Abstract
Background
Acute soft tissue injuries are common and costly. The best drug treatment for such injuries is not certain, although non‐steroidal anti‐inflammatory drugs (NSAIDs) are often recommended. There is concern about the use of oral opioids for acute pain leading to dependence. This is an update of a Cochrane Review published in 2015.
Objectives
To assess the benefits or harms of NSAIDs compared with other oral analgesics for treating acute soft tissue injuries.
Search methods
We searched the CENTRAL, 2020 Issue 1, MEDLINE (from 1946), and Embase (from 1980) to January 2020; other databases were searched to February 2019.
Selection criteria
We included randomised or quasi‐randomised controlled trials involving people with acute soft tissue injury (sprain, strain, or contusion of a joint, ligament, tendon, or muscle occurring within 48 hours of inclusion in the study), and comparing oral NSAIDs versus paracetamol (acetaminophen), opioid, paracetamol plus opioid, or complementary and alternative medicine. The outcomes were pain, swelling, function, adverse effects, and early re‐injury.
Data collection and analysis
Two review authors independently assessed studies for eligibility, extracted data, and assessed risk of bias. We assessed the quality of the evidence using GRADE methodology.
Main results
We included 20 studies, with 3305 participants. Three studies included children only. The others included predominantly young adults; approximately 60% were male. Seven studies recruited people with ankle sprains only. Most studies were at low or unclear risk of bias; however, two were at high risk of selection bias, three were at high risk of bias from lack of blinding, and five were at high risk of selective outcome reporting bias. Some evidence relating to pain relief was high certainty. Other evidence was either moderate, low or very low certainty, reflecting study limitations, indirectness, imprecision, or combinations of these. Thus, we are certain or moderately certain about some of the estimates, and uncertain or very uncertain of others.
Eleven studies, involving 1853 participants compared NSAIDs with paracetamol. There were no differences between the two groups in pain at one to two hours (1178 participants, 6 studies; high‐certainty evidence), at days one to three (1232 participants, 6 studies; high‐certainty evidence), and at day seven or later (467 participants, 4 studies; low‐certainty evidence). There was little difference between the groups in numbers of participants with minimal swelling at day seven or later (77 participants, 1 study; low‐certainty evidence). Very low‐certainty evidence from three studies (386 participants) means we are uncertain of the finding of little difference between the two groups in return to function at day seven or later. There was low‐certainty evidence from 10 studies (1504 participants) that NSAIDs may slightly increase the risk of gastrointestinal adverse events compared with paracetamol. There was low‐certainty evidence from nine studies (1679 participants) of little difference in neurological adverse events between the NSAID and paracetamol groups.
Six studies, involving 1212 participants compared NSAIDs with opioids. There was moderate‐certainty evidence of no difference between the groups in pain at one hour (1058 participants, 4 studies), and low‐certainty evidence for no difference in pain at days four or seven (706 participants, 1 study). There was very low‐certainty evidence of no important difference between the groups in swelling (84 participants, 1 study). Participants in the NSAIDs group were more likely to return to function in 7 to 10 days (542 participants, 2 studies; low‐certainty evidence). There was moderate‐certainty evidence (1143 participants, 5 studies) that NSAIDs were less likely to result in gastrointestinal or neurological adverse events compared with opioids.
Four studies, involving 240 participants, compared NSAIDs with the combination of paracetamol and an opioid. The applicability of findings from these studies is in question because the dextropropoxyphene combination analgesic agents used are no longer in general use. Very low‐certainty evidence means we are uncertain of the findings of no differences between the two interventions in the numbers with little or no pain at day one (51 participants, 1 study), day three (149 participants, 2 studies), or day seven (138 participants, 2 studies); swelling (230 participants, 3 studies); return to function at day seven (89 participants, 1 study); and the risk of gastrointestinal or neurological adverse events (141 participants, 3 studies).
No studies reported re‐injury rates.
No studies compared NSAIDs with oral complementary and alternative medicines,
Authors' conclusions
Compared with paracetamol, NSAIDs make no difference to pain at one to two hours and at two to three days, and may make no difference at day seven or beyond. NSAIDs may result in a small increase in gastrointestinal adverse events and may make no difference in neurological adverse events compared with paracetamol.
Compared with opioids, NSAIDs probably make no difference to pain at one hour, and may make no difference at days four or seven. NSAIDs probably result in fewer gastrointestinal and neurological adverse effects compared with opioids.
The very low‐certainly evidence for all outcomes for the NSAIDs versus paracetamol with opioid combination analgesics means we are uncertain of the findings of no differences in pain or adverse effects.
The current evidence should not be extrapolated to adults older than 65 years, as this group was not well represented in the studies
Plain language summary
Oral non‐steroidal anti‐inflammatory drugs compared with other oral pain killers for sprains, strains and bruises
Introduction and aims
Sprains, strains, and bruises are common injuries, and people with these injuries often require pain relief, given as a tablet or capsule that is swallowed (oral). Many types of oral painkillers are available to treat such injuries. We wanted to know whether there were any differences in people's pain, swelling, function, or unwanted side effects when sprains, strains, and bruises were treated with oral non‐steroidal anti‐inflammatory drugs (NSAIDs, e.g. ibuprofen) compared with paracetamol, opioids (e.g. codeine), complementary or alternative medicines, or combinations of these.
This is an update of a Cochrane review published in 2015.
What did we do?
We searched medical databases up to January 2020 for studies that compared NSAIDs with other painkillers in people with sprains, strains, and bruises. Study participants could be any age. We assessed the included studies to judge the reliability (certainty) of the evidence. We categorised the evidence as being of high, moderate, low, or very low certainty. High certainty means we are confident in the evidence, moderate certainty means we are fairly confident, low or very low certainty means that we are unsure or very unsure of the reliability of the evidence.
Results of our search and description of studies
We included 20 studies, with 3305 participants. Seven studies included people with ankle sprain only. Three studies included children only. Most of the participants of the other studies were young adults, and there were slightly more men than women. Few participants were aged over 65 years. Eleven studies compared NSAIDs with paracetamol, six studies compared NSAIDs with opioids, and four studies compared NSAIDs with paracetamol combined with an opioid. Studies reported outcomes at times varying from one hour after taking the medication, up to 10 to 14 days.
Main results
There is no difference between NSAIDs and paracetamol in pain after one to two hours, or after two to three days (high‐certainty evidence), and there may be no difference after a week or more (low‐certainty evidence). There is low‐certainty evidence that NSAIDs may make little difference to swelling after a week or more. We are uncertain whether NSAIDs make a difference to return to function after a week or more (very low‐certainty evidence). There is low‐certainly evidence that NSAIDs may slightly increase unwanted side effects related to the gut.
There is probably no difference between NSAIDs and opioids in pain at one hour (moderate‐certainly evidence), and there may be no difference four or seven days after taking medication (low‐certainty evidence). We are uncertain whether NSAIDs make a difference to swelling after 10 days (very low‐certainty evidence). There is low‐certainty evidence that NSAIDs may increase return to function in 7 to 10 days. There is moderate‐certainty evidence that NSAIDs probably result in fewer unwanted side effects, such as nausea and dizziness, compared with opioids.
The evidence suggests that there is little or no difference between NSAIDs and paracetamol combined with opioids in pain, swelling, return to function, or unwanted side effects. However, the evidence was very low certainty, so we are uncertain of these results.
No studies reported the risk of re‐injury after treatment.
We found no studies comparing NSAIDs with complementary or alternative medicines.
Conclusions
The body of evidence to date has found no difference between NSAIDs and other pain killers for pain relief for strains, sprains, and bruises in younger people. However, we need more, and better evidence on return to function and unwanted side effects in all age groups, particularly in older people.
Summary of findings
Background
Description of the condition
Acute soft tissue injuries are common; they cause 5% to 10% of emergency department attendances in the United Kingdom (Handoll 2007; Williams 1979). In Australia, over five million sports injuries occur annually (Cassell 2003; Medibank 2006), and in Germany, 3.1% of the population sustain a sports injury each year, most of which are acute soft tissue injuries (Schneider 2006).
The costs associated with these 'minor' injuries are substantial, with the lifetime cost of soft tissue injuries sustained in 2019 estimated at over $5 billion NZD in New Zealand (population: approximately five million; ACC 2020). The costs relate to treatment and time taken off work, with loss of income. Previously in the United Kingdom, lost productivity due to soft tissue injuries was estimated at over six million days/year (Nicholl 1995).
Acute soft tissue injuries include a number of conditions (sprain, strain, contusion, and haematoma) with similar well‐researched and understood pathology (Burke 2006). When the mechanical load on a tissue exceeds the tensile strength of the tissue, cell damage and haemorrhage occur. This initiates the inflammatory cascade (Burke 2006). Inflammation clears the necrotic cell debris after traumatic haemorrhage, providing a connective tissue framework for tissue regeneration (Martin 2005). Pain is the most common sequela of acute soft tissue injuries, and the main reason for the use of oral analgesics. Inflammation is the natural response to such injuries, and mediators of inflammation contribute to pain and swelling following injury. Inflammation and pain are worst in the first two days post‐injury, then decline rapidly (Almekinders 1986; Burke 2006; Obremsky 1994).
Description of the intervention
Analgesics are commonly prescribed, or used without prescription, for acute soft tissue injuries (Gøtzsche 2000; Motola 2004; Warner 2002). Traditional non‐selective non‐steroidal anti‐inflammatory drugs (NSAIDs) are the analgesic agents most often prescribed worldwide, as they have both analgesic and anti‐inflammatory effects (Gøtzsche 2000; Jones 1999; Motola 2004; Warner 2002). The use of NSAIDs for analgesia following an injury has been questioned, due to the high side effect profile of NSAIDs compared with that of other analgesic agents. For example, a short course (one week) of diclofenac has an associated mortality rate of 5.9 deaths per million users, compared with a rate of 0.2 per million users for paracetamol; thus, a nearly 30‐fold increased risk (Andrade 1998). The most common side effects of non‐selective NSAIDs are gastrointestinal. The incidence of these has been found to be twice as high in people receiving an NSAID for soft tissue injuries compared with a placebo (11% versus 5.5%); this equates to a number needed to treat for an additional harmful outcome (NNTH) of 19 (95% confidence interval (CI) 11 to 43; Jones 1998). NSAIDs can cause acute renal failure (Pérez Gutthann 1996), bronchospasm, hypersensitivity reactions (Amadio 1997; Brooks 1991), and psychological decompensation (Browning 1996). They have also been implicated in necrotising fasciitis, with excess risk in the first month of treatment (Rietveld 1995). Further information on adverse effects can be found in Appendix 1.
Other oral analgesic agents in common use are paracetamol (acetaminophen), and oral opioids. Opioids and paracetamol have no direct peripheral anti‐inflammatory effects. Opioids act centrally and peripherally on opioid receptors for their analgesic efficacy and other effects (Pathan 2012). The exact mechanism of paracetamol remains unclear, but likely involves a number of inter‐related central pain pathways, including prostaglandin, serotinergic, nitric oxide, and cannabinoid pathways (Sharma 2013). Biologically active, oral complementary and alternative medicines (CAM), such as glucosamine, have also been studied in the setting of musculoskeletal pain. Glucosamine may have inhibitory effects on cytokines involved in inflammation (Haghighat 2013).
Recently, increasing use of a subclass of NSAIDs, the selective cyclooxygenase isoenzyme type 2 (COX‐2) inhibitors, and the centrally acting, oral opioid analgesic tramadol, has renewed interest in the topic of this review, with the publication of several trials of oral analgesics in acute soft tissue injuries in the last few years (Dalton 2006; Diaz 2006a; Ekman 2002a; Ekman 2006; Hewitt 2007; Nadarajah 2006a; Petrella 2004a). While the selective COX‐2 inhibitors have fewer gastrointestinal side effects compared with non‐selective NSAIDs, this may be at the cost of more cardiovascular side effects, particularly with long‐term use. Little is known about the cardiovascular risk with the short‐term use of selective COX‐2 inhibitors for acute soft tissue injury (Burke 2006; Chan 2006; Farkouh 2004; Kearney 2006; Schnitzer 2004).
How the intervention might work
The pain and swelling that result from injury are mediated by an inflammatory process (Burke 2006). The rationale for using NSAIDs for acute soft tissue injury is that pain and swelling are due to inflammation, so NSAIDs will improve symptoms because they reduce inflammation (Baldwin 2003; Ivins 2006; Mehallo 2006). However, there are counter‐arguments to the concept that NSAIDs improve healing. The first is that in this setting, inflammation is integral to the healing process, and by reducing inflammation, healing may be impaired (Major 1992; Paoloni 2005). The second is that NSAIDs delay, but do not reduce, the inflammatory response to injury (Almekinders 1986; Jones 1999). This means the putative benefit of using an NSAID for acute soft tissue injuries may not be realised.
We describe the common side effects of NSAIDs in the previous section. Opioids are known to cause sedation, respiratory depression, nausea, vomiting, constipation, diuresis, and dysphoria, depending on which opioid receptors are most stimulated (Pathan 2012). Paracetamol in prescribed doses has not been shown to have more adverse events than placebo, when used for up to three months for osteoarthritis, other than elevation of liver function tests in approximately five per cent of people (Leopoldino 2019). This is consistent with the known hepatoxicity of paracetamol when taken in overdose (Park 2015).
Why it is important to do this review
This is an update of a Cochrane Review first published in 2015 (Jones 2015). Prior to 2015, narrative reviews reached different conclusions; some recommended NSAIDs for acute soft tissue injuries (Baldwin 2003; Ivins 2006; Mehallo 2006); while others argued that they may be harmful (Jones 1999; Major 1992; Paoloni 2005). Some reviews found the evidence inconclusive (Gøtzsche 2000; Hertel 1997). Contributing to this uncertainty were conflicting reports of the effect of NSAIDs on inflammation in both animal and human models (Almekinders 1986; Almekinders 1995; Bogatov 2003; Obremsky 1994; Rahusen 2004), and a paucity of evidence that NSAIDs are superior to other analgesics in clinical studies (De Gara 1982a; Yates 1984a). These initial reviews were criticised on the basis of the poor quality of included studies, non‐systematic methods (CRD 2007), and variable outcome reporting that hindered meta‐analysis (Ogilvie‐Harris 1995). Athough we successfully addressed some of these concerns, the previous version of this review still found a paucity of available evidence, and imprecise results for some outcomes. There is recent concern that oral opioid prescription in the acute setting is increasing, and is associated with the development of opioid dependence (Barnett 2017). With the publication of new studies of oral analgesic agents for acute soft tissue injuries, it was timely to update this systematic review of NSAIDs compared with other analgesics for these injuries.
Objectives
To assess the effects (benefits and harms) of oral non‐steroidal anti‐inflammatory drugs (NSAIDs) compared with other oral analgesics for treating acute soft tissue injuries.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials (RCTs) and quasi‐randomised (method of allocating participants to a treatment that is not strictly random, e.g. by date of birth, hospital record number, alternation) controlled trials comparing an oral non‐steroidal anti‐inflammatory drug (NSAID) with a different class of oral analgesic agent for the treatment of acute soft tissue injuries. We excluded cross‐over trials, which are inappropriate for short‐term conditions, and cluster‐randomised trials.
Types of participants
We included participants with an acute soft tissue injury. We defined this as follows:
soft tissue injury = sprain, strain, or contusion (haematoma) of a joint, ligament, tendon, or muscle; and
acute = injury occurring < 48 hours prior to inclusion in the study. We included studies with a clear majority of participants meeting this criterion (≥ 70%).
We had no restrictions based on age, sex, ethnicity, or study site.
We excluded studies if they focused on back pain, cervical spine injury, repetitive strain injuries, delayed onset muscle soreness, or primary inflammatory conditions (such as tendonitis or arthritis), as these conditions have either a different natural history, or reflect a different disease process.
Types of interventions
We considered oral analgesic agents commonly prescribed for treating acute soft tissue injuries, grouped by their local anti‐inflammatory effects.
We considered studies in which the intervention was to be completed within one month (30 days) of the injury, as by this time, most of the uncomplicated acute soft tissue injuries should have healed (Almekinders 1986; McClellan 2006). We included studies of oral NSAIDs versus oral comparators.
The groups for comparison were:
NSAID versus paracetamol (acetaminophen);
NSAID versus opioid;
NSAID versus combination analgesics (see below); and
NSAID versus complementary and alternative medicine; we planned to group these according to biological activity (Koithan 2009).
There are many combination analgesics containing different analgesics, with or without other agents (ANZCA 2005; Bandolier 2005). We grouped these analgesics according to anti‐inflammatory and opiate constituents if they were sufficiently similar (NSAID and opioid; NSAID and paracetamol; paracetamol and opioid; McNicol 2005). We only included comparisons of NSAID versus the paracetamol and opioid combination.
We excluded studies comparing COX‐2 selective NSAIDs versus non‐selective NSAIDs.
Types of outcome measures
When treating acute soft tissue injuries, pain, swelling, functional improvement, and adverse effects are of particular interest (Kellett 1986; Paoloni 2005; Weiler 1992). See Measures of treatment effect for further consideration on outcomes, including timing. More details of the measures listed below are provided in Measures of treatment effect.
We did not seek economic data for this review.
Primary outcomes
Pain
Our primary outcome measure was pain. Owing to its subjective nature, there is no standard method for reporting pain (IASP 2007). Consequently, different authors recorded pain in different ways, generally using categorical or visual analogue scales, and at different time points (Honig 1988).
Secondary outcomes
Swelling
We sought data for both subjectively reported and objectively measured swelling, which is considered a surrogate marker of inflammation. We collected both categorical and continuous data.
Function
We sought data for self‐reported assessment of function, functional impairment, and the proportion of people who had returned to function at prespecified time points.
Adverse effects
Potential adverse events of NSAIDs and other oral analgesics include gastrointestinal tract upset, renal disease, cardiovascular events, central nervous system side effects, respiratory depression, haematological abnormalities, skin photosensitivity, allergic reactions (rashes, throat swelling, wheeze, stridor), and necrotising fasciitis or soft tissue infections. We classified these events as serious if they led to death or admission to hospital (or the review authors thought it likely to lead to admission, if not stated in the report); required invasive intervention or monitoring (endoscopy, intermittent positive pressure ventilation, intramuscular or intravenous adrenalin); or needed resuscitation with crystalloid, colloid, or blood transfusion. We classed other adverse effects as minor.
Gastrointestinal adverse events were nausea, vomiting, dyspepsia, abdominal pain, peptic ulcer disease, gastrointestinal bleeding, hepatic dysfunction, diarrhoea, constipation, and other, if reported.
Neurological adverse effects were drowsiness or somnolence, dizziness or vertigo, headache, paraesthesia, seizure, and other, if reported.
Early re‐injury
We sought data on the recurrence of injury within three months, and time to re‐injury.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2020 Issue 1) in the Cochrane Library (searched 29 January 2020); MEDLINE Ovid (Medline, Epub Ahead of Print, In‐process & Other non‐indexed citations, Daily and Versions; 1946 to 28 January 2020); Embase (1980 to 29 January 2020); Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1937 to 12 February 2019); Allied and Complementary Medicine (AMED; 1985 to 12 February 2019); International Pharmaceutical Abstracts (1970 to 12 February 2019); the Physiotherapy Evidence Database (PEDro; 1929 to 11 March 2019); and SPORTDiscus (1985 to 12 February 2019). The initial search was run in February 2019 for all databases, and a top‐up search was run in January 2020 in the three main databases: CENTRAL, MEDLINE, and Embase. We did not place any restrictions based on language.
At the time of the search, CENTRAL was fully up‐to‐date with all records from the Bone Joint Muscle Trauma (BJMT) Group’s Specialised Register, and so it was not necessary to search this separately. For this update, we limited the searches were limited from the date of the previous searches to present: 2014 for CENTRAL, MEDLINE, and Embase, and 2012 for CINAHL, AMED, International Pharmaceutical Abstracts, and SPORTDiscus. Details of the search strategies used for the previous review are given in Jones 2015.
We also searched trial registries, ClinicalTrials.gov (19 February 2019), and the World Health Organization International Clinical Trials Registry platform (WHO ICTRP; 18 February 2019), for ongoing and recently completed trials.
In MEDLINE, we combined the subject‐specific strategy with the sensitivity‐maximising version of the Cochrane highly sensitive search strategy for identifying randomised trials (Lefebvre 2011). See Appendix 2 for details of all search strategies.
Searching other resources
We handsearched the reference lists of retrieved articles. We contacted authors of retrieved studies to obtain relevant unpublished data, such as summary statistics if the published report did not contain these, or to ascertain whether a potentially relevant trial met the review inclusion criteria when this was unclear. We also contacted experts in the field and pharmaceutical companies to identify unpublished trials.
Data collection and analysis
Selection of studies
Two review authors, who were not blinded to trial authors or results, independently assessed studies for eligibility. They resolved any disagreement by discussion. Where necessary, we attempted to contact authors for further information. We saved details of searches (database, host, years covered, date and results) and present them in Appendix 2.
Data extraction and management
Using a piloted form, two review authors independently extracted data for the listed outcomes. They resolved discrepancies by consensus, or adjudication by a third review author. Where necessary, we contacted trialists for additional and missing data.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias in the included studies using the 'Risk of bias' tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We graded each study's potential bias in each of the following domains: sequence generation, allocation concealment, blinding (treatment providers, participants, outcome assessors), incomplete outcome data (pain, swelling, function, adverse effects), selective outcome reporting, and 'other'. For each study, we described the domains as reported (or after discussion with the trial authors), and judged their risk of bias. Our judgements were 'low', 'unclear' or 'high' risk of bias. We judged bias as 'unclear' if there was insufficient detail to make a judgement. The two review authors resolved disagreements regarding the risk of bias for domains by consensus.
Measures of treatment effect
Pain, swelling, and return to function are time dependent, as are the effects of the interventions (medicines with different times of onset and duration of effect). Therefore, we analysed these outcomes at different time intervals from the onset of treatment, based on the pathophysiology of acute soft tissue injury, and pharmacology of interventions discussed above, to minimise the 'effect modification' of time on pain and swelling (Glasziou 2002). If a trial did not report a relevant outcome at one of the specified time intervals, we did not include data from that study in the meta‐analysis. We used 95% confidence intervals (CI) throughout.
Primary outcome
Pain
Some trials reported pain on a continuous scale, others used a categorical scale, and some used both. We analysed the meta‐analysis of continuous and categorical pain outcomes separately.
Continuous data
For acute pain, a standard linear 10‐cm visual analogue scale (VAS‐10) has been shown to be a valid measurement tool, regardless of the severity of pain (Myles 1999; Myles 2005). In comparison, chronic pain has been shown to be non‐linear, possibly due to changes in the pain experience over time (Lund 2005; Quiding 1983; Svensson 2000; Williams 2000). The minimum clinically important difference (MCID) in acute pain scores using a 100‐mm VAS scale is 13 mm, regardless of age and baseline pain severity, equivalent to a one‐point change on a five‐point categorical scale (Barden 2004; Bijur 2003; Falgarone 2005; Fosnocht 2005; Gallagher 2001; Gallagher 2002; Kelly 1998; Kelly 2001; Lee 2003; Powell 2001; Salo 2003). However, a more clinically meaningful change for people is 30 mm (Bergh 2001; Farrar 2003; Jensen 2005; Lee 2003).
VAS‐10 scores are skewed. The skew shifts with time as pain subsides (Geraci 2007; Rosen 2000). This may invalidate summarising mean data from VAS‐10 scores using parametric methods, and there are currently no tools available to pool data using medians (Altman 2000; Geraci 2007; Quiding 1983). However, according to the Central Limit Theorem, the distribution of means of samples of a skewed distribution, will approximate normal for sample sizes over 15 (Kirkwood 2003). This has proven to be robust in computer simulations (Dexter 1995; Philip 1990).
We used mean differences (95% CI) to summarise VAS‐10 scores across studies. We carried out all analyses on an intention‐to‐treat basis.
We derived dichotomous outcomes from VAS plots over time, a method developed to get around the issue of skew in single dose, postoperative pain studies (Moore 1996; Moore 1997). However, we considered this inappropriate for longer trials (Moore 2007), and now also consider it a poor reflection of the truth, even for single dose short‐term trials (Barden 2004).
Categorical data
For studies reporting analgesic effect using a categorical scale, we collapsed data into the proportion of participants experiencing 'good' or 'complete' pain relief, where possible. This method has previously been recommended to compare analgesics using five‐point scales (Moore 2005), as it facilitates analysis and interpretation, albeit at the cost of some lost information (Altman 2000; Cochrane 2002).
Similarly, if studies used different categorical scales, we collapsed them according to the following schedule.
3‐point: lowest two categories 'no pain relief', and one highest 'good pain relief'
4‐point: lowest three categories 'no pain relief', and one highest 'good pain relief'
5‐point: lowest three categories 'no pain relief', and two highest 'good pain relief'
6‐point: lowest four categories 'no pain relief', and two highest 'good pain relief'
7‐point: lowest four categories 'no pain relief', and three highest 'good pain relief'
8‐point: lowest five categories 'no pain relief', and three highest 'good pain relief'.
9‐point: lowest five categories 'no pain relief', and four highest 'good pain relief'.
10‐point: lowest six categories 'no pain relief', and four highest 'good pain relief'.
For all dichotomised data, we reported risk ratios (RR, 95% CI). We analysed outcomes on an intention‐to‐treat basis. For acute soft tissue injuries pain, RR is appropriate to report as event rates are high (typically > 50%) in this setting, and using odds ratios (OR) may lead to overestimation of the differences between interventions (Cukiernik 2007; Diaz 2006a).
We recognise that there are limitations of using RRs, which vary, depending on which intervention is chosen as the 'control', and are bounded by the event rate (Deeks 2001). Reflecting this lack of a standard approach (Deeks 2002), previous review authors have reported either OR or RR (Bandolier 2007; Manterola 2007; Wiffen 2005). Another alternative, risk difference (RD), depends on baseline risk, and is unlikely to be consistent between trials (Deeks 2001). We performed a sensitivity analysis for our results with RR by repeating the analysis with both OR and RD, checking for consistency, variance, and ease of interpretation (Deeks 2001; Deeks 2002).
Where trials reported categorical data as a mean with a standard deviation (SD), we only included studies with scales of 10 points or more (Bijur 2003; Herbison 2008).
We analysed pain at the following time points.
First 24 hours
Days one to three (the time of maximum pain related to acute injury (Jones 1998))
Days four to six (if reported (Jones 1998))
Days seven or more (pain expected to be minimal (Jones 1998), and analgesics often stopped (Kellett 1986))
Secondary outcomes
Swelling
We intended to combine trials that reported swelling using an objective measure, such as water displacement in mL, or circumference in cm (mean with SD given or calculable), in a meta‐analysis using the standardised mean difference (SMD, 95% CI). If trials reported subjective reduction in swelling, we treated this as dichotomised data.
We aimed to assess swelling at the following time points.
Day zero to three (bleeding due to initial tissue trauma)
Days four to six (maximum inflammatory response – data from animal studies)
Day seven or more (resolution of swelling in most cases)
Function
Where available, we presented data from self‐reported assessment of function and activities of daily living. However, the included trials usually reported function as 'Time to return of function' (from the time of injury to the time to return to full activity (work or sports)) and 'Functional impairment'. We dichotomised functional impairment on categorical scales, considering none or slightly clinically successful, and reported risk ratios (95% CI). If measured on a VAS, we calculated mean differences (95% CI). We took a difference of 15 mm to represent a clinically significant difference. We assessed 'Time to return to function' where possible, as proportions of people who had returned to function at the prespecified time intervals below.
Up to day seven
Days 7 to 14
After day 14
Adverse effects
We tabulated the presence or absence of major and minor adverse outcomes (described above) that occurred any time within three months (90 days) of the start of the study. We calculated risk ratios (95% CI).
Re‐injury
We intended to calculate risk ratios (95% CI) for the proportion of participants who reported that they had a recurrence of the index injury within three months. We planned to assess time to re‐injury, where possible, as the proportions of people who had re‐injured within the prespecified time intervals (up to day 15, days 15 to 30, after day 30); however, we found that included studies, or those retrieved for full text review, reported this outcome.
Unit of analysis issues
We did not include cluster‐randomised or cross‐over trials, a decision that minimised the unit of analyses issues in this review. We stratified analysis by different time points, to avoid the effect modification of time with respect to the outcomes measured. Some studies reported adverse effects at the event level rather than the participant level (some participants may have had more than one adverse event in the same system). We included data at the participant level, rather than the event level, in the analysis.
Multiple interventions
For trials investigating multiple interventions (for example, NSAID 1 versus NSAID 2 versus other analgesic), we combined the groups for comparison into a single pair‐wise comparison for the meta‐analysis (thus, NSAID 1 and NSAID 2 versus other analgesic), as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Dealing with missing data
At the study level, we tried to ensure that we found all relevant studies by using a comprehensive search strategy. From study authors, we sought outcome data that studies measured but did not report. Where possible, we calculated missing standard deviations from other data, such as standard errors, exact P values, and 95% confidence intervals, when presented in the trial reports. Had we imputed data from other sources, we intended to perform a sensitivity analysis, by calculating the treatment effect including and excluding the imputed data, to see whether this would alter the outcome of the analysis.
Where studies reported adverse events at the event level for the broad categories of gastrointestinal and neurological adverse events, we used participant‐level data for the most common adverse event within the broad categories in the analysis.
Assessment of heterogeneity
We displayed the results graphically, using forest plots with a summary statistic, in the absence of major clinical or statistical heterogeneity (lack of overlap of confidence intervals on the forest plots; Egger 2001; Egger 2001a). We assessed heterogeneity between trial results by examining the forest plots and calculating I² and Chi² statistics (Higgins 2003).
Assessment of reporting biases
We planned to assess reporting bias, using funnel plots when a single comparison included 10 or more studies (Higgins 2011b).
Data synthesis
We combined data using standard inverse variance methods and a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analysis where there was disparity in the dosing of the drugs under comparison (e.g. one drug was given at standard dose and the other was given at less than standard dose):
insufficient dosing (less than maximum dose) of at least one comparator drug or relative dose discrepancy between comparators (i.e. one drug at low dose of therapeutic range and the other at maximal dose) versus
equivalent dosing of all comparator drugs (defined by national formulary of country of study, or British National Formulary, if this was not available).
Where there were sufficient numbers of trials, we undertook subgroup analysis by the analgesic comparator (paracetamol, opioid, paracetamol plus opioid).
We used the test for subgroup differences available in Review Manager 5 for the fixed‐effect model, to determine if the results for subgroups were conclusive (Review Manager 2014).
Where possible in future, we plan to undertake subgroup analyses based on NSAID categories (COX‐2 selective versus non‐selective NSAIDs), and age (< 18 years, 18 to 65 years, and > 65 years).
Sensitivity analysis
We undertook sensitivity analyses to explore the effects of different risks of bias associated with sequence generation (low or unclear versus high), allocation concealment (low or unclear versus high), and blinding (low versus unclear or high).
'Summary of findings' tables
We summarised the results for the three comparisons for which there were data in 'Summary of findings' tables: NSAIDs versus paracetamol, NSAIDs versus opioid, and NSAIDs versus paracetamol plus opioid. We used the GRADE approach to assess the certainty of evidence related to seven key outcomes for each comparisons (Schünemann 2011). We used GRADEpro GDT to create the 'Summary of findings' tables and imported them into Review Manager 5 (GRADEpro GDT).
The outcomes were pain at < 24 hours; pain at 1 to 3 days (or 4 to 6 days if not available); pain at day 7 or later; swelling at day 7 or later; return to function at day 7 or later; gastrointestinal adverse events; and neurological adverse events. Although no studies reported on early re‐injury, we retained this as a key outcome, despite this increasing the number of outcomes to eight.
Results
Description of studies
Results of the search
For this update, we screened a total of 4353 records from the following databases: CENTRAL (854), MEDLINE (518), Embase (675), CINAHL (125), AMED (44), SPORTDiscus (48), International Pharmaceutical Abstracts (51), PEDro (444), the WHO ICTRP (621), and ClinicalTrials.gov (973). We searched CENTRAL, MEDLINE, and Embase to January 2020, and the other databases to February 2019. Our searches of other resources (reference lists) identified no additional studies that appeared to meet the inclusion criteria.
Once duplicates had been removed, we had a total of 2419 records. Two studies changed status since the previous version of this review. The PanAM study, which was ongoing in 2015, was published as Ridderikhof 2018, and 'Graham 2012', previously awaiting classification, has been published as Hung 2018.
We included four new trials Fathi 2015; Hung 2018; Le May 2017; Ridderikhof 2018, in addition to the previously included 16 trials (Abbott 1980; Aghababian 1986; Beveridge 1985; Bondarsky 2013; Bourne 1980; Clark 2007; Cukiernik 2007; Dalton 2006; Ekman 2006; Indelicato 1986; Jaffé 1978; Kayali 2007; Lyrtzis 2011; Man 2004; McCulloch 1985; Woo 2005). We excluded another 50 studies (see Excluded studies), two studies are ongoing (see Ongoing studies), and two are awaiting classification (see Studies awaiting classification).
Figure 1 illustrates details of the process of screening and selecting studies for inclusion in the review (including the database search results from the previous publication; Jones 2015).
1.
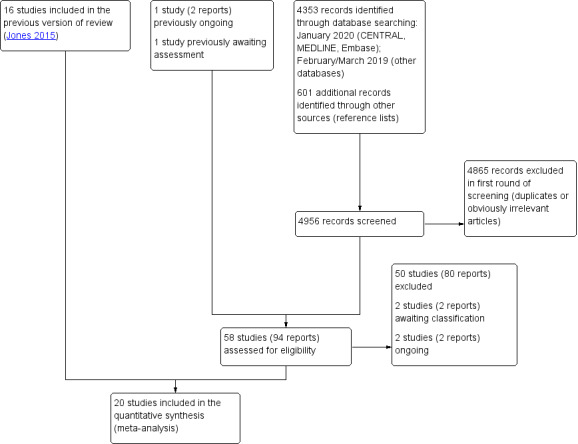
Study flow diagram for update
Results of contacting authors
We attempted to contact trialists when we needed clarification on study eligibility criteria for the review, or published data were insufficient to include in the quantitative analysis. We considered the published data sufficient for inclusion in just one included study (Jaffé 1978). We were unable to find current contact details for four included studies (Abbott 1980; Aghababian 1986; Beveridge 1985; Indelicato 1986), and two excluded studies (Buccelletti 2014; De Gara 1982). We received no reply from authors of six included studies (Bourne 1980; Dalton 2006; Fathi 2015; Kayali 2007; Lyrtzis 2011; Man 2004). We received replies from authors of eight included studies, five of whom provided the requested data (Bondarsky 2013; Cukiernik 2007; Clark 2007; Le May 2017; Ridderikhof 2018), and three who reported that the study data were no longer available (Hung 2018; McCulloch 1985; Woo 2005). Notably, we received adverse events data from Clark 2007 subsequent to the finalisation of the 2015 version of this review; we added these data to the current version (2020). We received replies from authors of two excluded studies, Le May 2013 provided data, while Yates 1984 reported that the study data were no longer available. We had contacted one pharmaceutical company previously, but they did not provide data relevant to this review (Ekman 2006).
Included studies
The 20 trials included a total of 3305 participants, 3287 for whom data were available for at least one outcome. For each trial, we present a summary of the condition, comparison, number randomised, number analysed for the outcome 'pain', and the number included in at least one outcome Table 4. Participants of seven trials had acute ankle sprains, and those of Jaffé 1978 had either ankle or wrist sprains. The participants of the other 12 trials were being treated for a variety of conditions; these were either solely or mainly soft tissue injuries. In all except one study, it was clear or likely that the majority of participants had an acute soft tissue injury. Aghababian 1986 did not state this explicitly, and Fathi 2015 did not specify that 'acute' was < 48 hours; however, as the setting was an emergency department in both studies, we considered it most likely that this was the case. We provide a full description of individual studies in the Characteristics of included studies table.
1. Summary of key characteristics of the included trials.
| Study ID | Condition^ | Comparison | No. randomised | No. in analyses (pain) | No. in analyses (at least 1 outcome) |
| Abbott 1980 | Acute soft tissue injury (76%) | NSAID vs combined* | 98 | 98 | 98 |
| Aghababian 1986 | Ankle sprain | NSAID vs combined* | 40 | 40 | 40 |
| Beveridge 1985 | Acute soft tissue injury | NSAID vs opioid | 68 | 0 | 63 |
| Bondarsky 2013 | Acute soft tissue injury (70%) | NSAID vs paracetamol | 60 | 60 | 60 |
| Bourne 1980 | Acute soft tissue injury | NSAID vs paracetamol | 60 | 0 | 55 |
| Clark 2007 | Children: Acute soft tissue injury | NSAID vs paracetamol NSAID vs opioid | 72 68 |
72 68 |
72 68 |
| Cukiernik 2007 | Children: ankle sprain | NSAID vs paracetamol | 80 | 76 | 77 |
| Dalton 2006 | Ankle sprain | NSAID vs paracetamol | 260 | 204 | 260 |
| Ekman 2006 | Ankle sprain | NSAID vs opioid | 706 | 706 | 706 |
| Fathi 2015 | Acute soft tissue injury (>80%) | NSAID vs opioid | 150 | 150 | 150 |
| Hung 2018 | Acute soft tissue injury (86%) | NSAID vs paracetamol | 521 | 453 | 519 |
| Indelicato 1986 | Acute soft tissue injury (and back pain) | NSAID vs combined* | 50 | 0 | 50 |
| Jaffé 1978 | Ankle/wrist sprain | NSAID vs combined* | 52 | 51 | 51 |
| Kayali 2007 | Ankle sprain | NSAID vs paracetamol | 100 | 100 | 100 |
| Le May 2017 | Children: Acute soft tissue injury | NSAID vs opioid | 134 | 134 | 134 |
| Lyrtzis 2011 | Ankle sprain | NSAID vs paracetamol | 90 | 86 | 90 |
| Man 2004 | Acute soft tissue injury (92%) | NSAID vs paracetamol | 39 | 39 | 39 |
| McCulloch 1985 | Ankle sprain | NSAID vs opioid | 86 | 0 | 84 |
| Ridderikhof 2018 | Acute soft tissue injury (96%) | NSAID vs paracetamol | 365 | 365 | 365 |
| Woo 2005 | Acute soft tissue injury (82%) | NSAID vs paracetamol | 206 | 206 | 206 |
| TOTAL | 8 = ankle (+ 1 wrist) sprain. Others included any acute soft tissue injury |
11 'vs paracetamol' 6 'vs opioid' 4 'vs combined' | 3368 | 2908 | 3287 |
^notes in brackets reflect trials in which a percentage of the population met the review inclusion criteria *combined = paracetamol plus opioid vs = versus
Five studies had three trial groups (Bondarsky 2013; Clark 2007; Hung 2018; Le May 2017; Ridderikhof 2018). The third group in three studies, Bondarsky 2013, Hung 2018, and Ridderikhof 2018, used a combination intervention of NSAID plus paracetamol, and Le May 2017 used a combination of NSAID plus opioid; we excluded these four groups from the review. Clark 2007 compared ibuprofen versus paracetamol versus codeine.
Ekman 2006,Man 2004, and Woo 2005 had four treatment groups; valdecoxib twice daily, valdecoxib once daily, tramadol, and placebo in Ekman 2006; and indomethacin, diclofenac, paracetamol, and a combination of diclofenac plus paracetamol in Man 2004 and Woo 2005. We merged data from the first two NSAID groups in the analyses for all three trials, and excluded the fourth group, either placebo or a combination of NSAID plus paracetamol, from all three trials.
We grouped the following description of studies by the comparisons listed in Types of interventions. There were no trials comparing NSAID versus complementary and alternative medicine. Note that Clark 2007 features in two comparisons: NSAID versus paracetamol, and NSAID versus opioid.
NSAID versus paracetamol
Eleven studies compared NSAID with paracetamol (Bondarsky 2013; Bourne 1980; Clark 2007; Cukiernik 2007; Dalton 2006; Hung 2018; Kayali 2007; Lyrtzis 2011; Man 2004; Ridderikhof 2018; Woo 2005). Data were available for analysis of at least one outcome for 1843 out of 1853 participants; Table 4.
One study received no funding (Woo 2005); two studies were sponsored by pharmaceutical companies (Bourne 1980; Dalton 2006); four studies were funded through competitive public good research grants (Clark 2007; Cukiernik 2007; Hung 2018; Ridderikhof 2018); and four studies did not state the source of funding (Bondarsky 2013; Kayali 2007; Lyrtzis 2011; Man 2004). An author of one study was an employee of a pharmaceutical company (Dalton 2006); the authors of five studies declared no relevant interests (Clark 2007; Hung 2018; Lyrtzis 2011; Man 2004; Ridderikhof 2018); and five studies made no statement of declaration of interests (Bondarsky 2013; Bourne 1980; Cukiernik 2007; Kayali 2007; Woo 2005).
Four studies, with 530 participants, studied exclusively ankle sprain (Cukiernik 2007; Dalton 2006; Kayali 2007; Lyrtzis 2011). The other seven studies included a mix of participants with mainly lower and upper extremity soft tissue injuries (Bondarsky 2013; Bourne 1980; Clark 2007; Hung 2018; Man 2004; Ridderikhof 2018; Woo 2005). Due to variable reporting in the studies, it was not possible to account for the exact numbers of participants with specific injuries in these studies; these included at least 77 participants with back or neck injuries, 66 with lacerations, and 70 with minor fractures (which were initially thought to be soft tissue injuries); injuries that were outside the criteria for the review, but whose data we were unable to disaggregate for analysis. Thus, we included the data from these participants (approximately 11%) in the review.
All studies reported the gender of the enrolled participants; 60% of participants were male. Participants of two studies (N = 152) were exclusively children aged 6 to 17 years (Clark 2007), and 8 to 14 years (Cukiernik 2007), with the remaining nine studies conducted exclusively in adults over 16 years of age. Two studies (N = 320), in which 80% of participants were white, reported ethnicity (Bondarsky 2013; Dalton 2006).
The studies took place in Canada (Clark 2007; Cukiernik 2007); Greece (Lyrtzis 2011); Hong Kong (Hung 2018; Man 2004; Woo 2005); Turkey (Kayali 2007); the United Kingdom (Bourne 1980); the Netherlands (Ridderikhof 2018); and the USA (Bondarsky 2013; Dalton 2006). The studies were carried out in a variety of locations, including general practice, emergency departments, student health centres, research facilities, sports medicine clinics, orthopaedic clinics, urgent care facilities, and rheumatology clinics.
Five studies compared ibuprofen with paracetamol (Bondarsky 2013; Bourne 1980; Clark 2007; Dalton 2006; Hung 2018); one compared naproxen with paracetamol (Cukiernik 2007); three compared diclofenac with paracetamol (Kayali 2007; Lyrtzis 2011; Ridderikhof 2018); and two separate studies, by the same group, compared indomethacin and diclofenac separately with paracetamol (Man 2004; Woo 2005). The doses of the medications varied across the studies. Submaximal dosing of paracetamol occurred in three studies (Bourne 1980; Kayali 2007; Lyrtzis 2011); and submaximal dosing of NSAID was present in two studies (Man 2004; Woo 2005). In Hung 2018, the initial dose was optimal for the analysis in the emergency department, but subsequent daily dosing was suboptimal.
All but one study reported suitable data for pain (Bourne 1980); three studies provided suitable data about swelling (Dalton 2006; Kayali 2007; Lyrtzis 2011); two provided suitable data on function (Bourne 1980; Cukiernik 2007); and 10 studies provided suitable data on adverse events for the meta‐analysis (Bondarsky 2013; Bourne 1980; Cukiernik 2007; Dalton 2006; Hung 2018; Kayali 2007; Lyrtzis 2011; Man 2004; Ridderikhof 2018; Woo 2005).
NSAID versus opioid
Six studies compared NSAIDs with opioids (Beveridge 1985; Clark 2007; Ekman 2006; Fathi 2015; Le May 2017; McCulloch 1985). Data were available for analysis for at least one outcome for 1205 out of 1212 participants (Table 4).
One study received no funding (Fathi 2015); one was funded by a pharmaceutical company (Ekman 2006); two were funded through competitive public good research grants (Clark 2007; Le May 2017); and two studies did not state the source of funding (Beveridge 1985; McCulloch 1985). An author of one study was an employee of a pharmaceutical company (Ekman 2006); the authors of three studies stated no relevant interests (Clark 2007; Fathi 2015; Le May 2017); and two studies made no statement of declarations of interest (Beveridge 1985; McCulloch 1985).
Two studies, with 792 participants, exclusively considered ankle sprain (Ekman 2006; McCulloch 1985). Beveridge 1985 included participants with a mixture of lower extremity (53 participants) and 'other' sites (10 participants) of soft tissue injury. Fathi 2015 included 150 participants with a mix of injury types, including 26 with lumbosacral or intervertebral disc problems. Clark 2007 did not specify the site or type of injury. The study authors of Le May 2017, which included approximately 40% fractures, provided data to us on the 134 participants with soft tissue injuries in multiple sites separately for inclusion in this review.
Five of the studies reported the gender of participants. Slightly under 60% were male (Beveridge 1985; Clark 2007; Ekman 2006; Fathi 2015; Le May 2017). McCulloch 1985 reported no difference in the ratio of male and female, but provided no data. Two of the studies (N = 202) randomised exclusively children aged 6 to 17 years (mean age of 12 years; Clark 2007; Le May 2017), one of which only enrolled participants during the approximately 30 hours per week when research staff were available (Le May 2017). One study enrolled participants aged between 16 and 64 years, with a mean age of 29 years (Ekman 2006); one enrolled participants aged between 18 and 45 years (Beveridge 1985); and one study enrolled participants older than 18 years, with no upper age limit (Fathi 2015). One did not state an age restriction; the mean age of those enrolled in this study was 32 years (McCulloch 1985). Only Ekman 2006 reported ethnicity of participants; 80% were white, 8% black, 3% Asian, and 9% other.
Three of the studies were single‐centre emergency department studies: one in the United Kingdom (McCulloch 1985), one in Canada (Clark 2007), and one in Iran (Fathi 2015). Another Canadian study was a multicentre emergency department study (Le May 2017). The study by Beveridge 1985 took place at a football club in the United Kingdom. Ekman 2006 was a multicentre study with 14 European and 73 American sites; it did not state whether these were hospital‐based, emergency or orthopaedic departments, or primary care facilities.
Three studies compared naproxen with dextropropoxyphene (Beveridge 1985), dihydrocodeine (Fathi 2015), and oxycodone (McCulloch 1985). McCulloch 1985 reported a four‐arm factorial trial, simultaneously comparing plaster immobilisation to Tubigrip™ bandage, as well as NSAID versus opioid. Clark 2007 compared ibuprofen with codeine phosphate, and Le May 2017 compared ibuprofen to morphine. Ekman 2006 compared two doses of valdecoxib (selective COX‐2 inhibitor) separately with tramadol. Submaximal dosing of tramadol was present in one study (Ekman 2006).
Four studies reported data sufficiently to be pooled for the outcome of pain (Clark 2007; Ekman 2006; Fathi 2015; Le May 2017), one reported swelling (McCulloch 1985), two reported on function (Beveridge 1985; Ekman 2006), and four reported adverse events (Beveridge 1985; Ekman 2006; Fathi 2015; Le May 2017).
NSAID versus combination analgesics (combination of paracetamol and opioid)
Four studies compared NSAIDs with combined analgesics (paracetamol and an opioid; Abbott 1980; Aghababian 1986; Indelicato 1986; Jaffé 1978). Data were available for analysis for at least one outcome for 239 out of 240 participants (Table 4).
Two studies were funded by pharmaceutical companies (Aghababian 1986; Indelicato 1986); two did not state the source of funding (Abbott 1980; Jaffé 1978). An author of one study was an employee of a pharmaceutical company (Jaffé 1978); the other three studies made no statement of declarations of interest.Abbott 1980; Aghababian 1986; Indelicato 1986).
Aghababian 1986 studied only ankle sprains; Jaffé 1978studied ankle or wrist injuries; and there was a mix of injuries in the remaining two studies (Abbott 1980; Indelicato 1986). In total, 25 participants had ankle injuries; 25 had other lower extremity injuries; 37 had upper extremity injuries; and for 12 participants, the site was not specified. Some participants in two studies had back injuries or inflammatory conditions that were outside the criteria for the review (Abbott 1980; Indelicato 1986). Since separate outcome data for eligible participants were not available, we included the data from these participants (approximately 15% of study populations) in the review (seeDifferences between protocol and review).
All studies referred to the gender of the enrolled participants: 72% were male. The age range of participants was 16 years to 66 years. No study reported ethnicity data.
The studies took place in the UK (Abbott 1980; Jaffé 1978), and the USA (Aghababian 1986; Indelicato 1986). The studies were carried out in a variety of centres, including general practice, emergency departments, armed forces medical centres, and university sports clinics.
Two studies compared NSAIDs with a combination of paracetamol and dextropropoxyphene. The NSAID was diflunisal in Jaffé 1978, and naproxen in Abbott 1980. The other two studies compared a single NSAID (diflunisal) versus a paracetamol and codeine combination (Aghababian 1986; Indelicato 1986). The doses of the medications varied across the studies. All studies used combination analgesics that contained submaximal doses of paracetamol.
We included three of the four studies in the pain analyses (Abbott 1980; Aghababian 1986; Jaffé 1978). Data from Indelicato 1986 were unavailable for analysis because of the way in which the results were reported (for example, no standard deviations were reported for continuous outcomes, and we were unable to obtain separate data for acute soft tissue injuries). For similar reasons, we included data from Abbott 1980 only in the analyses for swelling and function. We pooled data on adverse events from all four studies.
Excluded studies
We grouped the 50 excluded studies initially by comparison, then by condition studied, and trial design. We provide more details of the reasons for excluding the studies in the 'Characteristics of excluded studies' table.
NSAID versus paracetamol
Five mixed population studies reported insufficiently separate data on relevant participants (Buccelletti 2014; De Gara 1982; Moore 1999; Patel 1993; Yates 1984). We attempted to contact study authors for additional information. One author replied (Yates 1984); however, the original study data were no longer available. One study, Yilmaz 2019, reported intravenous dosing of NSAIDs and paracetamol; a route that was not specified for this review .
NSAID versus opioid
One study reported insufficiently separate data for participants with relevant injuries (Pagliara 1997); the authors did not respond to a request for data. One study enrolled the majority of participants after 48 hours of injury (Goswick 1983).
NSAID versus combination analgesics (combination of paracetamol and opioid)
One study was not randomised (Stableforth 1977); we were unable to disaggregate data for participants with relevant injuries in the other six studies (Buccelletti 2014; Hardo 1982; Muncie 1986; Sherry 1988; Simmons 1982; Sleet 1980). The study authors did not respond to requests for more data.
NSAID plus other analgesic versus NSAID alone
Four studies (Kolodny 1975; Le May 2013; Turturro 2003; Yazdanpanah 2011), and one ongoing study (NCT03025113) compared a combination of NSAID plus another oral analgesic agent with NSAID alone; Kolodny 1975 was also not randomised.
NSAID plus other analgesic versus combination analgesics
We excluded two studies (Chang 2017; Graudins 2016), and three ongoing studies that compared NSAIDs in combination with another analgesic agent with different combinations of analgesic agents (NCT02862977; NCT03173456; NCT03767933).
COX‐2 selective NSAID versus non‐selective NSAID
We excluded 10 studies comparing a COX‐2 selective NSAID with a non‐selective NSAID, because they did not compare an NSAID versus another oral analgesic agent (Cardenas‐Estrada 2009; Cauchioli 1994; D'Hooghe 1992; Diaz 2006; Ekman 2002; Ferreira 1992; Jenoure 1998; Nadarajah 2006; Petrella 2004; Pfizer 2005). We excluded a further six studies considering this comparison for additional reasons (Calligaris 1993; Costa 1995; Dougados 2007; Jenner 1987; Kyle 2008; NCT00954785).
Placebo and other comparisons
We excluded two studies that compared NSAID with placebo (Andersson 1983; Jorgensen 1986), and one non‐randomised study that compared a biologically active CAM with placebo (Feragalli 2017). One other study compared an opioid with another non‐NSAID analgesic; this study was also not randomised (Khoury 2018).
Wrong condition
Two ongoing studies were excluded as they are not recruiting people with acute soft tissue injuries (NCT01974609; NCT02373254).
Not RCT
Five other studies were excluded as they were not randomised controlled trials (Collopy 2012; Gyer 2012; Jenner 1987; van den Bekerom 2016; Whitehead 2016)
Ongoing studies
We identified two ongoing studies comparing NSAID with paracetamol (NCT02667730; NCT03222518); see Characteristics of ongoing studies for further information.
Studies awaiting classification
One trial comparing ibuprofen to another medication may have been completed in 2010, but we do not know the class of the comparator medication. Only the trial registration is available for this study (CTRI/2009/091/001067). One trial that has not yet started recruiting plans to compare diclofenac with a plant extract for pain and adverse effects when treating acute muscle strain (TCTR20160126001); seeCharacteristics of studies awaiting classification.
Risk of bias in included studies
Figure 2 and Figure 3 summarise the risk of bias for the included studies.
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
3.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Allocation
All 20 studies were randomised, although only eleven described an adequate method of sequence generation (Bondarsky 2013; Clark 2007; Cukiernik 2007; Ekman 2006; Fathi 2015; Hung 2018; Le May 2017; Lyrtzis 2011; Man 2004; Ridderikhof 2018; Woo 2005). Eight studies did not state the method of sequence generation, so the risk of bias was unclear (Abbott 1980; Aghababian 1986; Beveridge 1985; Dalton 2006; Indelicato 1986; Jaffé 1978; Kayali 2007; McCulloch 1985). Bourne 1980 did not describe the method of sequence generation. We judged it at high risk of bias as "an attempt was made to pair the patients for site and type of injury"; and thus, it may have been a quasi‐randomised study.
Eight studies reported adequate allocation concealment, with the use of sealed, opaque, or unmarked envelopes, or identical packaging (Abbott 1980; Bondarsky 2013; Clark 2007; Cukiernik 2007; Ekman 2006; Hung 2018; Le May 2017; Ridderikhof 2018). One study reported the use of envelopes, but did not provide further details (Woo 2005), and one used envelopes, but the study tablets were not identical (Fathi 2015). In these studies, and the eight studies that did not report the method of allocation concealment, we considered the risk of bias unclear (Aghababian 1986; Beveridge 1985; Dalton 2006; Jaffé 1978; Kayali 2007; Lyrtzis 2011; Man 2004; McCulloch 1985). Given the pairing of participants for site and type of injury, Bourne 1980 clearly did not conceal allocation; thus, we judged it to be at high risk of selection bias. The other study at high risk for selection bias was Indelicato 1986, as it was an open‐label study.
Blinding
Eleven studies had adequate blinding of outcome assessors, participants, and treatment providers, and we judged them at low risk of performance and detection bias (Bondarsky 2013; Clark 2007; Cukiernik 2007; Dalton 2006; Ekman 2006; Hung 2018; Jaffé 1978; Le May 2017; Man 2004; Ridderikhof 2018; Woo 2005). We judged one study at low risk of bias for blinding of treatment providers and outcome assessors, although not for participants, as it did not blind them (Abbott 1980). Two studies blinded only the participants (Beveridge 1985; Bourne 1980), and McCulloch 1985 only blinded the outcome assessors. Four studies did not state the method of blinding, and we considered these to be at unclear risk of bias (Aghababian 1986; Fathi 2015; Kayali 2007; Lyrtzis 2011). Indelicato 1986 was an open‐label design, and at high risk of bias for blinding.
Incomplete outcome data
We assessed attrition bias separately according to the specific outcomes specified in the protocol of the review. Fifteen studies were at low risk of attrition bias across all outcomes they measured (Abbott 1980; Aghababian 1986; Beveridge 1985; Bondarsky 2013; Bourne 1980; Clark 2007; Dalton 2006; Ekman 2006; Fathi 2015; Indelicato 1986; Jaffé 1978; Le May 2017; Man 2004; Ridderikhof 2018). Cukiernik 2007 was at low risk for three outcomes, but at unclear risk for swelling, because it did not present these data in a format that allowed accurate abstraction. Hung 2018 was at low risk for the outcomes of pain and adverse effects in the emergency department; unclear for function, as this was not reported; and high for adverse effects at one month, as less than 50% were followed to this point. Lyrtzis 2011 was at low risk for two outcomes, and unclear risk for adverse events, because of incomplete reporting of these. Kayali 2007 was at unclear risk of bias because of not reporting the follow‐up rate. One study was at high risk of bias because of a disproportionate and high dropout rate between the groups (McCulloch 1985).
Selective reporting
Fourteen studies were at low risk of reporting bias (Abbott 1980; Aghababian 1986; Beveridge 1985; Bondarsky 2013; Clark 2007; Cukiernik 2007; Fathi 2015; Jaffé 1978; Kayali 2007; Le May 2017; Man 2004; McCulloch 1985; Ridderikhof 2018; Woo 2005). Lyrtzis 2011 was at unclear risk because of the way it described adverse effects. We considered five studies to be at high risk, either for not reporting all prespecified outcomes at the prespecified time points (Bourne 1980; Hung 2018; Indelicato 1986), or for selectively reporting only a proportion of adverse events (Dalton 2006; Ekman 2006).
Other potential sources of bias
We judged that the most likely other source of bias would be performance bias, reflecting imbalance between intervention groups in the use of concomitant physical (rest, ice, compression, elevation, splintage), or pharmacological therapies during the studies. We considered seven studies at low risk of other bias (Bourne 1980; Clark 2007; Cukiernik 2007; Dalton 2006; Ekman 2006; Lyrtzis 2011; Ridderikhof 2018).
Reflecting either no or incomplete accounts of treatment other than the interventions, we judged 12 studies to be at unclear risk of other bias (Abbott 1980; Aghababian 1986; Bondarsky 2013; Fathi 2015; Hung 2018; Indelicato 1986; Jaffé 1978; Kayali 2007; Le May 2017; Man 2004; McCulloch 1985; Woo 2005). We considered Beveridge 1985 to be at high risk of other bias because of the imbalance in the use of rehabilitation therapy (exercises) between the intervention groups.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. NSAID compared with paracetamol for acute soft tissue injury.
| NSAID compared with paracetamol for acute soft tissue injury | ||||||
| Patient or population: people with acute soft tissue injury, such as ankle sprain Setting: various outpatient locations (e.g. emergency department, student health centre) Intervention: NSAID Comparison: paracetamol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with paracetamol | Risk with NSAIDs | |||||
|
Pain at < 24 hours (VAS: 0 to 100 mm: worst) Follow‐up: 1 to 2 hours |
The mean pain score ranged across paracetamol groups from 43 to 55 mm; and ‐12 to ‐19 mm mean reduction from baseline | The mean pain score in the NSAID groups was 0.12 mm lower (2.27 lower to 2.03 higher) | ‐ | 1178 (6 RCTs) | ⊕⊕⊕⊕ High | All studies included mixed STI populations. The confidence interval did not reach the MCID (13 mm). |
|
Pain at days 1 to 3 (VAS: 0 to 100 mm: worst) Follow‐up: 2 to 3 days |
The mean pain score ranged across paracetamol groups from 11.9 to 36.7 mm; and ‐12.7 to ‐18.3 mm mean reduction from baseline | The mean pain score in the NSAID groups was 1.5 mm higher (0.91 lower to 3.91 higher) | ‐ | 1232 (6 RCTs) | ⊕⊕⊕⊕ Higha | Two studies included ankle sprains and four included mixed STI populations. The confidence interval did not reach the MCID (13 mm). |
|
Pain on day 7 or later (VAS: 0 to 100 mm: worst) Follow‐up: 7 to 10 days |
The mean pain score ranged across control groups from 5.0 to 6.3 mm; with ‐54.4 mm mean reduction from baseline | The mean pain score in the NSAID groups was 1.55 mm higher (0.33 lower to 3.43 higher) | ‐ | 467 (4 RCTs) | ⊕⊕⊝⊝ Lowb | All four studies included ankle sprains; one included only children. The confidence interval did not reach the MCID (13 mm). |
|
Little or no swelling on day 7 or later Follow‐up: 10 days |
Study population | RR 0.84 (0.58 to 1.22) | 77 (1 RCT) | ⊕⊕⊝⊝ Lowd | The study included children with ankle sprains only. This lack of difference was also found by two more studies (290 participants) involving ankle sprains that presented continuous (volume and VAS) data |
|
| 639 per 1000c | 537 per 1000 (371 to 779) | |||||
|
Return to function on day 7 or latere Follow‐up: 9 to 14 days |
Study population | RR 0.99 (0.90 to 1.09) | 386 (3 RCTs) | ⊕⊝⊝⊝ Very lowg | Two studies included ankle sprains; one included children only, and one included a mixed STI population. | |
| 820 per 1000f | 812 per 1000 (738 to 894) | |||||
|
Gastrointestinal adverse events Follow‐up: 1 hour to 30 days |
Study population | RR 1.34 (0.97 to 1.86) | 1504 (10 RCTs) | ⊕⊕⊝⊝ Lowi | Four studies included ankle sprains, one included children only, and six included mixed STI populations. | |
| 75 per 1000h | 101 per 1000 (73 to 140) | |||||
| Neurological adverse events Follow‐up: 1 hour to 30 days | Study population | RR 0.85 (0.62 to 1.17) | 1679 (9 RCTs) | ⊕⊕⊝⊝ Lowj | Four studies included ankle sprains, one included children only, and five included mixed STI populations. | |
| 92 per 1000h | 78 per 1000 (57 to 108) | |||||
| Early re‐injury (within 3 months) | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; RR: risk ratio; STI: soft tissue injury | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
a One study (100 participants) at unclear risk of bias across all domains slightly favoured paracetamol, while five others at low risk of bias did not. The evidence was not downgraded for risk of bias or inconsistency as including this study did not impact the finding of no statistically significant or clinically important difference b We downgraded the evidence by one level for study limitations (three studies were at unclear risk of several biases, including selection bias), and one level for indirectness reflecting suboptimal dosing of paracetamol in two studies (although these favoured paracetamol), and of both comparators in one study. Although there was inconsistency, reflecting statistically significant heterogeneity (P = 0.04; I² = 63%) of the pooled results, we did not consider this a reason to further downgrade the evidence, given the lack of clinical significance of the individual results of these studies c Assumed risk = control group risk in the study reporting this outcome d We downgraded the evidence by one level for study limitations (the sole study reporting this outcome was at unclear risk of bias relating to incomplete data for this outcome), and one level for imprecision (wide confidence intervals) e Function assessed differently in each study: numbers with no disability at day 14; numbers resuming sporting activity at day 10; and numbers who had resumed normal activity at day 9 f Assumed risk = median control group risk in the studies reporting this outcome g We downgraded the evidence by two levels for study limitations (one study was at high risk of selection bias and one at high risk of reporting bias), and one level for imprecision. Of note is the suboptimal dosing of paracetamol in one study, and of both comparators in another study. h Assumed risk = average risk for those in the paracetamol groups i We downgraded the evidence two levels for imprecision, as the lower confidence level just passed the point of no difference and the upper confidence level indicates an important difference j We downgraded the evidence two levels for imprecision, as the confidence interval was wide and included both benefit and harm
Summary of findings 2. NSAID compared with opioid for acute soft tissue injury.
| NSAID compared with opioid for acute soft tissue injury | ||||||
| Patient or population: acute soft tissue injury Setting: various outpatient locations (e.g. emergency department, sports club) Intervention: NSAID Comparison: opioid | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with opioid | Risk with NSAID | |||||
|
Pain at < 24 hours (VAS: 0 to 100 mm: worst) Follow‐up: 1 hour |
The mean pain score ranged across opioid groups from 13 to 27.7 mm | The mean pain in the NSAID group was 0.49 mm lower (3.05 lower to 2.07 higher) | ‐ | 1058 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | Three studies included a mixed STI population, one in children; the other study involved ankle sprains. The confidence interval did not include the MCID (13 mm). |
|
Pain at days 4 to 6 (VAS: 0 to 100 mm: worst) Follow‐up: 4 days |
The mean pain score in the opioid group was 31.8 mm | The mean pain in the NSAID group was 2.9 mm lower (6.06 lower to 0.26 higher) | ‐ | 706 (1 RCT) | ⊕⊕⊝⊝ Lowb | There were no data for the earlier interim period (up to 3 days). The study included ankle sprains. The confidence interval did not include the MCID (13 mm). |
|
Pain on day 7 or later (VAS: 0 to 100 mm: worst) Follow‐up: 7 days |
The mean pain score in the opioid group was 15.1 mm | The mean pain in the NSAID group was 6.5 mm lower (9.31 lower to 3.69 lower) | ‐ | 706 (1 RCT) | ⊕⊕⊝⊝ Lowb | The study included ankle sprains. The confidence interval did not include the MCID (13 mm). |
|
Little or no swelling on day 7 or later Follow‐up: 10 days |
Study population | RR 1.14 (0.61 to 2.13) | 84 (1 RCT) | ⊕⊝⊝⊝ Very lowd | The study included ankle sprains | |
| 300 per 1000c | 342 per 1000 (183 to 639) | |||||
|
Return to function on day 7 or later (numbers returning to full function or returning to training) Follow‐up: 7 to 10 days |
Study population | RR 1.13 (1.03 to 1.25) | 749 (2 RCTs) | ⊕⊕⊝⊝ Lowb | One study included ankle sprains, and one a mixed STI population. | |
| 664 per 1000e | 750 per 1000 (684 to 830) | |||||
|
Gastrointestinal adverse events Follow‐up: 2 hours to 14 days |
Study population | RR 0.48 (0.36 to 0.62) | 1151 (5 RCTs) | ⊕⊕⊕⊝ Moderatef | Four studies included a mixed STI population, one in children; the other study involved ankle sprains. | |
| 205 per 1000e | 98 per 1000 (74 to 127) | |||||
|
Neurological adverse events Follow‐up: 2 hours to 14 days |
Study population | RR 0.40 (0.30 to 0.53) | 1151 (5 RCTs) | ⊕⊕⊕⊝ Moderatef | Four studies involved a mixed STI population, one in children; the other study involved ankle sprains. | |
| 203 per 1000e | 81 per 1000 (61 to 108) | |||||
| Early re‐injury (with 3 months) | See Comment | ‐ | ‐ | ‐ | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; RR: risk ratio; STI: soft tissue injury | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
a We downgraded the evidence one level for indirectness, 49% of the evidence and 67% of participants came from one study that used a COX‐2 selective NSAID that has been withdrawn from the market (Valdecoxib) b We downgraded the evidence two levels for indirectness, The evidence came from one study that used a COX‐2 selective NSAID that has been withdrawn from the market (Valdecoxib), and also used a suboptimal dose of opioid as a comparator c Assumed risk = control risk in this study d We downgraded the evidence by two levels for severe study limitations (the sole study reporting this outcome was at high risk of attrition bias relating to incomplete data for this outcome), and one level for imprecision (wide confidence interval) eAssumed risk = average control group risk in the studies reporting this outcome f We downgraded the evidence one level for indirectness, more than 80% of the evidence and 61% of participants came from one study that used a COX‐2 selective NSAID that has been withdrawn from the market (Valdecoxib)
Summary of findings 3. NSAID compared with combination (paracetamol and opioid) analgesic for acute soft tissue injury.
| NSAID compared with combination (paracetamol and opioid) analgesic for acute soft tissue injury | ||||||
| Patient or population: people with acute soft tissue injury, such as ankle sprain Setting: various outpatient locations (e.g. emergency department, GP practice) Intervention: NSAID Comparison: combination (paracetamol plus opioid) analgesic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with combination (paracetamol and opioid) analgesic | Risk with NSAID | |||||
|
Little or no pain at < 24 hours Follow‐up: first day |
See Comment | ‐ | 51 (1 RCT) | ⊕⊝⊝⊝ Very lowa | The study involved ankle or wrist sprain in adults. Just one trial participant, who was in the NSAID group, had little or no pain on the first day. | |
|
Little or no pain at 1 to 3 days Follow‐up: day 3 |
Study population | RR 1.49 (0.65 to 3.40) | 149 (2 RCTs) | ⊕⊝⊝⊝ Very lowc | One study included mixed soft tissue injuries (mainly acute), and the other, ankle and wrist sprain, both in adults. | |
| 108 per 1000b | 161 per 1000 (70 to 368) | |||||
|
Little or no pain on day 7 or later Follow‐up: 7 days |
Study population | RR 1.05 (0.88 to 1.25) | 138 (2 RCTs) | ⊕⊝⊝⊝ Very lowd | One study included mixed soft tissue injuries (mainly acute), and the other, ankle sprain, both in adults. | |
| 671 per 1000b | 705 per 1000 (591 to 839) | |||||
|
Swelling on day 7 or later Follow‐up: 7 days |
All three studies presented the means of small categorical scales, so we were unable to present these results in quantitative meta‐analysis. Two (N = 132) reported no significant between‐group differences at three time points (days 3, 5, and 7). One study (N = 98) reported that there was no difference between groups for this outcome on day 7. | ‐ | 230 (3 RCTs) | ⊕⊝⊝⊝ Very lowe | Two studies included mixed soft tissue injuries, and one ankle sprain. All three were in adults. | |
|
Return to function on day 7 or later Assessed as 'cure' Follow‐up: 7 days |
Study population | RR 1.28 (0.90 to 1.81) | 89 (1 RCT) | ⊕⊝⊝⊝ Very lowg | This study included mixed soft tissue injuries in adults (76% acute) | |
| 523 per 1000f | 669 per 1000 (470 to 946) | |||||
|
Gastrointestinal adverse events Follow‐up: 3 to 7 days |
Study population | RR 0.21 (0.03 to 1.74) | 141 (3 RCTs) | ⊕⊝⊝⊝ Very lowh | One study involved ankle sprain, one study involved ankle and wrist sprain, and the third study, mixed soft tissue injuries, all in adults. Just 4 events were reported |
|
| 56 per 1000b | 12 per 1000 (2 to 98) | |||||
|
Neurological adverse events Follow‐up: 3 to 7 days |
Study population | RR 0.52 (0.09 to 2.84) | 141 (3 RCTs) | ⊕⊝⊝⊝ Very lowh | One study involved ankle sprain, one study involved ankle and wrist sprain, and the third study, mixed soft tissue injuries, all in adults. Just 4 events were reported |
|
| 42 per 1000b | 22 per 1000 (4 to 120) | |||||
| Early re‐injury (with 3 months) | See Comment | ‐ | ‐ | ‐ | No study reported this outcome. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCID: minimal clinically important difference; RR: risk ratio; STI: soft tissue injury | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
a The available data (one participant experienced little or no pain) for this outcome are too limited to draw any conclusions or useful analysis. Nominally, we downgraded the evidence by one level for study limitations (unclear risk of selection bias and other bias), and two levels for imprecision (very few events, data from one small single study) b Assumed risk = average risk for those in combination groups c We downgraded the evidence by one level for study limitations (one study was at high risk of bias, reflecting lack of blinding of participants, and one was at unclear risk of selection bias, and other bias), one level for indirectness, since most of the weight of the evidence (87%) came from one study that had suboptimal dosing of both comparators, and two levels for imprecision (few events, wide confidence interval) d We downgraded the evidence by one level for study limitations (one study was at high risk of bias, reflecting lack of blinding of participants, and one was at unclear risk of several biases), one level for indirectness, since most of the weight of the evidence (88%) came from one study that had suboptimal dosing of both comparators, and one level for imprecision (wide confidence interval) e We downgraded the evidence two levels for risk of bias, as all three studies were either at high or unclear risk of bias across multiple domains, one level for indirectness, due to mixed study populations, and one level for imprecision, due to the small sample size f Assumed risk = control group risk in the study reporting this outcome g We downgraded the evidence by one level for study limitation (the study was at high risk of bias, relating to lack of participant blinding), two levels for indirectness, reflecting suboptimal dosing of comparators and the inadequate nature of the outcome, and one level for imprecision, due to the small sample size h We downgraded the evidence by two levels for study limitations (two studies were at high risk of bias for one or more domains, and the other was at unclear risk of bias for several domains), and two levels for imprecision (very few events, wide confidence interval)
In the following, the continuous outcome measure for pain is the visual analogue scale, scored 0 to 100 mm; higher scores equal greater pain.
In the following, we avoided potential unit of analysis issues when studies reported adverse events at the event level for the broad categories of gastrointestinal and neurological adverse events, by using participants‐level data for the most common adverse event within these broad categories in the analysis. This pertained to data from four trials, two of which appeared in the 2015 version of the review (Bourne 1980; Cukiernik 2007), and two of which are newly included (Hung 2018; Ridderikhof 2018). When checking through the studies for this problem, we realised that Dalton 2006 reported adverse events by participant rather than by event, and rectified this error.
NSAID versus paracetamol
Eleven studies (1853 participants) compared NSAID with paracetamol (Bondarsky 2013; Bourne 1980; Clark 2007; Cukiernik 2007; Dalton 2006; Hung 2018; Kayali 2007; Lyrtzis 2011; Man 2004; Ridderikhof 2018; Woo 2005).
Pain
There is high‐certainty evidence of no clinically important difference between NSAID and paracetamol in pain measured for up to two hours (mean difference (MD) ‐0.12 mm, 95% CI ‐2.27 to 2.03; 1178 participants, 6 studies; P = 0.91; Analysis 1.1). There was no evidence of heterogeneity (Chi² = 2.42, df = 5 (P = 0.79); I² = 0%). and subgroup analysis comparing the results of trials with adequate dosing of both comparators with suboptimal NSAID dosing did not show subgroup differences (test for subgroup differences Chi² = 0.96, df = 1 (P = 0.33); I² = 0%). Pooled data (N = 818) from two studies found an little difference between groups in the number of participants with little or no pain in the first two hours (risk ratio (RR) 0.94, 95% CI 0.82 to 1.08, P = 0.40; Analysis 1.2), although there was some evidence of heterogeneity (Chi² = 2.06, df = 1 (P = 0.15); I² = 51%).
1.1. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 1: Pain < 24 hours (VAS: 0 to 100 mm: worst)
1.2. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 2: Little or no pain < 24 hours
There is high‐certainty evidence of no clinically important difference between NSAID and paracetamol in pain measured at one to three days (MD 1.50 mm, 95% CI ‐0.91 to 3.91; 1232 participants, 6 studies; P = 0.22, with some evidence of heterogeneity (Chi² = 10.08, df = 5 (P = 0.07); I² = 50%); Analysis 1.3). Subgroup analysis comparing the results of trials with suboptimal and adequate dosing did not show subgroup differences (test for subgroup differences: Chi² = 4.28, df = 2 (P = 0.12); I² = 53.2%). Sensitivity analysis excluding two studies, Kayali 2007 and Lyrtzis 2011, at unclear risk of blinding did not substantially alter the result (MD ‐0.51 mm, 95% CI ‐3.58 to 2.56; P = 0.75). Pooled data (N = 894) from three studies found little difference between groups in the number of participants with little or no pain at day three (RR 1.10, 95% CI 0.96 to 1.27; P = 0.16; Analysis 1.4).
1.3. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 3: Pain on days 1 to 3 (VAS: 0 to 100 mm: worst)
1.4. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 4: Little or no pain on days 1 to 3
At day four, Dalton 2006 (N = 204), which had suboptimal dosing of both comparators, found no clinically important difference between the groups (MD ‐0.68 mm, 95% CI ‐6.09 to 4.73; Analysis 1.5). This study was at high risk of bias for selective outcome reporting, as it only sufficiently reported the per‐protocol population to include in the analysis. Bourne 1980 reported that there was no difference between the groups in pain at day five, but did not provide data for us to include in the meta‐analysis.
1.5. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 5: Pain on days 4 to 6 (VAS: 0 to 100 mm: worst)
There is low‐certainty evidence of no clinically important difference between NSAID and paracetamol in pain measured at day seven or beyond (MD 1.55 mm, 95% CI ‐0.33 to 3.43; 467 participants, 4 studies; P = 0.11; Analysis 1.6). There is evidence of heterogeneity (Chi² = 8.11, df = 3 (P = 0.04); I² = 63%), and subgroup analysis comparing the results of trials with adequate dosing of both comparators with those with suboptimal dosing of one or more comparators showed evidence of subgroup differences (test for subgroup differences: Chi² = 7.50, df = 2 (P = 0.02); I² = 73.3%). The studies with adequate dosing of both comparators or suboptimal paracetamol dosing favoured paracetamol, while the study with suboptimal dosing of both comparators, Dalton 2006, favoured NSAID. However, none of the observed differences in these subgroups or for individual studies were clinically important.
1.6. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 6: Pain on day 7 or later (VAS: 0 to 100 mm: worst)
Cukiernik 2007 (N = 76), which had adequate dosing of both comparators, found little difference between groups in the number of participants with little or no pain at day seven (RR 0.96, 95% CI 0.71 to 1.28; Analysis 1.7;).
1.7. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 7: Little or no pain on day 7 or later
Swelling
Overall, there was low‐certainty evidence of little between‐groups difference in swelling. Lyrtzis 2011 (N = 86) reported a statistically significant but clinically unimportant difference at day three in swelling, measured by volume, in favour of paracetamol (MD 4.30 mL, 95% CI 0.79 to 7.81; P = 0.02; Analysis 1.8). This study used a suboptimal dose of paracetamol, and was at unclear risk of bias for blinding. Dalton 2006 (N = 204), which used a subjective measure of swelling (100‐mm VAS) assessed by the investigator at day four, found no important difference between groups (MD ‐2.03 mm, 95% CI ‐7.71 to 3.65; P = 0.48; Analysis 1.9). This study used suboptimal dosing of both comparators, and was at high risk of bias for selective outcome reporting. Using the same measures at day seven or later, both of these studies found minimal difference between the two groups at day 9 and day 10 (Analysis 1.10).
1.8. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 8: Swelling on days 0 to 3
1.9. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 9: Swelling on days 4 to 6 (VAS: 0 to 100 mm: worst)
1.10. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 10: Swelling on day 7 or later
Another study, Cukiernik 2007 (N = 77), which used adequate dosing of both comparators, found little difference between groups in the numbers of participants with little or no swelling on day seven (22/41 versus 23/36; RR 0.84, 95% CI 0.58 to 1.22; Analysis 1.11).
1.11. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 11: Little or no swelling on day 7 or later
Two studies, both of which used suboptimal doses of paracetamol, reported means of small categorical scales. These studies reported no difference between the groups at day two (Kayali 2007), or at day five (Bourne 1980). Kayali 2007 also reported no difference at day 10, or at six weeks.
Function
Two studies (N = 131) reported the number of participants with better function within the first seven days of treatment. Cukiernik 2007, with adequate dosing of both comparators, used a four‐point scale of self‐assessed disability. Bourne 1980, with suboptimal paracetamol dosing, reported the number of participants returning to sporting activity. As the pooled analysis of these two studies shows substantial heterogeneity (Chi² = 4.82, df = 1 (P = 0.03); I² = 79%), we present the results of each trial separately in Analysis 1.12. Bourne 1980, which is at high risk of bias for allocation concealment, found in favour of NSAID whereas Cukiernik 2007 found no difference between the groups (18/41 versus 17/35; RR 0.90, 95% CI 0.56 to 1.47; P = 0.68).
1.12. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 12: Return to function within 7 days
The very low‐certainty evidence from three studies (N = 386) of minimal difference in the numbers of people returning to full activity by day seven or beyond means we are uncertain of these findings (RR 0.99, 95% CI 0.90 to 1.09; Analysis 1.13). There was no heterogeneity between the groups (Chi² = 0.88, df = 2 (P = 0.65); I² = 0%).
1.13. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 13: Return to function in 7 days or later
Two studies reported the mean time to return to normal activity, with no conclusive difference between the groups in either study. Kayali 2007 (N = 100), used a suboptimal dose of paracetamol, and was at unclear risk of bias for blinding (MD ‐0.18, 95% CI ‐0.79 to 0.43; Analysis 1.14). Dalton 2006 (N = 255), which used a suboptimal dose of both medications, reported a mean return to activity of 4.1 days in the NSAID group and 4.0 days in the paracetamol group.
1.14. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 14: Time to return to full activity (days)
Kayali 2007 (N = 100) reported no important clinical difference between groups in the range of motion of the injured ankle joint at six weeks (MD 0.70 degrees, 95% CI ‐0.62 to 2.02; P = 0.3; Analysis 1.15).
1.15. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 15: Range of motion (degrees)
One study sought data on time to return to function but did not report this outcome (Hung 2018).
Adverse effects
There is low‐certainty evidence of an increased risk of gastrointestinal adverse effects with NSAID compared with paracetamol (74/788 versus 54/716; RR 1.34, 95% CI 0.97 to 1.86; 1504 participants, 10 studies; Analysis 1.16). However, 95% CI include the possibility of no difference or a very small increased risk for paracetamol. There was no evidence of heterogeneity between studies (Chi² = 6.81, df = 8 (P = 0.56); I² = 0%). Subgroup analysis comparing the results of trials with adequate dosing of both medications with trials with suboptimal dosing of paracetamol, or NSAIDs, or both, also showed no evidence of subgroup differences (test for subgroup differences Chi² = 3.29, df = 3 (P = 0.35); I² = 8.8%). A sensitivity analysis excluding three studies (245 participants; Bourne 1980; Kayali 2007; Lyrtzis 2011) at unclear risk of bias for blinding found no evidence of a difference between the two groups (RR 1.16, 95% CI 0.79 to 1.69; P = 0.44).
1.16. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 16: Gastrointestinal adverse events
Ridderikhof 2018 (N = 365) gave all participants omeprazole to offset potential gastrointestinal adverse effects. This study reported approximately triple the rate of gastrointestinal adverse effects in all participants (23%) compared with other studies combined (8%). Ridderikhof 2018 found fewer participants with gastrointestinal adverse effects in the NSAID group on day three (37/183 versus 47/182; RR 0.78, 95% CI 0.54 to 1.14; P = 0.25). Due to possible confounding from the addition of omeprazole, we did not include these data in the pooled analysis.
There is low‐certainty evidence from nine studies (N = 1679) of little difference between groups for neurological adverse effects (64/876 verus 74/803; RR 0.85, 95% CI 0.62 to 1.17; P = 0.33; Analysis 1.17). There was no evidence of heterogeneity (Chi² = 1.70, df = 6 (P = 0.94); I² = 0%), and no evidence of subgroup differences based on dosing (test for subgroup differences (Chi² = 1.65, df = 3 (P = 0.65); I² = 0%).
1.17. Analysis.

Comparison 1: NSAIDs versus paracetamol, Outcome 17: Neurological adverse events
None of the studies reported any serious adverse events.
Early re‐injury
No studies reported any re‐injury events.
2. NSAIDs versus opioids
Six studies (1212 participants) compared NSAIDs with opioids (Beveridge 1985; Clark 2007; Ekman 2006; Fathi 2015; Le May 2017; McCulloch 1985).
Pain
Four studies (N = 1058) measured this outcome; two with adequate dosing (Clark 2007; Le May 2017), one with suboptimal doses of NSAID (Fathi 2015), and one with suboptimal doses of opioid (Ekman 2006).
There is moderate‐certainty evidence of no difference between groups for pain relief at 60 minutes on a 100‐mm VAS scale (MD ‐0.49 mm, 95% CI ‐3.05 to 2.07; 1058 participants, 4 studies; Analysis 2.1). There was little evidence of heterogeneity (Chi² = 4.57, df = 3 (P = 0.21); I² = 34%), and no evidence of a difference between subgroups (test for subgroup differences: Chi² = 1.44, df = 2 (P = 0.49), I² = 0%). In contrast, Le May 2017 (134 participants) found a higher proportion of people in the NSAID group had little or no pain at one hour (very low‐certainty evidence; Analysis 2.2).
2.1. Analysis.

Comparison 2: NSAID versus opioid, Outcome 1: Pain < 24 hours (VAS: 0 to 100 mm: worst)
2.2. Analysis.

Comparison 2: NSAID versus opioid, Outcome 2: Little or no pain < 24 hours
Only Ekman 2006 (N = 706), which used a suboptimal dose of the opioid, reported pain beyond the first 24 hours. Although favouring the NSAID, differences were clinically unimportant at both day four (MD ‐2.9 mm, 95% CI ‐6.06 to 0.26 mm; Analysis 2.3), and day seven (MD ‐6.50 mm, 95% CI ‐9.31 to ‐3.69 mm; Analysis 2.4).
2.3. Analysis.

Comparison 2: NSAID versus opioid, Outcome 3: Pain on days 4 to 6 (VAS: 0 to 100 mm: worst)
2.4. Analysis.

Comparison 2: NSAID versus opioid, Outcome 4: Pain on day 7 or later (VAS: 0 to 100 mm: worst)
Beveridge 1985 (N = 68), which presented pain data as the mean of a small categorical scale, measured daily for 14 days, and reported no difference between the groups on any day. (Data were not available to include in the analysis.)
Swelling
Two studies recorded this outcome, but data were only available for analysis from McCulloch 1985 (N = 84). There is very low‐certainty evidence of little difference between the two groups in the number of participants with swelling at day 10 (RR 1.14, 95% CI 0.61 to 2.13; Analysis 2.5). Beveridge 1985 (N = 68), reported swelling as the mean of a small categorical scale, measured daily for 14 days, and reported a small statistically significant difference (in the order of 5%) between the groups favouring NSAID at days two to six; however, the clinical importance of this difference is uncertain.
2.5. Analysis.

Comparison 2: NSAID versus opioid, Outcome 5: Swelling on day 7 or later
Function
There is low‐certainty evidence from two studies (N = 705), one with adequate dosing (Beveridge 1985), and one with suboptimal doses of opioid (Ekman 2006), of a greater return to function in the NSAID group (204/470 versus 78/235; RR 1.22, 95% CI 0.99 to 1.49; P = 0.06; Analysis 2.6), with no evidence of heterogeneity (Chi² = 0.91, df = 1 (P = 0.34); I² = 0%). The results were dominated by the data from Ekman 2006 (N = 642), which, unlike Beveridge 1985, was at low risk of detection bias. Likewise, a greater proportion of participants who took NSAIDs returned to function on or after day seven than of those who took opioids (366/484 versus 176/265; RR 1.13, 95% CI 1.03 to 1.25; P = 0.01; 2 studies, 749 participants; Analysis 2.7), with no evidence of heterogeneity (Chi² = 0.09, df = 1 (P = 0.76); I² = 0%); again, this result was dominated by the data from Ekman 2006 (N = 686).
2.6. Analysis.

Comparison 2: NSAID versus opioid, Outcome 6: Return to function within 7 days
2.7. Analysis.

Comparison 2: NSAID versus opioid, Outcome 7: Return to function in 7 days or later
McCulloch 1985, which was at high risk of bias for incomplete outcome data, found that participants treated with an NSAID had a statistically significant, though small, difference in step length for the affected versus the unaffected limb, compared with those who took opioids (reported difference between limbs 5.0 cm, 95% CI 0.7 to 10.26 cm less). McCulloch 1985 also reported no difference in ankle range of motion between NSAID and opioid‐treated participants.
Adverse effects
Five studies (N = 1151) measured adverse events, three with adequate dosing of both comparators (Beveridge 1985; Clark 2007; Le May 2017), one with suboptimal dosing of opioid (Ekman 2006), and one with suboptimal dosing of NSAID (Fathi 2015).
There is moderate‐certainty evidence of fewer gastrointestinal adverse effects with NSAID compared with opioid (78/658 versus 101/493; RR 0.48, 95% CI 0.36 to 0.62; P < 0.001; 1151 participants, 5 studies; Analysis 2.8). There was evidence of some heterogeneity (Chi² = 8.82, df = 4 (P = 0.07); I² = 55%), due to discordant results of one small study (Beveridge 1985). There was no evidence of subgroup differences, based on adequate dosing of the comparators (test for subgroup differences Chi² = 2.60, df = 2 (P = 0.27); I² = 23.1%); I² dropped to zero on the removal of Beveridge 1985.
2.8. Analysis.

Comparison 2: NSAID versus opioid, Outcome 8: Gastrointestinal adverse events
There is moderate‐certainty evidence of fewer neurological adverse effects with NSAID compared with opioid (68/658 versus 100/493; RR 0.40, 95% CI 0.30 to 0.53; P < 0.001; 1151 participants, 5 studies; Analysis 2.9), with no evidence of heterogeneity (Chi² = 1.27, df = 3 (P = 0.74); I² = 0%), and no evidence of subgroup differences (test for subgroup differences (Chi² = 0.87, df = 2 (P = 0.65); I² = 0%).
2.9. Analysis.

Comparison 2: NSAID versus opioid, Outcome 9: Neurological adverse events
Ekman 2006 also reported other system adverse effects; however, the study reported these at the individual type of adverse event level rather than the participant level. McCulloch 1985 reported that 18% of participants taking non‐selective NSAIDs had some sort of adverse event compared with 20% of those taking opioids, but did not report these events in sufficient detail to allow inclusion in the analyses.
None of the studies reported serious adverse events, although Clark 2007 treated one child successfully for an accidental overdose of opioid (this participant was withdrawn from the study).
Early re‐injury
No studies reported on re‐injury.
3. NSAID versus combination analgesics (paracetamol and opioid)
Four studies (240 participants) compared NSAIDs with combined analgesics (paracetamol plus an opioid; Abbott 1980; Aghababian 1986; Indelicato 1986; Jaffé 1978).
One study used suboptimal doses of both comparators (Abbott 1980). We anticipate that the other three studies used the standard doses as marketed, although the dose of paracetamol was suboptimal in all proprietary preparations combining paracetamol with opioid (Aghababian 1986; Indelicato 1986; Jaffé 1978).
The evidence for all reported outcomes was very low‐certainty meaning that we have very little confidence in the effect estimates. This primarily reflects the low numbers of participants available and thus very serious imprecision.
Pain
Jaffé 1978, the only study (N = 51) reporting on pain in the first 24 hours, found just one person, who was in the NSAID group, who experienced little or no pain (Analysis 3.1).
3.1. Analysis.

Comparison 3: NSAID versus combination analgesic (paracetamol + opioid), Outcome 1: Little or no pain < 24 hours
Pooled data (N = 149) from two studies showed little difference between the groups in the numbers of participants with little or no pain at days one to three (12/74 versus 8/74; RR 1.49, 95% CI 0.65 to 3.40; P = 0.34; Analysis 3.2). There was no evidence of heterogeneity (Chi² = 0.05, df = 1 (P = 0.82); I² = 0%). Abbott 1980 (N = 98), which was at high risk for blinding of treatment providers, found little difference between groups in the number of participants with little or no pain at day five (RR 1.33, 95% CI 0.78 to 2.29; P = 0.3; Analysis 3.3).
3.2. Analysis.

Comparison 3: NSAID versus combination analgesic (paracetamol + opioid), Outcome 2: Little or no pain on days 1 to 3
3.3. Analysis.

Comparison 3: NSAID versus combination analgesic (paracetamol + opioid), Outcome 3: Little or no pain on days 4 to 6
Pooled data (N = 138) from two studies showed little difference between the groups in the proportion of participants with little or no pain at day seven (49/68 versus 47/70; RR 1.05, 95% CI 0.88 to 1.25; with no evidence of heterogeneity (Chi² = 0.69, df = 1 (P = 0.41); I² = 0%; Analysis 3.4).
3.4. Analysis.

Comparison 3: NSAID versus combination analgesic (paracetamol + opioid), Outcome 4: Little or no pain on day 7 or later
We excluded data from three studies that reported means of small categorical scales from the meta‐analysis for pain (Abbott 1980; Aghababian 1986; Indelicato 1986). Indelicato 1986 (N = 50) reported no difference between the groups at all time points up to day seven. At day seven, Abbott 1980 (N = 98) found a statistically significant difference of 0.5 on a 4‐point scale favouring NSAID (this is of uncertain clinical significance), and Aghababian 1986 (N = 82) found no difference.
We also excluded data from one study that used a numeric rating scale (NRS), as more than 30% of the population had inflammatory conditions, and data for participants with injuries (N = 134) were insufficiently reported to enable us to include them in the meta‐analysis (Buccelletti 2014). The authors reported there was no difference between the groups at 30 minutes (P = 0.77), and 120 minutes (P = 0.48).
Swelling
Three studies measured swelling, but all used the means of small categorical scales, therefore, we just reported the results from the publications. Two reported that at days three, five, and seven, there were no significant differences between the groups (Aghababian 1986, N = 82; Indelicato 1986, N = 50). Abbott 1980 (N = 98) reported that no difference between groups on day seven.
Function
Abbott 1980 (N = 89) found an inconclusive difference between the groups in the number of participants 'cured' by day seven (RR 1.28, 95% CI 0.90 to 1.81; P = 0.17; Analysis 3.5). Abbott 1980 (N = 89) and Aghababian 1986 (N = 40) reported function as a mean limitation of movement on a small categorical scale on day seven; both reported no significant difference between groups.
3.5. Analysis.

Comparison 3: NSAID versus combination analgesic (paracetamol + opioid), Outcome 5: Return to function in 7 days or later
Adverse effects
These were reported by all four trials but we did not include the results from Abbott 1980 (N = 98) in the analyses as they reported at the event level rather than the participant level.
There was little evidence of a difference in the numbers of participants who developed gastrointestinal adverse events reported in three other trials (0/70 versus 4/70; RR 0.21, 95% CI 0.03 to 1.74; P = 0.15; 141 participants, 3 studies; Analysis 3.6).
3.6. Analysis.

Comparison 3: NSAID versus combination analgesic (paracetamol + opioid), Outcome 6: Gastrointestinal adverse events
Pooled data (N = 141) from the same three studies also found little difference between groups in neurological adverse effects (1/70 versus 3/71; RR 0.52, 95% CI 0.09 to 2.84; P = 0.45; Analysis 3.7).
3.7. Analysis.

Comparison 3: NSAID versus combination analgesic (paracetamol + opioid), Outcome 7: Neurological adverse events
Indelicato 1986 (N = 50) reported one participant with a rash in the NSAID group, and none in the paracetamol plus opioid group.
None of the studies reported any serious adverse events.
Early re‐injury
No studies reported any re‐injury events.
Subgroup analysis of the three different comparators
We undertook exploratory subgroup analyses to examine differences between the results of the three comparisons (NSAIDs versus paracetamol, versus opioids, and versus combined paracetamol and opioid analgesics). Sufficient data were available for pain in the first one to two hours of treatment, and gastrointestinal and neurological adverse effects.
Pain
Exploratory subgroup analysis, involving nine trials, of pain scores in the first one to two hours of treatment, measured on a 100‐mm VAS, showed no evidence of a difference between two subgroups (NSAID versus paracetamol; NSAID versus opioid): test for subgroup differences: Chi² = 0.05, df = 1 (P = 0.83); I² = 0% (Analysis 4.1; Figure 4).
4.1. Analysis.

Comparison 4: NSAID versus other oral analgesics, Outcome 1: Pain at < 24 hours (VAS: 0 to 100 mm: worst)
4.

Forest plot of comparison: 4 NSAID versus other oral analgesics, outcome: 4.1 Pain at < 24 hours (VAS: 0 to 100 mm: worst).
Adverse effects
Exploratory subgroup analysis, involving 17 studies, of gastrointestinal adverse effects showed a difference between the three groups: test for subgroup differences: Chi² = 24.69, df = 2 (P < 0.00001), I² = 91.9% (Analysis 4.2; Figure 5). This is consistent with the expected differences in gastrointestinal adverse effects profiles of paracetamol and opioids relative to NSAIDs.
4.2. Analysis.

Comparison 4: NSAID versus other oral analgesics, Outcome 2: Gastrointestinal adverse events
5.

Forest plot of comparison: 4 NSAID versus other oral analgesics, outcome: 4.2 Gastrointestinal adverse events.
Exploratory subgroup analysis, involving 16 studies, of neurological adverse effects showed a difference between the three groups: test for subgroup differences: Chi² = 12.73, df = 2 (P = 0.002), I² = 84.3% (Analysis 4.3; Figure 6). This is consistent with the expected differences in neurological adverse effects profiles of paracetamol and opioids relative to NSAIDs.
4.3. Analysis.

Comparison 4: NSAID versus other oral analgesics, Outcome 3: Neurological adverse events
6.

Forest plot of comparison: 4 NSAID versus other oral analgesics, outcome: 4.3 Neurological adverse events.
Notably, none of the studies reported any serious adverse effects, although one participant was withdrawn from Clark 2007 for an accidental overdose of opioid.
Subgroup analyses that were planned but not done
By age
There are insufficient studies and data to undertake formal subgroup analysis by age. The three studies comparing NSAIDs and paracetamol that exclusively enrolled participants younger than 18 years of age tested two comparisons: NSAID versus paracetamol (Clark 2007; Cukiernik 2007) and NSAID versus opioid (Clark 2007; Le May 2017). The youngest participant was six years old. Five other studies may have included participants who were under 18 years, but we were unable to disaggregate the data specific to paediatric participants (Ekman 2006; Jaffé 1978; Man 2004; McCulloch 1985; Woo 2005). No other studies enrolled paediatric participants.
Although some studies may have included participants over 65 years old, for all but one study, we were unable to disaggregate the data specific to them. Ridderikhof 2018, which did not specify an upper age limit, reported data separately for 28 of 365 included participants who were 60 years and older; they found no subgroup difference between the younger and older participants for pain.
The average age of participants enrolled in studies across all comparisons was between 20 and 35 years, and thus the results may not be generalised to older adults.
COX‐2 selective NSAIDs versus non‐selective NSAIDs
There were insufficient studies using different types of NSAID (COX‐2 selective and non‐selective) to undertake subgroup analysis.
NSAIDs versus complementary and alternative medicines
We identified no studies that explored this comparison, although one study awaiting classification may meet criteria for inclusion when completed TCTR20160126001
Discussion
Summary of main results
We included 20 studies; 11 studies compared non‐steroidal anti‐inflammatory drugs (NSAIDs) with paracetamol, six studies compared NSAIDs with opioids, and four compared NSAIDs with combined analgesics comprising paracetamol and an opioid. One study included all three comparators (Clark 2007). Although some evidence was high certainty or moderate certainty, the majority of the evidence was either low certainty, meaning that our confidence in the effect estimate is limited and the true effect may be substantially different from the estimate of the effect, or very low certainty, meaning that we have very little confidence in the effect estimate, and the true effect is likely to be substantially different from the estimate of effect.
We found no studies comparing NSAID versus oral complementary and alternative medicines.
NSAIDs versus paracetamol
Summary of findings table 1 summarises the findings for the outcomes for this comparison. The evidence for outcomes ranged from moderate certainty to very low certainty. There is high‐certainty evidence of no clinically important differences between the two groups (NSAID versus paracetamol) in pain, measured on a visual analogue scale, at one to two hours (1178 participants, 6 studies) and at one to three days (1232 participants, 6 studies). There was low‐certainty evidence of no clinically important difference between the two groups at day seven or later (467 participants, 4 studies). There is low‐certainty evidence of little difference between the two groups in the numbers of participants with no or little swelling at day seven or later (77 participants, 1 study; consistent data also from two studies with 290 participants). There is very low‐certainty evidence of no difference between groups in return to function at day seven or later (386 participants, 3 studies). Low certainty evidence (1504 participants, 10 studies) indicates that NSAID may increase the risk of gastrointestinal adverse events; however, the 95% CI includes the possibility of a very slight increase with paracetamol. Based on an assumed risk of gastrointestinal adverse events of 75 per 1000 participants in the paracetamol group, 26 more participants per 1000 had a gastrointestinal adverse event in the NSAID group (95% CI 2 fewer to 65 more). There is low‐certainty evidence that there may be little difference between NSAID and paracetamol in the risk of neurological adverse events (1679 participants, 9 studies). Based on an assumed risk of neurological adverse events on 92 per 1000 participants in the paracetamol group, 14 fewer participants per 1000 had a neurological adverse event in the NSAID group (95% CI 35 fewer to 16 more). None of the studies reported re‐injury.
NSAIDs versus opioids
Table 2 summarises the findings for the outcomes for this comparison. The evidence for outcomes ranged from moderate certainty to very low certainty.
There was moderate‐certainty evidence of no clinically important difference between NSAIDs and opioids for pain, measured on a visual analogue scale, at one hour (1058 participants, 4 studies). There was low‐certainty evidence of no clinically important differences between the groups at day four (706 participants, 1 study) or at day seven. The confidence intervals for all three time points were smaller than the minimum clinically detectable difference in pain score (13 mm on a 100‐mm VAS).
There was very low‐certainty evidence of little clinically important difference between the groups in swelling (1 study, 84 participants). There was low‐certainty evidence that participants in the NSAID group were more likely to return to function in 7 to 10 days (2 studies, 749 participants).
There was moderate‐certainty evidence from five studies (1143 participants) that those who took NSAIDs were less likely to develop either gastrointestinal or neurological adverse events compared with opioid. Based on an assumed risk of 205 per 100 participants in the opioid group, 107 fewer participants per 1000 had an gastrointestinal adverse event in the NSAID group (95% CI 78 to 131 fewer). Based on an assumed risk of 203 per 1000 participants in the opioid group, 122 fewer participants per 1000 had a neurological adverse event in the NSAID group (95% CI 95 to 142 fewer). None of the studies reported re‐injury.
NSAIDs versus combination analgesics (paracetamol and opioid)
Table 3 summarises the findings for the outcomes for this comparison, for which very limited data were available. The evidence for outcomes was very low certainty.
There is very low‐certainty evidence of little difference between the two groups in the numbers reporting little or no pain on day one (51 participants, 1 study), days one to three (149 participants, 2 studies), and at day seven or later (138 participants, 2 studies). The confidence intervals were wide at each time point and crossed the line of no effect, and thus included the potential for a better outcome for either intervention. No usable data were available from the three studies (230 participants) reporting on swelling; very low‐certainty evidence from these did not indicate a clinically important difference between groups at day seven. There was very low‐certainty evidence of little difference between groups for those who reported a 'cure' at day seven (1 study, 89 participants). There is very low‐certainty evidence (141 participants, 3 studies) relating to gastrointestinal adverse events, of which all four cases occurred in the combined paracetamol and opioid group; and for neurological adverse events, where three of the four cases occurred in the combined group. The wide confidence intervals of both pooled analyses also include the potential for fewer adverse events for the paracetamol and opioid combination and illustrate the uncertainty surrounding these findings. None of the studies reported re‐injury.
Overall completeness and applicability of evidence
NSAIDs versus paracetamol
The results for this comparison are from 11 small to large size studies, with data available for pooling from a maximum of 1679 participants (9 studies); these data were for neurological adverse events. Participants were children (mean age of 12 years) in two studies, and mainly young adults in the other nine studies. The participants were predominantly from North America, Europe, and Asia. Four studies involved people with ankle sprains only, while the other seven studies had mixed populations of various soft tissue injuries. Both sexes were well represented, although there was a male predominance of approximately 60%. Generalisation to other populations, including elderly populations, may be limited. The results are more limited for the outcome of return to function, because few studies reported data sufficiently to enable us to include them in all analyses. Although there was suboptimal paracetamol dosing in three studies, and suboptimal dosing of NSAIDs in three studies, this reflected local practice, and did not appear to affect the results. Thus, the evidence is likely to be applicable to current practice.
NSAIDs versus opioids
The results for this comparison are from three small, two moderate sized, and one large study. Most studies included various soft tissue injuries, but the largest trial, accounting for over half of the participants, included predominantly young, white adults with ankle sprains. Data available for pooling were from a maximum of 1151 participants (5 studies); these data were for gastrointestinal and neurological adverse events. Participants were children in one small and one moderate sized trial, and adults in the other four. Both sexes were well represented, although there was a male predominance of approximately 60%. Generalisation to other populations, including the elderly, may be limited. The particular COX‐2 selective NSAID (valdecoxib) used in the largest trial, was subsequently withdrawn from the market due to fears about cardiovascular toxicity; this limits the generalisability of the results for this comparison to some extent.
NSAIDs versus combination analgesics (paracetamol and opioid)
The results for this comparison came from four small studies, with data available for pooling from a maximum of 149 participants (2 studies); these data were for pain at days one to three. One study included ankle sprains; one included ankle and wrist injuries; and there was a mixture of injuries in the remaining two studies. The studies exclusively enrolled young adults, in a variety of acute care settings; they did not report ethnicity. There was also a strong male predominance in these studies (more than 70%). Generalisation of these results to other populations may not be appropriate. These studies used suboptimal paracetamol doses because of the proprietary analgesic formulations, which may not be relevant to current practice, as these dextropropoxyphene combination analgesic agents are no longer in general use.
Quality of the evidence
In our assessment of the certainty of the evidence, we downgraded for one of three reasons: study limitations, indirectness, and imprecision.
NSAIDs versus paracetamol
The reasons for our assessment of the certainty of evidence for each outcome displayed in Table 1 are presented in the footnotes. We assessed the evidence for pain at one or two hours and for pain at days one to three as high certainty. We downgraded the evidence for pain at day seven or later by two levels, to low certainty, because of study limitations and indirectness of the data.
We downgraded the evidence for swelling at day seven or later by two levels to low certainty, because of study limitations and imprecision. Although binary data for this outcome were from one study only, volume data available from two other studies provided consistent evidence. We downgraded the evidence for return to function at day seven or later by three levels to very low certainty, reflecting serious study limitations and imprecision. Finally, we downgraded the evidence for gastrointestinal and neurological adverse events by one level to moderate‐certainty because of imprecision.
NSAIDs versus opioids
The reasons for our assessment of the certainty of the evidence for each outcome displayed in Table 2 are presented in the footnotes. Half or more of the evidence for this comparison were from one large (706 participants) study that was at low risk of bias for all domains except for selective reporting of adverse events. However, because this study used a COX‐2 selective NSAID, which has been withdrawn from the market (Valdecoxib), and also used a suboptimal dosing of the opioid, we downgraded evidence for indirectness for outcomes it contributed data to. Thus, we downgraded the evidence for pain at less than 24 hours by one level to moderate quality because of indirectness. We downgraded pain at days four to six, and pain at day seven or later by two levels for indirectness to low certainty. We downgraded the evidence for swelling at day seven or later by three levels to very low certainty; this reflected downgrading for serious study limitations and imprecision. We downgraded the evidence for return to function at day seven or later by two levels to low certainty for serious indirectness. We downgraded the evidence for gastrointestinal and neurological adverse effects by one level for indirectness.
NSAIDs versus combination analgesics (paracetamol and opioid)
The reasons for our assessment of the certainty of the evidence for each outcome displayed in Table 3 are presented in the footnotes. Two of the four trials for this comparison were at high risk of bias; and the other two were at unclear risk of several biases including selection bias. Hence, we downgraded the evidence by either one or two levels for study limitations. Another reason for downgrading was imprecision, especially where there were very few events. Another reason for downgrading was indirectness, which for swelling, reflected the inadequacy of outcome measurement. Another source of indirectness, which would have also counted against all four studies if the evidence from these had not already been downgraded, was that the dextropropoxyphene combination analgesic agents used by these now relatively dated studies, are no longer in general use. We downgraded the evidence for all outcomes by a minimum of three levels, to very low certainty.
Potential biases in the review process
Although the search strategy was sensitive, it is still possible that we missed potentially relevant studies. To minimise bias in the review process, two review authors independently undertook study selection, data extraction, and assessment of risk of bias, using a standardised data extraction form, and resolving any discrepancies by consensus with a third author. Decisions to pool or not pool results, based on similarities or differences in subgroups, may have affected the results of the study. We preplanned subgroup analyses based on optimal and suboptimal dosing of comparator analgesics, and combined data when it was appropriate to do so, based on the test for subgroup differences.
We included some studies with participants who did not have soft tissue injuries for which separate data were available. We think this is unlikely to be an important source of bias. All studies included a population that had 70% or more with soft tissue injuries; and effective randomisation should mean that the proportion of participants with other conditions, such as minor fractures, should be balanced among the treatment groups. From a clinical perspective in the acute setting, at the time of giving analgesia, it may not be known whether the injury is one of soft tissue or bone, so we believe this is a pragmatic, clinically relevant approach to dealing with this issue.
In Ekman 2006, which was a study run by a pharmaceutical company, the number of adverse events reported in the published article was substantially lower than that contained in unpublished trial data (356 versus 416 adverse events). As Ekman 2006 reported only adverse effects with at least 2% incidence, this could have resulted in an underestimation of the incidence of rare but potentially important adverse events. Four studies reported combined gastrointestinal and neurological adverse events at the event level rather than the participant level (Bourne 1980; Cukiernik 2007; Fathi 2015; Hung 2018). For these studies, we included participant‐level data for the most common gastrointestinal or neurological adverse event. This may also have led to an underestimation of adverse events in this review.
Agreements and disagreements with other studies or reviews
A recent systematic review also reported no difference in analgesic efficacy between NSAIDs and paracetamol, although most of the analysis was narrative, with meta‐analysis restricted to four trials, and the outcome of pain was measured at rest (Ridderikhof 2019). There is also systematic review evidence that there is no difference in analgesic efficacy between COX‐2 selective and non‐selective NSAIDs when used for acute soft tissue injuries; in this setting, COX‐2 selective NSAIDs had fewer gastrointestinal adverse effects than non‐selective NSAIDs, although the quality of evidence was low (Jones 2010). Our review showed that NSAIDs have more gastrointestinal adverse effects than paracetamol. However, the certainty of evidence was low. Of note, is that this effect was not found in (Ridderikhof 2018), where all participants also received omeprazole, a proton pump inhibitor. Our review also showed that compared with opioid there were fewer gastrointestinal or neurological adverse effects when NSAID was used. This is consistent with other studies where opioid was used in the setting of acute soft tissue injury (Gong 2019; Graudins 2016).
Authors' conclusions
Implications for practice.
Compared with paracetamol, NSAIDs make no difference to pain at one to two hours and at two to three days (high‐certainty evidence) and may make no difference at day seven or beyond (low‐certainty evidence). There is low‐certainty evidence that NSAIDs may make little difference to swelling after a week or more. We are uncertain whether NSAIDs make a difference to return to function at a week or over (very low‐certainty evidence). There is low‐certainly evidence that NSAIDs may result in a small increase in gastrointestinal adverse events and may make no difference in neurological adverse events compared with paracetamol.
Compared with opioids, NSAIDs probably make no difference to pain at one hour (moderate‐certainly evidence) and may make no difference at days four or seven (low‐certainty evidence). We are uncertain whether NSAIDs make a difference to swelling at 10 days (very low‐certainty evidence). There is low‐certainty evidence that NSAIDs may increase return to function at 7 to 10 days follow‐up. There is moderate‐certainty evidence that NSAIDs probably result in fewer gastrointestinal and neurological adverse effects compared with opioids.
There was very low‐certainty evidence for all outcomes for the NSAIDs versus paracetamol with opioid combination analgesics comparison. Thus we are uncertain of the findings of no between‐groups differences in pain, swelling, return to function or adverse effects.
The current evidence should not be extrapolated to adults older than 65 years, as this group was not well represented in the studies.
Implications for research.
Further studies of analgesic efficacy between oral analgesics currently used for acute soft tissue injury in young adults are not a priority; none of the evidence thus far has shown a discernable difference between any of them for the outcome of pain. However, this review raises other questions. The evidence regarding return to function remains incomplete, while the evidence of more gastrointestinal adverse effects with NSAID compared with paracetamol is low certainty and there were no studies of COX‐2 specific NSAID compared with paracetamol. These should be the primary outcome of future research around pharmacological interventions for acute soft tissue injuries. Further research is also warranted in older people with these injuries, again with a focus on functional benefit and adverse effects.
Feedback
Feedback on the NSAIDs versus paracetamol comparison, April 2016
Summary
Comment: I looked at this review because it was relevant to my current interest. I rather agree with the overall conclusions, but I do have some concerns about the way they were reached, and I think they may be even weaker than suggested.
Very briefly:
The references of Man and Woo share authors, and it looks very much as if the Man 2004 study was an early version of the complete study by Woo 2005. The only difference is one of smaller numbers. The review authors may have checked to see that this was not the case, and that data have not been duplicated, but it was not clear that this is the case. It might have been more prudent to have used only the later, larger study. The diclofenac dose was 25 mg (as the potassium salt admittedly), but that is not a large dose.
Bondarsky 2013 had participants with acute musculoskeletal injury, but made no mention of soft tissue injury. In that study, a combination of ibuprofen 800 mg and paracetamol 1000 mg was no better than paracetamol alone, while ibuprofen alone was at least numerically better over much of the first two hours. That is perhaps a reflection of random chance with small numbers, but it may also reflect a failed trial or something going haywire.
That leaves the children's study of Clark 2007, where you chose only the soft tissue data, and excluded the fracture data, where ibuprofen was predictably better than paracetamol. Again, that's fine because your title is soft tissue injury. But doses were on a per kg basis.
Given all this, and the combining of final pain score and negative pain changes in a meta‐analysis, is there actually anything we can say? I would argue that the evidence we have doesn't even come up to that of very low, yet you call it moderate on a mean difference of 1.5 mm on a 100‐mm pain scale. And this is on the basis of a very light analysis. A more detailed analysis would probably cast these results in an even less favourable light.
I have modified the conflict of interest statement below to declare my interests:
I have received institutional grant support from RB relating to individual patient level analyses of trial data on ibuprofen in acute pain and the effects of food on drug absorption of analgesics (2013), and from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). I have received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015).
Reply
Thank you for your interest in this review and taking the time to provide feedback.
In response to the first issue, the studies by Man and Woo were sequential, with the Man 2004 study recruiting participants from September to October 2001, and the Woo 2005 study recruiting participants from January 2002 to June 2003 (see the respective Results sections in the published articles). So it is not the case that data from the same participants were included in both studies. We agree that the dose of Diclofenac used in this study was suboptimal; however, this study was conducted in Asia, where there is local concern about the upper GI adverse effects of NSAIDs, and this is the standard dose used at the study site. This potential limitation is addressed in the current review.
With respect to your second point, about Bondarsky 2013, we had provided the criteria for our review in correspondence with the supervising author prior to publication of the study (which we had identified some years earlier, and prior to publication, through a 2011 conference abstract). In that correspondence, there was an assumption that the 'musculoskeletal' injuries were soft tissue injuries suitable for inclusion in the review. However, we concede that we did not subsequently confirm this when the final article was published, an issue that we will address when this review is revised. With respect to the difference in pain scores between paracetamol and ibuprofen at different time points in Bondarsky 2013, the differences were not clinically important at any point in time, and there was wide overlap of the 95% confidence intervals at all time points. Thus, we would argue that these results are consistent with no clinically meaningful difference between the analgesics in this study.
As you state in your third point, about Clark 2007, those with fractures were not eligible for the current review. It is standard practice for children to be prescribed medication on a per kg basis. Additionally, one of our review authors is a paediatric emergency medicine specialist, who advised on the appropriate doses of agents for children; these were set prior to starting the review.
With respect to your final point, we used the GRADE tool to assess the quality of evidence, and have been explicit in describing how we came to our conclusion about the quality of evidence, using this tool. Such assessments will always have a degree of subjectivity.
Contributors
Feedback submitted by: Andrew Moore, University of Oxford Reply prepared by: Peter Jones (review contact author) Editors: Helen Handoll (Co‐ordinating Editor, Cochrane Bone, Joint and Muscle Trauma Group); Cathie Sherrington (Feedback Editor; Cochrane Bone, Joint and Muscle Trauma Group)
What's new
| Date | Event | Description |
|---|---|---|
| 18 April 2020 | New citation required and conclusions have changed | The evidence for lack of difference in analgesic effect between the various comparator groups was strengthened and the worse adverse event profile of the opioid group was confirmed. There was a change to the byline, as two of the original authors did not contribute to this update. |
| 18 April 2020 | New search has been performed | We updated the search to January 2020. We included 4 new trials. We added extra adverse event data for two previously included trials. We reported on the funding and conflict of interest statements in the included trials. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 26 April 2016 | Feedback has been incorporated | Feedback about the NSAIDs versus paracetamol comparison incorporated |
Acknowledgements
We thank the following people for support and valuable editorial feedback for this version of the review: Joanne Elliott, Helen Handoll, and Maria Clark. We thank the external referees, Dominic Aldington and Ewan McNicol, for their helpful feedback.
The following people were authors on the first iteration of this review, and their contribution should be recognised: Jennifer Miles‐Chan screened studies for inclusion, wrote to authors for clarification where data were incomplete, extracted data, and helped revise the manuscript, and Christopher Frampton provided statistical support.
This project was supported by a grant (NZD $2,500) from the Auckland District Health Board Charitable Trust, known as A+ Trust (A+4185) and the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. Stuart R Dalziel's time is supported by Cure Kids New Zealand. The views and opinions expressed therein are those of the authors, and do not necessarily reflect those of the Auckland District Health Board, Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Additional information on adverse events of NSAIDs
This section was compiled as part of the protocol for the original version of this review in 2009, and was not updated for the current version of the review.
Gastrointestinal
The erosive effects of non‐steroidal anti‐inflammatory drugs (NSAIDs) on the upper gastrointestinal tract are well recognised, and are the most commonly reported adverse outcome following NSAID use (Burke 2006). The incidence of peptic ulcer disease in chronic NSAID users (six months to two years) has been estimated to be as high as 20% (Wright 1995). Variation in the reported incidence relates to the type and quality of studies, with cohort studies reporting a lower rate than case‐control (Bollini 1992). As part of the Saskatchewan NSAID exposure‐outcome study, Singh reported 15% of NSAID users with a complaint of gastrointestinal tract upset, with a hospitalisation rate of 2.2% (Singh 1996). This represents a risk ratio (RR) of hospitalisation for upper gastrointestinal tract disease in NSAID users at three times that of non‐users. Less well recognised are NSAID‐related small and large intestinal effects, such as protein‐losing enteropathy and inflammation, which can be complicated by perforation, band fibrosis, and obstruction (Kaufman 1996). NSAIDs also have a hepatotoxic effect, with the rate of acute liver injury in current NSAID users at twice that of non‐users (García Rodríguez 1992). To date, there is only one published report on the risk of adverse gastrointestinal effects of very short‐course NSAIDs (1 to 12 days in the postoperative setting), with one peptic ulcer occurring in 750 NSAID participants (Merry 2004). Selective COX‐2 inhibitors are believed to have fewer gastrointestinal side effects than traditional non‐selective NSAIDs (Burke 2006; Schnitzer 2004). Opiate analgesics are known to cause constipation, and in extreme cases, subacute bowel obstruction (Gutstein 2006).
Renal
Both selective and non‐selective NSAID‐mediated inhibition of COX lead to disruption of renovascular autoregulation, and hence to a reduction in renal blood flow (Burke 2006). This is not evident in healthy individuals at rest, but becomes significant in both healthy exercising subjects (Walker 1994), and those with pre‐existing risk factors, such as sodium or volume depletion, renovascular disease, and critical illness (Brooks 1991). There are case reports of acute renal failure in association with NSAID use in previously healthy athletes (Seedat 1990; Vitting 1986), postoperative patients (Feldman 1997), and in association with binge drinking of alcohol (Johnson 1995). A population‐based, case‐control study found the risk of idiopathic acute renal failure was rare (2/100,000 person‐years) for non‐users of NSAIDs, but increased eight‐fold with NSAID use within the preceding month (Pérez Gutthann 1996).
Cardiovascular
Long‐term use of both selective COX‐2 inhibitors and traditional non‐selective NSAIDs is associated with an increased risk of cardiovascular disease, particularly ischaemic heart disease. However, there is no information on the effect of very short‐term use of NSAIDs on the heart (Chan 2006; Farkouh 2004; Kearney 2006).
Central nervous system
Opiate analgesics share central nervous system side effects (drowsiness, respiratory depression) distinct from non‐opiate analgesics (Gutstein 2006). However, lipid soluble NSAIDs can alter mood perception and cognition (Brooks 1991); there are case reports of depressive illness, two with paranoid features, related to short‐course oral NSAID use (one to three days), which were reproducible on re‐challenge with NSAIDs (Browning 1996).
Respiratory
One in 10 asthmatics may be NSAID‐sensitive, with the precipitation of bronchospasm (Szczeklik 1987). This may be life threatening in those with the triad of nasal polyps, asthma, and aspirin sensitivity (Amadio 1997).
Haematological
NSAIDs inhibit the second phase of platelet aggregation, detectable as an increase in bleeding time, although this usually remains within the normal range. Thrombocytopenia has been reported with most non‐selective NSAIDs (Todd 1988). This may become significant in people with impaired haemostasis, and subgroups of postoperative patients (Merry 2004). Agranulocytosis and aplastic anaemia are also reported, although the incidence is very low (Henry 1990). NSAIDs also reduce neutrophil chemotaxis and activation (Partsch 1990).
Dermatological
Photosensitivity is a recognised side effect of NSAID use, as are the rare, life‐threatening, Stevens‐Johnson syndrome and toxic epidermal necrolysis (Henry 1990).
Infection
NSAIDs have been associated with life‐threatening soft tissue infections, such as necrotising fasciitis. A direct causal link is not proven, although many authors have cautioned about the use of NSAIDs where infection is possible (Rietveld 1995).
Early re‐injury
As inflammation is integral to healing of soft tissue, some authors believe that NSAIDs may impair healing and lead to a risk of early re‐injury (Jones 1999; Major 1992; Paoloni 2005).
Appendix 2. Search strategies for this update
CENTRAL (CRS Web)
The CENTRAL search was run in two stages: the first search was run in February 2019 and a second top‐up search was run in January 2020.
Search 1
1. MeSH DESCRIPTOR Anti‐Inflammatory Agents, Non‐Steroidal EXPLODE ALL AND CENTRAL:TARGET (15882) 2. MeSH DESCRIPTOR Prostaglandin Antagonists EXPLODE ALL AND CENTRAL:TARGET (69) 3. MeSH DESCRIPTOR Cyclooxygenase Inhibitors EXPLODE ALL AND CENTRAL:TARGET (12379) 4. ((nonsteroid* adj antiinflammator*) or (nonsteroid* adj anti‐inflammator*)): AB,EH,KW,KY,MC,MH,TI,TO (4792) 5. ((non steroid* adj antiinflammator*) or (non steroid* adj anti‐inflammator*)): AB,EH,KW,KY,MC,MH,TI,TO (7933) 6. (NSAID*): AB,EH,KW,KY,MC,MH,TI,TO (4548) 7. (nonsteroid* adj analgesi*): AB,EH,KW,KY,MC,MH,TI,TO (433) 8. (non adj steroid* adj analgesi*): AB,EH,KW,KY,MC,MH,TI,TO (713) 9. (cox 2 inhib* or cyclooxygenase 2 inhib*): AB,EH,KW,KY,MC,MH,TI,TO (1340) 10. (rofecoxib or celecoxib or parecoxib or Imrecoxib or valdecoxib or etoricoxib or cimicoxib or deracoxib or tiracoxib or lumiracoxib or firocoxib or lefucoxib or *coxib* or nimesulide or acetaminophen or paracetamol or tramadol or codeine or dextropropoxyphene or *propoxyphene or hydrocodone or dihydrocodeine or oxycodone or meperidine or pethidine or morphine or methadone or diclofenac or aspirin or Sodium Salicylate or Salicylates or Salicyl* or diflunisal or etodolac or fenoprofen or flurbiprofen or ibuprofen or indomethacin or ketoprofen or suprofen or ketorolac or mefenamic acid or meloxicam or nabumetone or naproxen or oxaprozin or piroxicam or sulindac or tolmetin or niflumic acid or dipyrone or oxyphenbutazone or phenlybutazone or isoxazoles or sulphonamides): AB,EH,KW,KY,MC,MH,TI,TO (52125) 11. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 (57860) 12. MeSH DESCRIPTOR Soft Tissue Injuries EXPLODE ALL AND CENTRAL:TARGET (75) 13. MeSH descriptor Muscle, Skeletal AND CENTRAL:TARGET (6563) 14. MeSH DESCRIPTOR Ligaments EXPLODE ALL AND CENTRAL:TARGET (1141) 15. MeSH DESCRIPTOR Ligaments, Articular EXPLODE ALL AND CENTRAL:TARGET (1075) 16. MeSH DESCRIPTOR Tendons EXPLODE ALL AND CENTRAL:TARGET (1111) 17. MeSH DESCRIPTOR Tendon Injuries EXPLODE ALL AND CENTRAL:TARGET(746) 18. MeSH DESCRIPTOR Tendinopathy EXPLODE ALL AND CENTRAL:TARGET (586) 19. MeSH DESCRIPTOR Sprains and Strains AND CENTRAL:TARGET (296) 20. MeSH DESCRIPTOR Contusions AND CENTRAL:TARGET (98) 21. MeSH DESCRIPTOR Athletic Injuries AND CENTRAL:TARGET (548) 22. ((soft tissue or musc* or tendon* or ligament* or athlet* or sport*) and (trauma or injur*)): AB,EH,KW,KY,MC,MH,TI,TO (9806) 23. (sprain* or strain*): AB,EH,KW,KY,MC,MH,TI,TO (10286) 24. (bruis* or contus*): AB,EH,KW,KY,MC,MH,TI,TO (1349) 25. (tendinous or tendinopathy or tenosynovitis): AB,EH,KW,KY,MC,MH,TI,TO (856) 26. MeSH DESCRIPTOR Musculoskeletal Pain WITH QUALIFIER DT AND CENTRAL:TARGET (37) 27. #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26(22117) 28. #11 and #27 (1276) 29. 01/09/2014_TO_06/02/2019:CRSCREATED AND CENTRAL:TARGET (510832) 30. 28 and 29 (430)
Search 2
29. 06/02/2019_TO_29/01/2020:CRSCREATED AND CENTRAL:TARGET (348321) 30. 28 and 29 (424)
MEDLINE Ovid
The MEDLINE search was run in two stages: the first search was run in February 2019 and a second top‐up search was run in January 2020.
Search 1
1 exp Anti‐Inflammatory Agents, Non‐Steroidal/ (196028) 2 exp Prostaglandin Antagonists/ (2810) 3 exp Cyclooxygenase Inhibitors/ (124277) 4 ((nonsteroid* adj antiinflammator*) or (nonsteroid* adj anti‐inflammator*)).tw. (20314) 5 ((non steroid* adj antiinflammator*) or (non steroid* adj anti‐inflammator*)).tw. (16563) 6 nsaid*.tw. (23160) 7 (nonsteroid* adj analgesi*).tw. (72) 8 (non adj steroid* adj analgesi*).tw. (96) 9 (cox 2 inhib* or cyclooxygenase 2 inhib*).tw. (9645) 10 ("rofecoxib" or "celecoxib" or "parecoxib" or "Imrecoxib" or "valdecoxib" or "etoricoxib" or "cimicoxib" or "deracoxib" or "tiracoxib" or "lumiracoxib" or "firocoxib" or "lefucoxib" or "*coxib*" or "nimesulide" or "acetaminophen" or "paracetamol" or "tramadol" or "codeine" or "dextropropoxyphene" or "*propoxyphene" or "hydrocodone" or "dihydrocodeine" or "oxycodone" or "meperidine" or "pethidine" or "morphine" or "methadone" or "diclofenac" or "aspirin" or "Sodium Salicylate" or "Salicylates" or "Salicyl*" or "diflunisal" or "etodolac" or "fenoprofen" or "flurbiprofen" or "ibuprofen" or "indomethacin" or "ketoprofen" or "suprofen" or "ketorolac" or "mefenamic acid" or "meloxicam" or "nabumetone" or "naproxen" or "oxaprozin" or "piroxicam" or "sulindac" or "tolmetin" or "niflumic acid" or "dipyrone" or "oxyphenbutazone" or "phenlybutazone" or "isoxazoles" or "sulphonamides" or "acetylsalicyl*" or "prostaglandin synthase inhib*" or "meclofenamic acid" or "timicoxib").tw. (236006) 11 or/1‐10 (352913) 12 Soft Tissue Injuries/ (5015) 13 Muscle, Skeletal/ (132348) 14 exp Ligaments/ or exp Ligaments, Articular/ (37797) 15 exp Tendons/ (39222) 16 exp tendon injuries/ (22528) 17 exp tendinopathy/ (11317) 18 exp Sprains/ and Strains/ (5059) 19 Contusions/ (4759) 20 Athletic Injuries/ (25619) 21 ((soft tissue or musc* or tendon* or ligament* or athlet* or sport*) and (trauma or injur*)).tw. (111281) 22 (sprain* or strain*).tw. (673823) 23 (bruis* or contus*).tw. (15576) 24 (tendinous or tendinopathy or tenosynovitis).tw. (8102) 25 Musculoskeletal Pain/dt [Drug Therapy] (249) 26 or/12‐25 (992379) 27 11 and 26 (7695) 28 randomised controlled trial.pt. (476030) 29 controlled clinical trial.pt. (92909) 30 randomized.ab. (434190) 31 placebo.ab. (195285) 32 drug therapy.fs. (2083182) 33 randomly.ab. (305135) 34 trial.ab. (453314) 35 groups.ab. (1879270) 36 or/28‐35 (4374316) 37 exp animals/ not humans.sh. (4546384) 38 36 not 37 (3782438) 39 27 and 38 (1871) 40 (201409* or 201410* or 201411* or 201412*or 2015* or 2016* or 2017* or 2018* or 2019*).ed,dt. (4852612) 41 39 and 40 (380)
Search 2
40 (2019* or 2020*).ed,dt. (2135148) 41 39 and 40 (138)
Embase Ovid
The Embase search was run in two stages: the first search was run in February 2019 and a second top‐up search was run in January 2020.
Search 1
1 exp Nonsteroid Antiinflammatory Agent/ (688334) 2 exp Prostaglandin Synthase Inhibitor/ (505662) 3 exp Cyclooxygenase 2 Inhibitor/ (49884) 4 ((nonsteroid* adj antiinflammator*) or (nonsteroid* adj anti‐inflammator*)).tw. (24773) 5 ((non steroid* adj antiinflammator*) or (non steroid* adj anti‐inflammator*)).tw. (23546) 6 nsaid*.tw. (39739) 7 (nonsteroid* adj analgesi*).tw. (92) 8 (non adj steroid* adj analgesi*).tw. (148) 9 (cox 2 inhib* or cyclooxygenase 2 inhib*).tw. (12730) 10 ("rofecoxib" or "celecoxib" or "parecoxib" or "Imrecoxib" or "valdecoxib" or "etoricoxib" or "cimicoxib" or "deracoxib" or "tiracoxib" or "lumiracoxib" or "firocoxib" or "lefucoxib" or "*coxib*" or "nimesulide" or "acetaminophen" or "paracetamol" or "tramadol" or "codeine" or "dextropropoxyphene" or "*propoxyphene" or "hydrocodone" or "dihydrocodeine" or "oxycodone" or "meperidine" or "pethidine" or "morphine" or "methadone" or "diclofenac" or "aspirin" or "Sodium Salicylate" or "Salicylates" or "Salicyl*" or "diflunisal" or "etodolac" or "fenoprofen" or "flurbiprofen" or "ibuprofen" or "indomethacin" or "ketoprofen" or "suprofen" or "ketorolac" or "mefenamic acid" or "meloxicam" or "nabumetone" or "naproxen" or "oxaprozin" or "piroxicam" or "sulindac" or "tolmetin" or "niflumic acid" or "dipyrone" or "oxyphenbutazone" or "phenlybutazone" or "isoxazoles" or "sulphonamides" or "acetylsalicyl*" or "prostaglandin synthase inhib*" or "meclofenamic acid" or "timicoxib").tw. (355236) 11 or/1‐10 (855239) 12 Soft Tissue Injury/ (7894) 13 muscle injury/ or musculoskeletal injury/ (14882) 14 exp Ligament/ or exp Ligament Injury/ (68520) 15 exp Tendon/ (34226) 16 exp tendon injury/ (20581) 17 exp tendinitis/ (15233) 18 exp Sprain/ (5040) 19 contusion/ (7803) 20 Skin Bruising/ (4013) 21 Sport Injury/ (27738) 22 ((soft tissue or musc* or tendon* or ligament* or athlet* or sport*) and (trauma or injur*)).tw. (141778) 23 (sprain* or strain*).tw. (731779) 24 (bruis* or contus*).tw. (21274) 25 (tendinous or tendinopathy or tenosynovitis).tw. (10043) 26 Musculoskeletal Pain/dt [Drug Therapy] (1064) 27 or/12‐26 (997594) 28 11 and 27 (18497) 29 exp Randomized Controlled Trial/ or exp Single Blind Procedure/ or exp Double Blind Procedure/ or Crossover Procedure/ (599890) 30 (random* or RCT or placebo or allocat* or crossover* or 'cross over' or trial or (doubl* adj1 blind*) or (singl* adj1 blind*)).ti,ab. (1931516) 31 29 or 30 (2020966) 32 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (6047742) 33 31 not 32 (1787918) 34 28 and 33 (1835) 35 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).dc,yr. (8257465) 36 34 and 35 (560)
Search 2
35 (2019* or 2020*).dc,yr. (2271004) 36 34 and 35 (115)
CINAHL EBSCO
S1 (MH "Antiinflammatory Agents, Non‐Steroidal+") (27,363) S2 (MH "Prostaglandin Antagonists") (119) S3 (MH "Cox‐2 Inhibitors") (3,490) S4 nsaid* (4,831) S5 cox 2 inhib* or cyclooxygenase 2 inhib* (289) S6 rofecoxib or celecoxib or parecoxib or Imrecoxib or valdecoxib or etoricoxib or cimicoxib or deracoxib or tiracoxib or lumiracoxib or firocoxib or lefucoxib or *coxib* or nimesulide or acetaminophen or paracetamol or tramadol or codeine or dextropropoxyphene or *propoxyphene or hydrocodone or dihydrocodeine or oxycodone or meperidine or pethidine or morphine or methadone or diclofenac or aspirin or Sodium Salicylate or Salicylates or Salicyl* or diflunisal or etodolac or fenoprofen or flurbiprofen or ibuprofen or indomethacin or ketoprofen or suprofen or ketorolac or mefenamic acid or meloxicam or nabumetone or naproxen or oxaprozin or piroxicam or sulindac or tolmetin or niflumic acid or dipyrone or oxyphenbutazone or phenlybutazone or isoxazoles or sulphonamides or acetylsalicyl* or prostaglandin synthase inhib* or meclofenamic acid or timicoxib (46,211) S7 S1 or S2 or S3 or S4 or S5 or S6 (57,027) S8 (MH "Soft Tissue Injuries") (1,957) S9 (MH "Muscle, Skeletal+/IN") (2,310) S10 (MH "Ligament Injuries+") (6,596) S11 (MH "Tendon Injuries+") (6,066) S12 (MH "Tendinopathy+") (3,848) S13 (MH "Sprains and Strains+") (9,036) S14 (MH "Contusions and Abrasions") (1,197) S15 (MH "Athletic Injuries") (16,069) S16 ( soft tissue or musc* or tendon* or ligament* or athlet* or sport* ) AND ( trauma or injur* ) (62,701) S17 sprain* or strain* (35,502) S18 bruis* or contus* (3,478) S19 tendinous or tendinopathy or tenosynovitis (4,653) S20 (MH "Muscle Pain/DT") (110) S21 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 (104,461) S22 S7 AND S21 (1,160) S23 (MH "Clinical Trials+") (254,120) S24 PT Clinical trial (86,743) S25 TX clinic* n1 trial* (238,566) S26 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) (1,003,190) S27 TX randomi* control* trial* (163,216) S28 (MH "Random Assignment") (53,365) S29 TX random* allocat* (9,818) S30 TX placebo* (55,292) S31 (MH "Placebos") (11,134) S32 (MH "Quantitative Studies") (21,821) S33 TX allocat* random* (9,818) S34 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 (1,303,755) S35 S22 AND S34 (370) S36 EM 2012 OR EM 2013 OR EM 2014 OR EM 2015 OR EM 2016 OR EM 2017 OR EM 2018 OR EM 2019 (2,737,716) S37 S35 AND S36 (125)
AMED Ovid
1 exp Antiinflammatory agents nonsteroidal/ (384) 2 exp analgesics/ (1884) 3 ((nonsteroid* adj antiinflammator*) or (nonsteroid* adj anti‐inflammator*)).af. (262) 4 ((non steroid* adj antiinflammator*) or (non steroid* adj anti‐inflammator*)).af. (142) 5 nsaid*.af. (262) 6 (cox 2 inhib* or cyclooxygenase 2 inhib*).af. (83) 7 ("rofecoxib" or "celecoxib" or "parecoxib" or "Imrecoxib" or "valdecoxib" or "etoricoxib" or "cimicoxib" or "deracoxib" or "tiracoxib" or "lumiracoxib" or "firocoxib" or "lefucoxib" or "*coxib*" or "nimesulide" or "acetaminophen" or "paracetamol" or "tramadol" or "codeine" or "dextropropoxyphene" or "*propoxyphene" or "hydrocodone" or "dihydrocodeine" or "oxycodone" or "meperidine" or "pethidine" or "morphine" or "methadone" or "diclofenac" or "aspirin" or "Sodium Salicylate" or "Salicylates" or "Salicyl*" or "diflunisal" or "etodolac" or "fenoprofen" or "flurbiprofen" or "ibuprofen" or "indomethacin" or "ketoprofen" or "suprofen" or "ketorolac" or "mefenamic acid" or "meloxicam" or "nabumetone" or "naproxen" or "oxaprozin" or "piroxicam" or "sulindac" or "tolmetin" or "niflumic acid" or "dipyrone" or "oxyphenbutazone" or "phenlybutazone" or "isoxazoles" or "sulphonamides" or "acetylsalicyl*" or "prostaglandin synthase inhib*" or "meclofenamic acid" or "timicoxib").af. (1860) 8 1 or 2 or 3 or 4 or 5 or 6 or 7 (3621) 9 Soft tissue/ or Injuries/ (3863) 10 Muscle skeletal/ (4297) 11 ligaments/ or ligaments articular/ (1133) 12 exp ligaments/ or exp ligaments articular/ (2668) 13 exp Tendons/ (2240) 14 exp Tendon injuries/ (995) 15 exp tendinopathy/ (418) 16 exp "Sprains and strains"/ (995) 17 contusions/ (24) 18 Athletic injuries/ (4064) 19 ((soft tissue or musc* or tendon* or ligament* or athlet* or sport*) and (trauma or injur*)).af. (11943) 20 (sprain* or strain*).af. (4444) 21 (bruis* or contus*).af. (195) 22 (tendinous or tendinopathy or tenosynovitis).af. (743) 23 exp musculoskeletal pain/ (185) 24 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 (23049) 25 8 and 24 (143) 26 (2012* or 2013* or 2014* or 2015* or 2016* or 2017* or 2018* or 2019*).yr. (64829) 27 25 and 26 (44)
International Pharmaceutical Abstracts Ovid
1 ((nonsteroid* adj antiinflammator*) or (nonsteroid* adj anti‐inflammator*)).af. (4060) 2 ((non steroid* adj antiinflammator*) or (non steroid* adj anti‐inflammator*)).af. (1084) 3 nsaid*.af. (2700) 4 (nonsteroid* adj analgesi*).af. (12) 5 (non adj steroid* adj analgesi*).af. (4) 6 (cox 2 inhib* or cyclooxygenase 2 inhib*).af. (859) 7 ("rofecoxib" or "celecoxib" or "parecoxib" or "Imrecoxib" or "valdecoxib" or "etoricoxib" or "cimicoxib" or "deracoxib" or "tiracoxib" or "lumiracoxib" or "firocoxib" or "lefucoxib" or "*coxib*" or "nimesulide" or "acetaminophen" or "paracetamol" or "tramadol" or "codeine" or "dextropropoxyphene" or "*propoxyphene" or "hydrocodone" or "dihydrocodeine" or "oxycodone" or "meperidine" or "pethidine" or "morphine" or "methadone" or "diclofenac" or "aspirin" or "Sodium Salicylate" or "Salicylates" or "Salicyl*" or "diflunisal" or "etodolac" or "fenoprofen" or "flurbiprofen" or "ibuprofen" or "indomethacin" or "ketoprofen" or "suprofen" or "ketorolac" or "mefenamic acid" or "meloxicam" or "nabumetone" or "naproxen" or "oxaprozin" or "piroxicam" or "sulindac" or "tolmetin" or "niflumic acid" or "dipyrone" or "oxyphenbutazone" or "phenlybutazone" or "isoxazoles" or "sulphonamides" or "acetylsalicyl*" or "prostaglandin synthase inhib*" or "meclofenamic acid" or "timicoxib").af. (34455) 8 1 or 2 or 3 or 4 or 5 or 6 or 7 (37137) 9 ((soft tissue or musc* or tendon* or ligament* or athlet* or sport*) and (trauma or injur*)).af. (642) 10 (sprain* or strain*).af. (6614) 11 (bruis* or contus*).af. (217) 12 (tendinous or tendinopathy or tenosynovitis).af. (56) 13 9 or 10 or 11 or 12 (7447) 14 8 and 13 (287) 15 (2012* or 2013* or 2014* or 2015* or 2016* or 2017* or 2018* or 2019*).yr. (117190) 16 14 and 15 (51)
SPORTDiscus EBSCO
S1 DE "NONSTEROIDAL anti‐inflammatory agents" OR DE "ASPIRIN" OR DE "FLURBIPROFEN" OR DE "IBUPROFEN" OR DE "INDOMETHACIN" OR DE "KETOROLAC (Drug)" OR DE "NAPROXEN" OR DE "PHENYLBUTAZONE" OR DE "PIROXICAM" (1,715) S2 nsaid* (880) S3 cox 2 inhib* or cyclooxygenase 2 inhib* (143) S4 rofecoxib or celecoxib or parecoxib or Imrecoxib or valdecoxib or etoricoxib or cimicoxib or deracoxib or tiracoxib or lumiracoxib or firocoxib or lefucoxib or *coxib* or nimesulide or acetaminophen or paracetamol or tramadol or codeine or dextropropoxyphene or *propoxyphene or hydrocodone or dihydrocodeine or oxycodone or meperidine or pethidine or morphine or methadone or diclofenac or aspirin or Sodium Salicylate or Salicylates or Salicyl* or diflunisal or etodolac or fenoprofen or flurbiprofen or ibuprofen or indomethacin or ketoprofen or suprofen or ketorolac or mefenamic acid or meloxicam or nabumetone or naproxen or oxaprozin or piroxicam or sulindac or tolmetin or niflumic acid or dipyrone or oxyphenbutazone or phenlybutazone or isoxazoles or sulphonamides or acetylsalicyl* or prostaglandin synthase inhib* or meclofenamic acid or timicoxib(5,301) S5 S1 OR S2 OR S3 OR S4 (6,130) S6 DE "SOFT tissue injuries" (665) S7 DE "LIGAMENT injuries" OR DE "COLLATERAL ligament injuries" OR DE "CRUCIATE ligament injuries" OR DE "PATELLAR ligament injuries" (911) S8 DE "TENDON injuries" OR DE "ACHILLES tendon injuries" (1,153) S9 DE "TENDINOSIS" (190) S10 DE "SPRAINS" OR DE "AVULSION fractures" OR DE "SKIER'S thumb" (2,735) S11 DE "BRUISES" (454) S12 DE "SPORTS injuries" OR DE "ACHILLES tendinitis" OR DE "AEROBICS injuries" OR DE "AQUATIC sports injuries" OR DE "BASEBALL injuries" OR DE "BASKETBALL injuries" OR DE "BOXING injuries" OR DE "COMMOTIO cordis" OR DE "CRICKET injuries" OR DE "DELAYED onset muscle soreness" OR DE "EQUESTRIAN accidents" OR DE "FOOTBALL injuries" OR DE "GOLF injuries" OR DE "GYMNASTICS injuries" OR DE "HIKING injuries" OR DE "HOCKEY injuries" OR DE "HORSE sports injuries" OR DE "IN‐line skating injuries" OR DE "JOGGING injuries" OR DE "JUDO injuries" OR DE "JUMPER'S knee" OR DE "KARATE injuries" OR DE "MARTIAL arts injuries" OR DE "MOTORSPORTS injuries" OR DE "NETBALL injuries" OR DE "RACKET game injuries" OR DE "RUGBY football injuries" OR DE "RUNNING injuries" OR DE "SKATEBOARDING injuries" OR DE "SOCCER injuries" OR DE "TENNIS injuries" OR DE "TURF toe" OR DE "VAULTING injuries" OR DE "VOLLEYBALL injuries" OR DE "WALKING (Sports) injuries" OR DE "WEIGHT training injuries" OR DE "WINTER sports injuries" (18,667) S13 ( soft tissue or musc* or tendon* or ligament* or athlet* or sport* ) AND ( trauma or injur* ) (94,871) S14 sprain* or strain* (14,085) S15 bruis* or contus* (1,768) S16 tendinous or tendinopathy or tenosynovitis (2,397) S17 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 (108,229) S18 S5 AND S17 (623) S19 TX ( (clinic* N3 trial) or (controlled N3 trial) or (comparative N3 trial) or (placebo N3 trial) or (prospective N3 trial) or (randomi?ed N3 trial) ) or TX ( (clinic* N3 study) or (controlled N3 study) or (comparative N3 study) or (placebo N3 study) or (prospective N3 study) or (randomi?ed N3 study) ) (88,331) S20 (random* N7 allot*) or (random* N7 assign*) or (random* N7 basis*) or (random* N7 divid*) or (random* N7 order*) (12,338) S21 TX ( (singl* N7 blind*) or (doubl* N7 blind*) or (trebl* N7 blind*) or (tripl* N7 blind*) ) or TX ( (singl* N7 mask*) or (doubl* N7 mask*) or (trebl* N7 mask*) or (tripl* N7 mask*) ) (7,449) S22 TX (cross#over*) or TX (cross N1 over*) (6,324) S23 TX randomi?ed control* trial* (17,313) S24 TX ( (allocat* N3 condition*) or (allocat* N3 experiment*) or (allocat* N3 intervention*) or (allocat* N3 treatment*) or (allocat* N3 therap*) or (allocat* N3 control*) or (allocat* N3 group*) ) or TX ( (allot* N3 condition*) or (allot* N3 experiment*) or (allot* N3 intervention*) or (allot* N3 treatment*) or (allot* N3 therap*) or (allot* N3 control*) or (allot* N3 group*) ) or TX ( (assign* N3 condition*) or (assign* N3 experiment*) or (assign* N3 intervention*) or (assign* N3 treatment*) or (assign* N3 therap*) or (assign* N3 control*) or (assign* N3 group*) ) or TX ( (divid* N3 condition*) or (divid* N3 experiment*) or (divid* N3 intervention*) or (divid* N3 treatment*) or (divid* N3 therap*) or (divid* N3 control*) or (divid* N3 group*) ) (13,681) S25 TX placebo* (10,509) S26 S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 (108,655) S27 S18 AND S26 (208) S28 2012 to 2019 (48)
PEDro
Simple search
Search term (or terms):
*xib OR nimesulide OR paracetamol OR tramadol OR *phen OR *codone OR *codeine OR *idine OR morphine OR methadone OR diclofenac OR aspirin OR Salicyl* OR diflunisal OR etodolac OR *profen OR indomethacin OR ketorolac OR mefenamic OR *xicam OR nabumetone OR naproxen OR oxaprozin OR sulindac OR tolmetin OR niflumic OR dipyrone OR *butazone OR isoxazoles OR sulphonamides (444)
ClinicalTrials.gov
1. NSAID OR Non‐steroidal anti‐inflammatory OR Prostaglandin OR Cyclooxygenase OR Cox | Interventional Studies | soft tissue injury OR trauma OR tendon OR ligament OR sprain OR strain OR contusion OR bursitis OR bruise OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain | First posted from 11/01/2012 to 02/19/2019 (250)
2. rofecoxib OR celecoxib OR parecoxib OR Imrecoxib OR valdecoxib OR etoricoxib OR cimicoxib OR deracoxib OR tiracoxib OR lumiracoxib OR firocoxib OR lefucoxib OR nimesulide OR acetaminophen OR paracetamol OR tramadol OR codeine OR dextropropoxyphene | Interventional Studies | soft tissue injury OR trauma OR tendon OR ligament OR sprain OR strain OR contusion OR bursitis OR bruise OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain | First posted from 11/01/2012 to 02/19/2019 (177)
3. hydrocodone OR dihydrocodeine OR oxycodone OR meperidine OR pethidine OR morphine OR methadone OR diclofenac OR aspirin OR Sodium Salicylate OR Salicylates OR diflunisal OR etodolac OR fenoprofen OR flurbiprofen OR ibuprofen OR indomethacin | Interventional Studies | soft tissue injury OR trauma OR tendon OR ligament OR sprain OR strain OR contusion OR bursitis OR bruise OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain | First posted from 11/01/2012 to 02/19/2019 (343)
4. ketoprofen OR suprofen OR ketorolac OR mefenamic acid OR meloxicam OR nabumetone OR naproxen OR oxaprozin OR piroxicam OR sulindac OR tolmetin OR niflumic acid OR dipyrone OR oxyphenbutazone OR phenlybutazone OR isoxazoles OR sulphonamides | Interventional Studies | soft tissue injury OR trauma OR tendon OR ligament OR sprain OR strain OR contusion OR bursitis OR bruise OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain | First posted from 11/01/2012 to 02/19/2019 (68)
5. acetylsalicylic OR prostaglandin synthase OR meclofenamic acid OR timicoxib | Interventional Studies | soft tissue injury OR trauma OR tendon OR ligament OR sprain OR strain OR contusion OR bursitis OR bruise OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain | First posted from 11/01/2012 to 02/19/2019 (135)
WHO ICTRP
Advance search, all available registries
1. Condition: soft tissue injur* OR tendon OR ligament OR sprain* OR strain* OR contus* OR bruise OR buris* OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain Intervention: nsaid or non steroidal anti inflammatory or non‐steroidal anti‐inflammatory OR Cox OR Cyclooxygenase OR Prostaglandin 01/11/2012 and 18/02/2019 (146)
2. Condition: soft tissue injur* OR tendon OR ligament OR sprain* OR strain* OR contus* OR bruise OR buris* OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain Intervention: rofecoxib OR celecoxib OR parecoxib OR Imrecoxib OR valdecoxib OR etoricoxib OR cimicoxib OR deracoxib OR tiracoxib OR lumiracoxib OR firocoxib OR lefucoxib OR nimesulide OR acetaminophen OR paracetamol OR tramadol OR codeine OR dextropropoxyphene 01/11/2012 and 18/02/2019 (148)
3. Condition: soft tissue injur* OR tendon OR ligament OR sprain* OR strain* OR contus* OR bruise OR buris* OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain Intervention: hydrocodone OR dihydrocodeine OR oxycodone OR meperidine OR pethidine OR morphine OR methadone OR diclofenac OR aspirin OR Sodium Salicylate OR Salicylates OR diflunisal OR etodolac OR fenoprofen OR flurbiprofen OR ibuprofen OR indomethacin 01/11/2012 and 18/02/2019 (179)
4. Condition: soft tissue injur* OR tendon OR ligament OR sprain* OR strain* OR contus* OR bruise OR buris* OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain Intervention: ketoprofen OR suprofen OR ketorolac OR mefenamic acid OR meloxicam OR nabumetone OR naproxen OR oxaprozin OR piroxicam OR sulindac OR tolmetin OR niflumic acid OR dipyrone OR oxyphenbutazone OR phenlybutazone OR isoxazoles OR sulphonamides 01/11/2012 and 18/02/2019 (144)
5. Condition: soft tissue injur* OR tendon OR ligament OR sprain* OR strain* OR contus* OR bruise OR buris* OR tendinous OR tendinopathy OR tenosynovitis OR Musculoskeletal Pain Intervention: acetylsalicylic OR prostaglandin synthase OR meclofenamic acid OR timicoxib (4) 01/11/2012 and 18/02/2019 (4)
Data and analyses
Comparison 1. NSAIDs versus paracetamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain < 24 hours (VAS: 0 to 100 mm: worst) | 6 | 1178 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐2.27, 2.03] |
| 1.1.1 Adequate dosing of both comparators | 4 | 933 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐2.69, 1.80] |
| 1.1.2 Suboptimal dosing of NSAID | 2 | 245 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐3.94, 10.73] |
| 1.2 Little or no pain < 24 hours | 2 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 1.2.1 Adequate dosing of both comparators | 2 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 1.3 Pain on days 1 to 3 (VAS: 0 to 100 mm: worst) | 6 | 1232 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [‐0.91, 3.91] |
| 1.3.1 Adequate dosing of both comparators | 1 | 365 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐5.16, 3.96] |
| 1.3.2 Suboptimal dosing of paracetamol | 2 | 186 | Mean Difference (IV, Fixed, 95% CI) | 4.71 [0.83, 8.59] |
| 1.3.3 Suboptimal dosing of NSAID | 3 | 681 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐4.59, 3.72] |
| 1.4 Little or no pain on days 1 to 3 | 3 | 894 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.96, 1.27] |
| 1.4.1 Adequate dosing of both comparators | 2 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.28] |
| 1.4.2 Suboptimal dosing of NSAID | 1 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.88, 1.44] |
| 1.5 Pain on days 4 to 6 (VAS: 0 to 100 mm: worst) | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐6.09, 4.73] |
| 1.5.1 Suboptimal dosing of both comparators | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐6.09, 4.73] |
| 1.6 Pain on day 7 or later (VAS: 0 to 100 mm: worst) | 4 | 467 | Mean Difference (IV, Fixed, 95% CI) | 1.55 [‐0.33, 3.43] |
| 1.6.1 Adequate dosing of both comparators | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐2.47, 6.47] |
| 1.6.2 Suboptimal dosing of paracetamol | 2 | 186 | Mean Difference (IV, Fixed, 95% CI) | 2.71 [0.45, 4.97] |
| 1.6.3 Suboptimal dosing of both comparators | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐5.14 [‐10.30, 0.02] |
| 1.7 Little or no pain on day 7 or later | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.1 Adequate dosing of both comparators | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.8 Swelling on days 0 to 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.8.1 Suboptimal dose of paracetamol | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9 Swelling on days 4 to 6 (VAS: 0 to 100 mm: worst) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9.1 Suboptimal dosing of both comparators | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10 Swelling on day 7 or later | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10.1 Suboptimal dosing of paracetamol | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10.2 Suboptimal dosing of both comparators | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11 Little or no swelling on day 7 or later | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.11.1 Adequate dosing of both comparators | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.12 Return to function within 7 days | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.12.1 Adequate dosing of both comparators | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.12.2 Suboptimal dosing of paracetamol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.13 Return to function in 7 days or later | 3 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.09] |
| 1.13.1 Adequate dosing of both comparators | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.14] |
| 1.13.2 Suboptimal dosing of paracetamol | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.74, 1.38] |
| 1.13.3 Suboptimal dosing of both comparators | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.10] |
| 1.14 Time to return to full activity (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14.1 Suboptimal dosing of paracetamol | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.15 Range of motion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.15.1 Suboptimal dosing of paracetamol | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.16 Gastrointestinal adverse events | 10 | 1504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.97, 1.86] |
| 1.16.1 Adequate dosing of both comparators | 4 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.80, 1.82] |
| 1.16.2 Suboptimal dosing of NSAID | 2 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.21, 16.54] |
| 1.16.3 Suboptimal dosing of paracetamol | 3 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.10, 4.15] |
| 1.16.4 Suboptimal dosing of both comparators | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.24, 2.26] |
| 1.17 Neurological adverse events | 9 | 1679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.17] |
| 1.17.1 Adequate dosing of both comparators | 5 | 1119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.14] |
| 1.17.2 Suboptimal dosing of NSAID | 2 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.15, 13.34] |
| 1.17.3 Suboptimal dosing of paracetamol | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.18, 2.93] |
| 1.17.4 Suboptimal dosing of both comparators | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.16 [0.25, 106.34] |
Comparison 2. NSAID versus opioid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain < 24 hours (VAS: 0 to 100 mm: worst) | 4 | 1058 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐3.05, 2.07] |
| 2.1.1 Adequate dosing of both comparators | 2 | 202 | Mean Difference (IV, Fixed, 95% CI) | ‐3.37 [‐9.17, 2.44] |
| 2.1.2 Suboptimal dosing of opioid | 1 | 706 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐4.41, 3.41] |
| 2.1.3 Suboptimal dosing of NSAID | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐3.16, 5.16] |
| 2.2 Little or no pain < 24 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Pain on days 4 to 6 (VAS: 0 to 100 mm: worst) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 Suboptimal dosing of opioid | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4 Pain on day 7 or later (VAS: 0 to 100 mm: worst) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4.1 Suboptimal dosing of opioid | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Swelling on day 7 or later | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.6 Return to function within 7 days | 2 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.49] |
| 2.6.1 Adequate dosing of both comparators | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.16 [0.26, 103.27] |
| 2.6.2 Suboptimal dosing of opioid | 1 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.98, 1.46] |
| 2.7 Return to function in 7 days or later | 2 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.03, 1.25] |
| 2.7.1 Adequate dosing of both comparators | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.83, 1.72] |
| 2.7.2 Suboptimal dosing of opioid | 1 | 686 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.02, 1.25] |
| 2.8 Gastrointestinal adverse events | 5 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.36, 0.62] |
| 2.8.1 Adequate dosing of both comparators | 3 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.39, 1.82] |
| 2.8.2 Suboptimal dosing of opioid | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.32, 0.58] |
| 2.8.3 Suboptimal dosing of NSAID | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.13, 1.22] |
| 2.9 Neurological adverse events | 5 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.30, 0.53] |
| 2.9.1 Adequate dosing of both comparators | 3 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.18, 1.30] |
| 2.9.2 Suboptimal dosing of opioid | 1 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.30, 0.54] |
| 2.9.3 Suboptimal dosing of NSAID | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.03] |
Comparison 3. NSAID versus combination analgesic (paracetamol + opioid).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Little or no pain < 24 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Little or no pain on days 1 to 3 | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.65, 3.40] |
| 3.3 Little or no pain on days 4 to 6 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 Little or no pain on day 7 or later | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| 3.5 Return to function in 7 days or later | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6 Gastrointestinal adverse events | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.03, 1.74] |
| 3.7 Neurological adverse events | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.09, 2.84] |
Comparison 4. NSAID versus other oral analgesics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain at < 24 hours (VAS: 0 to 100 mm: worst) | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 NSAID vs paracetamol | 6 | 1178 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐2.27, 2.03] |
| 4.1.2 NSAID vs opioid | 4 | 1058 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐3.05, 2.07] |
| 4.2 Gastrointestinal adverse events | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 NSAID vs paracetamol | 10 | 1504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.97, 1.86] |
| 4.2.2 NSAID vs opioid | 5 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.36, 0.62] |
| 4.2.3 NSAID vs paracetamol + opioid | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.03, 1.74] |
| 4.3 Neurological adverse events | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 NSAID vs paracetamol | 9 | 1679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.17] |
| 4.3.2 NSAID vs opioid | 5 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.30, 0.53] |
| 4.3.3 NSAID vs paracetamol + opioid | 3 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.09, 2.84] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbott 1980.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 98 people from 3 groups of UK Armed Forces personnel in 1970s, UK, with soft tissue disorders Mean age = 26 years; 93% were male; ethnicity not reported; 76% were < 48 hours from injury to entry into the study Included: "recently suffered traumatic or sports induced soft tissue injury". No exclusions were given |
|
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | The dose of paracetamol was suboptimal in this combination analgesic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no description |
| Allocation concealment (selection bias) | Low risk | There was no description aside from "identical packaging" |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Quote: "identical individual dose envelopes, identical boxes" |
| Blinding (performance bias and detection bias) Participants | High risk | Quote: "single blind" Quote: "identical individual dose envelopes, identical boxes" ‐ but 1 contained 2 tablets, the other 1 tablet. The trial participants could determine which of the treatments they were taking (assuming informed consent and adequate information was given pre‐enrolment) |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | Quote: "Single blind" Quote: "identical individual dose envelopes, identical boxes" ‐ the treatment providers would not know which treatment they had dispensed |
| Incomplete outcome data (attrition bias) Pain | Low risk | The trial did not account for 2 participants, 1 in each group (2%) |
| Incomplete outcome data (attrition bias) Swelling | Low risk | The trial did not account for 2 participants, 1 in each group (2%) |
| Incomplete outcome data (attrition bias) Function | Low risk | The trial did not account for 2 participants, 1 in each group (2%) |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | The trial did not account for 2 participants, 1 in each group (2%) |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | There was no mention of use of RICE (Rest, Ice, Compression, Elevation) therapy, physiotherapy, or concomitant treatment or any effort to control these |
Aghababian 1986.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 40 adults presenting to 1 emergency department in the USA, who had sustained a grade II ankle sprain with moderate pain. The time from injury to entry into the trial was not stated, but as the setting was the emergency department, we considered that it was likely that most participants would have sustained their injury within 48 hours 55% were aged between 18 to 25 years, 25% were aged between 26 to 35 years, and 20% were aged between 36 to 51 years; 60% male; ethnicity not reported |
|
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: Merck Sharp and Dohme, West Point, Pennsylvania, USA Declarations of interest: not stated |
|
| Notes | The dose of paracetamol was suboptimal, and it was unclear whether allocation was concealed. All participants were given advice to rest, apply local cooling, and to elevate the limb. The study did not record compliance with either physical or pharmacological treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Qualifying patients were randomly allocated..." However, the trial did not detail the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | This was not mentioned |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | This was not mentioned |
| Blinding (performance bias and detection bias) Participants | Unclear risk | This was not mentioned |
| Blinding (performance bias and detection bias) Treatment providers | Unclear risk | This was not mentioned |
| Incomplete outcome data (attrition bias) Pain | Low risk | No participants were lost to follow‐up |
| Incomplete outcome data (attrition bias) Swelling | Low risk | No participants were lost to follow‐up |
| Incomplete outcome data (attrition bias) Function | Low risk | No participants were lost to follow‐up |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes investigated were reported |
| Other bias | Unclear risk | Most participants in both groups were treated with strapping or casts and were advised to non‐weight bear as tolerated. Rest and elevation was also advised. It was not reported how many in each group underwent these other treatments. Concommitant medication was not mentioned in the report |
Beveridge 1985.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 68 male football players at 1 club in England with acute soft tissue injuries (63 in analysis). All were < 24 hours of injury. There was no description of what the injuries were. There were 5 exclusions due to fractures (2 participants) and only mild pain (3 participants) Mean age = 21.4 years; 100% male; ethnicity was not stated |
|
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | The study sufficiently reported the outcome of number returning to training to include in the quantitative analysis, as well as the number of adverse effects. We imputed data for the number returning to training from figure 1 in the published manuscript We discussed the other outcomes in the qualitative analysis, as the means of 4‐point categorical scales were presented graphically without standard deviations The study medication doses were optimal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not stated |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not stated |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | The outcomes were assessed by the treatment providers who knew the treatment allocation, apart from participant self reporting of adverse effects |
| Blinding (performance bias and detection bias) Participants | Low risk | The participants were blinded to the treatment allocation, although it was not stated what steps were taken in order to maintain blinding |
| Blinding (performance bias and detection bias) Treatment providers | High risk | The outcomes were assessed by the treatment providers who knew the treatment allocation |
| Incomplete outcome data (attrition bias) Pain | Low risk | 93% (63/68) of participants completed follow‐up |
| Incomplete outcome data (attrition bias) Swelling | Low risk | 93% (63/68) of participants completed follow‐up |
| Incomplete outcome data (attrition bias) Function | Low risk | 93% (63/68) of participants completed follow‐up |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | 93% (63/68) of participants completed follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods were reported in the results |
| Other bias | High risk | 10 of 31 (32%) versus 3 of 32 (9%) participants in the naproxen group compared with the dextropropoxyphene group undertook rehabilitation exercises, which may have influenced the outcomes |
Bondarsky 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 60 of 90 included in this review. Adults with acute musculoskeletal pain presenting to the emergency department in New York, USA. All injuries were < 24 hours prior to enrolment. 40% had upper extremity, 25% had lower extremity, and 35% had back or neck injuries Mean (SD) age (90 adults) = 36 (15) years; 54% were male; the majority were white 66/90 (73%), 12/90 (13%) were Hispanic |
|
| Interventions | 1. Ibuprofen 800 mg single dose (N = 30) 2. Paracetamol 1000 mg single dose (N = 30) (3. Combination of Ibuprofen 800 mg + paracetamol 1000 mg single dose; N = 30) |
|
| Outcomes |
|
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | The doses of interventions were maximal. The sample was a convenience sample based on investigator presence in the emergency department For the analyses, we calculated (imputed) standard deviations from the 95% CI presented in the study report We did not include the data from the ibuprofen and paracetamol group in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study used a computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | The study used consecutively numbered opaque envelopes prepared by pharmacy |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | The outcome assessors were the treatment providers ‐ it is likely that the study adequately blinded them: "Four similarly appearing tablets" were given to all participants |
| Blinding (performance bias and detection bias) Participants | Low risk | Quote: "Four similarly appearing tablets" were given to all participants |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | Quote: "Four similarly appearing tablets" were given to all participants |
| Incomplete outcome data (attrition bias) Pain | Low risk | The study included all participants |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | The study included all participants |
| Selective reporting (reporting bias) | Low risk | The study reported all outcomes |
| Other bias | Unclear risk | There was no mention of physical therapies (ice, compression, elevation) |
Bourne 1980.
| Study characteristics | ||
| Methods | Probably quasi‐randomised ("an attempt was made to match the patients for site and type of injury") | |
| Participants | 60 students presenting to a Student Health Centre at Manchester University in the 1970s (UK). Included only participants with acute soft tissue injuries; > 80% were within 48 hours. Excluded those with fractures, dislocations, and lacerations. 30% were knee sprains, 25% were ankle sprains, 20% were other lower limb injuries, 20% were upper limb or torso injuries, and 5% were neck or back injuries Mean age (SD) = 20.4 (1.92) years; 88.3% male; ethnicity not reported |
|
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: a 'Boots' company representative supported the study. (It was unclear what support was given or the influence of the company on the design or reporting of the study, or both, or if there was any vested interest in the result.) This acknowledgement was not made in 1 of the 2 papers reporting this study (identical data). Declarations of interest: not stated |
|
| Notes | The study used slightly less than standard doses of paracetamol (900 mg versus 1000 mg) Presented data from pain and swelling as differences in scores (converted from a 4‐point categorical scale) over time at day 5 only; these data were not useable in the meta‐analysis. We received no response from the author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...randomly allocated to 2 treatment groups, but an attempt was made to pair the patients for site and type of injury." There was no description, but attempts to pair participants by key characteristics means that this was not fully random |
| Allocation concealment (selection bias) | High risk | Quote: "...randomly allocated to 2 treatment groups, but an attempt was made to pair the patients for site and type of injury" |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Quote: "Double blind" ‐ allocation does not appear to have been concealed from the study personnel |
| Blinding (performance bias and detection bias) Participants | Low risk | Quote: "Double blind" ‐ it appears that the participants were not aware of the study allocation |
| Blinding (performance bias and detection bias) Treatment providers | Unclear risk | Quote: "Double blind" ‐ allocation does not appear to have been concealed from the study personnel |
| Incomplete outcome data (attrition bias) Pain | Low risk | 55/60 (92%) participants were reported, although there was no ITT analysis |
| Incomplete outcome data (attrition bias) Swelling | Low risk | 55/60 (92%) participants were reported, although there was no ITT analysis |
| Incomplete outcome data (attrition bias) Function | Low risk | 55/60 (92%) participants were reported, although there was no ITT analysis |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | 55/60 (92%) participants were reported, although there was no ITT analysis |
| Selective reporting (reporting bias) | High risk | The trial selectively reported data for 2 outcomes at day 5 only |
| Other bias | Low risk | Quote: "All other drug therapy and physical treatment were excluded throughout the study" |
Clark 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 149 with a soft tissue injury included out of 336 children presenting to a tertiary children's hospital in Canada with acute musculoskeletal pain occurring in the preceding 48 hours Mean age = 12 years (range = 6 to 17 years); 202 (60%) male; ethnicity not reported The study did not specify the number of participants with particular types of soft tissue injury, although extremity, neck, and back injuries were included |
|
| Interventions |
|
|
| Outcomes |
|
|
| Funding and declarations of interest | Funding source: a research grant from the Eastern Ontario Research Institute supported the study Declarations of interest: The authors declared no relevant financial interests |
|
| Notes | Data were only available for 105 (70%) of participants with soft tissue injuries at 120 minutes. For the analyses, we calculated (imputed) standard deviations from the 95% CI presented in the study report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was computer generated with a block size of 9 |
| Allocation concealment (selection bias) | Low risk | The trial used sealed opaque envelopes |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Triage nurses gave the medication. All medication was purple in colour, grape favoured, and given in amber syringes covered with opaque plastic bags. The volumes of study drug per kilogram were similar but not identical |
| Blinding (performance bias and detection bias) Participants | Low risk | Triage nurses gave the medication. All medication was purple in colour, grape favoured, and given in amber syringes covered with opaque plastic bags. The volumes of study drug per kilogram were similar but not identical |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | Triage nurses gave the medication. All medication was purple in colour, grape favoured, and given in amber syringes covered with opaque plastic bags. The volumes of study drug per kilogram were similar but not identical |
| Incomplete outcome data (attrition bias) Pain | Low risk | Data were available on 105 (70%) participants with soft tissue injuries at 120 minutes |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | Data were available on 304 (90%) participants, which means that at least 86% of participants with soft tissue injuries were followed up for this outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes assessed were reported |
| Other bias | Low risk | Similar numbers of participants in each group received casts or splints for their injuries |
Cukiernik 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 77 of 80 children presenting to a tertiary children's hospital in Canada with an acute soft tissue injury of the ankle. All were included < 48 hours from injury. Mean age = 12 years (range = 8 to 14 years); 61% male; ethnicity not reported | |
| Interventions | The study randomised 80 participants; however, it did not state in the text how many it assigned to each group. The numbers below refer to the number analysed
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source:research grants from the Lawson Health Research Institute and the Children's Health Research Institute supported the study Declarations of interest: not stated |
|
| Notes | The study provided data for 77 (96%) participants. Information pertaining to the time from injury to inclusion in the study was not available in the manuscript, but the study authors provided this in October 2014 along with confirmation that adverse events were reported at the participant level. The study used standard doses of comparators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was undertaken using a Latin square with a block size of 10 |
| Allocation concealment (selection bias) | Low risk | The trial used sealed unmarked envelopes |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | The trial used opaque orange gel capsules |
| Blinding (performance bias and detection bias) Participants | Low risk | The trial used opaque orange gel capsules |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | The trial used opaque orange gel capsules |
| Incomplete outcome data (attrition bias) Pain | Low risk | 76/77 = 99% of participants were analysed at both time points (day 3 and day 14) |
| Incomplete outcome data (attrition bias) Swelling | Unclear risk | Data were not presented in a format that allowed accurate abstraction |
| Incomplete outcome data (attrition bias) Function | Low risk | 76/77 = 99% of participants were analysed at both time points (day 3 and day 14) |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | 76/77 = 99% of participants were analysed at both time points |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the methods were reported |
| Other bias | Low risk | Quote: "The patients and parents/legal guardians were also given written and oral instructions on RICE (Rest, Ice, Compression, Elevation) therapy." Although the proportion in each group that undertook the physical therapies as instructed was not stated, it was assumed in the manuscript that all did so |
Dalton 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 260 adults > 18 years old presenting to 42 centres in the USA (research facilities, family practice, sports medicine clinics, orthopaedic clinics, emergency and urgent care facilities, rheumatology clinics), who had sustained a grade I or II lateral ankle sprain < 24 hours prior. Participants had ≥ 40 mm of pain on walking on a 100‐mm VAS Mean age = 33 years; 46% male; 84% white | |
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: financial support for this study was provided by McNeil Consumer & Specialty Pharmaceuticals Declarations of interest: one author of the paper was a current employee of Novartis, Emeryville, CA, USA. |
|
| Notes | The published report reported only the per‐protocol analysis (N = 104 for paracetamol and N = 100 for ibuprofen) sufficiently (actual mean with SD presented for mean changes in pain) to include in the meta‐analysis. The least square means for the changes in pain scores for the ITT analysis (N = 128 for paracetamol and N = 127 for ibuprofen) were within 1 to 2 mm on 100‐mm scale of the least square means per‐protocol analysis, which was not clinically important. However, the direction of benefit was different In the per‐protocol analysis; it favoured NSAID, and in the ITT analysis, it favoured paracetamol Although the study reported adverse events for all 260 participants, only those events that happened in ≥ 1% and in > 1 participant were reported (which leads to the risk of missing rare but important events). Therefore, we included data where an adverse event was reported, but if no reported events then this cannot be assumed as there may have been 1. The study used slightly less than standard doses for both comparators |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive either..." The process of randomisation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Medicaction was given in a blister pack, and the daily dosing was similar (3 times daily). There was no mention of whether the medication looked the same or was otherwise unidentifiable |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | The trial mentioned "double blind" |
| Blinding (performance bias and detection bias) Participants | Low risk | The trial mentioned "double blind" |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | The trial mentioned "double blind" |
| Incomplete outcome data (attrition bias) Pain | Low risk | As explained in the Notes, we used the per‐protocol analysis (N = 204, 79%) in this review. However, the results from the per‐protocol and ITT analysis (N = 255, 98%) were very similar, and so we assessed the risk of bias as low |
| Incomplete outcome data (attrition bias) Swelling | Low risk | As explained in the Notes, we used the per‐protocol analysis (N = 204, 79%) in this review. However, the results from the per‐protocol and ITT analysis (N = 255, 98%) were very similar, and so we assessed the risk of bias as low |
| Incomplete outcome data (attrition bias) Function | Low risk | As explained in the Notes, we used the per‐protocol analysis (N = 204, 79%) in this review. However, the results from the per‐protocol and ITT analysis (N = 255, 98%) were very similar, and so we assessed the risk of bias as low |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | The intention‐to‐treat population was used to assess adverse events |
| Selective reporting (reporting bias) | High risk | Although the per‐protocol and intention‐to‐treat analyses showed very similar results, with only 1 to 2 mm differences in change in pain over time, the direction of the changes differed: NSAIDs were favoured in the per‐protocol analysis, and paracetamol was favoured in the intention‐to‐treat analysis. The intention‐to‐treat analysis was insufficiently reported to include in the meta‐analysis, which may bias the result slightly against paracetamol Only 'common adverse events' (those adverse events occurring in > 1 and > 1% of participants) were reported. 13 adverse effects were reported; however, "11.5% of patients reported adverse events" ‐ which approximates to 30 participants of the 260 included. It was highly likely that adverse events were selectively reported in this study |
| Other bias | Low risk | No other treatments were allowed |
Ekman 2006.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 706 included of 829 adults, 16 to 65 years old, presenting to 87 centres (14 in Europe and 73 in the USA), who had sustained a first or second degree lateral ankle sprain < 48 hours prior. Participants had ≥ 60 mm of pain on weight bearing on a 100‐mm VAS Mean age = 29 years (15 to 74); 58% male; 80% white The study excluded participants if a similar injury affecting the same joint had occurred within the past 6 months, if they had a complete rupture of the ankle ligaments (third‐degree sprain), or if the injury was part of a bilateral ankle injury or was concurrent with an ipsilateral knee injury. Bed rest, hospitalisation, surgery, or use of a non‐removable rigid cast were also criteria for exclusion. The study excluded participants if they had active gastrointestinal (GI), renal, or hepatic disease; upper GI ulceration within the past 30 days; or a history of epilepsy or a recognised risk for seizure, such as head trauma, metabolic disorders, alcohol or drug withdrawal, or central nervous system (CNS) infections. Also excluded: those treated with corticosteroids in the previous 8 weeks; had taken any analgesics in the previous 6 hours, or 24 hours in the case of long‐acting NSAIDs; or if they had a known hypersensitivity to NSAIDs, COX‐2 specific inhibitors, sulphonamides, or tramadol (aspirin ≤ 325 mg/d for cardiovascular prophylaxis and inhaled steroids were permitted) |
|
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: Pfizer Global Pharmaceuticals sponsored the study, and the company monitored the study sites, although they state they did not have access to data until after analysis. Declarations of interest: One or more of the authors declared a potential conflict of interest: All authors received speaker's fees and research funds from Pfizer Global Pharmaceuticals. One author was employed by Pfizer Global Pharmaceuticals |
|
| Notes | We combined data from the 2 valdecoxib groups for the analysis We calculated (imputed) standard deviations from the standard errors reported in this study We did not include data from the placebo group in this review The study used high doses of valdecoxib and a submaximal dose of tramadol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was computer generated with a 7‐block size. Prior to the start of the study, a computer generated the randomisation list |
| Allocation concealment (selection bias) | Low risk | Participants were randomised in the order in which they were enrolled; all capsules and tablets and dosing schedules were outwardly identical |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Quote: "Matching placebos ‐ all capsules and tablets and dosing schedules were outwardly identical." Emergency 'un‐blinding' was possible but not used |
| Blinding (performance bias and detection bias) Participants | Low risk | Quote: "Matching placebos ‐ all capsules and tablets and dosing schedules were outwardly identical." Emergency 'un‐blinding' was possible but not used |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | Quote: "Matching placebos ‐ all capsules and tablets and dosing schedules were outwardly identical." Emergency 'un‐blinding' was possible but not used |
| Incomplete outcome data (attrition bias) Pain | Low risk | Quote: "All randomised subjects received at least one dose of study medication, and were therefore, included in the ITT population" |
| Incomplete outcome data (attrition bias) Function | Low risk | At day 4, 439/468 (93%) of the valdecoxib group and 203/238 (85%) of the tramadol group were assessed for return to normal function At day 7, 453/468 (97%) of the valdecoxib group and 233/238 (98%) of the tramadol group were assessed for return to normal function |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | All participants were included in the analysis of adverse events; however, only those adverse events occurring in at least 2% of participants were reported (considered to be reporting rather than attrition bias) |
| Selective reporting (reporting bias) | High risk | Adverse effects were reported only in those with at least 2% incidence. In the published report, a total of 358 adverse events were reported, compared with 416 adverse events in the study synopsis from the PhMRA database (both only reported those with ≥ 2% incidence) |
| Other bias | Low risk | Quote: "Patients were permitted to receive traditional remedies such as RICE therapy as well as other nonpharmacologic interventions considered to be standard care, including crutches, cane, contrast baths, ankle taping or bracing, rigid double‐upright ankle brace, strengthening and proprioceptive exercises, transcutaneous electrical nerve stimulation (TENS), diathermy, massage therapy, ultrasound, and acupuncture." Similar numbers of participants in each group received such therapies |
Fathi 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Convenience sample of adults (aged > 18 years) with acute (not further defined) soft tissue injuries and pain score 3 to 7 out of 10 presenting to an urban academic hospital in Tehran, Iran. Included 150 of 179 potentially eligible participants. 89% were limb injuries (site of limb injury was not stated), 11% were back injuries
Mean age = 35.5 years; 57% were male; ethnicity not reported Excluded if concurrent multi‐trauma or non‐injury‐related pain, known opioid or NSAIDs allergy; narcotics addiction, history of chronic respiratory, renal, hepatic, or heart failure; people who had received analgesics before their ED presentation; pregnant women and people who were unable to understand or communicate because of language barrier or any other reason |
|
| Interventions | 1. Naproxen 250 mg single dose (N = 75) 2. Oxycodone 2 x 5 mg single dose (N = 75) |
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: no funding was received for the study Declarations of interest: all authors declared they had no conflict of interest |
|
| Notes | We included this study as participants were 'acute' although a specific time from injury was not explicitly stated. Converted pain scores and SD to scale equivalent to other studies by multiplying by 10. The SD of the mean differences from baseline to one hour in pain scores were imputed. The dose of NSAID was half the recommended does for acute pain (New Zealand Formulary) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random blocks of 4. Note: was a convenience sample and it is unclear how many potentially eligible participants were not approached to be in the study |
| Allocation concealment (selection bias) | Unclear risk | Used sealed envelope method. However, no mention that the tablets looked the same, and two oxycodone vs one naproxen. So, if knew protocol and felt number of tablets in envelope, then would know allocation |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Stated that participants, physicians, nurses, and research assistants blinded to group throughout the study. However, this may not have been the case, as allocation may not have been adequately concealed |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Stated that participants, physicians, nurses, and research assistants blinded to group throughout the study. However, this may not have been the case, as allocation may not have been adequately concealed |
| Blinding (performance bias and detection bias) Treatment providers | Unclear risk | Stated that participants, physicians, nurses, and research assistants blinded to group throughout the study. However, this may not have been the case, as allocation may not have been adequately concealed |
| Incomplete outcome data (attrition bias) Pain | Low risk | All participants followed‐up |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | All participants followed‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | No mention of concomitant therapies |
Hung 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 521 of 784 included in this review. Convenience sample of adults (aged > 16 years) presenting to an urban tertiary academic hospital in Hong Kong, between 09:00 and 15:00, Monday to Friday, with isolated soft tissue injury, without significant fracture. 75% of injuries were < 48 hours. 38% were lower limb injuries, 24% hand or finger injuries, 16% were back injuries, the rest were multiple or not specified
Mean age = 39 years; 64.5% were male; ethnicity not reported Excluded if contraindications to the use of paracetamol or ibuprofen, including a history of indigestion, gastroduodenal ulceration, bleeding disorders, recent anticoagulation, pregnancy, adverse reaction to paracetamol, NSAIDs or ibuprofen, cardiac failure, hepatic or kidney problems, rectal bleeding, or chronic NSAID consumption, asthma, chronic obstructive airways disease, or chronic pain syndromes. Analgesia in the four hours prior to recruitment, if they appeared to have other injuries or if they had a physical, visual, or cognitive impairment that might make the use of the visual analogue scale unreliable. |
|
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: a Direct Grant for Research from the Chinese University of Hong Kong (Reference Number: 2041095) and the Hong Kong College of Emergency Medicine Research Fund (Reference Number: 6902289) Declarations of interest: the authors declared that no competing interests exist |
|
| Notes | Standard deviations imputed from 95% confidence intervals provided in the published study The first dose of ibuprofen was adequate, however the subsequent daily dosing regimen was suboptimal at 1200 mg daily. The change in rest pain was slightly less than the change in activity pain for all groups, and is reported in this review (there was no difference between the groups for change in rest or activity pain). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random number table |
| Allocation concealment (selection bias) | Low risk | Generated by independent researcher with no involvement in the study otherwise |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | The research nurse, the physician, and the participant were blinded to the treatment allocations with the use of double placebo |
| Blinding (performance bias and detection bias) Participants | Low risk | The research nurse, the physician, and the participant were blinded to the treatment allocations with the use of double placebo |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | The research nurse, the physician, and the participant were blinded to the treatment allocations with the use of double placebo |
| Incomplete outcome data (attrition bias) Pain | Low risk | For pain in ED 100% follow‐up, for pain at day 3 83.9% follow‐up |
| Incomplete outcome data (attrition bias) Function | Unclear risk | This data were sought but not reported |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | In ED 99.6% follow‐up. Note that at 28 days only 40.2% follow‐up |
| Selective reporting (reporting bias) | High risk | The trial registry title and outcome state that cost‐effectiveness would be analysed, but this was not reported. In the conference presentation (15/9/09), time to return to function was sought at day 28 but not reported |
| Other bias | Unclear risk | There was no mention of RICE or other therapies. The study was funded from public good grant sources with no vested interest. |
Indelicato 1986.
| Study characteristics | ||
| Methods | Participants were randomly assigned to interventions; however, this was an open‐label study | |
| Participants | 50 men presenting during North American football season at University of Florida with soft tissue injury including back pain. 36 (72%) were treated within 48 hours of injury Age = 18 to 22 years; 100% male; ethnicity not reported |
|
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: the study was supported by a grant from Merck Sharp and Dohme, West Point, Pennsyvania, USA Declarations of interest: not stated |
|
| Notes | The paracetamol dose was suboptimal. The manufacturer of the study NSAID supported the study. The trial included participants with back injury; 41/49 met the inclusion criteria for this review (83%). Treatment started between 24 hours and 12 days of injury. Physical therapies rest, elevation, cold, or heat were at the discretion of the treating clinician. We were unable to contact authors for data. 1 treatment participant withdrew due to side effects, and their data were not included in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly divided into two groups of 25 each." The method of randomisation was not stated |
| Allocation concealment (selection bias) | High risk | Quote: "Open prospective study" |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | Quote: "Open prospective study" |
| Blinding (performance bias and detection bias) Participants | High risk | Quote: "Open prospective study" |
| Blinding (performance bias and detection bias) Treatment providers | High risk | Quote: "Open prospective study" |
| Incomplete outcome data (attrition bias) Pain | Low risk | 1 participant in the treatment group (diflunisal) was not included in analysis due to dropping out because of side effects |
| Incomplete outcome data (attrition bias) Swelling | Low risk | 1 participant in the treatment group (diflunisal) was not included in analysis due to dropping out because of side effects |
| Incomplete outcome data (attrition bias) Function | Low risk | 1 participant in the treatment group (diflunisal) was not included in analysis due to dropping out because of side effects |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | 1 participant in the treatment group (diflunisal) was not included in analysis due to dropping out because of side effects |
| Selective reporting (reporting bias) | High risk | The prespecified outcome of limitation of function was not reported in the results |
| Other bias | Unclear risk | Additional treatment was permitted 'as indicated' and included rest, ice, elevation, physiotherapy. It was not stated how many in each group had these treatments. Other medications were not permitted |
Jaffé 1978.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 52 people presenting to 6 different GPs in UK (England and Scotland) with moderate to severe unilateral sprain or strain of wrist or ankle < 24 hours (all meet criteria for acute soft tissue injury). No mention of exclusions Age range = 16 to 62; 55.7% male; ethnicity not reported |
|
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding Source: not stated Declarations of interest: one author was an employee of Merck Sharp and Dohme, Hoddesdon, England |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were allocated at random to one of two treatment groups." The study did not mention the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Twenty‐six patients were allocated originally to each treatment group." It was not stated if allocation was concealed |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Quote: "Double blind, double dummy" |
| Blinding (performance bias and detection bias) Participants | Low risk | Quote: "Double blind, double dummy" |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | Quote: "Double blind, double dummy" |
| Incomplete outcome data (attrition bias) Pain | Low risk | 51/52 participants were analysed |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | 51/52 participants were analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | Co‐interventions were not accounted for. The dose of the combination comparator was standard (however, it contained a lower than standard dose of paracetamol) |
Kayali 2007.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 100 adults ≥ 18 years old, presenting to a single hospital in Turkey with a first or second degree lateral ankle sprain sustained < 48 hours prior; participants had ≥ 45 mm of pain on weight bearing on a 100‐mm VAS Mean age = 28 years; 49% male; ethnicity not stated | |
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | The paracetamol dose was submaximal, while the diclofenac dose was maximal For the analyses, we calculated (imputed) standard deviations from the 95% CI presented in the study report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of 2 treatment groups." The method of randomisation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of 2 treatment groups." The method of allocation concealment was not stated |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | Double‐blind was mentioned, although details were not provided. One intervention was taken twice daily, and 1 intervention was taken 3 times daily, leaving potential for either participant or study personnel to work out which treatment was given |
| Blinding (performance bias and detection bias) Participants | Unclear risk | Double‐blind was mentioned, although details were not provided. One intervention was taken twice daily, and 1 intervention was taken 3 times daily, leaving potential for either participant or study personnel to work out which treatment was given |
| Blinding (performance bias and detection bias) Treatment providers | Unclear risk | Double‐blind was mentioned, although details were not provided. One intervention was taken twice daily, and 1 intervention was taken 3 times daily, leaving potential for either participant or study personnel to work out which treatment was given |
| Incomplete outcome data (attrition bias) Pain | Unclear risk | Rates of follow‐up were not mentioned |
| Incomplete outcome data (attrition bias) Swelling | Unclear risk | Rates of follow‐up were not mentioned |
| Incomplete outcome data (attrition bias) Function | Unclear risk | Rates of follow‐up were not mentioned |
| Incomplete outcome data (attrition bias) Adverse effects | Unclear risk | Rates of follow‐up were not mentioned |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Unclear risk | All participants received instruction about range of motion and stretching exercises, other physical therapies were not mentioned, and the proportion in each group that underwent rehabilitation exercises was not stated |
Le May 2017.
| Study characteristics | ||
| Methods | Randomised Controlled Trial | |
| Participants | 134 of 225 included in this review. Convenience sample of children (aged 6 to 17 years) with an acute soft tissue injury to either an upper or lower limb, which was neither obviously deformed nor neurovascularly compromised, with a self‐reported pain score > 29 mm on a 100‐mm VAS, presenting to one of three emergency departments during hours the research staff were available in specialist childrens' hospitals in: Montreal, Quebec; Edmonton, Alberta; and Ottawa, Ontario. Seventy percent of injuries were < 48 hours. 50% had lower limb and 50% had upper limb injuries. Sample of 501 participants with any musculoskeletal injury
Mean age = 11.9 years; 55% were male; ethnicity not reported Excluded if known allergy to morphine, ibuprofen, or artificial colouring; suspected child abuse; inability to self‐report pain; chronic pain requiring daily analgesics; NSAIDs or opioid use within 3 hours before triage; injury to > 1 limb; known hepatic or renal disease, or dysfunction, or both; known bleeding disorder; neurocognitive disability precluding assent and participation in the study; and a known history of sleep apnoea or loud snoring in the past 5 days |
|
| Interventions |
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: Canadian Institutes for Health Research Operational Grant Program (MOP 125943) Declarations of interest: all authors declared they had no relevant financial relationships or potential conflicts of interest |
|
| Notes | Study authors provided data specific to participants with soft tissue injuries < 48 hours for this review. Standard deviations imputed from the 95% CI provided in the tables of results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated by an independent biostatistician by use of a computer‐generated random listing of the arms using a prespecified seed. Recruited participants were randomly assigned by following a 2:2:1 ratio with a stratified permuted block design to receive either of the 3 arms' treatments. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was pharmacy‐controlled, with a sequentially numbered system. As such, only the research pharmacist at each site (not a team member) received the randomisation list directly from the biostatistician and kept it concealed. |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Participants and their parents, research nurses, and treating physicians were all blinded to the treatment received. |
| Blinding (performance bias and detection bias) Participants | Low risk | Participants and their parents, research nurses, and treating physicians were all blinded to the treatment received. |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | Participants and their parents, research nurses, and treating physicians were all blinded to the treatment received. |
| Incomplete outcome data (attrition bias) Pain | Low risk | Low at 60 min (100% assessed). This was the data point used for the review. There was an unclear risk of bias at 90 min, as only 71% assessed at this time, and high risk of bias at 120 min, as only 52% assessed at this time. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | No mention of RICE. Funding from public good source. Convenience sampling with three times as many missed during screening than included, so may be selection bias |
Lyrtzis 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 90 adults presenting to a single hospital in Greece, with an isolated grade II lateral ankle sprain sustained < 24 hours prior. Participants had ≥ 45 mm of pain on a 100‐mm VAS Mean age = 35 years; 64% male; ethnicity not stated | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: the authors declared, "No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article." |
|
| Notes | The study used a suboptimal dose of paracetamol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomised in two groups...using the Random Number Generator of SPSS statistical software" |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment of allocation to treatment group was not stated |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | There was no mention of blinding of outcome assessors |
| Blinding (performance bias and detection bias) Participants | Unclear risk | The manuscript stated that all participants were blinded to treatment group, although the method of blinding was not stated. The study compared a twice daily medication with a 3 times a day medication, so it is possible that participants may have been able to work out which treatment they received, depending on the study information provided to them |
| Blinding (performance bias and detection bias) Treatment providers | Unclear risk | There was no mention of blinding of the treatment providers, or of disguising the medications, so the treatment providers may have been able to recognise which treatment was given |
| Incomplete outcome data (attrition bias) Pain | Low risk | Data were available for 42/45 (93%) participants in the diclofenac group and 44/45 (98%) participants in the paracetamol group |
| Incomplete outcome data (attrition bias) Swelling | Low risk | Data were available for 42/45 (93%) participants in the diclofenac group and 44/45 (98%) participants in the paracetamol group |
| Incomplete outcome data (attrition bias) Adverse effects | Unclear risk | The manuscript mentioned 3 participants who withdrew because of adverse events. However, no mention was made of adverse events in the participants who completed the study |
| Selective reporting (reporting bias) | Unclear risk | The manuscript mentioned 3 participants who withdrew because of adverse events. However, no mention was made of adverse events in the participants who completed the study |
| Other bias | Low risk | No concomitant medications were permitted, and all participants received an explicit standard programme of rest, ice, compression, and elevation |
Man 2004.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 39 of 50 adults, ≥16 years old, presenting to a single emergency department in Hong Kong with a soft tissue injury following a traumatic mechanism; 57% were sprains, 31% were contusion or crush, and 12% were lacerations; 20% were ankle injuries, 29% were other lower limb injuries, and 51% were upper extremity injuries Mean age = 34 years; 68% male; ethnicity not reported | |
| Interventions |
|
|
| Outcomes |
|
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: no competing interests were declared |
|
| Notes | We combined diclofenac and indomethacin data for the NSAID group in the review analyses For the analyses, we calculated (imputed) standard deviations from the 95% CI presented in the study report We excluded the data from the diclofenac and paracetamol arm in this review The authors declared that there were no competing interests. The study used a submaximal dose of NSAID |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table undertook random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | This was not mentioned |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | This was a double‐blind study. A double‐dummy placebo design was used to account for some interventions being 3 times daily and some being 4 times daily |
| Blinding (performance bias and detection bias) Participants | Low risk | This was a double‐blind study. A double‐dummy placebo design was used to account for some interventions being 3 times daily and some being four 4 daily |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | This was a double‐blind study. A double‐dummy placebo design was used to account for some interventions being 3 times daily and some being 4 times daily |
| Incomplete outcome data (attrition bias) Pain | Low risk | All data were reported |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | It was reported that only 1 person suffered an adverse event in any group |
| Selective reporting (reporting bias) | Low risk | All outcomes that were assessed were reported |
| Other bias | Unclear risk | The numbers of participants receiving physiotherapy, other analgesics, or Chinese medicines differed between groups; however, there were few people in each group, so the differences were not statistically significant |
McCulloch 1985.
| Study characteristics | ||
| Methods | Randomised controlled trial. Single‐blind (observer). This was a complicated trial design as participants were randomised into 1 of 4 groups (tested intervention of plaster of paris versus Tubigrip™ for 10 days, as well as NSAID versus opioid simultaneously) | |
| Participants | 86 people, > 13 years, with inversion injury of ankle within 24 hours, presenting to the emergency department of a single institution in the UK Mean age = 32 years; male and female (unknown proportions); ethnicity not reported |
|
| Interventions | The study included 84/86 participants; it was not clear from the manuscript to which group they were randomised
|
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: not stated Declarations of interest: not stated |
|
| Notes | The study medication doses were appropriate There was significant attrition in this study. We analysed data as if those participants who were not available for assessment of swelling had no improvement in this outcome (denominators are 44 for the NSAID group and 40 for the opioid group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to receive either a below‐knee plaster or instructions for mobilisation exercises...in addition patients received either dihydrocodeine or naproxen." It was not stated how the randomisation was done for either the immobilisation or the medications |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Treatment allocation was predetermined by a set of 2 x 2 Latin square sequences of the 4 possible treatment combinations." There was no mention of whether or how the allocation was concealed |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | Quote: "All assessments at ten days were carried out by a single observer who was unaware of the treatment used" |
| Blinding (performance bias and detection bias) Participants | Unclear risk | There was no mention of attempts to conceal drug treatment made. The study medication was different with respect to number of tablets and frequency of dosing, and it may have been possible for participants to determine which treatment they had received |
| Blinding (performance bias and detection bias) Treatment providers | Unclear risk | There was no mention of attempts to conceal drug treatment made. The study medication was different with respect to number of tablets and frequency of dosing, and it may have been possible for participants to determine which treatment they had received |
| Incomplete outcome data (attrition bias) Swelling | High risk | 42% of the dihydrocodeine group dropped out and were not assessed; reasons were not given 25% of the naproxen group dropped out and were not assessed; reasons were not given. It was unclear how many were in the plaster of paris or Tubigrip™ and mobilise groups, respectively |
| Incomplete outcome data (attrition bias) Function | High risk | 42% of the dihydrocodeine group dropped out and were not assessed; reasons were not given 25% of the naproxen group dropped out and were not assessed; reasons were not given. It was unclear how many were in the plaster of paris or Tubigrip™ and mobilise groups, respectively |
| Incomplete outcome data (attrition bias) Adverse effects | High risk | 42% of the dihydrocodeine group dropped out and were not assessed; reasons were not given 25% of the naproxen group dropped out and were not assessed; reasons were not given. It was unclear how many were in the plaster of paris or Tubigrip™ and mobilise groups, respectively |
| Selective reporting (reporting bias) | Low risk | All outcomes assessed were reported |
| Other bias | Unclear risk | As this was a 4‐arm study with 2 types of physical therapy compared simultaneously with 2 medication types, the physical therapies were strictly controlled and divided equally between groups, although compliance with the physical therapies was not stated |
Ridderikhof 2018.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 365/547 included in this review. Consecutive sample of adults (aged >18 years) with non‐penetrating minor musculoskeletal trauma of an extremity, presenting to two university teaching hospital, two general practices, and one urgent care centre in the Netherlands. All injuries were < 48 hours of enrolment. Median age was 30 years for the paracetamol group and 29 years for the NSAID group; 55.9% were male; ethnicity not stated Excluded if previous treatment with analgesia for the same injury; self‐inflicted injury; presence of wound, joint dislocation, or more than one injury; presence of a fracture; daily use of acetaminophen or NSAID or other analgesia within two weeks before presentation; chronic pain; previous adverse reaction or known allergy to acetaminophen, non‐steroidal anti‐inflammatory drugs, or omeprazole; a known pregnancy; previous gastrointestinal haemorrhage or perforation after non‐steroidal anti‐inflammatory drug use; active or recurrent peptic ulceration, or peptic bleeding (two or more evident episodes); previous exacerbation of asthma after use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid; severe cardiac failure; liver cirrhosis; severe renal insufficiency (a known glomerular filtration rate < 30 mL/min); or physical, visual, or cognitive impairment or non‐Dutch speaking (unable to use numeric rating scale (NRS) pain scores or pain diary). |
|
| Interventions | 1. Diclofenac 50 mg three times daily for 3 days (N = 183) 2. Paracetamol 1 gm four times daily for 3 days (N = 182) (3. Diclofenac 50 mg three times daily for 3 days + Paracetamol 1 gm four times daily for 3 days; N = 182) Note: participants of both groups also received omeprazole 20 mg oral once daily for 3 days (N = 182) |
|
| Outcomes |
Outcomes not specified in this review
|
|
| Funding and declarations of interest | Funding source: Netherlands Organization for Health Research and Development, grant 836011015 Declarations of interest: none declared |
|
| Notes | The primary outcome reported differed from that stated in the protocol (90 minutes rather than 30 or 60 minutes, which were reported as secondary outcomes). The NRS scores were multiplied by 10 for comparison with 100‐mm VAS scores in this review. Two of the prespecified analyses were not reported (quality of life and economic evaluation). Standard deviations were imputed from the 95% confidence intervals presented in the study. The authors provided additional data for total number of adverse effects in each group for the period of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online randomisation module, blocks of 9, 1:1:1 ratio, stratified in subgroups < 60 ≥ 60 |
| Allocation concealment (selection bias) | Low risk | Double‐dummy design, identical appearances. Pre‐packaged and numbered according to randomisation sequence, by independent pharmacist |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | All participants, care providers, research assistants, and outcome assessors were blinded for assigned study medication during the complete study course |
| Blinding (performance bias and detection bias) Participants | Low risk | All participants, care providers, research assistants, and outcome assessors were blinded for assigned study medication during the complete study course |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | All participants, care providers, research assistants, and outcome assessors were blinded for assigned study medication during the complete study course |
| Incomplete outcome data (attrition bias) Pain | Low risk | > 80% included in all analyses |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | > 80% included in all analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes specified were reported |
| Other bias | Low risk | Similar numbers in both groups received RICE. The study was funded by a public good competitive research grant. Two of the prespecified analyses were not reported (quality of life and economic evaluation). All participants received omeprazole, in addition to the study medication. This was intended to reduce the gastrointestinal adverse effects of NSAID, and may have confounded the results for this outcome (the adverse event rate in this study was high for both groups compared to other studies). |
Woo 2005.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | 206 included of 300 adults, ≥ 16 years, presenting to a single emergency department in Hong Kong, with an isolated painful limb injury following trauma; 58% were sprains, 18% were contusions or crushes, 16% were wounds, 6% were fractures; 37% were upper limb, 35% were lower limb, and 28% were back or neck injuries Mean age = 37 years; 59% male; ethnicity not reported |
|
| Interventions |
|
|
| Outcomes |
|
|
| Funding and declarations of interest | Funding source: the authors reported that no outside funding or support for the study was received Declarations of interest: not stated |
|
| Notes | The study used a suboptimal dose for NSAIDs We combined diclofenac and indomethacin data for the NSAID group in the review analyses For the analyses, we calculated (imputed) standard deviations from the 95% CI presented in the study report Adverse event data were included when reported at the participant level We did not include the data from the diclofenac and paracetamol arm in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to 1 of the 4 treatment groups using a computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | An envelope was used, without description of adequate safeguards |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | This was a double‐blind study. A double‐dummy placebo design was used to account for some interventions being 3 times daily and some being 4 times daily |
| Blinding (performance bias and detection bias) Participants | Low risk | This was a double‐blind study. A double‐dummy placebo design was used to account for some interventions being 3 times daily and some being 4 times daily |
| Blinding (performance bias and detection bias) Treatment providers | Low risk | This was a double‐blind study. A double‐dummy placebo design was used to account for some interventions being 3 times daily and some being 4 times daily |
| Incomplete outcome data (attrition bias) Pain | Low risk | Only 7 (2%) participants withdrew or were lost to follow‐up |
| Incomplete outcome data (attrition bias) Adverse effects | Low risk | Only 7 (2%) participants withdrew or were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting |
| Other bias | Unclear risk | There were no other treatments allowed. There was no mention of non‐pharmacological treatments |
CI: confidence interval GPs: general practitioner ITT: intention‐to‐treat NSAID: non‐steroidal anti‐inflammatory drug SD: standard deviation VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersson 1983 | This RCT compared non‐selective NSAID to placebo (no active comparator), finding no difference in pain or swelling between the groups |
| Buccelletti 2014 | This pseudo‐randomised controlled trial without blinding compared NSAID to paracetamol + codeine for participants with either trauma (67%) or inflammatory pain (33%), with the outcome of pain at 30 and 120 minutes. Due to the high number of participants with inflammatory conditions, the primary analysis did not meet the inclusion criterion for the condition of interest for the currently review. A subgroup analysis was performed on the trauma participants alone (N = 134), finding no difference between the groups (P = 0.48 at 120 minutes). However, the actual pain scores were not reported, so these data were not able to be included in the review. The authors were contacted to provide results via email in May and September 2019, but the email provided in the manuscript was invalid. |
| Calligaris 1993 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review. Additionally, there were no useable data |
| Cardenas‐Estrada 2009 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| Cauchioli 1994 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| Chang 2017 | This RCT compared NSAID + paracetamol to combinations of paracetamol + opioid, so was not the appropriate intervention for this review |
| Collopy 2012 | This was a narrative review of pre‐hospital treatment of musculoskeletal injuries, so was not the appropriate study type for this review |
| Costa 1995 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review. Additionally, there were no useable data |
| D'Hooghe 1992 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| De Gara 1982 | This paper reported two studies, of which the second enrolled potentially eligible participants for the comparison NSAID versus paracetamol. However, it was not possible to disaggregate the data for this comparison for inclusion in the quantitative analysis. We had previously attempted to contact this author for the data, but were unsuccessful |
| Diaz 2006 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| Dougados 2007 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID versus placebo, which were not comparisons that we included in this review. Additionally, this study was on rotator cuff tendonitis with onset within 7 days, which places the population outside the remit of the current review |
| Ekman 2002 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| Feragalli 2017 | This study was not randomised, and compared a boswellic acid to placebo for ankle sprains, so the study design and comparison did not meet the criteria for this review |
| Ferreira 1992 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| Goswick 1983 | This RCT compared NSAIDs versus opioid versus placebo. The mean time from injury to entry into the study was 3 to 5 days. Since it is likely that the majority of participants were not enrolled before 48 hours, we excluded this study |
| Graudins 2016 | This RCT compared NSAID + paracetamol to NSAID to paracetamol + opioid, for acute limb injuries, which was not a comparison we included in this review. There was non‐inferiority between the groups at 30 minutes. |
| Gyer 2012 | This was a commentary article of a narrative review of the treatment of minor injuries, so was not a study type included in this review |
| Hardo 1982 | This was an open RCT in a primary care setting, which enrolled 201 participants within 72 hours of injury, and compared azapropazone with a paracetamol + dextropropoxyphene combination. It was not stated what proportion they enrolled within 48 hours, and of those enrolled, only 63% met the definition of acute soft tissue injury. On this basis, we excluded this study |
| Jenner 1987 | This article was a narrative review of five studies of a COX‐2 selective NSAID, which were not referenced and reported briefly in abstract form. None of the studies were randomised |
| Jenoure 1998 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| Jorgensen 1986 | This RCT compared a non‐selective NSAID to placebo, which was not a comparison that we included in this review |
| Khoury 2018 | This was not a randomised trial, so was not a study type that we included in this review. The comparison was between a non‐opioid, non‐NSAID, and opioid, a comparison not relevant to this review. |
| Kolodny 1975 | This study considered NSAID versus NSAID in combination with a centrally acting catecholamine reuptake inhibitor, which was not a comparison that we included in this review. Additionally, it was not a randomised trial |
| Kyle 2008 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review. Additionally, only 44% of participants had acute soft tissue injuries as defined in this review, and the time from injury to enrolment was > 48 hours in an unspecified number of participants |
| Le May 2013 | This RCT compared ibuprofen plus paracetamol versus ibuprofen alone, which was not a comparison that we included in this review |
| Moore 1999 | This RCT compared NSAID versus paracetamol in a heterogenous group of participants with pain from different conditions. It was not possible to determine how many of the 32% with musculoskeletal conditions had acute soft tissue injuries, and the times from onset of symptoms to enrolment were not stated. On this basis, we excluded this study |
| Muncie 1986 | This RCT compared paracetamol with diflunisal in 42 participants with mild to moderate pain. The setting was primary care, and around 50% had back pain, with an average time > 48 hours post‐injury. We attempted to contact the author for data on potentially eligible participants, but were unsuccessful |
| Nadarajah 2006 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| NCT00954785 | This RCT planned to compare a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review. Additionally, when we checked the trial registration (3 September 2014), the status had changed to 'withdrawn prior to enrolment' |
| NCT01974609 | This is an ongoing trial for treatment of carpal tunnel syndrome, so is not a condition included in this review |
| NCT02373254 | This study compared NSAID to paracetamol + opioid for ankle fractures, so the participants were not the type included in this review. The reference is to a clinical trials registry entry, no study results have been published |
| NCT02862977 | This RCT is not yet recruiting, but plans to compare NSAID + cyclobenzaprine with NSAID + cyclobenzaprine + caffeine. The comparison is not eligible for this review |
| NCT03025113 | This RCT is not yet recruiting, but plans to compare NSAID + cyclobenzaprine with cyclobenzaprine. The comparison is not eligible for this review |
| NCT03173456 | This RCT is recruiting, and plans to compare NSAID + paracetamol versus paracetamol + opioid (various different opioids and doses of NSAID). None of the comparisons will be eligible for this review |
| NCT03767933 | This RCT is recruiting, and plans to compare NSAID versus NSAID + paracetamol versus NSAID + paracetamol + opioid. Neither comparison is eligible for this review |
| Pagliara 1997 | This RCT compared NSAID versus opioid after trauma in participants with severe pain at baseline (> 7/10). Most participants had fractures, and only 16/120 (13%) may have met inclusion criteria for the current review. The paper did not mention time from injury to inclusion in the study. Based on the small number of potentially eligible participants, we excluded this study. We attempted to contact the author for data on potentially eligible participants, but were unsuccessful |
| Patel 1993 | Although we have been unable to obtain a copy of this article (we received no response from the journal), this RCT compared NSAID versus paracetamol in a paediatric population, with a large range of conditions characterised by pain, inflammation, fever, or a combination of these. The abstract indicated that these included soft tissue injuries or inflammation, but it is unlikely that separate data were available for these, or that the duration of injury was stated and was within the 48‐hour limit for this review |
| Petrella 2004 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| Pfizer 2005 | This RCT compared a COX‐2 selective NSAID versus a non‐selective NSAID, which was not a comparison that we included in this review |
| Sherry 1988 | This was a quasi‐RCT, comparing NSAID versus a combination of paracetamol + opioid. It was a mixed injury trial: 55% had sprains and were eligible for inclusion. Although their pain data were presented separately in a graph, the study authors did not define the error bars. The paper reported adverse effects for all injury groups together. In summary, there were no useable data. We attempted to contact the authors for data on potentially eligible participants, but were unsuccessful |
| Simmons 1982 | This study compared NSAID versus paracetamol + opioid combination. The proportion who would have met criteria for the current review was unknown, although it is likely to be small, as the enrolment included participants regardless of time of injury. It is also likely that the study included participants with non‐injury conditions |
| Sleet 1980 | This RCT compared NSAID versus paracetamol + opioid combination for participants with musculoskeletal trauma and fractures, burns, and soft tissue infections. Only about half of participants may have met the type of injury inclusion criteria for this review, although the paper did not state the time from onset of injury to enrolment. On this basis, we excluded the study |
| Stableforth 1977 | This study compared NSAID versus paracetamol + opioid combination. We excluded this study as it was not randomised |
| Turturro 2003 | This RCT compared NSAID versus NSAID, in combination with a centrally acting catecholamine reuptake inhibitor with a similar structure and action to tri‐cyclic antidepressants, which was not a comparison that we included in this review |
| van den Bekerom 2016 | This was a commentary article on the previous version of this systematic review, so was not a study type included in this review |
| Whitehead 2016 | This was a commentary article on the previous version of this systematic review, so was not a study type included in this review |
| Yates 1984 | This RCT compared NSAID versus paracetamol, but based on the published report, was insufficiently reported to include. We successfully contacted the author, who provided some additional information, but this was still insufficient to include in the quantitative analysis (the original data were lost): 59% of participants had acute soft tissue injuries; however, the time from injury to enrolment was not available |
| Yazdanpanah 2011 | This RCT compared NSAID versus NSAID + oral methocarbamol (versus NSAID + intramuscular methocarbamol), which were not comparisons that we included in this review |
| Yilmaz 2019 | This RCT compared intravenous NSAID to intravenous paracetamol, finding no difference in analgesic efficacy at 60 minutes. As this review considered only orally administered treatments, this study did not meet the criteria for inclusion in this review |
NSAID: non‐steroidal anti‐inflammatory drug RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
CTRI/2009/091/001067.
| Methods | RCT (open‐label) |
| Participants | Adults of either sex, between 18 and 60 years, with acute painful soft tissue injury |
| Interventions | Ibuprofen 400 mg three times daily for seven days IN‐PHARM‐002 80 mg three times daily for seven days |
| Outcomes | Pain: change in the VAS at end point compared to baseline; change in investigators global assessment of pain relief at the end point compared to baseline; Percent of participants requiring rescue medication. |
| Notes | This was a phase III pharmaceutical company trial. Further details of the experimental drug IN‐PHARM‐002 were not provided in the trial registry entry so it is unclear to what class it belongs. The study started in 2010 with a plan to recruit 204 participants, and expected duration of five months. No results have been published to date. Study contact: Rajendra C Rane (rane@instaspharma.com). |
TCTR20160126001.
| Methods | RCT |
| Participants | Adults, aged between 20 and 45 years, with acute muscle strain presenting to Payakkhaphumpisai Hospital, Mahasarakham, Thailand |
| Interventions | Diclofenac 25 mg, one capsule three times daily for seven days Prasaplai extract 200 mg, two capsules three times daily for seven days |
| Outcomes | Pain; adverse effects at six hours after taken, and at end of trial |
| Notes | Not recruiting yet. Target enrolment 370 participants. Study contact: Matoorada Wisai (doong06@hotmail.com) |
Characteristics of ongoing studies [ordered by study ID]
NCT02667730.
| Study name | Correlation between acute Analgesia and Long‐term Function following ankle injuries (CALF) |
| Methods | Pragmatic RCT |
| Participants | Recruitment target 160 adults (17 years to 60 years) eligible for care at Garrison Petawawa, Ontario, Canada with acute grade I to II ankle injuries < 48 hours |
| Interventions | Naproxen 500 mg twice daily for seven days Celecoxib 100 mg twice daily for seven days Acetominophen (paracetamol) 500 mg four times daily for seven days |
| Outcomes | Pain; swelling; function; proprioception three month follow‐up Health resource consumption one year follow‐up |
| Starting date | June 2015 (estimated completion was December 2019) |
| Contact information | Principal Investigator Koren Lui: koren.lui@forces.gc.ca |
| Notes | Trial registry updated on 9 August 2020 An interim analysis has been presented at a conference, and the abstract is available on ResearchGate www.researchgate.net/publication/321930772_Correlation_between_acute_Analgesia_Long_term_Function_following_Lateral_Ankle_Sprains |
NCT03222518.
| Study name | NSAIDs versus paracetamol versus paracetamol + NSAIDs in traumatic pain management |
| Methods | RCT |
| Participants | Recruitment target 1000 adults (> 18 yr) with acute pain of traumatic origin presenting to the emergency department of Monastir University Hospital, Tunisia |
| Interventions | Ketaprofen 50 mg three times daily for seven days Paracdetamol 1000 mg three times daily for seven days (Ketaprofen 50 mg three times daily plus paracetamol 1000 mg three times daily for seven days) |
| Outcomes | Treatment success "EN" (not further defined) seven‐day follow‐up Rescue analgesia seven‐day follow‐up Participant's satisfaction seven‐day follow‐up Quality of life (EQ5D questionnaire) Adverse effects seven‐day follow‐up |
| Starting date | August 2016 (estimated completion is 1 August 2020) |
| Contact information | Study contact: Prof Semir Noura: semir.nouira@rns.tn |
| Notes | Trail registry updated 11 February 2020 |
Differences between protocol and review
Additional changes in the 2020 update
Types of interventions
We clarified our intention to group complementary and alternative medicines according to their biological activity.
Dealing with missing data
We clarified that where studies reported adverse events at the event level for the broad categories of gastrointestinal and neurological adverse events, we used participant‐level data for the most common adverse event within the broad categories in the analysis.
Description of studies
We reported on the funding and conflict of interest statements in the included trials; we also presented these in the Characteristics of included studies.
Additional subgroup analysis (former secondary analysis)
As described in the 'Differences between protocol and review' for the 2015 version, we performed a pooled analysis across different types of analgesics, which had not been specified in the protocol. External feedback received on this version of the review questioned why we had completed this extra analysis, which remained unspecified in the Methods section and was not discussed in the review. Given our main intention had been to present a visual summary of the results of the three comparisons, we decided against conducting pooled analysis, and instead conducted exploratory subgroup analysis where there were sufficient data. We added this new subgroup analysis to Subgroup analysis and investigation of heterogeneity.
GRADE and 'Summary of findings' tables
We used GRADEpro to generate 'Summary of findings' tables; and changed our terminology to describe the evidence from quality to certainty.
We added neurological adverse effects, as this is of current interest, given the concern around overuse of oral opioids in this setting.
Changes in the 2015 version
Types of interventions
The search identified no studies of NSAID versus CAM; hence, we did not evaluate this comparison in the current review.
We clarified that the study comparisons of NSAID and paracetamol or opioid versus NSAID alone were ineligible.
Although set up as a subgroup analysis in the protocol, we clarified that direct comparisons of COX‐2 selective NSAIDs versus non‐selective NSAIDs was not in keeping with the stated intention of the review, i.e. to compare oral NSAIDs with other oral analgesic agents.
Searches
In November 2012, the search of MEDLINE was conducted via the OVID interface, and modified on the advice of the Cochrane Bone, Joint and Muscle Trama Group (Appendix 2).
Data management
Not all studies reported data sufficiently to include in all preplanned analyses at all time points for each comparison. Where available, we included data in the analyses. We calculated missing standard deviations from study data (confidence intervals (CI) or standard errors) for the comparisons of NSAID versus paracetamol, or NSAID versus opioid in all but one of the included studies; either from 95% CI (Bondarsky 2013; Clark 2007; Kayali 2007; Lyrtzis 2011; Man 2004; Woo 2005), or standard errors (Ekman 2006) provided in the study reports. The exception was Lyrtzis 2011. For the outcome, NSAID versus combination paracetamol and opioid, we were not able to use any continuous data in the analyses. We clarified in our review that our planned sensitivity analyses relating to imputed data was not done where missing standard deviations could be readily calculated from other statistics.
For one included study, it was not absolutely clear that ≥ 70% of the participants had acute soft tissue injuries within 48 hours of study entry, as specified in the methods, although we considered this most likely based on the emergency department setting (Aghababian 1986).
Some studies included participants outside the prespecified criteria for this review, those with wounds, minor closed fractures, and back or neck injuries. As we were unable to disaggregate the data for these participants, we decided to include the studies on the basis that a minority of participants (< 15%) were involved, and the back and neck injuries were all acute injuries rather than chronic pain conditions, which was again consistent with the aim of the review.
In the protocol, we had prespecified the acceptable risk of bias for inclusion of a study in the primary meta‐analysis for each type of bias (seeTable 9), and intended to undertake secondary analyses for trials that did not meet these criteria. In the review, we analysed all studies, and then undertook sensitivity analyses, excluding studies that did not meet the prespecified acceptable level of risk of bias. When the outcomes reported in studies with unclear risk of bias were objective (such as volumetric and tape measurements of swelling), we did not undertake sensitivity analysis. When the only study analysed was at a higher risk of bias than we had prespecified, we noted this in the body of the text, where pertinent.
We were unable to conduct our planned subgroup analyses (e.g. COX‐2 selective versus non‐selective NSAIDs; age groups < 18 years, 18 to 65 years, and > 65 years) because insufficient studies were available.
No study in any comparison group reported re‐injury within three months, so we could not assess this outcome.
We performed a pooled analysis across different types of analgesics, which was not specified in the protocol. We did this to summarise the main outcomes across all three comparisons. The time points chosen were the earliest possible for pain relief (within one to two hours of treatment on the first day, and at days one to three), as these are the times that analgesics are most likely to be taken, and subsequently, the pain from acute soft tissue injuries has subsided substantially for most people. For function, the time point chosen was at the end of treatment – at or after day seven.
2. Level of bias considerable acceptable to include data in the primary meta‐analysis for pain, swelling, function, and adverse effects.
| Domain | Acceptable risk of bias for inclusion in the first order (primary) meta‐analysis |
| Sequence generation | Randomised (Low or unclear risk of bias) |
| Allocation concealment | Allocation concealed (Low or unclear) |
| Blinding | Critical that participants, care providers, and assessors are blinded to treatment group (Low) |
| Incomplete outcome data | ≧ 70% follow‐up mandatory for inclusion, providing that reasons for missing data are not related to true outcome, and there is a balance in the number missing from each group (Low) |
| Selective outcome reporting | Where there has been selective outcome reporting, the study will be deemed at high risk of bias for that outcome and will be excluded for the meta‐analysis of that outcome (Low) |
| Other (stopped early, or claimed to be fraudulent) | (Low or unclear) Note: drug dose differences, length of follow‐up, and characteristics of participants (e.g. age) are specifically excluded from the risk of bias table in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). They are regarded as a potential source of bias, but will be addressed in the analysis by subgroup analysis, and considered in the grading and interpretation of evidence in a 'Summary of findings' table |
Contributions of authors
Peter Jones initiated and co‐ordinated the current update, conducted the search, screened studies for inclusion, wrote to authors for clarification where data were incomplete, extracted data, drafted the manuscript, and revised the manuscript. Rain Lamdin screened studies for inclusion, extracted data, and helped revise the manuscript. Stuart Dalziel screened studies for inclusion, extracted data, and helped revise the manuscript.
Contributions of editorial base
Helen Handoll: edited the review; advised on methodology and review content; and approved the final version for publication. Joanne Elliott: co‐ordinated the editorial process; advised on content; and edited the protocol. Maria Clarke: ran search update and edited the search methods section.
Sources of support
Internal sources
-
Department of Emergency Medicine, Auckland City Hospital, Auckland District Health Board, New Zealand
Provided salaried non‐clinical time, computer hardware, internet, library access, and email facility for review authors while working on this review
External sources
-
Auckland District Health Board Charitable Trust, New Zealand
Provided funding for consumables required in the production of this review
Declarations of interest
Peter Jones: employed by the Auckland District Health Board, and has received unrelated research support from the Health Research Council of New Zealand; the Green Lane Research and Education Fund; and the Auckland District Health Board Charitable Trust, known as A+ Trust.
Rain Lamdin: nothing to declare
Stuart R Dalziel: employed by the Auckland District Health Board; is an advisor for the Pharmaceutical Management Agency (PHARMAC), New Zealand; and receives research support (unrelated to this manuscript) from the Health Research Council of New Zealand; Cure Kids New Zealand; Auckland Medical Research Foundation; the Auckland District Health Board Charitable Trust, known as A+ Trust (New Zealand); and the National Health and Medical Research Council (NHMRC) (Australia).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abbott 1980 {published data only}
- Abbott CJ, Bouchier-Hayes TA, Hunt HA. A comparison of the efficacy of naproxen sodium and a paracetamol/dextropropoxyphene combination in the treatment of soft-tissue disorders. British Journal of Sports Medicine 1980;14(4):213-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aghababian 1986 {published data only}
- Aghababian RV. Comparison of diflunisal and acetaminophen with codeine in the management of grade 2 ankle sprain. Clinical Therapeutics 1986;8(5):520-6. [MEDLINE: 3094957] [PubMed] [Google Scholar]
Beveridge 1985 {published data only}
- Beveridge K. Treatment of sports injuries with naproxen sodium and dextropropoxyphene napsylate. Pharmatherapeutica 1985;4(6):393-8. [PMID: ] [PubMed] [Google Scholar]
Bondarsky 2013 {published and unpublished data}
- Bondarsky EE, Domingo AT, Matuza NM, Taylor MB, Thode HC Jr, Singer AJ. Ibuprofen vs acetaminophen vs their combination in the relief of musculoskeletal pain in the ED: a randomized, controlled trial. The American Journal of Emergency Medicine 2013;31(9):1357-60. [PMID: 23896011] [DOI] [PubMed] [Google Scholar]
- Bondarsky EE, Domingo AT, Matuza NM, Thode HC, Singer AJ. Ibuprofen versus acetominophen versus their combination in the relief of musculoskeletal pain in the emergency setting. Annals of Emergency Medicine 2011;58 Suppl 4:S255. [DOI] [PubMed] [Google Scholar]
- NCT01827475. Ibuprofen versus acetaminophen vs their combination in the relief of musculoskeletal pain in the emergency setting. clinicaltrials.gov/ct2/show/NCT01827475 (first received 09 April 2013).
- Singer A. Study results pre-publication [personal communication]. Email to: P Jones 11 December 2012.
Bourne 1980 {published data only}
- Bourne M, Bentley S. Enhanced recovery from sports injuries: a comparison of ibuprofen and paracetamol. British Journal of Clinical Practice 1980;6(Symp Suppl):72-7. [Google Scholar]
- Bourne MS. The effect on healing of analgesics and anti-inflammatory therapy. British Journal of Sports Medicine 1980;14:26-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2007 {published and unpublished data}
- Alvarez JCB. In children treated in an emergency hospital service for acute traumatic musculoskeletal pain, ibuprofen showed an analgesic efficacy greater than that of paracetamol and codeine [En ninos atendidos en un servicio hospitalario de urgencias por dolor agudo musculoesqueletico de origen traumatico, el ibuprofeno mostro una eficacia analgesica superior a la del paracetamol y la codeina]. Formacion Medica Continuada en Atencion Primaria 2007;14(7):439. [Google Scholar]
- Clark E, Plint AC, Correll R, Gaboury I, Passi B. A randomized, controlled trial of acetaminophen, ibuprofen, and codeine for acute pain relief in children with musculoskeletal trauma. Pediatrics 2007;119(3):460-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Plint A. Per-patient adverse events data for acute soft tissue injury patients [personal communication]. Email to: P Jones 25 November 2014.
Cukiernik 2007 {published and unpublished data}
- Cukiernik VA, Lim R, Warren D, Seabrook JA, Matsui D, Rieder MJ. Naproxen versus acetaminophen for therapy of soft tissue injuries to the ankle in children. Annals of Pharmacotherapy 2007;41(9):1368-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rieder MJ. Per patient data on adverse events [personal communication]. Email to: P Jones 30 October 2014.
Dalton 2006 {published data only}
- Dalton JD Jr, Schweinle JE. Randomized controlled noninferiority trial to compare extended release acetaminophen and ibuprofen for the treatment of ankle sprains. Annals of Emergency Medicine 2006;48(5):615-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00261560. A safety and effectiveness study of acetaminophen extended release (3900 mg/day) and ibuprofen (1200 mg/day) in the treatment of pain associated with ankle sprains. clinicaltrials.gov/ct2/show/NCT00261560 (first received 05 December 2005).
Ekman 2006 {published and unpublished data}
- Cawkwell G. Clarification of study data for outcomes in format appropriate for the review [personal communication]. Email to: P Jones 26 August 2008.
- Ekman EF, Ruoff G, Kuehl K, Ralph L, Hormbrey P, Fiechtner J, et al. Effective pain relief with valdecoxib following acute ankle sprain. In: European League Against Rheumatism Annual Congress; 2004 June 9-12; Berlin. Berlin (Germany) http://scientific.sparx-ip.net/archiveeular/?c=a&searchfor=valdecoxib&view=1&item=2004SAT0366, 2004. [SAT0366 (2004)]
- Ekman EF, Ruoff G, Kuehl K, Ralph L, Hormbrey P, Fiechtner J, et al. The COX-2 specific inhibitor Valdecoxib versus tramadol in acute ankle sprain: a multicenter randomized, controlled trial. American Journal of Sports Medicine 2006;34(6):945-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Pfizer Inc. Multicenter, double-blind, placebo-controlled, randomised, parallel group study to compare the efficacy and tolerability of valdecoxib vs. tramadol in treatment of acute ankle sprains. Protocol 872-IFL-0513-008. www.clinicalstudyresults.org/documents/company-study (accessed 4 August 2008).
Fathi 2015 {published data only (unpublished sought but not used)}IRCT201112208104N3
- Fathi M, Zare MA, Bahmani HR, Zehtabchi S. Comparison of oral oxycodone and naproxen in soft tissue injury pain control: a double-blind randomized clinical trial. American Journal of Emergency Medicine 2015;33(9):1205-8. [DOI] [PubMed] [Google Scholar]
- IRCT201112208104N3. Comparison of oxycodone and naproxen in pain control of acute soft tissue injuries. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201112208104N3 (accessed 30 January 2020).
Hung 2018 {published data only (unpublished sought but not used)}94738550
- Graham CA, Mak PSK, Man SY, Woo WK, Cattermole GN, Rainer TH. Oral paracetamol or ibuprofen or both for treating pain after soft tissue injuries: double-blind, randomised, controlled trial. In: The Fifth Mediterranean Emergency Medicine Congress; 2009 Sep 14-17; Valencia, Spain. 2009.
- Graham CA. Request for study data relevant to this review [personal communication]. Email to: P Jones 15 September 2019.
- Hung KKC, Graham CA, Lo RSL, Leung YK, Leung LY, Man SY, et al. Oral paracetamol and/or ibuprofen for treating pain after soft tissue injuries: single centre double-blind, randomised controlled clinical trial. PloS One 2018;13(2):e0192043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN94738550. Cost-effectiveness analysis of oral paracetamol and ibuprofen for treating pain after soft tissue limb injuries: double-blind, randomised controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN94738550 (first received 01 January 2005). [ISRCTN94738550]
Indelicato 1986 {published data only}
- Indelicato PA. Comparison of diflunisal and acetaminophen with codeine in the treatment of mild to moderate pain due to strains and sprains. Clinical Therapeutics 1986;8(3):269-74. [PMID: ] [PubMed] [Google Scholar]
Jaffé 1978 {published data only}
- Jaffé GV, Roylance PJ, Grimshaw JJ. A controlled study of diflunisal in sprains and strains. Current Medical Research and Opinion 1978;5(7):584-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kayali 2007 {published data only}
- Kayali C, Agus H, Surer L, Turgut A. The efficacy of paracetamol in the treatment of ankle sprains in comparison with diclofenac sodium. Saudi Medical Journal 2007;28(12):1836-9. [PMID: ] [PubMed] [Google Scholar]
- Kayali C, Agus H, Surer L. The efficacy of acetaminophen in the treatment of ankle sprains: comparison with diclofenac sodium. Journal of Bone and Joint Surgery. British Volume 2009;91(Suppl):163. [Google Scholar]
Le May 2017 {published and unpublished data}
- Gregoire G. Study data relevant to current review (acute soft tissue injury patients) [personal communication]. Email to: P Jones 3 October 2019.
- Le May S, Ali S, Plint A, Masse B, Neto G, Auclair MC, et al. A randomized controlled trial on oral analgesic utilization for children presenting with a musculoskeletal trauma in the emergency department. Canadian Journal of Emergency Medicine 2016;23:S54-5. [Google Scholar]
- Le May S, Ali S, Plint A, Masse B, Neto G, Auclair MC, et al. Oral analgesics utilization for children with musculoskeletal injury (OUCH Trial): an RCT. Pediatrics 2017;140(5):1-9. [DOI] [PubMed] [Google Scholar]
Lyrtzis 2011 {published data only}
- Lyrtzis C, Natsis K, Papadopoulos C, Noussios G, Papathanasiou E. Efficacy of paracetamol versus diclofenac for grade II ankle sprains. Foot & Ankle International / American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society 2011;32(6):571-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Man 2004 {published data only}
- Man SY, Woo WK, Lam PKW, Rainer TH. Feasibility study comparing oral paracetamol and oral non-steroidal anti-inflammatory drugs for treating pain after musculoskeletal injury: a randomized, double blind, controlled trial. Hong Kong Journal of Emergency Medicine 2004;11(2):78-84. [Google Scholar]
McCulloch 1985 {published data only}
- McCulloch PG, Holden P, Robson DJ, Rowley DI, Norris SH. The value of mobilisation and non-steroidal anti-inflammatory analgesia in the management of inversion injuries of the ankle. British Journal of Clinical Practice 1985;39(2):69-72. [PMID: 3872674] [PubMed] [Google Scholar]
- McCulloch PG. Request for study data that was unavailable [Personal communication]. Email to: P Jones 7 November 2014.
Ridderikhof 2018 {published and unpublished data}
- NL3815 (NTR3982). Paracetamol or NSAID's in minor musculoskeletal trauma. trialregister.nl/trial/3815 (first received 02 May 2013).
- Ridderikhof ML, Lirk P, Goddijn H, Vandewalle E, Schinkel E, Van Dieren S, et al. Acetaminophen or nonsteroidal anti-Inflammatory drugs in acute musculoskeletal trauma: a multicenter, double-blind, randomized, clinical trial. Annals of Emergency Medicine 2018;71(3):357-68. [DOI] [PubMed] [Google Scholar]
- Ridderikhof ML, Lirk P, Schep NW, Hoeberichts A, Goddijn WT, Luitse JSK, et al. The PanAM study: a multi-center, double-blinded,randomised, non-inferiority study of paracetamol versus non-steroidal anti-inflammatory drugs in treating acute musculoskeletal trauma. BMC Emergency Medicine 2013;13(19):1-9. [DOI] [PMC free article] [PubMed]
- Ridderikhof, ML. Total Adverse Events Data by Study End [personal communication]. Email to: P Jones 29 September 2019.
Woo 2005 {published data only}
- Rainer TH. Request for study data relevant to this review [personal communication]. Email to: P Jones 25 September 2019.
- Woo WW, Man SY, Lam PK, Rainer TH. Randomized double-blind trial comparing oral paracetamol and oral nonsteroidal antiinflammatory drugs for treating pain after musculoskeletal injury. Annals of Emergency Medicine 2005;46(4):352-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andersson 1983 {published data only}
- Andersson S, Fredin H, Lindberg H, Sanzen L, Westlin N. Ibuprofen and compression bandage in the treatment of ankle sprains. Acta Orthopaedica Scandinavica 1983;54(2):322-5. [DOI] [PubMed] [Google Scholar]
Buccelletti 2014 {published data only (unpublished sought but not used)}
- Buccelletti F, Marsiliani D, Zuccala G, Iacomini P, Proietti L, Pola E, et al. Paracetamol-codeine compared to ketorolac for pain control in the emergency department. European Review for Medical and Pharmacological Sciences 2014;18(20):3139-43. [PubMed] [Google Scholar]
Calligaris 1993 {published data only}
- Calligaris A, Scaricabarozzi I, Vecchiet L. A multicentre double-blind investigation comparing nimesulide and naproxen in the treatment of minor sport injuries. Drugs 1993;46(Suppl 1):187-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cardenas‐Estrada 2009 {published and unpublished data}
- Cardenas-Estrada E, Oliveira LG, Abad HL, Elayan F, Khalifa N, El-Husseini T. An open label randomized multicenter comparative study on celecoxib efficacy and safety versus non-selective NSAIDs in acute pain due to ankle sprain. Journal of International Medical Research 2009;37(6):1937-51. [DOI] [PubMed] [Google Scholar]
- NCT00446797. Open label randomized multicenter comparative study on celecoxib efficacy and safety versus non-selective NSAIDs in acute pain due to ankle sprain. clinicaltrials.gov/show/NCT00446797 (first received 13 March 2009).
- Pfizer Inc. A3191332. An open label randomized multicenter comparative study on celecoxib efficacy and safety versus non-selective NSAIDs in acute pain due to ankle sprain. www.clinicalstudyresults.org/documents/company-study (accessed 28 January 2009).
Cauchioli 1994 {published data only}
- Cauchioli CA. Comparative trial between etodolac and naproxen in the treatment of acute sports injuries. Arquivo Brasileiro de Medicina 1994;68(2):109-14. [Google Scholar]
Chang 2017 {published data only}
- Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA 2017;318(17):1661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02455518. Comparative efficacy of 4 oral analgesics. clinicaltrials.gov/ct2/show/NCT02455518 (first received 28 May 2015).
Collopy 2012 {published data only}
- Collopy KT, Kivlehan SM, Snyder SR. Managing unstable musculoskeletal injuries. EMS World 2012;41(2):36-43. [PubMed] [Google Scholar]
Costa 1995 {published data only}
- Costa JRB, Rehfeldt LCL, Da Silva SAG. Traumatic sprains and strains. Prospective blind study to compare the efficacy and safety of nimesulide and diclofenac. Revista Brasileira de Medicina 1995;52(4):366-8. [Google Scholar]
D'Hooghe 1992 {published data only}
- D'Hooghe M. Double-blind, parallel-group evaluation of etodolac and naproxen in patients with acute sports injuries. Clinical Therapeutics 1992;14(4):507-16. [PMID: ] [PubMed] [Google Scholar]
De Gara 1982 {published data only}
- De Gara C, Taylor M, Hedges A. Assessment of analgesic drugs in soft tissue injuries presenting to an accident and emergency department – a comparison of antrafenine, paracetamol and placebo. Postgraduate Medical Journal 1982;58(682):489-92. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz 2006 {published data only}
- Diaz JA, Cuervo C, Valderrama AM, Kohles J. Valdecoxib provides effective pain relief following acute ankle sprain. Journal of International Medical Research 2006;34(5):456-67. [PMID: ] [DOI] [PubMed]
- Pfizer Inc. Protocol VALA-0513-146. A multi-center, randomised, double-blind, parallel group study to compare the efficacy and tolerability of valdecoxib vs. diclofenac in ankle sprain. www.clinicalstudyresults.org/documents/company-study (accessed 27 April 2004).
Dougados 2007 {published data only}
- Dougados M, Le Henanff A, Logeart I, Ravaud P. Short-term efficacy of rofecoxib and diclofenac in acute shoulder pain: a placebo-controlled randomized trial. PLoS Clinical Trials 2007;2(3):e9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekman 2002 {published data only}
- Ekman EF, Fiechtner JJ, Levy S, Fort JG. Efficacy of celecoxib versus ibuprofen in the treatment of acute pain: a multicenter, double-blind, randomized controlled trial in acute ankle sprain. American Journal of Orthopedics 2002;31(8):445-51. [PMID: 12216965] [PubMed] [Google Scholar]
Feragalli 2017 {published data only}
- Feragalli B, Ippolito E, Dugall M, Cacchio M, Belcaro G, Cesarone MR, et al. Effectiveness of a novel boswellic acids delivery form (Casperome) in the management of grade II ankle sprains due to sport trauma – a registry study. European Review for Medical and Pharmacological Sciences 2017;21(20):4726-32. [PubMed] [Google Scholar]
Ferreira 1992 {published data only}
- Ferreira A, Espirandelli L, Peloso UC. Etodolac versus diclofenac in acute sports injuries [Etodolac versus diclofenaco em traumatismos esportivos agudos]. Arquivos Brasileiros de Medicina 1992;66(2):175-81. [0365-0723] [Google Scholar]
Goswick 1983 {published data only}
- Goswick C. Ibuprofen versus propoxyphene hydrochloride and placebo in acute musculoskeletal trauma. Current Therapeutic Research, Clinical and Experimental 1983;34(4):685-92. [Google Scholar]
Graudins 2016 {published data only}
- Graudins A, Meek R, Parkinson J, Egerton-Warburton D, Meyer A. A randomised controlled trial of paracetamol and ibuprofen with or without codeine or oxycodone as initial analgesia for adults with moderate pain from limb injury. Emergency Medicine Australasia 2016;28(6):666-72. [DOI] [PubMed] [Google Scholar]
Gyer 2012 {published data only}
- Gyer VF, Gloster A. Use of non-steroidal drugs for the treatment of sprains. Nursing Standard 2012;26(25):30. [DOI] [PubMed] [Google Scholar]
Hardo 1982 {published data only}
- Hardo HG, Templeton JS. A short-term randomised comparative study of Rheumox and distalgesic in outpatients suffering from painful soft tissue injuries/sprains and strains. British Journal of Clinical Practice 1982;36:112-5. [Google Scholar]
Jenner 1987 {published data only}
- Jenner PN. Nabumetone in the treatment of skin and soft tissue injury. American Journal of Medicine 1987;83(4B):101-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jenoure 1998 {published data only}
- Jenoure P, Gorschewsky O, Ryf C, Steigbugel M, Wetzel C, Frey W, et al. Randomised, double-blind, multicentre study of nimesulide vs. diclofenac in adults with acute sport injuries. Journal of Drug Assessment 1998;1:495-508. [Google Scholar]
Jorgensen 1986 {published data only}
- Jørgensen FR, Gøtzsche PC, Hein P, Jensen CM, Nielsen BM, Nielsen FM, et al. Naproxen (Naprosyn) and mobilization in the treatment of acute ankle sprains [Naproxen (Naprosyn) og mobilisering ved behandling af akut ankeldistorsion]. Ugeskrift for Laeger 1986;148(21):1266-68. [PubMed] [Google Scholar]
Khoury 2018 {published data only}
- Khoury M, Caspi S, Stalnikowics R, Peless E, Raiizman E, Salameh S. Emergency department administration of oxycodone by nurses treating musculoskeletal pain: an observational prospective study. Israel Medical Association Journal 2018;20(5):281-5. [PubMed] [Google Scholar]
Kolodny 1975 {published data only}
- Kolodny AL, Winter L Jr. Further clinical evaluations of nefopam hydrochloride, a new analgesic. Current Therapeutic Research, Clinical and Experimental 1975;17(6):519-24. [PMID: ] [PubMed] [Google Scholar]
Kyle 2008 {published data only}
- Kyle C, Zachariah J, Kinch H, Ellis G, Andrews C, Adekunle F. A randomised, double-blind study comparing lumiracoxib with naproxen for acute musculoskeletal pain. International Journal of Clinical Practice 2008;62(11):1684-92. [PMID: 19143855] [DOI] [PubMed] [Google Scholar]
Le May 2013 {published and unpublished data}
- Le May S, Gouin S, Fortin C, Messier A, Robert MA, Julien M. Efficacy of an ibuprofen/codeine combination for pain management in children presenting to the emergency department with a limb injury: a pilot study. Journal of Emergency Medicine 2013;44(2):536-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Le May S. Request for data relevant to this review [personal communication]. Email to: P Jones 6 September 2013.
- LeMay S, Gouin S, Robert MA, Messier A, Morin N. Combination of analgesics for pain management of children presenting to the emergency department for an acute musculoskeletal trauma. Academic Emergency Medicine 2010;17:S142. [Google Scholar]
- NCT01189773. Combined analgesia to control pain in children seen in emergency department for a trauma of a limb. clinicaltrials.gov/ct2/show/NCT01189773 (first received 27 August 2010).
Moore 1999 {published data only}
- Le Parc JM, Van Ganse E, Moore N, Wall R, Schneid H, Verrière F. Comparative tolerability of paracetamol, aspirin and ibuprofen for short-term analgesia in patients with musculoskeletal conditions: results in 4291 patients. Clinical Rheumatology 2002;21(1):28-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Moore N, Charlesworth A, Van Ganse E, LeParc JM, Jones JK, Wall R, et al. Risk factors for adverse events in analgesic drug users: results from the PAIN study. Pharmacoepidemiology and Drug Safety 2003;12(7):601-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Moore N, Van Ganse E, Le Parc JM, Wall R, Schneid H, Farhan M, et al. The PAIN study: Paracetamol, Aspirin and Ibuprofen New tolerability study. A large-scale, randomised clinical trial comparing the tolerability of aspirin, ibuprofen and paracetamol for short-term analgesia. Clinical Drug Investigation 1999;18(2):89-98. [Google Scholar]
- Moore N. Forty years of ibuprofen use. International Journal of Clinical Practice Supplement 2003;135:28-31. [PMID: ] [PubMed] [Google Scholar]
Muncie 1986 {published data only}
- Muncie HL Jr, King DE, DeForge B. Treatment of mild to moderate pain of acute soft tissue injury: diflunisal vs acetaminophen with codeine. Journal of Family Practice 1986;23(2):125-7. [PMID: ] [PubMed] [Google Scholar]
Nadarajah 2006 {published data only}
- Cawkwell G. Success II-G study data [personal communication]. Email to: P Jones 19 August 2008.
- Nadarajah A, Abrahan L, Lau FL, Hwang LJ, Fakir-Bolte C. Efficacy and tolerability of celecoxib compared with diclofenac slow release in the treatment of acute ankle sprain in an Asian population. Singapore Medical Journal 2006;47(6):534-42. [PMID: 6752024] [PubMed] [Google Scholar]
NCT00954785 {unpublished data only}
- NCT00954785. Etoricoxib in acute ankle ligament sprains. clinicaltrials.gov/show/NCT00954785 (first received 07 August 2009).
NCT01974609 {published data only}
- NCT01974609. Narcotic vs. non-narcotic pain study protocol. clinicaltrials.gov/ct2/show/NCT01974609 (first received 01 November 2013).
NCT02373254 {published data only}
- NCT02373254. NSAIDS versus opioids in acute SER II ankle fractures. clinicaltrials.gov/ct2/show/NCT02373254 (first received 26 February 2015).
NCT02862977 {published data only}
- NCT02862977. Efficacy and safety of the combination of ketoprofen and cyclobenzaprine and caffeine in osteomuscular treatment. clinicaltrials.gov/ct2/show/NCT02862977 (first received 11 August 2016).
NCT03025113 {published data only}
- NCT03025113. Efficacy and safety of the combination of ketoprofen and cyclobenzaprine in osteomuscular treatment. clinicaltrials.gov/ct2/show/NCT03025113 (first received 19 January 2017).
NCT03173456 {published data only}
- NCT03173456. Comparing five oral analgesics for treatment of acute pain in the ED. clinicaltrials.gov/ct2/show/NCT03173456 (first received 01 June 2017).
NCT03767933 {published data only}
- NCT03767933. Non-steroidal or opioid analgesia use for children with musculoskeletal injuries. clinicaltrials.gov/ct2/show/NCT03767933 (first received 07 December 2018).
Pagliara 1997 {published data only}
- Pagliara L, Tornago S, Metastasio J, Peretti G, Albisetti W, Thovez G, et al. Tramadol compared with diclofenac in traumatic musculoskeletal pain. Current Therapeutic Research, Clinical and Experimental 1997;58(8):473-80. [Google Scholar]
Patel 1993 {published data only}
- Patel R. Management of painful inflammatory disorders in paediatric practice. Indian Practitioner 1993;46(7):513-20. [Google Scholar]
Petrella 2004 {published data only}
- Petrella R, Ekman EF, Schuller R, Fort JG. Efficacy of celecoxib, a COX-2-specific inhibitor, and naproxen in the management of acute ankle sprain: results of a double-blind, randomized controlled trial. Clinical Journal of Sport Medicine 2004;14(4):225-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pfizer 2005 {unpublished data only}
- Pfizer Inc. A multicenter, randomized, open-label, parallel group study to compare the efficacy and tolerability of valdecoxib versus ketorolac in ankle sprain. Pharmaceutical Research and Manufacturers of America Database of Study Synopses 23 September 2005.
Sherry 1988 {published data only}
- Sherry E, Jack D, Henderson A. Post-traumatic pain relief on an out-patient basis: drug trial looking at injured skiers. Australian Journal of Science and Medicine in Sport 1988;20(1):18-20. [Google Scholar]
Simmons 1982 {published data only}
- Simmons RL, Owen S, Abbott CJ, Bouchier-Hayes TA, Hunt HA. Naproxen sodium and paracetamol/dextropropoxyphene in sports injuries – a multicentre comparative study. British Journal of Sports Medicine 1982;16(2):91-5. [PMID: 7104563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sleet 1980 {published data only}
- Sleet RA, Khan MA. Comparative study of mefenamic acid and dextropropoxyphene plus paracetamol in an accident and emergency department. Current Medical Research and Opinion 1980;7(2):77-84. [PMID: 7438771] [DOI] [PubMed] [Google Scholar]
Stableforth 1977 {published data only (unpublished sought but not used)}
- Stableforth PG. Mefenamic acid and dextropropoxyphene with paracetamol as analgesics in the accident department. Current Medical Research and Opinion 1977;5(2):189-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Turturro 2003 {published data only}
- Turturro MA, Frater CR, D'Amico FJ. Cyclobenzaprine with ibuprofen versus ibuprofen alone in acute myofascial strain: a randomized, double-blind clinical trial. Annals of Emergency Medicine 2003;41(6):818-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
van den Bekerom 2016 {published data only}
- den Bekerom MP. No difference in pain, swelling or function with NSAIDs compared with paracetamol for soft tissue injury. Evidence-based Nursing 2016;19(1):21. [DOI] [PubMed] [Google Scholar]
Whitehead 2016 {published data only}
- Whitehead PB. Oral NSAIDs versus other oral analgesic agents for acute soft tissue injury. International Journal of Evidence-based Healthcare 2016;14(3):138-9. [DOI] [PubMed] [Google Scholar]
Yates 1984 {published data only}
- Yates D. Request for data relevant to this review [personal communication]. Email to: P Jones October 2011.
- Yates DW, Laing GS, Peters K, Kumar K. Mild analgesics and the accident and emergency department – cost and safety more important than potency? Archives of Emergency Medicine 1984;1(4):197-203. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yazdanpanah 2011 {published data only}IRCT138904014238N1
- IRCT138904014238N1. Effect of oral and intramuscular injection of methocarbamol and placebo on muscle strain [Comparison effect of oral and intramuscular injection of methocarbamol and placebo on muscle strain]. apps.who.int/trialsearch/trial.aspx?trialid=IRCT138904014238N1 (first received 10 October 2010). [IRCT138904014238N1]
- Yazdanpanah P, Nikbakht S, Kheramin S, Hossini R. Effect of oral and intramuscular injection of methocarbamol and placebo on muscle strain pain. European Journal of Pain Supplements 2011;5(1):26. [Google Scholar]
Yilmaz 2019 {published data only}
- NCT03428503. Comparison of efficacy of intravenous paracetamol and dexketoprofen for acute traumatic musculoskeletal pain. clinicaltrials.gov/ct2/show/NCT03428503 (first received 09 February 2018).
- Yilmaz A, Sabirli R, Ozen M, Turkcuer I, Erdur B, Arikan C, et al. Intravenous paracetamol versus dexketoprofen in acute musculoskeletal trauma in the emergency department: a randomised clinical trial. American Journal of Emergency Medicine 2019;37(5):902-8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
CTRI/2009/091/001067 {unpublished data only}
- CTRI/2009/091/001067. An open label, randomized, parallel group, prospective, multicentre clinical trial for evaluation of efficacy and safety of IN-Pharm-002 in comparison with ibuprofen in adult patients wity acute painful soft tissue injury. ctri.nic.in/Clinicaltrials/showallp.php?mid1=1196&EncHid=&userName=CTRI/2009/091/001067 (registered on 17 February 2010).
TCTR20160126001 {published data only}
- TCTR20160126001. Efficacy and safety of extracted Prasaplai for relieving acute muscle strain. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20160126001 (first received 25 January 2016).
References to ongoing studies
NCT02667730 {published data only}
- NCT02667730. Correlation between acute analgesia and long-term function following ankle injuries. https://clinicaltrials.gov/ct2/show/NCT02667730 (first received 29 January 2016).
NCT03222518 {published data only}
- NCT03222518. NSAIDs versus paracetamol versus paracetamol + NSAIDs in traumatic pain management. https://clinicaltrials.gov/ct2/show/NCT03222518 (first received 19 July 2017).
Additional references
ACC 2020
- Accident Compensation Corporation (ACC, NZ). Average expense per claim by injury type in last 12 months. catalogue.data.govt.nz/dataset/average-expense-per-claim-by-injury-type-in-last-12-months#dataset-resources (accessed 19 June 2020).
Almekinders 1986
- Almekinders LC, Gilbert JA. Healing of experimental muscle strains and the effects of nonsteroidal antiinflammatory medication. The American Journal of Sports Medicine 1986;14(4):303-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Almekinders 1995
- Almekinders LC, Baynes AJ, Bracey LW. An in vitro investigation into the effects of repetitive motion and nonsteroidal antiinflammatory medication on human tendon fibroblasts. American Journal of Sports Medicine 1995;23(1):119-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Altman 2000
- Altman DG. Statistics in medical journals: some recent trends. Statistics in Medicine 2000;19(23):3275-89. [PMID: ] [DOI] [PubMed] [Google Scholar]
Amadio 1997
- Amadio P Jr, Cummings DM, Amadio PB. NSAIDs revisited: selection, monitoring, and safe use. Postgraduate Medicine 1997;101(2):257-60, 263-7, 270-1. [PMID: ] [DOI] [PubMed] [Google Scholar]
Andrade 1998
- Andrade SE, Martinez C, Walker AM. Comparative safety evaluation of non-narcotic analgesics. Journal of Clinical Epidemiology 1998;51(12):1357-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
ANZCA 2005
- Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute pain management: scientific evidence. www.anzca.edu.au/resources/books-and-publications/acutepain.pdf (accessed April 2008).
Baldwin 2003
- Baldwin Lanier A. Use of nonsteroidal anti-inflammatory drugs following exercise-induced muscle injury. Sports Medicine 2003;33(3):177-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bandolier 2005
- Bandolier Extra. Combination analgesics. www.medicine.ox.ac.uk/bandolier/Extraforbando/combo.pdf (accessed 31 July 2008).
Bandolier 2007
- Bandolier. Oxford league table of analgesics in acute pain. www.jr2.ox.ac.uk/bandolier/booth/painpag/Acutrev/Analgesics/Leagtab.html (accessed 31 July 2008).
Barden 2004
- Barden J, Edwards JE, Mason L, McQuay HJ, Moore RA. Outcomes in acute pain trials: systematic review of what was reported? Pain 2004;109(3):351-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Barnett 2017
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. New England Journal of Medicine 2017;376(7):663-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergh 2001
- Bergh I, Sjostrom B, Oden A, Steen B. Assessing pain and pain relief in geriatric patients with non-pathological fractures with different rating scales. Aging Clinical and Experimental Research 2001;13(5):355-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bijur 2003
- Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Academic Emergency Medicine 2003;10(4):390-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bogatov 2003
- Bogatov VB, Weinhold P, Dahners LE. The influence of a cyclooxygenase-1 inhibitor on injured and uninjured ligaments in the rat. American Journal of Sports Medicine 2003;31(4):574-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bollini 1992
- Bollini P, Garcia Rodriguez LA, Perez Gutthann S, Walker AM. The impact of research quality and study design on epidemiologic estimates of the effect of nonsteroidal anti-inflammatory drugs on upper gastrointestinal tract disease. Archives of Internal Medicine 1992;152(6):1289-95. [PMID: ] [PubMed] [Google Scholar]
Brooks 1991
- Brooks PM, Day RO. Nonsteroidal antiinflammatory drugs – differences and similarities. New England Journal of Medicine 1991;324(24):1716-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Browning 1996
- Browning CH. Nonsteroidal anti-inflammatory drugs and severe psychiatric side effects. International Journal of Psychiatry in Medicine 1996;26(1):25-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Burke 2006
- Burke A, Smyth EM, Fitzgerald GA. Analgesic-antipyretic agents. In: Brunton LL, Lazo JS, Parker KL, editors(s). Goodman and Gilman's The Pharmaceutical Basis of Therapeutics. 11 edition. NY: McGraw-Hill, 2006:671-716. [Google Scholar]
Cassell 2003
- Cassell EP, Finch CF, Stathakis VZ. Epidemiology of medically treated sport and active recreation injuries in the Latrobe Valley, Victoria, Australia. British Journal of Sports Medicine 2003;37(5):405-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2006
- Chan FK. Primer: managing NSAID-induced ulcer complications – balancing gastrointestinal and cardiovascular risks. Nature Clinical Practice. Gastroenterology & Hepatology 2006;3(10):563-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cochrane 2002
- Cochrane Collaboration open learning material for reviewers Version 11. Summary statistics for dichotomous outcome data. www.cochrane-net.org/openlearning/HTML/modA1-5.htm (accessed April 2008).
CRD 2007
De Gara 1982a
- De Gara C, Taylor M, Hedges A. Assessment of analgesic drugs in soft tissue injuries presenting to an accident and emergency department--a comparison of antrafenine, paracetamol and placebo. Postgraduate Medical Journal 1982;58(682):489-92. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks J, Altman DG. Chapter 16: Effect measures for meta-analysis of trials with binary outcomes. In: Egger M, Smith GD, Altman DG, editors(s). Systematic Reviews. 2nd edition. London: BMJ, 2001:313-5. [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575-600. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dexter 1995
- Dexter F, Chestnut DH. Analysis of statistical tests to compare visual analog scale measurements among groups. Anesthesiology 1995;82(4):896-902. [PMID: ] [DOI] [PubMed] [Google Scholar]
Diaz 2006a
Egger 2001
- Egger M, Dickersin K, Davey-Smith G. Chapter 3: Problems and limitations in conducting systematic reviews. In: Egger M, Smith GD, Altman DG, editors(s). Systematic Reviews in Healthcare: Meta-analysis in Context. 2nd edition. London: BMJ, 2001:43-65. [Google Scholar]
Egger 2001a
- Egger M, Smith GD. Chapter 2: Principles and procedures for systematic reviews. In: Egger M, Smith GD, Altman DG, editors(s). Systematic Reviews in Healthcare: Meta-analysis in Context. London: BMJ, 2001:23-42. [Google Scholar]
Ekman 2002a
- Ekman EF, Fiechtner JJ, Levy S, Fort JG. Efficacy of celecoxib versus ibuprofen in the treatment of acute pain: a multicenter, double-blind, randomized controlled trial in acute ankle sprain. American Journal of Orthopedics 2002;31(8):445-51. [PMID: ] [PubMed] [Google Scholar]
Falgarone 2005
- Falgarone G, Zerkak D, Bauer C, Messow M, Dougados M. How to define a Minimal Clinically Individual State (MCIS) with pain VAS in daily practice for patients suffering from musculoskeletal disorders. Clinical and Experimental Rheumatology 2005;23(2):235-8. [PMID: ] [PubMed] [Google Scholar]
Farkouh 2004
- Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 2004;364(9435):675-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Farrar 2003
- Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. Journal of Pain and Symptom Management 2003;25(5):406-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Feldman 1997
- Feldman HI, Kinman JL, Berlin JA, Hennessy S, Kimmel SE, Farrar J, et al. Parenteral ketorolac: the risk for acute renal failure. Annals of Internal Medicine 1997;126(3):193-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fosnocht 2005
- Fosnocht DE, Chapman CR, Swanson ER, Donaldson GW. Correlation of change in visual analog scale with pain relief in the ED. American Journal of Emergency Medicine 2005;23(1):55-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gallagher 2001
- Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Annals of Emergency Medicine 2001;38(6):633-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gallagher 2002
- Gallagher EJ, Bijur PE, Latimer C, Silver W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. American Journal of Emergency Medicine 2002;20(4):287-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
García Rodríguez 1992
- García Rodríguez LA, Pérez Gutthann S, Walker AM, Lueck L. The role of non-steroidal anti-inflammatory drugs in acute liver injury. BMJ 1992;305(6858):865-8. [PMID: 1422399] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geraci 2007
- Geraci M, Bottai M. Quantile regression for longitudinal data using the asymmetric Laplace distribution. Biostatistics 2007;8(1):140-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Glasziou 2002
- Glasziou PP, Sanders SL. Investigating causes of heterogeneity in systematic reviews. Statistics in Medicine 2002;21(11):1503-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gong 2019
- Gong J, Colligan M, Kirkpatrick C, Jones P. Oral paracetamol versus combination oral analgesics for acute musculoskeletal injuries. Annals of Emergency Medicine 2019;74(4):521-9. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 13 May 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Gutstein 2006
- Gutstein HB, Akil H. Opioid analgesics. In: Brunton LL, Lazo JS, Parker KL, editors(s). Goodman and Gilman's: The Pharmaceutical Basis of Therapeutics. 11 edition. New York: McGraw-Hill, 2006:547-90. [Google Scholar]
Gøtzsche 2000
- Gøtzsche PC. Non-steroidal anti-inflammatory drugs. BMJ 2000;320(7241):1058-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haghighat 2013
- Haghighat A, Behnia A, Kaviani N, Khorami B. Evaluation of Glucosamine sulfate and Ibuprofen effects in patients with temporomandibular joint osteoarthritis symptom. Journal of Research in Pharmacy Practice 2013;2(1):34-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handoll 2007
- Handoll HH, Gillespie WJ, Gillespie LD, Madhok R. Moving towards evidence-based healthcare for musculoskeletal injuries: featuring the work of the Cochrane Bone, Joint and Muscle Trauma Group. Journal of the Royal Society for the Promotion of Health 2007;127(4):168-73. [PMID: 17711062] [DOI] [PubMed] [Google Scholar]
Henry 1990
- Henry D. Assessing the benefits and risks of drugs. The example of NSAIDs. Australian Family Physician 1990;19(3):378-83, 385, 387. [PMID: ] [PubMed] [Google Scholar]
Herbison 2008
- Herbison P. Statistical advice [Personal communication] 15 September 2008.
Hertel 1997
- Hertel J. The role of nonsteroidal anti-inflammatory drugs in the treatment of acute soft tissue injuries. Journal of Athletic Training 1997;32(4):350-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Hewitt 2007
- Hewitt DJ, Todd KH, Xiang J, Jordan DM, Rosenthal NR, CAPSS-216 Study Investigators. Tramadol/acetaminophen or hydrocodone/acetaminophen for the treatment of ankle sprain: a randomized, placebo-controlled trial. Annals of Emergency Medicine 2007;49(4):468-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011a
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Honig 1988
- Honig S. Clinical trials in acute musculoskeletal injury states. An analysis of methodology. American Journal of Medicine 1988;84(5A):42-4. [PMID: 3376973] [DOI] [PubMed] [Google Scholar]
IASP 2007
- International Association for the Study of Pain (IASP). IASP pain terminology. www.iasp-pain.org/AM/Template.cfm?Section=General_Resource_Links&Template=/CM/HTMLDisplay.cfm&ContentID=3058#Painz (accessed 31 July 2008).
Ivins 2006
- Ivins D. Acute ankle sprain: an update. American Family Physician 2006;74(10):1714-20. [PMID: 17137000] [PubMed] [Google Scholar]
Jensen 2005
- Jensen MP, Martin SA, Cheung R. The meaning of pain relief in a clinical trial. Journal of Pain 2005;6(6):400-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnson 1995
- Johnson GR, Wen SF. Syndrome of flank pain and acute renal failure after binge drinking and nonsteroidal anti-inflammatory drug ingestion. Journal of the American Society of Nephrology 1995;5(9):1647-52. [PMID: 7780052] [DOI] [PubMed] [Google Scholar]
Jones 1998
- Jones P. NSAIDs in soft tissue injury: current practice vs. available evidence. Emergency Medicine Australasia 1998;10(4):361. [Google Scholar]
Jones 1999
- Jones PG. Analgesia in soft-tissue injury: current practice in Auckland is not supported by the available evidence. New Zealand Medical Journal 1999;112(1097):376-9. [PMID: ] [PubMed] [Google Scholar]
Jones 2010
- Jones P, Lamdin R. Oral cyclo-oxygenase 2 inhibitors versus other oral analgesics for acute soft tissue injury: systematic review and meta-analysis. Clinical Drug Investigation 2010;30(7):419-37. [PMID: 20527999] [DOI] [PubMed] [Google Scholar]
Kaufman 1996
- Kaufman HL, Fischer AH, Carroll M, Becker JM. Colonic ulceration associated with nonsteroidal anti-inflammatory drugs. Report of three cases. Diseases of the Colon and Rectum 1996;39(6):705-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kearney 2006
- Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006;332(7553):1302-8. [PMID: 16740558] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kellett 1986
- Kellett J. Acute soft tissue injuries – a review of the literature. Medicine and Science in Sports and Exercise 1986;18(5):489-500. [PMID: ] [PubMed] [Google Scholar]
Kelly 1998
- Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Academic Emergency Medicine 1998;5(11):1086-90. [PMID: 9835471] [DOI] [PubMed] [Google Scholar]
Kelly 2001
- Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emergency Medicine Journal 2001;18(3):205-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkwood 2003
- Kirkwood BR, Sterne JAC. Medical Statistics. 2nd edition. Oxford: Blackwell, 2003. [Google Scholar]
Koithan 2009
- Koithan M. Introducing complementary and alternative therapies. Journal for Nurse Practitioners 2009;5(1):18-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2003
- Lee JS, Hobden E, Stiell IG, Wells GA. Clinically important change in the visual analog scale after adequate pain control. Academic Emergency Medicine 2003;10(10):1128-30. [PMID: 14525749] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leopoldino 2019
- Leopoldino AO, Machado GC, Ferreira PH, Pinheiro MB, Day R, McLachlan AJ, et al. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database of Systematic Reviews 2019, Issue 2. Art. No: CD013273. [DOI: 10.1002/14651858.CD013273] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lund 2005
- Lund I, Lundeberg T, Sandberg L, Budh CN, Kowalski J, Svensson E. Lack of interchangeability between visual analogue and verbal rating pain scales: a cross sectional description of pain etiology groups. BMC Medical Research Methodology 2005;5:31. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Major 1992
- Major P. Should non-steroidal anti-inflammatory agents be used in acute soft tissue injuries? [Bor ikke-steroide antiinflammatoriske midler brukes ved akutte blotdelsskader?]. Tidsskrift for den Norske Laegeforening 1992;112(12):1614-5. [PMID: ] [PubMed] [Google Scholar]
Manterola 2007
- Manterola C, Vial M, Moraga J, Astudillo P. Analgesia in patients with acute abdominal pain. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD005660. [DOI: 10.1002/14651858.CD005660.pub2] [DOI] [PubMed] [Google Scholar]
Martin 2005
- Martin DJ, Palmer S. Soft tissue pain and physical therapy. Anaesthesia & Intensive Care Medicine 2005;6(1):23-5. [Google Scholar]
McClellan 2006
- McClellan CM, Greenwood R, Benger JR. Effect of an extended scope physiotherapy service on patient satisfaction and the outcome of soft tissue injuries in an adult emergency department. Emergency Medicine Journal 2006;23(5):384-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNicol 2005
- McNicol ED, Strassels S, Goudas L, Lau J, Carr DB. NSAIDS or paracetamol, alone or combined with opioids, for cancer pain. Cochrane Database of Systematic Reviews 2005, Issue 2. Art. No: CD005180. [DOI: 10.1002/14651858.CD005180] [DOI] [PubMed] [Google Scholar]
Medibank 2006
- Medibank Private. Safe sports report 2006. www.medibank.com.au/Client/Documents/Pdfs/Medi_Safe_Sports_Report_2006.pdf (accessed 31 July 2008).
Mehallo 2006
- Mehallo CJ, Drezner JA, Bytomski JR. Practical management: nonsteroidal antiinflammatory drug (NSAID) use in athletic injuries. Clinical Journal of Sport Medicine 2006;16(2):170-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Merry 2004
- Merry AF, Webster CS, Holland RL, Middleton NG, Schug SA, James M, et al. Clinical tolerability of perioperative tenoxicam in 1001 patients – a prospective, controlled, double-blind, multi-centre study. Pain 2004;111(3):313-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2-3):229-37. [PMID: 8880845] [DOI] [PubMed] [Google Scholar]
Moore 1997
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69(3):311-5. [PMID: 9085306] [DOI] [PubMed] [Google Scholar]
Moore 2005
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta-analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moore 2007
- Moore A. Advice on derivation of dichotomous data from graphs of VAS scores [personal communication]. Email to: P Jones 10 September 2007.
Motola 2004
- Motola D, Vaccheri A, Silvani MC, Poluzzi E, Bottoni A, De Ponti F, et al. Pattern of NSAID use in the Italian general population: a questionnaire-based survey. European Journal of Clinical Pharmacology 2004;60(10):731-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Myles 1999
- Myles PS, Troedel S, Boquest M, Reeves M. The pain visual analog scale: Is it linear or nonlinear? Anesthesia and Analgesia 1999;89(6):1517-20. [PMID: 10589640] [DOI] [PubMed] [Google Scholar]
Myles 2005
- Myles PS, Urquhart N. The linearity of the visual analogue scale in patients with severe acute pain. Anaesthesia and Intensive Care 2005;33(1):54-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nadarajah 2006a
- Nadarajah A, Abrahan L, Lau FL, Hwang LJ, Fakir-Bolte C. Efficacy and tolerability of celecoxib compared with diclofenac slow release in the treatment of acute ankle sprain in an Asian population. Singapore Medical Journal 2006;47(6):534-42. [PMID: ] [PubMed] [Google Scholar]
Nicholl 1995
- Nicholl JP, Coleman P, Williams BT. The epidemiology of sports and exercise related injury in the United Kingdom. British Journal of Sports Medicine 1995;29(4):232-8. [PMID: 8808535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Obremsky 1994
- Obremsky WT, Seaber AV, Ribbeck BM, Garrett WE Jr. Biomechanical and histologic assessment of a controlled muscle strain injury treated with piroxicam. American Journal of Sports Medicine 1994;22(4):558-61. [PMID: 7943524] [DOI] [PubMed] [Google Scholar]
Ogilvie‐Harris 1995
- Ogilvie-Harris DJ, Gilbart M. Treatment modalities for soft tissue injuries of the ankle: a critical review. Clinical Journal of Sport Medicine 1995;5(3):175-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Paoloni 2005
- Paoloni JA, Orchard JW. The use of therapeutic medications for soft-tissue injuries in sports medicine. Medical Journal of Australia 2005;183(7):384-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Park 2015
- Park BK, Dear JW, Antoine DJ. Paracetamol (acetaminophen) poisoning. BMJ Clinical Evidence 2015;2015:2101. [PMC free article] [PubMed] [Google Scholar]
Partsch 1990
- Partsch G, Schwarzer C, Eberl R. The effects of ibuprofen and diclofenac on the chemotaxis and adenosine triphosphate level of polymorphonuclear cells in vitro. Journal of Rheumatology 1990;17(5):583-8. [PMID: 2162960] [PubMed] [Google Scholar]
Pathan 2012
- Pathan H, Williams J. Basic opioid pharmacology: an update. British Journal of Pain 2012;6(1):11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrella 2004a
- Petrella R, Ekman EF, Schuller R, Fort JG. Efficacy of celecoxib, a COX-2-specific inhibitor, and naproxen in the management of acute ankle sprain: results of a double-blind, randomized controlled trial. Clinical Journal of Sport Medicine 2004;14(4):225-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Philip 1990
- Philip BK. Parametric statistics for evaluation of the visual analog scale. Anesthesia and Analgesia 1990;71(6):710. [PMID: 2240648] [DOI] [PubMed] [Google Scholar]
Powell 2001
- Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Annals of Emergency Medicine 2001;37(1):28-31. [PMID: 11145767] [DOI] [PubMed] [Google Scholar]
Pérez Gutthann 1996
- Pérez Gutthann S, Garcia Rodriguez LA, Raiford DS, Duque Oliart A, Ris Romeu J. Nonsteroidal anti-inflammatory drugs and the risk of hospitalization for acute renal failure. Archives of Internal Medicine 1996;156(21):2433-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Quiding 1983
- Quiding H, Häggquist SO. Visual analogue scale and the analysis of analgesic action. European Journal of Clinical Pharmacology 1983;24(4):475-8. [PMID: 6861862] [DOI] [PubMed] [Google Scholar]
Rahusen 2004
- Rahusen FT, Weinhold PS, Almekinders LC. Nonsteroidal anti-inflammatory drugs and acetaminophen in the treatment of an acute muscle injury. American Journal of Sports Medicine 2004;32(8):1856-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ridderikhof 2019
- Ridderikhof ML, Saanen J, Goddijn H, Van Dieren S, Van Etten-Jamaludin F, Lirk P, et al. Paracetamol versus other analgesia in adult patients with minor musculoskeletal injuries: asystematic review. Emergency Medicine Journal 2019;36(8):493-500. [DOI] [PubMed] [Google Scholar]
Rietveld 1995
- Rietveld JA, Pilmore HL, Jones PG, Theis JC, Bowie DA, Dempster AG, et al. Necrotising fasciitis: a single centre's experience. New Zealand Medical Journal 1995;108(995):72-4. [PMID: 7891945] [PubMed] [Google Scholar]
Rosen 2000
- Rosen DA, Morris JL, Rosen KR, Valenzuela RC, Vidulich MG, Steelman RJ, et al. Analgesia for pediatric thoracostomy tube removal. Anesthesia and Analgesia 2000;90(5):1025-8. [PMID: 10781447] [DOI] [PubMed] [Google Scholar]
Salo 2003
- Salo D, Eget D, Lavery RF, Garner L, Bernstein S, Tandon K. Can patients accurately read a visual analog pain scale? American Journal of Emergency Medicine 2003;21(7):515-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schneider 2006
- Schneider S, Seither B, Tönges S, Schmitt H. Sports injuries: population based representative data on incidence, diagnosis, sequelae, and high risk groups. British Journal of Sports Medicine 2006;40(4):334-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnitzer 2004
- Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004;364(9435):665-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Seedat 1990
- Seedat YK, Aboo N, Naicker S, Parsoo I. Acute renal failure in the "Comrades Marathon" runners. Renal Failure 1989-1990;11(4):209-12. [PMID: 2485484] [DOI] [PubMed] [Google Scholar]
Sharma 2013
- Sharma CV, Mehta V. Paracetamol: mechanisms and updates. Continuing Education in Anaesthesia, Critical Care & Pain 2013;14(4):153-8. [DOI: 10.1093/bjaceaccp/mkt049] [Google Scholar]
Singh 1996
- Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, Fries JF. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Archives of Internal Medicine 1996;156(14):1530-6. [PMID: 8687261] [PubMed] [Google Scholar]
Svensson 2000
- Svensson E. Concordance between ratings using different scales for the same variable. Statistics in Medicine 2000;19(24):3483-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Szczeklik 1987
- Szczeklik A. Adverse reactions to aspirin and nonsteroidal anti-inflammatory drugs. Annals of Allergy 1987;59(5 Pt 2):113-8. [PMID: ] [PubMed] [Google Scholar]
Todd 1988
- Todd PA, Sorkin EM. Diclofenac sodium. A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1988;35(3):244-85. [PMID: 3286213] [DOI] [PubMed] [Google Scholar]
Vitting 1986
- Vitting KE, Nichols NJ, Seligson GR. Naproxen and acute renal failure in a runner. Annals of Internal Medicine 1986;105(1):144. [PMID: 3717800] [DOI] [PubMed] [Google Scholar]
Walker 1994
- Walker RJ, Fawcett JP, Flannery EM, Gerrard DF. Indomethacin potentiates exercise-induced reduction in renal hemodynamics in athletes. Medicine and Science in Sports and Exercise 1994;26(11):1302-6. [PMID: 7837949] [PubMed] [Google Scholar]
Warner 2002
- Warner DC, Schnepf G, Barrett MS, Dian D, Swigonski NL. Prevalence, attitudes, and behaviors related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in student athletes. Journal of Adolescent Health 2002;30(3):150-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weiler 1992
- Weiler JM. Medical modifiers of sports injury. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) in sports soft-tissue injury. Clinics in Sports Medicine 1992;11(3):625-44. [PMID: ] [PubMed] [Google Scholar]
Wiffen 2005
- Wiffen PJ, Derry S, Moore RA, McQuay HJ. Carbamazepine for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD005451. [DOI: 10.1002/14651858.CD005451] [DOI] [PubMed] [Google Scholar]
Williams 1979
- Williams GJP, Sperryn PN. Sports Medicine. 2nd edition. London: Edward Arnold, 1979. [Google Scholar]
Williams 2000
- C Williams AC, Davies HT, Chadury Y. Simple pain rating scales hide complex idiosyncratic meanings. Pain 2000;85(3):457-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wright 1995
- Wright V. Historical overview of non-steroidal anti-inflammatory drugs. British Journal of Rheumatology 1995;34 Suppl 1:2-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yates 1984a
- Yates DW, Laing GS, Peters K, Kumar K. Mild analgesics and the accident and emergency department – cost and safety more important than potency? Archives of Emergency Medicine 1984;1(4):197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Jones 2009
- Jones P, Dalziel SR, Lamdin R, Miles J, Frampton C. Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007789. [DOI: 10.1002/14651858.CD007789] [DOI] [PubMed] [Google Scholar]
Jones 2015
- Jones P, Dalziel SR, Lamdin R, Miles-Chan JL, Frampton C. Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD007789. [DOI: 10.1002/14651858.CD007789.pub2] [DOI] [PubMed] [Google Scholar]


