Highlights
-
•
R t can be used to closely monitor the non-pharmaceutical interventions (NPI) of the COVID-19 epidemic.
-
•
Activity of the epidemic in Oman is examined.
-
•
Other factors, like the return of overseas students, have increased the epidemic activity.
-
•
Responses to NPI are different between migrants and natives.
Keywords: COVID-19 epidemiology, Reproductive number, Public health intervention
Abstract
Background
COVID-19’s emergence carries with it many uncertainties and challenges, including strategies to manage the epidemic. Oman has implemented non-pharmaceutical interventions (NPIs) to mitigate the impact of COVID-19. However, responses to NPIs may be different across different populations within a country with a large number of migrants, such as Oman. This study investigated the different responses to NPIs, and assessed the use of the time-varying reproduction number (Rt) to monitor them.
Methods
Polymerase chain reaction (PCR) laboratory-confirmed COVID-19 data for Oman, from February 24 to June 3, 2020, were used alongside demographic and epidemiological information. Data were arranged into pairs of infector–infectee, and two main libraries of R software were used to estimate reproductive number (Rt). Rt was calculated for both Omanis and non-Omanis.
Findings
A total of 13,538 cases were included, 44.9% of which were Omanis. Among all these cases we identified 2769 infector–infectee pairs for calculating Rt. There was a sharp drop in Rt from 3.7 (95% confidence interval [CI] 2.8–4.6) in mid-March to 1.4 (95% CI 1.2–1.7) in late March in response to NPIs. Rt then decreased further to 1.2 (95% CI 1.1–1.3) in late April after which it rose, corresponding to the easing of NPIs. Comparing the two groups, the response to major public health controls was more evident in Omanis in reducing Rt to 1.09 (95% CI 0.84–1.3) by the end of March.
Interpretation
Use of real-time estimation of Rt allowed us to follow the effects of NPIs. The migrant population responded differently than the Omani population.
Introduction
The emergence of a new infectious disease carries with it many challenges and uncertainties regarding its natural history, clinical course, transmissibility, and methods of control. With these in mind, the leading resort for control when there is no treatment or vaccine is to apply well-known and strict public-health measures to mitigate and prevent its spread (Davies et al., 2020a, Lai et al., 2020). These measures were implemented by the Chinese government after the reporting of 27 cases of corona virus disease 2019 (COVID-19) in Wuhan, in China’s Hubei province, on 30 December 2019. Measures included the isolation of confirmed and suspected cases, and restriction of movement inside the country (Kraemer et al., 2020). During the two months following the appearance of COVID-19, and despite the measures that were taken, international travel spread cases to 26 countries around the world (Wells et al., 2020), after which the World Health Organization (WHO) announced COVID-19 to be a worldwide pandemic (World Health Organization, 2020), with daily cases reaching 6,080,963 by the 1 June (European Centre for Disease Prevention and Control, 2020).
With the increasing number of cases and the introduction of multiple NPIs by most countries, there is a need to assess and monitor the transmissibility of the disease as a measurement of the effectiveness of control measures. The effective reproduction number, R t, defined as the average number of secondary cases from a partially susceptible population per infectious case (Delamater et al., 2019), is a universally applied indicator for assessing the effectiveness of control measures (Distante et al., 2020).
Oman is a country of 4.60 million, and has a heterogeneous population with migrants constituting 41% of the population (National Center for Statistical Information, 2020). The effects of the control and the dynamics of transmission of COVID-19 were expected to be different between these two populations—Omanis and non-Omanis. Similar situations that have led to increasing numbers of cases have been documented in many neighbouring countries of the Gulf Cooperation Council (GCC) (Ministry of public health, 2020, Ministry of health, 2020a) and also in Singapore (COVID-19 situation report, 2020).
The government of Oman responded to the COVID-19 pandemic much like most countries by implementing multiple NPIs in phases to control the disease, beginning in mid-March 2020. Examples ranged from restricting flights from infected countries, to closing schools and commercial activities (Table 1 ).
Table 1.
Timeline for preparation phases and non-pharmaceutical intervention taken in the sultanate to prevent and contain the COVID-19 outbreak.
| Time frame | Non-pharmaceutical interventions | |
|---|---|---|
| Phase 1: Preparatory phase | Mid-February to mid-March | Recommendation to avoid travelling to China unless absolutely necessary |
| Suspension of flights to and from China | ||
| Quarantine on arrivals from China | ||
| Quarantine application on arrivals from Singapore, South Korea, Japan, and Iran (February 2) | ||
| Suspension of flights from Iran | ||
| Avoidance of travel to the above countries | ||
| Suspension of flights to and from Italy | ||
| Quarantine on arrivals from Italy | ||
| Quarantine on arrivals from Egypt | ||
| Formation of the supreme committee (March 10) | ||
| Phase 2: Major public health control phase | Starting from mid-March to mid-April | Closure of schools and higher education institutions |
| Closure of port of entries (airports, seaports, and ground crossings) | ||
| Suspension of all public transport | ||
| Closure of mosques and all non-Muslim places of worship | ||
| Closure of all movie theatres | ||
| Prohibition of public gatherings and conferences | ||
| Closure of all stores in shopping malls, with the exception of food and consumer catering shops, clinics, pharmacies, and optical stores | ||
| Closure of traditional markets, such as Mutrah, Nizwa, Rustaq, and Sinaw, and closure of popular markets, such as Wednesday markets, Thursday markets, and Friday markets | ||
| Prohibition of the provision of food in restaurants and cafes, including those in hotels, with the exception of external requests | ||
| Suspension of sports activities of all kinds, and closure of sports and cultural clubs | ||
| Closure of gyms and health clubs, and men’s and women’s barbers/hairdressers and beauty shops | ||
| Reduction of the number of employees in workplaces in government agencies to no more than 30% of the total number of employees | ||
| Closure of all exchange shops, provided that the banks provide exchange services, | ||
| Return of all overseas students (March 23) | ||
| Activation of the role of the military and security services in reducing the movement of residents and citizens | ||
| Isolation of the Governorate of Muscat until April 1 | ||
| Closure of Muscat and Mutrah (April 10) | ||
| Closure of Jaalan Bani Bu Ali (April 16) | ||
| Phase 3: Gradual easing up | From mid-April to end of May | Expanded opening of some activities in grades (April 22 and May 18) |
| Allowing 50% of governmental employees to return to work (May 31) | ||
| Opening of Muscat governorate (May 29) |
In this study, we analysed the dynamics of COVID-19 infection transmissibility in Oman in the different populations (Omani and non-Omani), and the effects of the introduction of non-pharmaceutical measures on disease transmissibility.
Methods
Data source: national COVID-19 data
Data for laboratory-confirmed COVID-19 cases in Oman, from February 24 to June 3, 2020, were included in this study. Suspected cases of COVID-19 (as per the Ministry of Health case definition) (Ministry of health, 2020b) were confirmed by PCR testing. Subsequently, all confirmed cases underwent epidemiological investigation, generating the daily final line list, which included information on each patient’s demography (age, gender, residency, nationality), epidemiology (source of infection if known, date of onset, primary case [infector] or secondary case [infectee], designation [cluster or sporadic]). The type of surveillance, whether active (proactive case finding) or passive (regular reporting by health-care institutions), was also included in the data, with only the passive surveillance cases used in our study.
Time-varying reproduction number
The basic reproductive number before mitigation starts is called R 0. The reproduction number after mitigation starts, R t, is a measure of the transmissibility of the infection, and is defined as the average number of new infections one case can produce (Delamater et al., 2019). An R t of more than 1 means that the infection is spreading, with more cases generated, whereas an R t of less than 1 means that the spread of infection is decreasing. Theoretically, we need information about the generation time—defined as the time period between the infection of the index and the next case. However, this information is usually difficult to ascertain, and therefore information regarding the serial interval (defined as the interval between disease onset in the index and the next case) distribution in the data is used instead.
Using R software, we utilised two main libraries to estimate R t—Epicontact and EpiEstim (Nagraj et al., 2017, Cori, 2013).
The main function of the Epicontact library (Nagraj et al., 2017) is to arrange the data and help in estimating the distribution parameters of the serial interval for our data. The estimate-R function in the EpiEstim library (Cori, 2013) assumes a gamma distribution of the SI and models the transmission of the infection using a Poisson likelihood to calculate the instantaneous reproduction number.
We arranged our line list into two parts, as required by Epicontact library (Nagraj et al., 2017)—the main line list data and the contact data. The daily list of all new PCR-positive cases contains all the demographic and exposure data for each case, whereas the contact data contain information about the transmission of infection between each identifier number. As case-by-case transmission data are available for many cases on the line list, we used the Epicontact R library to find out the serial interval for our data and its distribution for the entire population, and classified these data by nationality.
We used the serial interval distribution calculated by the Epicontact library to estimate the R t for the entire population, classified by nationality, for the period between February 24 and June 3, 2020. We used the estimate_R function in the EpiEstim library to calculate R t, given the distribution of the serial interval (obtained by the Epicontacts library) and incidence time series. This was done through a sliding window of 7 days using the Poisson transmission model (Cori et al., 2013, Thompson et al., 2019).
The comparison of R t values between Omanis and non-Omanis was performed after extracting R t values from each estimate_R object and plotting the two time series against each other.
To investigate the differences in behaviour of transmission for the two groups, the epidemic curve trend was investigated according to cluster/sporadic types for Omanis versus non-Omanis using the geom_smooth function in a ggplot2 library (Wickham, 2016).
Statistical analysis
All data cleaning and statistical analyses were carried out using R software version 3.6.3 (R Core Team, 2020). Two R packages were used to estimate the time-varying reproduction number—Epicontacts and EpiEstim (Nagraj et al., 2017, Cori, 2013). Plots of incidence were produced using the R software’s ‘incidence library’ (Jombart et al., 2020). The comparison of Rt values for the different groups was performed using a simple line plot.
Results
As of June 3, 2020, a total of 13,538 PCR laboratory-confirmed COVID-19 cases had been included in this study. Only passive cases presented to the health institutions were included, with the active surveillance cases (totalling 1974) removed from the dataset. Of the included cases, 44.9% were Omanis, with the majority of these (71.1%) in the Muscat governorate, where Oman’s capital city, Muscat, is located. Among all included cases, 2769 infector–infectee pairs data were identified and included in the contact dataset. The median serial interval was estimated to be 6 (IQR 3–14).
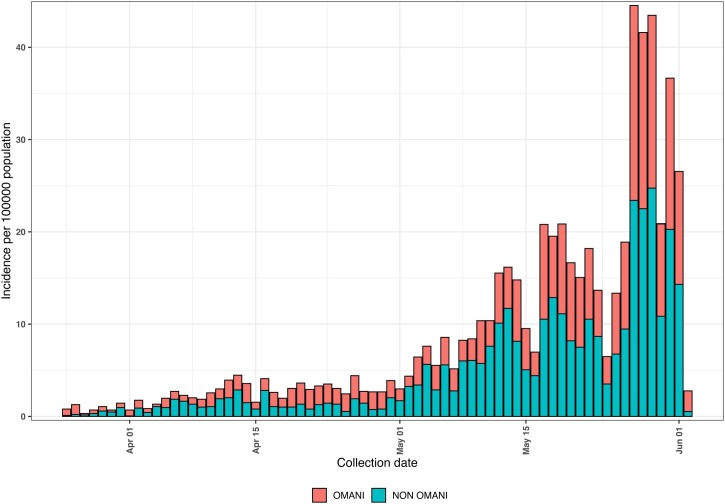
The daily epidemic curve, by date of onset, for passive laboratory-confirmed COVID-19 cases classified by nationality—Omani and non-Omani—is shown in (Figure 1 ). The first cases registered in Oman were in two women returning from a visit to Iran on February 24. There was a doubling of cases from the middle of March to the end of May, noticed in both nationality groups.
Figure 1.
Daily COVID-19 epidemic curve by date of collection for Omanis compared with non-Omanis, February 24 to June 3, 2020.
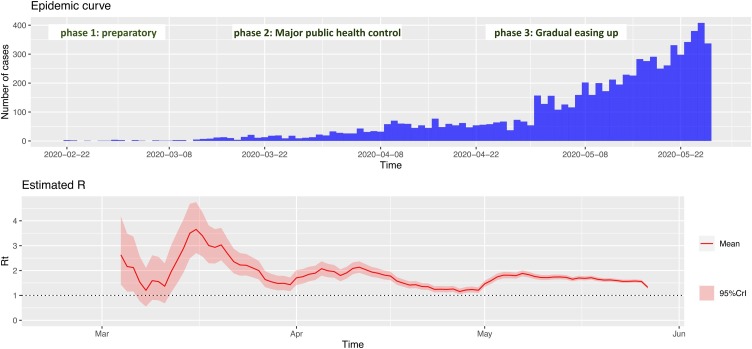
Figure 2 shows the epidemic curve with the corresponding reproduction numbers between February 24 and June 3, 2020 for the total population. There was a sharp drop in R t from 3.7 (95% CI 2.8–4.6) in mid-March to 1.4 (95% CI 1.2–1.7) towards the end of March. Subsequently, R t started to rise again and fluctuated between 2.1 (95% CI 1.8–2.1) at the start of April and 1.3 (95% CI 1.2–1.5) in mid-April. This effect corresponded with the implementation of phase 2 of NPIs (major public health control—Table 1). Figure 2 also shows that R t decreased to approximately 1.2 (95% CI 1.1–1.3) towards the end of April, when it started to rise again, corresponding with the start of phase 3 and the gradual easing of restrictions.
Figure 2.
Daily COVID-19 epidemic curve with corresponding reproduction number by date of onset for Oman, February 24 to June 3, 2020. The phases of public health control measures are also shown.
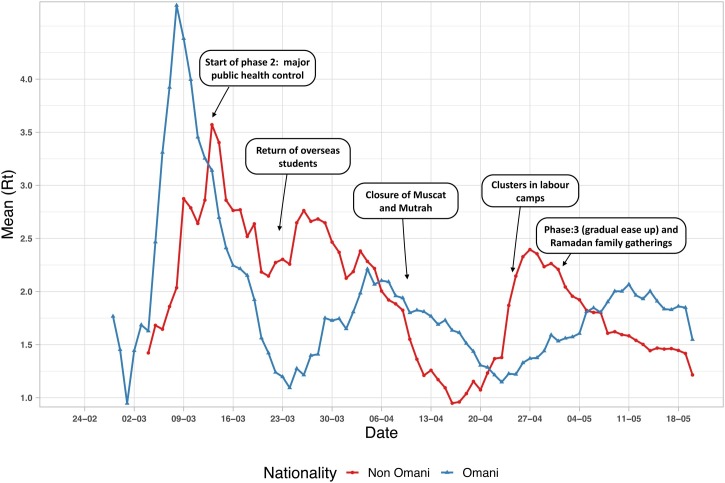
Analysis of the trend in R t for the different nationality groups is presented in Figure 3 , with the events corresponding with those in Table 1. Generally, there was a difference in R t trends between the two groups. The response to major public health control (phase 2) was more evident in Omanis in reducing R t to 1.09 (95% CI 0.84–1.3) by the end of March. Nevertheless, closure of Muscat and Mutrah (the old market area) led a marked reduction in R t for the non-Omani group, reaching 0.9 (95% CI 0.8–1.1) by mid-April.
Figure 3.
Rt trend variation between Omanis and non-Omanis, February 24 to June 3, 2020. The phases of public health control measures and events are also shown. The arrows indicate the start of public health control measures. The mean value corresponds to the start date of the 7-day moving window.
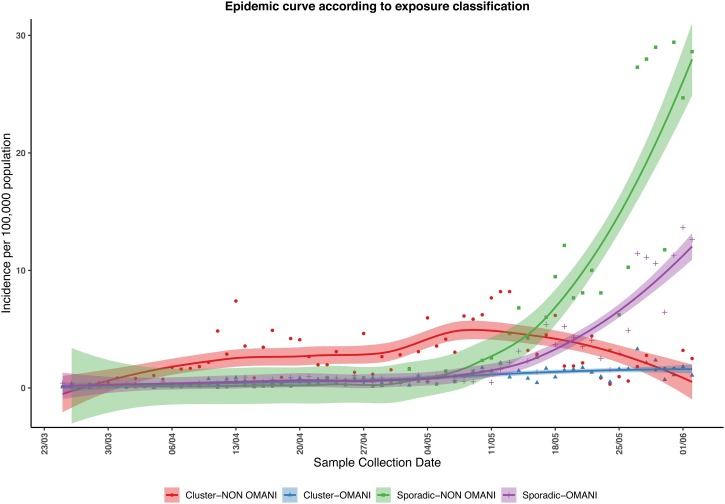
Figure 4 illustrates an explanation for the differences in R t between the two groups, showing a trend towards a cluster in the non-Omani population peaking around May 9. In addition, the number of cases belonging to a cluster tended to remain stable in the Omani group and to decline in the non-Omani group, while the number of sporadic cases not linked to a known cluster increased in both groups (Figure 4).
Figure 4.
The trend of incidence per 100,000 population (with 95% CI) for daily cases in Omanis and non-Omanis. After June 1, the number of cases that could not be linked to known clusters increased rapidly. A cluster of cases is where more than two cases can be linked to a common index case. A sporadic case is one that cannot be linked to an existing cluster.
Discussion
With the increasing numbers of COVID-19 cases in Oman, our study showed the feasibility of using the time-varying R t to assess and explain transmissibility dynamics and epidemic progression. We showed that there was a marked reduction in the reproduction number for COVID-19 infections in Oman in response to the major public health control introduced by the government. However, this reduction was less evident in the non-Omani group compared with the Omanis. Moreover, the closure of Muscat (specifically the old market area) drastically decreased R t, presumably because of of high proportion of immigrants in Muscat. The estimated median serial interval in our study was 6 (IQR 3–14). Previous studies have estimated this parameter to be 4.6 days (95% CrI: 3.5–5.9) and 4.9 days (95% CI: 3.6–6.2) (Nishiura et al., 2020, Zhao et al., 2020a).
The daily number of new cases is known to be influenced by testing capacity. Moreover, a study from China demonstrated that changes in reporting rates substantially affect estimates of R t (Zhao et al., 2020b). Thus, the initial R t is influenced by testing frequency, contact tracing, and reporting of mild cases outside hospital. For these reasons, R t will fluctuate from country to country. However, the use of R t in assessing the transmissibility dynamics and epidemic progression in this Oman study was a crucial tool for establishing the influences of mitigation measures.
The development of the pandemic in Oman has followed the trend seen in other countries that could not curb the outbreak before the spread of COVID-19 in the community. Oman introduced most of the same mitigation measures implemented in other countries—stopping international travel by closing the points of entry (airports, seaports, and ground crossings), closing schools and shops, sending public- and private-sector workers home, introducing the use of masks in public spaces, and restricting movement in districts with particularly high densities of people, such as the old markets in Muscat. A modelling study from China showed how the comprehensive package of mitigation interventions China implemented in Hubei province caused R t to decline rapidly to less than 1 in areas where NPIs were implemented (Leung et al., 2020). Another study based on data from Wuhan, China found that mitigation measures reduced the median number of infections by more than 92% (IQR 66–97) and by 24% (13–90) at the peak and at the post peak of the outbreak, respectively (Prem et al., 2020).
On the basis of exponential curve prediction, and the assumption that the duration of infection ranges from 15 to 20 days, a study from Italy estimated the R 0 to be between 2.8 and 3.3 (Remuzzi and Remuzzi, 2020). This number is similar to that reported for the initial phase of the infection outbreak in Wuhan (Leung et al., 2020), and slightly higher than the 2.2 reported by Li et al. in a more recent report (Li et al., 2020). These numbers are similar to those found in our study.
Throughout the outbreak, the number of cases has been higher in the migrant population compared with Omani nationals. This reflects differences in living conditions, where migrant workers often share dormitories, sleeping in close proximity—conditions that favour transmission of a respiratory virus.
Many of the disease clusters in the Omani population are present because Omanis tend to live in extended families (5–12 per household), thereby increasing the possible number of contacts for each primary case. A study from the UK found that a 74% reduction in the average daily number of contacts observed per participant (from 10.8 to 2.8) would be sufficient to reduce R t from 2.6 prior to lockdown to 0.62 (95% CI 0.37–0.89) after the lockdown, based on all types of contact (Jarvis et al., 2020). Another study from China also found that transmission of COVID-19 was prominent in family clusters (Jing et al., 2020).
The rise in R t by the end of March in our study was due to a considerable number of Omani students (roughly 8000) returning from abroad, even though they were placed in institutional or home quarantine. One study looked at the effects of travel restrictions in China and found that the travel quarantine imposed in Wuhan delayed the overall epidemic progression by only 3–5 days in mainland China, but had a more marked effect on an international scale, where case importation was reduced by nearly 80% until mid-February (Chinazzi et al., 2020). The travel restrictions in Oman were effective, except for food supply trucks, which continued over the border with the United Arab Emirates, and between the locked-down governorates.
The situation in Oman is complicated by having two major and socio-economically different population groups—Omani nationals and migrants. So far, countries with this population structure have been unable to control the outbreak as efficiently as countries with homogeneous populations, with Taiwan, South Korea, and Hong Kong as prime examples (Kim et al., 2020, Wang et al., 2020). In Singapore, the outbreak expanded rapidly once the infection spread into migrant worker dormitories (COVID-19 situation report, 2020), with 731 cases per 100,000 population confirmed by June 16. Qatar, another country with a large migrant population, has reported 3080 positive cases per 100,000 population since the start of the outbreak (Ministry of public health, 2020), while Saudi Arabia has reported 445 cases per 100,000 population (Ministry of health, 2020a).
Easing mitigation efforts in Oman increased the number of cases, and it can be argued that the opening of small businesses and shops happened too early. However, the overall situation was complex because many small shop owners and self-employed had had no income for months. Nevertheless, with this transmissibility and the way in which Oman handles communicable diseases, the crude mortality rate from COVID-19 in Oman remains comparably low, at 0.4%.
Isolation and contact tracing reduce the time during which cases are infectious in the community, thereby reducing R t. The overall impact of isolation and contact tracing, however, is uncertain and highly dependent on the number of asymptomatic cases (Kucharski et al., 2020). As case numbers rise, the burden of contact tracing and quarantining increases. A modeling study from the US indicated that, in such a situation, emphasis should be on physical distancing and contact tracing, whereas isolation should be prioritised for persons with a high risk of transmission to others (Peak et al., 2020).
An analysis of the four major clusters in South Korea estimated R t to be 1.5 (95% CI 1.4–1.6). The intrinsic growth rate was estimated to be 0.6 (95% CI 0.6–0.7), and the scaling of growth parameter was estimated to be 0.8 (95% CI 0.7–0.8), indicating sub-exponential growth dynamics for COVID-19. The results indicate an early sustained transmission of COVID-19 in South Korea, and support the implementation of social distancing measures to rapidly control the outbreak (Shim et al., 2020).
One model found that the effects of physical distancing strategies varied across age categories, with the reduction in incidence being highest among school children and older individuals, and the lowest among working-age adults. Children are at a similar risk of infection to the general population, but less likely to have severe symptoms; hence they should be considered in analyses of transmission and control (Bi et al., 2020). It is a limitation of this study that we did not stratify R t into different age groups (Davies et al., 2020b).
Another limitation of this study is that the testing capacity increased as the pandemic progressed. Using daily time series of COVID-19 incidence, epidemic curves of reported cases may not always reflect the true epidemic growth rate due to changes in testing rates, with limited diagnostic testing capacity during the early epidemic phase (Omori et al., 2020).
A third limitation of the study is the increasing number of sporadic cases by the end of the study period, indicating a lag in the identification and hence classification of the source of infection. This is likely due to the overwhelmed and fatigued public health workforce in the country. There is, therefore, a call for increasing and training this workforce to help it cope with current and future epidemics. In the short term, the introduction of advanced technologies (such as artificial intelligence and location tracking systems) will help public health professionals in this battle against COVID-19.
Conclusion
The use of real-time estimation of R t allowed us to follow the effects of the mitigation strategies adopted by the government. Our analysis shows that the migrant population behaves differently from the native population, and that the COVID-19 infection is spreading more rapidly in non-Omanis mainly because of their special living conditions.
Authors’ contributions
Adil Al Wahaibi conducted the statistical analysis and wrote the draft manuscript. Abdullah Al Manji wrote the introduction and Table 1. Amal Al-Maani, Fatma Alyaquobi, Khalid Said Al Harthy, Bader Al Rawahi, and Amina Al Jardani reviewed and edited the manuscript. Eskild Petersen wrote the discussion and contributed to the overall manuscript. Seif Al-Abri supervised the study and participated in all stages of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
This study was funded by the Ministry of Health’s Directorate General for Disease Surveillance and Control, as part of its operational research.
Ethical approval
This study was approved by the Directorate General for Disease Surveillance and Control. There was no need for patients’ consent because the study was anonymous and used data produced for public health purposes.
Acknowledgment
The authors would like to thank Lesley Carson for her editorial assistance in finalizing the manuscript.
References
- Bi Q., Wu Y., Mei S. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinazzi M., Davis J.T., Ajelli M. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020 doi: 10.1126/science.aba9757. eaba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cori A. R Package Version; 2013. EpiEstim: A Package to Estimate Time Varying Reproduction Numbers From Epidemic Curves; p. 1. [Google Scholar]
- Cori A., Ferguson N.M., Fraser C., Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178:1505–1512. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Singapore. COVID-19 Situation Report. https://covidsitrep.moh.gov.sg/ [Accessed 19 June 2020].
- Davies N.G., Kucharski A.J., Eggo R.M., Gimma A., Edmunds W.J. Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: a modelling study. Lancet Public Health. 2020;5(7):e375–e385. doi: 10.1016/S2468-2667(20)30133-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Eggo R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- Delamater P.L., Street E.J., Leslie T.F., Yang Y.T., Jacobsen K.H. Complexity of the basic reproduction number (R0) Emerg Infect Dis. 2019;25:1–4. doi: 10.3201/eid2501.171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distante C., Piscitelli P., Miani A. Covid-19 outbreak progression in Italian regions: approaching the peak by march 29th. medRxiv. 2020 doi: 10.1101/2020.03.30.20043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.ecdc.europa.eu/en/covid-19-pandemicCOVID-19. [Accessed 2 June 2020].
- Jarvis C.I., Van Zandvoort K., Gimma A. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18:124. doi: 10.1186/s12916-020-01597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Q.-L., Liu M.-J., Zhang Z.-B. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30471-0. S1473309920304710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jombart T., Kamvar Z.N., FitzJohn R. 2020. Incidence: Compute, Handle, Plot and Model Incidence of Dated Events. [DOI] [Google Scholar]
- Kim J.-H., An J.A.-R., Min P., Bitton A., Gawande A.A. How South Korea responded to the COVID-19 outbreak in Daegu. NEJM Catal. 2020:1. CAT.20.0159. [Google Scholar]
- Kraemer M.U.G., Yang C.-H., Gutierrez B. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020 doi: 10.1101/2020.03.02.20026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski A.J., Klepac P., Conlan A.J.K. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S., Ruktanonchai N.W., Zhou L. Effect of non-pharmaceutical interventions for containing the COVID-19 outbreak in China. Infect Dis (except HIV/AIDS) 2020 doi: 10.1101/2020.03.03.20029843. [DOI] [Google Scholar]
- Leung K., Wu J.T., Liu D., Leung G.M. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395:1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://covid19awareness.sa/en/home-pageHome Page. Prot. COVID-19. [Accessed 19 June 2020].
- Ministry of Health, Oman. Health care Workers Section. https://www.moh.gov.om/en/-56 [Accessed 16 June 2020].
- Ministry of Public Health, State of Qatar. COVID19 Home. https://covid19.moph.gov.qa/EN/Pages/default.aspx [Accessed 19 June 2020].
- Nagraj V.P., Jombart T., Randhawa N., Sudre B., Campbell F., Crellen T. 2017. Epicontacts: Handling, Visualisation and Analysis of Epidemiological Contacts.https://CRAN.R-project.org/package=epicontacts [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.ncsi.gov.om/Pages/NCSI.aspxGovernment of Oman. Main Page. [Accessed 12 February 2018].
- Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori R., Mizumoto K., Chowell G. Changes in testing rates could mask the novel coronavirus disease (COVID-19) growth rate. Int J Infect Dis. 2020;94:116–118. doi: 10.1016/j.ijid.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak C.M., Kahn R., Grad Y.H. Comparative impact of individual quarantine vs. active monitoring of contacts for the mitigation of COVID-19: a modelling study. Infect Dis (except HIV/AIDS). 2020 doi: 10.1101/2020.03.05.20031088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prem K., Liu Y., Russell T.W. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261–e270. doi: 10.1016/S2468-2667(20)30073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.N., Stockwin J.E., van Gaalen R.D. Improved inference of time-varying reproduction numbers during infectious disease outbreaks. Epidemics. 2019;29:100356. doi: 10.1016/j.epidem.2019.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323:1341. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- Wells C.R., Sah P., Moghadas S.M. Impact of international travel and border control measures on the global spread of the novel 2019 coronavirus outbreak. Proc Natl Acad Sci U S A. 2020;117:7504–7509. doi: 10.1073/pnas.2002616117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. Springer-Verlag; New York: 2016. ggplot2: Elegant Graphics for Data Analysis.https://ggplot2.tidyverse.org [Google Scholar]
- WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ [Accessed 2 June 2020].
- Zhao S., Gao D., Zhuang Z. Estimating the serial interval of the novel coronavirus disease (COVID-19): a statistical analysis using the public data in Hong Kong from January 16 to February 15, 2020. Review. 2020 doi: 10.21203/rs.3.rs-18805/v3. [DOI] [Google Scholar]
- Zhao S., Lin Q., Ran J. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]