Abstract
Purpose
The presence of SARS-CoV-2 RNA in anterior chamber fluid and/or the vitreous in patients with SARS-CoV-2 RNA on the ocular surface is unclear. Knowledge about the infectious state of intraocular structures could influence the daily work of ophthalmic surgeons.
Observations
We analyzed ocular samples from a patient who had succumbed to COVID-19 pneumonia for the prevalence of SARS-CoV-2 RNA. We detected viral RNA in the ocular-surface samples on one swab and in one excidate from the conjunctiva. Samples from the anterior chamber and vitreous revealed no SARS-CoV-2 RNA.
Conclusions
SARS-CoV-2 can effectively be inactivated with standard disinfection agents. The now proven absence of SARS-CoV-2 in intraocular fluids could influence how ophthalmic surgeons work. Without having to account for the risk of a contagion via the anterior chamber and/or vitreous body, the surgical staff would require no additional, more elaborate protection.
Keywords: SARS-CoV-2, Covid-19, Ocular surgery, Infectious diseases
1. Introduction
Since the outbreak of the novel coronavirus (SARS-CoV-2), the ocular surface has been suggested as a potential infection zone.1, 2, 3, 4 Whether the virus is also detectable in deeper eye tissues is not yet known. However, knowledge thereof could be particularly important for ophthalmic surgeons, since aqueous and vitreous fluid could act as an infectious medium while intraocular instruments are being changed, and aerosols particles containing SARS-CoV-2 could be dispersed. The lack of knowledge about SARS-CoV-2 RNA is present in the eye's deeper compartments has already led to different protective recommendations. Various professional associations have suggested wearing filtering face-piece (FFP) 3 masks and protective eye wear during surgical procedures like vitrectomies to account for possible aerosol production.5,6 Below, we report on the ocular findings from a 72-year-old patient who died of CoVid-19 pneumonia.
1.1. Medical history
On April 28, 2020, a 72-year-old female patient - already intubated and ventilated - was transferred from an external hospital to the intensive care unit at the University Medical Center Goettingen. She died the following day of severe septic shock and multiple organ failure due to CoVid-19 pneumonia. There was no documentation of eye involvement in her medical records.
2. Material and methods
A few hours after death, samples of various eye tissues were removed from the left eye during autopsy: a conjunctival swab, two conjunctival excidates, one anterior chamber fluid sample and one vitreous sample (1 ml each). The conjunctival swab was taken with a Copan Liquid Amies Elution Swab (eswab™, COPAN Diagnostics, CA, USA). One conjunctival excidate was carried in a container with 0.9% saline solution, the other conjunctival excidate was fixed in glutaraldehyde. All but the sample with glutaraldehyde were tested for the presence of SARS-CoV-2 viral RNA using the Genesig COVID-19 in vitro Real-Time diagnostic PCR assay (Primerdesign Ltd, Chandlers Ford, UK). The sample fixed in glutaraldehyde was analyzed under an electron microscope.
3. Results
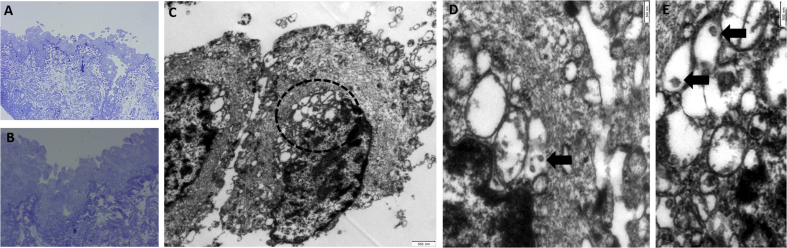
PCR-analysis revealed SARS-CoV-2 RNA in the conjunctival swab and conjunctival excidate. Samples from the aqueous and vitreous revealed no definitive RNA. The histological, electron microscopic analysis revealed virus – like components in the conjunctival excidate (Fig. 1).
Fig. 1.
(A,B) Conjuctival epithelium. Epithelial cells appear to be swollen and are focally detaching (Semithin Section). (C) Two swollen epithelial cells. Note the swollen bleb-like organelles adjacent to the nucleus (dotted line, TEM) (D,E). Higher Magnification reveals single virus-like particles (size: diameter 100-150 nm) within vesicles (arrows, TEM).
Higher Magnification reveals single virus-like particles within vesicles (arrows, TEM).
4. Discussion
In their letter published recently, Lu et al. already suspected that a SARS-CoV-2 infection could occur through the ocular surface.3 They based their assumption on reports from Guangfa Wang, an expert on the national pneumonia committee who had become infected during an inspection in Wuhan wearing an N95 mask but no eye protection.3 As angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) are the key proteins enabling SARS-CoV-2's entry into host cells,7 Zhang et al. analyzed several ocular tissues of 30 mice for the prevalence of the aforementioned proteins. The largest number was identified in the conjunctiva, leading them to conclude that it might act as a target issue for SARS-CoV-2 infection.4 Xie et al. analyzed the conjunctival swabs from 33 COVID-19 patients for SARS-CoV-2 positive PCR results. None of the patients in the study presented ocular symptoms, but viral RNA was detected in 2 of them.1 These findings concur with our results, as we also demonstrated SARS-CoV-2 RNA in the conjunctiva. Moreover, we have shown that our patient's intraocular fluids were not infected despite the ocular surface's proven infection. In addition to the fact that standard disinfection agents suffice to inactivate SARS-CoV-28, this information could affect how ophthalmic surgeons work. Without having to account for the risk of contagion via the anterior chamber and/or vitreous body, the surgical staff would require no additional, more elaborate protection. Regarding the histological analysis, electron microscopic imaging revealed virus - like components in the conjunctival excidate comparable to the SARS- CoV virus particles described by Goldsmith et al.9 in 2004, but in a much smaller number. Further pathological analyses will be necessary to support the results reported here.
Patient consent
Written consent to publish this case has not been obtained. This report does not contain any personal identifying information.
Funding
We acknowledge support by the Open Access Publication Funds of the Göttingen University.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Xie H.-T., Jiang S.-Y., Xu K.-K. SARS-CoV-2 in the ocular surface of COVID-19 patients. Eye Vis (Lond). 2020;7:23. doi: 10.1186/s40662-020-00189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napoli P.E., Nioi M., d'Aloja E., Fossarello M. The ocular surface and the coronavirus disease 2019: does a dual 'ocular route' exist? J Clin Med. 2020;9(5) doi: 10.3390/jcm9051269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C-w, Liu X-f, Jia Z-f. SARS-CoV-2 transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B.N., Wang Q., Liu T. Expression analysis of SARS-CoV-2 related ACE2 and TMPRSS2 in eye tissues. Zhonghua Yan Ke Za Zhi. 2020;56:E011. doi: 10.3760/cma.j.cn112142-20200310-00170. 0. [DOI] [PubMed] [Google Scholar]
- 5.Chao D. asrs_recommendations_conducting_vr_surgery_during_covid_19_pandemic. https://www.cosprc.ca/wp-content/uploads/2020/04/asrs_recommendations_conducting_vr_surgery_during_covid_19_pandemic.pdf
- 6.Price L. Vitreoretinal-surgery-management-guidance-070420. https://www.rcophth.ac.uk/wp-content/uploads/2020/04/Vitreoretinal-surgery-management-guidance-070420.pdf
- 7.Zhou P., Yang X.-L., Wang X.-G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fathizadeh H., Maroufi P., Momen-Heravi M. Protection and disinfection policies against SARS-CoV-2 (COVID-19) Inf Med. 2020;28(2):185–191. [PubMed] [Google Scholar]
- 9.Goldsmith C.S., Tatti K.M., Ksiazek T.G. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10(2):320–326. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]