Summary
Mosquitoes pose widespread threats to humans and other animals as disease vectors [1]. Day- vs. night-biting mosquitoes occupy distinct time-of-day niches [2, 3]. Here, we explore day- vs. night-biting female and male mosquitoes’ innate temporal attraction/avoidance behavioral responses to light and their regulation by circadian circuit and molecular mechanisms. Day-biting mosquitoes Aedes aegypti, particularly females, are attracted to light during the day regardless of spectra. In contrast, night-biting mosquitoes Anopheles coluzzii, specifically avoid ultraviolet (UV) and blue light during the day. Behavioral attraction/avoidance to light in both species change with time-of-day and show distinct sex and circadian neural circuit differences. Males of both diurnal and nocturnal mosquito species show reduced UV light avoidance in anticipation of evening onset relative to females. The circadian neural circuits of diurnal/day- and nocturnal/night-biting mosquitoes based on PERIOD (PER) and Pigment-Dispersing Factor (PDF) expression show similar but distinct circuit organizations between species. The basis of diurnal versus nocturnal behaviors is driven by molecular clock timing, which cycle in anti-phase between day- versus night-biting mosquitoes. Observed differences at the neural circuit and protein levels provide insight into the fundamental basis underlying diurnality versus nocturnality. Molecular disruption of the circadian clock severely interferes with light-evoked attraction/avoidance behaviors in mosquitoes. In summary, attraction/avoidance mosquito behaviors show marked differences between day- vs. night-biting mosquitoes, but both classes of mosquitoes are circadian and light regulated, which may be applied towards species-specific control of harmful mosquitoes.
Keywords: mosquito, circadian, light choice, light avoidance, phototaxis, Aedes, Anopheles, vector control
Graphical Abstract
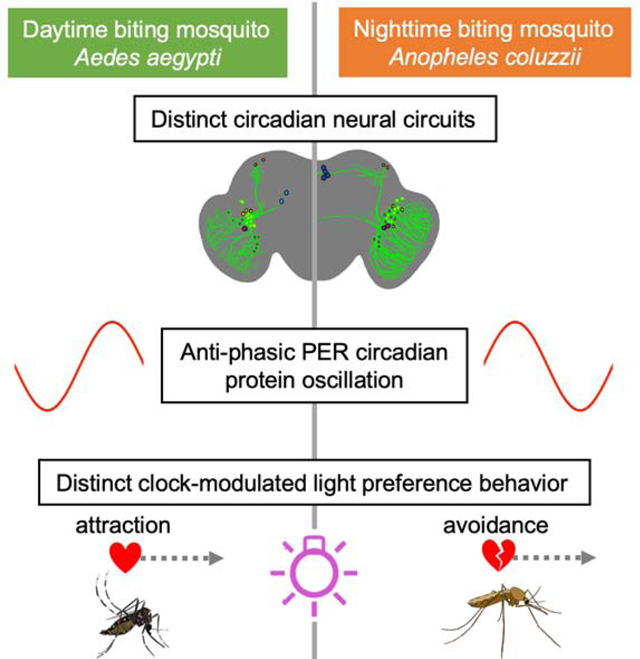
In Brief
Baik et al. show that diurnal vs. nocturnal mosquitoes have distinct attraction and avoidance to light that is controlled by the circadian clock. They find distinct features of circadian neural circuit and phasic oscillation of circadian protein in daytime-active vs. nighttime-active mosquitoes that may underlie diurnality versus nocturnality.
Results
Mosquito-spread diseases may have contributed to the deaths of half of all the people who have ever lived [1]. Toxic pesticides are environmentally harmful in contrast to relatively safe light-based insect control approaches. However, light-based insect controls do not typically take into consideration the day vs. night behavioral profiles that changes with daily light: dark cycles. Insects display a wide range of short wavelength light modulated behaviors, including attraction/avoidance [4–11]. It has been long assumed that insect responses to ultraviolet (UV) light are mediated by external photoreceptors including opsins in the eyes. Mosquitoes and flies additionally express non-opsin photoreceptors including the blue and UV light sensitive CRYPTOCHROME (CRY) [12]. Recent work in flies shows that CRY mediates a wide range of behavioral responses to blue and UV light, including circadian modulated attraction/avoidance [4–6].
Different mosquito species have evolved distinct circadian timing of behaviors according to their temporal/ecological niches. Some mosquito species are diurnal (i.e., Aedes aegypti) while others are nocturnal (i.e., Anopheles coluzzii). Numerous mosquito behaviors change with time-of-day, including flight activity, mating, oviposition, and biting [2, 3, 13–17]. Circadian clocks are light entrained and altered light timing disrupts circadian behaviors [2, 3, 10, 11, 13]. Despite their large impact on health and ecology, little is known about the basis of diurnality/nocturnality and behavioral timing in mosquitoes. We chose to investigate diurnal Aedes aegypti (Ae. aegypti) and nocturnal Anopheles coluzzii (An. coluzzii) mosquitoes based on comparative circadian interest and because both are anthropophilic mosquitoes that are major vectors of many human diseases.
Light-evoked attraction/avoidance behaviors in diurnal and nocturnal mosquitoes are species-, sex-, and spectra-dependent, and changes with time-of-day.
To measure behavioral attraction/avoidance to light in mosquitoes, we developed a custom designed arena (Figure 1A). 12 hr: 12 hr light: dark (LD) circadian entrained young adult diurnal (Ae. aegypti) and nocturnal (An. coluzzii) mosquitoes were presented with a choice of light-exposed versus shaded environments during the subjective daytime (Zeitgeber Time, ZT 0–12). Light intensity was set to 400 μW/cm2, which is a relatively high-intensity light that is within a natural physiological and environmental range. Light was continuously kept on during the subjective daytime (12 hrs; ZT 0–12) then turned off during the subjective nighttime (12 hrs; ZT 12–24), maintaining the prior LD entrainment and thus the circadian clock unperturbed.
Figure 1. UV light-evoked attraction/avoidance responses in diurnal and nocturnal mosquitoes are specie- and sex-dependent.
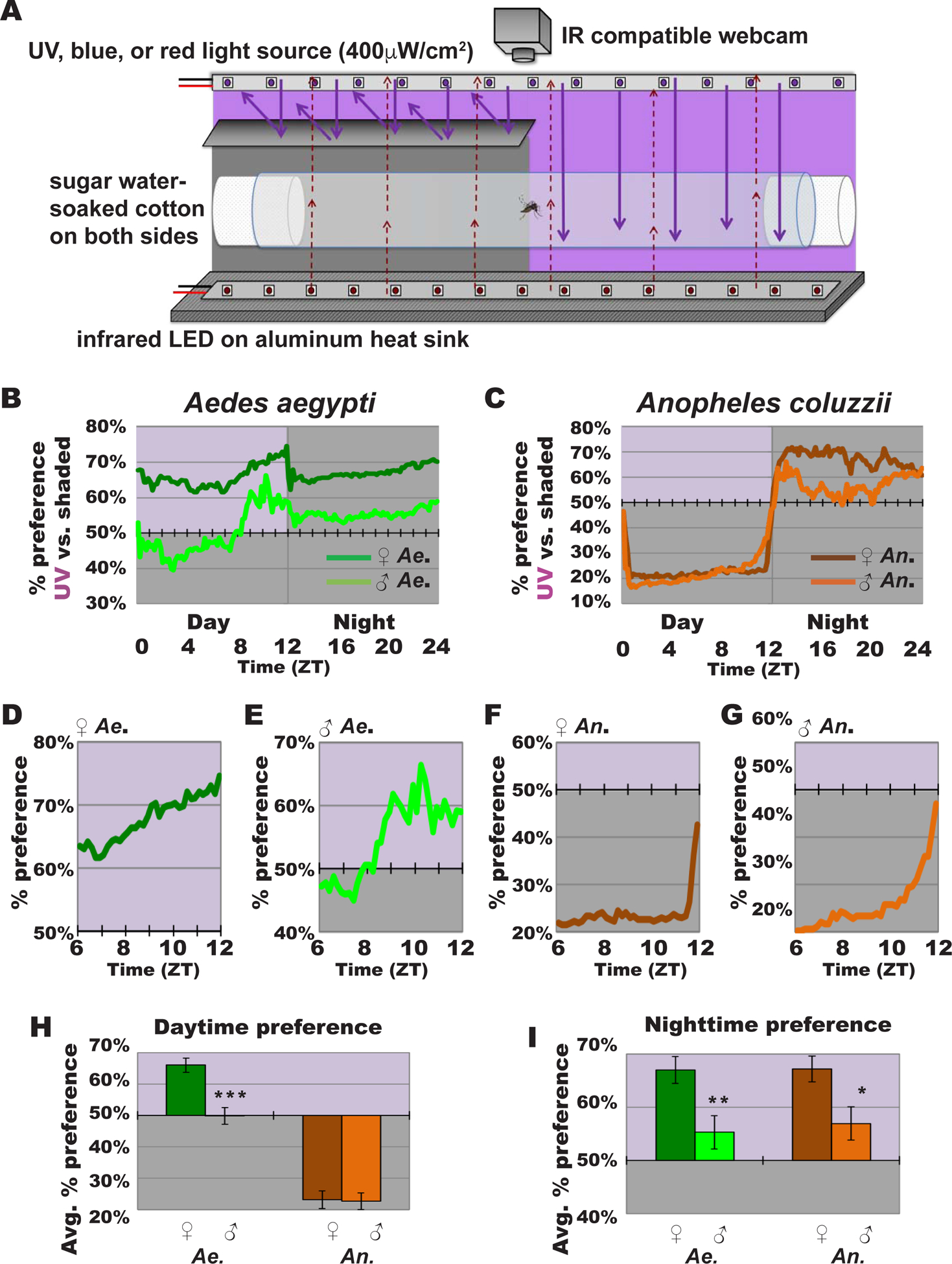
(A) Schematic of mosquito light-evoked attraction/avoidance preference behavioral assay setup.
(B-C) Attraction/avoidance behavior to UV light, measured by % of preference in UV-exposed versus shaded environment throughout 12 hr: 12 hr UV light: dark for (B) female Ae. aegypti (dark green; n=110) and male Ae. aegypti (light green; n=61), and (C) female An. coluzzii (brown; n=64) and male An. coluzzii (orange; n=47).
(D-G) Attraction/avoidance behavior to UV light, measured by % of preference in UV-exposed versus shaded-environment during Zeitgeber time (ZT) 6–12 for (D) female Ae. aegypti, (E) male Ae. aegypti, (F) female An. coluzzii, and (G) male An. coluzzii.
(H-I) Average attraction/avoidance behavioral preference to UV light-exposed versus shaded-environment by Ae. aegypti and An. coluzzii female and male mosquitoes for (H) daytime, light violet background indicates illuminated area; and (I) nighttime, light violet background indicates previously illuminated area. Data are represented as mean ± S.E.M. *p < 0.05; **p < 0.01; ***p < 0.001 vs. female. See also Figure S1.
Diurnal versus nocturnal mosquito species exhibit striking differences in their light-evoked attraction/avoidance behavior. Diurnal Ae. aegypti females are behaviorally attracted to UV light during the day (Figure 1B and 1H) and remain in the same general spatial area at night that they occupied previously during the day (Figure 1B, 1H, and 1I). In contrast, nocturnal An. coluzzii females strongly avoid UV light during most of the daytime and occupy the previously illuminated spatial area at night that they avoided during the day (Figure 1C, 1H, and 1I). Both species females shift behavioral attraction/avoidance as dusk approaches in anticipation of the simulated night (Figure 1B, 1C, 1D, and 1F). We observe this change in behavior despite the that the light stimulus remains constant during the daytime, indicating that this is likely a circadian-modulated behavior in anticipation of approaching nighttime. The “anticipatory” afternoon behavioral shift begins around mid-afternoon (~ZT7) for diurnal Ae. aegypti females (Figure 1B and 1D). In contrast, nocturnal An. coluzzii, females show sharp decreases in UV light avoidance starting about an hour before dusk (~ZT11) (Figure 1C and 1F).
Mosquitoes are highly sexually dimorphic; male mosquitoes’ primary needs are to feed on nectar and to mate, while females additionally need to seek blood-meal hosts and oviposition sites. Male mosquitoes swarm earlier in anticipation of females [14–16]. The timing of male swarm roughly coincides with or precedes the timing of female anticipatory behavioral shift we observed in UV light attraction/avoidance (Figure 1D and 1F) [14]. Thus, we considered the possibility of sex differences for light environmental preference. Diurnal Ae. aegypti males are attracted to UV light during the late subjective daytime (Figure 1B and 1E), but to a significantly lesser extent than females, which are attracted to UV light throughout the entire day. Nocturnal An. coluzzii males strongly avoid UV light, similar to An. coluzzii females (Figure 1C and 1H). Both species show sex-specific differences in timing of “anticipatory” behavioral shifts in attraction/avoidance to light approaching dusk. Ae. aegypti light attraction peaks earlier in males (~ZT10) than in females (~ZT 12) (Figure 1B, 1D, and 1E). Similarly, as dusk approaches An. coluzzii males show earlier behavioral shift in avoidance/attraction than females in anticipation of dusk (Figure 1C, 1F, and 1G). Sex-dependent differences persist even after the UV light is turned off, which simulates the subjective nighttime (ZT 12–24). Nighttime preference for previously UV light exposed environment is significantly higher in females, compared to males in both species (Figure 1I).
The color spectral preference of attraction/avoidance behavior varies between different insect species [4, 5, 8, 9, 18]. A diptera Drosophila melanogaster avoids short wavelength light during midday, but not long wavelength light. [4–6]. We examined spectral dependence of mosquito attraction/avoidance to light, using intensity-matched visible short wavelength blue light and visible long wavelength red light for comparison with UV light behavior at same light intensity (400 μW/cm2). Diurnal Ae. aegypti females are attracted to both blue and red lights during the day, comparable to their UV light attraction (Figure S1A, S1C, and S1E). In contrast, nocturnal An. coluzzii females, which strongly avoid UV light (Figure 1), avoid blue light during the day but to a significantly lesser extent than UV light (Figure S1B and S1E). Significantly different from their avoidance to blue and UV light, female An. coluzzii are attracted to red light (Figure S1D and S1E). During the nighttime, females of both species prefer environments with prior UV light exposure, significantly higher than their weaker nighttime preference for areas with prior blue or red light exposure (Figure S1F). We conclude that behavioral attraction/avoidance to light are wavelength-dependent and differ in both overall valence and anticipation of dusk between nocturnal and diurnal mosquito species.
Diurnal versus nocturnal mosquitoes have similar yet distinct circadian neuronal circuits.
We anatomically mapped the circadian neuronal network in the central brain of female diurnal and nocturnal mosquitoes. Insect circadian neuronal circuits are defined by the cyclic expression of highly conserved clock protein PERIOD (PER) that drives rhythmic changes in physiology and behavior. Pigment-Dispersing Factor (PDF) is a highly conserved neuropeptide co-expressed with PER in the small- and large-lateral ventral neurons (LNvs) which modulate circadian- and light-mediated behaviors such as locomotion, sleep, arousal, and light attraction/avoidance in Drosophila melanogaster [4, 5, 19–21]. Antibodies against Drosophila PER labels circadian neurons in a broad range of other insects [22–24]. The use of Drosophila PER or PDF antibodies has been validated in insects that are much more distantly related to Drosophila than mosquitoes are (i.e., in lepidopteran Antheraea pernyi silk moths and in hymenopteran Apis mellifera honeybees) while Drosophila melanogaster and mosquitoes are more closely related dipterans [23, 24]. Thus we reasoned that Drosophila PER and PDF antibodies can effectively label PER and PDF proteins in mosquitoes.
We find PER and PDF are co-expressed in the lateral ventral area in both Ae. aegypti and An. coluzzii female adult brains (Figure 2 and S2, and Table S1). These PDF+ and PER+ neurons can be further distinguished as large- (l-LNvs) and small-lateral ventral neurons (s-LNvs) (Figure 2 and S2). Other neuronal groups include putative dorsal neurons (DNs) (Figure 2 and S2). There are large neuronal arbors in the optic lobes and dorsal projections to the DNs from the PDF+ LNvs [21] (Figure 2, S2, S3E–H and S4E–H, Movie S1, and Movie S2). In An. coluzzii, the PDF+ LNv dorsal projections continue medially to the pars intercerebralis (PI) region; this projection pattern is not observed in Ae. aegypti (Figure 2, S3A–D and S4A–D). Another species-specific feature is that the contralateral projection of PDF+ LNvs crosses the midline in the early daytime in An. coluzzii, but not in Ae. aegypti (Figure 2, S3A–D and S4A–D). Approximately ~5 PER+/PDF− neurons in the medial-anterior region of Ae. aegypti female brains, which we call medial-anterior neurons (m-ANs) here, are not detected in An. coluzzii (Figure 2 and S2). Another species-specific neuronal group includes approximately ~7 PER+/PDF− neurons in the PI region in An. coluzzii, which are not detected in Ae. aegypti (Figure 2 and S2, and Table S1). Thus, there are both similar and species-distinct features of the circadian neuronal circuits of diurnal Ae. aegypti and nocturnal An. coluzzii.
Figure 2. Schematic representation of Aedes aegypti and Anopheles coluzzii circadian neuronal circuits.
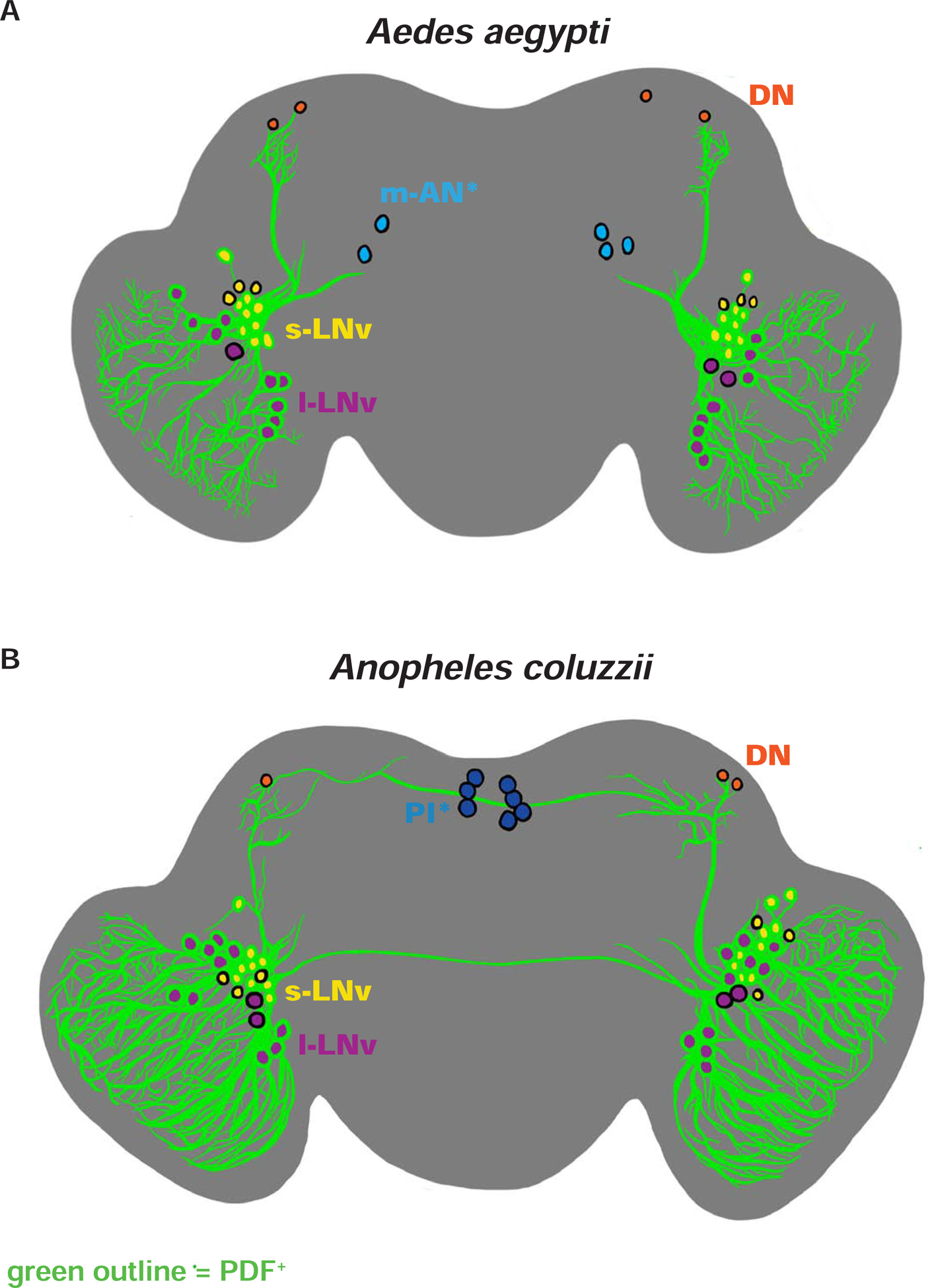
(A-B) Illustrations of representative adult female central brains and their neuronal expression of PER and/or PDF with projections depicted in black. Asterisk (*) indicates groups distinct for each species. PDF+ neurons are indicated with green outline. (A) Ae. aegypti s-LNv in yellow, l-LNv in violet, DNs in orange, and m-AN in light blue. (B) An. coluzzii s-LNv in yellow, l-LNv in violet, DNs in orange, and PI neurons in dark blue. See also Figure S2, Figure S3, Figure S4, Table S1, Video S1, and Video S2.
Molecular clock of diurnal versus nocturnal mosquitoes in PDF+ LNv circadian neurons oscillate in an anti-phasic manner.
To examine the timing of molecular clock underlying diurnal and nocturnal mosquitoes, we measured PER and PDF protein oscillation over 24 hrs at 6 hr intervals in the brains of Ae. aegypti and An. coluzzii female mosquitoes. PER protein cycles robustly in both Ae. aegypti and An. coluzzii circadian neurons. PER+/PDF+ LNv axonal projections undergo structural remodeling with time-of-day for both An. aegypti and An. coluzzii (Figure S3A–D and S4A–D). Notably, PER oscillates in opposite phases between diurnal Ae. aegypti versus nocturnal An. coluzzii PDF+ LNv circadian neurons (Figure 3). PER protein levels peak in late night/early day in PDF+ s-LNv and l-LNv of the diurnal mosquito Ae. aegypti (Figure 3A, 3C, and 3D). In contrast, PER protein levels peak in late day/early night in PDF+ s-LNv and l-LNv of the nocturnal mosquito An. coluzzii (Figure 3B, 3E, and 3F). In addition to PER oscillation, PDF protein levels in s-LNv and l-LNv of nocturnal An. coluzzii oscillate with its peak expression in early night (Figure S4I and S4J). In contrast, PDF protein levels peak in early day in s-LNv and l-LNv of diurnal Ae. aegypti, but its oscillation is less clear (Figure S3I and S3J). Diurnal and nocturnal mosquitoes have distinct circadian clock protein phases and circadian neuronal architecture in the brain that suggest a possible mechanism for diurnality and nocturnality.
Figure 3. Diurnal versus nocturnal mosquito PER expression in PDF+ LNv neurons oscillate in anti-phasic manner.
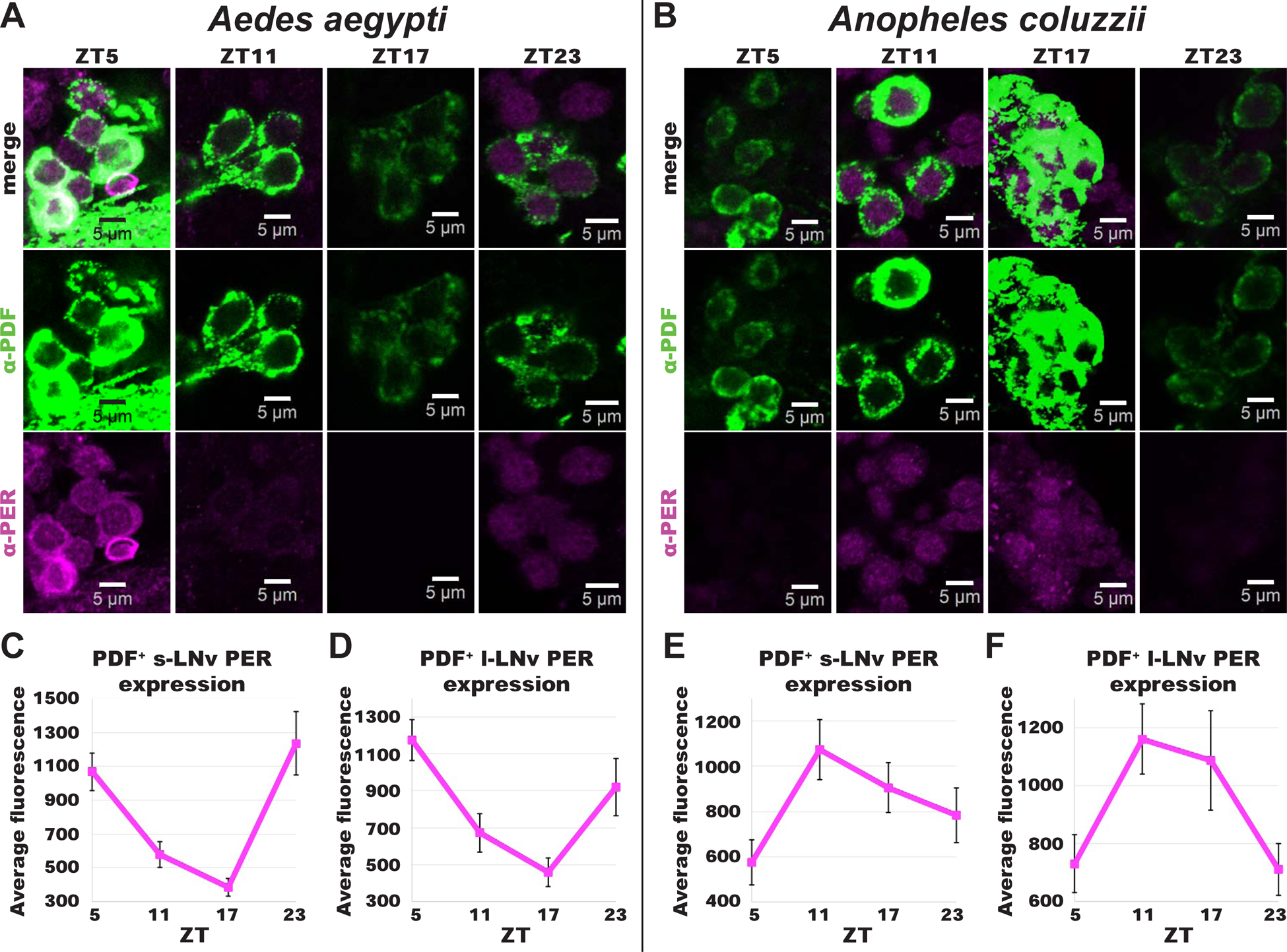
(A-B) Representative confocal images of adult female (A) Ae. aegypti and (B) An. coluzzii mosquito lateral ventral neurons (LNv) immunocytochemistry stained with α–PER (magenta) and α–PDF (green) antibodies at ZTs 5, 11, 17, and 23. *Scale bars indicate 5 μm.
(C-F) PERIOD expression levels over 24 hrs time for Ae. aegypti (ZT5, n=27; ZT11, n=17; ZT17, n=6, ZT23, n=7) (C) s-LNv and (D) l-LNv, and An. coluzzii (ZT5, n=13; ZT11, n=31; ZT17, n=9, ZT23, n=8) (E) s-LNv and (F) l-LNv. Data are represented as mean ± S.E.M. See also Figure S2, Figure S3, Figure S4, Video S1, and Video S2.
Constant light exposure disrupts circadian protein expression and the timing of behavioral attraction/avoidance to UV light in mosquitoes.
Constant light condition (LL) disrupts circadian clock gene expression and rhythmic behaviors in many animals, including mosquitoes [2, 3, 29, 30]. In Drosophila melanogaster, LL disrupts the core clock protein oscillation, behavioral valence, and circadian timing of attraction/avoidance to light via a circadian UV/blue light sensor, CRY [5, 6, 30]. In mosquitoes, LL condition disrupts the cycling of core circadian genes and clock modulation of behaviors including locomotor rhythm, anticipation behavior, and timing of oviposition [3, 10, 31–34].
By using LL circadian clock disruption, we tested whether the circadian clock modulates the valence and timing of behavioral attraction/avoidance to light. Following LD entrainment, female mosquitoes were exposed to constant UV light (UV LL) for 3–5 days. Then we measured circadian protein levels corresponding to species-specific PER peak times using anti-PER and anti-PDF immunocytochemistry. Similar to LL-induced disruption at mRNA level [34], PER protein levels were severely reduced in mosquito brains following UV LL compared to LD in both Ae. aegypti and An. coluzzii (Figure 4A–D). In many brains, PER protein levels in LNvs could not be quantified because there was no visible PER staining following UV LL (not shown).
Figure 4. Constant UV light exposure disrupts circadian protein expression and clock modulation of attraction/avoidance behavioral responses to UV light in mosquitoes.
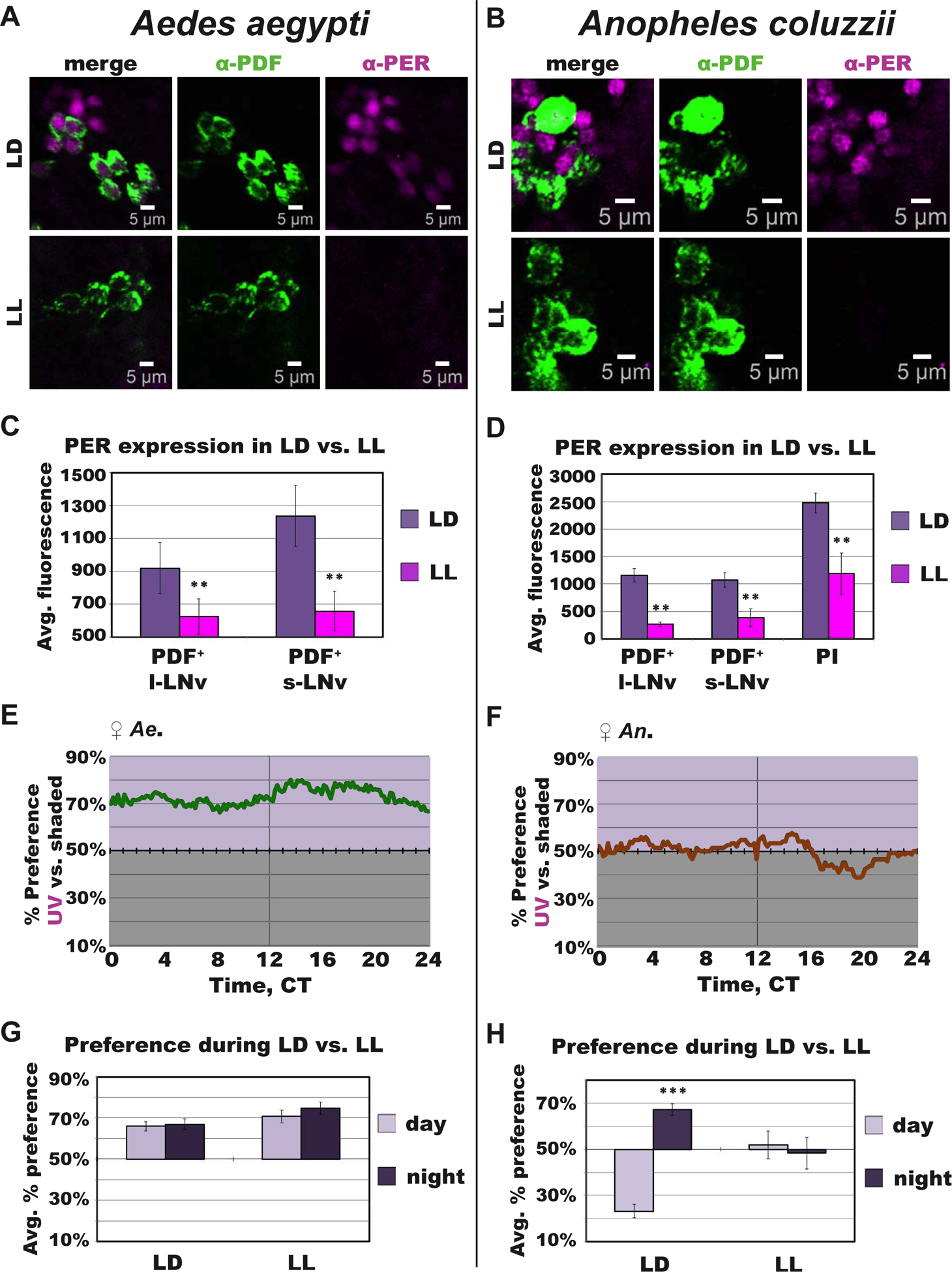
(A-B) Representative confocal images of anti–PER (magenta) and anti–PDF (green) immunocytochemistry stained adult female mosquito brains under 12 hr: 12 hr light: dark (LD) or following constant UV light (LL) exposure for (A) Ae. aegypti and (B) An. coluzzii. *Scale bars indicate 5 μm.
(C-D) Average fluorescence intensity of circadian neurons under LD (light violet) versus LL (magenta) conditions for Ae. aegypti ZT/CT 23 (LD, n=7; LL, n=13) (C) and An. coluzzii ZT/CT 11 (LD, n=31; LL, n=6) (D).
(E-F) Attraction/avoidance behavior to UV light, measured by % of preference in UV-exposed versus shaded during UV LL for female (E) Ae. aegypti and (F) An. coluzzii.
(G-H) Average attraction/avoidance behavioral preference to light-exposed versus shaded-environments during subjective daytime (light violet) versus nighttime (dark violet) under LD or LL conditions for (G) Ae. aegypti and (H) An. coluzzii female mosquitoes. Data are represented as mean ± S.E.M. *p < 0.05; **p < 0.01; ***p < 0.001 vs. LD or day. See also Figure S2.
Drosophila melanogaster has UV light avoidance that peaks in the midday, coinciding with their low locomotor activity “siesta”, followed by behavioral shift from avoidance to attraction in anticipation of dusk, similar to mosquitoes. In Drosophila, this shift in attraction/avoidance is disrupted in flies with core clock gene knockout, circadian neuronal silencing, or with LL-induced circadian clock disruptions [5, 6]. To examine the functional link between the circadian clock and behavioral attraction/avoidance to light, we measured the behavioral preference under the clock-disrupting UV LL condition. During UV LL, both mosquito species lack clear time-of-day dependent changes in UV attraction/avoidance behavior, including the anticipatory behavioral shift approaching dusk (Figure 4E–4H). Ae. aegypti females show attraction to UV light regardless of time-of-day (Figure 4E and 4G). An. coluzzii females show loss of day versus night differences in avoidance/attraction, and overall lack any clear valence for either light environments under UV LL condition (Figure 4F and 4H). LL-induced circadian clock disruption severely disrupted the timing of UV-evoked attraction/avoidance behavior in both diurnal and nocturnal mosquitoes, which strongly resembles findings in Drosophila using either the same LL protocol or tests of genetic clock gene nulls [5, 6].
Discussion
Different mosquito species have evolved to occupy distinct temporal niches, likely to minimize inter-species competition and optimize their chance of mating, biting, and overall survival. In addition to diurnality versus nocturnality, Ae. aegypti and An. coluzzii are behaviorally and ecologically unique. Diurnal Aedine mosquitoes are aggressive biters that maintain relatively high activity levels and take multiple blood meals within a same gonotropic cycle [3, 34, 35]. In contrast, nocturnal Anopheline mosquitoes are relatively quiescent especially during the day and mainly target defenseless hosts that are sleeping at night [2, 15]. The timing of species- and sex-specific behavioral attraction/avoidance to light we describe here coincides with the ecological timing of these mosquito species’ increased flight activity, mating, and host seeking behaviors [2, 3, 13–17]. In instance, the sex-specific difference in An. coluzzii behavioral attraction/avoidance to light are mainly observed in the late afternoon and nighttime, which is the most ecologically relevant and active time for this species.
We find that diurnal Ae. aegypti are attracted to a wide range of light spectra during the daytime while nocturnal An. coluzzii are strongly photophobic to short wavelength light. Our results suggest that the timing and spectra must be considered when targeting specific mosquitoes. For instance, the use of high intensity UV light during the day may not be effective in attracting nocturnal mosquitoes. Controlling the timing and light spectra may allow targeting of specific mosquito species using environmentally friendly light-based approaches [36].
A wide range of behaviors in mosquito and other insects are temporally modulated by light, including mating, seeking a blood-meal, biting, oviposition, flight activity, and sleep [2, 3, 13–17]. Light treatments that alter circadian function also disrupts biting, flight activity, and oviposition behaviors in mosquitoes [2, 3, 10, 11, 13]. Both Ae. aegypti and An. coluzzii exhibit behavioral shifts in attraction/avoidance in anticipation of dusk, despite no change in light stimulus itself. Our characterization of light-evoked attraction/avoidance behavior in mosquitoes shows timing features that suggest that these processes are under circadian regulation, similar to that of Drosophila melanogaster [4–6]. The disruption of circadian protein expression and loss of UV-evoked attraction/avoidance anticipatory behavior under clock-disrupting LL condition is clear evidence that UV attraction/avoidance is a circadian clock-modulated behavior. The timing of anticipatory behavioral shift in UV avoidance/attraction is distinct between diurnal versus nocturnal mosquitoes, supporting the idea that change in avoidance/attraction behavior may contribute to temporal niches occupied by each species.
In the more extensively studied circadian neural network, that of Drosophila melanogaster, there are ~150 circadian neurons [37]. In comparison, we find that Ae. aegypti and An. coluzzii has only about ~80–90 circadian neurons total. The largest difference between flies and mosquitoes is that both mosquitoes have only a few DNs (~90 DNs in flies vs. only ~4 in the two mosquito species tested). LNvs on the other hand, are much more abundant in both mosquitoes tested than in flies (~10–11 LNvs in flies vs. ~25–30 in mosquitoes). Another interesting group of neurons are the mosquito species-specific neuronal groups, m-AN of Ae. aegypti and PI neurons of An. coluzzii, which are not PER+ in Drosophila. PI neurons in many other insect species express PER (Reviewed in [27]). In Drosophila melanogaster, PI neurons are not PER+, yet are circadian rhythmic due to clock neuronal inputs [38, 39]. PDF is a critical neural peptide in circadian neuronal signaling working in close neuronal proximity. In Drosophila melanogaster, PDF+ s-LNv axon terminal structures undergo temporal oscillation to modulate circadian neural signaling [39, 40]. Similarly, we find that axon terminals of PDF+ LNvs of both mosquito species undergo structural remodeling according to time-of-day. Further investigation of the circadian neural circuit dynamics may reveal the principles of clock coding mechanism of mosquitoes.
In addition to circuit-level differences, PER protein oscillates in anti-phasic manner between diurnal and nocturnal mosquitoes. Anti-phasic oscillation is not observed at the DNA and mRNA levels [34, 35, 41, 42]. It has been demonstrated in cyanobacteria, Drosophila, and mice that post-translational modification of core circadian proteins modulate the protein stability, and phasic expression and nuclear translocation, which is essential in timing of the clock and its behavioral outputs [30, 43–49]. Our qualitative analysis suggests nuclear PER peaks at opposite times between diurnal versus nocturnal mosquitoes: PER peaks in the late nighttime (~ZT23) in Ae. aegypti versus in the late daytime (~ZT11) in An. coluzzii (Figure 3), which can be further confirmed with nuclear markers. Furthermore, the circadian clock in nonsuprachiasmatic nuclei neurons and periphery tissues cycle in opposite phases in diurnal versus nocturnal mammals [50–52]. These findings along with our findings of the circuit-level differences and anti-phasic oscillation of core circadian proteins point to a potential mechanism underlying diurnal versus nocturnal behaviors.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Prof. Todd C. Holmes (tholmes@uci.edu).
Materials Availability Statement
While this study did not generate new unique reagents, further information and requests for resources should be directed to and will be fulfilled by the Lead Contact.
Data and Code Availability Statement
The datasets generated during this study are available at Mendeley Data Repository (http://dx.doi.org/10.17632/f862zzmj8m.1).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mosquito rearing and maintenance
Mosquitoes were reared at 27°C, 70%–80% relative humidity with a photoperiod of 12 hrs:12 hrs light: dark cycle. Mosquito larvae were fed Tetramin Tropical fish food (Tetra GMBH, Melle Germany). All larvae were kept in plastic containers until pupation, then transferred into a small paper cup containing deionized water and moved to a 30cm2 Bugdorm mosquito cage to eclose. Emerged mosquitoes were fed 10% sucrose ad libitum and allowed to mate prior to all experiments.
METHOD DETAILS
Light Attraction/Avoidance Behavior Assay
All mosquitoes were reared in standard 12 hr: 12 hr light: dark (LD) schedule in 27°C, and 80% humidity in large cages with access to 10% sucrose diet. Adult mosquitoes (0–5 days post-eclosion) were entrained to LD schedule for minimum of 3 days prior to testing. Individual mosquitoes were each placed into 25mm diameter × 125 mm length Pyrex glass tubes (25mm diameter × 125mm length, TriKinetics) plugged with cotton “flugs” (Genesee) on either side. Flugs were soaked with 10% sucrose providing a food source, while simultaneously allowing airflow sufficient for multi-day survival of the mosquitoes in the tubes. Tubes containing individual mosquitoes were placed in humidity-, temperature-, and light-controlled incubator and allowed to acclimate for a full day. One half of the tubes were covered with infrared (IR) filters (LEE Filters 4 × 4” Infrared (87C) Polyester Filter), providing the mosquitoes with a choice of a shaded environment (IR filtered) versus light-exposed (not covered with IR filter) during the 12 hrs of light (daytime). Philips TL-D Blacklight ultraviolet light (UV) source with narrow peak wavelength of 365 nm and intensity of ~400 μW/cm2 was used for UV light. Blue (450 nm, Supernight) and red (630 nm, Supernight) LED strips set at ~400 μW/cm2 was used as blue and red light sources. Light intensities were determined by a Newport 843-R Power/Energy Meter with Newport 818-UV Sensor. Additionally, IR LED strips (Infrared 850 nm 3528 LED Strip Light, 78/m, 8mm wide, by the 5m Reel) placed on aluminum heat sink was placed under the entire setup which allowed dark imaging of mosquitoes. With each light source, same LD schedule as the LD entrainment schedule prior to experiment was continued to minimize any disturbance to the circadian time. For constant light (LL) light choice assay, the UV light was constantly left on. Webcam (Microsoft Q2F-00013 USB 2.0 LifeCam) took pictures at 10 min intervals for 3–5 days of experiment. Each mosquito’s preference in the light-exposed versus shaded side of the tube was analyzed by the ImageJ program. Preference for the light-exposed or the shaded-environment, quantified as preference %. Each experiment was repeated a minimum of three times for each group. Related to Figure 1, Figure 4, and Figure S1.
Immunocytochemistry
All mosquitoes were reared in standard 12 hr: 12 hr light: dark (LD) schedule at 27°C, and 80% humidity in large cages, with access to 10% sucrose diet. Mosquito brains were dissected 5–10 days post-eclosion at times specified in the manuscript. All dissections, staining, and imaging were carried out in an exact same manner for all conditions for fair comparison. Brains were dissected in 1X PBS in the dark with dim red light source, fixed in 4% paraformaldehyde (PFA) for 30 min, washed 3X 10 min in PBS-Triton-X 1%, incubated in blocking buffer (10% Horse Serum-PBS-Triton-X 0.5%) at room temperature before incubation with mouse α-PDF C7, monoclonal (1:10,000) and rabbit α-PER, polyclonal (1:1,000) antibodies overnight in 4° C. Primary antibody incubated brains were then washed 3X 10 min in PBS-Triton-X 0.5% then incubated in goat α-mouse-Alexa 488 (1:500) and goat α-rabbit-Alexa-594 (1:500) secondary antibodies in blocking buffer overnight in 4°C. Brains were washed 5X 15min in PBSTriton-X 0.5% before mounting in Vectashield mounting media (Vector Laboratories). Microscopy was performed using Zeiss LSM700 confocal microscope. All settings for confocal microscope was kept the same across all samples, including laser power, gain, objective (20x), averaging per frame (x2), etc. Then fluorescence intensities were analyzed and represented as raw images using Imaris and ImageJ (not manipulated by Photoshop or any other photo manipulation program). All representative images in this manuscript are also raw images, not manipulated for intensity, gain, etc. unless noted otherwise in the figure legend and normalized to background fluorescence in areas outside of the imaged brain. Each condition was carried out over a minimum of 3 separate tests to further minimize any experimental variability. Related to Figure 2, Figure 4, Figure S2, Figure S3, Figure S4, and Table S1.
QUANTIFICATION AND STATISTICAL ANALYSIS
Mosquito preference behavior was quantified as present or not present in light-exposed versus shaded sides of the tube. This binary preference for each 10 min time point over 3–5 days was averaged per individual mosquito (n= individual mosquito). Individual preferences were then further averaged for each group. Preference behavior statistical measurements including significance were analyzed by t test using Microsoft Excel and Sigma Plot. Related to Figure 1, Figure 4, and Figure S1.
For immunocytochemistry, fluorescence levels were analyzed using Imaris software (Bitplane). Spherical region of interest was selected for each cell based on anatomical location and size. Fluorescence was quantified for each region by the Imaris software. Each species time point was collected for minimum of three repetitions. Each n represents one brain. Reported quantification values reflect the average fluorescence intensity levels and error bars indicate S.E.M. Statistical measurements includeing significance was defined using t test using Microsoft Excel and SIgma Plot. Related to Figure 3, Figure 4, Figure S3 and Figure S4.
Supplementary Material
Video S1. 3D Rendering of PDF neurons and projections in Aedes aegypti female brain, Related to Figure 2 and Figure 3. 3D animation of anti-PDF stained Aedes aegypti female brain showing individual z-slice progressing from anterior to posterior, then posterior to anterior, followed by building of z-stack, and z-stacked brain rotated in multiple directions (+180° around x-axis and back, −180° around x-axis and back, −180° around the y-axis and back, −180° around y-axis and back).
Video S2. 3D Rendering of PDF neurons and projections in Anopheles coluzzii female brain, Related to Figure 2 and Figure 3. 3D animation of anti-PDF stained Anopheles coluzzii female brain showing individual z-slice progressing from anterior to posterior, then posterior to anterior, followed by building of z-stack, and z-stacked brain rotated in multiple directions (+180° around x-axis and back, −180° around x-axis and back, −180° around the y-axis and back, −180° around y-axis and back).
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-PDF C7, monoclonal | Developmental Studies Hybridoma Bank at U of Iowa | Cat# PDF-C7-C |
| Goat anti-mouse Alexa Fluor 488 | Invitrogen | Cat# A-11001 |
| Rabbit anti-PER, polyclonal | Michael Rosbash, Brandeis University | n/a |
| Goat anti-rabbit Alexa Fluor 594 | Invitrogen | Cat# A-11037 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Paraformaldehyde | Alfa Aesar/Fisher Scientific | Cat# AA433689L |
| Triton-X | Fisher Scientific | Cat# T8787 |
| PBS | VWR International | Cat# 12001–766 |
| Horse Serum | Fisher Scientific | Cat# MT35030CV |
| Glycerol | Fisher Scientific | Cat#G33–500 |
| TetraMin Tropical Tablets | Tetra GMBH, Melle, Germany | Product No. 16110 |
| Deposited Data | ||
| Raw and analyzed data | This paper | http://dx.doi.org/10.17632/f862zzmj8m.1 |
| Experimental Models: Organisms/Strains | ||
| Aedes aegypti (Orlando strain) | Leslie Vosshall, Rockefeller University | Orlando strain |
| Anopheles coluzzii (Ngousso strain) | Bradley White BEI Resources | BEI Resources # MRA-1279 |
| Software and Algorithms | ||
| SigmaPlot 11 | Systat Software, Inc | https://systatsoftware.com/products/sigmaplot/ |
| ImageJ | Fiji | https://imagej.nih.gov/ij/ |
| Imaris | Bitplane, Oxford Instruments | https://imaris.oxinst.com/ |
| iSpy64 | DeveloperInABox © | www.developerinabox.com |
| Other | ||
| Webcam | Microsoft | Cat# Q2F-00013 |
| Pyrex glass tubes | TriKinetics | Cat# PGT25×125 |
| Cotton Flugs | Genesee | Cat# 49–102 |
| Red light | Supernight | Cat# B00DTOAW3O |
| Blue light | Supernight | Cat# SYNCHKG048722 |
| UV light | Philips | Cat# TL-D/08 |
| Power adaptor, Power supply for LED strip | Lighting EVER | Cat# 5000028-US |
| Light Meter | Newport Corp. | Cat# 843-R |
| Light Sensor | Newport Corp. | Cat# 818-UV |
| IR filters | B & H Foto & Electronics Corp. | Cat# B&H # LE87C44 |
| Incubator | Percival Scientific, Inc. | Cat# DR-36VL |
Highlights.
Diurnal/nocturnal mosquitoes have distinct circadian neural circuit and PER cycling
Diurnal vs. nocturnal mosquitoes have distinct clock-modulated light preferences
Light preference is dependent on mosquito sex and species, time of day, and color
Circadian clock modulates timing and valence of mosquito light responses
Acknowledgments
We thank Annika Barber, Naoki Okamoto, and Anupama Dahanukar for helpful discussions; Guiyun Yan for generous access to insectary facilities and Adeela Syed for imaging support; and Janita Parpana, Duke Park, and Lillian Li for administrative support. T.C.H. is supported by R35 GM127102. L.S.B. acknowledges partial support by an individual NSF GRFP fellowship, ARCS Foundation Fellowship, and UCI Graduate Dean’s Dissertation Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
A.R. is founder of Sensorygen Inc, a startup working on natural odorants as insect repellents and food flavorings. All other authors have no financial interests to declare.
References
- 1.Whitfield J (2002). Portrait of a serial killer: a roundup of the history and biology of the malaria parasite. (Nature Publishing Group). [Google Scholar]
- 2.Jones MD, Hill M, and Hope AM (1967). The circadian flight activity of the mosquito Anopheles Gambiae: phase setting by the light regime. J Exp Biol 47, 503–511. [DOI] [PubMed] [Google Scholar]
- 3.Taylor B, and Jones MD (1969). The circadian rhythm of flight activity in the mosquito Aedes aegypti (L.). The phase-setting effects of light-on and light-off. J Exp Biol 51, 59–70. [DOI] [PubMed] [Google Scholar]
- 4.Baik LS, Fogle KJ, Roberts L, Galschiodt AM, Chevez JA, Recinos Y, Nguy V, and Holmes TC (2017). CRYPTOCHROME mediates behavioral executive choice in response to UV light. Proc Natl Acad Sci U S A 114, 776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baik LS, Recinos Y, Chevez JA, and Holmes TC (2018). Circadian modulation of light-evoked avoidance/attraction behavior in Drosophila. PLoS One 13, e0201927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baik LS, Recinos Y, Chevez JA, Au DD, and Holmes TC (2019). Multiple Phototransduction Inputs Integrate to Mediate UV Light–evoked Avoidance/Attraction Behavior in Drosophila. J. Biol. Rhythms, 0748730419847339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knop E, Zoller L, Ryser R, Gerpe C, Horler M, and Fontaine C (2017). Artificial light at night as a new threat to pollination. Nature 548, 206–209. [DOI] [PubMed] [Google Scholar]
- 8.Tokushima Y, Uehara T, Yamaguchi T, Arikawa K, Kainoh Y, and Shimoda M (2016). Broadband Photoreceptors Are Involved in Violet Light Preference in the Parasitoid Fly Exorista Japonica. PLoS One 11, e0160441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green C, and Cosens D (1983). Spectral responses of the tsetse fly, Glossina morsitans morsitans. J. Insect Physiol 29, 795–800. [Google Scholar]
- 10.Farnesi LC, Barbosa CS, Araripe LO, and Bruno RV (2018). The influence of a light and dark cycle on the egg laying activity of Aedes aegypti (Linnaeus, 1762) (Diptera: Culicidae). Mem Inst Oswaldo Cruz 113, e170362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheppard AD, Rund SS, George GF, Clark E, Acri DJ, and Duffield GE (2017). Light manipulation of mosquito behaviour: acute and sustained photic suppression of biting activity in the Anopheles gambiae malaria mosquito. Parasites & Vectors 10, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu B, Liu H, Zhong D, and Lin C (2010). Searching for a photocycle of the cryptochrome photoreceptors. Curr Opin Plant Biol 13, 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S, and Dimopoulos G (2008). Molecular analysis of photic inhibition of blood-feeding in Anopheles gambiae. BMC Physiol 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawadogo SP, Costantini C, Pennetier C, Diabate A, Gibson G, and Dabire RK (2013). Differences in timing of mating swarms in sympatric populations of Anopheles coluzzii and Anopheles gambiae s.s. (formerly An. gambiae M and S molecular forms) in Burkina Faso, West Africa . Parasit Vectors 6, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rund SS, Lee SJ, Bush BR, and Duffield GE (2012). Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J Insect Physiol 58, 1609–1619. [DOI] [PubMed] [Google Scholar]
- 16.Araripe LO, Bezerra JRA, Rivas G, and Bruno RV (2018). Locomotor activity in males of Aedes aegypti can shift in response to females’ presence. Parasites & Vectors 11, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumba LA, Okoth K, Deng AL, Githure J, Knols BG, Beier JC, and Hassanali A (2004). Daily oviposition patterns of the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) on different types of aqueous substrates. J Circadian Rhythms 2, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coombe P (1982). Visual behaviour of the greenhouse whitefly, Trialeurodes vaporariorum. J. Phys. Ent 7, 243–251. [Google Scholar]
- 19.Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, and Holmes TC (2008). Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol 18, 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. (2008). PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 60, 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renn SC, Park JH, Rosbash M, Hall JC, and Taghert PH (1999). A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99, 791–802. [DOI] [PubMed] [Google Scholar]
- 22.Giebultowicz JM (2000). Molecular mechanism and cellular distribution of insect circadian clocks. Annual Rev. Entomol 45, 769–793. [DOI] [PubMed] [Google Scholar]
- 23.Sauman I, and Reppert SM (1996). Circadian clock neurons in the silkmoth Antheraea pernyi: novel mechanisms of Period protein regulation. Neuron 17, 889–900. [DOI] [PubMed] [Google Scholar]
- 24.Bloch G, Solomon SM, Robinson GE, and Fahrbach SE (2003). Patterns of PERIOD and pigment-dispersing hormone immunoreactivity in the brain of the European honeybee (Apis mellifera): age- and time-related plasticity. J Comp Neurol 464, 269–284. [DOI] [PubMed] [Google Scholar]
- 25.Nässel DR, Shiga S, Mohrherr CJ, and Rao KR (1993). Pigment - dispersing hormone - like peptide in the nervous system of the flies Phormia and Drosophila: immunocytochemistry and partial characterization. J. Comp. Neurol 331, 183–198. [DOI] [PubMed] [Google Scholar]
- 26.Helfrich-Förster C, and Homberg U (1993). Pigment - dispersing hormone - immunoreactive neurons in the nervous system of wild - type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp. Neurol 337, 177–190. [DOI] [PubMed] [Google Scholar]
- 27.Helfrich-Forster C, Nitabach MN, and Holmes TC (2011). Insect circadian clock outputs. Essays Biochem. 49, 87–101. [DOI] [PubMed] [Google Scholar]
- 28.Helfrich-Forster C (1995). The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A 92, 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konopka RJ, Pittendrigh C, and Orr D (1989). Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J Neurogenet 6, 1–10. [DOI] [PubMed] [Google Scholar]
- 30.Zerr DM, Hall JC, Rosbash M, and Siwicki KK (1990). Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci 10, 2749–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillett J, and Haddow A (1957). Laboratory observations on the oviposition-cycle in the mosquito Aedes (Stegomyia) africanus Theobald. Annals of Tropical Medicine & Parasitology 51, 170–174. [DOI] [PubMed] [Google Scholar]
- 32.Nayar JK, and Sauerman DM Jr. (1971). The effect of light regimes on the circadian rhythm of flight activity in the mosquito Aedes taeniorhynchus. J Exp Biol 54, 745–756. [DOI] [PubMed] [Google Scholar]
- 33.Pandian RS (1994). Circadian rhythm in the biting behaviour of a mosquito Armigeres subalbatus (Coquillett). Indian J Exp Biol 32, 256–260. [PubMed] [Google Scholar]
- 34.Rivas GBS, Teles-de-Freitas R, Pavan MG, Lima JBP, Peixoto AA, and Bruno RV (2018). Effects of Light and Temperature on Daily Activity and Clock Gene Expression in Two Mosquito Disease Vectors. J Biol Rhythms 33, 272–288. [DOI] [PubMed] [Google Scholar]
- 35.Gentile C, Rivas GB, Meireles-Filho AC, Lima JB, and Peixoto AA (2009). Circadian expression of clock genes in two mosquito disease vectors: cry2 is different. J Biol Rhythms 24, 444–451. [DOI] [PubMed] [Google Scholar]
- 36.Liu YN, Liu YJ, Chen YC, Ma HY, and Lee HY (2017). Enhancement of mosquito trapping efficiency by using pulse width modulated light emitting diodes. Sci Rep 7, 40074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieger D, Shafer OT, Tomioka K, and Helfrich-Förster C (2006). Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. Journal of Neuroscience 26, 2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barber AF, Erion R, Holmes TC, and Sehgal A (2016). Circadian and feeding cues integrate to drive rhythms of physiology in Drosophila insulin-producing cells. Genes Dev 30, 2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavanaugh DJ, Geratowski JD, Wooltorton JR, Spaethling JM, Hector CE, Zheng X, Johnson EC, Eberwine JH, and Sehgal A (2014). Identification of a circadian output circuit for rest: activity rhythms in Drosophila. Cell 157, 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorostiza EA, Depetris-Chauvin A, Frenkel L, Pirez N, and Ceriani MF (2014). Circadian pacemaker neurons change synaptic contacts across the day. Curr Biol 24, 2161–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rund SS, Hou TY, Ward SM, Collins FH, and Duffield GE (2011). Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proceedings of the National Academy of Sciences 108, E421–E430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leming MT, Rund SS, Behura SK, Duffield GE, and O’Tousa JE (2014). A database of circadian and diel rhythmic gene expression in the yellow fever mosquito Aedes aegypti. BMC Genomics 15, 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirano A, Fu YH, and Ptacek LJ (2016). The intricate dance of post-translational modifications in the rhythm of life. Nat Struct Mol Biol 23, 1053–1060. [DOI] [PubMed] [Google Scholar]
- 44.Li YH, Liu X, Vanselow JT, Zheng H, Schlosser A, and Chiu JC (2019). O-GlcNAcylation of PERIOD regulates its interaction with CLOCK and timing of circadian transcriptional repression. PLoS Genet 15, e1007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiu JC, Ko HW, and Edery I (2011). NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell 145, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, and Kondo T (2005). Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308, 414–415. [DOI] [PubMed] [Google Scholar]
- 47.Shafer OT, Rosbash M, and Truman JW (2002). Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. J Neurosci 22, 5946–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nawathean P, and Rosbash M (2004). The doubletime and CKII kinases collaborate to potentiate Drosophila PER transcriptional repressor activity. Mol Cell 13, 213–223. [DOI] [PubMed] [Google Scholar]
- 49.Yagita K, Tamanini F, Yasuda M, Hoeijmakers JH, van der Horst GT, and Okamura H (2002). Nucleocytoplasmic shuttling and mCRY-dependent inhibition of ubiquitylation of the mPER2 clock protein. EMBO J 21, 1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, and Cooper HM (2018). Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359, eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemos DR, Downs JL, and Urbanski HF (2006). Twenty-four-hour rhythmic gene expression in the rhesus macaque adrenal gland. Mol. Endocr 20, 1164–1176. [DOI] [PubMed] [Google Scholar]
- 52.Ramanathan C, Nunez A, and Smale L (2008). Daily rhythms in PER1 within and beyond the suprachiasmatic nucleus of female grass rats (Arvicanthis niloticus). Neuroscience 156, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. 3D Rendering of PDF neurons and projections in Aedes aegypti female brain, Related to Figure 2 and Figure 3. 3D animation of anti-PDF stained Aedes aegypti female brain showing individual z-slice progressing from anterior to posterior, then posterior to anterior, followed by building of z-stack, and z-stacked brain rotated in multiple directions (+180° around x-axis and back, −180° around x-axis and back, −180° around the y-axis and back, −180° around y-axis and back).
Video S2. 3D Rendering of PDF neurons and projections in Anopheles coluzzii female brain, Related to Figure 2 and Figure 3. 3D animation of anti-PDF stained Anopheles coluzzii female brain showing individual z-slice progressing from anterior to posterior, then posterior to anterior, followed by building of z-stack, and z-stacked brain rotated in multiple directions (+180° around x-axis and back, −180° around x-axis and back, −180° around the y-axis and back, −180° around y-axis and back).
Data Availability Statement
The datasets generated during this study are available at Mendeley Data Repository (http://dx.doi.org/10.17632/f862zzmj8m.1).


