SUMMARY
During short-lived perturbations, such as inflammation, the gut microbiota exhibits resilience and reverts to its original configuration. Although microbial access to the micronutrient iron is decreased during colitis, pathogens can scavenge iron using siderophores. How commensal bacteria acquire iron during gut inflammation is incompletely understood. Curiously, the human commensal Bacteroides thetaiotaomicron does not produce siderophores, but grows under iron-limiting conditions using enterobacterial siderophores. Using RNAseq, we here identify B. thetaiotaomicron genes that were upregulated during Salmonella-induced gut inflammation and were predicted to be involved in iron uptake. Mutants in the xusABC locus (BT2063-2065) were defective for xenosiderophore-mediated iron uptake in vitro. In the normal mouse gut, the XusABC system was dispensable, while a xusA mutant colonized poorly during colitis. This work identifies xenosiderophore utilization as a critical mechanism for B. thetaiotaomicron to sustain colonization during inflammation and suggests a mechanism of how interphylum iron metabolism contributes to gut microbiota resilience.
Keywords: Gut inflammation, iron metabolism, siderophore, gut microbiota resilience, Bacteroidetes
eTOC blurb:
During inflammation, the mammalian body restricts microbial access to iron. Pathogens scavenge iron using siderophores; however, how commensal bacteria acquire iron during gut inflammation is incompletely understood. Zhu et al. report that commensal Bacteroides thetaiotaomicron survives iron restriction during colitis by utilizing siderophores produced by members of the Enterobacteriaceae family.
INTRODUCTION
Communities of organisms, such as gut-associated microbial communities, often exist in a stable equilibrium with minor fluctuations. Disturbances lead to transient changes in the systems’ function and composition, and the community may in time revert to the original equilibrium as the perturbation passes. Alternatively, the community may adopt a new equilibrium with properties similar to the original state. In both cases, communities exhibit a type of resilience (reviewed in (Gunderson, 2000; Lozupone et al., 2012; Sommer et al., 2017)). A timely return to the original state is referred to as engineering resilience. In contrast, the term ecological resilience refers to the amount of insult that can be tolerated before a system changes to an altered state.
Several extrinsic and intrinsic factors impact the mammalian gut microbiota. Changes in host diet and ingestion of drugs, in particular antimicrobial therapy, impact the diversity, composition, and function of gut microbial communities (reviewed in (Sommer et al., 2017; Spanogiannopoulos and Turnbaugh, 2018; Winter and Baumler, 2014)). Alterations in gut microbiota composition have been reported in human diseases, including inflammatory bowel disease, diabetes, and colorectal cancer (reviewed in (Pham and Lawley, 2014; Tamboli et al., 2004; Winter and Baumler, 2014)). Disease-associated states of the microbiota, frequently referred to as dysbiosis, exhibit permanent decreased species diversity.
Inflammation-associated dysbiosis of the gut microbiota is not merely a bystander effect, but negatively influences health of its host. Decreased diversity has been linked to decreased microbiota resilience against pathogens (Lupp et al., 2007; Stecher et al., 2007). In murine models, non-infectious colitis is communicable via the transfer of a dysbiotic microbiota into genetically susceptible hosts (Arthur et al., 2012; Garrett et al., 2010). Transfer of fecal microbiota from colorectal cancer patients into mice enhanced intestinal tumor formation (Wong et al., 2017). Targeted manipulation of the dysbiotic microbiota improved acute colitis and colitis-associated colorectal cancer (Zhu et al., 2019a; Zhu et al., 2018). Despite the importance of both engineering resilience and ecological resilience of the gut microbiota, our understanding of the molecular mechanisms that underlie microbiota resilience is limited.
Recent studies have revealed mechanisms of how gut bacteria respond to perturbations of their niches. During intestinal inflammatory episodes, the production of antimicrobial peptides is significantly increased as an effort to restrict the bloom of harmful organisms in the gut (Muniz et al., 2012). These peptides non-specifically target conserved molecular patterns in both pathogenic and commensal bacteria, raising the question of how the commensal community could remain stable for years (Faith et al., 2013). One of the mechanisms that allow members of the phylum Bacteroidetes to be resilient to host inflammation is that these bacteria modify their lipopolysaccharide (LPS), resulting in increased resistance to antimicrobial peptides (Cullen et al., 2015). In another example, changes in the host’s diet forces gut bacteria to adapt their carbon and energy metabolism (Desai et al., 2016). Gut microbes rely on dietary glycans provide essential substrates for growth, and consumption of a Western diet leads to a depletion of dietary glycans in the intestinal tract. The microbial community adapts to such changes by switching from dietary glycan degradation to foraging host-secreted mucus glycoproteins as nutrients (Desai et al., 2016).
Here, we utilized transcriptomic profiling to probe how a member of the Bacteroidetes phylum maintains fitness during enteric pathogen infection. We show that siderophore cross-feeding between different phyla of bacteria allows gut commensals to acquire iron in the inflamed gut. Our findings implicate iron metabolism as an important factor of gut microbiota resilience.
RESULTS
B. thetaiotaomicron genes related to iron metabolism are upregulated in response to infection with a pro-inflammatory pathogen.
To identify factors mediating commensal resilience in the inflamed intestine, we performed a transcriptome analysis of a defined minimal community upon challenge with a pro-inflammatory enteric pathogen, Salmonella enterica serovar Typhimurium (S. Tm). S. Tm is a common cause of bacterial foodborne gastroenteritis. In mice, experimental infection results in subacute intestinal inflammation. Groups of gnotobiotic mice were associated with two human isolates, B. thetaiotaomicron and Clostridium symbiosum, representing the predominant phyla in the human gut microbiota (Bacteroidetes and Firmicutes). After 7 days, we infected one group with the S. Tm wild-type strain while the mock treatment group received sterile LB broth. Two days later, we extracted total RNA from cecal contents and determined the transcriptome by high-throughput RNA sequencing (RNAseq) (Fig. 1A). We generated a Bacteroides-specific transcriptome by mapping unambiguous reads to the genome of B. thetaiotaomicron VPI-5482. S. Tm infection profoundly altered the gene expression profile of B. thetaiotaomicron in the murine cecum (Fig. 1B), with 364 genes being differentially transcribed in response to S. Tm infection (Fig. 1C; Table S1). The predicted primary amino acid sequence of several of these genes shared limited sequence similarity with proteins involved in iron uptake in other bacteria. This finding suggests that, as essential micronutrients including iron become limited during gut inflammation (Deriu et al., 2013; Raffatellu et al., 2009), B. thetaiotaomicron adapts its iron metabolism to optimize fitness during S. Tm infection. While bacterial pathogens employ a plethora of strategies to overcome nutritional immunity (Caza and Kronstad, 2013), our understanding of how commensal gut bacteria acquire iron during gut inflammation is incomplete. We thus focused on investigating iron metabolism in B. thetaiotaomicron.
Figure 1: Changes in the B. thetaiotaomicron transcriptome in response to Salmonella infection.
(A – C) Groups of gnotobiotic Swiss Webster mice were colonized with C. symbiosum ATCC14940 and B. thetaiotaomicron VPI-5482 Δtdk for 7 days. Mice were either intragastrically inoculated with S. Tm IR715 (N = 5) or remained uninfected (N = 5) for 2 days, and the cecal content was collected and the bacterial transcriptome assessed using RNA-seq. (A) Schematic representation of the experiment. (B) Principle coordinate plot of the B. thetaiotaomicron transcriptomes in mock-treated (black circles) and S. Tm-infected mice (red). (C) Volcano plot of differentially expressed genes in B. thetaiotaomicron in response to S. Tm infection. Genes downregulated by more than a 4-fold and P < 0.05 are shown in blue, genes upregulated by the same criteria are shown in red. Gene with predicted functions in iron metabolism are indicated by their gene locus number. See also Fig. S3.
Bacteroides strains isolated from the murine gut utilize enterobacterial siderophores in vitro
We first investigated iron uptake of B. thetaiotaomicron under laboratory conditions. One strategy used by bacterial pathogens to overcome iron limitation imposed by the host immune responses is to produce low molecular weight, high-affinity iron chelators termed siderophores. B. thetaiotaomicron utilizes heme iron for growth in vitro, but does not produce any known siderophores (Manfredi et al., 2015; Rocha et al., 1991; Verweijvanvught et al., 1988). Consequently, B. thetaiotaomicron grows poorly in TYG media supplemented with 200 μM of the metal chelating agent bathophenanthroline disulfonate (BPS) (Fig. 2A). BPS acts as a buffer to chelate free iron and addition of small amounts of iron salts (10 μM) does not restore growth (Fig. S1A). This growth defect is rescued by addition of 200 μM ammonium iron citrate, indicating that the inability to access iron, and not other trace metals, was responsible for diminished growth (Fig. S1B). In contrast, S. Tm produces two catecholate siderophores, enterobactin and salmochelin, and grows under iron-limiting, anaerobic conditions in vitro (Hantke et al., 2003; Muller et al., 2009; Pollack and Neilands, 1970)(Fig. 2A). Some bacterial species do not produce siderophores but instead utilize siderophores that are produced by other microbes, a phenomenon sometimes referred to as xenosiderophore utilization or siderophore piracy. We thus tested a small panel of enterobacterial siderophores for their ability to enhance growth of B. thetaiotaomicron in BPS-supplemented media. Consistent with a previous report (Rocha and Krykunivsky, 2017), growth in BPS-supplemented media was restored by the addition of purified, iron-laden enterobactin or salmochelin (Fig. 2B; Fig. S1C), suggesting that B. thetaiotaomicron may acquire iron through xenosiderophore uptake.
Figure 2: Utilization of enterobacterial siderophores by Bacteroides strains in vitro.
(A) B. thetaiotaomicron VPI-5482 Δtdk or S. Tm SL1344 were anaerobically cultured in haemin-containing tryptone yeast extract glucose (TYG) medium in the presence or absence of 200 μmol/L of iron chelator bathophenanthroline disulfonate (BPS) for 36 hours. Bacterial growth was assessed by measuring optical density of the culture at a wavelength of 600 nm (OD600). (B – C) Bacteroides strains were cultured in haemin-supplemented TYG medium before being subcultured in iron-limited (200 μmol/L of BPS) TYG medium supplemented with either 0.5 μM aerobactin, 0.5 μM 2,3-dihydroxybenzoic acid (2,3-DHBA), 0.5 μM enterobactin, or 2 μM salmochelin. Growth of B. thetaiotaomicron (B) and Bacteroides mouse isolates (C) was determined by measuring the optical density. See also Fig. S1–3. Bars represent the geometric mean ± 95% confidence interval. *, P < 0.05; **, P < 0.01 ***, P < 0.001; ns, not statistically significant.
To study xenosiderophore-mediated iron uptake in a more controlled setting, we depleted trace elements from media using a polyvalent ion exchange resin, added back trace elements except iron, and assessed growth of B. thetaiotaomicron (Fig. S2A). The apo form of both catecholate siderophores was unable to support growth of the B. thetaiotaomicron wild-type, while supplementation with ferric-enterobactin or ferric-salmochelin markedly increased growth (Fig. S2A). We performed analogous experiments with S. Tm mutants defective for catecholate siderophore production (entB mutant) and uptake (fepA iroN cirA mutant) as controls (Baumler et al., 1998; Klebba et al., 1982; Rabsch et al., 2003; Rabsch et al., 1999; Wookey and Rosenberg, 1978)(Fig. S2B). These experiments provide further evidence that B. thetaiotaomicron can overcome iron limitation by using xenosiderophores.
We then explored whether xenosiderophore utilization is a phenomenon specific to B. thetaiotaomicron. We isolated several Bacteroides species from our mouse vivarium and tested whether they utilize enterobactin or salmochelin during anaerobic growth in vitro (Fig. 2C). Addition of iron-laden enterobactin or salmochelin enhanced growth of murine Bacteroides vulgatus and other Bacteroides species in BPS-supplemented media. As such, Bacteroides exhibit varying capacities to utilize catecholate xenosiderophores to support growth under iron-limiting conditions.
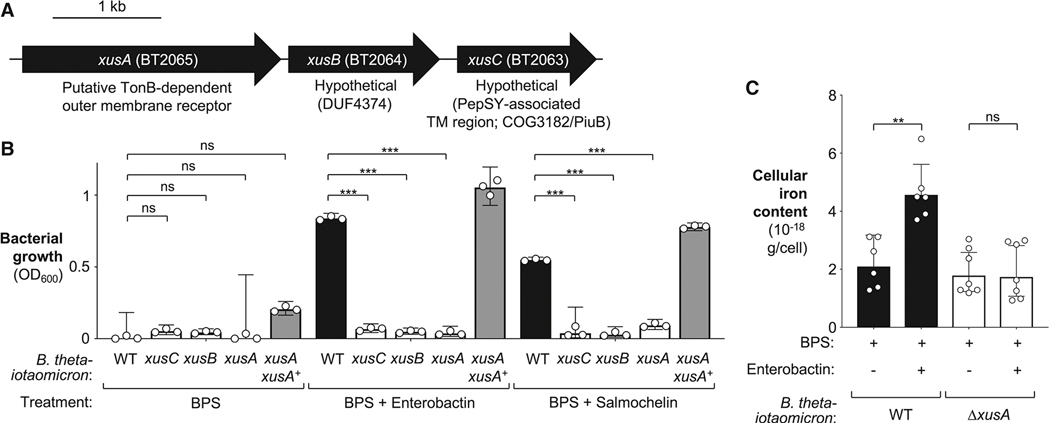
The xusABC genes are required xenosiderophore uptake in vitro.
We next sought to determine the genetic requirements for B. thetaiotaomicron to utilize enterobactin. Siderophore uptake in Gram-negative bacteria typically involves an outer membrane TonB-dependent receptor which transports iron-laden siderophores into the periplasmic space. In the periplasmic space, a siderophore binding protein relays the siderophore onto an ABC-transporter which transports the siderophore into the cytoplasm (Frost and Rosenberg, 1975; Pugsley and Reeves, 1976; Skare et al., 1993; Wookey and Rosenberg, 1978). In our transcriptome analysis, we identified several genes whose products might be involved in iron uptake or were predicted to be TonB-dependent receptors of unknown function. We generated a small library of clean deletion mutants in candidate genes and determined their fitness under iron-limiting conditions in comparison to the wild-type strain (Fig. S3). To quantify abundance, individual strains were marked with signature tags in a neutral locus in their genome (Goodman et al., 2009; Martens et al., 2008). In the presence of iron-laden enterobactin or salmochelin, the wild-type strain outgrew a mutant lacking the BT2063-2065 locus. Given its role in xenosiderophore utilization (Fig. S3), we renamed the genes in this locus xusABC (xenosiderophore utilization system; Fig. 3A).
Figure 3: Role of the B. thetaiotaomicron xusABC operon in xenosiderophore uptake in vitro.
(A). Schematic representation of the xusABC operon in B. thetaiotaomicron VPI-5482. (B) The indicated B. thetaiotaomicron strains were cultured in iron-limiting TYG medium supplemented with siderophores for 36 hours. The chelator bathophenanthroline disulfonate (BPS) was added at a concentration of 200 μmol/L. Enterobactin or salmochelin (50% iron saturation) were added at a final concentration of 0.5 μM and 2 μM, respectively. Growth was assessed by measuring optical density (OD600). (C) The B. thetaiotaomicron wild-type strain and an isogenic xusA mutant were cultured in iron-deprived media to exhaust endogenous iron before being subcultured in the presence of iron-laden enterobactin or vehicle. Inductively Coupled Plasma Mass Spectrometry was used to assess cellular iron levels. Bars represent the geometric mean ± 95% confidence interval. **, P < 0.01 ***, P < 0.001; ns, not statistically significant.
We next created clean, in-frame deletion mutants in xusA (BT2065), xusB (BT2064), and xusC (BT2063); the xusA strain was complemented by introducing the xusA gene under control of its native promoter in the BT3743-3744 intergenic region, a neutral locus in the B. thetaiotaomicron chromosome (Lim et al., 2017). The xusA mutant did not display any growth defects in rich media (Fig. S3B), indicating that the xusA mutant is not defective in general iron metabolism. Under iron-limiting conditions, growth of the wild-type and the mutant strains was impeded (Fig. 3B). Importantly, supplementation with purified, iron-laden enterobactin or salmochelin rescued the growth defect of the wild-type strain while the xusA, xusB, and xusC mutants did not benefit from siderophore supplementation (Fig. 3B; Fig. S1B and S2A). Genetic complementation of xusA restored the ability to grow in the presence of siderophores (Fig. 3B). Furthermore, supplementation with excess iron citrate reversed the growth defect of the xusA mutant in BPS-supplemented media (Fig. S1B and S2A).
The xusA gene is predicted to encode a putative TonB-dependent receptor. To monitor iron acquisition more directly, we cultured B. thetaiotaomicron in iron-deprived media to exhaust endogenous iron before we supplemented the culture with either vehicle or iron-laden enterobactin. We then employed Inductively Coupled Plasma Mass Spectrometry (ICP-MS) to assess cellular iron levels (Fig. 3C). Supplementation of the growth media with iron-laden enterobactin significantly increased the cellular iron levels of the wild-type strain compared to the iron-depleted growth condition. In contrast, cellular iron levels in the xusA mutant were unaffected by enterobactin availability in the growth media (Fig. 3C), suggesting that XusA is required for enterobactin-mediated iron uptake.
The B. thetaiotaomicron xusABC locus is required for colonization during murine S. Tm infection.
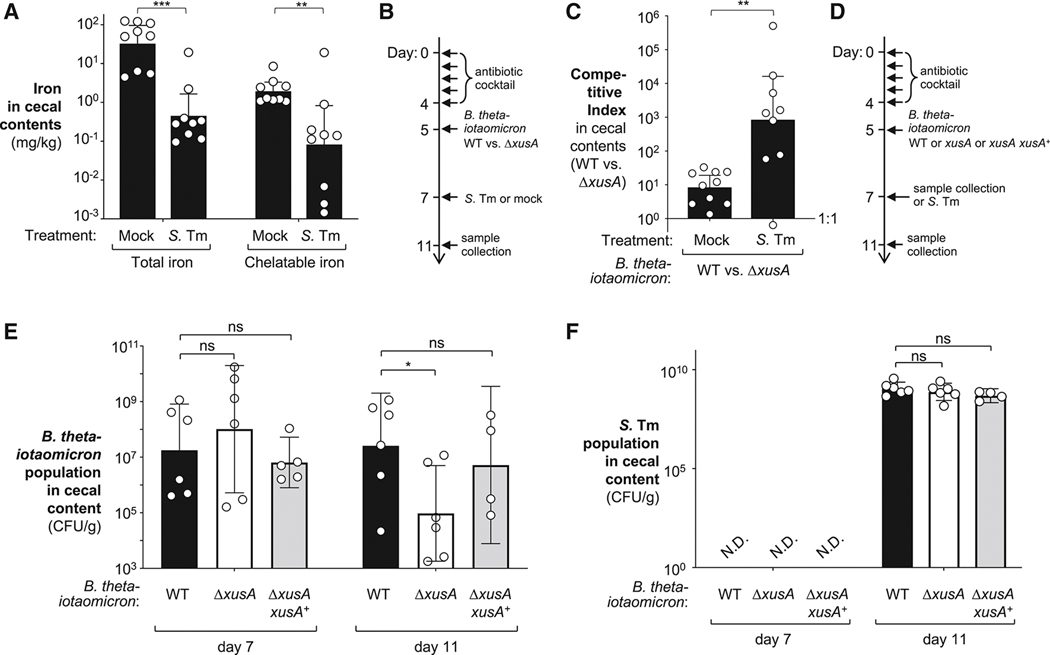
Next, we investigated the availability of iron in the intestinal contents during Salmonella infection. We intragastrically inoculated streptomycin-pretreated C57BL/6 mice with S. Tm or LB broth. After 4 days, we quantitated chelatable and total iron levels in the cecal contents by ICP-MS. Both iron pools decreased substantially during S. Tm infection when compared to mock-treated animals (Fig. 4A), suggesting that iron indeed becomes limited during S. Tm infection.
Figure 4: Role of xusABC locus in B. thetaiotaomicron colonization during murine S. Tm infection.
(A) Groups of streptomycin-treated C57BL/6 mice were either mock-treated (N = 9) or intragastrically inoculated with S. Tm SL1344 (N = 9). Four days after infection, the cecal contents were collected and separated into the chelatable and inaccessible iron fraction. The iron concentration was determined by ICP-MS. (B – C) Groups of C57BL/6 mice were treated with a cocktail of antibiotics, followed by intragastrical inoculation of an equal mixture of B. thetaiotaomicron wild-type strain (genomic signature tag 3; WZ433) and a xusA mutant (genomic signature tag 13; WZ647). Mice were mock-treated (LB, N = 10) or challenged with S. Tm SL1344 (N = 9) for 4 days. The abundance of each B. thetaiotaomicron strain in cecal contents was determined using qPCR targeting strain-specific signature tags. The competitive index was calculated as the ratio of the two strains in the cecal content, corrected by the ratio in the inoculum. A schematic representation of the experiment is shown in (B). Competitive index of B. thetaiotaomicron wild-type over xusA mutant in cecal contents (C). (D – F) Groups of C57BL/6 mice were treated with a cocktail of antibiotics, followed by intragastrical inoculation of either the B. thetaiotaomicron wild-type strain (Δtdk, GenR; N = 12), an isogenic xusA mutant (WZ777, GenR CmR, N = 12), or a complemented xusA mutant (WZ675, xusA xusA+, N = 9). After two days, half of the animals in each group were euthanized to assess the colonization levels of these strains at homeostatic conditions, while the remaining groups were challenged with S. Tm SL1344 for 4 days. A schematic representation of the experiment is shown in (D). Abundance of indicated B. thetaiotaomicron (E) and S. Tm (F) strains in cecal contents as determined by plating on selective agar. See also Fig. S4–6. Bars represent the geometric mean ± 95% confidence interval. *, P < 0.05; **, P < 0.01; ***; P < 0.001; ns, not statistically significant.
S. Tm relies on catecholate siderophores to overcome iron limitation during infection. Mutants unable to produce or utilize enterobactin or salmochelin are attenuated in mouse models of infection (Costa et al., 2016; Deriu et al., 2013; Diaz-Ochoa et al., 2016; Karlinsey et al., 2019; Nagy et al., 2013; Nagy et al., 2014; Raffatellu et al., 2009). To detect siderophore production in the murine intestinal contents, we developed a simple assay that relies on growth of a bacterial reporter strain in vitro. The isochorismatase EntB catalyzes the conversion of isochorismate to 2,3-dihydroxy-2,3-dihydrobenzoate, a precursor for 2,3-dihydroxybenzoic acid as well as the subsequent biosynthesis of enterobactin and salmochelin (Hantke et al., 2003). As such, entB mutants in Enterobacteriaceae are defective to produce these siderophores (Luke and Gibson, 1971; Young et al., 1971). In BPS-containing media, growth of a S. Tm entB mutant depends on exogenous supplementation with siderophores in a dose-dependent manner (Fig. S4A). We colonized two groups of gnotobiotic mice with B. thetaiotaomicron and infected one group with the S. Tm wild-type strain and one group with a mutant unable to produce catecholate siderophores (entB mutant)(Fig. S4B). We then incubated filter-sterilized homogenates of the intestinal contents of these mice with the reporter strain and monitored growth (Fig. S4). Only the homogenates obtained from mice infected with the S. Tm wild-type strain supported robust growth of the reporter strain, consistent with the idea that S. Tm produces siderophores when colonizing the murine large intestine.
We then explored whether xenosiderophore utilization is required for B. thetaiotaomicron to colonize the mammalian intestinal tract. As B. thetaiotaomicron does not readily colonize conventionally-raised mice (Lee et al., 2013), we pretreated C57BL/6 mice with a cocktail of antibiotics to facilitate engraftment of B. thetaiotaomicron (Curtis et al., 2014) (Fig. 4B). We colonized antibiotic-treated mice with an equal mixture of the B. thetaiotaomicron wild-type strain and a ΔxusA mutant (marked with unique signature tags; competitive colonization assay). After two days, animals were intragastrically infected with the S. Tm wild-type strain or mock-treated with LB broth. The abundance of each B. thetaiotaomicron strain in the cecal contents was determined four days post infection by qPCR using primers specific for genome-integrated signature tags. In mock-treated animals, the wild-type B. thetaiotaomicron strain and the ΔxusA mutant displayed similar levels of fitness (Fig. 4C). In contrast, the B. thetaiotaomicron wild-type strain outcompeted the ΔxusA mutant by more than 3 orders of magnitude in mice infected with S. Tm. These results suggest that xus-mediated iron uptake is necessary for optimal fitness of B. thetaiotaomicron during pathogen-induced colitis.
To more directly test the idea that xenosiderophore utilization contributes to gut colonization during colitis, we colonized two groups of antibiotic-treated mice with either the B. thetaiotaomicron wild-type strain, a ΔxusA mutant, or the complemented xusA mutant (ΔxusA xusA+) (Fig. 4D–F). After two days, half of the animals in each group were euthanized to assess the colonization levels of these strains at homeostatic conditions. The remaining animals were infected with S. Tm. Four days after infection, we determined the abundance of B. thetaiotaomicron and S. Tm by plating on selective media. Under steady state conditions, the B. thetaiotaomicron wild-type strain, the xusA mutant, and the complemented strain (xusA xusA+) were recovered at similar levels (Fig. 4E), consistent with our previous finding that the xusA gene is dispensable for fitness under homeostatic conditions (Fig. 4C). In contrast, the B. thetaiotaomicron ΔxusA mutant colonized the cecum of S. Tm-infected mice at significantly lower levels than the B. thetaiotaomicron wild-type strain (Fig. 4E). The colonization defect observed for the xusA mutant upon S. Tm infection was fully restored in the complemented strain. No significant differences in S. Tm gut colonization or markers of mucosal inflammation were noted (Fig. 4F; Fig. S5A). Similar results were obtained when the experiment was performed with B. thetaiotaomicron strains carrying genome-integrated signature tags (Fig. S5B–E).
Furthermore, we determined whether transcription of xusABC genes was induced as a result of S. Tm infection (Fig. S6). Mice pre-colonized with B. thetaiotaomicron were infected with S. Tm or mock-treated and RNA extracted from the colon content. mRNA levels of xusA, xusB, and xusC, normalized to the housekeeping gene gmk, were determined by RT-qPCR. Transcription of all three genes was markedly induced in S. Tm-infected animals (Fig. S6B). In this model, populations of other gut commensals are present (Fig. S6C–E), thus validating our initial transcriptomic analysis in gnotobiotic animals (Fig. 1). Collectively, these data suggest that utilization of xenosiderophores through the XusABC system is important for B. thetaiotaomicron to maintain efficient gut colonization in the setting of enteric pathogen infection.
Enterobacterial siderophores contribute to B. thetaiotaomicron fitness during S. Tm infection.
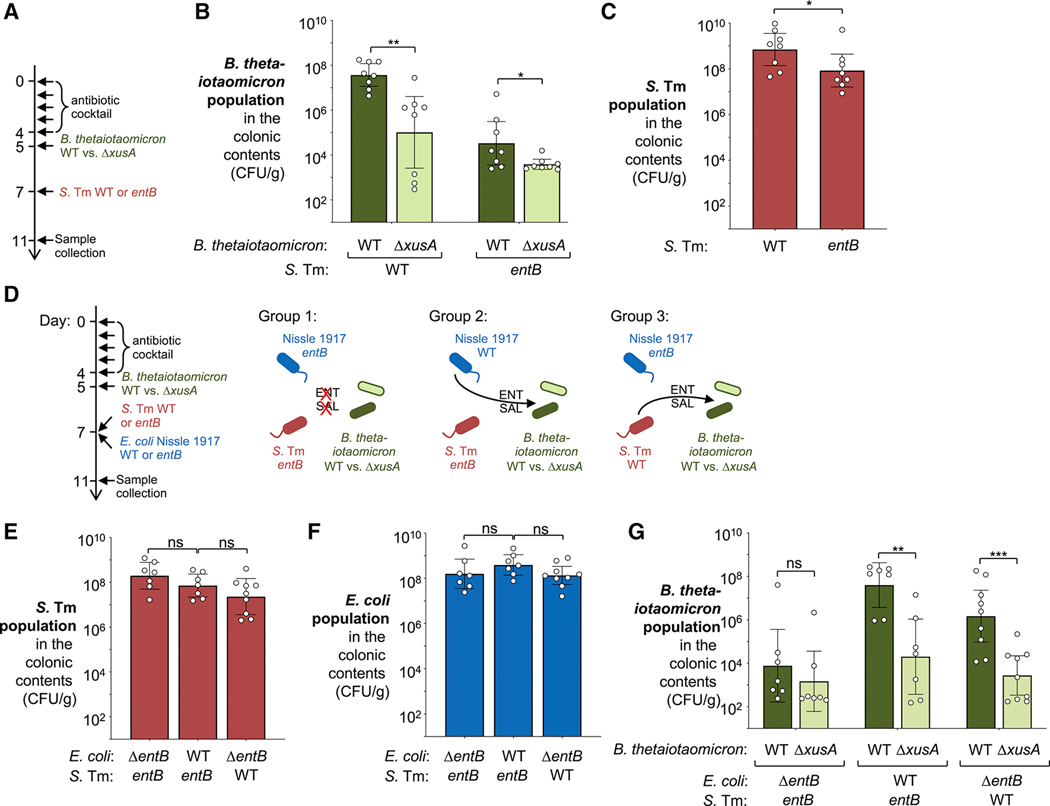
We next examined the origin of the siderophores used by the B. thetaiotaomicron XusABC system. S. Tm produces both enterobactin and salmochelin. As such, we predicted that the fitness defect of a B. thetaiotaomicron xusA mutant would be rescued when mice are infected by a S. Tm mutant unable to produce siderophores (S. Tm entB mutant) (Fig. 5A–C). As expected, the B. thetaiotaomicron xusA mutant colonized the colon of S. Tm-infected mice at significantly lower levels than the wild-type strain (~360-fold fitness defect; Fig. 5B). In contrast, the fitness defect mice infected with the S. Tm entB mutant was drastically reduced (8.7-fold fitness defect; Fig. 5B). This experiment suggests that during S. Tm infection, the siderophore pool accessed by B. thetaiotaomicron is primarily derived from S. Tm siderophores, with a minor contribution of siderophores from other members of the gut microbiota.
Figure 5: Contribution of enterobacterial siderophores to B. thetaiotaomicron fitness during infectious colitis.
(A – C) Groups of C57BL/6 mice were treated with a cocktail of antibiotics, followed by intragastrical inoculation of an equal mixture of the B. thetaiotaomicron wild-type strain (Δtdk, GenR) and a xusA mutant (WZ777, GenR CmR). Mice were then intragastrically inoculated with either S. Tm wild-type strain (SL1344; N = 8) or an entB mutant (WZ818; N = 8). (A) Schematic representation of the experiment. Four days after the S. Tm challenge, the abundance of B. thetaiotaomicron (B) and S. Tm (C) populations in the colonic contents was determined by plating on selective agar. (D – G) Groups of Lcn2-/mice were treated with a cocktail of antibiotics, followed by intragastrical inoculation of an equal mixture of the B. thetaiotaomicron wild-type strain (Δtdk, GenR) and a xusA mutant (WZ777, GenR CmR). Mice were then intragastrically inoculated with either an equal mixture of an S. Tm entB mutant (AR1258) and an E. coli Nissle 1917 entB mutant (WZ780) (N = 7, group 1), an equal mixture of an S. Tm entB mutant and the Nissle 1917 wild-type strain (WZ36) (N = 7, group 2), or an equal mixture of the S. Tm wild-type strain (IR715) and the Nissle 1917 entB mutant (WZ780) (N = 9, group 3). (D) Schematic representation of the experiment. Four days after the S. Tm challenge, the abundance of S. Tm (E), E. coli (F), and B. thetaiotaomicron (G) populations in the colonic contents was determined by plating on selective agar. See also Fig. S4. Bars represent the geometric mean ± 95% confidence interval. *, P < 0.05; **, P < 0.01; ***; P < 0.001; ns, not statistically significant.
Commensal Enterobacteriaceae family members produce siderophores that could be utilized by B. thetaiotaomicron for iron acquisition. E. coli is considered a member of the “core microbiota” shared by the majority of humans and E. coli strains are frequently isolated from human feces (Lozupone et al., 2012; Mitsuoka and Hayakawa, 1973; Penders et al., 2006). For example, E. coli Nissle 1917 strain produces and utilizes four distinct siderophores: enterobactin, salmochelin, yersiniabactin, and aerobactin and thus represents a useful tool for investigating iron metabolism in Enterobacteriaceae (Deriu et al., 2013). We hypothesized that siderophores produced by commensal E. coli could contribute to the siderophore pool utilized by commensal B. thetaiotaomicron during inflammatory conditions. To test this idea, we colonized antibiotic-treated mice with an equal mixture of B. thetaiotaomicron wild-type and the ΔxusA mutant as described above (competitive colonization assay) (Fig. 5D). Mice were then intragastrically inoculated with a Nissle 1917 ΔentB mutant as well as a S. Tm entB mutant (group 1). Because defects in siderophore production attenuates S. Tm virulence and gut colonization (Crouch et al., 2008; Raffatellu et al., 2009; Sassone-Corsi et al., 2016)(Fig. 5C), we used lipocalin-2-deficient (Lcn2) mice on the C57BL/6 background. Lipocalin-2, a protein released by neutrophils and epithelial cells, sequesters enterobactin, thus impeding bacterial iron acquisition through enterobactin. In Lcn2-deficient mice, the S. Tm entB mutant and the Nissle 1917 entB mutant efficiently colonized the large intestine (Fig. 5E and F). The B. thetaiotaomicron wild-type and the ΔxusA mutant were present in the colonic contents at low, but similar levels (Fig. 5G). This outcome suggests that in the absence of enterobacterial siderophore production, B. thetaiotaomicron was unable to benefit from siderophore utilization by the XusABC system. We then repeated this experiment and colonized groups of mice with either the Nissle 1917 wild-type strain and a S. Tm entB mutant (group 2) or a Nissle 1917 ΔentB mutant and the S. Tm wild-type strain (group 3) (Fig. 5D). We observed no differences in Nissle 1917 and S. Tm populations in the colonic contents (Fig. 5E and F). In both groups (group 2 and 3), the B. thetaiotaomicron wild-type population colonized the colon lumen at high levels, while the B. thetaiotaomicron ΔxusA mutant was unable to benefit from enterobacterial siderophore production (Fig. 5G). Taken together, these findings suggest that the B. thetaiotaomicron XusABC system only provides a fitness advantage in the context of siderophore production by Enterobacteriaceae family members.
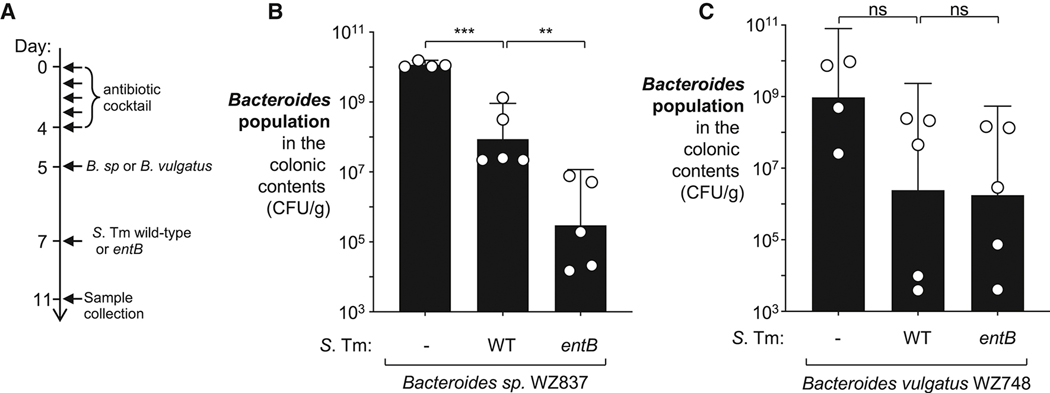
We also explored whether siderophore production by S. Tm enhances fitness of other members of the genus Bacteroides. We colonized antibiotic treated mice with two Bacteroides strains (B. vulgatus WZ748 and Bacteroides sp. WZ837) that utilize catecholate siderophores under iron-limiting conditions in the laboratory (Fig. 2C). These animals were then either infected with the S. Tm wild-type, an isogenic entB mutant, or mock-treated (Fig. 6A). Bacteroides sp. WZ837 was recovered at a significantly higher level in mice challenged by the S. Tm wild-type strain than those that had received the isogenic entB mutant (Fig. 6B), suggesting that this strain benefited from enterobacterial siderophores. In contrast, the B. vulgatus isolate showed no difference in colonization levels as a result of S. Tm siderophore production (Fig. 6C). This outcome suggests that members of the genus Bacteroides likely utilize divers, and possibly redundant, pathways for iron acquisition.
Figure 6: Contribution of siderophore utilization to fitness of Bacteroides isolates in vivo.
(A – E) Groups of C57BL/6 mice were treated with a cocktail of antibiotics, followed by intragastrical inoculation of B. sp (WZ837, GenR) or B. vulgatus (WZ748, GenR). Groups of mice either remained untreated (N = 4), or were intragastrically inoculated with the S. Tm wild-type strain (N = 5) or a S. Tm entB mutant (WZ818, N = 5). (A) Schematic representation of the experiment. Four days after the S. Tm inoculation, the abundance of B. sp (B) and B. vulgatus (C) populations in the colonic contents was determined by plating on selective agar. Bars represent the geometric mean ± 95% confidence interval. **, P < 0.01; ***, P < 0.001; ns, not statically significant.
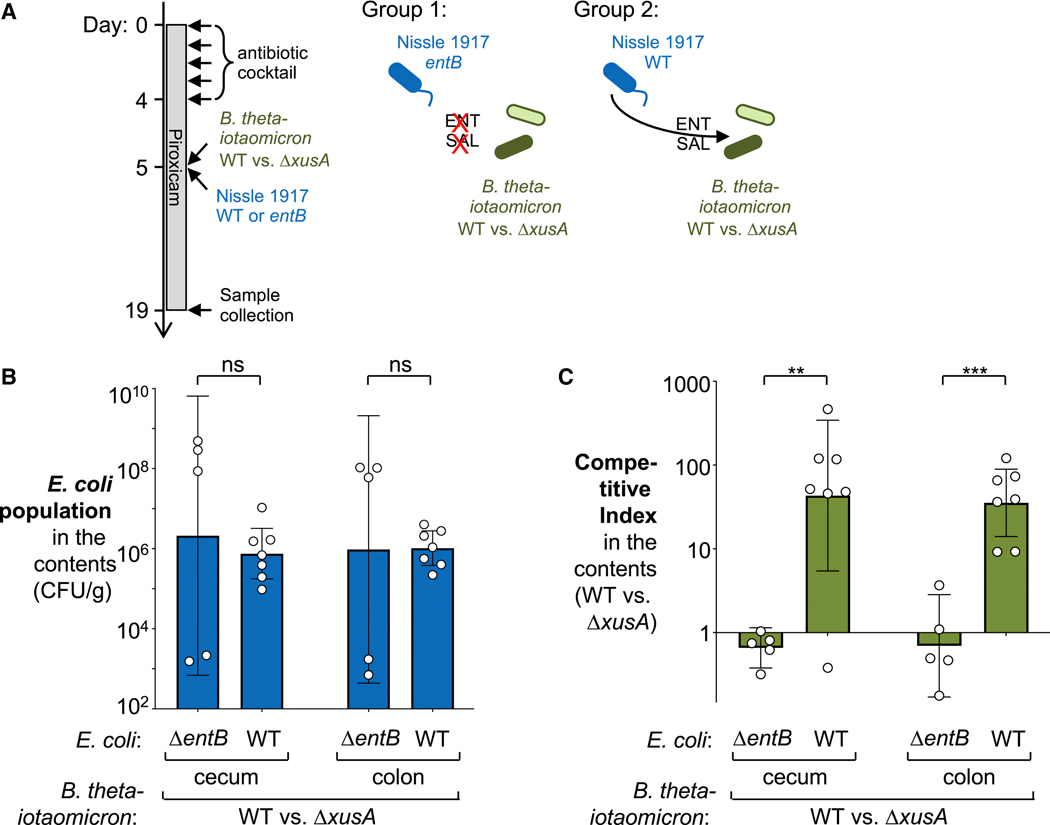
Enterobacterial siderophores enhance B. thetaiotaomicron fitness in mouse models of non-infectious colitis
Lastly, we determined whether cross-feeding of siderophores commensal E. coli and B. thetaiotaomicron also occurs in a setting of non-infectious colitis. Il10-deficient mice spontaneously develop colitis under laboratory conditions. Administration of oral non-steroidal anti-inflammatory drugs, such as piroxicam, accelerate this process (Hale et al., 2005). We treated Il10-deficient C57BL/6 mice with oral antibiotics as described above. One group of animals was then intragastrically inoculated with an equal mixture of the B. thetaiotaomicron wild-type and the ΔxusA mutant (competitive colonization assay), as well as a Nissle 1917 entB mutant (Fig. 7A). A second group was similarly inoculated with the two B. thetaiotaomicron strains as well as the Nissle 1917 wild-type strain. Both groups were treated with piroxicam throughout the entire experiment. Samples of intestinal contents were collected 14 days after colonization with B. thetaiotaomicron and E. coli, and bacterial populations were quantified by plating luminal contents on selective media (Fig. 7B and C). Both the Nissle 1917 and the isogenic entB mutant colonized the large intestine to similar levels (Fig. 7B). This observation is consistent with the idea that in the absence of enterobactin and salmochelin production, Nissle 1917 may rely on other siderophores such as aerobactin or yersiniabactin to acquire iron. Notably, the XusABC system only provided a fitness advantage in mice colonized by the Nissle 1917 wild-type strain (Fig. 7C). The XusABC system did not confer any fitness advantage in the absence of catecholate siderophore production by Nissle 1917 (entB mutant). No differences in inflammatory markers were observed in the two treatment groups (Fig. S7). This experiment demonstrates that cross-feeding of siderophores between B. thetaiotaomicron and commensal Enterobacteriaceae occurs in the inflamed intestinal tract.
Figure 7: Contribution of Enterobacterial siderophores to B. thetaiotaomicron fitness during non-infectious colitis.
(A – C) Groups of Il10−/− mice were treated with a cocktail of antibiotics to allow stable engraftment of B. thetaiotaomicron. Piroxicam was administered in the mouse diet throughout the experiment. Mice were intragastrically inoculated with an equal mixture of B. thetaiotaomicron wild-type strain (Δtdk, GenR) and a xusA mutant (WZ777, GenRCmR), plus a Nissle 1917 entB mutant (WZ780) (N = 5, group 1) or the same B. thetaiotaomicron mixture plus the Nissle 1917 wild-type strain (WZ36) (N = 7, group 2). Fourteen days after bacterial inoculation, Nissle 1917 and B. thetaiotaomicron abundance in intestinal contents was determined by plating on selective agar. (A) Schematic representation of the experiment. (B) E. coli population in intestinal content. (C) Competitive index of B. thetaiotaomicron wild-type over xusA mutant in intestinal content. See also Fig. S7. Bars represent the geometric mean ± 95% confidence interval. **, P < 0.01 ***, P < 0.001; ns, not statistically significant.
DISCUSSION
Resilience is an important feature of the gut microbiota in the context of inflammatory diseases. Bacteroides species are highly abundant in the microbiota of the mammalian large intestine and fulfill many pivotal functions for human health. For instance, anaerobic fermentation of complex dietary glycans by members of the Bacteroidetes phylum results in production of large quantities of short chain fatty acids, in particular propionate (Fischbach and Sonnenburg, 2011; Martens et al., 2014). Microbiota-derived propionate modulates T cell differentiation by inhibiting histone-deacetylase activity (Arpaia et al., 2013; Luu et al., 2018). In addition, propionate produced by B. thetaiotaomicron has been shown to inhibit growth of S. Tm in a mouse model of infection (Jacobson et al., 2018; Sorbara et al., 2019). It is therefore plausible that a more resilient microbial community enables the host to recover from enterobacterial pathogen challenges with faster kinetics and less severe host inflammatory responses.
The molecular mechanisms that mediate ecological or engineering resistance in the gut microbiota are beginning to be uncovered. Previous work suggests that Bacteroides species respond to the release of antimicrobial peptides by modifying their surface (Cullen et al., 2015). Furthermore, during nutrient limitation caused by the lack of dietary fiber, Bacteroides species resort to degrading host-derived glycoproteins as nutrient sources (Desai et al., 2016). Here, we present evidence that during inflammation-associated iron limitation, members of the Bacteroidetes phylum rely on the xenosiderophores enterobactin and salmochelin for iron acquisition. We identified a set of genes, xusABC, which are required for enterobactin and salmochelin utilization in B. thetaiotaomicron VPI-5482. XusABC-mediated xenosiderophore uptake was dispensable under homeostatic conditions, consistent with the idea that Bacteroides acquire iron from other sources, such as heme iron (Otto et al., 1990; Rocha et al., 1991) or transferrin (Manfredi et al., 2015), under these conditions. During colitis, microbial access to metals is limited, presumably by the release of lactoferrin and calprotectin (reviewed in (Hood and Skaar, 2012; Lopez and Skaar, 2018; Zhu et al., 2019b)). We found that the XusABC system was required for B. thetaiotaomicron VPI-5482 to efficiently maintain gut colonization during pathogen-induced (S. Tm infection) and non-infectious colitis (Il10/piroxicam-induced colitis).
Enterobactin and salmochelin are typically produced by Enterobacteriaceae family members. In our mouse models, the fitness advantage conferred by the XusABC system was markedly reduced when mice were colonized with E. coli and S. Tm mutants defective for enterobactin and salmochelin production (entB mutants; Fig. 5). This suggests that the experimentally introduced E. coli and S. Tm are a major source of enterobactin and salmochelin in our experiment. The XusABC system enhances colonization when iron accessibility is limited and siderophore-producing Enterobacteriaceae are present. The E. coli wild-type strain or S. Tm wild-type were each sufficient to permit B. thetaiotaomicron to benefit from xenosiderophore utilization through the XusABC system (Fig. 5). As such, both commensal and pathogenic Enterobacteriaceae can serve as the source of siderophores for B. thetaiotaomicron. Since B. thetaiotaomicron only relies on xenosiderophore utilization during specific environmental conditions that are conducive for siderophore production by Enterobacteriaceae, such as infection with an enterobacterial pathogen or non-infectious colitis (Raffatellu et al., 2009), it is tempting to speculate that may be little evolutionary pressure for B. thetaiotaomicron to maintain the ability to produce siderophores (Rocha et al., 1991; Verweijvanvught et al., 1988).
In vitro, the XusABC system was specific for two structurally related catecholate siderophores, enterobactin and salmochelin (Fig. 2). Salmochelin is a glucosylated derivative of enterobactin (Hantke et al., 2003). Since microbial siderophores exhibit vast structural variability (Hider and Kong, 2010), it is conceivable that other siderophores that are structurally similar to enterobactin and salmochelin could be utilized by the XusABC system. In the murine gut, enterobacterial enterobactin and salmochelin are likely to be the sole siderophores that allow B. thetaiotaomicron to acquire iron through the XusABC system since genetic ablation of enterobacterial enterobactin and salmochelin production rendered the XusABC system inefficacious (Fig. 5).
The XusABC system we identified is predicted to be composed of a putative TonB-dependent outer membrane transporter (XusA) and an inner membrane permease (XusC). The primary amino acid sequence of XusB may contain a signal that could lead to lipidation and export, suggesting that this protein may localize to the periplasm or possibly to the cell surface (Lauber et al., 2016; Wexler et al., 2018). Some aspects of the architecture of this system may be analogous to the FepABCDG system in E. coli and the FhuABCD system in Salmonella enterica. However, there is little sequence homology shared by the B. thetaiotaomicron XusABC components with known siderophore transport systems. Interestingly, utilization of the xenosiderophores requires the release of iron from the siderophore upon reaching the cytoplasm. Generally, there are two distinct mechanisms of iron release from siderophores. Because siderophores display significantly higher affinity towards iron (III) than iron (II), some bacterial species employ ferric reductases to reduce siderophore-bound iron (III) to iron (II) to facilitate its dissociation. Other bacterial species such as E. coli hydrolyze the siderophore through the action of esterases to release iron. Based on protein homology, we did not identify any ferric reductases or siderophore esterases in B. thetaiotaomicron, and how this bacterium releases iron from siderophores remains to be investigated.
In addition to xenosiderophores, Bacteroides use TonB-dependent outer membrane receptors to acquire corrinoids produced by other members of the gut microbiota (Goodman et al., 2009). B. thetaiotaomicron mutants defective for several redundant corrinoid uptake systems were less fit in colonizing the murine intestinal tract (Degnan et al., 2014; Wexler et al., 2018). In contrast to corrinoid uptake, which occurs under homeostatic conditions, xenosiderophore uptake was only relevant under inflammatory conditions. These examples of competition for corrinoids and siderophores highlight the complex nutritional interactions between members of the gut microbiota as it relates to micronutrient metabolism.
Nissle 1917 is an E. coli strain isolated from a soldier resistant to Shigella infection. It is currently a probiotic strain approved in certain European countries (Sonnenborn, 2016). It is effective in maintaining remission in a subset of ulcerative colitis patients (Kruis et al., 2004; Kruis et al., 1997; Rembacken et al., 1999). The molecular mechanisms associated with these probiotic properties are incompletely understood, but may be in part attributed to its ability to produce several distinct siderophores (Deriu et al., 2013). In mouse models of Salmonella infection, E. coli Nissle 1917 competes with the luminal Salmonella population for metals, such as iron and zinc, thus reducing pathogen burden and shedding (Deriu et al., 2013). Our data suggest that an additional mechanism may contribute to the probiotic potential of Nissle 1917. By producing catecholate siderophores, Nissle 1917 may enhance colonization by commensal Bacteroides during inflammatory episodes and thus increase microbiota resilience. In turn, increased commensal colonization may assist with pathogen clearance (Endt et al., 2010).
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY.
Further information and requests for resources and reagents should be directed to the Lead Contact, Sebastian E. Winter (Sebastian.Winter@UTSouthwestern.edu). All plasmids and bacterial strains generated in this study are available from the Lead Contact with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in the Key Resource Table. E. coli and S. Typhimurium strains were routinely grown in LB broth (10 g/L tryptone, 5 g/L yeast extract, 10 g/L sodium chloride) or on LB plates (LB broth, 15 g/L agar) at 37 ºC. When appropriate, antibiotics were added at the following concentrations: 100 μg/mL streptomycin (Strep), 100 μg/mL ampicillin, 50 μg/ml nalidixic acid (Nal), 100 μg/mL kanamycin (Kan), 50 μg/mL gentamycin (Gen) and 15 μg/mL chloramphenicol (Cm). B. thetaiotaomicron was grown anaerobically (90 % N2, 5 % CO2, 5 % H2; anaerobic chamber, Sheldon Manufacturing) in hemin supplemented brain-heart-infusion (BHI) media (0.8 % brain heart infusion from solid, 0.5 % peptic digest of animal tissue, 1.6 % pancreatic digest of casein, 0.5% sodium chloride, 0.2 % glucose, 0.25 % disodium hydrogen phosphate, 0.005 % haemin, pH 7.4) or hemin-supplemented TYG media (0.5 % tryptone, 0.5 % peptone, 0.5 % bacto yeast extract, 0.2 % glucose, 0.05 % cysteine, 0.1 M potassium phosphate, 1 ng/L vitamin K, 0.8 % CaCl2, 0.04 μg/L ferrous sulfate, 1 μg/L resazurin, 0.02 g/L magnesium sulfate heptahydrate, 0.4 g/L sodium bicarbonate, 0.08 g/L sodium chloride, pH = 7.2 ) for 24 h or on blood agar plates (37 g/L brain heart infusion, 15 g/L agar, 5 % [v/v] defibrinated blood) containing 50 μg/mL Gen or 15 μg/mL Cm for 2 days at 37 ºC. C. symbiosum was routinely cultured in pre-reduced thioglycollate medium (0.5 % yeast extract, 1.5 % pancreatic digest of casein, 0.5 % glucose, 0.05 % L-Cysteine, 0.25 % sodium chloride, 0.05 % sodium thioglycollate, 0.0001 % resazurin, 15 g/L agar, pH 7.1) or chopped meat broth (BD) in Hungate tubes. For in vitro dependence on iron-laden siderophores, single colonies of indicated strains were inoculated in hemin supplemented BHI media and grown anaerobically for 24 hours and sub-cultured (1 × 103 CFU/ml) into iron restricting media (BHI or TYG supplemented with 0.005 % protoporphyrin IX and 200 μmol/L bathophenanthroline disulfonate (BPS), pH 7.4) (Rocha and Krykunivsky, 2017) and further cultured anaerobically for 36 h. Iron-laden enterobactin and salmochelin (at 50 % saturation) were added at a final concentration of 0.5 μmol/L and 2 μmol/L, respectively. The apo-forms of these siderophores were added to media at identical concentrations. To demonstrate that the absence of iron, but not other trace elements, is responsible for the growth defect observed when BPS was supplemented, ammonium iron (III) citrate was supplemented at a concentration of 200 μmol/L.
To test whether the trace metal ions other than iron are responsible for supporting bacterial growth when BPS was supplemented, divalent ions were removed from a modified semi-defined media (1.5 g/L KH2PO4, 0.5 g/L NH4SO4, 0.9 g/L NaCl, 150 mg/L L-methionine, 5 μg/L vitamin B12, 1 mg/L resazurin, 1 g/L tryptone, 0.2% NaHCO3,) (Rocha and Smith, 2004) as previously described (Anderson et al., 2017; Yep et al., 2014). Briefly, the semi-defied media was mixed with 1 g/L Chelex 100 Resin (Biorad, USA) under rotary agitation at 4 °C overni ght. 1 g/L L-cysteine, 5 mg/L protoporphyrin IX, and 5 g/L glucose were added to the media and further incubated with additional Chelex 100 Resin (1 g/L) for 3 hours at 4 °C. The media was supplemented with divalent ions except iron (200 mg/L MgCl2 × 6 H2O, 100 mg/L CaCl2 × 2 H2O, 10 mg/L MnCl2 × 4 H2O, 10 mg/L CoCl2 × 6 H2O, 100 mg/L ZnCl2, 10 mg/L CuSO4, 10 mg/L Na2MoO4), and sterilized by passing through a 0.22 μm filter. Overnight cultures of B. thetaiotaomicron or S. Tm were washed twice using iron-free semi defined media to remove cell-associated iron before inoculation. The inoculum for these experiments was 104 CFU/ml.
For isolating members of phylum Bacteroidetes from the murine large intestinal tract, cecal and colonic contents of wild-type C57BL/6 mice were collected in phosphate buffered saline (PBS) supplemented with 0.05% L-cysteine. The contents were then plated on Bacteroides Bile Esculin Agar (Becton Dickinson, USA) and incubated anaerobically until colonies were observed. Single colonies were further purified using Bacteroides Bile Esculin Agar and the bacterial strains were identified by sequencing the V3-V4 region of 16S rDNA and comparing the sequences to the RDP database (https://rdp.cme.msu.edu/). The isolated Bacteroidetes strains were routinely propagated in hemin supplemented brain-heart-infusion (BHI) media. Of note, both Bacteroides isolates used in this manuscript (Bacteroides sp. WZ837 and Bacteroides vulgatus WZ748) are resistant to gentamycin.
Animal models.
C57BL/6J wild-type, Il10−/−, and Lcn2−/− mice, originally obtained from Jackson Laboratory (Bar Harbor), were bred under specific pathogen-free conditions at UT Southwestern Medical Center. Mice had ad libitum access to irradiated feed (16 % protein; Envigo) and autoclaved water, and were on a 12 h light-dark cycle. All animals were treatment naïve and healthy prior to our studies. Seven to nine-week-old male and female mice were semi-randomly assigned into treatment groups before the experiment.
Antibiotic cocktails (5 mg of each of ampicillin (Cayman Chemicals), metronidazole (Sigma), vancomycin (Chem Impex International) and neomycin (Sigma) per mouse) or mock treatment (water) were administered by oral gavage daily for 5 days. After antibiotic treatment, fecal pellets were collected and tested for bacterial growth on blood agar and blood agar supplemented with 50 μg/mL gentamycin. Only mice with no detectable bacterial growth on both media were included in the study to allow for quantification of experimentally-introduced Bacteroides strains in luminal content and feces. At day 5, mice were then inoculated with 3 × 109 CFU of the indicated B. thetaiotaomicron strains, or mouse Bacteroides isolates, or remained uninfected. In competitive colonization experiments, animals were inoculated with an equal mixture of 1.5 × 109 CFU of the B. thetaiotaomicron wild-type strain and 1.5 × 109 CFU of the indicated mutants. Two days later, mice were challenged by 1 × 109 CFU of the S. Typhimurium strain SL1344 for 4 days (Fig. 4 and 5A–C). For experiment involving E. coli Nissle 1917 and S. Typhimurium (Fig. 6D-G), animals were inoculated with an equal mixture of 1 × 109 CFU of the B. thetaiotaomicron wild-type strain, or 1 × 109 CFU of indicated B. thetaiotaomicron mutant at day 5. Two days after inoculation, mice were challenged with 1 × 109 CFU of indicated E. coli Nissle 1917 strain and 1 × 109 CFU of the indicated S. Typhimurium strain. For the non-infectious colitis model (Il10−/−)(Fig. 7), animals were inoculated with an equal mixture of 1 × 109 CFU of WT B. thetaiotaomicron strain, 1 × 109 CFU of indicated B. thetaiotaomicron mutant, and 1 × 109 CFU of indicated E. coli strain at day 5. Piroxciam (Sigma-Aldrich) was administered in the mouse feed (Envigo) at a concentration of 100 ppm for 19 days to accelerate colitis development.
For all experiments, fecal pellets were collected at the indicated time points. After euthanasia, cecal and colonic tissue was collected, flash frozen, and stored at −80 °C for subsequent mRNA analysis. For culture-dependent quantification of bacterial load, colonic and cecal contents were harvested in sterile PBS and the load of B. thetaiotaomicron, S. Tm, and E. coli were quantified by plating serial-diluted intestinal contents on selective agar. For culture-independent quantification, total DNA was extracted using QIAamp PowerFecal DNA Kit (Qiagen, CA) per manufacturer’s recommendations. The samples were eluted in 100 μl elution buffer and 2 μl was used to determine the bacterial load of the B. thetaiotaomicron and S. Typhimurium strains via qPCR with primers targeting strain-specific signature tags and primers specific for Enterobacteriaceae, respectively (Martens et al., 2008; Winter et al., 2013). Relevant primers are listed in Table S2.
Gnotobiotic mouse experiments.
Germ-free Swiss-Webster mice were maintained in plastic gnotobiotic isolators on a 12-hour light cycle. Mice were randomized and orally gavaged with 3×109 CFU of B. thetaiotaomicron and 3 × 109 CFU of C. symbiosum strains or remained uninfected. Seven days later, mice were challenged with 1 × 105 CFU of S. Typhimurium strain IR715 for 2 days. After euthanasia, cecal and colonic tissue was collected in RNAlater solution (Invitrogen, USA), flash frozen and stored at −80 °C for subsequ ent mRNA analysis. Total RNA was extracted as described above.
METHOD DETAILS
Iron-laden siderophores.
The iron-free siderophores enterobactin (Sigma-Aldrich) and salmochelin S4 (Genaxxon bioscience) were dissolved in dimethyl sulfoxide (DMSO) at the concentration of 2 mmol/L. The siderophores were filter-sterilized using 0.22 μm cellulose membrane (RPI Research Product International). Sterile 1 mmol/L ammonium Fe(III) citrate (Sigma-Aldrich) was incubated with the iron-free siderophores at 1:1 (v/v) overnight at 4 ºC to obtain a 1 mmol/L siderophore stock solution at 50% saturation.
Plasmids.
All plasmids used in this study are listed in the Key Resource Table. Suicide plasmids were routinely propagated in DH5α λpir. The flanking regions of the B. thetaiotaomicron genes BT_2063, BT_2065, BT_2063-65, BT_0496, BT_2479, BT2098-2100, BT_1950-52, BT_2409, BT_0502-04, BT_1219 were amplified and assembled into pExchange-tdk using the Gibson Assembly Cloning Kit (New England Biolab, Boston) to give rise to pWZ498, pWZ628, pWZ630, pWZ496, pWZ500, pWZ502, pWZ504, pWZ506, pWZ508, pWZ510, respectively. To complement the BT_2065 deletion, the flanking regions of the intergenic region between BT_3743 and BT_3744 were amplified and assembled into pExchange-tdk to give rise to pKI. Regions containing the promoter region and open reading frame of BT_2065 were amplified and assembled into pKI, yielding pWZ661. The flanking regions of the E. coli entB were amplified and ligated into pGP706 to generate pWZ517. The flanking regions of S. Typhimurium iroB, fepA, cirA, iroN genes were amplified and ligated into pGP706 to generate pWZ842, pWZ650, pWZ681, and pWZ626. S. Typhimurium entB was described previously (Tsolis et al., 1995) and was introduced into SL1344 by generalized phage transduction (Schmieger, 1972). Relevant plasmid inserts were verified by Sanger sequencing. In some instances, Nissle 1917 strains were marked by introducing the low-copy number plasmid pWSK129 through electroporation (WZ36 and WZ780) (Wang and Kushner, 1991).
Construction of mutants by allelic exchange.
All bacterial mutant strains constructed using the method below are listed in the Key Resource Table. For B. thetaiotaomicron mutants, suicide plasmid pExchange-tdk containing the flanking regions of genes of interest was conjugated into the B. thetaiotaomicron using S17-1 λpir as the conjugative donor strain. Exconjugants that had the suicide plasmid integrated into the recipient chromosome (single crossover) were recovered on blood plates containing appropriate antibiotics. FudR plates (blood plates supplemented with 200 μg/mL 5-fluoro-2-deoxy-uridine) were used to select for the second crossover event. To create the E. coli Nissle 1917 entB mutant, pWZ517 was conjugated into the E. coli Nissle 1917 using S17-1 λpir. Exconjugants that had the suicide plasmid integrated into the recipient chromosome (single crossover) were recovered on LB agar containing appropriate antibiotics. Sucrose plates (8 g/l nutrient broth base, 5 % sucrose, 15 g/l agar) were used to select for the second crossover event, thus creating WZ532. Deletion of the target gene was confirmed by PCR. Similar strategies were used to construct S. Typhimurium mutants lacking iroB, and fepA cirA iroN.
Transcriptional profiling of B. thetaiotaomicron in large intestine of gnotobiotic mice.
Total RNA of cecal contents was extracted and purified using RNeasy PowerMicrobiome Kit (Qiagen, CA) per the manufacturer’s recommendations. TruSeq Stranded Total RNA Library Prep kit (Illumina, CA) with Ribo-Zero Gold (‘epidemiology’) was used to construct single-end 150 bp RNAseq libraries depleted of host and bacterial ribosomes. Quantity and quality of total RNA and final libraries was determined using a Qubit 3 (Thermo Fisher) and TapeStation 4200 (Agilent, CA), respectively, before sequencing on an Illumina NextSeq 500 (Illumina, CA). Reads were trimmed using BBMap software suite and decontaminated by filtering against mouse genome (mm10, UCSC Genome Browser). Mapping against the B. thetaiotaomicron genome VPI-5482 was performed using Bowtie2 (Langmead and Salzberg, 2012; Langmead et al., 2019). Mapped reads were quantified using featureCounts software package (Liao et al., 2014) and differential expression analysis was performed using DESeq2 software (Love et al., 2014).
Targeted quantification of mRNA levels in intestinal tissue and contents.
Colonic or cecal tissue were homogenized in a bead beater (Biospec Products, Bartlesville) and RNA extracted using the TRI reagent method (Molecular Research Center, Cincinnati). DNA contamination was removed using the DNA-free Kit (Ambion, USA) per the manufacturer’s recommendations. RNA from intestinal contents was extracted using the RNeasy PowerMicrobiome Kit (Qiagen, USA) per manufacturer’s recommendation. TaqMan reverse transcription reagents (Invitrogen, USA) was used to generate cDNA. Real-time PCR was performed using Power SYBR Green Master Mix (Applied Biosystem, USA), data was acquired in a QuantStudio 6 Flex instrument (Life Technologies, USA). The final concentration of primers listed in Table S2 was 250 nM. Target gene transcription of each sample was normalized to the respective levels of Gapdh (mouse) or gmk (bacterial) mRNA.
Microbiota analysis.
RNA from the colon contents were extracted and reverse transcribed as described above. A 2 μl sample of the bacterial cDNA was used as the template for SYBR-green based real-time PCR reactions as described above. The primers used in this experiment were listed in Table S2. The gene copy number in the sample was determined based on a standard curve generated by using pSW196, pSW325, and pSW326 (Bacchetti De Gregoris et al., 2011). Plasmid preparations with a known DNA concentration were diluted (100-fold serial dilutions) and the threshold cycle value (Ct) determined by qPCR as described above. These Ct values were used to generate a standard curve.
Quantification of Bacteroides populations.
Quantification of B. thetaiotaomicron was done by two independent methods. In a culture-independent method, we used qPCR to quantify the copy numbers of B. thetaiotaomicron genomes using primers specific for genome-inserted signature tags. In this method, BT_0159, whose deletion bears no fitness cost in vitro or in vivo (Goodman et al., 2009), was replaced with a nonfunctional chloramphenicol resistance cassette fused with unique signature tags (Potvin et al., 2003) to generate signature-tagged, isogenic wild-type strains (WZ412, WZ413, WZ415, WZ418, and WZ433). The signature tags and the corresponding detection primers are listed in Table S2. A variable primer that hybridizes to each unique 20 bp tag and a universal primer that hybridizes to a position in the chloramphenicol resistance cassette (224 bp away from the signature tag) were used to detect tagged genomes by qPCR. To ensure the specificity of each signature tag, genomic DNA from B. thetaiotaomicron strains harboring each of the five signature tags were combined in pre-determined ratios and tested for the specificity of the tags using RT-qPCR assays (data not shown). Targeted mutations of genes involved in iron uptake were made in the background of WZ412, WZ413, WZ415, WZ418, or WZ433, respectively, to generate WZ534, WZ537, WZ541, WZ549, WZ551, WZ553, WZ555, WZ591, WZ636, WZ647, and WZ697. To detect the relative representation of each strain in vitro and in vivo, bacterial genomic DNA were extracted by directly boiling bacterial suspension in sterile water or using the QIAamp PowerFecal DNA isolation kit (Qiagen, USA) per the recommendations of the manufacturer. Tagged genomes were detected using the qPCR method as described above. 2 μL of 100-fold serial diluted, purified plasmid DNA standards prepared from each tagged cassette were included in each qPCR run as described above. A standard curve was generated using these standards and used to calculate the relative representation of each strain in each sample. This method was used for experiments shown in Fig. 4C, S3A, and S5B–E. For all other experiments, a culture-dependent method was used. Wild-type B. thetaiotaomicron VPI-5482 (Xu et al., 2003) is resistant to gentamycin. To differentially mark the ΔBT_2065 mutant (WZ777), a chloramphenicol resistant cassette derived from pACYC184 (Chang and Cohen, 1978) was inserted into the intergenic region of BT_3743 and BT_3744. Colonic or cecal contents were serially diluted in sterile PBS and plated on selective agar plates, followed by incubation in the anaerobic chamber. Colonization levels of mouse Bacteroides isolates were quantified using Gentamycin-supplemented blood agar plates as selective media.
Detection of enterobactin in intestinal contents.
Intestinal contents were harvested in sterile PBS and homogenized using Lysing Matrix B (MP Biomedicals, USA). The resulting supernatant was filter-sterilized and mixed with an equal volume of iron-limiting growth media (LB broth supplemented with 200 μmol/L BPS and 100 μmol/L Kanamycin) containing 2 × 104 CFU/ml of a reporter strain (S. Tm SL1344 entB). Sterile PBS or a serial dilution of iron-laden enterobactin were used as controls. The incubation was allowed to proceed aerobically for 12 hours and growth of the reporter strain was determined by measuring optical density of the culture at 600 nm (OD600).
Inductively Coupled Plasma Mass Spectrometry.
For measurement of intracellular iron concentration, 8–10 colonies of indicated strains were cultured in 3 ml of modified, semi-defined medium (1.5 g/L KH2PO4, 0.5 g/L NH4SO4, 0.9 g/L NaCl, 150 mg/L L-methionine, 5 μg/L vitamin B12, 20 mg/L MgCl2 × 6 H2O, 10 mg/L CaCl2 × 2 H2O, 1 mg/L MnCl2 × 4 H2O, 1 mg/L CoCl2 × 6 H2O, 1 mg/L resazurin, 1 g/L L-cysteine, 5 mg/L protoporphyrin IX, 5 g/L glucose, 1 g/L tryptone, 0.2% NaHCO3, and 200 μmol/L BPS, pH = 7.2) (Rocha and Smith, 2004) anaerobically for 24 hours, and subcultured (1:50) in 50 ml of modified semi-defined medium supplemented with 0.5 μmol/L iron-laden enterobactin for additional 36 hours before ICP-MS measurement. Cells were washed three times with 1 mM EDTA (pH = 8.0) to remove extracellular iron before acid digestion. For measurement of iron concentration in the large intestine, cecal contents were collected and the soluble and chelatable fractions were obtained by mixing the contents with H2O and 1 mM EDTA (pH = 8.0) respectively. The iron in the remaining contents was considered the inaccessible fraction. After collection, the samples were digested using freshly prepared 50% nitric acid (Thermo Fisher Scientific, USA). Incubation in nitric acid was allowed to proceed for two days to ensure complete digestion of the cells and dissolution of the metals to be analyzed. Nitric acid was evaporated by heating the vials in an oil-bath at 150 °C in a chemical fume hood and the remaining matter in each vial was re-dissolved in 3% nitric acid followed by sonication for 30 min to ensure a homogeneous dispersion of the iron to be analyzed. The solution was then centrifuged and filtered if needed to remove any particulates. The supernatant was analyzed for iron by inductively coupled plasma mass spectrometry (ICP-MS) using Agilent 7700x instrument (Agilent Technologies). The measurement was repeated three times for each sample.
Measurement of free iron in the ferene-S assay
Working solution containing chromogenic reagent ferene-s (NH4CH3CO2, 0.4 mol/L; (3-(2-Pyridyl)-5,6-di(2-furyl)1,2,4-triazine-5′,5′′-disulfonic acid disodium salt (Ferene-s), 0.005 mol/L; pH = 4.3) was prepared (Hedayati et al., 2018). 900 μL of working solution was mixed with 100 μL of sample and incubated at room temperature in the dark for 20 hours. Iron concentration was determined by measuring the absorbance at 595nm.
QUANTIFICATION AND STATISTICAL ANALYSIS.
Unless noted otherwise, data analysis was performed in GraphPad Prism v8.1.1. Values of bacterial population sizes, competitive indices, iron concentrations, and fold changes in mRNA levels were normally distributed after transformation by the natural logarithm. A two-tailed Student’s t-test was then applied to the ln-transformed data. Unless otherwise stated, *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not statistically significant. The exact number of independent samples (N) and other information regarding descriptive statistics, such as the definition of bar height and error bars, is listed in each figure legend. In all mouse experiments, N refers to the number of animals from which samples were taken. Sample sizes (e.g. the number of animals per group) were not estimated a priori since effect sizes in our system cannot be predicted. No predicted statistical outliers were removed since the presence or absence of these potential statistical outliers did not affect the overall interpretation. Mice that were euthanized early due to health concerns were excluded from analysis.
DATA AND CODE AVAILABILITY
The sequencing data has been deposited in the European Nucleotide Archive under the accession number PRJEB33026.
Supplementary Material
Changes in the B. thetaiotaomicron transcriptome during Salmonella infection. Related to Fig. 1.
Oligonucleotides used in this study. Related to STAR Methods.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER AND USAGE |
|---|---|---|
| Bacterial Strains | ||
| Bacteroides thetaiotaomicron VPI-5482 Δtdk (GenR) | (Koropatkin et al., 2008) | VPI-5482 |
| B. thetaiotaomicron Δtdk ΔBT0159::Cm-cassette-signature-tag-3 | This study | WZ433 |
| B. thetaiotaomicron Δtdk ΔBT0159::Cm-cassette-signature-tag-13 | This study | WZ412 |
| B. thetaiotaomicron Δtdk ΔBT0159::Cm-cassette-signature-tag-15 | This study | WZ413 |
| B. thetaiotaomicron Δtdk ΔBT0159::Cm-cassette-signature-tag-21 | This study | WZ415 |
| B. thetaiotaomicron Δtdk ΔBT0159::Cm-cassette-signature-tag-24 | This study | WZ418 |
| B. thetaiotaomicron WZ412 ΔBT_2063 (= ΔxusC) | This study | WZ534 |
| B. thetaiotaomicron WZ412 ΔBT_2064 (= ΔxusB) | This study | WZ697 |
| B. thetaiotaomicron WZ412 ΔBT_2065 (= ΔxusA) | This study | WZ647 |
| B. thetaiotaomicron WZ412 ΔBT_2063-65 | This study | WZ636 |
| B. thetaiotaomicron WZ412 ΔBT_0496 | This study | WZ541 |
| B. thetaiotaomicron WZ412 ΔBT_2479 | This study | WZ555 |
| B. thetaiotaomicron WZ413 ΔBT_2098-2100 | This study | WZ553 |
| B. thetaiotaomicron WZ413 ΔBT_1950-1952 | This study | WZ551 |
| B. thetaiotaomicron WZ413 ΔBT_2409 | This study | WZ549 |
| B. thetaiotaomicron WZ418 ΔBT_0502-04 | This study | WZ591 |
| B. thetaiotaomicron WZ415 ΔBT_1219 | This study | WZ537 |
| B. thetaiotaomicron WZ647 (BT_3743-3744)::BT_2065 (complemented) | This study | WZ675 |
| B. thetaiotaomicron Δtdk ΔBT_2065 (BT_3743-3744)::CmR | This study | WZ777 |
| Bacteroides vulgatus (mouse isolate) | This study | WZ748 |
| Bacteroides sp. (mouse isolate) | This study | WZ837 |
| Bacteroides sp. (mouse isolate) | This study | WZ838 |
| S. Tm wild-type strain (NaIR) | (Stojiljkovic et al., 1995) | IR715 |
| S. Tm wild-type strain (StrepR) | (Hoiseth and Stocker, 1981) | SL1344 |
| S. Tm IR715 entB::MudJ (KanR) | (Tsolis et al., 1995) | AR1258 |
| S. Tm SL1344 entB::MudJ (StrepR KanR) | This study | WZ818 |
| S. Tm SL1344 iroB (StrepR) | This study | WZ840 |
| S. Tm SL1344 iroN fepA cirA (StrepR) | This study | WZ692 |
| Clostridium symbiosum | ATCC | ATCC 14940 |
| E. coli wild-type strain (O6:K5:H1) | (Grozdanov et al., 2004) | Nissle 1917 |
| E. coli Nissle 1917 (pWSK129) (KanR) | This study | WZ36 |
| E. coli Nissle 1917 ΔentB | This study | WZ532 |
| E. coli Nissle 1917 ΔentB (pWSK129) (KanR) | This study | WZ780 |
| E. coli DH5α λpir; F- endA1 hsdR17 (r-m+) supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U189 φ80lacZΔM15 λpir | (Pal et al., 2005) | DH5α λpir |
| E. coli S17-1 λpir; zxx::RP4 2-(Tetr::Mu) (Kanr::Tn7) λpir | (Simon et al., 1983) | S17-1 λpir |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 5-fluoro-2-deoxy-uridine | Ark Pharm | Cat#AK-24802 |
| 5,5′-[3-(2-Pyridyl)-1,2,4-triazine-5,6-diyl]difuran-2-sulfonic acid disodium salt | Sigma | Cat#P4272 |
| Ammonium acetate | USB | Cat#11251 |
| Agar | Thermo Fisher | Cat# BP1423 |
| Ammonium Fe(III) citrate | Sigma | Cat# F5879 |
| Ampicillin | Cayman Chemical Company | Cat# 14417 |
| Bacteroides Bile Esculin Agar (BBE) | Becton Dickinson | Cat# 221836 |
| Bathophenanthroline disulfonic acid disodium salt hydrate | Alfa Aesar | Cat# B23244 |
| Brain-heart-infusion (BHI) media | Becton Dickinson | Cat# 237500 |
| Bacto yeast extract | Becton Dickinson | #212750 |
| CaCl2 x 2 H2O | Sigma | Cat#223506 |
| Chelex 100 Resin | Biorad | Cat#142-1253 |
| Chopped meat media | Remel | Cat# R05031 |
| Chloramphenicol | Fisher Bioreagents | Cat#BP904-100 |
| CoCl2 x 6 H2O | Sigma | Cat# 202185 |
| CuSO4 | Sigma | Cat#C1297 |
| L-Cysteine | Alfa Aesar | Cat#A10389 |
| Defibrinated horse blood | Hemostat | Cat# DHB500 |
| Difco LB agar-Miller | Becton Dickinson | Cat# 244520 |
| Difco LB broth-Miller | Becton Dickinson | Cat# 244620 |
| Dimethyl sulfoxide (DMSO) | Corning | Cat# MT-25950CQC |
| DNA-free DNA removal Kit | Invitrogen | Cat# AM1906 |
| EDTA Disodium Salt | RPI | Cat# E57020-1000.0 |
| Enterobactin, iron free | Sigma | Cat# E3910 |
| FeSO4 | Sigma | Cat#F8633 |
| Glucose | Fisher Chemical | Cat#D14-212 |
| Hemin | Sigma | Cat#51280 |
| Kanamycin | Fisher Chemical | Cat#BP906-5 |
| KH2PO4 | Fisher Chemical | Cat#P285 |
| L-cysteine | Alfa Aesar | Cat#A10389 |
| L-methionine | Sigma | Cat#M9625 |
| Metronidazole | Sigma | Cat# M3761 |
| MgCl2 x 6 H2O | Fisher Chemical | Cat#M33 |
| MgSO4 x 7 H2O | Amresco | Cat#10034-99-8 |
| MnCl2 x 4 H2O | Acros Organics | Cat#205891000 |
| NaCl | RPI | Cat#S23020 |
| NaHCO3 | Sigma | Cat#S6014 |
| Nalidixic acid | Fisher Bioreagents | Cat#BP908-25 |
| Neomycin trisulfate hydrate | Sigma | Cat# N1876 |
| (NH4)2SO4 | Sigma | Cat#A4915 |
| Nitric acid (TraceMetal™ Grade) | Fisher Chemicals | Cat# A509-P500 |
| Nutrient broth base | Becton Dickinson | Cat# 234000 |
| Menadione | Sigma | Cat#M9429 |
| Protoporphyrin IX | Enzo Life Sciences | Cat# ALX-430-041-G001 |
| Peptone | Becton Dickinson | Cat#211684 |
| Resazurin | Sigma | Cat#R7017 |
| Salmochelin S4, iron free | EMC Microcollections | Cat# SAL-S4 |
| Sodium molybdate dihydrate | Sigma | Cat#M-1003 |
| Streptomycin sulfate | VWR | Cat# 97061-528 |
| Sucrose | Fisher Science Education | Cat#S25590B |
| SYBR Green qPCR Master Mix | Life Technologies | Cat# 4309155 |
| TaqMan reverse transcription reagents | Invitrogen | Cat# N8080234 |
| Thioglycollate media | Sigma | Cat# 90404 |
| TRI reagent | Molecular research center | Cat# TR118 |
| Tryptone | Thermo Fisher | Cat#BP1421 |
| Vancomycin | Chem-Impex INT’L INC | Cat# 00315 |
| Vitamin B12 | Sigma | V2876 |
| Lysing Matrix B | MP Biomedicals | Cat# 6911050 |
| Zinc chloride | Sigma | Cat#208086 |
| Critical Commercial Assays | ||
| QIAamp PowerFecal DNA Isolation Kit | Qiagen | Cat# 12830-50 |
| RNeasy PowerMicrobiome Kit | Qiagen | Cat# 26000-50 |
| TruSeq Stranded Total RNA Library Prep Kit | Illumina | Cat# 20020598 |
| RNAlater™ Stabilization Solution | Thermo Fisher | Cat# AM7020 |
| TOPO cloning kit | Invitrogen | Cat# K457502 |
| Gibson Assembly Cloning Kit | NEB | Cat# E2611 |
| Q5 Hot Start 2x Master Mix | NEB | Cat# M0494L |
| DNA-free™ DNA Removal Kit | Invitrogen | Cat#AM1906 |
| Deposited Data | ||
| RNA-seq data | European Nucleotide Archive | PRJEB33026 |
| Experimental Models: Organisms/Strains | ||
| SPF C57BL/6 mice (wild-type) | The Jackson Laboratory | Cat# 000664 |
| SPF B6.129P2-Il10tm1Cgn/J (Il10-/-) | The Jackson Laboratory | Cat# 002251 |
| SPF B6.129P2-Lcn2tm1Aade/AkiJ (Lcn2-/-) | The Jackson Laboratory | Cat# 024630 |
| Wild-type Swiss Webster (ex-germ-free) | (Spiga et al., 2017) | N/A |
| Recombinant DNA | ||
| pKNOCK-bla-ermGb::tdk | (Koropatkin et al., 2008) | pExchange-tdk |
| pExchange-tdk::intergenic region of BT_3743 and BT_3744 | This study | pKI |
| ori(R6K) mobRP4 sacRB KanR | (Hughes et al., 2017) | pGP706 |
| ori(pSC101) lacZα KanR | (Wang and Kushner, 1991) | pWSK129 |
| pExchange-tdk:: BT_0159:: ΔBT0159::Cm-cassette-signature-tag-3 | This study | pWZ433 |
| pExchange-tdk:: BT_0159:: ΔBT0159::Cm-cassette-signature-tag-13 | This study | pWZ412 |
| pExchange-tdk:: BT_0159:: ΔBT0159::Cm-cassette-signature-tag-15 | This study | pWZ413 |
| pExchange-tdk:: BT_0159:: ΔBT0159::Cm-cassette-signature-tag-21 | This study | pWZ415 |
| pExchange-tdk:: BT_0159:: ΔBT0159::Cm-cassette-signature-tag-24 | This study | pWZ418 |
| Upstream and downstream regions of E. coli Nissle 1917 entB in pGP706 | This study | pWZ517 |
| Upstream and downstream regions of B. theta BT_2063 in pExchange-tdk | This study | pWZ498 |
| Upstream and downstream regions of B. theta BT_2065 in pExchange-tdk | This study | pWZ628 |
| Upstream and downstream regions of B. theta BT_2063-65 in pExchange-tdk | This study | pWZ630 |
| Upstream and downstream regions of B. theta BT_0496 in pExchange-tdk | This study | pWZ496 |
| Upstream and downstream regions of B. theta BT_2479 in pExchange-tdk | This study | pWZ500 |
| Upstream and downstream regions of B. theta BT_2098-2100 in pExchange-tdk | This study | pWZ502 |
| Upstream and downstream regions of B. theta BT_1950-52 in pExchange-tdk | This study | pWZ504 |
| Upstream and downstream regions of B. theta BT_2409 in pExchange-tdk | This study | pWZ506 |
| Upstream and downstream regions of B. theta BT_0502-04 in pExchange-tdk | This study | pWZ508 |
| Upstream and downstream regions of B. theta BT_1219 in pExchange-tdk | This study | pWZ510 |
| Upstream and downstream regions of S.Tm iroN in pGP706 | This study | pWZ626 |
| Upstream and downstream regions of S.Tm fepA in pGP706 | This study | WZ650 |
| Upstream and downstream regions of S.Tm cirA in pGP706 | This study | pWZ681 |
| Upstream and downstream regions of S.Tm iroB in pGP706 | This study | pWZ842 |
| Promoter and open reading frame of B. theta BT_2065 in pKI | This study | pWZ661 |
| 16S rDNA fragment of E. coli K-12 cloned into pCR2.1 | (Winter et al., 2013) | pSW196 |
| 16S rDNA fragment of a member of the order Clostridiales cloned into pCR2.1 | (Bacchetti De Gregoris et al., 2011; Winter et al., 2013) | pSW325 |
| 16S rDNA fragment of a Coriobacteriaceae family member cloned into pCR2.1 | (Bacchetti De Gregoris et al., 2011; Winter et al., 2013) | pSW326 |
| Software and Algorithms | ||
| Excel for Mac 2016 | Microsoft | N/A |
| Prism V8.1.1. | Graph Pad | https://www.graphpad.com/scientific-software/prism/ |
| DESeq2 V1.22.2 | (Love et al., 2014) | http://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| BBMap V36.20 | DOE Joint Genome Institute | https://github.com/BioInfoTools/BBMap/releases/tag/v36.20 |
| featureCounts V1.5.1 | (Liao et al., 2014) | http://subread.sourceforge.net/ |
| Bowtie2 V2. 29 | (Langmead and Salzberg, 2012) | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| MacVector V13.5.2 | MacVector | https://macvector.com/downloads.html |
| Oligonucleotides | ||
| Information regarding oligonucleotides used in this study is listed in Table S2. | ||
Highlights:
Microbial access to the micronutrient iron is decreased during gut inflammation
Bacteroides thetaiotaomicron acquires iron through siderophores from other bacteria.
XusABC system is required for B. thetaiotaomicron to use enterobactin and salmochelin
Xenosiderophores are critical for B. thetaiotaomicron colonization during inflammation
ACKNOWLEDGEMENTS
We thank Dr. Eric Hansen (UT Southwestern Medical Center) and Dr. Melissa Ellermann (UT Southwestern Medical Center) for helpful discussions. Work in S.E.W.’s lab was funded by the NIH (AI118807, AI128151), The Welch Foundation (I-1969-20180324), the Burroughs Wellcome Fund (1017880), and a Research Scholar Grant (RSG-17-048-01-MPC) from the American Cancer Society. Work in L.V.H.’s lab is supported by the Howard Hughes Medical Institute and by the NIH ( DK070855). W.Z. was supported by a Research Fellows Award from the Crohn’s and Colitis Foundation of America (454921). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the funding agencies. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
DECLARATION OF INTEREST
The corresponding author (SEW) is listed as an inventor on patent application WO2014200929A1, which describes a treatment to prevent the inflammation-associated expansion of Enterobacteriaceae. The other authors have no additional financial nterests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson MT, Mitchell LA, Zhao L, and Mobley HLT (2017). Capsule Production and Glucose Metabolism Dictate Fitness during Serratia marcescens Bacteremia. MBio. 8(3). Published online 2017/05/26 DOI: 10.1128/mBio.00740-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 504(7480), 451–455. Published online 2013/11/15 DOI: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. (2012). Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 338(6103), 120–123. Published online 2012/08/21 DOI: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti De Gregoris T, Aldred N, Clare AS, and Burgess JG (2011). Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods. 86(3), 351–356. Published online 2011/06/28 DOI: 10.1016/j.mimet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Baumler AJ, Norris TL, Lasco T, Voight W, Reissbrodt R, Rabsch W, and Heffron F. (1998). IroN, a novel outer membrane siderophore receptor characteristic of Salmonella enterica. J Bacteriol. 180(6), 1446–1453. Published online 1998/03/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caza M, and Kronstad JW (2013). Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front Cell Infect Microbiol. 3, 80 Published online 2013/12/07 DOI: 10.3389/fcimb.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AC, and Cohen SN (1978). Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 134(3), 1141–1156. Published online 1978/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LF, Mol JP, Silva AP, Macedo AA, Silva TM, Alves GE, Winter S, Winter MG, Velazquez EM, Byndloss MX, et al. (2016). Iron acquisition pathways and colonization of the inflamed intestine by Salmonella enterica serovar Typhimurium. Int J Med Microbiol. 306(8), 604–610. Published online 2016/10/21 DOI: 10.1016/j.ijmm.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch ML, Castor M, Karlinsey JE, Kalhorn T, and Fang FC (2008). Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol. 67(5), 971–983. Published online 2008/01/16 DOI: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH., Booth CJ., Yu H., and Goodman AL. (2015). Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science. 347(6218), 170–175. Published online 2015/01/13 DOI: 10.1126/science.1260580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MM, Hu Z, Klimko C, Narayanan S, Deberardinis R, and Sperandio V. (2014). The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe. 16(6), 759–769. Published online 2014/12/17 DOI: 10.1016/j.chom.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan PH, Barry NA, Mok KC, Taga ME, and Goodman AL (2014). Human gut microbes use multiple transporters to distinguish vitamin B(1)(2) analogs and compete in the gut. Cell Host Microbe. 15(1), 47–57. Published online 2014/01/21 DOI: 10.1016/j.chom.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, and Raffatellu M. (2013). Probiotic bacteria reduce salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 14(1), 26–37. Published online 2013/07/23 DOI: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, et al. (2016). A Dietary FiberDeprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 167(5), 1339–1353 e1321 Published online 2016/11/20 DOI: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ochoa VE, Lam D, Lee CS, Klaus S, Behnsen J, Liu JZ, Chim N, Nuccio SP, Rathi SG, Mastroianni JR, et al. (2016). Salmonella Mitigates Oxidative Stress and Thrives in the Inflamed Gut by Evading Calprotectin-Mediated Manganese Sequestration. Cell Host Microbe. 19(6), 814–825. Published online 2016/06/10 DOI: 10.1016/j.chom.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ, Heikenwalder M, et al. (2010). The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 6(9), e1001097 Published online 2010/09/17 DOI: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, et al. (2013). The long-term stability of the human gut microbiota. Science. 341(6141), 1237439 Published online 2013/07/06 DOI: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, and Sonnenburg JL (2011). Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe. 10(4), 336–347. Published online 2011/10/25 DOI: 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost GE, and Rosenberg H. (1975). Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 124(2), 704–712. Published online 1975/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, et al. (2010). Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 8(3), 292–300. DOI: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, Knight R, and Gordon JI (2009). Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe. 6(3), 279–289. Published online 2009/09/15 DOI: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson LH (2000). Ecological resilience - in theory and application. Annu Rev Ecol Syst. 31, 425–439. DOI: DOI 10.1146/annurev.ecolsys.31.1.425. [DOI] [Google Scholar]
- Hale LP, Gottfried MR, and Swidsinski A. (2005). Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm Bowel Dis. 11(12), 1060–1069. Published online 2005/11/25 DOI: 10.1097/01.mib.0000187582.90423.bc. [DOI] [PubMed] [Google Scholar]
- Hantke K, Nicholson G, Rabsch W, and Winkelmann G. (2003). Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A. 100(7), 3677–3682. Published online 2003/03/26 DOI: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayati M, Abubaker-Sharif B, Khattab M, Razavi A, Mohammed I, Nejad A, Wabler M, Zhou H, Mihalic J, Gruettner C, et al. (2018). An optimised spectrophotometric assay for convenient and accurate quantitation of intracellular iron from iron oxide nanoparticles. Int J Hyperthermia. 34(4), 373–381. Published online 2017/08/02 DOI: 10.1080/02656736.2017.1354403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider RC, and Kong X. (2010). Chemistry and biology of siderophores. Nat Prod Rep. 27(5), 637–657. Published online 2010/04/09 DOI: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- Hood MI, and Skaar EP (2012). Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 10(8), 525–537. Published online 2012/07/17 DOI: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, et al. (2018). A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection. Cell Host Microbe. 24(2), 296–307 e297 Published online 2018/07/31 DOI: 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlinsey JE, Stepien TA, Mayho M, Singletary LA, Bingham-Ramos LK, Brehm MA, Greiner DL, Shultz LD, Gallagher LA, Bawn M, et al. (2019). Genome-wide Analysis of Salmonella enterica serovar Typhi in Humanized Mice Reveals Key Virulence Features. Cell Host Microbe. 26(3), 426–434 e426 Published online 2019/08/27 DOI: 10.1016/j.chom.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebba PE, McIntosh MA, and Neilands JB (1982). Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 149(3), 880–888. Published online 1982/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, Kamm MA, Weismueller J, Beglinger C, Stolte M, et al. (2004). Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 53(11), 1617–1623. Published online 2004/10/14 DOI: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, and Stolte M. (1997). Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 11(5), 853–858. Published online 1997/11/14. [DOI] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods. 9(4), 357–359. Published online 2012/03/06 DOI: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Wilks C, Antonescu V, and Charles R. (2019). Scaling read aligners to hundreds of threads on general-purpose processors. Bioinformatics. 35(3), 421–432. Published online 2018/07/19 DOI: 10.1093/bioinformatics/bty648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber F, Cornelis GR, and Renzi F. (2016). Identification of a New Lipoprotein Export Signal in Gram-Negative Bacteria. MBio. 7(5). Published online 2016/11/01 DOI: 10.1128/mBio.01232-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, and Mazmanian SK (2013). Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 501(7467), 426–429. Published online 2013/08/21 DOI: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W. (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 30(7), 923–930. Published online 2013/11/15 DOI: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Lim B, Zimmermann M, Barry NA, and Goodman AL (2017). Engineered Regulatory Systems Modulate Gene Expression of Human Commensals in the Gut. Cell. 169(3), 547–558 e515 Published online 2017/04/22 DOI: 10.1016/j.cell.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CA, and Skaar EP (2018). The Impact of Dietary Transition Metals on Host-Bacterial Interactions. Cell Host Microbe. 23(6), 737–748. Published online 2018/06/15 DOI: 10.1016/j.chom.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15(12), 550 Published online 2014/12/18 DOI: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, and Knight R. (2012). Diversity, stability and resilience of the human gut microbiota. Nature. 489(7415), 220–230. Published online 2012/09/14 DOI: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke RK, and Gibson F. (1971). Location of three genes concerned with the conversion of 2,3-dihydroxybenzoate into enterochelin in Escherichia coli K-12. J Bacteriol. 107(2), 557–562. Published online 1971/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, and Finlay BB (2007). Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2(2), 119–129. Published online 2007/11/17 DOI: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Luu M, Weigand K, Wedi F, Breidenbend C, Leister H, Pautz S, Adhikary T, and Visekruna A. (2018). Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci Rep. 8(1), 14430 Published online 2018/09/28 DOI: 10.1038/s41598-018-32860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi P, Lauber F, Renzi F, Hack K, Hess E, and Cornelis GR (2015). New iron acquisition system in Bacteroidetes. Infect Immun. 83(1), 300–310. Published online 2014/11/05 DOI: 10.1128/IAI.02042-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Chiang HC, and Gordon JI (2008). Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 4(5), 447–457. Published online 2008/11/11 DOI: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Kelly AG, Tauzin AS, and Brumer H. (2014). The devil lies in the details: how variations in polysaccharide fine-structure impact the physiology and evolution of gut microbes. J Mol Biol. 426(23), 3851–3865. Published online 2014/07/16 DOI: 10.1016/j.jmb.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuoka T, and Hayakawa K. (1973). [The fecal flora in man. I. Composition of the fecal flora of various age groups]. Zentralbl Bakteriol Orig A. 223(2), 333–342. Published online 1973/03/01. [PubMed] [Google Scholar]
- Muller SI, Valdebenito M, and Hantke K. (2009). Salmochelin, the long-overlooked catecholate siderophore of Salmonella. Biometals. 22(4), 691–695. Published online 2009/02/14 DOI: 10.1007/s10534-009-9217-4. [DOI] [PubMed] [Google Scholar]
- Muniz LR, Knosp C, and Yeretssian G. (2012). Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front Immunol. 3, 310 Published online 2012/10/23 DOI: 10.3389/fimmu.2012.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy TA, Moreland SM, Andrews-Polymenis H, and Detweiler CS (2013). The ferric enterobactin transporter Fep is required for persistent Salmonella enterica serovar typhimurium infection. Infect Immun. 81(11), 4063–4070. Published online 2013/08/21 DOI: 10.1128/IAI.00412-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy TA, Moreland SM, and Detweiler CS (2014). Salmonella acquires ferrous iron from haemophagocytic macrophages. Mol Microbiol. 93(6), 1314–1326. Published online 2014/08/02 DOI: 10.1111/mmi.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto BR, Sparrius M, Verweij-van Vught AM, and MacLaren DM (1990). Iron-regulated outer membrane protein of Bacteroides fragilis involved in heme uptake. Infect Immun. 58(12), 3954–3958. Published online 1990/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, and Stobberingh EE (2006). Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 118(2), 511–521. Published online 2006/08/03 DOI: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- Pham TA, and Lawley TD (2014). Emerging insights on intestinal dysbiosis during bacterial infections. Curr Opin Microbiol. 17, 67–74. Published online 2014/03/04 DOI: 10.1016/j.mib.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack JR, and Neilands JB (1970). Enterobactin, an iron transport compound from Salmonella typhimurium. Biochem Biophys Res Commun. 38(5), 989–992. Published online 1970/03/12 DOI: 10.1016/0006-291x(70)90819-3. [DOI] [PubMed] [Google Scholar]
- Potvin E, Lehoux DE, Kukavica-Ibrulj I, Richard KL, Sanschagrin F, Lau GW, and Levesque RC (2003). In vivo functional genomics of Pseudomonas aeruginosa for high-throughput screening of new virulence factors and antibacterial targets. Environ Microbiol. 5(12), 1294–1308. Published online 2003/12/03. [DOI] [PubMed] [Google Scholar]
- Pugsley AP, and Reeves P. (1976). Iron uptake in colicin B-resistant mutants of Escherichia coli K-12. J Bacteriol. 126(3), 1052–1062. Published online 1976/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabsch W, Methner U, Voigt W, Tschape H, Reissbrodt R, and Williams PH (2003). Role of receptor proteins for enterobactin and 2,3-dihydroxybenzoylserine in virulence of Salmonella enterica. Infect Immun. 71(12), 6953–6961. Published online 2003/11/26 DOI: 10.1128/iai.71.12.6953-6961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabsch W, Voigt W, Reissbrodt R, Tsolis RM, and Baumler AJ (1999). Salmonella typhimurium IroN and FepA proteins mediate uptake of enterobactin but differ in their specificity for other siderophores. J Bacteriol. 181(11), 3610–3612. Published online 1999/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, et al. (2009). Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 5(5), 476–486. Published online 2009/05/21 DOI: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, and Axon AT (1999). Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 354(9179), 635–639. Published online 1999/08/31 DOI: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- Rocha ER, de Uzeda M, and Brock JH (1991). Effect of ferric and ferrous iron chelators on growth of Bacteroides fragilis under anaerobic conditions. FEMS Microbiol Lett. 68(1), 45–50. Published online 1991/11/01 DOI: 10.1016/0378-1097(91)90393-o. [DOI] [PubMed] [Google Scholar]
- Rocha ER, and Krykunivsky AS (2017). Anaerobic utilization of Fe(III)-xenosiderophores among Bacteroides species and the distinct assimilation of Fe(III)-ferrichrome by Bacteroides fragilis within the genus. Microbiologyopen. 6(4). Published online 2017/04/12 DOI: 10.1002/mbo3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha ER, and Smith CJ (2004). Transcriptional regulation of the Bacteroides fragilis ferritin gene (ftnA) by redox stress. Microbiology. 150(Pt 7), 2125–2134. Published online 2004/07/17 DOI: 10.1099/mic.0.26948-0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi M, Chairatana P, Zheng T, Perez-Lopez A, Edwards RA, George MD, Nolan EM, and Raffatellu M. (2016). Siderophore-based immunization strategy to inhibit growth of enteric pathogens. Proc Natl Acad Sci U S A. 113(47), 13462–13467. Published online 2016/11/09 DOI: 10.1073/pnas.1606290113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. (1972). Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 119(1), 75–88. Published online 1972/01/01 DOI: 10.1007/bf00270447. [DOI] [PubMed] [Google Scholar]
- Skare JT, Ahmer BM, Seachord CL, Darveau RP, and Postle K (1993). Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J Biol Chem. 268(22), 16302–16308. Published online 1993/08/05. [PubMed] [Google Scholar]
- Sommer F, Anderson JM, Bharti R, Raes J, and Rosenstiel P. (2017). The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 15(10), 630–638. Published online 2017/06/20 DOI: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- Sonnenborn U. (2016). Escherichia coli strain Nissle 1917-from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol Lett. 363(19). Published online 2016/09/14 DOI: 10.1093/femsle/fnw212. [DOI] [PubMed] [Google Scholar]
- Sorbara MT, Dubin K, Littmann ER, Moody TU, Fontana E, Seok R, Leiner IM, Taur Y, Peled JU, van den Brink MRM, et al. (2019). Inhibiting antibiotic-resistant Enterobacteriaceae by microbiota-mediated intracellular acidification. J Exp Med. 216(1), 84–98. Published online 2018/12/20 DOI: 10.1084/jem.20181639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanogiannopoulos P, and Turnbaugh PJ (2018). Broad collateral damage of drugs against the gut microbiome. Nat Rev Gastroenterol Hepatol. 15(8), 457–458. Published online 2018/05/13 DOI: 10.1038/s41575-018-0028-3. [DOI] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. (2007). Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 5(10), 2177–2189. Published online 2007/09/01 DOI: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli CP, Neut C, Desreumaux P, and Colombel JF (2004). Dysbiosis in inflammatory bowel disease. Gut. 53(1), 1–4. Published online 2003/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Baumler AJ, Stojiljkovic I, and Heffron F. (1995). Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J Bacteriol. 177(16), 4628–4637. Published online 1995/08/01 DOI: 10.1128/jb.177.16.4628-4637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweijvanvught AMJJ, Otto BR, Namavar F, Sparrius M, and Maclaren DM (1988). Ability of Bacteroides Species to Obtain Iron from Iron Salts, Heme-Compounds and Transferrin. Fems Microbiology Letters. 49(2), 223–228. [Google Scholar]
- Wang RF, and Kushner SR (1991). Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 100, 195–199. Published online 1991/04/01. [PubMed] [Google Scholar]
- Wexler AG, Schofield WB, Degnan PH, Folta-Stogniew E, Barry NA, and Goodman AL (2018). Human gut Bacteroides capture vitamin B12 via cell surface-exposed lipoproteins. Elife. 7. Published online 2018/09/19 DOI: 10.7554/eLife.37138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, and Baumler AJ (2014). Dysbiosis in the inflamed intestine: chance favors the prepared microbe. Gut Microbes. 5(1), 71–73. Published online 2014/03/19 DOI: 10.4161/gmic.27129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, et al. (2013). Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 339(6120), 708–711. Published online 2013/02/09 DOI: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK, et al. (2017). Gavage of Fecal Samples From Patients With Colorectal Cancer Promotes Intestinal Carcinogenesis in Germ-Free and Conventional Mice. Gastroenterology. 153(6), 1621–1633 e1626 Published online 2017/08/22 DOI: 10.1053/j.gastro.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Wookey P, and Rosenberg H. (1978). Involvement of inner and outer membrane components in the transport of iron and in colicin B action in Escherichia coli. J Bacteriol. 133(2), 661–666. Published online 1978/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, and Gordon JI (2003). A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 299(5615), 2074–2076. Published online 2003/03/29 DOI: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- Yep A, McQuade T, Kirchhoff P, Larsen M, and Mobley HL (2014). Inhibitors of TonB function identified by a high-throughput screen for inhibitors of iron acquisition in uropathogenic Escherichia coli CFT073. MBio. 5(2), e01089–01013 Published online 2014/02/27 DOI: 10.1128/mBio.01089-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young IG, Langman L, Luke RK, and Gibson F (1971). Biosynthesis of the iron-transport compound enterochelin: mutants of Escherichia coli unable to synthesize 2,3-dihydroxybenzoate. J Bacteriol. 106(1), 51–57. Published online 1971/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Miyata N, Winter MG, Arenales A, Hughes ER, Spiga L, Kim J, Sifuentes-Dominguez L, Starokadomskyy P, Gopal P, et al. (2019a). Editing of the gut microbiota reduces carcinogenesis in mouse models of colitis-associated colorectal cancer. J Exp Med. Published online 2019/07/31 DOI: 10.1084/jem.20181939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Spiga L, and Winter S. (2019b). Transition metals and host-microbe interactions in the inflamed intestine. Biometals. Published online 2019/02/23 DOI: 10.1007/s10534-019-00182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Winter MG, Byndloss MX, Spiga L, Duerkop BA, Hughes ER, Buttner L, de Lima Romao E, Behrendt CL, Lopez CA, et al. (2018). Precision editing of the gut microbiota ameliorates colitis. Nature. 553(7687), 208–211. Published online 2018/01/13 DOI: 10.1038/nature25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti De Gregoris T, Aldred N, Clare AS, and Burgess JG (2011). Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods. 86(3), 351–356. Published online 2011/06/28 DOI: 10.1016/j.mimet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, and Salzman N. (2008). Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 76(3), 907–915. Published online 2007/12/28 DOI: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis CC, Winter MG, Chanin RB, Zhu W, Spiga L, and Winter SE (2019). Host-derived metabolites modulate transcription of Salmonella genes involved in l-lactate utilization during gut colonization. Infect Immun. Published online 2019/01/09 DOI: 10.1128/IAI.00773-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, Santos RL, Dandekar S, Tsolis RM, and Baumler AJ (2008). T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect Immun. 76(5), 2008–2017. Published online 2008/03/19 DOI: 10.1128/IAI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, and Dobrindt U. (2004). Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J Bacteriol. 186(16), 5432–5441. Published online 2004/08/05 DOI: 10.1128/JB.186.16.5432-5441.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth SK, and Stocker BA (1981). Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 291(5812), 238–239. Published online 1981/05/21. [DOI] [PubMed] [Google Scholar]
- Hughes ER, Winter MG, Duerkop BA, Spiga L, Furtado de Carvalho T, Zhu W, Gillis CC, Buttner L, Smoot MP, Behrendt CL, et al. (2017). Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host Microbe. 21(2), 208–219. Published online 2017/02/10 DOI: 10.1016/j.chom.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatkin NM, Martens EC, Gordon JI, and Smith TJ (2008). Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure. 16(7), 1105–1115. Published online 2008/07/10 DOI: 10.1016/j.str.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods. 9(4), 357–359. Published online 2012/03/06 DOI: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W. (2014). featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 30(7), 923–930. Published online 2013/11/15 DOI: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, Restrepo NA, Hosking MP, Edwards RA, Battistoni A, et al. (2012). Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe. 11(3), 227–239. Published online 2012/03/20 DOI: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15(12), 550 Published online 2014/12/18 DOI: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, and Mathieu C. (2003). The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech. 14(1), 33–43. Published online 2003/08/07. [PMC free article] [PubMed] [Google Scholar]
- Pal D, Venkova-Canova T, Srivastava P, and Chattoraj DK (2005). Multipartite regulation of rctB, the replication initiator gene of Vibrio cholerae chromosome II. J Bacteriol. 187(21), 7167–7175. Published online 2005/10/21 DOI: 10.1128/JB.187.21.7167-7175.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin E, Lehoux DE, Kukavica-Ibrulj I, Richard KL, Sanschagrin F, Lau GW, and Levesque RC (2003). In vivo functional genomics of Pseudomonas aeruginosa for high-throughput screening of new virulence factors and antibacterial targets. Environ Microbiol. 5(12), 1294–1308. Published online 2003/12/03. [DOI] [PubMed] [Google Scholar]
- Rudi K, Skulberg OM, Larsen F, and Jakobsen KS (1997). Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Appl Environ Microbiol. 63(7), 2593–2599. Published online 1997/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Priefer U, and Puhler A. (1983). A Broad Host Range Mobilization System for In Vivo Genetic Engineering: Transposon Mutagenesis in Gram Negative Bacteria. Nature Biotechnology. 1, 784–791. DOI: doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- Spiga L, Winter MG, Carvalho TF, Zhu W, Hughes ER, Gillis CC, Behrendt CL, Kim J, Chessa D, Andrews-Polymenis H, et al. (2017). An oxidative central metabolism enables Salmonella to utilize microbiota-derived succinate. Cell Host Microbe. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojiljkovic I, Baumler AJ, and Heffron F. (1995). Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J Bacteriol. 177(5), 1357–1366. Published online 1995/03/01 DOI: 10.1128/jb.177.5.1357-1366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Baumler AJ, Stojiljkovic I, and Heffron F. (1995). Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J Bacteriol. 177(16), 4628–4637. Published online 1995/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, and Kushner SR (1991). Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 100, 195–199. Published online 1991/04/01. [PubMed] [Google Scholar]
- Wilson RP, Raffatellu M, Chessa D, Winter SE, Tukel C, and Baumler AJ (2008). The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol. 10(4), 876–890. Published online 2007/11/24 DOI: 10.1111/j.1462-5822.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, et al. (2013). Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 339(6120), 708–711. Published online 2013/02/09 DOI: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in the B. thetaiotaomicron transcriptome during Salmonella infection. Related to Fig. 1.
Oligonucleotides used in this study. Related to STAR Methods.
Data Availability Statement
The sequencing data has been deposited in the European Nucleotide Archive under the accession number PRJEB33026.