Abstract
Mitochondrial ATP generation by oxidative phosphorylation combines the stepwise oxidation by the electron transport chain (ETC) of the reducing equivalents NADH and FADH2 with the generation of ATP by the ATP synthase. Recent studies show that the ATP synthase is not only essential for the generation of ATP but may also contribute to the formation of the mitochondrial permeability transition pore (PTP). We present a model, in which the PTP is located within the c-subunit ring in the Fo subunit of the ATP synthase. Opening of the PTP was long associated with uncoupling of the ETC and the initiation of programmed cell death. More recently, it was shown that PTP opening may serve a physiologic role: it can transiently open to regulate mitochondrial signaling in mature cells, and it is open in the embryonic mouse heart. This review will discuss how the ATP synthase paradoxically lies at the center of both ATP generation and cell death.
Keywords: ATP synthase, Bioenergetics, Electron transport chain, Embryonic heart, Mitochondria, Permeability transition pore
1. Mitochondrial Energy Production
1.1. Metabolic Substrates and Energy Supply
Oxidative phosphorylation (OXPHOS) can be defined as the oxidation of metabolic substrates by cytosolic and then mitochondrial enzymes to release energy that is transferred to ATP, which is the basic energetic currency of the cell (Fig. 1). The main metabolic substrates for all cellular processes are carbohydrates, fatty acids, and proteins. Carbohydrates are transformed into glucose and, via the glycolytic pathway, pyruvate, which enters the mitochondrion where it is decarboxylated and acetylated by pyruvate dehydrogenase to produce acetyl coenzyme A (CoA). A second source of acetyl-CoA is the β-oxidation of fatty acids in the mitochondrial matrix. Proteins can be hydrolyzed into peptides and amino acids, which are then deaminated and converted into pyruvate or acetyl-CoA. In the mitochondrial matrix, acetyl-CoA enters the tricarboxylic acid (TCA, aka. citric acid, Krebs) cycle, which generates NADH and FADH2, the substrates for the mitochondrial ETC, which is located in the mitochondrial cristae membrane.
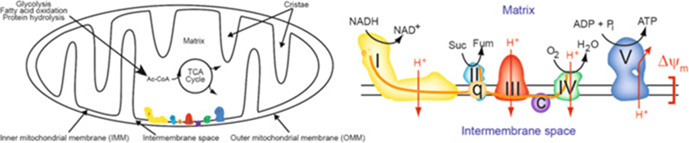
Fig. 1.
Model of oxidative phosphorylation and the electron transport chain. (Left) Macroscopic diagram of a mitochondrion and metabolic pathways. Mitochondria utilize carbons from the metabolism of glucose, fatty acids, proteins, and other metabolic pathways to feed the TCA cycle, which produces NADH. Mitochondrial structures that are indicated are as follows: the cristae, the inner mitochondrial membrane (IMM), the intermembrane space, the matrix, and the outer mitochondrial membrane (OMM). (Right) Microscopic diagram of the electron transport chain. The oxidation of NADH to NAD+ donates electrons (e−) to complex I (I, NADH-ubiquinone oxidoreductase/dehydrogenase), where they are eventually passed to ubiquinone (q, coenzyme Q). In the TCA cycle, succinate (Suc) is oxidized to fumarate (Fum) at complex II (II, succinate dehydrogenase, the electron carrier is FADH2), and electrons are passed to q. Electrons flow from q to complex III (III, ubiquinol/cytochrome c oxidoreductase/dehydrogenase) to cytochrome c (C) to complex IV (IV, cytochrome c oxidase), where they reduce O2 to water. Complexes I, III, and IV pump protons (H+) across the inner mitochondrial membrane to generate the membrane potential (Δψm) that complex V (V, ATP synthase, F1Fo ATPase) taps to synthesize ATP. The matrix and intermembrane space are indicated
1.2. The Electron Transport Chain
The mitochondrial ETC consists of five large, multi-protein complexes (Fig. 1). The proximal complexes (I, II, III, and IV) use the energy supplied by electron transport to generate an electrochemical gradient across the mitochondrial inner membrane (IMM), which complex V (also known as ATP synthase or F1Fo-ATP synthase) is used to produce ATP. The subunits of complexes I, III, IV, and V are encoded in both the nuclear and mitochondrial genome, while the four subunits of complex II are nuclear encoded (Wallace 1999).
Electrons enter the ETC at three sites: complex I, complex II, and ubiquinone (Fig. 1). Oxidation of NADH in the hydrophilic, matrix-protruding arm of complex I donates electrons that flow through a series of iron-sulfur-containing subunits to eventually reduce ubiquinone. Complex II of the ETC is also the succinate dehydrogenase enzyme of the TCA cycle, and the oxidation of succinate to fumarate reduces complex II, which then reduces ubiquinone. At least three other enzymes can also directly reduce the ubiquinone pool (Nicholls and Ferguson 2013). Ubiquinol (reduced ubiquinone) is then oxidized by complex III, which then uses these electrons to reduce cytochrome c. Cytochrome c in the intermembrane space then reduces complex IV, leading to the final redox reaction where complex IV reduces oxygen to water.
Energy released from the flow of electrons is used by complexes I, III, and IV to pump protons against their electrochemical gradient into the intermembrane space (Fig. 1). This gradient is called the electrochemical proton gradient (ΔμH, proportional to the proton motive force, Δp) that is the product of the electrical (Δψm) and proton (ΔpH) gradients across the IMM. Maintenance of this gradient is essential to OXPHOS (see below) and requires high capacitance of the inner mitochondrial membrane so that neither Δψm nor ΔpH are dissipated. Mitochondria prevent such leaks by tightly regulating any leak currents as well as the many ion channels and transporters that reside in the IMM (reviewed in Szabo and Zoratti 2014).
The major source of mitochondrial reactive oxygen species (ROS) is the ETC (Fig. 1), although other enzymes may play lesser roles. Slipping of electron flowing through complexes I and III can form superoxide anions in the matrix, while complex III can also release superoxide into the intermembrane space (Nickel et al. 2014). In addition, it was recently suggested that complex II can also produce ROS (Quinlan et al. 2012).
1.3. Electron Transport Chain Assembly and Respirasomes
The four proximal members of the ETC are large, multi-subunit protein complexes that must be assembled to function efficiently. Each requires additional assembly factors, and their assembly may be controlled by the assembly of the other complexes, but the exact mechanisms that regulate the assembly of each remain unclear. Assembly proceeds through sub-complexes, monomers, dimers, and higher-order homo- and hetero-oligomers (Fig. 2).
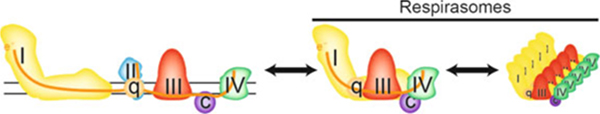
Fig. 2.
Electron transport chain assembly into respirasomes. When the individual complexes of the ETC transfer electrons by random collision, electron flux is less efficient (left). However, the assembly of complexes I, III, and IV with ubiquinone and cytochrome c into supercomplexes called respirasomes greatly increases the efficiency of electron transfer and oxygen consumption
An example of this assembly is complex I. In mammals, it is the largest and most complicated ETC complex and contains at least 44 subunits that combine to form a membrane-embedded hydrophobic domain and a hydrophilic domain containing a chain of the redox centers that protrudes into the matrix (Hirst 2013). It remains unclear how the assembly of these subunits into a functional complex I monomer is regulated, but additional assembly factors/proteins are required (Mimaki et al. 2012). In addition, its assembly can be stabilized if it is co-assembled into respirasome supercomplexes (Fig. 2) (Calvaruso et al. 2012).
Assembly of respirasomes adds an additional layer of complexity to ETC activity. Technical advances and the development of blue and clear-native electrophoresis show that complexes I, III, and IV (along with ubiquinone and cytochrome c) have the tendency to form respiratory active supercomplexes (Dudkina et al. 2008; Wittig et al. 2007). The formation of respirasomes appears to confer a bioenergetic advantage, because the close proximity of the complexes increases the efficiency of electron transfer (Cogliati et al. 2013; Genova and Lenaz 2013). Respirasomes are functionally active even after purifying them by blue native electrophoresis (Acin-Perez et al. 2008). It appears that complexes III and IV begin the process of the respirasome formation followed by the addition of partially assembled and still de-active complex I (Moreno-Lastres et al. 2012). Not only does respirasome formation increase ETC efficiency, but it also decreases ROS production, perhaps due to the more efficient flow of electrons through the chain (Maranzana et al. 2013). However, it is not clear whether respirasomes are permanent structures (solid model) or form depending on the bioenergetic requirements of the cell (plastic model) (Cogliati et al. 2013; Genova and Lenaz 2013; Lapuente-Brun et al. 2013).
1.4. ATP Synthase
The proximal four complexes of the ETC accept and transfer electrons from NADH and the TCA cycle, and subsequent proton translocation by complexes I, III, and IV from the mitochondrial matrix into the intermembrane space creates the proton motive force (Δp) to drive ATP synthesis by ATP synthase (complex V or F1Fo ATP synthase; Jonckheere et al. 2012). The monomer of the mammalian ATP synthase is a ~600 kDa protein complex of 15 subunits and consists of 2 functional units (Fig. 3): the membrane-embedded Fo subunit and the matrix facing F1 subunit. In bovine heart, Fo contains a ring of 8 very hydrophobic c-subunits and the subunits a, b, e, f, g, and A6L. A central stalk composed of the subunits δ, ε, and γ connects the c-subunit ring to the catalytic F1 unit, which consists of a hexamer of alternating α and β subunits, where ATP synthesis and hydrolysis occur. Finally, a lateral stalk or stator containing the subunits b, d, and F6 and the oligomycin-sensitivity conferring protein (OSCP) connects the lateral portion of Fo to the top of F1. Movement of protons between the c- and a-subunit causes rotation of the c-subunit ring and provides the energy necessary for F1 to synthesize ATP (Walker 2013).
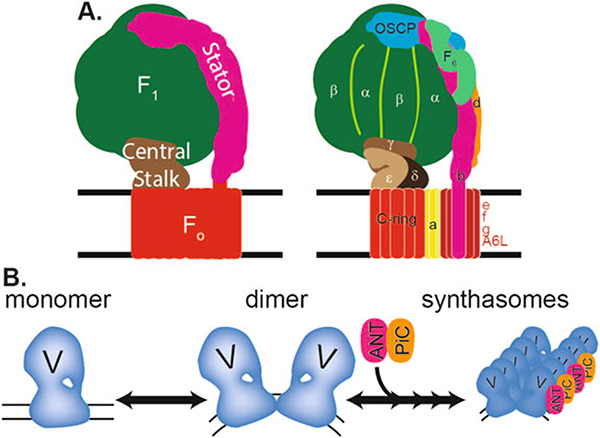
Fig. 3.
ATP synthase structure and assembly into synthasomes. (a) Diagrams of structure of an ATP synthase monomer. (Left) The structural components of the complex are F1 (green), Fo (orange), central stalk (brown), and stator (pink). (Right) The position of the 15 subunits in the complex is labeled (adapted from Dickson et al. 2006). The F1 component contains the α- and β-subunits. The Fo component contains the a-, b-, c-, e-, f-, g-, and A6L-subunits. The F1 and Fo components are connected by the central stalk (δ-, ε-, and γ-subunits) and the stator (b-, d-, F6-, and OSCP-subunits). (b) ATP synthase monomers combine to form dimers, which then assemble with adenine nucleotide translocase (ANT) and the phosphate carrier (PiC) into supercomplexes called synthasomes that increase the efficiency of energy (ATP) production and energy transfer into the sarcoplasm. Ribbons of synthasomes likely increase this efficiency and mold the cristae into tubular structures
ATP synthase monomers are enzymatically active, but recent studies show that in vivo the ATP synthase forms dimers and ribbons of even-numbered oligomers (Fig. 3) (Bornhovd et al. 2006; Davies et al. 2011; Wittig and Schagger 2008). Oligomerization of ATP synthase has been shown to shape the cristae membranes, and this may confer a physiologic advantage (Cogliati et al. 2013; Davies et al. 2011). For example, ATP synthase oligomerization is essential to build and to maintain the mitochondrial membrane potential and local proton charge to increase ATP synthase activity (Bornhovd et al. 2006). Since the mitochondrial ATP synthase harbors the PTP (see below), elucidating the functional regulation of monomers, dimers, and oligomers will provide important knowledge of how the ATP synthase transforms into the PTP (see below for detailed discussion).
The ATP synthase also forms supercomplexes with the mitochondrial creatine kinase (mtCK), adenine nucleotide translocase (ANT), and phosphate carrier (PiC) (Fig. 3). These “synthasomes” connect the machinery required for ATP generation (ATP synthase) to that of ADP/ATP and phosphate exchange (ANT, PiC) and energy transfer to the cytoplasm (ANT, mtCK), therefore creating a regulatory unit where energy production and exchange pathways meet (Chen et al. 2004; Saks et al. 2012).
2. The Permeability Transition Pore
The permeability transition was first described in the 1950s as an acute swelling and uncoupling of mitochondria when exposed to high calcium concentrations and phosphate, oxidative stress, or other conditions (Fig. 4; for more details of the history of the PT, see Bernardi 2013). The term permeability transition was introduced by Haworth and Hunter, who first described the pharmacological properties of the permeability transition pore (PTP) (Haworth and Hunter 1979; Hunter and Haworth 1979a, b). Their work and that of many others indicated that during PT, a large pore/ion channel opens to allow molecules of up to 1.5 kDa across the IMM.
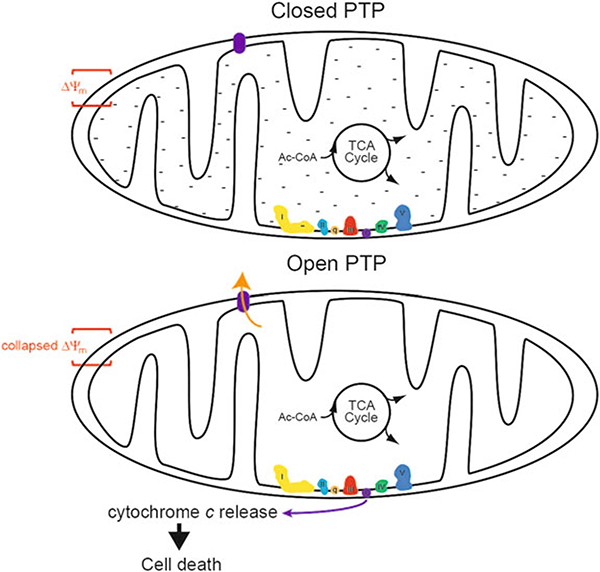
Fig. 4.
The mitochondrial permeability transition pore. Under most conditions, the PTP is closed (Upper), maintaining the high capacitance of the IMM, the relative negative charge of the matrix, and the mitochondrial membrane potential (Δψm). When the PTP is open (Lower), the capacitance of the IMM falls, the negative charge of the matrix dissipates, and Δψm collapses. This can also lead to release of cytochrome c, which, along with other factors, initiates cell death pathways. Note also that transient PTP opening can occur, and this does not lead to cell death and may serve a physiologic function
2.1. Physiologic Consequences of the Permeability Transition
For a long time, the opening of the PTP was considered to be a traumatic event leading to cell death (Fig. 4). Increased permeability of the IMM causes mitochondrial swelling due to the high osmotic pressure of the matrix, and this swelling leads to rupture of the outer mitochondrial membrane (OMM) and the release of proteins (cytochrome c, apoptosis inducing factor) from the mitochondria that push cell death pathways past the point of no return (Petit et al. 1997). In addition, the energetic failure due to uncoupling of the IMM and subsequent reversal of the ATP synthase reaction to consume ATP is also thought to play a role in cell death. Over the years, the PTP has been extensively studied for its role in ischemic injury in brain, heart, and other organs as well as in neurodegenerative conditions (Bonora et al. 2015). In the heart, data suggest that opening of the PTP during early reperfusion after ischemia is a harmful event that precipitates further damage to the myocardium (Griffiths and Halestrap 1993). Additional data suggest that cell death pathways may regulate or be regulated by the PTP, and the general consensus now is that uncontrolled opening of the PTP leads to cellular necrosis and, perhaps, apoptosis (Baines 2011; Bernardi 2013).
However, more recent data suggest that transient PTP opening can serve a physiologic purpose. Transient opening was described in the late 1990s (Huser and Blatter 1999; Ichas and Mazat 1998; Jouaville et al. 1998; Petronilli et al. 1999). In the heart, transient opening of the PTP during preconditioning can be protective, thus serving a physiological role even during injury (Hausenloy et al. 2004). This transient PTP opening in striated muscle mitochondria may be associated with transient increases in ROS (so-called superoxide flashes) which are proposed to serve as a signaling mechanism (Wang et al. 2008, 2012) Furthermore, it has been hypothesized that transient opening of the PTP releases mitochondrial matrix Ca2+ to maintain mitochondrial homeostasis (Elrod et al. 2010), although this function of the PTP has recently been questioned (De Marchi et al. 2014).
In the embryonic heart, the PTP appears to be open at early stages of myocyte differentiation (Beutner et al. 2014; Hom et al. 2011). The early heart derives most of its energy from anaerobic glycolysis (Porter et al. 2011), and the activity of the ETC is low (Beutner et al. 2014). At an early stage of development (mouse embryonic day 9.5), myocytes have an open PTP, low Δψm, and high levels of ROS, and closure of the PTP using CsA increases Δψm and decreases ROS, leading to further myocyte differentiation (Hom et al. 2011). These changes in PTP activity are associated with increased assembly and activation of the ETC at or after embryonic day 11.5 (Beutner et al. 2014).
2.2. Defining the Permeability Transition Pore
In the late 1980s, patch-clamping experiments defined the biophysical, electrophysiological, and pharmacological properties of the PTP and began to establish criteria for defining PTP activity (Table 1) (Kinnally et al. 1989; Petronilli et al. 1989; Sorgato et al. 1987). In 1987, the first patch-clamping recordings of liver mitochondrial inner membrane isolated from cuprizone-fed mice demonstrated an ~100 pS channel, although its relationship to the PTP remains unclear (Sorgato et al. 1987). Then, in 1989, a putative PTP was recorded by patch-clamping mitoplasts (Petronilli et al. 1989). Channel activity occurred at positive potentials of the patch pipette and was found either in whole organelle mode or in single channel recordings in the organelle-attached configuration. Gating was less common at negative potentials and prolonged openings, and fewer subconductance states at negative patch potentials were observed. The activity was slightly anion selective and had multiple conductance states ranging from 30 pS to a peak conductance of 1.3 nS, with the lower conductances attributed to substates of the larger channel openings. The mitochondrial multi-conductance channel (MCC) in mouse liver mitoplasts was also described in 1989 to have similar properties (Kinnally et al. 1989). During recordings, this channel initially had low activity that progressively increased with time. Rectification was observed at both positive and negative potentials, and channel activity was weakly cation selective with multiple conductances ranging from 10 to 1,000 pS.
Table 1.
Electrophysiological properties of the PTP and ATP synthase
| Preparation | Conductance | Selectivity | Rectification | Reference |
|---|---|---|---|---|
| PTP | ||||
| I MM, patch clamping, induced by cuprizone | ~100pS | Slight anion selectivity | Not determined | Sorgato et al. (1987) |
| Mitoplast. patch clamping | Multiple conductances (30 pS to 1.3 nS) | Slight anion selectivity | Positive rectification at positive potentials and negative rectification at negative potentials | Petronilli et al. (1989) |
| Mitoplast, patch clamping (mitochondrial muhiconductance channel) | Multiple conductances (10 pS to 1 nS) | Slight cation selectivity | Variable rectification at positive and negative potentials | Kinnally et al. (1989) |
| ATP synthase | ||||
| ATP synthase dimers in lipid bilayers | Peak conductance of 1-1.3 nS with multiple subconductance states | Not determined | Not determined | Giorgio et al. (2013) |
| ATP synthase monomers in liposomes, patch clamping | Multiple conductances (-800 pS) | Not determined | Not determined | Alavian et al. (2014) |
| Purified ATP synthase c-subunits in lipid bilayers | Multiple conductances (15 pS to 2 nS) | Relative cation selectivity | Not tested | Azarashvili et al. (2014) |
| Purified ATP synthase c-subunits in liposomes, patch clamping | Multiple conductances (lOOpS to 2 nS) | Slight cation selectivity | Slightly negative rectification | Alavian et al. (2014) |
These studies also delineated the activators and inhibitors that are used today to investigate the PTP (Table 2). The PTP can be induced by elevated mitochondrial matrix Ca2+, ROS, inorganic phosphate (Pi), fatty acids, and intracellular acidification. In contrast, it is inhibited by adenine nucleotides, divalent cations (Mg2+ > Mn2+ >Ba2+ >Sr2+), and acidification of the matrix. Early in these investigations, it was found that cyclosporine A (CsA), an immunosuppressant that was known to inhibit cyclophilin proteins, inhibits these large conductance channels in the IMM by binding to a site in the mitochondrial matrix (Szabo and Zoratti 1991). Sub-micromolar concentrations of CsA inhibited a Ca2+-activated, large conductance (1.3 nS) channel, but not the lower (~100 pS) substate conductances, suggesting that this activity might be due to a separate ion channel (Szabo and Zoratti 1991). In addition, PTP activity could be induced by atractyloside (an inhibitor of ANT; see below) and phenylarsine oxide (an oxidizing agent) and inhibited by bongkrekic acid (an inhibitor of ANT; see below) (Lenartowicz et al. 1991). It should be emphasized that many of these agents, such as CsA, alter the amount of matrix Ca2+ required to open the PTP; thus, they are said to sensitize or desensitize the PTP to Ca2+, but the mechanisms of this sensitization remain unknown.
Table 2.
Activators and inhibitors of the PTP
| Activators | Reference | Inhibitors | Reference |
|---|---|---|---|
| Acidification, intracellular | Gendron et al. (2001) | Acidification, matrix | Haworth and Hunter (1979) and Nicolli et al. (1993) |
| Atractyloside | Hunter and Haworth (1979a) | ATP/ADP/AMP | Haworth and Hunter (1979), Hunter and Haworth (1979a), and Hunter and Haworth (1979b) |
| Calcium | Hunter et al. (1976) | Bongkrekic acid | Hunter and Haworth (1979a) |
| Fatty acids | Hunter et al. (1976) | Cyclosporine A | Crompton et al. (1988) and Fournier et al. (1987) |
| ROS | Brookes et al. (2004) | Mg2+ and other divalent cations | Hunter and Haworth (1979b) |
| Inorganic phosphate | Hunter et al. (1976) | ||
| Phenylarsine oxide | Lenartowicz et al. (1991) | ||
| Polyhydroxybutyrate | Elustondo et al. (2013) | ||
| Polyphosphate | Seidlmayer et al. (2012) |
2.3. The Search for the Identity of the Permeability Transition Pore
Since the electrophysiological studies of the late 1980s, much effort has been made to define the molecular identity of the PTP. A long list of candidates has been proposed, but subsequent studies demonstrated that many of these factors were either not related to the PTP or merely regulated its function (summarized in Table 3). Potential PTP candidates included the targets of common inducers and inhibitors of the PTP, such as ANT and cyclophilin D (CypD). Additional proteins that were proposed to be the PTP because their function seemed to control its activity include hexokinase (HK), mtCK, PiC, TSPO (translocator protein of 18 kDa, previously called the peripheral benzodiazepine receptor), and VDAC (voltage-dependent anion channel). For the most part, these proteins were eliminated as candidates to form the pore of the PTP by experiments where their expression was deleted (summarized in Table 3). Finally, it has also been hypothesized that unfolded proteins in the IMM might form a nonspecific, high-conductance pore (He and Lemasters 2002), and other recent reports suggest that polyphosphate chains and polyhydroxybutyrate may, when in the presence of elevated Ca2+, regulate or participate in the formation of the PTP (Elustondo et al. 2013; Seidlmayer et al. 2012).
Table 3.
Proposed PTP and PTP regulatory molecules
| Protein/complex (Abbreviation, gene) | Evidence for role in PTP | Probable role in the PTP | |
| For | Against | ||
| Adenine nucleotide translocase (ANT, Slc25a4-6) | PTP-like activity of ANT in bilayers (Ruck et al. 1998), effect of ADP. ATP, ATR and bongkrekic acid on PTP activity (Haworth and Hunter 1979; Hunter and Haworth 1979a, b) | Deletion of ANTI and ANT2 (Slc25a4 and Slc25a5) does not eliminate PTP activity, but decreases its sensitivity to Ca2+ (Kokoszka et al. 2004) | Regulates PTP activity, perhaps through interactions with ATP synthase as part of the synthasome |
| ATP synthase | Binds to or associates with PTP regulatory molecules (ANT, CypD, mtCK. PiC; see text). PTP activity found in ATP synthase dimers and the C-subunit ring (Alavian et al. 2011; Giorgio et al. 2013) | The exact mechanisms of PTP regulation within ATP synthase remain unknown | Although ATP synthase dimers have been proposed to create the pore of the PTP. more evidence exists suggesting that the F0 C-ring does this, but how this is regulated remains unresolved |
| Creatine kinase, mitochondrial (mtCK, Cknit1A, 1B. 2) | Knockout of Ckmtl increases susceptibility to PTP (Datler et al. 2014) | Regulatory, connects energy-producing and energy- consuming mechanism (Saks et al. 2012) | |
| Cyclophilin D (CypD, Ppif) | Inhibition of CypD with CsA closes the PTP | Deletion of CypD does not eliminate PTP (Baines et al. 2005; Basso et al. 2005; Nakagawa et al. 2005; Schinzel et al. 2005) | Regulatory, binds to ATP synthase at OSCP but exact mechanism unknown |
| Hexokinase (Hk1-4) | Phosphorylation of glucose by mitochondrial bound HK 1 and 11 inhibits PTP (Azoulay-Zohar et al. 2004) | Regulatory, binds to VDAC, creates micro-compartments with high capacitive coupling to favor ADP/ATP exchange (Vyssokikh and Brdiczka 2003) | |
| Phosphate carrier (PiC, Slc25a3) | Binds to ANT and CypD (Leung et al. 2008) | Deletion of PiC does not eliminate PTP activity, but decreases its sensitivity to Ca2+ (Gutierrez-Aguilar et al. 2014: Kwong et al. 2014) | Regulates PTP activity, perhaps through interactions with ATP synthase as part of the synthasome |
| Polyphosphate and Polyhydro xybuty rate | The naturally occurring molecules increase PTP (Elustondo et al. 2013; Seidlmayer et al. 2012; Stotz et al. 2014) | Regulates PTP activity, perhaps through interactions with ATP synthase and the C-ring | |
| Spastic paraplegia 7 (Spg7) | Binds to CypD and VDAC. and its knockdown inhibits the PT (Shanmughapriya et al. 2015) | Regulates the PTP through currently unknown mechanisms. Its role in pore formation is unknown | |
| Translocator protein of 18 kDa (TSPO. Tspo) | TSPO was isolated with ANT and VDAC (McEnery etal. 1992) | Deletion of TSPO does not eliminate PTP activity (Sileikyte et al. 2014) | Probably plays no role in creating the PTP |
| Unfolded proteins | He and Lemasters (2002) | Unknown | |
| Voltage-dependent anion carrier (VDAC, Vdac 1-3) | Pathway for cytochrome c release (Shimizu et al. 2000) | Deletion of VDAC1, VDAC2, and VDAC3 does not eliminate PTP activity (Baines et al. 2007) | Interaction with HK or mtCK keeps VDAC in anion- selective state (Vyssokikh and Brdiczka 2003) |
Two particular IMM translocators (ANT and PiC) were purported to form the PTP. In the 1990s, ANT was the prime candidate (Beutner et al. 1998; Crompton et al. 1998; Halestrap and Davidson 1990), because the PTP activator atractyloside/carboxyatractyloside and the PTP inhibitor bongkrekic acid both inhibit the ADP/ATP translocase activity of ANT. Interestingly, these agents have different effects on the conformation of ANT (Brustovetsky and Klingenberg 1996; Haworth and Hunter 2000). In addition, several papers showed that the ANT can form channels with properties of the PTP, although issues with the purity of these preparations may have led to this conclusion (Brustovetsky and Klingenberg 1996; Ruck et al. 1998). Later, the PiC was proposed as an integral component of the PTP due to its binding to two proteins that regulate the PTP, ANT, and CypD (Leung et al. 2008). However, more recent studies have demonstrated by genetic deletion of ANT1 and 2 and of the PiC that these proteins are not essential to PTP formation, although these studies still support regulatory roles for these translocators (Gutierrez-Aguilar et al. 2014; Kokoszka et al. 2004; Kwong et al. 2014) (Table 3).
CypD, a peptidyl-prolyl cis/trans isomerase, is the most important regulator of the PTP and was a candidate to create the pore. However, deletion of CypD in mice did not eliminate PTP activity, although this did decrease the sensitivity of the PTP to Ca2+ and oxidative stress (Baines et al. 2005; Basso et al. 2005; Elrod and Molkentin 2013; Nakagawa et al. 2005; Schinzel et al. 2005). Although it is well known that CypD is the target of CsA, which is known to prevent its binding to target proteins, how this regulates the PTP and to what protein CypD binds to confer this activity remains controversial (see below). Interestingly, it was recently found that CypD binds to SPG7 (spastic paraplegia 7), an IMM protein that may link CypD to VDAC in the OMM (Shanmughapriya et al. 2015). SPG7 appears to be an important regulator of the PTP, but its role in creating the pore of the PTP remains untested.
3. ATP Synthase and the Permeability Transition Pore
3.1. ATP Synthase and the Permeability Transition Pore Interact
Metabolic pathways are known to regulate the PTP. For example, glycolysis in the cytoplasm can regulate PTP activity via the binding of hexokinase II to the OMM (Pasdois et al. 2013). In addition, since the ETC proteins make up the majority of the proteins in the IMM, it is logical to assume that the PTP is either physically associated with the ETC or derived from its components. In fact, electron transport chain activity regulates the PTP, as an increase in mitochondrial energization (Δψm) inhibits the PT, while de-energization/depolarization (a fall in Δψm) enhances it (Di Lisa et al. 2011; Haworth and Hunter 1979; Hunter and Haworth 1979a, b). Furthermore, inhibition of complex I using rotenone or metformin can inhibit the PTP when CypD is present at low levels, and it was proposed that CypD may bind to complex I (Li et al. 2012).
However, recent evidence has suggested an interaction between ATP synthase and the PTP. Many of the proteins that are known to regulate the activity of the PTP can also bind directly or indirectly to ATP synthase and/or CypD. First, CypD can bind directly to ATP synthase at the OSCP (oligomycin-sensitivity conferring protein) subunit (Giorgio et al. 2009, 2010). Second, CypD can bind to Bcl-2 (Eliseev et al. 2009), while Bcl-XL, an anti-apoptotic member of the Bcl-2 family, interacts with the β-subunit of ATP synthase and increases the efficiency of ATP production by decreasing a leak current in the IMM (Alavian et al. 2011; Chen et al. 2011).
Third, CypD binds to ANT and the PiC and disruption of this association affects PTP activity (Leung et al. 2008; Woodfield et al. 1998). This interaction is very interesting. As discussed above, ANT and the PiC interact with ATP synthase in synthasomes that also contain mtCK, which binds to ANT (Beutner et al. 1998; Leung et al. 2008; Saks et al. 2012; Vyssokikh et al. 2001; Woodfield et al. 1998). Furthermore, the interaction between ANT and VDAC may confer indirect associations between synthasomes and both HK and TSPO in the OMM (McEnery et al. 1992).
3.2. The Permeability Transition Pore Lies Within or Around ATP Synthase
In the last 2 years, more direct evidence supports the idea that the pore of the PTP is associated with ATP synthase (Alavian et al. 2014; Azarashvili et al. 2014; Bonora et al. 2013; Giorgio et al. 2013). In particular, work from the Bernardi group suggests that the PTP forms in the membrane surrounding dimers of ATP synthase (Giorgio et al. 2013). To extend their work showing that CypD binds to the stator of ATP synthase (Giorgio et al. 2009), they showed that this binding is specifically at the OSCP subunit and that decreasing the expression of OSCP decreases the association of CypD with ATP synthase (Giorgio et al. 2013). Furthermore, they demonstrated that a novel inhibitor of ATP synthase that binds to OSCP, benzodiazipine-423, inhibits the interaction of OSCP and CypD and, like CypD, opens the PTP in response to Ca2+. These data suggested that ATP synthase and in particular OSCP are somehow associated with and control the PTP. They also showed that conditions that promote ATP hydrolysis by ATP synthase decreased the sensitivity of the PTP to Ca2+ compared to ATP synthesis and that OSCP may control this Ca2+ sensitivity.
The Bernardi group purified ATP synthase monomers and dimers from native gels, incorporated these into lipid bilayers, and tested for PTP ion channel activity. It is important to note that monomer and dimer preparations contained no associated CypD, ANT, or VDAC, but that purified dimers did contain complexes I and III. No conductance was reportedly observed in monomer preparations, but dimers exhibited conductances of 1–1.3 nS with multiple subconductance states (Table 1). Conductance required the presence of Ca2+ and Pi, was induced by benzodiazepine-423, was inhibited by Mg2+ and adenine nucleotides, and was not affected by typical PTP sensitizing (atractyloside, phenylarsine oxide) and inhibiting (CsA, bongkrekic acid) agents. Additional data from this group suggested that dimerization was important for PTP formation, because deletion of the e- and d-subunits of yeast ATP synthase decreased PTP opening (Carraro et al. 2014). Based on these data, the authors proposed that the PTP forms at the membrane interface of the dimers and is perhaps related to changes in the lipid environment in that region upon PTP induction (Fig. 5a) (Bernardi 2013), but no further information has been published that clarifies this hypothesis.
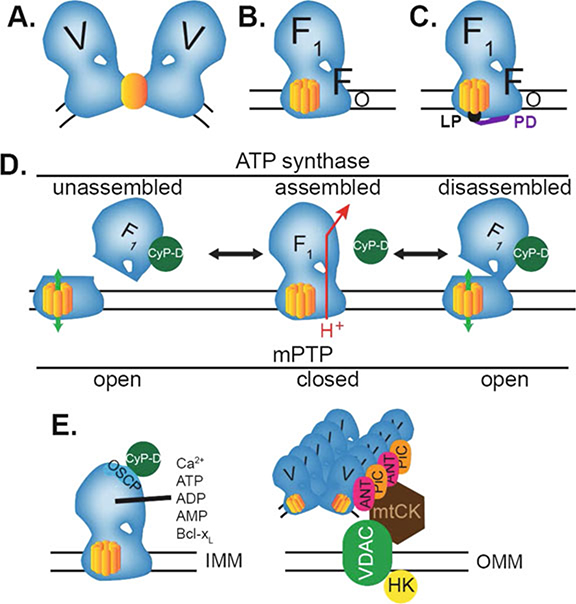
Fig. 5.
Models of the permeability transition pore derived from ATP synthase. (A–C) Three current models of the PTP as part of ATP synthase. In (A), the PTP lies within the membrane between a dimer of ATP synthase (V, modified from (Bernardi 2013). In (B), the PTP is the C-ring of the Fo component of ATP synthase. In (C), the lipid plug (LP) in the central pore of the C-ring is controlled by the p-side density (PD) to regulate C-ring conductance. (D) For ATP synthase assembly (left), F1 is added to the C-ring and other components of Fo. Assembly (center) allows the flow of protons (H+) between the C-ring and the stator, causing rotation of the C-ring, which provides energy for F1 to synthesize ATP. In this model, binding of CypD to ATP synthase during assembly promotes the presence of unassembled C-rings that create the open mPTP in the embryonic heart (left). Assembly and pore formation are reversible (double arrows), and CypD also causes disassembly of ATP synthase to create the pore in mature cells (right). (E) Control of PTP opening by CypD occurs due to its binding to OSCP, while Ca2+, adenine nucleotides (ATP, ADP, and AMP), and Bcl-xL may control PTP by binding to ATP synthase at other sites. Other proteins may regulate the PTP via the assembly of ATP synthase, ANT, and PiC into synthasomes that associate with mtCK in the intermembrane space, VDAC in the OMM, and HK attached to the OMM
3.3. The C-Ring of ATP Synthase Creates the Permeability Transition Pore
The data presented in the last two sections suggest that ATP synthase is related to the PTP in some way, but the exact molecular identity of the pore is not defined in these studies. However, additional reports in recent years suggest that the pore of the PTP lies within the c-subunit, a major membranous component of ATP synthase. Membranous pores are usually formed by integral membrane proteins, but, as outlined above, the only membrane-delimited IMM proteins (ANT, PiC) that have been proposed to form the PTP were not required for PTP in genetic deletion models. The ATP synthase subunits a, b, and c are highly hydrophobic membrane proteins and evolutionally conserved, making them candidates to form the PTP. However, the a-subunit is not required for the PTP, as it is absent in ρ0 cells that lack mitochondrial DNA, but do undergo PT (Masgras et al. 2012).
Attention has focused on the c-subunit because the c-subunits form a ring in the membrane and have ion-conducting properties. The known C-ring structures consist of 8 (bovine heart) and 10–15 (bacteria) c-subunits, and bacterial C-rings have a torus shape with a central hole (Pogoryelov et al. 2007). In the late 1990s, reconstitution experiments by the McGeoch laboratory showed that purified mammalian c-subunits have ion channel activity (McGeoch and Guidotti 1997; McGeoch et al. 2000; McGeoch and Palmer 1999). Exposure of these purified subunits to water induces a conformational change whereby a channel with a diameter of 3 nm (McGeoch and McGeoch 2008), close to the estimated size (2.3 nm) of the PTP pore, is formed (Crompton and Costi 1990). Finally, c-subunit homologues in the VO component of vacuolar H+-ATPases line a water-accessible pore structure and are involved in creating gap junctions between cells (Harrison et al. 2003; Jones et al. 1995; Peters et al. 2001).
A recent study published evidence in 2013 that the c-subunit was important for the PT (Bonora et al. 2013). When all three mammalian isoforms of the c-subunit (ATP5G1, ATP5G2, ATP5G3) were deleted in HeLa cells using siRNA, there was no effect on ATP production, as these cells are glycolytic. However, depletion of c-subunits prevented ionomycin-induced opening of the PTP, mitochondrial fragmentation, oxidative stress-induced mitochondrial depolarization, and release of cytochrome c. Overexpression of ATP5G1 had the opposite effect on PTP. Under conditions of Ca2+ overload (ionomycin) or oxidative stress, c-subunit depletion prevented cell death in HeLa cells and attenuated glutamate-induced cell death in primary cultures of cortical neurons.
PTP-like activity has been observed using mitochondrial proteins purified by chloroform/methanol extraction. In 2005, the Pavlov lab attributed such activity to polyhydroxybutyrate, although they later concluded that this preparation probably contained the c-subunit (Elustondo et al. 2013). In addition, work from the Saris lab over the last 15 years suggests pore-forming activities of the c-subunit. In the early 2000s, they isolated a phosphorylated form of the c-subunit from liver mitochondria and found that it induced, while a c-subunit antibody inhibited, the PT in isolated mitochondria and that dephosphorylation of this protein was associated with opening of the PTP (Azarashvili et al. 2002). Furthermore, this isolated fraction increased conductance of lipid membranes, an effect that was blocked by c-subunit antibodies (Azarashvili et al. 2002). These results were further explored in 2014, when they demonstrated that dephosphorylated c-subunit was more effective than phosphorylated c-subunit at inducing PT and that the c-subunit could bind Ca2+ (Azarashvili et al. 2014). Furthermore, the conductance of the c-subunit in lipid bilayers was found to be 15 pS to 2 nS, consistent with previous recordings of the PTP (Table 1).
Starting in 2004, the Jonas laboratory began to study the molecular identity of the PTP when they found that overexpression of the anti-apoptotic protein, Bcl-xL, in neurons increased cytoplasmic ATP levels yet decreased oxygen consumption, consistent with the idea that Bcl-xL overexpression increases mitochondrial bioenergetic efficiency (Alavian et al. 2011; Chen et al. 2011). Furthermore, Bcl-xL was found to bind to the β-subunit of ATP synthase to increase ATP synthesis, and its depletion or inhibition increased an IMM leak current in patch-clamping experiments (Alavian et al. 2011).
These data suggested that a regulated leak current lay within the ATP synthase and the Jonas and Porter laboratories began to study this further (Alavian et al. 2014). Patch clamping of purified ATP synthase subunits reconstituted in liposomes revealed that extremely pure c-subunit preparations with no other associated proteins formed channels in proteoliposomes. These channels had multi-conductance states that ranged from 100 pS to 2 nS, were slightly cation selective (Table 1), and were inhibited by c-subunit antibodies and high concentrations of ATP but not CsA. Additional patch clamp studies were performed on purified ATP synthase monomers that contained no CypD. In contrast to results reported by Giorgio et al. (Giorgio et al. 2013), these studies revealed that calcium and purified CypD induced an ~1 nS conductance (with multiple subconductance states, Table 1). Similar experiments, using mitoplasts, submitochondrial vesicles (purified IMMs), and urea-stripped submitochondrial vesiicles stripped of non-membrane-embedded proteins with urea, revealed multiple levels of regulation of this C-ring channel. The conductance in mitoplasts and submitochondrial vesicles had the expected properties of the PTP – activation by Ca2+ and inhibition by CsA. However, when the c-subunit is stripped of non-membrane-embedded proteins, submitochondrial vesicles were neither responsive to Ca2+ nor inhibited by CsA, suggesting that regulatory components were peripheral to the c-subunit and were removed by urea. Interestingly, the conductance associated with the C-ring could be inhibited with specific purified ATP synthase F1 components.
Additional experiments revealed that the C-ring expands upon exposure to calcium to open the PTP and that mutation of the c-subunit to make the C-ring larger increased its conductance and enhanced PTP opening and associated cell death in neurons. In contrast, depletion of the c-subunit attenuated PTP opening and cell death.
Combined, these data suggest a model whereby in the intact synthase complex the central stalk and F1 of the ATP synthase inhibit opening of the c-subunit channel; in contrast the C-ring of ATP synthase is exposed upon induction of the PTP (Fig. 5b, d). Release of the C-ring from ATP synthase was demonstrated by exposing mitochondria to calcium and immunocapturing the ATP synthase using an antibody directed at the F1 (Alavian et al. 2014). These studies showed that calcium exposure indeed destabilizes, in a CypD-dependent manner, the connection between the stalk and the c-subunit, disrupting protein/protein interaction between the c-subunit and F1. Furthermore, this dissociation was prevented when CsA or ADP was added to prevent PTP opening (Alavian et al. 2014).
4. A Current Model of the Permeability Transition Pore
Overall, the data presented above suggest a model, in which the PTP is contained within the C-ring of ATP synthase (Fig. 5b). In this model, induction of the PT disrupts ATP synthase to unmask the face of the C-ring to the matrix and expose its central pore. As molecules flow through this pore, the IMM depolarizes and the ETC uncouples (Figs. 4 and 5d). The PTP can be regulated by CypD due to its binding to OSCP on ATP synthase and by Ca2+, adenine nucleotides, and Bcl-xL at the level of ATP synthase (Fig. 5e). Furthermore, control of ATP synthase assembly and PTP opening by other proteins known to regulate this process (ANT, PiC, SPG7, mtCK, VDAC, and HK) occurs at the level of the synthasome where the ATP synthase forms connections to the latter three proteins in the intermembrane space or at the OMM.
However, this model does not explain all of the current data, and many other issues must be resolved to refine this model. The first issue is whether it is logical and possible for ATP synthase to disassemble and expose a pore within its central structure when the mitochondrion faces stressful situations. Experimental conditions in which this occurs are consistent with known matrix calcium concentrations; the 60 μM Ca2+ required to open the PTP is well within the range of physiological Ca2+ concentrations found within the mitochondrial matrix or adjacent to the mitochondria in Ca2+ microdomains (Csordas et al. 2001; Rizzuto et al. 2000, 2009; Rizzuto and Pozzan 2006; Schneggenburger and Neher 2005). In addition, the opening of the PTP is reversible (see above), so under physiologic conditions, PTP opening might only occur as a transient exposure of the C-ring pore with subsequent reconstitution, as has been observed for ATP synthase (Pedersen et al. 1978).
The inhibition of the C-ring pore by F1 components of ATP synthase (Alavian et al. 2014) may play a role in transient pore opening. Alternatively blockade of the PTP by F1 components may prevent PTP opening when ATP synthase becomes disassembled or has not yet assembled as it is forming (Havlickova et al. 2010), but further work will need to be done to address this issue. However, under certain conditions, this disassembly may become irreversible and safety mechanisms may fail, thus causing pathophysiologic PT and rupture of the OMM. Finally, it is also important to note that opening of only a few C-rings/PTPs would be required to depolarize the IMM, as this pore has such a large conductance, so even a low probability event of ATP synthase disruption could lead to devastating consequences for the mitochondrion.
Structural studies suggest that the center of the C-ring contains lipids that would prevent the observed conductance. However, a recent cryo-electron microscopic study suggests that this lipid plug lies close to the matrix side of the ring center and this may be attached to a portion of ATP synthase composed of the e-subunit (Zhou et al. 2015). Furthermore, a recent review of this work suggested that this p-side density (or death finger) could pull the lipid plug toward the matrix, opening the center of the C-ring to allow conductance (Fig. 5c) (Gerle 2016). Additional experiments must be done to explore this potential mechanism of C-ring activity.
Many questions are unanswered regarding the regulation of the PTP. Probably the most interesting question is: what is the mechanism, by which Ca2+ and ROS regulate the PTP? These molecules play the most central and powerful roles in this process, but where they bind or what they modify to regulate PTP opening is still completely unknown. Most likely, they affect the proteins described in this chapter, but other targets may be involved. For example, lipids such as cardiolipin likely play a major role in the assembly and control of both of these structures and provide a site for Ca2+-mediated regulation.
Despite the long-standing and justified idea that CypD plays a central role in PTP regulation, the mechanism, by which it does this, is unclear. It has recently been assumed that the binding of CypD to OSCP is important for this function; as suggested by Giorgio et al., CypD can induce PTP opening in purified ATP synthase preparations (Alavian et al. 2014), but it is not isolated with ATP synthase monomers or dimers (Alavian et al. 2014; Giorgio et al. 2013). Thus, its binding to OSCP/ATP synthase may be weak. It is possible that other, more important targets, such as ANT or SPG7, are as important as OSCP, but this remains to be determined. It is also unclear how the protein folding (peptidyl-prolyl, cis-trans isomerase) activity of CypD is important for its regulation of PTP function, as suggested by mutation studies (Lin and Lechleiter 2002).
The exact role of the other proteins that control the PTP (Table 3) remains unclear, and the mechanisms by which they exert their effects can only be surmised from incomplete data. Figure 5e suggests one possible model, but more work must be done to establish the importance of the association of the synthasome with mtCK, VDAC, and HK in the regulation of PTP. Furthermore, how the ANT inhibitors, atractyloside and bongkrekic acid, have opposite effects on the PTP is a mystery, but the answer probably lies in their ability to lock ANT into two totally different conformations, as discussed above. It is possible that defining the effects of these agents on ANT may also shed light on the role of CypD’s enzymatic activity, as it was reported that Ca2+ stabilization of ANT’s “c” conformation is related to its proline isomerization (Pestana et al. 2010).
5. Summary
OXPHOS and the mitochondrial ETC are essential for eukaryotic life as they produce the majority of ATP in most cells. However, disruption of mitochondrial function can lead to cell death, and the PTP plays a major role in both the energetic failure and the activation of programmed cell death pathways that lead to cellular demise. The molecular identity of the PTP has long been sought, and recent evidence suggests a model where ATP synthase, the major producer of ATP in the cell, also contains within its core the PTP. Further work must be done to delineate the exact nature of the pore of the PTP, how it is derived from ATP synthase, and what controls this process.
Acknowledgments
American Heart Association Grant 12GRNT12060233 to GAP. NIH NS045876; NS081746 to EAJ.
Contributor Information
Gisela Beutner, Division of Cardiology, Department of Pediatrics, University of Rochester Medical Center, 601 Elmwood Ave., Box 631, Rochester 14642, NY, USA.
Kambiz N. Alavian, Division of Brain Sciences, Department of Medicine, Imperial College London, London, UK
Elizabeth A. Jonas, Department of Internal Medicine, Section of Endocrinology, Yale University, New Haven, CT, USA
George A. Porter, Jr, Division of Cardiology, Department of Pediatrics, University of Rochester Medical Center, 601 Elmwood Ave., Box 631, Rochester 14642, NY, USA.
References
- Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA (2008) Respiratory active mitochondrial supercomplexes. Mol Cell 32:529–539 [DOI] [PubMed] [Google Scholar]
- Alavian KN, Li H, Collis L, Bonanni L, Zeng L, Sacchetti S, Lazrove E, Nabili P, Flaherty B, Graham M, Chen Y, Messerli SM, Mariggio MA, Rahner C, Mcnay E, Shore GC, Smith PJ, Hardwick JM, Jonas EA (2011) Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol 13:1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr, Jonas EA (2014) An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111:10580–10585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarashvili TS, Tyynela J, Odinokova IV, Grigorjev PA, Baumann M, Evtodienko YV, Saris NE (2002) Phosphorylation of a peptide related to subunit c of the F0F1-ATPase/ATP synthase and relationship to permeability transition pore opening in mitochondria. J Bioenerg Biomembr 34:279–284 [DOI] [PubMed] [Google Scholar]
- Azarashvili T, Odinokova I, Bakunts A, Ternovsky V, Krestinina O, Tyynela J, Saris NE (2014) Potential role of subunit c of F0F1-ATPase and subunit c of storage body in the mitochondrial permeability transition. Effect of the phosphorylation status of subunit c on pore opening. Cell Calcium 55:69–77 [DOI] [PubMed] [Google Scholar]
- Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V (2004) In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J 377:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP (2011) The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatr Cardiol 32:258–262 [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662 [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD (2007) Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9:550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P (2005) Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem 280:18558–18561 [DOI] [PubMed] [Google Scholar]
- Bernardi P (2013) The mitochondrial permeability transition pore: a mystery solved? Front Physiol 4:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner G, Ruck A, Riede B, Brdiczka D (1998) Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys Acta 1368:7–18 [DOI] [PubMed] [Google Scholar]
- Beutner G, Eliseev RA, Porter GA Jr (2014) Initiation of electron transport chain activity in the embryonic heart coincides with the activation of mitochondrial complex 1 and the formation of supercomplexes. PLoS One 9, e113330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora M, Bononi A, DE Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P (2013) Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12:674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora M, Wieckowski MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P (2015) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 34:1475–1486 [DOI] [PubMed] [Google Scholar]
- Bornhovd C, Vogel F, Neupert W, Reichert AS (2006) Mitochondrial membrane potential is dependent on the oligomeric state of F1F0-ATP synthase supracomplexes. J Biol Chem 281:13990–13998 [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287:C817–C833 [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Klingenberg M (1996) Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+. Biochemistry 35:8483–8488 [DOI] [PubMed] [Google Scholar]
- Calvaruso MA, Willems P, VAN DEN Brand M, Valsecchi F, Kruse S, Palmiter R, Smeitink J, Nijtmans L (2012) Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Hum Mol Genet 21:115–120 [DOI] [PubMed] [Google Scholar]
- Carraro M, Giorgio V, Sileikyte J, Sartori G, Forte M, Lippe G, Zoratti M, Szabo I, Bernardi P (2014) Channel formation by yeast F-ATP synthase and the role of dimerization in the mitochondrial permeability transition. J Biol Chem 289:15980–15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ko Y, Delannoy M, Ludtke SJ, Chiu W, Pedersen PL (2004) Mitochondrial ATP synthasome: three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J Biol Chem 279:31761–31768 [DOI] [PubMed] [Google Scholar]
- Chen YB, Aon MA, Hsu YT, Soane L, Teng X, Mccaffery JM, Cheng WC, Qi B, Li H, Alavian KN, Dayhoff-Brannigan M, Zou S, Pineda FJ, O’Rourke B, Ko YH, Pedersen PL, Kaczmarek LK, Jonas EA, Hardwick JM (2011) Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol 195:263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L (2013) Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155:160–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Costi A (1990) A heart mitochondrial Ca2(+)-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. Biochem J 266:33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Ellinger H, Costi A (1988) Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255:357–360 [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Virji S, Ward JM (1998) Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem 258:729–735 [DOI] [PubMed] [Google Scholar]
- Csordas G, Thomas AP, Hajnoczky G (2001) Calcium signal transmission between ryanodine receptors and mitochondria in cardiac muscle. Trends Cardiovasc Med 11:269–275 [DOI] [PubMed] [Google Scholar]
- Datler C, Pazarentzos E, Mahul-Mellier AL, Chaisaklert W, Hwang MS, Osborne F, Grimm S (2014) CKMT1 regulates the mitochondrial permeability transition pore in a process that provides evidence for alternative forms of the complex. J Cell Sci 127:1816–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KM, Strauss M, Daum B, Kief JH, Osiewacz HD, Rycovska A, Zickermann V, Kuhlbrandt W (2011) Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc Natl Acad Sci U S A 108:14121–14126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE Marchi E, Bonora M, Giorgi C, Pinton P (2014) The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell Calcium 56:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI Lisa F, Carpi A, Giorgio V, Bernardi P (2011) The mitochondrial permeability transition pore and cyclophilin D in cardioprotection. Biochim Biophys Acta 1813:1316–1322 [DOI] [PubMed] [Google Scholar]
- Dickson VK, Silvester JA, Fearnley IM, Leslie AG, Walker JE (2006) On the structure of the stator of the mitochondrial ATP synthase. EMBO J 25:2911–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudkina NV, Sunderhaus S, Boekema EJ, Braun HP (2008) The higher level of organization of the oxidative phosphorylation system: mitochondrial supercomplexes. J Bioenerg Biomembr 40:419–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseev RA, Malecki J, Lester T, Zhang Y, Humphrey J, Gunter TE (2009) Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J Biol Chem 284:9692–9699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Molkentin JD (2013) Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J 77:1111–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, Karch J, Gabel S, Farber J, Force T, Brown JH, Murphy E, Molkentin JD (2010) Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest 120:3680–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elustondo PA, Angelova PR, Kawalec M, Michalak M, Kurcok P, Abramov AY, Pavlov EV (2013) Polyhydroxybutyrate targets mammalian mitochondria and increases permeability of plasmalemmal and mitochondrial membranes. PLoS One 8, e75812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier N, Ducet G, Crevat A (1987) Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr 19:297–303 [DOI] [PubMed] [Google Scholar]
- Gendron MC, Schrantz N, Metivier D, Kroemer G, Maciorowska Z, Sureau F, Koester S, Petit PX (2001) Oxidation of pyridine nucleotides during Fas- and ceramide-induced apoptosis in Jurkat cells: correlation with changes in mitochondria, glutathione depletion, intracellular acidification and caspase 3 activation. Biochem J 353:357–367 [PMC free article] [PubMed] [Google Scholar]
- Genova ML, Lenaz G (2013) A critical appraisal of the role of respiratory supercomplexes in mitochondria. Biol Chem 394:631–639 [DOI] [PubMed] [Google Scholar]
- Gerle C (2016) On the structural possibility of pore-forming mitochondrial FF ATP synthase. Biochim Biophys Acta 1857:1191–1196 [DOI] [PubMed] [Google Scholar]
- Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, Forte MA, Bernardi P, Lippe G (2009) Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem 284:33982–33988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, Soriano ME, Basso E, Bisetto E, Lippe G, Forte MA, Bernardi P (2010) Cyclophilin D in mitochondrial pathophysiology. Biochim Biophys Acta 1797:1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, VON Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P (2013) Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A 110:5887–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EJ, Halestrap AP (1993) Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol 25:1461–1469 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Aguilar M, Douglas DL, Gibson AK, Domeier TL, Molkentin JD, Baines CP (2014) Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition. J Mol Cell Cardiol 72:316–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Davidson AM (1990) Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 268:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M, Durose L, Song CF, Barratt E, Trinick J, Jones R, Findlay JB (2003) Structure and function of the vacuolar H+-ATPase: moving from low-resolution models to high-resolution structures. J Bioenerg Biomembr 35:337–345 [DOI] [PubMed] [Google Scholar]
- Hausenloy D, Wynne A, Duchen M, Yellon D (2004) Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 109:1714–1717 [DOI] [PubMed] [Google Scholar]
- Havlickova V, Kaplanova V, Nuskova H, Drahota Z, Houstek J (2010) Knockdown of F1 epsilon subunit decreases mitochondrial content of ATP synthase and leads to accumulation of subunit c. Biochim Biophys Acta 1797:1124–1129 [DOI] [PubMed] [Google Scholar]
- Haworth RA, Hunter DR (1979) The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys 195:460–467 [DOI] [PubMed] [Google Scholar]
- Haworth RA, Hunter DR (2000) Control of the mitochondrial permeability transition pore by highaffinity ADP binding at the ADP/ATP translocase in permeabilized mitochondria. J Bioenerg Biomembr 32:91–96 [DOI] [PubMed] [Google Scholar]
- He L, Lemasters JJ (2002) Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett 512:1–7 [DOI] [PubMed] [Google Scholar]
- Hirst J (2013) Mitochondrial complex I. Annu Rev Biochem 82:551–575 [DOI] [PubMed] [Google Scholar]
- Hom JR, Quintanilla RA, Hoffman DL, de Mesy Bentley KL, Molkentin JD, Sheu SS, Porter GA Jr (2011) The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev Cell 21:469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA (1979a) The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys 195:453–459 [DOI] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA (1979b) The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys 195:468–477 [DOI] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA, Southard JH (1976) Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem 251:5069–5077 [PubMed] [Google Scholar]
- Huser J, Blatter LA (1999) Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J 343(Pt 2):311–317 [PMC free article] [PubMed] [Google Scholar]
- Ichas F, Mazat JP (1998) From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim Biophys Acta 1366:33–50 [DOI] [PubMed] [Google Scholar]
- Jonckheere AI, Smeitink JA, Rodenburg RJ (2012) Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis 35:211–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PC, Harrison MA, Kim YI, Finbow ME, Findlay JB (1995) The first putative transmembrane helix of the 16 kDa proteolipid lines a pore in the Vo sector of the vacuolar H(+)-ATPase. Biochem J 312(Pt 3):739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Ichas F, Mazat JP (1998) Modulation of cell calcium signals by mitochondria. Mol Cell Biochem 184:371–376 [PubMed] [Google Scholar]
- Kinnally KW, Campo ML, Tedeschi H (1989) Mitochondrial channel activity studied by patch-clamping mitoplasts. J Bioenerg Biomembr 21:497–506 [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, Macgregor GR, Wallace DC (2004) The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427:461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JQ, Davis J, Baines CP, Sargent MA, Karch J, Wang X, Huang T, Molkentin JD (2014) Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ 21:1209–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapuente-Brun E, Moreno-Loshuertos R, Acin-Perez R, Latorre-Pellicer A, Colas C, Balsa E, Perales-Clemente E, Quiros PM, Calvo E, Rodriguez-Hernandez MA, Navas P, Cruz R, Carracedo A, Lopez-Otin C, Perez-Martos A, Fernandez-Silva P, Fernandez-Vizarra E, Enriquez JA (2013) Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340:1567–1570 [DOI] [PubMed] [Google Scholar]
- Lenartowicz E, Bernardi P, Azzone GF (1991) Phenylarsine oxide induces the cyclosporin A-sensitive membrane permeability transition in rat liver mitochondria. J Bioenerg Biomembr 23:679–688 [DOI] [PubMed] [Google Scholar]
- Leung AW, Varanyuwatana P, Halestrap AP (2008) The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem 283:26312–26323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chauvin C, de Paulis D, de Oliveira F, Gharib A, Vial G, Lablanche S, Leverve X, Bernardi P, Ovize M, Fontaine E (2012) Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim Biophys Acta 1817:1628–1634 [DOI] [PubMed] [Google Scholar]
- Lin DT, Lechleiter JD (2002) Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J Biol Chem 277:31134–31141 [DOI] [PubMed] [Google Scholar]
- Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML (2013) Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal 19:1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masgras I, Rasola A, Bernardi P (2012) Induction of the permeability transition pore in cells depleted of mitochondrial DNA. Biochim Biophys Acta 1817:1860–1866 [DOI] [PubMed] [Google Scholar]
- Mcenery MW, Snowman AM, Trifiletti RR, Snyder SH (1992) Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A 89:3170–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgeoch JE, Guidotti G (1997) A 0.1–700 Hz current through a voltage-clamped pore: candidate protein for initiator of neural oscillations. Brain Res 766:188–194 [DOI] [PubMed] [Google Scholar]
- Mcgeoch JE, Mcgeoch MW (2008) Entrapment of water by subunit c of ATP synthase. J R Soc Interface 5:311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgeoch JE, Palmer DN (1999) Ion pores made of mitochondrial ATP synthase subunit c in the neuronal plasma membrane and Batten disease. Mol Genet Metab 66:387–392 [DOI] [PubMed] [Google Scholar]
- Mcgeoch JE, Mcgeoch MW, Mao R, Guidotti G (2000) Opposing actions of cGMP and calcium on the conductance of the F(0) subunit c pore. Biochem Biophys Res Commun 274:835–840 [DOI] [PubMed] [Google Scholar]
- Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT (2012) Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta 1817:851–862 [DOI] [PubMed] [Google Scholar]
- Moreno-Lastres D, Fontanesi F, Garcia-Consuegra I, Martin MA, Arenas J, Barrientos A, Ugalde C (2012) Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab 15:324–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652–658 [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ (2013) The oxygen electrode: monitoring proton current. Bioenergetics 4 Elsevier, Amsterdam [Google Scholar]
- Nickel A, Kohlhaas M, Maack C (2014) Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol 73:26–33 [DOI] [PubMed] [Google Scholar]
- Nicolli A, Petronilli V, Bernardi P (1993) Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by matrix pH. Evidence that the pore open-closed probability is regulated by reversible histidine protonation. Biochemistry 32:4461–4465 [DOI] [PubMed] [Google Scholar]
- Pasdois P, Parker JE, Griffiths EJ, Halestrap AP (2013) Hexokinase II and reperfusion injury: TAT-HK2 peptide impairs vascular function in Langendorff-perfused rat hearts. Circ Res 112: e3–e7 [DOI] [PubMed] [Google Scholar]
- Pedersen PL, Greenawalt JW, Reynafarje B, Hullihen J, Decker GL, Soper JW, Bustamente E (1978) Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol 20:411–481 [DOI] [PubMed] [Google Scholar]
- Pestana CR, Silva CH, Uyemura SA, Santos AC, Curti C (2010) Impact of adenosine nucleotide translocase (ANT) proline isomerization on Ca2 + -induced cysteine relative mobility/mitochondrial permeability transition pore. J Bioenerg Biomembr 42:329–335 [DOI] [PubMed] [Google Scholar]
- Peters C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409:581–588 [DOI] [PubMed] [Google Scholar]
- Petit PX, Zamzami N, Vayssiere JL, Mignotte B, Kroemer G, Castedo M (1997) Implication of mitochondria in apoptosis. Mol Cell Biochem 174:185–188 [PubMed] [Google Scholar]
- Petronilli V, Szabo I, Zoratti M (1989) The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett 259:137–143 [DOI] [PubMed] [Google Scholar]
- Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, DI Lisa F (1999) Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 76:725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoryelov D, Reichen C, Klyszejko AL, Brunisholz R, Muller DJ, Dimroth P, Meier T (2007) The oligomeric state of c rings from cyanobacterial F-ATP synthases varies from 13 to 15. J Bacteriol 189:5895–5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter GA Jr, Hom J, Hoffman D, Quintanilla R, de Mesy Bentley K, Sheu SS (2011) Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog Pediatr Cardiol 31:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, Brand MD (2012) Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J Biol Chem 287:27255–27264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T (2006) Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86:369–408 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Bernardi P, Pozzan T (2000) Mitochondria as all-round players of the calcium game. J Physiol 529(Pt 1):37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, DE Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P (2009) Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta 1787:1342–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruck A, Dolder M, Wallimann T, Brdiczka D (1998) Reconstituted adenine nucleotide translocase forms a channel for small molecules comparable to the mitochondrial permeability transition pore. FEBS Lett 426:97–101 [DOI] [PubMed] [Google Scholar]
- Saks V, Kuznetsov AV, Gonzalez-Granillo M, Tepp K, Timohhina N, Karu-Varikmaa M, Kaambre T, DOS Santos P, Boucher F, Guzun R (2012) Intracellular energetic units regulate metabolism in cardiac cells. J Mol Cell Cardiol 52:419–436 [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ (2005) Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A 102:12005–12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E (2005) Presynaptic calcium and control of vesicle fusion. Curr Opin Neurobiol 15:266–274 [DOI] [PubMed] [Google Scholar]
- Seidlmayer LK, Gomez-Garcia MR, Blatter LA, Pavlov E, Dedkova EN (2012) Inorganic polyphosphate is a potent activator of the mitochondrial permeability transition pore in cardiac myocytes. J Gen Physiol 139:321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmughapriya S, Rajan S, Hoffman NE, Higgins AM, Tomar D, Nemani N, Hines KJ, Smith DJ, Eguchi A, Vallem S, Shaikh F, Cheung M, Leonard NJ, Stolakis RS, Wolfers MP, Ibetti J, Chuprun JK, Jog NR, Houser SR, Koch WJ, Elrod JW, Madesh M (2015) SPG7 is an essential and conserved component of the mitochondrial permeability transition pore. Mol Cell 60:47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Shinohara Y, Tsujimoto Y (2000) Bax and Bcl-xL independently regulate apoptotic changes of yeast mitochondria that require VDAC but not adenine nucleotide translocator. Oncogene 19:4309–4318 [DOI] [PubMed] [Google Scholar]
- Sileikyte J, Blachly-Dyson E, Sewell R, Carpi A, Menabo R, DI Lisa F, Ricchelli F, Bernardi P, Forte M (2014) Regulation of the mitochondrial permeability transition pore by the outer membrane does not involve the peripheral benzodiazepine receptor (Translocator Protein of 18 kDa (TSPO)). J Biol Chem 289:13769–13781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato MC, Keller BU, Stuhmer W (1987) Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature 330:498–500 [DOI] [PubMed] [Google Scholar]
- Stotz SC, Scott LO, Drummond-Main C, Avchalumov Y, Girotto F, Davidsen J, Gomez-Garcia MR, Rho JM, Pavlov EV, Colicos MA (2014) Inorganic polyphosphate regulates neuronal excitability through modulation of voltage-gated channels. Mol Brain 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Zoratti M (1991) The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J Biol Chem 266:3376–3379 [PubMed] [Google Scholar]
- Szabo I, Zoratti M (2014) Mitochondrial channels: ion fluxes and more. Physiol Rev 94:519–608 [DOI] [PubMed] [Google Scholar]
- Vyssokikh MY, Brdiczka D (2003) The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim Pol 50:389–404 [PubMed] [Google Scholar]
- Vyssokikh MY, Katz A, Rueck A, Wuensch C, Dorner A, Zorov DB, Brdiczka D (2001) Adenine nucleotide translocator isoforms 1 and 2 are differently distributed in the mitochondrial inner membrane and have distinct affinities to cyclophilin D. Biochem J 358:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE (2013) The ATP synthase: the understood, the uncertain and the unknown. Biochem Soc Trans 41:1–16 [DOI] [PubMed] [Google Scholar]
- Wallace DC (1999) Mitochondrial diseases in man and mouse. Science 283:1482–1488 [DOI] [PubMed] [Google Scholar]
- Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H (2008) Superoxide flashes in single mitochondria. Cell 134:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jian C, Zhang X, Huang Z, Xu J, Hou T, Shang W, Ding Y, Zhang W, Ouyang M, Wang Y, Yang Z, Zheng M, Cheng H (2012) Superoxide flashes: elemental events of mitochondrial ROS signaling in the heart. J Mol Cell Cardiol 52:940–948 [DOI] [PubMed] [Google Scholar]
- Wittig I, Schagger H (2008) Structural organization of mitochondrial ATP synthase. Biochim Biophys Acta 1777:592–598 [DOI] [PubMed] [Google Scholar]
- Wittig I, Karas M, Schagger H (2007) High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol Cell Proteomics 6:1215–1225 [DOI] [PubMed] [Google Scholar]
- Woodfield K, Ruck A, Brdiczka D, Halestrap AP (1998) Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J 336(Pt 2):287–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Rohou A, Schep DG, Bason JV, Montgomery MG, Walker JE, Grigorieff N, Rubinstein JL (2015) Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. Elife 4, e10180. [DOI] [PMC free article] [PubMed] [Google Scholar]