Elevated IL-10 in aged mice suppresses immune responses and has implications for vaccine nonresponsiveness in the elderly.
Abstract
Aging results in profound immune dysfunction, resulting in the decline of vaccine responsiveness previously attributed to irreversible defects in the immune system. In addition to increased interleukin-6 (IL-6), we found aged mice exhibit increased systemic IL-10 that requires forkhead box P3–negative (FoxP3−), but not FoxP3+, CD4+T cells. Most IL-10–producing cells manifested a T follicular helper (Tfh) phenotype and required the Tfh cytokines IL-6 and IL-21 for their accrual, so we refer to them as Tfh10 cells. IL-21 was also required to maintain normal serum levels of IL-6 and IL-10. Notably, antigen-specific Tfh10 cells arose after immunization of aged mice, and neutralization of IL-10 receptor signaling significantly restored Tfh-dependent antibody responses, whereas depletion of FoxP3+ regulatory and follicular regulatory cells did not. Thus, these data demonstrate that immune suppression with age is reversible and implicate Tfh10 cells as an intriguing link between “inflammaging” and impaired immune responses with age.
INTRODUCTION
Declining adaptive immune function in the elderly leads to increased risk and severity of infection, poorer control of cancer, and impaired responses to vaccination (1). Paradoxically, aging is also characterized by a persistent low-grade immune activation (so-called inflammaging). Inflammaging is implicated in several deleterious processes in the elderly, including Alzheimer’s disease, cardiovascular diseases, and general frailty (2). High levels of circulating, proinflammatory interleukin-6 (IL-6) are associated with increased morbidity and mortality among older individuals (3).
Paradoxically, alongside increased “inflammaging” is the recent observation that serum levels of IL-10, a potent anti-inflammatory mediator, are also increased in aged individuals (4), although this is somewhat controversial. In addition, an IL-10 genotype (21082GG) that is associated with high production of IL-10 in Caucasians is more prevalent in centenarians than in younger individuals (65 to 73 years) (5) and similarly more prevalent in middle-aged controls than in age-matched patients with myocardial infarction (6). In line with this observation, elderly men with the highest serum levels of inflammatory cytokines or with the lowest levels of IL-10 had the highest risk of frailty-associated pathologies (7). Thus, IL-10 levels appear to play an important role in counter-regulation of inflammation to promote healthy aging. However, the links between inflammaging, IL-10, and immune suppression remain largely unexplored.
In contrast to its purported beneficial roles in aging, IL-10 limits protective responses to pathogens. IL-10 plays a deleterious role in chronic infections, in mice and humans, limiting microbial clearance (8). We recently showed that IL-10 fosters reactivation and replication of cytomegalovirus in the salivary glands of latently infected mice (9). Further, IL-10 dampens responses to acute infections or vaccines (10). In aged humans, the interferon-γ (IFN-γ):IL-10 ratio appears to be a critical predictor to protective immunity (11). Despite these proposed roles for IL-10 in controlling both systemic inflammation and vaccine responsiveness, little is known about the function of IL-10, its cellular source, or its molecular control in aged animals or humans.
IL-10 can be produced by many cells, including those of the innate immune system (notably multiple myeloid cell subsets), the adaptive immune system (T cells and B cells), and even nonimmune cells (e.g., keratinocytes and hepatocytes) (12). At baseline, in the spleen of young mice, the majority of IL-10 expression is localized to B cells and CD4+ T cells (both CD25+ and CD25−) (13). In aging, a few studies have investigated the cellular sources of IL-10 and suggested a role for memory CD4+ T cells. Early work showed increased IL-10 production by aged CD44hiCD4+ T cells (14). The proportion of IL-10+ influenza-specific CD4+ T cells increases in aged mice (15). In contrast, B cells capable of IL-10 production appear to be decreased in older individuals (16). Nonetheless, the major cellular source of increased IL-10 in aging remains unclear.
Here, we investigated the cellular sources of IL-10 in aging and the relationship between IL-10, inflammaging, and immune suppression in age. Notably, we found that CD4+FoxP3− cells were required for increased systemic IL-10 levels in aging. Further, these IL-10–producing T cells bore markers of T follicular helper (Tfh) cells and were present in both mice and humans. IL-21 was critical for promoting Tfh10 cells, whose absence drove dysregulated systemic inflammaging. Last, neutralization of IL-10, but not depletion of forkhead box P3–positive (FoxP3+) regulatory T cells (Tregs), restored vaccine-driven B cell responses in aged mice. Together, our data show that Tfh10 cells provide a critical link between inflammaging and aged-related immune suppression.
RESULTS
Aged mice have increased systemic levels of IL-10
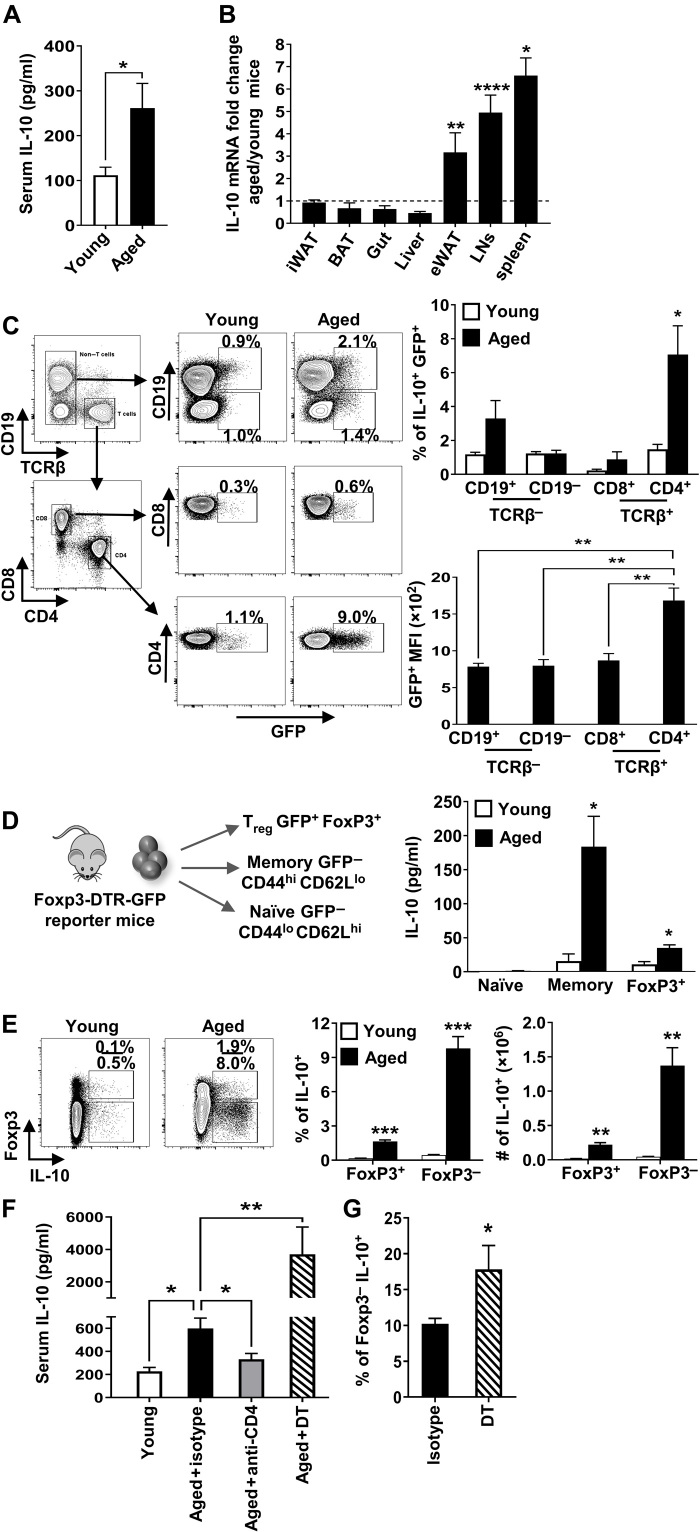
To determine whether IL-10 levels are increased in aged mice, we used a sensitive in vivo cytokine capture assay (IVCCA) to measure IL-10 in the serum of young versus aged mice (17). This approach reflects the actual in vivo steady-state levels of IL-10 as opposed to single-snapshot measurement, which is subject to more fluctuation. Notably, we found that IL-10 levels were increased two- to threefold in the serum of old compared to young mice (Fig. 1A). To assess the potential sources of this enhanced IL-10, we examined various lymphoid and nonlymphoid tissues and found an increase in IL-10 mRNA in the epididymal white adipose tissue, lymph nodes, and spleen of aged mice compared to young mice (Fig. 1B). These data show that the systemic levels of IL-10 are increased with age and that secondary lymphoid organs appear to be major contributors of augmented IL-10 expression in aging.
Fig. 1. Aged mice have increased systemic levels of IL-10.
(A) Serum IL-10 (means ± SEM) levels in young (4 months, n = 6) and aged (18 months, n = 5) mice, representative of four independent experiments. (B) Mean fold change in IL-10 mRNA gene expression (means ± SEM) from the spleen, liver, gut, lymph nodes (LNs), inguinal white adipose tissue (iWAT), epididymal white adipose tissue (eWAT), and brown adipose tissue (BAT) from individual young (2 months, n = 4 to 8) and aged (21 months, n = 5 to 9) C57BL/6 mice. Dashed line represents equal aged:young ratio. Data pooled from two independent experiments. (C) Splenocytes from young (1.5 months, n = 3) and aged (18 months, n = 5) IL-10gfp (VertX) mice were analyzed by flow cytometry. Upper graph shows the frequency of cells that are green fluorescent protein–positive (GFP+) (means ± SEM). Lower graph shows the average level of GFP expression in aged CD4+, CD8+, CD19+, and CD19− that are GFP+ (means ± SEM). (D) IL-10 levels (means ± SEM) from phorbol 12-myristate 13-acetate and ionomycin (P + I)–stimulated cells sorted from young (3.5 months, n = 4) and aged (24 months, n = 4) FoxP3–internal ribosomal entry site (IRES)–diphtheria toxin receptor (DTR)–GFP mice. (E) Gating strategy, frequencies, and numbers of FoxP3+ or FoxP3− that are IL-10+ from P + I–stimulated splenocytes from young (1.5 months, n = 4) and aged (23 months, n = 4) C57BL/6 mice (means ± SEM). (F) Serum IL-10 levels (means ± SEM) in young (2.5 months, n = 6) and aged (18 months, n = 14) C57BL/6 mice treated with anti-CD4 or isotype control or DT-treated FoxP3-DTR mice (19 months, n = 6). Data are pooled from two independent experiments. (G) Percentage of FoxP3− splenocytes that are IL-10+ (means ± SEM) from aged (18 months, n = 8) and DT-treated FoxP3-DTR (19 months, n = 6) mice. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001, Student’s t test. MFI, mean fluorescence intensity.
CD4+FoxP3− T cells are the major source of IL-10
To identify cells with enhanced IL-10 production in aged mice, we took advantage of IL-10–reporter (VertX) mice, which have an IL-10– internal ribosomal entry site (IRES)–enhanced green fluorescent protein (eGFP) cassette in the endogenous IL-10 locus (13). VertX mice allowed us to examine baseline IL-10 production directly ex vivo, in the absence of exogenous stimulation, as GFP levels in these mice directly correlate with IL-10 production (13). Direct ex vivo flow cytometric analysis of spleen cells in aged versus young VertX mice revealed a significantly increased frequency of GFP+ (IL-10+) cells in multiple cell types, but the largest increase was observed in CD4+ T cells (Fig. 1C). In addition, the level of IL-10 produced per cell was significantly higher in CD4+ T cells than in CD8+ T cells, CD19−TCRβ− cells, or CD19+ B cells (Fig. 1C). Because FoxP3+ Tregs are a well-known source of IL-10 in young mice and their frequency is increased in old mice (18), we next determined whether they were the major contributor to this increased IL-10 in aged mice. Staining for FoxP3 in VertX mice while maintaining GFP expression is technically infeasible; thus, we sorted naïve cells (CD4+CD44loCD62LhiFoxP3GFPneg), memory cells (CD4+CD44hiCD62LloFoxP3GFPneg), and Tregs (FoxP3GFPpos) from young and aged FoxP3–diphtheria toxin receptor (DTR)–GFP mice, stimulated the cells with phorbol 12-myristate 13-acetate (PMA) and ionomycin (P + I), and measured their production of IL-10 by enzyme-linked immunosorbent assay (ELISA). As expected, naïve CD4+ T cells produced little IL-10 whether they were from young or old mice (Fig. 1D). IL-10 production from FoxP3+ Treg was slightly increased in aged mice (~2-fold) (Fig. 1D). However, IL-10 production from aged FoxP3− memory T cells was increased >10-fold (Fig. 1D). Similarly, flow cytometric analysis of spleen cells of wild-type (WT) C57BL/6 mice showed that the frequency of IL-10–producing CD4+FoxP3+ cells was increased slightly with age, while IL-10–producing FoxP3−CD4+ T cells were ~10 times more frequent with age (Fig. 1E) and expressed the highest levels of IL-10 per cell (~2- to 3-fold) compared to their young counterparts and aged FoxP3+ cells (fig. S1A).
Together, these three independent approaches show that CD4+FoxP3− cells have the highest capacity for IL-10 production in the spleens of aged mice. In addition, they are required for the increased systemic levels of IL-10, as depletion of >95% of CD4+ T cells in the spleens of old mice nearly returned the serum levels of IL-10 to levels observed in young mice (Fig. 1F). In contrast, depletion of FoxP3+ T cells, using FoxP3-DTR mice, increased systemic IL-10 levels (Fig. 1F). Elevated levels of IL-10 in DT-treated FoxP3-DTR mice were associated with an increased frequency of IL-10–producing CD4+ T cells (Fig. 1G). Thus, FoxP3−, but not FoxP3+, CD4+ T cells are required for the increased systemic levels of IL-10.
Accrual of IL-10–producing CD4+FoxP3− T cells occurs in germ-free animals
Recent work has shown that the microbiome changes with age (19). Further, alterations in the microbiome can affect IL-10 production from CD4+FoxP3+ and FoxP3− T cells (20). To test whether the microbiome affects the accumulation of IL-10–producing cells, we aged several cohorts of mice in a germ-free facility. The accumulation of IL-10+CD4+FoxP3− cells was similar between age-matched mice housed under specific pathogen–free conditions and germ-free animals across a range of ages (fig. S1B). Further, the age-driven accrual of IL-10–producing cells was consistently observed at four different institutions, including the Cincinnati Children’s Hospital, the Indiana University/Purdue University Indianapolis, the University of Alabama-Birmingham, and the Research Center Borstel in Germany, and it is unlikely that the microbiomes of mice are the same at these different institutions. Therefore, age-driven changes to the microbiome do not appear to alter the accrual of IL-10–producing CD4+FoxP3− T cells.
IL-10–producing CD4+FoxP3− T cells in aged mice are predominantly Tfh cells
Several distinct subsets of FoxP3−CD4+ T cells have been reported to produce IL-10, predominantly T helper 2 (TH2) cells, type I regulatory (TR1) T cells, “exTH17” cells, and exTregs (21, 22). Although aged IL-10–producing cells expressed lymphocyte activation gene 3 (LAG3), it is unlikely that they were TR1 cells, as they lacked expression of CD49b (fig. S2A), an important marker on TR1 cells (23). Very few aged IL-10+CD4+ T cells were capable of IL-4 or IL-17A coproduction, ruling out the possibility that these were TH2 or TH17 cells (fig. S2B). Next, analysis of IL-17A fate-tracking mice (24) revealed that the frequency of exTH17 cells within IL-10+FoxP3−CD4+ T cells from aged mice was ~1% (fig. S2C). Analysis of exTregs using FoxP3−CreRosaloxstoploxdTomato mice (25) revealed that ~20% of the IL-10+CD4+ T cells were dTomato+GFP− “exTregs” in both young and aged mice (fig. S2D). Thus, none of TH2, TR1, exTH17, and exTreg make up the bulk of the IL-10–producing CD4+ T cells that accumulate in aged mice.
In our investigation of cytokine coproduction by IL-10–producing CD4+ T cells, we found that the frequency and total numbers of IL-10+ cells that coproduced IL-21 were significantly increased in aged compared to young mice (Fig. 2A). As IL-21 is typically produced by Tfh cells, we next assessed the frequency of IL-10+CD4+FoxP3− T cells that expressed CXCR5 and programmed cell death 1 (PD1), two canonical surface markers of Tfh cells, in conjunction with the transcription factor B cell lymphoma 6 (BCL6) (26). Notably, we found that most of IL-10+FoxP3−CD4+ T cells were CXCR5+ and PD1+ in old mice (Fig. 2B) and expressed BCL6 at levels comparable to IL-10−CXCR5+PD1+ Tfh cells in aged mice (fig. S3A). Further, there was a progressive age-related accrual of CXCR5+PD1+ Tfh cells, including those that produce IL-10 (fig. S3B). Because T follicular regulatory (Tfr) cells have been reported to expand with age (27), we considered the possibility that IL-10–producing Tfr cells may increase with age. However, we observed that IL-10–producing Tfr only marginally increased with age unlike IL-10–producing Tfh cells, which increased markedly with age (fig. S3C). Thus, most of the IL-10–producing T cells that accumulate with age bore markers of Tfh cells, so, for clarity, we will refer to them as Tfh10 cells.
Fig. 2. IL-10–producing FoxP3−CD4+ T cells in aged mice are predominantly Tfh cells.
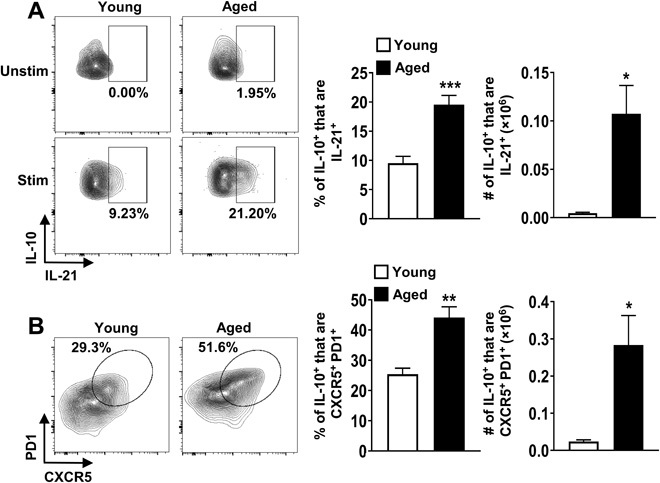
(A) Splenocytes from young (2 months, n = 6) and aged (18 months, n = 6) C57BL/6 mice were stimulated with P + I, stained with antibodies against TCRβ, CD8, FoxP3, IL-10, and IL-21, and analyzed by flow cytometry. The representative plots and graphs show the frequencies and total numbers of IL-21+ cells originating from FoxP3−IL10+ cells (means ± SEM). Data are representative of at least two independent experiments. (B) Splenocytes from young (2 months, n = 4) and aged (18 months, n = 4) C57BL/6 mice were stimulated as above and stained with antibodies against TCRβ, CD8, FoxP3, CXCR5, PD1, and IL-10 and analyzed by flow cytometry. The representative plots and graphs show the frequencies and total numbers of CXCR5+PD1+ cells originating from FoxP3−IL10+ cells (means ± SEM). *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001, Student’s t test.
IL-6 is required for Tfh10 generation and systemic increase of IL-10 in aged mice
We next examined the role of IL-6 in this system, as IL-6 has been reported to (i) control Tfh development (28), (ii) promote IL-10 production from CD4+ T cells (29), and (iii) act as a key inflammatory cytokine that is increased with age (30). To determine whether IL-6 promotes the accrual of Tfh10 cells with age, we aged IL-6−/− mice to ≥17 months and examined the proportion of Tfh10 cells in their spleens. While no difference in Tfh cells (including those that produce IL-10) was observed between young WT and IL-6−/− mice, aged IL-6−/− mice exhibited a marked reduction in the frequency of Tfh10 cells compared to aged WT mice (Fig. 3, A and B). These data show that IL-6 is required for the accrual of Tfh10 cells in aged mice.
Fig. 3. IL-6 is required for Tfh10 cells and for elevated levels of IL-10 in aged mice.
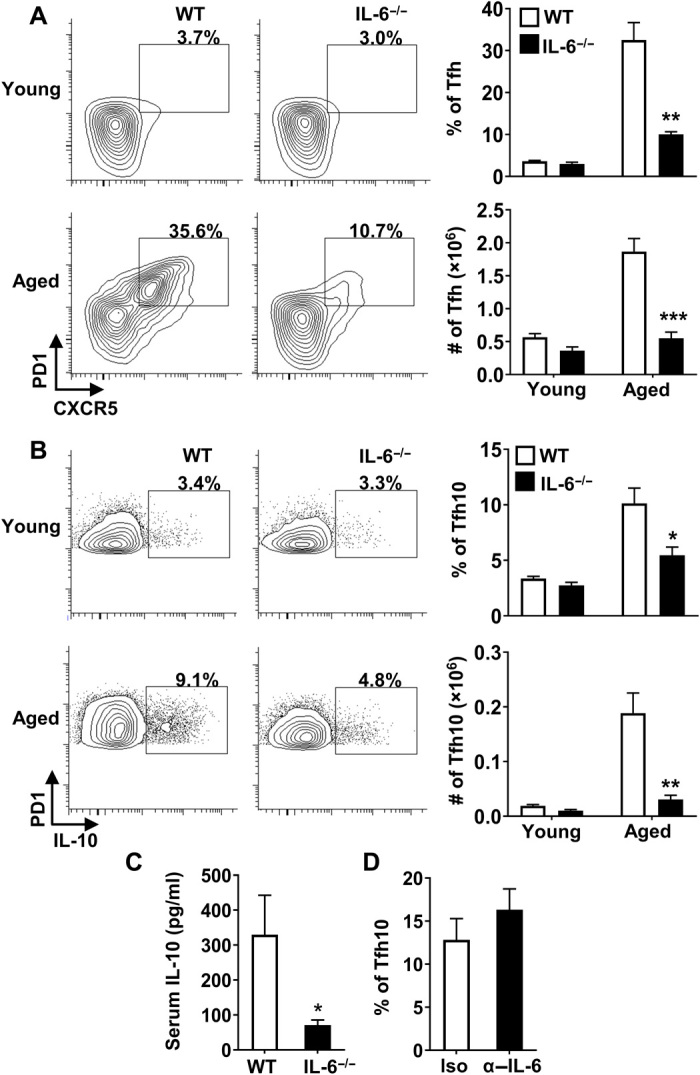
(A and B) Splenocytes from young (2 months, n ≥ 4 per group) and aged (17 months, n ≥ 4 per group) C57BL/6 or IL-6−/− mice were stimulated with P + I, stained with antibody against TCRβ, CD8, CXCR5, PD1, FoxP3, and IL-10 and analyzed by flow cytometry. The representative plots and bar graphs show the frequency and total number of FoxP3− that are (A) CXCR5+PD1+ and (B) those that produce IL-10 (means ± SEM). (C) Aged C57BL/6 (17 months, n = 9) and IL-6−/− (17 months, n = 9) mice were intravenously injected with biotinylated anti–IL-10 antibodies, serum was collected 24 hours later, and IL-10 levels were measured by ELISA. Graph shows the average serum IL-10 (means ± SEM). (D) Aged C57BL/6 mice were treated with isotype control (19 months, n = 6) or α–IL-6 blocking antibody (19 months, n = 8) on day 0 and euthanized on day 2. Splenocytes were stimulated with P + I, stained with antibody against TCRβ, CD8, PD1, FoxP3, and IL-10, and analyzed by flow cytometry. The representative bar graph shows the frequency of FoxP3− that are IL-10+ (means ± SEM). *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001, Student’s t test.
Consistent with Tfh10 cells being a major source of IL-10 in vivo, we found that systemic levels of IL-10 were significantly decreased in aged IL-6−/− mice (Fig. 3C). To determine whether IL-6 was required for the development or maintenance of IL-10–producing FoxP3−CD4+ T cells, we blocked IL-6 after Tfh cells were formed in aged mice and found that neutralization of IL-6 did not reduce the frequency or numbers of IL-10–producing cells (Fig. 3D). This was not because the anti–IL-6 antibody was not functional, as it was able to prevent development of TR1 cells in response to anti-CD3 injection in vivo as reported previously (29) (fig. S3D). Thus, IL-6 likely controls the initial development, rather than the maintenance, that contributes to the accrual of Tfh10 cells with age.
IL-21 promotes accumulation of Tfh10 cells and regulates the systemic IL-6/IL-10 balance
As IL-21 is a critical cytokine produced by Tfh cells (31), we next examined whether IL-6 promoted IL-21 production by CD4+ T cells. As expected, consistent with elevated Tfh cells with age, the proportion and absolute number of IL-21+CD4+ T cells was significantly increased in aged compared to young mice (Fig. 4A). Notably, in the absence of IL-6, the frequency and total numbers of IL-21–producing CD4+ T cells were completely abrogated (Fig. 4A). As IL-21 is also critical for the development and homeostasis of Tfh cells (32), we reasoned that IL-21 could contribute to the accrual of Tfh10 cells with age. Similar to aged IL-6−/− mice, the loss of IL-21 prevented age-driven accrual of Tfh cells (Fig. 4B), including those that produce IL-10 (Fig. 4C). Again, consistent with the loss of Tfh10 cells, levels of systemic IL-10 were reduced in aged IL-21−/− mice compared to aged WT controls (Fig. 4D). Notably, the levels of IL-6 were increased in IL-21–deficient aged mice (Fig. 4E). Together, these data show that IL-21 is critical to balance systemic inflammation (e.g., IL-6/IL-10 levels), likely by promoting the accrual of Tfh10 cells. As IL-6 and IL-21 have been reported to increase the inducible T cell co-stimulator (ICOS) and promote the survival of Tfh cells (33), we considered the possibility that increased levels of ICOS on aged Tfh cells could be contributing to Tfh10 accumulation. ICOS ligand (ICOS-L) neutralization drove a reduction in overall Tfh cell number but had no effect on IL-10–producing cells (fig. S4). These data show that IL-21 plays a key role in promoting accrual of Tfh10 cells with age, whose production of IL-10 likely feeds back to suppress IL-6.
Fig. 4. IL-21 contributes to accrual of Tfh10 cells and regulates the systemic IL-6/IL-10 balance.
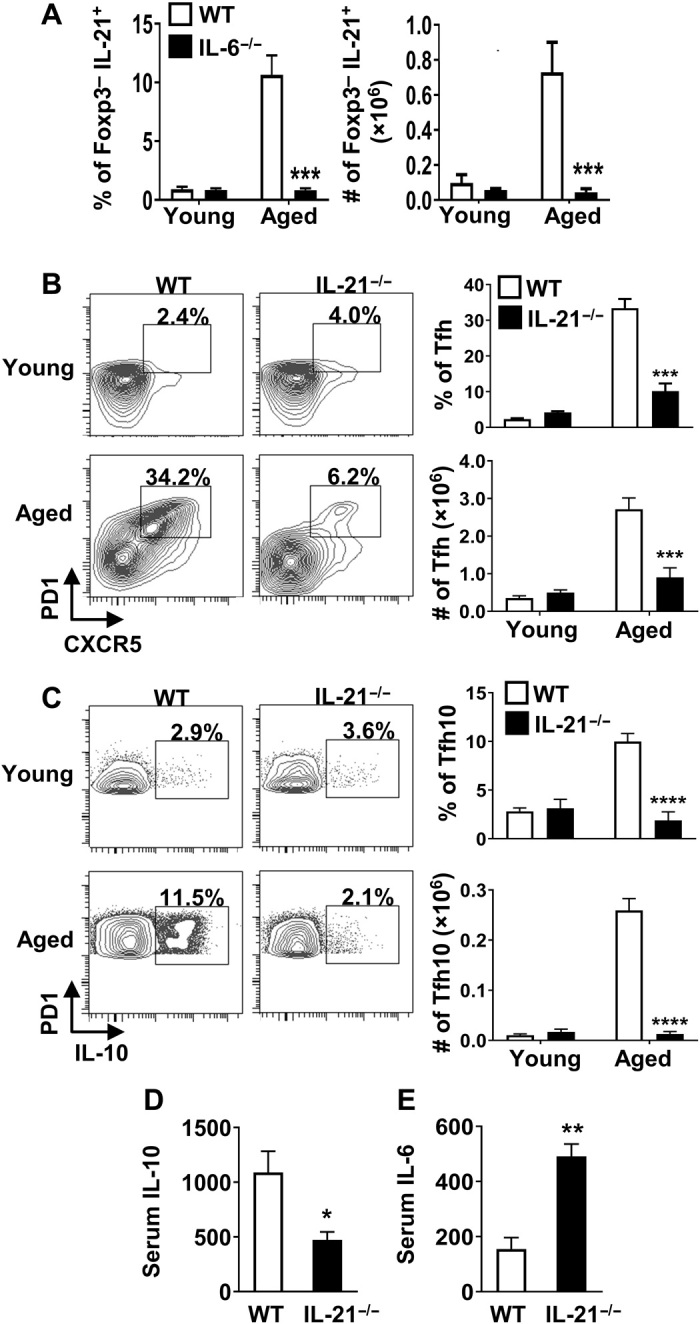
(A) Splenocytes from young (2.5 months, n ≥ 4 per group) and aged (16 months, n ≥ 4 per group) C57BL/6 or IL-6−/− mice were stimulated with P + I, stained with antibody against TCRβ, CD8, FoxP3, and IL-21, and analyzed by flow cytometry. Bar graphs show the frequency and total number of FoxP3− that are IL-21+ (means ± SEM). (B and C) Splenocytes from young (2 months, n ≥ 4 per group) or aged (17 months, n ≥ 3 per group) C57BL/6 or IL-21−/− mice were stimulated as above, stained with antibody against TCRβ, CD8, CXCR5, PD1, FoxP3, and IL-10, and analyzed by flow cytometry. The representative plots and bar graphs show the frequency and total number of FoxP3− cells that are (B) CXCR5+PD1+ and (C) those that produce IL-10 (means ± SEM). Data are pooled from two independent experiments. (D and E) Aged C57BL/6 (17 months, n = 4) and IL-21−/− (17 months, n = 3) mice were intravenously injected with biotinylated anti–IL-10 and anti–IL-6 capturing antibodies, serum was collected 24 hours later, and IL-10 and IL-6 levels were measured by ELISA. Graphs show the average serum (D) IL-10 and (E) IL-6 (means ± SEM). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001, Student’s t test.
IL-21 promotes repression of Bim in aged Tfh10 cells, leading to their enhanced survival
The accumulation of Tfh10 cells with age could be due to their increased proliferation and/or increased survival. The frequency of Tfh10 cells that stained positive for the proliferation marker Ki-67 actually decreased with age, ruling out the possibility that increased proliferation explains their accrual (Fig. 5A). Given our and others’ previous data implicating the proapoptotic molecule Bim in aged T cell survival (34), we examined the role of Bim in the survival of IL-10–producing CD4+ T cells. First, we found that ex vivo aged Tfh10 cells survived better than young Tfh cells (Fig. 5B). Second, Bim levels were reduced in IL-10–producing CD4+ T cells from aged compared to young mice (Fig. 5C). Third, the frequency and total number of FoxP3−CD4+ T cells that were IL-10+ were significantly increased in Bim-deficient mice, as early as 6 months of age (Fig. 5D). Given that IL-21 promotes accumulation of Tfh10 cells, we next determined whether IL-21 contributed to their reduced expression of Bim. IL-21 was critical to suppress the levels of Bim within Tfh10 cells, which likely contributes to their increased survival (Fig. 5E). Together, these data suggest that IL-21–driven suppression of Bim contributes to the accumulation of IL-10–producing cells by enhancing their survival.
Fig. 5. IL-21–driven repression of Bim in aged Tfh10 cells results in their enhanced survival.
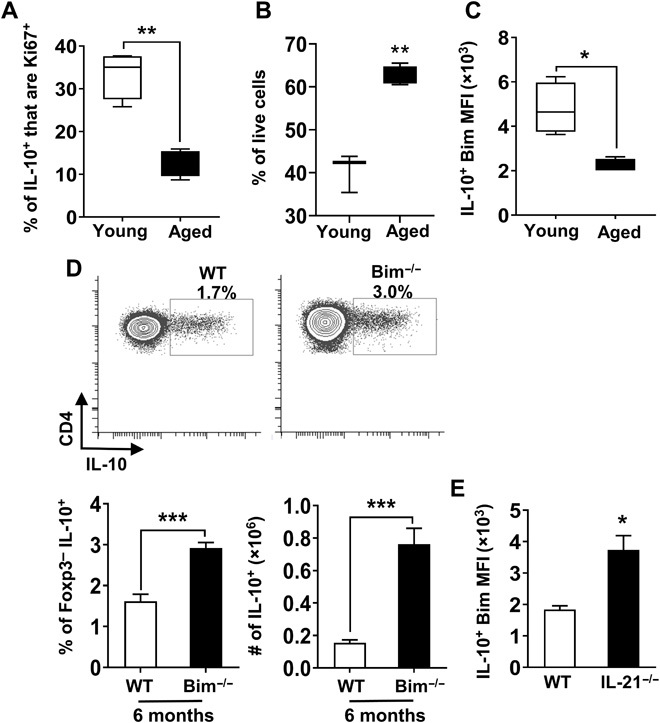
Splenocytes from young (1.5 months, n = 4) and aged (18 months, n = 4) mice were stimulated with P + I, stained, and analyzed by flow cytometry. (A) Graph shows the frequency of FoxP3− IL-10+ cells that are Ki67+ (means ± SEM). (B) Spleen cells were pooled from young (2 months, n = 9) and aged (18 months, n = 6) IL-10gfp (VertX) mice, and CXCR5+PD1+GFP+ cells were fluorescence-activated cell sorting (FACS)–sorted and cultured. After 15 hours in culture, cells were stained for FoxP3 and a viability dye. Graph shows the percentage of live cells in gated FoxP3− cells (means ± SEM). (C) Splenocytes from young (2 months, n = 4) and aged (18 months, n = 4) mice were stimulated as mentioned in (A). Graph shows the level of expression of Bim in FoxP3−IL-10+ cells (means ± SEM). (D) Splenocytes from 6-month-old WT C57BL/6 and Bim−/− mice (n = 6 per group) were stimulated as mentioned in (A), stained, and analyzed by flow cytometry. Plots and bar graphs show the frequency and total number of FoxP3− that are IL-10+ (means ± SEM). (E) Splenocytes from aged (17 months, n = 3 to 4 per group) C57BL/6 or IL-21−/− mice were stimulated as mentioned in (A), stained, and analyzed by flow cytometry. Graph shows the levels of expression of Bim in FoxP3−CXCR5+PD1+ that are IL-10+ cells (means ± SEM). *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001, Student’s t test.
IL-10 limits Tfh-dependent vaccine responses in aged mice
We next sought to determine physiologic relevance of age-driven Tfh10 cell accrual. As vaccine responsiveness is a major problem in elderly humans and Tfh cells are critical regulators of vaccine responses (35), we first examined the ability of Tfh cells to produce IL-10 in response to vaccination. We bred IL-10eGFP to FoxP3RFP mice to generate double-reporter mice, which allowed for efficient detection of IL-10 (GFP) and FoxP3 [red fluorescent protein (RFP)] while maintaining normal aged-driven Tfh10 accrual (fig. S5A). Old and young double-reporter mice were immunized with influenza nucleoprotein (NP) in alum, and their NP-specific CD4+ T cells were assessed by major histocompatibility complex (MHC) tetramer staining 8 days later. NP-specific CD4+ T cells emerged after immunization and were predominantly FoxP3− in both young and aged mice (Fig. 6A). When we gated on the NP-specific CD4+ T cells, there was a significant increase in the frequency of FoxP3−IL-10+ cells in aged compared to young mice (Fig. 6A). Further, Tfh cells made up the majority of IL-10–producing cells, and their numbers were significantly increased in old mice (Fig. 6A). Thus, antigen-specific Tfh10 cells emerge following vaccination in aged mice and are the dominant population of IL-10–producing T cells.
Fig. 6. IL-10–producing Tfh cells emerge after vaccination, and IL-10, but neither Tregs nor Tfr cells, limits Tfh-dependent vaccine responses in aged mice.
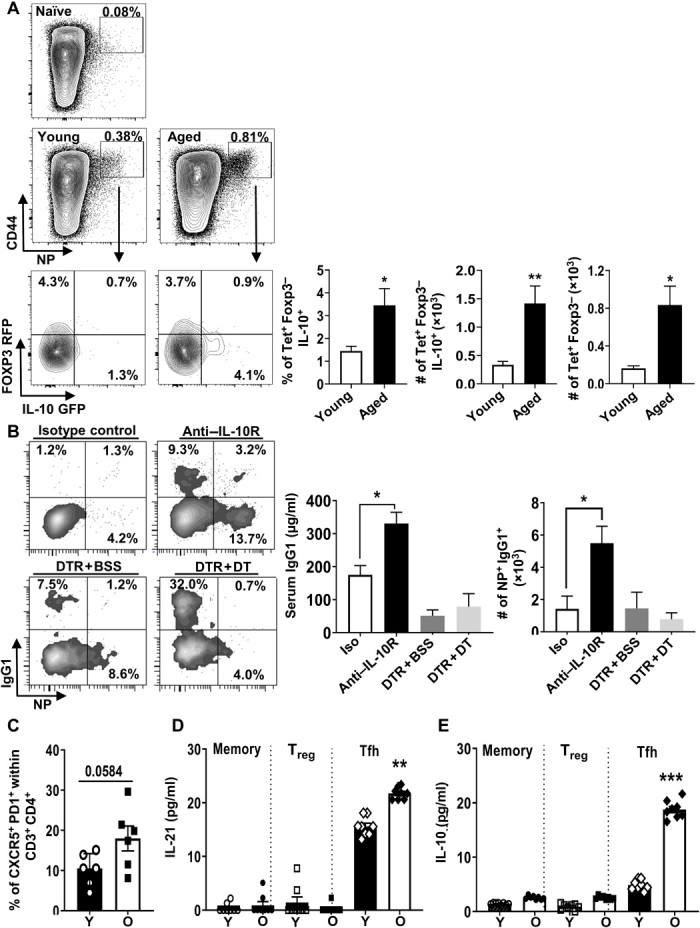
(A) Plots show IAb-NP tetramer staining in immunized and unimmunized young (3.5 months, n = 5) and aged (18 months, n = 5) IL-10GFP–FOXP3RFP dual-reporter mice. Graphs show the frequency and number of NP-specific FoxP3−CD4+ T cells that are IL-10+ and the number of NP-specific FoxP3−IL-10+ T cells that are CXCR5+PD1+ (means ± SEM). (B) Aged (17 months, n ≥ 7) WT C57BL/6 (isotype or anti–IL-10) and (20 months, n = 5) FoxP3−DTR [phosphate-buffered saline (PBS)– or DT-treated] mice were immunized with nitrophenol-keyhole limpet hemocyanin (NP-KLH) in alum. Plots display the frequency of germinal center (GC) B cells (NP-specific) gated on Fas+GL7+. Graphs show the frequency and number of B cells that are IgG1+NP+ (means ± SEM) and serum levels of immunoglobulin (Ig) specific for NP (IgG1) (means ± SEM). Data are pooled from two independent experiments. (C to E) Tfh10 cells accumulate during aging in humans. Human spleen cells from young (Y) (median, 18.8; range, 18 to 26 years; three males and five females) and old (O) (median, 62; range, 60 to 67 years; four males and four females) individuals were analyzed by flow cytometry. (C) The frequencies of CD3+CD4+CD45RO+FoxP3− that are CXCR5+PD1+ (means ± SEM). (D and E) CD4+ T cells were bead-purified by negative selection; FACS-sorted memory CD4+ T cells (CD45RO+) into Tfh cells (CD25−CD127+PD1+CXCR5+), Tregs (CD25+CD127−PD1−CXCR5−), and other memory cells (CD25−CD127+PD1−CXCR5−) were either stimulated in vitro with anti-CD3/CD28 beads or unstimulated, and cytokines were analyzed by Luminex (means ± SEM). Each individual is represented by a symbol. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001, Student’s t test. BSS, balanced salt solution.
We next reasoned that if Tfh10 cells were important for regulating vaccine responses, then limiting IL-10 signaling should affect vaccine responsiveness. To track antigen-specific B cell responses, we used a classic nitrophenol-keyhole limpet hemocyanin (NP-KLH) model. As reported before (27), we confirmed that aged mice displayed a significantly lower level of anti-NP antibody production, as well as significantly lower frequency and total numbers of NP-specific B cells compared to young mice (fig. S5B). Neutralization of IL-10 receptor (IL-10R) during NP-KLH immunization significantly restored anti-NP antibody production as well as the frequency and numbers of anti-NP–specific B cells to levels close to those observed in young mice (Fig. 6B). Although our data showed that both FoxP3+ Tregs and FoxP3+ Tfr cells were only minor producers of IL-10 (Fig. 1D and fig. S3C), we nevertheless assessed their contribution to vaccine responsiveness using FoxP3-DTR mice. Despite efficient depletion of Tfr cells and Tregs (fig. S5C), Treg/Tfr depletion failed to restore antibody responses to vaccination (Fig. 6B). Thus, IL-10 limits Tfh-dependent B cell responses in aged mice.
Tfh10 cells accumulate during aging in humans
Given the above data in mice showing accumulation of Tfh10 cells in aged mice, we next determined whether Tfh10 cells also accumulate in aged humans. As Tfh cells are mainly located and function in secondary lymphoid organs, we analyzed their proportion in the spleens of young and old deceased organ donors with no immunologic condition. The frequency of Tfh cells (CXCR5+PD1+) was increased in aged humans (Fig. 6C). Because flow cytometric analysis of cytokines is affected by cryopreservation, we used fluorescence-activated cell sorting (FACS) to purify (gating strategy; fig. S6) memory CD4+ T cells (CD45RO+) into Tfh cells (CD25−CD127+PD1+CXCR5+), Tregs (CD25+CD127−PD1−CXCR5−), and other non-Tfh memory cells (CD25−CD127+PD1−CXCR5−) and analyzed their production of IL-10 and IL-21 after in vitro restimulation with anti-CD3/CD28 beads. As expected, IL-21 production was largely limited to Tfh cells and was increased with age (Fig. 6D). Notably, the population with the highest production of IL-10 was the old Tfh cells (Fig. 6E). Thus, in agreement with our data from mice, Tfh10 cells accumulate in aged humans. These findings may explain the well-known age-related impairment in vaccine responsiveness in the elderly.
DISCUSSION
While there have been many associations between inflammaging and immune suppression, our data show a novel linkage between these two age-related phenomena. At first glance, the increased levels of IL-10 seem counterintuitive to the well-documented increased inflammation in aging. However, the concept that both increases in both pro- and anti-inflammatory cytokines can coexist has been known for many years (36). In addition, these increases in both pro- and anti-inflammatory cytokines likely coexist as part of a feedback loop in which proinflammatory responses elicit an anti-inflammatory response. We found that IL-6 (a hallmark of inflammaging) is critical to maintain the elevated levels of IL-10 in aged mice. Further, our data suggest that IL-10 production is a feedback mechanism to dampen, but not ameliorate, IL-6–driven inflammaging. IL-21 appears to be critical in maintaining this balance, as mice deficient in IL-21 have decreased IL-10 (and loss of Tfh10 cells) but increased IL-6. Future work will focus on mechanism(s) by which IL-21 controls this systemic balance between IL-6 and IL-10. Combined, these data provide intriguing insight into the complex interplay between pro- and anti-inflammation in aging.
Our data are consistent with pro- and anti-inflammation existing along a continuum that controls age-related disease states. For example, increased levels of IL-10 appear to be beneficial in limiting frailty, systemic inflammation, and organ dysfunction (37). On the other hand, IL-10 and a reduced IFN-γ:IL-10 ratio are correlated with cell-mediated immunity to influenza infection and are inversely correlated with the severity of infection (38) and also with seroconversion following influenza vaccination (39). Thus, on the basis of the association of IL-10 and longevity, it is likely that the suppressive effect of IL-10 on systemic inflammation may be more critical in protecting the aged population from inflammation-associated diseases that contribute substantially to mortality in the elderly (e.g., cancer and cardiovascular diseases). While the negative effect of IL-10 on response to immunization/infection likely contributes to mortality, it is offset by the notable benefit IL-10 offers to mitigate other chronic diseases of the elderly. Nonetheless, further work is necessary to understand the mechanism(s) underlying the beneficial versus pathologic effects of IL-10 in aging.
Our data strongly implicate Tfh10 cells as major contributors to the systemically increased levels of IL-10 in aged mice. Depletion of CD4+ T cells, but not FoxP3+ Treg, reduces systemic IL-10. Two cytokines, IL-6 and IL-21, critical for Tfh homeostasis, are required for the emergence of Tfh10 cells and systemic increased IL-10. Consistent with prior work, our data suggest a unique and requisite role for each cytokine in Tfh cell homeostasis (40). For instance, our data are consistent with IL-6 being required for the development, but not the maintenance, of Tfh10 cells. We saw a precipitous loss of Tfh10 cells in IL-6–deficient mice but no change in Tfh10 cells when IL-6 was neutralized after their formation, similar to a recent study in which IL-6 was neutralized during development of a Tfh response in aged mice (41). Part of this IL-6–driven developmental program involves induction of IL-21 production (42), which our and others’ data suggest may be required for the long-term maintenance of Tfh and Tfh10 cells (43). Our data also provide some molecular insights into the role of IL-21 on maintenance of Tfh10 cells in which we show that it is required to suppress their expression of Bim, which regulates their long-term survival. This concept of dual cytokines individually promoting TH cell development versus maintenance may be a common theme for TH cells, as a similar phenomenon was recently observed in TH2 cells with thymic stromal lymphopoietin (TSLP) and IL-4 (44).
Two recent papers have also described the existence of IL-10–producing Tfh cells: one in which the cells appear during chronic viral infection in mice (45) and another in which they were found in human tonsils (46). Neither appears to have the precise phenotype or function, as we describe. The Tfh10 cells arising during chronic viral infection appear to promote antibody responses, unlike our findings here. One possible explanation for these differences is that chronic viral infection alters the Tfh cells or other cells to facilitate their ability to enhance B cell responses. While the Tfh10 cells described in human tonsils appeared to be functionally similar to ours in that they suppressed antibody responses, this suppression was not IL-10 dependent, and these cells expressed markers distinct from those expressed on the Tfh10 cells we describe in aging. Nonetheless, these combined data further document the existence of Tfh10 cells, whose phenotype and function may vary across disease states (e.g., viral infection and aging), and suggest that further work is necessary to unravel mechanism(s) by which Tfh10 cells alter immune responses.
Our work is consistent with prior data showing an increase in Tfh cells in aged mice (15, 47). These prior studies suggested that subtle maturation defects in aged Tfh cells (slightly decreased levels of GL7, CXCR5, and ICOS) accounted for their decreased ability to provide help to B cells. However, the homing of Tfh cells to germinal centers (GCs) was not significantly affected compared to young mice when the data were corrected for GC size (15). Further, although these authors observed an increase in IL-10–producing CD4+ T cells after influenza infection in aged mice, this increase was not attributed to Tfh cells but rather to Tregs or Tfr cells (15). However, in our aged cohorts of mice, neither Tfr nor Treg appears to be substantial contributors to IL-10 production in vivo. Further, we showed that IL-10 neutralization, but not Treg/Tfr depletion, largely restored vaccine responsiveness in aged mice.
This IL-10 neutralization would have a similar effect in altering the IFN-γ:IL-10 balance as increasing proinflammatory stimuli to restore vaccine responsiveness in aged mice (48). In aged humans, a strong proinflammatory adjuvant MF59 was able to significantly boost antibody responses to influenza (49). In both studies, increases in IFN-γ–producing CD4+ T cell responses were observed. Combined, these data show that the aged immune system is amenable to restoration by manipulating the inflammatory environment. In agreement with and extension to the above observations, our data suggest that, instead of enhancing proinflammation, transient blockade of IL-10 could be a novel strategy to enhance vaccine responses in the elderly and, due to its transient nature, is unlikely to have untoward effects on autoimmunity, cardiovascular disease, or frailty.
Overall, our data add to the accumulating number of defects associated with the aging immune system, including, but not limited to, increased low-level inflammation (50, 51), decreased dendritic cell function and activation in GCs (52–54), cell-intrinsic defects in lymphocyte signaling (55, 56), and decreased numbers of naïve T cells (57, 58). Our data show that active suppression of immune responses also occurs with age and can be reversed to enhance immune responses to vaccination. These data have substantial implications for vaccination of elderly populations.
MATERIALS AND METHODS
Mice
Young C57BL/6 mice were purchased from Taconic Biosciences (Germantown, NY). Our age classifications were young (<6 months), middle age (12 to 15 months), and old (≥18 months) according to (59). Ages of mice used in each experiment are described in the figure legends. C57BL/6 mice were from the National Institute on Aging colony located at Charles River Laboratories (Wilmington, MA). FoxP3-IRES-DTR-GFP knock-in C57BL/6 mice (60) were a gift from A. Rudensky and were aged in house. Bim-deficient [Bim knockout (KO)] mice were originally a gift from P. Bouillet and A. Strasser and were bred in-house. IL-6–deficient (IL-6 KO) mice on the C57BL/6 background were aged in-house. IL-10–reporter (VertX) mice that have the IL-10–IRES-eGFP cassette in the endogenous IL-10 locus on the C57BL/6 background (13) were aged in-house. IL-10–reporter (VertX) mice were bred to FoxP3-IRES-mRFP mice (the Jackson laboratory) to generate IL-10 GFP FoxP3 RFP mice, which were aged in house.
Young, middle-aged, and old germ-free mice on the C57BL/6 background were maintained in isolator units in the Cincinnati Children's Hospital Medical Center (CCHMC) Gnotobiotic Mouse Facility. Young and aged FoxP3 fate-mapping mice (Foxp3CreRosa26dTomato) on the C57BL/6 background were provided by S.S.W. (CCHMC). IL-17A fate-tracking mice IL-17CreRosa26eYFP (24) on the C57BL/6 background were bred and aged under specific pathogen–free conditions in the animal facility of the Research Center Borstel, Germany. Young and aged IL-21–deficient (IL-21 KO) mice on the C57BL/6 background were bred, maintained, and aged in fully accredited facilities at the University of Alabama at Birmingham. Spleens (controls and IL-21 KO) were shipped overnight on ice and analyzed in Cincinnati. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the Cincinnati Children’s Hospital Research Foundation (IACUC 2016-0087).
Immunization, neutralization, and depletion treatments
For depletion of FoxP3+ Tregs in old FoxP3-DTR mice, 1.25 μg of DT per mouse was intraperitoneally injected, and mice were euthanized 2 days later. For CD4 T cell depletion, mice were injected with a single dose of 600 μg per mouse of anti-CD4 intraperitoneally (clone: YTS191, Bio X Cell) or isotype control (clone: LFT-2, Bio X Cell) and were euthanized 2 days later. For T cell–dependent immunization, mice were intraperitoneally immunized with 100 μg of NP-KLH (Biosearch Technologies) mixed with 50% (v/v) alum (Thermo Fisher Scientific) and euthanized 10 or 20 days later. For IL-10R neutralization, mice were injected with anti–IL-10R blocking antibody (clone: 1B1.3A, Bio X Cell) or rat immunoglobulin G1 (IgG1) isotype control (clone: HRPN, Bio X Cell) at days −1 (1 mg), 1 (250 μg), 3 (500 μg), 6 (500 μg), and 8 (250 μg) and were euthanized 10 days after immunization. For Treg depletion in NP-KLH model, mice were immunized as mentioned above, and DT was intraperitoneally administered at days −1 (1 μg per mouse), 2, 5, and 8 (0.25 μg per mouse). Mice were euthanized 10 days after immunization. For NP immunization, mice were immunized with recombinant A/PR/8/34 influenza NP (MyBioSource). Immunizations intraperitoneally injected contained 50 μg of NP mixed with 50% (v/v) alum. For IL-6 neutralization, mice were intraperitoneally injected with 300 μg of α–IL-6 (clone: MP5-20F3, Bio X Cell) or 300 μg of isotype control (clone: HRPN, Bio X Cell) on day 0 and euthanized on day 2. For ICOS-L neutralization, aged C57BL/6 mice were intraperitoneally injected with 150 μg of anti–ICOS-L (clone: HK5.3, Bio X Cell) or with rat IgG2A isotype control (clone: 2A3, Bio X Cell) on days 0, 3, 6, and 9 and then euthanized on day 12.
IVCCA and ELISAs
IL-6 and IL-10 IVCCA was performed as previously described (17) using biotinylated capture antibodies (Invitrogen). Briefly, young and aged C57BL/6 mice were intravenously injected with 10 μg of biotinylated anti–IL-6 (MP5-32C11, Invitrogen) and anti–IL-10 (JES5-16E3, Invitrogen) capture antibodies; mice were bled within 24 hours, and serum was collected. A luminescent ELISA was performed using anti–IL-6 (MP5-20F3, Invitrogen) or anti–IL-10 (JES5-2A5, BD Biosciences) as the coating antibody. For NP-specific antibody titers, 96-well plates were coated overnight at 4°C with NP30-BSA (bovine serum albumin) (Biosearch Technologies), followed by blockade of nonspecific binding by incubation for 1 to 2 hours at 25°C with 5% BSA. Serum samples were loaded into plates with eight serial dilutions (starting from 1:100 or 1:1000), followed by incubation for 2 hours at 25°C or overnight at 4°C. After samples were washed, horseradish peroxidase–conjugated goat antibody to mouse IgG1 (PA1-74421, Thermo Fisher Scientific) was added to plates, followed by incubation for 2 hours at 25°C. The reactions were developed by incubation for 15 min at 37°C with 50 μl of trimethylboron substrate (BioLegend) and were stopped by the addition of 25 μl of 10% H3PO4. The plates were read at 450 and 570 nm (for correction) with an ELISA reader.
Reverse transcription polymerase chain reaction
Samples from different tissues were homogenized, and total cellular RNA was extracted and quantified. Deoxyribonuclease-treated RNA was then used to synthesize complementary DNA. The primer sequences used for detection of IL-10 were 5′-GCTCTTACTGACTGGCATGAG-3′ and 5′-CGCAGCTCTAGGAGCATGTG-3′. Expression levels were normalized to S14 as an internal control gene. The primer sequences used for S14 detection were 5′-GAGGAGTCTGGAGACGACGA-3′ and 5′-TGGCAGACACCAAACACATT-3′. Quantitative real-time polymerase chain reaction was performed with Roche LightCycler 480 SYBR Green 1 Master Mix using the Roche LightCycler 480 II instrument (Roche Diagnostics). Each reaction was performed in triplicate.
Flow cytometry and cell sorting
Human studies
Collection of spleen samples was approved by the CCHMC Institutional Review Board as an exempt research, as individuals were not recruited for this study and all samples were deidentified. Only basic demographic information and cause of death were shared with the researchers. Spleen cells from young (median, 18.8; range, 18 to 26 years; three males and five females) and old (median, 62; range, 60 to 67 years; four males and four females) organ donors with no immunological condition were rested overnight in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 1% penicillin, streptomycin, and glutamine, and 0.5% Hepes at 37°C and 5% CO2. The cells were then washed with phosphate-buffered saline (PBS) and 2% FBS and stained for CD4, CXCR5, PD1, CD45RO (BioLegend), CD25 (BD Biosciences), CD3 (Invitrogen), and CD127 (Beckman Coulter) for 30 min in 4°C, fixed with 4% para-formaldehyde for 20 min in 4°C. Cells were stained for FoxP3 (Invitrogen) using Invitrogen FoxP3 permeabilization buffer and acquired on a flow cytometer. For sorting, CD4+ T cells were bead-purified by negative selection from spleen cells and surface-stained with antibodies against CD45RO, CD127, CD25, PD1, and CXCR5, and the following populations were sorted by FACS after gating on memory CD4+ T cells (CD45RO+): Tfh cells (CD25−CD127+PD1+CXCR5+), Tregs (CD25+CD127−PD1−CXCR5−), and non-Tfh memory cells (CD25−CD127+PD1−CXCR5−). A total of 10,000 cells were either stimulated in vitro with anti-CD3/CD28 beads at a 1:1 cell:bead ratio or unstimulated. After 16 hours, supernatants were collected and analyzed by Luminex.
Mouse studies
Spleens were harvested and crushed through 100-μm filters (BD Falcon) to generate single-cell suspensions. A total of 2 × 106 cells were plated, incubated with Fc block, and surface-stained with a combination of the following antibodies: CD4, CD8α, T cell receptor β (TCRβ), LAG3, and Fas (BD Biosciences); CD19, PD1, CXCR5, GL7, and CD49b (Invitrogen); and B220, IgG1, and CD44 (BioLegend). To identify the NP-specific CD4+ T cells, splenocytes were stained with MHC class II NP tetramer (National Institutes of Health). For intracellular staining, cells were intracellularly stained with antibodies against Bim (Cell Signaling Technology), Ki67, FoxP3 (Invitrogen), and BCL6 (BD Biosciences). For cytokine staining, cells were stimulated with PMA (25 ng/ml) and ionomycin (0.5 μg/ml) for 5 hours, in the presence of brefeldin A and monensin for the final 4 hours, fixed with 2% methanol-free formaldehyde for 1 hour, and then followed by intracellular staining for IL-10 (BioLegend), IL-17, and IL-4 (Invitrogen) using the Invitrogen FoxP3 permeabilization buffer.
For IL-21 staining, cells were fixed, permeabilized with FoxP3 perm buffer from Invitrogen, and incubated with IL-21R/Fc (R&D Systems) chimera for 45 min to 1 hour at 4°C. Cells were then washed with perm buffer and stained with Alexa Fluor 488– or Alexa Fluor 647–conjugated affinity-purified F(ab')2 fragment of goat anti-human Fcγ antibody (Jackson ImmunoResearch Laboratories) for 45 min to 1 hour at 4°C. Data were acquired on an LSRII, Fortessa II, or Fortessa I flow cytometer (BD Biosciences) and analyzed using FACSDiva software (BD Biosciences) or FlowJo software (FlowJo, Ashland, OR).
For sorting, spleen cells from young (3 months, n = 3) and old (≥18 months, n = 4) FoxP3-IRES-DTR-GFP mice were enriched for CD4+ T cells using the negative selection magnetic-activated cell sorting CD4+ T cell isolation kit II (Miltenyi Biotec, San Diego, CA). Enriched cells were stained with anti-CD4, anti-CD44, and anti-CD62L antibodies, and the following populations were sorted by a FACSAria (BD Biosciences): CD4+Foxp3GFP+ (Treg), CD4+FoxP3−GFP−CD44loCD62Lhi (naïve CD4+), and CD4+FoxP3−GFP−CD44hiCD62Llo (memory CD4+). All three populations were stimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml), and supernatants were collected after 15 hours.
Statistical analysis
Statistical analysis was performed with GraphPad Prism or Excel software. Statistical significance was determined by unpaired t test or analysis of variance (ANOVA) (as indicated in the figure legends), with a significance set at P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank members of the David Hildeman, Claire Chougnet, and Michael Jordan laboratories (V. Varghese, C. Castro-Rojas, R. Walters, S. Shanmuganad, K.-P. Li, C. Jackson, and V. Chaturvedi) for suggestions and help with mouse experiments. We also thank J. M. Kinder for help with the FoxP3 fate-mapping mice and C. Chan for help with adipose tissue IL-10 gene studies. We thank A. Herr and J. Miller for help with the influenza NP immunization study. We apologize to authors whom we were unable to cite because of citation limitations. Funding: This work was supported by funds from the NIH grants AG033057 (to D.A.H. and C.C.), AI132771 (to A. D.), DK114123, DK116868 (to T.A.), and AI049360 (to A.J.Z). Author contributions: M.A., J.R., I.O., A.M., S.M., and S.A.H. designed and performed experiments and analyzed data. J.T.I., A.S., and M.M.X. performed certain experiments and provided reagents and mice. M.A., S.A.H., T.A., G.S.D., S.D., H.S., E.M., A.J.Z., A.L.D., C.H., C.C., and D.A.H. wrote and edited the manuscript. E.M., N.S., and S.S.W. provided mice, reagents, and expertise for performing experiments and data analysis. All authors reviewed the manuscript. Competing interests: Patent application: Methods of improving vaccine responsiveness. Application for U.S. Patent. Attorney Docket No.: 47108-535001US. The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. The IL-21–deficient mice were generated under a material transfer agreement from the Mutant Mouse Resource and Research Centers (MMRRC). Requests for the IL-21–deficient mice should be submitted to A.J.Z. (azajac@uab.edu).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/31/eabb0806/DC1
REFERENCES AND NOTES
- 1.Lord J. M., The effect of ageing of the immune system on vaccination responses. Hum. Vaccin. Immunother. 9, 1364–1367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrucci L., Fabbri E., Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maggio M., Guralnik J. M., Longo D. L., Ferrucci L., Interleukin-6 in aging and chronic disease: A magnificent pathway. J. Gerontol. A Bio. Sci. Med. 61, 575–584 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lustig A., Liu H. B., Metter E. J., An Y., Swaby M. A., Elango P., Ferrucci L., Hodes R. J., Weng N. P., Telomere shortening, inflammatory cytokines, and anti-cytomegalovirus antibody follow distinct age-associated trajectories in humans. Front. Immunol. 8, 1027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lio D., Scola L., Crivello A., Colonna-Romano G., Candore G., Bonafè M., Cavallone L., Franceschi C., Caruso C., Gender-specific association between -1082 IL-10 promoter polymorphism and longevity. Genes Immun. 3, 30–33 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Lio D., Candore G., Crivello A., Scola L., Colonna-Romano G., Cavallone L., Hoffmann E., Caruso M., Licastro F., Caldarera C. M., Branzi A., Franceschi C., Caruso C., Opposite effects of interleukin 10 common gene polymorphisms in cardiovascular diseases and in successful ageing: Genetic background of male centenarians is protective against coronary heart disease. J. Med. Genet. 41, 790–794 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauley J. A., Barbour K. E., Harrison S. L., Cloonan Y. K., Danielson M. E., Ensrud K. E., Fink H. A., Orwoll E. S., Boudreau R., Inflammatory markers and the risk of hip and vertebral fractures in men: The osteoporotic fractures in men (MrOS). J. Bone Miner. Res. 31, 2129–2138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belkaid Y., Hoffmann K. F., Mendez S., Kamhawi S., Udey M. C., Wynn T. A., Sacks D. L., The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194, 1497–1506 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almanan M., Raynor J., Sholl A., Wang M., Chougnet C., Cardin R. D., Hildeman D. A., Tissue-specific control of latent CMV reactivation by regulatory T cells. PLOS Pathog. 13, e1006507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobber R., Tielemans M., Nagelkerken L., The in vivo effects of neutralizing antibodies against IFN-gamma, IL-4, or IL-10 on the humoral immune response in young and aged mice. Cell. Immunol. 160, 185–192 (1995). [DOI] [PubMed] [Google Scholar]
- 11.McElhaney J. E., Xie D., Hager W. D., Barry M. B., Wang Y., Kleppinger A., Ewen C., Kane K. P., Bleackley R. C., T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 176, 6333–6339 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Moore K. W., de Waal Malefyt R., Coffman R. L., O’Garra A., Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Madan R., Demircik F., Surianarayanan S., Allen J. L., Divanovic S., Trompette A., Yogev N., Gu Y., Khodoun M., Hildeman D., Boespflug N., Fogolin M. B., Gröbe L., Greweling M., Finkelman F. D., Cardin R., Mohrs M., Müller W., Waisman A., Roers A., Karp C. L., Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J. Immunol. 183, 2312–2320 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobbs M. V., Weigle W. O., Ernst D. N., Interleukin-10 production by splenic CD4+ cells and cell subsets from young and old mice. Cell. Immunol. 154, 264–272 (1994). [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre J. S., Masters A. R., Hopkins J. W., Haynes L., Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci. Rep. 6, 25051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Geest K. S., Lorencetti P. G., Abdulahad W. H., Horst G., Huitema M., Roozendaal C., Kroesen B.-J., Brouwer E., Boots A. M. H., Aging-dependent decline of IL-10 producing B cells coincides with production of antinuclear antibodies but not rheumatoid factors. Exp. Gerontol. 75, 24–29 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Finkelman F., Morris S., Orekhova T., Sehy D., The in vivo cytokine capture assay for measurement of cytokine production in the mouse. Curr. Protoc. Immunol. Chapter 6, Unit 6.28 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Raynor J., Lages C. S., Shehata H., Hildeman D. A., Chougnet C. A., Homeostasis and function of regulatory T cells in aging. Curr. Opin. Immunol. 24, 482–487 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J. Z., Abe F., Osawa R., Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 16, 90 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Round J. L., Mazmanian S. K., Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 107, 12204–12209 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagliani N., Vesely M. C. A., Iseppon A., Brockmann L., Xu H., Palm N. W., de Zoete M. R., Licona-Limón P., Paiva R. S., Ching T., Weaver C., Zi X., Pan X., Fan R., Garmire L. X., Cotton M. J., Drier Y., Bernstein B., Geginat J., Stockinger B., Esplugues E., Huber S., Flavell R. A., TH17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z.-Y., Sato H., Kusam S., Sehra S., Toney L. M., Dent A. L., Regulation of IL-10 gene expression in Th2 cells by Jun proteins. J. Immunol. 174, 2098–2105 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Gagliani N., Magnani C. F., Huber S., Gianolini M. E., Pala M., Licona-Limon P., Guo B., Herbert D. R., Bulfone A., Trentini F., di Serio C., Bacchetta R., Andreani M., Brockmann L., Gregori S., Flavell R. A., Roncarolo M.-G., Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19, 739–746 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Hirota K., Duarte J. H., Veldhoen M., Hornsby E., Li Y., Cua D. J., Ahlfors H., Wilhelm C., Tolaini M., Menzel U., Garefalaki A., Potocnik A. J., Stockinger B., Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12, 255–263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bittner-Eddy P. D., Fischer L. A., Costalonga M., Cre-loxP reporter mouse reveals stochastic activity of the Foxp3 promoter. Front. Immunol. 10, 2228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston R. J., Poholek A. C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A. L., Craft J., Crotty S., Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sage P. T., Tan C. L., Freeman G. J., Haigis M., Sharpe A. H., Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Rep. 12, 163–171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eto D., Lao C., DiToro D., Barnett B., Escobar T. C., Kageyama R., Yusuf I., Crotty S., IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLOS ONE 6, e17739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin J. O., Han X., Yu Q., Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J. Autoimmun. 40, 28–44 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volpato S., Guralnik J. M., Ferrucci L., Balfour J., Chaves P., Fried L. P., Harris T. B., Cardiovascular disease, interleukin-6, and risk of mortality in older women: The women’s health and aging study. Circulation 103, 947–953 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Nurieva R., Yang X. O., Martinez G., Zhang Y., Panopoulos A. D., Ma L., Schluns K., Tian Q., Watowich S. S., Jetten A. M., Dong C., Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Vogelzang A., McGuire H. M., Yu D., Sprent J., Mackay C. R., King C., A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Akiba H., Takeda K., Kojima Y., Usui Y., Harada N., Yamazaki T., Ma J., Tezuka K., Yagita H., Okumura K., The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 175, 2340–2348 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Chougnet C. A., Tripathi P., Lages C. S., Raynor J., Sholl A., Fink P., Plas D. R., Hildeman D. A., A major role for Bim in regulatory T cell homeostasis. J. Immunol. 186, 156–163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crotty S., Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Karp C. L., el-Safi S. H., Wynn T. A., Satti M. M., Kordofani A. M., Hashim F. A., Hag-Ali M., Neva F. A., Nutman T. B., Sacks D. L., In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J. Clin. Invest. 91, 1644–1648 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Couper K. N., Blount D. G., Riley E. M., IL-10: The master regulator of immunity to infection. J. Immunol. 180, 5771–5777 (2008). [DOI] [PubMed] [Google Scholar]
- 38.McElhaney J. E., Kuchel G. A., Zhou X., Swain S. L., Haynes L., T-cell immunity to influenza in older adults: A pathophysiological framework for development of more effective vaccines. Front. Immunol. 7, 41 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corsini E., Vismara L., Lucchi L., Viviani B., Govoni S., Galli C. L., Marinovich M., Racchi M., High interleukin-10 production is associated with low antibody response to influenza vaccination in the elderly. J. Leukoc. Biol. 80, 376–382 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Choi Y. S., Eto D., Yang J. A., Lao C., Crotty S., Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 190, 3049–3053 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsukamoto H., Senju S., Matsumura K., Swain S. L., Nishimura Y., IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age. Nat. Commun. 6, 6702 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nurieva R. I., Chung Y., Hwang D., Yang X. O., Kang H. S., Ma L., Wang Y. H., Watowich S. S., Jetten A. M., Tian Q., Dong C., Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linterman M. A., Beaton L., Yu D., Ramiscal R. R., Srivastava M., Hogan J. J., Verma N. K., Smyth M. J., Rigby R. J., Vinuesa C. G., IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207, 353–363 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rochman Y., Dienger-Stambaugh K., Richgels P. K., Lewkowich I. P., Kartashov A. V., Barski A., Khurana Hershey G. K., Leonard W. J., Singh H., TSLP signaling in CD4+ T cells programs a pathogenic T helper 2 cell state. Sci. Signal. 11, eaam8858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin G., Zander R., Schauder D. M., Chen Y., Weinstein J. S., Drobyski W. R., Tarakanova V., Craft J., Cui W., Single-cell RNA sequencing unveils an IL-10-producing helper subset that sustains humoral immunity during persistent infection. Nat. Commun. 9, 5037 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canete P. F., Sweet R. A., Gonzalez-Figueroa P., Papa I., Ohkura N., Bolton H., Roco J. A., Cuenca M., Bassett K. J., Sayin I., Barry E., Lopez A., Canaday D. H., Meyer-Hermann M., Doglioni C., Fazekas de St Groth B., Sakaguchi S., Cook M. C., Vinuesa C. G., Regulatory roles of IL-10-producing human follicular T cells. J. Exp. Med. 216, 1843–1856 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefebvre J. S., Lorenzo E. C., Masters A. R., Hopkins J. W., Eaton S. M., Smiley S. T., Haynes L., Vaccine efficacy and T helper cell differentiation change with aging. Oncotarget 7, 33581–33594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maue A. C., Eaton S. M., Lanthier P. A., Sweet K. B., Blumerman S. L., Haynes L., Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J. Immunol. 182, 6129–6135 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Della Cioppa G., Nicolay U., Lindert K., Leroux-Roels G., Clement F., Castellino F., Galli G., Groth N., Giudice G. D., Superior immunogenicity of seasonal influenza vaccines containing full dose of MF59® adjuvant: Results from a dose-finding clinical trial in older adults. Hum. Vaccin. Immunother. 8, 216–227 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Franceschi C., Bonafè M., Valensin S., Olivieri F., de Luca M., Ottaviani E., de Benedictis G., Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Fulop T., Witkowski J. M., Olivieri F., Larbi A., The integration of inflammaging in age-related diseases. Semin. Immunol. 40, 17–35 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Chougnet C. A., Thacker R. I., Shehata H. M., Hennies C. M., Lehn M. A., Lages C. S., Janssen E. M., Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J. Immunol. 195, 2624–2632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereira L. F., de Souza A. P., Borges T. J., Bonorino C., Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: Decreased stimulation by aged dendritic cells. Mech. Ageing Dev. 132, 187–194 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Li G., Smithey M. J., Rudd B. D., Nikolich-Zugich J., Age-associated alterations in CD8α+ dendritic cells impair CD8 T-cell expansion in response to an intracellular bacterium. Aging Cell 11, 968–977 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goronzy J. J., Li G., Yu M., Weyand C. M., Signaling pathways in aged T cells—A reflection of T cell differentiation, cell senescence and host environment. Semin. Immunol. 24, 365–372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller R. A., Calcium signals in T lymphocytes from old mice. Life Sci. 59, 469–475 (1996). [DOI] [PubMed] [Google Scholar]
- 57.Hale J. S., Boursalian T. E., Turk G. L., Fink P. J., Thymic output in aged mice. Proc. Natl. Acad. Sci. U.S.A. 103, 8447–8452 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linton P. J., Dorshkind K., Age-related changes in lymphocyte development and function. Nat. Immunol. 5, 133–139 (2004). [DOI] [PubMed] [Google Scholar]
- 59.J. M. Currer, K. Flurkey, D. E. Harrison, Mouse models in aging research, in The Mouse in Biomedical Research, J. G. Fox, S. W. Barthold, M. T. Davisson, C. E. Newcomer, F. W. Quimby, A. L. Smith, Eds. (Elsevier, ed. 2, 2007), vol. 3, pp. 637–672. [Google Scholar]
- 60.Kim J. M., Rasmussen J. P., Rudensky A. Y., Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8, 191–197 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/31/eabb0806/DC1