Introduction
In December 2019, a series of pneumonia cases of unknown etiology, which were epidemiologically linked to a seafood market, was reported in Wuhan, Hubei province, China. The disease has rapidly spread around the world, becoming a global pandemic. A coronavirus, which was named 2019 novel coronavirus (2019-nCov), was isolated from respiratory tract samples1 and later identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The name of the disease was officially changed to coronavirus disease 2019 (COVID-19).
Pulmonary involvement with alveolar damage and acute respiratory failure has been the most remarkable clinical condition of COVID-19 patients. However, there is increasing evidence that this virus also affects other organs, kidney injury being relatively common in this infection and associated with an increased mortality rate.2
In a large prospective cohort of 701 COVID-19 patients in China, 44% had proteinuria and 27% had hematuria on admission. During hospitalization, 5.1% of them developed acute kidney injury.2
We report a patient who developed systemic symptoms and renal involvement after being hospitalized because of a COVID-19 infection. A mesangial proliferation and IgA deposits in the glomeruli were seen on the kidney biopsy specimen.
Case Presentation
We report a 78-year-old man with a medical history of moderate alcohol consumption, essential hypertension treated with losartan, dyslipidemia, moderate aortic valve stenosis, and bladder cancer treated with transurethral resection and currently in remission. His baseline serum creatinine, measured 2 months before presentation, was 0.78 mg/dl. Proteinuria and microhematuria were negative on previous urinalysis.
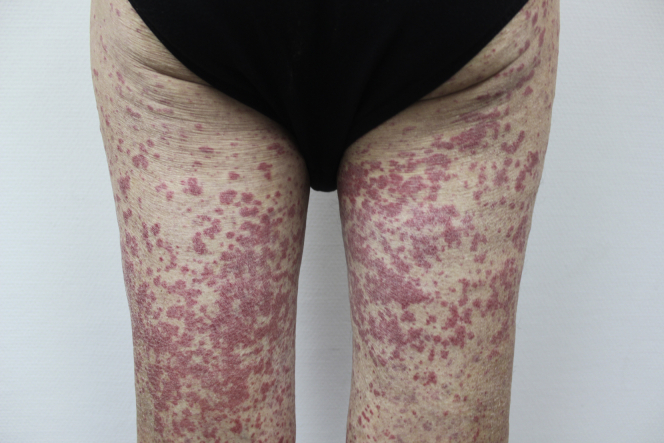
He was hospitalized on 4 April 2020 for bilateral pneumonia due to COVID-19, based on a positive reverse transcription−polymerase chain reaction test for SARS-CoV-2 in a nasopharyngeal swab. He was treated with hydroxychloroquine, lopinavir/ritonavir, dexamethasone, ceftriaxone, azithromycin, and tocilizumab because of respiratory failure and significant elevation of inflammatory parameters, including interleukin-6 (IL-6). He was discharged on 17 April 2020. Three weeks later, he returned to the emergency department with wrist arthritis and lower limb purpura (Figure 1). Cutaneous vasculitis was diagnosed during a skin biopsy, and consequently a short-term regimen of prednisone 40 mg/d was prescribed. In addition, proteinuria and hematuria with dysmorphic red blood cells were detected on urinalysis. The patient was promptly referred to our nephrology department.
Figure 1.
Purpuric papules of lower extremities with the typical distribution of Henoch− Schönlein purpura.
A physical examination showed a blood pressure of 160/90 mm Hg. His heart sounds were normal, with a systolic aortic murmur. He also had lower extremity pitting edema. The rest of the examination did not reveal anything unusual.
On admission, his serum creatinine was 1.35 mg/dl, and he had hypoalbuminemia, massive proteinuria (10 g/d), and hematuria with 60% of dysmorphic red blood cells. In addition, serologic testing for hepatitis C and HIV were negative, with positive hepatitis B surface antibody. Serum complement testing for C3 and C4 were normal. Antinuclear antibodies, anti-DNA antibodies, antineutrophil cytoplasmic antibodies, anti−glomerular basement membrane (GBM) antibodies, and cryoglobulin were all negative. Serum and urine protein electrophoresis and immunofixation were negative for monoclonal protein. The immunoglobulin value was normal, except for a mild hypogammaglobulinemia. A nasal swab for SARS-CoV-2 was repeated on this admission, with negative result but with a positive result for anti-SARS-CoV-2 by chemiluminescent immunoassay. Laboratory results are detailed in Table 1.
Table 1.
Laboratory results and urinalysis on presentation
| Laboratory test | Patient result | Reference |
|---|---|---|
| Glucose (mg/dl) | 71 | 76–110 |
| Urea (mg/dl) | 40 | 10–50 |
| Creatinine (mg/dl) | 1.35a | 0.70–1.30 |
| Sodium (mEq/L) | 139 | 135–145 |
| Potassium (mEq/L) | 4.3 | 3.5–5 |
| Calcium (mg/dl) | 8.3 | 8.1–10.4 |
| Total cholesterol (mg/dl) | 254a | 100–200 |
| Albumin (g/dl) | 3.2a | 3.4–4.8 |
| Serum IgG (mg/dl) | 396a | 700–1600 |
| Serum IgM (mg/dl) | 45.2 | 37–258 |
| Serum IgA (mg/dl) | 304 | 70–400 |
| Hemoglobin (g/dl) | 13.6 | 13–18 |
| WBC count (103 per μl) | 10.03 | 4.80–10.80 |
| Platelets (103 per μl) | 238 | 130–400 |
| Urinalysis | ||
| Protein excretion (g/24 h) | 10.7a | <0.30 |
| CCr (ml/min) | 70a | 71–151 |
| Urine RBCs (per hpf) | Hematuriaa | 0–2 |
| Urine dysmorphic RBCs | 60%a | Not detected |
CCr, creatinine clearance; hpf, high-power field; RBC, red blood cell; WBC, white blood cell.
Abnormal.
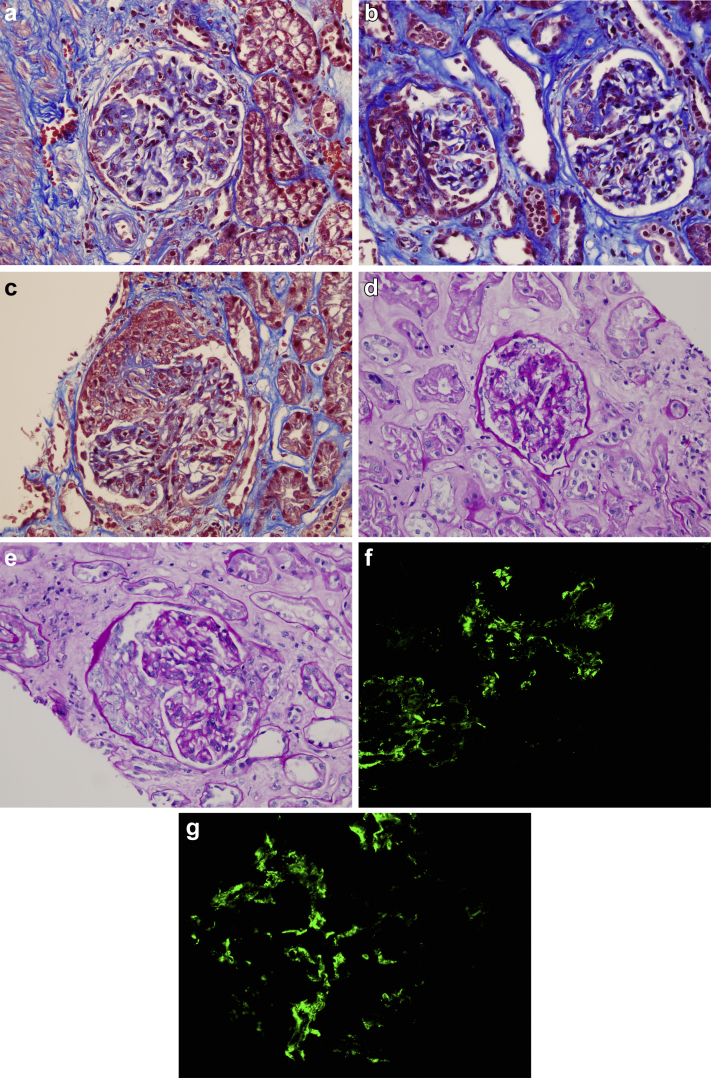
A kidney biopsy was performed to assess the patient’s renal disease. A total of 7 glomeruli were identified, 1 of which was sclerotic. Four glomeruli demonstrated segmental mesangial expansion with hypercellularity, and 1 them showed a synechia with Bowman capsule. Epithelial crescents were seen in 2 glomeruli, 1 of which obliterated the glomerular capillary lumens. No tubular or interstitial abnormalities were observed. Immunofluorescence microscopy showed IgA granular deposits in the glomerular mesangium (Figure 2). The biopsy findings were interpreted as an IgA vasculitis, also known as Henoch−Schönlein purpura (International Study of Kidney Diseases in Children [ISKDC] classification IIIb). Electron microscopy revealed electrondense mesangial deposits, with podocytes showing extensive pedicelar effacement. No viral particles were seen (Figure 3)
Figure 2.
Renal biopsy findings. Light microscopy study shows the following: (a) glomerulus with segmental mesangial expansion with hypercellularity (Masson’s trichrome stain; original magnification ×200). (b) Two glomeruli, both with mesangial expansion; the glomerulus on the left shows an epithelial crescent, and the one on the right shows a synechia with Bowman capsule (Masson’s trichrome stain; original magnification ×200). (c) Glomerulus with epithelial crescent that obliterates glomerular capillary lumens (Masson’s trichrome stain; original magnification ×200). (d) Glomerulus with segmental mesangial expansion with hypercellularity (periodic acid−Schiff [PAS] stain; original magnification ×40). (e) Glomerulus with mesangial expansion with hypercellularity that shows an epithelial crescent (PAS stain; original magnification ×40). (f,g) Immunofluorescence microscopy shows IgA granular deposits in the glomerular mesangium (immunofluorescence for IgA, anti−human-IgA antibodies marked with fluorescein; original magnification ×200).
Figure 3.
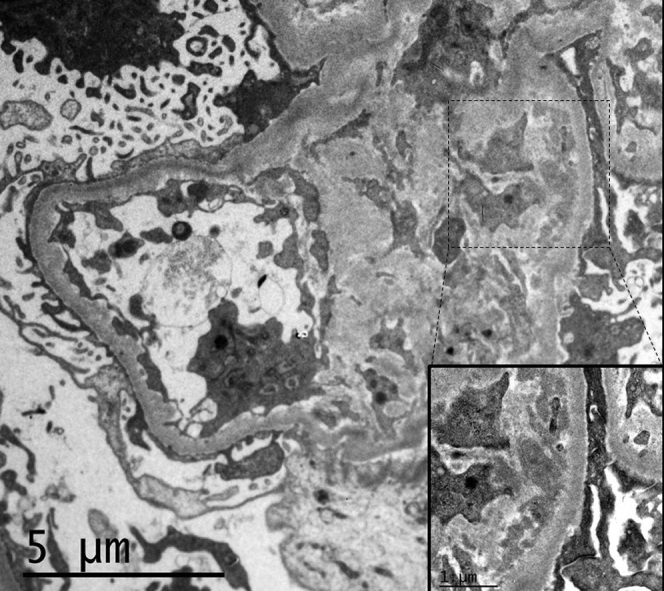
Kidney biopsy specimen. Electron microscopy reveals amorphous, electrodense mesangial deposits. Podocytes showed extensive pedicelar effacement. No viral particles were identified in the podocytes, endothelial or tubular cells (original magnification ×5000; inset, original magnification ×20,000).
Follow-Up
During hospitalization, the patient’s renal function declined to serum creatinine of 1.96 mg/dl (reference range 0.70−1.30 mg/dl) and decreased urine output. Based on the biopsy results and the clinical course, a decision was made to start methylprednisolone pulses plus rituximab. On discharge, serum creatinine had improved to 1.4 mg/dl, and the patient had good urine output. He persisted with gross proteinuria (6 g/d) and hematuria. The cutaneous purpura markedly improved. The patient has continued with oral prednisone upon discharge.
Discussion
We herein report a patient who developed cutaneous vasculitis, arthritis, and nephritic syndrome with hypertension, massive proteinuria (10 g/d), and mild acute kidney injury, after a COVID-19−related pneumonia. In addition, diffuse mesangial proliferation with IgA mesangial staining was confirmed in a kidney biopsy specimen, as well as crescentic deposits in 28% of the glomeruli. To our knowledge, this is the first reported case of IgA vasculitis related to COVID-19.
The first glomerular disease described in the setting of COVID-19 was collapsing glomerulopathy. All of these patients were reported to have severe acute kidney injury and massive proteinuria.3, 4, 5, 6
In a renal histopathological analysis of 26 post mortem findings of patients with COVID-19 in China, 1 case showed IgA staining in the mesangial area as well as capillary wall, which was shown to be associated with mesangial and subendothelial deposits by electron microscopy.7 The medical history and clinical manifestations of that case were not described.
IgA vasculitis (Henoch−Schönlein purpura) is a small-vessel vasculitis mediated by immune-complexes deposits containing IgA. It is usually preceded by respiratory tract infection, and its association with multiple microorganisms, including different viruses, is known.8 The main clinical manifestations are nonthrombocytopenic purpura, arthritis/arthralgia, and abdominal pain.8,9,S1,S2 Renal involvement occurs in 40% to 50% of patients,9,S2 being more severe among the adult population.S1
A recent meta-analysis of patients with IgA vasculitis showed that older age at onset, lower glomerular filtration rate, nephrotic or nephritic syndrome, and kidney biopsy with crescentic nephritis (ISKDC grades III−V) were significant risk factors associated with poor outcomes.S3
It has been proposed that increased levels of a circulating form of IgA1 with aberrant glycosylation (Gd-IgA1) plays a role in the pathogenic mechanism of IgA nephritis. This variant of IgA1 would be able to antigenically recognize structures of some microorganisms and to form circulating complexes. Through specific receptors, they would be deposited in the mesangium, inducing the activation of the mesangial cells, which would finally lead to renal damage. It has also been hypothesized that mucosal infections lead to upregulation of IL-6, which could lead to development of Gd-IgA1 by altering the glycosylation machinery,9 thereby perpetuating kidney damage.
In our patient’s case, he previously had a significant elevation of inflammatory biomarkers due to the bilateral COVID-19 pneumonia, with a polymerase chain reaction value of 129 mg/l (reference range 0−5 mg/l) and an IL-6 of 177 pg/ml (reference range 0−7 pg/ml). Consequently, according to our protocol, he received tocilizumab 600 mg and dexamethasone 10 mg i.v. for 5 days. We believe that a cytokine storm due to SARS-CoV-2 infection could have triggered an immunological disarrangement responsible for IgA vasculitis in our patient.
So far, the pathogenic mechanism of kidney damage associated with COVID-19 is not completely understood. The renal involvement is considered to be multifactorial. The possible underlying mechanisms are direct kidney infection through angiotensin converting enzyme−2 (ACE-2) receptors expressed in tubular cells7,S4,S5 and podocytes4,7,S5 as well as an indirect mechanism through the cytokine release syndrome characteristic of patients with COVID-19. Cytokine storm appears to be responsible for endothelialS6 and glomerular3,5,6 damage, as described in collapsing glomerulopathy cases reported so far.
Although future research will be needed to determine the role of SARS-CoV-2 in the pathogenesis of IgA vasculitis, its frequent association with respiratory viruses and the recent COVID-19−related pneumonia in our patient suggest that this case might be the first reported case of IgA vasculitis related to COVID-19 (Table 2).
Table 2.
Key teaching points
|
|
|
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Conclusion
We report a patient who developed an IgA vasculitis with nephritis after he had been hospitalized for COVID-19. This case highlights that SARS-CoV-2 could be the trigger for IgA nephritis, which indicates a need to determine a specific management for such cases. However, we cannot rule out that the IgA vasculitis is unrelated to the virus in this patient and was disclosed in the setting of COVID-19.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen C.P., Bourne T.D., Wilson J.D. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020;5:935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kissling S., Rotman S., Gerber C. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98:228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peleg Y., Kudose S., D'Agati V. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5:940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaillard F., Ismael S., Sannier A. Tubuloreticular inclusions in COVID-19-related collapsing glomerulopathy. Kidney Int. 2020;98:241. doi: 10.1016/j.kint.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J.J., Xu Y., Liu F.F. Association of the infectious triggers with childhood Henoch-Schönlein purpura in Anhui province, China. J Infect Public Health. 2020;13:110–117. doi: 10.1016/j.jiph.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Heineke M.H., Ballering A.V., Jamin A. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura) Autoimmun Rev. 2017;16:1246–1253. doi: 10.1016/j.autrev.2017.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.