Abstract
Invasive lobular breast carcinoma (ILC) accounts for 10% to 15% of breast cancers diagnosed annually. Evidence suggests that some aspects of endocrine treatment response might differ between invasive ductal carcinoma (IDC) and ILC, and that patients with ILC have worse long-term survival. We analyzed The Cancer Genome Atlas dataset and observed lower levels of ESR1 mRNA (P = 0.002) and ERα protein (P = 0.038) in ER+ ILC (n = 137) compared to IDC (n = 554), and further confirmed the mRNA difference in a local UPMC cohort (ILC, n = 143; IDC, n = 877; P < 0.005). In both datasets, the correlation between ESR1 mRNA and ERα protein was weaker in ILC, suggesting differential post-transcriptional regulation of ERα. In vitro, 17β-estradiol (E2) decreased the rate of degradation and increased the half-life of ERα in ILC cell lines, whereas the opposite was observed in IDC cell lines. Further, E2 failed to induce robust ubiquitination of ERα in ILC cells. To determine the potential clinical relevance of these findings, we evaluated the effect of 2 selective estrogen receptor downregulators (SERDs), ICI 182,780 and AZD9496, on ERα turnover and cell growth. While ICI 182,780 and AZD9496 showed similar effects in IDC cells, in ILC cell lines, AZD9496 was not as effective as ICI 182,780 in decreasing ERα stability and E2-induced proliferation. Furthermore, AZD9496 exhibited partial agonist activity in growth assays in ILC cell lines. Our study provides evidence for a distinct ERα regulation by SERDs in ILC cell lines, and therefore it is important to include ILC models into preclinical and clinical testing of novel SERDs.
Keywords: estrogen receptor, breast cancer, invasive lobular carcinoma, endocrine response
Invasive lobular breast carcinoma (ILC) accounts for 10% to 15% of breast cancers diagnosed annually, making it the second most common histological subtype of invasive breast cancer diagnosed in women (1). While most ILCs cluster with luminal A (LumA)-like tumors, they have clinical, pathological, and molecular characteristics that are distinct from the more common subtype invasive ductal breast carcinoma (IDC) (2–8). The hallmark of ILC tumors is loss of the adherens junction protein E-Cadherin, leading to a “single-file pattern” of tumor growth (5, 9). The majority of ILC tumors display favorable prognostic markers including estrogen receptor (ER) positivity and low Ki67 levels (3). Nevertheless, a subset of ILC tumors have been reported to be less responsive to endocrine therapy compared to stage-matched IDC tumors, and patients suffer from recurrence, often many years after the original diagnosis (4, 7, 10–12). Previous studies from our group have uncovered a unique estrogen response and de novo endocrine therapy resistance in human ILC cell lines (8, 13).
Very few studies have compared ERα protein levels between IDC and ILC, with little concordance between the existing data. Some studies have detected similar ERα levels (14, 15), while an analysis as part of a larger TCGA study reported decreased ERα levels in LumA ILC compared to LumA IDC tumors (16). In contrast, an additional study reported increased levels of ERα in ILC compared to IDC (17). However, the levels of ESR1 mRNA and the correlation between ERα mRNA expression and its protein abundance in IDC and ILC tumor samples have not previously been compared.
Regulation of ERα levels is fundamental in determining the receptor’s activity and response to therapy (18, 19). ERα protein turnover is suggested to play a role in the development and progression of breast cancer (reviewed in 20, 21). 17β-estradiol (E2), the natural ligand of ERα, induces the degradation of ER via the ubiquitin-proteasomal pathway in breast cancer cells (22–24). Overexpression of various proteins and altered post-translational modifications of ERα can stabilize the receptor by inhibiting its degradation (20, 25–27). There is evidence that increased stability of ERα results in increased ER activity, contributing to E2-induced proliferation and hormonal resistance in breast cancer cells (19, 27, 28). However, there is also evidence that ER degradation is uncoupled from transcriptional activity, and prior and recent studies showing that full ER degradation does not ensure complete ER antagonism (29–31).
Selective estrogen receptor downregulators (SERDs) induce degradation of ERα via the proteasomal machinery (32). Fulvestrant (ICI 182,780) is the only SERD currently approved for the treatment of metastatic breast cancer (33). Owing to its present limitations of dosing and intramuscular route of administration, there is a need for oral SERDs with improved bioavailability. AZD9496 is a newly developed orally bioavailable, nonsteroidal SERD (34, 35). AZD9496 displayed significant tumor growth inhibition in in vitro and in vivo, including in a series of endocrine resistant models (34, 36, 37). Recent results from a Phase 1 study in women with ER+/HER2– advanced breast cancer show that AZD9496 is well tolerated and has an acceptable safety profile (38). Since ILC is a distinct subtype of breast cancer displaying unique ER biology, evaluating the efficacy of AZD9496 in inducing ERα degradation and growth inhibition of ILC tumor models is critical to assessing the potential utility of this compound in the clinical management of patients with ILC.
In this study, we compared the levels of ESR1 mRNA and ERα protein, as well as the ERα mRNA-protein correlation, in IDC and ILC tumors from the TCGA dataset and in a local patient tumor tissue cohort. Our studies revealed significantly weaker correlation between ESR1 mRNA and ERα protein levels in ILC compared to IDC. Extensive analyses of ERα protein levels, E2-induced ERα protein expression and turnover in ER+ estrogen responsive human IDC (MCF-7, T47D, ZR-75-1) and ILC (BCK4, MDA-MB-134-VI, SUM44PE) cell lines showed that E2 decreased the rate of degradation and increased the half-life of ERα uniquely in the ILC models. Our studies suggest that E2 treatment failed to induce robust ubiquitination of ERα in the ILC cell line MDA-MB-134-VI, likely contributing to the extended ERα half-life. Furthermore, we found that the novel orally available SERD AZD9496 is less effective than ICI 182,780 in degrading ERα in ILC cell lines, and that it acts as a partial agonist in ILC but not in IDC models. In addition to pinpointing unique aspects of ERα biology in ILC, our data also underline the necessity of including models representing different histological breast cancer subtypes into development of novel SERDs.
Materials and Methods
TCGA dataset analysis
We utilized publicly available data from The Cancer Genome Atlas (TCGA, RRID:SCR_003193) (39, 40) to perform in silico analyses of ESR1 and PGR mRNA and ERα and PGR protein expression between ER+ ILC (n = 137) and IDC (n = 554). TCGA RNA-Seq expression data (transcripts per million [TPM]) were downloaded from the Gene Expression Omnibus database (GEO:GSE62944) (41). For protein expression, reverse-phase protein array (RPPA) data were downloaded as median-normalized, batch-corrected expression values from the TCGA website (Level 4, version 4.0) (42). The correlation between ESR1 mRNA and ERα protein levels were calculated as Pearson (r) correlations and comparisons of correlations was performed using Fisher r-to-z transformation, followed by a 2-tailed test using R (version 3.5.1).
Analysis of UPMC study population and design
ER immunohistochemistry H-scores and ESR1 and PGR mRNA levels were analyzed from tumor samples collected from patients with ER+ ILC (n = 143) and IDC (n = 877) treated at UPMC Magee Women’s Hospital, Pittsburgh (hereafter referred to as the “UPMC” dataset). ESR1 and PGR mRNA levels were quantified using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) as part of the Oncotype DX® (Genomic Health, Redwood City, CA) analysis, as described previously (43). Immunohistochemical (IHC) detection of ER was performed using the clone SP1 ER antibody (Ventana Medical Systems, Tucson, AZ [RRID:AB_ 2857956]) (44), and PR IHC was performed using the anti-PR (1E2) antibody (Ventana Medical Systems, Tucson, AZ [RRID:AB_2335976]) (45). The protein levels of the receptors were scored using a semiquantitative modified H-score method reported previously (46–48). The ESR1 mRNA and ERα protein correlation was calculated using Pearson correlation analysis, as described above.
Cell culture and reagents
BT474 (RRID:CVCL_0179) (49), HCC1500 (RRID:CVCL_1254) (50), MCF-7 (RRID:CVCL_0031) (51), MDA-MB-231 (MM-231) (RRID:CVCL_0062) (52), MDA-MB-330 (MM-330) (RRID:CVCL_0619) (53), T47D (RRID:CVCL_0553) (54), and ZR-75-1 (RRID:CVCL_0588) (55) were obtained from American Type Culture Collection (ATCC, Manassas, VA). BT474, HCC1500, T47D, and ZR-75-1 cells were maintained in RPMI-1640 (Product No: 11875119; Life Technologies, Carlsbad, CA) supplemented with 10% Fetal bovine serum (FBS). MCF-7, MDA-MB-231, and MDA-MB-330 cells were maintained in DMEM (Product No: 11965; Life Technologies) supplemented with 10% FBS. MDA-MB-134-VI (MM134, ATCC [RRID:CVCL_0617]) (56) and SUM44PE (SUM44, Asterand Bioscience, Detroit, MI [RRID:CVCL_3424]) (57) cells were cultured, as previously described (13). BCK4 cells kindly provided by Dr Britta Jacobsen (Anschutz Medical Campus, CO) were cultured, as detailed earlier (58). All cell lines were maintained in a 5% CO2 incubator at 37°C. Cell lines were authenticated by Arizona Research Laboratories (University of Arizona, Tucson, Arizona) and were tested to be mycoplasma negative. In hormone deprivation experiments, cells were grown in phenol red-free improved minimum essential medium (IMEM) supplemented with charcoal-stripped FBS (CSS) (12676, Life Technologies), as described earlier (13, 59). The cell lines were maintained in IMEM with 2% (SUM44), 5% (MCF-7, T47D, ZR-75-1, BCK4), or 10% (MDA-MB-134-VI) CSS. 17β-Estradiol (E2), 4-hydroxytamoxifen (4-OHT), cycloheximide, and MG132 were obtained from Sigma-Aldrich (St. Louis, MO). ICI 182,780 (Fulvestrant, ICI) was obtained from Tocris Bio-sciences (Bristol, UK). AZD9496 was provided by AstraZeneca, Macclesfield, United Kingdom. Additional experiments (Fig. S4A, B) (60) were performed using commercially available AZD9496 (Cayman Chemical). The stock solutions of the compounds were prepared in DMSO and the final concentration of DMSO in the working solutions were kept at less than 0.01%.
Immunoblotting
Whole cell protein extracts were prepared in RIPA buffer (50mM Tris [pH 7.4], 150 mM NaCl, 2 mM EDTA, 0.5% Na-deoxycholate, 50 mM NaF, 1% NP-40, and 0.1% sodium dodecyl sulfate [SDS]) containing protease-phosphatase inhibitors (Halt Phosphatase/Protease Inhibitor Cocktail, Thermo Fisher Scientific). The proteins were separated on SDS-PAGE and transferred to PVDF membrane. The primary antibodies used were: ERα (1:1000, 6F11, Novocastra/Leica, Germany [RRID:AB_876939]) (61), E-cadherin (1:500, HECD-1, Life Technologies [RRID:AB_211510]) (62), and β-Actin (1:5000, A5441, Sigma [RRID:AB_476744]) (63). Goat-anti mouse (RRID:AB_621842) (64) or rabbit (RRID:AB_621843) (65) IRDye800CW secondary antibody was obtained from LI-COR Biosciences (Lincoln, NE). The blots were scanned using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE) and quantified using Image Studio Lite Version 2 (LI-COR Biosciences).
Immunocytochemical staining for ERα
Cells grown in the above-mentioned standard culture conditions were pelleted and fixed with 10% neutral-buffered formalin and embedded in paraffin. Immunocytochemical detection of ER was performed on 5-μm sections using Clone SP1 ER antibody (RRID:AB_ 2857956) (44) and the iVIEW detection on the Benchmark XT system (Ventana, Tuscon, AZ) at the UPMC Magee Womens Hospital Histology Core.
RNA isolation, qRT-PCR, and DNA copy number analysis
Total RNA was prepared by illustra™ RNAspin Mini Isolation Kit (GE Healthcare Bio-Sciences, Buckinghamshire, UK), according to the manufacturer’s instructions. RNA was reverse-transcribed using iScript RT master mix (Bio-Rad, Hercules, CA). The genes of interest were amplified by quantitative PCR (qPCR) using SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA) using the following primer pairs: ESR1 Forward, GAGTATGATCCTACCAGACCCTTC; ESR1 Reverse, CCTGATCATGGAGGGTCAAATC; RPLP0 Forward, TAAACCCTGCGTGGCAATC; RPLP0 Reverse, TTGTCTGCTCCCACAATGAAA; GREB1 Forward, AAAT CGAGGATGTGGAGTG; GREB1 Reverse, TCTCACCAA GCAGGAGGAG; PGR Forward, TCGCCTTAGAAAGTGC TGTC; PGR Reverse, GCTTGGCTTTCATTTGGAACG; RNF148 Forward, CCAGGAGGCGAAGTCAAATAA; RNF148 Reverse, CCCTTGTTCATACTTGGGTAGAG; UBR1 Forward, ACTCTGGCTCGACATCTTATTG; UBR1 Reverse, CCCAACCTGTCTTCGGATTT; FBXO4 Forward, GGGATCTTCCTTCTTGGTCTTC; FBXO4 Reverse, GTATGCTCTTCCCTTGCTCTATC; UBE2E3 Forward, CGGGTTCTGTATATGAAGGTGG; and UBE2E3 Reverse, GATGACTCCCTGACTGTTGATG. qRT-PCR was performed on a CFX384 thermocycler (Bio-Rad, Hercules, CA), according to manufacturer’s instructions. Relative mRNA levels were calculated using the comparative cycle threshold method (ΔΔCt). ESR1 DNA copy number in MCF-7 and ILC cell lines were analyzed, as previously reported, using a NanoString based method (66).
Proliferation assays
Cells were hormone-deprived and plated in 96-well plates (IDC 5000–8000 cells/well; ILC 15000–18000 cells/well), allowed to attach overnight, and were treated with the specified ligands. After the indicated duration of treatment, cells were harvested, and cell proliferation was measured using FluoReporter Blue Fluorometric dsDNA Quantitation Kit (F2692; Life Technologies) according to the manufacturer’s instructions. Fluorescence was assessed using a VICTOR X4 plate reader (PerkinElmer, Waltham, MA). Data are presented as mean of 6 biological replicates ± SD. Each experiment was repeated 2 to 3 independent times, with similar trends of results.
Cycloheximide chase experiments
The half-life of ERα in cell lines was determined by cycloheximide (CHX) chase assay. IDC and ILC cell lines were maintained in complete growth medium or treated with vehicle (0.001% DMSO), 1 nM E2, 100 nM ICI 182,780, or 100 nM AZD9496 after hormone deprivation for 3 days. Cells were treated with CHX (50 μg/ml), with addition of CHX considered as time 0 (T= 0). Following addition of cycloheximide, cells were lysed at the times of 3, 6, 12, 24, and 48 hours. Lysates were separated by SDS-PAGE, and ERα levels were measured by immunoblotting as described above. ERα protein levels relative to β-actin were quantitated by densitometry using Odyssey imaging system (Licor, Lincoln, NE). Half-life of the protein was calculated using one-phase exponential decay curve using GraphPad Prism software.
Ubiquitination pathway PCR array
After hormone depriving for 3 days, MCF-7 and MDA-MB-134-VI cells were treated with 1nM E2 for 6 hours. Total RNA was isolated with RNeasy Mini kit (Qiagen, Valencia, CA). RNA was reverse transcribed using the RT2 First Strand kit (SA Biosciences, Frederick, MD). The expression of 84 ubiquitin pathway-related genes was analyzed using RT2 profiler PCR array PAHZ-079ZD (Human Ubiquitination Pathway Array, SA Biosciences) on the CFX96 thermocycler (BioRad, Hercules, CA), as per the manufacturer’s recommendations. The array was performed at 3 independent times and the data analyzed represents these biological repeats. Analysis for relative changes in gene expression was performed with SA Biosciences RT2 Profiler PCR Array Data Analysis software v3.5 using the comparative threshold cycle (ΔΔCt) method. Heat maps were generated using Multiple Experiment Viewer (MeV).
Ubiquitination assay
Ubiquitination Assay was performed as described previously, with modifications (31). Briefly, MCF-7 and MDA-MB-134-VI cell lines were hormone-deprived for 4 days and pretreated with proteasomal inhibitor MG132 (10 μM, Calbiochem, La Jolla, CA) for 30 minutes. Cells were then treated with Vehicle (0.01% DMSO) or 1 nM E2 for 5 hours. Following treatment, cells were lysed in buffer containing 50 mM Tris, pH 7.5, 150 mM NaCl, 10% glycerol, 2 mM EDTA, 50 mM NaF, and 1% NP-40. Lysates were precleared by incubation with normal rabbit-IgG (sc-2027) and Protein G-Plus Agarose beads (sc-2002, Santa Cruz Biotechnology, TX) for 2 hours at 4°C. Immunoprecipitations were carried out using anti-ERα antibody (HC-20; Santa Cruz Biotechnology [RRID:AB_631471]) (67) and Protein G-Plus Agarose beads. Beads were washed 4 times in lysis buffer and boiled in 2x SDS sample buffer. Proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). Membranes were boiled in transfer buffer for 10 minutes and probed for ubiquitinated ERα using anti-ubiquitin antibody (P4D1; Santa Cruz Biotechnology, TX [RRID:AB_628423]) (68), followed by goat-anti mouse IRDye800CW antibody (Licor, Lincoln, NE [RRID:AB_621842]) (64). The bands were visualized using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE).
Statistical analysis
Unless otherwise specified, statistical analyses were performed using GraphPad Prism 7 (San Diego, CA) software. The experiments were repeated a minimum of 2 independent times. Each figure is from a representative experiment and the number of independent repeats is indicated in the figure legend. In all experiments, statistical comparisons were made between control and treatment groups using unpaired t-test (2 groups), 1-way/2-way ANOVA (3 or more groups) with Bonferroni or Dunnett’s multiple comparison test, or Wilcoxon rank-sum tests, as indicated. P-values < 0.05 were considered as significant and are indicated by asterisks in figures (****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05).
Results
ILC tumors display discordant ESR1 mRNA levels and ERα protein expression compared to IDC tumors
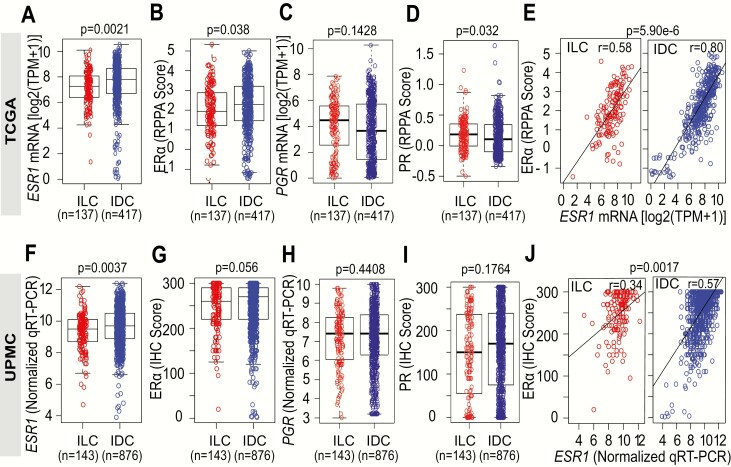
We utilized the TCGA dataset to compare ESR1 mRNA and ERα protein levels between ER+ ILC (n = 137) and ER+ IDC (n = 417) tumor samples. Tumor ESR1 mRNA and ERα protein levels had been assessed by RNA-Seq and RPPA, respectively. ER+ ILC tumors displayed significantly lower levels of ESR1 mRNA (P = 0.0021) (Fig. 1A) and ERα protein (P = 0.038) (Fig. 1B) compared to ER+ IDC tumors. Despite lower ESR1 mRNA and ERα protein levels in ILC, we did not detect significant decreases in PGR mRNA expression (Fig. 1C) and even slightly higher PGR protein level (Fig. 1D). Given the lower expression if ESR1 in ILC relative to IDC, we assessed the correlation between ESR1 mRNA and protein levels and observed that this was also significantly weaker in ER+ ILC (r = 0.58) compared to IDC (r = 0.80) (P = 5.9e-6) (Fig. 1E).
Figure 1.
Correlation of ESR1 mRNA and ERα protein levels in ER+ ILC and IDC tumors. A: ESR1 mRNA and RPPA ERα protein levels (B) in ER+ invasive lobular carcinoma (ILC) and ER+ invasive ductal carcinoma (IDC) samples analyzed from the TCGA data set. C: PGR mRNA and PGR protein levels (D) from the TCGA data set. E: Correlation between RPPA ERα protein and ESR1 mRNA levels between ILC and IDC samples analyzed from the TCGA data set using Pearson’s (r) correlation. P = 5.9e-6 for Wilcoxon rank-sum test comparison of Pearson correlations. F: ESR1 mRNA levels in IDC and ILC tumor samples as analyzed by qRT-PCR in the UPMC cohort. G: Immunohistochemical semiquantitation of ER in IDC and ILC tumor samples determined using a modified H-score. H: PGR mRNA by qRT-PCR and immunohistochemical semiquantitation of PGR (I) in IDC and ILC tumor samples determined using a modified H-score in the UPMC cohort. J: Correlation between ER IHC H-score and ESR1 mRNA levels between ILC and IDC samples as analyzed using Pearson’s coefficient (r) correlation. P = 0.0017 for Wilcoxon rank-sum test comparison of Pearson correlations.
Next, we analyzed ERα and PGR IHC H-scores and mRNA levels in tissue specimens from patients with ER+ ILC (n = 143) and IDC (n = 876) treated at UPMC Magee Women’s Hospital. Despite having significantly (P < 0.0037) lower ESR1 mRNA levels (Fig. 1F), ILC tumors displayed similar ERα IHC H-scores (ILC, H-score = 246) compared to IDC tumors (IDC, H Score = 252, P = 0.056) (Fig. 1G). Similar to our results from the TCGA analyses, PGR levels were not significantly different between ILC and IDC (Fig. 1H and 1I). In further concordance with the observations from the TCGA dataset, the correlation between ERα mRNA and protein levels was found to be significantly (P = 0.0017) weaker in ER+ ILC (r = 0.34) compared to ER+ IDC (r = 0.57) tumors (Fig. 1J). The consistently weaker ERα mRNA protein correlation in ILC compared to IDC samples suggests differences in post-transcriptional regulation of ERα, potentially due to increased protein synthesis, decreased protein degradation, or increased protein stability.
ILC cell lines are estrogen responsive and demonstrate higher ERα levels than IDC cell lines
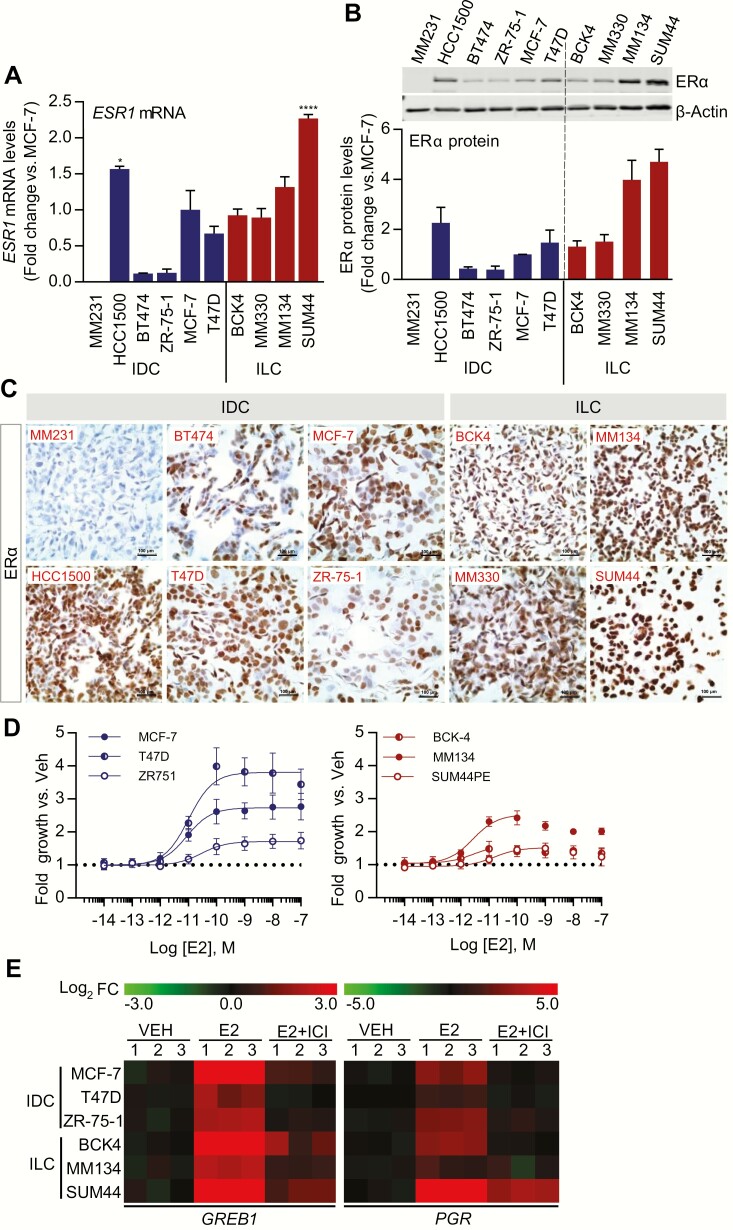
To assess any potential differences in the post-transcriptional regulation of ERα between ILC and IDC, we measured ERα protein levels, ESR1 mRNA expression and DNA copy number in a series of breast cancer cell lines. We included the 4 most commonly used ER+ IDC cell lines MCF-7, T47D, BT474, and ZR-75-1. We also included HCC1500 since it showed the highest ESR1 mRNA expression in the Cancer Cell Line Encyclopedia (CCLE) data set (Fig. S1A) (60). MDA-MB-231 served as an ER-negative IDC cell line control. For ILC, we used all currently available ER+ cell lines: MDA-MB-134-VI, SUM44 (69–71), and MDA-MB-330 with disrupted adherens junctions through α-catenin (CTNNA1) mutations (72) and BCK4, a recently described model of lobular disease of mucinous origin (58).
ESR1 mRNA analysis showed the lowest levels in BT474 and ZR-75-1 cells and the highest levels in MDA-MB-134-VI, SUM44, and HCC1500 (Fig. 2A). These findings are consistent with publicly available mRNA expression data from the CCLE and Marcotte et al (Fig. S1A, B) (60, 73). The observed higher mRNA expression was not a result of altered ESR1 DNA copy number (Fig. S1C) (60). In general, mRNA expression correlated well with ERα protein expression as measured by immunoblotting (Fig. 2B). We also performed immunohistochemical staining of ERα in cell line pellets and observed intense and mostly nuclear ERα staining in MDA-MB-134-VI and SUM44 cells with less heterogeneity compared to IDC cell line pellets (Fig. 2C).
Figure 2.
ERα protein levels and estrogen response in ILC and IDC cell line models. A: Expression of ESR1 mRNA in IDC and ILC cell lines. Cell lines were grown in standard culture conditions and ESR1 mRNA levels were quantified by qRT-PCR. Data are shown as mean ± SEM from 3 biological replicates. *P < 0.05; ****P < 0.0001; calculated by 2-way ANOVA followed by Dunnett’s multiple comparison test; comparing the ESR1 mRNA levels to that of MCF-7. B: Expression of ERα protein in IDC and ILC cell lines. Western blot analysis of ERα in total protein extracts from IDC and ILC cell lines. The protein quantity is expressed as average fold change versus MCF-7 cells. Results represent the mean ± SEM of 3 independent experiments. C: Pelleted, fixed, and paraffin-embedded cells were immunostained for ERα. Representative images taken at 40x objective are shown (scale bar = 100 μM). D: Effect of 17β-estradiol (E2) on growth of IDC and ILC cell lines. Hormone-deprived cell lines were treated with Vehicle (Veh, 0.01% DMSO) or increasing doses of E2 (10–14 to 10–7 M) for 6 (MCF-7, T47D) or 7 (ZR-75-1, BCK-4, MDA-MB-134-VI, SUM44) days and proliferation assessed by FluoReporter® Blue Fluorometric dsDNA Quantitation Kit. Data are shown as fold growth versus Veh control. Points represent the mean of 6 biological replicates; error bars denote SD. E: Heat maps depicting gene expression changes (log2 fold change [FC] vs Veh) after treatment with E2 ± ICI 182,780 in IDC and ILC cell lines. Cell lines were hormone-deprived and treated with Veh or 1 nM E2 ± 1 μM ICI 182,780 for 6 hours. GREB1 and PGR mRNA levels were quantified by qRT-PCR. Data expressed as log2 FC versus Veh from 3 biological replicates.
To assess endocrine response in selected IDC and ILC cell lines, cells were hormone deprived and stimulated with 17β-estradiol (E2). E2 induced the proliferation of all cell lines with EC50 values ranging between 1 and 30 pmol/L (Fig. 2D). In general, the magnitude of the E2 response is weaker in ILC lines despite the higher ERα levels, as we have previously described (8, 13). The expression of the classical E2-induced genes GREB1 and PGR was analyzed after treatment with 1 nM E2 ± 1 μM ICI 182,780 for 6 hours. In all cell lines, E2 induced the expression of GREB1, which was antagonized by the SERD, ICI 182,780 (Fig. 2E), confirming the ER-dependency of the effects. Thus, both the ILC and IDC cell lines used in our study were demonstrated to be ER responsive.
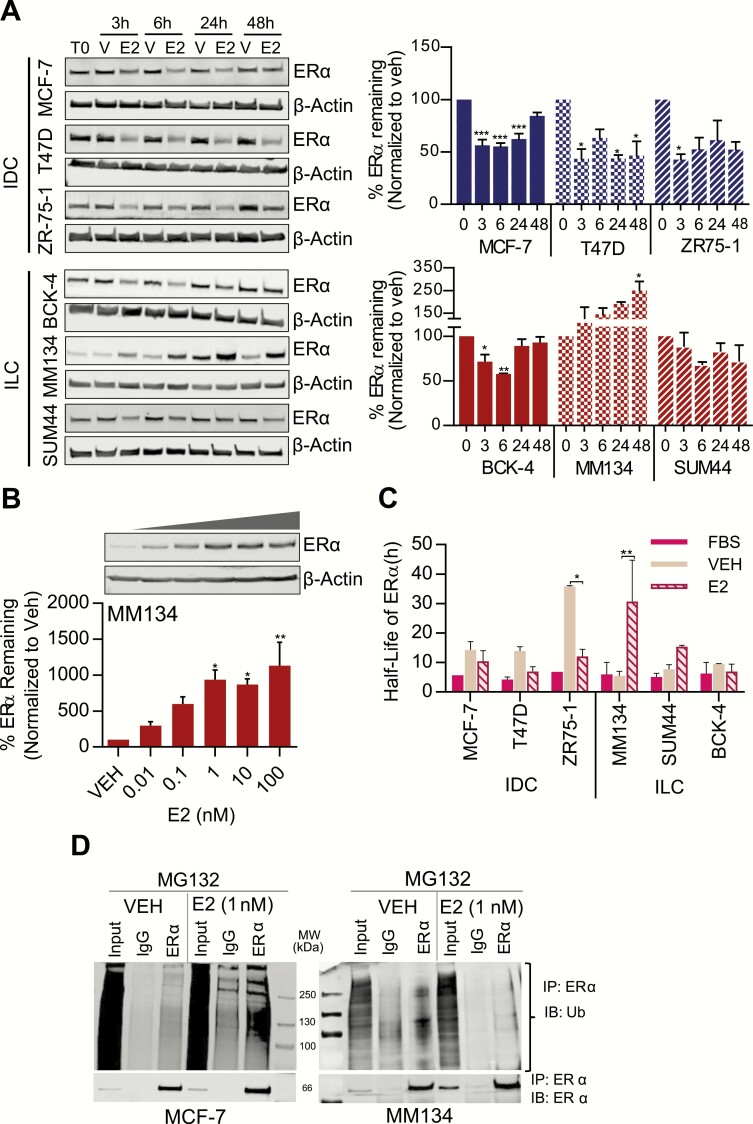
Differential estradiol-induced turnover of ERα in ILC and IDC cell lines
E2 stimulation has been reported to induce ERα turnover in breast cancer cells (22, 23). We examined the effect of E2 stimulation (1 nM) spanning several hours in IDC (MCF-7, T47D, and ZR-75-1) and ILC cell lines (BCK4, MDA-MB-134-VI, SUM44) and noted a differential response. Treatment with E2 decreased ERα protein levels in all 3 IDC cell lines. A 40% to 60% reduction in ERα protein levels in IDC cells was detected in time course experiments up to 24 hours after E2-treatment. After 48 hours of treatment with E2, the reduction in ERα levels continued at significant levels in T47D and at nonsignificant levels in MCF-7 and ZR-75-1 (Fig. 3A). In the ILC cell lines, ERα protein levels transiently decreased in the BCK4 (significantly) at 3 hours (29%) and 6 hours (43%), and SUM44 (nonsignificant trend) at 3 hours (34%) post-E2 treatment. In stark contrast, ERα levels paradoxically increased in MDA-MB-134-VI ILC cells upon E2 treatment (Fig. 3A). Furthermore, the increase in ERα protein levels in MDA-MB-134-VI cells was dose-dependent (Fig. 3B).
Figure 3.
17β-estradiol (E2)-induced changes in ERα protein levels, ubiquitination, and turnover in IDC and ILC cell lines. Cells were hormone-deprived and treated with Vehicle (V, 0.01% DMSO) or 1 nM E2 for varying time points (0, 3, 6, 24, 48 hours) (A) or increasing doses of E2 (0.01, 0.1, 1, 10, 100 nM) for 24 hours (B). ERα protein levels were assessed by immunoblotting. Protein levels are expressed as a percentage of ERα remaining as compared to the corresponding Veh-treated controls. Results represent the mean ± SEM of 3 experiments. *P < 0.05; **P < 0.01; ****P < 0.0001; calculated by 1-way ANOVA followed by Dunnett’s multiple comparison test. C: Half-life of ERα protein calculated by cycloheximide (CHX) chase assay. Cells were treated with CHX (50 µg/ml) in complete growth media or in combination with Veh or E2 (1 nM) after hormone deprivation. ERα protein bands were normalized to β-actin and then to the time (0 hour) control. Half-life of ERα protein was calculated based on 1-phase decay. *P < 0.05; **P < 0.01 calculated by 2-way ANOVA followed by Bonferroni multiple comparison test. D: Effect of E2 on ERα ubiquitination. MCF-7 and MDA-MB-134-VI cells were pretreated with 10 μM MG132 for 30 minutes followed by 5-hour treatment with Veh, or E2 (1 nM). ERα was immunoprecipitated (IP) from the total protein lysates, and ubiquitination was evaluated by IB using a ubiquitin (Ub)-specific antibody. Input lanes represent 5% of the amount of protein lysates used for IP. The bottom panel shows the blots re-probed with ERα specific antibody.
We next conducted cycloheximide chase studies to determine the contribution of protein degradation in regulating ERα protein levels in these cell lines. Consistent with the differential effects of E2 on ERα levels in IDC and ILC cells, E2 decreased the half-life of ERα protein in IDC cells lines and caused an increase in the half-life of ERα protein in both SUM44 and MDA-MB-134-VI cell lines following hormone deprivation (Fig. 3C).
Since E2-induced proteasomal degradation of ERα is preceded by polyubiquitination of the receptor (24), we assessed the ubiquitination of ERα in the presence of MG132, a proteasome inhibitor. We performed these studies in MDA-MB-134-VI cells, in which the observed effects of E2 on protein stability were the strongest among the ILC cells tested. In contrast to MCF-7 cells, we did not detect ubiquitination of ERα in MDA-MB-134-VI cells after treatment with E2 (Fig. 3D). Since this data suggested a potential deregulation of the ubiquitin pathway in MDA-MB-134-VI cells, we treated MCF-7 and MDA-MB-134-VI cells with E2 and analyzed the expression of 84 genes involved in the ubiquitin-proteasomal degradation pathway using an RT-PCR Array (Fig. S2A) (60). In MCF-7 cells, more than 70% of the ubiquitin pathway genes were found to be upregulated/unaltered after exposure to E2, though the fold-change induction of these genes was small. In contrast, in MDA-MB-134-VI cells, E2 treatment did not cause the increase in gene expression observed in MCF-7 cells but instead elicited moderate repression of many ubiquitin pathway genes—but again with small fold-changes (Fig. S2A-C) (60). These data suggest that in MDA-MB-134-VI cells, ERα protein has an extended half-life potentially in part due to decreased ubiquitination resulting in decreased protein degradation. Further, we compared expression of the top 20 E2 regulated ubiquitination pathway genes in MCF7 (Fig. S2B) (60) between ER+ IDC and ILC in TCGA. We observed that the majority (13/20) of these genes are significantly downregulated in ILC relative to IDC, suggesting that protein turnover may indeed be altered in these tumors (Fig. S3) (60); however, additional studies are needed to confirm a role for ubiquitination pathway in differential mRNA and the protein correlation between IDC and ILC (60).
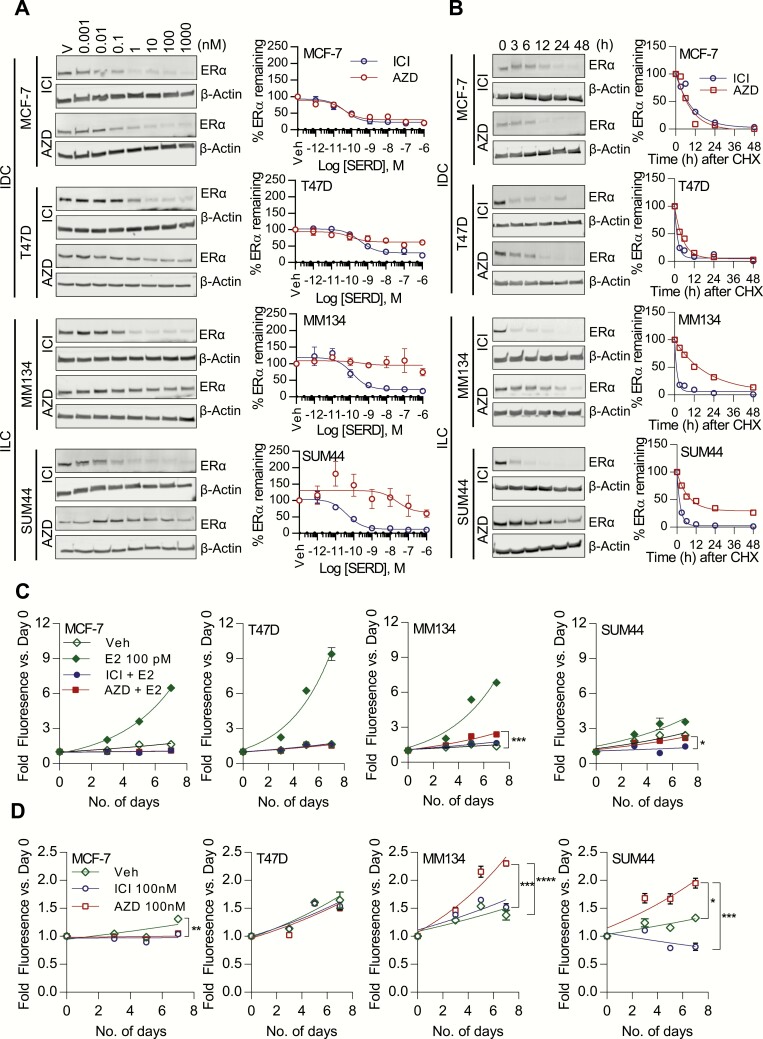
Oral SERD AZD9496 is less effective than ICI 182,780 in degrading ERα in ILC cells relative to IDC cell lines and displays agonist activity in cell proliferation assays
In addition to the subtype-specific effects in growth and ERα protein levels in response to E2, we next sought to evaluate any differences to SERDs comparing ILC and IDC cells. To this end, we determined the ability of the novel oral SERD AZD9496 in modulating ERα levels and inhibiting the growth of ILC (MDA-MB-134-VI, SUM44) and IDC (MCF-7, T47D) cell lines in comparison to ICI 182,780 (ICI). Hormone-deprived cells were treated with increasing doses of SERDs for 24 hours and ERα protein levels assessed by immunoblotting. Both ICI 182,780 and AZD9496 (10–12 to 10–6 M) were equally efficient in decreasing ERα levels in MCF-7 cells (IC50 ICI 182,780 = 4.4 × 10-10 M; AZD9496 = 3.5 × 10-10 M; Fig. 4A). In T47D cells, ICI 182,780 (10-10 to 10–6 M) induced a 15% to 80% reduction in ERα levels (IC50 = 3.7 × 10-10 M), while AZD9496 (10–11 to 10–6 M) induced a dose-dependent 15% to 50% reduction (IC50 = 6.3 × 10-10 M) (Fig. 4A). Similar to IDC cell lines, ICI 182,780 (10–11 to 10–6 M) robustly reduced ERα protein levels in both MDA-MB-134-VI and SUM44 cell lines (IC50 ICI 182,780 = 1.3 × 10-10 M and 4.4 × 10–11 M, respectively). Importantly, in striking contrast to IDC cell lines, AZD9496 was ineffective in downregulating ERα protein levels in both MDA-MB-134-VI and SUM44 cells (IC50 = 1.5 × 10–8 M and 1.0 × 10–7 M, respectively) except at the highest dose tested (10–6 M, 40% reduction) for SUM44 (Fig. 4A). Furthermore, in SUM44 cells, at lower doses of AZD9496, ERα protein levels were found to be stabilized, an effect similar to that evoked by 4-OHT (74).
Figure 4.
Oral SERD AZD9496 activity in blocking ILC and IDC growth, and ability to degrade ERα. A: Cells were hormone-deprived and treated with Vehicle (Veh, 0.01% DMSO) or increasing doses (10–12 to 10–6M) of SERDs ICI 182,780 (ICI) or AZD9496 (AZD) for 24 hours. ERα protein levels were assessed by immunoblotting. Protein levels are expressed as a percentage of ERα, remaining as compared to the corresponding Veh-treated controls. Results represent the mean ± SEM of 2 to 3 experiments. B: Half-life of ERα protein calculated by cycloheximide (CHX) chase assay. Cells were treated with CHX (50 µg/ml) in complete growth media or in combination with Veh, or ICI 182,780 (100 nM) or AZD (100 nM) after hormone deprivation. Cells were harvested after 0, 3, 6, 12, 24, and 48 hours. Protein lysates were prepared and ERα protein levels were assessed by Western blotting. ERα protein bands were normalized to β-actin and then to the time (0 hour) control. Half-life of ERα protein was calculated using GraphPad Prism software based on 1-phase decay. C, D: Hormone-deprived cells were treated with Veh, 100 nM ICI 182,780 or AZD with (C) or without (D) 100 pM E2 for 0, 3, 5, and 7 days, and proliferation was assessed by FluoReporter® Blue Fluorometric dsDNA Quantitation Kit. Data are shown as fold growth versus Day 0. Points represent mean of 6 biological replicates; error bars denote SD. **P < 0.01; ***P < 0.001; ****P < 0.0001; comparing the growth rate between treatment groups.
In order to measure the AZD9496-induced turnover of ERα, CHX chase assays were performed and ERα protein levels were measured after treatment with SERDs in the presence of cycloheximide. In MCF-7, the half-life of ERα was decreased at a similar rate by ICI 182,780 (t1/2 = 4.6 h) and AZD9496 (t1/2 = 3.9 h). A moderately increased half-life of ERα was observed in T47D after treatment with AZD9496 compared to ICI 182,780 (ICI 182,780 t1/2 = 1.4 h, AZD9496 t1/2 = 3.5 h) (Fig. 4B), consistent with the less dramatic reduction in protein levels, relative to MCF-7 (Fig. 4A). ERα protein was more stable in ILC cells in the presence of AZD9496, with a 4- to 10-fold increase in half-life (SUM44 ICI 182,780 t1/2 = 1.5 h, AZD9496 t1/2 = 4.6 h; MDA-MB-134-VI, ICI 182,780 t1/2 = 1.1 h, AZD9496 t1/2 = 13.5 h) compared to ICI 182,780 (Fig. 4B). This data supports a differential effect of AZD9496 between the IDC and ILC cell models.
Furthermore, we assessed the efficacy of AZD9496 in inhibiting the growth of ILC and IDC cell lines. E2-induced growth was completely blocked by both AZD9496 and ICI 182,780 in MCF-7 and T47D (Fig. 4C). Neither drug had any agonist activity in these cell lines in the absence of E2 (Fig. 4D). In MDA-MB-134-VI, ICI 182,780 completely repressed E2-induced growth (Fig. 4C), while AZD9496 only partially repressed E2-induced growth (ie, inhibition did not reach baseline growth conditions as seen in the IDC cell lines) (Fig. 4C). In the absence of E2, AZD9496 elicited more than 2-fold growth induction in MDA-MB-134-VI acting as a partial agonist (Fig. 4D). These data were repeated in an external laboratory (M.J.S., Fig. S4A) (60), where the unique partial agonist activity of AZD9496 (using a commercial source of the drug) was again observed in MDA-MB-134-VI. As previously reported by us (13), ICI 182,780 suppressed the growth of SUM44 in the presence and absence of E2, suggesting some ligand-independent ER activity (Fig. 4D); however, AZD9496 was not able to block the ligand-independent growth in SUM44 cells in the presence of E2 (Fig. 4C). In addition, similar to MDA-MB-134-VI cells, AZD9496 displayed agonist activities on growth in estrogen-deprived SUM44 cells (Fig. 4D). Cell proliferation experiments in hormone replete conditions performed in an independent laboratory (M.J.S.), again using a commercial source of AZD9496, confirmed the decreased antagonist activity of AZD9496 in MDA-MB-134-VI and SUM44 cells compared to MCF-7 cells (Fig. S4B [M.J.S.], C-E [S.O.]) (60).
Discussion
Resistance to endocrine therapy is a major problem in the management of ER+ breast cancer. Given that >90% of ILCs are ER+, and that a significant number of patients suffer from late recurrences due to resistance to endocrine therapy (4, 7, 10–13), it is critical to increase our understanding of the regulation of ERα expression and activity in ILC. In this study, we show that there is a weaker correlation between ESR1 mRNA transcripts and ERα protein levels in ILC compared to IDC patient samples, and we propose that altered E2-mediated degradation of ER proteins could potentially be playing a role in this observation. Clinical relevance is provided by our findings that the novel SERD AZD9496 demonstrates partial agonism in ILC cell lines, a surprising result that was not observed in the IDC cell lines tested in our study.
Our TCGA analyses demonstrate that both ESR1 mRNA and ERα protein (RPPA) levels are significantly lower in ILC tumors compared to IDC tumors, consistent with the data from the ILC TCGA working group (16). Determination of ERα expression levels using IHC in a local tumor cohort revealed similar ERα levels in both histological subtypes, consistent with previous reports using IHC (14, 15). This discrepancy is likely, at least in part, a result of using IHC rather than RPPA, in that the latter is more affected by tumor cellularity and heterogeneity (75). Importantly, irrespective of the differences in total ER detected in the TCGA (RPPA) and UPMC (IHC) studies, we observed a significantly weaker ESR1 mRNA-ERα protein correlation in ILC tumors compared to IDC in both cohorts. Although a linear proportional relationship between ER protein and ESR1 mRNA in breast tumors has been identified in multiple studies (76–78), nonlinear relationships have also been reported, for example, in a subset of tumors expressing low ESR1 mRNA levels (79). The weaker correlation between ESR1 mRNA and ERα protein that we observed in ILC may result from altered protein translation efficiency in ILC recently reported by us (80). Alternatively, or in parallel, there could be unique mechanisms of ERα degradation in place, which should be tested further in future studies.
The observed high expression of ERα in ILC cell lines is somewhat contradictory to our finding in human tumors. Potential explanations for this include the notorious difficulty of establishing ER+ cell lines, which is amplified by the slow growth of ILC cell lines. It is possible that currently available ILC models represent a minority of the heterogenous ILC cases, with the ability to propagate in vitro and in which ER is highly expressed; additional work is needed to fully characterize these models within the context of ILC heterogeneity. Another possible explanation is that many ILC tumors have a lower tumor cellularity compared to IDC owing to their unique growth pattern, and in this study we did not control for tumor cellularity.
Since it is well established that E2 regulates the steady state levels of ERα protein by inducing its degradation via the ubiquitin-proteasomal system, we characterized ERα expression in all currently available ER+ ILC cell line models (8) and 3 ER+ IDC cell lines in order to study ERα protein level changes in response to E2 stimulation (23, 24). As expected, E2 caused downregulation of ERα protein levels in the IDC cell lines MCF-7, T47D, and ZR-75-1 for up to 24 to 48 hours; however, there was heterogeneity in response in the ILC cell lines, with BCK4 showing ERα downregulation limited to early timepoints, no significant downregulation in SUM44, and increased in ERα protein levels in MDA-MB-134-VI. These data, supported by the measurement of ERα half-lives, suggested different mechanisms of ERα turnover in ILC versus IDC cells; however, this needs to be studied in more detail. Our data also suggests a heterogeneity of response to E2 in ERα levels within ILC, which needs to be considered in further studies and emphasizes the need for a generation of additional ILC models. Given that a lack of E-cadherin is the hallmark of ILC, it will be important in future studies to assess if E-cadherin plays a role in altering ERα half-life by assessing the effect of CDH1 overexpression and downregulation in ILC and IDC cell lines, respectively.
The lack of E2-mediated decrease of ERα in MDA-MB-134-VI was noted in a prior report (81), and our studies now reveal mechanistic insights for this prior observation, suggesting that E2 stimulation fails to activate ubiquitination and the subsequent proteasomal degradation of ERα in this ILC cell line model. Lack of ERα polyubiquitination has been suggested to be associated with breast cancer progression and endocrine therapy resistance (reviewed in 21). Along these same lines, E2-induced ERα degradation was also found to be absent in tamoxifen-resistant endometrial adenocarcinoma cell lines (82, 83). It is possible that the lack of ERα degradation could contribute to the previously reported tamoxifen-resistance in MDA-MB-134-VI (13). Of note, despite stabilized ERα levels, MDA-MB-134-VI cells remain responsive to E2 and have transcriptionally active ER. This data is in contrast with the idea that degradation of ERα is essential for E2-induced transcriptional activation of ER (24). We are currently leading a preoperative window trial of endocrine response in women with ILC (NCT02206984) where patients are randomized to receive either tamoxifen, fulvestrant, or anastrozole, and the change in Ki67 from baseline to post-treatment will be correlated with a number of other biomarkers, including ER levels, and downstream signaling.
To potentially link changes in ERα degradation with the response to SERDs, we compared the effects of ICI 182,780 (fulvestrant) to the novel potent and orally bioavailable SERD AZD9496. ICI 182,780 was equally efficacious in IDC and ILC cell lines, including in MDA-MB-134-VI cells that lacked E2-mediated degradation of ERα, rendering these cells as a great model for probing differences between E2 and SERD-mediated degradation of ERα. However, we made the unexpected discovery that AZD9496 acts as a partial agonist in the ILC cell lines tested. Given that SERDs are generally considered to lack agonist activity in breast cancer, this is the first report of AZD9496 displaying agonist activity in breast cancer cells. Of note, Weir et al have recently described an agonist activity of AZD9496 in uterine cells, an effect that was not seen with ICI 182,780 treatment (34). The agonist activity in ILC cells observed herein was accompanied by decreased effects on ERα protein degradation, but further studies are required to test if and how these observations are causatively linked. It is also possible that AZD9496 causes a unique recruitment of classical ERα co-factors in ILC cells, similar to what has been described as a mechanism for tamoxifen’s agonist activity (84, 85). A prime candidate is FoxA1, which has been shown to be more frequently altered in ILC compared to IDC, and has been suggested to play a role on endocrine resistant ILC (16, 86). Finally, given the recent studies showing that partial ER agonists cause increases in chromatin accessibility, ER dynamics in ILC cells should be further explored, as it is not currently known how intranuclear mobility of ER differs between ILC and IDC, and the relationship of this mobility to their response to novel SERDs (30).
In summary, our study adds to the existing evidence for unique ERα biology in ILC, which could contribute to endocrine resistance in some tumors. Further preclinical studies are warranted to understand the mechanisms underlying this unique biology and pharmacology. Importantly, our data calls for consideration of histological breast cancer subtypes in preclinical studies, as well as in clinical trials testing SERDs.
Acknowledgments
We thank Dr Elaine Alarid (University of Wisconsin-Madison) for her suggestions and help with the ubiquitination assay, Dr Britta Jacobson (University of Colorado, Denver) for the BCK4 cell line, and AstraZeneca for AZD9496. This project used the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core shared resource, which is supported in part by award P30CA047904.
Financial Support: This work was supported by the Susan G. Komen (PDF14301091 to S.S., Scientific Leadership awards for SO-SAC160073 and AVL-SAC110021, and CCR14300865 to R.C.J.), National Institutes of Health (F30CA203154 for K.L., K99CA193734 and R00CA193734 to M.J.S., and K99CA237736 to N.T.), Breast Cancer Research Foundation (S.O., A.V.L.), and Fashion Footwear of New York (FFANY for S.O.). This project used the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core shared resource, which is supported in part by award P30CA047904.
Additional Information
Disclosure Summary: The authors have a sponsored research agreement with AstraZeneca; however, this funding was obtained after completion of the studies described in this manuscript.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289(11):1421–1424. [DOI] [PubMed] [Google Scholar]
- 2. Joycelyn Jie Xin L, Guek Eng L. A review of invasive lobular carcinoma of the breast: should it be treated like invasive ductal carcinoma? Integr Cancer Sci Ther. 2016;3(5). [Google Scholar]
- 3. Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149–R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pestalozzi BC, Zahrieh D, Mallon E, et al. ; International Breast Cancer Study Group . Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26(18):3006–3014. [DOI] [PubMed] [Google Scholar]
- 5. Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol. 2010;27(1):49–61. [DOI] [PubMed] [Google Scholar]
- 6. McCart Reed AE, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and ‘omics. Breast Cancer Res. 2015;17(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barroso-Sousa R, Metzger-Filho O. Differences between invasive lobular and invasive ductal carcinoma of the breast: results and therapeutic implications. Ther Adv Med Oncol. 2016;8(4):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tasdemir N, Bossart EA, Li Z, et al. Comprehensive phenotypic characterization of human invasive lobular carcinoma cell lines in 2D and 3D cultures. Cancer Res. 2018;78(21):6209–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wahed A, Connelly J, Reese T. E-cadherin expression in pleomorphic lobular carcinoma: an aid to differentiation from ductal carcinoma. Ann Diagn Pathol. 2002;6(6):349–351. [DOI] [PubMed] [Google Scholar]
- 10. Engstrøm MJ, Opdahl S, Vatten LJ, Haugen OA, Bofin AM. Invasive lobular breast cancer: the prognostic impact of histopathological grade, E-cadherin and molecular subtypes. Histopathology. 2015;66(3):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Metzger Filho O, Giobbie-Hurder A, Mallon E, et al. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1-98 trial. J Clin Oncol. 2015;33(25):2772–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adachi Y, Ishiguro J, Kotani H, et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer. 2016;16:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sikora MJ, Cooper KL, Bahreini A, et al. Invasive lobular carcinoma cell lines are characterized by unique estrogen-mediated gene expression patterns and altered tamoxifen response. Cancer Res. 2014;74(5):1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van de Water W, Fontein DBY, van Nes JGH, et al. Influence of semi-quantitative oestrogen receptor expression on adjuvant endocrine therapy efficacy in ductal and lobular breast cancer—a TEAM study analysis. Eur J Cancer. 2013;49(2):297–304. [DOI] [PubMed] [Google Scholar]
- 15. Truin W, Roumen RMH, Siesling S, van de Vijver KK, Tjan-Heijnen VCG, Voogd AC. Estrogen and progesterone receptor expression levels do not differ between lobular and ductal carcinoma in patients with hormone receptor-positive tumors. Breast Cancer Res Treat. 2017;164(1):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ciriello G, Gatza ML, Beck AH, et al. ; TCGA Research Network . Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang B, Omoto Y, Iwase H, et al. Differential expression of estrogen receptor α, β1, and β2 in lobular and ductal breast cancer. Proc Natl Acad Sci U S A. 2014;111(5):1933–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fowler AM, Solodin N, Preisler-Mashek MT, Zhang P, Lee AV, Alarid ET. Increases in estrogen receptor-alpha concentration in breast cancer cells promote serine 118/104/106-independent AF-1 transactivation and growth in the absence of estrogen. Faseb J. 2004;18(1):81–93. [DOI] [PubMed] [Google Scholar]
- 19. Frech MS, Halama ED, Tilli MT, et al. Deregulated estrogen receptor alpha expression in mammary epithelial cells of transgenic mice results in the development of ductal carcinoma in situ. Cancer Res. 2005;65(3):681–685. [PMC free article] [PubMed] [Google Scholar]
- 20. Tecalco-Cruz AC, Ramírez-Jarquín JO. Mechanisms that increase stability of estrogen receptor alpha in breast cancer. Clin Breast Cancer. 2017;17(1):1–10. [DOI] [PubMed] [Google Scholar]
- 21. Tecalco-Cruz AC, Ramírez-Jarquín JO. Polyubiquitination inhibition of estrogen receptor alpha and its implications in breast cancer. World J Clin Oncol. 2018;9(4):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alarid ET, Bakopoulos N, Solodin N. Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Mol Endocrinol. 1999;13(9):1522–1534. [DOI] [PubMed] [Google Scholar]
- 23. Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci U S A. 1999;96(5):1858–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lonard DM, Nawaz Z, Smith CL, O’Malley BW. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5(6):939–948. [DOI] [PubMed] [Google Scholar]
- 25. Wei X, Xu H, Kufe D. MUC1 oncoprotein stabilizes and activates estrogen receptor alpha. Mol Cell. 2006;21(2):295–305. [DOI] [PubMed] [Google Scholar]
- 26. Rajbhandari P, Finn G, Solodin NM, et al. Regulation of estrogen receptor α N-terminus conformation and function by peptidyl prolyl isomerase Pin1. Mol Cell Biol. 2012;32(2):445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rajbhandari P, Schalper KA, Solodin NM, et al. Pin1 modulates ERα levels in breast cancer through inhibition of phosphorylation-dependent ubiquitination and degradation. Oncogene. 2014;33(11):1438–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fowler AM, Solodin NM, Valley CC, Alarid ET. Altered target gene regulation controlled by estrogen receptor-alpha concentration. Mol Endocrinol. 2006;20(2):291–301. [DOI] [PubMed] [Google Scholar]
- 29. Wardell SE, Marks JR, McDonnell DP. The turnover of estrogen receptor α by the selective estrogen receptor degrader (SERD) fulvestrant is a saturable process that is not required for antagonist efficacy. Biochem Pharmacol. 2011;82(2):122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guan J, Zhou W, Hafner M, et al. Therapeutic ligands antagonize estrogen receptor function by impairing its mobility. Cell. 2019;178(4):949–963.e18. [DOI] [PubMed] [Google Scholar]
- 31. Valley CC, Métivier R, Solodin NM, et al. Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor alpha N terminus. Mol Cell Biol. 2005;25(13):5417–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeh WL, Shioda K, Coser KR, Rivizzigno D, McSweeney KR, Shioda T. Fulvestrant-induced cell death and proteasomal degradation of estrogen receptor α protein in MCF-7 cells require the CSK c-Src tyrosine kinase. Plos One. 2013;8(4):e60889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Defriend DJ, Howell A, Nicholson RI, et al. Investigation of a new pure antiestrogen (ICI 182780) in women with primary breast cancer. Cancer Res. 1994;54(2):408–414. [PubMed]
- 34. Weir HM, Bradbury RH, Lawson M, et al. AZD9496: An oral estrogen receptor inhibitor that blocks the growth of ER-positive and ESR1-mutant breast tumors in preclinical models. Cancer Res. 2016;76(11):3307–3318. [DOI] [PubMed] [Google Scholar]
- 35. De Savi C, Bradbury RH, Rabow AA, et al. Optimization of a novel binding motif to (E)-3-(3,5-difluoro-4-((1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl)phenyl)acrylic acid (AZD9496), a potent and orally bioavailable selective estrogen receptor downregulator and antagonist. J Med Chem. 2015;58(20):8128–8140. [DOI] [PubMed] [Google Scholar]
- 36. Nardone A, Weir H, Delpuech O, et al. The oral selective oestrogen receptor degrader (SERD) AZD9496 is comparable to fulvestrant in antagonising ER and circumventing endocrine resistance. Br J Cancer. 2019;120(3):331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Toy W, Weir H, Razavi P, et al. Activating ESR1 Mutations Differentially Affect the Efficacy of ER Antagonists. Cancer Discov. 2017;7(3):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hamilton EP, Patel MR, Armstrong AC, et al. A first-in-human study of the new oral selective estrogen receptor degrader AZD9496 for ER+/HER2- advanced breast cancer. Clin Cancer Res. 2018;24(15):3510–3518. [DOI] [PubMed] [Google Scholar]
- 39. The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature. 2012;490(7418):61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. RRID:SCR_003193. [Google Scholar]
- 41. Rahman M, Jackson LK, Johnson WE, Li DY, Bild AH, Piccolo SR. Alternative preprocessing of RNA-sequencing data in the cancer genome atlas leads to improved analysis results. Bioinformatics. 2015;31(22):3666–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li J, Lu Y, Akbani R, et al. TCPA: a resource for cancer functional proteomics data. Nat Methods. 2013;10(11):1046–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kraus JA, Dabbs DJ, Beriwal S, Bhargava R. Semi-quantitative immunohistochemical assay versus oncotype DX(®) qRT-PCR assay for estrogen and progesterone receptors: an independent quality assurance study. Mod Pathol. 2012;25(6):869–876. [DOI] [PubMed] [Google Scholar]
- 44. RRID:AB_2857956. [Google Scholar]
- 45. RRID:AB_2335976. [Google Scholar]
- 46. McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109(8):716–721. [PubMed] [Google Scholar]
- 47. Flanagan MB, Dabbs DJ, Brufsky AM, Beriwal S, Bhargava R. Histopathologic variables predict Oncotype DX recurrence score. Mod Pathol. 2008;21(10):1255–1261. [DOI] [PubMed] [Google Scholar]
- 48. Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23(2):205–212. [DOI] [PubMed] [Google Scholar]
- 49. RRID:CVCL_0179. [Google Scholar]
- 50. RRID:CVCL_1254. [Google Scholar]
- 51. RRID:CVCL_0031. [Google Scholar]
- 52. RRID:CVCL_0062. [Google Scholar]
- 53. RRID:CVCL_0619. [Google Scholar]
- 54. RRID:CVCL_0553. [Google Scholar]
- 55. RRID:CVCL_0588. [Google Scholar]
- 56. RRID:CVCL_0617. [Google Scholar]
- 57. RRID:CVCL_3424. [Google Scholar]
- 58. Jambal P, Badtke MM, Harrell JC, et al. Estrogen switches pure mucinous breast cancer to invasive lobular carcinoma with mucinous features. Breast Cancer Res Treat. 2013;137(2):431–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sikora MJ, Johnson MD, Lee AV, Oesterreich S. Endocrine response phenotypes are altered by charcoal-stripped serum variability. Endocrinology. 2016;157(10):3760–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sreekumar S, Levine KM, Sikora MJ, et al. Data from: differential regulation and targeting of estrogen receptor α turnover in invasive lobular breast carcinoma. Figshare 2020. Deposited June 18, 2020. Doi: 10.6084/m9.figshare.1251080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. RRID:AB_876939. [Google Scholar]
- 62. RRID:AB_211510. [Google Scholar]
- 63. RRID:AB_476744. [Google Scholar]
- 64. RRID:AB_621842. [Google Scholar]
- 65. RRID:AB_621843. [Google Scholar]
- 66. Basudan A, Priedigkeit N, Hartmaier RJ, et al. Frequent ESR1 and CDK Pathway Copy-Number Alterations in Metastatic Breast Cancer. Mol Cancer Res. 2019;17(2):457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. RRID:AB_631471. [Google Scholar]
- 68. RRID:AB_628423. [Google Scholar]
- 69. Reis-Filho JS, Simpson PT, Turner NC, et al. FGFR1 emerges as a potential therapeutic target for lobular breast carcinomas. Clin Cancer Res. 2006;12(22):6652–6662. [DOI] [PubMed] [Google Scholar]
- 70. Riggins RB, Lan JP, Zhu Y, et al. ERRgamma mediates tamoxifen resistance in novel models of invasive lobular breast cancer. Cancer Res. 2008;68(21):8908–8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Christgen M, Derksen P. Lobular breast cancer: molecular basis, mouse and cellular models. Breast Cancer Res. 2015;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hollestelle A, Elstrodt F, Timmermans M, et al. Four human breast cancer cell lines with biallelic inactivating alpha-catenin gene mutations. Breast Cancer Res Treat. 2010;122(1):125–133. [DOI] [PubMed] [Google Scholar]
- 73. Marcotte R, Sayad A, Brown KR, et al. Functional Genomic Landscape of Human Breast Cancer Drivers, Vulnerabilities, and Resistance. Cell. 2016;164(1-2):293–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kiang DT, Kollander RE, Thomas T, Kennedy BJ. Up-regulation of estrogen receptors by nonsteroidal antiestrogens in human breast cancer. Cancer Res. 1989;49(19):5312–5316. [PubMed] [Google Scholar]
- 75. Meric-Bernstam F, Akcakanat A, Chen H, et al. Influence of biospecimen variables on proteomic biomarkers in breast cancer. Clin Cancer Res. 2014;20(14):3870–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lacroix M, Querton G, Hennebert P, Larsimont D, Leclercq G. Estrogen receptor analysis in primary breast tumors by ligand-binding assay, immunocytochemical assay, and northern blot: a comparison. Breast Cancer Res Treat. 2001;67(3):263–271. [DOI] [PubMed] [Google Scholar]
- 77. Gong Y, Yan K, Lin F, et al. Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol. 2007;8(3):203–211. [DOI] [PubMed] [Google Scholar]
- 78. Kim C, Tang G, Pogue-Geile KL, et al. Estrogen receptor (ESR1) mRNA expression and benefit from tamoxifen in the treatment and prevention of estrogen receptor-positive breast cancer. J Clin Oncol. 2011;29(31):4160–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bordeaux JM, Cheng H, Welsh AW, et al. Quantitative in situ measurement of estrogen receptor mRNA predicts response to tamoxifen. Plos One. 2012;7(5):e36559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Du T, Zhu L, Levine KM, et al. Invasive lobular and ductal breast carcinoma differ in immune response, protein translation efficiency and metabolism. Sci Rep. 2018;8(1):7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Reiner GC, Katzenellenbogen BS. Characterization of estrogen and progesterone receptors and the dissociated regulation of growth and progesterone receptor stimulation by estrogen in MDA-MB-134 human breast cancer cells. Cancer Res. 1986;46(3):1124–1131. [PubMed] [Google Scholar]
- 82. Horner-Glister E, Maleki-Dizaji M, Guerin CJ, Johnson SM, Styles J, White IN. Influence of oestradiol and tamoxifen on oestrogen receptors-alpha and -beta protein degradation and non-genomic signalling pathways in uterine and breast carcinoma cells. J Mol Endocrinol. 2005;35(3):421–432. [DOI] [PubMed] [Google Scholar]
- 83. Pole JC, Gold LI, Orton T, Huby R, Carmichael PL. Gene expression changes induced by estrogen and selective estrogen receptor modulators in primary-cultured human endometrial cells: signals that distinguish the human carcinogen tamoxifen. Toxicology. 2005;206(1):91–109. [DOI] [PubMed] [Google Scholar]
- 84. Grese TA, Sluka JP, Bryant HU, et al. Molecular determinants of tissue selectivity in estrogen receptor modulators. Proc Natl Acad Sci U S A. 1997;94(25):14105–14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295(5564):2465–2468. [DOI] [PubMed] [Google Scholar]
- 86. Desmedt C, Zoppoli G, Gundem G, et al. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol. 2016;34(16):1872–1881. [DOI] [PubMed] [Google Scholar]