glia, which are thought to be more sensitive to alcohol than GFAP-expressing astrocytes (Rintala et al., 2001). However, CIE/withdrawal did not alter GFAP in the cerebellum of non-tg mice (Table 1), which may indicate that neuronal GluR1 was the target of CIE/withdrawal in the non-tg mice rather than GluR1 in glial cells.
The reduction in GluR1, in the cerebellum of non-tg mice is consistent with studies showing that agonist binding at the AMPA receptor is reduced in the cerebellum of rats subjected to multiple cycles of alcohol exposure/withdrawal (Ulrichsen et al., 1996). A reduction in GluR1 and VGLUT could contribute to the reduced excitatory climbing fiber input to Purkinje neurons observed in rats exposed to chronic alcohol treatment (Rogers et al., 1980).
Glial proteins
In addition to the neuronal adaptations in cerebellum from alcohol naïve IL-6 tg mice, the level of the glial protein Glu Syn, which plays a key role in the production of both the inhibitory transmitter GABA and excitatory transmitter glutamate, was increased. Increased levels of Glu Syn could result in increased levels of synaptic glutamate, which could lead to aberrant excitability and glutamate toxicity.
CIE/withdrawal reduced the level of Glu Syn in the cerebellum from IL-6 tg mice, which along with reduced the levels of VGLUT1 and VGAT could lead to reduced levels of presynaptic excitatory and inhibitory transmitters and smaller synaptic responses. CIE/withdrawal increased Glu Syn levels in the cerebellum from the non-tg mice, an effect that could reflect compensatory mechanism to normalize for the CIE/withdrawal induced reduction in levels of GluR1 and VGLUT in that genotype. However, Glu Syn, in conjunction with high affinity glutamate transporters, which sequester glutamate into astrocytes, controls the concentration of glutamate at the synapse. If the increased levels of Glu Syn represented an over-compensation, it could result in increased levels of synaptic glutamate, which could lead to glutamate toxicity. One limitation of our studies is that it is unknown if/how the changes in Glu Syn levels would translate into changes in Glu Syn activity. Effects of alcohol on Glu Syn activity has been measured in several studies and show brain region dependent changes, typically a reduction, or no effect (Bell et al., 2016;Bondy and Guo, 1995;Das et al., 2016;Hemmingsen and Jorgensen, 1980). Glu Syn activity was reduced in the cerebellum of rats that drank alcohol (10% v/v in water) as the only liquid for 8 weeks, but this effect was not observed with 4 weeks of drinking, perhaps because of the low BALs (BALs at 4 weeks were 36 mg/dl) (Rouach et al., 1997). In cultured astrocytes, alcohol reduced Glu Syn activity without a corresponding decrease in protein levels (Davies and Vernadakis, 1984). A reduction in Glu Syn activity can lead to a reduction in synaptic GABA levels, a disruption of the GABA/glutamate balance and result in seizure activity (Chan et al., 2019). A reduction in Glu Syn activity can also play a role in glutamate and ammonia toxicity (Chao et al., 1992;Rose et al., 2013)
GLAST, the glutamate transporter localized in astrocytes that removes excess glutamate from synapses, was reduced in the cerebellum of the alcohol naïve IL-6 tg mice, which could result in glutamate neurotoxicity at older ages when cellular damage is observed in the CNS of the IL-6 tg mice (Brett, et al., 1995). In addition, alcohol drinking could be affected if other brain regions showed IL-6 induced reductions in GLAST. Studies of GLAST-deficient mutants indicate a role for GLAST in alcohol drinking (Holmes et al., 2013). CIE/withdrawal did not alter GLAST levels in either genotype, indicating GLAST was not a target of CIE/withdrawal under the conditions of our studies. Studies in other brain regions have also noted no effect of alcohol on GLAST levels (Alshehri et al., 2017;Das, et al., 2016;Davies and Vernadakis, 1984;Melendez et al., 2005)
Potential mechanisms
The mechanisms mediating the IL-6-induced changes in the levels of synaptic proteins observed in this study remain to be identified by future studies. However, components of the IL-6 signal transduction pathway (i.e., STAT3, c/EBP beta, p42/44 MAPK) have been shown to regulate expression of proteins examined in this study, raising the possibility that, at least for some proteins, effects of IL-6 and/or CIE/withdrawal on protein expression results from alterations in IL-6 signal transduction. The activated forms of STAT3, c/EBP beta and p42/44 MAPK were all upregulated in the cerebellum of the IL-6 tg mice (Gruol et al., 2020). Chronic exposure of cultured granule neurons to IL-6 elevated expression of c/EBP beta, consistent with the ability of IL-6 to upregulate the IL-6 signaling pathway (Gruol et al., 2011). The JAK/STAT pathway has been shown to contribute to negative regulation of GABAAR alpha-1 levels in the rodent hippocampus (Lund et al., 2008;Raible et al., 2015), and signaling through the JAK/STAT pathway has been shown to down regulate the level of GLAST (Martinez-Lozada et al., 2016;Raymond et al., 2011). Thus, the elevated levels of pSTAT3 in the cerebellum of the IL-6 tg mice could contribute to the reduced levels of GABAAR alpha-1 and GLAST observed in the cerebellum of the alcohol naïve IL-6 tg and non-tg mice in the current study. c/EBP beta has been shown to have binding sites in regions of the GABAAR alpha-6 promoter and to play a role in chronic alcohol induced reduction of GABAAR alpha-6 levels (Saba et al., 2005). CIE/withdrawal reduced c/EBP beta levels in the cerebellum of both IL-6 tg and non-tg mice (Gruol, et al., 2020). Thus, IL-6 induced elevated expression of c/EBP beta could mediate the elevated levels of GABAAR alpha-6 observed in the cerebellum of the alcohol naïve IL-6 tg mice, whereas the reduced c/EBP beta levels in the cerebellum of the CIE/withdrawn IL-6 tg and non-tg mice underlie reduced levels of GABAAR alpha-6.
Conclusion
Our studies show that there are multiple presynaptic and postsynaptic targets of IL-6 and CIE/withdrawal in the cerebellum. Targets of IL-6 are also targets of CIE/withdrawal, thus providing potential sites for interactions under conditions of CIE/withdrawal induced IL-6 production. Such interactions could have important consequences relative to the effects of alcohol exposure/withdrawal on behaviors mediated by the cerebellum such as motor control and those mediated by downstream circuits of other CNS regions that are connected to and influenced by cerebellar synaptic function.
Highlights.
IL-6 and alcohol alter synaptic protein expression in the cerebellum
Expression of the GABAAR α6 subunit is altered by both IL-6 and CIE/withdrawal
Synaptic protein expression is altered by interactions between IL-6 and CIE/withdrawal
Acknowledgements
Funding: This work was supported by National Institutes of Health Grants AA024484 and the Integrated Neuroscience Initiative on Alcoholism (INIA)-West grant AA020893, and The Scripps Research Institute’s Mouse Behavioral Assessment Core.
Abbreviations
- GABAaR alpha-1
alpha-1 subunit of GABAaR
- GABAAR alpha-5
alpha-5 subunit of GABAAR
- GABAAR alpha-6
alpha-6 subunit of GABAAR
- GABAAR
A type GABA receptor
- (BALs)
Blood alcohol levels
- CIE/withdrawal
chronic intermittent alcohol exposure/withdrawal paradigm
- GAD65/67
glutamic acid decarboxylase
- GluR
glutamate receptor
- Glu Syn
glutamine synthetase
- IL-6
interleukin-6
- IL-6 tg
interleukin-6 transgenic
- GLAST
Na+-dependent glutamate-aspartate transporter
- non-tg
non-transgenic littermate
- GluR1
subunit 1 of GluR
- VGAT
vesicular GABA transporter 1
- VGLUT
vesicular glutamate transporter 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest; The authors have no conflict of interest to declare.
Research Involving Animals: All animal procedures were performed in accordance with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
References
- Alfonso-Loeches S, Guerri C, Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain Crit Rev Clin Lab Sci, 48 (2011), 19–47 [DOI] [PubMed] [Google Scholar]
- Alshehri FS, Althobaiti YS, Sari Y, Effects of Administered Ethanol and Methamphetamine on Glial Glutamate Transporters in Rat Striatum and Hippocampus J Mol Neurosci, 61 (2017), 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS, The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer J Neurochem, 98 (2006), 641–653 [DOI] [PubMed] [Google Scholar]
- Baude A, Molnar E Latawiec D, McIlhinney RA, Somogyi P, Synaptic and nonsynaptic localization of the GluR1 subunit of the AMPA-type excitatory amino acid receptor in the rat cerebellum J Neurosci, 14 (1994), 2830–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice Alcohol Clin Exp Res, 28 (2004), 1829–1838 [DOI] [PubMed] [Google Scholar]
- Bell RL, Hauser SR, McClintick J, Rahman S, Edenberg HJ, Szumlinski KK, McBride WJ, Ethanol-Associated Changes in Glutamate Reward Neurocircuitry: A Minireview of Clinical and Preclinical Genetic Findings Prog Mol Biol Transl Sci, 137 (2016), 41–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Botta P, Weber JT, Carta M, De Ruiter M, De Zeeuw CI, Valenzuela CF, Hansel C, Alcohol impairs long-term depression at the cerebellar parallel fiber-Purkinje cell synapse J Neurophysiol, 100 (2008), 3167–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Borghese CM, Ruiz CI, Cullins MA, Da Costa A, Osterndorff-Kahanek EA, Homanics GE, Harris RA, Mutation of the inhibitory ethanol site in GABA(A) p1 receptors promotes tolerance to ethanol-induced motor incoordination Neuropharmacology, 123 (2017), 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA, Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies Addict Biol, 17 (2012), 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA, GABAA receptor alpha 1 and beta 2 subunit null mutant mice: behavioral responses to ethanol J Pharmacol Exp Ther, 305 (2003), 854–863 [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker DL, Iyer SV, Homanics G, Harris AR, Mice lacking Gad2 show altered behavioral effects of ethanol, flurazepam and gabaxadol Addict Biol, 15 (2010), 45–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL 2nd, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, Blednov YA, Harris RA, gamma-Aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions Biochem Pharmacol, 68 (2004), 1581–1602 [DOI] [PubMed] [Google Scholar]
- Bondy SC, Guo SX, Regional selectivity in ethanol-induced pro-oxidant events within the brain Biochem Pharmacol, 49 (1995), 69–72 [DOI] [PubMed] [Google Scholar]
- Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, Valenzuela CF, Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies Alcohol, 41 (2007), 187–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett FM, Mizisin AP, Powell HC, Campbell IL, Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes J Neuropathol Exp Neurol, 54 (1995), 766–775 [DOI] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L, Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6 Proc Natl Acad Sci U S A, 90 (1993), 10061–10065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta I, Chen CH, Schott A, Dorizan S, Khodakhah K, Cerebellar modulation of the reward circuitry and social behavior. Science, (2019), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF, Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability J Neurosci, 24 (2004), 3746–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF, Alcohol potently modulates climbing fiber-->Purkinje neuron synapses: role of metabotropic glutamate receptors J Neurosci, 26 (2006), 1906–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castejon OJ, Dailey ME, Immunohistochemistry of GluR1 subunits of AMPA receptors of rat cerebellar nerve cells Biocell, 33 (2009), 71–80 [PubMed] [Google Scholar]
- Chan F, Lax NZ, Voss CM, Aldana BI, Whyte S, Jenkins A, Nicholson C, Nichols S, et al. , The role of astrocytes in seizure generation: insights from a novel in vitro seizure model based on mitochondrial dysfunction Brain, 142 (2019), 391–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Tsang M, Weatherbee J, Molitor TW, Anderson WR, Peterson PK, Effects of transforming growth factor-beta on murine astrocyte glutamine synthetase activity. Implications in neuronal injury J Clin Invest, 90 (1992), 1786–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CS Stalder A, Samimi A, Campbell IL, Reactive gliosis as a consequence of interleukin-6 expression in the brain: studies in transgenic mice Dev Neurosci, 16 (1994), 212–221 [DOI] [PubMed] [Google Scholar]
- Chiu CS, Brickley S, Jensen K, Southwell A, McKinney S, Cull-Candy S, Mody I, Lester HA, GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum J Neurosci, 25 (2005), 3234–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr., The role of neuroimmune signaling in alcoholism Neuropharmacology, 122 (2017), 56–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar MS, Ethanol-Induced Cerebellar Ataxia: Cellular and Molecular Mechanisms Cerebellum (London, England), 14 (2015), 447–465 [DOI] [PubMed] [Google Scholar]
- Das SC, Althobaiti YS, Alshehri FS, Sari Y, Binge ethanol withdrawal: Effects on postwithdrawal ethanol intake, glutamate-glutamine cycle and monoamine tissue content in P rat model Behav Brain Res, 303 (2016), 120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Vernadakis A, Effects of ethanol on cultured glial cells: proliferation and glutamine synthetase activity Brain Res, 318 (1984), 27–35 [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Buck HM, Bordner K, Richey L, Jones ME, Deak T, Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure Alcohol Clin Exp Res, 38 (2014), 2186–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR, Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms J Neurosci, 14 (1994), 1834–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B, Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin Nat Neurosci, 1 (1998), 563–571 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LE, Crowe SF, Cognitive and emotional deficits in chronic alcoholics: a role for the cerebellum? Cerebellum (London, England), 12 (2013), 520–533 [DOI] [PubMed] [Google Scholar]
- Foltran F, Gregori D, Franchin L, Verduci E, Giovannini M, Effect of alcohol consumption in prenatal life, childhood, and adolescence on child development Nutr Rev, 69 (2011), 642–659 [DOI] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Becker HC, Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice Alcohol Clin Exp Res, 33 (2009), 1893–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Neuroimmune regulation of neurophysiology in the cerebellum Cerebellum (London, England), 12 (2013), 307–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, IL-6 regulation of synaptic function in the CNS Neuropharmacology, 96 (2015), 42–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Huitron-Resendiz S, Roberts AJ, Altered brain activity during withdrawal from chronic alcohol is associated with changes in IL-6 signal transduction and GABAergic mechanisms in transgenic mice with increased astrocyte expression of IL-6 Neuropharmacology, 138 (2018), 32–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Melkonian C, Huitron-Resendiz S, Roberts AJ, Alcohol alters IL-6 Signal Transduction in the CNS of Transgenic Mice with Increased Astrocyte Expression of IL-6 Cell Mol Neurobiol, (2020), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Melkonian C, Huitron-Resendiz S, Roberts AJ, Alcohol alters IL-6 Signal Transduction in the CNS of Transgenic Mice with Increased Astrocyte Expression of IL-6 Cellular and Molecular Neurobiology,, in press (2020), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Puro A, Hao C, Blakely P, Janneke E, Vo K, Neuroadaptive changes in cerebellar neurons induced by chronic exposure to IL-6 J Neuroimmunol, 239 (2011), 28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Vo K, Bray JG, Increased astrocyte expression of IL-6 or CCL2 in transgenic mice alters levels of hippocampal and cerebellar proteins Front Cell Neurosci, 8 (2014), 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyengesi E, Rangel A, Ullah F, Liang H, Niedermayer G, Asgarov R, Venigalla M, Gunawardena D, et al. , Chronic Microglial Activation in the GFAP-IL6 Mouse Contributes to Age-Dependent Cerebellar Volume Loss and Impairment in Motor Function Front Neurosci, 13 (2019), 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D, Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex Neuron, 33 (2002), 625–633 [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M, Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity Nat Neurosci, 8 (2005), 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Titley H, Grasselli G, Piochon C, Hansel C, Ethanol affects NMDA receptor signaling at climbing fiber-Purkinje cell synapses in mice and impairs cerebellar LTD J Neurophysiol, 109 (2013), 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen R, Jørgensen OS, Specific brain proteins during severe ethanol intoxication and withdrawal in the rat Psychiatry Res, 3 (1980), 1–11 [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH, Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain Proc Natl Acad Sci U S A, 94 (1997), 1500–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR, Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment Pediatrics, 118 (2006), e755–763 [DOI] [PubMed] [Google Scholar]
- Hioki H, Fujiyama F, Taki K, Tomioka R, Furuta T, Tamamaki N, Kaneko T, Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex Neuroscience, 117 (2003), 1–6 [DOI] [PubMed] [Google Scholar]
- Hirono M, Yamada M, Obata K, Ethanol enhances both action potential-dependent and action potential-independent GABAergic transmission onto cerebellar Purkinje cells Neuropharmacology, 57 (2009), 109–120 [DOI] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH, Glutamatergic targets for new alcohol medications Psychopharmacology (Berl), 229 (2013), 539–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortnagl H, Tasan RO, Wieselthaler A, Kirchmair E, Sieghart W, Sperk G, Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain Neuroscience, 236 (2013), 345–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Norenberg MD, Glutamine Synthetase: Role in Neurological Disorders Advances in neurobiology, 13 (2016), 327–350 [DOI] [PubMed] [Google Scholar]
- June HL, Harvey SC, Foster KL, McKay PF, Cummings R, Garcia M, Mason D, Grey C, et al. , GABA(A) receptors containing (alpha)5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: an extended ethanol reward circuitry J Neurosci, 21 (2001), 2166–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Alcohol Withdrawal and Cerebellar Mitochondria Cerebellum (London, England), 14 (2015), 421–437 [DOI] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Douglas JC, Wagoner G, Johnson JW, Xu J, Phelan PS, Drew PD, Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice Alcohol Clin Exp Res, 38 (2014), 384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JS, Nipper MA, Richardson BD, Jensen J, Helms M, Finn DA, Rossi DJ, Pharmacologically Counteracting a Phenotypic Difference in Cerebellar GABAA Receptor Response to Alcohol Prevents Excessive Alcohol Consumption in a High Alcohol-Consuming Rodent Genotype J Neurosci, 36 (2016), 9019–9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Novel GABAA receptor alpha subunit is expressed only in cerebellar granule cells J Mol Biol, 214 (1990), 619–624 [DOI] [PubMed] [Google Scholar]
- Kayakabe M, Kakizaki T, Kaneko R, Sasaki A, Nakazato Y, Shibasaki K, Ishizaki Y, Saito H, et al. , Motor dysfunction in cerebellar Purkinje cell-specific vesicular GABA transporter knockout mice Front Cell Neurosci, 7 (2013), 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir WJ Morrow AL, Differential expression of GABAA receptor subunit mRNAs in ethanol-naive withdrawal seizure resistant (WSR) vs. withdrawal seizure prone (WSP) mouse brain Brain Res Mol Brain Res, 25 (1994), 200–208 [DOI] [PubMed] [Google Scholar]
- Kros L, Lindeman S, Eelkman Rooda OHJ, Murugesan P, Bina L, Bosman LWJ, De Zeeuw CI, Hoebeek FE, Synchronicity and Rhythmicity of Purkinje Cell Firing during Generalized Spike-and-Wave Discharges in a Natural Mouse Model of Absence Epilepsy Front Cell Neurosci, 11 (2017), 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, LaVoie FIA, DiPette DJ, Singh US, Ethanol neurotoxicity in the developing cerebellum: underlying mechanisms and implications Brain sciences, 3 (2013), 941–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange I, Kasanova Z, Goossens L, Leibold N, De Zeeuw Cl, van Amelsvoort T, Schruers K, The anatomy of fear learning in the cerebellum: A systematic metaanalysis Neurosci Biobehav Rev, 59 (2015), 83–91 [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W, The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum J Neurosci, 12 (1992), 1063–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Bing YH, Chu CP, Cui X, Cui SB, Qiu DL, Su LD, Chronic Ethanol Consumption Impairs the Tactile-Evoked Long-Term Depression at Cerebellar Molecular Layer Interneuron-Purkinje Cell Synapses in vivo in Mice Front Cell Neurosci, 12 (2018), 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G, Alcohol-induced IL-1 β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation J Leukoc Biol, 94 (2013), 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC, Operant ethanol self-administration in ethanol dependent mice Alcohol, 48 (2014), 295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR, BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway Science signaling, 1 (2008), ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Effects of Ethanol on the Cerebellum: Advances and Prospects Cerebellum (London, England), 14 (2015), 383–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli L, Rossi P, Nieus T, D’Angelo E, Tonic activation of GABAB receptors reduces release probability at inhibitory connections in the cerebellar glomerulus J Neurophysiol, 101 (2009), 3089–3099 [DOI] [PubMed] [Google Scholar]
- Marcián V, Filip P, Bareš M, Brázdil M , Cerebellar Dysfunction and Ataxia in Patients with Epilepsy: Coincidence, Consequence, or Cause? Tremor Other Hyperkinet Mov (N Y), 6 (2016), 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Levey AI, Huganir RL, Price DL, AMPA glutamate receptor subunits are differentially distributed in rat brain Neuroscience, 53 (1993), 327–358 [DOI] [PubMed] [Google Scholar]
- Martinez-Lozada Z, Guillem AM, Robinson MB, Transcriptional Regulation of Glutamate Transporters: From Extracellular Signals to Transcription Factors Adv Pharmacol, 76 (2016), 103–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay PF, Foster KL, Mason D, Cummings R, Garcia M, Williams LS, Grey C, McCane S, et al. , A high affinity ligand for GABAA-receptor containing alpha5 subunit antagonizes ethanol’s neurobehavioral effects in Long-Evans rats Psychopharmacology (Berl), 172 (2004), 455–462 [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK, Prevalence of the GABAA receptor assemblies containing alpha1-subunit in the rat cerebellum and cerebral cortex as determined by immunoprecipitation: lack of modulation by chronic ethanol administration Brain Res Mol Brain Res, 67 (1999), 194–199 [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW, Ethanol exposure decreases glutamate uptake in the nucleus accumbens Alcohol Clin Exp Res, 29 (2005), 326–333 [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Pena G, Sieghart W, Ticku MK, Antibodies specific for GABAA receptor alpha subunits reveal that chronic alcohol treatment down-regulates alpha-subunit expression in rat brain regions J Neurochem, 61 (1993), 1620–1625 [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK, Chronic ethanol administration alters gamma-aminobutyric acidA receptor gene expression Mol Pharmacol, 42 (1992), 415–422 [PubMed] [Google Scholar]
- Moreno-Rius J, The cerebellum in fear and anxiety-related disorders Prog Neuropsychopharmacol Biol Psychiatry, 85 (2018), 23–32 [DOI] [PubMed] [Google Scholar]
- Morrow AL, Herbert JS, Montpied P, Differential effects of chronic ethanol administration on GABA(A) receptor alpha1 and alpha6 subunit mRNA levels in rat cerebellum Mol Cell Neurosci, 3 (1992), 251–258 [DOI] [PubMed] [Google Scholar]
- Nelson TE, Campbell IL, Gruol DL, Altered physiology of Purkinje neurons in cerebellar slices from transgenic mice with chronic central nervous system expression of interleukin-6 Neuroscience, 89 (1999), 127–136 [DOI] [PubMed] [Google Scholar]
- Nelson TE, Olde Engberink A, Hernandez R, Puro A, Huitron-Resendiz S, Hao C, De Graan PN, Gruol DL, Altered synaptic transmission in the hippocampus of transgenic mice with enhanced central nervous systems expression of interleukin-6 Brain Behav Immun, 26 (2012), 959–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, Gruol DL, Chronic interleukin-6 exposure alters electrophysiological properties and calcium signaling in developing cerebellar purkinje neurons in culture J Neurophysiol, 88 (2002), 475–486 [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K, Alcohol: effects on neurobehavioral functions and the brain Neuropsychol Rev, 17 (2007), 239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YP, Qiu YH, Lu JH, Wang JJ, Interleukin-6 protects cultured cerebellar granule neurons against glutamate-induced neurotoxicity Neurosci Lett, 374 (2005), 192–196 [DOI] [PubMed] [Google Scholar]
- Petrie J, Sapp DW, Tyndale RF, Park MK, Fanselow M, Olsen RW, Altered gabaa receptor subunit and splice variant expression in rats treated with chronic intermittent ethanol Alcohol Clin Exp Res, 25 (2001), 819–828 [PubMed] [Google Scholar]
- Phillips JR, Hewedi DH, Eissa AM, Moustafa AA, The cerebellum and psychiatric disorders Frontiers in public health, 3 (2015), 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering C, Avesson L, Lindblom J Liljequist S, Schioth HB, Identification of neurotransmitter receptor genes involved in alcohol self-administration in the rat prefrontal cortex, hippocampus and amygdala Prog Neuropsychopharmacol Biol Psychiatry, 31 (2007), 53–64 [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G, GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain Neuroscience, 101 (2000), 815–850 [DOI] [PubMed] [Google Scholar]
- Poltl A, Hauer B, Fuchs K, Tretter V, Sieghart W, Subunit composition and quantitative importance of GABA(A) receptor subtypes in the cerebellum of mouse and rat J Neurochem, 87 (2003), 1444–1455 [DOI] [PubMed] [Google Scholar]
- Qiu Z, Parsons KL, Gruol DL, Interleukin-6 selectively enhances the intracellular calcium response to NMDA in developing CNS neurons J Neurosci, 15 (1995), 6688–6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DJ, Frey LC, Del Angel YC, Carlsen J, Hund D, Russek SJ, Smith B, Brooks-Kayal AR, JAK/STAT pathway regulation of GABAA receptor expression after differing severities of experimental TBI Exp Neurol, 271 (2015), 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M, Li P, Mangin JM, Huntsman M, Gallo V, Chronic perinatal hypoxia reduces glutamate-aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway J Neurosci, 31 (2011), 17864–17871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Wen Y, Wilson A, Simpkins JW, Jung ME, Role of parvalbumin in estrogen protection from ethanol withdrawal syndrome Alcohol Clin Exp Res, 29 (2005), 1837–1844 [DOI] [PubMed] [Google Scholar]
- Riikonen J, Jaatinen P, Rintala J, Porsti I, Karjala K, Hervonen A, Intermittent ethanol exposure increases the number of cerebellar microglia Alcohol Alcohol, 37 (2002), 421–426 [DOI] [PubMed] [Google Scholar]
- Rintala J, Jaatinen P, Kiianmaa K, Riikonen J, Kemppainen O, Sarviharju M, Hervonen A, Dose-dependent decrease in glial fibrillary acidic protein-immunoreactivity in rat cerebellum after lifelong ethanol consumption Alcohol, 23 (2001), 1–8 [DOI] [PubMed] [Google Scholar]
- Robinson G, Most D, Ferguson LB, Mayfield J, Harris RA, Blednov YA, Neuroimmune pathways in alcohol consumption: evidence from behavioral and genetic studies in rodents and humans Int Rev Neurobiol, 118 (2014), 13–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Siggins GR, Schulman JA, Bloom FE, Physiological correlates of ethanol intoxication tolerance, and dependence in rat cerebellar Purkinje cells Brain Res, 196 (1980), 183–198 [DOI] [PubMed] [Google Scholar]
- Rose CF, Verkhratsky A, Parpura V, Astrocyte glutamine synthetase: pivotal in health and disease Biochem Soc Trans, 41 (2013), 1518–1524 [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Richardson BD, The Cerebellar GABAAR System as a Potential Target for Treating Alcohol Use Disorder Handb Exp Pharmacol, (2018), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach H, Houze P, Gentil M, Orfanelli MT, Nordmann R, Changes in some pro- and antioxidants in rat cerebellum after chronic alcohol intake Biochem Pharmacol, 53 (1997), 539–545 [DOI] [PubMed] [Google Scholar]
- Saab AS, Neumeyer A, Jahn HM, Cupido A, Simek AA, Boele HJ, Scheller A, Le Meur K, et al. , Bergmann glial AMPA receptors are required for fine motor coordination Science, 337 (2012), 749–753 [DOI] [PubMed] [Google Scholar]
- Saba L, Porcella A, Sanna A, Congeddu E, Marziliano N, Mongeau R, Grayson D, Pani L, Five mutations in the GABA A alpha6 gene 5’ flanking region are associated with a reduced basal and ethanol-induced alpha6 upregulation in mutated Sardinian alcohol non-preferring rats Brain Res Mol Brain Res, 137 (2005), 252257. [DOI] [PubMed] [Google Scholar]
- Saeed Dar M, Co-modulation of acute ethanol-induced motor impairment by mouse cerebellar adenosinergic A1 and GABA(A) receptor systems Brain Res Bull, 71 (2006), 287–295 [DOI] [PubMed] [Google Scholar]
- Samland H, Huitron-Resendiz S, Masliah E, Criado J, Henriksen SJ, Campbell IL, Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6 J Neurosci Res, 73 (2003), 176–187 [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS, Contributions of the GABAA receptor alpha6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the alpha6 gene J Neurosci, 26 (2006), 3357–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM, Colocalization of multiple GABA(A) receptor subtypes with gephyrin at postsynaptic sites J Comp Neurol, 420 (2000), 481–498 [DOI] [PubMed] [Google Scholar]
- Simonyi A, Zhang JP, Sun AY, Sun GY, Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons Neuroreport, 7 (1996), 2115–2118 [DOI] [PubMed] [Google Scholar]
- Stephens DN, King SL, Lambert JJ, Belelli D, Duka T, GABA(A) receptor subtype involvement in addictive behaviour Genes, brain, and behavior, 16 (2017), 149–184 [DOI] [PubMed] [Google Scholar]
- Stowell RD, Majewska AK, Acute ethanol exposure rapidly alters cerebellar and cortical microglial physiology Eur J Neurosci, (2020), [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LD, Sun CL, Shen Y, Ethanol acutely modulates mGluR1-dependent long-term depression in cerebellum Alcohol Clin Exp Res, 34 (2010), 1140–1145 [DOI] [PubMed] [Google Scholar]
- Thompson CL, Bodewitz G, Stephenson FA, Turner JD, Mapping of GABAA receptor alpha 5 and alpha 6 subunit-like immunoreactivity in rat brain Neurosci Lett, 144 (1992), 53–56 [DOI] [PubMed] [Google Scholar]
- Timmann D, Drepper J, Fringe M, Maschke M, Richter S, Gerwig M, Kolb FP, The human cerebellum contributes to motor, emotional and cognitive associative learning. A review Cortex, 46 (2010), 845–857 [DOI] [PubMed] [Google Scholar]
- Tyagarajan SK, Fritschy JM, Gephyrin: a master regulator of neuronal function? Nat Rev Neurosci, 15 (2014), 141–156 [DOI] [PubMed] [Google Scholar]
- Ulrichsen J, Bech B, Ebert B, Diemer NH, Allerup P, Hemmingsen R, Glutamate and benzodiazepine receptor autoradiography in rat brain after repetition of alcohol dependence Psychopharmacology (Berl), 126 (1996), 31–41 [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Jotty K, Mini-Review: Effects of Ethanol on GABAA Receptor-Mediated Neurotransmission in the Cerebellar Cortex--Recent Advances Cerebellum (London, England), 14 (2015), 438–446 [DOI] [PubMed] [Google Scholar]
- Wadleigh A, Valenzuela CF, Ethanol increases GABAergic transmission and excitability in cerebellar molecular layer interneurons from GAD67-GFP knock-in mice Alcohol Alcohol, 47 (2012), 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, et al. , Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice Eur J Neurosci, 10 (1998), 976–988 [DOI] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G, Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1 J Neurosci, 25 (2005), 6221–6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C, An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size Proc Natl Acad Sci U S A, 101 (2004), 7158–7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worst TJ, Vrana KE, Alcohol and gene expression in the central nervous system Alcohol Alcohol, 40 (2005), 63–75 [DOI] [PubMed] [Google Scholar]
- Wu CH, Frostholm A, De Blas AL, Rotter A, Differential expression of GABAA/benzodiazepine receptor subunit mRNAs and ligand binding sites in mouse cerebellar neurons following in vivo ethanol administration: an autoradiographic analysis J Neurochem, 65 (1995), 1229–1239 [DOI] [PubMed] [Google Scholar]
- Wu G, Liu H, Jin J, Hong L, Lan Y, Chu CP, Qiu DL, Ethanol attenuates sensory stimulus-evoked responses in cerebellar granule cells via activation of GABA(A) receptors in vivo in mice Neurosci Lett, 561 (2014), 107–111 [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Fukaya M, Hashimoto K, Yamasaki M, Tsujita M, Itakura M, Abe M, Natsume R, et al. , TARPs gamma-2 and gamma-7 are essential for AMPA receptor expression in the cerebellum Eur J Neurosci, 31 (2010), 2204–2220 [DOI] [PubMed] [Google Scholar]
- Yu W, Krook-Magnuson E, Cognitive Collaborations: Bidirectional Functional Connectivity Between the Cerebellum and the Hippocampus Front Syst Neurosci, 9 (2015), 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio-Bulcock PA, Homanics GE, Woodward JJ, Loss of Ethanol Inhibition of N-Methyl-D-Aspartate Receptor-Mediated Currents and Plasticity of Cerebellar Synapses in Mice Expressing the GluN1(F639A) Subunit Alcohol Clin Exp Res, 42 (2018), 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wei G, Wang Y, Jing L, Zhao Q, Gene expression profile analysis of rat cerebellum under acute alcohol intoxication Gene, 557 (2015), 188–194 [DOI] [PubMed] [Google Scholar]


