Key Points
Question
Can an intraoperative near-infrared fluorescence imaging technique be used to detect lung tumor margin via inhalation delivery of indocyanine green?
Findings
In this diagnostic study, the fluorescent signal of inhaled indocyanine green was observed throughout healthy lung tissue but was rarely detected in tumor tissue. Inhalation at a 20-fold lower dose of indocyanine green had a 2-fold higher efficiency for tumor margin detection compared with the intravenous injection of indocyanine green.
Meaning
Image-guided surgery based on low-dose indocyanine green inhalation appears to facilitate rapid, long-term visualization of the tumor margin of lung tumors.
Abstract
Importance
Identification of the tumor margin during surgery is important for precise minimal resection of lung tumors. Intravenous injection of indocyanine green (ICG) has several limitations when used for intraoperative visualization of lung cancer.
Objectives
To describe a technique for intraoperative visualization of lung tumor margin using ICG inhalation and evaluate the clinical applicability of the technique in mouse and rabbit lung tumor models as well as lung specimens of patients with lung tumors.
Design, Setting, and Participants
In lung tumor models of both mice and rabbits, the distribution of inhaled ICG in the lung tumor margin was investigated in vivo and ex vivo using a near-infrared imaging system. Lung tumor margin detection via inhalation of ICG was evaluated by comparing the results obtained with those of the intravenous injection method (n = 32, each time point for 4 mice). Based on preclinical data, use of ICG inhalation to help detect the tumor margin in patients with lung cancer was also evaluated (n = 6). This diagnostic study was conducted from May 31, 2017, to March 30, 2019.
Main Outcomes and Measures
The use of tumor margin detection by inhaled ICG was evaluated by comparing the inhaled formulation with intravenous administration of ICG.
Results
From 10 minutes after inhalation of ICG to 24 hours, the distribution of ICG in the lungs was significantly higher than that in other organs (signal to noise ratio in the lungs: 39 486.4; interquartile range [IQR], 36 983.74-43 592.5). Ex vivo and histologic analysis showed that, in both lung tumor models, inhaled ICG was observed throughout the healthy lung tissue but was rarely found in tumor tissue. The difference in the fluorescent signal between healthy and tumor lung tissues was associated with the mechanical airway obstruction caused by the tumor and with alveolar macrophage uptake of the inhaled ICG in healthy tissues. Inhalation at a 20-fold lower dose of ICG had a 2-fold higher efficiency for tumor margin detection than did the intravenous injection (2.9; IQR, 2.7-3.2; P < .001).
Conclusions and Relevance
The results of this study suggest that lung-specific inhalation delivery of ICG is feasible and may be useful for the intraoperative visualization of lung tumor margin in clinical practice.
This in vivo and ex vivo diagnostic study examines the use of inhaled indocyanine green for intraoperative detection of the margin of lung tumors.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide.1 Surgical resection is the preferred therapeutic option for long-term survival of patients with early-stage, non–small cell lung cancer.2 Precise minimal resection is the current treatment of choice in patients with compromised lung function who cannot tolerate lobectomy and in those without compromised function who have small, peripheral non–small cell lung cancer.3,4,5 Although an adequate surgical margin of the tumor must be determined to reduce local recurrence after surgery,6 defining the tumor margin during surgery is challenging. Several techniques for preoperative tumor localization with direct injection of various markers are used to identify tumor margin; however, these methods reveal the general location of the tumor—not the precise resection margins.7,8,9
Ishizawa et al10 reported intraoperative visualization of hepatocellular carcinoma using near-infrared (NIR) fluorescence after intravenous injection of indocyanine green (ICG). Since then, this intraoperative imaging technique has been used to visualize several types of cancer.11,12,13,14,15,16 More recently, Okusanya et al17 showed that metastatic pulmonary nodules can be identified via intraoperative NIR fluorescence imaging 24 hours after intravenous injection of ICG at a dose of 5.0 mg/kg.17 In a previous study, primary lung cancer tumors were visualized after the intravenous injection of ICG.18 Although intravenous injection of ICG facilitates the intraoperative visualization of lung tumors, it has limitations. First, to accurately display lung tumors, 2 mg/kg or more of ICG must be injected, so patients weighing 70 kg need to be injected with 140 to 350 mg of ICG (ie, 5.6-14 vials).10 Second, tumor detection should be performed 24 hours after ICG injection, which allows the ICG to be washed out from the healthy tissues.15,16 Third, intravenously injected ICG is distributed throughout the body, which may cause adverse effects, such as anaphylactic shock and hypotension, particularly at high doses.19 Therefore, a minimal effective dose and immediate imaging after lung-specific delivery are important in ICG-based intraoperative visualization of malignant lung tumors.
The use of inhalation drug delivery allows efficient localization of drugs into the lungs and airways. Therefore, inhalation has been a useful treatment modality for lung-specific diseases, such as asthma and chronic obstructive pulmonary disease.20,21,22,23 However, there is a limited number of studies on the potential of inhalation of fluorescent agents in intraoperative visualization of lung diseases. Noppen et al24,25 reported that parenchymal abnormalities in patients with pneumothorax can be detected with the inhalation of fluorescein. However, to our knowledge, no study has used fluorescence inhalation to determine lung tumor margin.
This study first examined an intraoperative NIR fluorescence imaging technique that may be used to detect lung tumor margin via inhalation delivery of ICG. Moreover, the associated mechanism of the method was examined, and the technique was compared with other existing tumor detection techniques. Furthermore, the use of this imaging technique in clinical settings was evaluated using data from preclinical and clinical studies.
Methods
The study was conducted from May 31, 2017, to March 30, 2019. Models using mice and rabbits, as well as examination of lung specimens of humans with lung cancer, were included. All surgical procedures, including animal care and handling, were approved by the Laboratory Animal Research Center, Korea University College of Medicine. We used a small number of animals (n = 4) in accordance with Reduction Regulation of Institutional Animal Care and Use Committee and because there was no reference for calculating the size of the sample. The present study was approved by the Korea University Guro Hospital Institutional Review Board; patients provided informed consent. This study followed the Standards for Reporting of Diagnostic Accuracy (STARD) reporting guideline for diagnostic studies.
Green fluorescent protein–tagged Lewis lung carcinoma cells were used to establish the lung tumor mouse models; C57BL/6 mice (16 females and 16 males) were used for tumor implantation (eMethods 1 in the Supplement). The lung tumor model was established 2 to 3 weeks after the intravenous administration of Lewis lung carcinoma (100 μL of 2 × 105 cells). The lung tumor models received inhalation ICG or intravenous injection of ICG, 1 mg/kg, and the distribution was detected by the NIR fluorescence imaging system at 1, 3, 6, and 24 hours.
We defined fluorescence signal as the fluorescence signal to noise ratio, which has been reported in previous studies.26,27,28 The signal to noise ratio was calculated using the following formula: Signal to Noise Ratio = Fluorescent Intensity of a Region of Interest / Background Intensity. As mentioned in previously published articles,12,29,30 the detection of lesions using fluorescent contrast agents is based on the difference in the fluorescent signal between tumor and healthy tissues. Therefore, we defined tumor margin detection efficiency using the following formulae: Tumor Margin Detection Efficiency in Intravenous Injection Group = Tumor Tissue Signal to Noise Ratio / Healthy Tissue Signal to Noise Ratio, and Tumor Margin Detection Efficiency in Inhalation Group = Healthy Tissue Signal to Noise Ratio / Tumor Tissue Signal to Noise Ratio. Three of us (K.N.H, C.K., and H.K.K.) reviewed images depicting different levels of tumor margin detection efficiency. All 3 observers selected images with tumor margin detection efficiency greater than or equal to 2.0 in which the margin could easily be distinguished by the fluorescent signal; hence, we justified that a tumor margin detection efficiency greater than or equal to 2.0 is sufficient for accurately distinguishing tumors from healthy tissue on the basis of fluorescent signals.
Twenty New Zealand rabbits aged 8 weeks (10 females and 10 males) with VX2 tumors, with the tumor model described in a previous study,31 were used in the present study. Four of those rabbits with VX2 tumors inhaled ICG, 1 mg/kg, and feasibility for tumor detection was assessed. Next, the rabbits were divided into 4 groups according to the ICG inhalation dose (0.1, 0.25, 0.5, and 1.0 mg/kg; n = 4 per group). The rabbits were anesthetized and intubated via tracheostomy. A mechanical ventilator was connected to the tracheostomy site, and anesthesia was maintained with isoflurane, 2% (Baxter Healthcare). The rabbits were placed in the lateral decubitus position, and a unilateral chest wall resection was performed to visualize the entire right lung. The nebulizer (Omron Healthcare) was positioned in the inspiratory limb of the ventilatory circuit, and the rabbits received the different doses of nebulized ICG for 4 minutes (0.25 mL/min). The distribution of ICG in the lungs of the rabbits was visualized in real time using an intraoperative color and NIR-fluorescence imaging system, and the lung tumor was surgically removed.
We evaluated the use of ICG inhalation for detection of tumor margin in human lungs by examining the tumor margin detection efficiency in lung specimens from 6 patients (2 men and 4 women; age, 58.2 years; interquartile range [IQR], 51-64 years) with lung cancer. The tumor diameter was 2.3 cm (IQR, 1.9-2.9 cm), and the tumor depth was 0.5 cm (IQR, 0.2-1.0 cm). Indocyanine green was delivered into the lung specimens via nebulization (eMethods 2 and eFigure 1 in the Supplement).
Statistical Analysis
GraphPad, version 8.3 for Windows (GraphPad Software) was used for all statistical analyses. A P value <.05 was considered statistically significant. Data are presented as median with IQR. The significant differences in the biodistribution of inhaled ICG in each organ were indicated using a repeated-measures analysis of variance test. The signal to noise ratio and tumor margin detection efficiency of the lung tumor tissue or normal tissue between intravenous injection and inhalation were analyzed using a 2-sample, paired t test. On the basis of the tumor margin detection efficiency in inhalation and intravenous injection results, we used G power to calculate the effect size d and the actual power.
Results
ICG Distribution
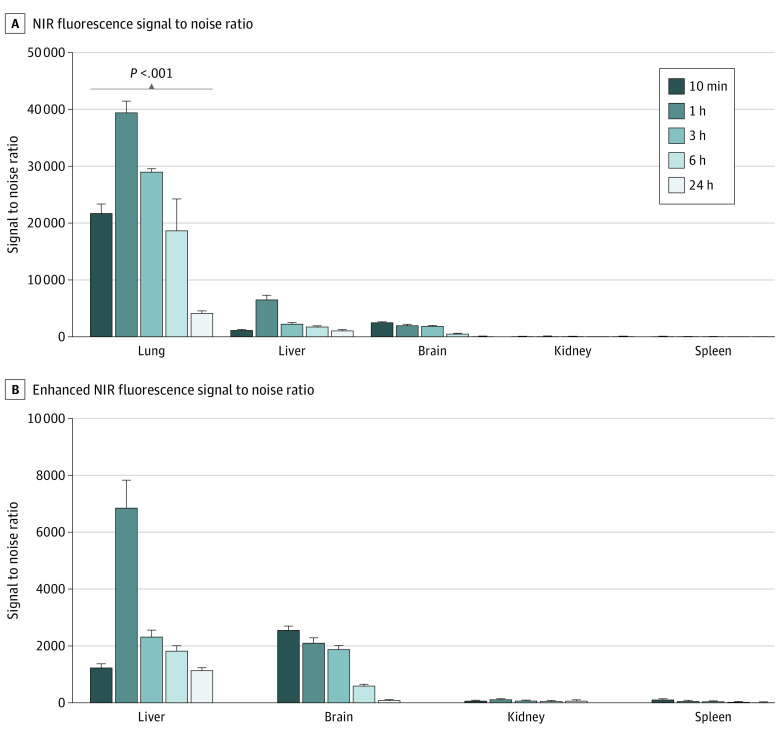
We evaluated whether inhalation delivery could facilitate efficient localization of ICG molecules in the lungs of healthy mice. Fluorescence images and quantification of signal to noise ratio in each organ revealed that the inhaled ICG accumulated preferentially in the lungs compared with the other organs (Figure 1; eFigure 2 in the Supplement). The signal to noise ratio in the lungs was determined at 10 minutes onward and was the highest at 1 hour postinhalation (39 486.4; IQR, 36 983.74-43 592.5). The signal to noise ratio was then reduced over time and reached 4251.1 (IQR, 3963.6-4574.9) after 24 hours but was significantly higher than that in other organs at all times measured. In addition to the lungs, the fluorescence signals in the liver and brain were evaluated, and the signal to noise ratios were 6059 (IQR, 7887.4-5148.1) in the liver and 2084 (IQR, 1905.0-2345.3) in the brain. These results suggest that inhaled ICG was preferentially retained in the lungs and may be used to visualize lung tissues via NIR fluorescence.
Figure 1. Biodistribution of Indocyanine Green (ICG) in Different Mouse Organs After ICG Inhalation.
A, Quantification of the near-infrared (NIR) fluorescence signal to noise ratio in the lungs, liver, brain, kidneys, and spleen of mice that inhaled ICG harvested after inhalation. B, The graph shows an enhanced view of the liver, brain, kidneys, and spleen at each time point. Error bars represent the median and interquartile ranges.
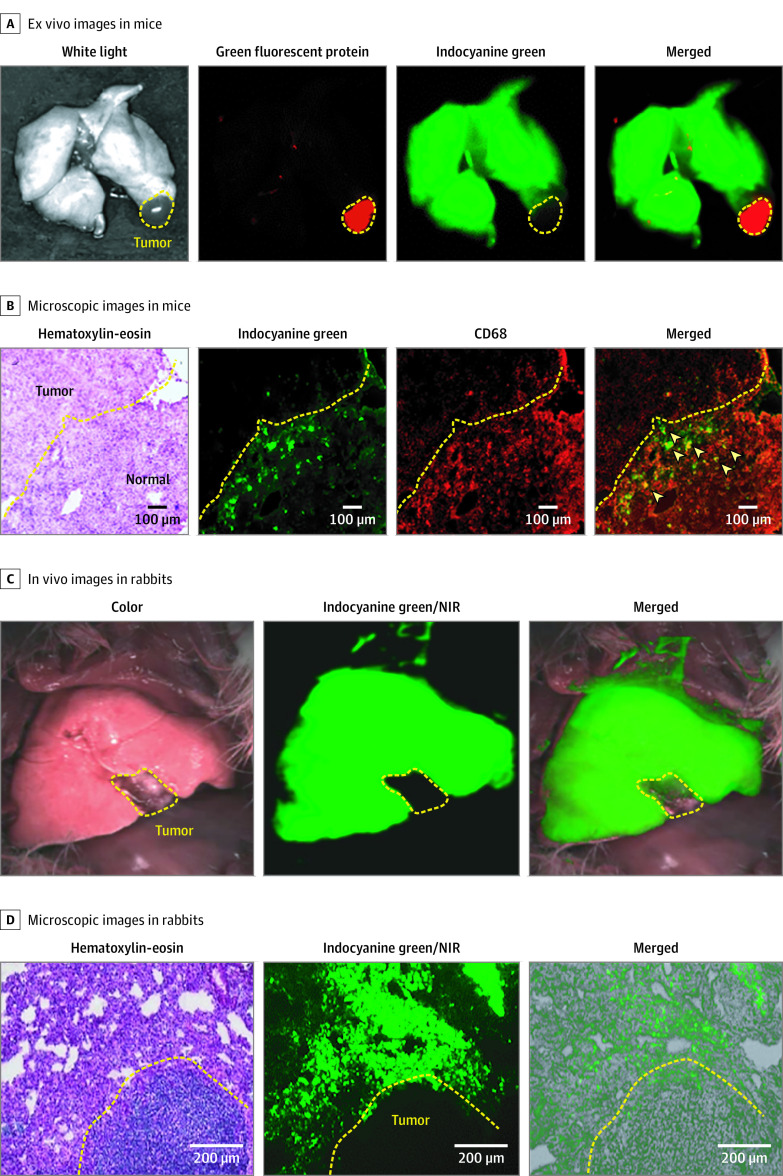
We examined whether lung tumor margin could be detected via fluorescence imaging after ICG inhalation in mice. Green fluorescent protein fluorescence was detected in the lung tumor, whereas ICG was found in the healthy tissue but not in the lung tumor. Ex vivo NIR fluorescence imaging revealed that the tumor tissues were able to be distinguished from healthy tissues (Figure 2A). Moreover, histologic analysis showed that most ICG molecules were located in the healthy tissues (Figure 2B). We further investigated whether alveolar macrophages, which play a role in the response to foreign substances in the lungs,32 sequestered ICG molecules in the healthy tissues. The expression of CD68, a macrophage marker, was mainly distributed in the healthy tissues rather than in the tumor. Colocalization of ICG and CD68 signals was found in the healthy tissues, suggesting that inhaled ICG remained in the healthy lung tissue via efficient macrophage uptake (Figure 2B).
Figure 2. Distribution of Inhaled Indocyanine Green in the Lungs of a Mouse and Rabbit Model With Lung Tumor.
A, Ex vivo images of the lung after indocyanine green inhalation in the mouse lung tumor model. The mouse model was established with Lewis lung carcinoma cell lines that were tagged with green fluorescent protein. The green fluorescent protein signal is represented with red, and the inhaled ICG signal is represented with green. The yellow dotted line indicates the tumor site. B, Microscopic images of the tumor margin with hematoxylin-eosin and CD68 (red) staining in the mouse lung tumor model. The arrowheads in the merged view indicate the alveolar macrophages. C, In vivo lung with bright-field, near-infrared (NIR) (green), and merged images (NIR with bright-field) in the rabbit lung tumor model after indocyanine green inhalation. The yellow dotted line indicates the tumor site. D, Microscopic images of the tumor margin with hematoxylin and eosin staining, NIR fluorescence (green), and NIR fluorescence merged with bright-field in the rabbit lung tumor model after indocyanine green inhalation. The yellow dotted line indicates the tumor site.
Furthermore, we evaluated the clinical applicability of ICG inhalation in a rabbit lung tumor model. The intraoperative NIR fluorescence imaging showed ICG fluorescence only in the healthy tissue, which facilitated visualization of the tumor margin and tumor resection at 1 hour postinhalation (Figure 2C; Video 1). Consistent with the mouse experiments, the fluorescence imaging and histologic analysis in the rabbits showed that inhaled ICG was localized exclusively in the healthy tissue (Figure 2D).
Video 1. Intraoperative Lung Tumor Margin Visualization Using Indocyanine Green (ICG) Inhalation .
Intraoperative tumor margin visualization and tumor resection after inhalation of ICG in a rabbit lung tumor model.
Fluorescent Assessment of Lung Tumor Margin
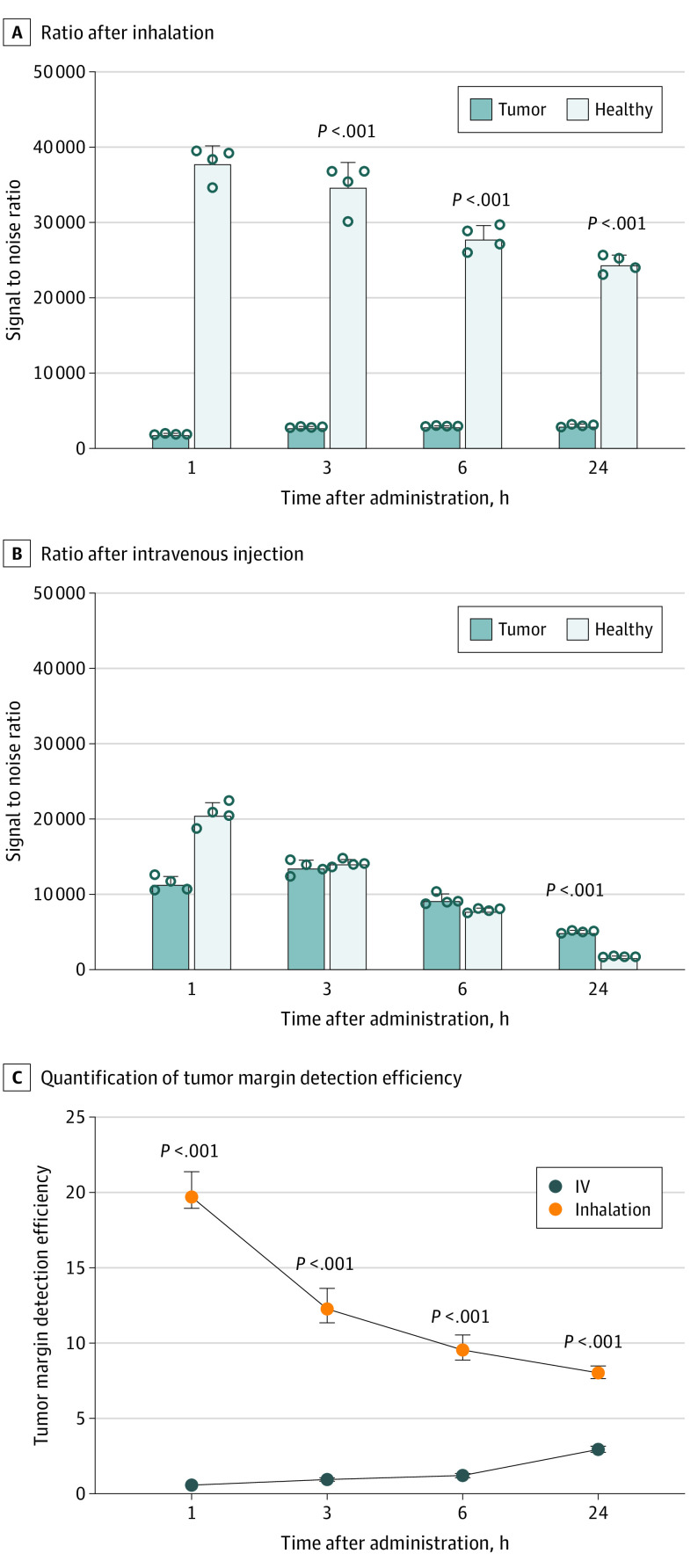
We investigated whether the inhalation delivery of ICG was more useful for detection of tumor margin than the systemic delivery.13,14,15,33 Fluorescence analysis of tumor and healthy tissues after ICG inhalation revealed a significantly higher signal to noise ratio in healthy tissue compared with the tumor tissue during the 24-hour period (1 hour: 37 939.9; IQR, 35 571.3-39 437.8 in healthy tissue; 1917.6; IQR, 1868.9-1981.9 in tumor tissue, P < .001) (Figure 3A). In contrast, when ICG was injected intravenously, the significant difference was observed only at 24 hours postinjection (24 hours: 5046.7; IQR, 4892.5-5226.5 in tumor tissue; 1747.54; IQR, 1685.7-1826.4 in healthy tissue, P < .001) (Figure 3B), suggesting that the tumor margin can be identified at least over a period of 6 hours after the administration of ICG.
Figure 3. Fluorescent Assessment of Lung Tumor Margin With Inhaled and Intravenously Injected Indocyanine Green (ICG).
Comparison of near-infrared fluorescence signal to noise ratio in tumor and normal tissues at different time points after inhalation (A) or intravenous injection (B) of ICG. Quantification of tumor margin detection efficiency is shown after inhalation and intravenous injection of ICG (C).
Analysis of the tumor margin detection efficiency of both delivery methods revealed that the efficiency of ICG inhalation was significantly higher than that of intravenous injection during the 24-hour period (P < .001). The highest tumor margin detection efficiency of inhalation (19.9; IQR, 18.9-20.5) and intravenous injection methods (2.8; IQR, 2.6-3.0) was observed at 1 hour and 24 hours postadministration, respectively (Figure 3C).
ICG Dose Optimization
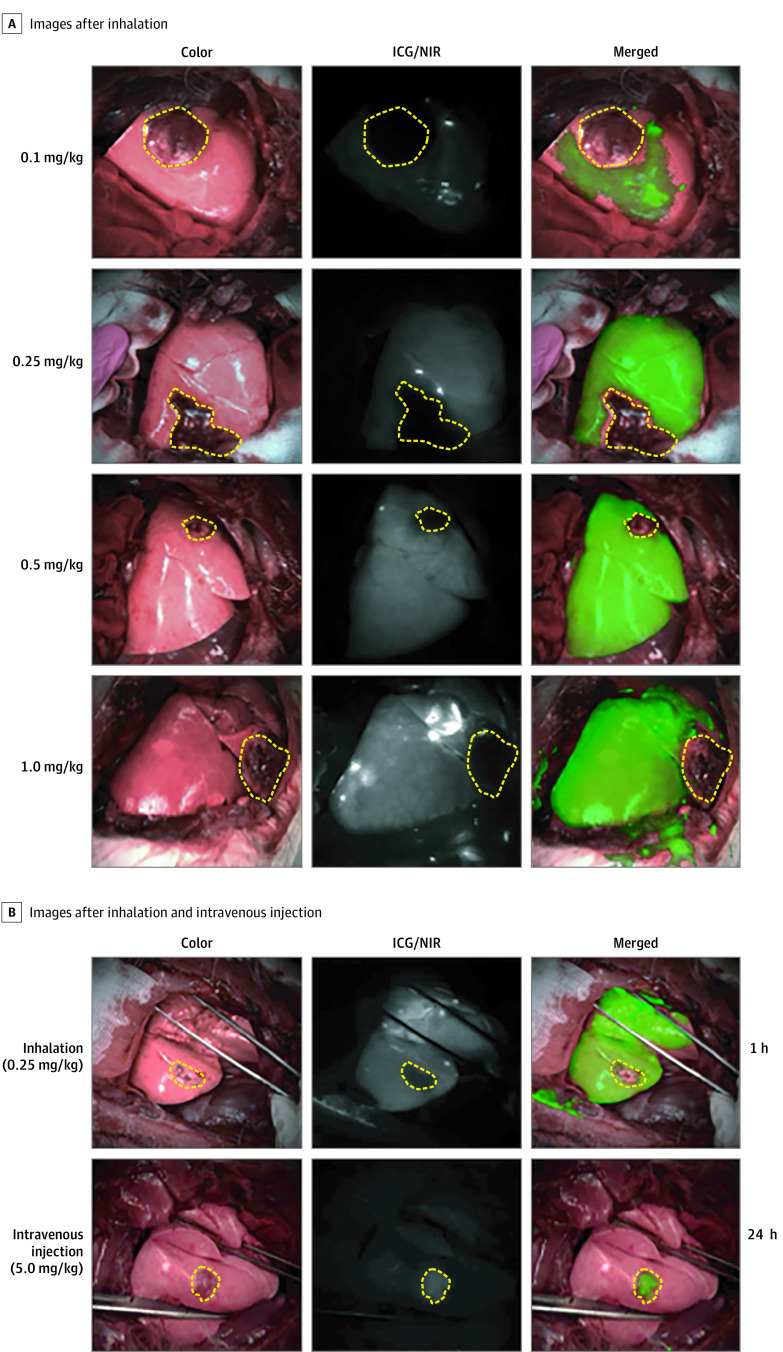
In vivo intraoperative NIR fluorescence imaging revealed that the tumor margin was clearly visualized with doses of ICG greater than or equal to 0.25 mg/kg (Figure 4A). Tumor margin detection efficiency analysis showed that higher doses resulted in greater contrast between tumor and healthy tissues, as expected (0.1 mg/kg: 2.3; IQR, 1.9-2.5; 0.25 mg/kg: 5.8; IQR, 5.4-6.0; 0.5 mg/kg: 7.0; IQR, 6.5-7.4; and 1 mg/kg: 9.4; IQR, 9.0-9.8) (Figure 4A; eFigure 3A in the Supplement). The tumor margin detection efficiency of ICG inhalation at a dose of 0.25 mg/kg was 2-fold higher than that of the intravenous injection of 5.0 mg/kg of ICG (2.9; IQR, 2.7-3.2; P < .001) (Figure 4B; eFigure 3B in the Supplement).
Figure 4. Dose Optimization of Indocyanine Green (ICG) for Detection of Tumor Margin in the Rabbit Model With VX2 Lung Tumor.
A, Images of the lung tumor margin with VX2 tumor in bright-field, near-infrared (NIR), and merged examples after the inhalation of ICG at different doses. B, Images of the lungs with VX2 tumor in bright-field, near-infrared (NIR), and merged examples after inhalation (0.25 mg/kg) and intravenous injection (5.0 mg/kg) of ICG. The yellow dotted line indicates the tumor site.
In addition, the inhalation technique allowed identification of a tumor with a depth of 0.5 cm, as observed on a computed tomographic (CT) scan (eFigure 4 in the Supplement and Video 2). Moreover, we noted that a 0.2-cm tumor was detectable with ICG inhalation (eFigure 5 in the Supplement).
Video 2. Intraoperative Subpleural Lung Tumor Margin Visualization Using Indocyanine Green (ICG) Inhalation .
Intraoperative subpleural tumor margin visualization and tumor resection after inhalation of ICG in a rabbit lung tumor model.
Tumor Margin Detection in Human Lung Specimens
Similar to the results observed in the mouse and rabbit models, the ICG fluorescence signal in human lung specimens was detected mainly in the noncancerous tissues, and the tumor margin detection efficiency was 2.9 (IQR, 2.7-3.3) (eTable in the Supplement). In all lung specimens, the tumor margin was clearly visualized using NIR fluorescent thoracoscopy (eFigure 1 in the Supplement).
Discussion
The intraoperative detection of tumor margin is a prerequisite for achieving minimal and precise resection of a tumor while preserving as much healthy tissue as possible. Near-infrared fluorescence imaging–guided surgery is performed not only to replace a surgeon’s hands, eyes, and intuition in clinical decision-making but also for improving the outcome of primary nodule or metastatic tumor resection.34 For these types of imaging-guided surgeries, clinicians have been using intravenous injection of ICG, 1.0 to 5.0 mg/kg.15,16,18 Compared with intravenous injection, inhalation is a lung-specific treatment that maximizes drug accumulation in the lungs while minimizing drug accumulation in other organs.22,23
Herein, our data suggest that ICG inhalation facilitates rapid and prolonged intraoperative detection of lung tumor margin at low doses 10 minutes to 24 hours after ICG inhalation. The ICG fluorescence signal observed in the healthy tissue of the lung contrasted with the tumor margin. This finding can be attributed to airflow obstruction caused by the tumor and destruction of alveoli in the tumor tissue. In addition, ICG was preferentially localized in the healthy tissue by macrophage phagocytosis (Figure 5). Although inhalation is a nonspecific delivery method, tumor margin detection efficiency obtained with the inhalation of ICG was 2-fold higher than that obtained with a 20-fold lower dose compared with the conventional intravenous injection method. This method of negative contrast imaging has been used in radiologic procedures, such as the barium swallow test for detecting gastric cancer or coronary angiography for identifying coronary atherosclerosis.35,36 Furthermore, we observed that tumors at a depth of 0.5 cm could be detected by ICG inhalation. As reported by Predina et al,37 the size of the smallest lung nodule detected with the intravenous injection of ICG was 0.2 cm. Our findings also showed that a 0.2-cm tumor apparently can be detected with ICG inhalation.
Figure 5. Intraoperative Detection of Tumor Margin by Inhalation of Indocyanine Green (ICG).
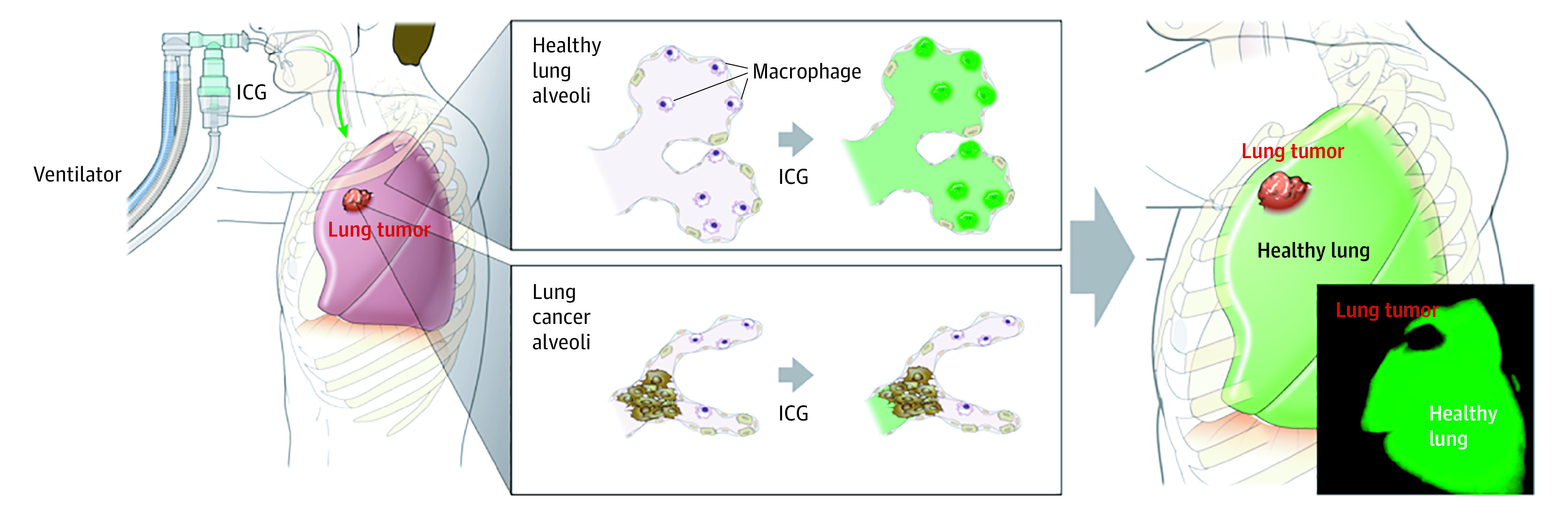
In healthy lung tissue, inhaled ICG was distributed into the alveoli and phagocytized by alveolar macrophages. However, the alveoli were destroyed in patients with lung tumor, and the inhaled ICG rarely diffused into the tumor tissues. Therefore, the tumor margin apparently can be detected after the inhalation of ICG during lung tumor surgery. The illustration was produced by studioMID and is published with permission.
Thus, the application of this study’s findings may aid in the detection of lung cancer during surgery and enable the surgeon to perform tumor resection more accurately; furthermore, use of ICG inhalation may improve the quality of life and survival rate of patients. This method may be useful in the surgical treatment of lung cancer for the following reasons. First, ICG inhalation maximizes accumulation of ICG in the lung while minimizing accumulation in other organs. In clinical settings, the maximum dosage of ICG recommended for intravenous injection is 5.0 mg/kg.17,37,38,39,40 The frequency of allergic reactions with doses below 0.5 mg/kg was reported as 0.003%, which increased significantly if the dose exceeded 5.0 mg/kg.41 In addition, inhalation may be applicable to patients with liver disfunction or kidney disfunction by administering a small amount of fluorescent contrast agent. Second, ICG in clinical studies must be injected intravenously 4 hours or 24 hours before surgery to detect cancer during surgery. Use of ICG inhalation has the advantage of direct administration to the lungs through the endotracheal tube during surgery.
Limitations
Although ICG inhalation-guided surgery appears to be a feasible and useful intraoperative technique, our study has several limitations. First, our research was limited to tumors with a depth of up to 1 cm from the pleural surface; other researchers have reported that the NIR image-guided surgery has depth limitations of 5 to 10 mm.9,14,15,16,18 Although the NIR fluorescence signal has depth limitations, it is a feasible intraoperative technique to provide a rapid intraoperative diagnosis, significantly decrease the total operative time, decrease the rate of conversion to open procedures, and allow for more complete removal of tumors, which has been demonstrated by intravenously injected ICG.9,13,14,17,34,38,39,42 In addition, compared with other soft tissues, such as skin and liver, the lungs undergo volumetric changes through respiratory movements. Thus, the tumor depth in the collapsed lung during surgery was much less than that measured from preoperative chest CT scan images. In addition, Newton et al39 suggested that ICG can detect lung tumors up to 1.4 cm in depth. Another study noted that intravenously injected ICG can detect lung tumors up to 1.3 cm in depth.9 Therefore, further studies on tumors at a depth greater than 1.0 cm from the pleural surface should be conducted. Furthermore, future research must assess the ability of ICG inhalation in the detection of ground glass lesions, which result in a partial filling of air spaces in the lungs by exudate, transudate, or solid components.
Second, in the rabbit experiment as shown in Video 1, the lack of a fluorescence signal was observed in the presence of atelectasis, which resulted in a false-positive result. Atelectasis is the collapse or closure of a lung, resulting in reduced or absent gas exchange,43 which is caused by various lung diseases such as inflammatory nodules, tuberculosis, asthma, chronic obstructive pulmonary disease, and bronchiectasis, resulting in mechanical alveolar obstruction.44 Clinically significant atelectasis is generally identified by preoperative chest radiography or CT scan.45 Therefore, based on the results of a preoperative CT scan, surgeons can accurately identify the tumor, benign lung lesion, or atelectasis in the operative field. In addition, chest physiotherapy bronchodilators, fiberoptic bronchoscopy, positive end-expiratory pressure, and surfactants have been described by many researchers as treatment modalities for atelectasis and shown to be effective in the resolution of atelectasis.46,47,48,49 Therefore, the treatment of patients with atelectasis confirmed using chest radiography or CT scan before surgery may be one of the ways to overcome the limitation of this study. In addition, to minimize the intraoperative atelectasis or nonventilated lung caused by single-lung ventilation that affects the distribution of ICG, it is recommended that ICG be inhaled immediately after intubation in the operating room and then followed by single-lung ventilation.46 Third, although ICG inhalation can be applied in clinical practice without significant safety concerns because it is approved by the US Food and Drug Administration, airway damage and hypersensitivity due to its inhalation must be further investigated.
Conclusions
An intraoperative NIR fluorescence imaging technique was developed to detect lung tumor margin after ICG inhalation. This technique appears to facilitate rapid and prolonged visualization of the tumor margin of a lung tumor on the pleural surface with a low dosage of ICG, which cannot be achieved using the intravenous injection method. We suggest that the ICG inhalation method will not only provide more accurate resection of lung tumors but also may improve patient safety during surgery.
eMethods 1. Organ Distribution of the Inhaled ICG in a Normal Mouse Model
eMethods 2. Human Study
eFigure 1. Detection of TM in the Resected Lung Tissue of Patients by ICG Nebulization
eFigure 2. Biodistribution of ICG in Different Mouse Organs After ICG Inhalation
eFigure 3. Fluorescent Assessment of Lung TM With Inhaled and Intravenously Injected ICG
eFigure 4. ICG Inhalation Can Detect Tumors at a Depth of 0.5 cm
eFigure 5. A 0.2 cm Tumor Could Be Detected With ICG Inhalation
eTable. Clinical and Demographic Characteristics of Patients With Lung Cancer
References
- 1.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1-19. doi: 10.1007/978-3-319-24223-1_1 [DOI] [PubMed] [Google Scholar]
- 2.Wolf AS, Richards WG, Jaklitsch MT, et al. . Lobectomy versus sublobar resection for small (2 cm or less) non–small cell lung cancers. Ann Thorac Surg. 2011;92(5):1819-1823. doi: 10.1016/j.athoracsur.2011.06.099 [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa S, Shimizu K, Mogi A, Kuwano H. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg. 2018;66(2):81-90. doi: 10.1007/s11748-017-0878-6 [DOI] [PubMed] [Google Scholar]
- 4.Petrella F, Diotti C, Rimessi A, Spaggiari L. Pulmonary metastasectomy: an overview. J Thorac Dis. 2017;9(suppl 12):S1291-S1298. doi: 10.21037/jtd.2017.03.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scanagatta P, Girelli L. Metastasectomy in pediatric patients: indications, technical tips and outcomes. J Thorac Dis. 2017;9(suppl 12):S1299-S1304. doi: 10.21037/jtd.2017.09.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narsule CK, Ebright MI, Fernando HC. Sublobar versus lobar resection: current status. Cancer J. 2011;17(1):23-27. doi: 10.1097/PPO.0b013e31820a51b6 [DOI] [PubMed] [Google Scholar]
- 7.Rho J, Lee JW, Quan YH, et al. . Fluorescent and iodized emulsion for preoperative localization of pulmonary nodules. Ann Surg. Published online April 8, 2019. doi: 10.1097/SLA.0000000000003300 [DOI] [PubMed] [Google Scholar]
- 8.Ujiie H, Kato T, Hu HP, et al. . A novel minimally invasive near-infrared thoracoscopic localization technique of small pulmonary nodules: a phase I feasibility trial. J Thorac Cardiovasc Surg. 2017;154(2):702-711. doi: 10.1016/j.jtcvs.2017.03.140 [DOI] [PubMed] [Google Scholar]
- 9.Abbas A, Kadakia S, Ambur V, Muro K, Kaiser L. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg. 2017;153(6):1581-1590. doi: 10.1016/j.jtcvs.2016.12.044 [DOI] [PubMed] [Google Scholar]
- 10.Ishizawa T, Fukushima N, Shibahara J, et al. . Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115(11):2491-2504. doi: 10.1002/cncr.24291 [DOI] [PubMed] [Google Scholar]
- 11.Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Intraoperative observation using a fluorescence imaging instrument during hepatic resection for liver metastasis from colorectal cancer. Hepatogastroenterology. 2012;59(113):90-92. doi: 10.5754/hge11223 [DOI] [PubMed] [Google Scholar]
- 12.Judy RP, Keating JJ, DeJesus EM, et al. . Quantification of tumor fluorescence during intraoperative optical cancer imaging. Sci Rep. 2015;5:16208. doi: 10.1038/srep16208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating J, Judy R, Newton A, Singhal S. Near-infrared operating lamp for intraoperative molecular imaging of a mediastinal tumor. BMC Med Imaging. 2016;16:15. doi: 10.1186/s12880-016-0120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keating JJ, Kennedy GT, Singhal S. Identification of a subcentimeter pulmonary adenocarcinoma using intraoperative near-infrared imaging during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg. 2015;149(3):e51-e53. doi: 10.1016/j.jtcvs.2014.10.081 [DOI] [PubMed] [Google Scholar]
- 15.Okusanya OT, DeJesus EM, Jiang JX, et al. . Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J Thorac Cardiovasc Surg. 2015;150(1):28-35.e1. doi: 10.1016/j.jtcvs.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Predina JD, Keating J, Patel N, Nims S, Singhal S. Clinical implications of positive margins following non–small cell lung cancer surgery. J Surg Oncol. 2016;113(3):264-269. doi: 10.1002/jso.24130 [DOI] [PubMed] [Google Scholar]
- 17.Okusanya OT, Holt D, Heitjan D, et al. . Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg. 2014;98(4):1223-1230. doi: 10.1016/j.athoracsur.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HK, Quan YH, Choi BH, et al. . Intraoperative pulmonary neoplasm identification using near-infrared fluorescence imaging. Eur J Cardiothorac Surg. 2016;49(5):1497-1502. doi: 10.1093/ejcts/ezv367 [DOI] [PubMed] [Google Scholar]
- 19.Laperche Y, Oudea MC, Lostanlen D. Toxic effects of indocyanine green on rat liver mitochondria. Toxicol Appl Pharmacol. 1977;41(2):377-387. doi: 10.1016/0041-008X(77)90039-4 [DOI] [PubMed] [Google Scholar]
- 20.Gagnadoux F, Hureaux J, Vecellio L, et al. . Aerosolized chemotherapy. J Aerosol Med Pulm Drug Deliv. 2008;21(1):61-70. doi: 10.1089/jamp.2007.0656 [DOI] [PubMed] [Google Scholar]
- 21.Molphy J, Dickinson JW, Chester NJ, Loosemore M, Whyte G. The effect of 400 μg inhaled salbutamol on 3 km time trial performance in a low humidity environment. J Sports Sci Med. 2017;16(4):581-588. [PMC free article] [PubMed] [Google Scholar]
- 22.Labiris NR, Dolovich MB. Pulmonary drug delivery; part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588-599. doi: 10.1046/j.1365-2125.2003.01892.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bräunlich J, Wirtz H. Oral versus nasal high-flow bronchodilator inhalation in chronic obstructive pulmonary disease. J Aerosol Med Pulm Drug Deliv. 2018;31(4):248-254. doi: 10.1089/jamp.2017.1432 [DOI] [PubMed] [Google Scholar]
- 24.Noppen M, Dekeukeleire T, Hanon S, et al. . Fluorescein-enhanced autofluorescence thoracoscopy in patients with primary spontaneous pneumothorax and normal subjects. Am J Respir Crit Care Med. 2006;174(1):26-30. doi: 10.1164/rccm.200602-259OC [DOI] [PubMed] [Google Scholar]
- 25.Noppen M, Stratakos G, Verbanck S, D’Haese J, Meysman M, Vincken W. Fluorescein-enhanced autofluorescence thoracoscopy in primary spontaneous pneumothorax. Am J Respir Crit Care Med. 2004;170(6):680-682. doi: 10.1164/rccm.200404-438CR [DOI] [PubMed] [Google Scholar]
- 26.Oh Y, Lee YS, Quan YH, et al. . Thoracoscopic color and fluorescence imaging system for sentinel lymph node mapping in porcine lung using indocyanine green-neomannosyl human serum albumin: intraoperative image-guided sentinel nodes navigation. Ann Surg Oncol. 2014;21(4):1182-1188. doi: 10.1245/s10434-013-3381-z [DOI] [PubMed] [Google Scholar]
- 27.Oh Y, Quan YH, Choi Y, et al. . Intraoperative combined color and fluorescent images-based sentinel node mapping in the porcine lung: comparison of indocyanine green with or without albumin premixing. J Thorac Cardiovasc Surg. 2013;146(6):1509-1515. doi: 10.1016/j.jtcvs.2013.02.044 [DOI] [PubMed] [Google Scholar]
- 28.Oh Y, Quan YH, Kim M, Kim BM, Kim HK. Intraoperative fluorescence image-guided pulmonary segmentectomy. J Surg Res. 2015;199(2):287-293. doi: 10.1016/j.jss.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 29.Gao RW, Teraphongphom NT, van den Berg NS, et al. . Determination of tumor margins with surgical specimen mapping using near-infrared fluorescence. Cancer Res. 2018;78(17):5144-5154. doi: 10.1158/0008-5472.CAN-18-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou X, Liu Y, Liu Q, et al. . Point-of-care ratiometric fluorescence imaging of tissue for the diagnosis of ovarian cancer. Theranostics. 2019;9(16):4597-4607. doi: 10.7150/thno.35322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi BH, Young HS, Quan YH, et al. . Real-time computed tomography fluoroscopy-guided solitary lung tumor model in a rabbit. PLoS One. 2017;12(6):e0179220. doi: 10.1371/journal.pone.0179220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambrecht BN. Alveolar macrophage in the driver’s seat. Immunity. 2006;24(4):366-368. doi: 10.1016/j.immuni.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 33.Keating J, Tchou J, Okusanya O, et al. . Identification of breast cancer margins using intraoperative near-infrared imaging. J Surg Oncol. 2016;113(5):508-514. doi: 10.1002/jso.24167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singhal S. The future of surgical oncology: image-guided cancer surgery. JAMA Surg. 2016;151(2):184-185. doi: 10.1001/jamasurg.2015.3604 [DOI] [PubMed] [Google Scholar]
- 35.Graboys TB, Biegelsen B, Lampert S, Blatt CM, Lown B. Results of a second-opinion trial among patients recommended for coronary angiography. JAMA. 1992;268(18):2537-2540. doi: 10.1001/jama.1992.03490180069028 [DOI] [PubMed] [Google Scholar]
- 36.Kelvin FM. Double contrast examination of the upper gastrointestinal tract. South Med J. 1979;72(6):661-666. doi: 10.1097/00007611-197906000-00009 [DOI] [PubMed] [Google Scholar]
- 37.Predina JD, Newton AD, Corbett C, et al. . A clinical trial of TumorGlow to identify residual disease during pleurectomy and decortication. Ann Thorac Surg. 2019;107(1):224-232. doi: 10.1016/j.athoracsur.2018.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim C, Vibert E, Azoulay D, et al. . Indocyanine green fluorescence imaging in the surgical management of liver cancers: current facts and future implications. J Visc Surg. 2014;151(2):117-124. doi: 10.1016/j.jviscsurg.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 39.Newton AD, Predina JD, Frenzel-Sulyok LG, Shin MH, Wang Y, Singhal S. Intraoperative near-infrared imaging can identify sub-centimeter colorectal cancer lung metastases during pulmonary metastasectomy. J Thorac Dis. 2018;10(7):E544-E548. doi: 10.21037/jtd.2018.06.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keating J, Newton A, Venegas O, et al. . Near-infrared intraoperative molecular imaging can locate metastases to the lung. Ann Thorac Surg. 2017;103(2):390-398. doi: 10.1016/j.athoracsur.2016.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pischik VG, Kovalenko A. The role of indocyanine green fluorescence for intersegmental plane identification during video-assisted thoracoscopic surgery segmentectomies. J Thorac Dis. 2018;10(suppl 31):S3704-S3711. doi: 10.21037/jtd.2018.04.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbett CJ, Frenzel Sulyok LG, Predina JD, et al. . Comparison of a short versus long stokes shift near-infrared dye during intraoperative molecular imaging. Mol Imaging Biol. 2020;22(1):144-155. doi: 10.1007/s11307-019-01434-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102(4):838-854. doi: 10.1097/00000542-200504000-00021 [DOI] [PubMed] [Google Scholar]
- 44.Woodring JH, Reed JC. Types and mechanisms of pulmonary atelectasis. J Thorac Imaging. 1996;11(2):92-108. doi: 10.1097/00005382-199621000-00002 [DOI] [PubMed] [Google Scholar]
- 45.Sobocińska M, Sobociński B, Jarzemska A, Serafin Z. Rounded atelectasis of the lung: a pictorial review. Pol J Radiol. 2014;79:203-209. doi: 10.12659/PJR.889983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedenstierna G, Rothen HU. Atelectasis formation during anesthesia: causes and measures to prevent it. J Clin Monit Comput. 2000;16(5-6):329-335. doi: 10.1023/A:1011491231934 [DOI] [PubMed] [Google Scholar]
- 47.Peroni DG, Boner AL. Atelectasis: mechanisms, diagnosis and management. Paediatr Respir Rev. 2000;1(3):274-278. doi: 10.1053/prrv.2000.0059 [DOI] [PubMed] [Google Scholar]
- 48.Schindler MB. Treatment of atelectasis: where is the evidence? Crit Care. 2005;9(4):341-342. doi: 10.1186/cc3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah PL, Herth FJ. Current status of bronchoscopic lung volume reduction with endobronchial valves. Thorax. 2014;69(3):280-286. doi: 10.1136/thoraxjnl-2013-203743 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Organ Distribution of the Inhaled ICG in a Normal Mouse Model
eMethods 2. Human Study
eFigure 1. Detection of TM in the Resected Lung Tissue of Patients by ICG Nebulization
eFigure 2. Biodistribution of ICG in Different Mouse Organs After ICG Inhalation
eFigure 3. Fluorescent Assessment of Lung TM With Inhaled and Intravenously Injected ICG
eFigure 4. ICG Inhalation Can Detect Tumors at a Depth of 0.5 cm
eFigure 5. A 0.2 cm Tumor Could Be Detected With ICG Inhalation
eTable. Clinical and Demographic Characteristics of Patients With Lung Cancer