Abstract
The aim of the present study was to assess antimicrobial effects of naringenin (NRG), luteolin (LUT), myricetin (MCT), and protocatechuic acid (PCA) identified in a Hibiscus rosa sinensis flower against two reference strains and five clinical isolates of Helicobacter pylori. NRG displayed the most growth inhibitory and bactericidal activities to seven bacterial strains including six strains resistant to one or several antibiotics, azithromycin (MIC, 16–32 mg/L), erythromycin (MIC, 32 mg/L), levofloxacin (MIC, 32 mg/L), and/or metronidazole (24–64 mg/L), followed by LUT and MCT, while PCA showed weak activities toward the strains. These constituents had similar antibacterial activities toward the seven tested strains suggesting that these constituents and the antibiotics do not have a common mechanism of anti-H. pylori activity. NRG, LUT, and MCT resulted in a high percentage of coccoid forms of H. pylori. NRG exhibited the highest anti-biofilm formation activity. MCT produced the strongest inhibition of urease activity followed by LUT and PCA, whereas the activity of NRG was similar to standard inhibitor thiourea. The four constituents had no significant toxicity to human cell lines. A global attempt to decrease utilization of antibiotics justifies the need for further research on H. rosa sinensis derived materials containing NRG, LUT, MCT, and PCA as potential products or lead compounds for the prevention or treatment of diseases caused by H. pylori infection.
Introduction
Helicobacter pylori infection is one of the major health concerns because of its high infection rate in humans. The bacterial infection is strongly associated with atrophy and intestinal metaplasia, active gastritis, peptic ulcer, and gastic cancer.1−3 Since H. pylori has been known as a human carcinogenic factor, several antibiotic combination regimens in addition to a proton pump inhibitor and/or bismuth have been often prescribed to achieve high eradication rates. However, the complex regimens often come with a number of adverse effects, such as nausea, vomiting, abdominal pain, headache, dizziness, bad taste, skin rash, fatigue, and diarrhea,4 as well as a cause of disturbance in gut microbiota balance, e.g., increase in the incidence of harmful bacteria such as Clostridium difficile,5,6 and the cost of combination therapy needs to be considered. In addition, the recurrence of H. pylori infection after successful eradication and increase of the infection rate of the multiple-drug resistant strains have often made recommended therapies less effective.5,7,8 The high prevalence of resistant H. pylori strains might be due to excessive and unregulated use of these antibiotics.7,8 These issues highlight a critical need for the development of new anti-H. pylori agents with a novel mechanism of action to establish an effective antibiotic-resistance management strategy based on all available information on the nature and extent of drug resistance in H. pylori.
Phytochemicals, especially those from consumable plants, have been suggested as potential alternatives for anti-H. pylori agents. This is an interesting and promising approach because plants contain a source of bioactive chemicals that have been perceived as relatively safe for humans by the public and often act at multiple and novel target sites, thereby decreasing the potential for resistance development.9 Many attempts have been undertaken to develop plant preparations and their constituents as potential sources of commercial antibacterial products for prevention or treatment of H. pylori infection. In particular, Hibiscus rosa sinensis L. (Malvaceae) is an evergreen shrub or small tree native to tropics and subtropics with varying colors of flowers, but the red flower variety is widely planted as a fence or hedge plant and used for medicine.10 All parts of the plant have been traditionally used in folk medicine. The phytochemical constituents, pharmacological effects, and medicinal uses of H. rosa sinensis have been well described.11,12 However, no information has been obtained to consider the potential use of H. rosa sinensis for managing and controlling drug-resistant strains of H. pylori.
The aim of the present study was to assess growth-inhibiting and bactericidal activities of constituents from H. rosa sinensis flower toward two reference strains and five antibiotic resistant isolates of H. pylori. H. pylori urease inhibition activity of the constituents was compared to that of thiourea, a potent H. pylori urease inhibitor. The effects of the constituents on biofilm formation and morphological transformation of the bacterial strains were also examined.
Results
Antibiotic Resistance
The antibacterial activities of five common antibiotics toward all H. pylori strains are recorded in Table 1. All five clinical strains showed resistance from two to four tested antibiotics, OX.64 resistant to azithromycin, erythromycin, levofloxacin, and metronidazole (MICs, 16–32 mg/L), OX.63 resistant to azithromycin, erythromycin, and metronidazole (MICs, 32–64 mg/L), OX.67 and OX.83 resistant to azithromycin and erythromycin (MICs, 64 mg/L), and OX.22 resistant to erythromycin and metronidazole (MICs, 32 mg/L). No strains showed resistance to amoxicillin. The two reference strains were susceptible to all five tested antibiotics, except for ATCC 43504 resistant to metronidazole.
Table 1. In Vitro Minimal Inhibitory Concentrations (MICs) and Bactericidal Concentrations (MBCs) of Five Antibiotics toward Test Strains of H. pylori by the Broth Dilution Bioassay.
| MICa (MBC) mg/L |
|||||||
|---|---|---|---|---|---|---|---|
| compound | ATCC 43504 | ATCC 51932 | OX.22 | OX. 63 | OX.64 | OX.67 | OX.83 |
| amoxicillin | 0.031 (0.063) | 0.063 (0.125) | 0.063 (0.25) | 0.063 (0.25) | 0.031 (0.125) | 0.031 (0.125) | 0.063 (0.25) |
| azithromycin | 0.5 (1.0) | 0.5 (1.0) | 0.5 (1.0) | 32 (128) | 16 (128) | 32 (128) | 32 (128) |
| erythromycin | 0.063 (0.125) | 0.063 (0.125) | 32 (128) | 32 (128) | 32 (128) | 32 (128) | 32 (128) |
| levofloxacin | 0.125 (0.25) | 0.125 (0.25) | 0.063 (0.5) | 0.063 (0.5) | 32 (128) | 0.063 (0.25) | 0.75 (1.25) |
| metronidazole | 32 (192) | 2 (4) | 32 (256) | 64 (256) | 32 (256) | 2 (4) | 1 (2) |
Bioassay-Guided Fractionation and Identification
H. rosa sinensis flower ethanol extract and its solvent soluble fractions were tested against H. pylori ATCC 43504 by paper-disc diffusion. The results indicated that the ethyl acetate-partition fraction (EtOAC fr.) showed the most growth inhibitory activity followed by the hexane-partition fraction (Hexane fr.). At concentrations of 10 and 5 mg/disc of EtOAC fr., the inhibition zone diameters were 32.0 and 20.3 mm, respectively. The EtOAC fr. was used to identify peak active fractions for the next steps in the isolation and purification.
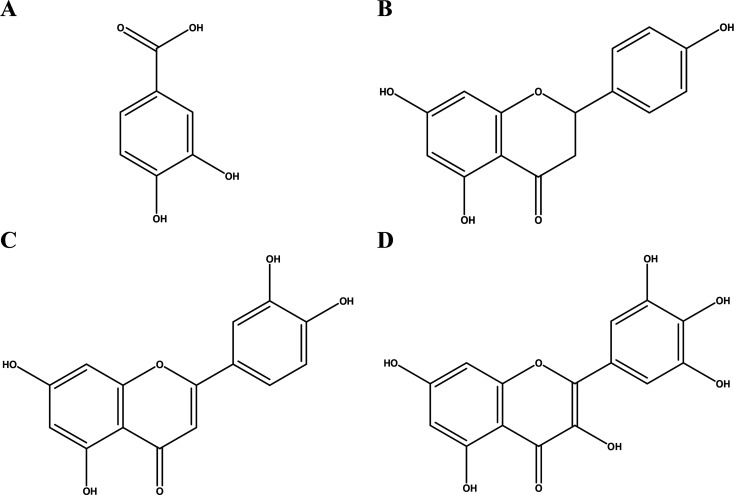
The four active principles were protocatechuic acid (PCA), naringenin (NRG), luteolin (LUT), and myricetin (MCT). Principle 1, PCA (Figure 1A) was obtained as a light brown powder and identified on the following evidence: UV (EtOH): λmax (nm) 265, melting point: 220–222 °C, ESI-HRMS, m/z 153.0195 [M–H]−, corresponding to molecular formula C7H6O4 with a degree of unsaturation of 5, and the data of 1H NMR, 13C NMR, and HBMC are shown in Table S1 and Figure S1A. Principle 2, NRG (Figure 1B) was gained as a white powder, UV (EtOH): λmax (nm) 288, melting point: 250–252 °C, ESI-HRMS, m/z 271.0627 [M–H]−, corresponding to molecular formula C15H12O5 with a degree of unsaturation of 10, and the data of 1H NMR, 13C NMR, and HBMC are displayed in Table S1 and Figure S1B. Principle 3, LUT (Figure 1C) was obtained as yellow crystals, UV (EtOH): λmax (nm) 346, melting point: 329–331 °C, ESI-HRMS, m/z 287.0530 [M–H]+, corresponding to molecular formula C15H10O6 with a degree of unsaturation of 11, and the data of 1H NMR, 13C NMR, and HBMC are presented in Table S1 and Figure S1C. Principle 4, MCT (Figure 1D) was obtained as a orange yellow powder, UV (EtOH): λmax (nm) 376, melting point: 356–358 °C, ESI-HRMS, m/z 319.0422 [M–H]+, corresponding to molecular formula C15H10O8 with a degree of unsaturation of 11, and the data of 1H NMR, 13C NMR, and HBMC are shown in Table S1 and Figure S1D. The interpretations of proton and carbon signals of compounds 1, 2, 3, and 4 were largely consistent with those of Tor et al.,16 Kyriakou et al.,17 Chaturvedula and Prakash,18 and He et al.,19 respectively.
Figure 1.
Structure of four principles. Protocatechuic acid (PCA) (A), naringenin (NRG) (B), luteolin (LUT) (C), and myricetin (MCT) (D).
Growth Inhibitory Activities of Test Compounds
The growth inhibitory activities of the four constituents toward antibiotic-susceptible strains ATCC 51932 and six antibiotic-resistant strains (ATCC 43504, OX.22, OX.63, OX64, OX.67, and OX.83) of H. pylori are recorded in Table 2. Based on MIC values, NRG has the most growth inhibitory activities (100 mg/L) followed by LUT and MCT (125 and 150 mg/L, respectively). The growth inhibitory activity of PCA was the least of any of the constituents (MICs, 250 mg/L). Interestingly, all the constituents were of similar growth-inhibiting activity toward both antibiotic-susceptible and -resistant strains, suggesting a lack of resistance to the compounds in the antibiotic resistant strains.
Table 2. In Vitro Minimal Inhibitory Concentrations (MICs) and Bactericidal Concentrations (MBCs) of H. rosa sinensis Flower Constituents toward Test Strains of H. pylori by the Broth Dilution Bioassay.
| MIC
(MBC) mg/L |
|||||||
|---|---|---|---|---|---|---|---|
| compound | ATCC 43504 | ATCC 51932 | OX.22 | OX. 63 | OX.64 | OX.67 | OX.83 |
| NRG | 100 (1000) | 100 (1000) | 100 (1000) | 100 (1000) | 100 (1000) | 100 (1000) | 100 (1000) |
| LUT | 125 (1250) | 125 (1250) | 125 (1250) | 125 (1250) | 125 (1250) | 125 (1250) | 125 (1250) |
| MCT | 150 (1250) | 150 (1250) | 150 (1250) | 150 (1250) | 150 (1250) | 150 (1250) | 150 (1250) |
| PCA | 250 (1500) | 250 (1500) | 250 (1500) | 250 (1500) | 250 (1500) | 250 (1500) | 250 (1500) |
Bactericidal Activities of Test Compounds
The MBCs of the four constituents toward the tested strains were likewise compared (Table 2). NRG showed the highest bactericidal activity (MBC, 1000 mg/L), while PCA showed the lowest activity (MBC, 1500 mg/L). LUT and MCT had similar bactericidal activity (MBCs, 1250 mg/L). All the compounds possessed similar toxicity to both antibiotic-susceptible and -resistant strains, indicating a lack of resistance in the resistant strains.
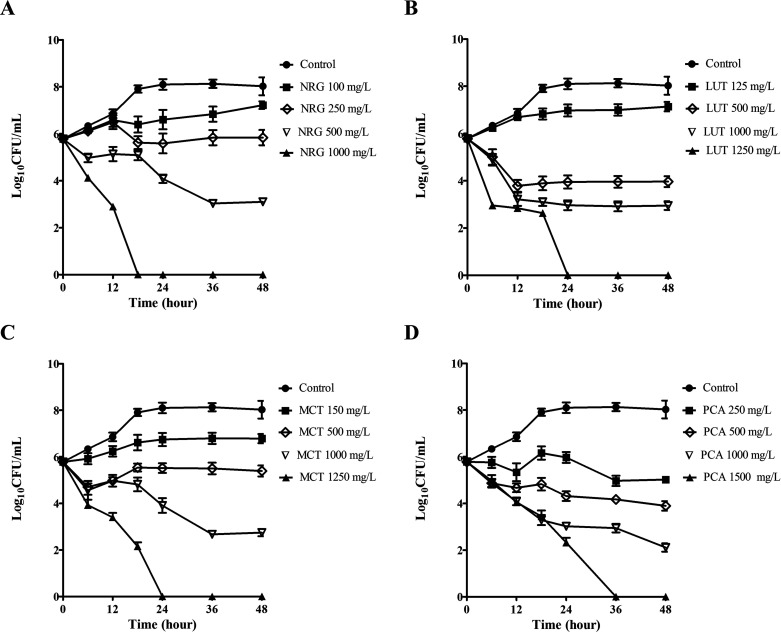
The time course of bactericidal activities of NRG, LUT, MCT, and PCA at various concentrations toward H. pylori ATCC 43504 was likewise investigated (Figure 2). The results revealed that the viable count of the bacteria was reduced in a dose-dependant manner. Treatment of H. pylori with NRG at five times the MIC reduced the viability of H. pylori by ∼4 log10 and ∼2.5 log10 over 36 and 24 h, respectively, while treatment with NRG at the MIC resulted in ∼1 log10 reduction in 48 h (Figure 2A). Treatment with LUT at four times the MIC resulted in ∼3 log10 reduction in 12 h (Figure 2B). Treatments with MCT at the MIC and eight times the MIC caused reductions of ∼2.7 and ∼4.2 log10 in 24 and 36 h, respectively (Figure 2C). Treatments with PCA at the MIC and four times the MIC resulted in reductions of ∼2 log10 and ∼6 log10 in 36 and 48 h, respectively (Figure 2D).
Figure 2.
Time-course bactericidal activity of H. rosa sinensis flower constituents at various concentrations ranging from MICs to MBCs toward H. pylori ATCC 43504. NRG (A), LUT (B), MCT (C), and PCA (D). The mean values (±SD) for the log number of CFU/mL were plotted.
Anti-Biofilm Formation Activity
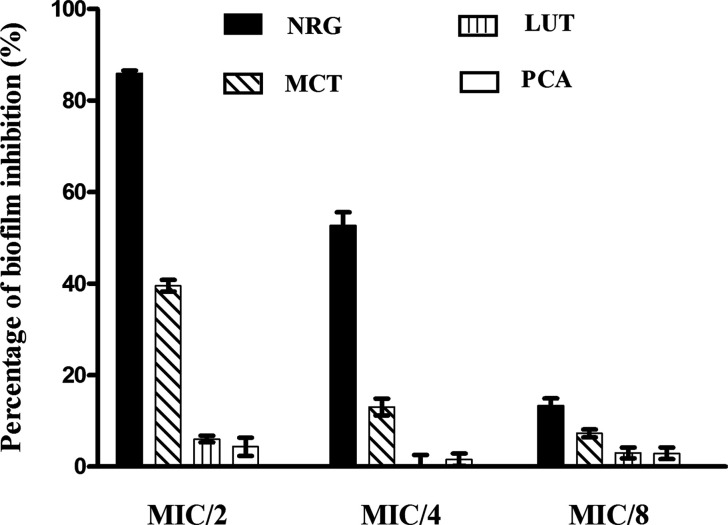
The inhibitory effect of the four compounds on biofilm formation by H. pylori ATCC 43504 is shown in Figure 3. NRG exhibited the pronounced antibiofilm activity, reducing H. pylori biofilm formation by 85.9 and 52.7% at concentrations of 1/2 × MIC (50 mg/L) and 1/4 × MIC (25 mg/L), respectively. MCT showed to reduce biofilm formation by 39.5% at a concentration of 1/2 × MIC (75 mg/L), while LUT and PCA did not show antibiofilm activity at test concentrations.
Figure 3.
Effect of H. rosa sinensis flower constituents at concentrations of sub-MICs on H. pylori ATCC 43504 biofilm formation 48 h of post-treatment. NRG (shaded square), MIC 100 mg/L; MCT (square with diagonal lines), MIC 150 mg/L; LUT (square with vertical lines), MIC 125 mg/L; PCA (unshaded square), MIC 250 mg/L.
Effect on Morphology of H. pylori
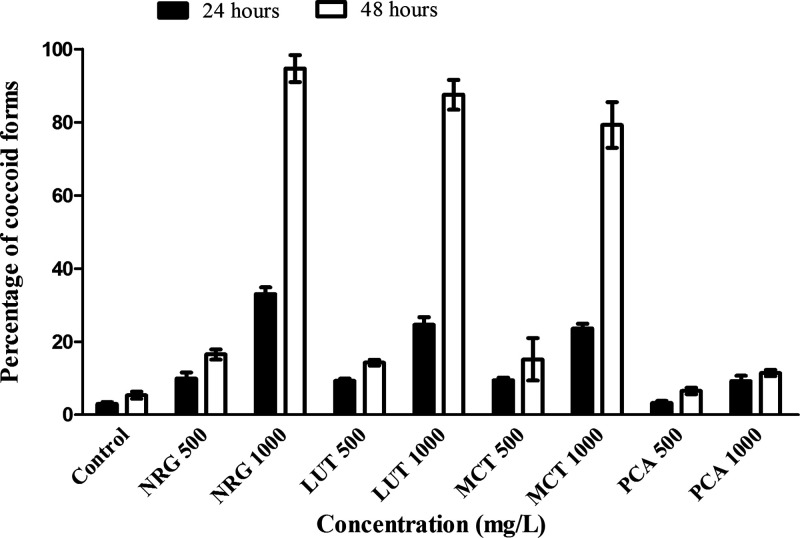
The proportion of the coccoid versus spiral form of H. pylori ATCC 43504 was determined at 500 and 1000 mg/L of the constituents for 24 and 48 h of post-treatment (Figure 4). The effect of concentration (F = 1186.99; df = 8, 45; P < 0.0001) and exposure time (F = 1765.93; df = 1, 45; P < 0.0001) on conversion of H. pylori to the coccoid form was significant. The concentration by exposure interaction was also significant (F = 295.94; df = 8, 45; P < 0.0001). NRG caused considerable conversion to the coccoid forms (95 versus 16.5% at 1000 and 500 mg/L), while PCA caused the lowest conversion (12% at 1000 mg/L), and the proportion of conversion caused by PCA at 500 mg/L was not significantly different from that in the control group (P > 0.05). LUT (87.5 versus 14.7% at 1000 and 500 mg/L) caused higher conversion to the coccoid forms than MCT (79 versus 15% at 1000 and 500 mg/L).
Figure 4.
Effect of H. rosa sinensis flower constituents at concentrations of 500 and 1000 mg/L on H. pylori ATCC 43504 morphology 24 (shaded square) and 48 h (unshaded square) of post-treatment.
Cytotoxicity
In order to determine the selectivity of the anti-H. pylori, the cytotoxic effect of the test materials on four human cell lines HeLa, MRF7, Jurkat, and fibroblast was examined (Table 3). NRG, LUT, MCT, and PCA showed no significant cytotoxicity toward these cell lines with CC50 values of 46.59–133.3, 5.12–49.83, 7.61–76.11, and >200 mg/L, respectively, compared with the positive control camptothecin (CC50 of 0.005–1.57 mg/L, P < 0.001).
Table 3. Cytotoxic Effect of Test Compounds on Four Human Cell Lines by the Sulforhodamine Assay.
| CC50 mg/L (95% CLa) |
||||
|---|---|---|---|---|
| compound | MCF7 | Jurkat | HeLa | Fibroblast |
| NRG | 93.74 (86.22–101.28) | 46.59 (44.24–48.90) | 65.18 (60.52–69.83) | 133.30 (124.94–141.63) |
| LUT | 8.64 (8.22–9.10) | 5.12 (4.35–5.90) | 8.36 (7.70–9.02) | 49.83 (45.54–54.13) |
| MCT | 31.69 (30.43–32.97) | 7.61 (7.24–8.01) | 36.81 (32.77–40.85) | 76.11 (72.65–79.57) |
| PCA | >200 | >200 | >200 | >200 |
| camptothecin | 0.005 (0.004–0.006) | 0.005 (0.004–0.006) | 0.890 (0.802–0.978) | 1.570 (0.731–2.410) |
CL denotes the confidence limit.
Urease Inhibitory Activity
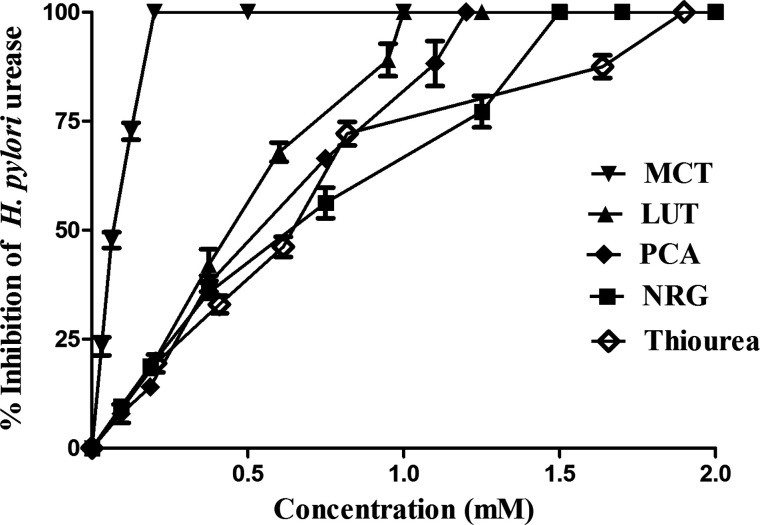
The three constituents MCT, LUT, and PCA exhibited stronger urease inhibitory activity than the positive compound thiourea (Table 4). MCT caused a steep dose–response curve of the percentage of urease inhibition (slope value of 2.26) and presented the strongest activity (IC50 at 0.063 mM) (Table 4) and complete inhibition at 0.402 mM (Figure 5). LUT was more effective than PCA (values of IC50, 0.402 and 0.486 mM, respectively). No significant difference in the inhibition was found between NRG and thiourea (values of IC50, 0.575 and 0.573 mM, respectively).
Table 4. Effect of Test Compounds and Thiourea on H. pylori ATCC 43504 Urease Activity by the Salicylate-Hypochlorite Method.
| compound | IC50 mM (95% CLa) | slope ± SE |
|---|---|---|
| MCT | 0.063 (0.062–0.065) | 2.26 ± 0.070 |
| LUT | 0.402 (0.397–0.430) | 2.16 ± 0.080 |
| PCA | 0.486 (0.467–0.506) | 2.00 ± 0.075 |
| NRG | 0.575 (0.553–0.599) | 1.38 ± 0.045 |
| thiourea | 0.573 (0.546–0.602) | 1.79 ± 0.093 |
CL denotes the confidence limit.
Figure 5.
H. pylori urease inhibitory activity of H. rosa sinensis flower constituents.
Discussion
Widespread use of antibiotics has resulted in the spreading of antimicrobial resistance that in turn caused low rates of eradication.5,7 Preparations and biochemicals derived from plants are potential antibacterial agents against H. pylori since some of them have multiple functions and act at multiple target sites.9 Numerous products derived from plants have high effectiveness against antibiotic-resistant strains of H. pylori, and they are likely to be a benefit to resistance management strategies. Previously, constituents glabridin and glabrene isolated from Glycyrrhiza glabra, licochalcone A from G. inflata, licoricidin and licoisoflavone B from G. uralensis were investigated to have in vitro growth inhibitory activities against both reference strains and clarithromycin- and amoxicillin-resistant strains,20 and glycyrrhetinic acid from G. glabra was highly effective toward reference strains and 29 isolates of H. pylori including five metronidazole-resistant strains and two clarithromycin-resistant strains.21 Interestingly, although the three medicinal licorices have been used as a crude drug since the ancient times in oriental countries, these H. pylori strains have not developed resistance to the constituents of the plants.20 Constituents 1,2,3,4,6-penta-O-galloyl-d-glucopyranose, methyl gallate, and paeonol derived from Paeonia lactiflora were observed to have growth-inhibiting and bactericidal activities on three H. pylori reference strains and four isolates resistant to amoxicillin, clarithromycin, metronidazole, or tetracycline.22 In the present study, the bioactive principles were identified as the phenolic acid protocatechuic PCA (1), the flavanone naringenin NRG (2), the flavone luteolin LUT (3), and flavonol myricetin MCT (4). NRG, LUT, and MCT showed the pronounced growth-inhibiting and moderate bactericidal activities against all test H. pylori strains, although the concentrations of these constituents were lower than those of the test antibiotics. These four constituents displayed growth-inhibiting and bactericidal activities toward six strains resistant to azithromycin, erythromycin, levofloxacin, and metronidazole (strain OX.64), azithromycin, erythromycin, and metronidazole (strain OX. 63), azithromycin and erythromycin (strains OX. 67 and OX.83), erythromycin and metronidazole (strain OX.22), and metronidazole (strain ATCC 43504). These findings that NRG, LUT, MCT, and PCA were equal in antibacterial activity toward both antibiotic-susceptible and -resistant strains of H. pylori suggest that these constituents and the macrolides azithromycin, erythromycin, fluoroquinolone levofloxacin, or nitroimidazole metronidazole do not share a common mode of action. Moreover, the four constituents exhibited no significant cytotoxicity toward HeLa, MRF7, Jurkat, and fibroblast cell lines suggesting that their anti-H. pylori activities might not be due to general toxicity. Previously, it was investigated that NRG, LUT, and PCA possessed potent growth inhibitory activities against several H. pylori reference strains23 or clinical strains.24 PCA was also reported to have growth inhibitory activity against 15 susceptible, 11 clarithromycin-resistant, and 9 metronidazole-resistant strains of H. pylori.25 LUT, known as a potential anticancer, antioxidant, and anti-inflammatory flavonoid,26,27 was reported to exhibit an inhibitory effect on arylamine N-acetyltransferase activity in H. pylori strains isolated from gastroduodenal disease patients.28 The antibacterial activity of LUT on methicillin-resistant Staphylococcus aureus (MRSA) was proven to be due to increasing cytoplasmic membrane permeability, disrupting the cell wall structure and inducing cell lysis.29 NRG was found to have a synergistic antibacterial effect with antibiotics ampicillin, methicillin, tetracycline, and vancomycin on MRSA.30 MCT was reported to possess growth inhibitoty and bactericidal activities against multidrug-resistant Burkholderia cepacia and a growth inhibitoty effect toward vancomycin-resistant enterococci and Klebsiella pneumoniae.(31) The present finding showed promise of developing novel and effective antibacterial agents even toward antibiotic-resistant strains of H. pylori from materials derived from H. rosa sinensis.
Investigations on antibacterial activities and the mechanisms of action of naturally occurring agents may contribute to development of novel and selective H. pylori control alternatives with novel target sites and safe with low or no side effects.22 Although the mechanisms of action of biochemicals such as phenolic acids, flavonoids, terpenoids, and alkaloids toward H. pylori have still remained not fully understood, major modes of action of flavonoids and phenolics suggested involving multiple bacterial cellular targets, such as reducing membrane fluidity,32 inactivating microbial adhesions and cell envelope transport proteins and disrupting cell membranes,33 changing membrane permeability and disrupting proton motive force and membrane-associated functions, and/or intracellular acidification, resulting in disruption of H+,K+-ATPase required for ATP synthesis of microbes.34 In addition, inhibitory effects on biofilm forming properties of H. pylori may be caused by antibacterial agents. Biofilm formation of H. pylori is one of the major reasons causing the failure of antibiotic treatment and infection recurrence.35 In the present study, the inhibitory effect of the constituents derived from H. rosa sinensis on biofilm formation by H. pylori was evaluated. Highly effective antibiofilm activity was observed for NRG, and weak antibiofilm activity was also recorded for MCT. NRG was reported to significantly inhibit bacterial motility36 that has been shown to play an important role in biofilm formation and attachment of cells to a surface, involve in cell–cell communication, and spread.37−40 It susgests that the inhibitory effect of NRG on biofilm forming acitivity of H. pylori may be due to its inhibition of bacterial motility and modulation of bacterial cell–cell communication activity. MCT was found to strongly inhibit biofilm formation in S. aureus strains that overexpress efflux protein genes.41
Moreover, morphological transformation caused by antimicrobial agents has also been considered in studies of modes of anti-H. pylori action. The coccoid form has lower levels of metabolism and synthesis of protein and DNA than the spiral form.42 The coccoid form may be a disadvantage for colonization and survival of the bacterium on the gastric mucosa.43,44 Coccoid forms are able to maintain their metabolic activities, pathogenicity, and may revert to spiral forms once they live in suitable conditions.43−45 Previously, it was reported that polyphenol 1,2,3,4,6-penta-O-galloyl-d-glucopyranose, and phenolic constituents methyl gallate and paeonol derived from the P. lactiflora root,22 and sesquiterpene lactone dehydrocostus lactone from Magnolia sieboldii leaves showed bactericidal properties against H. pylori and also induced significant conversion to coccoid cells.46 In the present study, a proportional relationship between the percentage of coccoid cells induced by the flavonoid constituents NRG, LUT, and MCT and their bactericidal activity was also observed. This suggests that the coccoid cells induced by the constituents contribute to the amount of dead bacteria. MCT was found to inhibit DNA synthesis in Proteus vulgaris.47
Urease plays important roles in survival, colonization, and pathogenesis of H. pylori in the harsh acidic environment of the stomach.48 It contributes to the persistence of the bacterial infection.49 Urease, therefore, is considered to be one of the most effective and promising targets for developing antibacterial agents against the pathogen.50,51 Many plant secondary metabolites have been pointed out as remarkable H. pylori urease inhibitors.50 In addition, their multiple effects toward metabolism activities of H. pylori were also investigated. For example, epigallocatechin gallate (EGCG) from the green tea plant showed to inhibit growth and urease activities and motility of H. pylori;52 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose and methyl gallate from the P. lactiflora root exhibited growth-inhibiting, bactericidal and urease inhibitory activities and induced morphological transformation of the pathogen.22 In the present finding, among the four anti-H. pylori constituents derived from H. rosa sinensis, MCT exhibited the highest urease inhibitory activity followed by LUT and PCA, whereas NRG has the weakest activity and was similar to the control thiourea. Flavonol MCT and flavone LUT were found to have high effects on H. pylori urease.53 PCA was recorded to effectively inhibit growth of several susceptible and drug-resistant H. pylori and show a morderate effect on urease activity of these bacterial strains,25 and flavanone NRG was found to show little effect on the urease activity.24
In conclusion, H. rosa sinensis flower derived preparations containing NRG, LUT, MCT, and PCA could be useful as sources of potential products for the prevention or treatment of diseases caused by H. pylori in light of their effects on antibiotic-resistant strains of H. pylori. The action of the constituents against H. pylori may be an indication of at least one of the pharmacological actions of the H. rosa sinensis flower. For practical use of H. rosa sinensis flower derived materials as effective and novel anti-H. pylori agents to process, further studies are needed to determine their in vivo activities and for human safety.
Materials and Methods
Instrumental Analyses
1H and 13C NMR spectra were recorded in (CD3)2SO or CD3OD using a Bruker AVANCE III 500 MHz spectrometer, and chemical shifts are given in δ (ppm). HRMS was performed using a Bruker MicroTOF-QII. Melting point was recorded using a Stuart Melting point/SMB30. UV spectra were obtained in ethanol with a T92+ spectrophotometer (PG Instruments, UK). Merck precoated silica gel plates (Kieselgel 60 F254) were used for analytical thin-layer chromatography (TLC). Merck preparative layer chromatography plates (PLC silica gel 60F254, 2 mm thickness) and Scharlau silica gel 60 (0.06–0.2 mm) (Scharlau, Spain) were used for isolation and purification.
Materials
Pure organic compounds naringenin (NRG) (98%), protocatechuic acid (PCA) (≥98%), luteolin (LUT) (≥98%), and myricetin (MCT) (≥98%) were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX). Levofloxacin (≥98%) and azithromycin (≥95%) were provided by Sigma-Aldrich. Erythromycin (≥92%), metronidazole (≥99%), and amoxicillin (≥98%) were provided by Santa Cruz Biotechnology Inc. Brucella broth (BB), brain heart infusion (BHI) broth, and newborn bovine serum (NBS) were provided by Becton, Dickinson and Company (Sparks, MD) and Hyclone (Longan, UT), respectively. All other chemicals and reagents used in this study were of analytical grade quality and available commercially.
Bacterial Strains and Culture Conditions
The two standard strains (ATCC 43504 and ATCC 51932) and five clinical isolates OX.22, OX.63, OX.64, OX.67, and OX.83 of H. pylori were used in this study. The clinical isolates were obtained in 2017 from the Microbiology Lab at OUCRU (Oxford University Clinical Research Unit, Ho Chi Minh City, Vietnam) from patients with duodenal and stomach ulcers. All strains were stored in BHI broth and 25% glycerol in a liquid nitrogen container until use. The bacterial strains were grown on Brucella agar supplemented with 10% NBS at 37 °C for 3 days in a microaerophilic jar using Oxoid CampyGen gas packs (Thermo Scientific, UK).
Human Cell Lines and Cell Cultures
HeLa cells (ATCC CCL2, cervix carcinoma cell line), MCF7 cells (ATCC HTB-22, human breast cancer cell line), and Jurkat cells (ATCC TIB-152, blood cancer cell line) were purchased from the American Type Culture Collection (Manassas, Rockville), and fibroblast cells derived from human foreskins were provided by the Lab of Molecular Biology Department of Genetics, University of Science, VNU-HCM. These cell lines were cultured in Eagle’s minimal essential medium (EMEM) supplemeted with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 20 mM HEPES, 0.025 μg/mL amphotericin B, 100 IU/mL penicillin, and 100 μg/mL streptomycin, at 37 °C and 5% CO2. HeLa, MCF7, and Jurkat cells used were between passages 4 and 20, and fibroblast cells used were between passages 2 and 5.
Extraction and Isolation
An air-dried powder (9 kg) of H. rosa sinensis red flower petals was pulverized and extracted with absolute ethanol followed by liquid–liquid fractionation with hexane, ethyl acetate, and water (Figure S2). Isolation of active fractions and principles was guided by a bioassay against H. pylori ATCC 43504. In brief, 5 and 10 mg of the extract or each fraction/paper disc (1 mm thickness, 6 mm diameter, Whatman, GE Healthcare UK Limited) were tested by the paper-disc diffusion method as previously described.54 The ethyl acetate-soluble fraction was most active against the bacterium and repeatedly subjected to column chromatography, TLC, and PLC to obtain four active principles. The isolation and purification of the four principles are briefly described in Figure S2.
Microbiological Assay
Minimal inhibitory concentrations (MICs), minimal bactericidal concentrations (MBCs), and time killing of the test materials toward all H. pylori strains were determined by the broth microdilution assay as reported previously.22,55
For MICs, serial dilutions of concentrations (25 to 2000 mg/L) of the test compounds were performed in 0.05 mL of 10% NBS-supplemented Brucella broth in sterile 96-well plates. The final concentration of DMSO in all assays was 2.5% or less. Subsequently, 0.05 mL of bacterial suspension (5 × 106 CFU/mL) of each strain from cultures on Brucella agar was added to each well. The plates were then incubated at 37 °C in microaerophilic jars and shaken at 50 rpm for 48 h. MICs are defined as the lowest concentrations that visibly inhibited bacterial growth using resazurin as an indicator. Different antibiotics, amoxicillin, metronidazole, erythromycin, levofloxacin, and azithromycin served as positive controls. Negative controls contained the DMSO solution.
MBC values of the test materials were identified following the MIC assays and carried out in 24-well plates with the brucella agar medium.55 An amount of 0.01 mL of each well that retained the blue color of the indicator was taken and dropped onto the agar medium. The agar plates were incubated in microaerophilic jars at 37 °C for 72 h. The lowest concentrations, which exhibited no growth on the subculture, were determined as MBC values.
The time-killing assay was performed in sterile 96-well plates with the test compounds at concentrations ranging from MICs to MBCs. Bacterial viability was measured after 0, 6, 12, 18, 24, 36, and 48 h by a plate colony count technique as described previously.22 The control without the test compounds was also performed.
Biofilm Formation Inhibitory Assay
The biofilm inhibition assay was carried out in 96-well plates by the crystal violet assay as reported previously.56,57 In brief, the bioassay was prepared like the bioassay of MIC identification with the H. pylori ATCC 43504 suspension (108 CFU/mL) and the test compounds at concentrations of sub-MICs (1/2 × MIC, 1/4 × MIC, and 1/8 × MIC). Blank and background wells, which contained DMSO and compounds, respectively, without the bacterial suspension, were similarly prepared. The biofilm formation was measured after 2 days by staining with crystal violet solution 0.1% at a wavelength of 595 nm using a Microlisa Plus microplate reader (Micro Lab Instruments, India).
Effect on Morphology of H. pylori
H. pylori ATCC 43504 was cultured in Brucella broth without or with the test materials at 500 and 1000 mg/L in microaerophilic conditions for 24 and 48 h. The bacterial suspension (15 μL) was evenly spread and fixed on slides and then stained with 0.3% methylene blue solution. The proportion of coccoid versus spiral-shaped bacteria was determined using a Euromex-BioBlue Lab microscope equipped with a Euromex camera (Netherlands). Counts of 200 bacteria from each slide were done as reported previously.42
Cytotoxicity Assay
The cytotoxicity of the four test materials to HeLa, MCF7, Jurkat, and fibroblast cells was evaluated by a sulforhodamine (SRB) assay described previously.58 In brief, cells were seeded onto 96-well plates at a densiy of 5 × 103 cells/well (HeLa), 104 cells/well (MCF7 and fibroblast), and 5 × 104 cells/well (Jurkat) for 24 h. The culture medium was then removed from the wells and replaced with a medium containing various concentrations of the test materials (0–200 mg/L) in DMSO. After incubation for 48 h, the cells were washed once with phosphate-buffered saline (PBS), fixed with cold trichloroacetic acid solution (50% w/v) for 20 min, and stained with 0.2% SRB for 20 min. After gently washing five times with acetic acid solution (1%), the protein-bound dye was solubilized in 10 mM Tris base solution. The absorbance was read at 492 nm by the microplate reader. Camptothecin was used as a positive control. Each treatment was performed in triplicate and repeated three times in independent experiments.
Inhibition of Urease In Vitro
Inhibition of H. pylori ATCC 43504 urease was determined by measuring ammonia production in 96-well plates by the salicylate-hypochlorite method with minor modification.59 In brief, the assay mixtures containing 0.25 μg of urease crude (0.04 urease units) in 0.1 mL of the EDTA-sodium phosphate buffer (pH 7.3) and the constituents at different concentrations were preincubated at 37 °C for 90 min with rotation at 50 rpm. An amount of 0.05 mL of urea solution (10 mM) in the sodium phosphate buffer was added to each well, and the plates were incubated in 30 min. Blank and background wells were similarly prepared but with inactive urease by heating at 100 °C in 30 min. Then stop solutions including 0.035 mL of solution A (146% Na salicylate + 0.1% sodium nitroprusside) and 0.065 mL of solution B (1.78% NaOH +11.57% Na citrate + 0.54% NaOCl) were added in sequence. The ammonia released by the urease activity was quantified by measuring the absorbance on the microplate reader at 625 nm. The protein content was determined using a Bradford protein assay kit. BSA was used as a protein standard. Thiourea served as a standard reference inhibitor. One unit of urease is defined as the amount of enzyme that releases 1 μM NH3 per minute, at 37 °C, pH = 7.3. Each treatment was performed in triplicate and repeated three times in independent experiments.
Statistical Analysis
MIC and MBC values of each test sample were results from at least three independent experiments performed in triplicate (n ≥ 9). Antimicrobial activity of the test constituents with MIC values of <0.1, 0.1–0.62, 0.62–1.25, 1.25–2.5, and >2.5 mg/mL was classified as extremely high, high, moderate, low, and no growth inhibition, respectively.60 Percentages of cytotoxicity and urease activity inhibition were calculated as described previously.58,61 Cytotoxicity to human cell lines and urease inhibition activity were presented as CC50 (50% cytotoxic concentration) and IC50 (molar concentration producing 50% inhibition of enzyme activity), respectively, and calculated using the GraphPad Prism 5 software program. The CC50 and IC50 values of the treatments would be declared significantly different if their 95% confidence limits did not overlap. The Bonferroni method was used to test for multiple comparisons among the treatments. The percentage of biofilm inhibition was calculated as follows (%): 100 – 100 × (ODtreatment – ODbackground)/(ODcontrol – ODblank).
Acknowledgments
This work is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-YS.06–2015.17.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01640.
Data of 1H NMR, 13C NMR, and HBMC of four principles derived from the H. rosa sinensis flower; 1H-13C HMBC correlations of PCA, NRG, LUT, and MCT; scheme of isolation of the four principles (PDF)
Author Contributions
H.T.T. and M.N.L.T. conceived and designed the experiments. All authors performed the experiments. H.T.T. and M.N.L.T. analyzed the data and wrote the manuscript. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Nguyen T. L.; Uchida T.; Tsukamoto Y.; Trinh D. T.; Ta L.; Mai B. H.; Le S. H.; Thai K. D.; Ho D. D.; Hoang H. H.; Matsuhisa T. Helicobacter pylori infection and gastroduodenal diseases in Vietnam: a cross-sectional, hospital-based study. BMC Gastroenterol. 2010, 10, 114. 10.1186/1471-230X-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. H.; Xiao S. D.; Megraud F.; Leon-Barua R.; Bazzoli F.; Van der Merwe S.; Vaz Coelho L. G.; Fock M.; Fedail S.; Cohen H.; Malfertheiner P. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J. Gastrointestin. Liver Dis. 2011, 20, 299–304. [PubMed] [Google Scholar]
- Binh T. T.; Tuan V. P.; Dung H. D. Q.; Tung P. H.; Tri T. D.; Thuan N. P. M.; Van Khien V.; Hoan P. Q.; Suzuki R.; Uchida T.; Trang T. T. H.; Yamaoka Y. Advanced non-cardia gastric cancer and Helicobacter pylori infection in Vietnam. Gut Pathog. 2017, 9, 46. 10.1186/s13099-017-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B.; Sharopov F.; Martorell M.; Rajkovic J.; Ademiluyi A.; Sharifi-Rad M.; Fokou P.; Martins N.; Iriti M.; Sharifi-Rad J. Phytochemicals in Helicobacter pylori Infections: What Are We Doing Now?. Int. J. Mol. Sci. 2018, 19, 2361. 10.3390/ijms19082361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollman N. Helicobacter pylori infection in the era of antibiotic resistance. Gastroenterol. Hepatol. 2016, 12, 122–125. [PMC free article] [PubMed] [Google Scholar]
- Siddiqui M. A.; Sadiq O.; Iqbal U.; Siddique O.; Jafri S. M.; Blumenkehl M. Incidence of Clostridium difficile in Patient Treated with Helicobacter pylori Eradication Therapy. Gastroenterology 2017, 152, S951. 10.1016/S0016-5085(17)33235-3. [DOI] [Google Scholar]
- Binh T. T.; Shiota S.; Nguyen L. T.; Ho D. D.; Hoang H. H.; Ta L.; Trinh D. T.; Fujioka T.; Yamaoka Y. The incidence of primary antibiotic resistance of Helicobacter pylori in Vietnam. J. Clin. Gastroenterol. 2013, 47, 233. 10.1097/MCG.0b013e3182676e2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek C.; Pham S. T.; Tran K. T.; Pham B. T.; Huynh L. V.; Luu N. B.; Le T. K.; Quek K.; Van Pham H. Antimicrobial susceptibility and clarithromycin resistance patterns of Helicobacter pylori clinical isolates in Vietnam. F1000Res. 2016, 5, 671. 10.12688/f1000research.8239.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I.; Ribnicky D. M.; Komarnytsky S.; Ilic N.; Poulev A.; Borisjuk N.; Brinker A.; Moreno D. A.; Ripoll C.; Yakoby N.; O’Neal J. M.; Cornwell T.; Pastor I.; Fridlender B. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. 10.1016/S0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- Jadhav V. M.; Thorat R. M.; Kadam V. J.; Sathe N. S. Traditional medicinal uses of Hibiscus rosa-sinensis. J. Pharm. Res. 2009, 2, 1220–1222. [Google Scholar]
- Vasudeva N.; Sharma S. K. Biologically Active Compounds from the Genus Hibiscus. Pharm. Biol. 2008, 46, 145–153. 10.1080/13880200701575320. [DOI] [Google Scholar]
- Missoum A. An update review on Hibiscus rosa sinensis phytochemistry and medicinal uses. J. Ayurdevic Herb. Med. 2018, 4, 135–146. [Google Scholar]
- García-Arata M. I.; Baquero F.; de Rafael L.; de Argila C. M.; Gisbert J. P.; Bermejo F.; Boixeda D.; Cantón R. Mutations in 23S rRNA in Helicobacter pylori conferring resistance to erythromycin do not always confer resistance to clarithromycin. Antimicrob. Agents Chemother. 1999, 43, 374–376. 10.1128/AAC.43.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. G.; Kim B. J. Antibiotic Resistance inHelicobacter pyloriInfection. Korean J. Helicobacter Up Gastrointest. Res. 2011, 11, 13–20. 10.7704/kjhugr.2011.11.1.13. [DOI] [Google Scholar]
- EUCAST . European Committee on Antimicrobial Susceptibility Testing, Breakpoint tables for interpretation of MICs and zone diameters. EUCAST Clinical Breakpoint Table; EUCAST, Version 5.0, 2015. [Google Scholar]
- Tor Y. S.; Yazan L. S.; Foo J. B.; Wibowo A.; Ismail I. S.; Yeap S. K. Induction of apoptosis in MCF-7 cells via oxidative stress generation, mitochondria dependent and caspase independent pathway by ethyl acetate extract of Dillenia suffruticosa and its chemical profile. PLoS One 2015, 10, e0127441 10.1371/journal.pone.0127441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakou E.; Primikyri A.; Charisiadis P.; Katsoura M.; Gerothanassis I. P.; Stamatis H.; Tzakos A. G. Unexpected enzyme-catalyzed regioselective acylation of flavonoid aglycones and rapid product screening. Org. Biomol. Chem. 2012, 10, 1739–1742. 10.1039/c2ob06784f. [DOI] [PubMed] [Google Scholar]
- Chaturvedula V. S.; Prakash I. Flavonoids from Astragalus propinquus. J. Chem. Pharm. Res. 2013, 5, 261–265. [Google Scholar]
- He D.; Gu D.; Huang Y.; Ayupbek A.; Yang Y.; Aisa H. A.; Ito Y. Separation and purification of phenolic acids and myricetin from Black Currant by high speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 3077–3088. 10.1080/10826070903320756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai T.; Marumo A.; Kaitou K.; Kanda T.; Terada S.; Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002, 71, 1449–1463. 10.1016/S0024-3205(02)01864-7. [DOI] [PubMed] [Google Scholar]
- Krausse R.; Bielenberg J.; Blaschek W.; Ullmann U. In vitro anti-Helicobacter pylori activity of Extractum liquiritiae, glycyrrhizin and its metabolites. J. Antimicrob. Chemother. 2004, 54, 243–246. 10.1093/jac/dkh287. [DOI] [PubMed] [Google Scholar]
- Ngan L. T. M.; Moon J. K.; Shibamoto T.; Ahn J. Y. Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and -resistant strains of Helicobacter pylori. J. Agric. Food Chem. 2012, 60, 9062–9073. 10.1021/jf3035034. [DOI] [PubMed] [Google Scholar]
- Moon S.; Lee J.; Kim K. T.; Park Y. S.; Nah S. Y.; Ahn D.; Paik H. D. Antimicrobial effect of 7-O-butylnaringenin, a novel flavonoid, and various natural flavonoids against Helicobacter pylori strains. Int. J. Environ. Res. Public Health. 2013, 10, 5459–5469. 10.3390/ijerph10115459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisignano C.; Filocamo A.; La Camera E.; Zummo S.; Fera M. T.; Mandalari G. Antibacterial activities of almond skins on cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013, 13, 103. 10.1186/1471-2180-13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. H.; Hsu C. C.; Yin M. C. In vitro anti-Helicobacter pylori activity of diallyl sulphides and protocatechuic acid. Phytother. Res. 2008, 22, 53–57. 10.1002/ptr.2259. [DOI] [PubMed] [Google Scholar]
- Lin Y. S.; Tsai P. H.; Kandaswami C. C.; Cheng C. H.; Ke F. C.; Lee P. P.; Hwang J. J.; Lee M. T. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial–mesenchymal transition in A431 epidermal cancer cells. Cancer Sci. 2011, 102, 1829–1839. 10.1111/j.1349-7006.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- Kim Y. S.; Kim S. H.; Shin J.; Harikishore A.; Lim J. K.; Jung Y.; Lyu H. N.; Baek N. I.; Choi K. Y.; Yoon H. S.; Kim K. T. Luteolin suppresses cancer cell proliferation by targeting vaccinia-related kinase 1. PLoS One 2014, 9, e109655 10.1371/journal.pone.0109655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J. G.; Hsia T. C.; Kuo H. M.; Li Y. C.; Lee Y. M.; Lin S. S.; Hung C. F. Inhibitory actions of luteolin on the growth and arylamine N-acetyltransferase activity in strains of Helicobacter pylori from ulcer patients. Toxicol. In Vitro 2001, 15, 191–198. 10.1016/S0887-2333(01)00015-7. [DOI] [PubMed] [Google Scholar]
- Joung D. K.; Lee Y. S.; Han S. H.; Lee S. W.; Cha S. W.; Mun S. H.; Kong R.; Kang O. H.; Song H. J.; Shin D. W.; Kwon D. Y. Potentiating activity of luteolin on membrane permeabilizing agent and ATPase inhibitor against methicillin-resistant Staphylococcus aureus. Asian Pac. J. Trop. Med. 2016, 9, 19–22. 10.1016/j.apjtm.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Ng’uni T.; Mothlalamme T.; Daniels R.; Klaasen J.; Fielding B. C. Additive antibacterial activity of naringenin and antibiotic combinations against multidrug resistant Staphylococcus aureus. Afr. J. Microbiol. Res. 2015, 9, 1513–1518. 10.5897/AJMR2015.7514. [DOI] [Google Scholar]
- Xu H.-X.; Lee S. F. Activity of plant flavonoids against antibiotic-resistant bacteria. Phytother. Res. 2001, 15, 39–43. . [DOI] [PubMed] [Google Scholar]
- Tsuchiya H.; Iinuma M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomed. 2000, 7, 161–165. 10.1016/S0944-7113(00)80089-6. [DOI] [PubMed] [Google Scholar]
- Cowan M. M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty K.; Lin Y. T.. Phenolic antimicrobials from plants for control of bacterial pathogens. In Food Biotechnology; 2nd ed.; Shetty K., Paliyath G., Pometto A., Levin R. E., Eds.; CRC Press: Boca Raton, FL, 2006, 1479–1503. [Google Scholar]
- Hathroubi S.; Servetas S. L.; Windham I.; Merrell D. S.; Ottemann K. M. Helicobacter pylori biofilm formation and its potential role in pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18 10.1128/MMBR.00001-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzoeva O. K.; Grishanin R. N.; Calder P. C. Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. 10.1016/S0944-5013(97)80034-1. [DOI] [PubMed] [Google Scholar]
- Abee T.; Kovács Á. T.; Kuipers O. P.; van der Veen S. Biofilm formation and dispersal in Gram-positive bacteria. Curr. Opin. Biotechnol. 2011, 22, 172–179. 10.1016/j.copbio.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Vikram A.; Jayaprakasha G. K.; Jesudhasan P. R.; Pillai S. D.; Patil B. S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. 10.1111/j.1365-2672.2010.04677.x. [DOI] [PubMed] [Google Scholar]
- Pratt L. A.; Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- Lemon K. P.; Higgins D. E.; Kolter R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J. Bacteriol. 2007, 189, 4418–4424. 10.1128/JB.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes L. A. A.; dos Santos Rodrigues J. B.; Magnani M.; de Souza E. L.; de Siqueira-Júnior J. P. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that over expresses efflux protein genes. Microb. Pathog. 2017, 107, 193–197. 10.1016/j.micpath.2017.03.033. [DOI] [PubMed] [Google Scholar]
- Cole S. P.; Kharitonov V. F.; Guiney D. G. Effect of nitric oxide on Helicobacter pylori morphology. J. Infect. Dis. 1999, 180, 1713–1717. 10.1086/315079. [DOI] [PubMed] [Google Scholar]
- Andersen A. P.; Elliott D. A.; Lawson M.; Barland P.; Hatcher V. B.; Puszkin E. G. Growth and morphological transformations of Helicobacter pylori in broth media. J. Clin. Microbiol. 1997, 35, 2918–2922. 10.1128/JCM.35.11.2918-2922.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. F.; Wang K. X. Cloning and expression of vacA gene fragment of Helicobacter pylori with coccoid form. J. Chin. Med. Assoc. 2004, 67, 549–556. [PubMed] [Google Scholar]
- Mazaheri Assadi M.; Chamanrokh P.; Whitehouse C. A.; Huq A. Methods for detecting the environmental coccoid form of Helicobacter pylori. Front. Public Health. 2015, 3, 147. 10.3389/fpubh.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-K.; Song H. E.; Lee H.-B.; Kim C.-S.; Koketsu M.; Ngan L. T. M.; Ahn Y.-J. Growth Inhibitory, Bactericidal, and Morphostructural Effects of Dehydrocostus Lactone from Magnolia sieboldii Leaves on Antibiotic-Susceptible and-Resistant Strains of Helicobacter pylori. PLoS One 2014, 9, e95530 10.1371/journal.pone.0095530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A.; Nishino C.; Enoki N.; Tawata S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. 10.1016/S0031-9422(00)84689-0. [DOI] [Google Scholar]
- Kusters J. G.; van Vliet A. H.; Kuipers E. J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev 2006, 19, 449–490. 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y.; Sheu B. S.; Wu J. J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. 10.1016/j.bj.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolo L. V.; de Souza A. X.; Horta L. P.; Araujo D. P.; de Fátima Â. An overview on the potential of natural products as ureases inhibitors: A review. J. Adv. Res. 2015, 6, 35–44. 10.1016/j.jare.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafarski P.; Talma M. Recent advances in design of new urease inhibitors: A review. J. Adv. Res. 2018, 13, 101–112. 10.1016/j.jare.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabe K.; Yamada M.; Oguni I.; Takahashi T. In vitro and in vivo activities of tea catechins against Helicobacter pylori. Antimicrob. Agents Chemother. 1999, 43, 1788–1791. 10.1128/AAC.43.7.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z. P.; Wang X. D.; Peng Z. Y.; Huang S.; Yang P.; Li Q. S.; Zhou L. H.; Hu X. J.; Wu L. J.; Zhou Y.; Zhu H. L. Molecular docking, kinetics study, and structure–activity relationship analysis of quercetin and its analogous as Helicobacter pylori urease inhibitors. J. Agric. Food Chem. 2012, 60, 10572–10577. 10.1021/jf303393n. [DOI] [PubMed] [Google Scholar]
- Lee H.-K.; Lee H.-B.; Kim C.-S.; Ahn Y.-J. Anti-Helicobacter pylori activity of methanol extracts from Korean native plant species in Jeju Island. Agric. Chem. Biotechnol. 2004, 47, 91–96. [Google Scholar]
- Ngan L. T. M.; Dung P. P.; Nhi N. V. T. Y.; Hoang N. V. M.; Hieu T. T.. Antibacterial activity of ethanolic extracts of some Vietnamese medicinal plants against Helicobacter pylori. In AIP Conference Proceedings; AIP Publishing, 2017, Vol. 1878, No. (1), , p. 020030. [Google Scholar]
- Windham I. H.; Servetas S. L.; Whitmire J. M.; Pletzer D.; Hancock R. E. W.; Merrell D. S. Helicobacter pylori biofilm formation is differentially affected by common culture conditions, and proteins play a central role in the biofilm matrix. Appl. Environ. Microbiol. 2018, 84, e00391–e00318. 10.1128/AEM.00391-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OˈToole G. A. Microtiter dish biofilm formation assay. J. Visualized Exp. 2011, 47, e2437–e2437. 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. N. T.; Ho-Huynh T. D. Selective cytotoxicity of a Vietnamese traditional formula, Nam Dia Long, against MCF-7 cells by synergistic effects. BMC Complement Altern. Med. 2016, 16, 220. 10.1186/s12906-016-1212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg H. S.; Goebel M. R.; Kleinert S. I.; Wünsch D.; Trautwein K.; Rabus R. Photometric determination of ammonium and phosphate in seawater medium using a microplate reader. J. Mol. Microbiol. Biotechnol. 2017, 27, 73–80. 10.1159/000454814. [DOI] [PubMed] [Google Scholar]
- Ngan L. T. M.; Moon J.-K.; Kim J.-H.; Shibamoto T.; Ahn Y.-J. Growth-inhibiting effects of Paeonia lactiflora root steam distillate constituents and structurally related compounds on human intestinal bacteria. World J. Microbiol. Biotechnol. 2012, 28, 1575–1583. 10.1007/s11274-011-0961-6. [DOI] [PubMed] [Google Scholar]
- Lin Y. T.; Kwon Y. I.; Labbe R. G.; Shetty K. Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl. Environ. Microbiol. 2005, 71, 8558–8564. 10.1128/AEM.71.12.8558-8564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.