Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic caused by a novel coronavirus that has been identified to belong to the β-coronavirus family.1 As the COVID-19 pandemic continues to evolve, more aspects of this illness are being defined and described. In the United States, the incidence of acute kidney injury (AKI) in patients hospitalized with COVID-19 has been reported to be around 37%.2 Different autopsy and kidney biopsy series have revealed acute tubular injury (ATI) to be the most common renal pathology lesion in these patients.3,S1
Although cases of collapsing glomerulopathy and thrombotic microangiopathy (TMA) with COVID-19 have been reported,4,5 an association between COVID-19 and crescentic glomerulonephritis (GN) has rarely been described.6 Herein, we report 2 cases of pauci immune GN with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, who clinically improved with treatment of COVID-19 and cautious use of immunosuppressants.
Case Presentation
Case 1
A 64-year-old African American man with a remote history of cryptogenic organizing pneumonia presented to the hospital with 2 weeks of progressive shortness of breath and dry cough. There was no history of fever or sick contacts. He was noted to be in hypoxic respiratory failure, with a respiratory rate of 23, and low oxygen saturation of 83% on room air, which improved to 100% on a 10-L non-rebreather mask. Otherwise, the patient was hemodynamically stable and afebrile. Laboratory findings revealed AKI with elevated serum creatinine (SCr) of 7.87 mg/dl (baseline SCr was unknown), and elevated inflammatory markers D-dimer 1353 ng/ml, ferritin 1985 ng/ml, and C-reactive protein 14.53 mg/dl. He was diagnosed with COVID-19 using reverse transcription−polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 on a nasopharyngeal swab; chest x-ray showed bilateral patchy opacities. Urinalysis revealed an active sediment with 55 red blood cells per high-power field, 65 white blood cells per high-power field, and significant proteinuria, with spot urine protein-to-creatinine ratio elevated at 5. Serum albumin was low at 2.8 g/dl. His respiratory status and kidney function progressively worsened, despite use of high-dose i.v. diuretics. The patient was initiated on intermittent hemodialysis treatment to optimize his condition for kidney biopsy, as well as to assist with volume overload and electrolyte derangements. COVID-19 was treated with convalescent plasma and intravenous tocilizumab, with negligible improvement in respiratory status. Serologies revealed normal serum complements (C3 and C4), a high perinuclear−antineutrophilic autoantibody (p-ANCA) titer (1:640) with a specific anti−myeloperoxidase antibody (MPO) titer of 32.5 units/ml, positive antinuclear antibody (1:160, homogenous pattern), anti−double strand DNA antibody of 112 IU/ml, and anti-ribonuclear protein (RNP) antibody of 1.1 AI. Anti−glomerular basement membrane antibody (anti-GBM) was not detected. A kidney biopsy was subsequently performed.
Kidney Biopsy Findings
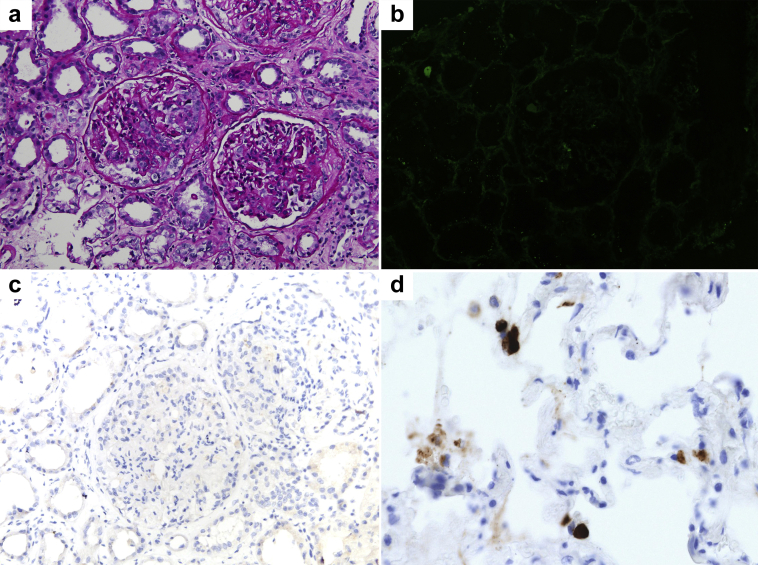
A total of 10 glomeruli were seen on light microscopy; 4 glomeruli showed small cellular crescents and/or segmental necrosis, and 3 contained fibrocellular (healing) crescents. There was moderate acute tubular necrosis. Immunohistochemistry staining for SARS-CoV-2 was negative. Immunofluorescence microscopy revealed nonspecific findings (Figure 1). On electron microscopy, there were no electron-dense deposits, and no viral particles were seen. (This case has been briefly described with emphasis on kidney biopsy findings, as a part of kidney biopsy series.7)
Figure 1.
Kidney biopsy findings. (a) Two glomeruli in the center reveal crescents: a cellular crescent in the glomerulus to the left, and a fibrocellular crescent to the right. Surrounding tubules reveal distension and flattening of the epithelium (periodic acid−Schiff stain, original magnification ×200). (b) Immunofluorescence staining for IgG reveals no significant staining in depicted glomerulus or surrounding tubular basement membranes (fluorescein isothiocyanate, original magnification ×200). (c) Representative section showing negative immunohistochemistry staining for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid protein after antigen retrieval (original magnification ×200). (d) Lung tissue from a known SARS-CoV-2−infected patient served as positive control for immunohistochemistry method (original magnification ×400).
Clinicopathologic Diagnosis
The clinicopatholic diagnosis was pauci-immune crescentic glomerulonephritis, in the setting of MPO-ANCA vasculitis.
Treatment
The patient received i.v. pulse dose corticosteroids (500 mg i.v. methylprednisolone daily for 3 days), followed by a dose of i.v. rituximab at a 1000-mg dose once the COVID-19 PCR result turned negative.
Clinical Outcome
The patient did not require mechanical ventilation. His kidney function started to improve after use of pulse dose corticosteroids, and hemodialysis was discontinued. Serum creatinine initially decreased and stabilized at 3.5 mg/dl; however, the hospital course was complicated by methicillin-sensitive Staphylococcus aureus bacteremia with new AKI, when SCr peaked at 4.89 mg/dl. The patient remained non-oliguric, with a decrease in SCr to 4.1 mg/dl and an MPO titer to 14 units/ml upon discharge. His SCr continues to decrease and has improved to 2.41 mg/dl approximately 1 month after receipt of rituximab. He is scheduled to receive a second dose of rituximab at completion of antibiotic therapy for bacteremia.
Case 2
A 46-year-old South Asian man with diabetes mellitus presented with fever, cough, diffuse purpuric rash, and AKI with an SCr of 2.9 mg/dl on admission. A few days prior, he was treated for pneumonia with azithromycin. The RT-PCR for SARS-CoV-2 was positive on nasopharyngeal swab, confirming the diagnosis of COVID-19, and he was initiated on hydroxychloroquine. Urinalysis showed 100 mg/dl of protein and moderate blood. Serum albumin was low at 2.1 g/dl. A skin biopsy revealed leukocytoclastic vasculitis. Kidney function worsened, with a peak SCr of 4.0 mg/dl. Serological evaluation for glomerular disease showed normal serum C3 and C4, an elevated proteinase 3 (PR3) level of 57.3 units/ml, elevated rheumatoid factor (320 IU/ml), and IgG kappa monoclonal band on serum immunofixation. A kidney biopsy was performed.
Kidney Biopsy Findings
Kidney biopsy findings were focal necrotizing glomerulonephritis with segmental glomerular thrombi, diffuse severe tubular epithelial injury, mild interstitial fibrosis, and moderate arteriosclerosis. Immunofluorescence microscopy showed trace segmental, finely granular, and mostly mesangial staining for IgA, IgM, and C3. No significant staining for IgG, C1q, kappa, or lambda light chains was noted. Rare mesangial dense deposits were seen on electron microscopy, but no viral particles were noted.
Clinicopathologic Diagnosis
The clinicopathologic diagnosis was PR3-ANCA−associated vasculitis (AAV) with focal necrotizing, pauci-immune glomerulonephritis.
Treatment
The patient was initiated on pulse dose corticosteroids (i.v. methylprednisolone, given as 1 g daily for 3 days) and received the first dose of rituximab (375 mg/m2 i.v.) during the hospital stay. Subsequently, he was transitioned to oral prednisone and completed his rituximab treatment after discharge.
Clinical Outcome
Two weeks after the initial dose of rituximab, the patient’s PR3 titer decreased to 28.8 units/ml, and SCr improved to 2.0 mg/dl. The most recent urinalysis has been negative for protein, with mild hematuria. The SCr has decreased to 1.27 mg/dl, at 12 weeks after initial diagnosis.
Table 1 summarizes clinical findings, demographics, and treatment strategies of our 2 cases and the already-published case6 of ANCA-associated GN with COVID-19.
Table 1.
Patient demographics, clinical findings, treatment, and outcomes
| Case no. | Age (yr) | Sex | Ethnicity | Comorbidities | Peak SCr (mg/dl) | Serum albumin | Positive serology | Lung involvement | Skin pathology | Kidney pathology | Renal replacement therapy | COVID-19 treatment | AAV treatment | Antibody titers on admission | Antibody titers 2 wk after rituximab |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | Male | African American |
None | 7.87 | 2.8 g/dl | MPO (p-ANCA) | Bilateral patchy infiltrates | None | Crescentic GN | Yes, hemodialysis | Tocilizumab, convalescent plasma | Glucocorticoids, rituximab | MPO: 32.5 U/ml | MPO: 14 U/ml |
| 2 | 46 | Male | South Asian | Diabetes mellitus | 4.0 | 2.1 g/dl | PR3 (c-ANCA) | Resolving peripheral ground glass opacities | Leukocytoclastic vasculitis | Focal necrotizing GN | No | Hydroxychloroquine, azithromycin | Glucocorticoids, rituximab | PR-3: 57.3 U/ml | PR-3: 28.8 U/ml |
| Ref 6 | 25 | Male | Iranian | None | 5.5 | NA | c-ANCA | Alveolar hemorrhage | None | Crescentic GN | No | Hydroxychloroquine, levofloxacin, i.v. Ig | Glucocorticoids, cyclophosphamide, plasmapheresis | c-ANCA (1:50) | NA |
c-ANCA, cytoplasmic antineutrophilic autoantibody; COVID-19, coronavirus disease 2019; GN, glomerulonephritis; p-ANCA, perinuclear antineutrophilic autoantibody; MPO, myeloperoxidase; NA: Not available; PR3, proteinase 3; Ref, reference; SCr, serum creatinine.
Discussion
Several mechanisms for the development of kidney injury in COVID-19 patients, including hemodynamic factors, viral tropism toward kidney tissue,8 and endothelial dysfunction leading to fibrinoid necrosis and development of micro thrombi, have been postulated.9 In addition to the direct cytopathic effect of SARS-CoV-2 on the glomeruli and renal tubules, there is also the indirect effect of cell-mediated immunity, the cytokine storm, and the cross-talk between organs, with possible systemic effects of the disease.S2
A series of publications have reported the development of a vasculitis-like illness in COVID-19 patients, with presentations ranging from vasculitis syndromesS3 to histologic findings of vasculitis seen on post mortem examination.S4
We describe 2 patients with ANCA-associated GN and severe AKI associated with COVID-19. Both patients are nonobese men, without any prior history of kidney disease or known ANCA vasculitis. The pulmonary findings in our 2 patients were deemed to be associated with COVID-19 illness and volume overload. Clinically, pulmonary ANCA disease was not suspected. Another case of cytoplasmic (c)−ANCA associated with glomerulonephritis in the setting of COVID-19 has been reported in a 25-year-old man from Iran.6
Although the association between SARS-CoV-2 infection and our patients with GN remains obscure, it is possible that cytokine storm, with immune system−related dysregulation in a uremic state, may have led to an altered response to infection (similar to the mechanism previously postulated for SARS-CoV infection),S5 further giving rise to AAV. In addition, it is possible that a specific host is prone to a certain type of kidney pathology in response to a “second hit.” Here, we postulate that the second hit is COVID-19.
Both MPO and PR3 are enzymes present on neutrophils, and autoantibodies to these enzymes can lead to pauci-immune GN, a mechanism previously demonstrated in a murine model in which i.v. injection of anti-MPO splenocyte resulted in the development of GN.S6 More recently, the role of these antibodies has been expanded with the evidence that neutrophil extracellular traps (NETs) serve as a source of autoantigens presenting MPO and PR3 to the immune system. The presence of NETs has been observed on kidney biopsy samples of patients with AAV,S7 and is postulated to be involved in COVID-19 pathogenesis.S8
Given the severity of renal AAV in our patients, cyclophosphamide and rituximab were considered standard-of-care treatment options in conjunction with glucocorticoid therapy. Immunosuppression with cyclophosphamide or rituximab during COVID-19 infection is rightfully of great concern in the medical community, and there is limited knowledge on outcomes of COVID-19 in patients on these background therapies. Rituximab leads to B-cell depletion and can abrogate a prompt and efficient antibody response to facilitate faster recovery from the virus. In addition, use of rituximab can lead to the inability to mount antibodies to a potential vaccine as well. However, for our patients, rituximab was considered as the choice of therapy based on its better tolerability and lesser side effects. Emerging reports of COVID-19 patients who had been receiving rituximab (or other anti-CD20 monoclonal antibodies) for their underlying immune-mediated conditions have demonstrated that these patients do not seem to have a worse course or outcome compared with those in the general population, with some even suggesting that rituximab may forestall the cytokine storm seen in COVID-19 and may improve outcomes.S9−S11 Furthermore, early and higher levels of antiviral antibody titers have been correlated with increased mortality in COVID-19 patients,S12 and patients with X-linked agammaglobulinemia (XLA) who suffer from full B-cell deficiency have shown full recovery from COVID-19 infection.S13
Anders et al.S14 suggest postponing maintenance rituximab during the surge of the pandemic to avoid not only the unnecessary immunosuppression but also unnecessary contact with other potentially infected patients and health personnel during the rituximab administration. Regardless, treatment might be still indicated in certain clinical settings. Although our first patient received rituximab after his COVID-19 PCR result turned negative (to promote recovery and to ensure immunologic memory from COVID-19), the second patient received it concurrently with corticosteroid therapy. Both of the patients had improvement in their COVID-19−related symptoms, as well as kidney recovery.
In summary, ANCA-associated GN can be associated with COVID-19. Because of the lack of scientific evidence related to COVID-19, management of diverse pathological entities arising in its setting is challenging. The existing literature on viral infection−related ANCA vasculitis reveals favorable outcomes with treatment of virus and ANCA disease using antiviral agent and immunosuppression concurrently; however, our major concern was worsening of infection with the use of immunosuppression, as no specific agent has been proved to be beneficial in treating COVID-19. All 3 patients with COVID-19 (2 patients in this series and 1 previously published case6) who developed ANCA glomerulonephritis responded well to immunosuppressive agents (Table 2). Interestingly, none of these patients had deterioration of SARS-CoV-2−related disease. Further research is still necessary to determine the optimal therapy for such conditions; however, based on our experience, it is noteworthy that immunosuppression, when indicated, can be used in COVID-19 patients, under close observation.
Table 2.
Teaching points
|
|
|
ANCA, antineutrophilic autoantibody; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Disclosure
KDJ serves as a consultant for Astex Pharmaceuticals and Natera. All the other authors declared no conflict of interest.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19). 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/summary.html Available at:
- 2.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasr S.H., Kopp J.B. COVID-19-associated collapsing glomerulopathy: an emerging entity. Kidney Int Rep. 2020;5:759–761. doi: 10.1016/j.ekir.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jhaveri K.D., Meir L.R., Flores Chang B.S. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98:509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moeinzadeh F., Dezfouli M., Naimi A. Newly diagnosed glomerulonephritis during COVID-19 infection undergoing immunosuppression therapy, a case report. Iran J Kidney Dis. 2020;14:239–242. [PubMed] [Google Scholar]
- 7.Sharma P., Uppal N.N., Wanchoo R. COVID-19 associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31:1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sardu C., Gambardella J., Morelli M.B. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9:E1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.