Abstract
Objective
We sought to analyze the safety and feasibility of elective sonolucent cranioplasty in the setting of extracranial-to-intracranial (EC-IC) bypass surgery to monitor bypass patency using ultrasound.
Methods
Patients who underwent direct EC-IC bypass surgery agreed to sonolucent cranioplasty at the time of surgery and received a sonolucent polymethyl methacrylate (PMMA) implant. Besides monitoring clinical outcome, all patients received transcranioplasty ultrasound (TCUS) on postoperative day 1 and at last follow-up. In addition, bypass patency was confirmed using catheter angiogram and fit of implant using computed tomography. Patient-rated outcome was assessed through phone questionnaire.
Results
EC-IC bypass surgery with PMMA cranioplasty was successful in all 7 patients with patent bypasses on postoperative angiogram. Direct TCUS was feasible in all patients, and bypass patency was monitored. There were no complications such as postoperative hemorrhagic/ischemic complications related to the bypass procedure in this patient population, as well as no complications related to the PMMA implant. Postoperative computed tomography showed favorable cosmetic results of the PMMA implant in both the pterional area for superficial temporal artery−middle cerebral artery bypasses and parietooccipital area for occipital artery−middle cerebral artery bypasses as confirmed by high-rated overall patient satisfaction with favorable cosmetic, pain, and sensory patient-rated outcomes.
Conclusions
In this study we were able to show that this novel technique is safe, allows for patency assessment of the EC-IC bypass using bedside TCUS technique, and is cosmetically satisfying for patients.
Key words: Extracranial-to-intracranial bypass, Real-time ultrasound monitoring, Sonolucent cranioplasty, Transcranioplasty ultrasound
Abbreviations and Acronyms: EC-IC, Extracranial-intracranial; EDAS, Encephaloduroarteriosynangiosis; MCA, Middle cerebral artery; OA, Occipital artery; PMMA, Polymethyl methacrylate; pSTA, Parietal STA branch; STA, Superficial temporal artery; TCUS, Transcranioplasty ultrasound
Introduction
Direct surgical revascularization in the form of extracranial-intracranial (EC-IC) bypass surgery provides immediate therapeutic benefit in the treatment of stenotic or occluded intracranial arteries for different indications.1 , 2 EC-IC bypass surgery is critical in the prevention of ischemic and/or hemorrhagic stroke across pathologies that characteristically display, or whose treatment may produce, diminished or obstructed cerebral blood flow such as moyamoya disease, occlusive cerebrovascular disease, intracranial aneurysms, vessel injury, and skull base tumors.3, 4, 5, 6, 7 This technique involves the transcranial bridging of the external and internal carotid arteries via anastomosis of grafted or branching donor vessels, most commonly performed connecting the superficial temporal artery (STA) to the middle cerebral artery (MCA).5 , 8 While EC-IC bypass facilitates the instantaneous restoration of blood flow and opportunity for direct vessel network augmentation, postoperative monitoring of bypass patency is imperative to detect potential anastomosis complications.9, 10, 11, 12 Conventional imaging modalities used include magnetic resonance angiography, computed tomography angiography (CTA), and catheter-based digital subtraction angiography; however, these techniques are time consuming, cannot be performed at the bedside, demand significant institutional resources, and expose the patient to additional risk in the form of radiation, contrast administration, and/or intravascular catheterization. For these reasons, alternative modalities are desired to optimize the safety, efficacy, and frequency of postoperative bypass imaging.
Ultrasonography represents a burgeoning, readily available imaging modality that provides noninvasive, frequent, real-time monitoring without radiation exposure. While the attenuative properties of autologous cranial bone prohibit widespread transcranial ultrasound use, the use of postoperative ultrasound to monitor bypass patency has been described by measuring the flow of the donor artery before obscured by the bone flap when the donor artery dives intracranially to the anastomosis site.9 However, neither the anastomosis nor the recipient cortical vessels are well visualized with this technique. The advantage of the recently Food and Drug Administration−approved polymethyl methacrylate (PMMA) cranioplasty implants (Longeviti Neuro Solutions, Hunt Valley, Maryland, USA) is to overcome the obscured view through the skull and may therefore offer advantages as an adjunct for EC-IC bypass procedures. Although reimplanting the autologous bone flap is currently standard in EC-IC bypass, first-in-human feasibility assessment using this sonolucent PMMA cranioplasty implants (Longeviti Neuro Solutions) has recently been described by our group with a favorable result.13
Here, we build on the existing research by describing the first consecutive case series analyzing the safety, feasibility, and patient satisfaction of elective sonolucent cranioplasty in the setting of EC-IC bypass surgery for the use of postoperative transcranioplasty ultrasound (TCUS) in monitoring of bypass patency and blood flow.
Materials and Methods
Data on EC-IC bypass patients with PMMA Clearfit cranioplasty from a single-surgeon, single-center series were prospectively collected and retrospectively analyzed after IRB approval. Both the bypass procedure and use of the PMMA cranioplasty implant, as opposed to the autologous bone flap, were discussed with the patient before surgery and written consent was obtained.
Bypass Technique
For the EC-IC bypasses in this study, standard direct single- or double-barrel superficial temporal artery to middle cerebral artery (STA-MCA), occipital artery−to−middle cerebral artery (OA-MCA), or occipital artery to middle cerebral artery with a descending branch of the lateral circumflex femoral artery as interposition graft (OA-DLCFA-MCA) was performed using 10-0 nylon sutures (BV 75-3, Ethilon, Cincinnati, Ohio, USA). All anastomoses were performed in an end-to-side fashion. For the double-barrel STA-MCA bypass, 2 separate anastomoses were performed using 2 different STA donor branches to 2 different M4 recipients.
Cranioplasty Procedure
For all 7 cases the PMMA cranioplasty implant (Longeviti Neuro Solutions, Hunt Valley, Maryland, USA) was used and affixed to the skull using the Stryker cranial plating system (Stryker, Kalamazoo, Michigan, USA).
Ultrasound Procedure
Postoperative ultrasound was performed by the senior author JKB and senior/chief neurosurgical residents, all with several years of experience in intraoperative or bedside ultrasound in all patients on postoperative day 1 and, if available, at the postoperative clinic visit at 2 weeks after surgery. For inpatient ultrasound the following ultrasound devices were used: 1) Butterfly iQ (Butterfly Network, Guilford, Connecticut, USA) with a probe size of 185 × 56 × 35 mm and 2-dimensional array, 9000 micromachined sensors curved and linear features; 2) Samsung HM70A (Samsung Medison, Seoul, South Korea) with PE2-4 phased array transducer; and 3) Philips Sparq (Philips Healthcare, Baltimore, Maryland, USA) with an L12-4 transducer. For clinic follow-up visits the Butterfly iQ portable device was used only. The following Butterfly iQ presets were used: vascular access and vascular deep vein in M-mode or color Doppler.
TCUS was performed over the skin using standard ultrasound gel to visualize the vasculature (especially the donor bypass vessel and anastomosis) and the surrounding brain parenchyma through the PMMA cranioplasty implant.
Patient-Rated Outcome
All patients were contacted by telephone for a postoperative survey regarding the cosmetic and clinical outcome after the bypass surgery with focus on the PMMA implant and ultrasound imaging technique (Table 1 ). The survey took place at 2 months after surgery. The questions were modified on the basis of a previous publication by Park et al.14 In addition, patients who had a bypass surgery before were asked to comment on overall cosmetic result compared with the PMMA implant.
Table 1.
Questionnaire for Patient-Rated Outcome on PMMA Cranioplasty and Ultrasound Experience
| Item | Description | Score |
|---|---|---|
| Cosmetic result compared with previous non-PMMA surgery | Compared with previous surgery cosmetic result of incision/bone window | 0 (same), 1 (worse) or 2 (better) |
| Craniotomy-related pain | Local pain, tenderness, or discomfort in the head, especially at or around the cranial bone flap | 0 (no symptoms) to 4 (severe pain, tenderness, or discomfort) |
| Sensory symptoms | Hypoesthesia or paresthesia in the head | 0 (no symptoms) to 4 (very unpleasant) |
| Cosmetic complaints | Disfiguring scar, bone edge dent, skin hollowing | 0 (very pleasant) to 4 (very unpleasant) |
| Overall patient satisfaction | Postoperative patient satisfaction related to the surgical approach based on a VAS | 0 (very unsatisfactory) to 100 (very satisfactory) |
| Ultrasound experience (I) | Does it hurt when provider performs ultrasound through skin? | 0 (no pain) to 4 (very unpleasant) |
| Was there a difference postoperatively in patient versus follow-up in clinic after recovery? | Free text | |
| Ultrasound experience (II) | Do you like the fact that you can see the bypass with the provider together at bedside? | 0 (no) to 1 (yes) |
Results
Seven patients underwent EC-IC bypass surgery with sonolucent cranioplasty at our hospitals between November 2019 and March 2020. The gender ratio was 1 male to 6 females. The mean age was 47 (range 37–64 years). The most common indication for bypass was moyamoya disease (86%, n = 6); one patient had medically refractory intracranial atheroocclusive disease. The decision for surgery was influenced in all cases by the presence of new or persistent transient ischemic attacks despite best medical management, with or without prior cerebral bypass surgery. Four patients had undergone previous bypass surgery (57%). In this series, all patients underwent low-flow augmentation bypasses to M4 recipients. The majority underwent STA-MCA anastomosis (71%, n = 5), one underwent OA-MCA (14%), and one received OA-descending lateral femoral circumflex artery−MCA interposition bypass (14%). Two of the STA-M4 MCA bypass patients received a double-barrel bypass (29%).
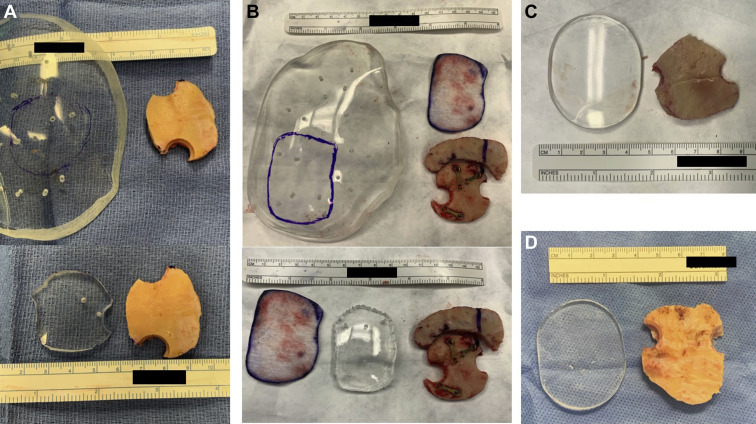
Two shapes of sonolucent PMMA implants were used in this series: 1) large, slightly curved piece used as raw material for cutting of a specific cranioplasty and 2) small, disk-shaped implant with greater curvature that could be used with minimal to no additional cutting or shaping (Figure 1 ). The latter is the product of surgeon-manufacturer collaboration to improve on the initial implant. The large implant was used in 4 patients (57%), and the small disk implant was used in 3 patients (43%). The large implant was used in both OA-involved surgeries (29%).
Figure 1.
Overview of evolution of different sonolucent polymethyl methacrylate (PMMA) implants used in this patient cohort. (A) Large, slightly curved piece used as raw material for cutting of a specific cranioplasty after outlining the size (top) and after cut out (bottom). (B) Similar to A, a large PMMA implant was cut to a specific size as needed to cover a cranial defect of a recraniotomy defect with additional craniotomy. A piece of telfa (white) was used to outline the size needed. (C and D) Small, disk-shaped implant curved (C) or flat (D) depending on the needs that could be used with minimal to no additional cutting or shaping.
Regardless of the size of initial implant, all fit well after they were shaped to the size of the cranial defect and achieved cosmetically favorable results both clinically and radiographically (Figures 2 and 3 ). The implant fit without step-off in all patients, and the slightly curved implant fit in continuity with the surrounding skull. The implant slightly thinner than the original bone flap was ideal in this bypass setting to avoid compressing the donor artery on its way to the anastomosis site on the surface of the brain (see Figures 2 and 3). Similar to bypass surgeries without a PMMA implant, we left a small area uncovered inferiorly to transmit the donor artery to pass from extracranial to intracranial. There were no intraoperative or postoperative complications related to the PMMA implant.
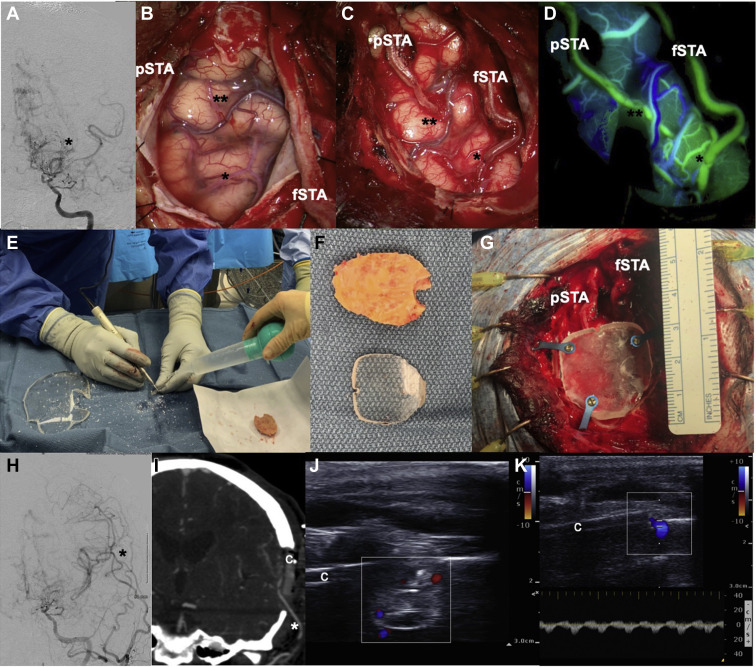
Figure 2.
A 41-year-old female patient with moyamoya disease presented with repeated transient ischemic attacks, and catheter angiogram (A) showed severe internal carotid artery narrowing with classic moyamoya disease vessel appearance (asterisk). She underwent left double-barrel superficial temporal artery−middle cerebral artery (MCA) bypass; intraoperative images are shown before (B), after the 2 anastomoses (C), and after indocyanine green angiography (D). (E−G) After the size of the PMMA cranioplasty was outlined, the implant was cut out using a craniotomy (E) and fixated using titanium plates and screws (G). Postoperative catheter angiogram (H) confirmed bypass patency (asterisk), and postoperative CTA in coronal reconstruction showed the PMMA implant and the patent bypass graft (I). Transcranioplasty Doppler ultrasound confirmed flow and bypass patency as well (J and K). 1 asterisk, frontal M4 MCA branch, 2 asterisks, temporal M4 MCA branch; c, PMMA implant; fSTA, frontal STA branch; pSTA, parietal STA branch.
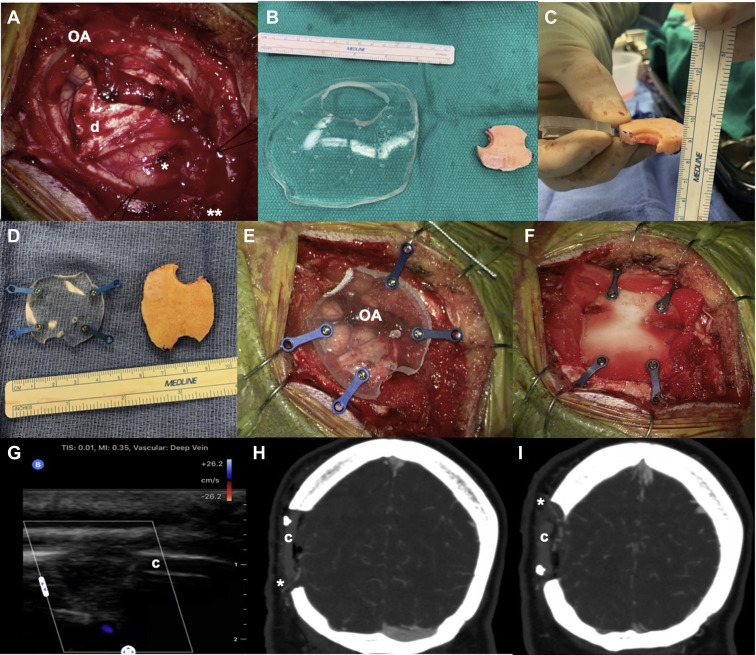
Figure 3.
This 42-year-old patient with moyamoya disease had a previous superficial temporal artery−middle cerebral artery (MCA) bypass and presented with new transient ischemic attacks despite previous surgery. The patient underwent an occipital artery (OA)-to-MCA bypass (A) using 1 of the distal OA branches for a direct bypass anastomosis (1 asterisk) and the second branch as an encephaloduroarteriosynangiosis (EDAS) (2 asterisks), as well as the dura for dural inversion technique (D). (B−D) A large, slightly curved polymethyl methacrylate implant was used as raw material for cutting of a specific cranioplasty after outlining the size. In this case the top and bottom portion of the implant were left open to allow enough space for the inflow and outflow artery of the direct bypass and EDAS (E) and was then fixated with titanium screws and plates (F) After only Duragen (Integra Lifesciences, Plainsboro Township, New Jersey, USA) was used to close the dura without compressing the graft. Postoperative transcranioplasty Doppler ultrasound (G), as well as computed tomography angiography in coronal reconstruction (H and I), confirmed bypass patency with inflow OA bypass graft (1 asterisk) exiting the distal EDAS branch (2 asterisks).
Postoperative bedside TCUS allowed for identification of the donor artery, anastomosis site, and recipient cortical artery using Doppler in real time (see Figures 2 and 3). Patency was confirmed in all cases using qualitative color-coded Doppler ultrasound and confirmed postoperative angiogram results (CTA or catheter angiogram) with an agreement/concordance rate of 100% in these 7 patients. Quantitative measurements were not performed. Onlay nonsuturable dural substitutes were used for all patients in this study to cover the dural opening without compressing the donor artery. These included Duragen (Integra Lifesciences, Plainsboro Township, New Jersey, USA) (n = 5) and DuraMatrix (Stryker, Kalamazoo, Michigan, USA) (n = 2). We did not see a significant image quality difference of the used dura substitutes in combination with the PMMA implant during postoperative TCUS imaging.
Clinical follow-up visits were performed in 3 patients and non in-person follow-up visits for 4 patients with a mean last follow up time of 48.6 days (range 6–106). In-office TCUS confirmed the postoperative findings with bypass patency in all cases (n = 3). The other 4 patients were not seen in the office yet, and telephone or virtual follow-up visits were performed due to the COVID-19 pandemic crisis.
Patient-reported outcome based on a phone questionnaire was available in 6 patients (response rate 86%) and showed an excellent overall patient satisfaction rating for all (n = 6, 100%) (Table 2 ). Three of the 6 patients had a previous bypass surgery and rated the cosmetic result better (n = 2) or the same (n = 1). Pain or sensory complaints due to the cranioplasty was low with 4 out of 6 patients rating the lowest pain level (0 out of 0–4 rating). Two patients rated mild pain (1 out of 0–4 rating) and mild numbness (1 and 2 out of 0–4 rating), which is mainly present at night in 1 of the 2 patients. Patients who only received inpatient ultrasound after the bypass procedure did not remember the ultrasound experience and were not able to answer these questions. The 3 patients with ultrasound follow-up all enjoyed the fact of seeing their bypass in real time with the provider, and 1 out of 3 complained of pain during the ultrasound as an inpatient, which was not present during follow-up ultrasound imaging. The other 2 patients did not experience pain during the inpatient or follow-up ultrasound imaging.
Table 2.
Patient-Rated Outcome on Polymethyl Methacrylate Cranioplasty and Ultrasound Experience
| Parameter and Score | Patient-Rated Outcome |
|---|---|
| Cosmetic result compared with previous non-PMMA surgery | Number = 3 |
| 0 (same) | 1 |
| 1 (worse) | 0 |
| 2 (better) | 2 |
| Craniotomy-related pain | Number = 6 |
| 0 (no symptoms) | 4 |
| 1 | 2 |
| 2 | 0 |
| 3 | 0 |
| 4 (severe pain, tenderness, or discomfort) | 0 |
| Sensory symptoms | Number = 6 |
| 0 (no symptoms) | 4 |
| 1 | 1 |
| 2 | 1 |
| 3 | 0 |
| 4 (very unpleasant) | 0 |
| Cosmetic complaints | Number = 6 |
| 0 (no symptoms) | 5 |
| 1 | 1 |
| 2 | 0 |
| 3 | 0 |
| 4 (very unpleasant) | 0 |
| Overall patient satisfaction | Number = 6 |
| Mean VAS | 100 |
| Ultrasound experience (I) | Number = 3 |
| 0 (no symptoms) | 2 (3 at follow-up) |
| 1 | 0 |
| 2 | 0 |
| 3 | 1 (0 at follow-up) |
| 4 (very unpleasant) | 0 |
| Ultrasound experience (II) | Number = 3 |
| 0 (no) | 0 |
| 1 (yes) | 3 |
Cost effectiveness was compared between PMMA versus native bone flap. In calculating the cost of each method, the cost of the cranioplasty and postoperative imaging was assessed. For the standard native bone flap procedures, postoperative catheter angiogram right after surgery or intraoperatively is performed followed by a CTA/CTP a few months later. A late catheter angiogram is performed 3–5 years afterward. Inpatient CTA/CTP and catheter angiography studies have a wide range of reported cost. The average CT head noncontrast is reported at approximately $1400 and cerebral catheter angiograms depending on the number of vessels with an average cost of $4800.15 , 16 On the basis of this calculation, the native bone procedure with follow-up would cost the patient approximately $11,000 (2 catheter angiograms and 1 CTA/CTP). However, especially in moyamoya disease patients with bilateral disease and progression, they may even require more imaging over a time period of 5 years. In the case of PMMA cranioplasty without imaging except TCUS, TCUS is performed at postoperative day 1 for a baseline assessment and then postoperative day 14 during the first clinic visit after surgery and then another long-term FU study a few years later. The cranioplasty cost can range from $500 to several thousand dollars depending on size.17 Since in this situation the smallest cranioplasty size is used (premade PMMA disks), the cost would be on the lower end. Notably, the cranioplasties are costs to the hospital and not the patient, unlike the imaging studies. For exclusive use of ultrasound for follow-up imaging in this case, the cost to the patient for follow-up imaging is approximately $900 (3 TCUS procedures).18 Also, these TCUS can be billed in the neurosurgical clinic setting and are revenues for the neurosurgery department instead of CTA and catheter angiogram, which is billed through diagnostic radiology.
Discussion
We have described the first case series of elective cranioplasty for ultrasound imaging of EC-IC bypass and demonstrated its safety, feasibility, and successful cosmetic outcome. Similar to our first published case, postoperative follow-ups to date indicate that there have been no implant-related complications to any patients across the performed bypass types.13 Favorable cosmesis was achieved per intraoperative review, CT imaging, and follow-up in anterior/lateral (STA-MCA bypasses) and posterior/lateral (OA-MCA and OA-DLCFA-MCA bypasses) craniotomies. These results suggest that minimal additional risk is associated with substitution of the autologous bone flap with a transparent PMMA cranioplasty, representing a viable reconstructive alternative with ultrasound imaging compatibility requiring little additional intraoperative time. Patency of the bypass assessment was feasible with transcranioplasty color-coded ultrasound and was confirmed with conventional imaging modalities.
Future follow-up of these patients will allow for better understanding of any intermediate- or long-term complications associated with the PMMA cranioplasty. Further, future investigations will focus on using ultrasound and Doppler to assess flow quantitatively in longitudinal follow-up settings. These data, as well as the potential ability to visualize developing collateral vessels, may shed light on both the timeline and potential promoting/inhibiting factors for vascular collateralization.
The feasibility of TCUS has only recently been explored with the incidental findings that alloplastic cranioplasty implants possess sonolucent attributes conducive to ultrasonography.19, 20, 21 Preliminary literature has displayed the value of using a sonolucent cranioplasty implant in monitoring a variety of intracranial variables including ventricular size, hematoma, and cerebral blood flow and vessel patency.13 , 22 Being highly portable and highly cost-effective, ultrasound imaging studies enable intraoperative and postoperative monitoring of longitudinal changes in patient vascularity and/or neurologic condition, being readily performed in an outpatient setting allowing comparison with immediate postoperative baseline. Additionally, alloplastic cranial implants provide ideal cosmetic outcomes by circumventing resorption complications commonly associated with autologous bone flap replacement.13 , 23, 24, 25 However, while there is clear potential benefit to TCUS, widespread use of the technique has yet to be achieved, commensurate with the novelty of its application.
Our patient-rated outcome showed favorable cosmetic results in this patient cohort. Although for EC-IC bypass the cranial defect is usually much smaller than in other patients requiring a cranioplasty, the 3 patients who had previous (non-PMMA) bypass surgery rated their PMMA cranioplasty cosmetic result the same or even better. As shown in previous studies, cosmetic results matter to patients, especially in the frontotemporal region.14 , 26 It has been shown that even a dent or irregularity of the cranioplasty may jeopardize the success of the surgery.26 It was discovered that TCUS imaging may be painful within the first days after surgery, which is explained by the postoperative pain over the wound incision, and should be kept in mind when performed at bedside. Our data showed that during outpatient follow-up there was no more pain after recovery from surgery and all patients enjoyed the opportunity to see the patent bypass together with the health care provider in real time.
For vascular neurosurgery, TCUS with Doppler has the potential to benefit both patient and providers in the future since it may add or replace invasive or x-ray−based imaging modalities and provides the provider with instant information at bedside in real-time inpatient and outpatient settings. Besides direct EC-IC bypass surgeries, indirect revascularization with sonolucent cranioplasty may glean benefit in the ability to assess vascular collateralization over time using ultrasound imaging. While doing this technique, the surgeon must keep in mind that titanium plates used to fixate the PMMA implant and skin closure using staples may cause ultrasonic artifacts interfering with TCUS assessment. With more experience using this technique in the future, we will be able to further optimize TCUS assessment and early detection of vascular complications at bedside. In addition, dural substitute use in patients assessed by TCUS is also an important topic and sonolucent quality of dural substitutes used after bypass surgery has not yet been systemically tested.
Regarding the cost analysis as presented, elective PMMA cranioplasty may reduce costs for the patient if only TCUS instead of catheter angiogram/CTA is used. Also, it is worth mentioning that cranioplasty itself is not charged to the patients but rather to the hospital and that TCUS can be billed in the clinical setting by the neurosurgeon performing the TCUS, increasing revenue for the neurosurgery department. All these calculations are currently hypothetical, but as more experience and data of this technique arise, it may be financially beneficial for the patient with reduced use of x-ray−based imaging modalities during follow-up.
While preliminary results are encouraging and opportunities for application are numerous, future research is necessary to better understand the reliability and efficacy of TCUS in both its present and prospective neurosurgical applications.
Limitations
Various limitations apply to this case series. All grafts were patent, and therefore the ability of TCUS to identify graft failure or discriminate other flow compromise could not be thoroughly assessed given no failure group. Detection of minor flow changes in patent grafts also needs to be further assessed, specifically the detection of partial decrease in flow (e.g., by extrinsic compression, kinking) or spasm. Therefore future work will aim to quantitatively assess patency via flow measurements, as done in Morton et al9 with native bone flaps. Further, while the results are promising, this case series represents preliminary work that will need to be built on and updated.
Conclusion
This preliminary work suggests cranial reconstruction with transparent sonolucent PMMA cranioplasty implants may offer benefit over autologous bone flap replacement by providing ultrasound compatibility for instant bedside ultrasound imaging. The present case series displayed the safety, feasibility, and cosmetic outcomes of elective sonolucent cranioplasty in the context of EC-IC bypass surgery for postoperative ultrasound monitoring of bypass patency. TCUS using elective sonolucent implants helps overcome the attenuative limitations of cranial bone, providing a cost-effective imaging technique for immediate and follow-up postoperative bypass patency surveillance. This early work will need to be built on as more experiences are added and results updated.
CRediT authorship contribution statement
Alex R. Flores: Data curation, Writing - original draft, Writing - review & editing. Visish M. Srinivasan: Conceptualization, Writing - original draft, Writing - review & editing. Jill Seeley: Data curation. Charity Huggins: Writing - original draft, Data curation. Peter Kan: Investigation. Jan-Karl Burkhardt: Conceptualization, Writing - original draft, Writing - review & editing.
Footnotes
Conflict of interest statement: Burkhardt, J.-K. is on the advisory board for Longeviti Neuro Solutions. All other authors have no conflicts of interest to disclose.
References
- 1.Baaj A.A., Agazzi S., Sayed Z.A., Toledo M., Spetzler R.F., van Loveren H. Surgical management of moyamoya disease: a review. Neurosurg Focus. 2009;26:E7. doi: 10.3171/2009.01.FOCUS08293. [DOI] [PubMed] [Google Scholar]
- 2.Morton R.P., Moore A.E., Barber J. Monitoring flow in extracranial-intracranial bypass grafts using duplex ultrasonography: a single-center experience in 80 grafts over 8 years. Neurosurgery. 2014;74:62–70. doi: 10.1227/NEU.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 3.Kawashima A., Andrade-Barazarte H., Jahromi B.R. Superficial temporal artery: distal posterior cerebral artery bypass through the subtemporal approach: technical note and pilot surgical cases. Oper Neurosurg (Hagerstown) 2017;13:309–316. doi: 10.1093/ons/opw033. [DOI] [PubMed] [Google Scholar]
- 4.Tayebi Meybodi A., Huang W., Benet A., Kola O., Lawton M.T. Bypass surgery for complex middle cerebral artery aneurysms: an algorithmic approach to revascularization. J Neurosurg. 2017;127:463–479. doi: 10.3171/2016.7.JNS16772. [DOI] [PubMed] [Google Scholar]
- 5.Wessels L., Hecht N., Vajkoczy P. Bypass in neurosurgery-indications and techniques. Neurosurg Rev. 2019;42:389–393. doi: 10.1007/s10143-018-0966-9. [DOI] [PubMed] [Google Scholar]
- 6.Ishishita Y., Tanikawa R., Noda K. Universal extracranial-intracranial graft bypass for large or giant internal carotid aneurysms: techniques and results in 38 consecutive patients. World Neurosurg. 2014;82:130–139. doi: 10.1016/j.wneu.2013.02.063. [DOI] [PubMed] [Google Scholar]
- 7.Grubb R.L., Jr., Powers W.J., Clarke W.R., Videen T.O., Adams H.P., Jr., Derdeyn C.P. Surgical results of the Carotid Occlusion Surgery Study. J Neurosurg. 2013;118:25–33. doi: 10.3171/2012.9.JNS12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman R., Lee M., Achrol A. Clinical outcome after 450 revascularization procedures for moyamoya disease. Clinical article. J Neurosurg. 2009;111:927–935. doi: 10.3171/2009.4.JNS081649. [DOI] [PubMed] [Google Scholar]
- 9.Morton R.P., Abecassis I.J., Moore A.E. The use of ultrasound for postoperative monitoring of cerebral bypass grafts: a technical report. J Clin Neurosci. 2017;40:169–174. doi: 10.1016/j.jocn.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Kawamata T., Kawashima A., Yamaguchi K., Hori T., Okada Y. Usefulness of intraoperative laser Doppler flowmetry and thermography to predict a risk of postoperative hyperperfusion after superficial temporal artery-middle cerebral artery bypass for moyamoya disease. Neurosurg Rev. 2011;34:355–362. doi: 10.1007/s10143-011-0331-8. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura M., Shimizu H., Mugikura S., Tominaga T. Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol. 2009;71:223–227. doi: 10.1016/j.surneu.2007.07.077. [DOI] [PubMed] [Google Scholar]
- 12.Mikami T., Suzuki H., Ukai R. Predictive factors for acute thrombogenesis occurring immediately after bypass procedure for moyamoya disease. Neurosurg Rev. 2020;43:609–617. doi: 10.1007/s10143-019-01086-4. [DOI] [PubMed] [Google Scholar]
- 13.Hadley C., North R., Srinivasan V., Kan P., Burkhardt J.K. Elective sonolucent cranioplasty for real-time ultrasound monitoring of flow and patency of an extra- to intracranial bypass. J Craniofac Surg. 2020;31:622–624. doi: 10.1097/SCS.0000000000006225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J., Son W., Kwak Y., Ohk B. Pterional versus superciliary keyhole approach: direct comparison of approach-related complaints and satisfaction in the same patient. J Neurosurg. 2018;130:220–226. doi: 10.3171/2017.8.JNS171167. [DOI] [PubMed] [Google Scholar]
- 15.Paul A.B., Oklu R., Saini S., Prabhakar A.M. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12:453–457. doi: 10.1016/j.jacr.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 16.How much does an angiogram cost? CostHelper. https://health.costhelper.com/angiograms.html Available at: Accessed 08/01/2020, 2020.
- 17.Asemota A., Santiago G.F., Zhong S., Gordon C.R. Comparative cost analysis of single and mutli-stage temporal deformity correction following neurosurgical procedures. J Craniofac Surg. 2018;29:130–138. doi: 10.1097/SCS.0000000000004107. [DOI] [PubMed] [Google Scholar]
- 18.2020 CPT Reimbursement Reference Guide. 2020. https://clarius.com/wp-content/uploads/2020/04/CPT-2020-MKTG-00084-Rev-3.pdf Available at: Accessed 08/01/2020, 2020.
- 19.Spena G., Guerrini F., Grimod G., Salmaggi A., Mazzeo L.A. Polymethyl methacrylate cranioplasty is an effective ultrasound window to explore intracranial structures: preliminary experience and future perspectives. World Neurosurg. 2019;127:e1013–e1019. doi: 10.1016/j.wneu.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Belzberg M., Shalom N.B., Yuhanna E. Sonolucent cranial implants: cadaveric study and clinical findings supporting diagnostic and therapeutic transcranioplasty ultrasound. J Craniofac Surg. 2019;30:1456–1461. doi: 10.1097/SCS.0000000000005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mursch K., Behnke-Mursch J. Polyether ether ketone cranioplasties are permeable to diagnostic ultrasound. World Neurosurg. 2018;117:142–143. doi: 10.1016/j.wneu.2018.06.064. [DOI] [PubMed] [Google Scholar]
- 22.Belzberg M., Shalom N.B., Lu A. Transcranioplasty ultrasound through a sonolucent cranial implant made of polymethyl methacrylate: phantom study comparing ultrasound, computed tomography, and magnetic resonance imaging. J Craniofac Surg. 2019;30:e626–e629. doi: 10.1097/SCS.0000000000005651. [DOI] [PubMed] [Google Scholar]
- 23.Korhonen T.K., Salokorpi N., Ohtonen P. Classification of bone flap resorption after cranioplasty: a proposal for a computed tomography-based scoring system. Acta Neurochir (Wien) 2019;161:473–481. doi: 10.1007/s00701-018-03791-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rashidi A., Sandalcioglu I.E., Luchtmann M. Aseptic bone-flap resorption after cranioplasty—incidence and risk factors. PLoS One. 2020;15:e0228009. doi: 10.1371/journal.pone.0228009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malcolm J.G., Mahmooth Z., Rindler R.S. Autologous cranioplasty is associated with increased reoperation rate: a systematic review and meta-analysis. World Neurosurg. 2018;116:60–68. doi: 10.1016/j.wneu.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Fischer C.M., Burkhardt J.K., Sarnthein J., Bernays R.L., Bozinov O. Aesthetic outcome in patients after polymethyl-methacrylate (PMMA) cranioplasty—a questionnaire-based single-centre study. Neurol Res. 2012;34:281–285. doi: 10.1179/1743132812Y.0000000007. [DOI] [PubMed] [Google Scholar]