Abstract
Three (60%) of five patients with coronavirus disease 2019 (COVID-19) had olfactory disorder. Two exhibited anosmia at the onset of COVID-19, while one had hyposmia 4 days after the onset of COVID-19. All patients with olfactory disorder were completely recovered with a mean recovery length of 11.3 days.
Keywords: Olfactory disorder, SARS-CoV-2, COVID-19
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a rapid global pandemic in 2020. As of the end of May, 2020, more than 5 million individuals have been infected with SARS-CoV-2 in 216 countries.1 In Taiwan, by the end of May, 429 laboratory-confirmed COVID-19 cases were reported to Taiwan Centers for Disease Control (CDC).2
SARS-CoV-2 is a human respiratory coronavirus and enters human cells through binding the host's angiotensin-converting–enzyme 2 (ACE2) receptor.3 High ACE2 expression has been identified in type II alveolar cells3 suggesting that the lungs are the major site of SARS-CoV-2 infection. ACE2 receptors also exist in the olfactory epithelium,3 providing SARS-CoV-2 with an entry site into the olfactory bulb and neurons. The invasion of SARS-CoV-2 into the olfactory bulb could cause the olfactory epithelium apoptosis and a decreased volume of the olfactory bulb,4 thereby possibly inducing the symptom of olfactory disorder. A case report indicated that COVID-19 patients could manifest the loss of olfactory function.5 According to two cross-sectional studies, the prevalence of olfactory disorder in COVID-19 patients ranged from 23.7% to 85.6%.6 , 7 Although several reports showed that olfactory disorder is one of the COVID-19-related symptoms, the clinical course of olfactory disorder in COVID19 patients has not been well studied. Therefore, we conducted this cohort study to characterize the clinical course of olfactory disorder in COVID-19 patients in Taiwan.
Methods
Study subjects
This cohort study included COVID-19 patients who were admitted to Taipei City Hospital (TCH) Yangming Branch between March 22 and April 3, 2020. The diagnosis of COVID-19 patients was confirmed by a positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR). All COVID-19 patients admitted to TCH were followed up until their discharge from hospital or the end of April 2020. This study was approved by the Institutional Review Board of TCH (no. TCHIRB-10904014-E).
Data collection
When COVID-19 patients were admitted to TCH, their demographic data and clinical symptoms (e.g., olfactory disorder) were recorded. Radiologic assessments and laboratory testing in COVID-19 patients were performed according to the World Health Organization's guidelines on clinical management of COVID-19 patients. Laboratory assessments on admission consisted of a complete blood cell count, tests of kidney and liver function, and measurement of electrolyte, C-reactive protein, and ferritin levels.
Clinical management
All COVID-19 patients admitted to TCH received supportive therapy, for example supplemental oxygen when saturations measured by pulse oximeters dropped below 92%. Patients' clinical symptoms, including olfactory disorder, were evaluated and recorded by their attending physicians and primary care nurses on a daily basis.
Respiratory samples (nasopharyngeal swabs and sputum) were collected at multiple time points during hospitalization and tested by RT-PCR for the presence of SARS-CoV-2. Deisolation was contingent on at least 3 consecutive negative PCR assay results at intervals of more than 24 h.
Statistical analysis
We first analyzed the patients' demographic data, clinical symptoms, and laboratory findings. Then, intergroup comparisons were examined according to individuals' olfactory functional status.
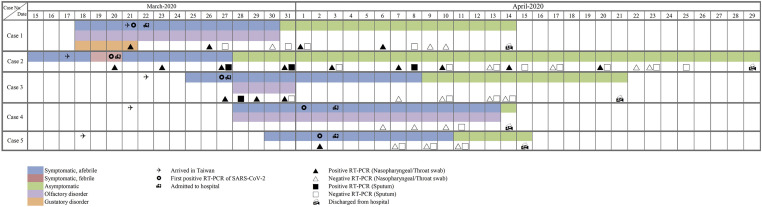
A timeline infographic was used to show the clinical course of olfactory disorder and the results of repeating RT-PCR assay in COVID-19 patients.
Result
Patients selection and epidemiologic features
Between March 22 and April 3, 2020, five Taiwanese patients infected with SARS-CoV-2 were admitted to TCH Yangming Branch, with a mean (SD) age of 25.6 (6.6) years. All patients reported having travelled to the UK (3 patients), the US (1 patient), and Germany (1 patient) 14 days prior to symptom onset. Four COVID-19 cases (80%) were identified through home quarantine screening, compared to one case (20%) through airport screening. Three of the five patients (60%) manifested olfactory disorder, including two anosmia and one hyposmia.
Characteristics and clinical features of COVID-19 patients
Characteristics and clinical features of COVID-19 patients are summarized in the Table 1 . Overall, the most common symptoms in COVID-19 patients were olfactory disorder (3 [60%]), cough (3 [60%]), rhinorrhea (3 [60%]), sore throat (2 [40%]), headache (2 [40%]), and chest tightness (2 [40%]). Patients with olfactory disorder were more likely to have gustatory disorder, rhinorrhea, and cough than those who did not suffer from this disorder.
Table 1.
Characteristics of COVID-19 patients, by the presence of olfactory disorder.
| Characteristics | No. (%) of patientsa |
||
|---|---|---|---|
| Total, n = 5 | With olfactory disorder, n = 3 | Without olfactory disorder, n = 2 | |
| Demographics | |||
| Age, yrs | |||
| Mean ± SD | 25.6 ± 6.6 | 23.3 ± 2.1 | 29.0 ± 11.3 |
| Sex | |||
| Female | 4 (80.0) | 3 (100.0) | 1 (50.0) |
| Male | 1 (20.0) | 0 | 1 (50.0) |
| Symptoms | |||
| Fever on admission | 1 (20.0) | 0 | 1 (50.0) |
| Body temperature | |||
| <37.5 °C | 3 (60.0) | 2 (66.7) | 1 (50.0) |
| 37.5–38.0 °C | 1 (20.0) | 1 (33.3) | 0 |
| >38.0 °C | 1 (20.0) | 0 | 1 (50.0) |
| Gustatory disorder | 1 (20.0) | 1 (33.3) | 0 |
| Rhinorrhea | 3 (60.0) | 2 (66.7) | 1 (50.0) |
| Headache | 2 (40.0) | 1 (33.3) | 1 (50.0) |
| Cough | 3 (60.0) | 3 (100.0) | 0 |
| Sore throat | 2 (40.0) | 1 (33.3) | 1 (50.0) |
| Palpitation | 2 (40.0) | 2 (66.7) | 0 |
| Chest tightness | 2 (40.0) | 1 (33.3) | 1 (50.0) |
| Diarrhea | 1 (20.0) | 0 | 1 (50.0) |
| Mylgia or arthralgia | 1 (20.0) | 1 (33.3) | 0 |
| Coexisting disorders | |||
| Diabetes | 1 (20.0) | 0 | 1 (50.0) |
| Radiologic findings | |||
| Abnormalities on chest X-ray | 1 (20.0) | 0 | 1 (50.0) |
| Laboratory findings | |||
| Blood leukocyte count, per mm3 (mean ± SD) | 5970.0 ± 2047.1 | 4593.3 ± 472.6 | 8035.0 ± 1449.6 |
| Lymphocyte count, per mm3 (mean ± SD) | 1817.1 ± 480.8 | 1700.2 ± 570.4 | 2127.5 ± 232.9 |
| Platelet count, per mm3 (mean ± SD) | 2,36,000 ± 46,076 | 2,29,000 ± 34,641 | 2,46,500 ± 75,660 |
| Haemoglobin level – g/dl (mean ± SD) | 14.8 ± 0.5 | 14.8 ± 0.6 | 14.8 ± 0.3 |
| C-reactive protein level ≥ 10 mg/liter | 1 (20.0) | 0 | 1 (50.0) |
| Sodium – mmol/liter, mean ± SD | 135.8 ± 4.1 | 136.0 ± 5.3 | 135.5 ± 3.5 |
| Potassium – mmol/liter, mean ± SD | 3.9 ± 0.2 | 3.9 ± 0.2 | 4.0 ± 0.4 |
| Ferritin > 150 ng/ml | 3 (60.0) | 1 (33.3) | 2 (100.0) |
COVID-19, coronavirus disease 2019; SD, standard deviation.
Unless stated otherwise.
Imaging and laboratory testing in the five COVID-19 patients showed that abnormal chest radiograph findings were found in one patient, while no patients presented a severe acute respiratory distress syndrome during hospitalization. Moreover, lymphopenia (<1.1 × 109/L) was present in 1 of 5 patients (20%), an elevated ferritin level (>150 ng/ml) in 2 of 5 (40%), and an elevated C-reactive protein level (>10 mg/L) in 1 of 5 (20%).
Clinical course of olfactory disorder and virologic features in patients infected with SARS-CoV-2
Figure 1 showed the clinical course of olfactory disorder and virologic features in COVID-19 patients. Two cases of olfactory disorder exhibited anosmia as the main symptoms at the onset of SARS-CoV-2 infection, while the other case had hyposmia 4 days after the onset of COVID-19. The mean recovery time (SD) for the patients with olfactory function was 11.3 (6.7) days. One patient with both anosmia and gustatory disorders suffered from olfactory dysfunction for 13 days. One of the other two patients with olfactory disorder recovered after 4 days, while the other after 17 days.
Figure 1.
Time course of olfactory disorder and RT-PCR assay in COVID-19 patients. Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, real-time reverse transcriptase–polymerase chain reaction; COVID-19, coronavirus disease 2019.
The median duration of viral shedding from the first to the last positive nasopharyngeal swab in all patients was 5 days (range, 1–35). Moreover, the median duration of viral shedding from the first to the last positive nasopharyngeal swab was 5 days (range, 1–17) in patients with olfactory disorder, compared to 18 days (range, 1–35) in those without this disorder. All patients with olfactory disorder fully restored olfactory function before hospital discharge.
Discussion
This case series cohort study found that the proportion of COVID-19 patients complicated with olfactory disorder was 60%. Two patients exhibited anosmia as the main symptom at the onset of SARS-CoV-2 infection, while one patient had hyposmia 4 days after the onset of COVID-19. All patients with olfactory disorder were completely recovered with a mean recovery length of 11.3 days.
The proportion of COVID-19 patients complicated with olfactory disorder in our present study (60%) was higher than that in a study conducted in Italy (23.7%)6 but lower than that in a multicenter European report (85.6%).7 Although respiratory symptoms, such as cough, sore throat, and fever, are those most commonly reported in COVID-19 patients,8 the findings of our study suggest that olfactory disorder is not uncommon in patients infected with SARS-CoV-2. Since SARS-CoV-2 is highly contagious in human, clinicians should pay special attention to the diagnosis of COVID-19 in suspected patients with olfactory disorder.
This study found that olfactory disorder could show up at the onset of SARS-CoV-2 infection or after the onset of COVID-19. Two COVID-19 patients in our study exhibited anosmia as the predominant symptom at the onset of SARS-CoV-2 infection, while one patient had the hyposmia after suffering from headache and cough during hospitalization. The findings of our study suggest that olfactory disorder could appear before or after the onset of COVID-19.
Sensory neuronal damage due to SARS-CoV-2 may account for the olfactory disorder in COVID-19 patients. SARS-CoV-2 is a neurotropic and neuroinvasive virus that enters human cells through ACE2 receptors.9 A previous animal study found that SARS-CoV could invade the olfactory nerves by binding with ACE2 receptors on the olfactory bulb.10 The invasion of SARS-CoV-2 into the olfactory bulb could cause the olfactory epithelium apoptosis and a decreased volume of the olfactory bulb,4 which may result in olfactory disorder.
Our present study found that the mean recovery time for patients with olfactory disorder was 11.3 days. Two thirds of the patients with olfactory disorder in our study had olfactory disorder for more than 4 days, which was comparable to that of a European study (67%).7 All patients with olfactory disorder in our study fully recovered their olfactory function before the RT-PCR results for SARS-CoV-2 turned negative.
This cohort study was the first to characterize the clinical course of olfactory disorder in COVID-19 patients. Nevertheless, the present study has three limitations. First, since Taiwan CDC successful contained the spreading of SARS-CoV-2 in the community, limited patients were diagnosed with SARS-CoV-2 infection during the COVID-19 pandemic.2 Limited COVID-19 cases in this study may preclude this analysis from estimating the precise prevalence of olfactory disorder in patients infected with SARS-CoV-2. However, consistent with a current report,7 the findings of our study suggest that olfactory disorder is not an uncommon symptom in COVID-19 patients. Second, the diagnosis of olfactory disorder in COVID-19 patients was not confirmed by the electrophysiologic test.9 Since SARS-CoV-2 is highly contagious, inessential invasive tests were not recommended while COVID-19 patients were under care. Finally, the external validity of our findings may be a concern because all of our patients were Taiwanese. The generalizability of our results to other non-Asian ethnic groups requires further verification.
In conclusion, this study findings show that olfactory disorder is not an uncommon symptom in COVID-19 patients and could emerge at the onset of SARS-CoV-2 infection or after the onset of general symptoms of COVID-19. Clinicians should consider the diagnosis of COVID-19 in patients with olfactory disorder.
Declaration of Competing Interest
No conflict of interest exists for the author.
Acknowledgements
The authors are grateful to the members of the Research Office for Health Data, Department of Education and Research, Taipei City Hospital, Taiwan for their valuable contributions in data management and statistical analysis. The authors gratefully acknowledge Chih-Yuan Shih for the significant contribution of the conception of the study.
References
- 1.World Health Organization Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/
- 2.Taiwan Centers for Disease Control and Prevention Surveillance of COVID-19. https://www.cdc.gov.tw/En
- 3.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020 Apr;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020 Jun;35(3):266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliezer M., Hautefort C., Hamel A.L., Verillaud B., Herman P., Houdart E. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 6.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 Jul 28;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 Aug;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doty R.L. Olfactory dysfunction and its measurement in the clinic. World J Otorhinolaryngol – Head Neck Surg. 2015;1(1):28–33. doi: 10.1016/j.wjorl.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]