Abstract
Background
Accurate prediction of preterm birth (PTB) is still difficult, mostly because of the multifactorial etiology of PTB. Previous studies have been mostly focused on the prediction of PTB in symptomatic women or those presenting with threatened preterm labor. We aimed to study whether complete blood count (CBC) parameters at 20‐30 weeks of pregnancy can predict asymptomatic PTB.
Methods
In this retrospective case‐control study, the preterm and term delivery groups were matched by propensity score‐matched (PSM) analysis. Baseline data and the CBC parameters examined at 20‐30 weeks of gestation were recorded.
Results
The combined marker of neutrophil‐to‐lymphocyte ratio (NLR), hemoglobin (HGB), and platelet distribution width (PDW) accurately predicts PTB at a cutoff value of 0.25, with sensitivity and specificity of 88.6% and 40.5% and negative and positive predictive value of 97.9% and 10.2%, respectively.
Conclusion
The combined marker of CBC parameters can supplement other markers to predict PTB about 10 weeks in advance. This combined marker had a very high negative predictive value for PTB. Therefore, in subjects with normal combined marker value, further screening tests for PTB may be eliminated unless clinical suspicion is high.
Keywords: complete blood count, Inflammation, neutrophil‐to‐lymphocyte ratio, pregnancy, preterm birth, preterm delivery, preterm labor, propensity score‐matched analysis
1. INTRODUCTION
Preterm birth (PTB), defined as birth before 37 complete weeks of gestation, is a very serious obstetric problem worldwide. It affects 5%‐18% of all deliveries in the world and represents an enormous health care burden. 1 , 2 It is the largest direct cause of both neonatal and child (under the age of 5 years) mortality. 3 In 2015, preterm birth complication (1.055 million) was the leading cause of death among the 5.9 million children under 5 years old worldwide. 4 In addition to its contribution to mortality, there is increasing evidence that PTB infants are at increased risk of developing some diseases such as respiratory distress syndrome, jaundice, temperature instability, feeding difficulties, developmental disabilities, and neurologic impairments. 5 , 6 , 7 Furthermore, the increased risk of some adult diseases, such as cardiovascular diseases, type 2 diabetes, stroke, asthma, and some behavior and psychiatric disorders, has also been linked to PTB. 8 , 9
The premature birth after spontaneous delivery and preterm premature rupture of membranes(PPROM) are collectively referred to as spontaneous PTB, and those after induction or cesarean labor (including maternal or fetal complications requiring delivery) are called iatrogenic PTB. 10 Accumulating evidence suggests that PTB is a complex syndrome resulting from the interplay of genetic, environmental, and lifestyle risk factors. It is associated with microbial‐induced inflammation, decidual hemorrhage and vascular disease, decidual senescence, disruption of maternal‐fetal tolerance, decline in progesterone action, uterine over‐distension, stress, and others. 11 Research so far has shown that accurate predictions of PTB, especially the use of a single biomarker to predict PTB, are still difficult, because of the multifactorial etiology of PTB. 6
Predicting PTB is still mostly dependent on subjective clinical experience in most countries. Such approach may increase unnecessary hospital admissions as well as unnecessary but potentially harmful interventions such as the use of steroids for fetal lung maturation and of tocolysis. 12 , 13 To improve the accuracy of threatened PTB diagnosis in symptomatic women, two different methods have been suggested: transvaginal ultrasound cervical length measurement and measurement of fetal fibronectin (fFN)/placental alpha microglobulin‐1(PAMG1)/phosphorylated insulin‐like growth factor binding protein‐1 (phIGFBP 1) in the cervicovaginal fluid(CVF). 14
Since their introduction nearly 28 years ago, cervical length screening and fFN levels have been widely used to predict PTB. 15 , 16 In recent years, however, some studies have shown low predictive accuracy of these two tests. A systematic review and meta‐analysis found that fFN should not be used as a screening test for asymptomatic pregnant women. For high‐risk asymptomatic pregnant women, and especially for women with multiple pregnancies, the predictive value of the test was also too low for clinical practice. 17 Another review and meta‐analysis did suggest that although management based on fFN results may reduce PTB, the evidence was found to be of low quality. 18 A prospective observational cohort study with 9410 nulliparous women of singleton pregnancies shows that quantitative vaginal fFN and serial transvaginal ultrasound cervical length had limited predictive value for PTB. The study does not support routine application of these tests in the clinic for those pregnancies. 19 Cervical phIGFBP‐1 has potential utility in identifying patients who had one episode of preterm labor but remained undelivered within 48 hours, based on a systematic review and meta‐analysis. 20 However, its overall predictive ability to identify symptomatic and asymptomatic women at risk of PTB is limited. 20 While the sensitivities of PAMG1 and phIGFBP1 were comparable in predicting spontaneous PTB within 7 days, the specificity of PAMG1 was significantly higher than phIGFBP1. 21 Another limitation of these studies is that they focused mostly on the prediction of PTB within 7 or 14 days in women presenting with threatened preterm labor. More recently, whole genome sequencing, whole exome sequencing, and cell‐free RNA (cfRNA) have been used to identify genes or RNA that may predict preterm delivery. 22 , 23 , 24 These new approaches hold promise in identifying specific biomarkers for spontaneous PTB. Validation in larger and blinded clinical trials in diverse ethnicities and different populations is needed however before they gain widespread clinical application. 22 , 23
It is well established that one highly significant risk factor for PTB is infection and inflammation. 24 One of the most simple, economic, and routine clinical tests during pregnancy is the complete blood count (CBC). CBC and its derived parameters, including neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR), have been recognized as inflammatory markers for low‐grade inflammatory diseases in recent years. 25 , 26 , 27 There is accruing evidence that NLR can have a significant association with inflammatory response observed in a variety of disorders, including cerebrovascular and heart diseases, 28 , 29 , 30 and can improve outcome prediction of such diseases when added to traditional risk scores and tools. 31 The present study examines whether CBC variables including inflammation‐related indicators at 20‐30 weeks of pregnancy can predict asymptomatic preterm delivery.
2. MATERIALS AND METHODS
2.1. Study design
This was a retrospective case‐control study. We reviewed the clinical data of all (15 387 total) pregnant women for preterm deliveries and term deliveries between January and December 2017 at Fujian Provincial Maternity and Children's Hospital, China. The preterm delivery group and term delivery group were matched by PSM analysis. The study was approved by the Medical Ethics Committee of the hospital (2019 No. 164).
2.2. Study participants
Pregnant women who delivered prematurely at 28‐36 weeks of gestation were included in the study group, and those who delivered at 37‐40 weeks of gestation were included in our control group. The inclusion criteria were singleton pregnancy, no uterine and placental abnormalities, no infection or chronic inflammatory diseases, no fever, no heart disease, no cervical insufficiency, no history of cervical cerclage, and no history of taking medications affecting platelet count. Exclusion criteria were gestational diabetes mellitus, hypertensive disorder complicating pregnancy, pre‐eclampsia, chorioamnionitis, severe anemia, thyroid disease, intrauterine growth retardation, and recurrent miscarriage. These pregnant women received CBC tests during 20‐30 weeks of gestation.
2.3. Clinical and laboratory data collection
Medical records were reviewed to collect baseline data including the maternal age, height and body mass index (BMI) before pregnancy, gravidity, parity, the gestational age at delivery, gestational age at CBC assessment, the delivery weight, and PPROM. CBC variables 20‐30 weeks of gestation were also recorded (CBC obtained during active childbirth was excluded). Sysmex‐SN3000 blood cell counter was used as a blood cell counting machine. CBC parameters used in the study were as follows: WBC, white blood cell; Neu#, neutrophil count; Lym#, lymphocyte count; Mon#, monocyte count; HGB, hemoglobin; HCT, hematocrit; RDW, red cell distribution width; PLT, platelet count; MPV, mean platelet volume; PCT, plateletcrit; PDW, platelet distribution width; NLR, neutrophil‐to‐lymphocyte ratio; LMR, lymphocyte‐to‐monocyte ratio; PLR, platelet‐to‐lymphocyte ratio.
2.4. Statistical analysis
Propensity score‐matched analysis was performed using R programming language (San Francisco, CA), according to the ratio of 1:2 cases. The premature deliveries and full‐term deliveries were matched in age, BMI, whether the pregnant woman had PPROM, and gestational age the pregnant women received CBC examinations. The nearest neighbor matching algorithm was used in the PMS analysis. The statistical software SPSS for Windows version 20.0 (SPSS Inc) was used to analyze whether there was a significant difference in CBC parameters between the two groups. The distributions of the data were determined by the Kolmogorov‐Smirnov test, and the results of the variables were expressed as means ± standard deviation or median (minimum—maximum). Independent sample Student's t test or Mann‐Whitney U test was performed to compare continuous numeric variables. The receiver operating characteristic (ROC) curve for each CBC parameter was plotted using MedCalc v.14.12.0 (MedCalc Software bvba). The best cutoff points for CBC parameters to discriminate preterm deliveries and term deliveries were evaluated by the area under the curve (AUC) of the ROC. The ability of each CBC parameter to identify PTB was assessed by comparing the AUC of the ROC. The combined marker value for discriminating PTB from term delivery was calculated by binary logistic regression. Two‐sided P‐values <.05 were considered statistically significant.
3. RESULTS
3.1. Study participants and baseline characteristics
A total of 1634 of the pregnant women fulfilled the inclusion criteria (Table 1). Of these pregnant women, 105 were diagnosed preterm labor, and 1529 were diagnosed for term labor. As presented in Table 1, 68.4% of the premature delivery group had PPROM, while only 29.2% of the 1529 full‐term deliveries had PPROM before labor. The incidence of PPROM was statistically significant between the two groups (preterm and term delivery group P < .001). In addition, clinical features such as BMI, age, and gestational age at assessment may have an impact on the results of CBC. After adjusting for these four clinical features with PSM analysis, a total of 105 and 210 pregnant women were retained in the study group and control group, respectively. The incidence of PPROM after the adjustment was not significantly different between the groups (P = .943). Delivery weight and gestational age at delivery were significantly lower in the preterm group than in the term group before and after PSM analysis, and the differences were significant (P < .001 for both). Gestational age at assessment of CBC parameters was not significantly different between the groups (about 24.8 weeks for both, P = .871).
TABLE 1.
Demographic and obstetric characteristics of the preterm delivery and control group before and after PSM analysis
| Pre‐PSM | Post‐PSM | |||||
|---|---|---|---|---|---|---|
| Preterm delivery (n = 105) | Term delivery (n = 1529) | P‐value | Preterm delivery (n = 105) | Term delivery (n = 210) | P‐value | |
| Maternal age (y) | 29.93 ± 4.039 | 29.59 ± 3.823 | .381 | 29.93 ± 4.039 | 30.07 ± 4.201 | .781 |
| Pre‐pregnancy IBM (kg/m2) | 20.17 ± 2.116 | 20.59 ± 8.425 | .619 | 20.17 ± 2.116 | 20.26 ± 2.316 | .760 |
| Gravidity (number) | 1.71 ± 0.917 | 1.76 ± 0.905 | .592 | 1.71 ± 0.917 | 1.90 ± 0.988 | .100 |
| Parity (number) | 0.33 ± 0.494 | 0.37 ± 0.527 | .502 | 0.33 ± 0.494 | 0.45 ± 0.611 | .075 |
| Gestational age at assessment (wk) | 24.75 ± 1.663 | 24.8 ± 1.608 | .779 | 24.75 ± 1.663 | 24.8 ± 1.487 | .871 |
| Gestational age at delivery(weeks) | 35.2 ± 1.477 | 39.25 ± 1.085 | .000 | 35.2 ± 1.477 | 39.06 ± 1.040 | .000* |
| Assessment to delivery interval (d) | 71.1 ± 15.0 | 99.2 ± 13.5 | .000 | 70.2 ± 15.2 | 97.0 ± 12.4 | .000* |
| Delivery weight (g) | 2573.1 ± 442.59 | 3338.9 ± 362.95 | .000 | 2573.1 ± 442.59 | 3344.9 ± 366.96 | .000* |
| PPROM | 64.8% (68/105) | 29.2% (447/1529) | .000 | 64.8% (68/105) | 65.2% (137/210) | .934 |
Abbreviation: PPROM, preterm premature rupture of membranes.
P < .05 was accepted as significant.
3.2. CBC parameters of the two groups
The CBC parameters of the two groups before and after PSM analysis are shown in Table 2. As indicated in the table, NLR, MLR, PLR, PDW, and lymphocytes were statistically different between the two groups before PSM analysis. After PSM analysis, the statistical differences between the first four indicators (NLR, MLR, PLR, and PDW) were more statistically significant (P‐value is smaller), in addition, HGB and MPV, HCT also reached statistical differences between the two groups after PSM analysis. The mean values of NLR, PDW, MPV, HGB, HCT, PLR were significantly higher (P < .001, P = .005, .037, .010, .017, respectively), and LMR and lymphocytes were significantly lower (P = .003, .016, .012, respectively) in preterm delivery group after PSM analysis.
TABLE 2.
Comparison of CBC parameters between the preterm delivery and control groups
| Pre‐PSM | Post‐PSM | |||||
|---|---|---|---|---|---|---|
| Preterm delivery (n = 105) | Term delivery (n = 1529) | P‐value | Preterm delivery (n = 105) | Term delivery (n = 210) | P‐value | |
| WBC count (103/mm3) | 10.18 ± 2.54 | 9.94 ± 2.13 | .350 | 10.18 ± 2.54 | 9.90 ± 1.95 | .317 |
| Neu# (103/mm3) | 7.58 ± 2.13 | 7.27 ± 1.82 | .098 | 7.58 ± 2.13 | 7.19 ± 1.66 | .106 |
| Lym# (103/mm3) | 1.80 ± 0.50 | 1.89 ± 0.44 | .029* | 1.80 ± 0.50 | 1.93 ± 0.42 | .012* |
| Mon# (103/mm3) | 0.65 ± 0.21 | 0.63 ± 0.17 | .180 | 0.65 ± 0.21 | 0.62 ± 0.16 | .195 |
| HGB (g/L) | 115.09 ± 9.42 | 113.52 ± 8.67 | .075 | 115.09 ± 9.42 | 112.31 ± 8.74 | .010 * |
| HCT (%) | 33.79 ± 2.51 | 33.33 ± 2.34 | .052 | 33.79 ± 2.51 | 33.10 ± 2.34 | .017* |
| RDW (%) | 13.28 ± 1.85 | 13.09 ± 0.80 | .292 | 13.28 ± 1.85 | 13.13 ± 0.80 | .415 |
| PLT (103/mm3) | 217.92 ± 51.32 | 219.75 ± 46.30 | .697 | 217.92 ± 51.32 | 219.56 ± 46.81 | .778 |
| MPV (fL) | 10.33 ± 1.00 | 10.18 ± 0.87 | .082 | 10.33 ± 1.00 | 10.10 ± 0.90 | .037 * |
| PCT (%) | 0.22 ± 0.05 | 0.22 ± 0.04 | .611 | 0.22 ± 0.05 | 0.22 ± 0.04 | .971 |
| PDW (%) | 12.03 ± 2.49 | 11.53 ± 2.01 | .046* | 12.03 ± 2.49 | 11.31 ± 1.95 | .005* |
| NLR | 4.42 ± 1.41 | 3.99 ± 1.21 | .000* | 4.42 ± 1.41 | 3.86 ± 1.09 | .000* |
| LMR | 2.94 ± 0.97 | 3.19 ± 0.91 | .007* | 2.94 ± 0.97 | 3.28 ± 0.98 | .003* |
| PLR | 128.75 ± 39.58 | 120.73 ± 33.30 | .045* | 128.75 ± 39.58 | 117.96 ± 31.32 | .016* |
Abbreviations: HCT, hematocrit; HGB, hemoglobin; LMR, lymphocyte‐to‐monocyte ratio; Lym#, lymphocyte count; Mon#, monocyte count; MPV, mean platelet volume; Neu#,neutrophil count; NLR, neutrophil‐to‐lymphocyte ratio; PCT, plateletcrit; PDW, platelet distribution width; PLR, platelet‐to‐lymphocyte ratio; PLT, platelet count; RDW, red cell distribution width; WBC, white blood cell.
P < .05 was accepted as significant.
3.3. ROC and combined marker
We also analyzed the ROC to compare the diagnostic usefulness of CBC parameters (Figure 1 and Table 3). ROC analysis suggested that NLR, HGB, and PDW had the highest area under the curve (AUC = 0.625, 0.604, and 0.588, respectively.) among the parameters of leukocytes, erythrocytes, and platelets in predicting preterm delivery. In addition, the combined marker of NLR, HGB, and PDW can accurately predict PTB at a cutoff value of 0.25 (AUC s 0.672 [95% CI is 0.618‐0.724]), with sensitivity and specificity of 88.6% and 40.5% and negative and positive prediction value of 97.9% and 10.2%, respectively.
FIGURE 1.
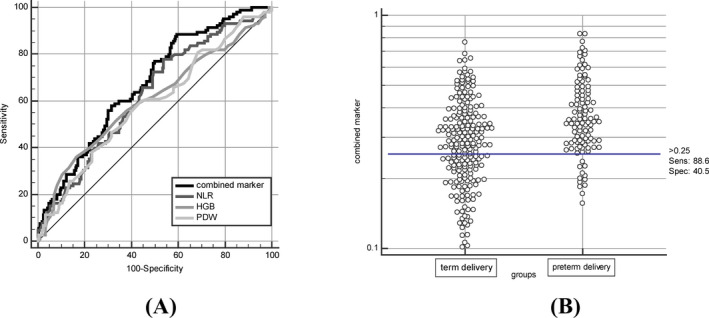
(A) Receiver operating characteristic (ROC) curve for NLR, HGB, PDW, and combined marker for the diagnosis of PTB. (B) Interactive dot diagram for the combined marker
TABLE 3.
Diagnostic sensitivity and specificity of CBC parameters in study subjects
| AUC (95% CI) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | +LR (95% CI) | ‐LR (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) | Cutoff value | |
|---|---|---|---|---|---|---|---|---|
| HGB | 0.604 (0.548‐0.658) | 36.2 (27.0‐46.1) | 83.3 (77.6‐88.1) | 2.17 (1.5‐3.2) | 0.77 (0.7‐0.9) | 14.2 (10.1‐19.8) | 94.5 (93.6‐95.2) | >119 |
| HCT | 0.591 (0.538‐0.650) | 51.4 (41.5‐61.3) | 69.1 (62.3‐75.2) | 1.66 (1.3‐2.2) | 0.7 (0.6‐0.9) | 11.3 (8.8‐14.3) | 94.9 (93.7‐95.9) | >34.2 |
| MPV | 0.569 (0.512‐0.624) | 61.0 (50.9‐70.3) | 51.9 (44.9‐58.8) | 1.27 (1.0‐1.6) | 0.75 (0.6‐1.0) | 8.8 (7.3‐10.7) | 94.6 (93.0‐95.8) | >10 |
| PDW | 0.588 (0.531‐0.643) | 60.0 (50.0‐69.4) | 55.7 (48.7‐62.5) | 1.35 (1.1‐1.7) | 0.72 (0.6‐0.9) | 9.4 (7.7‐11.4) | 94.8 (93.3‐96.0) | >11.2 |
| Lym | 0.598 (0.541‐0.652) | 68.6 (58.8‐77.3) | 49.5 (42.6‐56.5) | 1.36 (1.1‐1.6) | 0.63 (0.5‐0.9) | 9.4 (7.9‐11.1) | 95.4 (93.8‐96.6) | ≤1.95 |
| PLR | 0.575 (0.519‐0.630) | 74.3 (64.8‐82.3) | 41.4 (34.7‐48.4) | 1.27 (1.1‐1.5) | 0.62 (0.4‐0.9) | 8.8 (7.6‐10.2) | 95.5 (93.6‐96.8) | >108.17 |
| LMR | 0.615 (0.559‐0.669) | 31.4 (22.7‐41.2) | 87.1 (81.8‐91.4) | 2.44 (1.6‐3.8) | 0.79 (0.7‐0.9) | 15.7 (10.6‐22.7) | 94.3 (93.5‐95.0) | ≤2.31 |
| NLR | 0.625 (0.569‐0.679) | 78.1 (69.0‐85.6) | 46.2 (39.3‐53.2) | 1.45 (1.2‐1.7) | 0.47 (0.3‐0.7) | 10 (8.6‐11.5) | 96.5 (94.9‐97.6) | >3.56 |
| Combined marker | 0.672 (0.618‐0.724) | 88.6 (80.9‐94.0) | 40.5 (33.8‐47.4) | 1.49 (1.3‐1.7) | 0.28 (0.2‐0.5) | 10.2 (9.1‐11.5) | 97.9 (96.4‐98.8) | >0.25 |
4. DISCUSSION
In this PSM study, we investigated the ability of simple, noninvasive, and economic CBC tests to predict spontaneous PTB. At gestational age of 20‐30 weeks (mean of gestational age is 24 weeks), the mean values of NLR, PDW, MPV, HGB, HCT, PLR were significantly higher, and LMR and lymphocytes were significantly lower in preterm delivery group than in the term delivery group. Another major finding is that NLR, HGB, and PDW in white blood cell classification, red blood cell‐related parameters, and platelet parameters had the greatest predictive value for PTB, respectively. Furthermore, the combined marker of the above three parameters could better predict spontaneous PTB than a single marker.
4.1. PTB and inflammatory factors
Despite the wide range of etiology, it is well accepted that PTB is strongly associated with infection and inflammation. 32 Premature initiation was closely related to changes in inflammatory media and their signaling pathways. Previous studies have shown that inflammatory cytokines such as IL‐1, 33 TNF‐alpha, 34 IL‐6, 34 , 35 CRP, 35 and IP‐10 35 play an important role in the occurrence of PTB. NLR, a commonly available parameter that integrates information of the leukocyte differentials, has been proposed as a marker of systemic inflammation. 36 A study of HKDAGLA et al 37 showed that NLR was significantly higher in the preterm group than in the control group, which is consistent with our study. A key difference between our study and their previous investigation was the gestational age at assessment of CBC. Their blood routine examination time was after the onset of threatened PTB (31.1 ± 1.9 W in the preterm group). In our study, CBC obtained during active labor was not used. We used CBC values detected within 10 weeks prior to the hospitalization of the pregnant woman. (premature delivery group is 24.8 ± 1.7 W). Min‐A kim et al 38 analyzed CBC for predicting PTB in symptomatic pregnant women at an average gestational age of 28.6 ± 3.8 weeks. Their results also showed that NLR had predictive value of PTB (AUC = 0.665 CI: 0.586‐0.744 sensitivity: 0.52, specificity: 0.781, PPV: 0.768, NPV: 0.538, cutoff value: 5.47). Gezer et al 39 studied pregnant women with PTB symptoms between 34 and 37 weeks of gestation and found that NLR had predictive value for PTB with AUC:0.711 (0.662‐0.760), sensitivity: 0.651 (0.585‐0.712), specificity: 0.624 (0.548‐0.695), PPV: 0.69 (0.623‐0.751), NPV: 0.581 (0.508‐0.652), cutoff value: 6.2, LR + 1.73 (1.40‐2.14), and LR‐0.56 (0.45‐0.69).The preterm group they studied gave birth within 4.5 ± 11.1 days after CBC measurement, whereas in the present study, the interval between CBC measurement and delivery in the preterm group was 70.2 ± 15.2 days.
While the pathological mechanisms that NLR is higher in preterm delivery group compared to the term delivery group are still not fully elucidated, placental lesions consistent with maternal vascular underperfusion may be one pathway linking the rise of NLR to preterm delivery. NLR has been proved to have a significant association with systemic inflammatory response and demonstrated effective as prognostic markers in many diseases. And a study of Raffetti et al 40 showed that NLR can also reflect the severity of the underlying systemic inflammation and provide valuable prognostic information in HIV‐infected subjects. NLR was also indicated significantly higher in malaria group than in the healthy control group. 41 Furthermore, the dysregulation of soluble endoglin (angiogenic biomarker) was found in HIV‐infected pregnant women destined to deliver preterm. 42 And malaria infection in early pregnancy is associated with gestational length by obstructing placental vascular development detected at delivery. 43 Prospective studies are needed to investigate whether there are placental pathologic changes consistent with maternal vascular underperfusion, which may explain the association between NLR to preterm delivery.
In summary, previous studies have shown that NLR is of great value in predicting whether pregnant women with threatened PTB will eventually have a premature delivery. Our study further demonstrated that NLR has predictive value for PTB as early as 10 weeks before the onset of PTB symptoms.
4.2. PTB and MPV
MPV and PDW are two platelet volume indices that respond to platelet activation. Our study found that these two indicators, especially PDW, were significantly elevated in preterm delivery group and could be used as predictors of spontaneous PTB. Previous studies have shown that platelet activation occurs during PTB. 44 The platelets after activation may undergo morphological changes such as the formation of pseudopods, which causes the platelet volume to expand. The platelet volume in the circulation also becomes more heterogeneous. Such changes lead to increases in MPV and PDW, especially the latter. 45 MPV may increase in low‐grade inflammatory diseases. 46 Chronic inflammation that occurs without a clear contagious trigger is also associated with PTB. 32 PTB rates were significantly higher in pregnant women such as obesity 47 and gestational diabetes. 48 Moreover, diabetes, 49 fatty liver disease, 50 and obesity 49 were found to be associated with elevated MPV. We speculate that there may be low‐grade inflammation in pregnant women with spontaneous PTB.
4.3. PTB and HGB
Our study also demonstrated a statistically significant increase of HGB and HCT, especially HGB in the preterm delivery group, and their high levels had predictive values for PTB. A previous study of maternal HGB and PTB risk in the Chinese population also showed that having low HGB levels in first trimester and high HGB levels in the second trimester approximately doubled the PTB risk. 51 A possible explanation for high HGB status in the second trimester with higher PTB risk is insufficient plasma volume expansion during this period. This might lead to an increase in blood viscosity, which in turn may lead to poor blood flow in the placenta, thereby affecting the development of the fetus. 51
A study of hemorheological adaptation during pregnancy in a Latin American population showed that HCT decreased from pre‐pregnancy levels to a minimum at around 26 weeks gestation from 10 weeks on. Blood viscosity increased during the first trimester and then decreased to a minimum at about 26 weeks of gestation. 52 The time to evaluate CBC in pregnant women in our study was between 20‐30 weeks of gestation (mean gestational age, 24 weeks), during which blood viscosity is at a relatively low level. At this time, if the plasma volume expansion is insufficient, and the blood viscosity will rise. The blood flow in the placenta may be restricted, thus affecting the pregnancy outcome. HGB and HCT are often higher when plasma volume expansion is inadequate, which is a possible explanation for elevated HGB and HCT between 20‐30 weeks of gestation with higher risk of PTB.
4.4. Limitation
Our current study is retrospective, and its validity therefore very much depends on study group comparability. Although we used PSM analysis to procure comparability of some baseline characteristics between study and control groups, there may still be unknown confounding variables that were not balanced out, thus affecting the study conclusion. As the mechanism of PTB has not yet been fully elucidated and it is likely to be multifactorial, further large‐scale prospective studies are needed to confirm our findings.
5. CONCLUSIONS
Our study confirmed previous reports that NLR, PDW, and HGB are valuable markers to predict preterm delivery, and the diagnostic values of NLR, PDW, and HGB were superior to that of corresponding white blood cell count, platelet count, and red blood cell count. We found that the combined diagnostic marker from these three parameters could better predict preterm birth. When compared to NLR, PDW, or HGB alone, the combined diagnostic marker has the highest AUC (0.672) with 88.6% sensitivity and 40.5% specificity. To the best of our knowledge, no studies have explored the predictive value of CBC parameters in PTB as early as 10 weeks prior to preterm delivery. Current laboratory tests in routine clinical practice generally predict premature delivery by 1‐2 weeks. In addition, most of these tests use CVF as the specimens, which is known to have poor patient acceptability and compliance compared to blood sampling. Although emerging new methods hold promise in predicting PTB at earlier time, their clinical application needs to be validated by future large‐scale clinical trials. Until then, these simple and economic CBC parameters can be used as a valuable tool to predict PTB.
In summary, our study was the first to use combined diagnostic marker from NLR, PDW, and HGB to predict preterm delivery. This combined marker can supplement other markers to predict PTB about 10 weeks prior. The marker had a very high negative predictive value for PTB. Unless clinical suspicion is high, a normal combination marker value shall eliminate the need for further screening tests in an otherwise uneventful pregnancy.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENT
The authors thank all patients who participated in this study. This work was supported by Research Fund Project of Fujian Provincial Maternity and Children's Hospital, Grant/Award Number: 2018‐17.
Ma M, Zhu M, Zhuo B, et al. Use of complete blood count for predicting preterm birth in asymptomatic pregnant women: A propensity score‐matched analysis. J Clin Lab Anal. 2020;34:e23313 10.1002/jcla.23313
Funding information
Research fund project of Fujian Provincial Maternity and Children's Hospital, Grant/ Award Number: 2018‐17.
Contributor Information
Liangpu Xu, Email: xiliangpu@fjmu.edu.cn.
Jianying Yan, Email: Yanjy_2004@163.com.
REFERENCES
- 1. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162‐2172. [DOI] [PubMed] [Google Scholar]
- 2. Global, regional, and national disability‐adjusted life‐years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603‐1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post‐2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430‐440. [DOI] [PubMed] [Google Scholar]
- 4. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under‐5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027‐3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. 2013;132(4):741‐751. [DOI] [PubMed] [Google Scholar]
- 6. Georgiou HM, Di Quinzio MK, Permezel M, Brennecke SP. Predicting preterm labour: current status and future prospects. Dis Markers. 2015;2015:435014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vohr B. Long‐term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40(4):739‐751. [DOI] [PubMed] [Google Scholar]
- 8. Kajantie E, Strang‐Karlsson S, Evensen KAI, Haaramo P. Adult outcomes of being born late preterm or early term – what do we know? Semin Fetal Neonatal Med. 2019;24(1):66‐83. [DOI] [PubMed] [Google Scholar]
- 9. Bertagnolli M, Xie LF, Paquette K, et al. Endothelial colony‐forming cells in young adults born preterm: a novel link between neonatal complications and adult risks for cardiovascular disease. J Am Heart Assoc. 2018;7(14):e009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lucovnik M, Bregar AT, Steblovnik L, et al. Changes in incidence of iatrogenic and spontaneous preterm births over time: a population‐based study. J Perinat Med. 2016;44(5):505‐509. [DOI] [PubMed] [Google Scholar]
- 11. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kemp MW, Newnham JP, Challis JG, Jobe AH, Stock SJ. The clinical use of corticosteroids in pregnancy. Hum Reprod Update. 2016;22(2):240‐259. [DOI] [PubMed] [Google Scholar]
- 13. Lorthe E, Goffinet F, Marret S, et al. Tocolysis after preterm premature rupture of membranes and neonatal outcome: a propensity‐score analysis. Am J Obstetr Gynecol. 2017;217(2):212.e211‐212.e212. [DOI] [PubMed] [Google Scholar]
- 14. Di Renzo GC, Cabero Roura L, Facchinetti F, et al. Preterm labor and birth management: recommendations from the European Association of Perinatal Medicine. J Matern Fetal Neonatal Med. 2017;30(17):2011‐2030. [DOI] [PubMed] [Google Scholar]
- 15. Jackson GM, Ludmir J, Bader TJ. The accuracy of digital examination and ultrasound in the evaluation of cervical length. Obstetr Gynecol. 1992;79(2):214‐218. [DOI] [PubMed] [Google Scholar]
- 16. Lockwood CJ, Senyei AE, Dische MR, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325(10):669‐674. [DOI] [PubMed] [Google Scholar]
- 17. Faron G, Balepa L, Parra J, Fils JF, Gucciardo L. The fetal fibronectin test: 25 years after its development, what is the evidence regarding its clinical utility? A systematic review and meta‐analysis. J Matern Fetal Neonatal Med. 2018;1‐31. [DOI] [PubMed] [Google Scholar]
- 18. Berghella V, Saccone G. Fetal fibronectin testing for reducing the risk of preterm birth. Cochrane Database Syst Rev. 2019;7:Cd006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esplin MS, Elovitz MA, Iams JD, et al. Predictive accuracy of serial transvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Conde‐Agudelo A, Romero R. Cervical phosphorylated insulin‐like growth factor binding protein‐1 test for the prediction of preterm birth: a systematic review and metaanalysis. Am J Obstetr Gynecol. 2016;214(1):57‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Melchor JC, Khalil A. Prediction of preterm delivery in symptomatic women using PAMG‐1, fetal fibronectin and phIGFBP‐1 tests: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2018;52(4):442‐451. [DOI] [PubMed] [Google Scholar]
- 22. Zhang G, Feenstra B, Bacelis J, et al. Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med. 2017;377(12):1156‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ngo TTM, Moufarrej MN. Noninvasive blood tests for fetal development predict gestational age and preterm delivery. Science. 2018;360(6393):1133‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strauss JF 3rd, Romero R, Gomez‐Lopez N, et al. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstetr Gynecol. 2018;218(3):294‐314.e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serrano CV Jr, de Mattos FR, Pitta FG, Nomura CH, de Lemos J, Ramires JAF. Association between neutrophil‐lymphocyte and platelet‐lymphocyte ratios and coronary artery calcification score among asymptomatic patients: data from a cross‐sectional study. Mediators Inflamm. 2019;2019:6513847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sundararajan S, Kiernan MS, Couper GS, Upshaw JN, DeNofrio D, Vest AR. The neutrophil‐lymphocyte ratio and survival during left ventricular assist device support. J Card Fail. 2019;25(3):188‐194. [DOI] [PubMed] [Google Scholar]
- 27. Liu J, Li S, Zhang S, et al. Systemic immune‐inflammation index, neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio can predict clinical outcomes in patients with metastatic non‐small‐cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33(8):e22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil‐to‐lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res. 2019;10(2):137‐145. [DOI] [PubMed] [Google Scholar]
- 29. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil‐to‐lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;8(34):57489‐57494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen C, Cong BL, Wang M, et al. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr Med Res. 2018;7(2):192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil‐to‐lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. 2018;387:98‐102. [DOI] [PubMed] [Google Scholar]
- 32. Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S. Inflammation and preterm birth. J Leukoc Biol. 2016;99(1):67‐78. [DOI] [PubMed] [Google Scholar]
- 33. Nadeau‐Vallee M, Obari D, Quiniou C, et al. A critical role of interleukin‐1 in preterm labor. Cytokine Growth Factor Rev. 2016;28:37‐51. [DOI] [PubMed] [Google Scholar]
- 34. Dixon CL, Richardson L, Sheller‐Miller S, Saade G, Menon R. A distinct mechanism of senescence activation in amnion epithelial cells by infection, inflammation, and oxidative stress. Am J Reprod Immunol. 2018;79(3):e12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Le Ray I, Mace G, Sediki M, et al. Changes in maternal blood inflammatory markers as a predictor of chorioamnionitis: a prospective multicenter study. Am J Reprod Immunol. 2015;73(1):79‐90. [DOI] [PubMed] [Google Scholar]
- 36. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5‐14. [PubMed] [Google Scholar]
- 37. Daglar HK, Kirbas A, Kaya B, Kilincoglu F. The value of complete blood count parameters in predicting preterm delivery. Eur Rev Med Pharmacol Sci. 2016;20(5):801‐805. [PubMed] [Google Scholar]
- 38. Kim MA, Lee BS, Park YW, Seo K. Serum markers for prediction of spontaneous preterm delivery in preterm labour. Eur J Clin Invest. 2011;41(7):773‐780. [DOI] [PubMed] [Google Scholar]
- 39. Gezer C, Ekin A, Solmaz U, Sahingoz Yildirim AG, Dogan A, Ozeren M. Identification of preterm birth in women with threatened preterm labour between 34 and 37 weeks of gestation. J Obstetr Gynaecol. 2018;38(5):652‐657. [DOI] [PubMed] [Google Scholar]
- 40. Raffetti E, Donato F, Casari S, et al. Systemic inflammation‐based scores and mortality for all causes in HIV‐infected patients: a MASTER cohort study. BMC Infect Dis. 2017;17(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kotepui M, Phunphuech B, Phiwklam N, Chupeerach C, Duangmano S. Effect of malarial infection on haematological parameters in population near Thailand‐Myanmar border. Malar J. 2014;13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conroy AL, McDonald CR, Gamble JL, et al. Altered angiogenesis as a common mechanism underlying preterm birth, small for gestational age, and stillbirth in women living with HIV. Am J Obstetr Gynecol. 2017;217(6):684.e681‐684.e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moeller SL, Nyengaard JR, Larsen LG, et al. Malaria in early pregnancy and the development of the placental vasculature. J Infect Dis. 2019;220(9):1425‐1434. [DOI] [PubMed] [Google Scholar]
- 44. Erez O, Romero R, Hoppensteadt D, et al. Premature labor: a state of platelet activation? J Perinatal Med. 2008;36(5):377‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boos CJ, Balakrishnan B, Lip GY. The effects of coronary artery disease severity on time‐dependent changes in platelet activation indices in stored whole blood. J Thrombosis Thrombolysis. 2008;25(2):135‐140. [DOI] [PubMed] [Google Scholar]
- 46. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17(1):47‐58. [DOI] [PubMed] [Google Scholar]
- 47. Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309(22):2362‐2370. [DOI] [PubMed] [Google Scholar]
- 48. Billionnet C, Mitanchez D, Weill A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60(4):636‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 2009;63(10):1509‐1515. [DOI] [PubMed] [Google Scholar]
- 50. Madan SA, John F, Pitchumoni CS. Nonalcoholic fatty liver disease and mean platelet volume: a systemic review and meta‐analysis. J Clin Gastroenterol. 2016;50(1):69‐74. [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Li Z, Li H, et al. Maternal haemoglobin concentration and risk of preterm birth in a Chinese population. J Obstet Gynaecol. 2018;38(1):32‐37. [DOI] [PubMed] [Google Scholar]
- 52. Kametas N, Krampl E, McAuliffe F, Rampling MW, Nicolaides KH. Haemorheological adaptation during pregnancy in a Latin American population. Eur J Haematol. 2001;66(5):305‐311. [DOI] [PubMed] [Google Scholar]


