Abstract
Bovine coronaviruses (BoCVs) have been found in respiratory tissues in cattle and frequently associated with bovine respiratory disease (BRD); however, pathogenesis studies in calves are limited. To characterize the pathogenesis and pathogenicity of BoCV isolates, we used 5 different BoCV strains to inoculate colostrum-deprived calves, ~ 2–5 wk of age. Later, to determine if dual viral infection would potentiate pathogenicity of BoCV, calves were inoculated with BoCV alone, bovine viral diarrhea virus (BVDV) alone, or a series of dual-infection (BVDV–BoCV) schemes. A negative control group was included in all studies. Clinical signs and body temperature were monitored during the study and samples collected for lymphocyte counts, virus isolation, and serology. During autopsy, gross lesions were recorded and fixed tissues collected for histopathology and immunohistochemistry; fresh tissues were collected for virus isolation. Results suggest increased pathogenicity for isolate BoCV OK 1776. Increased body temperature was found in all virus-inoculated groups. Lung lesions were present in calves in all dual-infection groups; however, lesions were most pronounced in calves inoculated with BVDV followed by BoCV inoculation 6 d later. Lung lesions were consistent with mild-to-moderate interstitial pneumonia, and immunohistochemistry confirmed the presence of BoCV antigen. Our studies demonstrated that BVDV–BoCV dual infection may play an important role in BRD pathogenesis, and timing between infections seems critical to the severity of lesions.
Keywords: bovine coronavirus, bovine respiratory disease, bovine viral diarrhea virus, dual infection
Introduction
The term bovine respiratory disease complex (BRDC) refers to a range of clinical disease and lesions of the respiratory tract of cattle. BRDC has a multifactorial etiology and develops because of complex interactions among factors including environmental stressors, host predispositions, and both bacterial and viral pathogens.5 The diversity of diseases of BRDC has made it difficult to define the roles of individual factors, because several factors appear necessary but not sufficient to cause BRDC on their own. It has been proposed that either simultaneous or sequential infections with multiple pathogens, including both viruses and bacteria, are involved.
Bovine viral diarrhea viruses (BVDVs) and bovine coronaviruses (BoCVs) are 2 of the viruses implicated as contributors to BRDC.12,13 Seroprevalence rates for both are high among cattle in the United States.3,19,21,22 Acute uncomplicated infections with typical field strains of BVDV rarely result in clinical respiratory disease.10 Rather than being a primary respiratory pathogen, it is theorized that BVDV potentiates BRDC by 1 of 2 mechanisms: immunosuppression (requiring subsequent sequential infection) and synergism (requiring simultaneous infection).18,19 Initially BoCV was associated with enteric disease in calves and winter dysentery in adult cattle.23 More recently, respiratory BoCV infections have been associated with BRDC.4,14,24 Although BoCV has been isolated from cattle with BRDC clinical signs,13–15 it is also isolated from healthy cattle at a similar frequency.14,15 Therefore, the pathogenic role of BoCV in BRDC has not been clearly established. It has been suggested that BoCV should be studied “in the context of mixed infections.”8 Hence, we characterized the pathogenicity of 4 BoCV isolates and determined if sequential infections with BVDV and BoCV would result in clinical signs of respiratory disease as well as gross and microscopic lesions that were more severe than those produced by BoCV alone.
Materials and methods
Isolation and propagation of BoCV and BVDV strains
We used 4 field isolates of BoCV (OK 1776, OK An5, OK 43, OK 834) and a reference strain received from the National Veterinary Services Laboratory (BoCV NVSL; Ames, IA). The sources and studies from which the BoCV strains were derived have been described previously.15 The BoCV strains were propagated in human rectal tumor (HRT18G) monolayer cultures as described.15 This cell line was found free of bovine pestiviruses.2 The HRT18G cells were grown in Dulbecco modified Eagle medium (DMEM; ATCC) supplemented as described below.
The noncytopathic (NCP) BVDV-2 strain RS886, isolated from a persistently infected calf that appeared clinically normal, was used for in vivo studies.16 Acute infection of calves with this field strain resulted in pyrexia and a drop in peripheral blood lymphocytes. Two cytopathic BVDV strains from 2 different pestivirus species, BVDV genotype 1, subgenotype a (BVDV-1a) strain Singer, and BVDV-2a strain 296c, were used in virus neutralization tests.20 These isolates were propagated in bovine turbinate (BT) cells that were free of bovine pestiviruses.14 BT cells were grown in minimal essential medium (MEM; MilliporeSigma) supplemented as described below.
The media used for growing HRT18G or BT cells was supplemented with 10% fetal bovine serum (FBS), L-glutamine (final concentration, 1.4 mM; Gibco Life Technologies), and antibiotic–antimycotic solution (10 mL/L of 100×; Gibco Life Technologies). The FBS was free of bovine pestiviruses and antibodies against bovine pestiviruses.2
Endpoints for BoCV titration and serum neutralizations were based on observation of cytopathic effect (CPE). Endpoints for BVDV-2 strain RS886 titrations were determined using the N2 monoclonal antibody that binds the pestivirus E2 protein.2 Endpoints for virus neutralization tests using BVDV-1a Singer and BVDV-2a 296c were based on observation of CPE.3 To confirm that viral isolates from calves matched the viral inocula that each calf received, all isolates were sequenced as follows. Samples were processed, and virus isolation (VI) was conducted as described previously.1,14 Template preparation, PCR amplification, and phylogenetic analysis of BoCV and BVDV sequences were performed as described.14,19
Animal studies
All animal experiments were conducted at the National Animal Disease Center (Ames, IA) in accordance with the Animals Welfare Act Amended (7USC, 2131-2156). A brief outline of the experimental groups used in each of the in vivo studies is listed in Table 1. All calves were colostrum-deprived, housed under BL2 security, and fed a commercial ration. Basal temperatures were monitored using indwelling rumen probes (Advanced Telemetry Systems).1 BoCV challenge was by intranasal aerosol inoculation (LMA MAD300 nasal atomizer; Teleflex) as described previously.18 BVDV challenge was 4 mL of viral preparation (1 × 106 TCID/mL) by intranasal aerosol inoculation (LMA MAD300 nasal atomizer) as described previously.20
Table 1.
Summary of experimental design of bovine coronavirus (BoCV) and bovine viral diarrhea virus (BVDV) dual infections.
| Study 1 | ||||||
|---|---|---|---|---|---|---|
| Study purpose | Single infection. Comparison of BoCV strains. Determine if variation exists among BoCV strains and select BoCV strain from subsequent studies. |
|||||
| Groups | Control (n = 2) | 1A (n = 3) | 1B (n = 3) | 1C (n = 3) | 1D (n = 3) | 1E (n = 3) |
| Treatment | No virus | OK 1776* | OK An5* | OK 43* | OK 834* | NVSL† |
| Experimental design | All inoculations occurred on day 0 | |||||
| Study 2 | ||||||
| Study purpose | Dual infection. Determine outcome of sequential infections when second virus exposure occurs 3 d after first virus exposure. Viruses used: BoCV strain OK 1776, BVDV-2a strain RS886‡. |
|||||
| Groups | 2A (n = 2) | 2B (n = 3) | 2C (n = 3) | 2D (n = 2) | ||
| Treatment | Only BoCV | BoCV followed 3 d later by BVDV-2a | BVDV-2a followed 3 d later by BoCV | No virus control | ||
| Experimental design | day 0 | BoCV | BoCV | BVDV-2a | No virus | |
| day 3 | No virus | BVDV-2a | BoCV | No virus | ||
| Study 3 | ||||||
| Study purpose | Dual infection. Determine outcome of sequential infections when BVDV exposure occurs first followed by BoCV exposures at 6 or 9 d after BVDV exposure. Viruses used: BoCV strain OK 1776; BVDV-2a strain RS886. |
|||||
| Groups | 3E (n = 3) | 3F (n = 3) | 3G (n = 3) | 3H (n = 2) | ||
| Treatment | BVDV-2a followed 9 d later by BoCV | BVDV-2a followed 6 d later by BoCV | Only BVDV-2a | No virus control | ||
| Experimental design | day 0 | BVDV-2a | No virus | No virus | No virus | |
| day 3 | No virus | BVDV-2a | No virus | No virus | ||
| day 7 | No virus | No virus | BVDV-2a | No virus | ||
| day 9 | BoCV | BoCV | No virus | No virus | ||
| Study 4 | ||||||
| Study purpose | Dual infection. Determine outcome of sequential infections when BVDV exposure occurs first followed by BoCV exposures at 6 or 9 d after BVDV exposure. Viruses used: BoCV strain OK 1776; BVDV-2a strain RS886. |
|||||
| Groups | 4M (n = 4) | 4N (n = 4) | 4O (n = 2) | 4P (n = 2) | 4Q (n = 2) | |
| Treatment | BVDV-2a followed 9 d later by BoCV | BVDV-2a followed 6 d later by BoCV | BVDV-2a only | No virus control | BoCV only | |
| Experimental design | day 0 | BVDV-2a | No virus | BVDV-2a | No virus | No virus |
| day 3 | No virus | BVDV-2a | No virus | No virus | No virus | |
| day 9 | BoCV | BoCV | No virus | No virus | BoCV | |
Field isolate of BoCV provided by Oklahoma State University (Stillwater, OK).
Reference strain of BoCV provided by National Veterinary Services Laboratories (Ames, IA).
Field isolate of BVDV-2a provided by National Animal Disease Center (Ames, IA).
Blood samples for peripheral blood lymphocyte counts as well as VI and antibody testing were collected at various intervals by jugular venipuncture. Nasal swabs were collected by inserting a sterile cotton swab ~ 2.5 cm into the nasal cavity (nostril). Only one nostril was swabbed per sampling time, and sampling from nostrils was alternated to limit mucosal surface damage. Rectal swabs were collected by inserting a sterile cotton swab ~ 5 cm into the rectum.
The scoring criteria used for recording respiratory disease in calves can be found at https://www.vetmed.wisc.edu/fapm/svm-dairy-apps/calf-health-scorer-chs/.
Four successive studies were done (Suppl. Data). The design of each successive study was informed by the findings of the preceding studies.
Study 1: comparison of pathogenicity of BoCV strains
In the initial study to select the BoCV strain for sequential studies, 17 healthy colostrum-deprived Holstein calves, ~ 35 d of age, that tested negative for BVDV and for antibodies against BoCV, were assigned to 1 of 6 treatment groups for each of 5 different BoCV strains and a non-challenged control group (Table 1).
The 2 negative control calves were exposed to a mock inoculum consisting of clarified freeze–thaw lysate of non-infected HRT18G cells. Calves in the remaining 5 treatment groups (n = 3) were exposed to a clarified freeze–thaw lysate of HRT18G cells infected with 1 of 4 field isolates of BoCV (OK 1776, OK An5, OK 43, OK 834 )14 or BoCV NVSL. Following inoculation, calves were observed twice a day for signs of respiratory disease. Serum samples, nasal swabs, and rectal swabs were collected on days –1, 1, 3, 4, 5, 6, 7, 8, and 10. Calves were autopsied on day 17. At autopsy, lungs were photographed, and tissue samples were collected for microscopy as described in the “Microscopic evaluation” section of Materials and methods. Based on the data from study 1, the BoCV OK 1776 isolate was chosen for use in the dual-infection studies.
Study 2: BVDV–BoCV dual-infection study
Previous studies characterized the BVDV-2a RS886 isolate used in the next 3 studies.16 Ten colostrum-deprived calves, 3–5 wk of age, were assigned to 1 of 4 groups (2A–D; Table 1). The 2 calves in group 2A were exposed to BoCV at day 0; the 3 calves in group 2B were exposed to BoCV at day 0 followed 3 d later by exposure to BVDV-2a; the 3 calves in group 2C were exposed to BVDV-2a at day 0 followed 3 d later by exposure to BoCV; and the 2 calves in group 2D were given mock inoculation (freeze–thaw lysate of either HRT18G cells or MDBK cells) on days 0 and 4. Intranasal inoculum consisted of 4 mL of viral preparation (1 × 106 TCID/mL) delivered by intranasal atomization. One of the 2 calves in group 2D developed diarrhea caused by a bacterial infection and was removed from the study. Following inoculation, calves were observed twice a day for signs of respiratory disease. Serum samples and nasal swabs were collected on days –2, 2, 4, 6, 9, 11, 13, and 16. Calves were autopsied on day 17, which was 17 d post BoCV infection for calves in group 2A, 17 d post BoCV infection and 14 d post BVDV infection for calves in group 2B, and 17 d post BVDV infection and 14 d post BoCV infection for calves in group 2C. At autopsy, pictures were taken of the lungs, and tissue samples were collected for microscopy as described in the “Microscopy evaluation” section of Materials and methods.
Study 3: BVDV–BoCV dual-infection study
Eleven colostrum-deprived calves 3–5 wk of age were assigned to 1 of 4 groups (3E–H; Table 1). The 3 calves in group 3E were exposed to BVDV-2a at day 0 followed 9 d later by exposure to BoCV. The 3 calves in group 3F were exposed to BVDV-2a at day 3 followed 6 d later by exposure to BoCV. The 3 calves in group 3G were exposed to BVDV-2a at day 7, and a mock inoculum on day 9 that did not contain virus. The 2 calves in group 3H received a mock inoculum on days 0 (freeze–thaw lysate of non-infected MDBK cells) and 9 (freeze–thaw lysate of noninfected HRT18G cells). Inoculations consisted of 4 mL of viral preparation (1 × 106 TCID/mL) delivered by intranasal atomization. Following inoculation, calves were observed twice a day for signs of respiratory disease. Serum samples, nasal swabs, and whole blood were collected on days –2, 2, 4, 6, 9, 11, 13, 16, 18, 20, and 23. Calves were autopsied on day 24, which was 24 d post BVDV and 15 d post BoCV for calves in group 3E, 21 d post BVDV and 15 d post BoCV for calves in group 3F, and 17 d post BVDV for calves in group 3G. At autopsy, lungs were photographed, and tissue samples were collected for microscopy as described in the “Microscopy evaluation” section of Materials and methods.
Study 4: BVDV–BoCV dual-infection study
Fourteen colostrum-deprived calves, 3–5 wk of age, were assigned to 1 of 5 groups (4M–Q; Table 1). The 4 calves in group 4M were exposed to BVDV-2a at day 0 followed 9 d later by exposure to BoCV. The 4 calves in group 4N were exposed to BVDV-2a at day 3 followed 6 d later (day 9) by exposure to BoCV strain OK 1776. The 2 calves in group 4O were exposed to BVDV-2a at day 0 and mock inoculum (HRT18G cells lysate) at day 9. The 2 calves in group 4P were exposed to mock inoculum on days 0 (MDBK lysate) and 9 (HRT18G lysate). The 2 calves in group 4Q were exposed to mock inoculum on day 0 (MDBK lysate) and BoCV on day 9. Viral inoculations consisted of 4 mL of viral preparation (1 × 106 TCID/mL) delivered intranasally. Mock inoculations consisted of 4 mL of clarified freeze–thaw lysate of the appropriate cell line. Following inoculation, calves were observed twice a day for signs of respiratory disease. Serum samples, nasal swabs, and whole blood were collected on days –2, 2, 4, 6, 9, 11, 13, 16, 18, 20, and 23. Calves were autopsied on day 24, which was 24 d post BVDV and 15 d post BoCV for calves in group 4M, 21 d post BVDV and 15 d post BoCV for calves in group 4N, 24 d post BVDV for calves in group 4O, and 15 d post BoCV for calves in group 4Q. At autopsy, lungs were photographed, and tissue samples were collected for microscopy as described in the “Microscopy evaluation” section of Materials and methods.
Microscopic evaluation
For studies 1, 3, and 4, histopathology was done. Tissue specimens from mid-trachea, right main bronchus, right cranial lobe, right ventral caudal lobe, left cranial lobe, left ventral caudal lobe, colon, ileum, and mesenteric lymph nodes were collected from each calf, fixed in formalin, and processed routinely for histologic examination. If gross lesions were noted in areas unrelated to routine sampling, tissue was collected from those sites for histopathology. To increase the rigor of the analysis of the final study (study 4), histologic examination was done by one investigator (A.W. Confer) without the benefit of the accompanying information on gross findings or treatment group assignment of each calf. Each lung sample was scored on a scale of 0–4, where 0 = no lesions (normal lung), 1 = minimal pathologic changes, 2 = mild pathologic changes, 3 = moderate pathologic changes, and 4 = severe pathologic changes. Evaluation of tissue specimen changes was based on distribution and intensity, and included increased leukocytes in airway lumens, lamina propria, and alveolar septa; edema or fibrin exudation; necrosis or proliferation of pneumocytes; and interalveolar expansion. A morphologic diagnosis was given for each section that had a score of 1 or greater. The total respiratory histologic score for each calf (0–16) was the summation of the scores for each lung section examined.
For studies 1 and 4, immunohistochemistry (IHC) was done for BVDV and BoCV antigens. Six-microgram sections of paraffin-embedded, formalin-fixed tissue were cut, pronase treated, and stained by IHC for BoCV using monoclonal antibody lot WR99316 BC 28 HI.2C against N protein (developed by Dr. L.J. Saif, Ohio State University, Wooster, OH) and for BVDV using monoclonal antibody 3.12F1, which is directed against the GP48 BVDV protein (developed by Dr. J.T. Saliki, Oklahoma State University, Stillwater, OK).6,7 Antibodies in ascitic fluid were diluted 1:75,000 in phosphate-buffered saline solution containing Tween 20. Secondary and tertiary antibodies were rabbit anti-mouse IgG antibody diluted 1:500 and biotinylated goat anti-rabbit IgG (H&L), respectively (Zymed Laboratories). Streptavidin–horseradish peroxidase and nova red substrate (Vector Laboratories) were used to develop the color reaction. All reactions were conducted in a Model LV auto immunostainer (Dako). Positive control sections included BVDV-positive skin sections and BoCV-infected intestinal sections. For negative controls, duplicate positive sections of tissues were treated with normal mouse IgG before treatment with secondary and tertiary antibodies. Negative control tissues were treated for color development and examined microscopically as described above.
The distribution of antigen was documented in the respiratory, colonic, and lymph node sections for each calf. Antigen distribution was designated with respect to cell type or histologic location of a positive signal and was subjectively scored: 0 = no detectable antigen; + = weak, antigen faintly detected uniformly within the cytoplasm or in a stippled pattern; ++ = moderate, antigen readily detected uniformly within the cytoplasm; +++ = strong, antigen staining is intense within the cytoplasm.
Virus isolation
VI was conducted in nasal swabs (all studies), rectal swabs (study 1), and buffy coat samples (studies 2–4). HRT18G cells were used for BoCV VI; BT cells were used for BVDV VI as described previously.2,3,14 Briefly, 3 cell passages were performed in 70–80% confluent cell monolayers. Plates were incubated at 37°C with 5% CO2 and monitored daily for 72–96 h. Plates were read with a light microscope for the presence of CPE in the cell monolayer for BoCV or following an immunoperoxidase test as described previously for the NCP BVDV-2.2
Virus neutralization assay
Virus neutralization assay was performed on serum samples to detect and quantify the presence of neutralizing antibodies as described previously.1,2,14 Serum dilutions of 1:4–1:512 were incubated individually with 200 TCID/50 BVDV or BoCV. Following 90 min of incubation at 37°C in 5% with CO2, appropriate cells suspensions were added and incubated for 5 d at 37°C in 5% CO2. Plates were read with a light microscope for the presence of CPE in the cell monolayer for BoCV and cytopathic BVDV.
Results
Study 1: comparison of BoCV strains
VI from nasal and rectal swabs and/or the development of neutralizing antibodies indicated that all exposed calves became BoCV infected (Table 2). Clinical signs of respiratory disease were not observed in any of the calves infected with any of the 5 BoCV isolates. Although little difference in clinical presentation was observed among the calves infected with field strains of BoCV, there were differences between calves infected with BoCV field strains and those infected with BoCV NVSL. Between 98 and 130 h post-infection, most of the calves exposed to field strains of BoCV (90%) exhibited a rise in basal temperature that lasted 7–10 h, with the highest average temperature per treatment group of 39.5–40.2°C. Only 1 of 3 calves exposed to BoCV NVSL exhibited pyrexia during this period. Viremia in calves infected with BoCV field viruses, based on VI from nasal or rectal swabs, lasted 3–4 d. The day of first detection and number of days that virus was detected from nasal and rectal swabs were similar among field strain–inoculated calves. In contrast, virus could only be detected in samples collected from 1 of 3 calves exposed to the BoCV NVSL. Further isolation of virus was only positive with one day 10 nasal swab and one day 7 rectal swab. Although all calves exposed to virus developed antibodies by day 10 post-exposure, the antibody titers detected in calves exposed to BoCV NVSL were lower than the antibody titers detected in calves exposed to field strains (data not shown).
Table 2.
Study 1 (comparison of multiple bovine coronavirus (BoCV) strains based on clinical presentations).
| BoCV challenge strain | No. of calves with pyrexia | Mean highest temperature (°C) | Virus isolation | Average antibody level on day 10* | Tracheal and bronchial gross lesions | |
|---|---|---|---|---|---|---|
| Nasal swab | Rectal swab | |||||
| OK AN5 | 3/3 | 39.6 ± 0.2 | 1 calf/d 3–7 1 calf/d 3–6 1 calf/d 4–7 |
2 calves/d 3–7 1 calf/d 3–6 |
7.5 | 2/3 |
| OK 43 | 3/3 | 39.8 ± 0.1 | 2 calves/d 3–7 1 calf/d 3–6 |
2 calves/d 3–7 1 calf/d 3–6 |
6.6 | 0/3 |
| OK 834 | 2/3 | 40.2 ± 0.3 | 2 calves/d 3–7 1 calf/d 3–6 |
2 calves/d 3–7 1 calf/d 3–6 |
6.6 | 2/3 |
| NVSL | 1/3 | 39.5 ± 0.2 | 1 calf/d 10 | 1 calf/d 7 | 5.9 | 0/3 |
| OK 1776 | 2/3† | 39.5 ± 0.1 | 3 calves/d 3–7 | 2 calves/d 3–7 1 calf/d 3–6 |
7.7 | 2/3 |
| Control | 0/2 | No fever detected | None | None | No antibodies detected | 0/2 |
Reported as reciprocal of log base 2.
One animal disgorged the temperature probe.
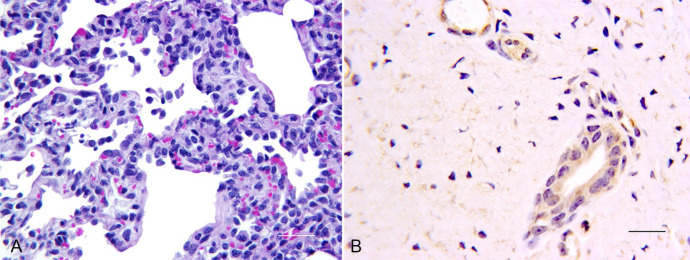
Gross lung lesions were inconsequential and precluded scoring or determination of percentage lung involvement. Five calves (2 in OK 1776, 1 in OK An5, and 2 in OK 834 groups) had mucofibrinous strands within the distal trachea and/or main bronchi. These lesions were most likely viral related; however, no histologic change was found in the underlying mucosa. Histologically, necrosis was seen in intestinal lymphoid tissue in association with OK An5, OK 43, and BoCV NVSL infections. Lesions in field strain BoCV–inoculated calves consisted of multifocal, minimal-to-mild, interstitial pneumonia characterized by minimal fibrin strands in alveoli, hypertrophy of pneumocytes, and increased pulmonary alveolar macrophages (Fig. 1A). The BoCV IHC signal was weakly positive in bronchial and tracheal epithelium and moderately to strongly positive in tracheal glands as well as lamina proprial and alveolar macrophages. Macrophages within lung, trachea, and lymph nodes were often strongly positive for BoCV antigen (Fig. 1B). Most isolates were associated with IHC-positive epithelia in small intestine and/or proximal colon. However, intestinal localization was not consistent. Because no consistent differences were observed among calves exposed to the 4 field strains, one strain (OK 1776) was selected for subsequent studies.
Figure 1.
Lesions and immunohistochemistry after bovine coronavirus (BoCV) strain OK 1776 infection. A. Interstitial pneumonia characterized by increased cellularity of alveolar septa and increased macrophages in alveoli. 200×. H&E. Bar = 20 µm. B. Anti-BoCV immunohistochemistry demonstrating positive BoCV signal in tracheal gland epithelium. 400×. Bar = 20 µm.
Study 2: BVDV–BoCV dual-infection study
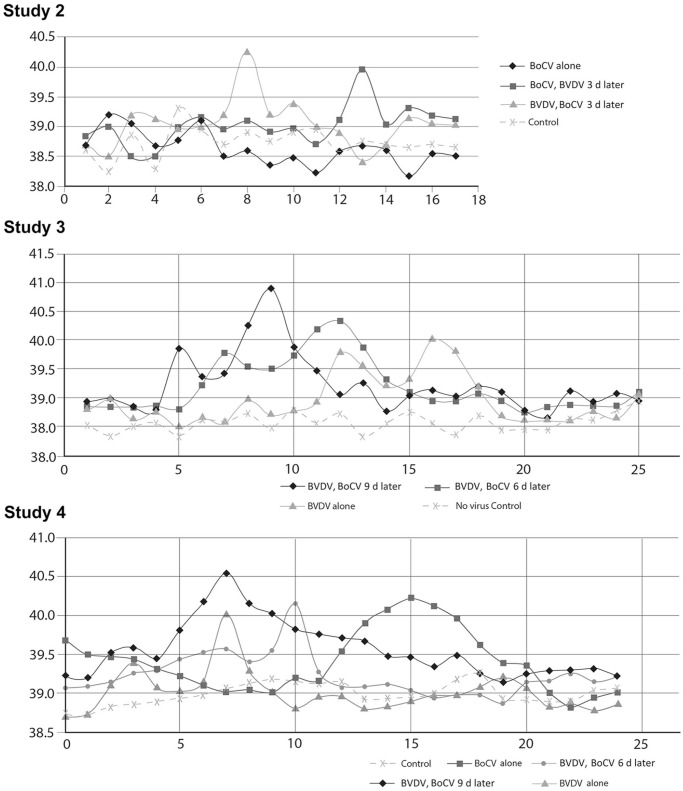
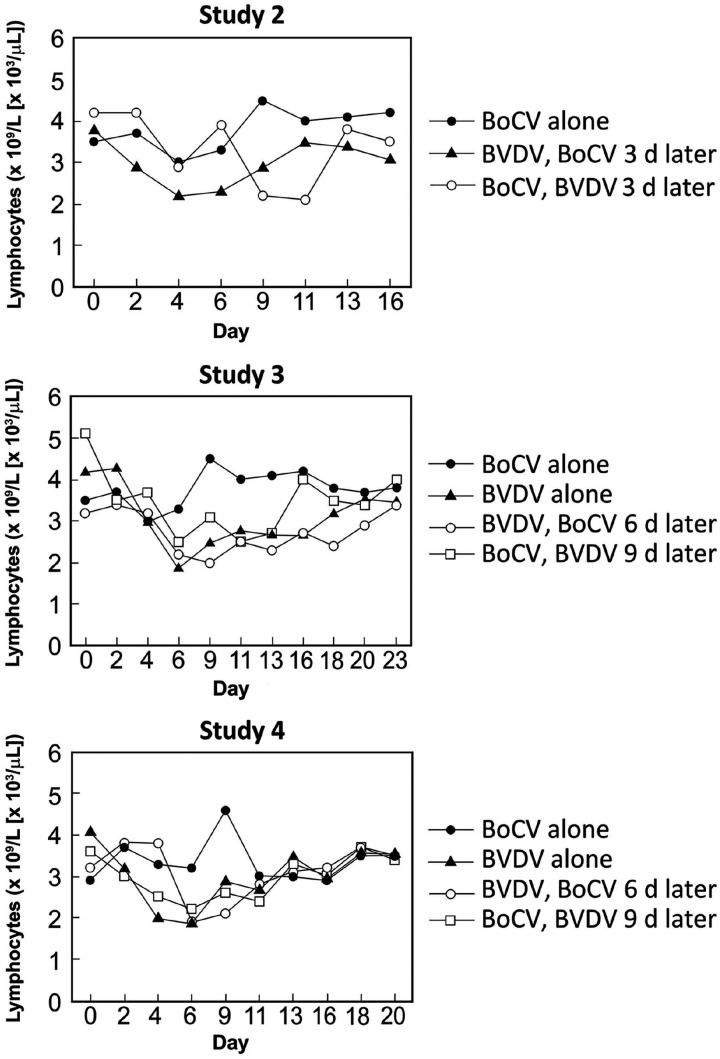
Pronounced pyrexia (> 40.0°C) was only observed in calves exposed to both BVDV and BoCV and corresponded to 8–9 d post BVDV exposure (Fig. 2A; Table 3). Changes were noted in peripheral blood lymphocyte counts over the course of the experiment in calves exposed to BoCV alone or in combination with BVDV (Fig. 3A; Suppl. Data). Peripheral blood lymphocyte counts in group 2A (BoCV only) were reduced on day 4 followed by a rebound to above baseline levels by day 9. The peripheral blood lymphocyte counts in group 2B calves (BoCV followed by BVDV) had a small reduction at day 4 but rebounded to near baseline level at day 6 followed by a pronounced decline at days 9 and 11 (5 and 7 d after BVDV, respectively). Peripheral blood lymphocyte counts for group 2C (BVDV followed by BoCV) had their biggest decrease on day 4, and values remained 20% lower than baseline until day 9. Peripheral blood lymphocyte counts were similar for groups 2B and 2C on days 11 and 13, with both groups having lower counts than calves in group 2A.
Figure 2.
Basal body temperatures over time in dual-infection studies. Panels show mean basal temperatures per day observed in dual-infection studies 2, 3, and 4. The y-axis is the mean temperature (°C) per group, and the x-axis is experimental day.
Table 3.
Study 2 (dual infection with bovine coronavirus [BoCV] strain OK 1776 and bovine viral diarrhea virus [BVDV] genotype 2, subgenotype a).
| Group | BoCV | BVDV | Lung lesions | Mean highest temperature (°C) | ||||
|---|---|---|---|---|---|---|---|---|
| VI | VN | VI | VN | |||||
| BC | NS | BC | NS | |||||
| 2A (BoCV only) | 0/2 | 1/2: d 2,4,6 post-BoCV exposure | 1/2: d 9 post-BoCV exposure | 0/2 | 0/2 | 0/2 | 0/2 | 39.1 ± 0.6 |
| 2B (BoCV followed 3 d later by BVDV) | 0/3 | 1/3: d 2,4 post-BoCV exposure 2/3: d 2,4,6 post-BoCV exposure |
1/3: d 6 post-BoCV exposure 2/3: d 9 post-BoCV exposure |
1/3: d 5,7 post-BVDV exposure 2/3: d 5,7,9 post-BVDV exposure |
1/3: d 5,7,9 post-BVDV exposure 1/3: d 7,9 post-BVDV exposure 1/3: d 5.7,9,11 post-BVDV exposure |
3/3: d 13 post-BVDV exposure | 3/3 | 40.0 ± 0.5 |
| 2C (BVDV followed 3 d later by BoCV) | 0/3 | 1/3: d 2,5 post-BoCV exposure 1/3: d 2,5,7 post-BoCV exposure 1/3: d 5,7,9 post-BoCV exposure |
3/3: d 13 BoCV exposure | 1/3: d 4,6,9 post-BVDV exposure 2/3: d 4,6 post-BVDV exposure |
0/3 | 1/3: d 13 post-BVDV exposure 2/3: d 17 post-BVDV exposure |
1/3 | 40.3 ± 0.8 |
| 2D (no virus) | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 39.2 ± 0.3 |
BC = buffy coat; NS = nasal swab; VI = virus isolation; VN = virus neutralization.
Figure 3.
Peripheral blood lymphocyte counts. Panels are group mean peripheral lymphocyte counts (× 109/L [× 103/µL]) in calves in dual-infection studies 2, 3, and 4.
BVDV was isolated from both buffy coat and nasal swab samples, whereas BoCV was only isolated from nasal swab samples (Table 3; Suppl. Data). BVDV was only isolated from nasal swab samples in the dual-infection treatment group in which calves were first inoculated with BoCV followed by BVDV. Genomic sequences of isolated viruses matched the sequences of viruses in inocula (data not shown). All calves seroconverted against both isolates in the dual-infected groups. One of the 2 calves in group 2A (BoCV alone) failed to seroconvert against BoCV. Seroconversion against BoCV occurred later in calves first infected with BVDV compared to the BoCV alone group 2A. Based on days of isolation, calves inoculated with both viruses had concurrent infections during at least part of the study. No lung lesions were observed in control calves or calves infected with BoCV alone. Lung lesions were observed in 3 of 3 calves in group 2B (BoCV followed 3 d later by BVDV) and 1 of 3 calves in group 2C (BVDV followed 3 d later by BoCV). Lung lesions consisted of pale, firm foci, 0.5–1.0 cm diameter, randomly scattered throughout the lungs, but particularly obvious in the ventral, caudal lung lobes. Histopathology was not done.
Study 3: BVDV–BoCV dual-infection study
Signs of mild respiratory disease was observed in 2 of 3 calves in group 3F (Table 4). Biphasic pyrexia of ≥ 39.5°C was observed in groups 3E–G. The highest temperatures were seen in calves from group 3E; calves in groups 3E and 3F had higher peak pyrexia than calves in group 3G. Conversely, calves in group 3G had the greatest decrease in peripheral blood lymphocytes, with calves in group 3E having the least and calves in group 3F falling in between (Fig. 3B). The greatest decrease in peripheral blood lymphocytes occurred 5–6 d post BVDV exposure.
Table 4.
Clinical signs in study 3 (dual infection with bovine coronavirus [BoCV] strain OK 1776 and bovine viral diarrhea virus [BVDV] genotype 2, subgenotype a).
| Group | Fever (no. of calves) | Mean highest temperature (°C) | Cough | Nasal discharge | Diarrhea | Blood in stool |
|---|---|---|---|---|---|---|
| 3E (BVDV d 0; BoCV d 9) | 3/3 | 41.1 ± 0.1 | 3/3 | 0/3 | 3/3, mild | 0/3 |
| 3F (BVDV d 3; BoCV d 9) | 3/3 | 40.6 ± 0.2 | 2/3 | 2/3 | 1/3 | 0/3 |
| 3G (BVDV d 7) | 3/3 | 40.2 ± 0.3 | 0/3 | 0/3 | 3/3 | 0/3 |
| 3H (no virus) | 0/2 | No fever detected | 0/2 | 0/2 | 0/2 | 0/2 |
BVDV was isolated only from buffy coat samples in single BVDV infections (Table 5; Suppl. Data). BVDV was isolated from both nasal swab and buffy coat samples in dual-infected calves. The dates of VI indicate that BVDV was replicating at the time of BoCV inoculation for both dual-infected groups. Genomic sequences of isolated viruses matched the sequences of viruses in inocula (data not shown). Based on VI, there appear to have been concurrent infections in group 3F calves but not in group 3E calves. Two of 3 calves in both groups 3E and 3F had lung lesions. Lung lesions consisted of pale, firm foci, 0.5–1.0 cm diameter, randomly scattered throughout the lungs but particularly obvious in the ventral, caudal lung lobes. Histologically, there were minimal-to-moderate, multifocal lesions of interstitial pneumonia that often were in peribronchial alveoli. Lesion scores indicated that group 3F had the highest mean score of 6.5 ± 3.7, whereas group 3E had a mean score of 3.5 ± 2.7, which was not significantly different between groups (p > 0.05).
Table 5.
Virus isolation, serology, and lung lesions in study 3 (dual infection with bovine coronavirus [BoCV] strain OK 1776 and bovine viral diarrhea virus [BVDV] genotype 2, subgenotype a).
| Group | BoCV | BVDV | Lung lesion | ||||
|---|---|---|---|---|---|---|---|
| VI | VN | VI | VN | ||||
| BC | NS | BC | NS | ||||
| 3E (BVDV d 0; BoCV d 9) | 0/3 | 2/3: d 2,4,7 post-exposure 1/3: d 2,4 post-exposure |
2/3: d 7 post-exposure 1/3: d 9 post-exposure |
1/3: d 6 post-exposure 1/3: d 6,9 post-exposure 1/3: d 4,6,9 post-exposure |
1/3: d 4,6 post-exposure 1/3: d 6,9 post-exposure |
3/3: d 13 post-exposure | 2/3 |
| 3F (BVDV d 3; BoCV d 9) | 0/3 | 3/3: d 2,4 post-exposure | 3/3: d 7 post-exposure | 1/3: d 9 post-exposure 1/3: d 6,9 post-exposure 1/3: d 9,11,13 post-exposure |
1/3: d 11 post-exposure 1/3: d 6,9 post-exposure |
3/3: d 16 post-exposure | 2/3 |
| 3G (BVDV d 7) | 0/2 | 0/2 | 0/2 | 2/2: d 10 post-exposure | 0/2 | 1/2: d 17 post-exposure 1/2: d 20 post-exposure |
0/2 |
| 3H (no virus) | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
VI = virus isolation; VN = virus neutralization.
Study 4: BVDV–BoCV dual-infection study
Pyrexia was observed in all groups except group 4P, which was the no-virus control group (Fig. 2C; Table 6). The highest average temperature was observed in group 4M (BVDV followed 9 d later by BoCV). In groups exposed to BVDV (groups 4M–O), pyrexia was first detected 3 d post-inoculation. In group 4Q (BoCV only), pyrexia was observed in only 1 of 2 calves beginning 3 d after exposure to BoCV. Cough and nasal discharge were observed in dual-infected calves but not in non-infected calves or calves infected only with BVDV or BoCV. No animals had titers against BVDV or BoCV at their day of birth. At day –3, 3 animals had titers against BoCV. However, by day 2, none of the 3 animals had a detectable titer. Two of the animals were not experimentally exposed to BoCV and did not have detectable antibodies against BoCV on any of the subsequent testing dates. One animal was experimentally exposed to BoCV and did not display BoCV antibodies after day 2 until the day 16 samples. The timing and level of detected BoCV antibodies was similar to the other animal in the experimental group (group N) that developed antibodies against BoCV. Although we have no explanations for the BoCV titers observed on day –3, subsequent samples collected from these 3 animals were consistent with other animals in their experimental group. Thus, these animals were not removed from the study, and seroconversion against BoCV was based on development of antibodies after day 2. Based on VI and/or seroconversion, viral replication occurred in all virus-exposed groups. VI was more successful with BVDV than BoCV in this study. Genomic sequences of isolated viruses matched the sequences of viruses in inocula (data not shown). However, IHC of tissues taken at autopsy was positive for all calves exposed to BoCV but negative for all calves exposed to BVDV.
Table 6.
Clinical signs in study 4 (dual infection with bovine coronavirus [BoCV] strain OK 1776 and bovine viral diarrhea virus [BVDV] genotype 2, subgenotype a).
| Group | Pyrexia | Mean highest temperature (°C) | Cough | Nasal discharge | Diarrhea | Ocular discharge |
|---|---|---|---|---|---|---|
| 4M (BVDV d 0; BoCV d 9) | 4/4 | 40.6 ± 0.1 | 4/4 | 2/4 | 1/4 | 1/4 |
| 4N (BVDV d 3; BoCV d 9) | 4/4 | 40.2 ± 0.2 | 2/4 | 1/4 | 0/4 | 0/4 |
| 4O (BVDV d 0) | 2/2 | 40.2 ± 0.1 | 0/2 | 0/2 | 0/2 | 0/2 |
| 4P (no virus) | 0/2 | No detectable fever | 0/2 | 0/2 | 0/2 | 0/1 |
| 4Q (BoCV d 9) | 1/2 | 40.3* | 1/2 | 0/2 | 0/2 | 0/1 |
No standard deviation calculated because only one animal exhibited pyrexia.
Calves only exposed to BoCV had a transient increase in peripheral blood lymphocytes (Fig. 3C; Suppl. Data), whereas calves only exposed to BVDV had a decrease in peripheral blood lymphocytes beginning on day 2. The maximum decrease averaged 50% on day 5 for the BVDV-inoculated calves. Although peripheral blood lymphocytes increased after day 6 for the BVDV-inoculated calves, they did not recover to baseline values during the experiment. Decreases in peripheral blood lymphocytes was observed in both dual-infection groups, and the extent of reduction was similar to that observed in calves infected only with BVDV. Peripheral blood lymphocyte counts in calves exposed to BoCV 6 d after BVDV inoculation returned to baseline levels by the end of the experiment. In contrast, peripheral blood lymphocyte counts did not recover to baseline levels in calves exposed to BoCV 9 d after inoculation with BVDV.
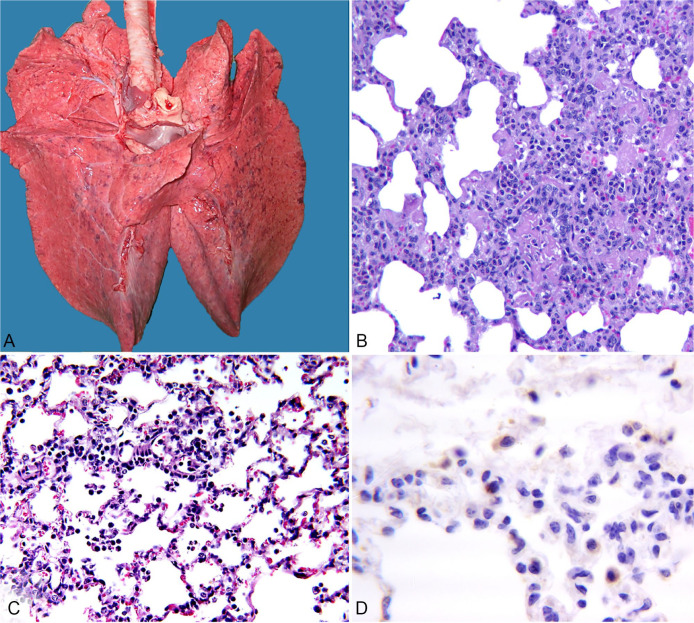
Lungs from control, BVDV only, and BoCV only groups were uniformly pink and well aerated with no obvious gross lesions (Table 7). In group 4N (BVDV followed by BoCV 6 d later), 1 calf (Fig. 4A) had pale, firm foci, 0.5–1.0 cm diameter, randomly scattered throughout the lungs but particularly obvious in the ventral, caudal lung lobes. Lungs from 2 other calves in that group had similar lesions, although not as numerous, and they had several, small (1–1.5 cm), dark, firm foci in ventral lungs. The fourth calf in that group had no obvious gross lesions. Only one calf had lung lesions in group 4M (BVDV followed by BoCV 9 d later). Lesions consisted of several 2-cm foci of consolidation in the right, ventral, middle lobe.
Table 7.
Virus isolation, serology, and lung lesions in study 4 (dual infection with bovine coronavirus [BoCV] strain OK 1776 and bovine viral diarrhea virus [BVDV] genotype 2, subgenotype a).
| Group | BoCV | BVDV | Lung lesions | |||||
|---|---|---|---|---|---|---|---|---|
| VN | VI | IHC | VN | VI | IHC | Gross | Histo | |
| 4M (BVDV d 0; BoCV d 9) | 2/4 | 1/4 | 4/4 | 4/4 | 4/4 | 0/4 | 1/4 | 6.8 ± 3.9 |
| 4N (BVDV d 3; BoCV d 9) | 3/4 | 0/4 | 4/4 | 4/4 | 4/4 | 0/4 | 3/4 | 11.4 ± 2.1 |
| 4O (BVDV d 0) | 0/2 | 0/2 | 0/2 | 1/2 | 1/2 | 0/2 | 0/2 | 4.0 ± 4.5 |
| 4P (no virus) | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 3.8 ± 0.4 |
| 4Q (BoCV d 9) | 2/2 | 1/2 | 2/2 | |||||
IHC = immunohistochemistry; VI = virus isolation; VN = virus neutralization.
Figure 4.
Lesions from one of the calves in group 4N (group exposed to bovine viral diarrhea virus [BVDV] and then bovine coronavirus [BoCV] 6 d later). A. Foci of 0.5–1.0 cm diameter are scattered randomly throughout the lungs. B. Moderate-to-marked infiltrates of neutrophils, lymphocytes, and macrophages within the alveolar septa, consistent with interstitial pneumonia. 200×. H&E. C Less severe interstitial pneumonia with numerous macrophages in alveoli. 200×. H&E. D. Anti-BoCV immunohistochemistry demonstrating positive BoCV signal in the cytoplasm of alveolar macrophages and interalveolar cells. 400×.
Although gross lesions were not evident in calves infected with only BoCV, microscopic lesions consistent with interstitial pneumonia were observed (Table 7). Microscopic lesions in 3 calves from group 4N and 1 calf from group 4M were characterized as multifocal, moderate-to-marked fibrinosuppurative bronchointerstitial pneumonia, with infiltration of neutrophils, lymphocytes, and macrophages (Fig. 4B, 4C). Lesion scores were significantly greater in group 4N (11.4 ± 2.1) compared to group 4M (4.4 ± 2.8; p = 0.003). IHC staining showed BoCV antigen in the tracheal glands, interstitial cells, and macrophages of calves in group 4N; BoCV antigen was observed in macrophages and to a lesser extent in bronchial epithelium of calves infected only with BoCV (Fig. 4D).
Discussion
We examined interactions between BoCV and BVDV, which are frequently isolated from cattle with BRD, to determine if dual infections could foster increased respiratory disease. The number of animals in each group was small, as is common when a study requires colostrum-deprived, BSL2 individually penned calves with no exposure to other pathogens before and throughout the study. Although the small numbers of animals preclude rigorous statistical analysis, these numbers were sufficient to detect patterns that were pursued in sequential studies. As stated above, 4 successive studies were done, and the design of each successive study was informed by the findings of the preceding studies. Although some dual-infection treatment groups were duplicated, different single infection controls were used in each study for purposes of comparison within that study. Observations of duplicated dual-infection treatment groups were consistent between studies.
Both the BoCV and the BVDV strains used in our studies were field strains. Clinical signs following single infection with either strain were limited to short-term pyrexia with little or no accompanying respiratory disease. It was observed, during single infections, that the 2 viral strains interacted differently with the immune system. Calves infected with the BoCV strain had a slight decrease in peripheral blood lymphocytes early in infection followed by a rebound to above baseline levels. In contrast, calves infected with BVDV had pronounced decreases in peripheral blood lymphocytes, and peripheral blood lymphocytes did not recover to baseline levels during the evaluation period. The isolation pattern of the virus following inoculation was different, with BoCV being isolated from nasal swabs and BVDV isolated from buffy coat samples. The impact of dual infection on peripheral tissues was also distinctly different.
Dual infections changed the isolation pattern of BVDV. In contrast to single infections in which BVDV was not isolated from nasal swabs, in dual infections, BVDV was isolated from nasal swabs. Typically, transmission of BVDV to naïve cohorts is limited, and BVDV is rarely isolated from nasal swabs.11 The isolation of BVDV from nasal swabs following dual infections suggests that BVDV may be transmitted at a higher rate in dual infections with BoCV than in single infections. This observation also illustrates problems inherent in attempting to determine prevalence of pathogens in BRD cases. If nasal swabs are the sample of choice, detection of BVDV could be dependent on whether dual BVDV–BoCV infections are present. Single acute BVDV infections or dual infections with other pathogens that do not potentiate BVDV nasal shedding could contribute to the clinical presentation but might not be detected using nasal swabs.
Previously, simultaneous infections, either in vivo or in vitro, with BVDV and bovine respiratory syncytial virus17 or with BVDV and transmissible gastroenteritis virus in pigs25 resulted in increased pathogenicity. Those studies suggested that BVDV had a synergistic effect with other viruses. The results of our studies indicate that BoCV and BVDV may act as potentiators for each other in development of respiratory disease. These results indicate that the window for potentiation differed between the 2 viruses. BoCV could potentiate respiratory disease when combined with BVDV exposure 3 d after BoCV inoculation. In contrast, respiratory disease was not seen in calves inoculated first with BVDV followed by BoCV 3 d later. However, respiratory disease was observed in calves inoculated with BoCV 6 or 9 d after BVDV inoculation. These results indicate that the window for BVDV potentiation occurred later following infection compared to BoCV. The window for BVDV potentiation corresponded to the period in which we observed the greatest decline in peripheral blood lymphocytes.20
Gross lesions within the lungs of dual-infected calves were multifocal and randomly distributed throughout the lungs in most cases. Histologically, lung lesions consisted of interstitial-to-bronchointerstitial pneumonia with inflammatory changes ranging from mononuclear infiltrates to fibrin and neutrophils in more severely affected lungs. Similar, less severe changes could be seen in several of the BVDV- or BoCV-inoculated calves. Similarly, mild bronchointerstitial pneumonia has been reported after experimental BVDV-2a infection.9 In our study, the BoCV isolates were from calves in which current BRD infections and/or clinical signs were present. To our knowledge, histopathologic changes associated directly with BoCV infection of the respiratory tract have not been described using isolates from the respiratory tract of naturally infected calves. Also, in our study, BoCV antigen was demonstrated in bronchial and tracheal epithelium, tracheal glands, alveolar interstitium, and macrophages, whereas BVDV was not detected using IHC. Therefore, the more severe lesions seen were most likely induced by BoCV in BVDV-compromised calves. Tracheal and bronchial epithelium were weakly positive for BoCV antigen; however, macrophages, especially in alveoli, were most consistently antigen positive. That finding suggests that BoCV may influence respiratory innate immunity, thus providing an environment that is fertile for bacterial secondary infection.
Although it is possible for cattle in production settings to be infected at exactly the same time with 2 different viruses, the more likely scenario is that viral infections are sequential. Our results indicate that clinical presentation and shedding may vary based on the sequence and timing of sequential infections. Therefore, the clinical presentation of respiratory disease may not only rest on the presence of multiple pathogens but also on the sequence of exposure to multiple pathogens. The complex etiology of BRD complicates the development and efficacy testing of vaccines to control BRD. Vaccine licensure requires demonstration that the vaccine reduces clinical presentation. Single-pathogen infection models for efficacy trials are problematic when clinical presentation in the field is related to synergy and potentiation among multiple pathogens. Therefore, sequential dual infections may have potential as BRD models for vaccine and therapy development and efficacy studies.
Supplemental Material
Supplemental material, Supplemental_material for Sequential exposure to bovine viral diarrhea virus and bovine coronavirus results in increased respiratory disease lesions: clinical, immunologic, pathologic, and immunohistochemical findings by Julia F. Ridpath, Robert W. Fulton, Fernando V. Bauermann, Shollie M. Falkenberg, Jenny Welch and Anthony W. Confer in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Patricia Federico and Kathy McMullen for technical support, Brian Conrad, Jeremy Spieker, and the NADC farm management group for animal husbandry, and Michael Marti for assistance in figure preparation.
Footnotes
Declarations of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication.
Funding: Funds for animal purchase and housing were provided by Zoetis Animal Health.
ORCID iDs: Julia F. Ridpath  https://orcid.org/0000-0001-7182-8161
https://orcid.org/0000-0001-7182-8161
Anthony W. Confer  https://orcid.org/0000-0002-1896-4399
https://orcid.org/0000-0002-1896-4399
Supplementary material: Supplementary material for this article is available online.
References
- 1. Bauermann FV, et al. Experimental infection of calves, sheep, goats and pigs with HoBi-like viruses by direct inoculation or exposure to persistently infected calves. Vet Microbiol 2015;181:289–293. [DOI] [PubMed] [Google Scholar]
- 2. Bauermann FV, et al. Antigenic relationships between bovine viral diarrhea virus 1 and 2 and HoBi virus: possible impacts on diagnosis and control. J Vet Diagn Invest 2012;24:253–261. [DOI] [PubMed] [Google Scholar]
- 3. Bauermann FV, et al. A serosurvey for ruminant pestivirus exposure conducted using cattle sera collected for brucellosis surveillance in the United States. J Vet Diagn Invest 2017;29:76–82. [DOI] [PubMed] [Google Scholar]
- 4. Boileau MJ, Kapil S. Bovine coronavirus associated syndromes. Vet Clin North Am Food Anim Pract 2010;26:123–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. St. Louis, MO: Elsevier, 2016:465–591. [Google Scholar]
- 6. Cho KO, et al. Evaluation of concurrent shedding of bovine coronavirus via the respiratory tract and enteric route in feedlot cattle. Am J Vet Res 2001;62:1436–1441. [DOI] [PubMed] [Google Scholar]
- 7. Confer AW, et al. Viral antigen distribution in the respiratory tract of cattle persistently infected with bovine viral diarrhea virus subtype 2a. Vet Pathol 2005;42:192–199. [DOI] [PubMed] [Google Scholar]
- 8. Ellis J. What is the evidence that bovine coronavirus is a biologically significant respiratory pathogen in cattle? Can Vet J 2019;60:147–152. [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis JA, et al. Lesions and distribution of viral antigen following an experimental infection of young seronegative calves with virulent bovine virus diarrhea virus type II. Can J Vet Res 1998;62:161–169. [PMC free article] [PubMed] [Google Scholar]
- 10. Evermann JF, et al. Clinical features. In: Goyal S, Ridpath JF, eds. Bovine Viral Diarrhea Virus: Diagnosis, Management and Control. Ames, IA: Blackwell, 2005:105–120. [Google Scholar]
- 11. Falkenberg SM, et al. Comparison of temperature fluctuations at multiple anatomical locations in cattle during exposure to bovine viral diarrhea virus. Livestock Sci 2014;164:159–167. [Google Scholar]
- 12. Fray MD, et al. The effects of bovine viral diarrhoea virus on cattle reproduction in relation to disease control. Anim Reprod Sci 2000;60-61:615–627. [DOI] [PubMed] [Google Scholar]
- 13. Fulton RW, et al. Detection and characterization of viruses as field and vaccine strains in feedlot cattle with bovine respiratory disease. Vaccine 2016;34:3478–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fulton RW, et al. Bovine coronaviruses from the respiratory tract: antigenic and genetic diversity. Vaccine 2013;31:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fulton RW, et al. Bovine coronavirus (BCV) infections in transported commingled beef cattle and sole-source ranch calves. Can J Vet Res 2011;75:191–199. [PMC free article] [PubMed] [Google Scholar]
- 16. Liebler-Tenorio EM, et al. Distribution of viral antigen and tissue lesions in persistent and acute infection with the homologous strain of noncytopathic bovine viral diarrhea virus. J Vet Diagn Invest 2004;16:388–396. [DOI] [PubMed] [Google Scholar]
- 17. Liu L, et al. Synergistic effects of bovine respiratory syncytial virus and non-cytopathic bovine viral diarrhea virus infection on selected bovine alveolar macrophage functions. Can J Vet Res 1999;63:41–48. [PMC free article] [PubMed] [Google Scholar]
- 18. McGill JL, et al. Bovine gamma delta T cells contribute to exacerbated IL-17 production in response to co-infection with bovine RSV and Mannheimia haemolytica. PLoS One 2016;11:e0151083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ridpath J. The contribution of infections with bovine viral diarrhea viruses to bovine respiratory disease. Vet Clin North Am Food Anim Pract 2010;26:335–348. [DOI] [PubMed] [Google Scholar]
- 20. Ridpath JF, et al. Comparison of acute infection of calves exposed to a high-virulence or low-virulence bovine viral diarrhea virus or a HoBi-like virus. Am J Vet Res 2013;74:438–442. [DOI] [PubMed] [Google Scholar]
- 21. Ridpath JF, et al. Prevalence and antigenic differences observed between bovine viral diarrhea virus subgenotypes isolated from cattle in Australia and feedlots in the southwestern United States. J Vet Diagn Invest 2010;22:184–191. [DOI] [PubMed] [Google Scholar]
- 22. Saif LJ. A review of evidence implicating bovine coronavirus in the etiology of winter dysentery in cows: an enigma resolved? Cornell Vet 1990;80:303–311. [PubMed] [Google Scholar]
- 23. Saif LJ. Coronaviruses of domestic livestock and poultry: interspecies transmission, pathogenesis and immunity, In: Perlman S, et al. , eds. The Nidoviruses. Washington, DC: ASM, 2008:279–296. [Google Scholar]
- 24. Saif LJ. Bovine respiratory coronavirus. Vet Clin North Am Food Anim Pract 2010;26:349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woods RD, et al. Bovine viral diarrhea virus isolated from fetal calf serum enhances pathogenicity of attenuated transmissible gastroenteritis virus in neonatal pigs. J Vet Diagn Invest 1999;11:400–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_material for Sequential exposure to bovine viral diarrhea virus and bovine coronavirus results in increased respiratory disease lesions: clinical, immunologic, pathologic, and immunohistochemical findings by Julia F. Ridpath, Robert W. Fulton, Fernando V. Bauermann, Shollie M. Falkenberg, Jenny Welch and Anthony W. Confer in Journal of Veterinary Diagnostic Investigation