Abstract
目的
在中国和欧洲人群中比较IgA肾病易感基因遗传多态性的种族差异。
方法
检索欧洲人群中已报道的IgA肾病易感基因遗传位点,验证其在中国人群(1 194例患者和902例对照)中的关联性,比较两个人群中显著相关位点的危险等位基因型及其基因型频率、效应OR值、人群归因风险百分比之间的差异。利用所有相关位点计算遗传危险度评分,比较其在亚欧人群中的分布,分析其与临床表型的关联。
结果
共有11个遗传座位上16个独立相关遗传位点与欧洲人群中IgA肾病的遗传易感性显著相关。93.75%(15/16)欧洲人相关遗传位点在中国人群中得到独立验证(P<0.05),且危险等位基因型在两个人群中一致。与欧洲人群相比,中国人群遗传位点拥有更高的危险等位基因型频率(P=3.09×10 -2)、OR值(P=1.94×10 -2)和人群归因风险百分比(P=3.03×10 -4)。中国人群IgA肾病患者与健康对照、东亚人群与欧洲人群相比遗传危险度评分更高(P值分别为3.60×10 -27和1.78×10 -163),东亚人群和欧洲人群各亚组之间差异无统计学意义。研究人群中IgA肾病遗传危险度评分与血浆IgA1水平、慢性肾脏病(chronic kidney disease,CKD)分期和Hass分级显著相关。
结论
IgA肾病相关易感基因在欧洲人群和中国人群相似,中国人群有更高遗传风险,提示亚洲人群中IgA肾病高患病率具有遗传基础。
Keywords: IgA肾病, 多态性, 单核苷酸, 中国人群, 欧洲人群
Abstract
Objective
To compare the genetic architecture of susceptibility variants of IgA nephropathy (IgAN) in Chinese and Europeans.
Methods
We selected the independent genome-wide significant variants of IgAN in European population as candidate variants. Their associations, risk alleles, risk allele frequencies, odds ratios and population attributable risk scores were derived and calculated, then compared with those in the current Chinese population, including 1 194 IgAN patients and 902 controls. Using the significant variants, genetic risk scores were calculated and compared between the East Asians and the Europeans. The correlation between the genetic risk scores and clinical manifestations was also evaluated.
Results
There were 16 independent single nucleotide polymorphisms (SNPs) located in 11 loci showing significantly association with susceptibility to IgAN in the Europeans. 93.75% (15/16) of them also showed significant associations in the Chinese (P<0.05). The effects of all the associated SNPs were in the same direction, either risk or being protective for IgAN, between the Chinese and the Europeans. On the contrary, remarkable higher risk allelic odds ratio (P=1.94×10 -2), higher risk allele frequency (P=3.09×10 -2), and higher population attributable risk (P=3.03×10 -4) were observed for most of the associated SNPs in the Chinese than in the Europeans. Furthermore, genetic risk scores were significantly larger in the Asian populations compared with the Europeans (P=1.78×10 -163). While there was no significance among the subpopulations in both the East Asians and the Europeans. Compared with the healthy controls, the genetic risk score in the IgAN patients was significantly larger (P=3.60×10 -27). Clinical analysis showed the genetic risk score was positively associated with serum levels of IgA and IgA1, phases of chronic kidney disease and Haas grades.
Conclusion
Our study provides further evidence in the shared genetic architecture between Chinese and Europeans, while diffe-rences with respect to the effect sizes and risk allele frequencies across ethnicities, contributing partially to the differences of disease prevalence.
Keywords: IgA nephropathy, Polymorphism, single nucleotide, Chinese, Europeans
IgA肾病(IgA nephropathy,IgAN)是全球最常见的原发性肾小球肾炎,患者在10~20年内将有5%~30%进展至终末期肾脏病。IgAN发病的家庭聚集现象和不同种族人群之间发病率差异提示遗传因素参与了IgAN的发病[1,2,3]。全基因组关联研究(Genome Wide Association Study,GWAS)发现多个IgAN相关的遗传座位,包括抗原提呈[主要组织相容性复合体(major histocompatibility complex,MHC)]、补体系统[补体因子H相关蛋白1/3(complement factor H-related protein 1/3,CFHR1/3)和整合素αM/αX(integrin αM/αX,ITGAM-ITGAX)]、调控IgA产生[肿瘤坏死因子配体超家族成员13(tumor necrosis factor ligand superfamily member 13,TNFSF13)和白血病抑制因子/抑瘤素M(leukemia inhibitory factor/oncostatin,LIF/OSM]、固有免疫[α防御素(α-defensin,DEFA)、胱天蛋白酶富集域家族成员9(caspase recruitment domain family member 9,CARD9)、ITGAM-ITGAX和鸟嘌呤核苷酸交换因子3(vav guanine nucleotide exchange factor 3,VAV3)][4,5,6,7,8],为探讨疾病致病通路提供了重要线索。然而,与欧洲人群相比,亚洲人群的IgAN患病率明显升高[3],提示IgAN患者在不同人群之间存在遗传异质性。在其他多基因遗传的复杂性疾病中,如系统性红斑狼疮多种族人群研究发现中国人群与欧洲人群之间遗传位点相似,但是中国人群具有更高的疾病遗传风险[9]。本研究拟通过检索欧洲人群中报道的IgAN易感基因遗传多态性,在中国人群中进行验证,并进一步比较中国人群与欧洲人群之间的遗传差异,旨在探讨IgAN不同种族人群发病率和表型差异的遗传基础。
1. 资料与方法
1.1. 研究对象
研究对象包括来自北京大学第一医院肾内科IgAN数据库中前期GWAS研究人群[5]、1000 Genome 3期人群中504名东亚人群和503名欧洲人群。
IgAN的GWAS研究人群为来自北方汉族的1 194 名IgAN患者[平均年龄(31.1±10.7)岁,男性百分比54%]和902名健康对照[平均年龄(31.5±8.4)岁,男性百分比67%]。IgAN患者纳入标准为:符合肾穿刺活检病理诊断,光镜下肾小球系膜细胞和系膜基质增生,免疫荧光下以IgA为主的免疫复合物颗粒样沉积于系膜区,并且至少在 2+以上,电子显微镜下系膜区有电子致密物沉积;排除标准为:通过临床表现以及实验室检查证实为其他系统疾病导致的继发性IgAN,包括慢性乙型肝炎、肝硬化、过敏性紫癜、系统性红斑狼疮等疾病。健康对照为尿液检查正常,且除外合并肾脏病及其他系统性疾病。收集IgAN患者肾穿刺活检时完整的基线临床病理资料,包括肾小球滤过率(estimated glomerular filtration rate,eGFR)、血肌酐、24小时尿蛋白定量、血浆IgA、血浆IgA1、血压、尿酸、血脂、Haas分级等信息。患者eGFR由EPI公式计算所得。IgAN数据库资料的使用获得北京大学第一医院生物医学研究伦理委员会批准,所有受试者均签署知情同意。
1.2. 单核苷酸多态性选择和基因分型
通过检索GWAS数据库(GWAS Catalog)和文献,目前3/5个IgAN的GWAS包含欧洲人群[4-5,7]。选取欧洲人群中与IgAN易感性相关的独立单核苷酸多态性(single nucleotide polymorphism,SNP)位点作为候选基因位点。
候选遗传多态性位点在IgAN研究人群中的基因分型从前期发表的GWAS数据中获取[5]。GWAS数据采用Illumina Human 610-Quad BeadChip进行基因分型,样本质控去除基因型分型成功率<95%、性别不一致、存在潜在亲缘关系或重复样本个体,位点质控去除基因型分型成功率<95%、次等位基因型频率<1%、不符合Hard-Weinberg平衡(P<1×10-4)、重复值的位点,人群分层采用主成分分析进行控制。质控后剩余1 194名IgAN患者和902名健康对照,498 329个SNP位点。对于原始GWAS数据中没有基因分型信息的SNP位点,通过对GWAS数据进行基因型填补(imputation)获取遗传关联信息。具体步骤如下:(1)从芯片数据中截取目标基因及其上、下游区段,整理成 PLINK 对应的二进制文件格式,利用UCSC 网站 LiftOver()在线平台将各芯片中SNP对应的物理位置转化为b37版本;(2)等位基因一致性检查:DNA 有正义和反义两条互补链,为了解决样本SNP 所在 DNA 链与模板数据库中DNA 所在链存在潜在不一致性的问题,将芯片分型数据统一对齐到正义链上;(3)单倍型估算:采用软件SHAPEIT2对1000 Genome亚洲人群对应目标基因片段分型数据进行单倍型估计;(4)基因型填补:IMPUTE2软件进行基因型填补;(5)基因型填补后按上述标准对样本和位点进行质控后,采用SNPTEST软件进行关联分析。
候选遗传多态性位点在1000 Genome 3期东亚人群和欧洲人群中的基因分型信息通过enseble数据库获取()。
1.3. 遗传危险度指标选择
对于单个SNP位点,根据其危险等位基因比值比(odds ratio,OR)和危险等位基因型频率(risk allele frequency,RAF)计算人口归因风险百分比(population attributable risk proportion,PARP),其公式为RAF(OR-1)/ [RAF(OR-1)+1]×100%[10]。对于所有SNP位点的加总效应,通过加权和非加权两种方法计算遗传危险度评分(genetic risk score,GRS)来评估[11,12]。非加权法遗传危险度评分(unweighted GRS,uwGRS) 计算公式为 Gi,加权法遗传危险度评分(weighted GRS,wGRS)计算公式为 ln(ORi)Gi。其中,m代表IgAN易感性相关SNP数目,Gi表示SNPi的危险等位基因型数目(即0、1或2),ORi表示SNPi的OR值。
1.4. 统计方法
GWAS数据采用PLINK软件(http://www.cog-genomics.org/plink2)进行基因型关联分析。GWAS数据Imputation采用IMPUTE2软件(http://mathgen.stats.ox.ac.uk/impute/impute_v2.html)进行,参考人群选用1000 Genome 3期人群中的亚洲人群,基因型关联分析采用SNPTEST软件(http://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html)进行。
组间统计分析采用SPSS 16.0软件进行,符合正态分布的计量资料组间比较采用配对t检验、独立样本t检验、方差分析和线性回归模型;非正态分布的计量资料组间比较采用Mann-Whitney U检验和Kruskal-Wallis H检验;计数资料采用卡方检验和Logistics回归分析。P <0.05为差异有统计学意义。
2. 结果
2.1. 中国IgAN患者易感SNP位点与欧洲人群相似
本研究中1 194例IgAN患者基线临床和病理资料结果见表1。目前,共有11个基因座位上的16个独立SNP位点与欧洲人群IgAN患者易感性显著相关,其中8个SNP位点位于人类白细胞抗原基因(human leukocyte antigen,HLA)域,包括VAV3(rs17019602)、CFHR3, 1-del (rs3766404和rs6677604)、HLA(rs3115573、rs7763262、rs9275224、rs2856717、rs9275596、rs9357155、rs1883414和rs3129269)、DEFA(rs10086568)、CARD9(rs4077515)、ITGAM-ITGAX(rs11150612)、TNFSF13(rs3803800)和HORMAD2(rs2412971)。其中,15个SNP位点在本研究中国人群中有基因分型数据,1个SNP位点rs10086568的基因型数据通过Imputation获得。上述16个SNP位点在本研究中国人群中均符合Hard-Weinberg平衡,结果显示93.75%(15/16)的SNP位点与中国人群IgAN遗传易感性显著相关(P<0.05),且与欧洲人群具有相同的危险等位基因型。但相关SNP位点危险等位基因型频率和效应值在中国人群和欧洲人群存在差异,特别是rs11150612A危险等位基因欧洲人群次等位基因型(0.36)是中国人群的主要等位基因型(0.75)(表2)。
1.
1 194例IgAN患者的基线临床资料
Baseline clinical manifestations of 1 194 IgA nephrpathy patients
| Characteristic | Median or percentage |
| IQR,interquartile range; eGFR, estimated glomerular filtration rate. | |
| Age/years, IQR | 32 (25, 39) |
| Gender, male/female | 651/543 |
| Systolic blood pressure/mmHg, IQR | 120 (110, 130) |
| Diastolic blood pressure/mmHg, IQR | 80 (70, 88) |
| Hypertension/% | 47.90 |
| Urinary protein (g/24 h)/% | |
| <1 | 33.93 |
| 1-2 | 27.52 |
| 2-3.5 | 15.60 |
| ≥3.5 | 20.95 |
| eGFR/[mL/(min·1.73 m2)], IQR | 85.63 (63.52, 106.63) |
| Haas classification/% | |
| Ⅰ | 38.19 |
| Ⅱ | 6.71 |
| Ⅲ | 38.43 |
| Ⅳ | 7.87 |
| Ⅴ | 8.80 |
2.
lgAN 易感基因遗传性位点在中国人群和欧洲人群中的分布比较
Comparison of associated SNPs in Chinese and European populations
 |
2.2. 中国人群较欧洲人群危险等位基因型频率、危险等位基因OR值及人群归因风险百分比更大
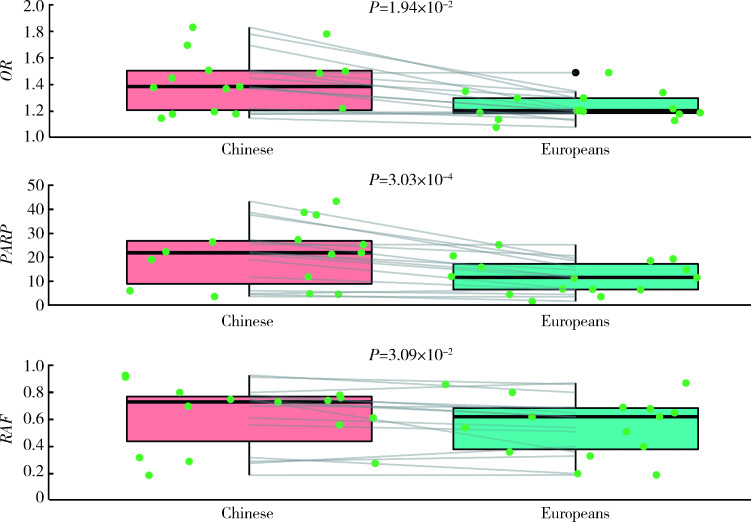
进一步分析与中国人群和欧洲人群IgAN易感性均显著相关的15个SNP的危险等位基因型频率和效应值的种族间差异发现,中国人群中危险等位基因型频率更高(P=3.09×10-2),危险等位基因型OR值更大(P=1.94×10-2),疾病归因风险百分比更高(P=3.03×10-4)(图1)。
1.
IgAN易感基因遗传性位点在中国人群和欧洲人群的疾病风险比较
Comparison of OR, PARP and RAF between the Chinese and Europeans
OR, odds ratio; PARP, population attributable risk proportion; RAF, risk allele frequency.
2.3. IgAN患者遗传危险度评分与血浆IgA1水平、慢性肾脏病分期和Haas分级相关
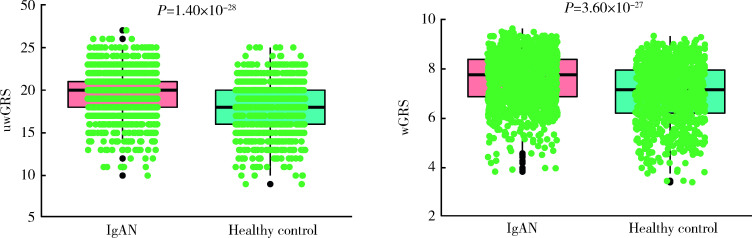
利用显著相关的SNP位点计算GRS,发现IgAN患者uwGRS和wGRS均比健康对照显著升高(P分别为1.40×10-28和3.60×10-27,图2)。进一步将相关SNP位点按其是否位于HLA区域组计算GRS,比较IgAN患者各GRS评分(非HLA-uwGRS、非HLA-wGRS、HLA-uwGRS、HLA-wGRS、uwGRS和wGRS)与临床病理表型的相关性。结果如表3所示,GRS与IgA1水平显著正相关,主要体现在非HLA区域SNP位点;GRS与慢性肾脏病(chronic kidney disease,CKD)分期和Hass分级显著相关,提示遗传因素参与病理损伤和肾功能进展。
2.
IgAN患者和健康对照遗传危险度评分比较
Comparison of genetic risk score between the IgAN patients and controls
uwGRS, unweighted genetic risk score; wGRS, weighted genetic risk score; IgAN, IgA nephropathy.
3.
lgAN 遗传风险评分与基线临床病理资料分析
Association of genetic risk score and clinical manifestations in lgA nephropathy
 |
2.4. 亚洲人群较欧洲人群遗传危险度评分更高
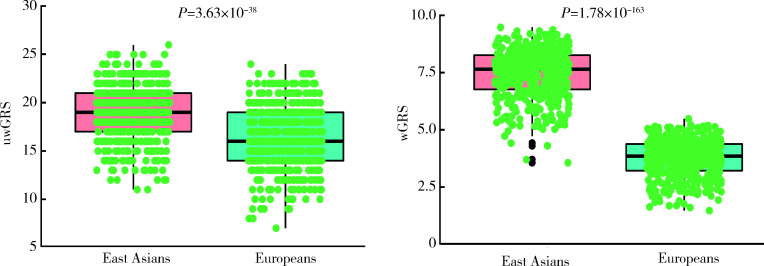
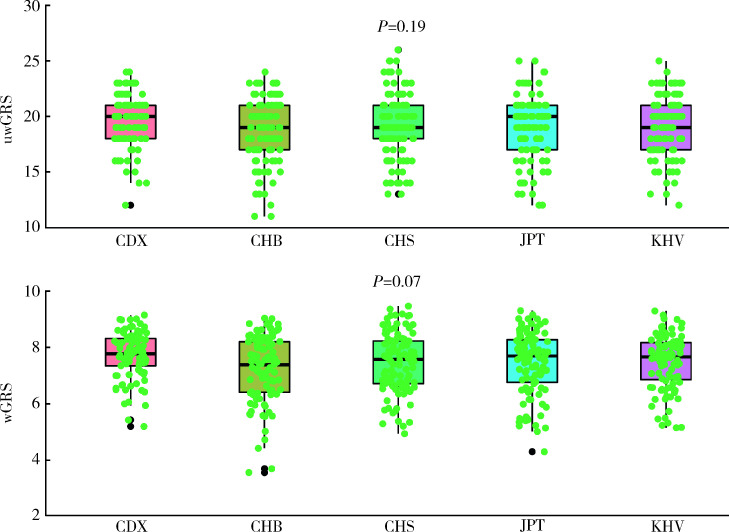
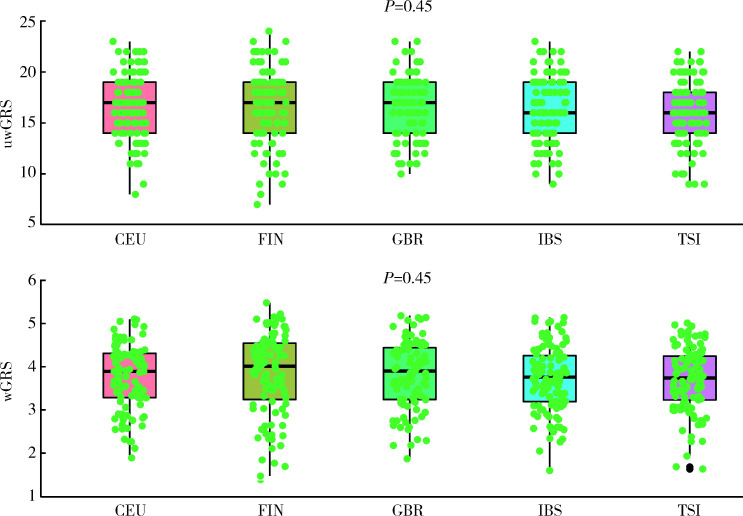
利用1000 Genome 3期人群数据计算东亚人群和欧洲人群GRS进行种族间比较发现,与IgAN患者患病率流行病学趋势相一致,东亚人群uwGRS和wGRS均显著高于欧洲人群(P值分别为3.63×10-38和1.78×10-163,图3),而东亚人群和欧洲人群各亚群之间差异未显示出统计学意义(P > 0.05,图4、5)。
3.
东亚人群和欧洲人群IgAN遗传危险度评分比较
Comparison of genetic risk score between East Asians and Europeans
uwGRS, unweighted genetic risk score; wGRS, weighted genetic risk score.
4.
东亚人群之间IgAN遗传危险度评分比较
Comparison of genetic risk score among subpopulations in East Asians
uwGRS, unweighted genetic risk score; wGRS, weighted genetic risk score; CDX, Chinese Dai in Xishuangbanna; CHB, Chinese Han in Beijing; CHS, Southern Han Chinese; JPT, Japanese in Tokyo; KHT, Kinh in Ho Chi Minh City.
5.
欧洲人群之间IgAN遗传危险度评分比较
Comparison of genetic risk score among subpopulations in Europeans
uwGRS, unweighted genetic risk score; wGRS, weighted genetic risk score; CEU, Utah residents with Northern and Western European Ancestry; FIN, Finnish in Finland; GBR, British in England and Scotland; IBS, Iberian population in Spain; TSI, Toscani in Italia.
3. 讨论
IgAN为全球范围内最常见的原发性肾小球肾炎,是我国尿毒症的主要病因。约80%的IgAN患者在20~30岁时发病,并且病情呈慢性进展,临床很难治愈。约1/3的患者在发病10年后进展到终末期肾脏病——尿毒症,只能依赖透析或肾移植维持生命,使得这群本应是社会劳动力核心的患者长期劳动力减弱或丧失,社会和家庭经济负担沉重[13,14]。IgAN的家族聚集现象和不同种族人群之间的发病率差异表明,遗传因素参与了IgAN的发病[1,2,3]。随着高通量基因分型技术的出现,基于大样本散发性人群进行的GWAS发现了多个IgAN的易感基因,包括适应性免疫反应(MHC)、补体系统(CFH、CFHR3,1和ITGAM-ITGAX)、IgA分子产生调控(TNFSF13、HORMAD2和ST6GAL1)和黏膜免疫(DEFA、CARD9、VAV3、ODF1-KLF10和UBR5)。据估算,IgAN的遗传度为50%~70%,目前发现的易感位点对IgAN遗传变异的解释度尚不足10%,提示仍有大量遗传因素未被发现。随着GWAS的开展,虽然扩大样本量将有助于进一步发现易感基因,但是单纯追求样本量的扩大越来越难,特别是对于IgAN这类人群发病率较低的疾病。多基因遗传复杂性疾病GWAS新近遗传学研究的一个关键发现是,大量遗传变异在不同种族人群中存在共性遗传位点,并且遗传危险评分与疾病流行病学发病率相一致。整合不同种族人群之间的遗传学研究为揭示发现疾病新的易感基因、揭示疾病发病机制之间的种族差异提供了重要线索[15,16,17]。然而,目前尚缺乏有关IgAN遗传学种族间差异的相关研究。
本研究基于已发现欧洲人群IgAN易感性相关位点,结合中国人群遗传信息探究了IgAN的种族间遗传差异。结果发现,在中国人群和欧洲人群,绝大部分(93.75%,15/16)遗传多态性位点的显著性和危险等位基因相一致,提示不同种族人群之间存在共同的遗传基础。通过Meta分析等研究策略,整合IgAN研究领域不同种族人群数据有望发现新的疾病易感基因。进一步比较发现,中国人群与欧洲人群相比,危险等位基因型频率、OR值和人群归因风险百分比均显著升高。IgAN患者与健康对照以及东亚人群与欧洲人群相比,GRS显著升高,并且与IgAN患者血浆IgA1水平、CKD分期和Hass分级显著正相关。IgA分子为IgAN发病机制研究中的核心分子[18,19],既往大量遗传学研究发现了多个与IgAN发病相关的易感基因,并从功能和表型上得以验证,提出遗传多态性影响IgA1分子糖基化,通过启动异常补体激活参与IgAN发生和发展的遗传发病机制假说[20,21,22]。本研究显示,遗传危险度评分与血IgA1分子水平、病理损伤程度和肾功能分期相关,为中国人更高的发病风险和更高的发病率提供了遗传学线索。
综上所述,本研究基于目前IgAN的遗传学发现,系统评价了遗传多态性位点在中国人群和欧洲人群之间的差异。不同种族人群之间存在共同的遗传基础,Meta分析利于扩大样本发现新的易感基因。此外,与欧洲人群相比,亚洲人群具有更高的遗传风险,为IgAN不同种族间发病率差异提供了来自遗传学层面的线索。后续将进一步在全基因组范围内整合和探究不同种族人群之间的遗传特点和差异。
本文编辑:赵波
Biography

张月苗,2017年毕业于北京大学,获得医学博士学位,为北京大学第一医院肾脏内科临床医师。现任全国幽门螺杆菌学组编委会编委、中国中药协会肾病中药发展研究专业委员会青年委员,被Autoimmune Disease、Clinical Science、Medicine、PLoS One等多个杂志邀请为审稿人。致力于IgA肾病遗传背景及发病机制、黏膜免疫异常与肾脏病发病以及幽门螺杆菌临床诊治研究。于2017年6—8月以访问学者身份赴香港大学学习。目前主持国家自然科学基金1项、北京市自然科学基金1项、北大医学青年科技创新培育基金1项,发表论文近20余篇,累计影响因子36.627。基于遗传学线索,发现IgA肾病的多个易感基因,及其通过调控Th17细胞和黏膜免疫参与IgA肾病发病的内在机制;基于临床随机对照研究,提出来自于黏膜的成浆细胞可能为CD20单抗美罗华(rituximab)治疗无效,而口服制剂nefecon通过在远端回肠和升结肠靶向释放糖皮质激素布地奈德显著降低IgA肾病患者尿蛋白水平的机制假说,并进行深入机制研究;检测发现IgA肾病患者中幽门螺杆菌的感染率约为55%,为黏膜免疫异常参与IgA肾病的发病和进展提供了线索,有望为IgA肾病黏膜免疫异常机制诊疗提供新的分子标志物,为靶向黏膜治疗提供新的证据和方向。
Funding Statement
国家自然科学基金(81800636)、北京市自然科学基金(7184253)和北大医学青年科技创新培育基金(BMU2017PY007)-中央高校基本科研业务费
Supported by the National Natural Science Foundation of China (81800636), Beijing Natural Science Foundation (7184253), and the Fundamental Research Funds for the Central Universities: Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (BMU2017PY007)
References
- 1.Scolari F. Familial IgA nephropathy. J Nephrol. 1999;12(4):213–219. [PubMed] [Google Scholar]
- 2.Scolari F, Amoroso A, Savoldi S, et al. Familial clustering of IgA nephropathy: further evidence in an Italian population. Am J Kidney Dis. 1999;33(5):857–865. doi: 10.1016/s0272-6386(99)70417-8. [DOI] [PubMed] [Google Scholar]
- 3.Kiryluk K, Li Y, Sanna-Cherchi S, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8(6):e1002765. doi: 10.1371/journal.pgen.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feehally J, Farrall M, Boland A, et al. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol. 2010;21(10):1791–1797. doi: 10.1681/ASN.2010010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gharavi AG, Kiryluk K, Choi M, et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43(4):321–327. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu XQ, Li M, Zhang H, et al. A genome-wide association study in Han Chinese identifies multiple susceptibility loci for IgA nephropathy. Nat Genet. 2012;44(2):178–182. doi: 10.1038/ng.1047. [DOI] [PubMed] [Google Scholar]
- 7.Kiryluk K, Li Y, Scolari F, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46(11):1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Foo JN, Wang JQ, et al. Identification of new susceptibility loci for IgA nephropathy in Han Chinese. Nat Commun. 2015;6:7270. doi: 10.1038/ncomms8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu J, Festen EA, Wijmenga C. Multi-ethnic studies in complex traits. Hum Mol Genet. 2011;20(R2):R206–R213. doi: 10.1093/hmg/ddr386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole P, MacMahon B Attributable risk percent in case-control studies. Br J Prev Soc Med. 1971;25(4):242–244. doi: 10.1136/jech.25.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prahalad S, Conneely KN, Jiang Y, et al. Susceptibility to childhood-onset rheumatoid arthritis: investigation of a weighted genetic risk score that integrates cumulative effects of variants at five genetic loci. Arthritis Rheum. 2013;65(6):1663–1667. doi: 10.1002/art.37913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlson EW, Chibnik LB, Kraft P, et al. Cumulative association of 22 genetic variants with seropositive rheumatoid arthritis risk. Ann Rheum Dis. 2010;69(6):1077–1085. doi: 10.1136/ard.2009.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365(9456):331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 15.Morris DL, Sheng Y, Zhang Y, et al. Genome-wide association meta-analysis in Chinese and European individuals identifies ten new loci associated with systemic lupus erythematosus. Nat Genet. 2016;48(8):940–946. doi: 10.1038/ng.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Ahlford A, Jarvinen TM, et al. Genes identified in Asian SLE GWASs are also associated with SLE in Caucasian populations. Eur J Hum Genet. 2013;21(9):994–999. doi: 10.1038/ejhg.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris AP, Le TH, Wu H, et al. Trans-ethnic kidney function association study reveals putative causal genes and effects on kidney-specific disease aetiologies. Nat Commun. 2019;10(1):29. doi: 10.1038/s41467-018-07867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao N, Hou P, Lv J, et al. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with di-sease progression. Kidney Int. 2012;82(7):790–796. doi: 10.1038/ki.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L, Zhai YL, Wang FM, et al. Variants in complement factor H and complement factor H-related protein genes, CFHR3 and CFHR1, affect complement activation in IgA nephropathy. J Am Soc Nephrol. 2015;26(5):1195–1204. doi: 10.1681/ASN.2014010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhai YL, Meng SJ, Zhu L, et al. Rare variants in the complement factor H-related protein 5 gene contribute to genetic susceptibility to IgA nephropathy. J Am Soc Nephrol. 2016;27(9):2894–2905. doi: 10.1681/ASN.2015010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, Guo WY, Shi SF, et al. Circulating complement factor H-related protein 5 levels contribute to development and progression of IgA nephropathy. Kidney Int. 2018;94(1):150–158. doi: 10.1016/j.kint.2018.02.023. [DOI] [PubMed] [Google Scholar]