Abstract
Early physical therapy models hold great promise for delivering high-value care for individuals with musculoskeletal pain. However, existing physical therapist practice and research standards are misaligned with value-based principles, which limits the potential for growth and sustainability of these models. This Perspective describes how the value proposition of early physical therapy can be improved by redefining harm, embracing a prognostic approach to clinical decision making, and advocating for system-wide guideline-adherent pain care. It also outlines the need to adopt a common language to describe these models and embrace new, rigorous study designs and analytical approaches to better understand where and how early physical therapy delivers value. The goal is to define a clear path forward to ensure physical therapists are aligned within health care systems to deliver on the American Physical Therapy Association’s vision of high-value care in a rapidly changing health care environment.
Keywords: Decision Making, Health Care Reform, Orthopedics, Pain Management, Quality of Health Care
The American Physical Therapy Association’s (APTA) Vision Statement focuses on improving quality, innovation, value, and access to physical therapy services. Since those guiding principles were adopted in 2013, the shift towards value-based purchasing has significantly changed the health care landscape in the United States by disrupting traditional treatment pathways. A notable example of pathway disruption driven by value-based purchasing is the bundling of services for joint replacement that accompanied the Comprehensive Care for Joint Replacement program in 2016.1 This program has fundamentally transformed perioperative and post-acute care pathways for Medicare beneficiaries undergoing joint replacement by incentivizing efficiency and utilization of high-quality, low-cost services.2
Physical therapists have increasingly become the target of innovative delivery models that aim to positively disrupt the traditional approach to musculoskeletal pain management.3 These models are not new4–6 but have gained renewed interest due to the growth of value-based purchasing and recent practice guidelines advocating earlier exposure to nonpharmacological care.3,7,8 They are unique because rather than modifying treatment content, these models alter timing of care, order of services, and mechanisms for accessing care to improve outcomes. In the traditional care model for musculoskeletal pain, patients receive physical therapy only after other treatment options have been exhausted (Fig. 1a). In contrast, early physical therapy models place treatment near the beginning of a care episode (Fig. 1b). The proposed value of early physical therapy is that it may not only resolve a patient’s symptoms but could also help avoid more invasive, costly, and lower value treatment options.9,10
Figure 1.
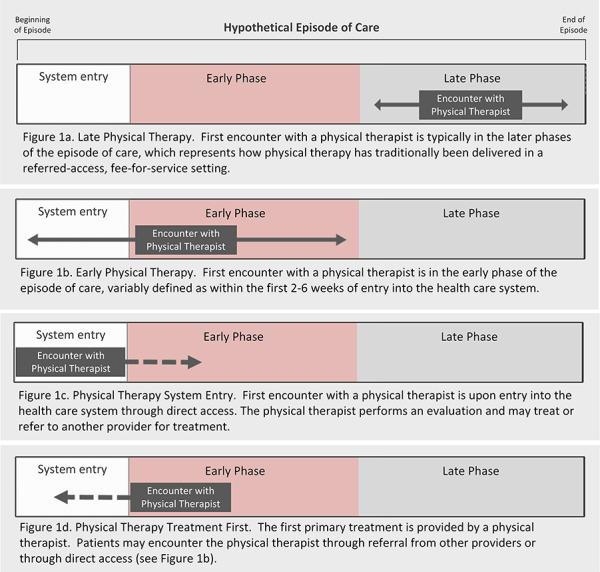
Hypothetical episode of care.
One form of early physical therapy is defined by how a patient accesses the health care system (ie, physical therapist system entry). The physical therapist system entry model moves the first point of care from the physician to the physical therapist, thus leveraging the physical therapists’ diagnostic and triage skills to improve outcomes and costs (Fig. 1c). This model is facilitated by legislative and third-party payer support for direct access to a physical therapist. In this role, physical therapists manage a patient’s overall care pathway, including treatment, consultation, or referral to other providers.
Another form of early physical therapy is defined by the type of treatment patients first receive for their musculoskeletal pain (ie, physical therapist treatment first). In the physical therapist treatment first model, patients receive services provided by a physical therapist before other services like advanced imaging, narcotics, or injection, regardless of how they initially access the health care system (Fig. 1d). Often, this model will colocate physical therapy at traditional system entry points, such as primary care or emergency departments, where patients will be immediately referred for initial treatment by a physical therapist when indicated.11–13
The success of early physical therapy models for the management of musculoskeletal pain is key to achieving the APTA Vision. These models will be most successful if they can demonstrate greater value than other care models and adapt to sustain value as the health care system changes. In this Perspective, we argue that existing physical therapist practice and research standards are misaligned with many value-based principles. To address these shortcomings, we propose new perspectives on clinical practice and research to more critically explore the benefits and drawbacks of early physical therapy. The overall goal of this Perspective is to define a clear path forward to ensure physical therapists are aligned within health care systems to deliver high-value care in a rapidly changing health care environment.
The Need for New Practice Standards
Current practice standards and conceptual approaches that allow physical therapists to thrive in a fee-for-service environment can alternatively threaten the impact, sustainability, and value added by early physical therapy. In the following sections, we describe how the traditional framework for clinical decision making limits the viability of early physical therapy models.
Outdated Concept of Harm
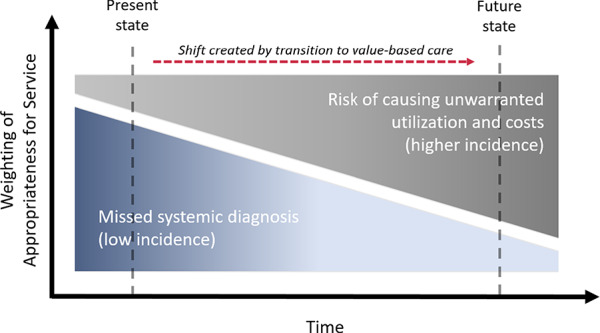
Historically, risk of harm from physical therapy as a point of entry into the health care system has focused on whether physical therapists can identify and appropriately refer patients with systemic conditions that cause or mimic musculoskeletal pain for evaluation and treatment.14 However, strong empirical evidence has established that patients are at no greater risk of a missed diagnosis when accessing the health care system through physical therapy than through any other provider.14–17 These data are encouraging, but emerging value-based care initiatives challenge us to consider other ways that health care might introduce harm. For instance, care pathways that lead to avoidable costs, excessive care episodes, or unwarranted care escalation can also cause harm, especially if escalation involves interventions with known risks like surgery or opioid prescription. Therefore, our concept of risk for harm must evolve from a relatively low threshold (ie, this pathway might lead to a missed diagnosis) to a much higher threshold (ie, this pathway might lead to unwarranted utilization and costs) (Fig. 2).
Figure 2.
Relationships between value-based physical therapy delivery models and traditional care.
In this paradigm, harm could occur if being treated by a physical therapist first simply delays the inevitable use of additional services, leads to unwarranted visits, or increases risk of care escalation. Recent studies suggest physical therapy is often not the last stop in the health care system for many patients with musculoskeletal pain.9,18 In some circumstances, use of physical therapy may even be associated with risk of higher downstream health care utilization and costs compared with other system entry points, although the literature lacks consensus on these risks. For example, Frogner and colleagues9 reported that patients with low back pain who saw a physical therapist first had a nearly 20% higher probability of hospitalization despite a lower probability of opioid prescription (89%), emergency department visits (15%), and advanced imaging (28%). Alternatively, Horn et al19 found a higher risk of advanced imaging among those seeing a physical therapist first compared with a primary care provider for neck pain. As early physical therapy models expand, their value proposition will be defined by how well they balance the potential benefits (eg, short-term cost savings, lower risk of opioid use) and harms (eg, risk of care escalation, delayed inevitable use of care) of treatment as well as how firmly we can establish the probability of those benefits and harms at the population and individual levels.
Misaligned Diagnostic- and Prognostic-Based Clinical Decision Framework for Value-Based Care
Currently, providers use a diagnostic-based, biomedical framework when making decisions on whether to provide physical therapy. In this framework, treatment is delivered because a diagnosis is amenable to physical therapy regardless of whether outcomes may be favorable. The predominant fee-for-service reimbursement model in the United States has facilitated this approach to decision making by providing little incentive to forego potentially wasteful care among those unlikely to benefit. As a result, physical therapy might often be utilized indiscriminately due to its low risk of adverse events and relatively low costs. Although this approach might be good for business under existing fee-for-service reimbursement models, it does little to prepare physical therapists to operate in a health care environment that emphasizes cost-effectiveness.
In contrast, value-based care favors a prognostic, or outcomes, framework for selecting treatment, and the decision to treat is dependent on probability of improvement or risk of poor outcomes. As system entry providers, physical therapists have the responsibility to identify a proper treatment pathway for a patient but not an equal imperative to be the provider delivering treatment. This model contrasts with referred access models where evaluation is coupled with treatment in all but very rare cases (ie, identification of a red flag). Left unaddressed, propagation of a diagnostic framework for treatment decision making reduces overall value by exposing patients to physical therapy even when they have a low probability of benefit.
Modernizing Physical Therapist Practice Standards for Value-Based Care
To address the need for new practice standards, we propose updated frameworks and tools to guide clinical decision making, and advocate for system-wide adoption of guideline adherent care so that physical therapists can better function in early provider roles.
Embracing Prognostic Clinical Decision Making Based on Benefit and Harm
Early physical therapy models have demonstrated promising outcomes in observational studies, including reductions in costs and utilization, when considering population-level effects.9,10,17,19 However, we have an opportunity to further improve the value of these models by identifying individuals who will optimally benefit from early physical therapy. Importantly, individuals most appropriate for physical therapy would be those with characteristics beyond the diagnosis that suggest physical therapy alone or in combination with other treatments would result in superior outcomes. For those with a generally positive prognosis regardless of treatment type, physical therapy would be most valuable only if it was less costly, more desirable, or less harmful than other treatments. In this context, “success” of early physical therapy will be equally measured by improvement in outcomes as a direct result of physical therapy treatment or the timely referral of individuals who are unlikely to benefit from physical therapist treatment.
Prospectively identifying risk of benefit or harm requires physical therapists to embrace a prognostic framework for clinical decision making. However, the lack of valid prognostic tools to help determine when escalation of care is appropriate is one of many barriers to embracing a prognostic framework.20 Multiple, often disparate prognostic tools exist, although none have been validated for this purpose and many suffer from marginal predictive accuracy.21 Accurate tools might assess psychological, social, and behavioral characteristics as well as key attributes related to the context of care.22,23 The most effective tools will be those that apply across diagnoses and can be deployed within the electronic health record (EHR).
Promoting System-Wide Adoption of Guideline-Adherent Care for Musculoskeletal Pain
As first point-of-care providers, physical therapists must build strong collaborative networks with other health care professionals, which is a central theme of the APTA Vision Statement.24 However, the value of care ultimately delivered by early physical therapy is strongly influenced by the extent to which providers in the network also adhere to evidence-based guidelines. Health services organizations, administrators, and providers share in the responsibility for minimizing risk of harm by conforming to evidence-based guidelines, especially when models of care value diagnostic testing or costly interventions like surgery. The value of early physical therapy models, especially among individuals with complex health care needs and multimorbidity, is substantially reduced if physical therapists are referring to other providers who do not adopt guideline-adherent care.
The Need for New Research Standards
Existing practice standards are not the only threat to value-based physical therapy models. In the following sections, we describe how common methods of conducting research and evaluating data also limit the development and sustainability of these models.
Familiar Research Designs Cannot Fully Answer Value-Based Questions
Researchers typically establish causality using a randomized controlled trial (RCT), which is considered the gold standard study design for causal inference. To date, RCTs have served physical therapists well because many research questions have focused on treatment efficacy. However, many of the characteristics that contribute to the high internal validity of traditional RCTs (eg, narrow study eligibility, rigorous controls over treatment assignment) may limit their utility for informing health policy, value propositions, and questions of treatment effectiveness.25 Therefore, we must broaden what study designs are acceptable for determining “best evidence” and informing health policy.
Practice-based or real-world data (RWD) can provide important insights on the effectiveness of value-based models in actual care settings if well designed.26 However, the observational and pragmatic clinical study designs that generate RWD represent lower quality designs on most evidence hierarchies, causing many to dismiss or underrate their potential contributions to advancing value-based care.27 Importantly, evidence to inform policy must meet appropriateness and generalizability standards, which tightly controlled efficacy trials rarely meet. Strict reliance on the traditional evidence hierarchy when judging the usefulness of evidence may leave physical therapists ill-equipped to establish and refine the value of their services.
Poor Methodological Rigor Beyond Randomization
Observational studies may have greater generalizability compared with RCTs if based on larger, more inclusive samples. However, making causal inferences with these designs is more challenging because outcomes could be due to treatment itself and/or unobserved confounders. Unobserved confounders are characteristics that can drive differences in treatment exposure or outcomes but are unknown to the researchers or known to researchers but unmeasured. Confounding of treatment effects by unobserved characteristics is of minimal concern in RCTs due to randomization. But unobserved confounding is a major concern in observational studies due to the lack of randomization. Within the last few decades, researchers have begun applying statistical methods that enable causal inferences to be posited in studies using observational health care data,28–30 and these methods have recently entered the rehabilitation literature.9,31–33 The appropriateness of these methods for establishing causation is hotly debated,34,35 and like any research methodology, interpreting results of these studies as causal depends on the rigor of the design and methods used.
The least rigorous form of these analyses includes descriptive comparisons or multivariate models adjusted for observed covariates that differ between groups receiving different treatments. Models using claims data with limited covariates are most likely to have unobserved confounding, whereas those that incorporate multiple sources of data (eg, claims plus EHR data plus survey data) and include all key confounders would likely provide the best causal effect estimates. More rigorous methods, such as propensity score matching,36 attempt to reduce the effects of confounding by matching (or weighting) individuals who receive different treatments on observed characteristics such as age, sex, or comorbidity burden. This method makes the treatment and comparison groups more similar, with outcomes then compared between matched (or weighted) groups. Use of propensity score methods is common in studies using observational data31–33,37 but rarely completely eliminates bias because it cannot account for unobserved confounders.
A less common approach to causal inference using RWD is instrumental variable (IV) analysis.30,38 IV analyses adjust for unobserved confounding by use of an “instrument,” which is a variable associated with treatment exposure but not the treatment outcome (ie, differential distance between provider seen and alternative provider who could have been seen).9 Comparing outcomes based on this “pseudo-randomization” is proposed to remove or limit the influence of selection bias and other unmeasured confounders. With a correctly specified instrument, IV analysis is viewed as the most defensible method for establishing causality using observational data.39 In some cases, IV analysis has produced treatment effects that are comparable with well-designed RCTs.40,41 However, good instruments are difficult to identify, and results can be generalized only to “marginal patients” whose value of the IV is influential in their treatment choice, thus making IV analyses for musculoskeletal pain uncommon.
Selection bias in observational studies represents a substantial threat to establishing strong and defensible evidence for value-based physical therapy models. Yet with the exception of a few published trials,42,43 much of the current evidence on early physical therapy models in the United States is concentrated in observational study designs with high risk of uncontrolled selection bias. To demonstrate, we conducted a narrative review of observational studies evaluating the effects of early physical therapy models in the United States during the last 25 years: 1 used IV analysis, 3 used propensity score analyses, 10 were covariate-adjusted only, and 2 did not control for covariates (Table). Among those that adjusted for covariates, the number of included covariates and covariate domains (eg, biological psychological, social,) were often limited. The relative benefit of value-based physical therapy models appears promising. However, variability in rigor among observational studies creates uncertainty regarding true effects.
Table 1.
Characteristics of Observational Studies on Value-Based Physical Therapy Modelsa
| Author | Design | Analysis | Intervention | Comparison | Results |
|---|---|---|---|---|---|
| Childs et al44 | Retrospective, observational cohort study | Covariate adjusted logistic regression models | Physical therapy within 14 d of primary index date | Physical therapy between 14 and 90 d from primary care index date | Early PT referral: lower 2-y utilization for physician visits, advanced imaging, spinal injections, spine surgery, emergency department visits, prescription medication use, opioid use, and 60% lower total LBP-related costs |
| Denninger et al17 | Retrospective, observational cohort study | Covariate adjusted mixed-effects model | DA | Medical referral | DA: lower total costs, fewer PT sessions and days in care, and lower PT costs, radiology costs, other costs, and total costs. Both groups had clinically important improvements in pain and disability, which were similar between groups. |
| Fritz et al45 | Retrospective, observational cohort study | Covariate adjusted logistic regression model | Physical therapy ≤14 d after primary care index date | Physical therapy ≥14 days after primary care index date | Early PT: decreased risk of advanced imaging, additional physician visits, surgery, injections, and opioid medications. Total medical costs for LBP lower for patients receiving early PT. |
| Fritz et al33 | Retrospective, observational cohort study | Propensity score matched groups; generalized linear model | Physical therapy first within 6 wk of index visit | Advanced imaging first within 6 wk of index visit | Advanced imaging recipients had higher odds of all utilization outcomes. Charges were higher with advanced imaging. |
| Frogner et al9 | Retrospective, observational cohort study | Two-stage residual inclusion (2SRI) instrumental variable models | PT first: patients saw PT as first point where a LBP diagnosis was recorded, or on index date | PT Later: patients visited PT at some point but not index date; no PT: never visited PT | PT first: lower probability of having an opioid, any advanced imaging services, and an Emergency Department visit, but higher probability of hospitalization. PT first had lower out-of-pocket costs, and costs shifted away from outpatient and pharmacy toward provider settings. |
| Gellhorn et al46 | Retrospective, observational cohort study | Covariate adjusted logistic regression models | Early group, <4 wk after primary index date | Delayed group: >3 mo after primary index date | Early PT: lower odds of undergoing surgery, receiving a lumbosacral injection, frequent physician office usage. |
| Horn et al19 | Retrospective, observational cohort study | Covariate adjusted logistic regression models | Patient seen by PT, chiropractor, or specialist at initial visit | Patient seen by PCP at initial visit | Initial consult by DC or PT had decreased odds of being prescribed opioids compared with PCP. Patients consulting with a DC had lower odds of advanced imaging and injections. Initiating care with a specialist or PT increased the odds of advanced imaging but only initiating care with specialist increased the odds of injections. |
| Horn et al47 | Retrospective, observational cohort study | Covariate adjusted generalized linear regression models | Early PT: Duration of symptoms <4 wk | Delayed PT: duration of symptoms >4 wk | Early PT: higher odds of achieving MCID on NDI and NPRS; greatest decrease in disability (2.27% change in NDI/$100, best value in decreasing pain (0.38 point change on the NPRS/$100; managed more efficiently with 3.44% change in NDI/visit and 0.57-point change in NPRS/visit. |
| Horn et al48 | Retrospective, observational cohort study | Covariate adjusted generalized linear regression models | PT within 14 d (early PT consultation) | PT between 15 and 90 d (delayed PT consultation) or between 91 and 364 d (late PT consultation) | Compared with early PT consultation, odds of receiving opioids (aOR = 2.79), spinal injection (aOR = 4.36,), undergoing an MRI (aOR = 4.68), X-ray (aOR = 2.97) or CT scan (aOR = 3.36) increased in patients in late PT consultation group. Similar increases in risk were found in delayed group (except CT and opioids). Compared with early PT consultation group, mean costs were $2172 ($557, $3786) higher in late PT contact group and $1063 ($138–$1988) higher in delayed PT group. |
| Karvelas et al49 | Prospective cohort study | Covariate adjusted generalized linear models | Early PT (initiated within 28 d of index visit) | Not initiating early PT | No difference in total spine RVUs between the 2 groups. Early PT group had greater PT RVUs and imaging RVUs. |
| Mitchell & de Lissovoy50 | Retrospective, observational cohort study | Covariate-adjusted linear regression models | DA | Physician referral | DA: fewer services and lower costs |
| Pendergast et al31 | Retrospective, observational cohort study | Covariate adjusted generalized linear models, propensity score matching | Self-referred | Physician-referred | Self-referred episodes: fewer PT visits and lower allowable amounts, after covariate adjustment, but did not differ in related health care utilization after PT. Similar results for propensity matched analysis. |
| Rundell et al32 | Prospective cohort study | Propensity score matched groups; covariate adjusted regression models | Early PT (initiated within 28 d of index visit) | Later PT (initiated within 3–6 mo of index visit) | Early PT: better functional status and less back pain at 12 mo. No difference between early PT groups at 3 and 6 mo. Odds of a 30% improvement in function or pain did not differ between groups at 12 mo. Early PT had increased odds of a 50% improvement in function at 12 mo. No difference between later groups at 12 mo. |
| Sun et al51 | Retrospective, observational cohort study | Covariate-adjusted linear regression models | Early PT (initiated within 90 d of index date) | Without early PT | Early PT: significant reduction in incidence of any opioid use between 91 and 365 d after index date. For patients who did use opioids, early PT was associated with an approximately 10% statistically significant reduction in amount of opioid use |
| Woods et al52 | Retrospective, observational cohort study | Unadjusted repeated-measures ANOVA | Early group received PT within 6 mo of initial injury | Delayed group received PT after 6 mo of initial injury | Early group displayed greater spine flexion, VO2 max, and lifting capacity. |
| Zigenfus et al53 | Retrospective, observational cohort study | GLM—General Factorial Analysis procedure with a restricted model | Early group received PT ≤2 d after pain onset | Delayed group received PT 8–197 d after pain onset | Fewer physician visits, restricted workdays, days away from work, shorter duration with early access |
aANOVA = analysis of variance; aOR = adjusted odds ratio; CT = computed tomography; DA = direct access; DC = chiropractor, LBP = low back pain; MCID = minimal clinically important difference; NDI = neck disability index; NPRS = numeric pain rating scale; PCP = primary care physician, PT = physical therapy; RVU = relative value unit.
Modernizing Physical Therapy Research Standards for Value-Based Care
Establishing high-quality evidence for the effectiveness of early physical therapy is perhaps the most important step towards widespread implementation and sustainability of these models. To establish high-quality evidence, we must first adopt a common language for describing these models, then embrace rigorous study designs and analytical approaches to better understand where and how early physical therapy delivers value.
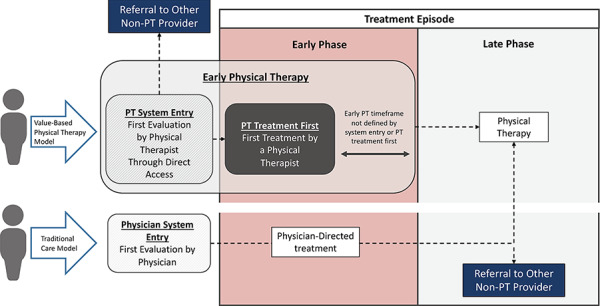
Adopting a Common Language Regarding the Process and Delivery of Care
Value-based physical therapy models comprise at least 3 distinct characteristics that could influence outcomes (Fig. 3): timing of care (ie, early physical therapy compared with late), mode of access (ie, direct or referred access), and order of treatment (ie, physical therapy first compared with other treatment). Reports in the literature often fail to capture each of these characteristics, suggesting the need for establishing common language. Common language will allow physical therapists to operationally define the different ways they deliver care, which will facilitate more effective communication about the value of their services. It will also allow for better recognition of physical therapists as care providers with triage and diagnostic capabilities who can oversee many facets of health care. Common language will also enable researchers to distinguish value delivered by different characteristics like timing of care, mode of access, and order of treatment. This is important because the influence of each characteristic has unique implications for clinical practice and policy. Conflating the influence of these characteristics might misrepresent the true mechanisms by which physical therapy delivers value and therefore where to concentrate training, resources, and health care policy.
Figure 3.
Change in determining harm associated with accessing physical therapy directly compared with referral.
Embracing New Research Designs to Support Value-Based Care
Study designs that evaluate health care consumer behavior in natural care settings will be most informative for clinical practice and policy. Pragmatic clinical trials are an evolution of RCTs that attempt to address the issue of poor generalizability by embedding randomized treatment into health care systems and relaxing many of the tight controls over utilization and decision making.54,55 Often, randomization will occur at the clinic or system level (ie, cluster randomization56), which minimizes disruption of the natural care setting. But these randomization designs are complex,57 and few health care systems are equipped to accommodate large-scale pragmatic trials because data capture relies heavily on the EHR. Nevertheless, pragmatic clinical trials are becoming more common42,58 and will provide important information on the optimal delivery of early physical therapy.
An additional consideration is wider use of noninferiority trial designs. For musculoskeletal pain conditions, the quest for trials that demonstrate treatment superiority has not led to actionable data and has contributed to high variability in available pain treatments. Clinical trials that compare common treatments for musculoskeletal pain often report small effect sizes and/or similar results between treatments.59,60 In some areas where it is well known that treatment effects are small or similar, noninferiority trials may provide important insights on how to efficiently deliver value-based treatment.60 If 2 treatments demonstrate approximately the same effects, treatment decisions can be made based on availability of resources, patient preference, or other aspects of quality such as patient satisfaction, safety, or costs. Importantly, failure to observe sufficient evidence for treatment effect differences in superiority trials does not prove 2 treatments are equal, so use of null trials for these purposes is not appropriate.61 Noninferiority trials are designed to account for factors that may inform clinical implementation, beyond generating an estimate of treatment effect, and therefore could provide substantial benefit in directing value-based care decisions especially for conditions with higher prevalence.62
Demanding Rigor in Observational Analyses
Observational analyses using RWD can answer important questions related to health care utilization and outcomes that trials cannot, making them an important part of the evidence hierarchy for value and health care policy questions. Indeed, it is largely observational analyses that are currently being used to pursue changes in reimbursement and reinforce the benefits of early physical therapy.3,63 However, recognizing the inherent bias in observational data, we stress the need to fully embrace methodologies that take a rigorous approach to minimizing bias. The first step is properly differentiating nonrandomized studies and understanding how varying design and statistical features contribute to bias. Clinicians and researchers are encouraged to use tools like the ROBINS-I64 that aid in assessing risk of bias in nonrandomized studies. For studies with higher risk of bias, we recommend caution when using the results to inform health policy and clinical practice.
When designing observational analyses, use of nonequivalent or control outcomes could help to strengthen the internal validity of the study.65 Control outcomes are not expected to change as the result of the treatment of interest but are expected to be sensitive to other contextually relevant threats to internal validity. For instance, we would not expect health care utilization for non-pain–related conditions, like hypertension, to change as a result of early physical therapy, but it could be impacted by overall health or health-seeking behavior. If utilization of services for hypertension changed after early physical therapy and rates differed from the control group, then we would suspect problems with unobserved confounding in the analysis.
Another consideration for strengthening design is more comprehensive use of covariates. The number and type of covariates available are often limited to measures that are collected because they are relevant to care delivery or payment, but these variables may not be sufficient to help establish causality. The growing availability of linked data resources, as well as the development of registries specifically designed to study care delivery (eg, the APTA Physical Therapy Outcomes Registry), will enhance our ability to design observational studies that better account for characteristics that introduce bias.
Investigations Beyond Low Back and Neck Pain
To date, much of the literature exploring value-based delivery of physical therapy has concentrated on low back and neck pain. Promising early results of value-based models may prompt the desire to extrapolate benefits to other conditions. Unfortunately, there is little empirical support for generalizing the benefits of these value-based physical therapy models to anatomical regions outside of the low back and neck. Physical therapists are therefore challenged to balance a desire to be good stewards of the profession with the need to demand high-quality evidence on behalf of patients. Studying these models in other musculoskeletal and nonmusculoskeletal conditions and determining whether value is universal across health care systems will significantly strengthen the value proposition of early physical therapy.51
A Path Forward for Value-Based Physical Therapy
The implementation of value-based care models and an epidemic of opioid abuse has provided a timely opportunity and captive public audience to promote physical therapy as a safe and effective first option for musculoskeletal pain management. But as the health care system adjusts to address the opioid crisis and low-value services are abandoned, physical therapists must be prepared to evaluate and refine their value. We conclude with actionable steps to better align physical therapist clinical practice and research with value-based care principles.
Defining Concepts
Use consistent descriptions of value-based care pathways that are differentiated, at a minimum, by timing, mode of access, and order of treatment.
Redefine success of early physical therapy pathways as the ability to improve patient-reported outcomes and/or avoid unwarranted care escalation, or the timely referral of individuals unlikely to benefit from physical therapy.
Delivery of Clinical Care
Use prognosis-based decision making for treatment and referral, with a focus on minimizing risk of unwarranted visits and care escalation.
Develop and validate prognostic tools that accurately predict a range of outcomes important to multiple stakeholders.
Engage with health systems, other provider groups, professional associations, and payers to promote and incentivize system-wide adherence to evidence-based pain management guidelines.
Conduct of Research
Encourage prospective investigations that determine the value conferred by the specific characteristics of timing, access, and order of treatment.
Consider pragmatic and noninferiority trials as viable options to traditional explanatory clinical trials.
Employ rigorous statistical approaches to control for potential bias in observational analyses.
Evaluate performance of value-based physical therapy models across different health conditions, health care systems, and geographic regions.
The health care system will continue to evolve in new and unexpected ways in response to the need for higher quality care. This evolution will present physical therapists with many opportunities to positively disrupt existing systems and promote their value as a nonpharmacological care provider for musculoskeletal pain. However, there will also be many challenges that could jeopardize value and threaten growth if existing practice and research standards do not evolve as well.
Author Contributions
Concept/idea/research design: T.A. Lentz, A.P. Goode, C.A. Thigpen, S.Z. George
Writing: T.A. Lentz, A.P. Goode, C.A. Thigpen, S.Z. George
Data collection: T.A. Lentz
Data analysis: T.A. Lentz
Project management: T.A. Lentz
Consultation (including review of manuscript before submitting): T.A. Lentz, A.P. Goode, C.A. Thigpen, S.Z. George
Funding
T.A. Lentz received support for this project from the Foundation for Physical Therapy with Promotion of Doctoral Studies (PODS I & II) Awards. A.P. Goode received support from the Duke Clinical and Translational Science Institute (home of the Clinical and Translational Science Award [UL1TR001117]) and the Duke Institute for Health Innovation.
Role of the Funding Source
The funders played no role in writing this Perspective.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Centers for Medicare Medicaid services (CMS), HHS. Medicare program: comprehensive care for joint replacement payment model for acute care hospitals furnishing lower extremity joint replacement services. Final rule. Fed Regist. 2015;80:73273–73554. [PubMed] [Google Scholar]
- 2. Siddiqi A, White PB, Mistry JB. et al. . Effect of bundled payments and health care reform as alternative payment models in total joint arthroplasty: a clinical review. J Arthroplasty. 2017;32:2590–2597. [DOI] [PubMed] [Google Scholar]
- 3. Innovative Collaborative Effort Between APTA , United Healthcare, and OptumLabs could introduce important changes to pain management policies. http://www.apta.org/PTinMotion/News/2018/07/10/OptumPilot2018/.
- 4. Rothstein JM. Direct access: beyond the diatribes. Phys Ther. 1991;71:181–182. [DOI] [PubMed] [Google Scholar]
- 5. Taylor TK, Domholdt E. Legislative change to permit direct access to physical therapy services: a study of process and content issues. Phys Ther. 1991;71:382–389. [DOI] [PubMed] [Google Scholar]
- 6. Overman SS, Larson JW, Dickstein DA, Rockey PH. Physical therapy care for low back pain. Monitored program of first-contact nonphysician care. Phys Ther. 1988;68:199–207. [DOI] [PubMed] [Google Scholar]
- 7. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qaseem A, Wilt TJ, McLean RM, Forciea MA. Clinical guidelines committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514–530. [DOI] [PubMed] [Google Scholar]
- 9. Frogner BK, Harwood K, Andrilla CHA, Schwartz M, Pines JM. Physical therapy as the first point of care to treat low back pain: an instrumental variables approach to estimate impact on opioid prescription, health care utilization, and costs. Health Serv Res. 2018;53:4629–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ojha HA, Wyrsta NJ, Davenport TE, Egan WE, Gellhorn AC. Timing of physical therapy initiation for nonsurgical management of musculoskeletal disorders and effects on patient outcomes: a systematic review. J Orthop Sports Phys Ther. 2016;46:56–70. [DOI] [PubMed] [Google Scholar]
- 11. Carvalho E, Bettger JP, Bowlby L, et al. . Integration of musculoskeletal physical therapy care in the patient-centred medical home (IMPaC): protocol for a single-site randomised clinical trial. BMJ Open. 2018;8:e022953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim HS, Strickland KJ, Mullen KA, Lebec MT. Physical therapy in the emergency department: a new opportunity for collaborative care. Am J Emerg Med. 2018;36:1492–1496. [DOI] [PubMed] [Google Scholar]
- 13. Fleming-McDonnell D, Czuppon S, Deusinger SS, Deusinger RH. Physical therapy in the emergency department: development of a novel practice venue. Phys Ther. 2010;90:420–426. [DOI] [PubMed] [Google Scholar]
- 14. Mintken PE, Pascoe SC, Barsch AK, Cleland JA. Direct access to physical therapy services is safe in a university student health center setting. J Allied Health. 2015;44:164–168. [PubMed] [Google Scholar]
- 15. Moore JH, McMillian DJ, Rosenthal MD, Weishaar MD. Risk determination for patients with direct access to physical therapy in military health care facilities. J Orthop Sports Phys Ther. 2005;35:674–678. [DOI] [PubMed] [Google Scholar]
- 16. Boissonnault WG, Ross MD. Physical therapists referring patients to physicians: a review of case reports and series. J Orthop Sports Phys Ther. 2012;42:446–454. [DOI] [PubMed] [Google Scholar]
- 17. Denninger TR, Cook CE, Chapman CG, McHenry T, Thigpen CA. The influence of patient choice of first provider on costs and outcomes: analysis from a physical therapy patient registry. J Orthop Sports Phys Ther. October 2017;42:1–26. [DOI] [PubMed] [Google Scholar]
- 18. Lentz TA, Beneciuk JM, George SZ. Prediction of healthcare utilization following an episode of physical therapy for musculoskeletal pain. BMC Health Serv Res. 2018;18:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horn ME, George SZ, Fritz JM. Influence of initial provider on health care utilization in patients seeking care for neck pain. Mayo Clin Proc Innov Qual Outcomes. 2017;1:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carey TS, Freburger J. Physical therapy for low back pain: what is it, and when do we offer it to patients? Ann Fam Med. 2014;12:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karran EL, McAuley JH, Traeger AC, et al. . Can screening instruments accurately determine poor outcome risk in adults with recent onset low back pain? A systematic review and meta-analysis. BMC Med. 2017;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boissoneault J, Mundt J, Robinson M, George SZ. Predicting low back pain outcomes: suggestions for future directions. J Orthop Sports Phys Ther. 2017;47:588–592. [DOI] [PubMed] [Google Scholar]
- 23. Katzan IL, Thompson NR, George SZ, Passek S, Frost F, Stilphen M. The use of STarT back screening tool to predict functional disability outcomes in patients receiving physical therapy for low back pain. Spine J. 2019;19:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Physical Therapy Association. Vision Statement for the Physical Therapy Profession and Guiding Principles to Achieve the Vision. http://www.apta.org/Vision/.
- 25. Malterud K, Bjelland AK, Elvbakken KT. Evidence-based medicine - an appropriate tool for evidence-based health policy? A case study from Norway. Health Res Policy Syst. 2016;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schneeweiss S, Eichler H-G, Garcia-Altes A, et al. . Real world data in adaptive biomedical innovation: a framework for generating evidence fit for decision-making. Clin Pharmacol Ther. 2016;100:633–646. [DOI] [PubMed] [Google Scholar]
- 27. Parkhurst JO, Abeysinghe S. What constitutes “good” evidence for public health and social policy-making? From hierarchies to appropriateness. Soc Epistemol. 2016;30:665–679. [Google Scholar]
- 28. Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. [DOI] [PubMed] [Google Scholar]
- 29. McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA. 1994;272:859–866. [PubMed] [Google Scholar]
- 30. Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19:537–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pendergast J, Kliethermes SA, Freburger JK, Duffy PA. A comparison of health care use for physician-referred and self-referred episodes of outpatient physical therapy. Health Serv Res. 2012;47:633–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rundell SD, Gellhorn AC, Comstock BA, Heagerty PJ, Friedly JL, Jarvik JG. Clinical outcomes of early and later physical therapist services for older adults with back pain. Spine J. 2015;15:1744–1755. [DOI] [PubMed] [Google Scholar]
- 33. Fritz JM, Brennan GP, Hunter SJ. Physical therapy or advanced imaging as first management strategy following a new consultation for low back pain in primary care: associations with future health care utilization and charges. Health Serv Res. 2015;50:1927–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hernán MA. The C-word: scientific euphemisms do not improve causal inference from observational data. Am J Public Health. 2018;108:616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Greenland S. For and against methodologies: some perspectives on recent causal and statistical inference debates. Eur J Epidemiol. 2017;32:3–20. [DOI] [PubMed] [Google Scholar]
- 36. Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiol Camb Mass. 2009;20:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33:2297–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson ML, Crown W, Martin BC, Dormuth CR, Siebert U. Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR good research practices for retrospective database analysis task force report--part III. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2009;12:1062–1073. [DOI] [PubMed] [Google Scholar]
- 40. Hernán MA, Alonso A, Logan R, et al. . Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiol Camb Mass. 2008;19:766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hernán MA, Robins JM. Instruments for causal inference: an epidemiologist’s dream? Epidemiology. 2006;17:360–372. [DOI] [PubMed] [Google Scholar]
- 42. Rhon DI, Miller RB, Fritz JM. Effectiveness and downstream healthcare utilization for patients that received early physical therapy versus usual care for low back pain: a randomized clinical trial. Spine. 2018;43:1313–1321. [DOI] [PubMed] [Google Scholar]
- 43. Fritz JM, Magel JS, McFadden M, et al. . Early physical therapy vs usual care in patients with recent-onset low back pain: a randomized clinical trial. JAMA. 2015;314:1459–1467. [DOI] [PubMed] [Google Scholar]
- 44. Childs JD, Fritz JM, Wu SS, et al. . Implications of early and guideline adherent physical therapy for low back pain on utilization and costs. BMC Health Serv Res. 2015;15:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fritz JM, Childs JD, Wainner RS, Flynn TW. Primary care referral of patients with low back pain to physical therapy: impact on future health care utilization and costs. Spine. 2012;37:2114–2121. [DOI] [PubMed] [Google Scholar]
- 46. Gellhorn AC, Chan L, Martin B, Friedly J. Management patterns in acute low back pain: the role of physical therapy. Spine. 2012;37:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Horn ME, Brennan GP, George SZ, Harman JS. Bishop MD. A value proposition for early physical therapist management of neck pain: a retrospective cohort analysis. BMC Health Serv Res. 2016;16:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Horn ME, Fritz JM. Timing of physical therapy consultation on 1-year healthcare utilization and costs in patients seeking care for neck pain: a retrospective cohort. BMC Health Serv Res. 2018;18:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karvelas DA, Rundell SD, Friedly JL, et al. . Subsequent health-care utilization associated with early physical therapy for new episodes of low back pain in older adults. Spine J. 2017;17:380–389. [DOI] [PubMed] [Google Scholar]
- 50. Mitchell JM, de Lissovoy G. A comparison of resource use and cost in direct access versus physician referral episodes of physical therapy. Phys Ther. 1997;77:10–18. [DOI] [PubMed] [Google Scholar]
- 51. Sun E, Moshfegh J, Rishel CA, Cook CE, Goode AP, George SZ. Association of Early Physical Therapy with long-term opioid use among opioid-naive patients with musculoskeletal pain. JAMA Netw Open. 2018;1:e185909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Woods CS, Kishino ND, Haider TT, Kay PK. Effects of subacute versus chronic status of low back pain patients’ response to a functional restoration program. J Occup Rehabil. 2000;10:229–233. [Google Scholar]
- 53. Zigenfus GC, Yin J, Giang GM, Fogarty WT. Effectiveness of early physical therapy in the treatment of acute low back musculoskeletal disorders. J Occup Environ Med. 2000;42:35–39. [DOI] [PubMed] [Google Scholar]
- 54. Concannon TW, Guise J, Dolor RJ, et al. . A national strategy to develop pragmatic clinical trials infrastructure. Clin Transl Sci. 2014;7:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weinfurt KP, Hernandez AF, Coronado GD, et al. . Pragmatic clinical trials embedded in healthcare systems: generalizable lessons from the NIH Collaboratory. BMC Med Res Methodol. 2017;17:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turner EL, Li F, Gallis JA, Prague M, Murray DM. Review of recent methodological developments in group-randomized trials: part 1-design. Am J Public Health. 2017;107:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cook AJ, Delong E, Murray DM, Vollmer WM, Heagerty PJ. Statistical lessons learned for designing cluster randomized pragmatic clinical trials from the NIH Health Care Systems Collaboratory Biostatistics and Design Core. Clin Trials. 2016;13:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bishop A, Ogollah RO, Jowett S, et al. . STEMS pilot trial: a pilot cluster randomised controlled trial to investigate the addition of patient direct access to physiotherapy to usual GP-led primary care for adults with musculoskeletal pain. BMJ Open. 2017;7:e012987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saper RB, Lemaster C, Delitto A, et al. . Yoga, physical therapy, or education for chronic low back pain: a randomized noninferiority trial. Ann Intern Med. 2017;167:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van de Graaf VA, Noorduyn JCA, Willigenburg NW, et al. . Effect of early surgery vs physical therapy on knee function among patients with nonobstructive meniscal tears: the escape randomized clinical trial. JAMA. 2018;320:1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hahn S. Understanding noninferiority trials. Korean J Pediatr. 2012;55:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mauri L, D’Agostino RB. Challenges in the design and interpretation of noninferiority trials. N Engl J Med. 2017;377:1357–1367. [DOI] [PubMed] [Google Scholar]
- 63. Hampson G, Towse A, Dreitlein WB, Henshall C, Pearson SD. Real-world evidence for coverage decisions: opportunities and challenges. J Comp Eff Res. 2018;7:1133–1143. [DOI] [PubMed] [Google Scholar]
- 64. Sterne JA, Hernán MA, Reeves BC, et al. . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;i4919:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dusetzina SB, Brookhart MA, Maciejewski ML. Control outcomes and exposures for improving internal validity of nonrandomized studies. Health Serv Res. 2015;50:1432–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]


