Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly contagious zoonotic pathogen that has exacted heavy public health, social and economic tolls. In February 2020, the World Health Organization acronymed the disease caused by SARS-CoV-2 as COVID-19, for coronavirus disease 2019. The number of confirmed COVID-19 infections, which has been detected in at least 103 countries, has reached 1,970,225 worldwide as of April 14, 2020 with 124,544 deaths, according to the U.S. Centers for Disease Control and Prevention (CDC).
Many cases of COVID-19 resolve quickly. However, the disease, which, like other respiratory pathogens that cause common cold symptoms is believed to be transmitted through respiratory droplets. Infection with COVID-19 can also lead to significant morbidity and death; this is particularly the case for cancer patients. Moreover, because the signs and symptoms of COVID-19 are easily misattributed to the sequelae of cancer itself, such as pulmonary embolism, or its treatment, such as nausea and diarrhea, diagnosis may be delayed or missed. Potential COVID-19 rule out criteria, based on the Wells' criteria for pulmonary embolism, another protean disease entity, are provided as a decision-making aid. This review summarizes the current understanding of the transmission, clinical presentation, diagnosis and differential diagnosis, pathogenesis, rationale to treat the cancer or not, treatment and prevention of COVID-19 with an emphasis on implications in cancer.
Keywords: COVID-19, SARS-CoV-2, Cancer
1. Introduction
SARS-CoV-2, the RNA virus responsible for the illness, which has been named COVID-19 for Coronavirus Disease-2019, the year it was diagnosed, has sent shockwaves and dominated the news cycle due to its pandemic spread from the point of origin in Wuhan, China, to the rest of the world. Declared a public health emergency of international concern (PHEIC) by the World Health Organization (WHO) [1], SARS-CoV-2 is the third highly pathogenic, novel zoonotic (bat) coronavirus (so-named because of the crownlike spikes on its surface) to have emerged: the first was SARS coronavirus (now named SARS-CoV-1) in 2002 with a fatality rate of 10.87% and the second was Middle East respiratory syndrome (MERS) in 2012 with a fatality rate of 34.77% where the camel was the intermediate host [2]. SARS-CoV-1, MERS-CoV, and SARS-CoV-2 belong to the Betacoronavirus genus, which are enveloped, positive-stranded RNA viruses whose approximately 30,000 nucleotide genome serves as an mRNA template for the translation of viral proteins [3]. The virion contains four proteins (Spike, Envelope, Membrane, and Nucleocapsid) and the host receptor with which the Spike surface glycoprotein of SARS-CoV-2 engages is angiotensin converting enzyme 2 (ACE2) [4]. The biology of SARS-CoV-2 is described in more detail in Fig. 1 .
Fig. 1.
Viral and host factors that influence the pathogenesis of SARS-CoV-2.
Top panel: Viral Factors. Bats are the reservoir of SARS-CoV-like viruses. SARS-CoV-2, an enveloped positive single-stranded RNA coronavirus (ssRNA), may originate from bats or unknown intermediate hosts and jump the species barrier to humans. The majority of the viral RNA located in the first open reading frame (ORF1a/b) encodes 16 non-structure proteins (nsp). The rest of the virus genome encodes four essential structural proteins, including the spike (S) glycoprotein, envelope (E) protein, matrix (M) protein, and nucleocapsid (N) protein and several accessory proteins. The S glycoprotein binds to angiotensin converting enzyme 2 (ACE2), which facilitates virus entry. Other virus factors may contribute to pathogenesis. Lower panel: The elderly and those with severe underlying comorbid conditions (e.g., cancer, immunosuppression, diabetes, cardiopulmonary disease) are more susceptible to SARS-CoV-2 infection and clinical deterioration with hospitalization. RBD, receptor-binding domain; HR1, heptad repeats 1; HR2, heptad repeats 2.
A zinc metallopeptidase enzyme, ACE-2, which is abundantly present in lung and gastrointestinal epithelial cells [5], not only mediates viral entry through receptor-mediated endocytosis [6] but also the efficiency of viral replication [7]. Its expression is upregulated with older age, smoking, the antihypertensives, angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), thiazolidinediones (TZDs), a class of oral antidiabetic drugs, and ibuprofen, risk factors, which may increase susceptibility to the COVID-19 virus infection and which are common in generally elderly multimorbid cancer patients [8].
The clinical spectrum of SARS-CoV-2 ranges from mild upper respiratory tract infection with fever, sore throat, headache, cough and potentially nausea and diarrhea (the majority of cases recover without serious complications) to severe pneumonia with sequelae that include acute respiratory distress syndrome (ARDS), cytokine storm and death [9]. Because SARS is an acronym for Severe Acute Respiratory Syndrome, digestive manifestations including inappetence, nausea, abdominal pain and diarrhea of COVID-19, resulting from binding of the virus to the ACE-2 receptor in the GI tract may precede respiratory symptoms [10]. Gastrointestinal manifestations are potentially underappreciated and overlooked as sentinel symptoms that herald the onset or persistence of disease, especially in cancer patients, and may contribute to delayed or missed opportunities for testing, diagnosis and containment.
Unlike SARS-CoV-1, which seems to have disappeared/died out [11], and MERS, which reappears only sporadically, SARS-CoV-2 is less lethal with a fatality rate between 2 and 4% (although this number is highly uncertain and debated) [12] but much more infective. Consequently, public panic and economic disruption have ensued, resulting in wartime-like mobilization efforts to mitigate its spread. Old age, smoking and comorbidities such as diabetes, morbid obesity, immunosuppression, frailty and cardiovascular disease appear to predispose to worse outcomes, possibly secondary to impaired T and B cell responses [13]. Notably, COVID-19 infection is associated with lymphopenia and delayed development of the adaptive immune response, which appears to correlate with prolonged virus clearance and more severe disease progression [14].
The first pillar of defense against infection is hand washing, avoidance of face touching and minimization of close contact (i.e., social distancing) with self-quarantine and self-isolation [15] in case of exposure or evidence of COVID-19 symptoms, respectively. The second prophylactic pillar is vaccination with specific viral antigens or mRNAs, which are not yet publicly available, although the company Moderna has reportedly started testing an mRNA vaccine in healthy volunteers and multiple other vaccination strategies/platforms appear to be in progress/under development [16].
The available evidence is limited, but clinical courses and outcomes of COVID-19 are likely to be worse in patients with cancer especially given the clear association between severity of disease and older age and higher levels of comorbidity [17]. The overall case fatality rate (CFR) for the general COVID-19-infected population is around 3.8%, but in cancer it rises to 7.6% overall and to 20% in Italy [18]. This CFR in cancer patients compares to 1.4% for no comorbid conditions, 13.2% for cardiovascular disease, 9.2% for diabetes, 8.4% for hypertension, and 8.0% for chronic respiratory disease [19].
The aim of this review is to summarize and condense the current understanding of the transmission, clinical presentation, diagnosis and differential diagnosis, pathogenesis, treatment and prevention of COVID-19 with a special focus on cancer.
2. Transmission and prevention
According to the World Health Organization (WHO) [20], SARS-CoV-2 is spread person-to-person mainly via aerosol inhalation from sneezing, coughing or exhalation [21] and via fomite-to-face contact since depending on the surface material the virus may remain viable and infectious for hours to days (Fig. 2 ) [22,23]. Fecal-oral transmission has also been hypothesized because diarrhea was a common feature with SARS and MERS, and diarrhea and other digestive issues have also been reported in patients with COVID-19 [24,25]. Notably, transmission of SARS-CoV-2 is not limited to symptomatic individuals i.e., those with fever, cough, sore throat, myalgias or dyspnea but also to asymptomatic or subclinically infected carriers of the virus, which is problematic from the perspective of disease control [26] and highlights the importance of containment measures, including isolation and quarantine. The basic reproduction number, R0, of SARS-CoV-2 is 2.2–3.8, which means that, on average, for every patient an additional 2.2–3.8 individuals are infected [27]. Because coronaviruses may persist on inanimate surfaces like metal, glass or plastic for up to 9 days, careful disinfection with ≥70% or greater ethanol for small surfaces or ≥ 0.1% sodium hypochlorite for larger surfaces is recommended.
Fig. 2.
Illustration of main transmission routes.
3. Diagnosis and clinical features
In the United States, the test of choice for SARS-CoV-2 is a nasopharyngeal swab specimen (or sputum if a productive cough is present) on which a reverse-transcriptase PCR (RT-PCR assay) or an enzyme-linked immunoassay (EIA) directed particularly at the envelope (E), RNA-dependent RNA polymerase (RdRp), spike protein (S) and nucleocapsid (N) genes is performed. The FDA has also approved an antibody test [28]. A positive test for SARS-CoV-2 in a symptomatic patient generally confirms the diagnosis of COVID-19, with the caveat that false positive and false negative tests have been documented. If initial testing is negative but a high index of suspicion and pretest probability for COVID-19 remains on the basis of patient signs and symptoms, then retesting is indicated. In patients with high indexes of clinical suspicion and equivocal or negative test results, the WHO recommends that lower respiratory tract specimens, which contain the highest viral loads, should be obtained since nasopharyngeal swabs may miss some infections [29]. According to CDC guidelines, disease is excluded on the basis of two consecutive negative tests respiratory tests separated by ≥24 h, however, in the presence of suggestive symptoms, rectal swabs may also be indicated since the ACE2 enzyme to which the virus binds is abundantly present in rectal epithelia cells [30].
The differential diagnosis for SARS-CoV-2 in cancer is extremely broad and includes conditions such as foreign body aspiration, toxicities from chemotherapy and radiation, tumor progression, post-obstructive pneumonia, malignant obstruction, atelectasis, pulmonary embolism, pneumonitis, pulmonary edema/fluid overload, immunotherapy-related pneumonitis, COPD exacerbation, Q fever, adenovirus, bocavirus, coronavirus 229E (HCoV 229E), coronavirus HKU1 (HCoV HKU1), coronavirus NL63 (HCoV NL63), coronavirus OC43 (HCoV OC43), human metapneumovirus (HMPV), influenza A, influenza A subtype H1N1/pdm09 influenza A subtypes H1 and H3, influenza B, parainfluenza virus 1–2–3–4 (PIV 1–4), respiratory syncytial virus A/B (RSV A/B), rhinovirus/enterovirus, (HRV/EV), Bordetella pertussis, Legionella pneumophila, and Mycoplasma pneumoniae [31].
The diagnosis of SARS-CoV-2 is complicated by the possibility of simultaneous coinfection with other respiratory viruses [32], which is especially true for immunosuppressed cancer patients whose susceptibility to microorganisms is increased. The heightened infectious risk for cancer patients underscores the importance of screening them at presentation with extended viral respiratory panel testing given that coinfection may impact management decisions since, conceptually at least, the morbidity of COVID-19 and the risk of severe illness should increase in the presence of a second or third virus.
Unlike infection with influenza, for example, COVID-19 signs and symptoms may vary considerably depending on the dose of viral inoculum, route of inoculation, concomitant medications and underlying health status [33] to include fever (87.9%), dry cough (67.7%), fatigue (38.1%), sputum production (33.4%), shortness of breath (18.6%), sore throat (13.9%), headache (13.6%), myalgia or arthralgia (14.8%), chills (11.4%), nausea or vomiting (5.0%), nasal congestion (4.8%), diarrhea (3.7%), hemoptysis (0.9%), and conjunctival congestion (0.8%) [34]. With an incubation period of 2 to 11 days after exposure [35], presymptomatic or minimally symptomatic infection may majorly drive transmission especially since detected viral loads are similar in both symptomatic and asymptomatic patients [36,37]. Populations of concern include the elderly, smokers, vapers and dual users, those of any age with pre-existing chronic medical conditions, those receiving particular medications or therapies, which upregulate the ACE-2 receptor or suppress the immune system and those from lower socioeconomic classes, a conglomeration of factors, which are often present in cancer patients, as depicted in Fig. 3 .
Fig. 3.
Venn diagram depicting risk factors for COVID-19 severity, which overlap with cancer risk factors.
While the surveillance focus for COVID-19 is on the respiratory tract, enteric symptoms are a potentially underappreciated, overlooked and misattributed manifestation of disease, as stated earlier, and this is especially the case for cancer patients, where gastrointestinal toxicity occurs routinely from chemotherapy i.e., cisplatin/carboplatin/oxaliplatin, irinotecan, 5-fluorouracil, ifosfamide, from targeted agents i.e., erlotinib, imatinib, bortezomib, temsirolimus, sunitinib, regorafenib-sorafenib, and bevacizumab [38] and from locally advanced or metastatic disease. Therefore, abdominal complaints in cancer patients, which are potentially but not automatically attributable to underlying disease, justify further investigation, especially if persistent, worsening or new, particularly because SARS-CoV-2 transmission may occur via the fecal–oral route [39].
Abnormal laboratory findings in COVID-19 include lymphopenia (70%), prolonged prothrombin time (58%), elevated lactate dehydrogenase (40%), elevated AST and ALT (4–22%), elevated highly sensitive (hs) CRP and elevated procalcitonin; however, because these parameters routinely fall well outside of the normal reference range in cancer patients, it is difficult to confirm or refute the presence of disease on this basis alone. Chest radiographs and chest CT abnormalities are similarly non-specific since the most common features, multifocal ground-glass opacities and consolidation, mimic other pneumonias [40]. Significant antibody production is observed after infection, but it is unknown whether this helps or harms since antibody-dependent enhancement (ADE) may potentiate viral entry and the induction of a severe inflammatory response [41].
Universal screening of cancer patients for COVID-19 is desirable but logistically impossible for the foreseeable future since diagnostic tests are in short supply and simply not always readily available [42]; hence, COVID-19 rule out criteria are proposed in Table 1 as a potential decision-making aide- mémoire, which separates patients into low- and high-risk groups, by analogy to the Wells' criteria for pulmonary embolism [43,44].
Table 1.
Proposed COVID-19 rule out criteria.
| COVID-19 suspicion index (SI) | Score | |
|---|---|---|
| Clinical signs and symptoms e.g., fever, cough, dyspnea of COVID-19 | No 0 | Yes +3 |
| COVID-19 is #1 diagnosis OR equally likely | No 0 | Yes +3 |
| Close or direct contact with a suspected COVID-19 patient | No 0 | Yes +3 |
| New lab abnormalities e.g., lymphopenia suggestive of COVID-19 | No 0 | Yes +1 |
| New onset or worsening diarrhea | No 0 | Yes +1 |
| Clinical probability of COVID-19 | Score |
|---|---|
| Low | 0–1 |
| High | ≥3 |
Preventive measures focus on self-isolation, social distancing with a 6-ft (2-m) separation [45], frequent hand washing with soap and water and/or use of hand sanitizers, patient isolation during clinical care, use of masks to help prevent aerosol transmission and flushing with the lid closed to control so-called “toilet plume” [46]. In an ASCO guidance, immunocompromised cancer patients are advised to minimize exposure to sick contacts and large crowds. For healthcare personnel the use of personal protective equipment such as N95 masks, FFP3 masks, gowns, eye protection, gloves, and gowns is mandated [47].
4. Vaccination and immunity
Vaccination efforts and the related topic of whether those who have recovered from COVID-19 develop protective immunity have drawn great attention. The latter has implications on whether people who test positive for SARS-CoV-2 antibodies can be safely assumed to be immune and at negligible risk of contracting or transmitting the disease. There have been case reports of patients who have recovered from COVID-19 and had recurrence of RT-PCR positivity approximately one month after initial diagnosis, with only one patient exhibiting significant clinical symptoms and another having a mild intermittent cough [48]. But while not zero, the risk of transmissibility or recurrence of symptomatic disease in recovered patients has yet to be quantified, and the paucity of currently available reports of recurrence in the setting of a pandemic suggests that it is low. A separate practical question will be whether antibody-based tests prove to have sufficient sensitivity and specificity to identify people who had asymptomatic infections, developed immunity, and can return to normal activities without jeopardizing disease containment efforts.
Immunity may be due to antibodies, cell mediated immunity, or a combination of the two. Previous experience with using plasma from convalescent patients to treat severe cases of the first SARS and MERS as well as limited experience with COVID-19 suggests that antibody mediated immunity alone is clinically beneficial even during acute infection [49]. Safety concerns about antibodies have been raised based on preclinical studies of SARS-CoV vaccination in ferrets showing hepatotoxicity [50] and of vaccination against feline infectious peritonitis virus (another coronavirus) leading to more severe disease when kittens were subsequently challenged with the virus [51]. Although animal models may not be representative of human host-pathogen interactions, the nature of SARS-CoV and SARS-CoV-2 antibodies are likely different as cross-neutralization was not observed in-vitro [52], and experience with convalescent plasma has not borne evidence of antibody mediated enhancement of infection in acutely infected patients, the potential risk deserves attention if vaccination is proposed for the entire population. T cell responses are also readily observable in patients who recover from coronavirus infections [53] and memory T cell responses alone were protective in mice [54] with the potential advantage of longer persistence of memory T cell responses compared to humoral immunity. When clinical data on vaccine candidates becomes available, cancer patients may face different considerations surrounding vaccination than the general population, particularly patients with hematologic malignancies being treated with agents targeting B cells who would derive greater benefit from vaccines eliciting cell mediated than antibody responses.
5. Pathogenesis and pathology relating to ACE2 and RAS signaling
The ACE-2 enzyme, a key regulator of the renin-angiotensin system (RAS) [55] to which the virus binds through its surface spike proteins is particularly abundant in the digestive tract, lungs, kidney, heart and blood vessels, where pathology from SARS-CoV-2 occurs [56]. A peptidase that catalyzes the conversion of angiotensin II (Ang-II), referred to as “the quintessential perpetrator of inflammation” [57] to angiotensin 1–7 (Ang 1–7), ACE-2 mediates antiproliferative and vasodilatory functions that oppose the vasoconstrictive and inflammatory functions of angiotensin converting enzyme (ACE) [58].
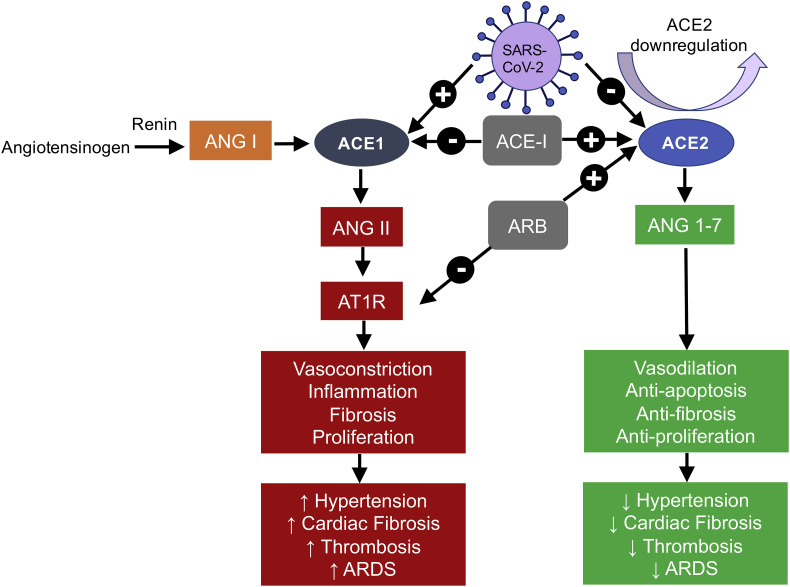
The binding of SARS-CoV-2 to ACE-2 leads to downregulation of ACE-2 expression potentially through increased internalization and shedding from the cell surface with decreased Ang-(1–7) generation and increased Ang II levels as a consequence [59]. This unfavorably shifts the balance of the renin angiotensin system (RAS) from the vasoprotective ACE-2/Ang(1–7) axis to the ACE/Ang II/Angiotensin 1 (AT1) receptor axis and drives a proinflammatory, profibrotic and proliferative response [60], as shown in Fig. 4 .
Fig. 4.
Alterations in renin-angiotensin system components with SARS-CoV-2 infection and ACEI/ARB use.
+ sign: promotes or upregulates.
– sign: inhibits or downregulates.
ACE1:Angiotension converting enzyme 1.
ACE2: Angiotensin converting enzyme 2.
ACE–I: ACE inhibitor.
ARB: Angiotensin receptor blocker.
ANG: Angiotensin.
AT1R: Angiotensin 1 receptor.
Fang et al. [61] contend that because thiazolidinediones, ibuprofen and angiotensin converting enzyme (ACE) inhibitors and angiotensin II type-I receptor blockers (ARBs) substantially increase the expression of ACE-2, they facilitate SARS-CoV-2 infection and, therefore, the risk of severe and fatal COVID-19. In contrast, AlGhatrif et al [62]. present a diametrically opposed hypothesis: that downregulated ACE-2 signaling is responsible for SARS-CoV-2-induced acute lung injury (ALI)/ acute respiratory distress syndrome (ARDS) and cytokine storm and that ACE-Is and ARBs are beneficial precisely because they increase ACE2 expression and activity. Furthermore, according to AlGhatrif et al, lower ACE-2 levels and, hence, higher baseline oxidative stress and inflammation [63,64], are present in older comorbid individuals, such as cancer patients, which renders them more susceptible to severe COVID-19 than younger non-comorbid individuals with increased ACE-2 levels and lower baseline inflammation, as shown in Fig. 5 . Furthermore, low ACE-2 may promote tumor progression and, conversely, ACE-2 overexpression is associated with antiangiogenesis and tumor regression [65]. In summary, then, despite the concerns and controversy [66] surrounding the use and continuation of ACEIs/ARBs during the SARS-CoV-2 epidemic, it is likely that the pros outweigh the cons especially in cancer patients due to their potential antitumor and anti-COVID-19 effects [67].
Fig. 5.
Schematic of inflammatory profile in comorbid cancer patients and non-cancerous non-comorbid patients with regard to COVID-19 infection.
In line with RAS involvement, emerging data suggest that SARS-CoV-2 infection may induce serious cardiovascular injury or exacerbate existing cardiovascular disease. Cardiovascular sequela includes heart failure, arrythmias, disseminated intravascular dissemination (DIC), and troponin elevation, which may closely correlate with disease severity and the likelihood of in-hospital death [68].
Liu et al. [69] propose a mechanism whereby the virus, which lowers hemoglobin (Hb) levels [70], binds to the porphyrin of heme and displaces iron, thereby compromising the oxygen-carrying capacity of red blood cells and exacerbating the hypoxemia. Since chloroquine and the experimental anticancer agent, RRx-001, also bind to porphyrins, they may competitively interfere with binding by the virus.
6. Rationale for continuation or discontinuation of cancer therapy
The benefit-risk calculus that informs the decision whether and how to treat with anticancer therapy falls into a “gray zone” about which no consensus exists, leading to a therapeutic dilemma. On the one hand, Zhang et al [71] in Annals of Oncology reported a strong association in 28 patients, 7 of them (25%) with lung cancer, between antineoplastic therapy in the past 14 days and severe effects of COVID-19 (HR = 4.079, 95% CI 1.086–15.322, P = 0.037); on this basis, the authors recommend treatment interruption, dose reduction or substitution of cytotoxic chemotherapy with non-immunosuppressive options (e.g., checkpoint inhibitors) if available, especially in the case of lung cancer patients that are already prone to develop respiratory infections and complications [72]. Similarly, heavily immunosuppressed patients, such as those who have undergone hematopoietic stem cell transplantation are also particularly susceptible to viral respiratory infections. These findings are supported by a nationwide analysis of data [73] in China from 1590 COVID-19 patients, 18 of which were diagnosed with cancer. This 18 patient cohort experienced a higher incidence of severe events (39% vs 8%; P = 0.0003) and the administration of chemotherapy or surgery was found to have increased the risk of death and/or intensive care unit admission even after adjusting for age, sex and comorbidities (odds ratio (OR) 5.4, 95% CI 1.8–16.2; P = 0.0026) [74] While these studies are limited by small sample sizes, the data suggests that cancer predisposes to more severe disease. Therefore, since in-person contact increases the risk of transmission, several institutions have mandated real-time video or telephone interactions, alternatively referred to as telehealth [75], postponed surgeries, biopsies, endoscopies scans and routine investigations, when possible, and in line with ESMO guidelines [76] encouraged conversion from the intravenous to the oral route e.g., 5-fluorouracil to capecitabine, etoposide and vinorelbine.
On the other hand, the immediate existential threat of progressive disease, for which death is an impending, imminent certainty rather than a remote possibility in the absence of treatment, likely outweighs the theoretical risk of SARS-CoV-2 infection. Even in lower risk disease, for example, in situ or localized prostate, breast and head and neck cancer, delayed treatment is potentially conducive to tumor development and progression and thus may unfavorably impact prognosis [77]. Hanna et al. have proposed a triage strategy [78], which prioritizes treatment for those patients with 1) imminent risk of early mortality from acute leukemias, aggressive lymphomas, metastatic germ cell tumors 2) oncologic emergencies such as spinal cord compression 3) chemoradiotherapeutic-responsive cancers such as head and neck, cervical and anal cancers and 4) neoadjuvant or adjuvant therapy-responsive tumor types such as stage III colon cancer and deprioritizes visits for surveillance and survivorship.
However, in the absence of a “one size fits all” consensus recommendation, which is unlikely, since cancer is so genetically diverse and heterogeneous, the decision-making process and the subsequent treatment plan are individualized and to be determined (TBD) on case-by-case basis, taking into account multiple factors including the risk of cancer recurrence if therapy is delayed, modified or interrupted, the type of therapy (e.g., surgery, radiation, chemotherapy, checkpoint inhibitors and stem cell transplantation), extent of comorbidities, concomitant medications, patient preferences, physician-patient relationship, race, age, the number of cycles of therapy completed, and treatment tolerance.
In terms of specific cancer-related conditions, ASCO makes the following heavily qualified recommendations:
-
•
Growth factor prophylaxis for neutropenia and neutropenic fever even at lower levels of risk (~10%) as well as empiric antibiotics for acute care
-
•
Erythropoietin-stimulating agents for anemia prophylaxis and transfusion when necessary depending on the patient context and underlying comorbidities
6.1. Treatment
Based on the high transmissibility of the virus [79], the main non-pharmacologic countermeasures to mitigate or delay the impact of COVID-19 include rigorous hand hygiene, use of facemasks, respiratory etiquette i.e., coughing or sneeze into the upper sleeve or elbow, not the hands, flushing with the lid down to prevent bioaerosolization, as well as quarantine, stay at home policies and workplace and school closures, which have upended the social, cultural, political and economic status quo.
No specific treatment or vaccine is currently available although promising activity has been reported for hydroxychloroquine, chloroquine, arbidol, remdesivir, convalescent sera, and favipiravir. The mainstay of medical therapy includes symptomatic care, such as supplemental oxygen, antibiotics, and hemodynamic and mechanical ventilatory support, if indicated for septic shock/multiple organ failure and respiratory failure, respectively [80]. Over 300 active clinical treatment trials are underway [81]. These include vaccines as well as a number of different agents, some with promising preliminary data as mentioned above and also those with potential anti-cancer activity, which will hopefully serve a double purpose, first to treat COVID-19 and second as an adjunct to bridge the time gap until the patient is recovered and the primary antineoplastic is started/restarted as shown in Table 2 .
Table 2.
Examples of potential treatments of COVID-19 with anticancer activity [88].
| Agent | Description | Anti-COVID Mechanism(s) | Anti-cancer Mechanism(s) | Toxicities |
|---|---|---|---|---|
| Chloroquine/hydroxychloroquine | Approved antimalarials | Changes the pH of endosomes and believed to prevent viral entry, transport and post-entry events | Autophagy inhibition | Retinopathy Cardiotoxicity |
| Ribavirin plus interferon | Approved nucleoside inhibitor and cytokine | Inhibition of viral replication | Interferon: immunostimulation | Leukopenia and anemia Depression Influenza-like symptoms |
| Mesenchymal Stem Cells | Experimental non-hematopoietic progenitor cells | Reduction of cytokine storm | Drug delivery | Safe |
| Tocilizumab | Approved interleukin 6 receptor inhibitor | Reduction of cytokine storm | Apoptosis induction | Skin and soft tissue infections Hepatotoxicity Hypercholesterolemia Neutropenia Anaphylaxis |
| Thalidomide | Approved anti-multiple myeloma | Reduction of the inflammatory cytokine surge | Antiangiogenesis | Neuropathy Somnolence Rash Fatigue Constipation |
| RRx-001 | Phase 3 immunomodulator for SCLC | Hypoxic nitric oxide generation Nrf2 activation Reduction of pulmonary hypertension |
Tumor associated macrophage (TAM) stimulation | Localized discomfort on injection |
7. Conclusions
The alarming spread of the COVID-19 pandemic has disproportionately affected cancer patients, an at-risk population both from the standpoint of increased disease severity and disruption to care, which includes widespread suspension of clinical trials in the United States that are already fraught with barriers to enrollment and participation [82] [83].Because the symptoms of COVID-19 are non-specific, underlying symptoms from the cancer e.g., dyspnea, cough, fever, fatigue, diarrhea etc., which overlap with those from the viral infection, may obscure and delay the diagnosis. Hence, if the COVID-19-specific Rapid Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) test is not readily available and/or in short supply, which is currently the case, diagnosis will depend on the maintenance of a high index of clinical suspicion especially in advanced cancer patients who check all the boxes for risk factors such as older age, frailty, disability, immunosuppression, generalized systemic inflammation, and multiple comorbidities (e.g., hypertension, diabetes, and cardiorenovascular diseases) that predispose to severe disease and death.
These comorbidities are commonly treated with renin angiotensin system blockers, such as angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs), which increase levels of ACE2. The continued use of ACEIs/ARBs is the centerpiece of an intense debate because, on the one hand, SARS-CoV-2 co-opts ACE2 for target cell entry, but on the other, ACE2 overexpression may counterbalance vasoconstriction and profibrotic processes and thereby reduce the incidence or mortality associated with COVID-19–associated ALI or acute respiratory distress syndrome.
Another controversy involves whether or not to continue cancer treatment given the high transmissibility potential of the virus; however, since no expert consensus recommendations have been issued to date and prognosis, stage and responses to therapy are highly heterogeneous, the risk-benefit tradeoff and subsequent treatment plan are highly individualized and context dependent.
Currently the focus of treatment is infection control, appropriate symptomatic care and oxygen therapy. No approved medication or vaccine has been developed but promising activity has been reported for hydroxychloroquine, chloroquine, arbidol, remdesivir, convalescent sera, and favipiravir and several repurposed agents with antitumor properties are under investigation including thalidomide and RRx-001 which may hopefully bridge the gap from the time COVID-19 is first diagnosed until the primary anticancer therapy is restarted.
Finally, multiple comparisons have been made between the all-out mobilization efforts to combat COVID-19 with the massive scale-up of human and material resources that occurred during World War II [84,85]. In the words of Winston Churchill, Prime Minister of Great Britain from 1940 to 1945, whose intrepid fighting spirit, iron will, and intransigent defiance of tyranny galvanized the resolve of an entire nation to fight on in the face of seemingly impossible odds, oncologists on the frontlines that have answered the call should “never worry about action only inaction”.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.https://www.medscape.com/viewarticle/924596
- 2.Meo S.A., Alhowikan A.M., Al-Khlaiwi T., Meo I.M., Halepoto D.M., Iqbal M., Usmani A.M., Hajjar W., Ahmed N. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur. Rev. Med. Pharmacol. Sci. 2020 Feb;24(4):2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 3.Chan J.F., Kok K.H., Zhu Z., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes. Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuba K., Imai Y., Penninger JM., et al. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol. 2008;93(5):543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Kang Z., Gong H., et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes [Epub ahead of print] BioRxiv. 2020 doi: 10.1101/2020.01.30.927806. [DOI] [Google Scholar]
- 6.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes S. COVID-19 and angiotensin drugs: help or harm? Medscape. March 25, 2020 https://www.medscape.com/viewarticle/927542 [Google Scholar]
- 9.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published online ahead of print, 2020 Feb 24] [published correction appears in Lancet Respir Med. 2020 Feb 28;:] Lancet Respir. Med. 2020;S2213–2600(20) doi: 10.1016/S2213-2600(20)30079-5. 30079–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. published online March 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiheng X. Chance missed, but still there! Memoirs at the 10th anniversary of 2003 SARS outbreak. J. Thorac. Dis. 2013 Aug;5(Suppl. 2):S90–S93. doi: 10.3978/j.issn.2072-1439.2013.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature. 2020 Mar;579(7799):321. doi: 10.1038/d41586-020-00751-9. [DOI] [PubMed] [Google Scholar]
- 13.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. 2004 Dec;10(12 Suppl):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng H., Zhang M., Yang C., et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tognotti E. Lessons from the history of quarantine, from plague to influenza a. Emerg. Infect. Dis. 2013;19(2):254–259. doi: 10.3201/eid1902.120312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins
- 17.https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
- 18.Onder G., Rezza G., Brusaferro S. March 23, 2020. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. Published online. [DOI] [PubMed] [Google Scholar]
- 19.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat. Rev. Clin. Oncol. 2020;17(5):268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . Jan 29, 2020. Advice on the Use Of Masks in the Community, during Home Care and in Healthcare Settings in the Context of the Novel Coronavirus (2019-nCoV) Outbreak. [Google Scholar]
- 21.Rodriguez-Morales A.J., MacGregor K., Kanagarajah S., Patel D., Schlagenhauf P. Going global - travel and the 2019 novel coronavirus. Travel Med. Infect. Dis. 2020;101578 doi: 10.1016/j.tmaid.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.https://www.nejm.org/doi/full/10.1056/NEJMc2004973
- 23.Lu C.W., Liu X.F., Jia Z.F. nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224:e39) doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holshue M.L., et al. First Case of 2019 Novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothe C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cascella M., Rajnik M., Cuomo A., et al. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): 2020 Jan. Features, evaluation and treatment coronavirus (COVID-19) [Updated 2020 Mar 20] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration 2020. https://www.fda.gov/media/136622/download
- 29.Hase R., Kurita T., Muranaka E., Sasazawa H., Mito H., Yano Y. A case of imported COVID-19 diagnosed by PCR-positive lower respiratory specimen but with PCR-negative throat swabs. Infect Dis (Lond). 2020 Apr 2:1–4. doi: 10.1080/23744235.2020.1744711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cipriano M., Ruberti E. Giacalone a (march 26, 2020) gastrointestinal infection could be new focus for coronavirus diagnosis. Cureus. 2020;12(3):e7422. doi: 10.7759/cureus.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bordi L., Nicastri E., Scorzolini L., et al. Differential diagnosis of illness in patients under investigation for the novel coronavirus (SARS-CoV-2), Italy, February 2020. Euro. Surveill. 2020;25(8) doi: 10.2807/1560-7917.ES.2020.25.8.2000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding Q., Lu P., Fan Y., Xia Y., Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 2020 Mar 20;10.1002(jmv.25781) doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geiben-Lynn R., Greenland J.R., Frimpong-Boateng K., Letvin N.L. Kinetics of recombinant adenovirus type 5, vaccinia virus, modified vaccinia Ankara virus, and DNA antigen expression in vivo and the induction of memory T-lymphocyte responses. Clin. Vaccine Immunol. 2008;15:691–696. doi: 10.1128/CVI.00418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- 35.Su S., Wong G., Shi W., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368(6490) doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boussios S., Pentheroudakis G., Katsanos K., Pavlidis N. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann. Gastroenterol. 2012;25(2):106–118. [PMC free article] [PubMed] [Google Scholar]
- 39.Yeo C., Sanghvi K., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. 2020;5:P335–P337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am. J. Roentgenol. 2020 Mar 4:1–7. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 41.Zheng M., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. February 18, 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kline J.A., et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J. Thromb. Haemost. 2008 May;6(5):772–780. doi: 10.1111/j.1538-7836.2008.02944.x. [DOI] [PubMed] [Google Scholar]
- 44.Douma R.A., Gibson N.S., Gerdes V.E., Büller H.R., Wells P.S., Perrier A., et al. Validity and clinical utility of the simplified Wells rule for assessing clinical probability for the exclusion of pulmonary embolism. Thromb. Haemost. 2009 Jan;101(1):197–200. [PubMed] [Google Scholar]
- 45.Management of ill travellers at points of entry—international airports, seaports and ground crossings—in the context of COVID-19 outbreak. World Health Organization website; 16, 2020. Published February. [Google Scholar]
- 46.Knowlton S.D., Boles C.L., Perencevich E.N., et al. Bioaerosol concentrations generated from toilet flushing in a hospital-based patient care setting. Antimicrob. Resist. Infect. Control. 2018;7:16. doi: 10.1186/s13756-018-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.United States Centers for Disease Control and Prevention (CDC) Strategies for Optimizing the Supply of PPE. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html
- 48.Lan L., Xu D., Ye G., et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;27 doi: 10.1001/jama.2020.2783. Published online February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan K., Liu B., Li C., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020 Apr 6;202004168 doi: 10.1073/pnas.2004168117. e-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weingartl H., Czub M., Czub S., et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 2004;78(22):12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gershwin L.J. Adverse reactions to vaccination: from anaphylaxis to autoimmunity. Vet. Clin. North Am. Small Anim. Pract. 2018 Mar;48(2):279–290. doi: 10.1016/j.cvsm.2017.10.005. (Epub 2017 Nov 29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends In Immunol. 2 April 2020 doi: 10.1016/j.it.2020.03.007. Available online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li C.K., Wu H., Yan H., Ma S., Wang L., Zhang M. T cell responses to whole SARS coronavirus in humans. J. Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Channappanavar R., Fett C., Zhao J., et al. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. Sep 2014;88(19):11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burrell L.M., Johnston C.I., Tikellis C., Cooper M.E. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol. Metab. 2004 May-Jun;15(4):166–169. doi: 10.1016/j.tem.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li G., Hu R., Zhang X. Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens. Res. 2020;43(6):588–590. doi: 10.1038/s41440-020-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 58.Liu C.X., Hu Q., Wang Y., et al. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol. Med. 2011;17:59–69. doi: 10.2119/molmed.2010.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuba K., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marshall R.P. The pulmonary renin-angiotensin system. Curr. Pharm. Des. 2003;9(9):715–722. doi: 10.2174/1381612033455431. [DOI] [PubMed] [Google Scholar]
- 61.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.AlGhatrif M., Cingolani O., Lakatta E.G. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol. April 03, 2020;5(7):747–748. doi: 10.1001/jamacardio.2020.1329. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Backhaus A. March 22, 2020. Coronavirus: Why it’s So Deadly in Italy. Accessed. [Google Scholar]
- 64.Lakatta E.G. The reality of getting old. Nat. Rev. Cardiol. 2018;15(9):499–500. doi: 10.1038/s41569-018-0068-y. [DOI] [PubMed] [Google Scholar]
- 65.Xu J., Fan J., Wu F., et al. The ACE2/angiotensin-(1–7)/mas receptor axis: pleiotropic roles in cancer. Front. Physiol. 2017;8:276. doi: 10.3389/fphys.2017.00276. Published 2017 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.South A.M., Tomlinson L., Edmonston D., et al. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020;16(6):305–307. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinter M., Jain R.K. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl. Med. 04 Oct 2017;9(410) doi: 10.1126/scitranslmed.aan5616. eaan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease [published online ahead of print, 2020 Mar 28] Int. J. Cardiol. 2020;S0167–5273(20) doi: 10.1016/j.ijcard.2020.03.063. 31593-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu W., Li H. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11938173.v5. [DOI] [Google Scholar]
- 70.Lippi G., et al. 2020 Apr 11. Hematol Transfus Cell Ther. [Google Scholar]
- 71.Zhang L., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;0(Issue 0) doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perlin E., Bang K.M., Shah A., et al. The impact of pulmonary infections on the survival of lung cancer patients. Cancer. 1990;66:593–596. doi: 10.1002/1097-0142(19900801)66:3<593::aid-cncr2820660331>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 73.Liang W., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet. Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sidaway P. COVID-19 and cancer: what we know so far. Nat. Rev. Clin. Oncol. 2020;17(6):336. doi: 10.1038/s41571-020-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Centers for Medicare & Medicaid Services . Telehealth Services; March 2020. Medicare Learning Network. MLN901705. [Google Scholar]
- 76.https://www.esmo.org/covid-19-and-cancer
- 77.Nakayama H., Kanemoto A., Kikuchi K., Matsuki K., Tomobe M., Tsukamoto S., Takeshima H., Oshiro Y., Sugahara S., Tokuuye K. Delayed radiotherapy for patients with localized prostate cancer: validation by propensity score matching. Anticancer Res. 2013 Apr;33(4):1629–1633. [PubMed] [Google Scholar]
- 78.Hanna T.P., Evans G.A., Cancer Booth C.M. COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat. Rev. Clin. Oncol. 2020 April 2;17(5):268–270. doi: 10.1038/s41571-020-0362-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.https://www.ecdc.europa.eu/sites/default/files/documents/RRA-seventh-update-Outbreak-of-coronavirus-disease-COVID-19.pdf
- 80.https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html
- 81.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 82.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-guidance-conducting-clinical-trials
- 83.BHR de Paula, Araújo I., Bandeira L., NMPB Barreto, Doherty G.J. Recommendations from national regulatory agencies for ongoing cancer trials during the COVID-19 pandemic de Paula BHR, Araújo I, Bandeira L, Barreto NMPB, Doherty GJ. Recommendations from national regulatory agencies for ongoing cancer trials during the COVID-19 pandemic. Lancet Oncol. 2020 Apr 8;S1470–2045(20):30226. doi: 10.1016/S1470-2045(20)30226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDermott A. PREVIEW inner workings: Molecular biologists offer ‘wartime service’ in the effort to test for COVID-19 McDermott A. Inner Workings: Molecular biologists offer “wartime service” in the effort to test for COVID-19. Proc Natl Acad Sci U S A. 2020;117(18):9656–9659. doi: 10.1073/pnas.2006240117. https://blog.pnas.org/2020/04/preview-inner-workings-molecular-biologists-offer-wartime-service-in-the-effort-to-test-for-covid-19/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.https://fortune.com/2020/04/05/coronavirus-history-world-war-ii-warnings-lessons-predictions-covid-19/