Abstract
Objective:
To evaluate aortic disease progression and reintervention following an initial thoracic aortic dissection in pathogenic variant carriers.
Methods:
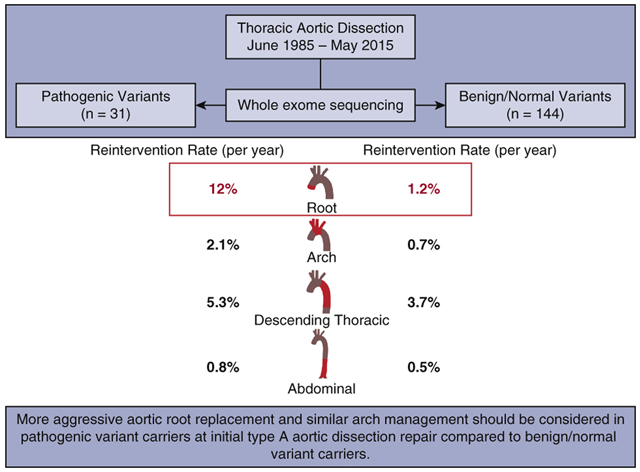
Of 175 participants diagnosed with thoracic aortic dissection, 31 had a pathogenic variant (pathogenic group) across 6 genes (COL3A1, FBN1, LOX, PRKG1, SMAD3, TGFBR2) identified by whole exome sequencing. Those with benign or normal variants (benign/normal group, n=144) comprised the control group. Clinical data was collected through medical record review (1985-2018) and supplemented with the National Death Index database (December 2018).
Results
The entire cohort (n=175) consisted of 108 type A aortic dissections (TAAD) and 67 type B aortic dissections, similarly distributed between groups. The pathogenic group was significantly younger (43- vs. 56-years-old, p<0.0001) and had significantly more aortic root replacements and similar extents of arch replacement at initial TAAD repair. The median follow-up time was 7.5 (4.6, 12) years. After initial treatment, the pathogenic group required significantly more aortic reinterventions (median 1 vs. 0, p<0.0001) and mean cumulative aortic reinterventions for each patient (10-year: 1 vs. 0.5, p=0.029). Both incidence rate (12%/year vs. 1.2%/year, p=0.0001) and cumulative incidence of reinterventions (9-year: 70% vs. 6%, p<0.0001) for the preserved native aortic root were significantly higher in the pathogenic group, but were similar for the preserved native aortic arch and distal aorta between groups. Ten-year survival was similar in the pathogenic and benign/normal groups (92% vs. 85%).
Conclusions:
Aggressive aortic root replacement and similar arch management should be considered in pathogenic variant carriers at initial TAAD repair compared to benign/normal variant carriers.
Graphical Abstract:
A summary of the key findings and their implications: Compared to the patients with benign and normal genetic variants, patients with pathogenic variants had a significantly higher rate of reinterventions only of the native aortic root after initial thoracic aortic dissection.
INTRODUCTION
Approximately 30% of patients presenting with a thoracic aortic aneurysm and dissection have an underlying genetic predisposition,1 which can be syndromic, such as Marfan syndrome (MFS) or Loeys-Dietz syndrome (LDS), or nonsyndromic, as with ACTA2, MYLK, and MYH11 mutations.2 To date, there is strong or definitive evidence for 11 genes associated with thoracic aortic aneurysm or dissection.3 Among patients with familial nonsyndromic thoracic aortic aneurysm and dissection, it is estimated that 30% have a pathogenic variant in one or more of these genes.1,4 The majority of causative variants have been identified in genes encoding proteins that function in the extracellular matrix, smooth muscle cell contraction and metabolism, and the transforming growth factor-β pathway, including FBN1, COL3A1, and LOX; ACTA2, MYH11, MYLK, and PRKG1; and TGFBR1, TGFBR2, TGFb2, and SMAD3, respectively.3
We recently performed research whole exome sequencing among patients with a history of thoracic aortic dissection and reported that pathogenic variants were identified in 10.8% of cases in 6 genes (COL3A1, FBN1, LOX, PRKG1, SMAD3, TGFBR2).5 There is a lack of evidence on how to manage patients with pathogenic variants at the time of the initial aortic event, such as an acute type A aortic dissection, and surveillance of the dissected aorta during follow-up. The objective was to evaluate aortic disease progression and subsequent reintervention following an initial thoracic aortic dissection in pathogenic compared to benign or normal variant carriers. We hypothesized that patients with pathogenic variants would require more late reinterventions after an initial thoracic aortic dissection.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board at Michigan Medicine and all subjects provided informed consent.
Study Population
In all patients who presented with a thoracic aortic dissection between 1985 and 2015, pathogenic variants were analyzed in 11 genes by whole exome sequencing5 and identified in 31 cases (pathogenic group) in 6 genes (COL3A1, FBN1, LOX, PRKG1, SMAD3, TGFBR2). The control group (n=144) was comprised of benign or normal variant carriers (benign/normal group). Patients with variants of unknown significance were excluded from this study. The final cohort consisted of 175 patients. The pathogenic group was further matched to patients with benign/normal variants (n=31) based on diagnosis (type A versus type B aortic dissection), extent of dissection, sex, and closest age. The results of the matched cohorts are reported as supplemental tables and figures.
Data Collection of Clinical Outcomes
Investigators systematically reviewed electronic medical records to verify demographics, genetic testing results, diagnoses, comorbidities, medications, operative characteristics, reintervention data, and imaging data. The National Death Index database through December 31, 20186 was utilized as well as electronic medical record review to obtain data on long-term survival.
Late reinterventions were defined as open and endovascular (TEVAR) aortic repair following initial aortic dissection for the expansion of the native aorta. At Michigan Medicine, we followed the AHA/ACC guidelines for the interventions of thoracic aortic aneurysms. If patients had a segment of the aorta replaced during the initial aortic event or prior, such as a total aortic root replacement, then those patients were not at risk of reintervention for the dilation of that specific segment of the aorta, such as an aortic root procedure for root aneurysm. Only patients with a native aorta were included in the analysis of incidence rate and cumulative incidence of reintervention for the different segments of the aorta, including the aortic root, arch, descending thoracic aorta, and abdominal aorta.
Statistical Analysis
Continuous variables are presented as median (25 percentile, 75 percentile) and categorical variables are reported as n (%) in frequency tables. Univariate comparisons between pathogenic carriers and benign/normal carriers were performed using chi-square tests or Fischer’s exact test for categorical data and Wilcoxon rank sum tests for continuous data. The incidence rate of reintervention was calculated based on the patients at risk who still had a significant portion of native aorta remaining after the initial dissection. As patients may experience death before reintervention was indicated, cumulative incidence curves adjusting for death as the competing risk were generated to assess cumulative incidence of first reintervention for different aortic segments. Cox proportional hazard regression was performed to calculate the hazard ratio (HR) for reoperation after initial aortic dissection by adjusting for group, age at dissection, arch procedure, type A aortic dissection, and hypertension. To account for all possible late reinterventions on any portion of the aorta, rather than only the first reintervention, a mean cumulative function curve was generated to describe the cumulative number of reinterventions in any of the aortic segments over time. Crude survival curves since initial aortic dissection were estimated using the non-parametric Kaplan-Meier method. Cox proportional hazard regression was performed to calculate the HR for mortality after initial aortic dissection by adjusting group, age at dissection, gender, type A aortic dissection, and hypertension. The growth rate of the aorta measured by imaging studies was calculated using mixed effect models, and the interaction between year and group is also tested in these models: reported in the supplemental material for the matched cohorts. All statistical calculations used SAS 9.4 (SAS Institute, Cary, NC) and were considered significant at p<0.05.
RESULTS
Pathogenic Variants
In the 31 patients with pathogenic variants, the variants were identified in FBN1 in 22 (71%) patients, and other genes in 9 (29%) patients, including PRKG1 (n=2), TGFBR2 (n=2), SMAD3 (n=3), COL3A1 (n=1), and LOX (n=1) (Supplemental Table 1).
Demographics
The median age for the entire cohort at the time of the initial aortic dissection was 55-years-old and 64% were male. There were 108 type A aortic dissections (TAAD) and 67 type B aortic dissections (TBAD) with the dissection types similarly represented between groups.
The pathogenic group was significantly younger at the time of the initial aortic dissection (43- vs. 56-years-old) and had less hypertension (26% vs. 70%) and dyslipidemia (10% vs. 33%) compared to the benign/normal group. There were eight patients undergoing prior aortic root replacement for an aortic root aneurysm [five (16%) pathogenic and three (2%) benign/normal variant carriers]; all eight patients had acute type B aortic dissections. All other comorbidities were similar between groups (Table 1).
Table 1.
Demographics
| Total (n=175) |
Pathogenic (n=31) |
Benign/Normal (n=144) |
p-value | |
|---|---|---|---|---|
| Age at dissection (years) | 55 (46, 65) | 43 (30, 51) | 56 (47, 66) | <0.0001 |
| Sex, male | 112 (64) | 14 (45) | 98 (68) | 0.02 |
| Body mass index (kg/m2) | 29 (25, 33) | 27 (24, 30) | 29 (25, 34) | 0.09 |
| Hypertension | 109 (62) | 8 (26) | 101 (70) | <0.0001 |
| Dyslipidemia | 50 (29) | 3 (10) | 47 (33) | 0.01 |
| Coronary artery disease | 16 (9) | 2 (7) | 14 (10) | 0.74 |
| Smoking history | 79 (45) | 10 (32) | 69 (48) | 0.11 |
| COPD | 9 (5) | 0 (0) | 9 (6) | 0.36 |
| Diabetes | 6 (3) | 0 (0) | 6 (4) | 0.59 |
| History of stroke | 2 (1) | 0 (0) | 2 (1) | 1.00 |
| Chronic kidney disease | 6 (3) | 0 (0) | 6 (4) | 0.59 |
| Peripheral arterial disease | 2 (1) | 0 (0) | 2 (1) | 1.00 |
| Prior cardiac surgery | 16 (9) | 6 (19) | 10 (7) | 0.04 |
| Root replacement | 8 (5) | 5 (16) | 3 (2) | 0.005 |
| Aortic aneurysm | ||||
| Root | 61 (35) | 17 (55) | 44 (31) | 0.01 |
| Ascending | 78 (45) | 14 (45) | 64 (44) | 0.94 |
| Arch | 32 (18) | 4 (13) | 28 (19) | 0.39 |
| Descending | 54 (31) | 7 (23) | 47 (33) | 0.27 |
| Aortic dissection | ||||
| Type A | 108 (62) | 21 (68) | 87 (60) | 0.45 |
| Type B | 67 (38) | 10 (32) | 57 (40) | 0.45 |
| Condition at time of dissection | ||||
| Acute stroke | 4 (2) | 2 (7) | 2 (1) | 0.15 |
| Acute myocardia infarction | 2 (1) | 1 (3) | 1 (1) | 0.32 |
| Acute renal failure | 6 (3) | 1 (3) | 5 (4) | 1.00 |
| Acute paralysis | 0 (0) | 0 (0) | 0 (0) | - |
| Medications at time of dissection | ||||
| Beta blocker | 46 (26) | 6 (19) | 40 (28) | 0.33 |
| ACE inhibitor | 23 (13) | 3 (10) | 20 (14) | 0.77 |
| Calcium channel blocker | 15 (9) | 0 (0) | 15 (10) | 0.08 |
| Angiotensin II receptor blocker | 6 (3) | 0 (0) | 6 (4) | 0.59 |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data.
ACE = angiotensin converting enzyme; COPD=chronic obstructive pulmonary disease
Initial Management of Thoracic Aortic Dissection
For the entire cohort, 68% (n=119) of patients underwent aortic repair (open or TEVAR) at the time of initial aortic dissection. Ninety-eight percent of all acute (n=105) and chronic TAADs (n=3) were managed with open aortic repair. Two patients with a TAAD (acute, n=1 and chronic, n=1) were medically managed. Sixty-eight percent of initial TAAD repairs were performed at the University of Michigan (from 1993 to 2015) while the remaining repairs (32%) were performed at an outside hospital (from 1985 to 2014). Ten TBAD patients underwent TEVAR for impending rupture, three had proximal descending replacement via thoracotomy, and nine had endovascular fenestration/stenting for malperfusion. Sixty-seven percent (45/67) of TBADs were only medically managed. (Table 2).
Table 2.
Extent of Aortic Replacement at Time of Aortic Dissection
| Total (n=175) |
Pathogenic (n=31) |
Benign/Normal (n=144) |
p-value | |
|---|---|---|---|---|
| Type A (n=108) | 108 | 21 | 87 | |
| Root | 0.002 | |||
| Root preservation | 63 (58) | 6 (29) | 57 (65) | |
| Root replacement | 45 (42) | 15 (71) | 30 (35) | |
| Ascending replacement | 106 (98) | 21 (100) | 85 (98) | 1.00 |
| Arch replacement | 0.12 | |||
| None/Hemiarch | 78 (72) | 18 (86) | 60 (69) | |
| Zone 1/2/3 | 30 (28) | 3 (14) | 27 (31) | |
| Frozen elephant trunk | 5 (5) | 1 (5) | 4 (5) | 1.00 |
| Institution of procedures* | 0.006 | |||
| Michigan Medicine | 72 (68) | 9 (43) | 63 (74) | |
| Outside hospital | 34 (32) | 12 (57) | 22 (26) | |
| Type B (n=67) | 67 | 10 | 57 | |
| Proximal descending replacement | 3 (5) | 1 (10) | 2 (4) | 0.39 |
| TEVAR | 10 (15) | 1 (10) | 9 (16) | 1.00 |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data.
TEVAR = thoracic endovascular aortic repair.
Two patients were medically managed were not included.
Among patients undergoing open TAAD repair, the pathogenic group had significantly more aortic root replacement (71% vs. 35%), but similar extents of arch replacement (hemiarch, zone 1-3 arch replacement with replacement of 1-4 arch branch vessels) and stent graft placement in the descending thoracic aorta (frozen elephant trunk) as the benign/normal group (Table 2). For the entire cohort, after the initial aortic dissection and surgical repair including previous aortic repairs, 70% of patients (total, n=122; pathogenic variant carrier, n=11and benign/normal variant carrier, n=111) had native aortic roots that were potentially at risk of reintervention for aortic root aneurysm. Eighty-two percent of patients (total, n=143; pathogenic, n=28 and benign/normal, n=115) had hemiarch or no arch replacement, and therefore had a partial or whole native aortic arch that was potentially at risk for reintervention due to an aortic arch aneurysm. One-hundred percent (175/175) of patients had native descending thoracic and abdominal aortas that were potentially at risk for thoracic aneurysm, thoraco-abdominal aortic aneurysm, and abdominal aortic aneurysm.
Long-term outcomes
The median follow-up time for the entire cohort was 7.5 (4.6, 12) years (range: 0.2-33.6 years) with a total of 1613 patient-years. In the pathogenic group, the median follow-up time was 9.5 (5.1, 19.8) years (range: 0.9-33.6 years) while the median follow-up time for the benign/normal group was 7.2 (4.5, 11.6) years (range: 0.2-29.7 years). In the survival analysis (study end, January 2018), 100% of patients had follow-up.
Late reinterventions
Overall, 68 (39%) patients required a late aortic reintervention following TAAD (n=45) repair and TBAD (n=23). The pathogenic group was significantly more likely to require more reinterventions per patient (1 [0,2] vs. 0 [0,1]), including one reintervention (71% vs. 32%) as well as multiple reinterventions (42% vs. 10%) compared to the benign/normal group. The pathogenic group required the first late aortic reintervention a median of 6.5 (2.0, 10.3) years following the initial aortic dissection while the benign/normal group required the first late aortic reintervention a median of 2.1 (0.5, 7.7) years following the initial aortic dissection (Table 3).
Table 3.
Late Reinterventions for Aortic Expansion After Aortic Event
| Total (n=175) |
Pathogenic (n=31) |
Benign/Normal (n=144) |
p-value | |
|---|---|---|---|---|
| Reinterventions per patient * | 0 (0, 1) | 1 (0, 2) | 0 (0, 1) | <0.0001 |
| One Reintervention (n (%)) | 68 (39) | 22 (71) | 46 (32) | 0.0001 |
| Two Reinterventions (n (%)) | 28 (16) | 13 (42) | 15 (10) | 0.0001 |
| Three Reinterventions (n (%)) | 5 (3) | 4 (13) | 1 (1) | 0.004 |
Reinterventions per patient was expressed as median (25% percentile, 75% percentile)
The incidence rate of aortic root reintervention for native root aneurysm was increased 10-fold in the pathogenic group compared to the benign/normal group (12%/year vs. 1.2%/year, p=0.0001) (Table 4). On the contrary, the incidence rate of reintervention of the aortic arch, descending thoracic aorta, and abdominal aorta for native aortic aneurysm was not significantly different between groups (Table 4).
Table 4.
Late Reinterventions for Aortic Expansion of Different Aortic Segments After Initial Aortic Dissection
| Pathogenic | Benign/Normal | ||||
|---|---|---|---|---|---|
| At Risk # (total pt years) |
Reintervention Rate # (%/year) |
At Risk # (total pt years) |
Reintervention Rate # (%/year) |
p-value | |
| Root | 11 (59) | 7 (12) | 111 (847) | 10 (1.2) | 0.0001 |
| Arch | 28 (334) | 7 (2.1) | 115 (905) | 6 (0.66) | 0.07 |
| Descending | 31 (302) | 16 (5.3) | 144 (982) | 36 (3.7) | 0.29 |
| Abdominal | 31 (357) | 3 (0.8) | 144 (1185) | 6 (0.5) | 0.75 |
The reintervention rate was calculated by using only first reintervention for each segment of the aorta for each patient. Root reintervention includes sternotomy aortic root replacement. Arch reintervention includes sternotomy arch replacement. Descending reintervention includes open thoracotomy or thoracoabdominal incision and TEVAR for thoracic or thoracoabdominal aneurysm repair. Abdominal reintervention includes isolated abdominal aortic aneurysm repair.
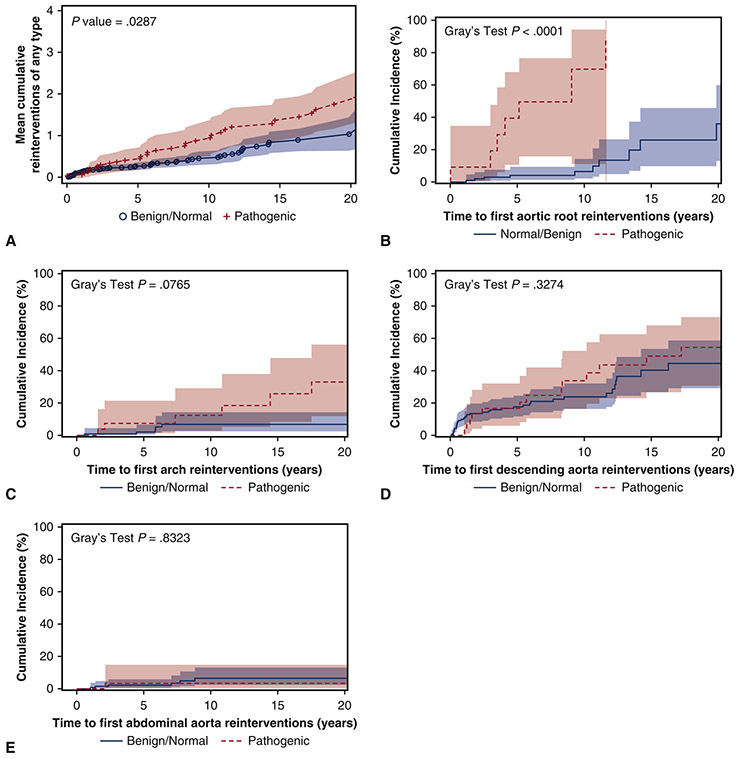
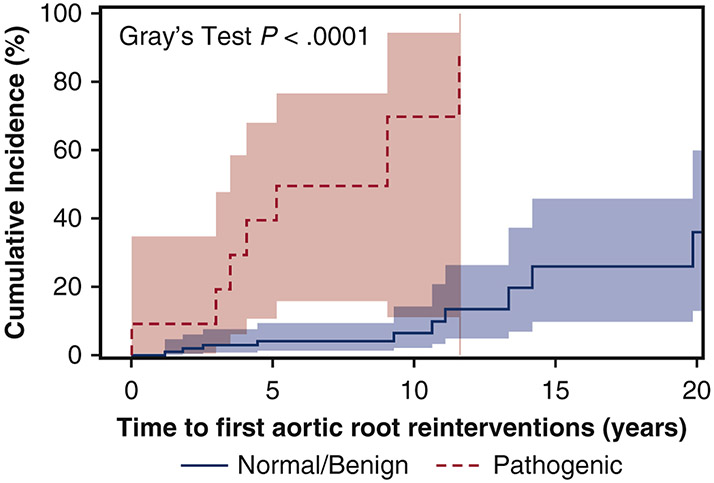
After initial aortic dissection, the mean cumulative reinterventions for each patient (including all aortic reinterventions) in the pathogenic group was significantly higher than the reintervention rate in the benign/normal group. (Figure 1A) At 10 years, the pathogenic group had an average of one reintervention/patient of any aortic reintervention, compared to 0.5 reintervention/patient in the benign/normal variant group. The cumulative incidence of first aortic root reintervention for aortic root aneurysm was significantly greater in the pathogenic group than the benign/normal variant group (9-years: 70% (11%, 94%) vs. 6% (2%, 14%), p<0.0001), but not for the aortic arch, descending thoracic aorta, or abdominal aorta (Figure 1 B-E). Multivariable Cox model analysis also confirmed that being a pathogenic variant carrier was a significant risk factor for reoperation of the native aortic root after the initial event of a thoracic aortic dissection (HR=13 [95% CI: 3.4, 51], p=0.0002), but not for reoperation of the native arch, descending thoracic aorta, or abdominal aorta. (Table 5) Hypertension was a significant risk factor for reoperation of the native arch (HR=5.8). (Table 5)
Figure 1.
A) The mean cumulative reinterventions per patient after thoracic aortic dissection was significantly higher in the pathogenic group than the benign/normal group. All aortic reinterventions were used to calculate the mean cumulative incidence. The cumulative incidence (CI) of first aortic root reintervention (B) was significantly higher in the pathogenic group (9-year CI: 70% vs. 6%). However, the cumulative incidence of first aortic arch reintervention (C), first descending thoracic aorta reintervention (D), and first abdominal aortic reintervention (E) were not significantly different between groups. Death was used as a competing factor.
Table 5.
Risk factors for reoperation across all segments of the aorta and late mortality (multivariable Cox proportional hazard regression)
| HR (95% CI) | p-value | |
|---|---|---|
| Root reoperation (among native root patients) | ||
| Pathogenic | 13.1 (3.4, 51.1) | 0.0002 |
| Age | 1.0 (1.0, 1.1) | 0.62 |
| Type A dissection | 1.3 (0.4, 4.2) | 0.62 |
| Hypertension | 0.5 (0.2, 1.7) | 0.28 |
| Arch reoperation (among native arch patients) | ||
| Pathogenic | 3.8 (0.7, 21.3) | 0.13 |
| Age | 1.0 (0.9, 1.0) | 0.64 |
| Type A dissection | 6.1 (0.7, 51.7) | 0.10 |
| Hypertension | 5.8 (1.5, 22.7) | 0.01 |
| Descending reoperation (stratified by type A) | ||
| Pathogenic | 1.7 (0.8, 3.8) | 0.17 |
| Age | 1.01 (0.98, 1.03) | 0.66 |
| Aggressive arch replacement | 1.3 (0.6, 3.0) | 0.55 |
| Hypertension | 1.4 (0.7, 2.7) | 0.38 |
| Abdominal reoperation (stratified by arch procedure) | ||
| Pathogenic | 0.8 (0.1, 9.4) | 0.85 |
| Age | 1.0 (0.9, 1.1) | 0.92 |
| Type A dissection | 1.0 (0.2, 4.8) | 0.97 |
| Hypertension | 1.6 (0.2, 10.4) | 0.64 |
| Late mortality | ||
| Pathogenic | 1.8 (0.4, 7.0) | 0.42 |
| Female sex | 3.4 (1.4, 8.3) | 0.008 |
| Age | 1.1 (1.0, 1.1) | <0.0001 |
| Hypertension | 1.3 (0.5, 3.5) | 0.65 |
| Type A dissection | 1.4 (0.5, 3.8) | 0.46 |
CI=confidence interval; HR=hazard ratio
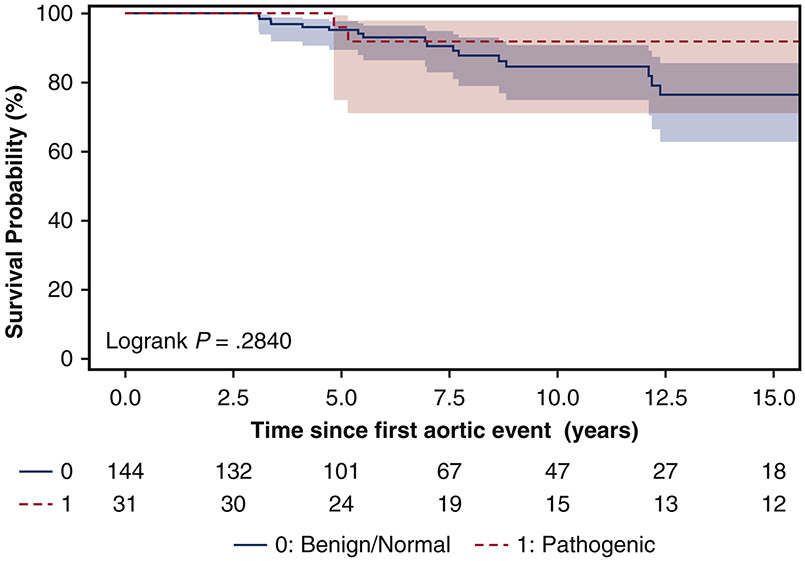
Survival
The 10-year survival was similar between the pathogenic (92% [95% CI: 71%, 98%]) and benign/normal groups (85% [95% CI: 75%, 91%], p=0.28) (Figure 2). Significant risk factors for late mortality after the initial aortic dissection were female sex (HR=3.4, 95% CI: [1.4, 8.3], p=0.008) and age at time of aortic dissection (HR=1.1, [95% CI: 1.0, 1.1], p<0.0001); while pathogenic variant carrier status was not a significant risk factor for late mortality, although the hazard ratio was 1.8 [95% CI: 0.4, 7.0]. (Table 5)
Figure 2.
Kaplan-Meier survival analysis for pathogenic and benign/normal variants carriers following a thoracic aortic dissection. The 10-year survival was similar between pathogenic (92% [95% CI: 71%, 98%]) and benign/normal groups 85% [95% CI: 75%, 91%].
Matched Cohort
The short- and long-term outcomes in the matched cohort (n=62) were consistent with the results of the entire cohort described above (Supplemental Tables 2-5, Supplemental Figures 1-3).
DISCUSSION
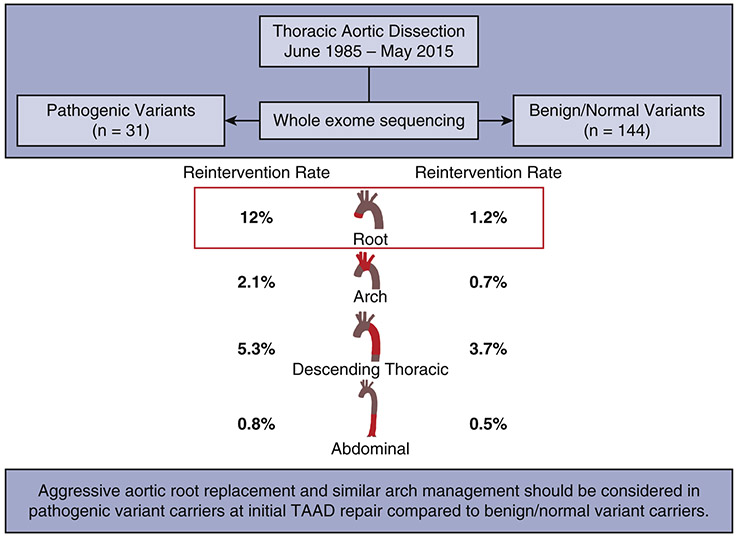
In this study, patients with a history of thoracic aortic dissection were grouped based on pathogenic variant carrier status identified from whole exome sequencing. With a clearly identified genotype in patients, pathogenic variants versus benign/normal variants, we found that the pathogenic group had significantly more reinterventions for aortic aneurysms of the native aorta after an initial aortic dissection compared to the benign/normal group. Specifically, the incidence rate of reinterventions (12%/year vs. 1.2%/year) and cumulative incidence of reinterventions for late native aortic root aneurysm was significantly higher in the pathogenic group than the benign/normal group with a HR of 13 in the Cox model; however, the incidence rates and cumulative incidence of late reinterventions of the native aortic arch, descending thoracic aorta, and abdominal aorta were not significantly different between groups. (Figure 3, Video)
Figure 3.
A summary of the key findings and their implications: Compared to the patients with benign and normal genetic variants, patients with pathogenic variants had a significantly higher rate of reinterventions only of the native aortic root after initial thoracic aortic dissection.
VIDEO:
Discussion of the late outcomes (reintervention and survival) in aortic dissection patients with pathogenic genetic variants and its clinical implications.
It is always a question of how much surgeons should do with the dissected aortic root and arch during the initial TAAD repair - when should a total aortic root or total aortic arch replacement be performed to save the patient’s life and also prevent future reinterventions. This question is even more challenging for those patients with known genetic mutations, such as FBN1 mutations in MFS, TGFBR1 or 2 mutations in LDS, and PRKG1 mutations as well as others. The majority of studies regarding late outcomes of the dissected aorta have been performed in MFS patients,7-9 which is more common than other connective tissue diseases, as seen in this study, in which 71% of pathogenic variants were in FBN1. Most surgeons agree that patients with connective tissue disease, such as Marfan, Loeys-Dietz, and Ehlers-Danlos syndromes (COL3A1 mutations), and ELN mutations, should have their aortic root replaced during an acute TAAD repair.7,10 However, the extent of arch replacement in patients with known pathogenic variants during an acute TAAD repair remains controversial. Some recommend conservative arch replacement if no other indications exist for total arch replacement,11 while others recommend a total arch replacement for all Marfan syndrome patients based on the diagnosis of MFS alone.12
In this study, there were 11 pathogenic and 111 benign/normal variant carriers with a native aortic root after the initial aortic dissection and surgical repair. Overall, the incidence rate of reinterventions was 10 times greater (12%/year vs. 1.2%/year) in the pathogenic group than the benign/normal group. (Table 4) In the pathogenic group (n=31), five patients had an aortic root replacement before they had a TBAD and 15 patients had an aortic root replacement during the acute TAAD repair, most likely for an existing aortic root aneurysm. By the end of the study period, all patients with a TAAD and pathogenic variant had undergone an aortic root replacement within 12 years except one patient. This patient had a COL3A1 mutation and was alive 8.2 years after the initial acute type A aortic dissection repair. If we just focused on the reinterventions in TAAD patients after initial TAAD repair, 83% (5/6) TAAD patients at risk had reintervention of the aortic root in the pathogenic group compared to only 12% (7/57) in the benign/normal group within 14 years. The cumulative incidence of first reintervention of the aortic root was also significantly higher in the pathogenic group (Figure 1B). Similarly, the mean cumulative reinterventions for each patient (including all reinterventions) was significantly higher in the pathogenic group, especially in the first 10 years (Figure 1A). The analysis of the matched cohort had similar findings (Supplemental Material). Our findings supported the recommendation for aggressive total aortic root replacement in patients with pathogenic variants during acute TAAD repair including: FBN1, TGFBR1/2, SMAD3, PRKG1, COL3A1, and LOX.
It is interesting that patients with the mutations noted above and other mutations in the TGF-β pathways (such as TGFB2, TGFB3, SMAD2, SMAD3 SMAD4) frequently develop aortic root aneurysm first.13-17 In our lab and Harry Dietz’s lab, we both found the defective differentiation of the smooth muscle cells from the second heart field due to SMAD3 mutation in a human induced pluripotent stem cell model and Tgfbr1 mutation in mouse model.14,18 This evidence also supports aggressive aortic root replacement in patients with pathogenic variants during acute TAAD repair. If possible, a valve-sparing aortic root replacement should be considered.
A practical question is that during the presentation of acute aortic dissection, frequently we do not know if patients have a pathogenic genetic variant, nor do we wait for the results of gene sequencing to perform an emergent operation; therefore, how does this study help surgeons make a decision regarding the aortic root? From our previous study5, we found that if patients have a positive family history of thoracic aortic disease (aortic aneurysm or dissection), are younger than 50-years-old, and have no history of smoking or hypertension, then they have a very high risk of carrying a pathogenic genetic variant. Therefore, we would recommend aggressive aortic root replacement at time of acute type A aortic dissection repair in patients with a family history of aortic disease, younger than 50 years, and no history of smoking or hypertension. This information can be obtained before surgery in most patients with aortic dissection. If the patients already carry a diagnosis of MFS and LDS or are suspected to have those syndromic diseases based on clinical presentation, we strongly recommend aggressive aortic root replacement. If possible, a valve-sparing aortic root replacement is a good choice for those young patients.
On the contrary, our findings did not support aggressive arch replacement in patients with pathogenic variants. The incidence rate and cumulative incidence of first reintervention for the native aortic arch in patients who had no arch replacement or hemiarch replacement was similar between groups (Table 4, Figure 1C). The incidence rate and cumulative incidence of first reintervention for the descending thoracic and abdominal aorta were also similar between groups, even with 20-year follow-up (Figures 1 D-E). The multivariable Cox model also showed being a pathogenic variant carrier was not a significant risk factor for reoperation of the native aortic arch, descending thoracic, or abdominal aorta (Table 5). When considering all reinterventions of all aortic segments, we observed more reinterventions over time in the pathogenic group. Noticeably, the pathogenic group had more root reinterventions in the first 10 years compared to the benign/normal group. After 10 years, the difference in mean cumulative reinterventions for each patient between groups became smaller. (Figure 1A) These findings do not support a total arch replacement in all patients with a pathogenic variant without other indications. In our practice, we perform an aggressive arch replacement during acute TAAD repair if the patient has an intimal tear at the arch, arch aneurysm (>4 cm) that cannot be resected by a hemiarch replacement, or an arch branch vessel dissection with malperfusion syndrome.11 We do not use a pathogenic variant diagnosis alone as an indication for aggressive arch replacement. Moreover, we also reported that MFS is not an independent risk factor for reintervention of the distal aorta after acute TAAD repair11, which is consistent with the findings reported in this study.
The imaging studies in our matched cohorts, mainly CTA, showed significant growth of the aortic arch, descending thoracic aorta, and abdominal aorta after initial aortic dissection, which is consistent with previous findings7-9, indicating aortic dissection alone is a risk factor of distal aortic growth. There were no significant differences of distal aortic growth between groups (Supplemental Figure 1). This finding is consistent with the similar reintervention rate for the distal aorta (arch, descending thoracic, and abdominal aorta) between groups, which further supports that aortic dissection alone is a more important factor for late distal aortic growth than a pathogenic variant. Therefore, pathogenic variant status alone should not be used as an indication for aggressive arch replacement. Because of significant growth and reinterventions of the distal aorta in the entire cohort, we recommend regular surveillance with imaging studies for all patients with a history of thoracic aortic dissection. The frequency of imaging studies could be similar in patients with and without pathogenic variants and adjusted to the growth rate of the dissected aorta for each individual patient.
The long-term survival was surprisingly good in the pathogenic group and not significantly different than the benign/normal group. With a median follow-up time of 9.5 years, the 10-year survival in the pathogenic group remained 92% (Figure 2), which is likely due to young age at time of dissection (median age: 43 years) and otherwise healthy. These results support that we should aggressively treat the aortic disease in patients with pathogenic variants. The hazard ratio of pathogenic variants was 1.8 in the Cox proportional hazard model, though not significant, most likely due to the small sample size of the pathogenic group. This finding indicated that pathogenic variants could be a potential risk factor for late mortality.
There are several limitations in our study. It is a strength that we divided the groups based on whole exome sequencing data and the identified genotype of each patient; and the controls (benign/normal group) were without any known pathogenic variants3. However, the sample size for the pathogenic group was relatively small and could be insufficient to detect significant differences between groups. The pathogenic variants were identified mainly (71%) in FBN1 (Marfan syndrome). This reflects our practice that the most common connective disease with aortic dissection is MFS with FBN1 mutations. The finding of this study may be more applicable to patients with MFS. Finally, this study was retrospective and has all of the limitations of a retrospective study design. The rate of reinterventions could have been underestimated as our follow-up for reintervention was not 100% complete by January 2018.
CONCLUSION
Pathogenic variant carriers had a significantly higher rate of later reintervention of the preserved native aortic root compared to the benign/normal variant carriers. However, the reintervention rate of the distal aorta, including the aortic arch, descending thoracic aorta, and abdominal aorta were not significantly different between groups. Our findings support aggressive aortic root replacement but similar management of the aortic arch at time of type A aortic dissection repair for pathogenic variant carriers compared to benign/normal variant carriers.
Supplementary Material
Supplemental Figure 1: There were a total of 380 images (372 CT and 8 MRI) reviewed, with an average of six images per patient. For the matched cohort, including both pathogenic and benign/normal groups, there was significant growth of the preserved native aortas in all years of follow-up including growth of the aortic arch (0.49 mm/year), descending thoracic aorta (1.3 mm/year), and abdominal aorta (0.98 mm/year), all p<0.0001). If there was no significant growth of the dissected aorta, the growth rate should not be significantly different from 0 mm/year. The aortic root had significant growth in the first eight years, (0.39 mm/year, p=0.004), but not in all years (0.53 mm/year, p=0.10) due to almost all patients with pathogenic variant had their aortic root replaced within the first 10 years after dissection. There was no significant difference in growth in any of the aortic segments between groups overtime, although the pathogenic group had a nonsignificant trend of faster aortic growth at each of the aortic segments, including the aortic root (A) (0.85 mm/year vs. 0.41 mm/year, p=0.34), the aortic arch (B) (0.63 mm/year vs. 0.34 mm/year, p=0.18), the descending thoracic aorta (C) (1.49 mm/year vs. 1.13 mm/year, p=0.37), and the abdominal aorta (D) (1.18 mm/year vs. 0.75 mm/year, p=0.17). If the aortic segment was replaced, then the measurement of the replaced aortic segment for that specific patient was terminated. (Red: Benign/Normal variant carrier, Blue: Pathogenic variant carrier)
Supplemental Figure 2: A) The mean cumulative reinterventions per patient after thoracic aortic dissection was significantly higher in the pathogenic group than the benign/normal group, (p=0.039). All aortic reinterventions were used to calculate the mean cumulative incidence. The cumulative incidence of first aortic root reintervention (B) was significantly higher in the pathogenic group. However, the cumulative incidence of first aortic arch reintervention (C), first descending thoracic aorta reintervention (D), and first abdominal aortic reintervention (E) were not significantly different between groups. Death was used as a competing factor.
Supplemental Figure 3. Kaplan-Meier survival analysis for pathogenic and benign/normal variants carriers following a thoracic aortic dissection. The 10-year survival was similar between pathogenic (92% [95% CI: 71%, 98%]) and benign/normal groups (87% [95% CI: 54%, 97%].
Central Picture:
The cumulative incidence of native aortic root reintervention after aortic dissection.
Central Message:
More aggressive aortic root replacement and similar arch management should be considered at time of initial type A aortic dissection repair in pathogenic compared to benign/normal variant carriers.
Perspective Statement:
The incidence rate and cumulative incidence of native aortic root reintervention was increased significantly in pathogenic variant carriers compared to benign/normal variant carriers, but not of aortic arch and distal aorta reintervention, suggesting aggressive root management but similar arch management in pathogenic variant carriers at the time of the initial type A aortic dissection repair.
ACKNOWLEDGEMENT:
The authors would like to thank the support from the Cardiovascular Health Improvement Project (CHIP) and Department of Cardiac Surgery at the Frankel Cardiovascular Center, Michigan Medicine.
Sources of Funding: Dr. Yang is supported by the NHLBI of NIH K08HL130614 and R01HL141891, Phil Jenkins and Darlene & Stephen J. Szatmari Funds.
Dr. Willer is supported NIH R01-HL127564, R35-HL135824, R01-HL142023, R01-HL-109942, R01-HL109946.
GLOSSARY OF ABBREVIATIONS
- CI
confidence interval
- CT
computed tomography
- HR
hazard ratio
- LDS
Loeys-Dietz syndrome
- MFS
Marfan syndrome
- MRA
magnetic resonance angiography
- TAAD
type A aortic dissection
- TBAD
type B aortic dissection
- TEVAR
thoracic endovascular aortic repair
- VUS
variant of unknown significance
Footnotes
Conflict of Interest: None.
Date and Number of IRB Approval: HUM00052866 and HUM00094409
REFERENCES
- 1.Milewicz DM, Regalado E. Heritable Thoracic Aortic Disease Overview. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews((R)). Seattle (WA)1993. [Google Scholar]
- 2.Pomianowski P, Elefteriades JA. The genetics and genomics of thoracic aortic disease. Ann Cardiothorac Surg. 2013;2:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renard M, Francis C, Ghosh R, Scott AF, Witmer PD, Ades LC, et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J Am Coll Cardiol. 2018;72:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownstein AJ, Kostiuk V, Ziganshin BA, Zafar MA, Kuivaniemi H, Body SC, et al. Genes Associated with Thoracic Aortic Aneurysm and Dissection: 2018 Update and Clinical Implications. Aorta (Stamford). 2018;6:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolford BN, Hornsby WE, Guo D, Zhou W, Lin M, Farhat L, et al. Clinical Implications of Identifying Pathogenic Variants in Individuals With Thoracic Aortic Dissection. Circ Genom Precis Med. 2019;12:e002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention; National Center for Health Statistics. National Death Index. Available at http://www.cdc.gov/nchs/ndi/index.htm. December 27, 2017. [Google Scholar]
- 7.Rylski B, Bavaria JE, Beyersdorf F, Branchetti E, Desai ND, Milewski RK, et al. Type A aortic dissection in Marfan syndrome: extent of initial surgery determines long-term outcome. Circulation. 2014;129:1381–1386. [DOI] [PubMed] [Google Scholar]
- 8.Kari FA, Russe MF, Peter P, Blanke P, Rylski B, Euringer W, et al. Late complications and distal growth rates of Marfan aortas after proximal aortic repair. Eur J Cardiothorac Surg. 2013;44:163–171. [DOI] [PubMed] [Google Scholar]
- 9.Kimura N, Aizawa K, Kawahito K, Itagaki R, Yamaguchi A, Misawa Y, et al. Outcomes of Early-Onset Acute Type A Aortic Dissection- Influence of Etiologic Factors. Circ J. 2019;83:285–294. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Norton EL, Hobbs R, Farhat L, Wu X, Hornsby WE, et al. Short- and Long-term Outcomes of Aortic Root Repair and Replacement in Patients Undergoing Acute Type A Aortic Dissection Repair: 20-Year Experience. J Thorac Cardiovasc Surg. 2019;157:2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang B, Norton EL, Shih T, Farhat L, Wu X, Hornsby WE, et al. Late outcomes of strategic arch resection in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2019;157:1313–1321 e1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachet J Commentary: The importance of being earnest and ... eclectic. J Thorac Cardiovasc Surg. 2019;157:1322–1323. [DOI] [PubMed] [Google Scholar]
- 13.Gillis E, Van Laer L, Loeys BL. Genetics of thoracic aortic aneurysm: at the crossroad of transforming growth factor-beta signaling and vascular smooth muscle cell contractility. Circ Res. 2013;113:327–340. [DOI] [PubMed] [Google Scholar]
- 14.MacFarlane EG, Parker SJ, Shin JY, Kang BE, Ziegler SG, Creamer TJ, et al. Lineage-specific events underlie aortic root aneurysm pathogenesis in Loeys-Dietz syndrome. J Clin Invest. 2019;129:659–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton EL, Gordon D, Yang B. Managing the aorta in patients with a PRKG1 mutation. J Thorac Cardiovasc Surg. 2019;157:e107–e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asher SB, Chen R, Kallish S. Mitral valve prolapse and aortic root dilation in adults with hypermobile Ehlers-Danlos syndrome and related disorders. Am J Med Genet A. 2018;176:1838–1844. [DOI] [PubMed] [Google Scholar]
- 17.Guo DC, Regalado ES, Gong L, Duan X, Santos-Cortez RL, Arnaud P, et al. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ Res. 2016;118:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong J, Qiu P, Chen E, Yang B. Abstract 698: Functional Analysis of Smad3 Deficiency in VSMCs Derived From Crispr/cas9-modified Human Induced Pluripotent Stem Cells. Arterioscler Thromb Vasc Biol. 2018;38.Suppl_1.698.29563115 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: There were a total of 380 images (372 CT and 8 MRI) reviewed, with an average of six images per patient. For the matched cohort, including both pathogenic and benign/normal groups, there was significant growth of the preserved native aortas in all years of follow-up including growth of the aortic arch (0.49 mm/year), descending thoracic aorta (1.3 mm/year), and abdominal aorta (0.98 mm/year), all p<0.0001). If there was no significant growth of the dissected aorta, the growth rate should not be significantly different from 0 mm/year. The aortic root had significant growth in the first eight years, (0.39 mm/year, p=0.004), but not in all years (0.53 mm/year, p=0.10) due to almost all patients with pathogenic variant had their aortic root replaced within the first 10 years after dissection. There was no significant difference in growth in any of the aortic segments between groups overtime, although the pathogenic group had a nonsignificant trend of faster aortic growth at each of the aortic segments, including the aortic root (A) (0.85 mm/year vs. 0.41 mm/year, p=0.34), the aortic arch (B) (0.63 mm/year vs. 0.34 mm/year, p=0.18), the descending thoracic aorta (C) (1.49 mm/year vs. 1.13 mm/year, p=0.37), and the abdominal aorta (D) (1.18 mm/year vs. 0.75 mm/year, p=0.17). If the aortic segment was replaced, then the measurement of the replaced aortic segment for that specific patient was terminated. (Red: Benign/Normal variant carrier, Blue: Pathogenic variant carrier)
Supplemental Figure 2: A) The mean cumulative reinterventions per patient after thoracic aortic dissection was significantly higher in the pathogenic group than the benign/normal group, (p=0.039). All aortic reinterventions were used to calculate the mean cumulative incidence. The cumulative incidence of first aortic root reintervention (B) was significantly higher in the pathogenic group. However, the cumulative incidence of first aortic arch reintervention (C), first descending thoracic aorta reintervention (D), and first abdominal aortic reintervention (E) were not significantly different between groups. Death was used as a competing factor.
Supplemental Figure 3. Kaplan-Meier survival analysis for pathogenic and benign/normal variants carriers following a thoracic aortic dissection. The 10-year survival was similar between pathogenic (92% [95% CI: 71%, 98%]) and benign/normal groups (87% [95% CI: 54%, 97%].