Abstract
Species of genus Chlamydia are important pathogens of animals, with a worldwide distribution and broad host range. Some species, such as Chlamydia psittaci, also pose a zoonotic disease risk. Abortion is one of the many diseases that has been associated with chlamydial infections in animals, with most attention focused on the economic impacts to sheep production. The role of chlamydia in equine abortions is unknown. Using the family-specific 23S ribosomal RNA (rRNA) Chlamydiaceae real-time PCR, we tested 169 formalin-fixed, paraffin-embedded fetal membrane samples from 162 equine abortion cases collected between 2000 and 2018 in Switzerland. Two equine abortion cases (1.2%) tested positive for Chlamydiaceae. Further analyses by the species-specific 23S rRNA ArrayMate microarray and sequencing of a fragment of the 16S rRNA gene revealed C. abortus and C. psittaci. In both cases, equine herpesvirus 1 was also present, which might have been the abortion cause, alone or in synergy with Chlamydia. The prevalence of abortigenic chlamydial species in equine abortion cases in our study was significantly lower than rates described elsewhere. Zoonotic chlamydial agents present in equine fetal membranes nevertheless should be considered a potential risk to humans during foaling, abortion, or stillbirth.
Keywords: Chlamydiaceae, DNA, equine abortion, immunohistochemistry, placenta, real-time PCR
Introduction
The genus Chlamydia is comprised of human and animal pathogens, the latter with a diverse host range that includes domesticated animals and wildlife.3 Our growing knowledge of the diversity of chlamydial species and the animal hosts they infect29 is matched only by the diverse manifestations of disease to which chlamydial infections have been linked, including: 1) ocular infections, which may be asymptomatic or cause mild conjunctivitis through to corneal opacity; 2) respiratory diseases such as rhinitis and pneumonia; 3) systemic diseases such as polyarthritis; and 4) urinary and reproductive tract diseases, the latter of which may lead to a series of fertility-related sequelae.3 Abortions induced by C. abortus infection in ewes cause significant economic losses and are considered the most important effects globally.19 These infections are also important given the associated risk to human health, with reports that pregnant women are at zoonotic infection risk.11
Chlamydial infection as a cause of abortion and premature death of foals has emerged in the Thoroughbred racing industry in Australia.15–18 Notably, an awareness of these infections came initially through a cluster of cases of human chlamydiosis, with linked individuals presenting with symptoms consistent with atypical pneumonia.8 Investigations into these cases revealed that the individuals had encountered the abnormal placental membranes of a mare that had birthed a foal that died prematurely. Molecular testing revealed that the equine samples were positive for the well-known avian and zoonotic agent, C. psittaci, with molecular typing indicating that these strains were closely related to Australian parrot and human isolates belonging to the 6BC clade of this bacterial pathogen.15 Subsequent investigations within one Thoroughbred breeding operation in southern Australia found C. psittaci PCR positivity in 20% of equine reproductive loss events between January and December 2016.18 During 2016–2018, C. psittaci was also identified as a cause of respiratory disease in 15 neonatal foals from 13 different farms in this same region. Of these farms, 6 had a history of abortion associated with C. psittaci infection.18 Although these data point to a significant and previously unrecognized role for this chlamydial pathogen in equine reproductive loss, an actual causal role in disease has not been proven.
The prevalence and knowledge of the potential role of chlamydial infections in equine disease elsewhere are unclear. Retrospective investigations of equine abortions in Hungary in 1998–2000 revealed high positivity for chlamydiae (64 of 77; 83%) with chlamydial infections considered to be the probable abortigenic agent in 11 (14%) cases.28 That study used a combination of immunohistochemistry (IHC), Ziehl–Neelsen staining, and PCR screening, the latter confirming C. psittaci as the infecting chlamydial species. A German study found positivity for C. abortus in equine placental tissue specimens, with a mixed infection by C. suis, a pig pathogen, detected in 3 samples by species-specific PCRs.22 Previous studies in Switzerland have failed to find any evidence of chlamydiae as a cause of equine abortion in at least 2 studies, although both studies pre-dated the use of modern sensitive and discriminatory molecular assays for detection of chlamydiae in veterinary specimens.13,25 Beyond these studies, the respiratory pathogen, C. pneumoniae, has also been isolated previously from the respiratory tract of a horse with a serous nasal discharge.30
The emergence of concern over the potential role of chlamydial infections in equine abortions prompted by reports in Australia raises serious questions over the prevalence of these pathogens in equine reproductive loss worldwide. A scarcity of recent data exists on the role of these pathogens in equine reproductive disease in Europe and elsewhere, in part because detection of chlamydial pathogens is unlikely to be included as part of the routine veterinary diagnostic routine for equine abortion cases. Given this shortfall in knowledge, we investigated archived equine abortion cases collected between 2000 and 2018 in Switzerland for the presence of chlamydiae using established Chlamydiaceae-specific IHC and sensitive and specific PCR-based assays.
Materials and methods
Equine abortion cases and DNA extraction
The pathology archives from the veterinary faculties of Zurich and Bern were searched for equine abortion cases investigated between 2000 and 2018. Search terms “abortion”, “stillbirth”, and “neonatal” were used for age categories, and only cases with placenta available were selected. This search discovered 169 formalin-fixed, paraffin-embedded (FFPE) tissue blocks from 162 equine abortion cases. The blocks contained 153 placental and 13 amniotic membranes; 3 blocks contained umbilical tissue. Of the 162 equine abortion cases, 106 cases were retrieved from the Zurich archive and 56 from Bern. For Chlamydia-positive cases, all FFPE blocks available were retrieved from the archive and further investigated as described below. Corresponding pathology reports were retrieved from the archives.
DNA extraction of 169 FFPE blocks was performed (QIAamp DNA FFPE tissue kit; Qiagen) following the manufacturer’s instructions and as described previously.4 Extracted DNA was examined (NanoDrop-1000; WITec) to determine DNA quantity and quality. Extracted DNA samples containing > 120 ng/µL were diluted 1:10 in molecular biology–grade water prior to real-time PCR (rtPCR) to prevent amplification of inhibition by high DNA concentration.
PCR analysis for Chlamydiaceae DNA
All DNA samples were screened for the presence of Chlamydiaceae DNA by using a rtPCR assay targeting the 23S rRNA gene (Chlamydiaceae family specific)12 including primers Ch23S-F, Ch23S-R, and probe Ch23S-p (Microsynth) as described previously.10 The internal amplification control eGFP amplified with primers eGFP-1-F, eGFP-10-R, and probe eGFP-Hex (Microsynth)4 was added to each reaction. The PCR was conducted on an ABI 7500 fast thermocycler (Thermo Fisher Scientific). All samples were tested in duplicate, and samples with a cycle threshold of < 38 in duplicate PCR reactions were considered positive.
Chlamydial species determination and sequencing of Chlamydiaceae-positive PCR products
The 3 samples positive in the Chlamydiaceae family–specific rtPCR were further analyzed using a species-specific 23S rRNA gene ArrayMate microarray assay (Alere Technologies), as established previously.5
In parallel, the 3 positive samples were further investigated by a conventional PCR targeting a 278-bp fragment of the Chlamydiales 16S rRNA gene (16S-IGF/IGR-PCR) using primers 16S-IGF and 16S-IGR1,24 modified from a protocol described previously.12 Thermocycling was performed (Biometra TRIO thermal cycler; Analytik Jena), and PCR products were analyzed by gel electrophoresis on 1.5% agarose gel. For sequencing, amplicons of the 16S rRNA PCR were purified (GeneJET PCR purification kit–250 preps, or GeneJET gel extraction kit–50 preps; Thermo Fisher Scientific) according to the manufacturer’s instructions. Purified DNA was Sanger sequenced (Microsynth).27 Consensus sequences were generated by de novo assembly (Geneious Prime v2019.2.1; Qiagen). Sequences were compared to known sequences in the NCBI database by BLAST analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Chlamydial antigen detection by IHC
All 3 samples positive for Chlamydiaceae by rtPCR were examined by IHC according to protocols published previously,2,4 and using the Chlamydiaceae family–specific mouse monoclonal antibody clone ACI-P targeting the chlamydial lipopolysaccharide (Progen Biotechnik).
Histologic examination
For the 169 FFPE blocks containing placental membranes, 2-μm sections were prepared and stained with hematoxylin and eosin (H&E). All H&E slides of the placental membranes were examined at magnifications of 200× and 400× (BH 2 light microscope; Olympus Tokyo) and qualitatively assessed for the presence of necrosis, inflammation, hemorrhage, autolysis, and mineralization.
Results
Description of abortion cases
Most of the abortion cases (153 of 162) originated from mares based in Switzerland; only 9 cases were submitted from Germany. Most of the Swiss abortion cases were submitted from mares located in the cantons of Zurich (33), Bern (31), Aargau (18), or Thurgau (9). The remaining 53 cases were equally distributed within the remaining cantons. Aborting mares include the following: warmblood (46 of 162; 28.4%), pony (20 of 162; 12.3%), cold-blooded (16 of 162; 9.9%), Thoroughbred (15 of 162; 9.3%), or other breeds or unknown (65 of 162; 40.1%). The gestation duration was available from the pathology reports in 88 of 162 mares (54.3%), and most of the mares aborted in the sixth gestation month. Most of the abortion cases occurred in January–April, consistent with the breeding seasonality peak during springtime in the Northern Hemisphere.
Review of the pathology reports revealed the following infectious abortion causes in 33 of 162 (20.4%) cases: equine herpesvirus (EHV-1; 13 of 33; 39.4%), Streptococcus equi subsp. zooepidemicus (7 of 33; 21.2%), Escherichia coli (3 of 33; 9.1%), Rhodococcus equi (1 of 32; 3%), Actinomyces sp. (1 of 32; 3%), and fungal organisms (1 of 32; 3%). In 3 (9.1%) cases, a mixed infection with several infectious agents was detected, and 4 (12.1%) additional cases were of infectious origin, but the etiologic agent remained inconclusive. An infectious abortion cause was excluded in 69 (42.6%) cases; the remaining 60 (37%) cases were of unknown etiology.
Detection of Chlamydia
Three samples (2 placentae, 1 umbilicus) from 2 of 162 (1.2%) abortion cases (cases 1, 2) were positive for Chlamydiaceae by rtPCR (Table 1). In case 2, fetal organs including liver, lung, spleen, kidney, adrenal glands, heart, thymus, brain (cerebrum), intestine, and thyroid glands were available, and they tested negative by rtPCR for Chlamydiaceae. Both species identification methods revealed C. abortus in case 1 and C. psittaci in case 2. Both positive cases originated from warmblood mares from the canton of Bern, which aborted in March 2004. The gestation length was unknown in both cases. Coinfection with EHV-1 and R. equi was diagnosed in case 1, and with EHV-1 in case 2.
Table 1.
Details of equine abortion cases positive by real-time PCR (rtPCR) for Chlamydiaceae.
| Case | FFPE tissue | rtPCR (mean Ct value) | DNA ArrayMate microarray* | 16S rRNA and sequencing (identity %; GenBank accession) | IHC for Chlamydiaceae |
|---|---|---|---|---|---|
| 1 | Placenta | 32 | UT | C. abortus (100; MN702805) | Positive |
| Umbilicus | 34 | C. abortus | C. abortus (100; MN702806) | Positive | |
| 2 | Placenta | 32 | C. psittaci | C. psittaci (100; MN702807) | Positive |
Ct = cycle threshold; FFPE = formalin-fixed, paraffin-embedded; IHC = immunohistochemistry; UT = undetermined.
Alere Technologies.
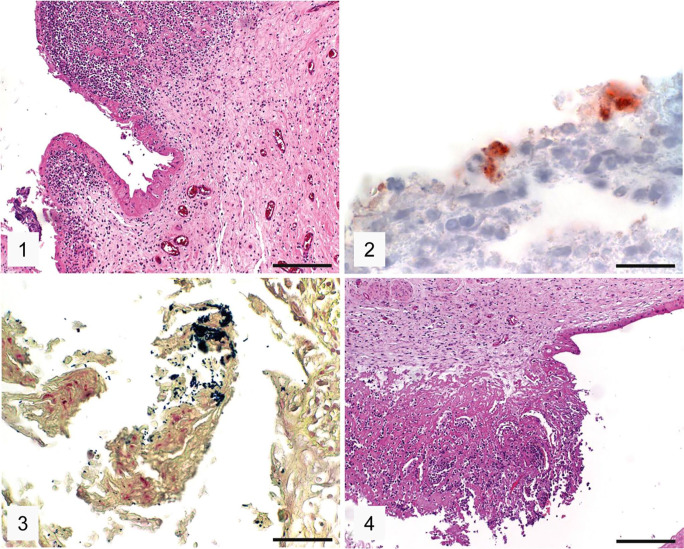
Histologic examination of the C. abortus–positive case (case 1) revealed moderate, multifocal necrosis and severe diffuse purulent placentitis (Fig. 1). The presence of Chlamydiaceae was confirmed by IHC in the placenta: intracytoplasmic granular positive immunolabeling consistent with chlamydial inclusions was present in trophoblasts (Fig. 2). The Gram staining of the placenta showed gram-positive coccoid bacteria consistent with R. equi (Fig. 3). There were moderate multifocal-to-coalescing necrosis and severe diffuse purulent omphalitis in case 1 (Fig. 4). Mineralization was present in both the placenta and the umbilicus. Necrosuppurative placentitis and omphalitis were diagnosed. The original cell culture of the placenta had resulted in the isolation of EHV-1, although histologic lesions typical of an EHV-1 infection were not evident.
Figures 1–4.
Placenta and umbilicus of the Chlamydia abortus–positive equine abortion case 1. Figure 1. Necrosuppurative placentitis. H&E. Bar = 200 µm. Figure 2. Positive granular intracytoplasmic immunolabeling (red) for Chlamydiaceae in trophoblasts of the placenta. IHC, hematoxylin counterstain. Bar = 20 µm. Figure 3. Presence of gram-positive coccoid bacteria (dark blue) consistent with Rhodococcus equi in the placenta. Gram stain. Bar = 50 µm. Figure 4. Necrosuppurative omphalitis. H&E. Bar = 200 µm.
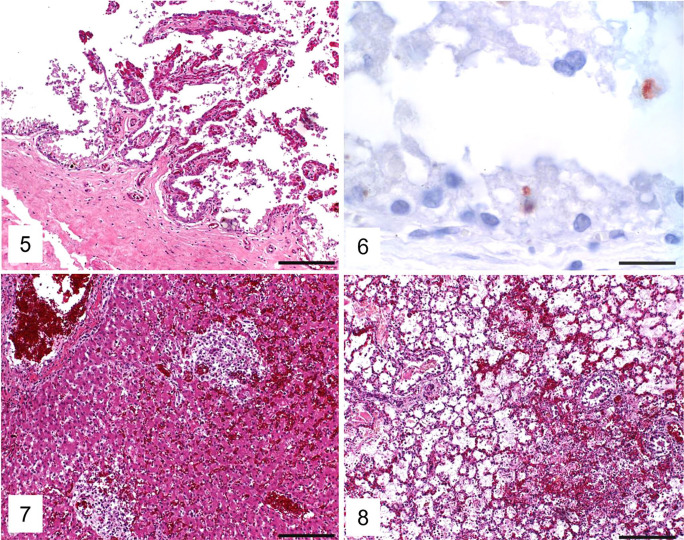
Histologic examination of the C. psittaci–positive case 2 revealed multifocal necrotizing placentitis and hyperemia (Fig. 5); the presence of Chlamydiaceae in trophoblasts was confirmed by IHC (intracytoplasmic granular positive immunolabeling; Fig. 6). In fetal organs, multifocal necrotizing hepatitis (Fig. 7), multifocal necrotizing and bronchointerstitial pneumonia with fibrin exudation into alveoli (Fig. 8), and multifocal necroses in the adrenal gland and the thymus with additional lymphoid depletion in the latter were present. Acute hemorrhages were present in the lung (Fig. 8) and kidney. Eosinophilic intranuclear inclusion bodies characteristic for herpesviruses were seen in the liver, lung, adrenal glands, and thymus. The heart, brain (cerebrum), intestine, and thyroid glands were unremarkable histologically.
Figures 5–8.
Placenta and fetal liver and lung from the Chlamydia psittaci–positive equine abortion case 2. Figure 5. Mild multifocal necrotizing placentitis and hyperemia of villi. H&E. Bar = 200 µm. Figure 6. Positive granular intracytoplasmic immunolabeling (red) for Chlamydiaceae in placental trophoblasts. IHC, hematoxylin counterstain. Bar = 20 µm. Figure 7. Multifocal hepatic necrosis. H&E. Bar = 200 µm. Figure 8. Multifocal-to-coalescing necrotizing bronchointerstitial pneumonia with fibrin in alveoli and acute alveolar hemorrhage. H&E. Bar = 200 µm.
Discussion
Our finding of chlamydiae in 2 of 162 (1.2%) equine abortion cases was lower than expected when considering the reports of C. psittaci epizootics in Australia18 and the Hungarian studies.28 Assigning a causative role for chlamydial infections in the equine abortion cases detected in our study and other cases28 is problematic given the high rate of coinfections by other potential abortigenic agents. We detected a variety of potential infectious abortigenic agents in our Swiss equine abortion material, including EHV-1, S. equi subsp. zooepidemicus, E. coli, R. equi, Actinomyces sp., and fungi, many at prevalence rates similar to or significantly higher than the chlamydial positivity detected. These results mirrored those of others28 who reported an overall higher chlamydial positivity rate, with C. psittaci confirmed as the sole equine abortigenic agent in only the minority of cases, whereas most chlamydial cases were found to be coinfections with viruses and other bacteria. The Australian study results stand in contrast to the European studies, with mixed infections with EHV-1 or other bacteria detected in only ~ 10% of the C. psittaci–positive specimens.18
Histopathologic results of both cases may provide a partial answer to the question of disease pathogenesis. Our C. abortus–positive case (case 1) had histologic features of bacterial etiology, namely necrosuppurative placentitis and omphalitis. The presence of chlamydial inclusion bodies was confirmed by IHC; this case was also positive for EHV-1 and for R. equi, which was confirmed by Gram staining of the placenta. The presence of both bacterial pathogens (R. equi and C. abortus) in placental lesions strongly indicates their relevance, whereas histologic lesions typical of an EHV-1 infection were not present.
Our C. psittaci–positive case (case 2) was also positive by IHC in the placenta, but multifocal necrotizing placentitis was less characteristic of a chlamydial abortion. The Australian C. psittaci–induced abortion cases had mild, diffuse, interstitial placentitis; the inflammatory infiltrate consisted mainly of neutrophils and macrophages.15 A lymphohistiocytic placentitis was also reported previously.28 In contrast, severe organ lesions were observed in several fetal tissues of our C. psittaci–positive case, in particular, multifocal necroses also containing intralesional viral inclusion bodies typical of EHV infection. In our case, C. psittaci might have played a bystander role whereas EHV seemed more likely as the abortion-inducing agent. Although these observations provided some insight, further work is required to demonstrate the pathogenic potential of chlamydial infections in equine reproductive disease individually and alongside other abortigenic infectious agents.
Our 2 samples that were positive for chlamydiae were identified as containing C. abortus (prevalence 0.6%) or C. psittaci (prevalence 0.6%). C. abortus was previously detected in placental tissues of mares22 but little data exists on the prevalence and pathogenic potential of this pathogen in horses. C. abortus is one of the most common infectious causes of abortion in sheep and goats in Switzerland.9 It is tempting to speculate that the C. abortus infection detected in our case may have been the result of exposure to sympatric populations of C. abortus–infected ruminants. Similar transmission risks are suspected in the chlamydial epidemiology of other domesticated animals in Switzerland, particularly at the farm–wildlife interface.14
Questions can also be asked about the potential source of the C. psittaci strain that we detected. In the Australian epizootic study, high infection pressure generated by C. psittaci–shedding, free-living Australian parrots carrying highly virulent strains of the 6BC clade (ompA genotype A) may have led to repeated infection spillover.6,15,18 Whereas free-living parrots can be excluded as an infection source in Switzerland, free-roaming pigeons are present and they can be infected with C. psittaci or C. abortus.26 A Swiss study21 found high rates of PCR positivity in feral pigeons, with molecular typing revealing that the strains were ompA genotype B, a genotype endemic to European feral pigeons and genetically distinct from strains found infecting parrots.20 None of the pigeons were positive for C. abortus. Interestingly, more fine-detailed molecular analyses found that the strains in Swiss pigeons shared 99% identity with another C. psittaci ompA B strain detected in the placenta of an equine abortion case from Queensland, Australia,16 distinct from the larger epizootic in southern Australia.18 Unfortunately, C. psittaci typing was not possible in our study because preparation and storage of FFPE blocks lead to physical and chemical changes of tissue DNA, reducing the length of amplifiable PCR fragments.5,7 Therefore, sequence analysis in our 2 Chlamydiaceae-positive cases was limited to short amplicons of the 16S rRNA gene, which did not allow comparative studies and phylogenetic analysis with other C. psittaci sequences. Until other sources can be identified, it is nevertheless likely that the C. psittaci equine infection in our study was the result of exposure to infected birds such as pigeons, a factor that was also speculated in the Hungarian study.28
Our investigation of retrospective FFPE material might be limited because of DNA fragmentation, possibly resulting in false-negative results. However, a similar approach has previously proven useful in related studies using FFPE materials.5,7 Moreover, the initial rtPCR for Chlamydiaceae used for screening is highly sensitive and is based on the amplification of a short (111 bp) fragment of the highly conserved 23S rRNA gene. Based on these results, it appears that chlamydiae are a potentially rare cause of equine abortions in Switzerland. Although studies are certainly warranted in other jurisdictions, our results and the absence of other such reports in the literature suggest that there may be unidentified and unique factors in the epidemiology of chlamydial infections in Australian horses that would explain why epizootics noted there have not been reported elsewhere. It is probably unnecessary to include chlamydial testing as part of the routine investigation of equine reproductive losses just yet. The zoonotic potential of chlamydiae should not be underestimated, however, with appropriate “One Health” approaches required to ensure that the risk to human health is reduced.23
Acknowledgments
We thank R. Güttinger, B. Senn, and S. Wunderlin from the Institute of Veterinary Pathology for technical assistance. The laboratory work was partly performed using the logistics of the Center for Clinical Studies at the Vetsuisse Faculty of the University of Zurich. We thank A. Polkinghorne for assistance in editing this manuscript.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Nicole Borel  https://orcid.org/0000-0002-1556-9262
https://orcid.org/0000-0002-1556-9262
References
- 1. Blumer C, et al. Chlamydiae in free-ranging and captive frogs in Switzerland. Vet Pathol 2007;44:144–150. [DOI] [PubMed] [Google Scholar]
- 2. Blumer S, et al. Waddlia, Parachlamydia and Chlamydiaceae in bovine abortion. Vet Microbiol 2011;152:385–393. [DOI] [PubMed] [Google Scholar]
- 3. Borel N, et al. A review on chlamydial diseases in animals: still a challenge for pathologists? Vet Pathol 2018;55:374–390. [DOI] [PubMed] [Google Scholar]
- 4. Borel N, et al. Chlamydiae in human intestinal biopsy samples. Pathog Dis 2018;76:fty081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borel N, et al. Direct identification of chlamydiae from clinical samples using a DNA microarray assay: a validation study. Mol Cell Probes 2008;22:55–64. [DOI] [PubMed] [Google Scholar]
- 6. Branley J, et al. Australian human and parrot Chlamydia psittaci strains cluster within the highly virulent 6BC clade of this important zoonotic pathogen. Sci Rep 2016;6:30019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burach F, et al. Chlamydiaceae and Chlamydia-like organisms in the koala (Phascolarctos cinereus)—organ distribution and histopathological findings. Vet Microbiol 2014;172:230–240. [DOI] [PubMed] [Google Scholar]
- 8. Chan J, et al. An outbreak of psittacosis at a veterinary school demonstrating a novel source of infection. EcoHealth 2017;3:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chanton-Greutmann H, et al. Aborte beim kleinen Wiederkäuer in der Schweiz: Untersuchungen während zwei Ablammperioden (1996–1998) unter besonderer Beachtung des Chlamydienabortes [Abortion in small ruminants in Switzerland: investigations during two lambing seasons (1996–1998) with special regard to chlamydial abortions]. Schweiz Arch Tierheilkd 2002;144:483–492. German. [DOI] [PubMed] [Google Scholar]
- 10. Ehricht R, et al. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol Cell Probes 2006;20:60–63. [DOI] [PubMed] [Google Scholar]
- 11. Essig A, Longbottom D. Chlamydia abortus: new aspects of infectious abortion in sheep and potential risk for pregnant women. Curr Clin Microbiol Rpt 2015;2:22–34. [Google Scholar]
- 12. Everett KD, et al. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol 1999;49:415–440. [DOI] [PubMed] [Google Scholar]
- 13. Forster JL, et al. Absence of chlamydia as an aetiological factor in aborting mares. Vet Rec 1997;141:424. [DOI] [PubMed] [Google Scholar]
- 14. Hoffmann K, et al. Prevalence of chlamydial infections in fattening pigs and their influencing factors. PLoS One 2015;10:e0143576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jelocnik M, et al. Multilocus sequence typing identifies an avian-like Chlamydia psittaci strain involved in equine placentitis and associated with subsequent human psittacosis. Emerg Microbes Infect 2017;6:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jelocnik M, et al. Molecular evidence to suggest pigeon-type Chlamydia psittaci in association with an equine foal loss. Transbound Emerg Dis 2018;65:911–915. [DOI] [PubMed] [Google Scholar]
- 17. Jelocnik M, et al. Detection of a range of genetically diverse chlamydiae in Australian domesticated and wild ungulates. Transbound Emerg Dis 2019;66:1132–1137. [DOI] [PubMed] [Google Scholar]
- 18. Jenkins C, et al. An epizootic of Chlamydia psittaci equine reproductive loss associated with suspected spillover from native Australian parrots. Emerg Microbes Infect 2018;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Longbottom D, et al. Evaluation of the impact and control of enzootic abortion of ewes. Vet J 2013;195:257–259. [DOI] [PubMed] [Google Scholar]
- 20. Magnino S, et al. Chlamydial infections in feral pigeons in Europe: review of data and focus on public health implications. Vet Microbiol 2009;135:54–67. [DOI] [PubMed] [Google Scholar]
- 21. Mattmann P, et al. Chlamydiaceae in wild, feral and domestic pigeons in Switzerland and insight into population dynamics by Chlamydia psittaci multilocus sequence typing. PLoS One 2019;14:e0226088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pantchev A, et al. Detection of all Chlamydophila and Chlamydia spp. of veterinary interest using species-specific real-time PCR assays. Comp Immunol Microbiol Infect Dis 2010;33:473–484. [DOI] [PubMed] [Google Scholar]
- 23. Polkinghorne A, et al. New evidence for domesticated animals as reservoirs of chlamydia-associated community-acquired pneumonia. Clin Microbiol Infect 2019;25:131–132. [DOI] [PubMed] [Google Scholar]
- 24. Pospischil A, et al. Evidence for Chlamydia in wild mammals of the Serengeti. J Wildl Dis 2012;48:1074–1078. [DOI] [PubMed] [Google Scholar]
- 25. Pospischil A, et al. Ursachen pränataler Fohlenverluste in der Schweiz [Causes of prenatal foal loss in Switzerland]. Schweiz Arch Tierheilkd 1992;134:401–409. German. [PubMed] [Google Scholar]
- 26. Sachse K, et al. More than classical Chlamydia psittaci in urban pigeons. Vet Microbiol 2012;157:476–480. [DOI] [PubMed] [Google Scholar]
- 27. Staub E, et al. Novel Chlamydia species isolated from snakes are temperature-sensitive and exhibit decreased susceptibility to azithromycin. Sci Rep 2018;8:5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szeredi L, et al. High prevalence of chlamydial (Chlamydophila psittaci) infection in fetal membranes of aborted equine fetuses. Vet Res Commun 2005;29:37–49. [DOI] [PubMed] [Google Scholar]
- 29. Taylor-Brown A, Polkinghorne A. New and emerging chlamydial infections of creatures great and small. New Microbes New Infect 2017;18:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wills JM, et al. Characterisation of Chlamydia psittaci isolated from a horse. Vet Microbiol 1990;24:11–19. [DOI] [PubMed] [Google Scholar]