Abstract
Senescence is a tumor suppression mechanism that is induced by several stimuli, including oncogenic signaling and telomere shortening, and controlled by the p53/p21WAF1 signaling pathway. Recently, a critical role for secreted factors has emerged, suggesting that extracellular signals are necessary for the onset and maintenance of senescence. Conversely, factors secreted by senescent cells may promote tumor growth. By using expression profiling techniques, we searched for secreted factors that were overexpressed in fibroblasts undergoing replicative senescence. We identified WNT16B, a member of the WNT family of secreted proteins. We found that WNT16B is overexpressed in cells undergoing stress-induced premature senescence and oncogene-induced senescence in both MRC5 cell line and the in vivo murine model of KRasV12–induced senescence. By small interfering RNA experiments, we observed that both p53 and WNT16B are necessary for the onset of replicative senescence. WNT16B expression is required for the full transcriptional activation of p21WAF1. Moreover, WNT16B regulates activation of the phosphoinositide 3-kinase (PI3K)/AKT pathway. Overall, we identified WNT16B as a new marker of senescence that regulates p53 activity and the PI3K/AKT pathway and is necessary for the onset of replicative senescence. [Cancer Res 2009;69(24):9183–91]
Introduction
Replicative senescence is a tumor suppression mechanism characterized by an irreversible growth arrest (1). This phenomenon, first observed after extended culture in vitro by Leonard Hayflick and Paul Moorhead in 1961, is due to telomere shortening (2). When telomeres become too short, DNA damage sensor proteins are recruited, inducing the stabilization and activation of the p53 tumor suppressor protein and its effector p21WAF1 (CDKN1A), an inhibitor of cell cycle progression (3). Several other stress-inducing factors can initiate a similar process independently of telomere shortening. This process, termed stress-induced premature senescence (SIPS), can be induced by events such as DNA damage (4), oxidative stress (5), epigenetic modifications (6), or cytokine stimulation (7). Moreover, premature senescence can be initiated in response to oncogenic stress. For example, the so-called onco-gene-induced senescence (OIS) can occur in response to the activating G12V mutation in Ras proteins (8). Depending on the type of inducer, cellular senescence is initiated through two complementary tumor suppressor pathways, p53 or p16INK4A, both pathways leading to irreversible cell cycle arrest (9).
More recently, it has been established that secreted factors also contribute to the induction and maintenance of senescence. Plasminogen activator inhibitor-1 (PAI-1) is required to induce replicative senescence in fibroblasts in an autocrine fashion (10). Insulin-like growth factor binding protein (IGFBP)-5, IGFBP-7, interleukin (IL)-8, IL-6, and CXC chemokine receptor 2 were also described as having a role in senescence (7, 11–14). IL-8 and IL-6 are part of an inflammatory network induced by B-RAFV600E oncogene activation that stimulates the senescent cells in a cell-autonomous manner for the maintenance of senescence. In the absence of one of these factors, cells bypass senescence. Thus, activation of both intracellular and extracellular signals is necessary for the induction and maintenance of cellular senescence.
Although they are characterized by growth arrest, senescent cells remain metabolically active and acquire specific properties, such as resistance to apoptosis and altered gene expression (15). They express specific markers that allow for their identification both in vitro and in vivo. The senescence-associated β-galactosidase activity (SA-β-Gal) assay, based on the lysosomal β-galactosidase enzyme that is active at pH 6 in senescent cells, is generally accepted as a marker of senescence (16, 17).
As a tumor suppression mechanism, senescence is associated with the early stage of tumor development. Indeed, it was shown that senescent cells accumulate in premalignant lesions (18). Hence, development of tools to detect the accumulation of senescent cells in vivo may allow for the identification of neoplasia at an early stage. The specificity of SA-β-Gal staining for senescent cells is underscored by the requirement of invasive detection techniques. PAI-1, IL-6, and IL-8 are candidate biomarkers of senescence because they are soluble factors overexpressed by senescent cells. However, their expression is not specific to senescence and is related to inflammation or other stimuli.
In the present study, we identified WNT16B as a new secreted factor overexpressed in cells undergoing senescence using two models: fibroblastic cell lines and the in vivo OIS model of KRasV12–induced premalignant lesions. In fibroblasts, WNT16B inhibition prevented activation of the AKT pathway and abrogated p53 transcriptional activity to delay replicative senescence. Thus, WNT16B is a newly uncovered factor secreted by senescent cells in vitro and in vivo that regulates the onset of replicative senescence.
Materials and Methods
Cell culture and retroviral gene transfer.
MRC5 fibroblasts and Phoenix-ampho cells (Nolan Lab, Stanford University) were maintained in DMEM supplemented with 10% fetal bovine serum at 37°C. For retroviral gene transfer, the packaging cell line Phoenix-ampho was transfected with Lipofectamine reagent in combination with PLUS Reagent (Invitrogen) for 3 h according to the manufacturer’s instructions. The medium containing the viral particles was recovered, filtered through 0.45-μm low-binding protein filters (Pall Corp.), and used to infect MRC5 fibroblasts in the presence of hexadimethrine bromide (Sigma). MRC5-infected cells were selected with G418 (800 μg/mL; Invitrogen) or puromycin (1 μg/mL; Sigma) for 5 to 7 d. WNT16B was cloned from Human Placenta Marathon-Ready cDNA (Clon-tech) into the pBabe.puro vector. For gene-specific knockdown, WNT16B short hairpin RNA (shRNA), p53 shRNA (p53kd), and control shRNA were all cloned into pSuper.Retro.Puro (OligoEngine). The respective sequences have all been previously described (19, 20). The telomerase-immortalized MRC5-hTERT cell line was obtained by infection with pLXSN-hTERT, a vector featuring the human telomerase catalytic subunit, as previously described (21, 22).
Premature senescence and SA-β-Gal assays.
To induce premature senescence, MRC5 fibroblasts were either exposed to 200 mmol/L hydrogen peroxide (H2O2; Sigma), infected with the retroviral vector pBabe.puro.HRasV12 (8, 23), or irradiated with X-rays (25 Gy). SA-β-Gal activity was detected in fibroblasts and in K-RasV12–induced lung adenomas as previously described (16, 17).
Immunohistochemistry.
Analysis of the expression of WNT16B in mouse tumor samples was performed by immunohistochemical staining of lungs from activated 7- to 9-mo-old K-Ras+/G12Vgeo mice (18). Lungs were dissected, fixed in 10% buffered formalin (Sigma), and embedded in paraffin. Consecutive 3-μm-thick sections were stained with H&E and processed for immunohistochemical staining using rat monoclonal antibody against Ki-67 (clone TEC3; DakoCytomation), rabbit polyclonal against p16INK4A (M-156; Santa Cruz Biotechnology), rat monoclonal against p19ARF (5-C3-1; Santa Cruz Biotechnology), and rabbit polyclonal against WNT16B (LSA9630; MBL International Corp.).
Colony formation assay and cell counting.
For colony formation, MRC5 fibroblasts were infected with shRNA or cDNA constructs, exposed to G418 or puromycin for 1 wk, seeded at 5 × 103 cells per well onto six-well plates, and stained with methylene blue 3 to 4 wk after. Cell proliferation after shRNA infection was determined using Alamar Blue (Biosource). After exposure to the dye for 2 h, fluorescence was read at 590 nm and cell numbers were determined using a standard curve.
RNA isolation and reverse transcription-PCR analysis.
Cells were lysed with Trizol reagent (Invitrogen) and total RNA was isolated according to the manufacturer’s instructions. RNA integrity was assessed by spectrophotometry with A260/A280 ratios of >1.7. RNA (1–5 μg) was subjected to reverse transcription using SuperScript III First-Strand Synthesis SuperMix (Invitrogen). Obtained cDNA was subjected to either PCR (Taq polymerase from New England Biolabs) or real-time PCR using Power SYBR Green PCR Master Mix (Applied Biosystems) with the Mx3005P Real-Time PCR System (Stratagene). Gene expression was calculated following normalization to 18S levels by the comparative CT (cycle threshold) method. Primer sequences are provided in Supplementary Materials and Methods.
Immunoblotting.
Western blot analysis was performed as previously described (24). The following antibodies were used: anti-WNT16, anti-p53 (DO-1), and anti–α-tubulin from BD Pharmingen; anti-p21WAF1 from Calbiochem; anti-Rb, anti–phosphorylated extracellular signal-regulated kinase (ERK), anti–phosphorylated p53, and anti–phosphorylated AKT from Cell Signaling; anti–β-actin from Sigma; and anti-p16 from Santa Cruz Biotechnology.
Trichloroacetic acid protein precipitation.
The same numbers of cells from each condition were cultivated in DMEM serum-free medium for 24 h. Culture medium was filtered through 0.45-μm low-binding protein filters. Proteins were precipitated by addition of 1 volume of trichloroacetic acid (TCA; Sigma) to 4 volumes of culture medium. Protein pellets were washed twice with cold acetone and then resuspended in SDS-PAGE loading buffer. Proteins were analyzed by Western blot.
RNA-mediated interference.
Stealth RNA-mediated interference (RNAi; Invitrogen) was used for WNT16B (siWNT16B) and p53 downregulation (siP53). The universal RNAi negative control (Invitrogen) was used (siCT). Transfections were carried out using Lipofectamine RNAiMAX (Invitrogen).
Phosphoinositide 3-kinase inhibition.
Subconfluent MRC5 fibroblasts expressing WNT16B were exposed to LY294002 (50 μmol/L; Cell Signaling) for 12 h in complete culture medium. Cells were harvested and proteins were analyzed by Western blot.
Results
Microarray analysis identifies WNT16 in senescent fibroblasts.
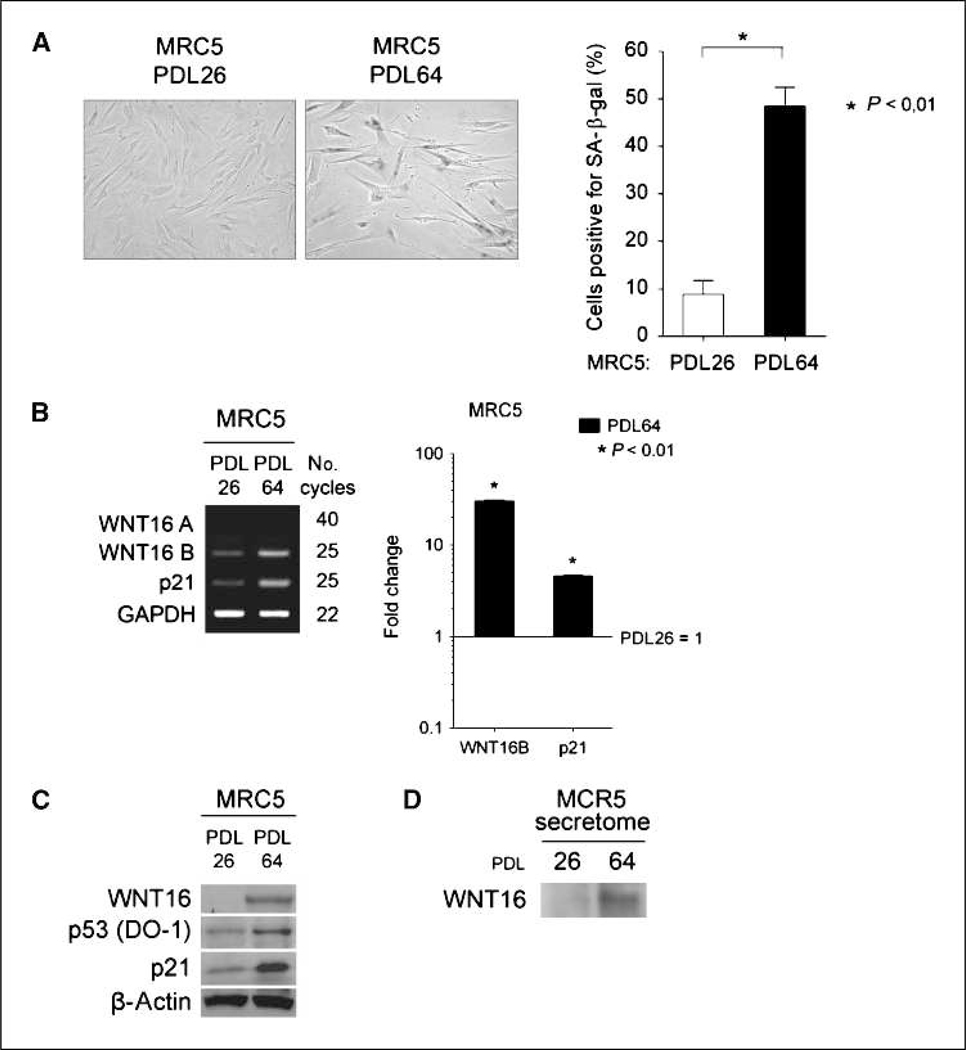
Gene expression profiling experiments were conducted to identify genes differentially expressed during replicative senescence. As described in Supplementary Materials and Methods, we compared young MRC5 fibroblasts at population doubling 28 (PDL28) to senescent MRC5 fibroblasts at PDL63. Induction of senescence in aged fibroblasts was confirmed with the SA-β-Gal assay (Fig. 1A). In total, 177 gene probes were upregulated and 338 gene probes were downregulated in senescent versus young MRC5 fibroblasts (Supplementary Table S1). Genes were classified based on mapping to KEGG pathways. Upregulated genes were enriched in three KEGG pathways: the wingless-type mouse mammary tumor virus (MMTV) integration site (WNT) signaling pathway, cytokine-cytokine receptor interaction, and the p53 signaling pathway (Supplementary Table S2). Repressed genes were enriched in three KEGG pathways: cell cycle, cytokine-cytokine receptor interaction, and DNA replication (Supplementary Table S3). Along with p53 transcriptional targets (CDKN1A, PERP, and WIG1), genes encoding secreted factors (WNT16, MMP10, and MMP3) were among the most strongly increased in senescent cells (Table 1). Strikingly, WNT16 was the gene most induced in senescent fibroblasts. WNT16 belongs to the WNT family of secreted proteins that are mainly involved in development and tumorigenesis (25). However, WNT proteins were recently described to be involved in aging and senescence (6, 26, 27). Thus, we hypothesized that WNT16 overexpression may play a role in replicative senescence.
Figure 1.
Expression of WNT16 in young (PDL26) and replicative senescent (PDL64) MRC5 human fibroblasts. A, MRC5 fibroblasts PDL26 and PDL64 were stained for SA-β-Gal expression and quantified. Columns, mean; bars, SD. B, left, WNT16A, WNT16B, and p21 mRNA expression was analyzed by RT-PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used as control. Right, WNT16B and p21 mRNA expression by qRT-PCR. mRNA expression in MRC5 PDL26 fibroblasts was normalized to 1. Columns, mean; bars, SD. C, Western blot analysis of WNT16, p53, and p21 protein expression. β-Actin expression was used as a loading control. D, TCA precipitation of proteins from the culture medium of MRC5 fibroblasts and Western blot analysis of WNT16 protein secretion.
Table 1.
Summary of the most highly expressed genes in replicative senescent cells identified by microarray
| Gene | Description | Fold change in replicative senescent cells |
|---|---|---|
| WNT16 | Wingless-type MMTV integration site, member 16 | 6.3 |
| MMP10 | Matrix metalloproteinase 10 (stromelysin 2) | 6.3 |
| MMP3 | Matrix metalloproteinase 3 (stromelysin 1) | 5.0 |
| CDKN1A | CDK inhibitor 1A (p21, Waf1, Cip1) | 3.4 |
| PERP | TP53 apoptosis effector | 2.7 |
| WIG1 | p53 target zinc finger protein | 2.2 |
NOTE: See Supplementary Tables S1–S3 for complete list. Data deposited in NCBI’s Gene Expression Omnibus as GSE15919.
WNT16B is specifically overexpressed in replicative senescence.
The WNT16 gene spans four exons with alternative exons 1a and 1b (28). Each exon 1 is associated with a unique promoter allowing transcription of two different mRNA isoforms: WNT16A and WNT16B. WNT16B was previously described as ubiquitously expressed in human tissues, whereas WNT16A was only detected in the pancreas (28). The probe used in the microarray was common to both variants. To discriminate between WNT16A and WNT16B, we analyzed mRNA expression by reverse transcription-PCR (RTPCR) or quantitative RT-PCR (qRT-PCR) using specific primers. No expression of WNT16A was detected in MRC5 fibroblasts, whereas a more than 10-fold increase of WNT16B was observed in replicative senescent cells (Fig. 1B). Specificity of WNT16A primers was verified using a WNT16A cDNA (data not shown). At the protein level, WNT16 protein expression was induced in replicative senescent MRC5 fibroblasts (Fig. 1C). The WNT16A and WNT16B isoforms are both recognized by the WNT16 antibody used and cannot be distinguished by Western blotting because they have very close molecular weights (41 and 42 kDa, respectively). However, because the WNT16B variant is more highly expressed than WNT16A, and it has been suggested that WNT16A mRNA may not be translated into protein in vivo (29), we concluded that the isoform detected by Western blot was WNT16B. WNT16B protein was detected in the supernatant of MRC5 fibroblasts, confirming that it is a secreted protein (Fig. 1D). Moreover, the detected amount increased in replicative senescence, consistent with microarray and PCR data. WNT16B increase was also observed in WI-38 and IMR90 fibroblasts undergoing replicative senescence (data not shown). Specific activation by the replicative senescence pathway was assessed in immortalized MRC5 fibroblasts (MRC5-hTERT). In MRC5-hTERT fibroblasts, the percentage of SA-β-Gal–positive cells was low and comparable with young fibroblasts, confirming that telomerase activation prevents the induction of senescence (Supplementary Fig. S4A). In addition, WNT16B expression was lower in MRC5-hTERT cells than in replicative senescent fibroblasts and comparable with the level of expression observed in young MRC5 fibroblasts, which suggests that telomere shortening is necessary for WNT16B accumulation (Supplementary Fig. S4B and C).
WNT16B is overexpressed in SIPS.
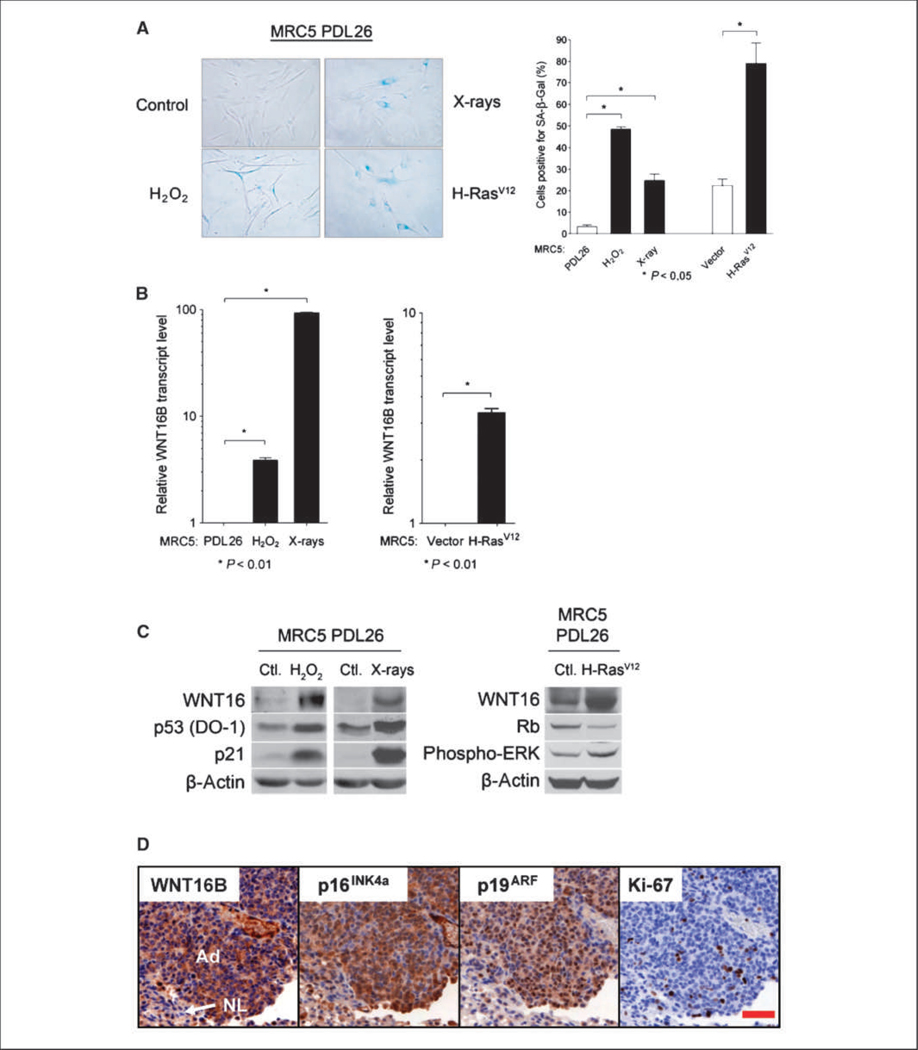
Whereas replicative senescence is related to telomere shortening and cellular aging, a pre-mature senescence phenotype can be induced in response to stress that is meant to prevent cellular transformation. We investigated the expression of WNT16B in such SIPS. MRC5 fibroblasts were exposed to H2O2 or X-rays. Both stresses are known to induce premature senescence through different signaling pathways (4, 5, 8). Senescence was verified using the SA-β-Gal assay after 96 hours (H2O2) or after 48 hours (X-rays; Fig. 2A) and accompanied by increased WNT16B expression (Fig. 2B and C), which was more pronounced following irradiation. As in replicative senescence, no increase of WNT16A was measured (data not shown).
Figure 2.
Expression of WNT16B in SIPS and OIS. A, MRC5 fibroblasts were stained for SA-β-Gal expression and quantified. Columns, mean; bars, SD. B, WNT16B mRNA expression was analyzed by qRT-PCR in MRC5 PDL26 fibroblasts exposed to H2O2 or X-rays (left) or infected with a retrovirus coding for H-RasV12 (right). mRNA expression in MRC5 PDL26 fibroblasts was normalized to 1. Columns, mean; bars, SD. C, left and middle, Western blot analysis of WNT16B, p53, and p21 protein expression in MRC5 PDL26 fibroblasts exposed to H2O2 or X-rays; right, WNT16, Rb, and phosphorylated ERK protein expression was analyzed in MRC5 PDL26 fibroblasts infected with a retrovirus coding for H-RasV12. β-Actin was used as a loading control. D, expression of WNT16B during OIS in vivo. Immunohistochemical analysis of WNT16B, p19ARF, p16INK4A, and Ki-67 in serial sections of K-RasV12–induced lung adenomas. Note strong positive staining of WNT16B in the cytoplasm of most tumor cells [adenomas (Ad)] but weak or negative staining in the pocket of nontumoral cells located in the bottom left corner [normal lung (NL)]. Most tumor cells showed nuclear staining of p19ARF often with intense signal in the nucleoli. A fraction of tumor cells gave positive nuclear staining for p16INK4A, whereas the cytoplasmic staining could be nonspecific. Scale bar, 50 μm.
WNT16 is activated in OIS in vitro and in vivo.
As a tumor suppression mechanism, premature senescence is triggered by oncogenic stress. One well-described example of OIS is the activation of the Ras oncogene family (8). We explored WNT16B expression in OIS by infecting MRC5 fibroblasts with a virus coding for the activated H-RasV12 oncogene. As expected, oncogene activation induced premature senescence characterized by SA-β-Gal 6 days after retroviral infection (Fig. 2A), as well as WNT16B mRNA and protein expression (Fig. 2B and C). We further analyzed the activation of WNT16B in OIS in vivo using an orthotopic murine lung cancer model driven by K-RasV12 (30). Mice expressing oncogenic K-RasV12 develop multifocal lung tumors. Tumors develop initially as preneoplastic lesions (adenomas), which contain a high percentage of senescent cells as shown by the expression of p16INK4a and SA-β-Gal activity (18). By immunostaining, we observed that WNT16B is overexpressed in K-RasV12–induced adenomas as compared with the surrounding normal lung tissue (Fig. 2D). These results indicate that WNT16B is a marker of OIS in vitro and in vivo.
WNT16B is necessary for the onset of replicative senescence.
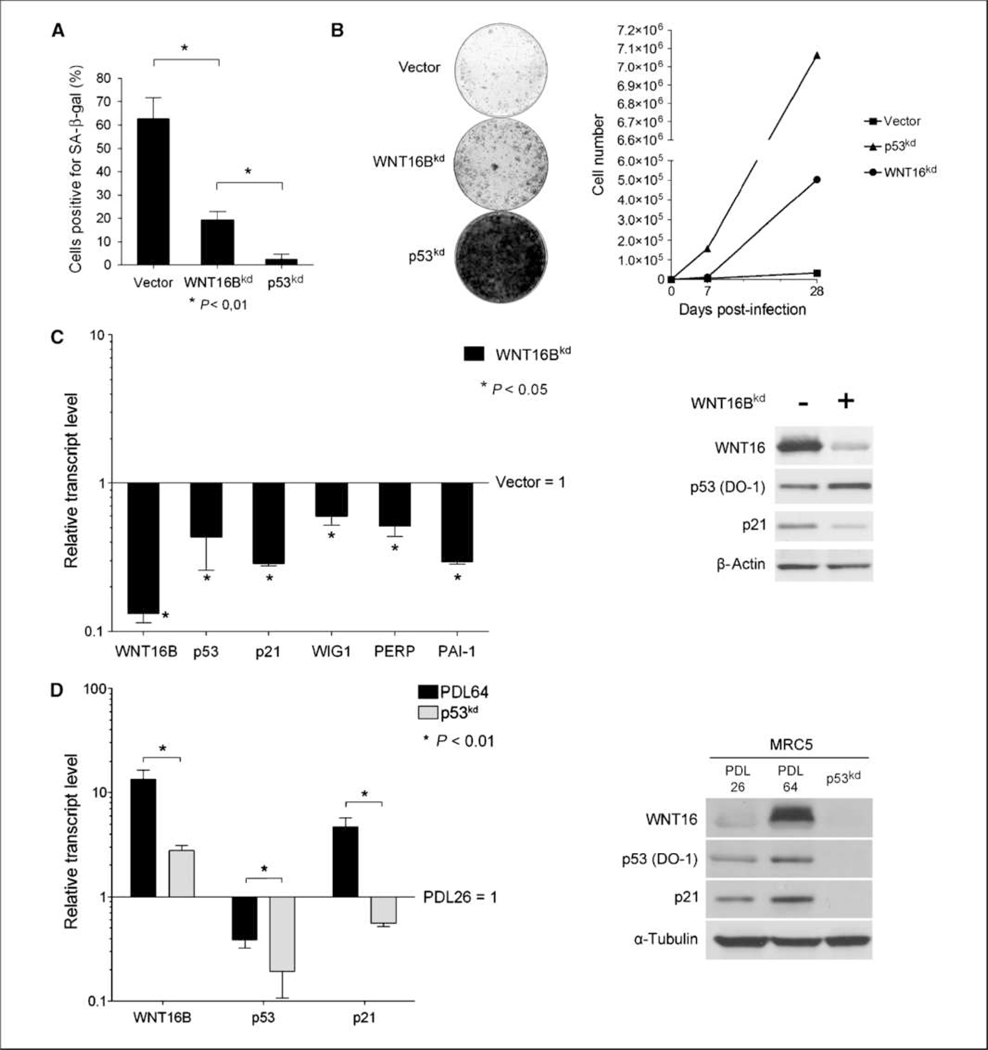
To investigate the role of WNT16B in replicative senescence, pre-senescent MRC5 fibroblasts (PDL58) were infected with retroviral vectors that targeted WNT16B or p53 (as a control for preventing senescence) for suppression through shRNA-mediated RNA interfere-nce. Cells infected with a control vector underwent replicative senescence by PDL64 as confirmed by SA-β-Gal positivity (Fig. 3A) and proliferation arrest (Fig. 3B). Cells infected with WNT16B shRNA (WNT16Bkd) bypassed senescence, as observed by increased proliferation (Fig. 3B) and low SA-β-Gal (Fig. 3A). WNT16Bkd MRC5 fibro-blasts proliferated beyond the replicative senescence checkpoint but to a lesser degree than the p53kd MRC5 fibroblasts (Fig. 3A and B). Quantitative PCR analysis showed that genes known to be trans-activated by p53 during replicative senescence were expressed at a lower level in WNT16Bkd cells than vector-infected cells: p21WAF1, PAI-1, WIG1, and PERP (Fig. 3C, left). Downregulation of WNT16B also prevented the accumulation of p21WAF1 protein (Fig. 3C, right). Intriguingly, in WNT16Bkd fibroblasts, p53 mRNA expression was decreased, whereas a slight increase was observed at the protein level. These results suggest that p53 activity in replicative senescence is dependent on WNT16B expression. To go further into the relationship between WNT16B and p53, we analyzed WNT16B expression in MRC5 p53kd cells. p21WAF1 and WNT16B mRNA and protein expression were significantly lower than in replicative senescent cells (Fig. 3D). Expression at the mRNA level of all known p53 effector genes (e.g., p21WAF1) was lower in p53kd cells compared with young MRC5 fibroblasts, confirming that their expression in replicative senescence is dependent on p53 transcriptional activity (Fig. 3D, left; data not shown). Similar results were obtained in presenescent fibroblasts expressing E6, an oncoprotein from human papilloma virus 16, which induces p53 degradation and inactivation and prevents the onset of senescence (data not shown). Overall, these results highlight that WNT16B is necessary for the onset of replicative senescence by modulating the transcription of p53 target genes, such as p21WAF1.
Figure 3.
Inhibition of WNT16B or p53 expression in presenescent fibroblasts. A, control senescent cells (PDL64), WNT16Bkd cells, and p53kd MRC5 fibroblasts were stained for SA-β-Gal activity and quantified. Columns, mean; bars, SD. B, left, 5,000 control senescent cells (PDL64), WNT16Bkd cells, and p53kd cells were seeded into 10-cm2 plates for 3 wk and stained with methylene blue; right, proliferation curves of control senescent cells (PDL64), WNT16Bkd cells, and p53kd cells. C, left, WNT16B, p53, p21, WIG1, PERP, and PAI-1 mRNA expression was analyzed by qRT-PCR in WNT16Bkd MRC5 fibroblasts. mRNA expression in MRC5 PDL26 fibroblasts was normalized to 1. Columns, mean; bars, SD. Right, WNT16, p53, and p21 protein expression was analyzed using α-tubulin as a loading control. D, left, WNT16B, p53, and p21 mRNA expression in young (PDL26), senescent (PDL64), or p53kd MRC5 fibroblasts was analyzed by qRT-PCR. mRNA expression in MRC5 PDL26 fibroblasts was normalized to 1. Columns, mean; bars, SD. Right, WNT16, p53, and p21 protein expression was analyzed using α-tubulin as a loading control.
WNT16B regulates p21WAF1 transcription.
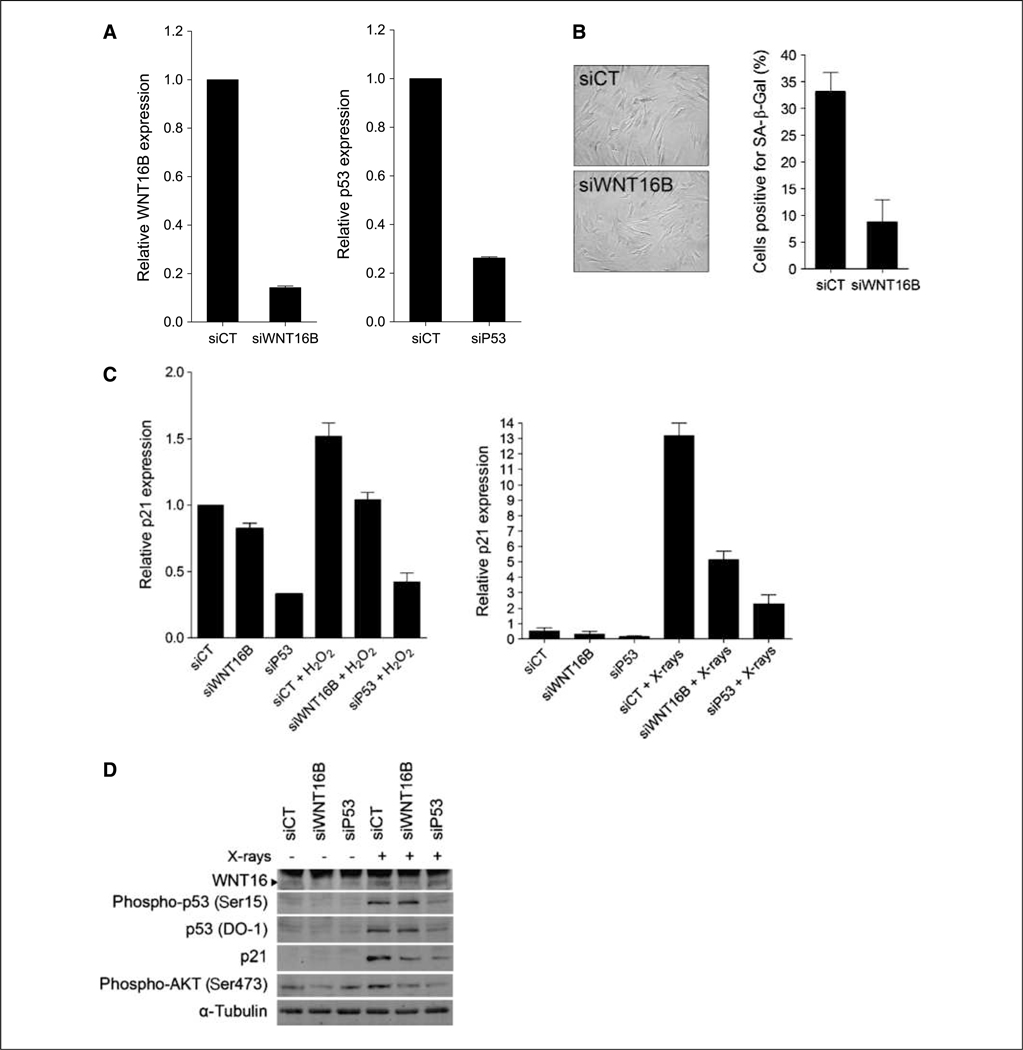
To further characterize the relationship between WNT16B and p53, we investigated the consequences of WNT16B depletion on p53 activity in two models of SIPS (i.e., X-rays and H2O2 exposures). WNT16B expression was transiently inhibited by transfecting a small interfering RNA (siRNA) into MRC5-hTERT cells (Fig. 4A). Twenty-four hours after transfection, siCT, siWNT16B, and siP53 cells were exposed to X-rays or H2O2 and the percentage of senescent cells was determined. Although no difference was observed after X-rays (data not shown), siWNT16B prevented the onset of senescence by H2O2 as shown by a lower percentage of SA-β-Gal–positive cells (Fig. 4B). Without treatment, the level of p21WAF1 mRNA was reduced in both siWNT16B and siP53 cells, with a stronger decrease in siP53 cells (Fig. 4C). Induction of p21WAF1 mediated by exposure to H2O2 was abrogated by downregulation of WNT16B (Fig. 4C, left), in accordance with the lower percentage of SA-β-Gal–positive cells. As expected, p21WAF1 was not induced by H2O2 in siP53 cells. Although siWNT16B did not affect senescence mediated by X-rays, it did partially abrogate transactivation of p21WAF1 (Fig. 4C, right). p21WAF1 mRNA level in irradiated siWNT16B cells is still higher than in nonirradiated cells and may be sufficient to induce senescence. Expression of p21WAF1 protein followed that of mRNA (Fig. 4D). Both p53 and phosphorylated p53 proteins accumulated after X-rays. Intriguingly, the level of p53 was still elevated after radiation in siWNT16B cells, indicating that p53 activation by X-rays maybe independent of WNT16B. Altogether, these results suggest that WNT16B plays a role downstream of p53 to modulate the expression of p21WAF1 in cells undergoing senescence.
Figure 4.
Regulation of p21 transcription by WNT16B. A, qRT-PCR analysis of WNT16B and p53 expression in MRC5-hTERT cells transfected with a control siRNA (siCT) and a siRNA targeting WNT16B (siWNT16B) or p53 (siP53). B, MRC5-hTERT fibroblasts expressing siCT or siWNT16B and treated with H2O2 were stained for SA-β-Gal expression and quantified. Columns, mean; bars, SD. C, qRT-PCR analysis of p21 expression after H2O2 treatment (left) or X radiations (right). MRC5-hTERT cells were transfected with siCT, siWNT16B, or siP53. D, Western blot analysis of WNT16, p53, and p21 expression and p53 and AKT activation after X radiations. α-Tubulin was used as a loading control. The film was overexposed to detect WNT16 basal expression and evaluate the siWNT16B efficiency. Note the presence of a cross-reacting band seen only in hTERT-immortalized fibroblasts.
WNT16B overexpression limits the life span of MRC5 fibroblasts.
We asked whether WNT16B expression was able to accelerate the onset of senescence. When overexpressed in young MRC5 fibroblasts, WNT16B resulted in an increased percentage of cells positive for SA-β-Gal, a decrease in cell proliferation, and a decrease in bromodeoxyuridine incorporation (Supplementary Fig. S5). Activation of p53 as a transcription factor was not apparent because no increase of p21WAF1 protein level was detected (Supplementary Fig. S5D, left; Fig. 5B). On the contrary, WNT16B over-expression induced moderate p16INK4A mRNA and protein expression (Supplementary Fig. S5D, right; data not shown). These results suggest that the overexpression of WNT16B in young fibro-blasts may limit the ability of cells to proliferate through activation of the cyclin-dependent kinase (CDK) inhibitor p16INK4A.
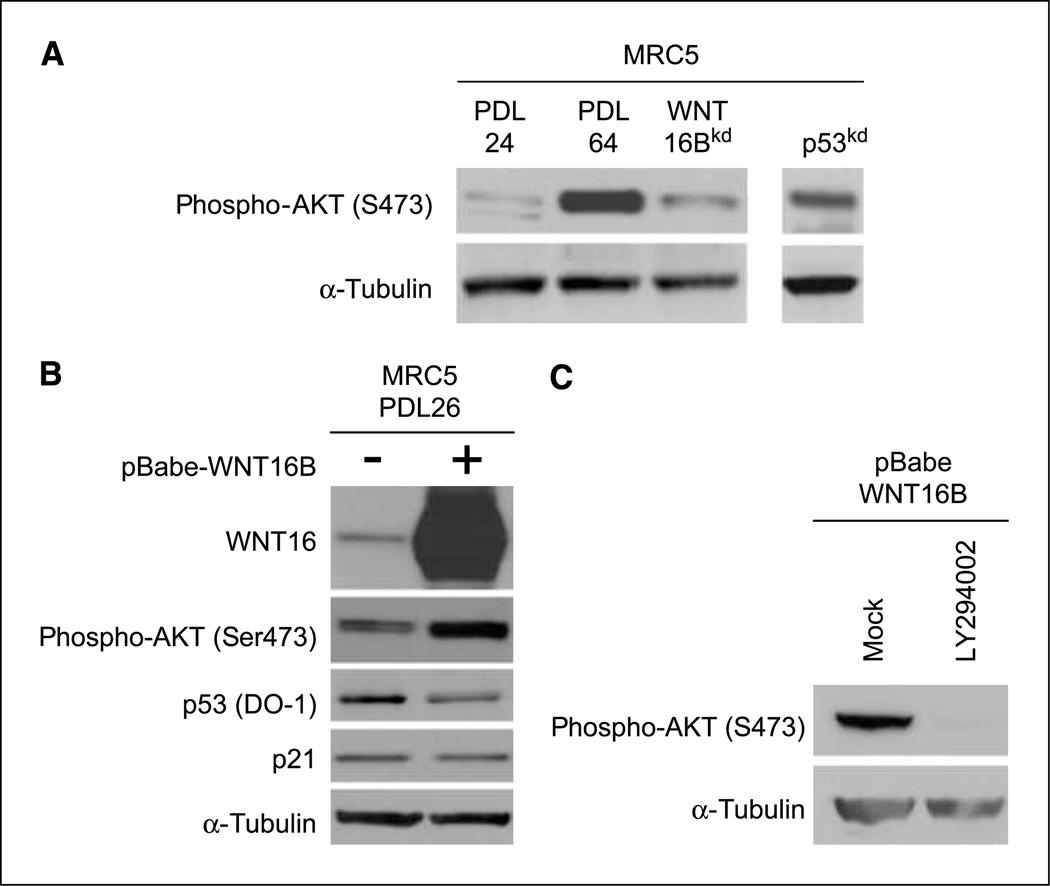
Figure 5.
Regulation of AKT by WNT16B. A, Western blot analysis of AKT activation in dividing (PDL28), senescent (PDL64), WNT16Bkd, and p53kd MRC5 fibroblasts. α-Tubulin was used as a loading control. B, Western blot analysis of AKT activation and WNT16, p53, and p21 protein expression in pBabe and pBabe-WNT16B MRC5 fibroblasts. α-Tubulin was used as a loading control. C, analysis of AKT activation in pBabe-WNT16B MRC5 fibroblasts by Western blot after treatment with LY294002.
WNT16B regulates the AKT pathway in replicative senescence.
To better understand the role of WNT16B in replicative senescence, downstream pathways were explored. WNT proteins mainly signal through the canonical WNT/β-catenin pathway, which was previously described as being activated by WNT16B in leukemia (20, 29). On WNT stimulation, β-catenin accumulates in the nucleus to transactivate target genes, such as MYC and CyclinD1. By combining fractionation, immunofluorescence, and quantitative PCR experiments, we did not observe any hallmark of β-catenin activation on WNT16B overexpression. These results are consistent with the observation that β-catenin is not activated during replicative senescence (data not shown). Some WNT proteins may also signal through the AKT pathway (31, 32). AKT was activated in replicative senescence and this activation was dependent on both WNT16B and p53 expression (Fig. 5A). AKT is also activated in WNT16Bexpressing MRC5 fibroblasts (Fig. 5B). Moreover, AKT activation was dependent on phosphoinositide 3-kinase (PI3K) activity and was abrogated through treatment with the specific PI3K inhibitor LY294002 (Fig. 5C). Interestingly, AKT was also activated after irradiation in MRC5-hTERT, and this activation was also dependent on both WNT16B and p53 expression (Fig. 4D). Overall, these results show that WNT16B regulates the PI3K/AKT pathway in cellular senescence.
Discussion
Replicative senescence is a permanent state of proliferative arrest resulting from telomere shortening (33). In replicative senescence, telomere shortening triggers a DNA damage response and the activation of the p53 transcription factor (34). In turn, p53 transactivates target genes, such as the CDK inhibitor p21WAF1, to induce cell cycle arrest. As observed by expression profiling experiments done by us and others (35), senescent cells are characterized by the downregulation of genes involved in cell cycle progression and DNA replication (Supplementary Table S3), whereas p53 pathway components are overexpressed (Supplementary Table S2). We identified components of the WNT pathway as being activated in replicative senescence and WNT16B as the most overexpressed gene. The WNT proteins are a family of secreted factors that can signal through either β-catenin–dependent or β-catenin–independent pathways (36). The WNT pathways have been associated with senescence reversal and cellular transformation (WNT2; ref. 6). The WNT pathways may also be involved in the activation of senescence because continuous exposure to WNT3A induces premature senescence in mouse fibroblasts (26). Our work provides a new insight into the functions of WNT pathways in promoting cellular senescence.
WNT16B was initially described as an oncogene able to activate β-catenin in pre-B acute lymphoblastoid leukemia or to signal in a β-catenin–independent manner in basal cell carcinoma (20, 29). In the present study, we observed that WNT16B does not activate βcatenin but the AKT pathway. This can be related to what is observed with WNT5A and WNT10B (37, 38). Indeed, WNT5A can activate the PI3K/AKT pathway, which inhibits β-catenin function and suppresses cell growth in colorectal cancer cells (37). Thus, WNT16B, WNT10B, and WNT5A belong to a subfamily of WNT ligands capable of antagonistic activities depending on which signaling pathway is stimulated.
WNT16B was upregulated in SIPS caused by exposure to H2O2, X-rays, or oncogenic H-RasV12. Thus, WNT16B is a bona fide marker of cellular senescence. Along with p21WAF1 and p16INK4A, WNT16B can be used to validate the senescence phenotype together with the SA-β-Gal assay. We further validated WNT16B as a marker of senescence in vivo in K-RasV12–induced lung adenomas. This model also associates WNT16B with the initial steps of tumorigenesis and suggests that this new marker could be useful as a diagnostic tool for the early detection of tumor formation. Indeed, because WNT16B is a secreted factor, we could expect that the protein would be detectable in body fluids (e.g., in blood) and allow a noninvasive examination (39).
Both p53 and WNT16B are necessary for the onset of replicative senescence. When p53 is inactivated, senescence is bypassed and WNT16B gene fails to be upregulated. Similarly, WNT16B knockdown delays the onset of senescence and does not allow the transcriptional activation of p53 target genes. Although WNT16B has never been described as a p53 transcriptional target, a bioinformatic analysis identified putative p53 response elements in the WNT16B promoter (data not shown). However, further experiments are needed to determine whether WNT16B is a direct transcriptional target of p53. Alternatively, WNT16B may be necessary for the maintenance of the p53 transcriptional activity in senescence. Indeed, our results show that despite the activation of p53 in WNT16Bkd cells, p21WAF1 is not fully activated. How is WNT16B regulating the p53 pathway? One candidate WNT16Bdependent pathway highlighted here is the PI3K/AKT pathway. Indeed, acute loss of the phosphatase and tensin homologue, an inhibitor of AKT activation in mouse fibroblasts, is responsible for the induction of senescence through the upregulation of ARF and stabilization of the p53 protein (40–42). AKT activation is responsible for the accumulation of reactive oxygen species that are essential to allow the activation of the p53 pathway and the onset of senescence (42). Thus, we propose that in replicative senescence WNT16B activation is dependent on telomere shortening and subsequent p53 activation. WNT16 may be necessary for the full and maintained transcriptional activity of p53 perhaps through activation of the AKT pathway. The relationship between WNT16B and p53 is different from that described with PAI-1, which is necessary for the onset of senescence but has no effect on p53 activity (10).
Overall, our study describes the secreted factor WNT16B as a marker and an actor of cellular senescence in vitro and in vivo.
Supplementary Material
Acknowledgments
Grant support: Institut National de la Sante et de la Recherche Medicale, “Agir a dom,” l’Association pour la Recherche contre le Cancer, and Marie Curie International Reintegration (EU) grant MIRG-CT-2006-042148. R. Binet, D. Ythier, and D. Larrieu are recipients of doctoral fellowships from La Fondation pour la Recherche Médicale (Prix Mariane Josso), INCa, and the French Ministry of Education and Research, respectively. R. Pedeux was a recipient of an International Association for the Study of Lung Cancer fellowship.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
We thank Thibault Voeltzel for providing MRC5 fibroblasts, Dr. Stephanie Corde and Antoine Dorenlot for their help in the X-radiation experiments, and Dr. Teck Teh for providing the pEGFP/IRES-WNT16A plasmid.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Deng Y, Chan S, Chang S. Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 2008;8:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayflick L The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965;37:614–36. [DOI] [PubMed] [Google Scholar]
- 3.d’Adda di Fagagna F Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer 2008; 8:512–22. [DOI] [PubMed] [Google Scholar]
- 4.Di Leonardo A, Linke S, Clarkin K, Wahl G. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev 1994;8:2540–51. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Ames B. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci U S A 1994;91:4130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye X, Zerlanko B, Kennedy A, Banumathy G, Zhang R, Adams P. Downregulation of Wnt signaling is a trigger for formation of facultative heterochromatin and onset of cell senescence in primary human cells. Mol Cell 2007;27:183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acosta J, O’Loghlen A, Banito A, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008;133:1006–18. [DOI] [PubMed] [Google Scholar]
- 8.Serrano M, Lin A, McCurrach M, Beach D, Lowe S. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88:593–602. [DOI] [PubMed] [Google Scholar]
- 9.Itahana K, Campisi J, Dimri G. Mechanisms of cellular senescence in human and mouse cells. Biogerontology 2004;5:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Kortlever R, Higgins P, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 2006;8:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K, Seu Y, Baek S, et al. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell 2007;18:4543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wajapeyee N, Serra R, Zhu X, Mahalingam M, Green M. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 2008;132:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuilman T, Michaloglou C, Vredeveld L, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008;133:1019–31. [DOI] [PubMed] [Google Scholar]
- 14.Coppé J, Patil C, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008;6:2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang E Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res 1995;55:2284–92. [PubMed] [Google Scholar]
- 16.Dimri G, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995;92:9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itahana K, Campisi J, Dimri G. Methods to detect biomarkers of cellular senescence: the senescence-associated β-galactosidase assay. Methods Mol Biol 2007;371:21–31. [DOI] [PubMed] [Google Scholar]
- 18.Collado M, Gil J, Efeyan A, et al. Tumour biology: senescence in premalignant tumours. Nature 2005; 436:642. [DOI] [PubMed] [Google Scholar]
- 19.Brummelkamp T, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science 2002;296:550–3. [DOI] [PubMed] [Google Scholar]
- 20.Mazieres J, You L, He B, et al. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene 2005;24:5396–400. [DOI] [PubMed] [Google Scholar]
- 21.Halbert C, Demers G, Galloway D. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J Virol 1992;66:2125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKenzie K, Franco S, May C, Sadelain M, Moore M. Mass cultured human fibroblasts overexpressing hTERT encounter a growth crisis following an extended period of proliferation. Exp Cell Res 2000;259:336–50. [DOI] [PubMed] [Google Scholar]
- 23.Pedeux R, Sengupta S, Shen J, et al. ING2 regulates the onset of replicative senescence by induction of p300-dependent p53 acetylation. Mol Cell Biol 2005;25: 6639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larrieu D, Ythier D, Binet R, et al. ING2 controls the progression of DNA replication forks to maintain genome stability. EMBO Rep 2009;10:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nusse R Wnt signaling in disease and in development. Cell Res 2005;15:28–32. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Fergusson M, Castilho R, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007;317:803–6. [DOI] [PubMed] [Google Scholar]
- 27.Maiese K, Li F, Chong Z, Shang Y. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther 2008;118:58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fear M, Kelsell D, Spurr N, Barnes M. Wnt-16a, a novel Wnt-16 isoform, which shows differential expression in adult human tissues. Biochem Biophys Res Commun 2000;278:814–20. [DOI] [PubMed] [Google Scholar]
- 29.Teh M, Blaydon D, Ghali L, et al. Role for WNT16B in human epidermal keratinocyte proliferation and differentiation. J Cell Sci 2007;120:330–9. [DOI] [PubMed] [Google Scholar]
- 30.Guerra C, Mijimolle N, Dhawahir A, et al. Tumor induction by an endogenous K-Ras oncogene is highly dependent on cellular context. Cancer Cell 2003;4:111–20. [DOI] [PubMed] [Google Scholar]
- 31.Dissanayake S, Wade M, Johnson C, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem 2007;282:17259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medrano E Wnt5a and PKC, a deadly partnership involved in melanoma invasion. Pigment Cell Res 2007;20:258–9. [DOI] [PubMed] [Google Scholar]
- 33.Bodnar A, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998;279:349–52. [DOI] [PubMed] [Google Scholar]
- 34.Herbig U, Jobling W, Chen B, Chen D, Sedivy J. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21 (CIP1), but not p16(INK4a). Mol Cell 2004;14:501–13. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Cohen S. Smurf2 up-regulation activates telomere-dependent senescence. Genes Dev 2004;18: 3028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logan C, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004;20: 781–810. [DOI] [PubMed] [Google Scholar]
- 37.Ying J, Li H, Yu J, et al. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/β-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res 2008;14:55–61. [DOI] [PubMed] [Google Scholar]
- 38.Yoshikawa H, Matsubara K, Zhou X, et al. WNT10B functional dualism: β-catenin/Tcf-dependent growth promotion or independent suppression with deregulated expression in cancer. Mol Biol Cell 2007;18:4292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang H, Schiffer E, Song Z, et al. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci U S A 2008;105:11299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Trotman L, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005;436:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007;445:656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nogueira V, Park Y, Chen C, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 2008;14:458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.