Abstract
Pancreatic neuroendocrine tumors (panNETs) are often inoperable at diagnosis. The mTORC1 inhibitor everolimus has been approved for the treatment of advanced NETs. However, the regular development of resistance to everolimus limits its clinical efficacy. We established two independent everolimus-resistant panNET (BON1) cell lines (BON1 RR1, BON1 RR2) to find potential mechanisms of resistance. After 24 weeks of permanent exposure to 10 nM everolimus, BON1 RR1 and BON1 RR2 showed stable resistance with cellular survival rates of 96.70% (IC50=5200 nM) and 92.30% (IC50=2500 nM), respectively. The control cell line showed sensitivity to 10 nM everolimus with cellular survival declining to 54.70% (IC50=34 nM). Both resistant cell lines did not regain sensitivity over time and showed persistent stable resistance after a drug holiday of 13 weeks. The mechanisms of resistance in our cell line model included morphological adaptations, G1 cell cycle arrest associated with reduced CDK1(cdc2) expression and decreased autophagy. Cellular migration potential was increased and indirectly linked to c-Met activation. GSK3 was over-activated in association with reduced basal IRS-1 protein levels. Specific GSK3 inhibition strongly decreased BON1 RR1/RR2 cell survival. The combination of everolimus with the PI3Kα inhibitor BYL719 re-established everolimus sensitivity through GSK3 inhibition and restoration of autophagy. We suggest that GSK3 over-activation combined with decreased basal IRS-1 protein levels and decreased autophagy may be a crucial feature of everolimus resistance, and hence a possible therapeutic target.
Keywords: panNETs, stable everolimus resistance, GSK3, combination treatment
1. Introduction
Pancreatic neuroendocrine tumors (panNETs) are a type of GastroEnteroPancreatic neuroendocrine tumor (GEP-NETs) (Yao, et al. 2008). Due to their slow and frequently asymptomatic growth, panNETs are often advanced when diagnosed, such that curative surgery is no longer an option (Briest and Grabowski 2014). For this reason, the 10-year survival for panNETs is only 44%, in spite of various advances in treatment modalities (Aristizabal Prada and Auernhammer 2018; Auernhammer, et al. 2017; Ekeblad, et al. 2008; Halfdanarson, et al. 2008). Common treatments for advanced disease such as biotherapy, chemotherapy, molecular-targeted therapy and peptide radioreceptor therapy (PRRT) remain limited in their efficacy, and novel treatment options are urgently needed (Aristizabal Prada and Auernhammer 2018; Auernhammer et al. 2017; Frilling, et al. 2014).
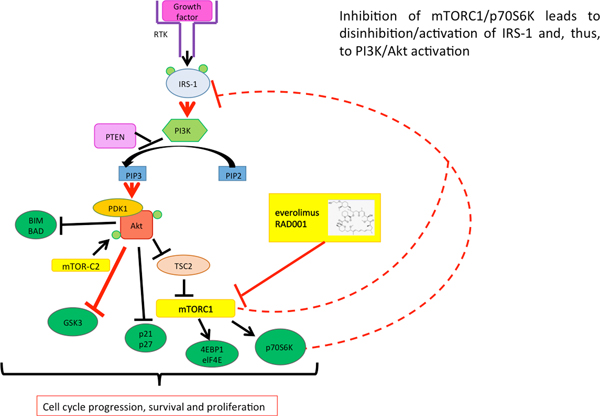
One of the most important receptor tyrosine kinase (RTK)-induced oncogenic signaling cascades in GEP-NETs is the mTORC1-p70S6K signaling pathway, which can be inhibited by the mTORC1 inhibitor everolimus (Fig. 1) (Shipkova, et al. 2016; Wolin 2013; Zaytseva, et al. 2012). The PI3K-Akt-mTORC1 axis is frequently over-activated in cancers, including panNETs, causing increased cellular survival, proliferation and protein synthesis (Briest and Grabowski 2014; Pavel and Sers 2016). Accordingly, the mTORC1 inhibitor everolimus has shown anti-tumor potential in several clinical phase 3 trials in patients with NETs (RADIANT-2, RADIANT-3, RADIANT-4) (Pavel, et al. 2011; Yao, et al. 2016a; Yao, et al. 2016b; Yao, et al. 2011), and has been approved for the treatment of pancreatic, intestinal and pulmonary NETs in many countries (Chan, et al. 2017; Pavel and Sers 2016). Unfortunately, only around 5 % of patients show partial remission in response to everolimus treatment, although many more patients show stable disease. However, most tumors eventually start to progress even if everolimus treatment is continued (Yao et al. 2011). Thus, secondary resistance seems to occur in most patients, although primary resistance may also be a problem (Capozzi, et al. 2015; He, et al. 2016; Vandamme, et al. 2016; Wagle, et al. 2014; Zatelli, et al. 2016). Advanced panNET patients are treated with a fixed dose of 10 mg everolimus daily (NCT00510068) (Yao, et al. 2014; Yao, et al. 2013): the majority develops treatment resistance within one year of treatment (Capurso, et al. 2012; Yao et al. 2013). Consequently, reversible adaptive resistance to everolimus in panNET cell lines, and combinatory regimes to overcome acquired resistance, have been the object of previous studies (Passacantilli, et al. 2014; Vandamme et al. 2016; Zitzmann, et al. 2010). However, long-term resistance to everolimus treatment has only been little studied to date (Capurso et al. 2012; Passacantilli et al. 2014; Vandamme et al. 2016), and thus there is an unmet need to better understand the mechanisms of resistance and to establish predictive biomarkers for resistance to everolimus, and hence appropriate treatments. Activation of autophagy by mTORC1 inhibitors has been reported as one potential mechanism for developing mTORC1 inhibitor resistance and for promoting tumor cell growth (Avniel, et al. 2018; Avniel-Polak, et al. 2016). Autophagy seems to play a controversial role in cancer: on the one hand, autophagy might offer a protective mechanism in some cancer cells promoting acquired drug resistance (Avniel-Polak et al. 2016; Sui, et al. 2013). On the other hand, autophagy might also induce autophagic cell death, separate from apoptosis (Sui et al. 2013).
Fig. 1.
Proposed mechanism of action of the mTORC1-inhibitor everolimus. Everolimus inhibits the mTORC1 complex and thereby down-regulates the mTORC1/p70S6K signaling cascade, preventing the cell from cell cycle progression, survival and proliferation. Inhibition of mTORC1/p70S6K leads to disinhibition/activation of IRS-1 and thereby activation of PI3K/Akt, which in turn inhibits GSK3 by phosphorylation.
Novel insights into the mechanisms of resistance in response to everolimus treatment could provide a rationale for dual-targeting approaches in order to overcome such acquired or intrinsic resistance (Avniel et al. 2018; Capozzi et al. 2015; Capurso et al. 2012; Passacantilli et al. 2014; Vandamme et al. 2016). Although the study group around Vandamme et al. treated BON1 and QGP1 cell lines over 24 weeks with increasing doses of everolimus up to 1 μM according to the “pulse selection model” (McDermott, et al. 2014), BON1 and QGP1 cells only showed reversible resistance and regained sensitivity to everolimus after a drug holiday of 10–12 weeks (Vandamme et al. 2016).
Therefore, our aim was to establish a clinically relevant panNET cell line model from BON1 cells, demonstrating stable, i.e. consistent and reliable resistance to everolimus in order to study the mechanisms of long-term resistance and explore novel combinatory treatment options to overcome such resistance.
Besides exploring PI3K-Akt signaling as a potential pathway of resistance (O’Reilly, et al. 2006; Passacantilli et al. 2014; Vandamme et al. 2016; Zitzmann et al. 2010), we also investigated GSK3 signaling. GSK3α/β is a direct effector protein of Akt: activated Akt phosphorylates and inhibits GSK3α/β (Porta, et al. 2014). GSK3 has been implicated in metabolism since GSK3 is inactivated by insulin leading to disinhibition/activation of glycogen synthase. Moreover, it seems to play a role in diseases such as diabetes, Alzheimer’s disease and psychiatric diseases, including bipolar disorder and schizophrenia (Maurer, et al. 2014). GSK3α/β has shown controversial effects on cell death and survival, including pro- and anti-apoptotic effects (Maurer et al. 2014). Activated GSKβ has also been reported to target cell cycle proteins such as Cyclin D for degradation (Maurer et al. 2014). On the one hand, treatment with different GSK3 inhibitors (CHIR99021, 6-bromoindirubin-3’oxime-, 1-azakenpaullone) and siRNA stimulated proliferation of rat insulinoma INS-1E cells (Mussmann, et al. 2007). Conversely, the non-specific GSK3α/β inhibitor lithium chloride inhibited neuroendocrine tumor growth in vitro and in vivo (Greenblatt, et al. 2010; Lubner, et al. 2011); however, in a clinical phase 2 study with 15 patients with low-grade NETs (8 carcinoid tumors, 5 islet cell tumors) the non-specific GSK3α/β inhibitor lithium chloride caused no objective responses, and pre- and post-treatment tumor biopsies showed no consistent GSK3β inhibitory effects due to insufficient phosphorylation of GSK3β in patients’ serum levels (Lubner et al. 2011). To assess the effects of more specific GSK3α/β inhibition on NETs, we have recently investigated the selective GSK3α/β inhibitor AR-A014418 in pancreatic, pulmonary and midgut NET cells and reported a significant inhibitory effect on cell viability and strong GSK3α/β phosphorylation/inhibition (Aristizabal Prada, et al. 2017). Biochemical studies have focused on GSK3β inhibition, while other studies have also shown the importance of GSK3α in drug resistance (McCubrey, et al. 2014). Thus, GSK3 appears to play a pleiotropic role in regulating cell proliferation.
We have used these everolimus-resistant cell lines to demonstrate combinatorial treatments that reversed everolimus resistance via GSK3 inhibition in vitro. In due course other cell lines can be studied to explore whether they also develop stable resistance and to compare the intrinsic mechanisms. In a pilot study we transferred our everolimus-resistant cell line model to an orthotopic intrapancreatic xenograft mouse model providing the basis for translating the in vitro approach to an in vivo situation.
2. Material and Methods
A. Cell Culture
The human panNET cell line BON1 (Evers, et al. 1991) was kindly obtained from Prof. R. Göke (Marburg, Germany). The BON1 cell line was analysed and certified by the German Biological Resource Centre DSMZ (DSMZ, Braunschweig, Germany) using short tandem repeats (STRs). BON1 cells were grown in DMEM/F12 (1:1) (Life Technologies, Karlsruhe, Germany), supplemented with 10% FBS (Biochrom, Berlin, Germany), 1% penicillin/streptomycin (Life Technologies) and 0.4% amphotericin B (Biochrom, Berlin, Germany) at 5% CO2 and 37 °C and tested mycoplasma-free for all experiments reported. BON1 is a permanent cell line derived from a lymph node metastasis of a human carcinoid tumor of the pancreas and presents phenotypic NET characteristics (Grozinsky-Glasberg, et al. 2012).
B. Experimental Design
In order to yield everolimus resistance in panNET cells, BON1 cells (passage 13) were treated continuously with a constant low dose of 10 nM everolimus for 24 weeks. A dose of 10 nM everolimus was chosen since the plasma levels of everolimus in patients on daily therapy with 10 mg everolimus range between around 8 nM (Cmin after 2 weeks of daily exposure to 10 mg everolimus) and 59 nM (C2h after 12 weeks of daily exposure to 10 mg everolimus) (Budde, et al. 2016). Every three days medium was exchanged and everolimus treatment renewed. The concentrations were not increased over time. To determine commonalities in resistance mechanisms in BON1 cells, we have established two independent everolimus-resistant BON1 cell lines (BON1 RR1 and BON1 RR2) in parallel and a control cell line (BON1 Control DMSO) to control for adaptive strategies of long-term in vitro culturing and passaging as such. BON1 is the initial parental cell line, from which the development of resistance started. To obtain a sensitive control cell line (BON1 Control DMSO), BON1 were continuously vehicle-treated with a comparable concentration of DMSO (0,00005%) over 24 weeks. We compare our results to the BON1 Control DMSO cell line. In order to assess the stability and reliability of the acquired resistance phenotype, all cells were frozen in liquid nitrogen (minimum for 48 h) and thawed, followed by drug withdrawal (McDermott et al. 2014). Resistance was defined as at least a doubling of the IC50, compared to the sensitive control cell line (BON1 Control DMSO), after treatment with 10 nM everolimus for 144 h (McDermott et al. 2014).
C. Inhibitors
The mTORC1 inhibitor everolimus (RAD001), the GSK3 inhibitor AR-A014418 and the PI3K Inhibitor BYL719 were acquired from Sigma-Aldrich St. Louis (MO, USA). All substances were dissolved in dimethyl-sulfoxide (DMSO, Sigma-Aldrich) and stored at −20°C. The stock solutions of everolimus, AR-A014418 and Byl719 were 20 mM in DMSO.
D. Cell Viability Assessment
BON1 cells were seeded at ~1,500 cells/96-well for 24h in complete medium. Then, the cells were treated with different concentrations of everolimus (from 1 nM to 2 μM), BYL719 (from 1 μM to10 μM), or AR-A014418 (from 5 μM to 20 μM) alone, or they were treated with everolimus (from 1 nM to 10 nM) in combination with BYL719 (from 1 μM to 10 μM) or in combination with AR-A014418 (from 5 μM to 20 μM). The BYL719 concentrations we chose are potentially clinicially relevant since in a clinical study assessing population pharmacokinetics and pharmacodynamics of BYL719 in patients with advanced solid malignancies median BYL719 plasma concentrations between 2 μM and 11 μM have been found with 1 month daily oral therapy with therapeutically-relevant doses of 300mg - 450mg BYL719 2 h to 8 h post-dose (De Buck, et al. 2014). For everolimus alone the two final DMSO controls were equal to 1μM everolimus (0.005% DMSO final concentration) and 2μM everolimus (0.01% DMSO final concentration), respectively, depending on the experiment. For the higher dose BYL719/everolimus combination treatment the final DMSO control was equal to 1:2000 (Byl719) + 1:2,000,000 (everolimus) = 0.05005% final DMSO concentration. For the lower dose BYL719/everolimus combination treatment the final DMSO control was equal to 1:4000 (Byl719) + 1:2,000,000 (everolimus) = 0.02505% final DMSO concentration. For AR-A014418/everolimus combination treatment the final DMSO control was equal to 1:1000 (AR-A014418) + 1:2,000,000 (everolimus) = 0.10005% final DMSO concentration. The final DMSO concentrations were never higher than 0.1% and had no significant effect on cell viability. The metabolic activity of living cells was assessed by the “Cell Titer Blue®” cell viability assay (Promega, Madison, WI, USA) after 72 h and 144 h of incubation with the different agents. Cells were incubated for 4 h with “Cell Titer Blue®” solution and fluorescence was measured at 560/590 nm using a GLOMAX plate reader (Promega, Madison, WI, USA).
E. Cell Cycle Analysis by Flow cytometric analysis (FACS)
Cell cycle phase distribution was analysed (Riccardi and Nicoletti 2006) (BD Accuri C6 Analysis). First, cells were cultured in 6-well plates for 24 h, before medium was replaced by antibiotic-free medium and incubated with 10 nM everolimus. 72 h later, cells were collected, washed and resuspended in 300 μl propidium iodide (Sigma-Aldrich). After 8h, 20,000 events from each sample were analysed.
F. Caspase-3/−7 Activity Assay
To measure the activity of Caspase-3/−7 we used the Apo-One homogeneous Caspase-3/7- Assay kit (Promega): 10,000 cells were seeded per well, incubated for 24 h without treatment or with respective doses of BYL719 (5–10 μM) or everolimus (10 nM) and Caspase-3/7 activity was assessed according to the manufacturer’s instructions. We compared all results of the BYL719- or everolimus-treated cell lines to the vehicle-treated cell lines. For each experiment the vehicle represented the highest DMSO concentration present in the respective experiment. The exact DMSO concentrations are detailed under D. Cell Viability Assessment.
G. Protein Extraction and Western Blotting
The cells were seeded into 10 cm-plates and grown for 24 h in complete medium for Western blot analysis. Following medium exchange, cells were incubated with 10 nM everolimus, either alone or in combination with BYL719 or AR-A014418. We compared all results to the vehicle-treated cells. For each experiment the vehicle represented the highest DMSO concentration present in the respective experiment. The exact DMSO concentrations are detailed under D. Cell Viability Assessment. The incubation times were up to 72 h. Western blotting was conducted as described previously (Reuther, et al. 2016). A cell line screening to determine commonalities between both resistant cell lines (BON1 RR1 and BON1 RR2) in protein expression was performed using the following primary antibodies: pIRS-1 Ser612 (#3203) (1:1000), IRS-1 (#3407) (1:1000), pAkt (Ser473) (#4060) (1:5000), Akt (#2920) (1:5000), pTORC1 (S151) (#3359) (1:1000), TORC1 (#2587) (1:000), pp70S6K (T387/389) (#9205) (1:2000), p70S6K (#9234) (1:5000), pS6 (S240/244) (#5354) (1:50000), S6 (#2217) (1:5000), p4EBP1 (Ser65) (#9451) (1:1000), 4EBP1 (#9644) (1:10000), pCDK1 (Tyr15) (#4539) (1:1000), CDK1 (#9116) (1:2000), pEGFR (Y1068) (#3777) (1:1000), EGFR (#4267) (1:5000), pMet (Y1234/5) (#3077) (1:2000), Met (#3127) (1:5000), pGSK3 (S21/9) (#9331) (1:2000), GSK3 (#5676) (1:10000), LC3-A (#4599) (1:1000), Caspase 3 (#9662) (1:1000), cleaved Caspase 3 Asp175 (#9661) (Cell Signaling, Danvers, USA) (1:1000), Actin (A5441) (Sigma, St.Louis, USA) (1:50000), Bcl2 (610539) (BD Transduction Laboratories, New Jersey, USA) (1:1000), CgA (ab68271) (abcam, Cambridge, UK) (1:100000).
H. Cell Migration Assay
Cells were seeded at densities of 120,000 (BON1) cells per chamber in culture inserts (Ibidi, Munich, Germany). After 24 h, the inserts were removed, and the cells treated with 10 nM everolimus for 48 h. We compared the four cell lines BON1, BON1 Control DMSO, BON1 RR1 and BON1 RR2 to each other after treatment with 10 nM Everolimus. In this case the BON1 Control DMSO cell line functioned as control and was compared to the resistant cell lines. After 0 h and 48 h of incubation with everolimus, photographs of the gap between the two cell layers were taken [Zeiss, Axiovert 135 TV (microscope) and Zeiss, AxioCam MRm (camera)] and analysed.
I. Statistical Analysis
The results are displayed as mean ± standard deviation of the mean (SD) of at least three independently performed experiments. Each cell viability experiment consisted of at least 6 samples per substance concentration and incubation period. A priori tests considering the normal distribution and homogeneity of variances were performed applying the Kolmogorov-Smirnov-Test and Levenés Test of the SPSS statistical package SPSS (version 13.0 for Windows, SPSS Inc (2005), Chicago, USA). When parametric criteria were met, an ANOVA comparison of means with a post hoc Tukey test or a two-tailed t-test was performed; when non-parametric criteria were met the Kruskal-Wallis followed by the Mann-Whitney test was performed. When the one group was normalised to 100%, comparisons were performed as one-sample tests.
To identify potential synergistic effects between BYL719 and everolimus on cell viability, we used Linear Mixed Effects Models. BYL719, everolimus (each in three different doses) and the interactions between both were considered as fixed effects, the trial as a random effect. If a significant negative interaction between BYL719 and everolimus on cell survival was found, we concluded a synergistic effect. One model was estimated for each cell line. These computations were done with R 3.3.3 (GNU General Public License; R Foundation, Vienna, Austria). For the analysis of the baseline differences in cell viability and Caspase 3/7 assay we used linear mixed effects models too. While the cell line was considered as fixed, the trial was considered as random.
Statistical significance was assessed at p<0.05, besides for the Western blot analysis, where due to the exploratory character and the sample size, significance was assessed at p<0.1.
J. Pilot mouse study: Establishment of an orthotopic intrapancreatic everolimus-resistant tumor xenograft mouse model
BON1 RR2 (2×106 in 20 μl medium) were implanted into the pancreas of 12 week-old female SCID mice (weight 20g) (n=4) as described by Soares et al. (Soares, et al. 2014) by minimally invasive surgery, with minor changes. This technique was previously established by us to generate the orthotopic BON1 xenograft mouse model. Briefly, SCID mice were anaesthetised by isoflurane and intraperitoneal injection of ketamine (70 mg/kg). After local shaving and disinfection the abdominal area was opened by a 1cm incision along the Linea Alba. The entire pancreatic body was then exposed on sterile gauze and retracted laterally on saline-soaked cotton tips in such way that enough solid pancreatic tissue was available for cell injection, before it was placed back into the abdominal cavity. Tumor growth was monitored once a week by a preclinical 1T PET/MRI (nanoScan, Mediso, Hungary) using a whole-body coil and T1-weighted gradient-echo and T2-weighted spin echo sequences. The metabolic activity of the engrafted tumor was estimated by 18FDG-PET/MRI, somatostatin receptor 2 (SSTR2) levels were assessed by 68Ga-DOTATOC PET-CT (15 MBq injected i.v. respectively). When the tumors had reached a size of ~1000mm3, the experiment was terminated. Animal experiments were performed in accordance with the national and local guidelines for animal welfare and approved by the ethics committee of the state Berlin. All in vivo experiments were performed at the Berlin Experimental Radionuclide Imaging Center (BERIC), Charité - Universitätsmedizin Berlin, Germany.
3. Results
Following 24 weeks of permanent exposure to 10 nM everolimus, we have, for the first time, been able to develop two independent stable everolimus-resistant panNET cell lines (BON1 RR1 and BON1 RR2). Both resistant cell lines did not regain sensitivity over time, and showed stable resistance after a drug holiday of 13 weeks. Moreover, both cell lines showed a GSK3 over-activation as a crucial commonality.
A. Stable resistance
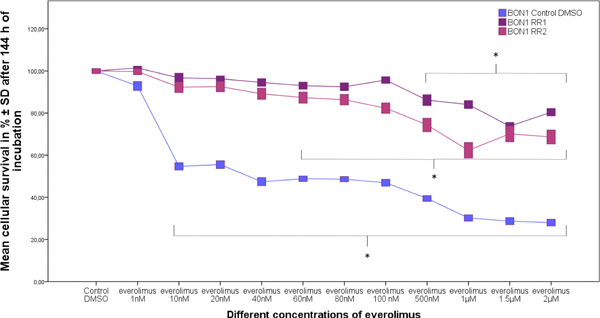
After 24 weeks of permanent everolimus exposure, both cell lines (BON1 RR1 and BON1 RR2) showed strong reliable resistance to everolimus, whereas BON1 Control DMSO cells displayed no resistance (Fig. 2). We compared dose-response curves of the resistant cell lines to the dose-response curves of the non-resistant cell lines with 1 nM - 2 μM everolimus and found resistance, i. e. at least a doubling of the IC50 in the resistant cell lines, compared to the sensitive controls, after 144 h everolimus treatment. IC50 calculations showed a 153-fold increase (IC50 = 5200 nM) in BON1 RR1 and a 74-fold increase (IC50 = 2500 nM) in BON1 RR2 cells when compared to the BON1 Control DMSO cells (IC50 = 34 nM) after 144 h everolimus treatment. BON1 Control DMSO cells demonstrated a significant decrease in cellular survival after re-application of 10 nM everolimus for 144 h, whereas BON1 RR1 and BON1 RR2 cells showed a significant decrease in cellular viability only at 500 nM and 60 nM, respectively (Fig. 2). The resistance was evaluated after freezing and thawing the cells again to confirm stable resistance. We performed additional dose-response curves after a drug holiday of 13 weeks and again found persistent stable resistance with an IC50 > 2000 nM in BON1 RR1 and BON1 RR2 cells.
Fig. 2.
Effects of everolimus on cell survival of resistant (BON1 RR1 and BON1 RR2) and sensitive (BON1 Control DMSO) cell lines after 144 h of incubation with everolimus (1 nM – 2 μM). The arithmetic means and standard deviation of at least three independent experiments are shown. Statistically significantly different results in comparison to BON1 Control DMSO are shown, considering p < 0,05 as significant (*).
B. Morphologic changes, cell growth and neuroendocrine identity
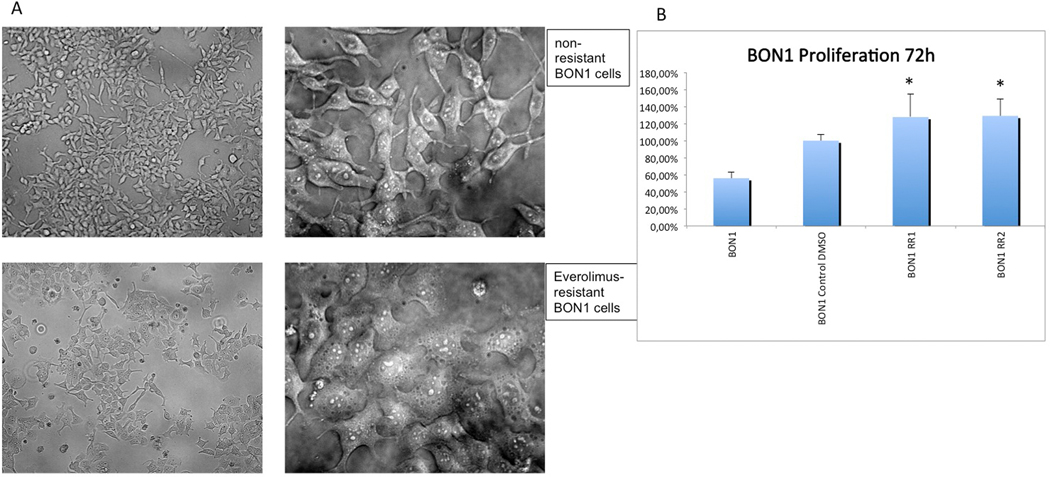
Everolimus-sensitive BON1 cells showed an oval shape with fibroblastic-like cellular extensions in some cells and regular cluster formation (Fig. 3A). Morphologic characteristics of everolimus-resistant cells displayed an amorphous, flattened cellular shape with lysosome-like bubbles and clumping conglomerates (Fig. 3A). The viable cell number of the resistant cell lines was slightly but significantly increased compared to the sensitive control cell line after 72 h without treatment (p < 0.001), suggesting more rapid proliferation (Fig. 3B).
Fig. 3.
Everolimus resistant cells show morphologic changes. (A) Everolimus-resistant cells (BON1 RR1 and BON1 RR2) displayed an amorphous, flattened cellular shape with lysosome-like bubbles and clumping conglomerates. (B) The viable cell number of the resistant cell lines was slightly but significantly increased compared to the sensitive control cell line after 72 h without treatment (p < 0.001), suggesting more rapid proliferation.
We investigated the neuroendocrine identity of the resistant cell lines by assessing chromogranin A (CgA) by Western blot techology (Suppl. Fig. 1) and found strong expression of CgA in both resistant cell lines.
C. Heterogeneity of BON1 RR1 and BON1 RR2
We observed inhibition of mTORC1/p70S6K signaling to a differential extent between BON1 RR1 and BON1 RR2 after re-application of everolimus (Supplementary Fig. 1). By using two independently-established everolimus-resistant cell lines, we have attempted to reduce if not totally eliminate the bias due to tumor heterogeneity in response to long-term everolimus treatment.
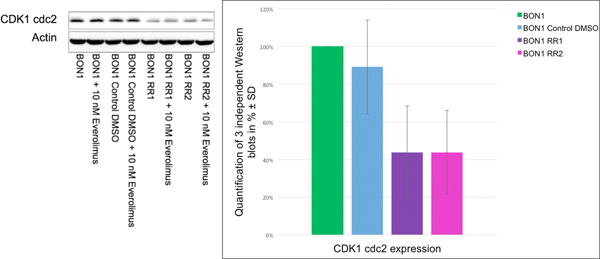
D. Everolimus resistance: G1 phase cell cycle arrest associated with reduced baseline expression of CDK1(cdc2) and of the autophagy marker LC3A
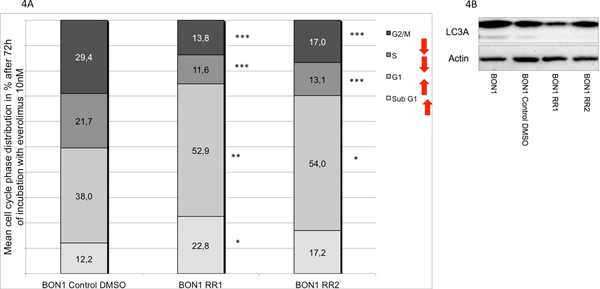
Both resistant cell lines displayed a significant increase in sub-G1 phase and G1 phase in response to everolimus (Fig. 4A). Associated with the sub G1 increase and G1 phase cell cycle arrest, we found a decreased baseline CDK1(cdc2) expression and reduced baseline expression of the autophagy marker LC3A demonstrating reduced autophagy in BON1 RR1 and BON1 RR2 cells (Fig. 4B and Fig. 5).
Fig. 4.
(A) Cell cycle analysis via FACS showed a significant increase in sub-G1 population and G1 phase cell cycle arrest in both resistant cell lines (BON1 RR1 and BON1 RR2) after 72 h everolimus treatment. The arithmetic means and standard deviation of at least three independent experiments are shown. Statistically significantly different results in comparison to BON1 Control DMSO are shown, considering p<0,05 = *; p<0,01 = **; p<0,001 = ***.(B) Basal LC3A expression level measured by Western blot analysis was decreased in BON1 RR1 and BON1 RR2, compared to BON1 and BON1 Control DMSO. A representative blot out of three independently performed experiments is shown.
Fig. 5.
Western blot analysis via densitometry of CDK1(cdc2) showed a baseline decrease in CDK1(cdc2) expression levels in BON1 RR1 and BON1 RR2 cell lines, compared to the sensitive control cells. A representative blot out of three independently performed experiments is shown. For densitometry analysis the arithmetic means and standard deviation of at least three independent experiments are shown.
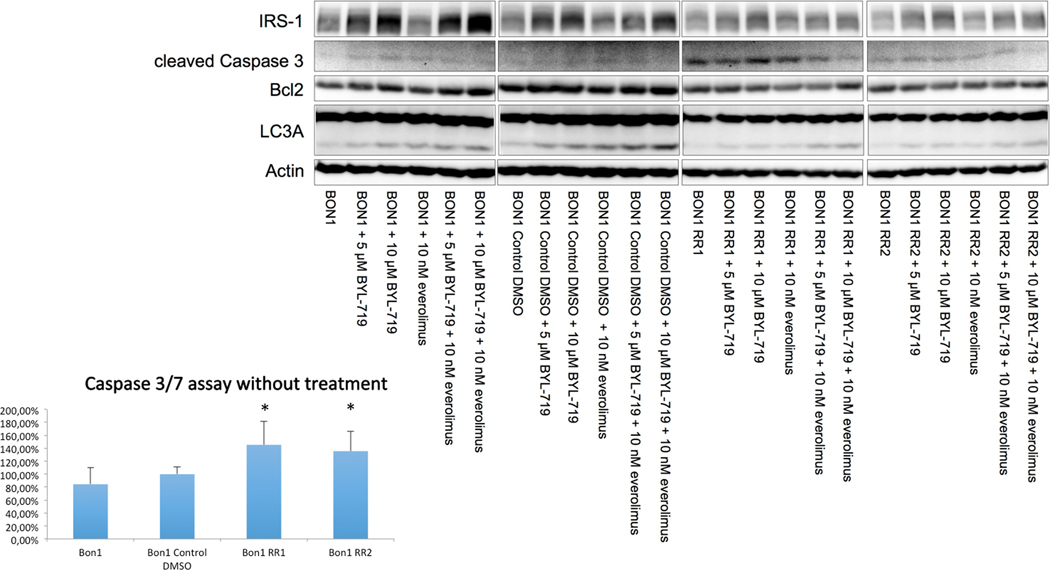
E. Everolimus resistance increased baseline apoptosis
Baseline cleaved caspase 3 as a marker of apoptosis was slightly increased in the resistant cell lines, compared to hardly detectable cleaved caspase 3 in the sensitive controls, indicating slightly stronger baseline apoptosis in the resistant cells (Fig 6). In order to show these results more clearly, we additionally performed the more sensitive Caspase 3/7 assay and found the same results with significantly increased baseline apoptosis, in both resistant cell lines, compared to the sensitive controls (p < 0.01) (Fig. 6). Basal expression of the anti-apoptotic marker Bcl2 was much lower in the resistant cell lines compared to the sensitive controls, and showed a lesser increase in response to BYL719 and everolimus treatment (Fig 6).
Fig. 6.
Western blot analysis showed a baseline decrease in IRS-1 protein levels, an increase in cleaved Caspase 3 and a decrease in anti-apoptotic Bcl-2 as a sign of increased apoptosis, a decrease in LC3A as a sign of decreased autophagy. Caspase 3/7 assay showed significantly increased apoptosis in both resistant cell lines, compared to the sensitive control cell line (p < 0.01).
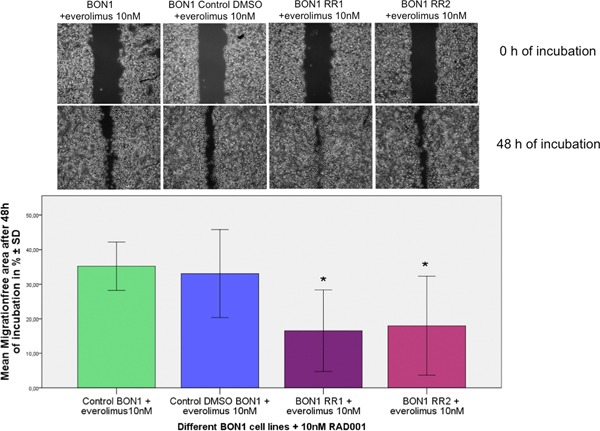
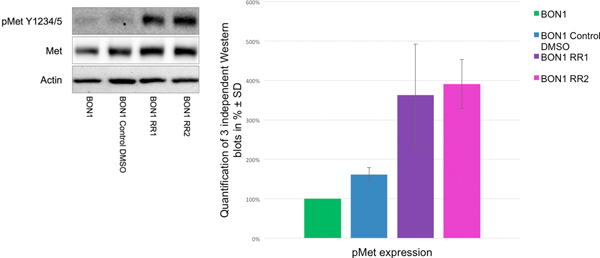
F. Everolimus resistance increased migration potential and basal c-Met activation
The migration assay reflected a significant increase in the migration potential of BON1 RR1/RR2 cells, compared to the sensitive control cell line, after treatment with 10 nM everolimus (Fig. 7). Both resistant cell lines displayed stronger baseline activation of c-Met when compared to the control cells (Fig. 8).
Fig. 7.
Everolimus-resistant cells showed a significantly increased migration potential with a significantly smaller migration gap after 48 h of everolimus treatment. The arithmetic means and standard deviation of at least three independent experiments are shown. Statistically significantly different results in comparison to Bon1 Control DMSO are shown, considering p<0,05 = *.
Fig. 8.
Western blot analysis via densitometry of phosphorylated/activated c-Met and total c-Met in resistant (BON1 RR1 and BON1 RR2) and sensitive control cells showed an increase in phosphorylated/activated c-Met in the resistant cell lines. A representative blot out of three independently performed experiments is shown. For densitometry analysis the arithmetic means and standard deviation of at least three independent experiments are shown.
G. Everolimus resistance: Baseline GSK3α/β (GSK3) over-activation, and strong BON1 RR1/RR2 cell viability reduction by specific GSK3 inhibition
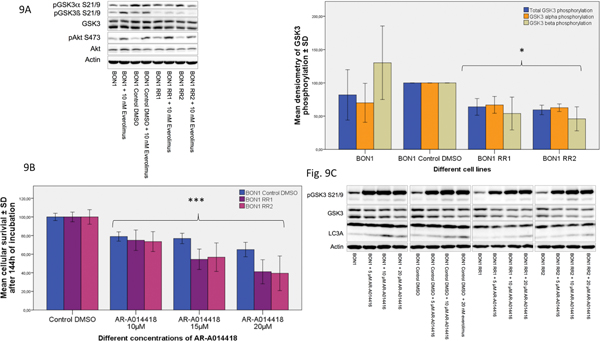
As GSK3 has been shown to be implicated in resistance to radio-, chemo- and molecular-targeted therapy (Martelli, et al. 2014; McCubrey et al. 2014; Shimura 2011), we examined long-term treatment effects of everolimus on GSK3 (Fig. 9A). GSK3 is inhibited by phosphorylation. BON1 RR1/RR2 cells displayed a pronounced baseline decrease in phosphorylated/inhibited GSK3, and thus GSK3 over-activation when compared to the sensitive control cell line (Fig. 9A). However, levels of activated and total Akt were similar in resistant and non-resistant cells with and without treatment (Fig. 9A). Akt was phosphorylated/activated in all resistant and sensitive cell lines after 24 h stimulation with everolimus (Fig. 9A).
Fig. 9.
GSK3 over-activation in the resistant cell lines (A) Both everolimus-resistant cell lines showed significantly enhanced activation of GSK3α/β (GSK3) compared to the sensitive control cell line (BON1 Control DMSO) and a significantly stronger activation of GSK3β compared to the parental BON1 cells, but stable phosphorylation/activation of AKT after 24 h of incubation with everolimus. A representative blot out of three independently performed experiments is shown. Statistically significantly different results (*) in comparison to BON1 Control DMSO are shown; (B) Single substance treatment of AR-A014418 (10 μM – 20 μM) significantly decreased cellular survival in all cell lines tested after 144 h of incubation, although much stronger in BON1 RR1 and BON1 RR2, compared to the sensitive control cell line. The arithmetic means and standard deviation of at least three independent experiments are shown. Statistically significant differences in comparison to either single substance treatment are shown, considering p<0,001 = ***; (C) Western blot analysis showed inhibition of GSK3 and an increase in the autophagy marker LC3A in response to the selective GSK3α/β inhibitor AR-A014418.
GSK3 inhibition by the specific GSK3 inhibitor AR-A014418 caused a pronounced decrease in BON1 RR1/RR2 cell viability and - to a much lesser extent - in sensitive control cells (Fig. 9B). In both resistant and in the sensitive control cell line, Western blot analyses showed clear inhibition of GSK3 and an increase in the autophagy marker LC3A in response to AR-A014418 treatment (Fig. 9C). However, combination treatment of everolimus plus AR-A014418 did not enhance the inhibitory effect of single AR-A014418 treatment on cell viability in resistant cells (Supplementary Fig. 2).
We also performed Western blots on the combination of everolimus plus AR-A014418 and, while single AR-A014418 treatment led to strong GSK3α inhibition in the resistant cell lines, combination treatment caused no stronger GSK3α inhibiton compared to single AR-A014418 treatment (Suppl. Fig. 2); this may help explain the absence of a synergistic effect.
H. Everolimus resistance: Lower baseline IRS-1 protein levels
Interestingly, baseline IRS-1 protein levels showed a decrease in BON1 RR1 and BON1 RR2 cells compared to parental BON1 and BON1 Control DMSO cells (Fig. 6).
For pIRS-1 the differences between the different cell lines were minor according to the Western blot quantification. Western blot quantifications for IRS-1 and pIRS-1 are shown in Supplementary Fig. 3. The effects of BYL719 and everolimus treatment on IRS-1 are described below.
Total mTORC1 showed no clear differences between resistant cells and non-resistant cells (Supplementary Fig. 4). No activated pmTORC1 could be detected in our cell lines.
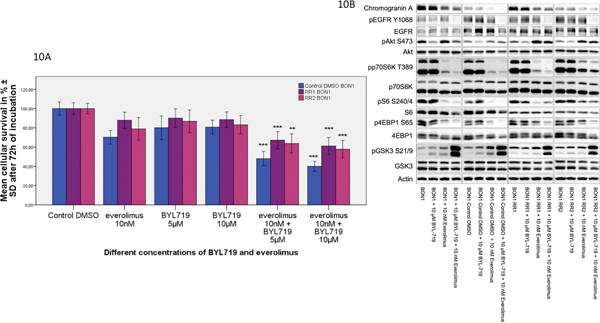
I. Dual-targeting approach with the PI3Kα inhibitor BYL719 overcame everolimus resistance and restored sensitivity through GSK3 inhibition, increased autophagy and decreased apoptosis
Combination treatment with the PI3Kα inhibitor BYL719 plus everolimus overcame everolimus resistance and restored treatment sensitivity (Fig. 10A). A significantly stronger decrease in cellular survival after combination treatment compared to single substance treatment could be observed in both resistant cell lines (Fig. 10A). We moreover studied additional dose response relationships combining low doses of 1–10 nM everolimus with 1–5 μM BYL719 and found significant synergistic effects at almost all drug doses tested in all cell lines, including both resistant cell lines (*p< 0.05) (Supplementary Figures 5, 6, 7, 8). This synergistic inhibitory effect on cell survival after the BYL719/everolimus combination treatment was associated with inhibition of GSK3, EGFR, p70S6K and 4EBP1 signaling, as well as showing an increase in IRS-1 protein levels in BON1 RR1/RR2 cells (Fig. 6, Fig. 10B). In both sensitive cell lines, everolimus alone already led to strong GSK3 phosphorylation/inhibition. In BON1 RR1/RR2 cells, single application of everolimus led to lower or no phosphorylation/inhibition of GSK3, but the combination treatment induced stronger inhibition of GSK3, compared to each drug separately. A different response of IRS-1 protein levels to everolimus and BYL719 treatment between resistant and non-resistant cell lines was observed: while sensitive control cells showed a slight increase in IRS-1 protein levels after everolimus treatment, the resistant cells showed a lesser or no IRS-1 increase (Supplementary Fig. 3A, Fig. 6). In the non-resistant cells BYL719 treatment alone was accompanied by increased IRS-1 protein expression, while in the resistant cells a lesser, but nevertheless clear, increase in IRS-1 protein levels was observed in response to BYL719 treatment (Supplementary Fig. 3A, Fig. 6).
Fig. 10.
Dual-targeting approach with the PI3Kα inhibitor BYL719 overcame acquired everolimus resistance and restored sensitivity through inhibition of GSK3, EGFR, p70S6K and 4EBP1 signaling. (A) Combination treatment of everolimus plus BYL719 showed a significantly enhanced inhibitory effect on cell viability compared to single substance treatment in all tested cell lines. Cells were incubated with BYL719 (5 μM and 10 μM) and everolimus (10 nM) alone and in combination for 72 h. The arithmetic means and standard deviation of at least three independent experiments are shown. Statistically significantly different results in comparison to either single substance treatment are shown, considering p<0,05 = *; p<0,01 = **; p<0,001 = ***. (B) Protein levels of components from the EGFR-Akt (pEGFR, EGFR, pAkt, Akt), mTORC1-p70S6K (pp70S6, p70S6K, pS6, S6, p4EBP, 4EBP1) and GSK3 (pGSK3, GSK3) signaling pathway are displayed. Inhibition of GSK3, EGFR, p70S6K and 4EBP1 signaling was observed after combination treatment. A representative blot out of three independently performed experiments is shown.
The BYL719/everolimus combination treatment showed a strong increase in IRS-1 expression in the non-resistant BON1 and BON1 Control DMSO cells, and a lesser, but still clear, increase in IRS-1 protein levels in the resistant cells (Supplementary Fig. 3A, Fig. 6).
We studied the differences concerning cell death mechanisms between resistant and non-resistant cell lines in response to BYL719/everolimus treatment: We found that in BON1 RR1/RR2 cells BYL719/everolimus combination treatment was accompanied by an increase in the autophagy marker LC3A, compared to each drug separately, indicating an increase in autophagy in response to the BYL719/everolimus combination treatment (Fig. 6).
In the sensitive control cells, the Caspase 3/7 assay showed apoptosis-induction at 24 h with BYL719 treatment, but not in BON1 RR1/RR2; everolimus treatment had no influence on apoptosis induction, while the everolimus/BYL719 combination treatment significantly decreased apoptosis in BON1 RR1/RR2 cells compared to each drug separately, but had no significant influence on apoptosis induction in the sensitive control cells (Supplementary Fig. 9). This was consistent with a decrease in cleaved Caspase 3 in the resistant cell lines after BYL719/everolimus combination treatment, compared to treatment with each drug separately.
J. Transferability of the resistant cell line model to an orthotopic intrapancreatic xenograft mouse model
In an in vivo pilot study we were able to transfer our resistant cell line model to an orthotopic xenograft SCID mouse model with intrapancreatic tumor growth (n=4). Supplementary Figures 10A-C show an example of the growth of an intrapancreatic BON1 RR2 tumor in a SCID mouse: 14 days after tumor cell injection into the pancreas a detectable tumor developed and reached a size of ~1000 mm3 around 4 weeks after first detection by MRI (Suppl. Fig. 10A-C). Tumor size and tissue morphology was determined by T2w imaging. The resistant tumors showed a similar growth rate and tumor morphology as the native BON1 tumors. When the tumors grew bigger, necrotic areas in the tumor core increased (Suppl. Fig. 10A-C) towards the end of the experiment. The glucose uptake (standard uptake value (SUV)) calculated from 18FDG-PET confirmed that the tumors were metabolically active (Suppl. Fig. 10E). As expected, 68Ga DOTATOC PET scanning showed no uptake in the resistant tumors, confirming the absence of somatostatin receptor-2 (Suppl. Fig. 10F).
4. Discussion
A. Stable resistance and morphologic changes
In our BON1 in vitro model, permanent long-term treatment with a constant low dose of 10 nM everolimus over 24 weeks caused stable resistance to everolimus. In contrast to the study of Vandamme et al., who found a complete reversal of resistance after a drug holiday of 10–12 weeks (Vandamme et al. 2016), we demonstrated stable persistent resistance after a drug holiday of 13 weeks.
Everolimus-resistant BON1 cells have previously been described as developing an elongated shape with fewer cell-to-cell contacts (Passacantilli et al. 2014), or to cause no morphologic changes (Vandamme et al. 2016). We, however, found an amorphous, flattened cellular shape with lysosome-like bubbles and clumping conglomerates as a morphologic feature of everolimus-resistant BON1 cells. The differences in stability of resistance and morphologic appearance between the studies may be explained by experimental design differences: Passacantilli et al. treated panNETs cells permanently only for 8 weeks with 10 nM everolimus and did not confirm stable resistance (Passacantilli et al. 2014); Vandamme et al. followed a pulsed-selection experimental design (McDermott et al. 2014) by permanently augmenting everolimus doses up to 1 μM, which might be less clinically relevant due to the application of high drug doses out of the clinically relevant range (around 8 nM - 59 nM) (Budde et al. 2016; Vandamme et al. 2016).
Since BON1 RR1 and BON1 RR2 have both been developed on the same genetic background and BON1 cells have a higher proliferative index than the typical well differentiated neuroendocrine tumor, we aimed to confirm our model in the novel slow growing human pancreatic NET cell line NT-3 (Benten, et al. 2018). Unfortunately, due to the slow growing nature of NT-3 cells, these experiments are still ongoing now for more than 12 months. The initial results showed a gradual increase in proliferation over time in the presence of doses up to four times of the IC50 value (4nM) of the parental cell line, and we observed similar changes in morphology as in resistant BON1 cells with a switch to a flattened and more pitted appearance under continuous everolimus exposure (unpublished results).
B. Heterogeneity of BON1 RR1 and BON1 RR2
Tumor heterogeneity has been suggested as a possible mechanism of everolimus resistance (Vandamme et al. 2016) and could be the driving force of sub-clone selection of the most resistant phenotype in response to treatment (Marusyk, et al. 2014; Vandamme et al. 2016). Our data are compatible with this theory since both resistant cell lines established in parallel do not show a completely identical phenotype with slightly different effects of everolimus on p70SK phosphorylation in both cell lines. The novelty of our work lies in its ability to elicit common features of both independently established everolimus-resistant cell lines in order to reduce the bias due to tumor heterogeneity.
C. Everolimus resistance: G1 phase cell cycle arrest, reduced baseline autophagy and increased baseline apoptosis
Consistent with an increase in G1 phase cell cycle arrest and sub-G1 population in the resistant cell lines in response to everolimus we observed a baseline decrease in CDK1(cdc2) expression. CDK1(cdc2) is a key regulator of the mitotic transition (Nurse 1990) and its inactivation causes exit from mitosis and stabilises G1 phase (Potapova, et al. 2009). The G1 phase cell cycle arrest/sub-G1 increase in the resistant cell lines might be associated with the observed decrease in baseline autophagy. Inhibition of autophagy has shown to be linked to a G1 phase cell cycle arrest, sub-G1 increase, and apoptosis induction in different cancer entities (Almasi, et al. 2018; Choi, et al. 2013; Liu, et al. 2014). Nevertheless, the decrease in autophagy seems unexpected in the context of resistance since mTORC1 inhibitor-induced autophagy has previously been described as a potential mechanism of promoting tumor cell resistance: inhibition of autophagy increased everolimus efficacy in NET cells (Avniel et al. 2018; Avniel-Polak et al. 2016). The decrease in the autophagy marker LC3A observed in the resistant cell lines might be a mechanism of adaption to the permanent induction of autophagy in the presence of the mTORC1 inhibitor everolimus. This might indicate a sensitive balance of the dual effects of autophagy as a protective mechanism for the cancer cell on the one hand and as a mediator of cell death on the other (Sui et al. 2013).
Since apoptosis negatively correlates with autophagy (Avniel-Polak et al. 2016; Liao, et al. 2012; Mejias-Pena, et al. 2017; Sui et al. 2013) the decrease in autophagy in the resistant cell lines might cause the observed increase in apoptosis. All these effects might be due to GSK3 over-activation. GSK3 over-activation, which is discussed below in detail, has previously been reported to be directly associated with G1 cell cycle arrest, decreased autophagy and increased apoptosis (Aristizabal Prada et al. 2017; Giancotti 2014; Li, et al. 2011; Maurer et al. 2014; Weikel, et al. 2016; Zhang, et al. 2016) and to be a crucial feature of cancer cell resistance to chemotherapy, radiotherapy and targeted therapies (Domoto, et al. 2016; McCubrey, et al. 2016).
D. Everolimus resistance increased migration potential and c-Met activation
BON1 RR1 and BON1 RR2 cells displayed significantly increased migration potential in response to everolimus treatment and baseline c-Met over-activation. Temsirolimus-(mTORC1 inhibitor-)resistant renal cell carcinoma cells also showed enhanced migration potential (Juengel, et al. 2014). Mis-regulation, overexpression or gene amplification of the receptor tyrosine kinase c-Met have been identified to play a crucial role in tumor invasion, motility and metastasis formation associated with poor prognosis (Camp, et al. 1999; Chen, et al. 1999; Di Renzo, et al. 1992; Ma, et al. 2003; Song, et al. 2010; Takeo, et al. 2001). However, in previous studies we demonstrated that specific c-Met inhibition by either knock-down experiments with siRNA or the specific c-Met inhibitor INC280 could neither decrease cellular viability nor cellular migration in NET cells in vitro (Reuther et al. 2016). C-Met activation might be rather indirectly associated with cellular migration potential in NET cells in vitro through activation of alternative pathways, such as the PI3K-Akt-mTORC1 pathway (Engelman, et al. 2007).
E. Everolimus resistance: Baseline GSK3 over-activation and decrease in IRS-1 protein levels
A feedback activation of receptor tyrosine kinases such as the insulin receptor due to mTORC1 inhibition has previously been reported (Chandarlapaty, et al. 2011). The feedback activation of PI3K-Akt signaling by the mTORC1-inhibitor everolimus via disinhibition/activation of IRS-1 has been discussed as a possible cause of resistance in NETs (Fig. 1, red arrows) (O’Reilly et al. 2006; Vandamme et al. 2016; Zitzmann et al. 2010). We have indeed found that pro-survival signaling of the PI3K-Akt pathway through Akt phosphorylation/activation upon everolimus re-application persisted in BON1 RR1 and BON1 RR2, as described previously in long-term everolimus-treated panNET cells (Passacantilli et al. 2014). Therefore, we speculate that there was permanent PI3K/Akt activation in the presence of persisting everolimus treatment during the development of resistance. Constitutively active PI3K has previously been reported to induce a reduction in IRS-1/2 protein levels (Pirola, et al. 2003; Wada 2009). Therefore, IRS-1 downregulation observed in the resistant cell lines might be a compensatory escape mechanism in response to chronic PI3K/Akt over-activation, similar to the mechanism of development of insulin resistance (Pirola et al. 2003). Different IRS-1-induced signaling pathways have previously been reported to inhibit GSK3 (Cohen and Frame 2001; Maurer et al. 2014; Vandamme et al. 2016; Wada 2009). Therefore, lower basal IRS-1 protein levels in the resistant cells may lead to lower GSK3 inhibition and thus stronger GSK3 activation. Since basal Akt activity or expression was not changed in the resistant cells, compared to the sensitive controls, it is unlikely that GSK3 is over-activated through modulation of the Akt pathway, but more likely through an alternative pathway. These mechanisms need to be investigated in future studies in NETs.
Various studies have demonstrated an association between IRS-1/GSK3α/β deregulation and tumorigenesis (McCubrey et al. 2016; Wada 2009). GSK3 over-activation may promote the development of everolimus resistance, as recently reviewed for GSK3 in the context of resistance to chemotherapy, radiotherapy and targeted therapies (Domoto et al. 2016; McCubrey et al. 2016). Similar effects were observed in transfected MCF-7 breast cancer cells, where a constitutively-active GSK3 construct showed resistance to the mTORC1 inhibitor rapamycin (McCubrey et al. 2014).
In order to prove the importance of constitutively-active GSK3 in everolimus-resistant panNET cells, we treated BON1 RR1/RR2 cells with the selective GSK3 inhibitor AR-A014418 and found a striking decrease in cellular viability in BON1 RR1/RR2, and to a lesser extent in sensitive control cells, associated with an increase in the autophagy marker LC3A. However, unexpectedly, combination treatment of AR-A014418 plus everolimus did not enhance the inhibitory effects of single AR-A014418 treatment. The absence of synergism in the AR-A014418/everolimus combination treatment may be explicable by GSK3α inhibition being similar in response to the combination treatment compared to single treatment with AR-A014418 in the resistant cell lines.
F. Dual-targeting approach with the PI3Kα inhibitor BYL719 overcame acquired everolimus resistance
In a previous study on different NET cell lines, the selective PI3Kα inhibitor BYL719 showed synergistic anti-proliferative effects in combination with everolimus through strong GSK3 inhibition (Nölting, et al. 2017). In contrast to AR-A014418/everolimus combination treatment, the combination of everolimus with BYL719 was synergistic at low therapeutically-relevant doses in both resistant cell lines and restored everolimus sensitivity through more potent GSK3 inhibition, compared to each drug separately; this was associated with IRS-1 up-regulation, increased autophagy associated with decreased apoptosis, and an inhibition of EGFR, 4EBP1 and p70S6K. This is consistent with our previous study showing that selective GSK3 inhibition led to inhibition of EGFR, p70S6K and 4EBP1 and enhanced the autophagy marker LC3A in BON1 cells (Aristizabal Prada et al. 2017). It has previously been reported that inhibition of GSK3 restored autophagy (Aristizabal Prada et al. 2017; Weikel et al. 2016; Zhang et al. 2016) and re-sensitised different tumor entities to chemotherapy, radiotherapy or targeted therapy (Domoto et al. 2016).
Similar combination approaches using PI3K inhibitors (BEZ235 or BKM120), although without taking GSK3 signaling into account, have previously been shown to overcome reversible everolimus resistance (Passacantilli et al. 2014; Vandamme et al. 2016). BYL719 is currently being investigated in a late phase 3 clinical study (SOLAR-I) for breast cancer (NCT02437318) and will probably enter clinical use. Moreover, a phase 1b clinical study is currently evaluating the safety and efficacy of BYL719 plus everolimus in advanced breast cancer, renal cell cancer and pancreatic tumors including panNETs (NCT02077933). Therefore, we provide novel data on the potentially clinically relevant PI3Kα inhibitor BYL719. GSK3 inhibition by BYL719 might be considered as second-line treatment in everolimus-resistant panNETs if indeed permanent GSK3 over-activation, potentially in combination with decreased IRS-1 protein levels, can be demonstrated as a central mechanism of resistance in vivo.
5. Conclusions and Perspectives
In this study, we established two stable everolimus-resistant panNET (BON1) cell lines. The mechanisms of resistance may include G1 cell cycle arrest, decreased CDK1/cdc2 expression, reduced autophagy, increased apoptosis and migration potential potentially due to permanent GSK3 over-activation compatible with downregulation of IRS-1 protein levels. The dual-targeting approach with the PI3Kα inhibitor BYL719 restored treatment sensitivity through GSK3 inhibition, IRS-1 up-regulation and increased autophagy in the resistant cells. In a pilot study, we have already transferred our resistant cell line model to an orthotopic intrapancreatic tumor xenograft mouse model, and this may represent a valid basis to validate our in vitro findings of this first stable everolimus-resistant cell line model and translate it into clinical practice.
Supplementary Material
Supplementary Fig. 1 Heterogeneity of the two independent resistant panNET cell lines and neuroendocrine identity. Distinct protein levels of activated/phosphorylated p70S6K were seen in BON1 RR1 and BON1 RR2 cells after 24 h of incubation with everolimus. In BON1 RR2 a slight phosphorylation/activation of p70S6K persisted after everolimus treatment. Western blot analysis of CgA showed strong CgA expression in BON1, BON1 Control DMSO, BON1 RR1 and BON1 RR2 cells as a sign of neuroendocrine identity. A representative blot out of at least three independently performed experiments is shown.
Supplementary Fig. 2 Combination treatment of everolimus plus AR-A014418 showed no significantly higher efficacy, when compared to the respective single substance treatments. The arithmetic means and standard deviation of at least three independent experiments are shown. Western blots on the combination of everolimus plus AR-A014418 showed no stronger inhibiton of GSK3α after combination treatment, compared to single AR-A014418 treatment, in the resistant cell lines.
Supplementary Fig. 3 Western blot quantification of IRS-1 protein levels and pIRS-1 of four independently performed Western blots shows down-regulation of IRS-1 protein levels in the resistant cell lines. Sensitive BON1 and BON1 Control DMSO cells showed a slightly stronger increase in IRS-1 protein levels after everolimus treatment, compared to the resistant cells according to the Western blot quantification. In addition, in the non-resistant cells BYL719 treatment was accompanied by increased IRS-1 protein expression, while in the resistant cells a lesser, but nevertheless a clear increase in IRS-1 protein levels was observed in response to BYL719 treatment. The BYL719/everolimus combination treatment showed a strong increase of IRS-1 expression in the non-resistant BON1 and BON1 Control DMSO cells, and a lesser, but still clear increase in IRS-1 protein levels in the resistant cells.
For pIRS-1 levels the differences between the resistant and sensitive cell lines were minor.
Suppl. Fig. 4 Western blot analysis showed similar mTORC1 expression in the resistant and sensitive cell lines, while pmTORC1 could not be detected in the cell lines investigated.
Suppl. Fig. 5, 6, 7, 8 Significant synergistic effects of BYL719 plus everolimus at low therapeutically-relevant doses in BON1 (Suppl. Fig. 5), BON1 Control DMSO (Suppl. Fig. 6), BON1 RR1 (Suppl. Fig. 7) and BON1 RR2 (Suppl. Fig. 8) cell lines. Matrix of the cell line proliferation together with the mean is shown. Each graph shows the vehicle-treated control (grey), BYL719 (green), everolimus (red) and the combination of both (blue). In the columns, the Byl719 concentration, in the rows, the everolimus concentration is increasing. The symbol * indicates synergism, assessed by the linear mixed effects model.
Suppl. Fig. 9 Caspase 3/7 assay in all cell lines after BYL719/everolimus combination treatment: Caspase 3/7 assay showed a significant decrease in apoptosis in response to BYL719/everolimus combination treatment in the resistant cell lines, but not in the sensitive cell lines.
Suppl. Fig. 10 Imaging of the orthotopic intrapancreatic everolimus-resistant tumor xenograft mouse model by preclinical PET/MRI: BON1 RR2 cells were inoculated into the pancreas of a 12 week old SCID mouse: axial T2w (A-C) and coronal T1w (D) images confirm tumor growth (arrow). The tumor was first detected in the pancreas 14 days after inoculation (A) and monitored during growth after 28 days (B) and 48 days (C). On day 48 the tumor reached 1000 mm3 and showed normal development of necrotic areas in the tumor core (hypointense areas). Fused 18FDG –PET/MRI (E) confirms high FDG uptake (SUV: .4 %ID/g) reflecting a strong metabolic activity and Ga68DOTATOC-PET/MRI in coronal view (F) shows no Ga68DOTATOC uptake due to absent SSTR2.
8. Acknowledgement
We thank Jakob Albrecht (Department of Nuclear Medicine, Charité Berlin) for performing the implantation of Everolimus-resistant BON cells into SCID mice and Berlin Experimental Radionuclide Imaging Center (BERIC), Charité - Universitätsmedizin Berlin, Germany for in vivo experiments.
7. Funding
ET Aristizabal Prada has received a scholarship from FAZIT-Stiftung (http://www.fazit-stiftung.de). This work was supported by a Young Investigator Research Grant (YING) to SN, project number RAFI 029, from Novartis Pharma GmbH, Nürnberg, Germany. This research was supported, in part, by the Intramural Research Program of the NIH, NICHD.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work. CJA and ABG have received research contracts (Ipsen, Novartis), lecture honoraria (Ipsen, Novartis) and advisory board honoraria (Novartis). JS has received research contracts (Novartis) and lecture honoraria (Novartis, Ipsen). SN has received a research contract (Novartis) and lecture honoraria (Ipsen).
9. References
- Almasi S, Kennedy BE, El-Aghil M, Sterea AM, Gujar S, Partida-Sanchez S & El Hiani Y 2018. TRPM2 channel-mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway. J Biol Chem 293 3637–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aristizabal Prada ET & Auernhammer CJ 2018. Targeted therapy of gastroenteropancreatic neuroendocrine tumours: preclinical strategies and future targets. Endocr Connect 7 R1–R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aristizabal Prada ET, Weis C, Orth M, Lauseker M, Spoettl G, Maurer J, Grabowski P, Grossman A, Auernhammer CJ & Nölting S 2017. GSK3alpha/beta: A Novel Therapeutic Target for Neuroendocrine Tumors? Neuroendocrinology. [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ, Spitzweg C, Angele MK, Boeck S, Grossman A, Nölting S, Ilhan H, Knosel T, Mayerle J, Reincke M, et al. 2017. Advanced neuroendocrine tumours of the small intestine and pancreas: clinical developments, controversies, and future strategies. Lancet Diabetes Endocrinol. [DOI] [PubMed] [Google Scholar]
- Avniel S, Leibowitz G, Doviner V, Gross DJ & Grozinsky-Glasberg S 2018. Combining chloroquine with RAD001 inhibits tumor growth in a NEN mouse model. Endocr Relat Cancer. [DOI] [PubMed] [Google Scholar]
- Avniel-Polak S, Leibowitz G, Riahi Y, Glaser B, Gross DJ & Grozinsky-Glasberg S 2016. Abrogation of Autophagy by Chloroquine Alone or in Combination with mTOR Inhibitors Induces Apoptosis in Neuroendocrine Tumor Cells. Neuroendocrinology 103 724–737. [DOI] [PubMed] [Google Scholar]
- Benten D, Behrang Y, Unrau L, Weissmann V, Wolters-Eisfeld G, Burdak-Rothkamm S, Stahl FR, Anlauf M, Grabowski P, Mobs M, et al. 2018. Establishment of the First Well-differentiated Human Pancreatic Neuroendocrine Tumor Model. Mol Cancer Res 16 496–507. [DOI] [PubMed] [Google Scholar]
- Briest F & Grabowski P 2014. PI3K-AKT-mTOR-signaling and beyond: the complex network in gastroenteropancreatic neuroendocrine neoplasms. Theranostics 4 336–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde K, Zonnenberg BA, Frost M, Cheung W, Urva S, Brechenmacher T, Stein K, Chen D, Kingswood JC & Bissler JJ 2016. Pharmacokinetics and pharmacodynamics of everolimus in patients with renal angiomyolipoma and tuberous sclerosis complex or lymphangioleiomyomatosis. Br J Clin Pharmacol 81 958–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp RL, Rimm EB & Rimm DL 1999. Met expression is associated with poor outcome in patients with axillary lymph node negative breast carcinoma. Cancer 86 2259–2265. [DOI] [PubMed] [Google Scholar]
- Capozzi M, Caterina I, De Divitiis C, von Arx C, Maiolino P, Tatangelo F, Cavalcanti E, Di Girolamo E, Iaffaioli RV, Scala S, et al. 2015. Everolimus and pancreatic neuroendocrine tumors (PNETs): Activity, resistance and how to overcome it. Int J Surg 21 Suppl 1 S89–94. [DOI] [PubMed] [Google Scholar]
- Capurso G, Fendrich V, Rinzivillo M, Panzuto F, Bartsch DK & Delle Fave G 2012. Novel molecular targets for the treatment of gastroenteropancreatic endocrine tumors: answers and unsolved problems. Int J Mol Sci 14 30–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DL, Segelov E & Singh S 2017. Everolimus in the management of metastatic neuroendocrine tumours. Therap Adv Gastroenterol 10 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J & Rosen N 2011. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BK, Ohtsuki Y, Furihata M, Takeuchi T, Iwata J, Liang SB & Sonobe H 1999. Overexpression of c-Met protein in human thyroid tumors correlated with lymph node metastasis and clinicopathologic stage. Pathol Res Pract 195 427–433. [DOI] [PubMed] [Google Scholar]
- Choi HS, Jeong EH, Lee TG, Kim SY, Kim HR & Kim CH 2013. Autophagy Inhibition with Monensin Enhances Cell Cycle Arrest and Apoptosis Induced by mTOR or Epidermal Growth Factor Receptor Inhibitors in Lung Cancer Cells. Tuberc Respir Dis (Seoul) 75 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P & Frame S 2001. The renaissance of GSK3. Nat Rev Mol Cell Biol 2 769–776. [DOI] [PubMed] [Google Scholar]
- De Buck SS, Jakab A, Boehm M, Bootle D, Juric D, Quadt C & Goggin TK 2014. Population pharmacokinetics and pharmacodynamics of BYL719, a phosphoinositide 3-kinase antagonist, in adult patients with advanced solid malignancies. Br J Clin Pharmacol 78 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo MF, Olivero M, Ferro S, Prat M, Bongarzone I, Pilotti S, Belfiore A, Costantino A, Vigneri R, Pierotti MA, et al. 1992. Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene 7 2549–2553. [PubMed] [Google Scholar]
- Domoto T, Pyko IV, Furuta T, Miyashita K, Uehara M, Shimasaki T, Nakada M & Minamoto T 2016. Glycogen synthase kinase-3beta is a pivotal mediator of cancer invasion and resistance to therapy. Cancer Sci 107 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeblad S, Skogseid B, Dunder K, Oberg K & Eriksson B 2008. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res 14 7798–7803. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. 2007. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316 1039–1043. [DOI] [PubMed] [Google Scholar]
- Evers BM, Townsend CM Jr., Upp JR, Allen E, Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC & Thompson JC 1991. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology 101 303–311. [DOI] [PubMed] [Google Scholar]
- Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, Kwekkeboom D, Lau WY, Klersy C, Vilgrain V, et al. 2014. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 15 e8–21. [DOI] [PubMed] [Google Scholar]
- Giancotti FG 2014. Deregulation of cell signaling in cancer. FEBS Lett 588 2558–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt DY, Ndiaye M, Chen H & Kunnimalaiyaan M 2010. Lithium inhibits carcinoid cell growth in vitro. Am J Transl Res 2 248–253. [PMC free article] [PubMed] [Google Scholar]
- Grozinsky-Glasberg S, Shimon I & Rubinfeld H 2012. The role of cell lines in the study of neuroendocrine tumors. Neuroendocrinology 96 173–187. [DOI] [PubMed] [Google Scholar]
- Halfdanarson TR, Rabe KG, Rubin J & Petersen GM 2008. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 19 1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Chen D, Ruan H, Li X, Tong J, Xu X, Zhang L & Yu J 2016. BRAFV600E-dependent Mcl-1 stabilization leads to everolimus resistance in colon cancer cells. Oncotarget 7 47699–47710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengel E, Makarevic J, Reiter M, Mani J, Tsaur I, Bartsch G, Haferkamp A & Blaheta RA 2014. Resistance to the mTOR inhibitor temsirolimus alters adhesion and migration behavior of renal cell carcinoma cells through an integrin alpha5- and integrin beta3-dependent mechanism. Neoplasia 16 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kong D, Bao B, Ahmad A & Sarkar FH 2011. Induction of cancer cell death by isoflavone: the role of multiple signaling pathways. Nutrients 3 877–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J & Tabas I 2012. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab 15 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Shi X, Zhou X, Wang D, Wang L & Li C 2014. Effect of autophagy inhibition on cell viability and cell cycle progression in MDAMB231 human breast cancer cells. Mol Med Rep 10 625–630. [DOI] [PubMed] [Google Scholar]
- Lubner SJ, Kunnimalaiyaan M, Holen KD, Ning L, Ndiaye M, Loconte NK, Mulkerin DL, Schelman WR & Chen H 2011. A preclinical and clinical study of lithium in low-grade neuroendocrine tumors. Oncologist 16 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma PC, Kijima T, Maulik G, Fox EA, Sattler M, Griffin JD, Johnson BE & Salgia R 2003. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res 63 6272–6281. [PubMed] [Google Scholar]
- Martelli AM, Buontempo F & Evangelisti C 2014. GSK-3beta: a key regulator of breast cancer drug resistance. Cell Cycle 13 697–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F & Polyak K 2014. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature 514 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Preiss F, Brauns-Schubert P, Schlicher L & Charvet C 2014. GSK-3 - at the crossroads of cell death and survival. J Cell Sci 127 1369–1378. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Rakus D, Gizak A, Steelman LS, Abrams SL, Lertpiriyapong K, Fitzgerald TL, Yang LV, Montalto G, Cervello M, et al. 2016. Effects of mutations in Wnt/beta-catenin, hedgehog, Notch and PI3K pathways on GSK-3 activity-Diverse effects on cell growth, metabolism and cancer. Biochim Biophys Acta 1863 2942–2976. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, Montalto G, D’Assoro AB, Libra M, Nicoletti F, et al. 2014. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 5 2881–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M, Eustace AJ, Busschots S, Breen L, Crown J, Clynes M, O’Donovan N & Stordal B 2014. In vitro Development of Chemotherapy and Targeted Therapy Drug-Resistant Cancer Cell Lines: A Practical Guide with Case Studies. Front Oncol 4 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias-Pena Y, Estebanez B, Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, de Paz JA, Gonzalez-Gallego J & Cuevas MJ 2017. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging (Albany NY) 9 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, Burk U, Onichtchouk D, Dohrmann C & Austen M 2007. Inhibition of GSK3 promotes replication and survival of pancreatic beta cells. J Biol Chem 282 12030–12037. [DOI] [PubMed] [Google Scholar]
- Nölting S, Rentsch J, Freitag H, Detjen K, Briest F, Mobs M, Weissmann V, Siegmund B, Auernhammer CJ, Aristizabal Prada ET, et al. 2017. The selective PI3Kalpha inhibitor BYL719 as a novel therapeutic option for neuroendocrine tumors: Results from multiple cell line models. PLoS One 12 e0182852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P 1990. Universal control mechanism regulating onset of M-phase. Nature 344 503–508. [DOI] [PubMed] [Google Scholar]
- O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. 2006. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Research 66 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passacantilli I, Capurso G, Archibugi L, Calabretta S, Caldarola S, Loreni F, Delle Fave G & Sette C 2014. Combined therapy with RAD001 e BEZ235 overcomes resistance of PET immortalized cell lines to mTOR inhibition. Oncotarget 5 5381–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavel ME, Hainsworth JD, Baudin E, Peeters M, Horsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, et al. 2011. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 378 2005–2012. [DOI] [PubMed] [Google Scholar]
- Pavel ME & Sers C 2016. WOMEN IN CANCER THEMATIC REVIEW: Systemic therapies in neuroendocrine tumors and novel approaches toward personalized medicine. Endocr Relat Cancer 23 T135–t154. [DOI] [PubMed] [Google Scholar]
- Pirola L, Bonnafous S, Johnston AM, Chaussade C, Portis F & Van Obberghen E 2003. Phosphoinositide 3-kinase-mediated reduction of insulin receptor substrate-1/2 protein expression via different mechanisms contributes to the insulin-induced desensitization of its signaling pathways in L6 muscle cells. J Biol Chem 278 15641–15651. [DOI] [PubMed] [Google Scholar]
- Porta C, Paglino C & Mosca A 2014. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol 4 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova TA, Daum JR, Byrd KS & Gorbsky GJ 2009. Fine tuning the cell cycle: activation of the Cdk1 inhibitory phosphorylation pathway during mitotic exit. Mol Biol Cell 20 1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther C, Heinzle V, Spampatti M, Vlotides G, de Toni E, Spottl G, Maurer J, Nolting S, Goke B & Auernhammer CJ 2016. Cabozantinib and Tivantinib, but Not INC280, Induce Antiproliferative and Antimigratory Effects in Human Neuroendocrine Tumor Cells in vitro: Evidence for ‘Off-Target’ Effects Not Mediated by c-Met Inhibition. Neuroendocrinology 103 383–401. [DOI] [PubMed] [Google Scholar]
- Riccardi C & Nicoletti I 2006. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc 1 1458–1461. [DOI] [PubMed] [Google Scholar]
- Shimura T 2011. Acquired radioresistance of cancer and the AKT/GSK3beta/cyclin D1 overexpression cycle. J Radiat Res 52 539–544. [DOI] [PubMed] [Google Scholar]
- Shipkova M, Hesselink DA, Holt DW, Billaud EM, van Gelder T, Kunicki PK, Brunet M, Budde K, Barten MJ, De Simone P, et al. 2016. Therapeutic Drug Monitoring of Everolimus: A Consensus Report. Ther Drug Monit 38 143–169. [DOI] [PubMed] [Google Scholar]
- Soares KC, Foley K, Olino K, Leubner A, Mayo SC, Jain A, Jaffee E, Schulick RD, Yoshimura K, Edil B, et al. 2014. A preclinical murine model of hepatic metastases. J Vis Exp 51677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Li M, Tretiakova M, Salgia R, Cagle PT & Husain AN 2010. Expression patterns of PAX5, c-Met, and paxillin in neuroendocrine tumors of the lung. Arch Pathol Lab Med 134 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, et al. 2013. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4 e838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S, Arai H, Kusano N, Harada T, Furuya T, Kawauchi S, Oga A, Hirano T, Yoshida T, Okita K, et al. 2001. Examination of oncogene amplification by genomic DNA microarray in hepatocellular carcinomas: comparison with comparative genomic hybridization analysis. Cancer Genet Cytogenet 130 127–132. [DOI] [PubMed] [Google Scholar]
- Vandamme T, Beyens M, de Beeck KO, Dogan F, van Koetsveld PM, Pauwels P, Mortier G, Vangestel C, de Herder W, Van Camp G, et al. 2016. Long-term acquired everolimus resistance in pancreatic neuroendocrine tumours can be overcome with novel PI3K-AKT-mTOR inhibitors. Br J Cancer 114 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A 2009. GSK-3 inhibitors and insulin receptor signaling in health, disease, and therapeutics. Front Biosci (Landmark Ed) 14 1558–1570. [DOI] [PubMed] [Google Scholar]
- Wagle N, Grabiner BC, Van Allen EM, Amin-Mansour A, Taylor-Weiner A, Rosenberg M, Gray N, Barletta JA, Guo Y, Swanson SJ, et al. 2014. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med 371 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikel KA, Cacicedo JM, Ruderman NB & Ido Y 2016. Knockdown of GSK3beta increases basal autophagy and AMPK signalling in nutrient-laden human aortic endothelial cells. Biosci Rep 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin EM 2013. PI3K/Akt/mTOR pathway inhibitors in the therapy of pancreatic neuroendocrine tumors. Cancer Lett 335 1–8. [DOI] [PubMed] [Google Scholar]
- Yao J, Wang JY, Liu Y, Wang B, Li YX, Zhang R, Wang LS & Liu L 2014. A randomized phase II study of everolimus for advanced pancreatic neuroendocrine tumors in Chinese patients. Med Oncol 31 251. [DOI] [PubMed] [Google Scholar]
- Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, et al. 2016a. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. 2008. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 26 3063–3072. [DOI] [PubMed] [Google Scholar]
- Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EG, et al. 2016b. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JC, Phan AT, Jehl V, Shah G & Meric-Bernstam F 2013. Everolimus in advanced pancreatic neuroendocrine tumors: the clinical experience. Cancer Res 73 1449–1453. [DOI] [PubMed] [Google Scholar]
- Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, et al. 2011. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatelli MC, Fanciulli G, Malandrino P, Ramundo V, Faggiano A, Colao A & Group N 2016. Predictive factors of response to mTOR inhibitors in neuroendocrine tumours. Endocr Relat Cancer 23 R173–183. [DOI] [PubMed] [Google Scholar]
- Zaytseva YY, Valentino JD, Gulhati P & Evers BM 2012. mTOR inhibitors in cancer therapy. Cancer Lett 319 1–7. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hou B, Yu S, Chen Q, Zhang N & Li H 2016. HGF alleviates high glucose-induced injury in podocytes by GSK3beta inhibition and autophagy restoration. Biochim Biophys Acta 1863 2690–2699. [DOI] [PubMed] [Google Scholar]
- Zitzmann K, Ruden J, Brand S, Goke B, Lichtl J, Spottl G & Auernhammer CJ 2010. Compensatory activation of Akt in response to mTOR and Raf inhibitors - a rationale for dual-targeted therapy approaches in neuroendocrine tumor disease. Cancer Letters 295 100–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Heterogeneity of the two independent resistant panNET cell lines and neuroendocrine identity. Distinct protein levels of activated/phosphorylated p70S6K were seen in BON1 RR1 and BON1 RR2 cells after 24 h of incubation with everolimus. In BON1 RR2 a slight phosphorylation/activation of p70S6K persisted after everolimus treatment. Western blot analysis of CgA showed strong CgA expression in BON1, BON1 Control DMSO, BON1 RR1 and BON1 RR2 cells as a sign of neuroendocrine identity. A representative blot out of at least three independently performed experiments is shown.
Supplementary Fig. 2 Combination treatment of everolimus plus AR-A014418 showed no significantly higher efficacy, when compared to the respective single substance treatments. The arithmetic means and standard deviation of at least three independent experiments are shown. Western blots on the combination of everolimus plus AR-A014418 showed no stronger inhibiton of GSK3α after combination treatment, compared to single AR-A014418 treatment, in the resistant cell lines.
Supplementary Fig. 3 Western blot quantification of IRS-1 protein levels and pIRS-1 of four independently performed Western blots shows down-regulation of IRS-1 protein levels in the resistant cell lines. Sensitive BON1 and BON1 Control DMSO cells showed a slightly stronger increase in IRS-1 protein levels after everolimus treatment, compared to the resistant cells according to the Western blot quantification. In addition, in the non-resistant cells BYL719 treatment was accompanied by increased IRS-1 protein expression, while in the resistant cells a lesser, but nevertheless a clear increase in IRS-1 protein levels was observed in response to BYL719 treatment. The BYL719/everolimus combination treatment showed a strong increase of IRS-1 expression in the non-resistant BON1 and BON1 Control DMSO cells, and a lesser, but still clear increase in IRS-1 protein levels in the resistant cells.
For pIRS-1 levels the differences between the resistant and sensitive cell lines were minor.
Suppl. Fig. 4 Western blot analysis showed similar mTORC1 expression in the resistant and sensitive cell lines, while pmTORC1 could not be detected in the cell lines investigated.
Suppl. Fig. 5, 6, 7, 8 Significant synergistic effects of BYL719 plus everolimus at low therapeutically-relevant doses in BON1 (Suppl. Fig. 5), BON1 Control DMSO (Suppl. Fig. 6), BON1 RR1 (Suppl. Fig. 7) and BON1 RR2 (Suppl. Fig. 8) cell lines. Matrix of the cell line proliferation together with the mean is shown. Each graph shows the vehicle-treated control (grey), BYL719 (green), everolimus (red) and the combination of both (blue). In the columns, the Byl719 concentration, in the rows, the everolimus concentration is increasing. The symbol * indicates synergism, assessed by the linear mixed effects model.
Suppl. Fig. 9 Caspase 3/7 assay in all cell lines after BYL719/everolimus combination treatment: Caspase 3/7 assay showed a significant decrease in apoptosis in response to BYL719/everolimus combination treatment in the resistant cell lines, but not in the sensitive cell lines.
Suppl. Fig. 10 Imaging of the orthotopic intrapancreatic everolimus-resistant tumor xenograft mouse model by preclinical PET/MRI: BON1 RR2 cells were inoculated into the pancreas of a 12 week old SCID mouse: axial T2w (A-C) and coronal T1w (D) images confirm tumor growth (arrow). The tumor was first detected in the pancreas 14 days after inoculation (A) and monitored during growth after 28 days (B) and 48 days (C). On day 48 the tumor reached 1000 mm3 and showed normal development of necrotic areas in the tumor core (hypointense areas). Fused 18FDG –PET/MRI (E) confirms high FDG uptake (SUV: .4 %ID/g) reflecting a strong metabolic activity and Ga68DOTATOC-PET/MRI in coronal view (F) shows no Ga68DOTATOC uptake due to absent SSTR2.