Abstract
This study aimed to determine the effects of prenatal exposure to angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), particularly when exposure is limited to the first trimester of pregnancy, on adverse maternal and neonatal outcomes. A systematic search was performed on four databases, that is, PubMed, Scopus, Web of Science, and Cochrane Library, to identify relevant articles published up to December 31, 2019. Included studies were limited to original investigations assessing the association between prenatal exposure to ACEIs/ARBs and adverse pregnancy outcomes. Odds ratios were used as a summary effect measure. Pooled‐effect estimates of each outcome were calculated by the random‐effects meta‐analysis. The main outcomes included overall and specific congenital malformations, low birth weight, miscarriage, elective termination of pregnancy, stillbirth, and preterm delivery. Of 19 included articles involving a total of 4 163 753 pregnant women, 13 studies reported an increased risk of, at least, one adverse pregnancy outcome in pregnant women who were exposed to ACEIs/ARBs. Meta‐analysis revealed a significant association between overall congenital malformations and first trimester‐only exposure to ACEIs/ARBs (OR = 1.94, 95% CI = 1.71‐2.21, P < .0001). Cardiovascular malformations, miscarriage, and stillbirth also provided a significant relation with ACEI/ARB exposure. In conclusion, prenatal exposure to ACEIs/ARBs in the first trimester of pregnancy was found to be associated with an increased risk of adverse pregnancy outcomes. Women of reproductive age should be aware of the potential teratogenic risks of these drugs if they become pregnant.
Keywords: adverse pregnancy outcome, angiotensin II receptor blocker, angiotensin‐converting enzyme inhibitor, congenital malformation
Abbreviations
- ACEI
angiotensin‐converting enzyme inhibitor
- ACR
assumed comparator risk
- ARB
angiotensin II receptor blocker
- CI
confidence interval
- CNS
central nervous system
- CVS
cardiovascular system
- ETOP
elective termination of pregnancy
- FDA
Food and Drug Administration
- GRACE
good research for comparative effectiveness
- LBW
low birth weight
- OAH
other antihypertensive medications
- OR
odds ratio
- PRISMA
preferred reporting items for systematic reviews and meta‐analyses
- RAAS
renin‐angiotensin‐aldosterone system
- RevMan
review manager
- RoB 2
a revised tool for assessing risk of bias in randomized trials
- RR
risk ratio
1. INTRODUCTION
Angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), an alternative for ACEI‐intolerant patients, are commonly used for the treatment of cardiovascular disease whereby the renin‐angiotensin‐aldosterone system (RAAS) is involved in its pathophysiology. 1 , 2 ACEIs/ARBs modulate the RAAS by either inhibiting an enzyme responsible for the conversion of angiotensin I to angiotensin II or by antagonizing the effects of angiotensin II at its receptors. As such, ACEIs/ARBs are beneficial to enhanced natriuresis, reduced afterload, and deferral of cardiovascular remodeling, making them useful for various cardiovascular conditions, such as hypertension, heart failure, and postmyocardial infarction. 3 , 4 , 5 ACEIs/ARBs are, thus, one of the most widely prescribed drug classes, with hundreds of thousands of patients worldwide who are exposed to each year. 6 , 7
In 1980s‐1990s, there were a series of cases reported to the US Food and Drug Administration (FDA) indicating that ACEIs/ARBs are teratogens when being used in the second and third trimesters of pregnancy. 8 Evidence suggests a relationship between neonatal adverse outcomes (ie, oligohydramnios and other adverse outcomes secondary to impaired fetal kidney development) and ACEI/ARB exposure. 9 , 10 A “black box” warning issued by the US FDA in 1992 has raised awareness of the teratogenic potential of ACEIs/ARBs, and the second and third trimesters of pregnancy are considered a contraindication to the use of ACEIs/ARBs accordingly. 11 In the 2013 Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy, it has been recommended not to use ACEIs/ARBs in women of reproductive age if there is no compelling reason. 12 Despite such a feature in the labeling of the potentially teratogenic medications, several cases of fetal exposure to ACEIs/ARBs have been reported thereafter. 13 , 14 , 15 ACEI/ARB exposure during pregnancy is still highly prevalent in many settings. 16 , 17
Up to the present time, it still remains unclear whether ACEIs/ARBs are teratogenic if exposure to these drugs is only limited to the first trimester of pregnancy. 18 , 19 , 20 Several epidemiologic studies report inconsistent results on the teratogenic effects of first‐trimester ACEI/ARB exposure in humans. 17 , 21 , 22 Given the increasing incidence of hypertension and conditions in which ACEIs/ARBs are often indicated, systematic investigations on the potential teratogenic consequences of ACEI/ARB exposure during early pregnancy are highly needed to provide more concrete guidance for the use of ACEIs/ARBs in women of reproductive age. 23 , 24 Should ACEI/ARB exposure during the first trimester of pregnancy be considered nonteratogenic, female patients of childbearing potential could be safely prescribed either an ACEI or an ARB, provided that they are advised of the risks involved and can switch the drug to other alternatives within a few weeks after conception. On the other hand, if exposure to ACEIs/ARBs during the first trimester of pregnancy is associated with an increased risk of congenital malformations or adverse maternal outcomes, the use of ACEIs/ARBs in women of reproductive age should be discouraged, particularly given the availability of alternative medications to treat their conditions. 25
The objective of the present study was to determine the effects of prenatal exposure to ACEIs/ARBs, particularly when exposure is limited to the first trimester of pregnancy, on adverse maternal and neonatal birth outcomes by means of systematic review and meta‐analysis.
2. METHODS
This study conformed to the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines. 26 The study protocol was prospectively registered at the PROSPERO international prospective register of systemic reviews in health and social care (CRD42019140107).
2.1. Search strategy and eligibility criteria
Initial literature searches were systematically performed in four major search engines, that is, PubMed, Scopus, Web of Science, and Cochrane Library, in September 2019, and a repeated search was updated on December 31, 2019. The terms related to adverse pregnancy outcomes (including congenital malformations, teratogens, fetus, and pregnancy) and ACEIs/ARBs (including all generic drug names based on Micromedex) were used to develop a comprehensive search strategy, with no language restriction, to identify all relevant articles. A number of medical subject headings were combined using the ‘OR’ operator; the results of the two searches (ie, adverse pregnancy outcomes and ACEIs/ARBs) were combined with the ‘AND’ operator. The reference lists of selected articles were screened manually in search of additional articles, if any.
Relevant studies were selected based on the following criteria: (a) a study involved pregnant women; (b) there was ACEI/ARB exposure during pregnancy; and (c) either adverse maternal outcomes or neonatal birth outcomes, or both, were reported. Included studies were limited to original investigation performed on humans. No restriction was made with respect to study design or subjects’ underlying conditions. Studies lacking a control group (eg, case reports, case series, or expert opinion) and review articles (including systematic reviews) were excluded. All studies deemed suitable were retrieved and reviewed independently by two authors to determine study eligibility. Study selection was carried out by two authors independently; disagreements were resolved through discussion and consensus.
2.2. Data extraction and quality assessment
Two authors independently extracted data from original full‐text articles using a standardized data collection form. The data extracted included (a) first author, (b) publication year, (c) study design, (d) study setting/location, (e) study period, (f) stage of pregnancy, (g) number of participants, (h) exposure (ie, ACEIs or ARBs), (i) control (ie, exposure to other antihypertensive drugs or nonexposure), and (j) outcome of interest (ie, overall and specific congenital malformations, low birth weight (LBW) (birth weight < 2500 g), miscarriage or spontaneous abortion, elective termination of pregnancy (ETOP), stillbirth, and preterm delivery). In cases where data were missing in an original publication or required clarification, attempts were made by e‐mail contact with the corresponding author.
The quality of included studies was evaluated using the standardized Good Research for Comparative Effectiveness (GRACE) checklist for observational studies and a revised tool for assessing the risk of bias in randomized trials (RoB 2) for randomized‐controlled trials. 27 , 28
2.3. Data analyses
The exposure of interest was maternal exposure to ACEIs/ARBs during any trimesters of pregnancy or during the first trimester only, and the outcome of interest was adverse pregnancy outcomes, including both maternal and neonatal outcomes. The first trimester‐only exposure was defined as any use of ACEIs/ARBs from the last menstrual period to the third month of pregnancy. An exposure cohort was defined as a group of pregnant women who were exposed to ACEIs/ARBs (ACEI/ARB group), while a control cohort was those who were exposed to other antihypertensive medications (OAH group) or those with no exposure to any antihypertensive drugs (nonexposure group). Extracted relevant data were tabulated in a 2 × 2 contingency table. Odds ratios (ORs) were used as a summary measure for meta‐analysis of dichotomous outcomes. Risk ratios (RRs) were calculated from ORs using the following formula for ease of interpretation: RR = OR/ [1 – ACR × (1 – OR)]; given that the assumed comparator risk (ACR) is the risk that the outcome of interest occurred in the control group.
Pooled‐effect estimates of each outcome of interest were calculated by the Mantel‐Haenszel random‐effects meta‐analysis. A cumulative meta‐analysis was conducted to determine whether each study added to the pool affected the overall estimate changes. Statistical heterogeneity among included studies was assessed using the Cochran's Q test and the percentage of total variability across studies due to heterogeneity (I 2 value). Subgroup analyses were conducted to determine the impact of ACEI/ARB exposure on adverse maternal and neonatal birth outcomes when exposure was limited to the first trimester of pregnancy.
Potential bias from small‐study effects (eg, publication bias) was assessed through visual examination of funnel plots displaying the log OR of individual studies on the horizontal axis and its standard error on the vertical axis. 29 A rank correlation test and a linear regression test were applied to identify any potential publication bias in a meta‐analysis with 10 or more included studies. 30 , 31 Sensitivity analyses on the impact of study design, drug classes, and exclusion of a single study from meta‐analysis as well as the impact of fixed‐effect or random‐effects models on summary measures were performed.
All tests were two‐tailed; P < .05 was considered statistically significant. Quantitative syntheses of the data were done in Review Manager (RevMan) version 5.3. Cumulative meta‐analyses were performed in chronological order using a standard software package (Stata, version 16.0; StataCorp). Formal tests for funnel plot asymmetry were performed using the jamovi project (2019), jamovi version 1.0 (Computer Software), retrieved from https://www.jamovi.org.
3. RESULTS
Of 3427 potentially relevant records identified through the systematic search, 49 full‐text articles were retrieved and examined for eligibility. A total of 19 articles, published between 1992 and 2018, were included for data extraction, with 18 articles that enabled quantitative analysis (Figure 1). Characteristics of 19 studies are presented in Table 1, 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 : 15 are observational cohort studies, three are case‐control studies, and one is a randomized‐controlled trial, all of which were classified as ‘sufficient quality’ or ‘low risk of bias’ studies (Table S1). Relevant studies were conducted in North America (n = 9), Europe (n = 9), or Australia (n = 2). Data syntheses involved a total of 4 163 753 pregnant women, with 7075 exposed to ACEIs/ARBs, 25 379 to other antihypertensive drugs, and 3 782 450 nonexposed individuals. Around two thirds of studies included in qualitative analysis (13/19) reported an increased risk of, at least, one adverse pregnancy outcome of interest in pregnant women with ACEI/ARB exposure (Table S2).
FIGURE 1.
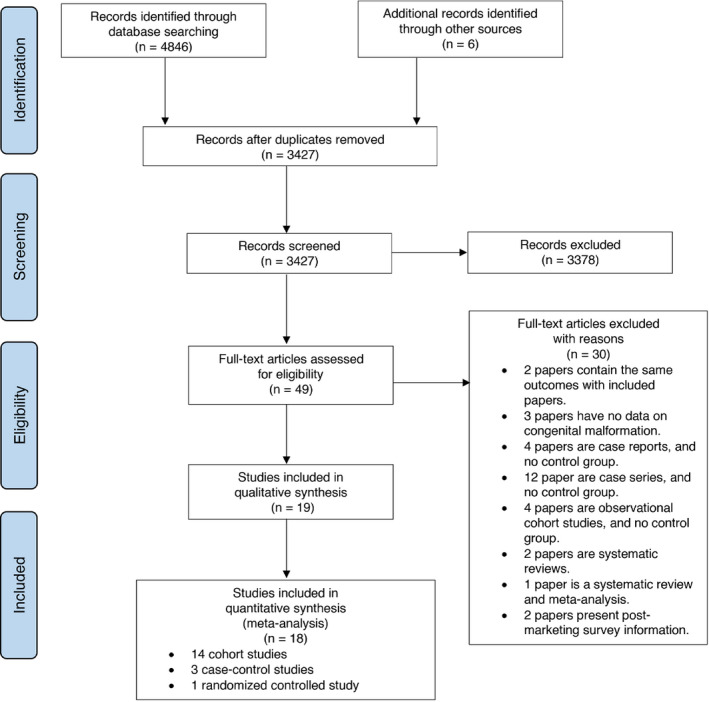
Flow diagram
TABLE 1.
Study characteristics
| Study | Year | Study design | Stage of pregnancy | Locations | Periods | Exposure | Comparator |
|---|---|---|---|---|---|---|---|
| Ahmed et al 32 | 2018 | Retrospective cohort | First trimester | Australia | 2005‐2012 | ACEIs/ARBs | Methyldopa |
| Banhidy et al 33 | 2011 | Case‐control | Any trimesters | Hungary | 1980‐1996 | Captopril | OAH; Nonexposure |
| Bateman et al 34 | 2017 | Retrospective cohort | First trimester | United States | 2000‐2010 | ACEIs | Nonexposure |
| Caton et al 35 | 2009 | Case‐control | First trimester | United States | 1997‐2003 | ACEIs/ARBs | OAH; Nonexposure |
| Chintamaneni et al 36 | 2018 | Retrospective cohort | Any trimesters | United States | 2003‐2014 | ACEIs (mostly Lisinopril) | Nonexposure |
| Colvin et al 37 | 2014 | Retrospective cohort | Any trimesters | Australia | 2002‐2005 | ACEIs | Nonexposure |
| Cooper et al 38 | 2006 | Retrospective cohort | First trimester | United States | 1985‐2000 | ACEIs | OAH; Nonexposure |
| Cournot et al 39 | 2006 | Prospective cohort | First trimester | France | n/a | ACEIs | Nonexposure |
| Diav‐Citrin et al 40 | 2011 | Prospective cohort | First trimester | Israel; Italy | 1994‐2007; 1990‐2008 | ACEIs | OAH; Nonexposure |
| Fisher et al 41 | 2017 | Case‐control | First trimester | United States | 1997‐2011 | ACEIs/ARBs | OAH; Nonexposure |
| Hoeltzenbein et al 42 | 2018a | Prospective cohort | First trimester 1 | Germany | 2000‐2014 | ACEIs | Methyldopa; Nonexposure |
| Hoeltzenbein et al 43 | 2018b | Prospective cohort | First trimester 1 | Germany | 2000‐2014 | ARBs | Methyldopa; Nonexposure |
| Lennestal et al 44 | 2009 | Retrospective cohort | First trimester | Sweden | 1995‐2006 | ACEIs/ARBs | OAH; Nonexposure |
| Li et al 45 | 2011 | Retrospective cohort | All trimesters; First trimester; Second or third trimester | United States | 1995‐2008 | ACEIs | OAH; Nonexposure |
| Malm et al 46 | 2008 | Retrospective cohort | First trimester | Finland | 1996‐2001 | ACEIs | OAH; Nonexposure |
| Moretti et al 47 | 2012 | Prospective cohort | First trimester | Canada | n/a | ACEIs/ARBs | OAH; Nonexposure |
| Piper et al 48 | 1992 | Retrospective cohort | All trimesters | United States | 1983‐1988 | ACEIs | n/a |
| Porta et al 49 | 2011 | Randomized‐control 2 | First trimester | Italy, USA, UK, Denmark, Sweden | 2001‐2008 | Candesartan | Nonexposure |
| Vasilakis‐Scaramozza et al 50 | 2013 | Retrospective cohort | First trimester | United Kingdom | 1991‐2002 | ACEIs | OAH; Nonexposure |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; n.a., not available; OAH, other antihypertensive medications.
No longer than gestational week 20.
Data derived from three randomized, placebo‐controlled trials (ie, DIRECT‐Prevent 1, DIRECT‐Protect 1, and DIRECT‐Protect 2).
Meta‐analysis of 17 included studies found a significant association between overall congenital malformations and prenatal exposure to ACEIs/ARBs (OR = 2.16, 95% CI = 1.72‐2.71, P < .0001, calculated RR = 2.06; Table 2). A cumulative meta‐analysis demonstrated that the addition of subsequent studies had little effect on the OR, but simply narrowed the 95% CI (Figure S1). The significant relationship still existed when analysis was limited to studies with the first trimester‐only exposure (OR = 1.94, 95% CI = 1.71‐2.21, P < .0001, calculated RR = 1.91; Figure 2). The cumulative meta‐analysis displaying results accumulated over successive studies is shown in Figure S2. Cardiovascular system (CVS), central nervous system (CNS), and urogenital malformations were found to be associated with ACEI/ARB exposure during pregnancy (OR = 2.96, 95% CI = 2.57‐3.39, P < .0001, calculated RR = 2.87; OR = 2.02, 95% CI = 1.08‐3.78, P = .03, calculated RR = 2.01; OR = 4.57, 95% CI = 2.11‐9.89, P = .0001, calculated RR = 4.35, respectively). The significant association between ACEI/ARB exposure and CVS malformations was still present when analysis was limited to studies with the first trimester‐only exposure (OR = 3.02, 95% CI = 2.60‐3.51, P < .0001, calculated RR = 2.92; Figure 3).
TABLE 2.
Adverse pregnancy outcomes following ACEI/ARB exposure compared with control
| Outcomes | Studies included | Exposure | Heterogeneity | Effect measure | ||||
|---|---|---|---|---|---|---|---|---|
| ACEIs/ARBs | Control | χ 2 | I 2 | OR | 95% CI | p value | ||
| Exposure in any trimesters | ||||||||
| Congenital malformations | ||||||||
| Overall | 17 | 538/6935 | 166295/3804799 | 0.0002 | 64% | 2.16 | (1.72, 2.71) | <.00001 |
| CVS | 9 | 244/5828 | 56389/3372581 | 0.71 | 0% | 2.96 | (2.57, 3.39) | <.0001 |
| CNS | 3 | 22/5014 | 5475/1800439 | 0.14 | 49% | 2.02 | (1.08, 3.78) | .03 |
| Urogenital | 2 | 7/141 | 1352/96903 | 0.81 | 0% | 4.57 | (2.11, 9.89) | .0001 |
| LBW | 3 | 101/639 | 27499/475076 | 0.001 | 85% | 2.30 | (1.20, 4.41) | .0004 |
| Miscarriage | 6 | 149/1180 | 254/3070 | 0.39 | 4% | 1.63 | (1.30, 2.05) | <.0001 |
| ETOP | 6 | 118/1180 | 145/3070 | 0.003 | 73% | 2.54 | (1.41, 4.59) | .02 |
| Stillbirth | 8 | 15/1474 | 24/4690 | 0.42 | 0% | 2.36 | (1.17, 4.76) | .02 |
| Preterm delivery | 9 | 321/1478 | 39071/478072 | <0.00001 | 95% | 1.69 | (1.04, 2.76) | <.00001 |
| Exposure in the first trimester only | ||||||||
| Congenital malformations | ||||||||
| Overall | 14 | 400/6071 | 107994/3252689 | 0.41 | 4% | 1.94 | (1.71, 2.21) | <.00001 |
| CVS | 7 | 213/4992 | 49733/2882376 | 0.72 | 0% | 3.02 | (2.60, 3.51) | <.0001 |
| CNS | 3 | 16/4684 | 5250/1785430 | 0.08 | 61% | 1.88 | (0.73, 4.83) | .19 |
| Urogenital | 1 | 1/46 | 6/977 | — | — | 3.60 | (0.42, 30.51) | .24 |
| LBW | 1 | 21/140 | 46/316 | — | — | 1.04 | (0.59, 1.81) | .90 |
| Miscarriage | 6 | 149/1180 | 254/3070 | 0.39 | 4% | 1.63 | (1.30, 2.05) | <.0001 |
| ETOP | 6 | 118/1180 | 145/3070 | 0.003 | 73% | 2.54 | (1.41, 4.59) | .02 |
| Stillbirth | 8 | 15/1474 | 24/4690 | 0.42 | 0% | 2.36 | (1.17, 4.76) | .02 |
| Preterm delivery | 7 | 200/979 | 394/3312 | 0.0008 | 74% | 1.26 | (0.84, 1.91) | .26 |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CI, confidence interval; CNS, central nervous system; CVS, cardiovascular system; ETOP, elective termination of pregnancy; LBW, low birth weight; OR, odds ratio.
FIGURE 2.
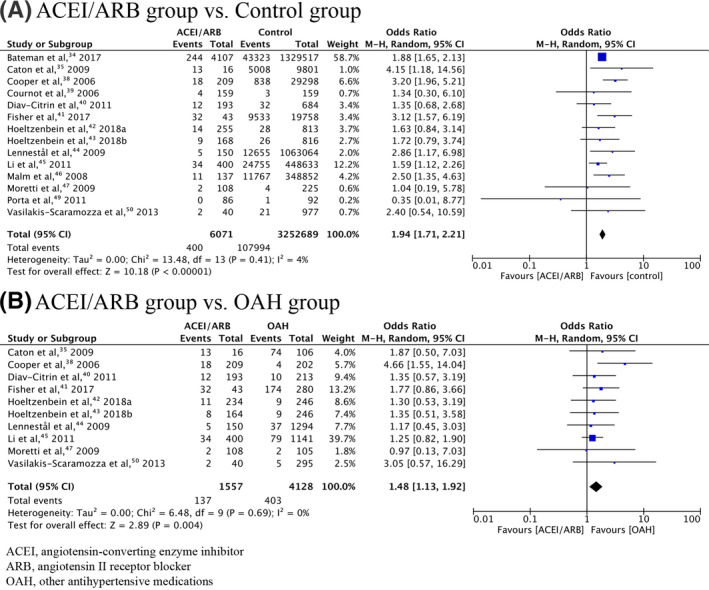
Forrest plot of overall congenital malformations in first trimester‐only exposure to ACEI/ARB
FIGURE 3.
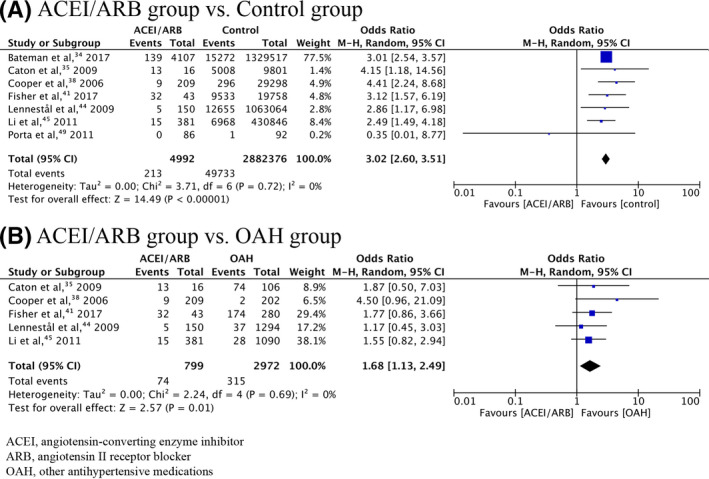
Forrest plot of CVS malformations in first trimester‐only exposure to ACEI/ARB compared with control and OAH
Other outcome measures that enabled analysis included LBW, miscarriage, ETOP, stillbirth, and preterm delivery, all of which were significantly associated with prenatal exposure to ACEIs/ARBs (Table 2). Miscarriage, ETOP, and stillbirth were also significantly related to ACEI/ARB exposure in the only first trimester of pregnancy (OR = 1.63, 95% CI = 1.30‐2.05, P < .0001, calculated RR = 1.55; OR = 2.54, 95% CI = 1.41‐4.59, P = .02, calculated RR = 2.37; OR = 2.36, 95% CI = 1.17‐4.76, P = .02, calculated RR = 2.34, respectively).
When comparing exposure to ACEIs/ARBs to nonexposure, the significant results were more or less similar to what was observed in the overall findings (Table S3). When comparing ACEI/ARB exposure to OAH exposure, the significant associations for most outcomes of interest were still existent when the analysis was limited to studies with the first trimester‐only exposure (Table S4).
Funnel plot asymmetries, indicative of the evidence of small‐study effects, were observed in the meta‐analyses of all the outcomes of interest, except for stillbirth (Figure S3). The formal tests suggested no significant asymmetry of the funnel plot for the effect estimate of overall congenital malformations (Rank correlation test, Kendall's Tau = −0.176, P = .349; Linear regression test, Z = −1.302, P = .193). When sensitivity analyses were applied, little changes on effect estimates were observed across all the outcomes of interest, indicative of robustness in the overall findings (Table S5). Prenatal exposure to ACEIs, but not ARBs, was found to be significantly associated with overall congenital malformations, LBW, miscarriage, ETOP, and preterm delivery.
4. DISCUSSION
To the best of our knowledge, this systematic review and meta‐analysis includes the largest dataset in the literature for the purpose of examining the associations between prenatal exposure to ACEIs/ARBs and adverse pregnancy outcomes, including both adverse maternal outcomes and neonatal birth defects. The first trimester‐only exposure to ACEIs/ARBs, previously presumably thought to be safe, 22 was found to be significantly associated with adverse pregnancy outcomes, including overall and CVS congenital malformations. The overall results of this study may raise concerns about the potential dangers of ACEI/ARB use during early pregnancy.
The adverse pregnancy outcomes that occur following in utero exposure to ACEIs/ARBs may result either directly from the drugs or from underlying maternal illnesses. When the ACEI/ARB group was compared to the OAH group, the effect size was smaller than when it was compared to nonexposure. It is also possible that ACEIs/ARBs may be prescribed more often than other antihypertensive drug classes in hypertensive patients with diabetes because of their proven efficacy against the progression of diabetic nephropathy. 51 , 52 A hypertensive or diabetic disorder in pregnancy may itself be associated with adverse pregnancy outcomes without drug specificity and, thus, may act as a confounder in some observational studies included in our analysis. 53 , 54 , 55 Moreover, patients with such underlying conditions tend to be older and may exhibit other comorbidities, including obesity, which may also be related to an elevated risk of adverse pregnancy outcomes. 56 , 57 Therefore, it should be kept in mind that there was a likelihood of the present meta‐analyses being confounded by some of these factors, for which some included studies might not adequately control.
Assumed the observed adverse pregnancy outcomes ascribed mainly to the drugs, the increased teratogenic risk could be conceivably attributed to inhibition of RAAS, a system that plays a key role in the embryogenic and fetal development of several organs/systems. 9 , 58 , 59 , 60 Not only does fetal RAAS blockade syndrome occur following ACEI/ARB exposure during the second and third trimesters of pregnancy it also may occur in those who are exposed to ACEIs/ARBs at the beginning of pregnancy. 15 , 61 Although there are unknown biologic mechanisms underlying adverse birth outcomes, inhibition of angiogenesis has been postulated to be a possible mechanism for the CVS malformations. 62 Given limited knowledge on how ACEIs/ARBs might interfere with embryonic development during the critical period for organogenesis, further research is warranted to gain a better understanding of underlying mechanisms whereby the drugs might result in adverse pregnancy outcomes. Moreover, differential effects of ACEI/ARB exposure in the first trimester as compared to the second and third trimesters need further investigations.
Although it remains uncertain whether the elevated risk of adverse pregnancy outcomes observed in our analysis is specific to ACEIs/ARBs or related to maternal underlying conditions, this systematic review and meta‐analysis largely supports the current recommendations stating that women of reproductive age should be treated with ACEIs/ARBs only if absolutely indicated. 17 Our findings may raise concerns about the potentially deleterious effects of prenatal exposure to ACEIs/ARBs during the first trimester of pregnancy. Given that numerous pregnancies are unplanned, there are formidable practical difficulties in avoiding first‐trimester ACEI/ARB exposure if the drugs are customarily used in female patients of reproductive age. 63 , 64 Clinical practitioners should treat those with the potential to become pregnant with the least teratogenic drug available. 25 , 65 Women of reproductive age whose condition is best treated with ACEIs/ARBs should be advised about the potential teratogenic risks of these drugs if they become pregnant. Effective contraception must be assured. However, if female patients inadvertently become pregnant while taking ACEIs/ARBs, clinical practitioners should instruct them to abruptly stop taking the drugs and offer alternatives. 66 The only one randomized‐controlled trial included in our analysis suggested no significant association of adverse pregnancy outcomes with drug exposure when the patients discontinued an ARB within an estimated 8 weeks from the last menstrual period. 51 It is reasonable to postulate that very short‐term cumulative exposure to ACEIs/ARBs during early pregnancy would be associated with better pregnancy outcomes; however, further investigation is required.
The results of this study should be interpreted with caution. First, asymmetric funnel plots, indicative of the evidence of small‐study effects (eg, publication bias), were observed in the meta‐analyses of most outcomes of interest. The formal tests for funnel plot asymmetry (either the Begg's rank correlation test or the Egger's linear regression test) are prone to type II errors (or false negative) in small meta‐analyses and, thus, the possibility of small‐study effects or publication bias cannot be ruled out. 67 Although search terms being used were broad without being limited to specific study designs, it is conceivable that our analysis might have missed some pertinent studies which are, for example, only available in other databases (eg, Embase) or even be unpublished. 68 , 69 Positive studies reporting a teratogenic effect of the drugs may be more likely to be published than studies with null results. 70 However, sensitivity analyses demonstrated no or little change on effect estimates, indicating the robustness of the results. Selective publications of studies may be of less concern to the validity of the present systematic review and meta‐analysis. 71 , 72 Second, it has been widely acknowledged in the literature that several observational studies on pregnancy outcome after drug exposure during early pregnancy often ignore left truncation and competing risks, leading to biased crude rates of miscarriage. 73 Moreover, ETOP rates may reflect patients’ anxiety, including misunderstanding of drug risk, rather than the toxic effects of a drug. As a result, the meta‐analysis might misestimate the effects of prenatal exposure to ACEIs/ARBs, particularly when exposure is limited to the first trimester of pregnancy, on some outcomes of interest. Pharmacovigilance with regard to the exposure of newly pregnant women to their current medications will further provide more evidence on the association between ACEI/ARB use during the early stage of pregnancy and adverse pregnancy outcomes.
In conclusion, this comprehensive and quantitative analysis of the evidence available to date suggests an increased risk of adverse pregnancy outcomes, including congenital malformations, with prenatal exposure to ACEIs/ARBs, regardless of the trimester of pregnancy. Prescription of ACEIs/ARBs in women with the potential to become pregnant should be discouraged provided that there are alternative drugs with a more favorable risk/benefit profile to treat a condition. Large observational studies that are properly designed to adequately account for the role of confounders are necessary to confirm the results of this study. Further investigations are required to reveal possible pathogenic pathways leading to adverse pregnancy outcomes, particularly congenital birth defects, if confirmed, in those with first‐trimester exposure to ACEIs/ARBs.
ETHICAL APPROVAL STATEMENT
This study is exempt from ethical review and received the certificate of exemption from the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University.
PATIENT CONSENT STATEMENT
Not applicable.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS' CONTRIBUTIONS
All authors contributed to the study conception and design. Data collection and analysis were performed by NB and NK. The first draft of the manuscript was written by NB and the manuscript was finalized by NK. All authors read and approved the final manuscript.
Supporting information
Figure S1
Figure S2
Figure S3
Table S1
Table S2
Table S3
Table S4
Table S5
ACKNOWLEDGMENTS
This study was partially supported by Chiang Mai University.
Buawangpong N, Teekachunhatean S, Koonrungsesomboon N. Adverse pregnancy outcomes associated with first-trimester exposure to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers: A systematic review and meta-analysis. Pharmacol Res Perspect. 2020;e00644 10.1002/prp2.644
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
DATA AVAILABILITY STATEMENT
All data used to support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin‐converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol. 2018;71(13):1474‐1482. [DOI] [PubMed] [Google Scholar]
- 2. Mirabito Colafella KM, Bovee DM, Danser AHJ. The renin‐angiotensin‐aldosterone system and its therapeutic targets. Exp Eye Res. 2019;186:107680. [DOI] [PubMed] [Google Scholar]
- 3. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119‐177. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129‐2200. [DOI] [PubMed] [Google Scholar]
- 5. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 6. Bian B, Kelton CM, Guo JJ, Wigle PR. ACE Inhibitor and ARB utilization and expenditures in the Medicaid fee‐for‐service program from 1991 to 2008. J Manag Care Pharm. 2010;16(9):671‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahmoudpour SH, Asselbergs FW, Souverein PC, de Boer A, Maitland‐van der Zee A. H. Prescription patterns of angiotensin‐converting enzyme inhibitors for various indications: a UK population‐based study. Br J Clin Pharmacol. 2018;84(10):2365‐2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tabacova S, Little R, Tsong Y, Vega A, Kimmel CA. Adverse pregnancy outcomes associated with maternal enalapril antihypertensive treatment. Pharmacoepidemiol Drug Saf. 2003;12(8):633‐646. [DOI] [PubMed] [Google Scholar]
- 9. Chevalier RL. Mechanisms of fetal and neonatal renal impairment by pharmacologic inhibition of angiotensin. Curr Med Chem. 2012;19(27):4572‐4580. [DOI] [PubMed] [Google Scholar]
- 10. Nadeem S, Hashmat S, Defreitas MJ, et al. Renin angiotensin system blocker fetopathy: a midwest pediatric nephrology consortium report. J Pediatr. 2015;167(4):881‐885. [DOI] [PubMed] [Google Scholar]
- 11. Dusetzina SB, Higashi AS, Dorsey ER, et al. Impact of FDA drug risk communications on health care utilization and health behaviors: a systematic review. Med Care. 2012;50(6):466‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hypertension in pregnancy . Report of the american college of obstetricians and gynecologists' task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122‐1131. [DOI] [PubMed] [Google Scholar]
- 13. Alwan S, Polifka JE, Friedman JM. Angiotensin II receptor antagonist treatment during pregnancy. Birth Defects Res A Clin Mol Teratol. 2005;73(2):123‐130. [DOI] [PubMed] [Google Scholar]
- 14. Polifka JE. Is there an embryopathy associated with first‐trimester exposure to angiotensin‐converting enzyme inhibitors and angiotensin receptor antagonists? A critical review of the evidence. Birth Defects Res A Clin Mol Teratol. 2012;94(8):576‐598. [DOI] [PubMed] [Google Scholar]
- 15. Lallemant M, Prévost S, Nobili F, et al. Prenatal hypocalvaria after prolonged intrauterine exposure to angiotensin II receptor antagonists. J Renin Angiotensin Aldosterone Syst. Oct‐Dec. 2018;19(4):1470320318810940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowen ME, Ray WA, Arbogast PG, Ding H, Cooper WO. Increasing exposure to angiotensin‐converting enzyme inhibitors in pregnancy. Am J Obstet Gynecol. 2008;198(3):291.e1‐291.e5. [DOI] [PubMed] [Google Scholar]
- 17. Bullo M, Tschumi S, Bucher BS, Bianchetti MG, Simonetti GD. Pregnancy outcome following exposure to angiotensin‐converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension. 2012;60(2):444‐450. [DOI] [PubMed] [Google Scholar]
- 18. Postmarketing surveillance for angiotensin‐converting enzyme inhibitor use during the first trimester of pregnancy–United States, Canada, and Israel, 1987–1995. MMWR Morb Mortal Wkly Rep. 1997;46(11):240‐242. [PubMed] [Google Scholar]
- 19. Gersak K, Cvijic M, Cerar LK. Angiotensin II receptor blockers in pregnancy: a report of five cases. Reprod Toxicol. 2009;28(1):109‐112. [DOI] [PubMed] [Google Scholar]
- 20. Karthikeyan VJ, Ferner RE, Baghdadi S, Lane DA, Lip GY, Beevers DG. Are angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers safe in pregnancy: a report of ninety‐one pregnancies. J Hypertens. 2011;29(2):396‐399. [DOI] [PubMed] [Google Scholar]
- 21. Burrows RF, Burrows EA. Assessing the teratogenic potential of angiotensin‐converting enzyme inhibitors in pregnancy. Aust N Z J Obstet Gynaecol. 1998;38(3):306‐311. [DOI] [PubMed] [Google Scholar]
- 22. Walfisch A, Al‐maawali A, Moretti ME, Nickel C, Koren G. Teratogenicity of angiotensin converting enzyme inhibitors or receptor blockers. J Obstet Gynaecol. 2011;31(6):465‐472. [DOI] [PubMed] [Google Scholar]
- 23. Lastra G, Syed S, Kurukulasuriya LR, Manrique C, Sowers JR. Type 2 diabetes mellitus and hypertension: an update. Endocrinol Metab Clin North Am. 2014;43(1):103‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134(6):441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74(3):283‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dreyer NA, Velentgas P, Westrich K, Dubois R. The GRACE checklist for rating the quality of observational studies of comparative effectiveness: a tale of hope and caution. J Manag Care Spec Pharm. 2014;20(3):301‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019:366;l4898. [DOI] [PubMed] [Google Scholar]
- 29. Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046‐1055. [DOI] [PubMed] [Google Scholar]
- 30. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmed B, Tran DT, Zoega H, Kennedy SE, Jorm LR, Havard A. Maternal and perinatal outcomes associated with the use of renin‐angiotensin system (RAS) blockers for chronic hypertension in early pregnancy. Pregnancy Hypertens. 2018;2018(14):156‐161. [DOI] [PubMed] [Google Scholar]
- 33. Banhidy F, Acs N, Puho EH, Czeizel AE. Chronic hypertension with related drug treatment of pregnant women and congenital abnormalities in their offspring: a population‐based study. Hypertens Res. 2011;34(2):257‐263. [DOI] [PubMed] [Google Scholar]
- 34. Bateman BT, Patorno E, Desai RJ, et al. Angiotensin‐converting enzyme inhibitors and the risk of congenital malformations. Obstet Gynecol. 2017;129(1):174‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caton AR, Bell EM, Druschel CM, et al. Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension. 2009;54(1):63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chintamaneni S, Duan L, Hekimian A, Qattan M, Lee MS. Exposure to angiotensin‐converting enzyme inhibitors in pregnancy and the risk of low birth weight and congenital cardiac malformation. J Am Coll Cardiol. 2018;71(11 Suppl):A570. [Google Scholar]
- 37. Colvin L, Walters BNJ, Gill AW, et al. The use of angiotensin converting enzyme inhibitors during the first trimester of pregnancy. J Pharmacovigil. 2014;2(3).1000129. [Google Scholar]
- 38. Cooper WO, Hernandez‐Diaz S, Arbogast PG, et al. Major congenital malformations after first‐trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443‐2451. [DOI] [PubMed] [Google Scholar]
- 39. Cournot MP, Vial T, Carlier P, et al. Angiotensin‐converting enzyme (ACE) inhibitors during first trimester of pregnancy: a french prospective collaborative study. Drug Saf. 2006;29(10):911‐1010. [Google Scholar]
- 40. Diav‐Citrin O, Shechtman S, Halberstadt Y, et al. Pregnancy outcome after in utero exposure to angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Reprod Toxicol. 2011;31(4):540‐545. [DOI] [PubMed] [Google Scholar]
- 41. Fisher SC, Van Zutphen AR, Werler MM, et al. Maternal antihypertensive medication use and congenital heart defects: updated results from the national birth defects prevention study. Hypertension. 2017;69(5):798‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoeltzenbein M, Tissen‐Diabaté T, Fietz A‐K, et al. Increased rate of birth defects after first trimester use of angiotensin converting enzyme inhibitors‐treatment or hypertension related? An observational cohort study. Pregnancy Hypertens. 2018;13:65‐71. [DOI] [PubMed] [Google Scholar]
- 43. Hoeltzenbein M, Tissen‐Diabaté T, Fietz A‐K, et al. Pregnancy outcome after first trimester use of angiotensin AT1 receptor blockers: an observational cohort study. Clin Res Cardiol. 2018;107(8):679‐687. [DOI] [PubMed] [Google Scholar]
- 44. Lennestal R, Otterblad Olausson P, Kallen B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol. 2009;65(6):615‐625. [DOI] [PubMed] [Google Scholar]
- 45. Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. 2011;343(oct18 1):d5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malm H, Artama M, Gissler M, et al. First trimester use of ACE‐inhibitors and risk of major malformations. Reprod Toxicol. 2008;26(1):67. [Google Scholar]
- 47. Moretti ME, Caprara D, Drehuta I, et al. The fetal safety of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers. Obstet Gynecol Int. 2012;2012:658310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Piper JM, Ray WA, Rosa FW. Pregnancy outcome following exposure to angiotensin‐converting enzyme inhibitors. Obstet Gynecol. 1992;80(3 Pt 1):429‐432. [PubMed] [Google Scholar]
- 49. Porta M, Hainer JW, Jansson S‐O, et al. Exposure to candesartan during the first trimester of pregnancy in type 1 diabetes: experience from the placebo‐controlled DIabetic REtinopathy Candesartan Trials. Diabetologia. 2011;54(6):1298‐1303. [DOI] [PubMed] [Google Scholar]
- 50. Vasilakis‐Scaramozza C, Aschengrau A, Cabral HJ, Jick SS. Antihypertensive drugs and the risk of congenital anomalies. Pharmacotherapy. 2013;33(5):476‐482. [DOI] [PubMed] [Google Scholar]
- 51. Lv X, Zhang Y, Niu Y, Song Q, Zhao Q. Comparison of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on cardiovascular outcomes in hypertensive patients with type 2 diabetes mellitus: a PRISMA‐compliant systematic review and meta‐analysis. Medicine (Baltimore). 2018;97(15):e0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thomopoulos C, Parati G, Zanchetti A. Effects of blood‐pressure‐lowering treatment on outcome incidence in hypertension: 10‐Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta‐analyses of randomized trials. J Hypertens. 2017;35(5):922‐944. [DOI] [PubMed] [Google Scholar]
- 53. Bellizzi S, Ali MM, Abalos E, et al. Are hypertensive disorders in pregnancy associated with congenital malformations in offspring? Evidence from the WHO Multicountry cross sectional survey on maternal and newborn health. BMC Pregnancy Childbirth. 2016;16(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaplinski M, Taylor D, Mitchell LE, Hammond DA, Goldmuntz E, Agopian AJ. The association of elevated maternal genetic risk scores for hypertension, type 2 diabetes and obesity and having a child with a congenital heart defect. PLoS ONE. 2019;14(5):e0216477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nasri HZ, Houde Ng K, Westgate MN, Hunt AT, Holmes LB. Malformations among infants of mothers with insulin‐dependent diabetes: Is there a recognizable pattern of abnormalities? Birth Defects Res. 2018;110(2):108‐113. [DOI] [PubMed] [Google Scholar]
- 56. Correa A, Marcinkevage J. Prepregnancy obesity and the risk of birth defects: an update. Nutr Rev. 2013;71(Suppl 1):S68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Persson M, Cnattingius S, Villamor E, et al. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. BMJ. 2017;357:j2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. Expression of AT2 receptors in the developing rat fetus. J Clin Invest. 1991;88(3):921‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mao C, Shi L, Xu F, Zhang L, Xu Z. Development of fetal brain renin‐angiotensin system and hypertension programmed in fetal origins. Prog Neurogibol. 2009;87(4):252‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Price RL, Carver W, Simpson DG, et al. The effects of angiotensin II and specific angiotensin receptor blockers on embryonic cardiac development and looping patterns. Dev Biol. 1997;192(2):572‐584. [DOI] [PubMed] [Google Scholar]
- 61. Plazanet C, Arrondel C, Chavant F, Gubler MC. Fetal renin‐angiotensin‐system blockade syndrome: renal lesions. Pediatr Nephrol. 2014;29(7):1221‐1230. [DOI] [PubMed] [Google Scholar]
- 62. Khakoo AY, Sidman RL, Pasqualini R, Arap W. Does the renin‐angiotensin system participate in regulation of human vasculogenesis and angiogenesis? Cancer Res. 2008;68(22):9112‐9115. [DOI] [PubMed] [Google Scholar]
- 63. Wellings K, Jones KG, Mercer CH, et al. The prevalence of unplanned pregnancy and associated factors in Britain: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal‐3). Lancet (London, England). 2013;382(9907):1807‐1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ameyaw EK, Budu E, Sambah F, et al. Prevalence and determinants of unintended pregnancy in sub‐Saharan Africa: A multi‐country analysis of demographic and health surveys. PLoS ONE. 2019;14(8):e0220970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kernaghan D, Duncan AC, McKay GA. Hypertension in pregnancy: a review of therapeutic options. Obstet Med. 2012;5(2):44‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Webster K, Fishburn S, Maresh M, Findlay SC, Chappell LC. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ. 2019;366:l5119. [DOI] [PubMed] [Google Scholar]
- 67. Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. CMAJ. 2007;176(8):1091‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Golder S, Loke YK, Wright K, Norman G. Reporting of adverse events in published and unpublished studies of health care interventions: a systematic review. PLOS Med. 2016;13(9):e1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Page MJ, McKenzie JE, Kirkham J, et al. Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions. Cochrane Database Syst Rev. 2014;(10):Mr000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ziai H, Zhang R, Chan AW, Persaud N. Search for unpublished data by systematic reviewers: an audit. BMJ Open. 2017;7(10):e017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schmucker CM, Blümle A, Schell LK, et al. Systematic review finds that study data not published in full text articles have unclear impact on meta‐analyses results in medical research. PLoS ONE. 2017;12(4):e0176210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van Driel ML, De Sutter A, De Maeseneer J, Christiaens T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. J Clin Epidemiol. 2009;62(8):838‐844.e3. [DOI] [PubMed] [Google Scholar]
- 73. Meister R, Schaefer C. Statistical methods for estimating the probability of spontaneous abortion in observational studies – analyzing pregnancies exposed to coumarin derivatives. Reprod Toxicol. 2008;26(1):31–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Table S1
Table S2
Table S3
Table S4
Table S5
Data Availability Statement
All data used to support the findings of this study are available from the corresponding author upon reasonable request.


