Abstract
Background: Decreased bone mineral density (BMD) is a concern in patients with congenital adrenal hyperplasia (CAH) due to lifelong glucocorticoid replacement. Studies till date have yielded conflicting results. We wanted to systematically evaluate the available evidence regarding BMD in adult patients with CAH.
Methods: We searched Medline, Embase and Cochrane Central Register of Controlled Trials to identify eligible studies. Studies comparing BMD in CAH patients with age- and sex-matched controls were included. Age <16 years and absence of controls were exclusion criteria. Two authors independently reviewed abstracts, read full-text articles, extracted data, assessed risk of bias using Newcastle-Ottawa scale, and determined level of evidence using Grading of Recommendations Assessment, Development, and Evaluation methodology.
Results: Nine case-control studies with a total sample of 598 (cases n = 254, controls n = 344) met eligibility criteria. Median age was 31 years (IQR 23.9–37) and 65.7% were female. Total body BMD (Mean Difference [MD]-0.06; 95%CI −0.07, −0.04), lumbar spine BMD (MD −0.05; 95%CI −0.07, −0.03) and femoral neck BMD (MD −0.07; 95%CI −0.10, −0.05) was lower in cases compared to controls. Lumbar spine T-scores (MD −0.86; 95%CI −1.16, −0.56) and Z-scores (MD −0.66; 95%CI −0.99, −0.32) and femoral neck T-scores (MD −0.75 95%CI −0.95, −0.56) and Z-scores (MD −0.27 95%CI −0.58, 0.04) were lower in cases.
Conclusion: BMD in adult patients with CAH was lower compared to controls. Although insufficient data precludes a dose-response relationship between glucocorticoid dose and BMD, it would be prudent to avoid overtreatment with glucocorticoids.
Keywords: bone mineral density, 21-hydroxylase deficiency, osteopenia, osteoporosis, glucocorticoids
Introduction
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders of adrenal steroid biosynthesis (1, 2). 21-hydroxylase deficiency (21OHD) due to CYP21A2 mutation is the most common cause of CAH accounting for 95–99% of all cases (3–5), followed by 11β-hydroxylase deficiency, 17α-hydroxylase/17,20-lyase deficiency, 3β-hydroxysteroid dehydrogenase type 2 deficiency, P450 oxidoreductase deficiency, lipoid adrenal hyperplasia, and cholesterol side chain cleavage enzyme deficiency (4, 6–8). Clinically classic 21OHD is characterized by glucocorticoid deficiency and adrenal androgen excess with or without additional mineralocorticoid deficiency (2). Incidence of classic CAH varies between 1:14000 and 1:18000 live births of which 75% are salt-wasting (SW) type and rest are simple virilizing (SV) (2). Non-classic 21OHD is more common with a reported incidence between 1:200 and 1:1000 livebirths, but even more common in certain ethnicities (9, 10). The treatment goals are to prevent adrenal crisis and optimize growth, sexual maturation, and reproductive function which is accomplished by replacing glucocorticoid and mineralocorticoid in sufficient doses (1, 2). This will decrease the associated excessive adrenocorticotropic hormone (ACTH) secretion from the pituitary gland and prevents hyperandrogenism. The balance between replacing deficient hormones and preventing hyperandrogenism can be difficult to achieve without overtreatment and its attendant risk of growth retardation and other clinical metabolic manifestations of glucocorticoid excess such as obesity, insulin resistance, diabetes, and hypertension (3, 4, 11, 12).
Decreased bone mineral density (BMD) and osteoporosis have been an important concern in patients with CAH due to lifelong glucocorticoid replacement. Glucocorticoids are a well-known secondary cause of osteoporosis and increased fracture risk has been established by various epidemiological studies (13, 14). Glucocorticoids have direct and indirect effects on bone leading to initial increased resorption and later decease in bone formation which results in microarchitectural distortion and increased fracture risk (15–17). It has also been postulated that glucocorticoids can cause secondary hyperparathyroidism by reducing intestinal calcium absorption and raising renal calcium excretion.
Gonadal and adrenal androgens are stimulators of osteoblast proliferation and differentiation in both males and females (18). Dehydroepiandrosterone sulphate (DHEAS) and other adrenal androgens affect bone metabolism throughout life and particularly during adrenarche, with a main effect on cortical bone (19). Some studies suggest that children with classic CAH fail to have a physiological rise in DHEAS levels during childhood, effectively accounting for absence of a typical adrenarche (20). Low DHEAS as a result of blunted response in adrenals of CAH patients and due to the glucocorticoid effect can affect the growth and osteoblastic function in these patients and might be the cause of low BMD.
However, there have been conflicting results in the published literature regarding BMD in CAH patients. Some studies reported normal BMD (21–27) while other studies reported low BMD in all or some sites (3, 28–44) or even high BMD (45). Thus, the aim of this systematic review and meta-analysis was to review the available literature to assess if adult patients with CAH are at risk of decreased BMD compared to age- and sex-matched controls.
Methods
Eligibility Criteria
Included studies had to have an exposure group consisting of patients aged 16 years or older with CAH, a control group consisting of age- and sex-matched controls without CAH, with standard BMD measures as outcomes of primary interest. Only clinical trials (RCTs and CCTs) and case-control studies were considered adapt to be included in this meta-analysis, eliminating any other study design such as studies with no age- and sex-matched controls, animal studies, reviews, editorials, news articles, case reports, case series, opinion pieces, and conference abstracts.
Search Strategy
PubMed/Medline, Embase and Cochrane Central Register of Controlled Trials (CENTRAL) were searched from inception to 31st July, 2019 using the following keywords: “Congenital Adrenal Hyperplasia,” “CAH” “21-Hydroxylase,” “11β-hydroxylase,” “3β-hydroxysteroid dehydrogenase,” “17α-hydroxylase,” “Osteoporosis,” “Osteopenia,” “Bone mineral density,” “Bone densitometry,” “BMD” and “Bone metabolism.” For details, please see Supplemental Table 1. No language restrictions were applied. The reference lists of included studies and non-included reviews were manually searched to identify additional studies.
Study Screening and Data Extraction
Four authors undertook the systematic review (SR, VG, AN, and HF) with SR coordinating the review. Two authors (SR and VG) independently ran the searches, screened the titles and abstracts as well as reviewed full-text copies to identify eligible articles. SR and VG independently performed data extraction using a standardized data extraction form (Microsoft Excel, Microsoft Inc, 2016). Data was extracted regarding the following variables: first author, type of study, country, year of publication, number of cases and controls, age, gender, whether the diagnosis of CAH was genetically confirmed or not, phenotype of CAH, body site, modality, and results of BMD measurements including T- and Z-scores, type, and average daily dose of glucocorticoid used for treatment, levels of bone turnover markers, type, and number of fractures, body mass index, vitamin D, 17-hydroxyprogesterone, androstenedione, testosterone, dehydroepiandrosterone, and dehydroepiandrosterone sulphate (DHEAS). Bone turnover markers are the collagen breakdown products and other molecules that are produced by osteoclasts and osteoblasts during bone resorption and bone formation. Markers that are specific to bone formation include serum bone-specific alkaline phosphatase (BALP), osteocalcin, and N-terminal propeptide of type I procollagen (P1NP), whereas markers specific to bone resorption include urinary N-terminal telopeptide of type I collagen (NTX), pyridinoline cross-links and serum C-terminal telopeptide of type I collagen (CTX).
An attempt was made to contact authors for clarification and additional data, if indicated. If such data could not be obtained, a decision was made to discuss its potential impact on the results. Disagreements were resolved by discussion and consensus. If consensus could not be reached, the last author (HF) made the final decision. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines were followed (46).
Data Synthesis
RevMan 5.3 was used to conduct data analysis. Results were calculated as mean differences with 95% confidence intervals (95%CI) for continuous data. I2 statistic was used to assess statistical heterogeneity between studies and significant heterogeneity was assumed if I2 was >40%. Meta-analyses using fixed-effect modeling were performed when the outcome data were found to be sufficiently clinically homogeneous. In the presence of significant heterogeneity, data was synthesized and presented qualitatively.
Assessment of Risk of Bias of Individual Studies
The risk of bias of individual studies was assessed independently by two authors (SR and VG) using a combination of the Newcastle-Ottawa Scale (NOS) (47), Agency for Healthcare Research and Quality (AHRQ) standards (48) and the reviewers' interpretation of study quality. NOS was chosen due to its validity, interrater reliability and ease of use in case-control studies. NOS allows assignment of scores under three domains and eight sub-domains while AHRQ standards allow conversion of NOS scores to a quality assessment of studies as good, fair or poor. We interpreted good quality studies to be at a low risk of bias, fair quality studies to be at moderate risk of bias and poor-quality studies to be at high risk of bias. Publication bias was assessed graphically using funnel plots.
Grading of the Body of Evidence
The quality of evidence was assessed independently by two authors (SR ad VG) using Grading of Recommendation, Assessment Development and Evaluation (GRADE) methodology (49) where evidence is graded on a quality continuum of high, moderate, low, and very low. GRADE methodology involves assigning a baseline quality of evidence based on study design which is usually low for observational studies and high for randomized trials with no significant limitations. Thereafter, there are five reasons for possible downgrading based on risk of bias, inconsistency, imprecision, indirectness, and publication bias. Reasons for possible upgrading include large magnitude of effect, dose-response gradient or if all plausible confounding would reduce the demonstrated effect or increase the effect if no effect was observed.
Results
Description of Studies
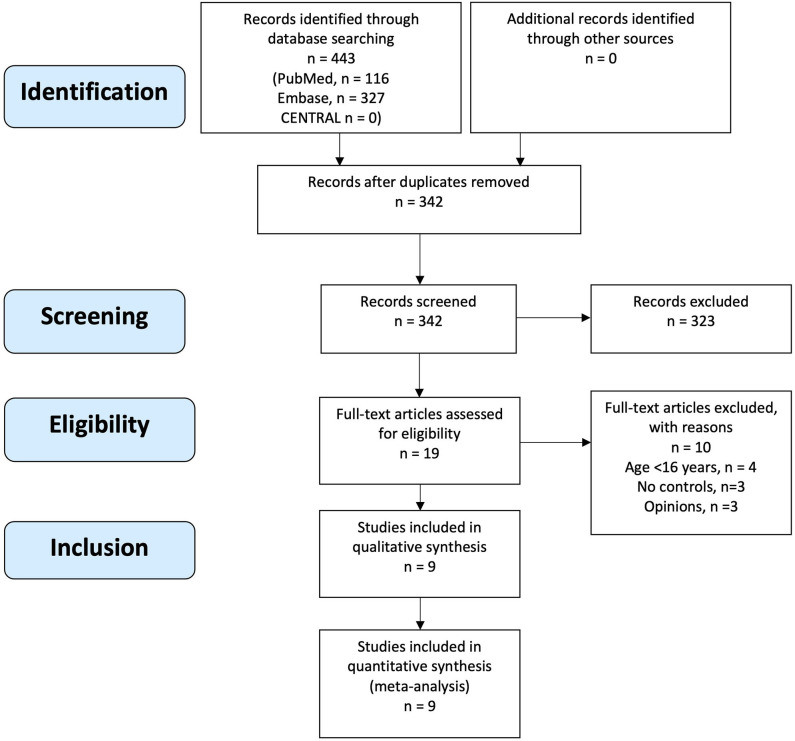
The initial search yielded 443 records of which 342 records were screened after removal of duplicates. Nineteen articles underwent full text review, of which nine articles met eligibility criteria and were included (Figure 1). Manual search of reference lists of included articles and non-included reviews did not yield any additional eligible articles.
Figure 1.
PRISMA flow diagram of study eligibility assessment and inclusion.
Included Studies
All nine included articles were case-control studies (23, 25, 33–37, 42, 43) with a total sample size of 598 (cases n = 254, controls n = 344). Median age of participants was 31 years (interquartile range 23.9–37) and 65.7% (393/598) were female. In all nine studies, cases were patients with CAH exposed to glucocorticoid use and controls were sex- and age-matched individuals without CAH. Outcomes were one or more measures of BMD. The characteristics of included studies are presented in Table 1.
Table 1.
Characteristics of included studies of bone mineral density in adult patients with congenital adrenal hyperplasia and controls.
| Study first author and year of publication | Study location | Total sample size | Number CAH | Age CAH | Sex CAH | BMI CAH | Number controls | Age controls | Sex controls | BMI controls | Genetic diagnosis; CAH variant | DXA | BMD (g/cm2) cases vs controls | T-score cases vs controls | Z-score cases vs controls |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceccato 2016 (42) | Italy | 76 | 38 | 31 ± 7 y | 24M 14F | 25.6 ± 5.9 | 38 | 31 ± 7 | 24M 14F | 23 ± 3.4 | Y 21OHD SW n = 21 SV n = 12 NC n = 5 | Hologic | TB: (-) LS: 0.961+/-0.1 vs. 1.02+/−0.113 FN: (-) | TB: (-) LS: (-) FN: (-) | TB: (-) LS: −0.1+/−1.0 vs. −0.3+/−1.1 FN: (-) |
| Falhammar 2007 (36) | Sweden | 122 | 61 | 24 y (18–29) 35 y (30–63) | F | 22.4 (17.7-41.8) 24.4 (20.7-48.3) | 61 | 24 y (18–29) 35 y (30–63) | F | 21.9 (17.5–33.8) 23.7(19.4–39.9) | Y 21OHD SW n = 27 SV n = 28 NC n = 6 | Mainly Lunar (Hologic n = 3) | TB: 1.128+/−0.016 vs. 1.186+/−0.015 LS: 1.130+/−0.025 vs. 1.237+/−0.025 FN: 0.941+/−0.025 vs. 1.039+/−0.028 | TB: 0.03+/−0.20 vs. 0.77+/−0.19 LS: −0.57+/−0.20 vs. 0.32+/−0.21 FN: −0.27+/−0.19 vs. 0.45+/−0.23 | TB: 0.0772+/−0.145 vs. 0.933+/−0.137 LS: −0.736+/−0.15 vs. 0.520+/−0.232 FN: −0.567+/−0.165 vs. 0.227+/−0.212 |
| Falhammar 2013 (37) | Sweden | 62 | 30 | 35.7 ± 11.4 y | M | 26.4 ± 4.7 | 32 | 36.5 ± 11.9 | M | 24.5 ± 3.6 | Y 21OHD SW n = 17 SV n = 11 NC n = 2 | Lunar | TB: 1.17+/– 0.11 vs. 1.27+/– 0.10 LS: 1.16+/−0.20 vs. 1.23+/−0.16 FN: 0.96+/−0.13 vs. 1.05+/−0.14 | TB: (-) LS: (-) FN: (-) | TB: (-) LS: (-) FN: (-) |
| Guo 1996 (23) | United Kingdom | 22 | 11 | 38.3 ± 14.3 y | 6F 5M | NR | 11 | 38.3 ± 16.3 y | 6F 5M | NR | Y 21OHD SW = 3, SV = NR, NC = NR; 11OHD n = 2 | Lunar | TB: 1.14+/−0.11 vs. 1.176+/−0.19 LS: 1.196+/−0.13 vs. 1.054+/−0.19 FN: 0.95+/0.18 vs. 0.93+/−0.15 | TB: (-) LS: (-) FN: (-) | TB: (-) LS: (-) FN: (-) |
| Hagenfeldt 2000 (33) | Sweden | 26 | 13 | 23.9 ± 0.8 y | F | 26.2 ± 1.7 | 13 | 22.3 ± 0.4 | F | 20.7 ± 0.3 | Y 21OHD SW, n = 12 SV, n = 1 | Lunar | TB: 1.12+/−0.02 vs. 1.13+/−0.02 LS: 1.14+/−0.04 vs. 1.13+/−0.03 FN: (-) | TB: (-) LS: (-) FN: (-) | TB: (-) LS: (-) FN: (-) |
| King 2006 (35) | USA | 34 | 26 | SW: 39 y (21–51) SV: 51 y, (32–71) NC: 9 y | F | 24.5 (16.5–40.5) | 9 | 49 (21–70) | F | 19.5 (17.3–28.3) | Y 21OHD, SW, n = 11 SV, n = 15 | Hologic | TB: 1.05+/−0.12 vs. 1.2+/−0.14 LS: 0.96+/−0.11 vs. 1.13+/−0.19 FN:(-) | TB: (-) LS: −0.61+/−1.26 vs. 1.14+/−1.59 FN: (-) | TB: (-) LS: −0.15+/−1.22 vs. 2.33+/−1.21 FN: (-) |
| Raizada 2016 (43) | India | 27 | 15 | 27.5 ± 6.2y | F | 28.9 ± 5.5 | 15 | 27.2 ± 5.2 | F | 27.8 ± 4.9 | N SW, n = 2 SV, n = 13 | Hologic | TB: (-) LS: 0.96+/−0.09 vs. 1.02+/−0.08 FN: 0.84+/−0.09 vs. 0.92+/−0.07 | TB: (-) LS: −0.7 (−2.2 to 0.7) vs. −0.35 (−1.3 to 1.5) FN: −0.9 (−0.18 to 0.5) vs. 0.0 (−1 to 0.9) | TB: (-) LS: (-) FN: (-) |
| Sciann-amblo 2006 (34) | Italy | 168 | 30 | M: 22.8 ± 0.9 y F: 23.1± 0.8 y | 15F 15M | 24.3 (19.8–28.6) | 138 | 22.8 ± 0.9 | 84 F 54 M | 24.3 (19.3–45.2) | Y 210HD, SW, n = 24 SV, n = 6 | Lunar | TB: 1.125+/−0.023 vs. 1.154+/−0.009 LS: 1.201+/−0.040 vs. 1.161+/−0.016 FN: | TB: (-) LS: (-) FN: (-) | TB: (-) LS: (-) FN: (-) |
| Stikkel-broeck 2003 (25) | Nether-lands | 60 | 30 | M: 21.7 ± 2.4 y, F: 20.6± 2.9 y | 15F 15M | 25 ± 3.6 | 30 | 21.9 ± 2.4 | 15F 15M | 22.3 ± 1.9 | Y 21OHD, SW, n = 24 SV, n = 3 NC, n = 3 | Hologic | TB: 1.11+/−0.06 vs. 1.14+/−0.07 LS:1.01+/−0.08 vs. 1.05+/−0.09 FN: 0.95+/−0.15 vs. 0.89 (0.78–1.23) | TB: (-) LS: (-) FN: (-) | TB: (-) LS: (-) FN: (-) |
F, females; M, males; Y, yes; N, no; yrs, years; BMD, bone mineral density; TB, total body; LS, lumbar spine; FN, femoral neck; NR, Not reported; (-) reported for cases only precluding comparisons and conclusions; 21OHD, 21-hydroxylase deficiency; 11OHD, 11-hydroxylase deficiency; SW, salt wasting; SV, simple virilizing; NC, non-classic; NR, Not reported.
Methodological Quality
Three studies were found to be of poor quality (34, 42, 43), one (23) of fair quality and the rest were found to be of good quality (25, 33, 35–37) (Table 2). This was based on an initial score calculated using the NOS (47) which was then converted to a quality measure based on AHRQ standards (48). The reviewers then assigned high, moderate or low risk of bias to studies of good, fair, and poor quality, respectively.
Table 2.
Risk of bias assessment of included studies based on NOS and AHRQ.
| Study | Selection | Comparability | Exposure | Quality | Risk of bias |
|---|---|---|---|---|---|
| Ceccato 2016 (42) | 4 | 2 | 1 | Poor | High |
| Falhammar 2007 (36) | 4 | 1 | 2 | Good | Low |
| Falhammar 2013 (37) | 4 | 1 | 2 | Good | Low |
| Guo 1996 (23) | 2 | 2 | 2 | Fair | Moderate |
| Hagenfeldt 2000 (33) | 4 | 1 | 2 | Good | Low |
| King 2006 (35) | 4 | 2 | 2 | Good | Low |
| Raizada 2016 (43) | 3 | 1 | 1 | Poor | High |
| Sciannamblo 2006 (34) | 4 | 1 | 1 | Poor | High |
| Stikkelbroeck 2003 (25) | 4 | 1 | 1 | Good | Low |
36.NOS-Newcastle Ottawa Scale. A study can be awarded a maximum of one star for each numbered item within the SELECTION and EXPOSURE categories while a maximum of two stars can be given for COMPARABILITY. The number items are as follows SELECTION 1. Is the case definition adequate? (a. yes with independent validation* b. yes record linkage based on self-reports c. no description) 2. Representativeness of Cases (a. consecutive or obviously representative series* b. potential for selection biases or not stated) 3. Selection of Controls (a. Community controls* b. Hospital Controls c.no description) 4. Definition of controls (a. no history of disease* b. no description of source) COMPARABILITY 5. Comparability of cases and controls on the basis of design or analysis (a. study controls for ___(select the most important factor)* b. Study controls for any additional factor*) EXPOSURE 6. Ascertainment of Exposure (a. secure record like surgical records* b. structured interview blinded to case/control status* c. interview not blinded to case/control status d. written self-report or medical record only. e. no description) 7. Same method of ascertainment for cases and controls (a. yes* b.no) 8. Non-response rate (a. same rate for both groups* b. non-respondents described c. rate different and no designation) 37AHRQ-Agency for healthcare research and quality standards. Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain; Fair quality 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain; Poor quality 0 or 1 star in selection domain OR 0 star in comparability domain OR 0 OR 1 star in outcome/exposure domain.
Glucocorticoid Type and Mean Dose
The type of glucocorticoid used, average daily glucocorticoid dose and the results of included studies are shown in Table 3. Studies used between two to four different types of glucocorticoids with a proportion of patients in each study on more than one type of glucocorticoid for variable duration. Prednisolone was the most commonly used glucocorticoid. The average daily dose of glucocorticoid varied between 9.66 and 22 mg/m2/day hydrocortisone equivalents dose. For studies that reported individual patient glucocorticoid doses (23) or average daily dose without adjusting for body surface area (33), the average daily dose adjusted for body surface area was calculated using mean population adult body surface area for United Kingdom (50) and Sweden (51).
Table 3.
Glucocorticoid type and hydrocortisone equivalent dose of patients with congenital adrenal hyperplasia in the included studies.
| First author and year | Type of glucocorticoids used for treatment and number of patients on each type | Averagea daily hydrocortisone equivalent dose (mg/m2/day) | Comments |
|---|---|---|---|
| Ceccato 2016 (42) | Prednisolone, n = 1 Hydrocortisone, n = 3 Dexamethasone, n = 34 |
10+/−5 | Cumulative glucocorticoid dose was not related to bone metabolism or BMD. |
| Falhammar 2007 (36) | Prednisolone, n = 30 Hydrocortisone, n = 17 Cortisone, n = 5 Dexamethasone, n = 7 Combination of two, n = 2 |
16.9+/−0.9 | No correlations were found between BMD and the current glucocorticoid dose. |
| Falhammar 2013 (37) | Prednisolone, n = 18 Hydrocortisone/Cortisone, n = 8 |
17.4+/−5.2 | No correlations were found between BMD and the current glucocorticoid dose. Patients on prednisolone had lower BMD than those on hydrocortisone/cortisone. |
| Guo 1996 (23) | Prednisolone, n = 2 Hydrocortisone, n = 3 Cortisone, n = 1 Dexamethasone, n = 5 |
17.4 | The total glucocorticoid dose in the previous 2 years was not correlated with BMD |
| Hagenfeldt 2000 (33) | Prednisolone, n = 5 Cortisone, n = 1 Dexamethasone, n = 5 Triamcinolone, n = 1 Cortisone+Pred, n = 1 |
17.5 | Negative correlation was found between BMD and the calculated index of accumulated post-menarcheal glucocorticoid dose |
| King 2006 (35) | Prednisone, n = 13 Cortisol, n = 10 Dexamethasone, n = 3 |
SW CAH: 22 (8–38) SV CAH: 17 (11–46) LS T-score < −1: 22 (13–28) LS T-score > −1: 15 (8–46) |
There were not higher cortisol equivalents per body surface area among the osteopenic CAH patients compared to CAH patients with normal BMD |
| Raizada 2016 (43) | Prednisolone, n = 1 Dexamethasone, n = 14 (Pred+Dex, n = 6 Hydrocortisone+Dex, n = 5) |
NR | Steroid doses not reported due to missing records |
| Sciannamblo 2006 (34) | Hydrocortisone (NR) Dexamethasone (NR) |
F:15.3+/−1 M:17.1+/−1.1 | No correlations were found between BMD and current glucocorticoid dose, nor the mean dose of the previous 7 yrs |
| Stikkelbroeck 2003 (25) | NR | F: 9.66+/−2.83 M: 13.16+/−2.66 | No significant correlations were found between cumulative glucocorticoid doses in the last 0.5, 2, or 5 yr and bone parameters. |
NR, not reported; BMD, bone mineral density.
average presented as mean+/- standard deviation or mean+/- standard error of mean or median(range).
Meta-Analyses of BMD
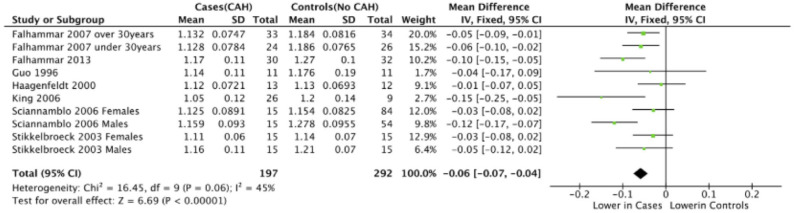
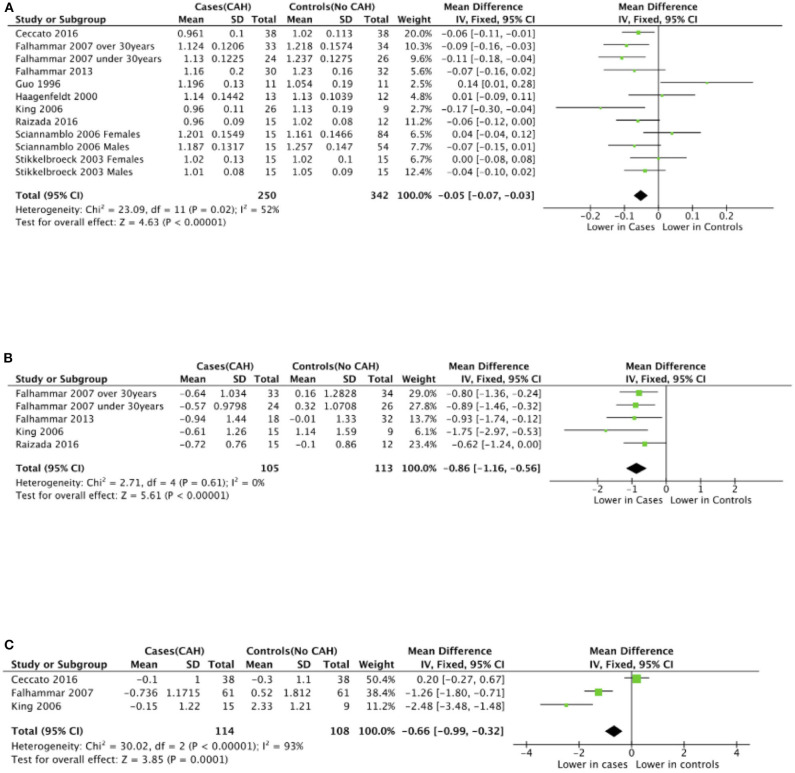
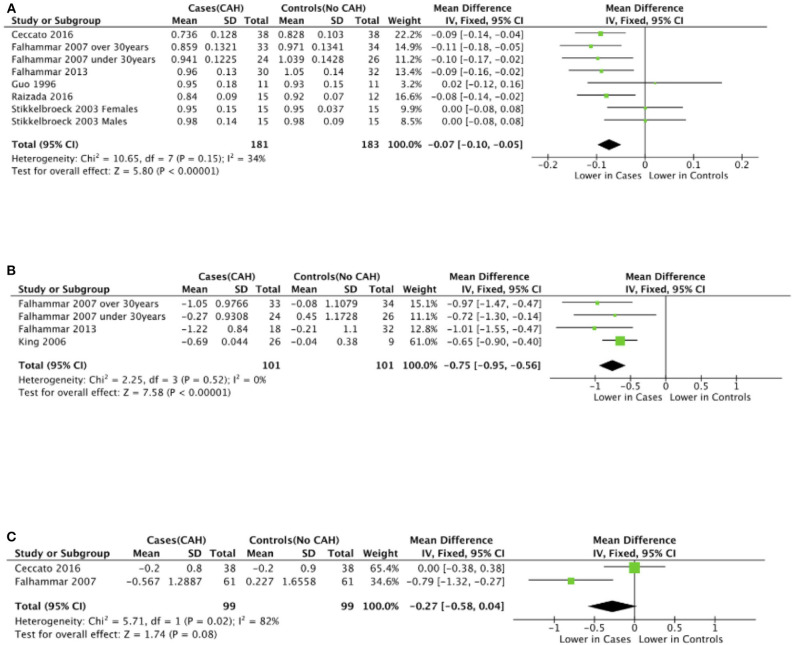
Seven studies reported total body BMD (Figure 2) (23, 25, 33–37), nine studies reported lumbar spine BMD (Figure 3) (23, 25, 33–37, 42, 43), six studies reported femoral neck bone mineral density, (Figure 4) (23, 25, 36, 37, 42, 43) one study reported total femur BMD (42) and one study reported forearm BMD (43) Meta-analyses were performed for data variables where more than one study reported values for cases and controls. As some studies provided mean values for subgroups (males/females, <30 years/ >30 years) without an overall mean value, these subgroups were included as separate studies in the meta-analyses to minimize bias. Meta-analysis of the studies showed decreased BMD (g/cm2) at all commonly measured sites in CAH compared to controls with a mean difference for total body BMD of −0.06 g/cm2 (95%CI −0.07, −0.04), lumbar spine BMD of −0.05 g/cm2 (95%CI −0.07, −0.03), and femoral neck BMD of −0.07 g/cm2 (95%CI −0.10, −0.05). The T-scores and Z-scores (SD) at lumbar spine and femoral neck were also lower in cases compared to controls with a mean difference for lumbar spine T-score of −0.86 (95%CI −1.16, −0.56), lumbar spine Z-score of −0.66 (95% CI −0.99, −0.32), femoral neck T-score of −0.75 (95% CI −0.95, −0.56), femoral neck Z-score of −0.27 (95% CI −0.58, 0.04) (Figures 2–4, Table 5).
Figure 2.
Meta-analysis of total body bone mineral density (g/cm2) in patients with congenital adrenal hyperplasia compared to matched controls.
Figure 3.
Meta-analysis of lumbar spine bone mineral density in patients with congenital adrenal hyperplasia compared to matched controls. (A) shows bone mineral density in g/cm2, (B) T-scores (SD), and (C) Z-scores (SD).
Figure 4.
Meta-analysis of femoral neck bone mineral density in patients with congenital adrenal hyperplasia compared to matched controls. (A) shows bone mineral density in g/cm2, (B) T-scores (SD), and (C) Z-scores (SD).
Meta-Analysis of Bone Turnover Markers
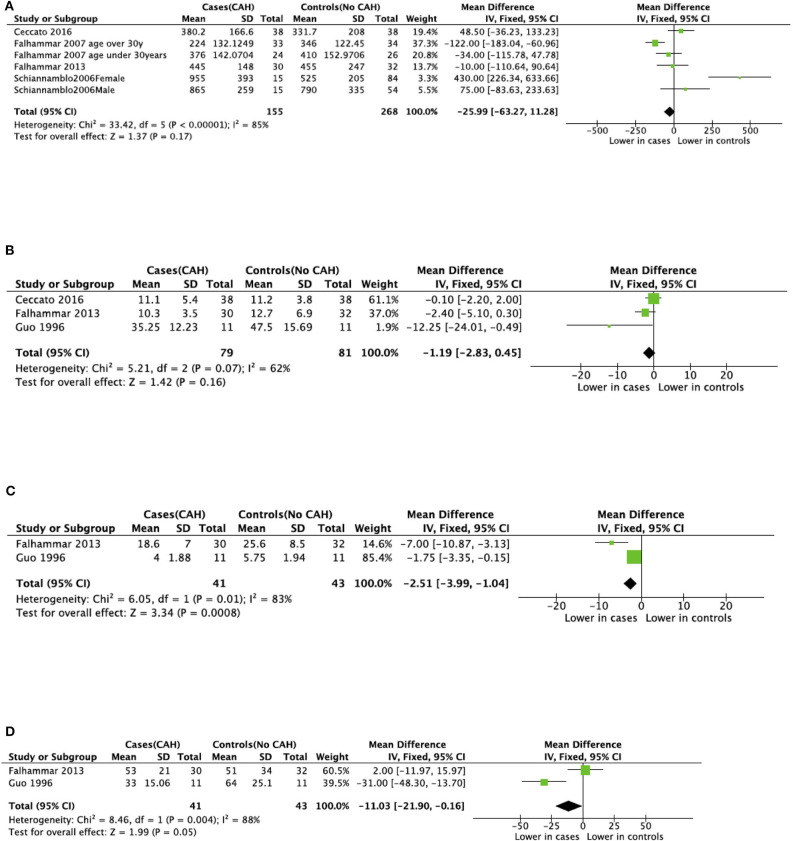
There was variable reporting and results of bone turnover markers in the included studies as shown in Table 4. CTX was reported in four studies (34, 36, 37, 42), BALP was reported in four studies (23, 36, 37, 42), osteocalcin and NTX were reported in two studies (23, 37). Meta-analyses of bone turnover markers showed that osteocalcin and NTX were lower in cases compared to controls whilst there was no significant difference in CTX and BALP between cases and controls (Figure 5). None of the studies reported P1NP or pyridinoline cross-links.
Table 4.
Bone turnover markers in included studies of patients with congenital adrenal hyperplasia.
| First author and year of publication | CTX (ng/L) | BALP (units/L)a | Osteocalcin (mcg/L) | Urinary NTX/creatinine (nmol/mmol) | ||||
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |
| Ceccato 2016 (42) | 380.2+/−166.6 | 331.7+/−208 | 11.1+/−5.4 | 11.2+/−3.8 | NR | NR | NR | NR |
| Falhammar 2007 (36) | <30y:376+/−29 >30y:224+/−23 | <30Y:410+/−30 >30Y: 346+/−21 | NR | NR | NR | NR | NR | NR |
| Falhammar 2013 (37) | 445+/−148 | 455+/−247 | 10.3+/−3.5 | 12.7+/−6.9 | 18.6+/−7 | 25.6+/−8.5 | 53+/−21 | 51+/−34 |
| Guo 1996 (23) | NR | NR | 32 (19–58) | 45 (25–75) | 4 (1–7) | 5.6 (2.8–9) | 32 (10–58) | 58 (30–110) |
| Raizada 2016 (43) | NR | NR | 120 | NR | NR | NR | NR | NR |
| Sciannamblo 2006 (34) | F: 900 (330–1,690) M: 850 (430–1,330) | F:460 (90–1,090) M:600 (310–1,650) | NR | NR | NR | NR | NR | NR |
NR, not reported.
Units varied between studies: microg/l(Ceccato 2016), microkat/L(Falhammar 2007,2013), U/L(Guo 1996), IU/L Raizada 2016).
Figure 5.
Meta-analysis of bone markers in patients with congenital adrenal hyperplasia (CAH) compared to age- and sex-matched controls. (A) Serum C-terminal telopeptide of type I collagen (CTX). (B) Serum Bone-specific alkaline phosphatase (BALP). (C) Serum Osteocalcin. (D) Urinary N-terminal telopeptide of type I collagen (NTX).
Publication Bias
According to the Cochrane Handbook of Systematic Reviews for Interventions (version 6.0) “tests for funnel plot asymmetry should be used only when there are at least 10 studies included in the meta-analysis, because when there are fewer studies the power of the tests is low” and “none of the recommended tests for funnel plot asymmetry is implemented in RevMan.” As we used RevMan to perform all our meta-analyses and our systematic review includes nine studies, we are unable to comment on the possibility of publication bias.
Sensitivity Analyses
The results of the meta-analyses remained unchanged when the analyses were limited to subgroups of female patients (Supplemental Figures 1–3).
Grading of Evidence
The overall evidence was of low quality given the observational design of the included studies (Table 5). The evidence was downgraded for reduction in total body T- and Z-score, lumbar spine T- and Z-score as well as femoral neck T- and Z-score to very low quality due to imprecision of the effect size estimates resulting from wide confidence intervals. The evidence was not downgraded for reduction in total body BMD and lumbar spine BMD despite I2 > 40 due to clinical homogeneity of the included studies and the narrow confidence intervals around the effect size estimates. The evidence was not downgraded for risk of bias, indirectness, inconsistency, or publication bias. None of the studies met criteria for upgradation of quality of evidence for any of the outcomes.
Table 5.
Summary of findings of included studies.
| Outcome measures | Results: absolute effects | Results: relative effects mean difference (95%CI) | Number of participants (studies) | Quality of evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Cases | Controls | |||||
| Total Body BMD | Mean total body BMD ranged from 1.05 to 1.17 | Mean total body BMD ranged from 1.13 to 1.278 | −0.06 (−0.07, −0.04) | 489 (7) | Low | |
| Total Body T-score | Mean total body T-score ranged from 0.03 to 0.09 | Mean total body T-score ranged from 0.73 to 0.77 | −0.68 (−1.04, −0.33) | 117 (1) | Very low1 | Only one study reported Total Body T-scores in two age-based subgroups (<30 years and >30 years) |
| Total Body Z-score | Mean total body Z-score was 0.0772 | Mean total body Z-score was 0.933 | −0.86 (−1.25, −0.46) | 50 (1) | Very low2 | Only one study reported Total Body Z-scores in <30 years subgroup |
| Lumbar Spine BMD | Mean Lumbar Spine BMD ranged from 0.948 to 1.201 | Mean Lumbar Spine BMD ranged from 0.991 to 1.257 | −0.05 (−0.07, −0.03) | 592 (9) | Low | |
| Lumbar Spine T-score | Mean Lumbar Spine T-score ranged from −0.94 to −0.57 | Mean Lumbar Spine T-score ranged from −0.1 to 1.14 | −0.86 (−1.16, −0.56) | 218 (4) | Very low3 | |
| Lumbar Spine Z-score | Mean Lumbar Spine Z-score ranged from −0.736 to −0.1 | Mean Lumbar Spine Z-score ranged from −0.5 to 2.33 | −0.66 (−0.99, −0.32) | 222 (3) | Very low4 | |
| Femoral Neck BMD | Mean Femoral Neck BMD ranged from 0.726 to 0.98 | Mean Femoral Neck BMD ranged from 0.8 to 1.05 | −0.07 (−0.10, −0.05) | 364 (6) | Low | |
| Femoral Neck T-score | Mean Femoral Neck T-score ranged from −1.22 to −0.27 | Mean Femoral Neck T-score ranged from −0.27 to 0.45 | −0.75 (−0.95, −0.56) | 202 (3) | Very low5 | |
| Femoral Neck Z-score | Mean Femoral Neck Z-score ranged from −0.9 to −0.2 | Mean Femoral Neck Z-score ranged from −0.3 to −0.2 | −0.27 (−0.58, 0.04) | 198 (2) | Very low6 | |
Population, Adults(>18 years) with Congenital Adrenal Hyperplasia (CAH); Exposure, Therapeutic glucocorticoid use; Control, Age and Sex-matched adults without Congenital Adrenal Hyperplasia; Outcome, Risk of Osteopenia and/or Osteoporosis; BMD, Bone Mineral Density; CI, Confidence Intervals; GRADE, Grading of Recommendations Assessment Development and Evaluation. Footnotes about differences in subgroups age and gender based for each variable to be done 1,2,3,4,5,6: Level of Evidence downgraded from Low to Very Low for imprecision of effect size estimates secondary to small sample sizes and wide confidence intervals.
Discussion
This is the first systematic review and meta-analysis analyzing BMD in adult patients with CAH. The meta-analysis found consistently lower BMD at all commonly measured sites in individuals with CAH compared to age-and sex-matched controls.
The lower BMD in patients with CAH has been attributed to glucocorticoid overtreatment and the resulting catabolic effects of systemic glucocorticoids on bone in some studies (32, 33). The normal physiological cortisol production rate is estimated to be 9–11 mg/m2 (52), while the average glucocorticoid dose in the included studies was 9.66–22 mg/m2 hydrocortisone equivalents dose. Thus, most patients with CAH seemed to be on supraphysiological doses of glucocorticoids and this may be the main cause for low BMD. Hagenfeldt et al. found that the calculated index of accumulated post menarcheal glucocorticoid was the strongest determinant for all bone variables except BMD of spine and was calculated by multiplying the daily glucocorticoid dose expressed as cortisol equivalents with actual age minus age at menarche and dividing the result with the body surface area at the time of the study (33). King et al. suggested an association with oversuppression of adrenal steroidogenesis and decreased BMD in CAH patients (35). Similarly, Falhammar et al. found in both females and males with CAH subnormal levels of testosterone and DHEAS suggesting overtreatment with glucocorticoids which might have been a reason for low BMD (36, 37). However, most of the studies in our systematic review did not show any relationship between BMD and glucocorticoid dose. We could not perform a dose response analysis as some studies only had the current dose of glucocorticoids (36, 37) and those calculating a glucocorticoid dose over several years estimated the total dose from certain timepoint (23, 25, 33, 34, 42), (i.e., no study had the life-time dose exposure of glucocorticoids). Hence, we cannot draw any conclusions regarding dose response relationship.
Areal BMD as measured using DXA can be largely affected by size of bone leading to overestimation in larger bones and underestimation in smaller bones (53). CAH patients were 5–12 cm shorter than controls in studies included in our review. It has been shown that patients with CAH fail to achieve optimal adult height due to several factors (54). Excess adrenal androgens can result in accelerated linear growth and premature fusion of the epiphyses, ultimately compromising adult stature. Furthermore, treatment of CAH with glucocorticoids, even at replacement doses, especially around time of puberty has been associated with poor growth with resultant short stature in these patients (54). In our systematic review, two studies (36, 42) reported BMD corrected for height, two studies reported BMD corrected for BMI (35, 43) and the rest reported uncorrected BMD. In those correcting for height, Ceccato et al. found that only femoral neck BMD was lower in CAH patients compared to controls (42) while Falhammar et al. found that BMD remained lower in all studied sites in CAH patients compared to controls (36). King et al. and Raizada et al. found no association between BMD and BMI (35, 43). Given that only two of the included studies reported BMD corrected for height, it is not possible to draw any conclusions regarding the impact of height on differences in BMD observed between cases and controls.
Only one study reported BMD at forearm and there was no difference between cases and controls (43) However a recent study by El-Maouche et al. showed patients with classic CAH had lower BMD than patients with non-classic CAH, with the greatest difference at the forearm and DHEAS was the only independent significant predictor of BMD at the forearm, whole body and spine (19). We need further studies measuring cortical bone density to understand the etiology of these differences on cortical bone in CAH patients.
Sexual steroids increase osteoblast activity, decrease the formation, and activity of osteoclasts, stimulate longitudinal growth of long bones during puberty (55). The excessive estrogen action in pediatric CAH patients from peripheral conversion of androgens causes advanced maturation of the epiphyseal plate, resulting in increased bone age and acquisition of peak bone mass compared with healthy children (56). DHEAS correlates with bone turnover before peak bone mass (57), which may represent a direct effect on bone metabolism or the role of DHEAS as a substrate for conversion to other sex steroids. One of the aims of glucocorticoid therapy in CAH is to suppress the hyperandrogenism, however, often the androgen levels are lower than in controls (11, 37). Low levels of androgen as a result of glucocorticoid effect and failure of typical adrenarche in classical CAH patients have been shown to cause bone loss due to an imbalance between bone resorption and bone formation (58). Thus, androgens are important in both maintenance of bone mass and peak bone mass accrual.
Bone turnover markers were reported in 6 of the included studies with varied results. Osteocalcin and NTX were lower in cases than controls whereas CTX and BALP were not significantly different between cases and controls (23, 34, 36, 37, 42, 43). These conflicting findings could be due to variable age of study populations, assay differences, and different glucocorticoid preparations and doses.
No association was found between genotypes of CAH and BMD in three studies (33, 36, 37). The risk of fractures in CAH patients was reported by three of the included studies (36, 37, 43). No fractures were reported in any of the CAH cases by Raizada et al. (43). On the other hand, more fractures were found in CAH women than control (36), while no difference in fracture frequency could be demonstrated in males with CAH (37). However, fragility fractures usually occur in older age and very few patients with CAH above the age of 50 years were included in the studies in this review. Low BMD is strongly associated with the risk of fractures but it is well-recognized that different risk factors, such as age, history of a prior fragility fracture, steroid use and many others are independent contributors to the risk of fractures (59). Moreover, fractures can also occur due to sports activities, especially hazardous, which were more common in females but not in males with CAH (60, 61). One study reported that the fractures had often occurred during sport activities, however, the underlying trauma was not recorded systematically (37). There are also other studies reporting on fractures in CAH but then there was no control group making it difficult to assess the risk (19, 39, 44, 62). Thus, future studies examining fractures, how they occurred (fragility vs. traumatic fractures) in patients with CAH compared to controls are required.
Our systematic review and meta-analysis had several limitations. The relatively small total sample size of 598 makes it difficult to draw firm conclusions about the outcomes. As all included studies were retrospective observational studies, the possibility of results having been influenced by unknown confounders cannot be ruled out. Individual patient data was not assessed and numerical data from one study (23) was deduced from graphs possibly introducing measurement bias. However, the latter is unlikely to have influenced the direction and magnitude of results given the small sample size (n = 22) of the study. Despite the fact that only studies with CAH patients 16 years and older and controls were included, the study cohorts were still quite clinically heterogenous due to different genotypes of CAH, inclusion of both sexes, inclusion of different age groups and different DXA scans used to measure BMD. These factors could also have resulted in moderate degree of statistical heterogeneity as evidenced from the I2 statistic. Not all variables related to bone health were reported in each study and all the factors related to BMD could not be assessed. However, in all studies the controls were measured on the same DXA scan as their cases and all results point in the same direction reassuring that the conclusions are valid. Finally, inability to perform a glucocorticoid dose-response analysis and the low to very low quality of evidence for the reported outcomes in the included studies limits the strength of our conclusions.
Future Directions
Bone health is an important issue. Well-designed, long-term prospective studies that assess all relevant measures of bone health in homogenous populations (age, sex, race, genotype) in relation to treatment are needed. Randomized studies are not possible instead structured long-term follow up are warranted, assessing glucocorticoid dose over time, and bone age during childhood years. Better endpoints than BMD would be fractures or markers of bone quality/strength but very few studies in CAH have investigated those, hence more studies are needed. The growth pattern (i.e., accelerated growth indicating insufficient dose and androgen excess or the opposite would also give important information). BMD in relation to glucocorticoid dose and hormonal control during puberty could give important insights to improve treatment strategies since puberty is thought to be the time in life for maximum bone mineral accrual. The timing of achieving peak bone mass in patients with CAH is lacking and timing of puberty is on average often earlier in this patient group. More data on peak bone mass in relation to puberty would be valuable. Bone markers would also be of importance to follow over time.
Conclusion
BMD seemed to be consistently low in CAH patients compared to controls. Although we could not analyse the dose-response relationship between glucocorticoid dose and BMD, it would be prudent to avoid overtreatment. The current Endocrine Society Guidelines recommends BMD screening in any patient with CAH with a prolonged period of higher-than-average glucocorticoid dosing, or in patients who have had a non-traumatic fracture (2). The current systematic review and meta-analysis may support a more liberal BMD screening and monitoring in patients with CAH.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
SR, VG, and HF: project conception and protocol preparation. SR and VG: idenfication of articles, data extraction, and data analysis. SR, VG, AN, and HF: manuscript preparation and review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This project was supported by grants from the Magnus Bergvall Foundation (Grant numbers 2017-02138, 2018-02566, and 2019-03149).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.00493/full#supplementary-material
References
- 1.Falhammar H, Thoren M. Clinical outcomes in the management of congenital adrenal hyperplasia. Endocrine. (2012) 41:355–73. 10.1007/s12020-011-9591-x [DOI] [PubMed] [Google Scholar]
- 2.Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2018) 103:4043–88. 10.1210/jc.2018-01865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. (2010) 95:5110–21. 10.1210/jc.2010-0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet. (2017) 390:2194–210. 10.1016/S0140-6736(17)31431-9 [DOI] [PubMed] [Google Scholar]
- 5.Gidlof S, Falhammar H, Thilen A, von Dobeln U, Ritzen M, Wedell A, et al. One hundred years of congenital adrenal hyperplasia in Sweden: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. (2013) 1:35–42. 10.1016/S2213-8587(13)70007-X [DOI] [PubMed] [Google Scholar]
- 6.Al Alawi AM, Nordenstrom A, Falhammar H. Clinical perspectives in congenital adrenal hyperplasia due to 3beta-hydroxysteroid dehydrogenase type 2 deficiency. Endocrine. (2019) 63:407–21. 10.1007/s12020-018-01835-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulsari K, Falhammar H. Clinical perspectives in congenital adrenal hyperplasia due to 11beta-hydroxylase deficiency. Endocrine. (2017) 55:19–36. 10.1007/s12020-016-1189-x [DOI] [PubMed] [Google Scholar]
- 8.Dean B, Chrisp GL, Quartararo M, Maguire AM, Hameed S, King BR, et al. P450 oxidoreductase deficiency: a systematic review and meta-analysis of genotypes, phenotypes and their relationships. J Clin Endocrinol Metab. (2019) 105:dgz255. 10.1210/clinem/dgz255 [DOI] [PubMed] [Google Scholar]
- 9.Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. (2018) 180:R127–45. 10.1530/EJE-18-0712 [DOI] [PubMed] [Google Scholar]
- 10.Hannah-Shmouni F, Morissette R, Sinaii N, Elman M, Prezant TR, Chen W, et al. Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi jews and caucasians. Genet Med. (2017) 19:1276–9. 10.1038/gim.2017.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjold A, Hagenfeldt K, et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. (2007) 92:110–16. 10.1210/jc.2006-1350 [DOI] [PubMed] [Google Scholar]
- 12.Falhammar H, Frisen L, Hirschberg AL, Norrby C, Almqvist C, Nordenskjold A, et al. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. (2015) 100:3520–8. 10.1210/JC.2015-2093 [DOI] [PubMed] [Google Scholar]
- 13.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. (2000) 15:993–1000. 10.1359/jbmr.2000.15.6.993 [DOI] [PubMed] [Google Scholar]
- 14.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. (2002) 13:777–87. 10.1007/s001980200108 [DOI] [PubMed] [Google Scholar]
- 15.Chappard D, Legrand E, Basle MF, Fromont P, Racineux JL, Rebel A, et al. Altered trabecular architecture induced by corticosteroids: a bone histomorphometric study. J Bone Miner Res. (1996) 11:676–85. 10.1002/jbmr.5650110516 [DOI] [PubMed] [Google Scholar]
- 16.Dalle Carbonare L, Arlot ME, Chavassieux PM, Roux JP, Portero NR, Meunier PJ. Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosis. J Bone Miner Res. (2001) 16:97–103. 10.1359/jbmr.2001.16.1.97 [DOI] [PubMed] [Google Scholar]
- 17.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. (1998) 102:274–82. 10.1172/JCI2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasperk CH, Wakley GK, Hierl T, Ziegler R. Gonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitro. J Bone Miner Res. (1997) 12:464–71. 10.1359/jbmr.1997.12.3.464 [DOI] [PubMed] [Google Scholar]
- 19.El-Maouche D, Collier S, Prasad M, Reynolds JC, Merke DP. Cortical bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol. (2015) 82:330–7. 10.1111/cen.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sellers EP, MacGillivray MH. Blunted adrenarche in patients with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Res. (1995) 21:537–44. 10.1080/07435809509030471 [DOI] [PubMed] [Google Scholar]
- 21.Girgis R, Winter JS. The effects of glucocorticoid replacement therapy on growth, bone mineral density, and bone turnover markers in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. (1997) 82:3926–9. 10.1210/jcem.82.12.4320 [DOI] [PubMed] [Google Scholar]
- 22.Fleischman A, Ringelheim J, Feldman HA, Gordon CM. Bone mineral status in children with congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. (2007) 20:227–35. 10.1515/JPEM.2007.20.2.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo CY, Weetman AP, Eastell R. Bone turnover and bone mineral density in patients with congenital adrenal hyperplasia. Clin Endocrinol. (1996) 45:535–41. 10.1046/j.1365-2265.1996.00851.x [DOI] [PubMed] [Google Scholar]
- 24.Mora S, Saggion F, Russo G, Weber G, Bellini A, Prinster C, et al. Bone density in young patients with congenital adrenal hyperplasia. Bone. (1996) 18:337–40. 10.1016/8756-3282(96)00003-8 [DOI] [PubMed] [Google Scholar]
- 25.Stikkelbroeck NM, Oyen WJ, van der Wilt GJ, Hermus AR, Otten BJ. Normal bone mineral density and lean body mass, but increased fat mass, in young adult patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. (2003) 88:1036–42. 10.1210/jc.2002-021074 [DOI] [PubMed] [Google Scholar]
- 26.Christiansen P, Molgaard C, Muller J. Normal bone mineral content in young adults with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. (2004) 61:133–6. 10.1159/000075588 [DOI] [PubMed] [Google Scholar]
- 27.Gussinye M, Carrascosa A, Potau N, Enrubia M, Vicens-Calvet E, Ibanez L, et al. Bone mineral density in prepubertal and in adolescent and young adult patients with the salt-wasting form of congenital adrenal hyperplasia. Pediatrics. (1997) 100:671–4. 10.1542/peds.100.4.671 [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann A, Sido PG, Schulze E, Al Khzouz C, Lazea C, Coldea C, et al. Bone mineral density and bone turnover in romanian children and young adults with classical 21-hydroxylase deficiency are influenced by glucocorticoid replacement therapy. Clin Endocrinol. (2009) 71:477–84. 10.1111/j.1365-2265.2008.03518.x [DOI] [PubMed] [Google Scholar]
- 29.Cameron FJ, Kaymakci B, Byrt EA, Ebeling PR, Warne GL, Wark JD. Bone mineral density and body composition in congenital adrenal hyperplasia. J Clin Endocrinol Metab. (1995) 80:2238–43. 10.1210/jcem.80.7.7608286 [DOI] [PubMed] [Google Scholar]
- 30.de Almeida Freire PO, de Lemos-Marini SH, Maciel-Guerra AT, Morcillo AM, Matias Baptista MT, de Mello MP, et al. Classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency: a cross-sectional study of factors involved in bone mineral density. J Bone Miner Metab. (2003). 21:396–401. 10.1007/s00774-003-0434-6 [DOI] [PubMed] [Google Scholar]
- 31.Paganini C, Radetti G, Livieri C, Braga V, Migliavacca D, Adami S. Height, bone mineral density and bone markers in congenital adrenal hyperplasia. Horm Res. (2000) 54:164–8. 10.1159/000053253 [DOI] [PubMed] [Google Scholar]
- 32.Jaaskelainen J, Voutilainen R. Bone mineral density in relation to glucocorticoid substitution therapy in adult patients with 21-hydroxylase deficiency. Clin Endocrinol. (1996) 45:707–13. 10.1046/j.1365-2265.1996.8620871.x [DOI] [PubMed] [Google Scholar]
- 33.Hagenfeldt K, Martin Ritzen E, Ringertz H, Helleday J, Carlstrom K. Bone mass and body composition of adult women with congenital virilizing 21-hydroxylase deficiency after glucocorticoid treatment since infancy. Eur J Endocrinol. (2000) 143:667–71. 10.1530/eje.0.1430667 [DOI] [PubMed] [Google Scholar]
- 34.Sciannamblo M, Russo G, Cuccato D, Chiumello G, Mora S. Reduced bone mineral density and increased bone metabolism rate in young adult patients with 21-hydroxylase deficiency. J Clin Endocrinol Metab. (2006) 91:4453–8. 10.1210/jc.2005-2823 [DOI] [PubMed] [Google Scholar]
- 35.King JA, Wisniewski AB, Bankowski BJ, Carson KA, Zacur HA, Migeon CJ. Long-term corticosteroid replacement and bone mineral density in adult women with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab. (2006) 91:865–9. 10.1210/jc.2005-0745 [DOI] [PubMed] [Google Scholar]
- 36.Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjold A, Hagenfeldt K, et al. Fractures and bone mineral density in adult women with 21-hydroxylase deficiency. J Clin Endocrinol Metab. (2007) 92:4643–9. 10.1210/jc.2007-0744 [DOI] [PubMed] [Google Scholar]
- 37.Falhammar H, Filipsson Nystrom H, Wedell A, Brismar K, Thoren M. Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. (2013) 168:331–41. 10.1530/EJE-12-0865 [DOI] [PubMed] [Google Scholar]
- 38.Bachelot A, Plu-Bureau G, Thibaud E, Laborde K, Pinto G, Samara D, et al. Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res. (2007) 67:268–76. 10.1159/000098017 [DOI] [PubMed] [Google Scholar]
- 39.Koetz KR, Ventz M, Diederich S, Quinkler M. Bone mineral density is not significantly reduced in adult patients on low-dose glucocorticoid replacement therapy. J Clin Endocrinol Metab. (2012) 97:85–92. 10.1210/jc.2011-2036 [DOI] [PubMed] [Google Scholar]
- 40.Nermoen I, Bronstad I, Fougner KJ, Svartberg J, Oksnes M, Husebye ES, et al. Genetic, anthropometric and metabolic features of adult Norwegian patients with 21-hydroxylase deficiency. Eur J Endocrinol. (2012) 167:507–16. 10.1530/EJE-12-0196 [DOI] [PubMed] [Google Scholar]
- 41.Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. (2012) 97:4429–38. 10.1210/jc.2012-2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceccato F, Barbot M, Albiger N, Zilio M, De Toni P, Luisetto G, et al. Long-term glucocorticoid effect on bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. (2016) 175:101–6. 10.1530/EJE-16-0104 [DOI] [PubMed] [Google Scholar]
- 43.Raizada N, Jyotsna VP, Upadhyay AD, Gupta N. Bone mineral density in young adult women with congenital adrenal hyperplasia. Indian J Endocrinol Metab. (2016) 20:62–6. 10.4103/2230-8210.172283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riehl G, Reisch N, Roehle R, Claahsen van der Grinten H, Falhammar H, Quinkler M. Bone mineral density and fractures in congenital adrenal hyperplasia: findings from the dsd-LIFE study. Clin Endocrinol. (2019) 92:284–94. 10.1111/cen.14149 [DOI] [PubMed] [Google Scholar]
- 45.Arisaka O, Hoshi M, Kanazawa S, Numata M, Nakajima D, Kanno S, et al. Preliminary report: effect of adrenal androgen and estrogen on bone maturation and bone mineral density. Metabolism. (2001) 50:377–379. 10.1053/meta.2001.21678 [DOI] [PubMed] [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses. (2019). Available online at: http://www.ohri.ca/Programs/clinical_epidemiology/default.asp
- 48.Borge TC, Aase H, Brantsaeter AL, Biele G. The importance of maternal diet quality during pregnancy on cognitive and behavioural outcomes in children: a systematic review and meta-analysis. BMJ Open. (2017) 7:e016777. 10.1136/bmjopen-2017-016777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October (2013). The GRADE Working Group (2013). Available online at: guidelinedevelopment.org/handbook
- 50.Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLoS ONE. (2010) 5:e8933. 10.1371/journal.pone.0008933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersen K, Rasmussen F, Neovius M, Tynelius P, Sundstrom J. Body size and risk of atrial fibrillation: a cohort study of 1.1 million young men. J Intern Med. (2018) 283:346–55. 10.1111/joim.12717 [DOI] [PubMed] [Google Scholar]
- 52.Kraan GP, Dullaart RP, Pratt JJ, Wolthers BG, Drayer NM, De Bruin R. The daily cortisol production reinvestigated in healthy men. The serum and urinary cortisol production rates are not significantly different. J Clin Endocrinol Metab. (1998) 83:1247–52. 10.1210/jcem.83.4.4694 [DOI] [PubMed] [Google Scholar]
- 53.Liu XS, Cohen A, Shane E, Yin PT, Stein EM, Rogers H, et al. Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res. (2010) 25:2229–38. 10.1002/jbmr.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin-Su K, Harbison MD, Lekarev O, Vogiatzi MG, New MI. Final adult height in children with congenital adrenal hyperplasia treated with growth hormone. J Clin Endocrinol Metab. (2011) 96:1710–7. 10.1210/jc.2010-2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonfig W, Bechtold S, Schmidt H, Knorr D, Schwarz HP. Reduced final height outcome in congenital adrenal hyperplasia under prednisone treatment: deceleration of growth velocity during puberty. J Clin Endocrinol Metab. (2007) 92:1635–9. 10.1210/jc.2006-2109 [DOI] [PubMed] [Google Scholar]
- 56.White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. (2000) 21:245–91. 10.1210/er.21.3.245 [DOI] [PubMed] [Google Scholar]
- 57.Walsh JS, Henry YM, Fatayerji D, Eastell R. Hormonal determinants of bone turnover before and after attainment of peak bone mass. Clin Endocrinol. (2010) 72:320–7. 10.1111/j.1365-2265.2009.03606.x [DOI] [PubMed] [Google Scholar]
- 58.Lindberg MK, Vandenput L, Moverare Skrtic S, Vanderschueren D, Boonen S, Bouillon R, et al. Androgens and the skeleton. Minerva Endocrinol. (2005) 30:15–25. [PubMed] [Google Scholar]
- 59.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. (2014) 25:2359–81. 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frisen L, Nordenstrom A, Falhammar H, Filipsson H, Holmdahl G, Janson PO, et al. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J Clin Endocrinol Metab. (2009) 94:3432–9. 10.1210/jc.2009-0636 [DOI] [PubMed] [Google Scholar]
- 61.Falhammar H, Nystrom HF, Thoren M. Quality of life, social situation, and sexual satisfaction, in adult males with congenital adrenal hyperplasia. Endocrine. (2014) 47:299–307. 10.1007/s12020-013-0161-2 [DOI] [PubMed] [Google Scholar]
- 62.Falhammar H, Claahsen-van der Grinten H, Reisch N, Slowikowska-Hilczer J, Nordenstrom A, Roehle R, et al. Health status in 1040 adults with disorders of sex development (DSD): a European multicenter study. Endocr Connect. (2018) 7:466–78. 10.1530/EC-18-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.