Abstract
This study assesses the presence of SARS-CoV-2 in the breast milk of 18 US women infected with SARS-CoV-2.
Concern has been raised that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be transmitted to infants by breastfeeding. A number of organizations advise that women infected with SARS-CoV-2 may choose to breastfeed with protections to prevent transmission of the virus through respiratory droplets. Of 24 case reports on breast milk samples from women infected with SARS-CoV-2, viral RNA was detected in 10 samples from 4 women.1,2,3,4,5,6 In some cases, environmental contamination or retrograde flow from an infected infant could not be ruled out. Detection of viral RNA by reverse transcriptase–polymerase chain reaction (RT-PCR) does not equate with infectivity. To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk. However, potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed.
Methods
Beginning in March 2020, women residing anywhere in the US who reported being symptomatic, having been exposed to an infected person, or having a confirmed SARS-CoV-2 infection and who were currently breastfeeding were invited to participate in the study using a variety of methods including media awareness, website, and clinician referral. Only women who tested positive by RT-PCR tests were included. The University of California San Diego Institutional Review Board approved the study, and women provided oral and written informed consent. Clinical data were collected by phone interview. Breast milk samples were self-collected and mailed to the study center according to a standard protocol. In some cases, women also provided stored samples collected prior to enrollment (eAppendix in the Supplement).
A quantitative RT-PCR assay for SARS-CoV-2 in breast milk was established and validated. Tissue culture methods to detect replication-competent SARS-CoV-2 in breast milk were developed (eAppendix in the Supplement).
Additionally, conditions of Holder pasteurization commonly used in human milk banks were mimicked by adding SARS-CoV-2 (200 × median tissue culture infectious dose 50% [TCID50]) to breast milk samples from 2 different control donors who provided milk samples prior to onset of the pandemic. The samples were heated to 62.5 °C for 30 minutes and then cooled to 4 °C. Following this procedure, the samples were added to the tissue culture. Nonpasteurized aliquots of the same 2 milk-virus mixtures were cultured in parallel.
SPSS version 25 and Prism version 8.4.3 (GraphPad) were used for analyses.
Results
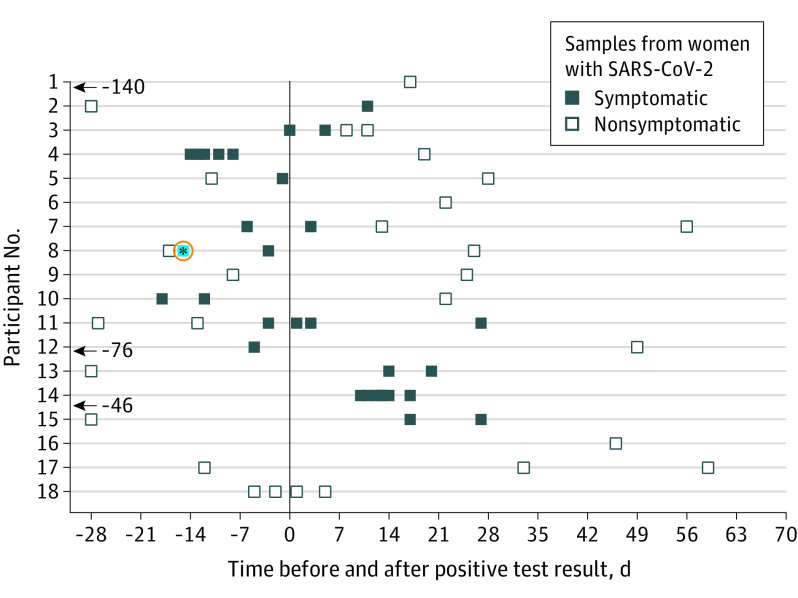
Between March 27 and May 6, 2020, we enrolled 18 women who had confirmed SARS-CoV-2 infection (77.7% White non-Hispanic, mean age, 34.4 years [SD, 5.2 years]). Their offspring ranged in age from newborn to 19 months. Women provided between 1 and 12 samples, with a total of 64 samples collected at varying time points before and after the positive SARS-CoV-2 RT-PCR test result. All but 1 woman had symptomatic disease (Figure). One breast milk sample had detectable SARS-CoV-2 RNA. The positive sample was collected on the day of symptom onset; however, 1 sample taken 2 days prior to symptom onset and 2 samples collected 12 and 41 days later tested negative for viral RNA. The breastfed infant was not tested. No replication-competent virus was detectable in any sample, including the sample that tested positive for viral RNA.
Figure. Breast Milk Sampling Relative to Time of Positive SARS-CoV-2 Test Result.
All samples were tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA by reverse transcriptase–polymerase chain reaction (RT-PCR). The blue data point outlined in red represents a participant who had tested positive by RT-PCR but negative by infectivity assay.
Following Holder pasteurization, viral RNA was not detected by RT-PCR in the 2 samples that had been spiked with replication-competent SARS-CoV-2, nor was culturable virus detected. However, virus was detected by culture in nonpasteurized aliquots of the same 2 milk-virus mixtures.
Discussion
Although SARS-CoV-2 RNA was detected in 1 milk sample from an infected woman, the viral culture for that sample was negative. These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant. Furthermore, when control samples spiked with replication-competent SARS-CoV-2 virus were treated by Holder pasteurization, no replication-competent virus or viral RNA was detectable. These findings are reassuring given the known benefits of breastfeeding and human milk provided through milk banks. Limitations include the small sample size, nonrandom sample with possible selection bias, self-report of RT-PCR positivity, and self-collection of milk samples, some before the standard protocol was instituted.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
eAppendix. Recruitment and Sample Collection
References
- 1.Chen H, Guo J, Wang C, et al. . Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809-815. doi: 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y, Liu C, Dong L, et al. . Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG. Published online May 5, 2020. doi: 10.1111/1471-0528.16276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa S, Posteraro B, Marchetti S, et al. . Excretion of SARS-CoV-2 in human breast milk. Clin Microbiol Infect. Published online June 2, 2020. doi: 10.1016/j.cmi.2020.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groß R, Conzelmann C, Müller JA, et al. . Detection of SARS-CoV-2 in human breastmilk. Lancet. 2020;395(10239):1757-1758. doi: 10.1016/S0140-6736(20)31181-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam PCK, Ly KM, Kernich ML, et al. . Detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID-19). Clin Infect Dis. Published online May 30, 2020. doi: 10.1093/cid/ciaa673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Wang J, Li W, Zhou Z, Liu S, Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020;14(2):193-198. doi: 10.1007/s11684-020-0772-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Recruitment and Sample Collection