Abstract
Investigations of 2 cases of high mortality in cull sows and feeder pigs from a buying station in Ohio and cull sows at an abattoir in Tennessee were conducted at the Iowa State University Veterinary Diagnostic Laboratory. The animals were presented as weak, lethargic, and some with high fever. Rapidly escalating mortality was reported to be as high as 30–50% within groups at the buying station over 8–10 d, and 30–40% over 5–7 d at the abattoir. Splenomegaly and red lymph nodes were the most consistent macroscopic findings, with scant fibrinous polyserositis observed in one sow. The microscopic lesions of vasculitis, fibrin thrombi, fibrinosuppurative polyserositis, and intralesional bacteria were consistent with acute bacterial septicemia. Bacterial culture isolated Streptococcus equi subsp. zooepidemicus (S. zooepidemicus) from multiple organs, including spleen, lung, and kidney. PCR tests were negative for African swine fever virus, classical swine fever virus, Erysipelothrix rhusiopathiae, porcine reproductive and respiratory syndrome virus, porcine circovirus 2, and Salmonella spp. Porcine circovirus 3 was inconsistently detected at low levels by PCR, with a lack of associated lesions. Next-generation sequencing identified S. zooepidemicus and porcine partetravirus in the serum sample of the feeder pig from the buying station. Phylogenetic analysis of the szP gene indicated that the S. zooepidemicus isolates from Ohio and Tennessee are in genotype VI. We conclude that the cause of these high mortality events in swine was S. zooepidemicus septicemia.
Keywords: septicemia, Streptococcus equi subsp. zooepidemicus, swine
We describe herein 2 cases of high mortality in cull sows and feeder pigs, the first at a swine buying station in Ohio and the second at an abattoir in Tennessee, that were submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU VDL; Ames, IA) in late September to early October 2019 (Table 1). The buying station in Ohio reported rapidly escalating mortality, with average daily losses of 5–10% and cumulative mortality approaching 30–50% in different groups over the previous 8–10 d. The attending veterinarian reported affected animals with rapid progression of lethargy, weakness, high fever, reluctance to move, cutaneous erythema, prostration, and bloody nasal discharge. This clinical presentation was observed in previously healthy cull sows and feeder pigs received at the buying station, which housed >1,000 animals. The reported postmortem findings consistently described enlarged red lymph nodes, with splenomegaly in some animals.
Table 1.
Summary of the clinical history, macroscopic and microscopic findings, bacteriology, and molecular assay results on high mortality cases in swine from an Ohio buying station and Tennessee abattoir.
| Ohio buying station | ||||
|---|---|---|---|---|
| First submission | Second submission | Tennessee abattoir | ||
| Cull sow 1 | Cull sow 2 | Feeder pig | Cull sows 3, 4 | |
| Clinical history | Severe lethargy, weakness, high fever, cutaneous erythema, prostration, and bloody nasal discharge with 10–50% mortality rate within 8–10 d. | Ataxia, open-mouth breathing, and rapid death with high mortality of 30–40% within 5–7 d. | ||
| Macroscopic findings | Enlarged and red lymph nodes, ± splenomegaly. | Severe splenomegaly. | Moderate splenomegaly. | Enlarged and red submandibular lymph nodes, ± fibrinous exudate on epicardium and splenic capsule. |
| Microscopic findings | No remarkable findings in heart, lung, kidney, spleen, tonsil, or lymph node. | Moderate degree of autolysis in lung, lymph node, liver, kidney, spleen. | Mild-to-moderate acute multifocal suppurative nephritis and myocarditis with intralesional cocci. Moderate splenic congestion. |
Moderate acute fibrinosuppurative perisplenitis with presence of vasculitis and intralesional gram-positive cocci (1 of 2). Mild multifocal-random acute fibrinosuppurative hepatitis (1 of 2). Moderate multifocal acute necrotizing fibrinosuppurative lymphadenitis (1 of 2). Mild-to-moderate chronic lymphoplasmacytic enterocolitis (2 of 2). |
| Bacterial culture* | High growth of S. zoo (spleen and lung). Moderate growth of mixed E. coli (spleen and lung). Moderate growth of coagulase-negative staphylococci (lung). A single colony of S. Derby (spleen). |
High pure growth of S. zoo (spleen, liver). |
High-to-moderate growth of S. zoo (lung, spleen, liver). Moderate growth of hemolytic E. coli (intestine). Low growth of S. Adelaide (intestine). |
High pure growth of S. zoo (splenic capsule [1 of 2], kidney [1 of 2]). Low growth of S. zoo (spleen [2 of 2], lung [2 of 2]). Low growth of S. suis (lung [1 of 2]). |
| Molecular assays | PCRs: ASFV, CSFV, ERY, PCV-2 not detected in spleen; PRRSV, IAV not detected in lung; PCV-3 detected in the spleen (Ct: 31.6). | PCRs: ASFV, CSFV not detected in the spleen. | PCRs: ASFV, CSFV not detected in the spleen. NGS: S. zoo and porcine partetravirus detected in serum. |
PCRs: ASFV, CSFV, PCV-2, SAL not detected in the spleen (2 of 2); PCV-3 detected in the spleen (1 of 2; Ct: 26.2). |
ASFV = African swine fever virus; CSFV = classical swine fever virus; ERY = Erysipelothrix rhusiopathiae; NGS = next-generation sequencing; PCRs = polymerase chain reactions; PCV = porcine circovirus; S. Adelaide, S. Derby = Salmonella enterica subsp. enterica serovars Adelaide and Derby, respectively; SAL = Salmonella spp.; S. suis = Streptococcus suis; S. zoo = Streptococcus equi subsp. zooepidemicus.
E. rhusiopathiae not isolated with enrichment broth in all submitted cases.
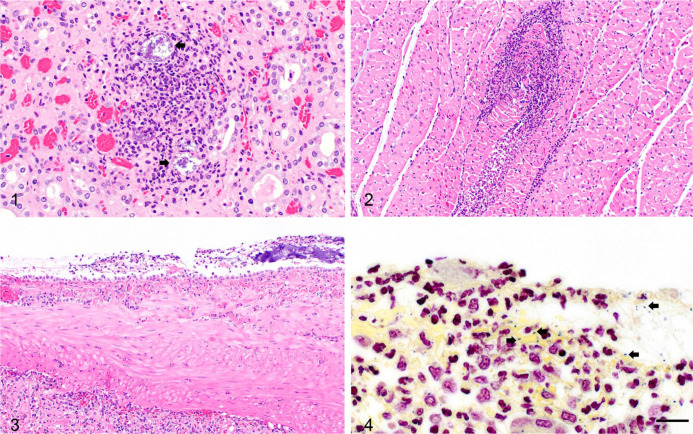
The samples received at the ISU VDL from the Ohio buying station were comprised of an initial limited set of tissues from sow 1. With persistent mortality and no clear cause of death, another submission was received 3 d later. This consisted of samples of fresh and formalin-fixed tissues (heart, lung, tonsil, lymph node, liver, kidney, and spleen) from sow 2 in a group with >30% mortality, and fresh and formalin-fixed tissues (brain, lung, heart, lymph node, tonsil, spleen, liver, kidney, skeletal muscle, and intestine) and serum from a feeder pig in a group experiencing nearly 50% mortality. The portion of spleens from both sows received was moderately to markedly enlarged, thick, firm, and exuded dark blood; tissues including lung, heart, lymph node, kidney, and tonsil were autolyzed and congested. The euthanized, very lethargic feeder pig had a moderately enlarged spleen. Histopathology performed on the fixed tissues of the sows was unremarkable, with moderate autolysis. The histopathology on the feeder pig tissues revealed lesions consistent with bacterial septicemia, including fibrin thrombi in the lung and liver, and suppurative lesions in the heart and kidney with intralesional bacteria (Figs. 1, 2).
Figures 1–4.
Microscopic changes of Streptococcus equi subsp. zooepidemicus septicemia in a feeder pig and sow. Figure 1. Acute suppurative nephritis of a feeder pig from the Ohio buying station, comprised of mostly neutrophils and fewer macrophages, with scattered karyorrhectic and karyolytic cellular debris admixed with scant fibrin. Note the large colonies of bacteria (arrows) within the inflammatory focus. H&E. 400×. Figure 2. Cardiac muscle of the feeder pig is effaced by similar suppurative inflammatory infiltrates with degeneration and necrosis of cardiac myofibers. H&E. 200×. Figure 3. The spleen from sow 3 from the Tennessee abattoir is covered by fibrinosuppurative exudate characterized by aggregates of degenerate neutrophils and necrotic cellular debris admixed with fibrin. H&E. 200×. Figure 4. Intracellular and extracellular gram-positive, paired cocci (arrows) are scattered within the fibrinous exudate on the splenic capsule; sow 3. Gram. 1,000×.
Given the impact and the repeated finding of splenic enlargement, African swine fever virus (ASFV) and classical swine fever virus (CSFV; Pestivirus C) testing was performed on individual animals and reported as negative by PCR. The spleen sample from sow 1 was positive for porcine circovirus 3 (PCV-3) by real-time PCR, with a cycle threshold (Ct) value of 31.6. However, no associated gross or microscopic changes of glomerulonephritis or dermatitis with necrotizing vasculitis that had previously been described in PCV-3–infected pigs were observed.22 Influenza A virus (IAV) was detected at a high Ct of 35.1 from the lung of sow 1. The abrupt mortality within 2–3 d of arrival and often within hours of initial clinical signs, along with the lack of coughing or other respiratory signs, suggested that influenza was unlikely the cause of mortality. Porcine reproductive and respiratory syndrome virus (PRRSV; Betaarterivirus suid), PCV-2, and Erysipelothrix rhusiopathiae were not detected by real-time PCR in sow 1 samples.
Additional communication with the practitioner eliminated the possibility of gastric ulcers as the cause of deaths and reaffirmed a lack of respiratory signs in the various groups transiently housed at the facility. A comprehensive bacterial culture setup was performed using nonselective agars (standard sheep blood agar; blood agar containing 4% agar to inhibit spread of Proteus, with and without Staphylococcus aureus nurse colonies), selective agars and broths (Tergitol-7 agar, Hektoen enteric agar, Erysipelothrix enrichment broth, Columbia CNA [colistin and nalidixic acid] agar, and Packer agar), and incubations at various atmospheric conditions (i.e., aerobic, anaerobic, 5% CO2) at 35°C for 2–6 d in an attempt to isolate a wide range of potential bacterial agents from multiple tissues including the intestine, lung, liver, and spleen. On sow 1 tissues, the bacterial cultures yielded a mixed growth of a moderate amount of Escherichia coli, a moderate amount of staphylococci, and a large amount of β-hemolytic streptococci, which were initially deemed as insignificant as they did not fit the characteristics of any of the bacterial differentials typical for swine systemic cases (i.e., Salmonella spp., Streptococcus suis, E. rhusiopathiae, Glaesserella (Haemophilus) parasuis, and Actinobacillus suis). However, a pure population and large amounts of clear, mucoid colonies surrounded by a clear zone of β-hemolysis was obtained from lung and spleen of the feeder pig and liver and spleen of sow 2. The colonies were confirmed as Streptococcus equi subsp. zooepidemicus (S. zooepidemicus) by classical biochemical tests (acid produced from salicin, sorbitol, and sucrose but not from inulin, mannitol, raffinose, or trehalose) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics). In light of these findings, the bacterial culture plates from the spleen and lung of sow 1 were re-examined, and S. zooepidemicus was identified from both tissues within the initial mixed cultures. Additional screening for clostridial agents by a fluorescent antibody test (FAT) was performed on the spleens of sow 2 and the feeder pig. A few positive fluorescent signals for Clostridium novyi were detected in the spleen of the feeder pig only. Given the inconsistency of detection of clostridia by FAT, and the lack of typical necrohemorrhagic and emphysematous myositis and cellulitis of gas gangrene in swine, the detection of C. novyi was likely associated with postmortem overgrowth of bacilli.
A second case involving cull sows from an abattoir in Tennessee that held ~2,200 animals was received 1 wk after the initial mortality event was reported at the buying station in Ohio. These sows had a similar history of high spiking mortality (30–40%) within 5–7 d while in lairage. Ataxia, open-mouth breathing, and rapid death were observed clinically. Fever was not observed. Postmortem examinations performed on multiple animals at the abattoir by the submitter described enlarged, red submandibular lymph nodes in the acutely dead animals, and lesions suggestive of septicemic changes including fibrinous exudate on the epicardium and splenic capsule in animals that survived a short course of clinical disease. Fresh and formalin-fixed tissues (brains, hearts, lungs, livers, kidneys, spleens, lymph nodes, intestines, and colons) from 2 animals were submitted to the ISU VDL (sows 3, 4). In sow 3, fibrinous exudate was observed grossly on the splenic surface. Sow 4 had red lymph nodes and splenomegaly with rounded edges. Histopathology corroborated the gross findings of fibrinosuppurative polyserositis in sow 3, with intracellular and extracellular gram-positive paired cocci observed within the fibrinous exudate overlying the splenic and renal capsule (Figs. 3, 4). The blood vessels within the splenic capsule occasionally contained fibrin thrombi with neutrophilic vasculitis. There were a few random foci of fibrinosuppurative hepatitis. The lymph node of sow 3 was effaced by multifocal areas of inflammation and necrosis comprised of degenerate neutrophils, macrophages, and karyorrhectic and karyolytic cellular debris admixed with abundant fibrin consistent with acute necrotizing fibrinosuppurative lymphadenitis. S. zooepidemicus was isolated from the spleen and lung of both sows and from the kidney of sow 3, mostly in pure culture with light-to-heavy growth. Splenic tissue of both sows tested negative by PCR for ASFV, CSFV, PCV-2, and Salmonella spp. Enrichment culture for E. rhusiopathiae was negative. The spleen sample from sow 3 was positive for PCV-3 by PCR (Ct: 26.2), with no lesions suggestive of PCV-3 infection.22
Antimicrobial susceptibility testing was performed by a microbroth dilution test panel (BOPO7F, Sensititre; Thermo Fisher Scientific) following Clinical and Laboratory Standards Institute guidelines8 on 3 S. zooepidemicus isolates, 2 from the buying station in Ohio and 1 from the abattoir in Tennessee. The test was repeated twice for each isolate. Quality control strains (Streptococcus pneumoniae ATCC 49619 and Mannheimia haemolytica ATCC 33369) were within the expected range for the antimicrobials included in the panel. The minimum inhibitory concentrations revealed that these isolates were susceptible to a variety of antimicrobials commonly used to treat streptococcal disease in swine, including ceftiofur, enrofloxacin, penicillin, tiamulin, and tilmicosin (Suppl. Table 1).
Given the consistent culture results, rapid onset of prostration and death, and the gross and microscopic lesions consistent with bacterial septicemia in the feeder pig, a laboratory diagnosis of S. zooepidemicus septicemia was made for both submissions from the Ohio buying station. Next-generation sequencing on the serum sample received from the buying station identified S. zooepidemicus and porcine partetravirus (Parvoviridae, Parvovirinae, known previously as hokovirus). The clinical significance of this virus is unlikely given the clinical history of multiple ages of pigs from different sources that were affected and died within 2–3 d of arrival. Findings from specimens received from the abattoir were even more clear-cut; both sows had lesions consistent with systemic bacterial infection, and S. zooepidemicus was isolated from lung, spleen, and kidney in pure or nearly pure growth.
Diseases caused by S. zooepidemicus are most commonly detected in horses29 and dogs,16 and reported in other animal species including cats,6 ruminants,27 camelids,9 guinea pigs,26 and nonhuman primates.20 S. zooepidemicus is a commensal organism in the upper respiratory and lower genital tracts in horses11,13; dogs can be asymptomatic carriers of this bacterium.5 In addition, this organism is a known zoonotic pathogen, with reports of transmission from horses and dogs to humans.1,23 In horses, opportunistic infection can occur resulting in bronchopneumonia, endometritis, neonatal septicemia, and abscess formation.29 Sporadic chronic upper respiratory tract infections occur in dogs infected with S. zooepidemicus as well as a severe acute form of hemorrhagic pleuropneumonia characterized by sudden onset of fever, dyspnea, and hemorrhagic nasal discharge.2,14,24,25 In swine, a severe outbreak of this bacterial infection was reported in Sichuan Province, China in 1975.18 Only 6 clinical cases of S. zooepidemicus infection have been diagnosed in swine since 2010 at the ISU VDL. The prevalence of this organism in North American swine is unknown.
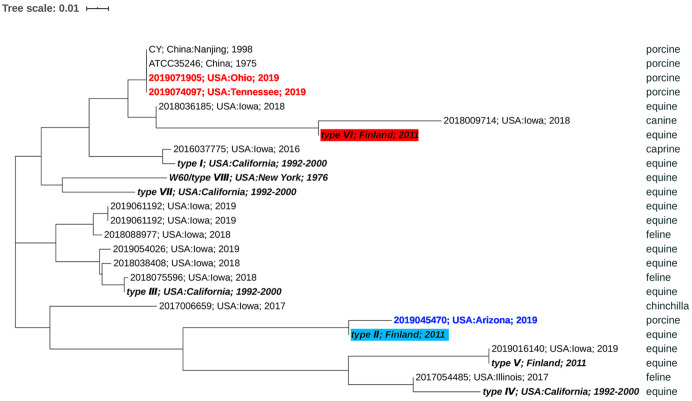
A phylogenetic analysis of the M-like surface protein gene (szP) was conducted from 15 sequences of S. zooepidemicus isolated at ISU VDL from 2016 to 2019. The sequences included the 2 swine isolates obtained from the buying station in Ohio and the abattoir in Tennessee described above along with 1 additional unrelated sporadic septicemic swine isolate from Arizona and 6 equine, 3 feline, 1 canine, 1 caprine, and 1 chinchilla isolates. The sequencing analysis was performed (MiSeq; Illumina) using a 250 read length of paired-end strategy. The allelic profiles of the gene szP were extracted using the 8 types of this gene described previously23 as reference sequences. Nucleotide sequence alignment was performed using ClustalW (http://www.clustal.org/), and a phylogenetic tree based on the szP gene was constructed by the neighbor-joining method using MEGA X15 (Fig. 5). The szP gene has been shown to be highly variable30 and can be used to genotype various strains of S. zooepidemicus.21,23 Our isolates from Ohio and Tennessee belong to genotype VI, and they clustered together with ATCC 35246,18 the strain that caused the severe disease outbreak in swine in China in 1975. Aside from the S. zooepidemicus canine isolate from 2018, all other sequences from our laboratory appeared to be of different genotypes than our 2 recent pig isolates, including the additional unrelated swine isolate from Arizona that was grouped in genotype II. The similarity of the isolates from the buying station in Ohio and the abattoir in Tennessee suggests that there may be an epidemiologic relationship that warrants further investigation. Additionally, the observation that these recent strains causing high mortality in swine were in the same genotype VI as the 1975 outbreak in China raises the question of whether this genotype of S. zooepidemicus is uniquely virulent in swine or if there are other factors that may play a role in this fatal outcome.
Figure 5.
Phylogenetic analyses were performed based on the szP gene with variable regions that have frequently been used to differentiate strains of Streptococcus equi subsp. zooepidemicus. Strains from the Ohio buying station (2019071905) and the Tennessee abattoir (2019074097; red) belong to genotype VI and clustered together with ATCC 35246, a strain that caused the death of > 300,000 pigs in China in 1975. These 2 strains also clustered closely to S. zooepidemicus recovered from a canine case isolated at the ISU VDL, but distant to 12 other strains from the ISU VDL that were isolated during 2016–2019 from equine, feline, caprine, chinchilla, and swine samples from Arizona (blue).
The pathogenesis of S. zooepidemicus infection in domestic animals is incompletely understood. However, bacterial virulence factors and the host immune status appear to have roles in the development of disease. M-like protein (SzP) is a bacterial surface protein that serves as a virulence factor for infection and also displays antiphagocytic properties.19 The expression of fibronectin-binding protein, a type of bacterial invasin and adhesin, can promote cell adhesion for S. zooepidemicus and contribute to biofilm formation.31 S. zooepidemicus is nonmotile, and its mechanism of penetration through the mucus layer is still unclear. In vitro experiments have demonstrated the ability of S. zooepidemicus to survive within epithelial cells, which could contribute to the persistent and recurrent infection described in horses.28 In addition, S. zooepidemicus isolates with increased capsular production have been linked with enhancement of antiphagocytic ability in experimentally infected mice and are often obtained from horses with opportunistic respiratory tract infection.3,12 Interestingly, instability of capsular expression has been reported on primary culture of S. zooepidemicus in contrast to the robust capsular production in the closely related S. equi subsp. equi.29 In our cases of swine infection, mucoid colonies were observed on the bacterial culture plates, indicating increased capsular production by our S. zooepidemicus isolates.
Multiple routes of infection have been suggested in clinical cases of S. zooepidemicus infection. Bacterial invasion through oral abrasion is reported as the most common route of infection in guinea pigs with cervical lymphadenitis caused by S. zooepidemicus.4 The infection routes in sporadic cases of S. zooepidemicus in llamas and other camelids are thought to be through wound contamination and oral ingestion.9 Several outbreaks of S. zooepidemicus pneumonia in dog shelters and kennels have been reported with the introduction of an asymptomatic carrier into a naïve canine population as the proposed source of infection and the subsequent spread of the bacteria though respiratory secretion and environmental contamination.24,25 The route of infection in the cases of our cull sows and feeder pigs is uncertain. Predisposing conditions are often required for disease manifestation of S. zooepidemicus infection, such as environmental stressors, overcrowding, poor ventilation, high humidity, changes in temperature, and alteration of the mucus layer.3,7 Many of these conditions are potential risk factors for pigs in transit to slaughter, as in the cases of our report. Infections by certain primary respiratory viral agents, such as IAV, can be exacerbated when coinfected with S. zooepidemicus.17 In our case series, IAV was detected in one pig, but is likely not related to the S. zooepidemicus infection given the lack of respiratory signs and airway changes typical of IAV infection in pigs. The mechanism by which streptococcal bacteria become septic is uncertain; potential pathways include invasion through the extracellular matrix of the lamina propria and gaining access to the vasculature, tropism of the bacteria for vascular endothelial cells of the serosa, and liberation of bacterial toxins that cause vascular injury and permeability changes resulting in disseminated intravascular coagulation.10 In our case of swine high mortality, the question of whether this bacterium is an opportunist or a primary pathogen, as well as the septicemic process, is unknown.
Our cases of peracute disease and high mortality in previously healthy swine warrant further investigation into the prevalence of S. zooepidemicus in the U.S. swine herd, the route of infection and pathogenesis, and the risk factors that may predispose to this devastating disease outcome in infected pigs. The outbreaks described herein illustrate the potential for a substantial economic impact of S. zooepidemicus infection in the swine industry. A better understanding of the nature of this pathogen in swine is crucial for the development of future methods of diagnosis, prevention, and control of this disease.
Supplemental Material
Supplemental material, Supplemental_material for Cases of high mortality in cull sows and feeder pigs associated with Streptococcus equi subsp. zooepidemicus septicemia by Panchan Sitthicharoenchai, Rachel Derscheid, Kent Schwartz, Nubia Macedo, Orhan Sahin, Xuhua Chen, Ganwu Li, Rodger Main and Eric Burrough in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank the staff at the Iowa State University Diagnostic Laboratory for their assistance throughout the process of this case series.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Panchan Sitthicharoenchai  https://orcid.org/0000-0002-4412-7121
https://orcid.org/0000-0002-4412-7121
Eric Burrough  https://orcid.org/0000-0003-4747-9189
https://orcid.org/0000-0003-4747-9189
Supplementary material: Supplementary material for this article is available online.
References
- 1. Abbott Y, et al. Zoonotic transmission of Streptococcus equi subsp. zooepidemicus from a dog to a handler. J Med Microbiol 2010;59:120–123. [DOI] [PubMed] [Google Scholar]
- 2. Acke E, et al. Isolation of Streptococcus zooepidemicus from three dogs in close contact with horses. Vet Rec 2010;167:102–103. [DOI] [PubMed] [Google Scholar]
- 3. Anzai T, et al. Comparison of the phenotypes of Streptococcus zooepidemicus isolated from tonsils of healthy horses and specimens obtained from foals and donkeys with pneumonia. Am J Vet Res 2000;61:162–166. [DOI] [PubMed] [Google Scholar]
- 4. Barthold SW, et al. Guinea pig. In: Pathology of Laboratory Rodents and Rabbits. 4th ed. Wiley, 2016:213–252. [Google Scholar]
- 5. Bjurström L, Linde-Forsberg C. Long-term study of aerobic bacteria of the genital tract in breeding bitches. Am J Vet Res 1992;53:665–669. [PubMed] [Google Scholar]
- 6. Blum S, et al. Outbreak of Streptococcus equi subsp. zooepidemicus infections in cats. Vet Microbiol 2010;144:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chalker VJ, et al. The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet Microbiol 2003;95:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. 5th ed. CLSI, 2018. CLSI standard VET01. [Google Scholar]
- 9. Corpa JM, et al. Streptococcus equi subspecies zooepidemicus septicemia in alpacas: three cases and review of the literature. J Vet Diagn Invest 2018;30:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 2000;13:470–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erol E, et al. Beta-hemolytic Streptococcus spp. from horses: a retrospective study (2000–2010). J Vet Diagn Invest 2012;24:142–147. [DOI] [PubMed] [Google Scholar]
- 12. Gilmour MI, et al. Ozone-enhanced pulmonary infection with Streptococcus zooepidemicus in mice. The role of alveolar macrophage function and capsular virulence factors. Am Rev Respir Dis 1993;147:753–760. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman AM, et al. Association of microbiologic flora with clinical, endoscopic, and pulmonary cytologic findings in foals with distal respiratory tract infection. Am J Vet Res 1993;54:1615–1622. [PubMed] [Google Scholar]
- 14. Jaeger G, et al. Haemorrhagic pneumonia in sled dogs caused by Streptococcus equi subsp. zooepidemicus—one fatality and two full recoveries: a case report. Acta Vet Scand 2013;55:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar S, et al. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 2018;35:1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamm CG, et al. Streptococcal infection in dogs: a retrospective study of 393 cases. Vet Pathol 2010;47:387–395. [DOI] [PubMed] [Google Scholar]
- 17. Larson LJ, et al. Efficacy of the canine influenza virus H3N8 vaccine to decrease severity of clinical disease after cochallenge with canine influenza virus and Streptococcus equi subsp. zooepidemicus. Clin Vaccine Immunol 2011;18:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma Z, et al. Complete genome sequence of Streptococcus equi subsp. zooepidemicus strain ATCC 35246. J Bacteriol 2011;193:5583–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma Z, et al. Interaction between M-like protein and macrophage thioredoxin facilitates antiphagocytosis for Streptococcus equi ssp. zooepidemicus. PLoS One 2012;7:e32099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mätz-Rensing K, et al. Outbreak of Streptococcus equi subsp. zooepidemicus infection in a group of rhesus monkeys (Macaca mulatta). J Med Primatol 2009;38:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicholson ML, et al. Analysis of immunoreactivity to a Streptococcus equi subsp. zooepidemicus M-like protein to confirm an outbreak of poststreptococcal glomerulonephritis, and sequences of M-like proteins from isolates obtained from different host species. J Clin Microbiol 2000;38:4126–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palinski R, et al. A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. J Virol 2016;91:e01879-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pelkonen S, et al. Transmission of Streptococcus equi subspecies zooepidemicus infection from horses to humans. Emerg Infect Dis 2013;19:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pesavento PA, et al. A clonal outbreak of acute fatal hemorrhagic pneumonia in intensively housed (shelter) dogs caused by Streptococcus equi subsp. zooepidemicus. Vet Pathol 2008;45:51–53. [DOI] [PubMed] [Google Scholar]
- 25. Priestnall S, et al. Streptococcus zooepidemicus: an emerging canine pathogen. Vet J 2011;188:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rigby C. Natural infections of guinea-pigs. Lab Anim 1976;10:119–142. [DOI] [PubMed] [Google Scholar]
- 27. Sharp MW, et al. S. zooepidemicus infection and bovine mastitis. Vet Rec 1995;137:128. [DOI] [PubMed] [Google Scholar]
- 28. Skive B, et al. Streptococcus equi subsp. zooepidemicus invades and survives in epithelial cells. Front Cell Infect Microbiol 2017;7:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Timoney JF. The pathogenic equine streptococci. Vet Res 2004;35:397–409. [DOI] [PubMed] [Google Scholar]
- 30. Walker RL, Runyan CA. Identification of variations in SzP proteins of Streptococcus equi subspecies zooepidemicus and the relationship between protein variants and clinical signs of infection in horses. Am J Vet Res 2003;64:976–981. [DOI] [PubMed] [Google Scholar]
- 31. Yi L, et al. Contribution of fibronectin-binding protein to pathogenesis of Streptococcus equi ssp. zooepidemicus. Pathog Dis 2013;67:174–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_material for Cases of high mortality in cull sows and feeder pigs associated with Streptococcus equi subsp. zooepidemicus septicemia by Panchan Sitthicharoenchai, Rachel Derscheid, Kent Schwartz, Nubia Macedo, Orhan Sahin, Xuhua Chen, Ganwu Li, Rodger Main and Eric Burrough in Journal of Veterinary Diagnostic Investigation