Abstract
Voluntary wheel running is widely used as a physical activity (PA) model in rodents, but most studies investigate the beneficial effects of this intervention in socially isolated mice. Social isolation stress (SIS) is associated with vulnerability to oxidative stress and reduced mitochondrial activity. Thus, the aim of this study was to investigate the effects of free access to a running wheel for 21 days on the various markers of the cellular redox/antioxidant status as well as mitochondrial function of mice subjected to SIS or maintained in groups of 3 in the homecage. SIS increased thiobarbituric acid reactive substance (TBARS) levels in the cerebral cortex, and PA intervention was not able to reverse such alteration. PA reduced TBARS levels in the liver of grouped mice and gastrocnemius of socially isolated mice. PA increased nonprotein thiol (NPSH) levels in the cerebral cortex of grouped mice. Furthermore, socially isolated mice presented lower glutathione peroxidase (GPx) activity in the cerebellum and gastrocnemius, and glutathione reductase (GR) activity in the cerebral cortex and liver. By contrast, SIS induced higher GPx activity in the cerebral cortex and heart. PA reduced GPx (cerebral cortex) and GR (cerebral cortex and liver) activities of socially isolated mice. SIS caused higher activity of mitochondrial complexes I and II in the cerebral cortex, and the PA paradigm was not able to alter this effect. Interestingly, the PA produced antidepressant-like effect at both SIS and control groups. In conclusion, the results showed the influence of SIS for the effects of PA on the antioxidant status, but not on the mitochondrial function and emotionality.
Abbreviations: DTNB, 5,5-dithiobis-2-nitrobenzoic acid; ANOVA, Analysis of variance; CHP, Cumene peroxide; Ethylenediamine tetraacetic acid (EDTA), Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (also known as Egtazic acid, EGTA); GPx, Glutathione peroxidase; GR, Glutathione reductase; MDA, Malondialdehyde; NADH, Nicotinamide adenine dinucleotide; NADPH, Nicotinamide adenine dinucleotide phosphate; NPSH, Nonprotein thiols groups; GSSG, Oxidized glutathione; PA, Physical activity; GSH, Reduced glutathione; SIS, Social isolation stress; TST, Tail suspension test; TBARS, Thiobarbituric acid reactive species
Keywords: Glutathione, Mitochondria, Oxidative stress, Physical activity, Voluntary wheel running, Social isolation stress
1. Introduction
Physical inactivity, also known as sedentarism, is a primary cause of most chronic diseases (Booth et al., 2012; Garber et al., 2011). Conversely, physical activity (PA) produces a number of beneficial health effects, including a reduction in the risk of several diseases related to oxidative stress and mitochondrial dysfunction, such as cancer, osteoporosis, obesity, cardiovascular disabilities, dementias, depression, and anxiety, among others (Booth et al., 2012; Kessels et al., 2018; Langsetmo et al., 2012; Rosa Jr et al., 2017; Schuch et al., 2016; Stoner et al., 2016; Stonerock et al., 2015; Thuné-Boyle et al., 2012; Zhang et al., 2018).
Running wheel exposure is commonly employed as a voluntary PA model in rodents, since this protocol is uncomplicated, easy, and results in a quantifiable measure of PA (Sherwin, 1998). Although there is no consensus on the ground mechanisms controlling running wheel activity (Novak et al., 2012), use of a running wheel increases the average rodent life expectancy by nearly 10% (Holloszy, 1998); produces antidepressant- and anxiolytic-like effects (Cunha et al., 2013; Mazur et al., 2017), which are paralleled to adult neurogenesis and neuronal survival (van Praag et al., 1999a, van Praag et al., 1999b); cardioprotection (Bronikowski et al., 2003; Naderi et al., 2015); hepatoprotection (Bay et al., 2017; Zolfagharzadeh and Roshan, 2013); and skeletal muscle trophism (Brooks et al., 2018; Takigawa et al., 2019). Studies have indicated that running wheel exposition exerts its positive effects on health through a variety of biological pathways, but the exact mechanisms underlying its influences on health are not fully determined. In this regard, evidence suggests that the running wheel improves mitochondrial function, enhances the antioxidant system, and reduces oxidative stress in experimental models of pathologies (Aguiar et al., 2014; Cunha et al., 2013; Dantas de Lucas et al., 2014; Naderi et al., 2015; Navarro et al., 2004; Wright et al., 2007).
Most studies analyzing the health impact of running wheels employed socially isolated animals during the entire experimental protocol, or at least over certain periods of time (e.g., during training). This procedure allows the monitoring of individual variables associated to PA, such as distance travelled, time spent on the running wheel, and speed. The literature data extensively reports that social isolation stress (SIS) or lack of social support may be associated with negative health outcomes, increasing the risks for mortality (Broadhead et al., 1983; House et al., 1988). In accordance with this, preclinical studies indicated that SIS induces behavioral abnormalities such as anxiety (Butler et al., 2014; Skelly et al., 2015) and depression (Zanier-Gomes et al., 2015), cognitive dysfunction (Li et al., 2016), cardiomyopathy (Sonei et al., 2017), impairment in the response to stroke and ischemia (Karelina et al., 2009a, Karelina et al., 2009b; O'Keefe et al., 2014; Venna et al., 2014), obesity and metabolic disturbance (Koshoridze et al., 2016; Sun et al., 2014), oxidative stress (reviewed by Filipović et al., 2017; Mumtaz et al., 2018; Shao et al., 2015; Zlatković et al., 2014a, Zlatković et al., 2014b), and mitochondrial dysfunction (Zhuravliova et al., 2009). Additionally, lack of social support is etiologically related to clinical diseases, including anxiety and depression (Elmer and Stadtfeld, 2020; Santini et al., 2020); and cardiovascular diseases (Knox and Uvnäs-Moberg, 1998), among others. The impacts of SIS on the beneficial effects of PA are not completely understood. This way, SIS could confound data interpretation on the health impacts of the running wheel as a rodent model of PA. For instance, SIS prevents the voluntary wheel running-induced proliferation of hippocampal progenitor cells in rats (Leasure and Decker, 2009), suggesting that several benefits of PA may be impacted by sociability conditions.
Although mitochondria play a pivotal role in cells by providing energy for cellular hemostasis and activities, under stress-related conditions, such as SIS, mitochondria may produce excessive amounts of reactive oxygen species, trigger apoptotic pathways, and initiate immune-inflammatory responses. As a consequence, cell damage and death can take place, suggesting that mitochondria could be an important target in the abnormalities induced by SIS (Zhuravliova et al., 2009). Here, we analyzed the effects of free access of mice (socially isolated or grouped) to voluntary wheel running for 21 days on the following: cellular antioxidant defenses (glutathione system); lipid peroxidation index; mitochondrial enzyme activities in the central nervous system tissues, such as cerebellum and cerebral cortex, and peripheral tissues, such as skeletal muscle gastrocnemius, heart, and liver; as well as the mice's behavior.
2. Material and methods
2.1. Animals
Male Swiss mice (8–10 weeks, 30–40 g) were obtained from the animal facility of Federal University of Santa Catarina (UFSC), and housed isolated or in groups of three animals per plastic cage under controlled conditions of light (07:00 to 19:00 h), and temperature (21 ± 1 °C). Mice were allowed to freely access standard laboratory food and tap water. Animals were randomly distributed into experimental groups. All manipulations were carried out between 14:00–17:00 h, being the animals used only once. All procedures in this study were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of the Institution (CEUA PP00282). All efforts were made to minimize animal suffering, and to reduce the number of animals used in the experiments.
2.2. Voluntary wheel running paradigm
Physically active mice had access to homecages (isolated mice 28 × 17 × 13 cm or grouped mice 42 × 34 × 17 cm) containing a running wheel of 12.6 cm in diameter that was attached to the cage, as performed by Cunha et al. (2013). The control group (sedentary) was placed in cages lacking the running wheel. On the 21st experimental day, physically active animals were removed from their homecages, and housed for a period of 24 h in a new cage, without access to the running wheel. Animals were subsequently euthanized by decapitation and cerebral cortex, cerebellum, heart, skeletal muscle gastrocnemius and liver were dissected for biochemical analyses. Using another set of animals the same PA protocol was applied. Subsequently, mice were housed in a new cage without the running wheel for a period of 24 h before exposure to the open field, and, 1 h later, to the tail suspension test (TST).
2.3. Evaluation of the distance travelled and time of activity in the running wheel
The distance travelled and time spent on the running wheel were measured through a magnetic counter attached to the running wheel. These parameters were counted in the cages containing 1 or 3 animals. In the cages containing 3 animals, one third of the values obtained in the magnetic counter was used, corresponding to the average PA for each animal.
2.4. Tissue preparation
For the analyses of oxidative stress-related parameters, the cerebral cortex, cerebellum, heart, skeletal muscle gastrocnemius and liver were obtained. The analyses included nonprotein thiol groups (NPSH), thiobarbituric acid reactive species (TBARS), and glutathione peroxidase (GPx) and glutathione reductase (GR) activities. The tissues were homogenized (1:10 w/v) in phosphate buffer (50 mM, pH 7.4), and the homogenates centrifuged at 16,000 ×g, 4 °C for 20 min. The supernatants were used for the determination of GPx and GR activities and to quantify the levels of NPSH and TBARS.
For the activities of mitochondrial complexes I and II, the gastrocnemius muscle and the cerebral cortex were homogenized in 20 volumes of 50 mM potassium phosphate buffer (pH 7.4), containing 0.3 M sucrose, 5 mM 3-(N-morpholino)propanesulfonic acid (MOPS), 1 mM egtazic acid (EGTA), and 0.1% bovine serum albumin. The homogenates were centrifuged at 1000 g for 10 min at 4 °C. The pellet containing nuclei and cell debris was discarded and the resulting supernatants, containing a suspension of preserved organelles, including mitochondria, were kept at −70 °C until enzyme activity was determined. The maximal period between homogenate preparation and enzyme activity measurement was <5 days.
2.5. Non-protein thiol levels measurement
The determination of NPSH was followed according to the method of Ellman (1959), with slight modifications (de Oliveira et al., 2013). An aliquot of tissue homogenate was supplemented on ice with 10% trichloroacetic acid (TCA), mixed, and centrifuged at 15,000g for 5 min. The supernatant was used for NPSH determination. The reaction media contained 800 mM sodium phosphate buffer (pH 7.4), and 5 mM 5,50-dithiobis-2-nitrobenzoic acid (DTNB). After sample addition, the color development, resulting from the reaction between DTNB and thiols, reaches a maximum within 5 min, and it is stable for >30 min. The absorbance was determined at 412 nm after 10 min. Results were calculated as nanomoles of NPSH per milligram of protein. The DTNB molar extinction coefficient (ɛ) was 13.6 × 103 M−1 cm−1.
2.6. TBARS levels analysis
The thiobarbituric acid reactive species (TBARS) assay is probably the oldest and one of the most widely used methods evaluating malondialdehyde (MDA), as an index of lipid peroxidation. TBARS levels were determined in tissue homogenates as described by Ohkawa et al. (1979), in which MDA forms an adduct with two thiobarbituric acid molecules, producing a pink color species that strongly absorbs at 532–535 nm (Dasgupta and Klein, 2014). Briefly, the samples were incubated at ~100 °C for 60 min in acetic acid buffer containing 0.45% sodium dodecyl sulfate, and 0.6% thiobarbituric acid. After centrifugation, the reaction product was determined at 532 nm, based on a standard curve made of a known concentration of 1,1,3,3-tetramethoxypropane. The results were presented as nmol/mg protein.
2.7. Glutathione peroxidase (GPx) activity evaluation
The activity of GPx was evaluated by the method described by Wendel (1981), where the reaction medium contained 0.1 M potassium phosphate buffer (pH 7.0), 1 mM ethylenediamine tetraacetic acid (EDTA), 1 mM GSH, 0.225 mM nicotinamide adenine dinucleotide phosphate (NADPH), and 0.2 U/ml GR. The reaction was started by the addition of cumene peroxide (CHP) to a final concentration of 1.0 mM. The GPx enzyme present in the sample reduces CHP using GSH as the electron donor, which results in CHP alcohol, and oxidized glutathione (GSSG). The GSSG formed was rapidly reduced by the added GR with the proportional consumption of NADPH, which can be measured spectrophotometrically at 340 nm.
2.8. Glutathione reductase (GR) activity assay
The GR activity was measured by the method of Carlberg and Mannervik (1985), where the reaction medium contained 0.1 M phosphate buffer, pH 7.0, 1 mM EDTA, and 0.225 mM NADPH. When adding 1 mM GSSG to the reaction media, the GR present in the sample reduces GSSG to GSH, thereby consuming NADPH. This NADPH consumption can be quantified by reading the absorbance at 340 nm.
2.9. Measurement of the respiratory chain enzyme activities
The activity of mitochondrial complex I was determined spectrophotometrically according to Cassina and Radi (1996), and adapted by our group (Remor et al., 2019). The tissue homogenates were added to the reaction media containing 0.2 mM NADH, 0.5 mM ferricyanide, and 5 mM rotenone. The rate of reduced nicotinamide adenine dinucleotide (NADH)-dependent ferricyanide reduction was followed at 420 nm (30 °C, ɛ = 1 mM−1 cm−1).
The activity of succinate:cytochrome c oxidoreductase (complex II-CoQ-complex III) was obtained according to Fischer et al. (1985), and slightly modified, as detailed in a previous report (Latini et al., 2005). Before use, the homogenates were submitted to 3 cycles of freezing and thawing. The complex II was activated by a pre-incubation medium (50 mM potassium phosphate buffer (pH 7.4), 20 mM sodium succinate, and 10 pM 2,6-dichlorophenolindophenol (DCPIP)), for 20 min, at 37 °C. The complex II activity was estimated at a final concentration of 50 pM DCPIP, 2 mM potassium cyanide, 2 pg/ml rotenone, and 2.5 mM sodium azide. Enzymatic activity was observed at 600 nm (25 °C, ɛ = 19.1 mM−1 cm−1) in a microplate reader Infinite M200 TECAN. The complex II activity was calculated from the initial change in absorbance.
The activities of the respiratory chain complexes were calculated as nmol/min/mg protein.
2.10. Protein measures
Protein content was estimated as previously described by Bradford (1976), using bovine serum albumin as a standard.
2.11. Behavior tests
2.11.1. Tail suspension test
The TST has become one of the most widely used tests for assessing antidepressant-like activity in mice. The test is based on the fact that animals subjected to the short-term inescapable stress of being suspended by their tail will develop an immobile posture. The total duration of immobility, induced by the tail suspension, was measured according to the original method (Steru et al., 1985). Mice, both acoustically and visually isolated, were suspended 50 cm above the floor by adhesive tape that was placed approximately 1 cm from the tip of the tail. Immobility time was recorded during a 6 min period. Mice were considered immobile only when they hung passively and completely motionless (Cunha et al., 2008).
2.11.2. Open field test
To assess the possible effects of PA and/or SIS on locomotor and exploratory activity, mice were evaluated in the open-field paradigm as previously described (Cunha et al., 2008). Mice were individually placed in a wooden box (40 × 60 × 50 cm) with the floor divided into 12 equal rectangles. The number of rectangles crossed by the animal with its four paws (crossing), and the rising of the front paws (rearing), were registered during a period of 6 min. The number of crossings was considered as indicative of locomotor activity, and the number of rearings was considered as the exploratory activity.
2.12. Statistical analysis
Data were presented as means + S.E.M. (standard error of the mean). Pairwise comparisons between experimental and control groups were performed by Student's t-test, otherwise, two-way analysis of variance (ANOVA) was followed by Newman-Keuls post-hoc test, when appropriate. The two investigated factors were sociability (isolated x grouped) and voluntary wheel running exposition (sedentary x PA). A Pearson's correlation analysis was performed to identify any possible relationship between the oxidative stress-related parameters and the distance travelled, or time spent on the voluntary wheel running. A value of P < 0.05 was considered significant.
3. Results
3.1. The effects of social isolation on the activity parameters in the running wheel
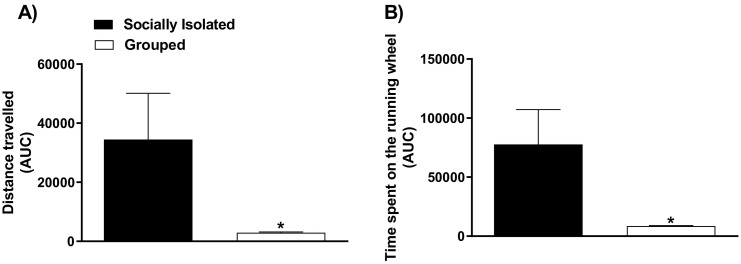
Socially isolated mice travelled greater distance in the running wheel, as compared to grouped animals (Fig. 1A). Furthermore, socially isolated mice spent longer time on the running wheel, as compared to grouped animals (Fig. 1B). The student's t-test revealed significant differences between experimental groups for the travelled distance and for the time spent on the running wheel, as evaluated by the area under the curve (t(10) = 2.02; p < 0.05; and t(10) = 2.24; p < 0.05, respectively).
Fig. 1.
The effect of housing conditions (socially isolated or grouped animals) on the distance travelled and time spent during the voluntary physical activity. The effect of housing conditions on the area under the curve of the distance travelled (Panel A) or the time spent on the running wheel (Panel B) over 21 days. Data are presented as mean of the area under curve of six mice/group. ⁎⁎p < 0.01 as compared to sedentary socially isolated mice (Student's t-test).
3.2. Lipid peroxidation in the socially isolated or grouped mice exposed to running wheel
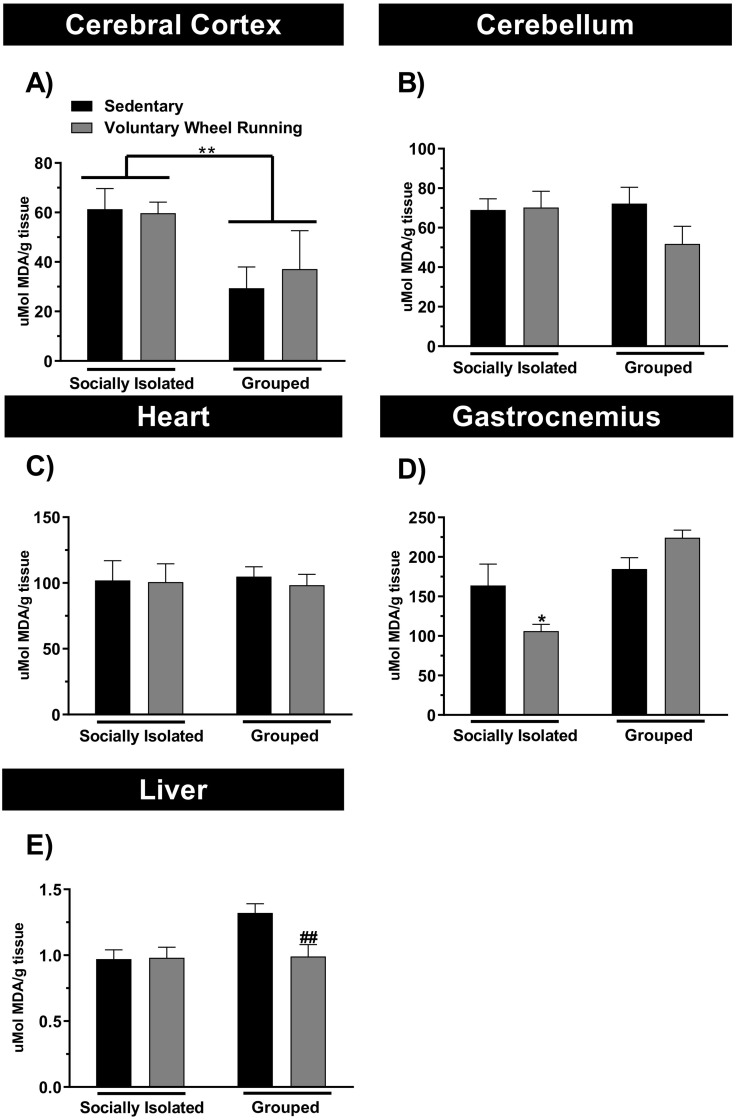
In the present study we also investigated the effect of free access of animals to voluntary wheel running on a lipoperoxidation index, TBARS. We evaluated the TBARS levels in the cerebral cortex, cerebellum, heart, skeletal muscle and liver from socially isolated or grouped animals and/or exposed to running wheel (Fig. 2A-E). Socially isolated mice presented higher TBARS levels in the cerebral cortex, as compared to grouped animals, and voluntary PA in the running wheel was unable to reverse this alteration. The two-way ANOVA showed a significant effect for sociability (F(1,20) = 7.313; p < 0.05), but not for PA and sociability × PA interaction.
Fig. 2.
The effect of physical activity and/or chronic social isolation on the lipoperoxidation index. The effect of physical activity on the TBARS levels in the cerebral cortex (Panel A), cerebellum (Panel B), heart (Panel C), gastrocnemius (Panel D) and liver (Panel E) of the socially isolated or grouped mice. Data are presented as mean + SEM of six mice/group. ⁎p < 0.05, ⁎⁎p < 0.01 as compared to sedentary socially isolated mice. #p < 0.05 as compared to sedentary grouped mice (two-way ANOVA followed by Newman-Keuls post-hoc test).
Interestingly, the socially isolated mice exposed to the running wheel presented lower TBARS levels in the gastrocnemius, as compared to sedentary socially isolated mice (Fig. 2D). The two-way ANOVA showed a significant effect for sociability (F(1,20) =17.41; p < 0.01), and sociability × PA interaction (F(1,20) = 8.59; p < 0.01), but not for PA.
Additionally, animals in the presence of running wheel presented decreased TBARS levels in the liver of grouped animals, as compared to sedentary grouped animals (Fig. 2E). The two-way ANOVA showed a significant effect for sociability (F(1,20) = 5.50; p < 0.05), PA (F(1,20) = 4.78; p < 0.05), and sociability × PA interaction (F(1,20) = 5.03; p < 0.05). The two-way ANOVA showed no significant changes in TBARS levels in the heart and skeletal muscle.
3.3. The effects of running wheel on NPSH levels of socially isolated or grouped mice
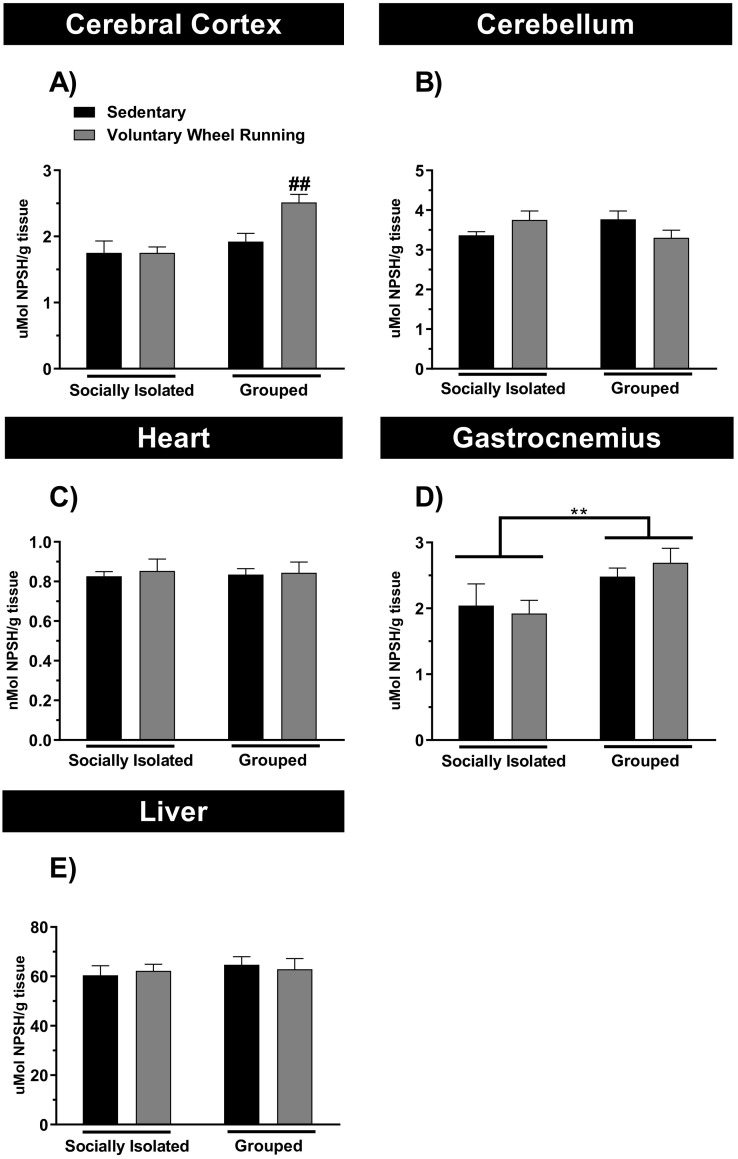
In the present study we also investigated the effect of free access of animals to voluntary wheel running on NPSH levels, being 95% comprised of GSH (Heisinger and Wait, 1989; DeLucia et al., 1975; Cohn and Lyle, 1964). NPSH was evaluated in the cerebral cortex, cerebellum, heart, skeletal muscle, and liver from socially isolated or grouped animals and/or exposed to running wheel (Fig. 3A-E). The main effect revealed significant differences for NPSH levels in the gastrocnemius between socially isolated and grouped mice (Fig. 3D). The two way ANOVA showed a significant effect for sociability (F(1,20) = 9.59; p < 0.05), but not for PA (F(1,20) = 0.045; p = 0.84), or sociability × PA interaction (F(1,20) = 0.76; p = 0.39).
Fig. 3.
The effect of physical activity and/or chronic social isolation on the non-protein thiols (NPSH) levels. The effect of physical activity on the NPSH levels in the cerebral cortex (Panel A), cerebellum (Panel B), heart (Panel C), gastrocnemius (Panel D) and liver (Panel E) of the socially isolated or grouped mice. Data are presented as mean + SEM of six mice/group. ⁎⁎p < 0.01 as compared to sedentary socially isolated mice (two-way ANOVA followed by Newman-Keuls post-hoc test).
The results depicted in the Fig. 3A showed that PA protocol leads to increased NPSH levels in the cerebral cortex of grouped mice, as compared to sedentary group. The two-way ANOVA showed a significant effect for sociability (F(1,20) = 12.04; p < 0.01), PA (F(1,20) = 4.87; p < 0.05), and sociability × PA interaction (F(1,20) = 4.76; p < 0.05). However, we did not observed alterations in the NPSH levels in other body tissues analyzed following SIS and/or PA paradigm.
3.4. The effect of running wheel on the activity of GPx and GR in distinct body tissues of socially isolated or grouped mice
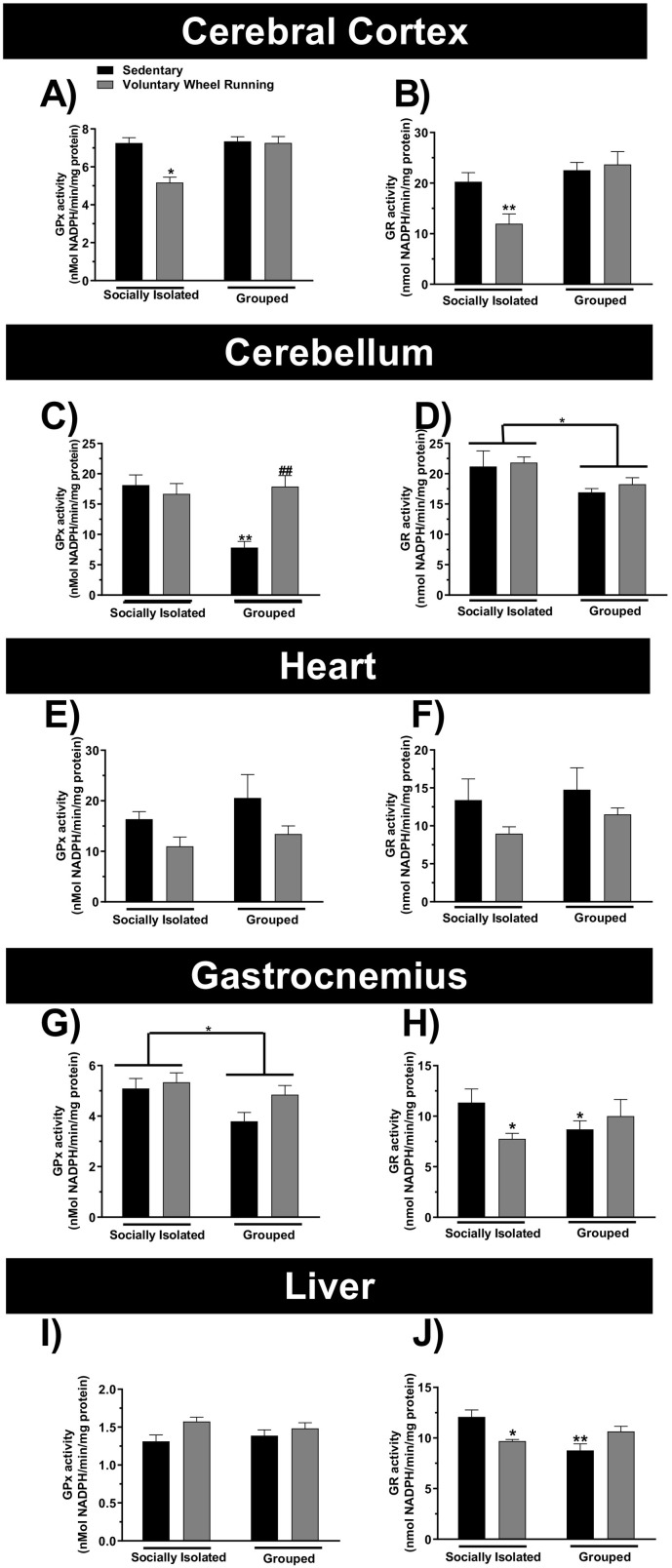
In the present study we also investigated the effect of PA paradigm induced by free access of mice to running wheel on the activity of GPx and GR enzymes, as evaluated in the cerebral cortex, cerebellum, heart, skeletal muscle and liver (Fig. 4A-J) of socially isolated or grouped mice.
Fig. 4.
Physical activity and/or chronic social isolation induce alterations on the glutathione peroxidase (GPx) or glutathione reductase (GR) activities. The effect of physical activity on the GPx or GR activities in the cerebral cortex (Panels A and B, respectively), cerebellum (Panels C and D, respectively), heart (Panels E and F, respectively), gastrocnemius (Panels G and H, respectively), liver (Panels I and J, respectively) of the socially isolated or grouped mice. ⁎p < 0.05, ⁎⁎p < 0.01 as compared to sedentary socially isolated mice. #p < 0.05 as compared to sedentary grouped mice (n = 6; two-way ANOVA followed by Newman-Keuls post-hoc test).
Among the sedentary animals, grouped mice presented lower GPx and GR activities in the cerebellum, as shown in Fig. 4C [sociability (F(1,20) = 8.23; p < 0.05), PA(F(1,20) = 7.34; p < 0.05), and sociability × PA interaction (F(1,20) = 13.12; p < 0.01)] and Fig. 4D [sociability (F(1,20) = 12.41; p < 0.05), PA (F(1,20) = 3.26; p = 0.086), and sociability × PA interaction (F(1,20) = 5.54; p < 0.05)]. Furthermore, grouped mice without running wheel access presented lower GPx and GR activities in the gastrocnemius, as shown in Fig. 4G [sociability (F(1,20) = 5.84; p < 0.05), PA (F(1,20) = 3.08; p = 0.09), and sociability × PA interaction (F(1,20) = 1.19; p = 0.29)], and Fig. 4H [sociability (F(1,17) = 0.04; p = 0.064), PA (F(1,17) = 1.30; p = 0.36), and sociability × PA interaction (F(1,17) = 4.0011; p < 0.01)]. Additionaly, grouped mice presented lower GR activity in the liver, as shown in Fig. 4J [sociability (F(1,20) = 4.76; p < 0.05), PA (F(1,20) = 0.22; p = 0.65), and sociability × PA interaction (F(1,20) = 15.24; p < 0.05)].
PA protocol decreased GPx and GR activities in the cerebral cortex of socially isolated animals, as shown in Fig. 4A [sociability (F(1,20) = 31.15; p < 0.05), PA (F(1,20) = 5.04; p < 0.05), and sociability × PA interaction (F(1,20) = 3.69; p = 0.099)], and Fig. 4B [sociability (F(1,20) = 12.41; p < 0.05), PA (F(1,20) = 3.26; p = 0.086), and sociability × PA interaction (F(1,20) = 5.54; p < 0.05)]. PA protocol also decreased GPx activity in the heart of mice, as shown in Fig. 4E [sociability (F(1,20) = 1.46; p = 0.24), PA (F(1,20) = 5.23; p < 0.05), and sociability × PA interaction (F(1,19) = 0.11; p = 0.75)]. Mice that were socially isolated and exposed to running wheel presented decreased GR activity in the liver, as shown in Fig. 4J. In contrast, mice submitted to PA presented increased GPx activity in the cerebellum of grouped animals, as shown in Fig. 4C.
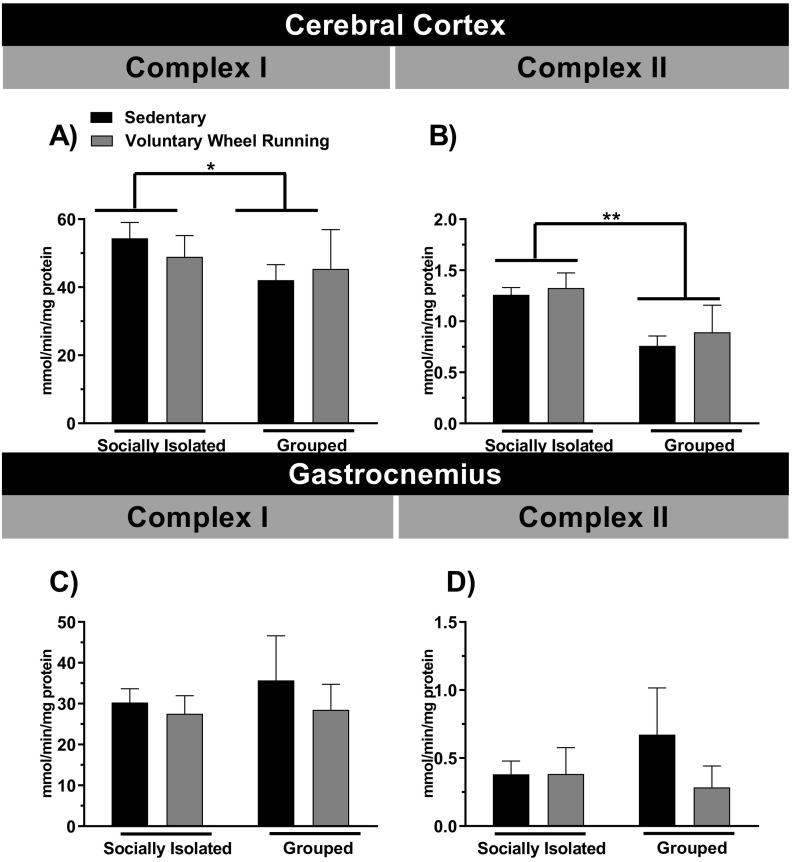
3.5. The effect of running wheel on the activity of mitochondrial complexes I and II in cerebral cortex and gastrocnemius muscle of socially isolated or grouped mice
In the present study we also investigated the effect of free access of mice to the voluntary wheel running on the activity of mitochondrial complexes I and II of the electron transport chain in the cerebral cortex and skeletal muscle gastrocnemius (Fig. 5A-D) of socially isolated or grouped animals. The results showed that the individualized animals had a higher activity of mitochondrial complexes I and II in the cerebral cortex, as shown in Fig. 5A [sociability (F(1,20) = 7.04; p < 0.05), but not for PA (F(1,20) = 0.13; p = 0.73), or sociability × PA interaction (F(1,20) = 2.17; p = 0.16)], and Fig. 5B [sociability (F(1,20) = 4.70; p < 0.05), PA (F(1,20) = 1.17; p = 0.29), and sociability × PA interaction (F(1,20) = 4.82; p < 0.05)]. The PA did not alter the activities of mitochondrial complexes I and II in the socially isolated or grouped animals.
Fig. 5.
The effect of physical activity and/or chronic social isolation on the complex I and II activities of mice. The effect of physical activity on the complex I or II activities in the cerebral cortex (Panels A and B, respectively) and gastrocnemius (Panels C and D, respectively), of the socially isolated or grouped mice. *p < 0.05 and ⁎⁎p < 0.01 as compared to sedentary socially isolated mice (n = 5–6; two-way ANOVA followed by Newman-Keuls post-hoc test).
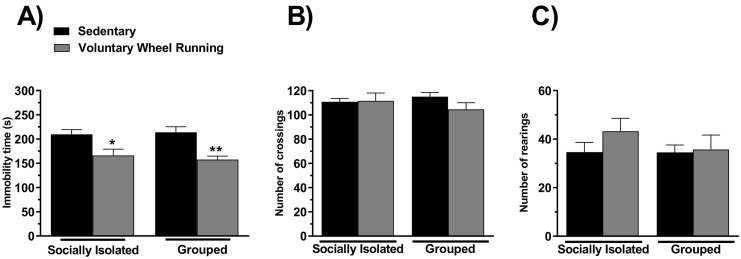
3.6. The effect of running wheel on the immobility time in the tail suspension test and the behavior in the open field of mice socially isolated or grouped mice
We also evaluated the effect of free access of mice to the running wheel on the immobility time in the TST of socially isolated or grouped animals. The results showed that both, individualized and grouped animals exposed to the running wheel, presented lower immobility time in the TST, as compared to respective control groups (Fig. 6A). A two-way ANOVA showed significant differences for PA (F(1,32) = 12.20, P < 0.05), but not for sociability (F(1,32) = 0.027, p = 0.87) and PA × sociability interaction (F(1,32) = 0.012, p = 0.91).
Fig. 6.
Voluntary wheel running induces antidepressant-like effect in the socially isolated and grouped animals. The effect of physical activity on the immobility time in the TST (Panel A) and the crossings and rearings in the open field test (Panels B and C, respectively) of the socially isolated or grouped mice. *p < 0.05 and ⁎⁎p < 0.01 as compared to sedentary socially isolated mice (n = 9; two-way ANOVA followed by Newman-Keuls post-hoc test).
The ambulation and exploratory behavior in the open field were also investigated in the present study. The number of crossings and rearings in the open field was not altered by free access to the running wheel (Fig. 6B-C).
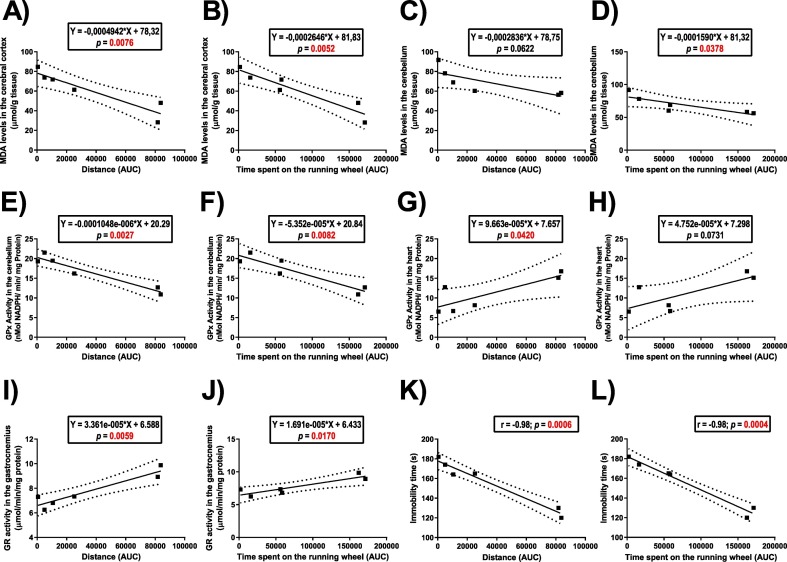
3.7. Analysis of correlation between oxidative stress-related parameters or immobility time in the TST and distance travelled or time spent on the running wheel
Fig. 7 depicts the significant correlations between the oxidative stress-related parameters or behavior and the distance travelled or time spent on the running wheel. The Pearson's test showed a positive correlation between MDA levels in the cerebral cortex with the distance travelled and the time spent on the running wheel (P < 0.05, Fig. 7A-B). The distance travelled and the time spent on the running wheel tended to be correlated with MDA levels in the cerebellum (Fig. 7C-D). Furthermore, NPSH levels did not correlated with PA parameters (P > 0.05, data not shown).
Fig. 7.
Correlation between the oxidative stress-related parameters or behavior and the distance travelled or time spent on the running wheel of socially isolated mice. The correlation between distance travelled or time spent on the voluntary wheel running with: 1) TBARS levels in the cerebral cortex (Panels A and B), and cerebellum (Panels C and D); 2) GPx activity in the cerebellum (Panels E and F) and heart (Panels G and H); 3) GR activity in the gastrocnemius (Panels G and H); and the immobility time in the tail suspension test (Panels I and J).
The GPx activity in the cerebellum was negatively correlated with the distance travelled and the time spent on the running wheel (P < 0.05, Fig. 7E-F). Furthermore, GPx activity in the heart was correlated with the distance travelled in the running wheel (P < 0.05, Fig. 7G) and tended to correlate with the time spent on the running wheel (Fig. 7H). The Pearson's test also showed a positive correlation of GR activity in the gastrocnemius muscle with the distance travelled and the time spent on the running wheel (P < 0.05, Fig. 7I-J).
The complex I and II activities in the cerebral cortex and gastrocnemius muscle did not correlate with the distance travelled and the time spent on the voluntary wheel running (P > 0.05, data not shown).
Finally, the immobility time in the TST was negatively correlated with the distance travelled and the time spent on the running wheel (P < 0.05, Fig. 7K-L). Furthermore, the number of crossings and rearings in the open field did not correlate with the distance travelled and the time spent on the voluntary wheel running (P > 0.05, data not shown).
4. Discussion
Social isolation stress (SIS) profoundly affects the daily activity of individuals and is considered an important risk component for morbidity and mortality in humans (Cacioppo et al., 2015). In particular, the COVID-19 pandemic has brought the impact of SIS to the world in the last months (Oliveira and Rossi, 2020). Imposed isolation by the COVID-19 pandemic is a unifamiliar and unpleasant experience that involves separation from friends and family, and a departure from usual, everyday routines (Onyeaka et al., 2020). In line with this, studies indicate that SIS in rodents is a stressful paradigm, since it produces a negative impact on behavior and endocrine responses, such as increased corticosterone, adrenocorticotropic hormone (ACTH), and catecholamine serum levels (Grippo et al., 2007a, Grippo et al., 2007b, Grippo et al., 2007c; Weiss et al., 2004). The results of a systematic review suggested that PA interventions are associated with decreased SIS among community-dwelling older adults, suggesting an important protective role of PA on the negative effects of SIS (Robins et al., 2018). Many activity/exercise programs have been designed for elderly persons in a bid to reduce isolation and its consequences (Dickens et al., 2011; Landeiro et al., 2017). However, in the present study we showed that grouped animals travelled lower distances in the running wheel, as compared to socially isolated mice. Pitzer et al., 2016a, Pitzer et al., 2016b demonstrated that housing conditions (C57BL6 mice individualized or housed in groups of 3 per cage) did not alter the distance travelled in the running wheel (Pitzer et al., 2016a, Pitzer et al., 2016b). The difference observed in the distance travelled could be explained by distinct mouse strains. In the present study, we used Swiss mice, a strain very sensitive to stressful events, but presenting little running wheel activity compared to other strains such as C57BL6 mice. Interestingly, Swiss mice are more dominant and aggressive than C57BL6 mice (Bisazza, 1979, Bisazza, 1981). The lower distance travelled in the running wheel by grouped Swiss mice, compared to socially isolated mice, could be dependent on the agonistic interaction for dominance and territorialism. Observations, such as those made by Bisazza (1981), suggest that male mice of strains with a high propensity to fight (e.g., Swiss) may greatly benefit from individual housing (Kappel et al., 2017; Poole and Morgan, 1973). Grouped animals tend to fight repeatedly, leading to wounds and to a painful distress condition. Regarding this point, individually housed rats engaged in slightly higher locomotor activity than grouped animals during the active phase (Stranahan et al., 2006). Interestingly, a study reported that CD1 mice, housed in standard polycarbonate cages (5 mice per cage) containing a running wheel, increased their aggressive behavior, disrupting stereotyped behavior and the linearity in dominance hierarchy (Howerton et al., 2008). In the present study, grouped animals without access to the running wheel did not present wounds or signs of distress. However, two mice of the sociability group with free access to the running wheel presented wounds on their backs, suggesting fights between animals may have intensified, which can be restricted to mice exposed to the running wheel. However, body weight change (a stress-related parameter) was not altered by the presence of a running wheel in the grouped or socially isolated mice (data not shown). Similar to our results, studies reported that SIS for 28 days did not alter the body weight of C57BL/6J mice of 12 or 20 weeks (Sun et al., 2014; Ieraci et al., 2016). Further experiments are required to clarify the effect of voluntary wheel running in the homecage of grouped Swiss mice on stress-related parameters and aggressiveness.
Here, we also investigated the impact of SIS on the PA paradigm (voluntary wheel running exposition) effect on oxidative stress-related parameters and mitochondrial function. Specific brain structures (cerebral cortex and cerebellum) and peripheral tissues, such as skeletal muscle gastrocnemius, liver, and heart, were selected for this study. The biochemical analysis in the cerebral cortex is justified due to its implications for cognition and affective behavior, being more sensitive to oxidative stress induced by SIS than other brain tissues such as the hippocampus (Zlatković et al., 2014a). The cerebellum was also a site chosen for biochemical analysis because the cerebellum is critical to social and affective processing and regulation (Van Overwalle et al., 2014; Hoche et al., 2016; Guell et al., 2018). The peripheral tissues (heart, liver, and skeletal muscle gastrocnemius) were chosen because SIS has been considered a risk factor for cardiovascular disease (Xia and Li, 2018) and overweight with hepatic hypertrophy (Sakakibara et al., 2012). Furthermore, the skeletal muscle was chosen for biochemical determinations because it is a primary tissue target of the PA paradigm using voluntary wheel running (Manzanares et al., 2018).
Chronic stress may disturb redox-status via increased reactive oxygen species, leading to oxidative damage to cellular macromolecules. Oxidative damage can be used as a biomarker of negative health outcomes, usually assessed by measuring lipid peroxidation end products, such as MDA, a reactive aldehyde produced by lipid peroxidation of polyunsaturated fatty acids (Ayala et al., 2014; Sahin and Gümüşlü, 2004). Here, the results suggested that SIS induced lipid peroxidation in the cerebral cortex by 21 days. No changes were observed in the cerebellum, gastrocnemius, and heart of mice. A study performed by Huong et al. (2005) showed that socially isolated mice by 6-8 weeks presented increased TBARS levels in the brain (Huong et al., 2005). Interestingly, socially isolated rats had higher TBARS levels in the prefrontal cortex and hippocampus, compared to socialized rats (Famitafreshi and Karimian, 2018; Zlatković et al., 2014c). It is noteworthy that the cerebellum is a brain structure presenting higher antioxidant capacity and is more resistant to oxidative stress than the cerebral cortex (Latini et al., 2007; Vandresen-Filho et al., 2015). Additionally, we also demonstrated that SIS decreased the TBARS levels in the liver of mice. A study pointed out that rats exposed for 6 weeks to chronic SIS increased cytosolic TBARS levels in the liver (Stanisavljevic et al., 2017). Discrepancies between our results and literature data might be due to the different species used. Furthermore, in the future we intend to carry out experiments using more specific techniques for lipid peroxidation, since TBARS is an unspecific method that is subject to a number of criticisms (Forman et al., 2015). Measuring the levels of isoprostanes and/or MDA-thiobarbituric acid adduct by HPLC would bring more specificity and precision (Forman et al., 2015). Oxidative protein and DNA modifications are also interesting end points to be analyzed in future studies. In line with this, rats that underwent SIS presented higher protein carbonyl levels (Zlatković et al., 2014a) and DNA damage (Krolow et al., 2013) in the brain, suggesting that oxidation of both proteins and DNA could be an important target for SIS.
In the present study, PA intervention using voluntary wheel running was unable to prevent the increase in TBARS levels in the cerebral cortex induced by SIS. Interestingly, free exposition of mice to the running wheel decreased TBARS levels in the liver of grouped mice and in the gastrocnemius skeletal muscle of socially isolated mice. Conversely, rats exposed to voluntary wheel running for 4 weeks did not alter the TBARS levels in the liver, skeletal muscle soleus, and heart (Škop et al., 2015). Similar to our results, Judge et al. (2005) demonstrated the absence of significant differences in lipid peroxidation (4-hydroxy-2-nonenal-modified proteins and TBARS levels) on the hearts of rats physically exercised in the running wheel, compared to sedentary rats (Judge et al., 2005).
Glutathione (GSH) has a pivotal role as an antioxidant defense, either by reacting directly with reactive oxygen and nitrogen species, or by acting as an essential cofactor for the enzymes glutathione S-transferase and GPx (Deponte, 2013). In line with this, thiol groups of GSH serve as electron donors in the reduction of disulfide bonds of cytoplasmic proteins, leading to GSSG build up (Pompella et al., 2003). Since about 85–95% of non-protein sulfhydryl groups (NPSH) are composed of GSH, this method was used as an indirect determination of GSH, and also served as index for the redox status of the cell (Heisinger and Wait, 1989; DeLucia et al., 1975; Cohn and Lyle, 1964). Studies have demonstrated that SIS decreases GSH levels in several tissues. For instance, a study demonstrated that SIS decreased GSH levels in the brain and heart of rats (Sonei et al., 2017). In addition, SIS for 6–8 weeks also depleted brain GSH content in mice (Huong et al., 2005). Chronic SIS also decreased GSH levels in the liver and blood of rats by 21 or 84 days (Mohale and Chandewar, 2012). The present study found statistical differences in NPSH levels on the gastrocnemius, but not on the other tissues, between socially isolated and grouped mice.
The present study is, to the best our knowledge, the first to investigate the effects of the running wheel paradigm on GSH homeostasis, which was investigated in several tissues of socially isolated and grouped mice. The present study showed that the PA paradigm in grouped mice lead to an increase in cerebral cortex NPSH levels compared to the sedentary group, however PA did not alter the NPSH levels in the peripheral tissues analyzed. Similar to our results, GSH levels in the heart were not altered following the running wheel paradigm (Judge et al., 2005). Contrasting to these results, the levels of GSH in the liver of physically active male and female rats were greater than the sedentary group (Yamamoto et al., 2002). These dissimilar responses regarding hepatic GSH levels could be due to different animal strains/species or differences in the experimental protocol. We intend to further investigate how GSH levels are relevant to SIS in the context of PA in future studies.
It is known that GSH is a cofactor for GPx in the reduction of lipid hydroperoxides to their corresponding alcohols, as well as in the reduction of hydrogen peroxide to water, releasing GSSG as the by-product (Bhabak and Mugesh, 2010; Flohe et al., 1973). GR then reduces GSSG, regenerating GSH to complete the catalytic cycle (Deponte, 2013). In the present study, SIS increased GPx activity in the cerebellum and gastrocnemius muscle, while GR activity was increased in the cerebellum, gastrocnemius muscle, and liver. Studies are controversial in relation to how SIS modulates the GSH system. For instance, SIS by 21 days impaired GSH-dependent protection, since the stress paradigm decreased GSH levels and increased GPx activity and immunocontent in the rat prefrontal cortex (Todorović and Filipović, 2017a, Todorović and Filipović, 2017b; Zlatković et al., 2014a). Furthermore, chronic SIS decreased the activity, but not the immunocontent, of GPx in the hippocampus of rats (Djordjevic et al., 2010). Chronic SIS decreased GSH content and GR activity in the liver of rats, but GPx activity remained unaltered (Todorović et al., 2016).
The PA protocol abrogated the increase in GPx and GR activities induced by SIS in the peripheral tissues, such as the gastrocnemius and liver. Additionally, we showed that PA exposition decreased GPx and GR activities in the cerebral cortex of socially isolated animals, suggesting a central nervous system adaptation to the PA paradigm. In the present study, the PA protocol did not alter the heart, GPx, or GR activities. Similar to our results, a study showed that the running wheel paradigm does not alter GSH levels, or GPx and GR activities in the heart (Judge et al., 2005). Additionally, rats exposed to the running wheel for 4 weeks did not have altered GPx activity in the liver, skeletal muscle soleus, or heart (Škop et al., 2015).
Emerging evidence suggests that mitochondrial function is an early target of stress (Gardner and Boles, 2005, Gardner and Boles, 2008; Jeanneteau et al., 2018; Picard et al., 2018; Ridout et al., 2016). Mitochondria are ubiquitous organelles in eukaryotic cells responsible for orchestrating cellular energy production in the form of ATP via protein complexes mediating oxidative phosphorylation. The NADH ubiquinone oxidoreductase (complex I) is the first enzyme of the respiratory chain. It oxidizes NADH, which is generated through the Krebs cycle in the mitochondrial matrix, and uses the two electrons to reduce ubiquinone to ubiquinol. The succinate ubiquinone oxidoreductase (complex II) catalyzes the oxidation of succinate to fumarate and the reduction of ubiquinone to ubiquinol. These two enzyme complexes are the gateway to oxidative phosphorylation, and can serve as indicators of mitochondrial function. Furthermore, previous studies demonstrated the pivotal role of these mitochondrial complexes in social behavior (Hollis et al., 2015). Interestingly, the cerebral cortex is more sensitive than the hippocampus to the effects of SIS with regards to mitochondrial function, taking into account that: 1) cerebral cortex, but not hippocampus, of rats exposed to SIS by 21 days presented increased cytochrome c release from mitochondria into the cytosol (Filipović et al., 2011); and 2) SIS did not alter the activity of both respiratory chain complexes I/III and II in the hippocampus of juvenile rats (Krolow et al., 2012). For this reason, we performed activity analyses of mitochondrial complexes in the cerebral cortex of animals. Furthermore, the skeletal muscle gastrocnemius was another tissue chosen to have its mitochondrial complex activity analyzed, based on the peripheral and direct association with voluntary wheel running, as this protocol stimulates its contraction. We did not analyze the enzymatic activity of mitochondria complexes in cardiac tissue because studies have reported that rats exposed to lifelong running wheel had displayed no effects on aerobic respiration parameters, such as the rate of oxygen consumption (state 4 or state 3) or respiratory control ratio, in the heart tissue (Servais et al., 2003; Judge et al., 2005).
In the present study, we demonstrated that socially isolated mice increased the activity of both complex I and II in the cerebral cortex, but not in the skeletal muscle gastrocnemius. However, studies indicated that SIS could be associated with impaired respiratory chain complex II, which can lead to reactive oxygen species formation, oxidative damage, and ATP depletion in both the brain and heart of rats (Sonei et al., 2017; Zhuravliova et al., 2009). The differences in the activity of complex II found in the present study in relation to literature data could be associated with species, age, and SIS protocol. Although there are no reports investigating the effects of free exposure of rodents to the running wheel on changes in the mitochondrial function induced by SIS, there is evidence indicating that the running wheel paradigm could modulate mitochondrial activity (Herbst and Holloway, 2015; Marques-Aleixo et al., 2015; Stolle et al., 2018). In the present study, we demonstrated that individually housed animals presented higher activity of mitochondrial complexes I and II in the cerebral cortex, but not in the gastrocnemius muscle. The PA paradigm was unable to alter the activity of mitochondrial complexes I and II. Interestingly, isolated brain mitochondria of C57BL/6 mice submitted to SIS, in combination with running wheel for 6 weeks, presented increased mitochondrial complex I activity and abrogated the inhibition of this complex induced by rotenone incubation, while the effects of MPP+ were not altered (Aguiar et al., 2014; Aguiar Jr et al., 2014). Furthermore, rats exposed to voluntary wheel running increased the activities of complex I and II in the soleus skeletal muscle (Hedges et al., 2019). The differences in the activities of mitochondrial complexes could be associated with differences in the strain and species.
Given that stress is the main triggering factor of major depressive disorder, we investigated the effect of SIS and PA on the immobility time in the TST. The results showed that the PA protocol produced a reduction in the immobility time of mice submitted to the TST, an antidepressant-like profile. The antidepressant-like effect of the voluntary wheel running paradigm has previously been shown (Cunha et al., 2013; Duman et al., 2008). However, under the effect of SIS, the antidepressant-like effect may limit the interpretation of data, precluding further discussions and conclusions. In the present study, the behavior of mice in the TST was not dependent of social condition, since SIS was unable to alter the immobility time. Similar to our results, rats submitted to SIS for 1, 2, or 3 weeks did not present depressive-like effects in the forced swimming test (FST; Gorlova et al., 2018). Furthermore, a study showed that SIS increased the immobility time of Wistar rats in the FST, which was not observed for Wistar-Kyoto rats, suggesting that the effect of SIS can be strain-specific (Mileva and Bielajew, 2015). Interestingly, a study reported that predisposed Wistar rats presenting a background of increased immobility time in the FST presented a depressive-like profile when submitted to SIS for 21 days. The same effect was not observed in non-predisposed Wistar rats, suggesting that predisposition to the effects of stress is a determining factor for behavior despair following SIS (Zanier-Gomes et al., 2015). Moreover, several reports also revealed that SIS significantly increased the immobility time of NMRI and C57BL/6J mice or Sprague-Dawley rats submitted to the FST (Chan et al., 2017; Cho et al., 2017; Dávila-Hernández et al., 2018; Haj-Mirzaian et al., 2015, Haj-Mirzaian et al., 2019). The discrepancies of our results in the behavior despair test, compared to the literature data, could be due to several factors, including biological factors such as strain, age, body weight, gender, and individual differences between animals.
We also investigated the correlation between distance travelled, or time spent on the running wheel, and oxidative stress-related parameters, as well as the mitochondrial function in the socially isolated mice. The TBARS levels were negatively correlated with the distance travelled and the time spent on the running wheel. Interestingly, a tendency of negative correlation between cerebellar TBARS levels and distance travelled or time spent on the running wheel was pointed out in the present study. These data suggest that PA could be protective against lipid peroxidation induced by SIS. Furthermore, the NPSH levels did not correlate with the distance travelled or the time spent on the running wheel. However, the literature data reported that GSH levels of the liver and brain were correlated with distance travelled in the running wheel (Yamamoto et al., 2002). Additionally, the present results also demonstrated that cerebellar GPx activity was negatively correlated with the distance travelled, or the time spent on the running wheel. Moreover, the heart GPx activity was positively correlated with the distance travelled. GR activity in the gastrocnemius was also positively correlated with distance travelled or time spent on the running wheel. These data indicated that PA could modulate the GSH-dependent enzymatic antioxidant system. Furthermore, a negative correlation between the immobility time in the FST and distance travelled and the time spent on the running wheel was reported in the present study, suggesting that PA was an important tool for clinical depression management.
5. Conclusion
Collectively, our results demonstrate that SIS produces mitochondrial functional alterations and oxidative stress, since it increases lipid peroxidation and alters the activity of the antioxidant enzymatic system dependent on GSH in peripheral and central tissues. Therefore, we further showed that a voluntary wheel running-induced decrease in oxidative stress is dependent, at least in part, on housing conditions. In Table 1 , we summarized main neurochemical findings in mice following SIS and/or PA paradigms in voluntary wheel running.
Table 1.
Summary of the main neurochemical findings in mice following social isolation and/or physical activity paradigm in the running wheel.
| Cerebral Cortex |
Cerebellum |
Heart |
Gastrocnemius |
Liver |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Social isolation | Running wheel | Social isolation | Running wheel | Social isolation | Running wheel | Social isolation | Running wheel | Social isolation | Running wheel | |
| MDA | ↑ | – | – | – | – | – | – | ↓ | – | ↓ |
| NPSH | – | ↑ | – | – | – | – | ↓ | – | – | – |
| GPX | – | ↓ | ↑ | – | – | – | ↑ | – | – | – |
| GR | – | ↓ | ↑ | – | – | ↓ | ↑ | ↓ | ↑ | ↓ |
| Complex I | ↑ | – | – | – | – | – | – | – | – | – |
| Complex II | ↑ | – | – | – | – | – | – | – | – | – |
Acknowledgments
This work was supported by CNPq (National Council for Research and Development, #403120/2012–8, #462333/2014-0, #306204/2014-2, #310113/2017–2, #150082/2018–5); ALD, AL, AFB and ALSR are recipients of CNPq Research Productivity Fellowship; FINEP Research Grant “Rede Instituto Brasileiro de Neurociência” (IBN-Net #01.06.0842-00); INCT Excitotoxicity and Neuroprotection; FAPESC (Foundation for the Support of Scientific and Technological Research in the State of Santa Catarina); MPC received a post-doctoral PNPD scholarships from CAPES (Coordination for the Improvement of Higher Education Personnel, Brazil).
References
- Aguiar A.S., Jr., Tristão F.S., Amar M., Chevarin C., Glaser V., de Paula Martins R., Moreira E.L., Mongeau R., Lanfumey L., Raisman-Vozari R., Latini A., Prediger R.D. Six weeks of voluntary exercise don’t protect C57BL/6 mice against neurotoxicity of MPTP and MPP(+) Neurotox. Res. 2014;25:147–152. doi: 10.1007/s12640-013-9412-5. [DOI] [PubMed] [Google Scholar]
- Aguiar A.S., Stragier E., da Luz Scheffer D., Remor A.P., Oliveira P.A., Prediger R.D., Latini A., Raisman-Vozari R., Mongeau R., Lanfumey L. Effects of exercise on mitochondrial function, neuroplasticity and anxio-depressive behavior of mice. Neuroscience. 2014;271:56–63. doi: 10.1016/j.neuroscience.2014.04.027. [DOI] [PubMed] [Google Scholar]
- Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014;2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay M.L., Gehl J., Pedersen B.K., Hojman P. Voluntary wheel running reduces the acute inflammatory response to liver carcinogen in a sex-specific manner. Cancer Prev. Res. (Phila.) 2017;10:719–728. doi: 10.1158/1940-6207.CAPR-17-0075. [DOI] [PubMed] [Google Scholar]
- Bhabak K.P., Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc. Chem. Res. 2010;43:1408–1419. doi: 10.1021/ar100059g. [DOI] [PubMed] [Google Scholar]
- Bisazza A. A comparison of aggressive behaviour in isolated and dominant male mice. Bolletino Zool. 1979;46:63–66. [Google Scholar]
- Bisazza A. Social organization and territorial behaviour in three strains of mice. Bolletino Zool. 1981;48:157–167. [Google Scholar]
- Booth F.W., Roberts C.K., Laye M.J. In: Comprehensive Physiology. Terjung R., editor. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2012. Lack of exercise is a major cause of chronic diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Broadhead W.E., Kaplan B.H., James S.A., Wagner E.H., Schoenbach V.J., Grimson R., Heyden S., Tibblin G., Gehlbach S.H. The epidemiologic evidence for a relationship between social support and health. Am. J. Epidemiol. 1983;117:521–537. doi: 10.1093/oxfordjournals.aje.a113575. [DOI] [PubMed] [Google Scholar]
- Bronikowski A.M., Carter P.A., Morgan T.J., Garland T., Ung N., Pugh T.D., Weindruch R., Prolla T.A. Lifelong voluntary exercise in the mouse prevents age-related alterations in gene expression in the heart. Physiol. Genomics. 2003;12:129–138. doi: 10.1152/physiolgenomics.00082.2002. [DOI] [PubMed] [Google Scholar]
- Brooks M.J., Hajira A., Mohamed J.S., Alway S.E. Voluntary wheel running increases satellite cell abundance and improves recovery from disuse in gastrocnemius muscles from mice. J. Appl. Physiol. 2018;124:1616–1628. doi: 10.1152/japplphysiol.00451.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T.R., Ariwodola O.J., Weiner J.L. The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Front. Integr. Neurosci. 2014;7 doi: 10.3389/fnint.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo S., Grippo A.J., London S., Goossens L., Cacioppo J.T. Loneliness: clinical import and interventions. Perspect. Psychol. Sci. 2015;10:238–249. doi: 10.1177/1745691615570616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Cassina A., Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch. Biochem. Biophys. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- Chan J.N.-M., Lee J.C.-D., Lee S.S.P., Hui K.K.Y., Chan A.H.L., Fung T.K.-H., Sánchez-Vidaña D.I., Lau B.W.-M., Ngai S.P.-C. Interaction effect of social isolation and high dose corticosteroid on neurogenesis and emotional behavior. Front. Behav. Neurosci. 2017;11 doi: 10.3389/fnbeh.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.-W., Jung S.-Y., Lee S.-W., Lee S.-J., Seo T.-B., Kim Y.-P., Kim D.-Y. Treadmill exercise ameliorates social isolation-induced depression through neuronal generation in rat pups. J. Exerc. Rehabil. 2017;13:627–633. doi: 10.12965/jer.1735180.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn W.H., Lyle J. A fluorometric assay for glutathione. Anal. Biochem. 1964;8:217. doi: 10.1016/0003-2697(66)90286-7. [DOI] [PubMed] [Google Scholar]
- Cunha M.P., Machado D.G., Bettio L.E.B., Capra J.C., Rodrigues A.L.S. Interaction of zinc with antidepressants in the tail suspension test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1913–1920. doi: 10.1016/j.pnpbp.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Cunha M.P., Oliveira ágatha, Pazini F.L., Machado D.G., Bettio L.E.B., Budni J., Aguiar A.S., Martins D.F., Santos A.R.S., Rodrigues A.L.S. The antidepressant-like effect of physical activity on a voluntary running wheel. Med. Sci. Sports Exerc. 2013;45:851–859. doi: 10.1249/MSS.0b013e31827b23e6. [DOI] [PubMed] [Google Scholar]
- Dantas de Lucas R., Caputo F., Mendes de Souza K., Sigwalt A.R., Ghisoni K., Lock Silveira P.C., Remor A.P., da Luz Scheffer Dé, Antonacci Guglielmo L.G., Latini A. Increased platelet oxidative metabolism, blood oxidative stress and neopterin levels after ultra-endurance exercise. J. Sports Sci. 2014;32:22–30. doi: 10.1080/02640414.2013.797098. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Klein K. Antioxidants in Food, Vitamins and Supplements. Elsevier; 2014. Methods for measuring oxidative stress in the laboratory; pp. 19–40. [Google Scholar]
- Dávila-Hernández A., Zamudio S.R., Martínez-Mota L., González-González R., Ramírez-San Juan E. Antidepressant effects of acupoint stimulation and fluoxetine by increasing dendritic arborization and spine density in CA1 hippocampal neurons of socially isolated rats. Neurosci. Lett. 2018;675:48–53. doi: 10.1016/j.neulet.2018.03.057. [DOI] [PubMed] [Google Scholar]
- de Oliveira J., Moreira E.L.G., Mancini G., Hort M.A., Latini A., Ribeiro-do-Valle R.M., Farina M., da Rocha J.B.T., de Bem A.F. Diphenyl diselenide prevents cortico-cerebral mitochondrial dysfunction and oxidative stress induced by hypercholesterolemia in LDL receptor knockout mice. Neurochem. Res. 2013;38:2028–2036. doi: 10.1007/s11064-013-1110-4. [DOI] [PubMed] [Google Scholar]
- DeLucia A.J., Mustafa M.G., Hussain M.Z., Cross C.E. Ozone interaction with rodent lung. III. Oxidation of reduced glutathione and formation of mixed disulfides between protein and nonprotein sulfhydryls. J. Clin. Invest. 1975;55:794–802. doi: 10.1172/JCI107990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta BBA - Gen. Subj. 2013;1830:3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Dickens A.P., Richards S.H., Greaves C.J., Campbell J.L. Interventions targeting social isolation in older people: a systematic review. BMC Public Health. 2011;11 doi: 10.1186/1471-2458-11-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic J., Djordjevic A., Adzic M., Radojcic M.B. Chronic social isolation compromises the activity of both glutathione peroxidase and catalase in hippocampus of male wistar rats. Cell. Mol. Neurobiol. 2010;30:693–700. doi: 10.1007/s10571-009-9493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman C.H., Schlesinger L., Russell D.S., Duman R.S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Elmer T, Stadtfeld C. Depressive symptoms are associated with social isolation in face-to-face interaction networks. Sci. Rep. 2020;10:1444. doi: 10.1038/s41598-020-58297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famitafreshi H., Karimian M. Socialization alleviates burden of oxidative-stress in hippocampus and prefrontal cortex in morphine addiction period in male rats. Curr. Mol. Pharmacol. 2018;11:254–259. doi: 10.2174/1874467210666170919161045. [DOI] [PubMed] [Google Scholar]
- Filipović D., Zlatković J., Inta D., Bjelobaba I., Stojiljkovic M., Gass P. Chronic isolation stress predisposes the frontal cortex but not the hippocampus to the potentially detrimental release of cytochrome c from mitochondria and the activation of caspase-3. J. Neurosci. Res. 2011;89:1461–1470. doi: 10.1002/jnr.22687. [DOI] [PubMed] [Google Scholar]
- Filipović D., Todorović N., Bernardi R.E., Gass P. Oxidative and nitrosative stress pathways in the brain of socially isolated adult male rats demonstrating depressive- and anxiety-like symptoms. Brain Struct. Funct. 2017;222:1–20. doi: 10.1007/s00429-016-1218-9. [DOI] [PubMed] [Google Scholar]
- Fischer J.C., Ruitenbeek W., Berden J.A., Trijbels J.M., Veerkamp J.H., Stadhouders A.M., Sengers R.C., Janssen A.J. Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin. Chim. Acta Int. J. Clin. Chem. 1985;153:23–36. doi: 10.1016/0009-8981(85)90135-4. [DOI] [PubMed] [Google Scholar]
- Flohe L., Günzler W.A., Schock H.H. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- Forman H.J., Augusto O., Brigelius-Flohe R., Dennery P.A., Kalyanaraman B., Ischiropoulos H., Mann G.E., Radi R., Roberts L.J., 2nd, Vina J., Davies K.J. Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015;78:233–235. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- Garber C.E., Blissmer B., Deschenes M.R., Franklin B.A., Lamonte M.J., Lee I.-M., Nieman D.C., Swain D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Gardner A., Boles R. Is a “mitochondrial psychiatry” in the future? A review. Curr. Psychiatr. Rev. 2005;1:255–271. [Google Scholar]
- Gardner A., Boles R.G. Symptoms of somatization as a rapid screening tool for mitochondrial dysfunction in depression. Biopsychosoc. Med. 2008;2 doi: 10.1186/1751-0759-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlova A.V., Pavlov D.A., Zubkov E.A., Morozova A.Yu., Inozemtsev A.N., Chekhonin V.P. Three-week isolation does not Lead to depressive-like disorders in rats. Bull. Exp. Biol. Med. 2018;165:181–183. doi: 10.1007/s10517-018-4125-7. [DOI] [PubMed] [Google Scholar]
- Grippo A.J., Cushing B.S., Carter C.S. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom. Med. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Lamb D.G., Carter C.S., Porges S.W. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol. Psychiatry. 2007;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A.J., Gerena D., Huang J., Kumar N., Shah M., Ughreja R., Carter C.S. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X., Schmahmann J.D., Gabrieli J., Ghosh S.S. Functional gradients of the cerebellum. Elife. 2018;7 doi: 10.7554/eLife.36652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Mirzaian A., Amiri S., Kordjazy N., Rahimi-Balaei M., Haj-Mirzaian A., Marzban H., Aminzadeh A., Dehpour A.R., Mehr S.E. Blockade of NMDA receptors reverses the depressant, but not anxiogenic effect of adolescence social isolation in mice. Eur. J. Pharmacol. 2015;750:160–166. doi: 10.1016/j.ejphar.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Haj-Mirzaian A., Nikbakhsh R., Ramezanzadeh K., Rezaee M., Amini-Khoei H., Haj-Mirzaian A., Ghesmati M., Afshari K., Haddadi N.-S., Dehpour A.R. Involvement of opioid system in behavioral despair induced by social isolation stress in mice. Biomed. Pharmacother. 2019;109:938–944. doi: 10.1016/j.biopha.2018.10.144. [DOI] [PubMed] [Google Scholar]
- Hedges C.P., Bishop D.J., Hickey A.J.R. Voluntary wheel running prevents the acidosis-induced decrease in skeletal muscle mitochondrial reactive oxygen species emission. FASEB J. 2019;33:4996–5004. doi: 10.1096/fj.201801870R. [DOI] [PubMed] [Google Scholar]
- Heisinger J.F., Wait E. The effects of mercuric chloride and sodium selenite on glutathione and total nonprotein sulfhydryls in the kidney of the black bullhead (Ictalurus melas) Comparative Biochemistry and Physiology Part C: Comparative Pharmacology. 1989;94:139–142. [Google Scholar]
- Herbst E.A.F., Holloway G.P. Exercise training normalizes mitochondrial respiratory capacity within the striatum of the R6/1 model of Huntington’s disease. Neuroscience. 2015;303:515–523. doi: 10.1016/j.neuroscience.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Hoche F., Guell X., Sherman J.C., Vangel M.G., Schmahmann J.D. Cerebellar contribution to social cognition. Cerebellum. 2016;15:732–743. doi: 10.1007/s12311-015-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis F., van der Kooij M.A., Zanoletti O., Lozano L., Cantó C., Sandi C. Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl. Acad. Sci. U. S. A. 2015;112:15486–15491. doi: 10.1073/pnas.1512653112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy J.O. Longevity of exercising male rats: effect of an antioxidant supplemented diet. Mech. Ageing Dev. 1998;100:211–219. doi: 10.1016/s0047-6374(97)00140-1. [DOI] [PubMed] [Google Scholar]
- House J.S., Landis K.R., Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Howerton D.L., Garner J.P., Mench J.A. Effects of a running wheel–igloo enrichment on aggression, hierarchy linearity, and stereotypy in group-housed male CD1 (ICR) mice. Appl. Anim. Behav. Sci. 2008;115:90–103. [Google Scholar]
- Huong N.T.T., Murakami Y., Tohda M., Watanabe H., Matsumoto K. Social isolation stress-induced oxidative damage in mouse brain and its modulation by majonoside-R2, a Vietnamese ginseng saponin. Biol. Pharm. Bull. 2005;28:1389–1393. doi: 10.1248/bpb.28.1389. [DOI] [PubMed] [Google Scholar]
- Ieraci A., Mallei A., Popoli M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016;2016:6212983. doi: 10.1155/2016/6212983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F., Barrère C., Vos M., De Vries C.J.M., Rouillard C., Levesque D., Dromard Y., Moisan M.-P., Duric V., Franklin T.C., et al. The stress-induced transcription factor NR4A1 adjusts mitochondrial function and synapse number in prefrontal cortex. J. Neurosci. 2018;38:1335–1350. doi: 10.1523/JNEUROSCI.2793-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge S., Jang Y.M., Smith A., Selman C., Phillips T., Speakman J.R., Hagen T., Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005;289:R1564–R1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- Kappel S., Hawkins P., Mendl M. To group or not to group? Good practice for housing male laboratory mice. Animals. 2017;7:88. doi: 10.3390/ani7120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K., Norman G.J., Zhang N., DeVries A.C. Social contact influences histological and behavioral outcomes following cerebral ischemia. Exp. Neurol. 2009;220:276–282. doi: 10.1016/j.expneurol.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Karelina K., Norman G.J., Zhang N., Morris J.S., Peng H., DeVries A.C. Social isolation alters neuroinflammatory response to stroke. Proc. Natl. Acad. Sci. 2009;106:5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels E., Husson O., Van der Feltz-Cornelis C.M. The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr. Dis. Treat. 2018;14:479–494. doi: 10.2147/NDT.S150464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox S.S., Uvnäs-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology. 1998;23:877–890. doi: 10.1016/s0306-4530(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Koshoridze N., Kuchukashvili Z., Menabde K., Lekiashvili S., Koshoridze M. Alterations in brain creatine concentrations under long-term social isolation (experimental study) Georgian Med. News. 2016:70–77. [PubMed] [Google Scholar]
- Krolow R., Noschang C., Arcego D.M., Pettenuzzo L.F., Weis S.N., Marcolin M.L., Huffell A.P., Mota C.S., Dalmaz C. Isolation stress exposure and consumption of palatable diet during the prepubertal period leads to cellular changes in the hippocampus. Neurochem. Res. 2013;38:262–272. doi: 10.1007/s11064-012-0915-x. [DOI] [PubMed] [Google Scholar]
- Krolow R., Noschang C., Weis S.N., Pettenuzzo L.F., Huffell A.P., Arcego D.M., Marcolin M., Mota C.S., Kolling J., Scherer E.B., Wyse A.T., Dalmaz C. Isolation stress during the prepubertal period in rats induces long-lasting neurochemical changes in the prefrontal cortex. Neurochem. Res. 2012;37:1063–1073. doi: 10.1007/s11064-012-0709-1. [DOI] [PubMed] [Google Scholar]
- Landeiro F., Barrows P., Nuttall Musson E., Gray A.M., Leal J. Reducing social isolation and loneliness in older people: a systematic review protocol. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsetmo L., Hitchcock C.L., Kingwell E.J., Davison K.S., Berger C., Forsmo S., Zhou W., Kreiger N., Prior J.C. Physical activity, body mass index and bone mineral density—associations in a prospective population-based cohort of women and men: the Canadian Multicentre Osteoporosis Study (CaMos) Bone. 2012;50:401–408. doi: 10.1016/j.bone.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini A., Rodriguez M., Borba Rosa R., Scussiato K., Leipnitz G., Reis de Assis D., da Costa Ferreira G., Funchal C., Jacques-Silva M.C., Buzin L., Giugliani R., Cassina A., Radi R., Wajner M. 3-Hydroxyglutaric acid moderately impairs energy metabolism in brain of young rats. Neuroscience. 2005;135:111–120. doi: 10.1016/j.neuroscience.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Latini A., Scussiato K., Leipnitz G., Gibson K.M., Wajner M. Evidence for oxidative stress in tissues derived from succinate semialdehyde dehydrogenase-deficient mice. J. Inherit. Metab. Dis. 2007;30:800–810. doi: 10.1007/s10545-007-0599-6. [DOI] [PubMed] [Google Scholar]
- Leasure J.L., Decker L. Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus. 2009;19:907–912. doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- Li M., Du W., Shao F., Wang W. Cognitive dysfunction and epigenetic alterations of the BDNF gene are induced by social isolation during early adolescence. Behav. Brain Res. 2016;313:177–183. doi: 10.1016/j.bbr.2016.07.025. [DOI] [PubMed] [Google Scholar]
- Manzanares G., Brito-da-Silva G., Gandra P.G. Voluntary wheel running: patterns and physiological effects in mice. Braz. J. Med. Biol. Res. 2018;52 doi: 10.1590/1414-431X20187830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Aleixo I., Santos-Alves E., Balça M.M., Rizo-Roca D., Moreira P.I., Oliveira P.J., Magalhães J., Ascensão A. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience. 2015;301:480–495. doi: 10.1016/j.neuroscience.2015.06.027. [DOI] [PubMed] [Google Scholar]
- Mazur F.G., Oliveira L.F.G., Cunha M.P., Rodrigues A.L.S., Pértile R.A.N., Vendruscolo L.F., Izídio G.S. Effects of physical exercise and social isolation on anxiety-related behaviors in two inbred rat strains. Behav. Process. 2017;142:70–78. doi: 10.1016/j.beproc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Mileva G.R., Bielajew C. Environmental manipulation affects depressive-like behaviours in female Wistar-Kyoto rats. Behav. Brain Res. 2015;293:208–216. doi: 10.1016/j.bbr.2015.07.035. [DOI] [PubMed] [Google Scholar]
- Mohale D.S., Chandewar A.V. Effect of Social Isolation on Oxidative Stress and Transaminase Level. Asian J. Biomed. and Pharm. Sci. 2012;2:41–44. [Google Scholar]
- Mumtaz F., Khan M.I., Zubair M., Dehpour A.R. Neurobiology and consequences of social isolation stress in animal model-A comprehensive review. Biomed. Pharmacother. 2018;105:1205–1222. doi: 10.1016/j.biopha.2018.05.086. [DOI] [PubMed] [Google Scholar]
- Naderi R., Mohaddes G., Mohammadi M., Ghaznavi R., Ghyasi R., Vatankhah A.M. Voluntary exercise protects heart from oxidative stress in diabetic rats. Adv. Pharm. Bull. 2015;5:231–236. doi: 10.15171/apb.2015.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A., Gomez C., López-Cepero J.M., Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Novak C.M., Burghardt P.R., Levine J.A. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci. Biobehav. Rev. 2012;36:1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- O’Keefe L.M., Doran S.J., Mwilambwe-Tshilobo L., Conti L.H., Venna V.R., McCullough L.D. Social isolation after stroke leads to depressive-like behavior and decreased BDNF levels in mice. Behav. Brain Res. 2014;260:162–170. doi: 10.1016/j.bbr.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira G.M., Rossi M.I.D. COVID-19, social isolation and human stress comparative behavior & welfare. New York Science Journal. 2020;13:14–22. [Google Scholar]
- Onyeaka H.K., Zahid S., Patel R.S. The unaddressed behavioral health aspect during the coronavirus pandemic. Cureus. 2020;12 doi: 10.7759/cureus.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M., McEwen B.S., Epel E.S., Sandi C. An energetic view of stress: focus on mitochondria. Front. Neuroendocrinol. 2018;49:72–85. doi: 10.1016/j.yfrne.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer C., Kuner R., Tappe-Theodor A. Voluntary and evoked behavioral correlates in inflammatory pain conditions under different social housing conditions. PAIN Rep. 2016;1 doi: 10.1097/PR9.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer C., Kuner R., Tappe-Theodor A. Voluntary and evoked behavioral correlates in neuropathic pain states under different social housing conditions. Mol. Pain. 2016;12 doi: 10.1177/1744806916656635. (174480691665663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompella A., Visvikis A., Paolicchi A., De Tata V., Casini A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- Poole T.B., Morgan H.D.R. Differences in aggressive behaviour between male mice (Mus musculus L.) in colonies of different sizes. Anim. Behav. 1973;21:788–795. doi: 10.1016/s0003-3472(73)80105-8. [DOI] [PubMed] [Google Scholar]
- Remor A.P., da Silva R.A., de Matos F.J., Glaser V., de Paula Martins R., Ghisoni K., da Luz Scheffer D., Andia D.C., Portinho D., de Souza A.P., et al. Chronic metabolic derangement-induced cognitive deficits and neurotoxicity are associated with REST inactivation. Mol. Neurobiol. 2019;56:1539–1557. doi: 10.1007/s12035-018-1175-9. [DOI] [PubMed] [Google Scholar]
- Ridout K.K., Carpenter L.L., Tyrka A.R. The cellular sequelae of early stress: focus on aging and mitochondria. Neuropsychopharmacology. 2016;41:388–389. doi: 10.1038/npp.2015.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins L.M., Hill K.D., Finch C.F., Clemson L., Haines T. The association between physical activity and social isolation in community-dwelling older adults. Aging Ment. Health. 2018;22:175–182. doi: 10.1080/13607863.2016.1242116. [DOI] [PubMed] [Google Scholar]
- Rosa F., Jr., Pedro Fuentes J., Pertile Remor A., Ghisoni K., César Lock Silveira P., Costa V., Luiz Prim R., da Luz Scheffer D., Silva Aguiar A., Jr., Antonacci Guglielmo L.G., et al. A tennis-based health program for middle-aged men who are at risk for heart disease. Integr. Obes. Diabetes. 2017;3 [Google Scholar]
- Sahin E., Gümüşlü S. Alterations in brain antioxidant status, protein oxidation and lipid peroxidation in response to different stress models. Behav. Brain Res. 2004;155:241–248. doi: 10.1016/j.bbr.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Sakakibara H., Suzuki A., Kobayashi A., Motoyama K., Matsui A., Sayama K., Kato A., Ohashi N., Akimoto M., Nakayama T., Shimoi K. Social isolation stress induces hepatic hypertrophy in C57BL/6J mice. J. Toxicol. Sci. 2012;37:1071–1076. doi: 10.2131/jts.37.1071. [DOI] [PubMed] [Google Scholar]
- Santini Z.I., Jose P.E., York Cornwell E., Koyanagi A., Nielsen L., Hinrichsen C., Meilstrup C., Madsen K.R., Koushede V. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health. 2020;5:e62–e70. doi: 10.1016/S2468-2667(19)30230-0. [DOI] [PubMed] [Google Scholar]
- Schuch F.B., Vancampfort D., Richards J., Rosenbaum S., Ward P.B., Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Servais S., Couturier K., Koubi H., Rouanet J.L., Desplanches D., Sornay-Mayet M.H., Sempore B., Lavoie J.M., Favier R. Effect of voluntary exercise on H2O2 release by subsarcolemmal and intermyofibrillar mitochondria. Free Radic. Biol. Med. 2003;35:24–32. doi: 10.1016/s0891-5849(03)00177-1. [DOI] [PubMed] [Google Scholar]
- Shao Y., Yan G., Xuan Y., Peng H., Huang Q.J., Wu R., Xu H. Chronic social isolation decreases glutamate and glutamine levels and induces oxidative stress in the rat hippocampus. Behav. Brain Res. 2015;282:201–208. doi: 10.1016/j.bbr.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Sherwin C.M. Voluntary wheel running: a review and novel interpretation. Anim. Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Skelly M.J., Chappell A.E., Carter E., Weiner J.L. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: possible role of disrupted noradrenergic signaling. Neuropharmacology. 2015;97:149–159. doi: 10.1016/j.neuropharm.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škop V.C., Malínská H., Trnovská J., Hüttl M., Cahová M., Blachnio-Zabielska A., Baranowski M., Burian M., Oliyarnyk O., Kazdová L. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonei N., Amiri S., Jafarian I., Anoush M., Rahimi-Balaei M., Bergen H., Haj-Mirzaian A., Hosseini M.-J. Mitochondrial dysfunction bridges negative affective disorders and cardiomyopathy in socially isolated rats: pros and cons of fluoxetine. World J. Biol. Psychiatry. 2017;18:39–53. doi: 10.3109/15622975.2016.1149218. [DOI] [PubMed] [Google Scholar]
- Stanisavljevic A., Peric I., Pantelic M., Filipovic D.M. Olanzapine alleviates oxidative stress in the liver of socially isolated rats. Can. J. Physiol. Pharmacol. 2017;95:634–640. doi: 10.1139/cjpp-2016-0598. [DOI] [PubMed] [Google Scholar]
- Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stolle S., Ciapaite J., Reijne A.C., Talarovicova A., Wolters J.C., Aguirre-Gamboa R., van der Vlies P., de Lange K., Neerincx P.B., van der Vries G., et al. Running-wheel activity delays mitochondrial respiratory flux decline in aging mouse muscle via a post-transcriptional mechanism. Aging Cell. 2018;17 doi: 10.1111/acel.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner L., Rowlands D., Morrison A., Credeur D., Hamlin M., Gaffney K., Lambrick D., Matheson A. Efficacy of exercise intervention for weight loss in overweight and obese adolescents: meta-analysis and implications. Sports Med. 2016;46:1737–1751. doi: 10.1007/s40279-016-0537-6. [DOI] [PubMed] [Google Scholar]
- Stonerock G.L., Hoffman B.M., Smith P.J., Blumenthal J.A. Exercise as treatment for anxiety: systematic review and analysis. Ann. Behav. Med. 2015;49:542–556. doi: 10.1007/s12160-014-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan A.M., Khalil D., Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat. Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Choi E.Y., Magee D.J., Stets C.W., During M.J., Lin E.-J.D. Metabolic effects of social isolation in adult C57BL/6 mice. Int. Sch. Res. Not. 2014;2014:1–9. doi: 10.1155/2014/690950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa K., Matsuda R., Uchitomi R., Onishi T., Hatazawa Y., Kamei Y. Effects of long-term physical exercise on skeletal muscles in senescence-accelerated mice (SAMP8) Biosci. Biotechnol. Biochem. 2019;83:518–524. doi: 10.1080/09168451.2018.1547625. [DOI] [PubMed] [Google Scholar]
- Thuné-Boyle I.C.V., Iliffe S., Cerga-Pashoja A., Lowery D., Warner J. The effect of exercise on behavioral and psychological symptoms of dementia: towards a research agenda. Int. Psychogeriatr. 2012;24:1046–1057. doi: 10.1017/S1041610211002365. [DOI] [PubMed] [Google Scholar]
- Todorović N., Filipović D. Prefrontal cortical glutathione-dependent defense and proinflammatory mediators in chronically isolated rats: modulation by fluoxetine or clozapine. Neuroscience. 2017;355:49–60. doi: 10.1016/j.neuroscience.2017.04.044. [DOI] [PubMed] [Google Scholar]
- Todorović N., Filipović D. The antidepressant- and anxiolytic-like effects of fluoxetine and clozapine in chronically isolated rats involve inhibition of hippocampal TNF-α. Pharmacol. Biochem. Behav. 2017;163:57–65. doi: 10.1016/j.pbb.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Todorović N., Tomanović N., Gass P., Filipović D. Olanzapine modulation of hepatic oxidative stress and inflammation in socially isolated rats. Eur. J. Pharm. Sci. 2016;81:94–102. doi: 10.1016/j.ejps.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K., Mariën P., Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- van Praag H., Christie B.R., Sejnowski T.J., Gage F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vandresen-Filho S., Martins W.C., Bertoldo D.B., Mancini G., De Bem A.F., Tasca C.I. Cerebral cortex, hippocampus, striatum and cerebellum show differential susceptibility to quinolinic acid-induced oxidative stress. Neurol. Sci. 2015;36:1449–1456. doi: 10.1007/s10072-015-2180-7. [DOI] [PubMed] [Google Scholar]
- Venna V.R., Xu Y., Doran S.J., Patrizz A., McCullough L.D. Social interaction plays a critical role in neurogenesis and recovery after stroke. Transl. Psychiatry. 2014;4:e351. doi: 10.1038/tp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss I.C., Pryce C.R., Jongen-Rêlo A.L., Nanz-Bahr N.I., Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Wendel A. Glutathione peroxidase. Methods Enzymol. 1981;77:325–333. doi: 10.1016/s0076-6879(81)77046-0. [DOI] [PubMed] [Google Scholar]
- Wright D.C., Han D.-H., Garcia-Roves P.M., Geiger P.C., Jones T.E., Holloszy J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Xia N., Li H. Loneliness, social isolation, and cardiovascular health. Antioxid. Redox Signal. 2018;28:837–851. doi: 10.1089/ars.2017.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Ohkuwa T., Itoh H., Sato Y., Naoi M. Effect of gender differences and voluntary exercise on antioxidant capacity in rats. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP. 2002;132:437–444. doi: 10.1016/s1532-0456(02)00097-2. [DOI] [PubMed] [Google Scholar]
- Zanier-Gomes P.H., de Abreu Silva T.E., Zanetti G.C., Benati É.R., Pinheiro N.M., Murta B.M.T., Crema V.O. Depressive behavior induced by social isolation of predisposed female rats. Physiol. Behav. 2015;151:292–297. doi: 10.1016/j.physbeh.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xu L., Zhang X., Yao Y., Sun Y., Qi L. Effects of different durations of aerobic exercise intervention on the cardiovascular health in untrained women: a meta-analysis and meta-regression. J. Sports Med. Phys. Fitness. 2018;58:1525–1536. doi: 10.23736/S0022-4707.17.07029-3. [DOI] [PubMed] [Google Scholar]
- Zhuravliova E., Barbakadze T., Zaalishvili E., Chipashvili M., Koshoridze N., Mikeladze D. Social isolation in rats inhibits oxidative metabolism, decreases the content of mitochondrial K-Ras and activates mitochondrial hexokinase. Behav. Brain Res. 2009;205:377–383. doi: 10.1016/j.bbr.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Zlatković J., Todorović N., Bošković M., Pajović S.B., Demajo M., Filipović D. Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol. Cell. Biochem. 2014;393:43–57. doi: 10.1007/s11010-014-2045-z. [DOI] [PubMed] [Google Scholar]
- Zlatković J., Bernardi R.E., Filipović D. Protective effect of Hsp70i against chronic social isolation stress in the rat hippocampus. J. Neural Transm. 2014;121:3–14. doi: 10.1007/s00702-013-1066-1. [DOI] [PubMed] [Google Scholar]
- Zlatković J., Todorović N., Tomanović N., Bošković M., Djordjević S., Lazarević-Pašti T., Bernardi R.E., Djurdjević A., Filipović D. Chronic administration of fluoxetine or clozapine induces oxidative stress in rat liver: a histopathological study. Eur. J. Pharm. Sci. 2014;59:20–30. doi: 10.1016/j.ejps.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Zolfagharzadeh F., Roshan V.D. Pretreatment hepatoprotective effect of regular aerobic training against hepatic toxicity induced by doxorubicin in rats. Asian Pac. J. Cancer Prev. 2013;14:2931–2936. doi: 10.7314/apjcp.2013.14.5.2931. [DOI] [PubMed] [Google Scholar]