Abstract
Previous report has confirmed the beneficial effects of α-mangostin (α-MG), a major and representative xanthone distributed in mangosteen (Garcinia mangostana) on the cisplatin-induced rat model. However, the molecular mechanisms related to its renoprotection have not been elucidated exhaustively. The present study investigated the protective effect of α-MG against cisplatin-induced cytotoxicity in the human embryonic kidney (HEK293) cell model. In this study, α-MG prevented cisplatin-induced cell death, accompanied with the decreased levels of malondialdehyde and increased glutathione content. Particularly, α-MG significantly suppressed the overproduction of reactive oxygen species (ROS), restored the activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), and downregulated the c-JUN N-terminal kinase (JNK) pathways following cisplatin challenge. Subsequently, the cleavage of caspases and poly-ADP-ribose polymerase (PARP) implicating ROS-mediated apoptosis pathways induced by cisplatin was effectively inhibited by α-MG. In conclusion, our findings provided a rationale for the development of α-MG to attenuate cisplatin-induced nephrotoxicity.
1. Introduction
Despite cisplatin, a class of cell cycle nonspecific widely anticancer drug with a unique structure–activity, which is widely used for treating various solid tumors, its clinical application is limited due to severe adverse effects for normal organism.1 Nephrotoxicity is the major dose-dependent and longstanding cumulative side reaction after cisplatin injection. Primary targets of cisplatin in kidney are proximal tubules, which induces the overproduction of oxidative stress, caspase activation, and even cell apoptosis and necrosis. Peroxidative damage caused by reactive oxygen species (ROS) is the initial process of cisplatin-induced renal failure. Briefly, cisplatin treatment interrupts the balance between oxidants and antioxidants, contributing to the formation of oxidative stress in the kidney.2 Hereby, it raises an urgent problem on how to reduce the nephrotoxicity caused by cisplatin, exert an antitumor effect, and improve the patients’ chemotherapy quality.
Garcinia mangostana L. (Clusiaceae) is a tropical tree native to Southeast Asia known as mangosteen, whose pericarps of the fruit have a long history of clinical application in the traditional Chinese medicinal practices for centuries.3 α-MG (Figure 1A) as a representative xanthone of secondary metabolites in the medicinal plant was discovered to produce various biological effects in multiple reports, such as hepatoprotection,4 neuroprotection,5 antibiofilm,6,7 anti-inflammation,8 antioxidant,9 anticancer,10 and antimalarial.11 Actually, it is noteworthy that reports have proven the beneficial properties of α-MG in diabetic nephropathy in rats,12 and studies associated with cisplatin-induced nephrotoxicity have not been elucidated exhaustively. Based on the early study in vivo, which has demonstrated the renoprotection by α-MG against cisplatin-induced kidney injury,13 the present one studied the beneficial properties of α-MG with the cisplatin-induced HEK293 cell model, which focused on oxidative injuries and apoptosis and intensively elaborated what multiple signaling pathways α-MG act on.
Figure 1.
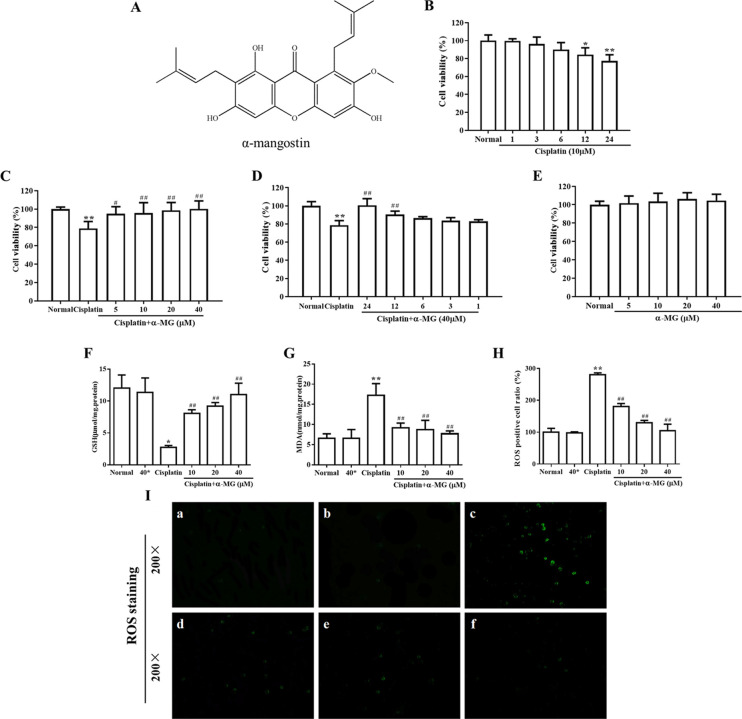
α-MG ameliorates cisplatin-induced cytotoxicity in HEK293 cells. (A) Chemical structure of α-mangostin (α-MG). α-MG ameliorates cisplatin-induced cytotoxicity in HEK293 cells. (B) Cytotoxicity effects of cisplatin on HEK293 cells. The cells were incubated with 20 μM cisplatin in varying durations (1–24 h). (C) α-MG exerts protective effects against cisplatin-induced decrease in cellular viability. HEK293 cells were pretreated with α-MG (5–40 μM) for 24 h and then exposed to cisplatin for 24 h. (D) Cells were pretreated with α-MG (40 μM) and then cultured in the presence of cisplatin (1–24 h). (E) Cells were incubated with α-MG (5–40 μM) alone for 24 h. Cell viability was measured by the MTT assay. α-MG (0–40 μM) dose-dependently attenuates cisplatin-induced lipid peroxidation in HEK293 cells. (F, G) Levels of (F) GSH and (G) MDA were determined using commercial kits. Cells pretreated with α-MG (0–40 μM) for 24 h attenuate cisplatin-induced accumulation of intracellular ROS. (H) Quantitative analysis of ROS. Data are represented as means ± SD; *p < 0.05, **p < 0.01 compared to the control group; #p < 0.05, ##p < 0.01 compared with the cisplatin group. (I) Representative images indicated the intracellular ROS assay. Green fluorescence shows positive cells. Note: (a) normal group, (b) 40 μM α-MG separate treatment group, (c) cisplatin separate treatment group, (d) 10 μM α-MG and cisplatin coprocessing group, (e) 20 μM α-MG and cisplatin coprocessing group, and (f) 40 μM α-MG and cisplatin coprocessing group.
2. Results
2.1. α-MG Ameliorates Cisplatin-Induced Cytotoxicity in HEK293 Cells
Cell viability was performed to access the renoprotective effect of α-MG on cisplatin-treated HEK293 cells by the MTT reduction assay. As shown in Figure 1B, cultured HEK293 cells were treated with cisplatin in varying periods of time, and the results indicated that cisplatin alone evidently decreased the cell viability in a time-dependent manner. For instance, cells incubated with 20 μM cisplatin for 24 h showed 78.9% cell viability than normal cells (p < 0.01). To evaluate whether α-MG exerts protective properties, HEK293 cells were pretreated with gradient concentrations of α-MG with cells being exposed to 20 μM cisplatin for extra 24 h (Figure 1C). Further, α-MG dramatically elevated cell viability of cisplatin-treated cells to 100.5 and 90.3% at 24 and 12 h, respectively (p < 0.01) (Figure 1D). The results demonstrated that α-MG significantly reversed the cisplatin-induced cytotoxic effect in a time- and dose-dependent manner (p < 0.05 or p < 0.01). Additionally, α-MG pretreatment did not affect the cell viability of normal HEK293 cells (Figure 1E).
2.2. α-MG Attenuates Cisplatin-Induced Oxidation in HEK293 Cells
As shown in Figure 1F, acute incubation with 20 μM cisplatin triggered significantly depletion of the GSH level in HEK293 cells (2.7 ± 0.3 μmol/mg protein) as compared with that in the normal cells (11.2 ± 3.1 μmol/mg protein). However, prior treatment with α-MG increased the reduced GSH content in a dose-dependent manner (p < 0.01). The level of lipid peroxidation after pretreatment or without α-MG in cisplatin-evoked HEK293 cells is shown in Figure 1G. The cellular level of MDA significantly increased after the exposure to cisplatin (17.2 ± 2.9 nmol/mg protein), whereas pretreatment with α-MG effectively inhibited the overproduction compared to that exposed to cisplatin alone (p < 0.01). These data emphasized the evidence of α-MG for effective antioxidant activity against cisplatin-induced oxidative damage in HEK293 cells.
2.3. α-MG Attenuates Cisplatin-Induced Accumulation of Intracellular ROS
Oxidative stress-induced cell injury is a vital molecular mechanism in the pathogenesis process of cisplatin-triggered cytotoxicity. ROS directly acted on cell components, including lipids, proteins, and DNA, ultimately destroyed their interior structure. As shown in Figure 1I, the intracellular ROS levels of HEK293 cells were significantly elevated when treated with cisplatin alone compared with untreated one. Meanwhile, the pretreatment with α-MG and cisplatin remarkably reduced the excessive ROS generation (p < 0.01), suggesting that the antioxidant activity of α-MG affected the numerous ROS induced by cisplatin.
2.4. α-MG Alleviates Cisplatin-Induced Activation of Caspase Signal Pathways
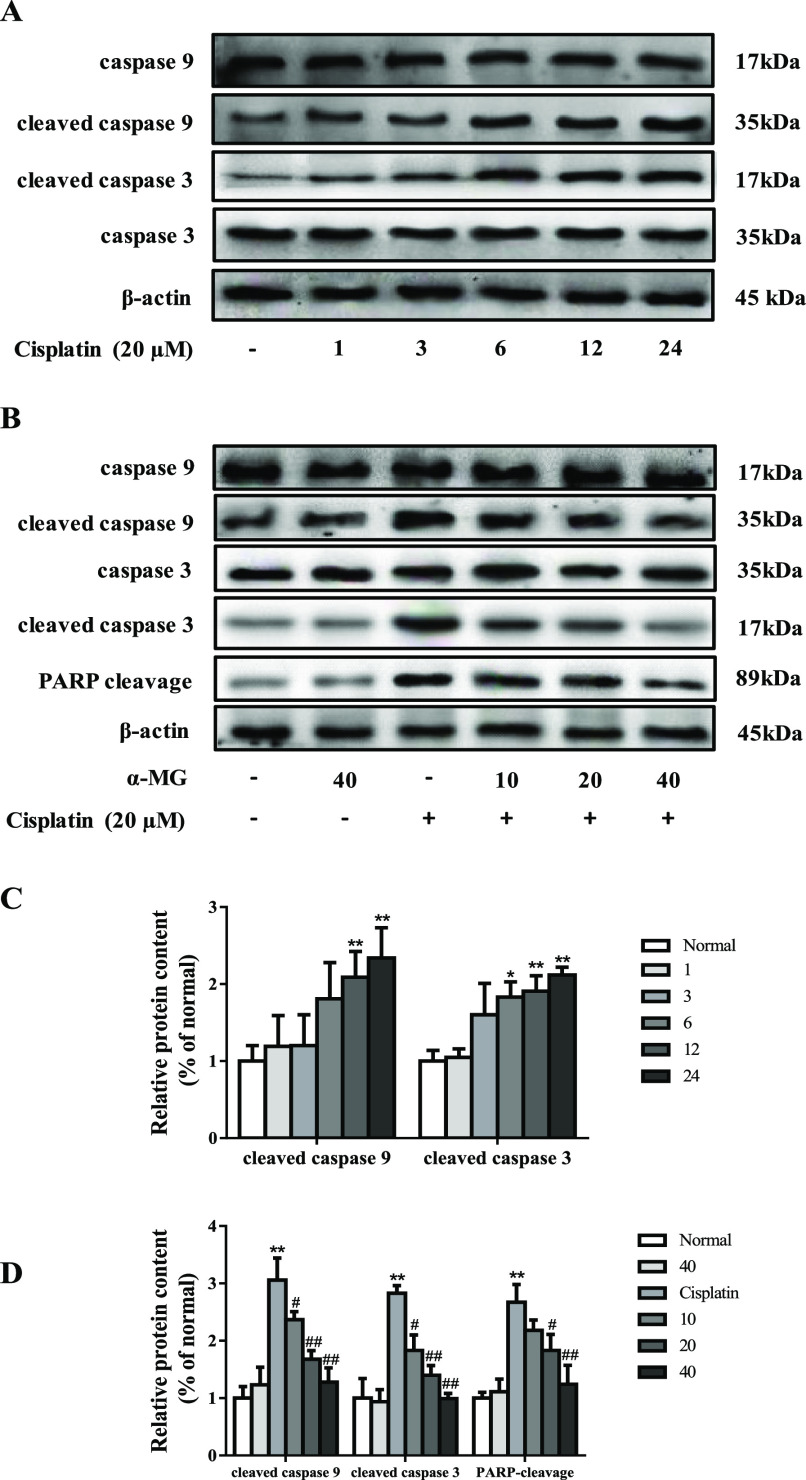
As depicted in Figure 2A, exposure to cisplatin with 40 μM at varying periods of time significantly elevated cleaved caspase 3 and caspase 9 in a time-dependent manner (p < 0.05 or p < 0.01). As shown in Figure 2B, incubation of cells with 20 μM cisplatin markedly increased the activation of cleaved caspase 9 and cleaved caspase 3 in a dose-dependent manner, respectively (p < 0.01). However, α-MG significantly suppressed activities of cleaved caspase 9 and caspase 3 compared to cisplatin alone (p < 0.05 or p < 0.01). In addition, pretreatment with cisplatin for 24 h elevated poly-ADP-ribose polymerase (PARP) cleavage activity in comparison to untreated cells (p < 0.01). Conversely, as expected, α-MG reduced the intracellular PARP cleavage release (p < 0.05 or p < 0.01) following cisplatin exposure. Together, we proposed that α-MG exerts inhibitory properties against cisplatin-triggered activation of caspase cascades.
Figure 2.
α-MG alleviates cisplatin-induced activation of caspases. (A) Time course of activation of caspases caused by cisplatin and western blotting analysis. The intensities of caspase 9, cleaved caspase 9, caspase 3, and cleaved caspase 3 were standardized to that of β-actin. (B) α-MG suppressed cisplatin-induced caspase activation in HEK293 cells in a dose-dependent manner. (C) Quantitative analysis of scanning densitometry for caspase 9, cleaved caspase 9, caspase 3, cleaved caspase 3, and PARP cleavage after being exposed to cisplatin with 40 μM at varying periods of time. (D) Quantitative analysis of scanning densitometry for caspase 9, cleaved caspase 9, caspase 3, cleaved caspase 3 and PARP cleavage after adding cisplatin and different doses of α-MG. Data are represented as means ± SD; *p < 0.05, **p < 0.01 compared to the control group; #p < 0.05, ##p < 0.01 compared with the cisplatin group.
2.5. α-MG Regulates the Expressions of Cisplatin-Induced Release of Bcl-2 Family Members
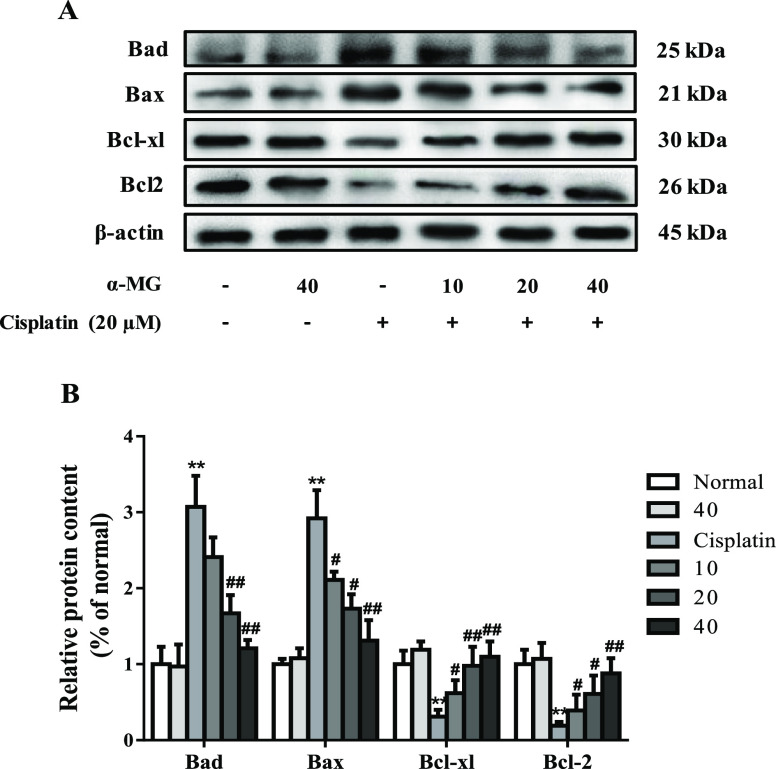
Once cisplatin enters the tubular cell, it exerts toxicity, which culminates in apoptosis response of the cells. In this study, we performed the western blotting assay to investigate the protective mechanism of α-MG against cisplatin caused HEK293 cell apoptosis. As shown in Figure 3A, after exposure to cisplatin alone for 24 h, the expressions of proapoptotic proteins Bax and Bad were significantly increased as simultaneously accompanied with the reduced levels of Bcl-2 and Bcl-xl with respect to untreated HEK293 cells (p < 0.01). In contrast, α-MG suppressed cisplatin-induced Bax and Bad release and maintained a balance between proapoptosis and antiapoptosis, compared with those of cisplatin-treated cells (p < 0.05 or p < 0.01). These data demonstrated that α-MG may act as a potential target for cisplatin-induced cellular apoptosis by inhibition of proapoptotic family protein release.
Figure 3.
α-MG regulates the expressions of cisplatin-induced release of Bcl-2 family members. (A) Protein levels of Bad, Bax, Bcl-xl, and Bcl-2 were determined by the western blotting assay. (B) Quantification of target proteins. All data are represented as means ± SD; *p < 0.05, **p < 0.01 compared to the control group; #p < 0.05, ##p < 0.01 compared with the cisplatin group.
2.6. α-MG Reverses Cisplatin-Induced PI3K/Akt Suppression and JNK Activation
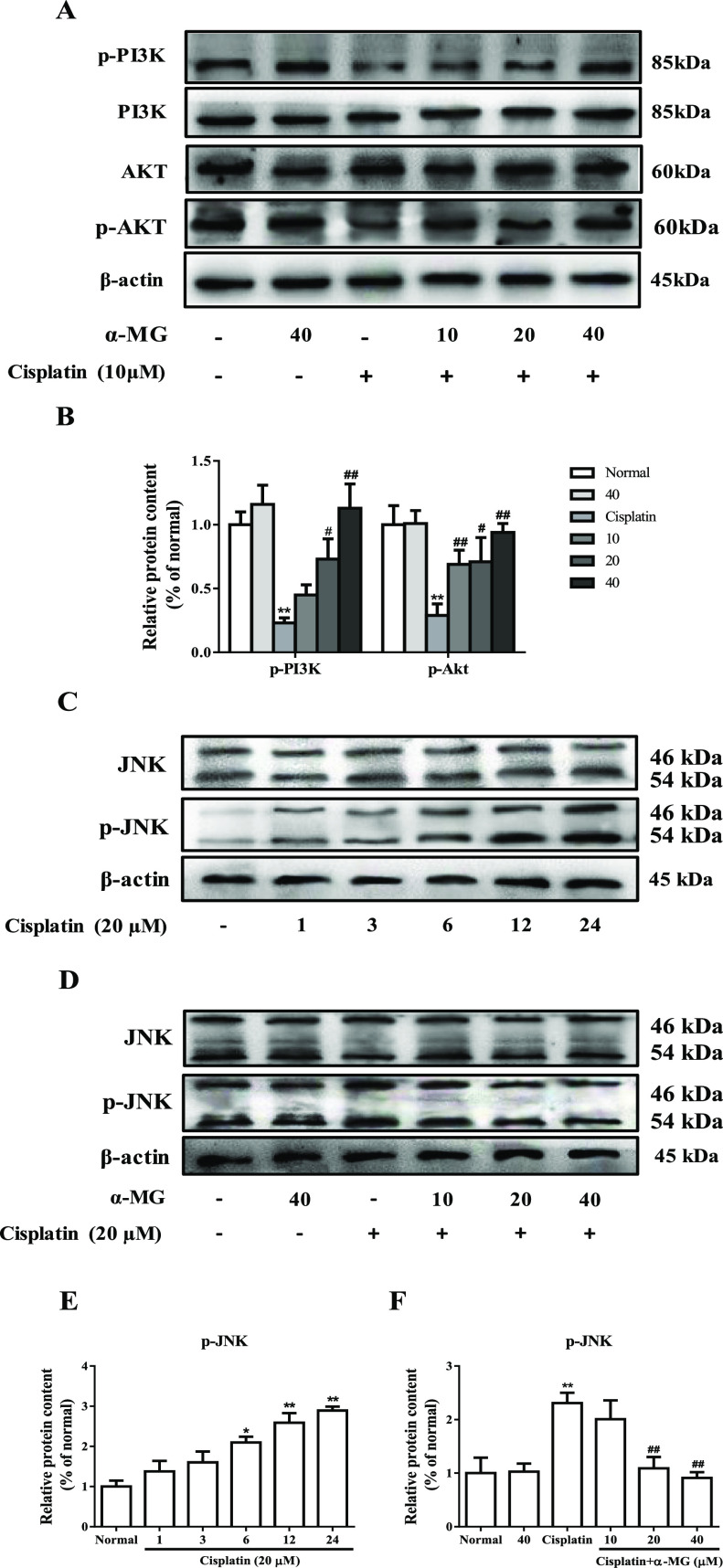
Previous study showed that cisplatin can lead to the inhibition of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) activities in cisplatin-induced nephrotoxicity.17 To explore whether the PI3K/Akt pathway affect the protection of α-MG cisplatin-induced cytotoxicity, we investigated the relative protein expression levels in the PI3K/Akt signal pathways by western blotting analysis. As depicted in Figure 4A, cisplatin exposure alone remarkably decreased the expressions of p-PI3K and p-Akt in comparison to normal HEK293 cells (p < 0.01). Treatment of α-MG dose-dependently attenuated the depleted levels of p-PI3K and p-Akt (p < 0.05 or p < 0.01). These findings revealed that α-MG may act as a PI3K/Akt activator to enhance the cytoprotective effect for cisplatin-induced cell apoptosis. In addition, we examined the effect of α-MG on the expression of p-JNK and JNK (c-JUN N-terminal kinase) in response to cisplatin exposure. As shown in Figure 4C, the expression of p-JNK was increased in HEK293 cells treated with cisplatin for different durations, which suggested that cisplatin could stimulate the JNK pathway in a time-dependent manner (p < 0.05 or p < 0.01). To further investigate the protective mechanism of α-MG, HEK293 cells were treated with α-MG and cisplatin together to analyze the protein expression levels of p-JNK. Interestingly, α-MG significantly extenuated activation of JNK caused by cisplatin in a dose-dependent manner (p < 0.01). These findings indicated that α-MG protects HEK293 cells from cisplatin-induced JNK activation.
Figure 4.
α-MG reverses cisplatin-induced PI3K/Akt suppression and JNK activation. (A) Western blotting analysis of PI3K, p-PI3K, Akt, and p-Akt proteins. (B) Quantitative analysis of PI3K/Akt. (C) Time course of activation of JNK caused by cisplatin. (D) α-MG suppressed the expression of JNK in a dose-dependent manner. (E, F) Quantitative analysis of p-JNK. All data are represented as means ± SD; *p < 0.05, **p < 0.01 compared to the control group; #p < 0.05, ##p < 0.01 compared with the cisplatin group.
3. Discussion
Cisplatin is a major antineoplastic drug for the treatment of various solid tumors, but it has dose- and time-dependent nephrotoxicity. Increasing evidence suggested that cisplatin-induced nephrotoxicity occurs through multiple molecular signal pathways. Among these, more and more evidence showed that oxidative stress and apoptosis were most implicated in cisplatin-induced cytotoxicity.18 Moreover, α-MG exerts beneficial antioxidant and antiapoptosis activities in various experimental animal models.19 Unlike antioxidative and antiapoptosis, it was revealed that the α-MG exerts the alleviative effect against cisplatin-induced nephrotoxicity in vivo(13) due to its antifibrotic and anti-inflammatory activities, but little is known about the underlying protective multifactorial molecular mechanisms. Therefore, α-MG was proposed as a mechanistically targeted preventive approach to reduce cisplatin nephrotoxicity in vitro. Here, we revealed the potential beneficial effect of α-MG against cisplatin-induced oxidative stress and apoptosis on HEK293 cells. Our results showed that cisplatin inhibited HEK293 cell viability by inducing oxidative damage, further stimulating apoptosis through an extrinsic apoptosis pathway, with involvement of caspase family members’ activation and PARP cleavage, which are consistent with previous reports.20 Moreover, cisplatin-induced intracellular stress also suppressed PI3K/Akt pathways while causing JNK activation.
In the presence of cisplatin, ROS is initially produced via mitochondria and the NADPH oxidase system and is implicated in the pathogenesis of cisplatin nephrotoxicity. Further, ROS directly acts on cell components, such as lipids and proteins, and destroys their structure and antioxidant system. Previous report proved that cisplatin triggers excessive intracellular ROS accumulation, resulting in production of oxidative stress, even death in the kidney tubular cell.21 Additionally, cisplatin disturbed antioxidant defense mechanisms followed an apparent decrease in GSH and increase in the MDA content. In addition, abnormal lipid peroxidation and hypoxia damage could result in the excessive free radicals or ROS in the model of cisplatin-induced acute renal injury. Our results showed that cisplatin increased intracellular ROS levels, which is similar with the previous study that cisplatin can lead to accumulation of ROS, and further activated ER stress, even cellular death.22 To mitigate the toxic and side effects of cisplatin’s toxic metabolites, many potential targets have been evaluated in which the supplementation with natural antioxidant has been considered for the nephroprotective strategy.23 Our study showed that α-MG remarkably reduced the generation of intracellular ROS, increased MDA, and elevated the content of GSH in HEK293 cells in a dose-dependent manner, suggesting that α-MG protection in cisplatin-induced cytotoxicity is mediated by suppression of ROS-induced oxidative damage.
Cisplatin-induced oxidative stress contributes to the activation of apoptosis at various concentrations of cisplatin.24 Lines of evidence have shown that the caspase pathway was involved in cisplatin-mediated nephrotoxicity in vivo and in vitro.25,26 Briefly, cisplatin ultimately triggered intrinsic apoptotic pathways via activating caspase cascade and caspase-independent pathways. Oxidative stressors and DNA fragments initiated the mitochondrial pathway by releasing cytochrome c and resulted in caspase 9 activation. Caspase 9 activates caspase 3, which is a primary apoptotic regulatory factor causing nuclear death and other changes in regard to apoptosis. PARP was able to shear by caspase 3, and its cleavage is regarded as a symbol of cellular apoptosis. Hence, PARP inhibition might be an important mechanism responsible for the renoprotection. As anticipated, in the present study, cisplatin exposure increased the protein expression of caspase 9, caspase 3, and PARP cleavage in HEK293 cells. On the other hand, α-MG intervention could effectively block the release of caspase 9, caspase 3, and PARP cleavage and dramatically increase the cell viability of HEK293. Previous studies also provided evidence that Bcl-2 family members regulated activation of caspases through suppressing cytochrome c release from the mitochondria in a cell model.27 Under apoptotic conditions, Bax was activated and accumulated at the mitochondrial outer membrane, leading to the release of proapoptotic factors.28 Bad, a proapoptotic member of the Bcl-2 family, has the ability to directly interact and bind to Bcl-2 and Bcl-xl, resulting in the blocking of their survival function in the presence of cisplatin stimuli.29 Therefore, antiapoptosis measures have been conducted for the renoprotective strategy. In agreement with previous reports, our data showed that cisplatin triggered the overexpressions of Bax and Bad, along with reduced levels of Bcl-2 and Bcl-xl in HEK293 cells. However, we observed a prominent increase of Bcl-2 and Bcl-xl when the cells were treated with α-MG for 24 h. These data suggested that α-MG suppressed the induction of mitochondrial-mediated apoptotic pathways triggered cisplatin by regulating Bcl-2 family members.
The PI3K/Akt pathway is a well-known critical signaling pathway that regulates multiple cellular processes, including cell proliferation, growth, survival, metabolism, and motility.30 The knockout of PI3K exhibited excessive apoptosis accumulated in renal tubular epithelial cells, suggesting a critical role of the PI3K/Akt pathway in the maintenance of renal function in cisplatin-induced acute kidney injury.31 In the present study, the protein levels of p-PI3K and p-Akt were markedly downregulated after cisplatin treatment in HEK293 cells, while α-MG reversed these changes in a dose-dependent manner, suggesting that α-MG enhanced the survival rate of HEK293 cells in part via activating the PI3K/Akt pathway.
Cisplatin was recently shown to activate ERK and p38 proteins, both in vitro and in vivo.32 JNK is one of the three main serine/threonine kinases pertaining to MAPK family, which participates in cell proliferation and differentiation and is widely associated with cellular apoptosis and death. Our previous findings in vivo have proved that activation of JNK (phospho-JNK) aggravated kidney dysfunction, causing intracellular apoptosis after cisplatin challenge, which clarified a mechanistic part of JNK in the cisplatin-induced renal toxicity model.33 Consistent with previous conclusion, reduction of JNK activation by α-MG may represent another potential mechanism for preventing cisplatin-mediated cytotoxicity.
In summary, it was determined that the salutary effect of α-MG against cisplatin-induced renal cytotoxicity was multifactorial. As depicted in Figure 5, our study demonstrated that α-MG reverses cisplatin-induced renal cytotoxicity in HEK293 cells through inhibition of ROS-mediated apoptosis and modulation of the PI3K/Akt and JNK signaling pathways. These effects presumably illustrated the antioxidant and antiapoptotic properties of α-MG.
Figure 5.
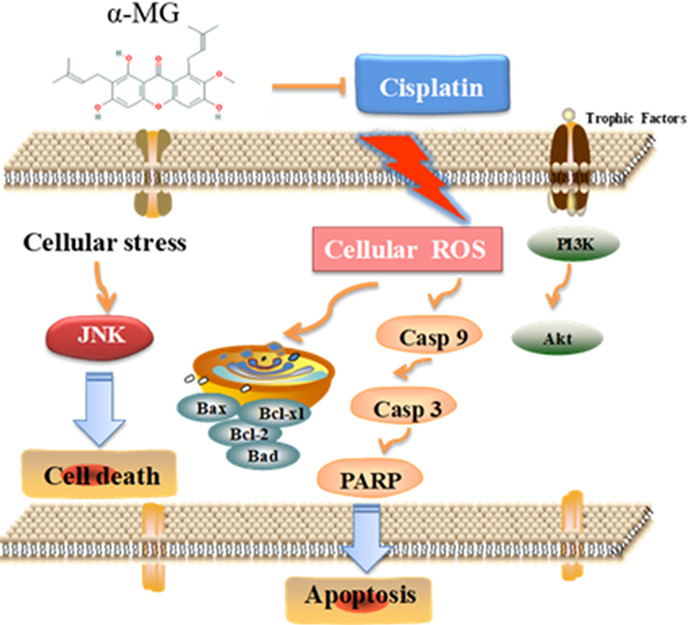
Underlying molecular mechanism related to the ameliorative effects of α-mangostin, a dietary xanthone, against cisplatin-induced nephrotoxicity in HEK293 cells.
4. Materials and Methods
4.1. Chemicals and Reagents
Cisplatin were manufactured by Sigma-Aldrich (St. Louis, MO, USA). DMSO and MTT were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), antibiotic (10,000 U/mL penicillin and 10,000 μg/mL streptomycin), and fetal bovine serum (FBS) were purchased from HyClone (Logan, UT, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Biotopped Technology Co., Ltd. (Beijing, China). The commercial assay kits of GSH and MDA were bought from Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China). Antibodies against rabbit proteins such as anticaspase 3, anticaspase 8, anticaspase 9, anticleaved PARP, anti-PI3K, anti-Akt, and anti-JNK were acquired from Cell Signaling Technology (Danvers, MA, USA). Antibody anti-rabbit-β-actin was accessed by Proteintech (Rosemont, USA). Antibodies rabbit anti-Bax and rabbit anti-Bcl-2 were obtained from Abcam (Cambridge, UK). 2′,7′-Dichlorofluorescein diacetate (DCFDA) were purchased from Wanleibio (Shenyang, China).The remaining reagents were analytically pure and provided by Beijing Chemical Works (Beijing, China).
4.2. Preparation of α-MG from Mangosteen Pericarp
Mangosteen was purchased from Carrefour in Changchun and authenticated by Prof. Wei Li (College of Chinese Medicinal Material, Jilin Agricultural University, Changchun, China). The voucher specimen (no. 160517) was stored in the Jilin Agricultural University, Chinese herbal medicine laboratory. The pericarp of the fruits was parched at a temperature of 50 °C, smashed into a homogeneous type using a disintegrator (HX-200A, Yongkang Hardware and Medical Instrument Plant, China) and then filtrated (30–40 mesh). The desiccative mangosteen pericarp powder (20 g) was extracted with 200 mL of 85% ethanol for 5 min twice by the smashing tissue extraction (STE) method.4 The purity of α-MG is more than 98%.
4.3. Culture of HEK293 Cells and Drug Treatment
HEK293, an epithelial cell derived from human embryonic kidney cell line, was purchased from ATCC Cell Bank and conventionally cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The incubator environment is 37 °C under a 5% CO2 atmosphere. All experiments were performed when cells were grown to 80% confluence in DMEM. Then, cells were treated with various concentrations of α-MG 24 h prior to the injection of cisplatin (20 μM) for the indicated stimulation time course.
4.4. MTT Assay
Cell viability was assessed by the MTT assay, a quantitative assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, to assure that only cells with viability over 95% were used. Cytotoxicity of cisplatin or/and α-MG was screened, and HEK293 cells were seeded in 96-well plates at 1 × 104 cells per well and treated with cisplatin (20 μM) in varying periods of time (0, 1, 3, 6,12, and 24 h) or with α-MG (24 h) for gradient concentrations (5, 10, 20, and 40 μM). For the protective treatment, the cells were pretreated with α-MG for different concentrations (5, 10, 20, and 40 μM) for 24 h and copretreated with cisplatin for another 24 h. Cells were washed twice with PBS followed by MTT (5 mg/mL). After that, the cells were incubated for 3 h, and then the solution was aspirated and the formed formazan salt was used as the solvent in 150 μL of DMSO per well. The precipitate in each well was dissolved for 5 min, and the optical density (OD) was read at 490 nm using a microplate reader (SPECTROstar Nano).
4.5. Analysis of Cell Oxidative Stress Indicators
Cells were pretreated with α-MG (5, 10, 20, and 40 μM) for 24 h and then treated with or without cisplatin (20 μM) for 24 h. Subsequently, cells were washed with 1.0 mL of 100 mM phosphate buffer (pH 7.4). The level of GSH was determined by commercially available diagnostic kits according to manufacturer’s programs (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China).
4.6. Measurement of Cell Lipid Peroxidation
The MDA content was measured using a commercial thiobarbituric acid reactive substances (TBARS) assay kit according to the manufacturer’s protocols.14 Briefly, HEK293 cells were hatched in a 6-well plate for 24 h until the cell density is 1 × 105, and then the cells were incubated with α-MG (5, 10, 20, 40 μM) or/and cisplatin (20 μM). After treatments, cells were scraped, pelleted, and collected in 1 mL of phosphate-buffered saline (PBS) solution (pH 7.4) and homogenized (3 min setting on ice) to cell lysates. MDA reacts with thiobarbituric acid to form a colored product and was measured at an absorbance of 532 nm.
4.7. Determination of ROS Accumulation
The relative levels of intracellular ROS were determined by a fluorometric assay (DCFH-DA assay).15 HEK293 cells were hatched in a 6-well plate for 24 h, and then the cells were incubated with α-MG (5, 10, 20, and 40 μM) or/and cisplatin (20 μM). After incubation, treated HEK293 cells were incubated with a 10 μM DCFH-DA probe at 37 °C with 5% CO2 in dark for 30 min. The medium was discarded, and cells were washed by PBS twice. Then, the generation of ROS was determined by the fluorescence intensity under a fluorescence microscope (Leica TCS SP8, Solms, Germany).
4.8. Western Blotting Analysis
After treatments, HEK293 cell extracts were collected and the total protein concentration was determined using the BCA protein assay kit (Beyotime Biotechnology, China).16 Equal amounts of protein (15 μg) were loaded on the 12% SDS-polyacrylamide gel electrophoresis and electroblotted onto a PVDF membrane. The membranes were blocked with 5% BSA in TBS with 0.1% Tween 20 for 2 h at room temperature and then incubated overnight at 4 °C with indicated primary antibodies followed by secondary antibodies conjugated with horseradish peroxidase (HRP) for 1 h at room temperature. Protein–antibody complexes were detected using an emitter-coupled logic (ECL) substrate (Pierce Chemical Co., Rockford, IL, USA). Protein band intensities were quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
4.9. Statistical Analysis
All data were expressed as means ± standard deviation (SD) derived from at least three separate experiments and analyzed with a two-tailed test or a one-way analysis of variance (ANOVA). The p values of less than 0.05 or 0.01 in differences between groups were considered to be significant. Statistical graphs were produced using software of GraphPad Prism 6.0.4 software (Graphpad Software, Inc., San Diego, USA).
Acknowledgments
This work was supported by the grants of Jilin Science and Technology Development Plan (nos. 20200301037RQ and 20190103092JH) and the Open Fund of Key Laboratory of Biotechnology and Bioresources Utilization (KF202004).
Glossary
Abbreviations
- α-MG
α-mangostin
- ROS
reactive oxygen species
- GSH
glutathione
- MDA
malondialdehyde
- Bax
B-associated X
- Bcl-2
B-cell lymphoma 2
- PI3K
phosphatidylinositol 3-kinase
- Akt
protein kinase B
- JNK
c-JUN N-terminal kinase
- PARP
poly-ADP-ribose polymerase
- STE
smashing tissue extraction
- P
polymerase
Author Contributions
∥ Q.L. and X.-T.Y. contributed equally to this work and considered as co-first authors.
The authors declare no competing financial interest.
References
- Nho J.-H.; Jung H.-K.; Lee M.-J.; Jang J.-H.; Sim M.-O.; Jeong D.-E.; Cho H.-W.; Kim J.-C. Beneficial Effects of Cynaroside on Cisplatin-Induced Kidney Injury In Vitro and In Vivo. Toxicol. Res. 2018, 34, 133–141. 10.5487/TR.2018.34.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Lee D.; Kang K. S.; Song J. H.; Choi Y.-K. Inhibition of Intracellular ROS Accumulation by Formononetin Attenuates Cisplatin-Mediated Apoptosis in LLC-PK1 Cells. Int. J. Mol. Sci. 2018, 19, 813. 10.3390/ijms19030813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im A.-R.; Kim Y.-M.; Chin Y.-W.; Chae S. Protective effects of compounds from Garcinia mangostana L. (mangosteen) against UVB damage in HaCaT cells and hairless mice. Int. J. Mol. Med. 2017, 40, 1941–1949. 10.3892/ijmm.2017.3188. [DOI] [PubMed] [Google Scholar]
- Yan X.-t.; Sun Y.-s.; Ren S.; Zhao L.-c.; Liu W.-c.; Chen C.; Wang Z.; Li W. Dietary α-Mangostin Provides Protective Effects against Acetaminophen-Induced Hepatotoxicity in Mice via Akt/mTOR-Mediated Inhibition of Autophagy and Apoptosis. Int. J. Mol. Sci. 2018, 19, 1335. 10.3390/ijms19051335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X.-M.; Li L.-D.; Duan C.-L.; Li Y.-J. Neuroprotective effect of α-mangostin on mitochondrial dysfunction and α-synuclein aggregation in rotenone-induced model of Parkinson’s disease in differentiated SH-SY5Y cells. J. Asian Nat. Prod. Res. 2017, 19, 833–845. 10.1080/10286020.2017.1339349. [DOI] [PubMed] [Google Scholar]
- Phuong N. T. M.; Van Quang N.; Mai T. T.; Anh N. V.; Kuhakarn C.; Reutrakul V.; Bolhuis A. Antibiofilm activity of α-mangostin extracted from Garcinia mangostana L. against Staphylococcus aureus. Asian Pac. J. Trop. Med. 2017, 10, 1154–1160. 10.1016/j.apjtm.2017.10.022. [DOI] [PubMed] [Google Scholar]
- Meepagala K. M.; Schrader K. K. Antibacterial activity of constituents from mangosteen (Garcinia mangostana) fruit pericarp against several channel catfish pathogens. J. Aquat. Anim. Health 2018, 30, 179–184. 10.1002/aah.10021. [DOI] [PubMed] [Google Scholar]
- Cui J.; Hu W.; Cai Z.; Liu Y.; Li S.; Tao W.; Xiang H. New medicinal properties of mangostins: analgesic activity and pharmacological characterization of active ingredients from the fruit hull of Garcinia mangostana L. Pharmacol., Biochem. Behav. 2010, 95, 166–172. 10.1016/j.pbb.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Mahabusarakam W.; Proudfoot J.; Taylor W.; Croft K. Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin. Free Radical Res. 2009, 33, 643–659. 10.1080/10715760000301161. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Yu G.; Shen Y. The naturally occurring xanthone α-Mangostin induces ROS-mediated cytotoxicity in non-small scale lung cancer cells. Saudi J. Biol. Sci. 2018, 25, 1090–1095. 10.1016/j.sjbs.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjahjani S. Antimalarial activity of Garcinia mangostana L rind and its synergistic effect with artemisinin in vitro. BMC Complementary Altern. Med. 2017, 17, 131. 10.1186/s12906-017-1649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Duan W.; Nizigiyimana P.; Gao L.; Liao Z.; Xu B.; Liu L.; Lei M. Alpha-mangostin attenuates diabetic nephropathy in association with suppression of acid sphingomyelianse and endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2018, 496, 394–400. 10.1016/j.bbrc.2018.01.040. [DOI] [PubMed] [Google Scholar]
- Pérez-Rojas J. M.; Cruz C.; García-López P.; Sánchez-González D. J.; Martínez-Martínez C. M.; Ceballos G.; Espinosa M.; Meléndez-Zajgla J.; Pedraza-Chaverri J. Renoprotection by α-Mangostin is related to the attenuation in renal oxidative/nitrosative stress induced by cisplatin nephrotoxicity. Free Radical Res. 2009, 43, 1122–1132. 10.1080/10715760903214447. [DOI] [PubMed] [Google Scholar]
- Mi X.-j.; Hou J.-g.; Jiang S.; Liu Z.; Tang S.; Liu X.-x.; Wang Y.-p.; Chen C.; Wang Z.; Li W. Maltol Mitigates Thioacetamide-induced Liver Fibrosis through TGF-β1-mediated Activation of PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1392–1401. 10.1021/acs.jafc.8b05943. [DOI] [PubMed] [Google Scholar]
- Li R.-y.; Zhang W.-z.; Yan X.-t.; Hou J.-g.; Wang Z.; Ding C.-b.; Liu W.-c.; Zheng Y.-n.; Chen C.; Li Y.-r.; Li W. Arginyl-fructosyl-glucose, a Major Maillard Reaction Product of Red Ginseng, Attenuates Cisplatin-Induced Acute Kidney Injury by Regulating Nuclear Factor κB and Phosphatidylinositol 3-Kinase/Protein Kinase B Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5754–5763. 10.1021/acs.jafc.9b00540. [DOI] [PubMed] [Google Scholar]
- Zhang J.-j.; Wang J.-q.; Xu X.-y.; Yang J.-y.; Wang Z.; Jiang S.; Wang Y.-p.; Zhang J.; Zhang R.; Li W. Red Ginseng Protects against Cisplatin-induced Intestinal Toxicity by Inhibiting Apoptosis and Autophagy via the PI3K/AKT and MAPK Signaling Pathways. Food Funct. 2020, 11, 4236–4248. 10.1039/d0fo00469c. [DOI] [PubMed] [Google Scholar]
- Ju S. M.; Kang J. G.; Bae J. S.; Pae H. O.; Lyu Y. S.; Jeon B. H. The Flavonoid Apigenin Ameliorates Cisplatin-Induced Nephrotoxicity through Reduction of p53 Activation and Promotion of PI3K/Akt Pathway in Human Renal Proximal Tubular Epithelial Cells. J. Evidence-Based Complementary Altern. Med. 2015, 1. 10.1155/2015/186436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian P.; Yan L.-J.; Li Y.-Q.; Yang H.-T.; Duan H.-Y.; Wu J.-T.; Fan X.-W.; Wang S.-L. Cyanidin ameliorates cisplatin-induced cardiotoxicity via inhibition of ROS-mediated apoptosis. Exp. Ther. Med. 2018, 15, 1959–1965. 10.3892/etm.2017.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P.; Ongsakul M.; Proudfoot J.; Croft K.; Beilin L. Mangostin inhibits the oxidative modification of human low density lipoprotein. Free Radical Res. 2009, 23, 175–184. 10.3109/10715769509064030. [DOI] [PubMed] [Google Scholar]
- Arany I.; Kaushal G. P.; Portilla D.; Megyesi J.; Price P. M.; Safirstein R. L.. Cellular mechanisms of nephrotoxicity. In Clinical Nephrotoxins; Springer: 2008, 155–170, 10.1007/978-0-387-84843-3_8. [DOI] [Google Scholar]
- Pabla N.; Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Quan Z.; Gu J.; Dong P.; Lu J.; Wu X.; Wu W.; Fei X.; Li S.; Wang Y.; Wang J.; Liu Y. Reactive oxygen species-mediated endoplasmic reticulum stress and mitochondrial dysfunction contribute to cirsimaritin-induced apoptosis in human gallbladder carcinoma GBC-SD cells. Cancer Lett. 2010, 295, 252–259. 10.1016/j.canlet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Han M.-S.; Han I.-H.; Lee D.; An J. M.; Kim S.-N.; Shin M.-S.; Yamabe N.; Hwang G. S.; Yoo H. H.; Choi S.-J.; Kang K. S.; Jang H.-J. Beneficial effects of fermented black ginseng and its ginsenoside 20(S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J. Ginseng Res. 2016, 40, 135–140. 10.1016/j.jgr.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. P.; Tadagavadi R. K.; Ramesh G.; Reeves W. B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibalová L.; Šereš M.; Rusnák A.; Ditte P.; Labudová M.; Uhrík B.; Pastorek J.; Sedlák J.; Breier A.; Sulová Z. P-glycoprotein depresses cisplatin sensitivity in L1210 cells by inhibiting cisplatin-induced caspase-3 activation. Toxicol. In Vitro 2012, 26, 435–444. 10.1016/j.tiv.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Park J. Y.; Choi P.; Kim T.; Ko H.; Kim H.-K.; Kang K. S.; Ham J. Protective Effects of Processed Ginseng and Its Active Ginsenosides on Cisplatin-Induced Nephrotoxicity: In Vitro and in Vivo Studies. J. Agric. Food Chem. 2015, 63, 5964–5969. 10.1021/acs.jafc.5b00782. [DOI] [PubMed] [Google Scholar]
- Martinou J.-C.; Youle R. J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell J. F.; Billen L. P.; Bindner S.; Shamas-Din A.; Fradin C.; Leber B.; Andrews D. W. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 2008, 135, 1074–1084. 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Zha J.; Harada H.; Yang E.; Jockel J.; Korsmeyer S. J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-XL. Cell 1996, 87, 619–628. 10.1016/S0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Luo J.; Manning B. D.; Cantley L. C. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 2003, 4, 257–262. 10.1016/S1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- Kuwana H.; Terada Y.; Kobayashi T.; Okado T.; Penninger J. M.; Irie-Sasaki J.; Sasaki T.; Sasaki S. The phosphoinositide-3 kinase gamma-Akt pathway mediates renal tubular injury in cisplatin nephrotoxicity. Kidney Int. 2008, 73, 430–445. 10.1038/sj.ki.5002702. [DOI] [PubMed] [Google Scholar]
- Arany I.; Megyesi J. K.; Kaneto H.; Price P. M.; Safirstein R. L. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am. J. Physiol.: Renal Physiol. 2004, 287, F543. 10.1152/ajprenal.00112.2004. [DOI] [PubMed] [Google Scholar]
- Ma Z.-n.; Liu Z.; Wang Z.; Ren S.; Tang S.; Wang Y.-p.; Xiao S.-y.; Chen C.; Li W. Supplementation of American ginseng berry extract mitigated cisplatin-evoked nephrotoxicity by suppressing ROS-mediated activation of MAPK and NF-κB signaling pathways. Food Chem. Toxicol. 2017, 110, 62–73. 10.1016/j.fct.2017.10.006. [DOI] [PubMed] [Google Scholar]