SUMMARY
Nuclear pore complex components (Nups) have been implicated in transcriptional regulation, yet what regulatory steps are controlled by metazoan Nups remains unclear. We identified the presence of multiple Nups at promoters, enhancers, and insulators in the Drosophila genome. In line with this binding, we uncovered a functional role for Nup98 in mediating enhancer-promoter looping at ecdysone-inducible genes. These genes were found to be stably associated with nuclear pores before and after activation. Although changing levels of Nup98 disrupted enhancer-promoter contacts, it did not affect ongoing transcription but instead compromised subsequent transcriptional activation or transcriptional memory. In support of the enhancer-looping role, we found Nup98 to gain and retain physical interactions with architectural proteins upon stimulation with ecdysone. Together, our data identify Nups as a class of architectural proteins for enhancers and supports a model in which animal genomes use the nuclear pore as an organizing scaffold for inducible poised genes.
Graphical Abstract
In Brief
Nuclear pore components (Nups) are known to interact with chromatin, but the precise mechanistic roles of Nups in gene regulation remain incompletely defined. Now, findings from Pascual-Garcia et al. reveal that silent genes can be bound by nuclear pores to promote enhancer-promoter looping upon activation.
INTRODUCTION
The nuclear pore complex (NPC) is a massive protein complex that forms the nuclear envelope (NE)-spanning channel, which allows selective nucleo-cytoplasmic transport of macromolecules (Knockenhauer and Schwartz, 2016; Raices and D’Angelo, 2012). It consists of approximately 30 different subunits, called nucleoporins (Nups). In addition to its canonical transport role, the NPC also functions in regulation of gene expression via physical interactions with chromatin. Multiple studies have demonstrated chromatin binding of specific Nups in a variety of genomes and have implicated NPC components in diverse aspects of gene regulation (Ptak and Wozniak, 2016; Sood and Brickner, 2014). These chromatin-binding roles of Nups are likely to be highly relevant to the control of gene expression programs in developing organisms, yet it is currently unclear what specific regulatory mechanisms are carried out by Nups at their gene targets in animal cells.
Nups in both yeast and metazoa have been most commonly linked to regulation of actively transcribing genes. Initially, the potential relationship between NPC and chromatin was suggested by cytological observations of de-condensed chromatin enriched in the vicinity of nuclear pores (Blobel, 1985). This has led to the idea that NPCs may contribute to setting up active expression states or to establishing boundaries between different chromatin states. Consistently, genomic binding sites of Nups are often enriched for active genes in yeast and metazoans (Capelson et al., 2010; Casolari et al., 2004; Kalverda et al., 2010; Liang et al., 2013; Rohner et al., 2013). Inducible yeast genes such as INO1, GAL1, and HXK1 re-localize to the NPC upon activation, and this association has been shown to promote expression and contribute to transcriptional regulation (Ahmed et al., 2010; Cabal et al., 2006; Light et al., 2010; Schmid et al., 2006; Taddei et al., 2006).
In animal cells, the relationship between chromatin and the NPC is further complicated by the phenomenon of dynamic Nups binding to genomic sites in the nuclear interior (Capelson et al., 2010; Kalverda et al., 2010; Liang et al., 2013; Vaquerizas et al., 2010). In contrast to stable Nups, which form the core structure of the NPC and remain stably associated with the NE during interphase, dynamic Nups are able to move on and off the nuclear pore (Rabut et al., 2004). Thus, in addition to being recruited to the NPC, genes can be regulated by dynamic Nups independently from the nuclear pore transport channel. Conceptually, this brings up the general question as to what regulatory function Nups exert on genes independently of their location. It has been proposed that active genes are recruited to nuclear pores to facilitate efficient export of generated mRNA (Blobel, 1985; Köhler et al., 2008; Kurshakova et al., 2007; Rodríguez-Navarro et al., 2004). But it is less clear how this reason would apply to genes regulated by Nups in the nucleoplasm, suggesting that Nups may have an additional, transport-independent role in regulation of the genome.
Nup98 is a dynamic Nup that can interact with chromatin off and on the nuclear pore, and that has been repeatedly implicated in transcriptional regulation in animal cells (Griffis et al., 2002; Liang et al., 2013; Light et al., 2013; Panda et al., 2014; Pascual-Garcia et al., 2014). In Drosophila, Nup98 binds to a subset of active genes and is required for full transcriptional activation of ecdysone-inducible genes Eip74 and Eip75 in larval development (Capelson et al., 2010). Yeast and human homologs of Nup98 have been shown to be involved in a process known as transcriptional memory, in which recently transcribed genes are primed for a more robust future activation (Light et al., 2013). But interestingly, genome-wide analysis of Drosophila Nup98 that could distinguish between nucleoplasmic and NPC-bound Nup98 revealed a puzzling pattern: although nucleoplasmic Nup98 targets primarily active genes, binding of the NPC-bound Nup98 is not enriched for transcribing loci and includes many silent genes (Kalverda et al., 2010). This observation is also supported by reported roles of NPC components in gene-silencing processes (Jacinto et al., 2015; Van de Vosse et al., 2013). Thus, the precise processes of transcriptional or epigenetic regulation, controlled by Nup98 and by genomic binding to the NPC remain to be elucidated.
To address these questions, we investigated genome-wide binding behavior of several Drosophila Nups, both stable and dynamic, and identified widespread presence of nuclear pore proteins at both promoters and regulatory regions such as enhancers. Our studies led us to test and identify a functional role of Nups in long-range contacts between enhancers and promoters. Together, our findings introduce a previously unrecognized role of metazoan nuclear pores in functioning as scaffolds for topological genome organization and suggest that in animal cells, nuclear pores stabilize enhancer-promoter loops, which contributes to transcriptional memory.
RESULTS
Nup98 Binds to DNase-Hypersensitive Regions, Including Enhancers and Insulators, in the Fly Genome
To obtain a Nup98 genome-wide binding profile of high resolution, we carried out chromatin immunoprecipitation sequencing (ChIP-seq) analysis of Nup98 in Drosophila S2 cells, using previously characterized antibodies (Capelson et al., 2010). The ChIP signal produced by this antibody was previously shown to be specific to Nup98 (Panda et al., 2014; Pascual-Garcia et al., 2014). The Nup98 ChIP-seq analysis resulted in the identification of 2,214 high-confidence genomic binding sites of Nup98 (Table S1), which included gene promoters and non-promoter regions. Because Nups have been linked to open chromatin states (Mendjan et al., 2006; Pascual-Garcia et al., 2014), we compared obtained Nup98 ChIP-seq profile with the previously characterized distribution of DNase-hypersensitive (DHS) sites, which are genomic regions with an absent or destabilized nucleosome (Shlyueva et al., 2014). We observed a strong correlation between Nup98-binding sites and DHS sites genome-wide (Figures 1A and S1B), with the overwhelming majority of Nup98 sites co-localizing with DHS sites.
Figure 1. Nup98 Binds Genomic Regulatory Elements, Including Enhancers and Insulators.
(A) Enrichment heatmaps of Nup98 ChIP-seq compared with DHS sequencing (DHS-seq) in S2 cells (Shlyueva et al., 2014), sorted by Nup98 occupancy around center of Nup98 binding peaks.
(B) Enrichment heatmaps of the non-promoter binding peaks of Nup98 ChIP-seq compared with enhancers (STARR-seq; Shlyueva et al., 2014) and ChIP-seq of insulators proteins(Wood et al., 2011; Yang et al., 2013), sorted by STARR-seq peak strength.
(C) Distribution of Nup98 ChIP-seq binding peaks relative to genomic elements, obtained from comparisons in (B).
(D) Representative genome browser (GB) view of a gene locus with Nup98 ChIP-seq, DHS-seq, and STARR-seq tracks. E and P mark the classified enhancer and promoters, respectively.
(E) Top DNA-binding motifs enriched among binding sites of Nup98, with corresponding p values and percentages of Nup98-binding sites with a given motif.
DHS sites in a variety of genomes are known to exist at three types of regions, namely, promoters, enhancers, and insulators (Tsompana and Buck, 2014). To determine the nature of non-promoter binding sites of Nup98, we compared the non-promoter/non-transcription start site (TSS) binding sites of Nup98 to known locations of enhancer and insulator elements. Enhancers have been recently mapped in the fly genome by the self-transcribing active regulatory region sequencing (STARR-seq) approach, and STARR-seq-identified (STARR) enhancers exhibit strong enrichment of the classic enhancer marks, including DHS sites and H3K27acetyl histone modification (Arnold et al., 2013). We observed a striking correlation between non-promoter binding sites of Nup98 and STARR enhancers (Figures 1B and S1B), suggesting that non-promoter binding sites of Nup98 commonly represent enhancers.
Additionally, we compared the non-promoter binding sites of Nup98 to genome-wide distribution of Drosophila architectural proteins dCTCF and Cp190, which are known to function in chromatin insulation (Van Bortle et al., 2014). The majority of non-promoter binding sites of Nup98 displayed a robust co-localization with dCTCF and Cp190 (Figures 1B, S1A, and S1B), which is consistent with proposed roles of architectural proteins in both insulator and enhancer functions (Pombo and Dillon, 2015). Together, although the majority of Nup98-binding sites were found to occur at promoters (74%), 17% fell into the category of enhancers (defined as non-promoter regions overlapping with STARR-seq signal), while an additional 6% corresponded to predicted insulators (defined as non-overlapping with STARR-seq, but overlapping with insulator proteins) (Figure 1C). Interestingly, we frequently detected Nup98 at both a promoter and an intronic STARR enhancer of the same gene (Figure 1D).
Generally, we found Nup98-binding sites to be enriched for promoter-associated consensus DNA motifs, including DREF, Motif 6, and E-box (Down et al., 2007; Ohler, 2006) (Figure 1E). The most significantly enriched DNA motif of Nup98 binding is the nuclear receptor (NR)-like motif, which shares similarity with consensus DNA motifs of nuclear hormone receptors such as the Drosophila ecdysone receptor (EcR) (Bernardo et al., 2009), which agrees with the previously identified role of Nup98 in regulation of ecdysone-inducible genes (Capelson et al., 2010). On the other hand, the non-promoter binding sites of Nup98 were found to be enriched for DNA motifs linked to insulator and enhancer function (Figure 1E), such as consensus binding motifs of CTCF, Su(Hw), and GAGA Factor (GAF), also known as Trithorax-like (Trl) (Ohtsuki and Levine, 1998) (Figure 1E). Interestingly, the GAGA binding motif is also over-represented in the genomic binding of human Nup98 (Liang et al., 2013), supporting evolutionary conservation.
Nuclear Pore Proteins Are Recruited to Enhancers in Fly Tissues
To determine whether we could similarly detect Nups at enhancers in vivo, we used isolated brains from third instar Drosophila larvae for ChIP-seq analysis of several Nups, including Nup98, Elys, and Nup93. Elys is the only Nup with a conserved DNA-binding domain and has been shown to bind to nucleosomes in vitro (Franz et al., 2007; Zierhut et al., 2014). Nup93 is a highly stable Nup (Rabut et al., 2004) that is part of the Nup93-Nup155 NPC scaffold sub-complex (Vollmer and Antonin, 2014) and thus would represent genomic binding of the actual nuclear pore. Our generated antibodies to Drosophila Elys and Nup93 were validated by immunofluorescence staining with RNAi knockdown of the respective protein in S2 cells (Figure S2A). To determine the distribution of Nups relative to active enhancers versus silenced chromatin, we concurrently performed ChIP-seq for H3K27acetyl and H3K27tri-methyl (H3K27Me3) histone modifications in the same tissue.
Our ChIP-seq analysis yielded highly reproducible binding patterns of the three Nups (Figures 2A and S2B; Tables S2, S3, and S4). As predicted by the ability of Elys to bind chromatin directly, ChIP-seq of Elys demonstrated widespread genome-wide binding (Figure 2A). ChIP-seq of Nup98 and Nup93 yielded considerably fewer binding peaks than Elys, yet the majority of Nup98 and Nup93 peaks were found to overlap with Elys (91% and 79% of Nup98 and Nup93 peaks, respectively; Figure S2C). These distribution patterns suggest that Elys may carry a widespread function in chromatin structure independently from other Nups, but that it likely co-functions with Nup98 and Nup93 in their chromatin-associated activities. Overall, our ChIP-seq analysis yielded 762 and 2,214 high-confidence binding sites of Nup98 in the brain tissues and S2 cells, respectively, which is comparable with the previously reported 811 binding sites of Nup98, identified by DamID in Kc cells (Kalverda et al., 2010), and the approximate 500 binding bands, identified by immunofluorescence on polytene chromosomes (Capelson et al., 2010).
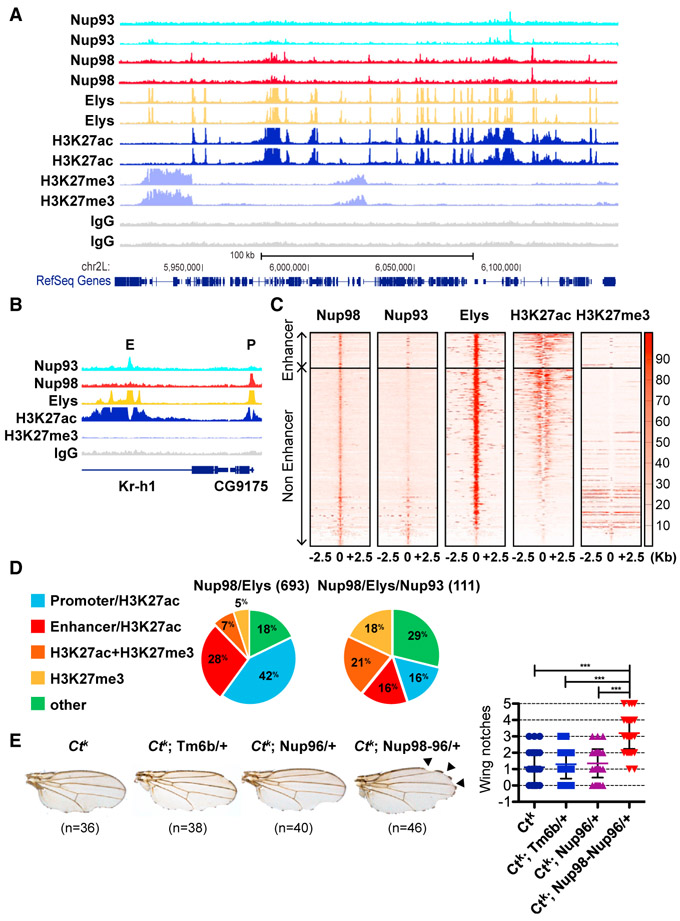
Figure 2. Nuclear Pore Proteins Are Recruited to Enhancers in Fly Tissues.
(A) A representative 200 kb GB snapshot (26AB on chromosome 2L), showing two independent replicates of Nup93, Nup98, Elys, H3K27acetyl (H3K27Ac), H3K27Me3, and control IgG ChIP-seq on brains from wandering third-instar larvae.
(B) GB view of a representative locus, showing binding peaks of Nups to regions, classified as either enhancer (E) or promoter (P). Enhancers are H3K27Ac peaks that are more than 1 kb away from promoter/TSS regions.
(C) Enrichment heatmaps of ChIP-seq-identified binding peaks for Nup98, Nup93, Elys, H3K27Ac, and H3K27me3, relative to centers of Nup98 binding peaks, sorted by H3K27Ac peak strength.
(D) Distribution of Nup98/Elys and Nup98/Elys/Nup93 common peaks (number in parenthesis is total number of peaks).
(E) Examples of wings from the listed genotypes in the ctK genetic background, demonstrating the enhancing effect of the Nup98 allele on the ctK wing-nicking phenotype (black arrows). The nicks/notches were quantified along the anterior half of the wing margin (p < 0.001 by Bonferroni method).
We observed a high level of genome-wide correlation of the H3K27acetyl signal to binding of Nup98 and Elys (Figures 2A-2C and S2D-S2F). The H3K27acetyl modification marks both active enhancers and promoters (Kharchenko et al., 2011; Rada-Iglesias et al., 2011), but we distinguished enhancers as regions of H3K27 acetylation that are not promoter associated (Figure 2B). Our analysis identified a subset of Nup98-binding sites, which correspond to enhancers (Figures 2D and S2F), supporting our conclusions on genomic distribution of Nup98 in S2 cells (Figure 1). Among the shared Nup98/Elys binding peaks, 28% are found at active enhancers (Figure 2D). Interestingly, genomic binding of the stable Nup93 was found to include a substantial amount of the silenced H3K27Me3-marked loci or loci, dually marked with H3K27Me3 and H3K27acetyl (Figures S2D-S2F), which may stem either from dually modified nucleosomes or from a mixed population of cells within the larval brain. Consistently, Nup93 displayed a genome-wide correlation to H3K27Me3 (Figures 2C and S2D-S2F), and the common Nup98/Elys/Nup93 sites, which are the highest confidence binding sites of actual nuclear pores, display roughly equal proportions of H3K27acetyl and H3K27Me3 regions (Figure 2D). This is supported by previous DamID studies, which found no enrichment for active genes among NPC-binding sites (Kalverda et al., 2010) and by the recent link of Nups to the function of polycomb group (PcG) proteins (Jacinto et al., 2015). These patterns confirm that unlike intranuclear Nups, which are strongly enriched for active chromatin, stable nuclear pores associate with genes in active or silent state.
The widespread presence of nuclear pore proteins at enhancers in the fly genome (Figures 1 and 2), as well as co-localization of Nup98 with architectural proteins (Figure 1), led us to hypothesize that Nups are involved in mediating long-range genome interactions, particularly enhancer-promoter contacts. To probe this idea, we used a genetic assay for enhancer-promoter communication, the cutK (ctK) allele (Herz et al., 2012; Hoover et al., 1992). The ctK allele carries an insertion of the gypsy insulator into the cut locus, such that the insulator prevents full activation of the cut gene by the wing margin enhancer and results in a “cut” phenotype, characterized by notches in the wing margin. Introduction of the heterozygous loss-of-function allele of the Nup98 gene resulted in a striking increase in the number of notches in the wing margin relative to the control genotypes (Figure 2E), indicative of a more impaired ability of the enhancer to communicate with the promoter. Although the Nup98 gene codes for both Nup98 and Nup96 proteins, the loss-of-function allele that disrupts only Nup96, Nup96339 (Presgraves et al., 2003), had no effect on the wing margin phenotype of the ctK allele (Figure 2E). Thus, this phenotype appears to be specific to Nup98, suggesting that Nup98 plays a role in promoter-enhancer communication.
Nup98 Is Required for Enhancer-Promoter Contacts at Ecdysone-Inducible Genes
Potential role of Nups in long-range genomic contacts has been previously suggested by the reported involvement of the yeast Nup Mlp1 in the formation of the 5′-3′ transcriptional gene loop (Tan-Wong et al., 2009). Thus, we aimed to determine whether metazoan Nups mediate enhancer-promoter or other long-range contacts of their target genes. To address this question, we analyzed the 3D folding of the ecdysone-inducible Eip74 and E23 genes, in response to transcriptional induction with ecdsyone (20-hydroxyecdysone [20E]). The Eip74 gene was found to exhibit extensive binding of Nup98 at promoters and intronic STARR enhancers in S2 cells (Figure 3A). Interestingly, we detected binding of Nup98 at the promoters and ecdysone-induced enhancers (those visible in STARR-seq +20E track) in untreated S2 cells, suggesting that Nup98 pre-binds to these regions before actual induction.
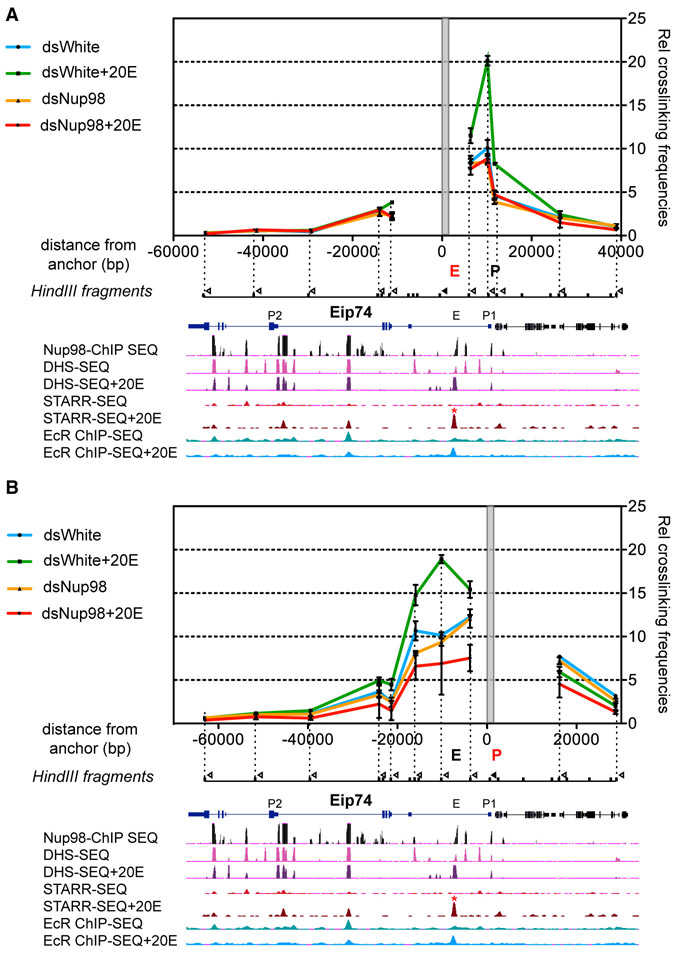
Figure 3. Nup98 Is Required for the Enhancer-Promoter Contact of Ecdysone-Inducible Genes.
(A and B) Long-range genomic interaction analysis by 3C at the Eip74 locus in S2 cells, ± ecdysone/20E treatment. The relative level of each ligation product (relative crosslinking or interaction frequencies) for each indicated condition is plotted against the genomic distance away from the anchor point (marked by gray rectangle). Below the graph is GB view of the Eip74 locus, with Nup98 ChIP-seq, DHS-seq, STARR-seq, and EcR ChIP-seq binding tracks before and after 20E treatment (Shlyueva et al., 2014). The analyzed enhancer is marked with an asterisk. Black tick marks indicate Hind III restriction sites, vertical dotted lines correspond to the fragments investigated in 3C assay, and hollow triangles indicate the primers tested. The primer of the anchor point is represented by a black triangle, at the enhancer (A) or the promoter (B). Data were collected from S2 cells, treated with dsRNAs against either Nup98 or control dWhite, ± 20E treatment for 4 hr. Error bars represent SEM from three independent experiments.
See also Figure S3.
To test potential role of Nup98 in scaffolding genomic loops, we carried out chromosome conformation capture (3C) assays in S2 cells with and without ecdysone/20E. Upon addition of 20E, the first intronic enhancer of Eip74 showed increased binding of EcR (Figure 3A). Using this enhancer as the anchor point in the 3C assay, we observed a strong enhancement of its contact with the Eip74 promoter upon addition of 20E to the control (White double-stranded RNA [dsRNA]-treated) cells (Figure 3A). This is expected given the proposed role of enhancer-promoter loop formation as part of transcriptional activation (Cavalli and Misteli, 2013). Strikingly, depletion of Nup98 using RNAi resulted in a drastic reduction of the ecdysone-induced enhancer-promoter contact, with the interaction frequency between these loci collapsing back to pre-induced levels (Figure 3A). These results indicate that Nup98 is necessary for the formation of ecdysone-induced enhancer-promoter loop. We obtained the same result using the promoter of Eip74 as the anchor point in the 3C assay (Figure 3B), which demonstrated a loss of the induced contact between the promoter and the first intronic enhancer upon depletion of Nup98. We did not detect a contact between the promoter and any of the 3′ regions of the gene, leading us to conclude that at least in the context of certain genes, metazoan Nups mediate enhancer-promoter looping as opposed to 5′-3′ looping. Furthermore, as for Eip74, we observed robust Nup98 ChIP-seq binding peaks at both the promoter and enhancer of another ecdysone-inducible gene E23 (Figure S3A). Using promoter as the anchor, the 3C assay similarly demonstrated a reduced frequency of contact between the E23 promoter and the Nup98-bound enhancer upon Nup98 depletion and ecdysone stimulation (Figure S3A). Additionally, we have previously reported a requirement of Nup98 in the expression of the Hph gene, which is constitutively transcribed in S2 cells (Pascual-Garcia et al., 2014). Thus, we tested and identified a modest but reproducible reduction in the interaction frequency of the constitutive enhancer-promoter contact for this gene in Nup98-depleted cells, using the 3C assay (Figure S3B). These results support the notion that facilitation of transcription-associated enhancer-promoter contacts is a general function of Nup98.
In addition to ecdysone-inducible genes, Hox genes have been shown to be transcriptional targets of Nup98 in both Drosophila and mammalian cells (Oka et al., 2016; Pascual-Garcia et al., 2014). In agreement with these findings, we detected binding of Nup98 within the Bithorax Hox gene cluster, including DHS sites in the Ultrabithorax (Ubx) intron and in the regulatory bxd region in S2 cells (Figure 3SC). The Bithorax region is transcriptionally silenced in S2 cells via extensive binding of PcG proteins (Kharchenko et al., 2011), but it is folded into a particular topological conformation in these cells (Lanzuolo et al., 2007). Using the DHS site in the bxd region as the anchor, we detected several high-frequency long-range contacts along the Ubx gene by 3C but did not see any pronounced change in these contacts upon Nup98 depletion (Figure 3SC). We conclude that although Nup98 binds to silenced genes such as Hox genes or un-induced Eip74 gene, it appears to preferentially scaffold activation-induced long-range contacts. Furthermore, the lack of effect on long-range contacts of the silenced Bithorax locus demonstrates that the effect of Nup98 depletion on Eip74 looping is not a consequence of widespread chromatin folding defects.
Drosophila Nup98 Plays a Role in Transcriptional Memory
Because Nup98 was found to be required for the ecdysone-induced enhancer-promoter loop, we asked whether Nup98 regulates the transcriptional output of ecdysone-inducible genes. Interestingly, we found that like other inducible genes (Hampsey et al., 2011; Light et al., 2013), ecdysone-inducible genes exhibit transcriptional memory. Treatment of S2 cells with ecdysone results in transcriptional activation of Eip74 and E23, which increases during the first 4 hr, as assessed by RT-qPCR (Figures 4A-4C). Removal of ecdysone leads to expected repression of these genes, which remain repressed during the 24 hr of recovery, while subsequent re-induction with ecdysone leads to a more robust transcriptional activation of Eip74 and E23, as assessed by mRNA levels (Figures 4A-4C).
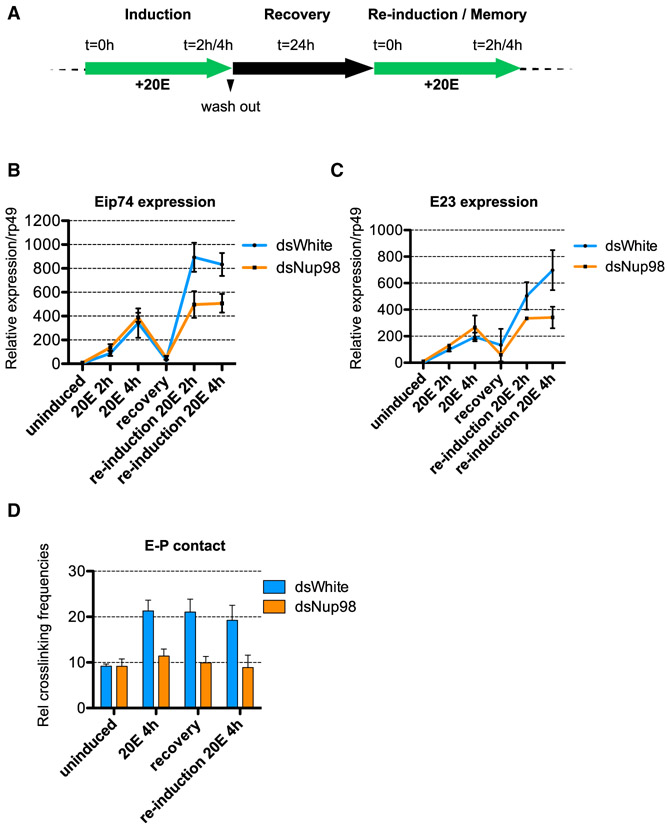
Figure 4. Nup98 and the Enhancer-Promoter Loop Facilitate Transcriptional Memory.
(A) Time course of induction and re-induction with ecdysone/20E. For initial induction, S2 cells were treated with 5 μM of 20E, and indicated time points were collected. Induced cells were cultured in fresh media without 20E for 24 hr, with the recovery time point collected at the end of this period, then reinduced with 5 μM of 20E for indicated time.
(B and C) RT-qPCR analysis of the mRNA levels of Eip74 (B) and E23 (C), in S2 cells, treated as indicated (at time points in A) and normalized relative to rp49. Error bars represent SEM from three independent experiments.
(D) Plot of the 3C relative interaction frequencies between Eip74 enhancer (anchor point, shown in Figure 3A) and promoter (at time points as in A), with and without Nup98 depletion, showing the presence of the induced enhancer-promoter contact during the recovery period. Error bars represent SEM from three independent biological replicates.
See also Figure S4.
Surprisingly, we found that RNAi-mediated depletion of Nup98 (Figure 4SA) did not impact transcriptional activation dynamics of the first induction, as measured by RT-qPCR (Figures 4B and 4C). This was particularly unexpected given that at the same time point of ecdysone treatment, the contact of the enhancer with the promoter of the Eip74 and E23 genes is impaired in Nup98-depleted conditions (Figures 3 and S3A). These results suggest that the observed effect of Nup98 on looping is not a consequence of upstream defects in transcriptional initiation. In support of this conclusion, Nup98 depletion did not affect either total or nuclear protein levels of EcR before or after ecdysone treatment (Figure S4B), indicating that the effect of Nup98 on looping of Eip74 and E23 is not due to indirect effects of Nup98 on levels or import of EcR. Intriguingly, these findings further imply that increased formation of the enhancer-promoter loop can be uncoupled from transcriptional activation.
In contrast to the first transcriptional induction, Nup98-depleted cells displayed a significant defect in the primed activation dynamics of the re-induction. The transcriptional re-activation after a period of repression occurred less robustly and less rapidly in cells treated with RNAi against Nup98 versus the control, at both Eip74 and E23 genes (Figures 4B and 4C). These results indicate that Nup98 is required for marking recently transcribed genes as part of epigenetic transcriptional memory, as has been reported for Nup98 homologs in yeast and human cells (Light et al., 2013). But combined with our results presented above, these findings suggest that stabilization of required enhancer-promoter contacts by Nup98 contributes to transcriptional memory. In support of this notion, we found that the ecdysone-induced enhancer-promoter contact of the Eip74 gene persists even after the 24 hr recovery period of transcriptional repression (Figure 4D). This Nup-mediated stabilization of the enhancer-promoter contact may promote the rapid activation dynamics of later inductions.
Overexpression of Nup98 Similarly Disrupts Enhancer-Promoter Looping, but Not Transport
Previously, we have found that overexpression of Nup98 in fly tissues behaves genetically similarly to the RNAi-mediated knockdown of Nup98 in the context of certain phenotypes (Pascual-Garcia et al., 2014). For example, both overexpression and knockdown of Nup98 in the fly haltere result in lower levels of Ubx expression (Pascual-Garcia et al., 2014). On the basis of these observations, we tested whether overexpression of Nup98 in S2 cells can recapitulate the effects of Nup98 depletion on transcriptional behavior of ecdysone-inducible genes (Figures 5A and 5B). Importantly, unlike depletion, overexpression of Nup98 is not expected to cause defects in nucleo-cytoplasmic transport. Consistently, we did not observe any mRNA export defect in S2 cells, transfected with myc-tagged Nup98, using fluorescence in situ hybridization (FISH) with oligo-dT probes (Figure 5SA). Furthermore, we did not detect any changes in total or nuclear levels of EcR during any of the time points of our time course upon overexpression of Nup98 (Figure 5SB). Overexpression of myc-Nup98 in S2 cells, which results in approximately 2-fold increase in Nup98 levels, exhibited an essentially identical transcriptional phenotype as the Nup98 depletion (Figures 5A and 5B). Overexpression of Nup98 impaired the robust activation dynamics of the re-induction of the Eip74 gene (Figure 5A), supporting the idea that the role of Nup98 in transcriptional memory is independent of its transport functions.
Figure 5. Increased Levels of Nup98 Disrupt Enhancer-Promoter Looping and Transcriptional Memory.
(A) RT-qPCR analysis of the Eip74 mRNA levels in S2 cells, transfected with Nup98-Myc (pNup98-Myc) or control empty plasmids (at time points as in Figure 4A), normalized to rp49 and plotted against time. Error bars represent SEM from three independent experiments.
(B) Western blot on S2 cells, transfected with Nup98 or control, ± 20E for 4 hr, stained with indicated antibodies and Ponceau S.
(C and D) ChIP-seq with antibodies to endogenous Nup98 in control/normal versus Nup98 overexpressing S2 cells, represented by a plot of binding enrichment, oriented around the center of normal Nup98 binding peaks (C), and by enrichment heatmaps of Nup98 ChIP-seq peaks, sorted by control Nup98 ChIP-seq signal (D).
(E) 3C interaction analysis at the Eip74 locus in S2 cells, transfected with Nup98-Myc or control, ± 20E for 4 hr. Relative crosslinking frequencies are plotted against genomic distance away from the enhancer (E) anchor point (marked by gray rectangle), with schematic and notation as in Figure 3. Error bars represent SEM of three independent experiments.
Nup98 has been shown to be involved in self-interactions (Qiu et al., 2015; Xu and Powers, 2013) and the self-interacting properties of its intrinsically disordered FG domains can lead to formation of hydrogels in vitro and presumably in vivo (Schmidt and Görlich, 2015). We hypothesized that overexpression of Nup98 can pheno-copy its depletion via aberrant oligomerization of Nup98, which may disrupt is normal chromatin binding. In support of this hypothesis, we observed a striking spreading of Nup98 around its normal binding sites in Nup98-overexpressing S2 cells, using ChIP-seq analysis with antibodies to endogenous Nup98 (Figures 5C and 5D; Table S5). Furthermore, the binding intensity of Nup98 at its normal binding peaks appeared reduced in the overexpressing cells (Figure 5C). These results support the idea that overexpression of Nup98 leads to mis-localized chromatin binding of Nup98 and provide a possible explanation for the similarity of transcriptional phenotypes between depletion and overexpression of Nup98. Consistently with the transcriptional phenotype, we found that overexpression of Nup98 also resulted in a loss of ecdysone-induced enhancer-promoter contact at the Eip74 locus, as assessed by 3C using either the enhancer (Figure 5E) or the promoter (Figure S5C) as the anchor points. Together, these findings reinforce the notion that Nup98 mediates activation-induced enhancer looping and does so independently of its transport functions.
Metazoan Nuclear Pores Bind Inducible Genes Independently of Transcriptional State
As discussed above, metazoan NPC components can interact with the genome at the nuclear periphery (via the NE-embedded NPCs) and in the nuclear interior (via nucleoplasmic Nups). Thus we assessed whether nuclear re-localization to or from the NPC is part of the regulation of ecdysone-inducible genes by Drosophila Nups. Previously, using DNA FISH, we have shown that multiple target genes of Nup98 reside in the nuclear interior of S2 cells (Capelson et al., 2010). In contrast, we found that un-induced Eip74 and E23 genes preferentially associate with the nuclear periphery, as assessed by DNA FISH in untreated S2 cells (Figure 6A). FISH-detected Eip74 and E23 loci were preferentially detected in zone 1 (nuclear periphery), quantified as previously described (Capelson et al., 2010). Given that these genes exhibit multiple peaks of Nup98 binding (Figures 3 and S3A), this association is highly likely to represent their presence at the NPCs. We assessed nuclear localization of the Eip74 locus throughout our time course (Figure 4A) and in all cases observed preferential targeting of Eip74 to the nuclear periphery/NPC and minimal (3%–16% of cells) localization in the interior zone 3 (Figure 6B). These findings reveal that ecdysone-inducible genes reside at the NPC before transcriptional activation and appear to remain associated with it through subsequent activation and establishment of transcriptional memory. Furthermore, we found that depletion of Nup98 results in increased localization of the Eip74 and E23 loci to the interior zone 3 (now elevated to 18%–26% of cells) (Figure 6C), suggesting that Nup98 is involved in anchoring these genes to the NPC. We similarly observed preferential localization of Eip74 to the nuclear periphery of polytenized cells of salivary glands in third instar wandering larvae (Figure 6D), indicating that this locus is associated with the NPC across different cell types.
Figure 6. Metazoan Nuclear Pores Scaffold Inducible Genes Independently of Transcriptional State.
(A) FISH analysis of nuclear localization of ecdysone-inducible genes in S2 cells, using probes against Eip74 and E23 (red) and co-stained with LaminDm0 (green) and Hoechst (blue). For assaying nuclear location, the nucleus was divided into three zones of equal volume, with zone 1 being the most peripheral and zone 3 most interior. The percentage of cells with FISH signal assigned into each zone is plotted to the right (scored cell number in parenthesis). Red dashed line indicates 33% distribution (random), and scale bar is set at 5 μm.
(B) FISH analysis with Eip74 probe, performed at indicated time points (described in Figure 4A, plus a time point of 24 hr induction).
(C) FISH analysis with Eip74 and E23 probes on S2 cells treated with dsRNAs against Nup98, co-stained with LaminDm0 and Hoechst.
(D) FISH analysis with Eip74 probe (white arrow), performed on polytenized nuclei from salivary glands of third instar wandering larvae, co-stained with LaminDm0 and Hoechst. Orthogonal views were generated, as shown, to quantify and plot percentage of cells with signal co-localization between Lamin and FISH probe, which were scored as peripheral location (number of scored nuclei in parenthesis).
The constitutive binding of ecdysone-inducible genes to the NPC suggests that the transcriptional regulation of these genes may rely on being at the NPC or require other NPC components. Thus we tested whether depletion of other Nups, such as the nuclear basket Mtor or the stable Nup93, similarly contribute to transcriptional regulation of Eip74 and E23. RNAi depletion of Mtor did not affect transcriptional behavior of ecdysone-inducible genes (Figures S6A and S6C), but similarly to depletion of Nup98, depletion of Nup93 was found to result in reduced levels of re-induction (Figures S6B and S6C). Interestingly, depletion of Nup93 also resulted in a somewhat compromised first induction with ecdysone, suggesting that Nup93 may be involved in a distinct aspect of the transcriptional response, but that similarly to Nup98, it is required for transcriptional memory. To further address the role of NPC targeting, we tested whether overexpression of Nup98, which is missing its C-terminal NPC-targeting domain (Nup981–578) (Kalverda et al., 2010), can recapitulate the effects of full-length Nup98 overexpression. We found that overexpression of Nup981–578 results in a partially compromised memory response of Eip74 during the re-induction (Figure S6D). Our interpretation of this result is that being anchored to the NPC contributes to regulation of ecdysone-inducible genes.
Nup98 Gains and Maintains Interactions with Architectural Proteins upon Ecdysone Stimulation
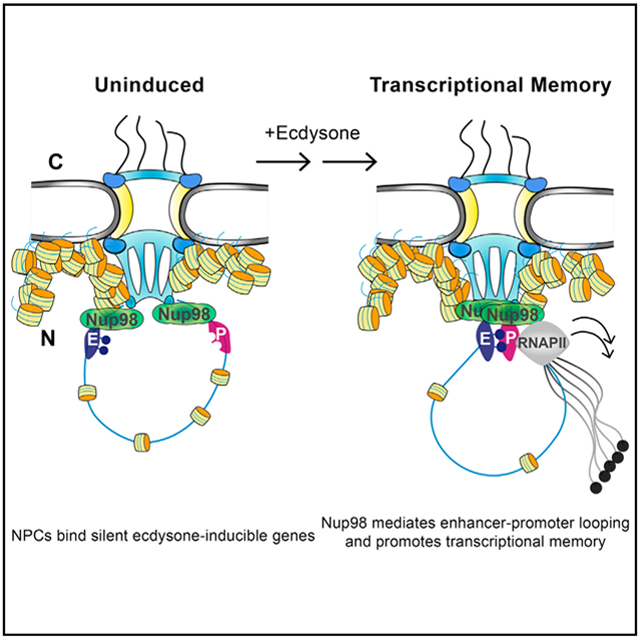
Taken together, our findings support a model in which silenced or poised genes are scaffolded by the NPC to mediate appropriate enhancer-promoter contacts upon activation and to stabilize such contacts as part of epigenetic memory of developing organisms (Figure 7A). Because Nup98 is already present at the Eip74 and E23 loci before expression, one possible implication of this model is that upon ecdysone treatment, Nup98 gains new physical interactions that would promote its role in enhancer-promoter communication (Figure 7A). To this end, we tested interactions of Nup98 with known architectural proteins, DNA-binding motifs of which were enriched in Nup98 ChIP-seq (Figure 1F), as well as with key machinery of the ecdysone transcriptional response, by co-immunoprecipitation (co-IP) analysis. We found that Nup98 gains an interaction with EcR only upon ecdysone stimulation, as opposed to ecdysone-independent interaction with another Nup, Mtor (Figure 7B). This interaction appears to be specific, as we did not detect an interaction between Nup98 and a histone methyl transferase (HMT) Trithorax-related (Trr) before or after ecdysone treatment (Figure 7B), although Trr is known to co-function with EcR in gene activation (Sedkov et al., 2003). Strikingly, we detected a dramatic increase in interactions between Nup98 and architectural proteins CTCF, GAF, and Su(Hw), upon ecdysone stimulation, while interactions of Nup98 with an insulator protein Cp190 were found to be constitutive (Figures 7B and 7C). Because a well-described function of architectural proteins is to mediate spatial genome organization (Gómez-Díaz and Corces, 2014), these findings provide further evidence to the role of Nup98 in promoting enhancer-promoter looping.
Figure 7. Nup98 Gains and Retains Interactions with Architectural Proteins upon Ecdsyone Treatment.
(A) Model for the role of Nup98 in enhancer-promoter communication and transcriptional priming of inducible genes at the NPC. Nup98 is present at both enhancer and promoter regions before activation and upon induction with ecdysone, Nup98 mediates formation of the enhancer-promoter contact, which is propagated epigenetically to contribute to faster re-activation.
(B and C) Co-IP analysis with anti-Nup98 (B), anti-CTCF, and anti-EcR (C) antibodies on S2 cell extracts, ± 20E for 4 hr, western-blotted with the indicated antibodies.
(D) Co-IP analysis with anti-Nup98 antibody on S2 cell extracts at the recovery and re-induction time points (defined in Figure 4A), western-blotted with the indicated antibodies.
See also Figure S6.
If the observed gained interactions between Nup98 and architectural proteins or EcR play a role in stabilization of the enhancer-promoter contacts to promote transcriptional memory, they may be expected to persist during the recovery period of transcriptional repression. As expected, we observed a similarly robust interaction between Nup98 and EcR, CTCF, Su(Hw), and GAF during the recovery and the re-induction time points (Figure 7D), indicating that Nup98 maintains interactions with architectural proteins. The prolonged maintenance of these interactions is consistent with the identified Nup98-mediated stabilization of the enhancer-promoter contact during transcriptional repression (Figure 4D).
DISCUSSION
Together, our results identify a specific transcriptional function for Nup98 in enhancer looping and reveal Nups as a class of architectural proteins that in addition to cohesins, CTCF, and Mediator components (Kagey et al., 2010; Merkenschlager and Nora, 2016) can promote enhancer-promoter interactions. Interestingly, the NPC has been recently shown to associate with the Mediator complex and to depend on a specific Mediator subunit, Med31, for binding the GAL1 gene in yeast (Schneider et al., 2015). Our genome-wide analysis and identification of physical interactions with architectural proteins further suggest that Nups may contribute to other topological contacts, such as those of insulators. This notion is further supported by previous links of Nups to insulators in both yeast and fly cells (Dilworth et al., 2005; Ishii et al., 2002; Kalverda and Fornerod, 2010).
Somewhat surprisingly, we have found that similarly to depletion of Nup98, Nup98 overexpression appears to disrupt the enhancer-promoter contact and transcriptional memory. This is likely due to the involvement of Nup98 in self-interactions, which can lead to aberrant oligomerization and mis-localized chromatin binding of Nup98 that prevent its participation in productive contacts (Figure 5). The biophysical properties of the intrinsically disordered FG domains of Nup98 involve the ability to polymerize and phase-separate (Schmidt and Görlich, 2015). Given recent implications of intrinsically disordered and phase-separating proteins in transcriptional regulation and nuclear structure (Dunker et al., 2015; Kwon et al., 2013), it will be particularly interesting to determine whether these properties of the FG domains underlie the identified functions of Nup98 in enhancer-promoter looping or in epigenetic memory of transcription.
The implications of our findings are potentially far reaching in the context of developmental gene regulation. Establishment and maintenance of appropriate enhancer-promoter contacts may be a general mechanism by which Nups contribute to regulation of gene expression during cell differentiation. Mutations in multiple Nups lead to defects in differentiation of specific cell lineages in the fly, zebrafish, and mouse models (D’Angelo et al., 2012; Davuluri et al., 2008; Mondal et al., 2014). The identified roles of the NPC in genome architecture may represent one of the mechanisms by which Nups contribute to tissue-specific processes. In addition to ecdysone-inducible genes, our chromatin binding analysis identified Hox genes as a recurrent target of NPC binding, and we have previously identified a physical association between Nup98 and an epigenetic regulator of Hox gene activity, Trithorax (Trx) (Pascual-Garcia et al., 2014). Trx and its mammalian homologs are HMTs responsible for methylation of H3 lysine K4 (Nakamura et al., 2002; Rickels et al., 2016), which is in line with the previously demonstrated dependence of promoter-associated H3K4 di-methylation levels on Nup98 in yeast and human cells (Light et al., 2013). It remains to be determined how enhancer-promoter looping is integrated with H3K4 di-methylation in transcriptional memory (D’Urso et al., 2016), but an intriguing possibility is the potential role of Nup98-stabilized enhancer-promoter contacts in Trx-mediated epigenetic regulation during development.
Our results also provide an example of uncoupling activation-induced enhancer-promoter contacts from transcriptional activation. A common model for how enhancers function proposes that enhancers loop over to contact their target promoters to drive transcriptional activation (Cavalli and Misteli, 2013), implying a direct cause to effect relationship from looping to transcription. But our data suggest that this relationship is not always this linear and can involve stabilization of enhancer-promoter loops that are not required for ongoing transcription. The increased enhancer-promoter interaction described in our study appears to function as part of a memory system through a period of repression.
Furthermore, our genome-wide analysis of Nup binding revealed that nuclear pores target both active and silenced loci in similar proportions (Figure 2). PcG-bound regions, marked by the H3K27Me3 mark, appear to be common targets of the stable NPCs, which is supported by a previous report of Nup153 playing a role in PcG-mediated gene silencing (Jacinto et al., 2015). On the basis of the rest of our findings, we propose that a specific subset of genes associates with NPCs independently of expression, for the purposes of stabilizing possible or future activation-induced long-range contacts. Although the transcriptional memory function of Nup98 is not limited to NPC targets and has been shown to apply to intranuclear genes (Light et al., 2013), it is possible that NPC binding may be particularly beneficial for genes with complex regulatory landscapes, such as ecdysone-inducible or Hox genes, where pre-scaffolding and epigenetic maintenance of enhancer-promoter architecture is most critical.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources should be directed to and will be fulfilled by the corresponding author, Dr. Maya Capelson (capelson@mail.med.upenn.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Lines
Drosophila S2 cells were cultured at 25°C in Schneider’s medium (DGRC) supplemented with 10% heat inactivated fetal bovine serum (GIBCO) and antibiotics.
Fly Lines
Drosophila genetic stocks used in this study include Bloomington stocks 28433 (P(EP)Nup98-96g2120), 4951 (Nup98-96339), 171 (ctk) and 5 (Oregon-R).
METHOD DETAILS
Transfection and RNAi Conditions
For ecdysone induction experiments, 20-hydroxyecdysone (Sigma) (hereafter 20E), dissolved in ethanol was added at 5 μM and cells were collected at different time points according to the experimental setup. Double-stranded RNAs (dsRNA) were synthesized with Megascript T7 kit (Ambion) using PCR-ed DNA template from fly genomic DNA or cDNA. RNAi-treated cells were obtained by incubating 7.5 μL of Fugene HD (Promega) with 10 μg of dsRNA per million of cells for 3 days. Nup98 overexpressed cells were obtained by transfecting the pNup98-Myc expression plasmid (Pascual-Garcia et al., 2014) combined with TransIT-Insect reagent (Mirus) at a 1:2 ratio, according to manufacturer’s instructions. For transcriptional memory experiments, cells were treated with the designated dsRNA for 72 hr (or Nup98-myc and empty vectors for 48 h) prior to ecdysone induction. Cells were induced with 20E at 5 μM and harvested at indicated time points during maximum of 24 hr (Induction 1). For recovery time points, cells were washed in normal media and re-transfected with the appropriate dsRNA (or Nup98-myc and empty vectors). After 24 hr of recovery in normal media, time point samples were harvested (Recovery), and cells were re-treated with 20E and harvested at indicated time points (Re-induction).
Fly Genetics and Wing Dissections
To assess phenotypic effect on ctK, ctK females were crossed to Nup98g2120/TM6b or Nup96339/TM6b males and the resulting male progeny were scored for wing margin phenotype. To analyze the cut wing phenotype, progeny flies of listed genotypes were immersed in dissection solution (60% Ethanol, 40% Sodium DL-lactate solution (Sigma)) and incubated at room temperature for at least 24 hr. Dissected wings were then flattened on glass slides and images were taken on Nikon Eclipse 80i and processed using Nikon software (NIS-Elements D3.0). The wing margin nicks were quantified by counting the number of gaps in the anterior row of the bristles along the wing margin.
Antibody Generation
Polyclonal antibodies to Nup98 were previously described (Capelson et al., 2010). Antibodies to Drosophila Elys (CG14215) and Nup93 (CG11092, LD21129 cDNA) were generated following a similar procedure as (Capelson et al., 2010). Briefly, C-terminal fragment of Elys, amino acids 1766-2110, was cloned into pDEST15 vector, expressed in BL21 cells and purified via the GST tag, using glutathione Sepharose beads. N-terminal fragment of Nup93, amino acids 1-179, was cloned into pETG-41A vector, expressed in BL21 cells and purified via the HIS tag, using Ni-NTA agarose beads. Purified recombinant proteins were sent to the Pocono Rabbit Farm & Laboratory for production of rabbit antisera for Elys and guinea pig antisera for Nup93. Obtained sera was affinity purified using the original antigen, coupled to CNBr-activated Sepharose (Sigma), similarly to (Capelson et al., 2010). Additional used antibodies and their sources include anti-EcR (DDA2.7, Developmental Studies Hybridoma Bank), anti-Tubulin (E7, Developmental Studies Hybridoma Bank), anti-Mtor (12F10, a gift from K. M. Johansen), anti-LaminDm0 (ADL101, Developmental Studies Hybridoma Bank), anti-Lamin (a gift from P.A. Fisher), anti-Nxf1 (a gift from E. Izaurralde), anti-Trr (a gift from A. Mazo), anti-H3 (ab1791, a gift from S.L. Berger), anti-CTCF, anti-CP190, anti-SuHw, anti-GAF (gifts from V.G. Corces), anti-H3K27Acetyl (Active Motif, 39133), anti-H3K27Me3 (Active Motif, 39155), mAb414 (Covance, MMS-120P) and anti-DIG-Rhodamine (Roche, 11 207 741 910).
Protein Extract and Cellular Fractionation
Protein extracts were prepared by lysing 1.5x106 S2 cells in RIPA buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP40 and 0.1% SDS) with 1mM of PMSF and Complete protease inhibitor cocktail (Roche) on ice for 20 min followed by brief centrifugation to remove debris. The cellular fractionation was performed with 1.0x107 cells per condition. Cells were re-suspended in swelling buffer (15 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl and 0.10% Igepal), incubated on ice for 30 min and homogenized with a 25 g needle. Lysate was centrifuged; supernatant corresponds to the cytoplasmic fraction and the pellet was washed twice with cold swelling buffer with no detergent to subsequently being re-suspended in RIPA buffer for 15 min. The re-lysed pellet was then centrifuged and the supernatant saved as the nuclear fraction. Protein concentration was quantified using BCA protein assay kit (ThermoFisher) and equal amounts of proteins were loaded and separated in SDS-PAGE gels. When detecting Nup98, EcR, Tubulin, Nxf1 or Lamin, we used 6% gels whereas 16% gels were used for H3 blots. Proteins were transferred to nitrocellulose membranes and incubated overnight at 4°C using primary antibodies at the following dilutions: rabbit anti-Nup98 1:4000, mouse anti-EcR (DDA2.7) 1:500, mouse anti-Tubulin (E7) 1:200, rabbit anti-Nxf1 1:5000, mouse anti-Lamin Dmo 1:5000 and rabbit anti-H3 1:10000. Membranes were then incubated with HRP secondary antibodies for 2 hr at room temperature and detected using ECL-Plus western blotting reagent (Amersham Biosciences).
Immunoprecipitation (IP)
Nuclei were purified from 1.5x107 cells by re-suspending in hypotonic buffer buffer (10mM HEPES pH 7.9, 1.5 mM MgCl2, 10mMKCl and 0.5mMEGTA) with 1mMPMSF and Complete protease inhibitor cocktail (Roche), followed by 10 min incubation on ice. Pelleted nuclei were incubated in lysis buffer (20mMHEPES pH 7.9, 25% Glycerol, 0.42MNaCl, 1.5mMMgCl2 and 0.2mMEGTA) with 1mMPMSF and Complete protease inhibitor cocktail (Roche) for 30 min on ice and then diluted 1:3 with lysis buffer without NaCl. Protein extracts were pre-cleared and incubated overnight with the appropriate antibodies: 15 μL of rabbit anti-Nup98 antibody, 10 μL of rabbit anti-CTCF antibody, 25 μL of rabbit anti-EcR antibody or 10 μL of the applicable IgG control. Finally, the antibody-protein complexes were incubated with 40 μL (as 50% slurry) of pre-blocked (PBS with 1% BSA) Rec-protein A-Sepharose 4B beads (Life technologies) for 1.5 hr. The beads were washed 4 times with wash buffer (50 mM Tris-HCl pH 8, 150 mM NaCl, 0.2% Igepal and 1 mM EDTA) and boiled in SDS-loading buffer for western blot analyses. Proteins were then resolved on 6% SDS-PAGE gels and transferred to nitrocellulose membranes. The primary antibodies used in western blotting were as follows: rabbit anti-Nup98 1:4000, mouse anti-EcR (DDA2.7) 1:500, mouse anti-Mtor 1:500, rabbit anti-Trr 1:1000, rabbit anti-CP190 1:10000, rabbit anti-CTCF 1:5000, rabbit anti-SuHw 1:5000 and rabbit anti-GAF 1:5000.
RNA Extraction and Expression Analysis
Total RNA was isolated from 0.5 x106 S2 cells using 1mL Trizol (Ambion). 1 μg of the extracted RNAs were used for first-strand cDNA synthesis using one-step RT-PCR kit (QIAGEN). To measure mRNA levels, Quantitative real-time PCRs (qPCRs) were carried out on obtained cDNA using gene-specific primers (Table S6). Each RT-qPCR was repeated at least three times, the values were normalized to the rp49 transcript and the error bars represented the standard deviation or error of the mean.
Chromosome Conformation Capture (3C)
3C experiments were performed as detailed in (Bernardo et al., 2014). Around 30 x106 S2 cells were cross-linked with 2% formaldehyde for 10 min at room temperature and the reaction was quenched with 0.125 M glycine. Samples were re-suspended in 10 mL of lysis buffer (10 mM Tris-HCl pH 8.0, 1 mM MgCl2, 10 mM NaCl, 0.2% Igepal) for 10 min in ice, followed by two series of 25 strokes using a Dounce homogenizer with 10 min incubation on ice in between. 10x106 of cells were then re-suspended in appropriate NEB 1.2X restriction enzyme buffer and incubated at 37°C for 90 min at 900 rpm with 5 μL of 20% SDS. We then added 50 μL of 12% Triton X-100/1X T4 Ligase buffer, incubated for 90 more min in the same conditions and digested overnight with 500U of Hind III or EcoRI while shacking at 900 rpm. Pellets were re-digested the next day with 100U of Hind III for 2 hr and the reaction was stopped by adding 40 μL of 20% SDS at 37°C for 30 min at 900 rpm. Samples before and after digestion reaction were taken to assess restriction efficiencies as described in Ea et al., 2017. We performed the ligation in diluted conditions by adding 6.125 mL of 1.15X of T4 ligation buffer, 375 μL of 12% Triton X-100 and incubating for 60 min at 37°C prior to addition of 2000U of T4 ligase and incubation for 5 hr at 16°C, followed by 30 min at room temperature (RT). After ligation, cross-linking was reversed at 65°C overnight with 400 μg of Proteinase K freshly prepared. DNA was purified with RNase A, phenol:chloroform extraction and ethanol precipitation and the DNA pellet was dissolved in 150 μL of 10 mM Tris-HCl pH 7.5.
Concentrations of ligation products were adjusted to 25 ng/μl by comparing to a reference sample of genomic DNA of known concentration. To measure interaction frequencies of 3C products, three independent experiments were conducted to amplify the ligated products using Power SYBR Green (Applied biosystems) using the recommended protocol. 3C primers and primers to assess digestion efficiencies were designed using the 3PD software described in (Fröhler and Dieterich, 2009). For each reaction, 2 μL of the 3C template was used and measured relative to control template generated from BAC DNA clones: BACN14N24 (Eip74), BACN04G20 (E23), BACR04K01 (Hph) or BACR28H1 (Ubx). All interactions were normalized to internal primers, not spanning any Hind III or EcoRI sites, at the rp49 locus (BAC#RP98-15I7). Primers are listed in Table S6.
Chromatin Immunoprecipitation (ChIP)
ChIP experiments from brains or S2 cells were done as described in (Agrawal and Shashidhara, 2014; Capelson et al., 2010) respectively with some modifications. In summary, each ChIP was performed with 60 brains from wandering third-instar larvae (Oregon-R) or 10x106 cells. Brains or cells were fixed in 1% formaldehyde for 10 min at room temperature. Cross-linking was stopped with glycine at a final concentration of 125 μM and then washed once in PBS and once in lysis buffer (140 mM NaCl, 15 mM HEPES pH 7.6, 1 mM EDTA, 0.5 mM EGTA, 0.1% sodium deoxycholate, 1% Triton X-100, 0.5 mM DTT, and 2X protease inhibitor cocktail). Brains or cells were re-suspended in 400 μL lysis buffer plus 0.1% SDS and DNA was sheared to enrichment around 100bp on a Diagenode Bioruptor. Fragmentation results were confirmed with High Sensitivity ChIP (Agilent technologies). Chromatin was immunoprecipitated overnight at 4°C with rabbit anti-Nup98, guinea pig anti-Nup93, rabbit anti-Elys, rabbit anti-H3K27Ac, rabbit anti-H3K27Me3 and IgG controls. All ChIP assays were performed with 1 - 5 μg of antibody and 45 μL (50% slurry) of pre-blocked M280 Dynabeads (Life technologies). Beads were washed once with each of the following wash buffers and twice with TE buffer for 5 min of rotation at 4°C: Low-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8, 150 mM NaCl), High-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8, 0.5 M NaCl), LiCl buffer (0.25 M LiCl, 1% Igepal, 1% NaDOC, 1 mM EDTA, 10 mM Tris-HCl, pH 8),TE buffer (10 mM Tris-HCl, pH 8, 1 mM EDTA). The DNA was eluted, reverse cross-linked, and purified by phenol chloroform extraction and ethanol precipitation. Immunoprecipitated DNA was prepared for Illumina sequencing using the NEBNext Ultra DNA Library Prep Kit for Illumina and the Index Primers Sets 1 and 2 NEBNext Multiplex Oligos for Illumina (New England Biolabs). DNA was quantified using Qubit dsDNA High Sensitivity Assay Kit (Life technology) and sequenced on an Illumina NextSeq 500 according to manufacturers protocol.
Immunofluorescence
Immunofluorescence of S2 cells was performed as described here (http://www.jove.com/details.php?id=1982). Rabbit anti-Nup98 was used at 1:400 dilution, guinea pig anti-Nup93 at 1:200 dilution, rabbit anti-Elys at 1:200 dilution, rabbit anti-Lamin at 1:1000 and mAb-414 at 1:500 dilution. Images were taken on Leica DM6000B and processed using Leica deconvolution software.
Fluorescence and poly(A+) In Situ Hybridizations
Fluorescence in situ hybridization (FISH) experiments in S2 cells were performed as detailed in (Liang et al., 2013). Cells were fixed and stained as explained in the immunofluorescence procedures, fixed again with 4% paraformaldehyde for 10 min, permeabilized in 0.1MHCl, 0.7% Triton X-100 on ice for 15 min and denatured in 50% formamide, 2X SSC pH 7.2 at 80°C for 30 min. BAC DNA clones, BACN14N24 and BACN04G20 were used as Eip74 and E23 templates respectively for DIG-labeled FISH probes using DIG-Nick translation mix (Roche), and 400 ng of the resulting products were used in each hybridization. Each probe was combined with 5 μL human Cot-I DNA (Invitrogen) and 10 μg salmon sperm DNA, precipitated, re-suspended and incubated with 5 μL 100% formamide for 30 min at 37°C, denatured for 8 min at 85°C, incubated for 1 hr at 37°C and combined with 5 μL 2X FISH hybridization buffer (4X SSC, 20% dextran sulfate, 2 mg/ml BSA). Cells were then incubated with corresponding probes overnight at 42°C, washed 3 times with 50% formamide/2X SSC (pH 7.2) and 2X SSC at 42°C and stained with anti-DIG antibody conjugated with Rodhamine at 1:200 dilution for 2 hr. For the three zone scoring, we used Fiji software to determine the zones and the relative position of the FISH spots. The nucleus was divided into three zones of equal volume, with zone 1-2-3 representing the most peripheral to the most interior zones, respectively.
FISH analysis in intact polytene chromosomes was performed following protocol in (Capelson et al., 2010) with some modifications. Salivary glands from third instar larvae were dissected and fixed with 8% Acetic Acid and 2% paraformaldehyde for 120 - 180 s and transferred into a drop of 2% paraformaldehyde on a sigma-coated coverslip before being gently pushed into a poly-L-lysinated slide. The slides were then submerged into blocking solution (PBS + 0.1% Tween20 + 3% BSA) for 1 hr and incubated overnight with rabbit anti-Lamin at 1:200 dilution. For in situ hybridization the tissues were post-fixed in 3.7% paraformaldehyde in PBS for 15 min at 37°C in a humidified chamber. The slides were then incubated in 2X SSC for 45 min at 70°C in a water bath and the DNA was denatured in 100 mM NaOH with 0.5% Triton X-100, washed in 2X SSC and dehydrated by passing twice through 70% ethanol and 96% ethanol. 1 μg of the corresponding probe was prepared as described previously and hybridized at 37°C overnight. After hybridization, the slides were washed 4 times in 2X SSC at 42°C for 5 min and incubated with anti-DIG antibody conjugated with Rodhamine at 1:100 dilution for 2 hr at RT.
The poly(A+) in situ hybridization experiments were done as described in (Herold et al., 2001). Cytospin S2 cells were fixed with 4% of paraformaldehyde in PBS for 10 min. After fixation, cells were permeabilized for 10 min in PBS containing 0.5% Triton X-100. To detect poly(A+) RNA, cells were incubated for 15 min at 37°C in pre-hybridization buffer (2X SSC, 20% formamide, 0.2% BSA, 1mg/ml of yeast tRNA). For hybridization, the coverslips were transferred to a humidified chamber and covered with 20 μL of hybridization buffer (2X SSC, 20% formamide, 10% dextran sulfate, 0.2% BSA, 1mg/ml of yeast tRNA) supplemented with 0.1 pmol/μl oligo (dT)40 fluorescently end labeled with Cy3 molecules. After 3 hr of hybridization at 37°C, cells were washed 3 times with 2X SSC/20% formamide and 2X SSC at 42°C and 2 times with 2X SSC and PBS at RT. Immunofluorescence was performed subsequently as previously described. The image-processing package Fiji was used to determine the poly (A+) relative intensity of the nucleus versus cytoplasm. In all in situ hybridization studies, three-dimensional image stacks were recorded with Leica DM6000B and processed with Leica deconvolution software.
QUANTIFICATION AND STATISTICAL ANALYSIS
ChIP-Seq Analysis
Sequencing reads were converted to FASTQ formatted files using the NCBI SRA Toolkit (Li et al., 2009) and mapped to Drosophila dm3 genome using Bowtie2 (version 2.1.0) (Langmead and Salzberg, 2012). Reads from culture cells or brains ChIP-seq libraries were quality trimmed by a minimum Phred quality of 3 at both ends and a minimum length of 30bp and Bowtie2 (version 2.1.0) (Langmead and Salzberg, 2012) was then used to map reads to Drosophila dm3 genome, allowing one mismatch in the seed alignment and reporting only the single best alignment whose mapping quality (MAPQ) is greater than 10. Duplicated reads and mitochondria reads were removed. The accession number for the ChIP-seq data reported in this paper is GEO: GSE94922.
Peak Calling and Data Visualization
For each biological replicate, significant regions of enrichment (peaks) were called using MACS2 (version 2.1.0) (Feng et al., 2012) (FDR < 0.05). Correlation heatmap and Principal Component Analysis were implemented by R package DiffBind (version 1.16.3) (Stark and Brown). Tracks were visualized using HOMER (version 4.7.2) (Heinz et al., 2010) and IGV (version 2.3.72) (Thorvaldsdóttir et al., 2013). For each IP, peaks from all replicates were merged into a final peak set using HOMER, keeping only peaks that were found in at least two replicates. Peak intersection between IP treatments was determined by BEDtools (version 2.25.0) (Quinlan and Hall, 2010). HOMER was again used for peak annotation. Enhancer regions in brain datasets were inferred as H3K27ac peaks that were neither promoter-TSS nor TTS. Tag enrichment heatmaps across a 5 kb region around Nup98, Nup93 or Elys peak center were generated for each IP treatment using HOMER and visualized using R (version 3.2.3) (Ihaka and Gentleman, 1996). To determine if the amount of overlap between two ChIP-seq peak datasets is more than we would expect given their coverage and the size of the genome, a one-tailed Fisher’s exact test was performed (implemented in BEDtools Fisher).
GEO: GSE47691 datasets for DHS-SEQ and STARR-SEQ were downloaded from (Shlyueva et al., 2014), and GEO: GSE30740 datasets for dCTCF and CP190 from (Wood et al., 2011; Yang et al., 2013).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-Nup98 | Capelson et al., 2010 | N/A |
| Rabbit polyclonal anti-Elys | This study | N/A |
| Guinea pig polyclonal anti-Nup93 | This study | N/A |
| Mouse monoclonal anti-EcR | Developmental Studies Hybridoma Bank | clone DDA2.7 |
| Mouse monoclonal anti-Tubulin | Developmental Studies Hybridoma Bank | clone E7 |
| Mouse monoclonal anti-Mtor | gift from Prof. K.M. Johansen (Iowa State University) | clone 12F10 |
| Mouse monoclonal anti-Lamin | Developmental Studies Hybridoma Bank | clone ADL101 |
| Rabbit polyclonal anti-Lamin | gift from Prof. P.A. Fisher (Stony Brook University) | N/A |
| Rabbit polyclonal anti-Nxf1 | gift from Prof. E. Izaurralde (MPI for Developmental Biology) | N/A |
| Rabbit polyclonal anti-Trr | gift from Prof. A. Mazo (Thomas Jefferson University) | N/A |
| Rabbit polyclonal anti-histone H3 | gift from Prof. S.L. Berger (University of Pennsylvania) | Abcam ab1791; RRID: AB_302613 |
| Rabbit polyclonal anti-CTCF | gift from Prof. V.G. Corces (Emory University) | N/A |
| Rabbit polyclonal anti-CP190 | gift from Prof. V.G. Corces (Emory University) | N/A |
| Rabbit polyclonal anti-SuHw | gift from Prof. V.G. Corces (Emory University) | N/A |
| Rabbit polyclonal anti-GAF | gift from Prof. V.G. Corces (Emory University) | N/A |
| Rabbit polyclonal anti-histone H3K27Acetyl | Active Motif | #39133; RRID: AB_2561016 |
| Rabbit polyclonal anti-histone H3K27Me3 | Active Motif | #39155; RRID: AB_2561020 |
| Mouse monoclonal anti-Nuclear Pore Complex proteins | Covance | clone MAb414 |
| Sheep polyclonal anti-Digoxigenin-AP | Roche | #11 207 741 910 |
| Bacterial and Virus Strains | ||
| E. Coli: BL21 competent | NEB | C2530H |
| Biological Samples | ||
| Drosophila third-instar larvae brains | This study | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 20-Hydroxyecdysone | Sigma-Aldrich | H5142 |
| TransIT-Insect Reagent | Mirus Bio LLC | MIR6100 |
| Fugene HD | Promega | E2311 |
| MEGAscript T7 Transcription kit | Thermo Fisher Scientific | AM1334 |
| OneStep RT-PCR kit | QIAGEN | cat#210212 |
| Power SYBR Green PCR Master mix | Applied Biosystems | cat#4367659 |
| DIG-Nick Translation mix | Roche | #11 745 816 910 |
| Protein A Agarose | Thermo Fisher Scientific | cat#15918014 |
| Protein G Agarose | Thermo Fisher Scientific | cat#15920010 |
| Dynabeads M-280 Sheep anti-Rabbit | Thermo Fisher Scientific | cat#11204D |
| Dynabeads M-280 Sheep anti-Mouse | Thermo Fisher Scientific | cat#11203D |
| Critical Commercial Assays | ||
| NEBNext Ultra DNA Library Prep Kit for Illumina 96 rxn | NEB | E7370L |
| NEBNext Multiplex Oligos for Illumina | NEB | Set1: E7335L |
| (Index Primers Set 1 and Set 2) 96 rxn | Set2: E7500L | |
| NextSeq 500/550 High Output v2 kit (75 cycles) | Illumina | FC-404-2005 |
| Deposited Data | ||
| ChIP-seq raw and analyzed data | This study | GSE94922 |
| Experimental Models: Cell Lines | ||
| S2-DRSC | Drosophila Genomics Resource Center | Flybase: FBrf0024118 |
| Experimental Models: Organisms/Strains | ||
| Nup98-96 allele: | Bloomington Drosophila | BDSC: 28433 |
| y[1] w[*]; P{w[+mC] = EP}Nup98-96 [G2120]/TM3, Sb[1] Ser[1] | Stock Center | |
| Nup98-96 allele: | Bloomington Drosophila | BDSC: 4951 |
| Nup98-96[339]/TM3, Sb[1] | Stock Center | |
| Gypsy insertion CtK: y[1] ct[K]; bw[1] | Bloomington Drosophila Stock Center | BDSC: 171 |
| Oregon-R | Bloomington Drosophila Stock Center | BDSC: 5 |
| Oligonucleotides | ||
| 3C primers | IDT | see Table S6 |
| Digestion efficiency primers | IDT | see Table S6 |
| Expression analysis primers | IDT | see Table S6 |
| dsRNA primers | IDT | see Table S6 |
| oligo-(dT)40-Cy3 | IDT | |
| Recombinant DNA | ||
| pNup98-Myc | Pascual-Garcia et al., 2014 | N/A |
| p-Myc (pAWM) | Carnegie Institution for Science | https://emb.carnegiescience.edu/Drosophila-gateway-vector-collection |
| pDEST15 | Thermo Fisher Scientific | cat#11802014 |
| pETG-41-A | EMBL | https://www.embl.de/pepcore/pepcore_services/strains_vectors/vectors/gateway_vectors/ |
| cDNA LD21129 | Drosophila Genomics Resource Center | Flybase: FBcl0172220 |
| BAC DNA clone BACN14N24 | BACPAC Resource Center | EDROSBAC-14N24 |
| BAC DNA clone BACN04G20 | BACPAC Resource Center | EDROSBAC-4G20 |
| BAC DNA clone BACR04K01 | BACPAC Resource Center | RP98-4K1 |
| BAC DNA clone BACR28H1 | BACPAC Resource Center | RP98-28H1 |
| BAC DNA clone BAC RP98-15I7 | BACPAC Resource Center | RP98-15I7 |
| Software and Algorithms | ||
| 3C primer design (3PD) | Fröhler and Dieterich, 2009 | http://3pd.mdc-berlin.de/ |
| Fiji | https://fiji.sc/ | |
| Leica Application Suite X | Leica software | http://www.leica-microsystems.com/products/microscope-software/software-for-life-science-research/las-x-powerful-and-flexible/ |
| NIS-Elements | Nikon software | http://www.nikonmetrology.com/en_US/Products/Software/Imaging-Software/NIS-Elements-Microscope-Imaging-Software |
| Prism | GraphPad software | https://www.graphpad.com/scientific-software/prism/ |
| NCBI SRA Toolkit | https://www.ncbi.nlm.nih.gov/sra/docs/toolkitsoft/ | |
| Bowtie2 | Langmead and Salzberg, 2012 | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| MACS2 | Feng et al., 2012 | https://github.com/taoliu/MACS |
| R | The R Project for Statistical Computing | https://www.r-project.org/ |
| R package DiffBind | Stark and Brown, 2011 | https://bioconductor.org/packages/release/bioc/html/DiffBind.html |
| HOMER | Heinz et al., 2010 | http://homer.salk.edu/homer/ |
| IGV | Thorvaldsdóttir et al., 2013 | http://software.broadinstitute.org/software/igv/ |
| BEDtools | Quinlan and Hall, 2010 | http://bedtools.readthedocs.io/en/latest/ |
Highlights.
Nuclear pore proteins (Nups) bind promoters and enhancers in Drosophila cells and tissues
Nup98 mediates enhancer-promoter looping of inducible genes
Inducible genes stably associate with nuclear pores in their silent and active states
Nup98 gains and retains interactions with architectural proteins upon induction
ACKNOWLEDGMENTS
Weare indebted to Drs. T.J. Bernardo (Albert Einstein College of Medicine) and T. Forné (Institut de Génétique Moléculaire de Montpellier) for input on the 3C technique. We thank the Developmental Studies Hybridoma Bank, the Bloomington Drosophila Stock Center, and the Drosophila Genomics Resource Center for reagents. The Mtor, Trr, Nxf1, and H3 antibodies were a gift from Drs. K.M. Johansen, A. Mazo, E. Izaurralde, and S.L. Berger, respectively. CTCF, CP190, SuHw, and GAF were a generous contribution from Dr. V.G. Corces. M.C. is supported by the Charles E. Kaufman Foundation and by Research Scholar Grant RSG-15-159-01-CSM from the American Cancer Society.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and six tables and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2017.02.020.
REFERENCES
- Agrawal P, and Shashidhara LS (2014). ChIP for Hox proteins from Drosophila imaginal discs. Methods Mol. Biol 1196, 241–253. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Brickner DG, Light WH, Cajigas I, McDonough M, Froyshteter AB, Volpe T, and Brickner JH (2010). DNA zip codes control an ancient mechanism for gene targeting to the nuclear periphery. Nat. Cell Biol 12, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold CD, Gerlach D, Stelzer C, Boryń LM, Rath M, and Stark A (2013). Genome-wide quantitative enhancer activity maps identified by STARR-seq. Science 339, 1074–1077. [DOI] [PubMed] [Google Scholar]
- Bernardo TJ, Dubrovskaya VA, Jannat H, Maughan B, and Dubrovsky EB (2009). Hormonal regulation of the E75 gene in Drosophila: identifying functional regulatory elements through computational and biological analysis. J. Mol. Biol 387, 794–808. [DOI] [PubMed] [Google Scholar]
- Bernardo TJ, Dubrovskaya VA, Xie X, and Dubrovsky EB (2014). A view through a chromatin loop: insights into the ecdysone activation of early genes in Drosophila. Nucleic Acids Res. 42, 10409–10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G (1985). Gene gating: a hypothesis. Proc. Natl. Acad. Sci. U S A 82, 8527–8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, Buc H, Feuerbach-Fournier F, Olivo-Marin JC, Hurt EC, and Nehrbass U (2006). SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature 441, 770–773. [DOI] [PubMed] [Google Scholar]
- Capelson M, Liang Y, Schulte R, Mair W, Wagner U, and Hetzer MW (2010). Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell 140, 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolari JM, Brown CR, Komili S, West J, Hieronymus H, and Silver PA (2004). Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell 117, 427–439. [DOI] [PubMed] [Google Scholar]
- Cavalli G, and Misteli T (2013). Functional implications of genome topology. Nat. Struct. Mol. Biol 20, 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo MA, Gomez-Cavazos JS, Mei A, Lackner DH, and Hetzer MW (2012).A change in nuclear pore complex composition regulates cell differentiation. Dev. Cell 22, 446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Urso A, Takahashi YH, Xiong B, Marone J, Coukos R, Randise-Hinchliff C, Wang JP, Shilatifard A, and Brickner JH (2016). Set1/COMPASS and Mediator are repurposed to promote epigenetic transcriptional memory. eLife 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davuluri G, Gong W, Yusuff S, Lorent K, Muthumani M, Dolan AC, and Pack M (2008). Mutation of the zebrafish nucleoporin elys sensitizes tissue progenitors to replication stress. PLoS Genet. 4, e1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth DJ, Tackett AJ, Rogers RS, Yi EC, Christmas RH, Smith JJ, Siegel AF, Chait BT, Wozniak RW, and Aitchison JD (2005). The mobile nucleoporin Nup2p and chromatin-bound Prp20p function in endogenous NPC-mediated transcriptional control. J. Cell Biol 171, 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Down TA, Bergman CM, Su J, and Hubbard TJ (2007). Large-scale discovery of promoter motifs in Drosophila melanogaster. PLoS Comput. Biol 3, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker AK, Bondos SE, Huang F, and Oldfield CJ (2015). Intrinsically disordered proteins and multicellular organisms. Semin. Cell Dev. Biol 37, 44–55. [DOI] [PubMed] [Google Scholar]
- Ea V, Court F, and Forne T (2017). Quantitative analysis of intra-chromosomal contacts: the 3C-qPCR method. Methods Mol. Biol 1589, 75–88. [DOI] [PubMed] [Google Scholar]
- Feng J, Liu T, Qin B, Zhang Y, and Liu XS (2012). Identifying ChIP-seq enrichment using MACS. Nat. Protoc 7, 1728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C, Walczak R, Yavuz S, Santarella R, Gentzel M, Askjaer P, Galy V, Hetzer M, Mattaj IW, and Antonin W (2007). MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 8, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhler S, and Dieterich C (2009). 3PD: rapid design of optimal primers for chromosome conformation capture assays. BMC Genomics 10, 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Díaz E, and Corces VG (2014). Architectural proteins: regulators of 3D genome organization in cell fate. Trends Cell Biol. 24, 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis ER, Altan N, Lippincott-Schwartz J, and Powers MA (2002). Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol. Biol. Cell 13, 1282–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampsey M, Singh BN, Ansari A, Lainé JP, and Krishnamurthy S (2011). Control of eukaryotic gene expression: gene loops and transcriptional memory. Adv. Enzyme Regul 51, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, and Glass CK (2010). Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold A, Klymenko T, and Izaurralde E (2001). NXF1/p15 heterodimers are essential for mRNA nuclear export in Drosophila. RNA 7, 1768–1780. [PMC free article] [PubMed] [Google Scholar]
- Herz HM, Mohan M, Garruss AS, Liang K, Takahashi YH, Mickey K, Voets O, Verrijzer CP, and Shilatifard A (2012). Enhancer-associated H3K4 monomethylation by Trithorax-related, the Drosophila homolog of mammalian Mll3/Mll4. Genes Dev. 26, 2604–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover KK, Gerasimova TI, Chien AJ, and Corces VG (1992). Dominant effects of suppressor of Hairy-wing mutations on gypsy-induced alleles of forked and cut in Drosophila melanogaster. Genetics 132, 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, and Gentleman R (1996). R: a language for data analysis and graphics. J. Comput. Graph. Stat 5, 299–314. [Google Scholar]
- Ishii K, Arib G, Lin C, Van Houwe G, and Laemmli UK (2002). Chromatin boundaries in budding yeast: the nuclear pore connection. Cell 109, 551–562. [DOI] [PubMed] [Google Scholar]
- Jacinto FV, Benner C, and Hetzer MW (2015). The nucleoporin Nup153 regulates embryonic stem cell pluripotency through gene silencing. Genes Dev. 29, 1224–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. (2010). Mediator and cohesin connect gene expression and chromatin architecture. Nature 467, 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalverda B, and Fornerod M (2010). Characterization of genome-nucleoporin interactions in Drosophila links chromatin insulators to the nuclear pore complex. Cell Cycle 9, 4812–4817. [DOI] [PubMed] [Google Scholar]
- Kalverda B, Pickersgill H, Shloma VV, and Fornerod M (2010). Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell 140, 360–371. [DOI] [PubMed] [Google Scholar]
- Kharchenko PV, Alekseyenko AA, Schwartz YB, Minoda A, Riddle NC, Ernst J, Sabo PJ, Larschan E, Gorchakov AA, Gu T, et al. (2011). Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature 471, 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knockenhauer KE, and Schwartz TU (2016). The nuclear pore complex as a flexible and dynamic gate. Cell 164, 1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler A, Schneider M, Cabal GG, Nehrbass U, and Hurt E (2008). Yeast Ataxin-7 links histone deubiquitination with gene gating and mRNA export. Nat. Cell Biol 10, 707–715. [DOI] [PubMed] [Google Scholar]
- Kurshakova MM, Krasnov AN, Kopytova DV, Shidlovskii YV, Nikolenko JV, Nabirochkina EN, Spehner D, Schultz P, Tora L, and Georgieva SG (2007). SAGA and a novel Drosophila export complex anchor efficient transcription and mRNA export to NPC. EMBO J. 26, 4956–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Kato M, Xiang S, Wu L, Theodoropoulos P, Mirzaei H, Han T, Xie S, Corden JL, and McKnight SL (2013). Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 155, 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzuolo C, Roure V, Dekker J, Bantignies F, and Orlando V (2007). Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nat. Cell Biol 9, 1167–1174. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, and Durbin R; 1000 Genome Project Data Processing Subgroup (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Franks TM, Marchetto MC, Gage FH, and Hetzer MW (2013). Dynamic association of NUP98 with the human genome. PLoS Genet. 9, e1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light WH, Brickner DG, Brand VR, and Brickner JH (2010). Interaction of a DNA zip code with the nuclear pore complex promotes H2A.Z incorporation and INO1 transcriptional memory. Mol. Cell 40, 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light WH, Freaney J, Sood V, Thompson A, D’Urso A, Horvath CM, and Brickner JH (2013). A conserved role for human Nup98 in altering chromatin structure and promoting epigenetic transcriptional memory. PLoS Biol. 11, e1001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendjan S, Taipale M, Kind J, Holz H, Gebhardt P, Schelder M, Vermeulen M, Buscaino A, Duncan K, Mueller J, et al. (2006). Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol. Cell 21, 811–823. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M, and Nora EP (2016). CTCF and Cohesin in genome folding and transcriptional gene regulation. Annu. Rev. Genomics Hum. Genet 17, 17–43. [DOI] [PubMed] [Google Scholar]
- Mondal BC, Shim J, Evans CJ, and Banerjee U (2014). Pvr expression regulators in equilibrium signal control and maintenance of Drosophila blood progenitors. eLife 3, e03626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, Dubois G, Mazo A, Croce CM, and Canaani E (2002). ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol. Cell 10, 1119–1128. [DOI] [PubMed] [Google Scholar]
- Ohler U (2006). Identification of core promoter modules in Drosophila and their application in accurate transcription start site prediction. Nucleic Acids Res. 34, 5943–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, and Levine M (1998). GAGA mediates the enhancer blocking activity of the eve promoter in the Drosophila embryo. Genes Dev. 12, 3325–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, Mura S, Yamada K, Sangel P, Hirata S, Maehara K, Kawakami K, Tachibana T, Ohkawa Y, Kimura H, and Yoneda Y (2016). Chromatin-prebound Crm1 recruits Nup98-HoxA9 fusion to induce aberrant expression of Hox cluster genes. eLife 5, e09540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D, Pascual-Garcia P, Dunagin M, Tudor M, Hopkins KC, Xu J, Gold B, Raj A, Capelson M, and Cherry S (2014). Nup98 promotes antiviral gene expression to restrict RNA viral infection in Drosophila. Proc. Natl. Acad. Sci. U S A 111, E3890–E3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Garcia P, Jeong J, and Capelson M (2014). Nucleoporin Nup98 associates with Trx/MLL and NSL histone-modifying complexes and regulates Hox gene expression. Cell Rep. 9, 433–442. [DOI] [PubMed] [Google Scholar]
- Pombo A, and Dillon N (2015). Three-dimensional genome architecture: players and mechanisms. Nat. Rev. Mol. Cell Biol 16, 245–257. [DOI] [PubMed] [Google Scholar]
- Presgraves DC, Balagopalan L, Abmayr SM, and Orr HA (2003). Adaptive evolution drives divergence of a hybrid inviability gene between two species of Drosophila. Nature 423, 715–719. [DOI] [PubMed] [Google Scholar]
- Ptak C, and Wozniak RW (2016). Nucleoporins and chromatin metabolism. Curr. Opin. Cell Biol 40, 153–160. [DOI] [PubMed] [Google Scholar]
- Qiu JJ, Zeisig BB, Li S, Liu W, Chu H, Song Y, Giordano A, Schwaller J, Gronemeyer H, Dong S, and So CW (2015). Critical role of retinoid/rexinoid signaling in mediating transformation and therapeutic response of NUP98-RARG leukemia. Leukemia 29, 1153–1162. [DOI] [PubMed] [Google Scholar]
- Quinlan AR, and Hall IM (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabut G, Doye V, and Ellenberg J (2004). Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat. Cell Biol 6, 1114–1121. [DOI] [PubMed] [Google Scholar]
- Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, and Wysocka J (2011). A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raices M, and D’Angelo MA (2012). Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat. Rev. Mol. Cell Biol 13, 687–699. [DOI] [PubMed] [Google Scholar]
- Rickels R, Hu D, Collings CK, Woodfin AR, Piunti A, Mohan M, Herz HM, Kvon E, and Shilatifard A (2016). An evolutionary conserved epigenetic mark of polycomb response elements implemented by Trx/MLL/COMPASS. Mol. Cell 63, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro S, Fischer T, Luo MJ, Antúnez O, Brettschneider S, Lechner J, Pérez-Ortín JE, Reed R, and Hurt E (2004). Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell 116, 75–86. [DOI] [PubMed] [Google Scholar]
- Rohner S, Kalck V, Wang X, Ikegami K, Lieb JD, Gasser SM, and Meister P (2013). Promoter- and RNA polymerase II-dependent hsp-16 gene association with nuclear pores in Caenorhabditis elegans. J. Cell Biol 200, 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Arib G, Laemmli C, Nishikawa J, Durussel T, and Laemmli UK (2006). Nup-PI: the nucleopore-promoter interaction of genes in yeast. Mol. Cell 21, 379–391. [DOI] [PubMed] [Google Scholar]
- Schmidt HB, and Görlich D (2015). Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity. eLife 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Hellerschmied D, Schubert T, Amlacher S, Vinayachandran V, Reja R, Pugh BF, Clausen T, and Köhler A (2015). The nuclear pore-associated TREX-2 complex employs mediator to regulate gene expression. Cell 162, 1016–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedkov Y, Cho E, Petruk S, Cherbas L, Smith ST, Jones RS, Cherbas P, Canaani E, Jaynes JB, and Mazo A (2003). Methylation at lysine 4 of histone H3 in ecdysone-dependent development of Drosophila. Nature 426, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyueva D, Stelzer C, Gerlach D, Yáñez-Cuna JO, Rath M, Boryń LM, Arnold CD, and Stark A (2014). Hormone-responsive enhancer-activity maps reveal predictive motifs, indirect repression, and targeting of closed chromatin. Mol. Cell 54, 180–192. [DOI] [PubMed] [Google Scholar]
- Sood V, and Brickner JH (2014). Nuclear pore interactions with the genome. Curr. Opin. Genet. Dev 25, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R, and Brown G (2011). DiffBind: Differential binding analysis of ChIP-seq peak data (University of Cambridge, Cancer Research UK-Cambridge Institute; ). [Google Scholar]
- Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, and Gasser SM (2006). Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 441, 774–778. [DOI] [PubMed] [Google Scholar]
- Tan-Wong SM, Wijayatilake HD, and Proudfoot NJ (2009). Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex. Genes Dev. 23, 2610–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdóttir H, Robinson JT, and Mesirov JP (2013). Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform 14, 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsompana M, and Buck MJ (2014). Chromatin accessibility: a window into the genome. Epigenetics Chromatin 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bortle K, Nichols MH, Li L, Ong CT, Takenaka N, Qin ZS, and Corces VG (2014). Insulator function and topological domain border strength scale with architectural protein occupancy. Genome Biol. 15, R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Vosse DW, Wan Y, Lapetina DL, Chen WM, Chiang JH, Aitchison JD, and Wozniak RW (2013). A role for the nucleoporin Nup170p in chromatin structure and gene silencing. Cell 152, 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquerizas JM, Suyama R, Kind J, Miura K, Luscombe NM, and Akhtar A (2010). Nuclear pore proteins nup153 and megator define transcriptionally active regions in the Drosophila genome. PLoS Genet. 6, e1000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer B, and Antonin W (2014). The diverse roles of the Nup93/Nic96 complex proteins - structural scaffolds of the nuclear pore complex with additional cellular functions. Biol. Chem 395, 515–528. [DOI] [PubMed] [Google Scholar]
- Wood AM, Van Bortle K, Ramos E, Takenaka N, Rohrbaugh M, Jones BC, Jones KC, and Corces VG (2011). Regulation of chromatin organization and inducible gene expression by a Drosophila insulator. Mol. Cell 44, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, and Powers MA (2013). In vivo analysis of human nucleoporin repeat domain interactions. Mol. Biol. Cell 24, 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sung E, Donlin-Asp PG, and Corces VG (2013). A subset of Drosophila Myc sites remain associated with mitotic chromosomes colocalized with insulator proteins. Nat. Commun 4, 1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierhut C, Jenness C, Kimura H, and Funabiki H (2014). Nucleosomal regulation of chromatin composition and nuclear assembly revealed by histone depletion. Nat. Struct. Mol. Biol 21, 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.