Abstract
Background
We describe key characteristics, interventions, and outcomes of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak within an inpatient geriatric psychiatry unit at the University of Washington Medical Center – Northwest.
Methods
After identifying 2 patients with SARS-CoV-2 infection on March 11, 2020, we conducted an outbreak investigation and employed targeted interventions including: screening of patients and staff; isolation and cohorting of confirmed cases; serial testing; and enhanced infection prevention measures.
Results
We identified 10 patients and 7 staff members with SARS-CoV-2 infection. Thirty percent of patients (n = 3) remained asymptomatic over the course of infection. Among SARS-CoV-2 positive patients, fever (n = 5, 50%) and cough (n = 4, 40%) were the most common symptoms. Median duration of reverse transcription polymerase chain reaction (RT-PCR) positivity was 25.5 days (interquartile range [IQR] 22.8-41.8) among symptomatic patients and 22.0 days (IQR 19.5-25.5) among asymptomatic patients. Median initial (19.0, IQR 18.7-25.7 vs 21.7, IQR 20.7-25.6) and nadir (18.9, IQR 18.2-20.3 vs 19.8, IQR 17.0-20.7) cycle threshold values were similar across symptomatic and asymptomatic patients, respectively.
Conclusions
Asymptomatic infection was common in this cohort of hospitalized, elderly individuals despite similar duration of SARS-CoV-2 RT-PCR positivity and cycle threshold values among symptomatic and asymptomatic patients.
Key Words: Inpatient psychiatry, COVID-19, Hospital outbreak, Elderly, Coronavirus
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus causing coronavirus disease 2019 (COVID-19), was initially described in Wuhan, China in late 2019.1 Infection with SARS-CoV-2 typically causes fever, cough, and shortness of breath, although the clinical spectrum can vary from asymptomatic carriage to critical illness.2, 3, 4, 5, 6 Older individuals have been disproportionately affected by COVID-19, with most deaths occurring in persons over the age of 60.6, 7, 8, 9, 10
SARS-CoV-2 has now spread to nearly every country in the world,11 and on March 11, 2020, the World Health Organization declared the COVID-19 outbreak to be a global pandemic.12 The first known case of COVID-19 in the United States was diagnosed in Snohomish County, Washington on January 20, 2020,13 and on February 28, 2020 Public Health – Seattle & King County identified an outbreak of COVID-19 within a Seattle-area long-term care (LTC) facility.10 Since that time, there have been over 64,151 cases of SARS-CoV-2 and 1,716 deaths due to COVID-19 in Washington State.14 While persons over the age of 60 represent 19% of diagnosed SARS-CoV-2 cases in Washington State, they account for 89% of COVID-19-related deaths.14 Outbreaks within LTC facilities have been central to the Washington State COVID-19 epidemic10 , 15; however, there have been no published reports of SARS-CoV-2 outbreaks within hospital-based settings. We describe key characteristics, interventions, and outcomes of a SARS-CoV-2 outbreak within an inpatient geriatric psychiatry unit at the University of Washington Medical Center – Northwest (UWMC – NW).
Index cases
On March 2, 2020, a 74-year-old man with a history of dementia, congestive heart failure, prior stroke, and hypertension was admitted to the UWMC-NW Geropsychiatric Center from a skilled nursing facility (SNF) due to increasing agitation. On March 7, 2020, he developed a fever to 39.2°C; laboratory evaluation revealed a white blood cell count of 5.33 THOU/μL, absolute lymphocyte count of 0.93 THOU/μL, and a negative influenza and respiratory syncytial virus reverse transcription polymerase chain reaction (RT-PCR). He continued to have intermittent fevers, and on March 11, 2020, he tested positive for SARS-CoV-2 by nasopharyngeal RT-PCR. He was transferred to the acute care unit, where a chest x-ray showed bilateral patchy opacities. During his acute care stay, he required intermittent supplemental oxygen but denied respiratory symptoms. On March 15, 2020, he was able to transfer back to the geriatric psychiatry unit in stable condition on droplet and contact isolation precautions.
Concurrently, on March 11, 2020, a 71-year-old man with a history of dementia, psychosis, and hypertension, who had been admitted to the Geropsychiatric Center on February 1, 2020, developed a fever to 38.6°C and new hypoxemia. Chest x-ray revelated a left lung opacity, and he tested positive for SARS-CoV-2 by nasopharyngeal RT-PCR. He was admitted to the acute care unit, where he developed worsening hypoxemia. His family elected to pursue comfort measures only, and he passed away on March 20, 2020.
Methods
Study design and setting
We conducted a prospective outbreak investigation of the novel SARS-CoV-2 virus within the UWMC – NW Geropsychiatric Center from March 11, 2020 to May 4, 2020. The Geropsychiatric Center, which is split between 2 physically distinct locked units (East and West), provides inpatient psychiatric care for individuals 60 years of age or older, specializing in dementia, depression, anxiety, and psychosis. In general, patients with more advanced dementia and higher care needs are admitted to the East unit, while higher functioning patients are admitted to the West unit. During periods of standard operating procedures, including the time period immediately preceding this outbreak, residents are permitted to ambulate freely and socialize within their locked unit. Residents typically eat together in a shared dining room and often spend considerable time in shared lounge spaces. While staff rotate between units on a daily basis, there is no comingling of patients between the East and West units.
Outbreak investigation
Following the diagnosis of 2 Geropsychiatric Center patients with SARS-CoV-2 infection on March 11, 2020 (day 0), all patients within these units were screened for SARS-CoV-2 on March 12, 2020 (day 1). All Geropsychiatric Center staff members were similarly offered SARS-CoV-2 screening on March 18 (day 7) and 19 (day 8). Symptomatic staff members had access to additional SARS-CoV-2 testing through the UW Medicine Employee Testing Clinic. Repeat surveillance testing for SARS-CoV-2 positive patients was performed at 3- to 7-day intervals until 2 negative results, separated by at least 24 hours, were obtained. Duration of SARS-CoV-2 RT-PCR positivity was defined as the number of days between a patient's initial positive RT-PCR test and the first of 2 consecutive negative RT-PCR tests.
All samples were analyzed at the University of Washington (UW) Virology Laboratory using either the UW SARS-CoV-2 real-time RT-PCR assay or the Hologic SARS-CoV-2 real-time RT-PCR assay.7 , 16 The UW SARS-CoV-2 real-time RT-PCR assay targets 2 distinct regions of the SARS-CoV-2 N gene and produces 2 cycle threshold (Ct) values per test, one for each amplified region of the N gene; whereas the Hologic SARS-CoV-2 real-time RT-PCR assay targets 2 conserved regions of the SARS-CoV-2 ORF1ab gene but produces only one Ct value, as amplification of both targets is recorded in the same channel.16 Both tests use a Ct cut off <40 to indicate viral detection. For this report, Ct values from the UW SARS-CoV-2 assay are reported as a mean of the N1 and N2 cycle thresholds.
Clinical symptoms, medical history, and radiographic findings for patients were obtained by review of the medical record. A symptom screen and review of medical history was performed over the phone for staff members who tested positive for SARS-CoV-2.
Statistical analysis
Descriptive statistics were calculated to summarize clinical characteristics and SARS-CoV-2 PCR cycle thresholds. All statistical analyses were preformed using R Studio (© R Foundation for Statistical Computing, 2016).
Ethics
Data presented in this study were obtained through investigation of a hospital-based disease outbreak. As such, approval by an institutional review board was not required, and informed consent was not obtained.
Results
Screening results and baseline characteristics
Following the identification of the 2 index SARS-CoV-2 cases on March 11, 2020 (day 0), the Geropsychiatric Center stopped admitting new patients, and the remaining 22 patients were screened for the virus. An initial round of screening on March 12 (day 1) identified 4 SARS-CoV-2 positive patients, and a second round of screening, performed on day 4, identified 3 additional SARS-CoV-2 positive patients. A third and fourth round of screening on days 5 and 7 resulted in no new SARS-CoV-2 diagnoses. One patient, who discharged from the Geropsychiatric Center on March 11 (day 0), returned to the emergency department on March 21 (day 10) with respiratory symptoms and tested positive for SARS-CoV-2.
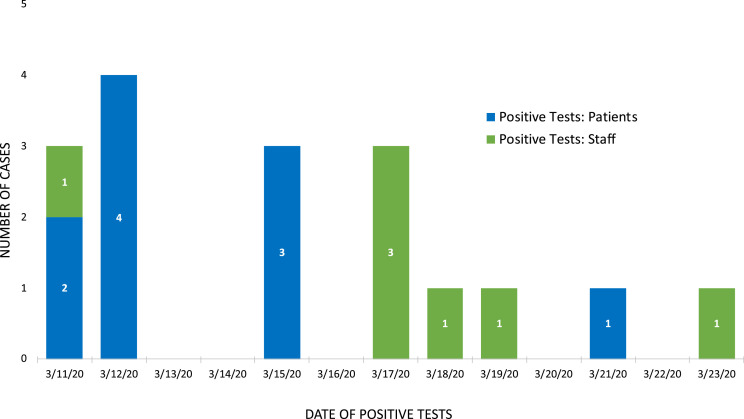
In total, 10 new cases of SARS-CoV-2 infection were detected among 25 patients in the Geropsychiatric Center from March 11 through 18, 2020 (Fig 1 ). All cases occurred among residents of the East unit, with 6 cases occurring among 3 sets of roommate pairs. The mean age of patients with and without SARS-CoV-2 infection was 77.8 years (standard deviation [SD] 8.5) and 71.7 years (SD 9.0), respectively. Among both groups, 40% of patients were female. Pre-existing medical conditions were common and are outlined in Table 1 .
Fig 1.
Date of diagnosis for confirmed cases of SARS-CoV-2 among patients and staff in the UWMC – NW Geropsychiatry Center. Positive patients are shown in blue. Positive staff members are shown in green.
Table 1.
Characteristics of SARS-CoV-2 positive and negative Geropsychiatric Center patients and SARS-CoV-2 positive staff
| SARS-CoV-2 positive patients (n = 10) | SARS-CoV-2 negative patients (n = 15) | SARS-CoV-2 positive staff (n= 7) | ||
|---|---|---|---|---|
| Age in years (SD) | 77.8 (8.5) | 71.7 (9.0) | 47.1 (9.1) | |
| Female sex (%) | 4 (40) | 6 (40) | 4 (57) | |
| Any symptoms (%) | 7 (70) | 4 (27) | 6 (86) | |
| Fever ≥38C | 5 (50) | 0 (0) | 2 (29) | |
| Cough | 4 (40) | 1 (7) | 5 (71) | |
| Dyspnea | 2 (20) | 0 (0) | 1 (14) | |
| Nasal congestion and/or sore throat | 2 (20) | 3 (20) | 5 (71) | |
| Myalgias | 1 (10) | 1 (7) | 2 (29) | |
| GI symptoms | 3 (30) | 1 (7) | 2 (29) | |
| Required supplemental O2 (%) | 3 (30) | 0 (0) | 0 (0) | |
| Intubated (%) | 0 (0)* | 0 (0) | 0 (0) | |
| Pre-existing medical condition (%) | 10 (100) | 14 (93) | 0 (0) | |
| Dementia | 9 (90) | 6 (40) | 0 (0) | |
| Heart disease | 4 (40) | 8 (53) | 0 (0) | |
| Hypertension | 9 (90) | 7 (47) | 0 (0) | |
| Diabetes | 2 (20) | 6 (40) | 0 (0) | |
| Underlying lung disease | 1 (10) | 0 (0) | 0 (0) | |
| Chronic kidney disease | 0 (0) | 1 (7) | 0 (0) | |
| Hyperlipidemia | 3 (3) | 8 (53) | 0 (0) | |
| Immunosuppressed | 0 (0) | 0 (0) | 0 (0) | |
| New abnormality on chest x-ray (%)† | 2 (67) | 0 (0) | n/a | |
| Death (%) | 1 (10) | 0 (0) | 0 (0) | |
One patient died of respiratory failure but was not intubated due to “do not intubate” status. RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Number of chest x-rays performed: SARS-CoV-2 positive patients n = 3; SARS-CoV-2 negative patients n = 1; SARS-CoV-2 positive staff n = 0.
Symptoms
Four (40%) SARS-CoV-2 positive patients were asymptomatic at the time of screening, with 3 (30%) remaining asymptomatic throughout the course of infection. A list of symptoms experienced over the course of SARS-CoV-2 infection is outlined in Table 1, with fever (n = 5, 50%) and cough (n = 4, 40%) being the most common. One death (10%) occurred in the SARS-CoV-2 positive group, with no deaths occurring in the SARS-CoV-2 negative group during the follow-up period.
Follow-up viral testing and cycle thresholds
Follow-up SARS-CoV-2 testing was performed at 3- to 7-day intervals for 9 of the 10 SARS-CoV-2 positive patients with the goals of removing isolation precautions, reducing personal protective equipment (PPE) usage, and supporting appropriate discharge planning. The patient who died of COVID-19 did not undergo follow-up testing. Among the 9 patients with follow-up testing data, the median duration of RT-PCR positivity was similar when comparing symptomatic (25.5, interquartile range [IQR] 22.8-41.8) and asymptomatic (22.0, IQR 19.5-29.0) patients (Table 2 ).
Table 2.
Cycle thresholds and duration of RT-PCR positivity among symptomatic and asymptomatic SARS-CoV-2 positive Geropsychiatric Center patients
| Patient | Ever symptomatic (y/n) | Initial cycle threshold | Nadir cycle threshold | Duration of RT-PCR positivity (days) |
|---|---|---|---|---|
| 1 | Yes | 30.75 | 30.75 | 26 |
| 2 | Yes | 19.00 | 19.00 | n/a* |
| 3 | No | 21.70 | 21.70 | 17 |
| 4 | Yes | 35.90 | 21.60 | 50 |
| 5 | Yes | 18.85 | 18.85 | 11 |
| 6 | Yes | 18.60 | 18.60 | 25 |
| 7 | No | 19.75 | 19.75 | 22 |
| 8 | Yes | 20.65 | 15.80 | 47 |
| 9 | No | 29.40 | 14.20 | 29 |
| 10 | Yes | 17.75 | 17.75 | 22 |
No follow-up RT-PCR testing available. RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Ct values for the initial positive SARS-CoV-2 RT-PCR among the 10 patients ranged from 17.8 to 35.9 (median 20.2, IQR 18.9-27.5), and were similar when comparing symptomatic (median 19.0, IQR 18.7-25.7) and asymptomatic patients (median 21.7, IQR 20.7-25.6). Nadir Ct values were also similar across symptomatic (median 18.9, IQR 18.2-20.3) and asymptomatic groups (median 19.8, IQR 17.0-20.7; Table 2).
Staff
Between March 11 (day 0) and March 23, 2020 (day 12), 7 UWMC – NW Geropsychiatric Center staff members tested positive for SARS-CoV-2, 6 (86%) of whom were symptomatic at the time of testing (Fig 1). One tested positive through routine screening provided to 56 asymptomatic Geropsychiatric Center employees, indicating the prevalence of infection was 1.8% among asymptomatic staff. The mean age of SARS-CoV-2 positive employees was 47 years of age (SD 9.1), 57% (n = 4) were female, and none reported underlying medical conditions (Table 1). Among symptomatic employees, symptoms started between March 9 and March 20, 2020, and only 1 staff member (14%) was ever symptomatic at work. This person developed pleuritic chest pain on March 12 (day 1) and subsequently did not return to work until at least 7 days had elapsed from symptoms onset and 72 hours had elapsed following symptoms resolution, in accordance with UWMC – NW Employee Health policy. The most common symptoms reported by staff are outlined in Table 1.
Infection control measures
All positive SARS-CoV-2 tests occurred in patients on the East unit of the Geropsychiatric Center, with no cases occurring among patients on the West unit. Following a second round of screening on day 4, patients who remained negative for SARS-CoV-2 were cohorted in the West unit. The East unit was designated a COVID-19 ward for the remaining patients. Positive patients were initially isolated in single or double patient rooms with a trained observer monitoring donning and doffing of PPE (mask, goggles or face shield, gown, gloves) at the door of each room. On April 2, the East unit was converted into a “hot zone,” allowing SARS-CoV-2 patients access to an activity/dining room. The “hot zone” was marked with floor tape, and staff were required to don and doff PPE upon entry and exit. Within the “hot zone,” staff perform hand hygiene and changed an outer pair of gloves between patients but were allowed to wear the same gown, eye protection, and mask until they left the “hot zone,” or until these items became soiled. SARS-CoV-2 positive patients were not required to wear masks or other PPE in the “hot zone” and were allowed to interact with other positive patients.
During the follow-up period, patients were removed from precautions and transferred to the West unit after 2 negative SARS-CoV-2 PCR results, separated by at least 24 hours. Patients on the West unit were allowed to access common dining areas, but tables were spread over 6-feet apart, and entrance to the common areas was restricted to a small number of patients at a time. When possible, patients were kept in single rooms, and double room beds were spaced apart to the extent possible.
In addition to cohorting of patients, an outside environmental services team was brought in to perform extensive cleaning on both the East and West units, and the hand sanitizer in the Geropsychiatric Center was switched from benzalkonium chloride 0.13% to alcohol-based sanitizer, per the Centers for Disease Control and Prevention guidelines.17 No modifications to the airflow system were made on either unit. Optional prolonged-use masking was implemented for all UWMC-NW staff members on April 1, and mandatory masking took effect on April 27. Due to the cognitive and psychiatric comorbidities of the Geropsychiatric Center patients, universal masking of patients was logistically challenging and not enforced. No visitors were allowed on either unit for the duration of the outbreak.
Discussion
To the best of our knowledge, this is the first report of a SARS-CoV-2 outbreak within a hospital-based setting. This outbreak highlights 3 key findings from the SARS-CoV-2 pandemic thus far, including 1) the vulnerability of elderly persons to COVID-19, 2) the high rate of asymptomatic infection among elderly patients, and 3) the prolonged duration of PCR positivity.
The high rate of morbidity and mortality from SARS-CoV-2 infection among elderly individuals, particularly those with comorbidities, has been well described.6, 7, 8, 9, 10 King County, Washington emerged as the early epicenter of the SARS-CoV-2 epidemic in the United States, driven in large part by a sizeable outbreak among elderly residents of a LTC facility.10 Since that time, there has been extensive community transmission within the Seattle area, and approximately 200 LTC facilities have reported a case of SARS-CoV-2.10 , 15 , 18 We highly suspect that SARS-CoV-2 was introduced into the UWMC – NW Geropsychiatric Center by a patient coming from a local SNF, where cases of SARS-CoV-2 had been identified. Shared patient rooms, dining, and activity space within the East unit of the Geropsychiatric Center, as well as the ability of patients to ambulate and socialized freely before the identification of the SARS-CoV-2 outbreak likely allowed for rapid dissemination of SARS-CoV-2. Although community transmission was occurring in King County at the time of Geropsychiatric Center outbreak (7.8% of county-wide SARS-CoV-2 tests were positive on day 0 of the outbreak),14 the fact that no staff were symptomatic at work prior to outbreak identification, no cases of SARS-CoV-2 were observed within the West unit, where SARS-CoV-2 positive staff had rotated, and 6 of the 10 patient cases occurred among roommate pairs, supports the hypothesis that the virus was introduced by a SARS-CoV-2 positive patient and transmitted primarily through comingling and shared rooms on the East unit.
Despite the advanced age and medical vulnerability of our cohort, 30% of SARS-CoV-2 patients showed no symptoms of COVID-19 disease. Asymptomatic disease has been well described in the general population,2 , 3 , 19 and there is accumulating evidence that asymptomatic infection among elderly persons may be a major contributor to institutional outbreaks.20 , 21 A recent study published by colleagues at the UW described a similarly high proportion of asymptomatic infection among older adults, with 3 of 4 residents who tested positive for SARS-CoV-2 at a senior assisted living facility showing no signs or symptoms of infection.22 Another study at a Seattle area SNF found that 57% of the 23 residents who tested positive for SARS-CoV-2 were asymptomatic at the time of screening.15 However, 10 of the 13 SNF residents who were asymptomatic at the time of screening ultimately developed symptoms.15
Our findings are unique in that they demonstrate a high proportion of asymptomatic SARS-CoV-2 infection in an elderly population over time, with 40% of patients being asymptomatic at the time of screening and 30% remaining asymptomatic over several weeks of follow-up. Paired with the results of other local studies,15 , 20 , 22 our findings suggest that a symptom-based approach to screening in older adults may not be sufficient, particularly in congregate living or hospitalized populations. It is worth noting that only 1 SARS-CoV-2 positive staff members was asymptomatic, despite wide-spread screening of asymptomatic Geropsychiatric Center employees. The lower proportion of staff members with asymptomatic infection may speak toward the difficulty of performing symptom screening in patients with dementia, or perhaps to differences in the immunologic response between younger and older individuals.
Despite a lack of symptoms, it is notable that the median duration of SARS-CoV-2 RT-PCR positivity was 22 days among asymptomatic patients. It is also notable that Ct values were similar across symptomatic and asymptomatic groups. These results indicate that our asymptomatic patients had large quantities of viral RNA in their nasopharynx and may have experienced a prolonged duration of viral shedding. Given the common initial exposure among all geropsychiatric patients, it is unclear if any of the asymptomatic patients transmitted the virus to others; however, asymptomatic transmission has been described, and recent data suggest there is viable virus in specimens collected from asymptomatic patients.2 , 19 , 21 Our findings are in keeping with those from a recent study of asymptomatic and presymptomatic SARS-CoV-2 infections in a SNF in King County, Washington, where authors found no difference in cycle threshold values between symptomatic, presymptomatic and asymptomatic patients involved in a COVID-19 outbreak.15 , 21 The approximate duration of RT-PCR positivity observed in our investigation is slightly longer than that described in other studies.23, 24, 25
Our report has several limitations. First, we performed follow-up surveillance screening on SARS-CoV-2 negative patient within the Geropsychiatric Center for 7 days following identification of the index cases and may have missed asymptomatic infections that arose late in the incubation period. Second, our ability to identify symptoms in our geriatric cohort is limited by their mental status. While signs and symptoms such as cough, fever, and hypoxemia were easily identified by medical staff, more subjective complaints may have been missed. Based on epidemiologic factors, we are highly suspicious that SARS-CoV-2 was introduced to the Geropsychiatric Center from a patient who was symptomatic on the East unit for at least 4 days prior to diagnosis, after arriving from a SNF with known SARS-CoV-2 cases. However, given community wide transmission of SARS-CoV-2 at the time of the outbreak, we cannot say this with complete certainty, and a detailed history of possible community exposures was not obtained from staff. However, no staff members were symptomatic at work prior to the identification of cases and a staff-initiated outbreak is thought to be less likely. Finally, we are unable to determine the exact duration of RT-PCR positivity, as there were variable intervals between follow-up SARS-CoV-2 tests. Further data are needed to better understand the duration of SARS-CoV-2 RT-PCR positivity and how that correlates with viral shedding in older adults.
Conclusions
Our report highlights SARS-CoV-2 as an important cause of health care-acquired infection, particularly among older adults. In our cohort of hospitalized, elderly individuals, asymptomatic infection was common despite similar SARS-CoV-2 RT-PCR Ct values among symptomatic and asymptomatic patients. Our findings suggest that asymptomatic individuals can remain SARS-CoV-2 RT-PCR positive for a prolonged period of time, and a symptom-based approach to screening in older adults may not be sufficient, particularly in an outbreak setting. Our data support the widespread use of testing to screen all individuals in or entering institutional and hospital settings.
Acknowledgments
We would like to acknowledge the UWMC – NW Geropsychiatric Center patients. We thank all UWMC – NW Geropsychiatric Center staff for their role in addressing this outbreak.
Footnotes
Conflicts of interest: Authors report no conflicts of interest.
Funding: M.C. was supported in this work by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK007742-22). G.S. was supported in this work by a training grant from the National Institute of Allergy and Infectious Diseases (5T32AI007044-43).
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ling Z, Xu X, Gan Q, et al. Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. Eur J Radiol. 2020;126 doi: 10.1016/j.ejrad.2020.108956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. COVID-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMichael TM, Currie DW, Clark S, et al. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johns Hopkins Coronavirus Resource Center. Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2020. Available at: https://coronavirus.jhu.edu/map.html. Accessed March 30, 2020.
- 12.World Health Organization. Rolling updates on coronavirus disease (COVID-19): World Health Organization. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed March 30, 2020.
- 13.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washington State Department of Health. 2019 novel coronavirus outbreak (COVID-19): Washington State Department of Health. 2020. Available at: https://www.doh.wa.gov/emergencies/coronavirus. Accessed August 12, 2020.
- 15.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UW Medicine. SARS-CoV-2 (COVID-19) qualitative PCR: University of Washington Department of Lab Medicine. 2020. Available at: https://testguide.labmed.uw.edu/public/view/NCVQLT. Accessed March 31, 2020.
- 17.Centers for Disease Control and Prevention. Hand hygiene recommendations: guidance for healthcare providers about hand hygiene and COVID-19. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene.html. Accessed August 13, 2020.
- 18.Fields A, Cornwell P. Coronavirus killed hundreds at Washington state's long-term care facilities. Widespread testing may finally be near. The Seattle Times. 2020 [Google Scholar]
- 19.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roxby AA, Greninger AL, Hatfield KM, et al. Outbreak Investigation of COVID-19 among residents and staff of an independent/assisted living community for older adults in Seattle, Washington. JAMA Intern Med. 2020;180:1101–1105. doi: 10.1001/jamainternmed.2020.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roxby AC, Greninger AL, Hatfield KM, et al. Detection of SARS-CoV-2 among residents and staff members of an independent and assisted living community for older adults—Seattle, Washington. MMWR Morb Mortal Wkly Rep. 2020;69:416–418. doi: 10.15585/mmwr.mm6914e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing Y-H, Ni W, Wu Q, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]