Abstract
While emotional dysregulation is a broad construct, the current paper adopts a narrow approach to facilitate translational neuroscience research on pediatric anxiety. The paper first presents data on an adapted version of the antisaccade task and then integrates these data into a research framework. Data on an adapted version of the antisaccade task were collected in 57 youth, including 35 seeking treatment for an anxiety disorder. Associations were examined between performance on the antisaccade task and (a) age, (b) performance on other cognitive-control tasks (i.e., the stop-signal delay and flanker tasks), and (c) level of anxiety symptoms. Better performance on the antisaccade task occurred in older relative to younger subjects and correlated with better performance on the flanker task. Across the 57 youth, higher levels of anxiety correlated with shorter latency for correct antisaccades. These data can be placed within a three-step framework for translational neuroscience research. In the first step, a narrow index of emotion dysregulation is targeted. In the second step, this narrow index is linked to other correlated indicators of the same underlying narrow latent construct. In the third and final step, associations are examined with clinical outcomes and response to treatment.
Keywords: antisaccade task, anxiety eye movements, inhibitory control, latency
Inhibitory control, the ability to modulate prepotent responses, supports effective emotion regulation (Fox & Calkins, 2003; Nigg, 2017). Perturbed inhibitory control occurs in children with various problems associated with emotional dysregulation (Meyer et al., 2018; Moser, Moran, Schroder, Donnellan, & Yeung, 2013; Musser, Galloway-Long, Frick, & Nigg, 2013; Nigg, 2013; Troller-Renfree, Nelson, Zeanah, & Fox, 2016; Weinberg et al., 2016). While most childhood psychiatric conditions involve reduced cognitive control, anxiety is one of the few clinical problems linked to excessive inhibitory control (Henderson, Pine, & Fox, 2015; Moser et al., 2013). Of note, as compared to behavioral measures, physiologic measures, such as the error-related negativity (ERN), generate stronger evidence of such excessive inhibitory control, with the strongest evidence arising for error-related processes (Moser et al., 2013). Through an adaptation of the antisaccade task, the current study addresses the need for more behavioral measures of inhibitory control related to error processing in anxiety. Using this task, our first analyses examine relations between anxiety and inhibitory control in the context of a task generating very high error rates. However, the primary study goal is to evaluate task performance as it relates to age and performance on two other inhibitory control tasks. The paper then uses these data as a stepping-stone to discuss a broader framework for studies of emotion dysregulation.
Emotion dysregulation can be viewed as a transdiagnostic vulnerability to psychopathology, and to anxiety disorders specifically (Aldao, Gee, De Los Reyes, & Seager, 2016; Beauchaine, 2015; Cole, Hall, & Hajal, 2017; Hofmann, Sawyer, Fang, & Asnaani, 2012; Jazaieri, Morrison, Goldin, & Gross, 2015). In this context, emotion dysregulation is often operationalized as the inability to modulate affective states in the service of goal-directed behavior; such goal-directed behavior is influenced by both bottom-up, stimulus-driven factors and top-down, goal-related factors. A view of emotion dysregulation as a transdiagnostic construct is supported by neuroimaging research. This research links altered subcortical-cortical functional activity and connectivity to both internalizing and externalizing disorders (Beauchaine & Zisner, 2017; Cardinale et al., 2018; Gold et al., 2016; Kircanski et al., 2018; Pagliaccio et al., 2015; Tone, Garn, & Pine, 2016). Consistent with these data, contemporary theories suggest that transdiagnostic, emotion dysregulation symptoms may in part emerge from compromised top-down cognitive control (Beauchaine, 2015; Beauchaine & Zisner, 2017); this could result from dysfunction in ventrolateral and dorsal lateral prefrontal regions involved in cognitive control of emotion (Ochsner, Silvers, & Buhle, 2012) via regulation of subcortical regions (e.g., amygdala; Hiser & Koenigs, 2018; Kohn et al., 2014). However, the specific role that cognitive-control dysfunction plays in emotion dysregulation symptoms remains unclear. Quantifying this specific role is difficult due to a lack of experimental studies targeting narrow mechanisms. Inhibitory control represents one such mechanism whereby aberrant patterns, in the form of both reduced and excessive inhibitory control, may support dysregulated affective states in externalizing and internalizing disorders, respectively.
Inhibitory control involves the suppression of automatic behaviors, thoughts, or emotions (Aron, 2011; Bari & Robbins, 2013). While this capacity is quantified in many paradigms, the antisaccade task possesses unique advantages (Hallett, 1978). For example, the antisaccade task assesses inhibitory behavior via eye movements, which can be more reliable than button-presses (Hedge, Powell, & Sumner, 2018; Price et al., 2015). Moreover, the relatively simple circuitry mediating eye movements tightly connects brain function to cognition and behavior (Everling & Fischer, 1998; Hikosaka, 2007; Hikosaka et al., 2018; Nieuwenhuis, Broerse, Nielen, & de Jong, 2004). The antisaccade task assesses inhibition of reflexive “prosaccades.” Subjects are required to make “antisaccades,” directed to the hemifield opposite from where a target appears, after first inhibiting the automatic tendency to make prosaccades toward the target (see Figure 1). Considerable work maps the neural circuitry mediating antisaccades, where closely aligned neural systems support target encoding, inhibition, and response execution (Everling & Fischer, 1998; Nieuwenhuis et al., 2004). For saccades, target encoding and execution both occur in the visual domain; in contrast, for button-presses, target encoding occurs in the visual system, but responses occur in a different domain under somatomotor-system control. Incorrect responses and prolonged antisaccade latencies reflect difficulty in deploying inhibitory-control processes, thus providing quantifiable measures of inhibitory control.
Figure 1.
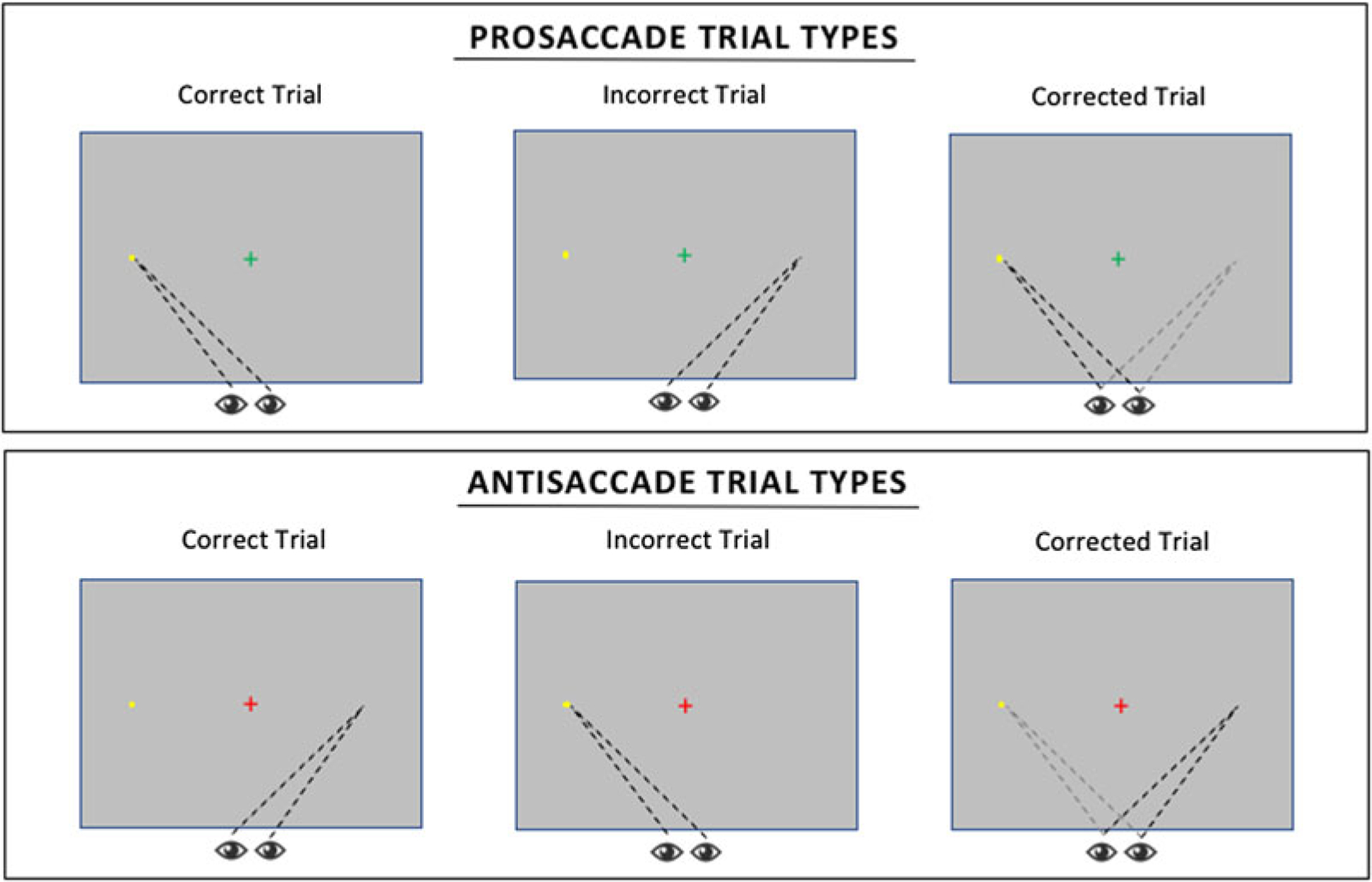
Schema of eye gaze patterns for correct, incorrect, and corrected prosaccade and antisaccade trials.
Most research on antisaccade performance in developmental psychopathology examines impulsivity (Aichert et al., 2012; Bari & Robbins, 2013; Loe, Feldman, Yasui, & Luna, 2009). These studies find reduced inhibitory control in youth with high as compared to low impulsivity. No prior study uses the antisaccade task in youth seeking treatment for an anxiety disorder, but findings for tasks assessing inhibitory control via button-press are mixed, with evidence of both increased and decreased inhibitory control in pediatric anxiety (Henderson et al., 2015). Finally, a few studies examine saccade metrics in anxiety, but these studies either examine adults or embed emotional stimuli within pro-and antisaccade trials (Derakshan, Ansari, Hansard, Shoker, & Eysenck, 2009; Hardin et al., 2009; Mueller, Hardin, Korelitz, et al., 2012; Mueller, Hardin, Mogg, et al., 2012). Studies are needed in anxious youth, given strong age differences in antisaccade task performance (Geier & Luna, 2012; Luna, Garver, Urban, Lazar, & Sweeney, 2004; Luna, Velanova, & Geier, 2008). Furthermore, studies are also needed using tasks without emotional content, to avoid the confounding of saccade performance through the known effects of emotional content on eye movements in pediatric anxiety (Price et al., 2015). The current study adapts a standard “nonemotional” version of the antisaccade task used in prior research on developmental psychopathology.
Finally, studying inhibitory control in the context of errors may be critical to gain a clear understanding of atypical inhibitory control associations with anxiety. The most consistent evidence of excessive inhibitory control in anxiety derives from studies of the ERN, an event-related potential. Whereas disorders associated with impulsivity manifest reduced inhibitory control in the form of a small ERN, disorders associated with anxiety manifest enhanced inhibitory control in the form of a large ERN (Moser et al., 2013; Nigg, 2013; Troller-Renfree et al., 2016). The current study adapts the antisaccade task by mixing pro-and antisaccades within blocks; this facilitates the exploration of error-related processes in anxiety. Many versions of the antisaccade task employed in developmental studies use block designs (Luna et al., 2004). While this reduces task difficulty, it complicates assessments of error-related processes. This is because performance on individual trials is contaminated by context, such that inhibitory control is more easily deployed on blocked antisaccade trials, as compared to antisaccade trials that follow prosaccade trials. Thus, by mixing pro-and antisaccades within blocks, we examine in anxiety aspects of error processing less contaminated by context.
While the study examines relations between inhibitory capacity and pediatric anxiety, large samples are needed to adequately examine behavioral correlates of developmental psychopathology (Abend et al., 2018; Miyake & Friedman, 2013; Moser et al., 2013). As a result, the current paper lays the groundwork for such a future, large-scale project by evaluating task validity, specifically by examining associations of antisaccade performance with age, and with performance on other inhibitory-control tasks. First, previous research finds strong age-related differences in antisaccade performance (Luna et al., 2004, 2008); errors and latencies are higher in children than adolescents, with effect size differences that are larger than in studies on inhibitory control correlates of anxiety. As a result, the study tests the hypotheses that antisaccade performance is better in older relative to younger participants. Second, task validity is evaluated by examining correlations among multiple measures of inhibitory control (Hedge et al., 2018; Miyake & Friedman, 2012), testing our hypothesis that measures of inhibitory-control performance on the antisaccade task will be correlated with performance on other established tasks of inhibitory control (i.e., the flanker task and stop-signal delay task).
Method
Participants
A total of 57 youth (M = 12.80, SD = 3.11 years, 42.1% male) were recruited using the same methods employed in prior work from our group (White, Moore, et al., 2017). These methods involve community outreach to identify treatment-seeking and healthy youth. This sample includes youth with a primary diagnosis of an anxiety disorder (n = 35) or youth with no psychiatric diagnosis (healthy volunteers; n = 22). Prior to participation, diagnostic status was confirmed by a doctoral-or master’s-level clinician (reliability k > 0.7) using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime version (Kaufman et al., 1997). Following administration of the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime version, all diagnoses were reviewed and confirmed by a board-certified child and adolescent psychiatrist (D.S.P.). Exclusionary criteria included IQ<70, a diagnosis of autism spectrum disorder, posttraumatic stress disorder, schizophrenia, depression, use of any substance with psychoactive effects within 3 months of participation, neurological disorder, a history of trauma, or significant medical illness. In addition, participants presenting with significant externalizing symptoms requiring treatment were excluded, due to ethical restrictions on withholding treatment for such symptoms. As a result, patients included only youth with clinically significant anxiety in need of immediate treatment but not other forms of psychopathology requiring treatment. All study procedures were approved by the National Institute of Mental Health Institutional Review Board. Parents provided written informed consent and youth provided written assent prior to participation. Participants received monetary compensation for participation.
Experimental tasks
The main experimental task consisted of a mixed event version of the antisaccade task. The task began with a practice block during which participants received feedback on their performance on 8 antisaccade and 8 prosaccade trials. After the practice block, participants completed 3 experimental blocks each with 16 antisaccade and 16 prosaccade trials totaling 48 antisaccade and 48 prosaccade trials across the entire task. Each trial started with a preparatory period during which an instructional fixation cross was presented for either 2 or 6 s. The color of the fixation cross indicated the trial type that would follow. A green fixation cross corresponded with prosaccade trials during which the participant was instructed “green means go so you are going to go ahead and look at the yellow flash” and red fixation crosses corresponded with antisaccade trials during which the participant was instructed “red means stop, so don’t look at the yellow flash— instead, look to the exact opposite location.” The preparatory period was followed by a brief blank screen (200 ms) and a response period in which the yellow visual target was presented for 1 s. The location of the cue was pseudorandomized across trials such that there were equal numbers of antisaccade and prosaccade trials with the visual cue presented ±630 pixels or ±315 pixels from the central fixation. The order of the prosaccade and antisaccade trials were fully randomized within each block.
Eye gaze data was collected using the EyeLink 1000 Plus eye tracking camera. Participants underwent a standard calibrating procedure during which participants sat in a fixed chair within 520 mm of the desktop mounted eye tracking camera. Eye level was consistent across all participants, and real-time binocular eye movement data was captured continuously throughout the task at 1000 Hz per second, through a 25 mm lens with a 1920 × 1080 pixel display resolution. The dimensions of the screen were also consistent for each participant at 475 mm × 270 mm.
All eye gaze data were processed using Eyelink Dataviewer (SR Research). For each trial, saccades were extracted and coded using a 600 × 600 pixel area of interest (AOI) centered on the coordinates of the visual cue location and the coordinates of the mirror of the visual cue. For prosaccade trials, saccades that ended in the AOI surrounding the visual cue were coded as correct saccades, and saccades that ended in the AOI surrounding the mirror location of the visual cue were coded as incorrect saccades. For antisaccade trials, saccades that ended in the AOI surrounding the mirror location of the visual cue were coded as correct saccades, and saccades ending in the AOI surround the visual cue were coded as incorrect saccades. All saccades that included a blink were filtered out and saccades <100 ms in duration were treated as an express saccade (Fischer & Ramsperger, 1984) and censored from all analyses. Participants with >25% trials of missing eye gaze data were excluded from analyses (4 healthy volunteers and 8 youth with a primary diagnosis of an anxiety disorder) resulting in a final sample of 45 participants (Table 1).
Table 1.
Sample characteristics by diagnostic group
| Characteristics Mean (SD) or number (%) | Healthy comparisons | Anxiety diagnosis | p value |
|---|---|---|---|
| Demographic variables | |||
| N | 19 | 27 | |
| Age (years) | 13.09 (3.31) | 12.37 (3.03) | .45 |
| Sex (male) | 9 (47.37%) | 9 (33.33%) | .34 |
| IQ | 109.94 (14.67) | 109.56 (13.24) | .93 |
| Symptom ratings | |||
| SCARED parent report | 4.14 (5.61) | 30.23 (11.14) | <.001 |
| SCARED youth report | 7.36 (6.74) | 31.19 (12.90) | <.001 |
Note: SCARED, Screen for Child Anxiety Related Emotional Disorders.
Comparison tasks
A subset of participants (n = 22) completed two additional inhibitory-control tasks. The first task was the stop-signal delay task (Logan & Cowan, 1984) during which participants were instructed to complete a go and stop task concurrently. For the go portion of the task, participants were presented with either an X or an O and instructed to press the right button when presented with an X and left button when presented with an O. On 25% of trials, the presentation of the go stimuli was paired with an auditory stop cue. Participants were instructed that when they hear the 1000 Hz tone stop signal they should withhold their response to the go stimuli. It is important to note that the auditory stop signal was always presented after the go stimuli, and the amount of time that passed between the onset of the go signal and onset of the stop signal (stop-signal delay; SSD) varied as a function of participants’ performance. Initially the SSD was set to 250 ms. For each stop-signal trial, if the previous stop-signal trial was successfully performed (e.g., the go response was withheld), the SSD increased by 50 ms, making the subsequent trial more difficult. If the previous stop-signal trial was unsuccessfully performed (e.g., the go response was not withheld), the SSD decreased by 50 ms, making subsequent trials easier. This performance-dependent adjustment of the SSD is designed to ensure 50% correct responding to stop trials. Within our sample, the average percentage correct stop trials was 51.61% (SD = 5.84%). Participants completed 5 blocks of 88 trials for a total of 440 trials (330 go trials and 110 stop trials). Prior to completion of the experimental blocks, participants completed a 16-trial practice training with feedback to learn the go responses and a 16-trial practice that included the stop signal. This training ensured that participants understood task instructions prior to beginning the experimental task. For measures of inhibitory-control efficiency, we extract the average reaction time (RT) to go trials, the average SSD for stop trials, as well as the stop-signal reaction time; this was calculated by subtracting the average SSD from the average go RT.
The second comparison inhibitory-control task was the flanker task (Eriksen, 1995). During the flanker tasks, participants were presented with five side-by-side arrows centered on the screen. Participants were instructed to press a left or right arrow button as quickly as possible to indicate the direction that the central arrow is facing. Trials included congruent trials, where the flanking arrows were pointing in the same direction as the central arrow, and incongruent trials, where the flanking arrows were pointing in the opposite direction than the central arrow. Participants first completed a practice training with feedback on 6 congruent and 6 incongruent trials randomly presented. The experimental task consisted of 4 blocks each with 60 trials (30 congruent, 30 incongruent). The trials were pseudorandomized to ensure equal numbers of congruent and incongruent trials were presented within each experimental block. Response accuracy on the congruent trials was high (M = 95.04%, SD = 4.65%) and within expected range for the incongruent trials (M = 74.64%, SD = 16.34%). For measures of inhibitory-control efficiency, we additionally extracted reaction time metrics for responses to congruent and incongruent trials as well as the difference between congruent RT and incongruent RT.
Symptom measures
Anxiety symptoms were assessed dimensionally using the parent-and youth-report versions of the Screen for Child Anxiety Related Emotional Disorders (Birmaher et al., 1997)). Parent-report scores ranged from 0 to 54 (M = 20.16, SD =15.89) and youth-report scores ranged from 0 to 54 (M = 21.78, SD = 16.13). Total score on the parent and child reports were significantly correlated (r = .38; p < .05).
Results
Task performance
Eye gaze data was used to code each trial as either (a) correct, if the initial saccade was to the correct location on the screen; (b) incorrect, if the initial saccade was to the incorrect location on the screen; or (c) corrected, if an initial incorrect saccade was followed by a saccade to the correct location (see Figure 1 and Table 2). Examining measures of global performance, most prosaccade trials were correct trials (96.94%), whereas only 37.31% of antisaccade trials were correct saccades. Paired sample t tests confirmed a smaller proportion of correct trials for antisaccade trials compared to prosaccade trials, t (44) = 15.85, p < .001 (Figure 2). While the majority of antisaccade trials consisted of an initial incorrect saccade, on average, 61.88% of these error trials were eventually corrected. As a result, on average, 36.52% (SD = 15.87%) of all antisaccade trials were corrected trials and 26.17% of all antisaccade trials were incorrect trials, which is comparable to error rates in previous studies of community samples (Hutton & Ettinger, 2006).
Table 2.
Descriptive statistics for count of trial types for prosaccade and antisaccade trials
| Mean (SD) | Range | Skew | |
|---|---|---|---|
| Prosaccade trials | |||
| PS correct | 43.89 (3.19) | 36–48 | −0.80 |
| PS corrected | 1.18 (1.23) | 0–6 | 1.72 |
| PS incorrect | 0.20 (0.41) | 0–1 | 1.55 |
| PS missing | 2.73 (2.89) | 0–11 | 1.11 |
| Antisaccade trials | |||
| AS correct | 15.11 (10.32) | 0–41 | 0.68 |
| AS corrected | 14.89 (7.19) | 1–33 | 0.42 |
| AS incorrect | 10.27 (7.45) | 0–31 | 0.74 |
| AS missing | 7.73 (4.59) | 0–17 | 0.33 |
Note: PS, prosaccade. AS, antisaccade.
Figure 2.
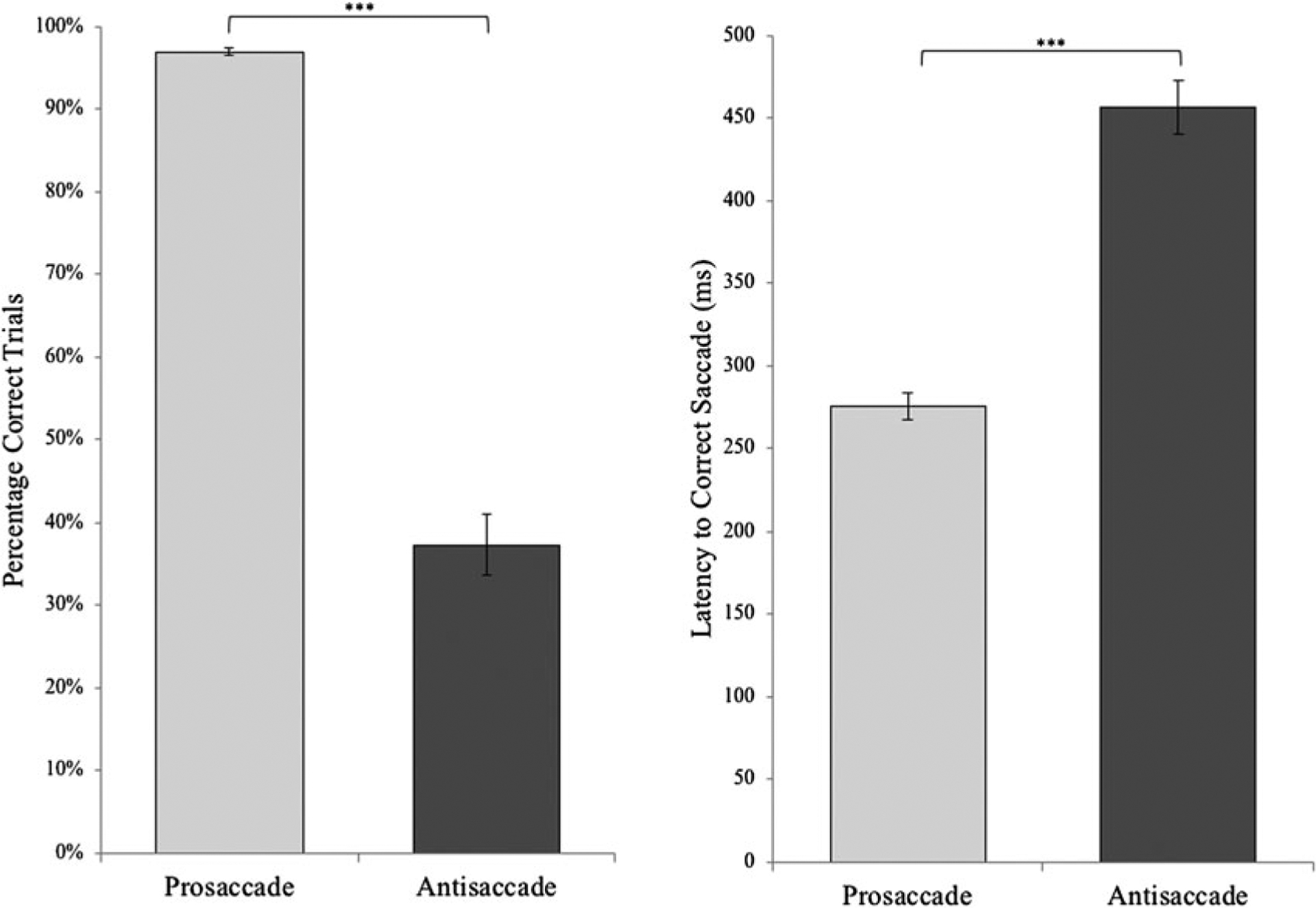
Average percentage correct and latency to correct saccade for prosaccade and antisaccade trials. ***p < .001. Error bars depict the standard error.
For each saccade, we extracted the duration of time that elapsed from the start of the trial to the end of the saccade. This allows us to capture the latency to initial correct saccades for both correct antisaccade and correct prosaccade correct trials, latency to errors (i.e., incorrect saccades) for incorrect antisaccade trials, and the time to correct an initial incorrect saccade for corrected antisaccade trials (Table 3). We were unable to extract a measure of error latency for two subjects as there were no incorrect antisaccade error trials. As expected, paired sample t tests revealed that correct latencies were shorter for prosaccade trials compared to antisaccade trials, t (43) = 11.74, p < .001, and within antisaccade trials, error latencies were shorter than correct trials, t (41) = 10.53, p < .001 (Figure 2). Finally, for corrected antisaccade trials, 365.05 ms elapsed between the initial incorrect saccade to the correct saccade, on average.
Table 3.
Descriptive statistics for measures of global performance and saccade latency on prosaccade and antisaccade trials
| Mean (SD) | Range | Skew | |
|---|---|---|---|
| Global performance | |||
| Prosaccade trials | |||
| % correct | 96.94% (2.78) | 87.50%–100% | −1.82 |
| Antisaccade trials | |||
| % correct | 37.31% (24.40) | 0%a–87.23% | 0.45 |
| Error rate | 26.17% (19.88) | 0%–91.18% | 0.99 |
| Correction rate | 61.88% (20.17) | 3.13%–100% | −0.34 |
| Saccade latency | |||
| Prosaccade trials | |||
| Correct latency | 274.56 (52.55) | 201.59–474.26 | 2.43 |
| Antisaccade trials | |||
| Correct latency | 456.57 (108.99) | 202.0–886.50 | 1.37 |
| Error latency | 272.27 (67.43) | 192.00–468.93 | 1.80 |
| Time to correct | 365.05 (77.78) | 182.18–547.33 | 0.55 |
Note: % correct, number of correct trials / total number of trials. Error rate, number of incorrect trials / total number of trials. Correction rate, number of corrected trials / (number of corrected trials + number of incorrect trials).
Only one participant had 0% correct antisaccade trials.
We next examined correlations among task measures (Table 4). With the exception of correct latency for antisaccade trials, all latency measures were positively correlated, suggesting that correct latency is a unique measure. Measures of global performance on antisaccade and prosaccade trials demonstrated predicted patterns of associations. That is, across subjects, a higher percentage of correct prosaccade trials was associated with faster latency to correct saccades for correct prosaccade trials (r = −.33, p = .03). Of particular importance, we found that measures of inhibitory-control performance during antisaccade trials were intercorrelated as hypothesized. That is, higher percentage of correct antisaccade trials was associated with improved performance across antisaccade measures, including decreased error rate (r = −.76, p < .001), increased correction rate (r = .47, p = .001), and slower latency to error (r = .42, p = .01). Increased correction rate was also associated with decreased error rate (r = −.89, p < .001), faster correct antisaccade latency (r = −.42, p = .004), and faster time to correct an incorrect antisaccade (r = −.37, p = .01). Finally, increased error rate was also associated with slower latency to correct antisaccade (r = .41, p = .01).
Table 4.
Correlations among task measures
| PS % correct | AS % correct | AS error rate | AS correction rate | PS correct latency | AS correct latency | AS error latency | |
|---|---|---|---|---|---|---|---|
| PS % correct | |||||||
| AS % correct | −0.25 | ||||||
| AS error rate | 0.13 | −0.76*** | |||||
| AS correction rate | −0.14 | 0.47** | −0.89*** | ||||
| PS correct latency | −0.33* | 0.27 | −0.12 | 0.01 | |||
| AS correct latency | 0.26 | −0.20 | 0.41** | −0.42** | 0.36* | ||
| AS error latency | −0.04 | 0.42** | −0.26 | 0.14 | 0.74*** | 0.21 | |
| AS time to correct | −0.08 | −0.19 | 0.29 | −0.37* | 0.40** | 0.27 | 0.34* |
Note: PS, prosaccade. AS, antisaccade.
p < .05.
p < .01.
p < .001.
External correlates of antisaccade performance
Next, we sought to examine associations between performance on the task and external correlates related to inhibitory processing. We conducted correlation analyses to interrogate the relationship between task measures and age (Table 5). As predicted, we found that older age was associated with improved performance across antisaccade trials (percentage correct antisaccade trials: r = .47, p = .001; error rate: r = −.54, p < .001; correction rate: r = .47, p = .001). Older age was also associated with reduced latency to correct for corrected antisaccade trials (r = −.48, p = .001) and decreased latency for correct antisaccades (r = −.29, p = .05).
Table 5.
Associations between antisaccade task performance measures with external correlates of inhibitory control
| PS % correct | AS % correct | AS error rate | AS correction rate | PS correct latency | AS correct latency | AS error latency | AS time to correct | |
|---|---|---|---|---|---|---|---|---|
| Age | −0.19 | 0.47* | −0.55** | 0.48* | −0.14 | −0.29 | −0.12 | −0.48** |
| SST SSRTa | 0.00 | −0.21 | 0.32 | −0.29 | 0.23 | 0.01 | 0.32 | 0.22 |
| SST average GoRTa | −0.10 | −0.29 | 0.38 | −0.33 | 0.08 | −0.02 | −0.11 | 0.22 |
| SST average SSDa | −0.15 | −0.16 | 0.13 | −0.06 | −0.15 | −0.12 | −0.34 | 0.01 |
| Flanker congruent RTa | 0.15 | −0.67** | 0.69*** | −0.51* | 0.08 | 0.01 | 0.05 | 0.32 |
| Flanker incongruent RTa | 0.16 | −0.61** | 0.69*** | −0.53* | 0.01 | −0.06 | −0.01 | 0.16 |
| Flanker congruent RT-incongruent RTa | 0.14 | −0.38 | 0.54** | −0.46* | −0.12 | −0.15 | −0.10 | −0.13 |
Note: PS, prosaccade. AS, antisaccade. SST, stop-signal delay task. SSRT, stop-signal reaction time. GoRT, go response reaction time. SSD, stop-signal delay. RT, reaction time,
n = 22.
p < .05.
p < .01.
p < .001.
For the subset of participants (n = 22) who completed the two comparison inhibitory-control tasks, we examined correlations across tasks (Table 5). We observed strong associations between the percentage of correct antisaccade trials, antisaccade error rate, and antisaccade correction rate, with reaction time on the flanker task (however, associations between percentage correct antisaccade trials and difference between reaction time to congruent and incongruent flanker stimuli was nonsignificant, r = −.38, p = .08). The direction of associations was as expected. That is, increases in both the percentage of correct antisaccade trials and antisaccade correction rate were associated with faster reaction times and a decrease in the difference between congruent and incongruent reaction times, whereas higher antisaccade error rate was associated with slower reaction times and a greater difference between congruent and incongruent reaction times. While we did not observe statistically significant associations with performance on the stop-signal delay task, the observed Pearson’s correlation coefficients are within the range of the expected effect size (r ≈.30) and in the expected direction. Given the small sample size (n = 22), we expect that with additional participants one would be adequately powered to detect effect sizes of this size.
Associations with anxiety
We next investigated whether performance on the antisaccade tasks differed as a function of having a primary anxiety diagnosis. Results of independent t tests revealed no differences on percentage correct prosaccade, t (43) = 0.08, p = .94, or antisaccade trials, t (43) = 0.41, p = .68. Similarly, there were no group differences for the antisaccade error rate, t (43) = 0.30, p = .76, or correction rate, independent t test, t (43) = 0.22, p = .83. Examining group difference in latency measures, no differences emerged between healthy volunteers and patients with an anxiety diagnosis on any of the four latency measures, including correct latency on antisaccade trials, t (42) = 1.67, p = .10. Given associations between age and antisaccade performance, coupled with participants spanning a wide age range, we conducted univariate analyses of covariance examining mean differences across healthy volunteers and patients after controlling for age. Results were consistent with those observed above with one exception. After controlling for age, group differences arose for correct latency on antisaccade trials, F (1, 43) = 5.07, p = .03; patients exhibited faster latency to correct antisaccades, indicative of better inhibitory control (Figure 3).
Figure 3.
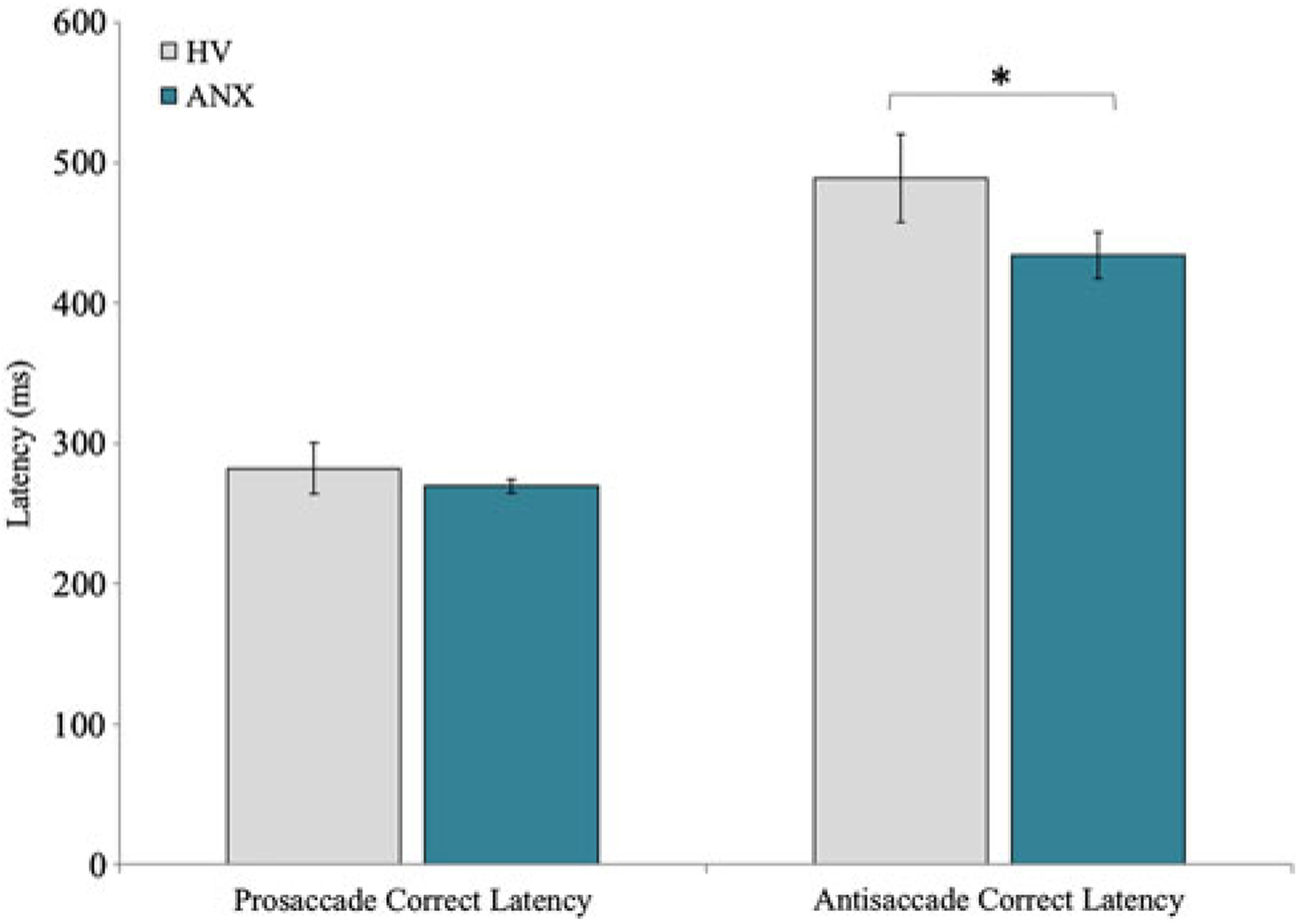
Latency to correct prosaccade and antisaccades for healthy volunteers (HV) and patients with a primary diagnosis of anxiety (ANX). *p < .05 for univariate analysis of covariance controlling for age. Error bars depict the standard error.
Correlations between dimensionally assessed anxiety across our whole sample revealed no association between performance on prosaccade or antisaccade trials as assessed through global performance measures or latency (all ps > .05; Table 6). We repeated these analyses controlling for age in a series of partial correlations. Results of these partial correlations revealed anxiety reported by both parent, r (38) = −.36, p = .02, and child, r (38) = −.34, p = .03, to negatively correlate with saccade latency during correct antisaccades. Thus, as anxiety symptoms increased, latency to a correct antisaccade decreased, again consistent with improved inhibitory control. No other significant partial correlations emerged for associations between anxiety and antisaccade task performance measures (Table 6).
Table 6.
Associations between anxiety and antisaccade task performance measures
| Raw bivariate correlations | Partial correlations controlling for age | |||
|---|---|---|---|---|
| Parent-reported anxiety | Youth-reported anxiety | Parent-reported anxiety | Youth-reported anxiety | |
| PS % correct | −0.08 | −0.11 | −0.24 | −0.25 |
| AS % correct | −0.17 | −0.07 | 0.02 | −0.06 |
| AS error rate | 0.28 | 0.06 | 0.13 | 0.09 |
| AS correction rate | −0.28 | −0.03 | −0.11 | 0.01 |
| PS correct latency | −0.14 | −0.10 | −0.19 | −0.07 |
| AS correct latency | −0.22 | −0.29 | −0.36* | −0.34* |
| AS error latency | −0.24 | −0.24 | −0.26 | −0.21 |
| AS time to correct | 0.07 | −0.08 | −0.08 | −0.04 |
Note: PS, prosaccade. AS, antisaccade.
p < .05.
Discussion
First, results from the current study are discussed. Next, we use these results as a foundation for a three-stage framework on emotion dysregulation. This framework emphasizes the utility of narrowly focused behavioral studies, such as the current one, in linking cognitive neuroscience and developmental psychopathology research on emotion dysregulation.
The current study
The first key finding relates to overall task performance: across all participants, antisaccade trials generated fewer correct responses and longer latencies than prosaccade trials. Moreover, accuracy correlated with latency, suggesting the presence of an inhibitory-control dimension. Second, expected associations also emerged with age and with performance on the flanker task. Third, preliminary support arose for association between task performance and anxiety. While anxiety was unrelated to accuracy, both anxiety diagnosis and symptoms related to faster latency to correct antisaccades.
Some overall performance findings were relatively novel. Most important, the current version of the antisaccade task is more difficult than other versions using a block-design version of the paradigm (Luna et al., 2004, 2008). These typically generate accuracy rates above 50%. In the current study, 61% of antisaccade trials involved a corrected response, whereby an errant response was initiated, before being corrected with the execution of a correct antisaccade. This high rate likely arises from the use of a fully randomized within-block design. Of note, this high proportion of corrected trials demonstrates subjects’ consistent engagement in the task, whereupon they continued to adjust their response immediately after initiating an error. Overall, this design appears to be well suited for an examination of task performance within a context where error rates are high.
Two external validators suggest that the task engaged inhibitory-control processes effectively, much like block-design versions of the task. First, research on a range of inhibitory-control measures demonstrates consistent age-related associations (Luna et al., 2004, 2008; White, Moore, et al., 2017). Performance on such measures generally improves throughout adolescence. Multiple indices from the current study correlated with age, such that adolescents performed better than children for both accuracy and latency measures. These relatively strong associations may reflect the relative difficulty of the antisaccade task, where performance might be expected to improve through adolescence. Second, performance on the antisaccade task correlated with performance on the flanker task. While correlations between the stop and antisaccade tasks were nonsignificant, the direction and magnitude of these associations were as expected (Hedge et al., 2018; Miyake & Friedman, 2012), such that better inhibitory control on one task related to better performance on the other. Thus, overall patterns suggested engagement of a latent inhibitory-control factor across the three paradigms.
Only preliminary support emerged concerning the presence of relations between inhibitory control and anxiety. No group differences emerged between the healthy comparisons and patients, considered as distinct groups. Moreover, accuracy measures were uncorrelated with continuous measures of anxiety. Nevertheless, in analyses controlling for age, both anxiety diagnosis as well as parent-and child-rated anxiety were associated with latency for correct antisaccade trials: individuals with elevated anxiety symptoms demonstrated faster latencies to correct antisaccades. This suggests that higher levels of anxiety may relate to increasingly high levels of inhibitory control, in the context of a paradigm that generates high rates of error commission. Such findings can be placed in a rich but complex past literature relating anxiety to inhibitory control.
Nevertheless, our findings are consistent with one strain of prior literature. This literature suggests that anxiety symptoms are associated with increases in cognitive-control processing (Henderson et al., 2015; Moser et al., 2013; Nigg, 2013). In particular, the current data demonstrate an association between increased symptoms of emotion dysregulation, in the form of anxiety, and increased inhibitory-control efficiency, a sign of enhanced cognitive-control capacity. However, such findings also do not easily fit with other existing theories grounded in a different large body of work. Specifically, other theories link increased symptoms of emotion dysregulation in various psychopathological domains to decreased inhibitory-control efficiency and other signs of reduced cognitive-control capacity. Most of this literature finds decreased inhibitory control in relation to externalizing symptoms (Beauchaine & Zisner, 2017; Venables et al., 2018) although some studies do note associations with internalizing symptoms (Henderson et al., 2015).
These seeming contradictions might be understood by considering the heterogeneous nature of both psychopathology and cognitive control. In the current study, subjects were recruited to express a particular, relatively narrow form of anxiety, uncomplicated by medication use or other serious forms of psychopathology. Moreover, cognitive control was assessed with a task optimized to generate large error rates, thereby engaging a specific form of cognitive control. While a broad literature links developmental psychopathology to reduced cognitive control, anomalous findings in the current study may apply specifically to the relatively pure forms of anxiety and areas of cognitive control targeted in the current study. In the cognitive domain, such forms may be closely related to error processing. As such, the current findings suggest that particular forms of anxiety are associated with a tendency to engage cognitive-control strategies in the context of error-laden activities. In this particular instance, perturbed emotion dysregulation in a cognitive-control domain would manifest in an anomalous way: as an excessive deployment of control capacities, and such excessive deployment in psychopathology is a relatively unusual pattern in the broader literature on emotion dysregulation, whereby emotional dysregulation involved reduced cognitive-control capacities. From this broader perspective, the current findings suggest that apparent contradictions in the literature on psychopathology and cognitive control might be resolvable by narrowing the focus of investigation, in terms of both psychopathological and cognitive-control domains.
In past literature, the associations with anxiety tend to vary across measures of inhibitory control and developmental stage and are particularly inconsistent for behavioral measures compared to psychophysiological measures (Henderson et al., 2015, Moser et al., 2013; White, Moore, et al., 2017), the current antisaccade paradigm could fill a need for more measures in this domain. The most consistent findings arise for the ERN, an electrophysiological measure, where meta-analysis demonstrates an association in the medium to large range (Moser et al., 2013). Of note, in the same studies generating this difference in physiology, meta-analysis finds no evidence of relationships between anxiety and behavior. Thus, electrophysiology is more sensitive than behavior to between-group differences, with the data suggesting greater engagement of error-related processes in high-relative to low-anxiety subjects. Other data dissociate anxiety from impulse control disorders, where both ERN and behavior suggest impaired inhibitory control (Nieuwenhuis et al., 2004; Troller-Renfree et al., 2016).
Finally, still other data suggest developmental moderation of the association between anxiety and ERN. Specifically, in young children, anxiety may relate to reduced inhibitory control and a small ERN, which may transform with development to yield the more consistent pattern of findings relating anxiety to increased inhibitory control (Meyer et al., 2018). Thus, not only might findings vary by the specific psychiatric and cognitive profile that is examined in a study, but they might vary as a function of developmental stage and level at which cognition is assessed using electrophysiology as opposed to behavior. Compared with relatively consistent electrophysiology results, findings for behavior are inconsistent, with evidence of negative, positive, and no associations (Henderson et al., 2015; Moser et al., 2013; White, Moore, et al., 2017), Again, this emphasizes the need for more sensitive behavioral measures targeting error-related processes related to inhibitory control.
Study conclusions should be tempered by limitations, many of which relate to inherent weaknesses in the case-control design. Thus, generalizability is limited by referral biases, and score distributions on key variables can be biased, through a categorical recruitment approach, for constructs that are continuously distributed in the community. Because our recruitment strategy was aimed to oversample for clinical levels of anxiety in medication-free, treatment-seeking youth with no other primary diagnosis, we are limited in our ability to interrogate the effects of comorbid symptoms (e.g., externalizing symptoms) on the association between anxiety and inhibitory control. Given both the high overlap of emotion dysregulation symptoms, such as irritability, with anxiety symptoms across psychiatric illness (Brotman, Kircanski, & Leibenluft, 2017; Gilliom & Shaw, 2004; Rhee, Lahey, & Waldman, 2015) and previous work demonstrating an association between externalizing behaviors and inhibitory control (Venables et al., 2018), it is essential for future work to recruit a large transdiagnostic sample within which a large variance in both externalizing and internalizing symptoms can be observed and statistical approaches can be employed to test for both independent and interactive effects of these symptoms on inhibitory control. In contrast, as outlined below, the current study is seen as a first step toward a broader framework, which emphasizes translation through studies of therapeutics. The current design recruits treatment-seeking medication-free patients; this facilitates research on treatment. This is because perturbed performance is demonstrated in a sample recruited using the same means that would be required to evaluate a novel treatment. Such a novel treatment could be designed to address a form of perturbed inhibitory control manifesting in aberrant task performance among such treatment-seeking patients. Another major limitation concerns the sample size. While typical of many studies examining associations between task performance and symptoms, our sample size is at the lower bound of acceptable. Power analyses indicate that a sample of 84 participants would be necessary to detect a correlation of .30 (α = 0.05, power = .80). Finally, while hypothesized associations between inhibitory control and anxiety were found, these associations only arose for one task parameter after controlling for age, potentially raising concerns regarding multiple testing and Type 1 error. Due to these limitations, the current findings might most appropriately be considered an initial step in methods development and hypothesis generation for a broader research framework.
An emotion dysregulation framework
While neuroscience research has flourished, there remains a serious need for a framework through which neuroscience findings can influence outcome prediction or treatment. The current study fits within a three-step framework aimed at leveraging cognitive neuroscience advances specifically to address clinical needs. The framework first refines tasks to target narrow behaviors. The second step adopts a latent-variable approach focused on multiple indicators of these narrow behavioral constructs. A latent-variable approach uses measured variables (e.g., latency to correct antisaccade, stop-signal reaction time, and reaction time to incongruent vs. congruent flanker trials) to extract an unmeasured (i.e., latent) factor capturing the common variance among the measured variables and thus, the shared underlying construct. Within this framework, the use of latent-variable analysis, such as confirmatory factor analysis, would leverage multiple measured inhibitory-control variables across tasks that differ in behavioral goals, response modality, and task context. By extracting only what is common among these measures, a latent factor is viewed to be a purer measure through the minimization of noise due to indiosyncratic task impurities. As a result, this statistical approach overcomes barriers posed by the relatively small effect sizes observed when quantifying the relationship between narrow and complex behaviors assessed in clinical research. This maximizes sensitivity to link cognitive neuroscience and clinical constructs. Finally, the framework attempts to maximize clinical impact through longitudinal investigation of developmental trajectories of inhibitory control and anxiety paired with experimental work developing novel treatments targeting inhibitory control.
The framework begins by targeting narrow cognitive neuroscience constructs, targeting inhibitory control as opposed to emotion regulation or cognitive control. Whereas emotion regulation encompasses many phenomena, cognitive control refers to subcomponents of this broader construct. Inhibitory control represents an even narrower behavioral component in the cognitive-control domain. As part of this focused approach, the framework uses behavioral paradigms derived from neuroscience, such as the antisaccade task, to bridge basic and developmental psychopathology research. Lack of progress in translational neuroscience may at least partly derive from differences in scale for the constructs targeted in neuroscience and clinical studies. Whereas neuroscience targets simple constructs, clinical disorders manifest with complex behaviors and heterogenous presentations. Neuroscience research has enjoyed greater success in delineating mechanisms generating behavior when mapping circuits regulating simple as opposed to complex behaviors such as clinical entities (LeDoux & Daw, 2018; LeDoux & Pine, 2016; Luna et al., 2008). Specifically, research finds greater progress by targeting antisaccades as opposed to emotion dysregulation (Hikosaka, 2007; Hikosaka et al., 2018). As such, linking performance on the antisaccade task to clinical constructs creates a bridge connecting findings in neuroscience to clinical research on emotion regulation.
This narrow-focus approach faces problems when it fails to acknowledge the small effect sizes that are typically found when testing relationships between cognitive task performance and clinical variables. Many of the cognitive tasks that are employed were designed orginally in the context of experimental research, where the main goal was to maximize experimental effects resulting in robust and highly reliable task effects. These highly reliable effects often come at the cost of reducing intersubject variability (Hedge, Powell, & Sumner, 2018). Therefore, when using these cognitive paradigms within the context of clinical research, where the aim is to uncover individual differences, the ability to detect individual differences using correlational analyses (such as those used in the present study) can be undermined due to high reliability of task performance and low between-subject variability. This is consistent with research on pediatric anxiety disorders and other emotional conditions, where effect sizes for cognitive task performance are rarely more than medium. Meaningful correlations typically fall in the r = .30 to r = .40 range, the range found in the current study (Bari & Robbins, 2013; Derakshan et al., 2009; White, Sequeira, et al., 2017). The second step of the current framework leverages novel methods to target narrow constructs, given small effect sizes. Recent research on inhibitory control suggests that latent-variable approaches mitigate the problems associated with such small effects by minimizing the noise resulting from task-or measurement-specific effects (Miyake & Friedman, 2012). Findings from the current study lay the groundwork for such a latent-variable approach, as expected patterns of intercorrelations emerged among the tasks.
As a third step, the current framework utilizes a latent factor of inhibitory control in longitudinal and experimental research. For example, prior work on adolescent depressive symptoms suggests that reductions in inhibitory control at one study wave predict increased depressive symptoms at subsequent waves. Findings in the current study might suggest an opposite trajectory in anxiety, and results from the ERN support this possibility. Specifically, unlike for depressive symptoms, relatively high levels of inhibitory control at one study wave might predict increases in anxiety symptoms over time. In addition, experimental work shows that it is possible to alter levels of inhibitory control through training. Thus, future experimental work on anxiety might utilize such training regimens to develop novel treatments, as has been done for other cognitive perturbations (i.e., attention bias to threat) in pediatric anxiety. Of note, findings from such longitudinal research carry major implications for experimental research. If inhibitory-control findings in anxiety resemble those in depression, such that reductions in inhibitory control early in life are predictive in increased anxiety symptoms later in life, manipulations that increase inhibitory control would be therapeutic. However, if anxiety is uniquely associated with enhanced inhibitory control, then such manipulations might be contraindicated.
The current study and proposed framework lay the groundwork for future large-scale translational work aimed at assessing a focused narrow target of emotion dysregulation: inhibitory control. Preliminary results support the use of a mixed-event antisaccade task as a measure of inhibitory control and suggest that anxiety symptoms may be related to increased inhibitory-control capacity. Following the three steps outlined in our broader framework for translation research of emotion dysregulation, researchers can leverage inhibitory control behavioral paradigms derived from neuroscience, such as the antisaccade task, and advanced statistical methods to bridge basic and developmental psychopathology research. Such research would provide much needed power and clarity to our understanding of atypical cognitive-control processes as it relates to anxiety disorders and ultimately may inform advances in addressing clinical needs such as outcome prediction or treatment.
Financial support.
Supported by the NIMH Intramural Research Program (ZIAMH002781), conducted under NIH Clinical Study Protocols 01-M-0192 (ClinicalTrials.gov identifier: NCT00018057), and by Bench-to-Bedside Award 479969.
References
- Abend R, de Voogd L, Salemink E, Wiers RW, Perez-Edgar K, Fitzgerald A, … Bar-Haim Y (2018). Association between attention bias to threat and anxiety symptoms in children and adolescents. Depression and Anxiety, 35, 229–238. doi: 10.1002/da.22706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aichert DS, Wostmann NM, Costa A, Macare C, Wenig JR, Moller HJ, … Ettinger U (2012). Associations between trait impulsivity and prepotent response inhibition. Journal of Clinical and Experimental Neuropsychology, 34, 1016–1032. doi: 10.1080/13803395.2012.706261 [DOI] [PubMed] [Google Scholar]
- Aldao A, Gee DG, De Los Reyes A, & Seager I (2016). Emotion regulation as a transdiagnostic factor in the development of internalizing and externalizing psychopathology: Current and future directions. Development and Psychopathology, 28(4, Pt. 1), 927–946. doi: 10.1017/S0954579416000638 [DOI] [PubMed] [Google Scholar]
- Aron AR (2011). From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biological Psychiatry, 69, e55–e68. doi: 10.1016/j.biopsych.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, & Robbins TW (2013). Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. doi: 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Future directions in emotion dysregulation and youth psychopathology. Journal of Clinical Child and Adolescent Psychology, 44, 875–896. doi: 10.1080/15374416.2015.1038827 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, & Zisner A (2017). Motivation, emotion regulation, and the latent structure of psychopathology: An integrative and convergent historical perspective. International Journal of Psychophysiology, 119, 108–118. doi: 10.1016/j.ijpsycho.2016.12.014 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SM (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 545–553. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Brotman MA, Kircanski K, & Leibenluft E (2017). Irritability in children and adolescents. Annual Review of Clinical Psychology, 13, 317–341. doi: 10.1146/annurev-clinpsy-032816-044941 [DOI] [PubMed] [Google Scholar]
- Cardinale EM, Breeden AL, Robertson EL, Lozier LM, Vanmeter JW, & Marsh AA (2018). Externalizing behavior severity in youths with callous–unemotional traits corresponds to patterns of amygdala activity and connectivity during judgments of causing fear. Development and Psychopathology, 30, 191–201. doi: 10.1017/S0954579417000566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PM, Hall SE, & Hajal NJ (2017). Emotion dysregulation as a vulnerability to psychopathology In Beauchaine TP & Hinshaw SP (Eds.), Child and adolescent psychopathology (3rd ed., pp. 346–386). Hoboken, NJ: Wiley. [Google Scholar]
- Derakshan N, Ansari TL, Hansard M, Shoker L, & Eysenck MW (2009). Anxiety, inhibition, efficiency, and effectiveness: An investigation using antisaccade task. Experimental Psychology, 56, 48–55. doi: 10.1027/1618-3169.56.1.48 [DOI] [PubMed] [Google Scholar]
- Eriksen CW (1995). The flankers task and response competition: A useful tool for investigating a variety of cognitive problems. Visual Cognition, 2, 101–118. doi: 10.1080/13506289508401726 [DOI] [Google Scholar]
- Everling S, & Fischer B (1998). The antisaccade: A review of basic research and clinical studies. Neuropsychologia, 36, 885–899. doi: 10.1016/S0028-3932(98)00020-7 [DOI] [PubMed] [Google Scholar]
- Fischer B, & Ramsperger E (1984). Human express saccades: Extremely short reaction times of goal-directed eye movements. Experimental Brain Research, 57, 191–195. doi: 10.1007/BF00231145 [DOI] [PubMed] [Google Scholar]
- Fox NA, & Calkins SD (2003). The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion, 27, 7–26. doi: 10.1023/A:1023622324898 [DOI] [Google Scholar]
- Geier CF, & Luna B (2012). Developmental effects of incentives on response inhibition. Child Development, 83, 1262–1274. doi: 10.1111/j.1467-8624.2012.01771.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliom M, & Shaw DS (2004). Codevelopment of externalizing and internalizing problems in early childhood. Development and Psychopathology, 16, 313–333. 10.1017/S0954579404044530 [DOI] [PubMed] [Google Scholar]
- Gold AL, Shechner T, Farber MJ, Spiro CN, Leibenluft E, Pine DS, & Britton JC (2016). Amygdala-cortical connectivity: Associations with anxiety, development, and threat: 2015 ADAA Scientific Research Symposium: Anxiety, development, and connectivity. Depression and Anxiety, 33, 917–926. doi: 10.1002/da.22470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE (1978). Primary and secondary saccades to goals defined by instructions. Vision Research, 18, 1279–1296. doi: 10.1016/0042-6989(78)90218-3 [DOI] [PubMed] [Google Scholar]
- Hardin MG, Mandell D, Mueller SC, Dahl RE, Pine DS, & Ernst M (2009). Inhibitory control in anxious and healthy adolescents is modulated by incentive and incidental affective stimuli. Journal of Child Psychology and Psychiatry, 50, 1550–1558. doi: 10.1111/j.1469-7610.2009.02121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge C, Powell G, & Sumner P (2018). The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behavior Research Methods, 50, 1166–1186. doi: 10.3758/s13428-017-0935-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, & Fox NA (2015). Behavioral inhibition and developmental risk: A dual-processing perspective. Neuropsychopharmacology, 40, 207–224. doi: 10.1038/npp.2014.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O (2007). Basal ganglia mechanisms of reward-oriented eye movement. Annals of the New York Academy of Sciences, 1104, 229–249. doi: 10.1196/annals.1390.012 [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Kim HF, Amita H, Yasuda M, Isoda M, Tachibana Y, & Yoshida A (2018). Direct and indirect pathways for choosing objects and actions European Journal of Neuroscience. Advance online publication. doi: 10.1111/ejn.13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J, & Koenigs M (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biological Psychiatry, 83, 638–647. doi: 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Fang A, & Asnaani A (2012). Review: Emotion Dysregulation Model of Mood and Anxiety Disorders. Depression and Anxiety, 29, 409–416. doi: 10.1002/da.21888 [DOI] [PubMed] [Google Scholar]
- Hutton SB, & Ettinger U (2006). The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology, 43, 302–313. doi: 10.1111/j.1469-8986.2006.00403.x [DOI] [PubMed] [Google Scholar]
- Jazaieri H, Morrison AS, Goldin PR, & Gross JJ (2015). The role of emotion and emotion regulation in social anxiety disorder. Current Psychiatry Reports, 17. doi: 10.1007/s11920-014-0531-3 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, … Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kircanski K, White LK, Tseng W-L, Wiggins JL, Frank HR, Sequeira S, … Brotman MA (2018). A latent variable approach to differentiating neural mechanisms of irritability and anxiety in youth. JAMA Psychiatry, 75, 631. doi: 10.1001/jamapsychiatry.2018.0468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, & Habel U (2014). Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. NeuroImage, 87, 345–355. doi: 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, & Daw ND (2018). Surviving threats: Neural circuit and computational implications of a new taxonomy of defensive behaviour. Nature Reviews Neuroscience, 19, 269–282. doi: 10.1038/nrn.2018.22 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, & Pine DS (2016). Using neuroscience to help understand fear and anxiety: A two-system framework. American Journal of Psychiatry, 173, 1083–1093. doi: 10.1176/appi.ajp.2016.16030353 [DOI] [PubMed] [Google Scholar]
- Loe IM, Feldman HM, Yasui E, & Luna B (2009). Oculomotor performance identifies underlying cognitive deficits in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 48, 431–440. doi: 10.1097/CHI.0b013e31819996da [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, & Cowan WB (1984). On the ability to inhibit simple and choice reaction time responses: A model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10, 276–291. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, & Sweeney JA (2004). Maturation of cognitive processes from late childhood to adulthood. Child Development, 75, 1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x [DOI] [PubMed] [Google Scholar]
- Luna B, Velanova K, & Geier CF (2008). Development of eye-movement control. Brain and Cognition, 68, 293–308. doi: 10.1016/j.bandc.2008.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey-Newman D, Kujawa A, Olino TM, Dyson M, & Klein DN (2018). Early temperamental fearfulness and the developmental trajectory of error-related brain activity. Developmental Psychobiology, 60, 224–231. doi: 10.1002/dev.21605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21, 8–14. doi: 10.1177/0963721411429458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Moran TP, Schroder HS, Donnellan MB, & Yeung N (2013). On the relationship between anxiety and error monitoring: A meta-analysis and conceptual framework. Frontiers in Human Neuroscience, 7. doi: 10.3389/fnhum.2013.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Hardin MG, Korelitz K, Daniele T, Bemis J, Dozier M, … Ernst M (2012). Incentive effect on inhibitory control in adolescents with early-life stress: An antisaccade study. Child Abuse & Neglect, 36, 217–225. doi: 10.1016/j.chiabu.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Hardin MG, Mogg K, Benson V, Bradley BP, Reinholdt-Dunne ML, … Ernst M (2012). The influence of emotional stimuli on attention orienting and inhibitory control in pediatric anxiety. Journal of Child Psychology and Psychiatry, 53, 856–863. doi: 10.1111/j.1469-7610.2012.02541.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Galloway-Long HS, Frick PJ, & Nigg JT (2013). Emotion regulation and heterogeneity in attention-deficit/hyperactivity disorder. Journal of the Amerian Academy of Child & Adolescent Psychiatry, 52, 163–171. doi: 10.1016/j.jaac.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Broerse A, Nielen MM, & de Jong R (2004). A goal activation approach to the studyof executive function: An application to antisaccade tasks. Brain and Cognition, 56, 198–214. doi: 10.1016/j.bandc.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Nigg JT (2013). Attention deficits and hyperactivity–impulsivity: What have we learned, what next? Development and Psychopathology, 25(4, Pt. 2), 1489–1503. doi: 10.1017/S0954579413000734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2017). Annual Research Review: On the relations among self‐regulation, self‐control, executive functioning, effortful control, cognitive control, impulsivity, risk‐taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, 58, 36–83. doi: 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). A synthetic review and evolving model of the cognitive control of emotion: Functional imaging studies of emotion regulation. Annals of the New York Academy of Sciences, 1251, E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, … Barch DM (2015). Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. Journal of Abnormal Psychology, 124, 817–833. doi: 10.1037/abn0000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, … Amir N (2015). Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment, 27, 365–376. doi: 10.1037/pas0000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Lahey BB, & Waldman ID (2015). Comorbidity among dimensions of childhood psychopathology: Converging evidence from behavior genetics. Child Development Perspectives, 9, 26–31. doi: 10.1111/cdep.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone EB, Garn CL, & Pine DS (2016). Anxiety regulation: A developmental psychopathology perspective In Cicchetti D (Ed.), Developmental psychopathology: Vol. 2. Developmental neuroscience (3rd ed, pp. 523–556). Hoboken, NJ: Wiley. [Google Scholar]
- Troller-Renfree S, Nelson CA, Zeanah CH, & Fox NA (2016). Deficits in error monitoring are associated with externalizing but not internalizing behaviors among children with a history of institutionalization. Journal of Child Psychology and Psychiatry, 57, 1145–1153. doi: 10.1111/jcpp.12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Foell J, Yancey JR, Kane MJ, Engle RW, & Patrick CJ (2018). Quantifying inhibitory control as externalizing proneness: A cross-domain model. Advance online pubication. Clinical Psychological Science. [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, & Hajcak G (2016). Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology, 53, 372–385. doi: 10.1111/psyp.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, Moore TM, Calkins ME, Wolf DH, Satterthwaite TD, Leibenluft E, … Gur RE (2017). An evaluation of the specificity of executive function impairment in developmental psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry, 56, 975–982. doi: 10.1016/j.jaac.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, Sequeira S, Britton JC, Brotman MA, Gold AL, Berman E, … Pine DS (2017). Complementary features of attention bias modification therapy and cognitive-behavioral therapy in pediatric anxiety disorders. American Jorunal of Psychiatry, 174, 775–784. doi: 10.1176/appi.ajp.2017.16070847 [DOI] [PMC free article] [PubMed] [Google Scholar]


