On a wide battery of cognitive tasks predicted to be hippocampally dependent, monkeys with hippocampal damage performed normally.
Abstract
The theory that the hippocampus is critical for visual memory and relational cognition has been challenged by discovery of more spared hippocampal tissue than previously reported in H.M., previously unreported extra-hippocampal damage in developmental amnesiacs, and findings that the hippocampus is unnecessary for object-in-context memory in monkeys. These challenges highlight the need for causal tests of hippocampal function in nonhuman primate models. Here, we tested rhesus monkeys on a battery of cognitive tasks including transitive inference, temporal order memory, shape recall, source memory, and image recognition. Contrary to predictions, we observed no robust impairments in memory or relational cognition either within- or between-groups following hippocampal damage. These results caution against over-generalizing from human correlational studies or rodent experimental studies, compel a new generation of nonhuman primate studies, and indicate that we should reassess the relative contributions of the hippocampus proper compared to other regions in visual memory and relational cognition.
INTRODUCTION
It is almost canon that the human hippocampus is necessary for some types of memory and relational reasoning, and that hippocampal dysfunction produces anterograde amnesia (1–7). However, three recent reports highlight major challenges to dominant theories of hippocampal function. First, anatomical study of the famous amnesic patient H.M., whose memory loss is usually attributed to hippocampal damage (5), found that more of his hippocampus was spared and histologically intact than was previously reported (8) (H.M.’s spared volume: left = 2.02 cm3, right = 1.96 cm3; age-matched intact hippocampus volume: 3.04 cm3). Instead of selective hippocampal damage, the histological examination confirmed previously observed (9) widespread damage to other temporal lobe structures such as the entorhinal cortex and perirhinal cortex, a focal orbital frontal lesion, atrophy of the mammillary bodies and thalamus, and greater loss of white matter integrity than was normal for a man his age (8, 10). Second, the previously reported selective hippocampal damage in developmental amnesic patients (6) was recently found to be accompanied by mammillary body and anterior thalamus damage (11). The extent of this nonhippocampal damage correlated better with memory loss than did the hippocampal damage. Third, formation of new memories in the object-in-scene task, one of the most accepted tests of episodic memory used with nonhuman primates, was found to be unaffected by lesions of the hippocampus itself (12). By contrast, performance in this task is robustly impaired by fornix transections (13) and mammillary body damage (14). These studies and others have prompted the proposal that we need to recalibrate our understanding of the relative contributions of the hippocampus and other brain areas in memory and relational cognition (15, 16).
There is a concerning lack of clear causal evidence for a critical role of the hippocampus in visual memory, episodic memory, recollection, or relational cognition in nonhuman primates. The most empirically supported function of the primate hippocampus is largely limited to allocentric spatial memory (17, 18), which does not encompass the wide deficits seen in cases of human amnesia often attributed to hippocampal damage. Most evidence about hippocampal function comes from correlational studies in humans and studies conducted with rodents that use rodent-specific methods. Many early findings of memory loss following hippocampal aspirations in primates may have been caused by unintended damage to white matter pathways or additional structures such as the rhinal cortex, amygdala, and parahippocampal gyrus (19). More selective, fiber-sparing, excitotoxic approaches have yielded mixed results, with some laboratories reporting moderate memory deficits (20, 21) but others reporting intact memory despite substantial hippocampal damage (22–24). Because it runs counter to the dominant theory, and because “negative” results are difficult to publish, it is likely that studies finding no effects of hippocampal damage are underreported. If the dominant theory that hippocampal damage produces deficits in relational cognition and visual memory is correct, then the lack of an established nonhuman primate model of hippocampal-based amnesia or relational deficits is concerning.
To address the need for causal evidence on the role of the hippocampus in nonhuman primates, we evaluated the performance of five rhesus monkeys with selective hippocampal damage on a battery of relational and memory tests, using both within-subjects comparisons and between-subjects comparisons to five matched, unoperated, control monkeys. Selective hippocampal lesions were created by injection of excitotoxin, thus sparing white matter tracks, and resulted in substantial damage that compared favorably to other published studies of hippocampal lesions in nonhuman primates (Fig. 1, table S1, and fig. S1). Our battery of tests covered a wide range of cognitive processes including transitive inference (TI) (25), temporal order memory for both trial-unique sequences (26) and habitually learned sequences (27), shape recall (28), source memory (29), several variations of image recognition (30–32), and perceptual classification (33–35). Table 1 lists the tasks, the cognitive faculty on which they are proposed to rely, a publication that behaviorally characterizes the task in monkeys, a publication suggesting what role the hippocampus might play, and the predicted result based on the existing literature.
Fig. 1. Excitotoxic injections of N-methyl-d-aspartate produced selective hippocampal lesions.
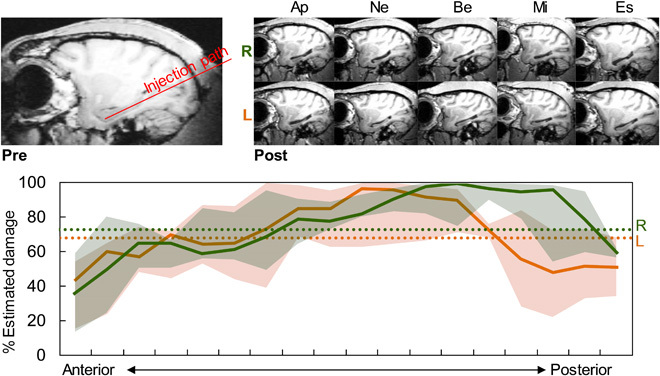
(Top) Left: Presurgery sagittal MRI showing intact hippocampus and injection path. Right: Postsurgery sagittal MRIs of both right (R) and left (L) hemispheres from five monkeys showing shrunken hippocampi. (Bottom) Percent estimated cell loss for each hemisphere along the anterior-posterior axis. Solid lines are group medians, shaded areas are first and third quartiles, and dotted lines are overall means for each hemisphere.
Table 1. Cognitive tests used to assess hippocampal (HP) function.
| Test | Cognitive faculty | Behavioral demonstration | Relevant HP citation(s) | Prediction |
| Transitive inference | Relational representations | (25) | (39); (40) | Impaired |
| Temporal order | One-trial order memory | (1) | (7) | Impaired |
| Simultaneous chaining | Habitual order memory | (27); (46) | (46) | Impaired 2-item probes |
| Shape recall | Item recollection | (28) | (6) | Impaired |
| Item recognition | Familiarity/recollection | (32) | (2); (22) | Altered false alarms |
| Serial position curve | Transfer to long-term memory |
(30) | (24); (70) | Spared |
| Source memory | Recollection of study details | (29) | (4); (52) | Impaired |
| Image classification | Visual perception, generalization, discrimination |
(31) | (53) | Spared |
RESULTS
Transitive inference
TI involves inferring unobserved relations based on observed relations (e.g., Ben is taller than Rob. Rob is taller than Vicky. Therefore, Ben must be taller than Vicky). TI tasks are often solved using order-based strategies such as mentally arranging items in a spatial representation, “A>B>C, therefore A>C” (36). The use of order-based strategies is indicated by increased accuracy and, sometimes, decreased response latency with increasing distance between items in a test pair, known as the symbolic distance effect (SDE). These ordered representations are thought to rely on the hippocampus (37). In humans, functional magnetic resonance imaging (fMRI) studies have found hippocampal activation during presentation of critical nonadjacent test pairs, with the degree of activation correlated to performance (38). TI performance is impaired after hippocampal system damage in humans (39), pigeons (37), and rodents (40). Monkeys with full ablation of the entorhinal cortex, the major input to the hippocampus, are also impaired (41), suggesting that the hippocampus might be involved.
In the present study, monkeys learned six overlapping pairs of object discriminations (A + B-, B + C- … F + G-) pseudo-randomly intermixed in 150-trial sessions until they performed above 80% correct on each adjacent training pair. During testing, critical novel nonadjacent test pairs that had not been presented in training (AC, BD, … EG) were intermixed with training pairs as nondifferentially reinforced probe trials for four sessions, with each test pair appearing once per session (Fig. 2A). Monkeys were trained and tested on three unique seven-item TI sets: one trained and tested prelesion (prelesion set), one trained prelesion and tested postlesion (cross-lesion set), and one trained and tested postlesion (postlesion set). If the hippocampus is critical for TI and the formation of ordered representations, selective hippocampal lesions should impair monkeys’ ability to infer the relation of the nonadjacent pairs and should abolish the SDE.
Fig. 2. Hippocampal damage did not affect TI performance.
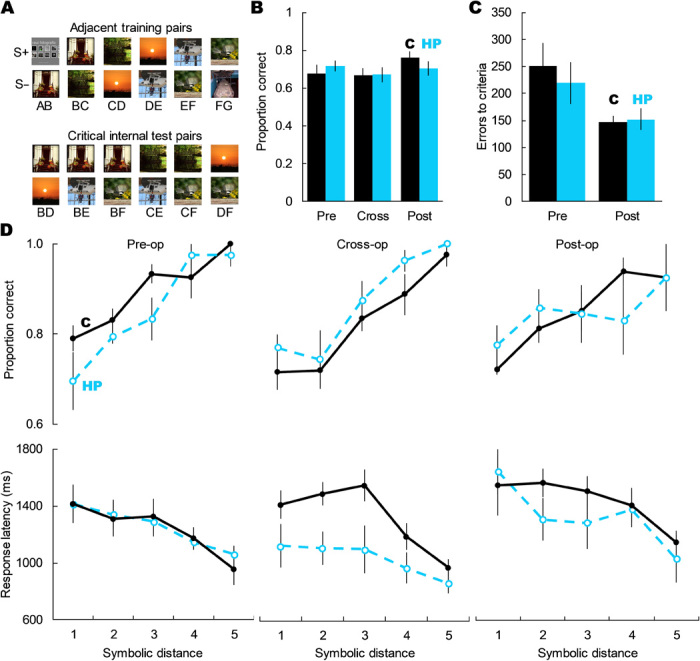
(A) Examples of a TI set with the six adjacent training pairs and the six critical internal test pairs. (B) Hippocampal damage did not impair choice of the higher ranked item on critical internal test trials; main effect of group: F1,8 = 0.73, P = 0.795; main effect of TI set: F2,16 = 0.635, P = 0.543; group × TI set: F2,16 = 0.295, P = 0.756. (C) Hippocampal damage did not affect errors to criterion on either the pre- or postlesion TI sets; main effect of group: F1,8 = 0.130, P = 0.728; group × TI set: F1,8 = 0.531, P = 0.487. (D) Hippocampal damage did not alter how monkeys solved TI tests, as measured by the SDE (accuracy: main effect of group: F1,8 = 0.049, P = 0.830; main effect of SDE: F4,32 = 56.66, P < 0.001; group × TI set: F2,16 = 3.43, P = 0.058; SDE × TI set: F8,64 = 0.99, P = 0.446; SDE × group × TI set: F8,64 = 0.63, P = 0.749; response latency: main effect of group: F1,8 = 1.254, P = 0.295; main effect of SDE: F4,32 = 20.07, P < 0.001; group × TI set: F2,16 = 2.75, P = 0.094; SDE × TI set: F8,64 = 1.18, P = 0.346; SDE × group × TI set: F8,64 = 0.84, P = 0.574). Error bars represent SEM. Stimuli images from Flickr under a Creative Commons CC BY 2.0 Generic License.
Hippocampal lesions did not impair TI accuracy on internal test pairs (Fig. 2B) and did not significantly attenuate the SDE for either accuracy or response latency (Fig. 2D), nor did hippocampal damage slow learning of TI premise pairs in the postlesion set (Fig. 2C). Because end anchor items (A and G) were either always (item A) or never (G) reinforced, test pairs containing these images may be easier to solve than internal pairs, resulting in an artificial SDE. Thus, we also analyzed the SDE using only the internal test pairs and still found an SDE that did not differ between groups after surgery. There was no three-way interaction between symbolic distance, group, and image set for either accuracy (F4,32 = 0.58, P = 0.682) or latency (F4,32 = 0.04, P = 0.746). For accuracy, we still found a robust SDE effect (fig. S2; main effect of symbolic distance: F2,16 = 12.99, P < 0.001) but no main effect of group (F1,8 = 0.84, P = 0.387) or group × SDE interaction (F2,16 = 1.27, P = 0.308). For latency, the effect of SDE did not reach statistical significance (fig. S2; F2,16 = 2.58, P = 0.107), possibly due to the reduced number of symbolic distances afforded by looking at only the internal test pairs. There was still no main effect of group (F1,8 = 1.44, P = 0.265) or group × SDE interaction (F2,16 = 1.49, P = 0.256). Together, this evidence demonstrates that the primate hippocampus is not critical for learning new TI image sets or for solving critical nonadjacent test trials. Further, because the SDE did not change reliably as a result of hippocampal damage, it appears that monkeys lacking the hippocampus continued to use the same relational, order-based strategy for TI.
Memory for temporal order
The hippocampus has been identified as critical for remembering the order in which events occurred, a key component of episodic memory (7, 42). In rodents, selective hippocampal lesions spared recognition of whether odors had been presented, but impaired memory for the order in which those odors were encountered (7, 42). Studies of humans also implicate the hippocampus in memory for the relative order in which events occurred (43). Monkeys with transections of the fornix, the major output pathway of the hippocampus, were significantly impaired at selecting the more recent of two images studied in a list of five (44), implying involvement of the hippocampus proper.
As in the monkey study using fornix transections (44), we tested memory for the order in which images were seen on a touch screen. After touching five images in temporal sequence, monkeys had to select the image that had occurred earlier from among two test images (26, 45). Lists of images were trial unique. Across trials, tests consisted of all possible pairs of list positions and could be grouped for analysis based on the number of intervening images or symbolic distance (SD; e.g., 2,4 = SD 1; 1,5 = SD 3). As with TI, monkeys show an SDE that indicates a spatial relational representation of order (26), allowing us to evaluate both changes in overall accuracy and changes in how monkeys solve the task. If the primate hippocampus is critical for remembering temporal order, monkeys with selective hippocampal damage should be impaired in overall accuracy and the SDE should be attenuated.
Monkeys with hippocampal lesions were impaired transiently, if at all, in memory for temporal order. Monkeys with hippocampal damage performed accurately and showed a robust SDE postoperatively (Fig. 3B). This is in contrast to analogous studies in rats in which accuracy fell to chance and the SDE disappeared after hippocampal damage (7, 42). We did observe two potential group differences, but both need to be interpreted with caution. First, there was a significant three-way interaction between symbolic distance, surgical time point, and group (F1.981,11.35 = 7.196, P = 0.006). Although intriguing, concluding that this is a reliable effect is contradicted by the postoperative data, in which we saw no robust lesion effects or interactions (Fig. 3B). Further, monkeys with hippocampal damage were still able to perform accurately and still showed a robust SDE postoperatively. The best performing monkey across all symbolic distances had hippocampal damage (Monkey Ap; see Fig. 1 and table S1). Second, monkeys with hippocampal damage showed lower accuracy than control monkeys on the first postoperative session (fig. S3; main effect of group for session 1: F1,32 = 8.45, P = 0.007), but this difference did not persist into the second postoperative session or beyond (fig. S3; main effect of group for sessions 2 to 5: all F1,32 < 2.97, all P > 0.095). See the Supplementary Materials for expanded statistics and a discussion that explores the possibility that the hippocampus may play a role in the temporal order memory task that can rapidly be replaced by neural reorganization or other compensation. Because a transient effect was not our a priori hypothesis, more investigation will be needed to determine whether this is a true transient impairment or a type I error. Regardless, a memory loss that abates after one testing session is inconsistent with amnesia. Although “end items” are less of a concern for tests of temporal order than with tests of TI, we also analyzed postoperative performance while excluding all trials containing the first or last list item. Using only internal list items, monkeys still showed a significant main effect of symbolic distance (F1,8 = 22.46, P = 0.001), but no main effect of group (F1,8 = 0.09, P = 0.766) or interaction (F1,8 = 0.14, P = 0.720). Overall, monkeys with substantial and selective hippocampal damage showed high accuracy and clear SDEs when reporting temporal order.
Fig. 3. Hippocampal damage did not reliably impair memory for temporal order.
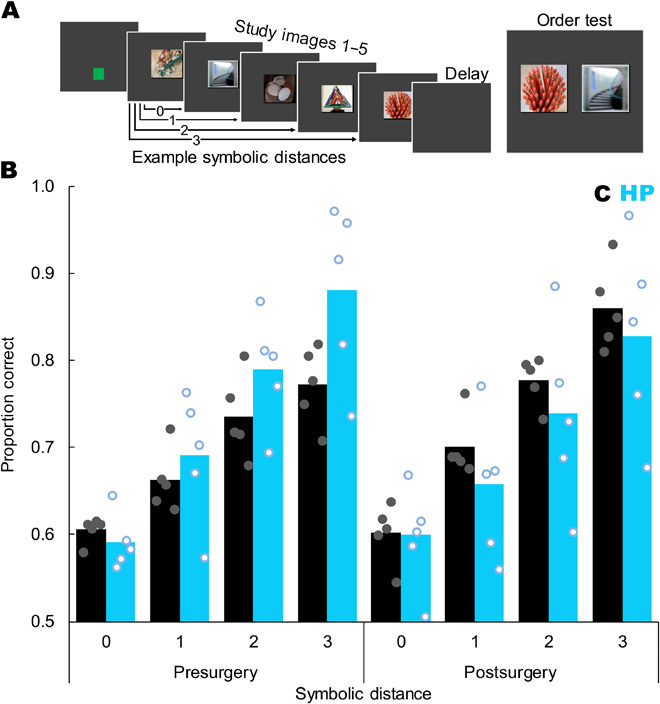
(A) Trial sequence showing a green start square, five study images, a retention delay, and then a test in which the monkey had to touch the image that had appeared first during study. Example symbolic distances are indicated by arrows. The depicted test shows images 5 and 2, for a symbolic distance of 2. (B) Proportion correct as a function of symbolic distance, surgical time point, and group (control = black bars and filled gray dots, hippocampal = blue bars and open blue dots). Postoperatively, monkeys showed a strong SDE (F3,24 = 84.30, P < 0.001) but there was no difference between the groups in overall accuracy (t8 = 0.67, P = 0.520), no interaction of symbolic distance and group (F3,24 = 0.50, P = 0.689), and no group difference at any individual symbolic distance (all t8 < 1.15, all P > 0.284). Bars represent group means, each dot represents one monkey, and dots are jittered to allow visualization of individual performance. Chance is 0.5. Compare figure 2 of (7). Stimuli images from Flickr under a Creative Commons CC BY 2.0 Generic License.
Simultaneous chaining
In simultaneous chaining (SC), subjects learn to touch a set of simultaneously presented images in a fixed order, regardless of spatial location, and then flexibly report the relative ordinal position of nonadjacent images either from the same list or from different lists. The ability to flexibly sort images by ordinal position across lists has been proposed as a test of declarative memory and is theorized to depend on the hippocampus (46). After subjects learn two arbitrary lists through trial and error (list 1: A1➔B1➔C1➔D1➔E1; list 2: A2➔B2➔C2➔D2➔E2), they are tested with nondifferentially reinforced probe trials containing nonadjacent images from the same list (e.g., A1➔E1) or different lists (e.g., B2➔D1) and are required to report the relative order of the two images (47). In humans and monkeys, performance on these two-image probe tests reliably produces an SDE both within and between lists, suggesting a representation of ordered relations that reflects knowledge of ordinal position, rather than rote item-item association chains (46).
In the present study, monkeys learned by trial and error to select the five color images in each list in a predefined order (Fig. 4A; list 1: A1➔B1➔C1➔D1➔E1; list 2: A2➔B2➔C2➔D2➔E2; etc.). After monkeys learned four lists, they received nondifferentially reinforced two-image probe tests randomly intermixed with normal five-image lists. Probe trials consisted of all combinations of two images from the lists presented in two randomly selected screen locations. They were either within-list tests (e.g., A1, C1) or between lists tests (e.g., A3, C4). Probe test pairs can be sorted by symbolic distance (Fig. 4B; e.g., A, E = SD 3; A, B = SD 0). The literature suggests that hippocampal damage will reduce accuracy and disrupt the SDE when monkeys are required to flexibly report the order of nonadjacent items, and that this impairment will be greater for between-list pairs than within-list pairs.
Fig. 4. Hippocampal lesions did not impair SC.
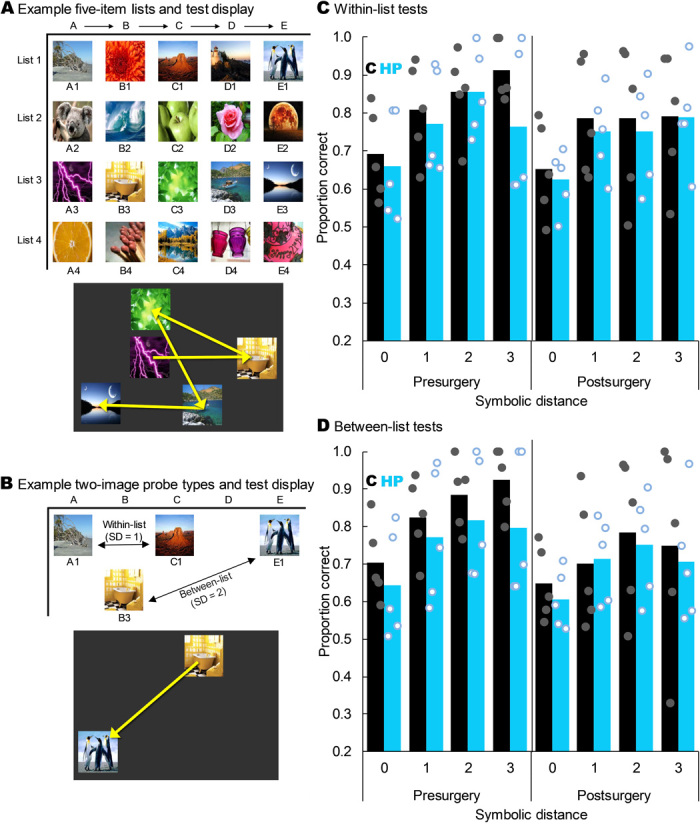
(A) Four example five-item lists and how one list might appear on the screen at test. The yellow arrows indicate the order in which the monkeys had to touch the images to earn food; these arrows were not shown to the monkeys. (B) Example two-image probe tests showing a within-list test at an SD of 1 and a between-list test at an SD of 2. The yellow arrow on the example screen shows the order in which the monkeys had to touch the images to earn food and was not visible during test. (C) Proportion correct on within-list probe tests as a function of SD, group, and experimental time point. Monkeys with hippocampal damage did not perform less accurately than controls overall (t8 = −0.833, P = 0.429) or at any individual SD (all t8 < 0.54, all P > 0.603). All monkeys showed a robust SDE (F3,24 = 25.303, P < 0.001), but there was no main effect of lesion group or interaction of group with any factor (all P > 0.05). Bars represent group means, and dots represent individual monkeys jittered along the x axis to help visualize individual performance. (D) As in (C) but for between-list tests. All monkeys showed a robust SDE (F1.549,12.390 = 19.626, P < 0.001), but there was no main effect of group or interaction of group with any factor (all P > 0.05). Stimuli images from Flickr under a Creative Commons CC BY 2.0 Generic License.
Hippocampal lesions did not impair performance on the five-item lists that had been learned preoperatively (main effect of surgical time point: F1,8 = 0.060, P = 0.812; main effect of group: F1,8 = 1.576, P = 0.245; time point × group interaction: F1,8 = 2.521, P = 0.151), indicating that lesions did not impair memory for routinized lists. Critically, and contrary to the prediction in the literature, hippocampal damage did not impair postoperative performance with either within-list or between-list nonadjacent pairs. Monkeys still showed a robust SDE for both within- and between-list probe pairs postoperatively (Fig. 4, C and D). As with the tests of TI and temporal order, we also analyzed postoperative performance while excluding all trials containing the first or last list item. Using only internal list items, monkeys still showed a significant main effect of symbolic distance for both between list probes (F1,8 = 58.68, P < 0.001) and within list probes (F1,8 = 12.48, P = 0.008), but no main effects of group (between: F1,8 = 0.08, P = 0.777; within: F1,8 = 083, P = 0.780) or group × SDE interactions (between: F1,8 = 1.18, P = 0.309; within: F1,8 = 0.9178, P = 0.366; see the Supplementary Materials for expanded analyses and discussion). Together, these results demonstrate that the hippocampus is not necessary for executing well-learned lists from long-term memory or for flexibly ordering items according to ordinal position from memory, regardless of whether they come from the same or different lists. Furthermore, the consistent SDE pre- and postsurgery suggests that monkeys did not use an alternative strategy to solve SC probe tests postsurgery.
Shape recall
Some humans with hippocampal damage can recognize items that are presented to them but are impaired at recalling information about previously seen items from memory (6). We tested whether monkeys with selective hippocampal damage were similarly impaired using a shape reproduction test (28) modeled after human recall tests (6). On each trial, monkeys saw a simple shape composed of one blue block and one or two connected red blocks. After a short retention interval, the blue block reappeared on screen and the monkeys earned food by reproducing the remaining one or two blocks by touching the location those blocks should occupy from among two or four possible locations, respectively (Fig. 5). To push the monkeys to the limits of their performance and thus increase the chance of detecting an effect of hippocampal damage if one existed, we also tested monkeys in a condition in which they had to reproduce two red blocks when all surrounding distractor locations were available, and the retention interval contained a competing cognitive load that was expected to interfere with working memory (31). If the hippocampus is necessary for recall, then monkeys with selective hippocampal damage should be impaired on shape reproduction and this impairment should be most severe when working memory is compromised by cognitive distraction.
Fig. 5. Hippocampal damage did not impair shape recall.
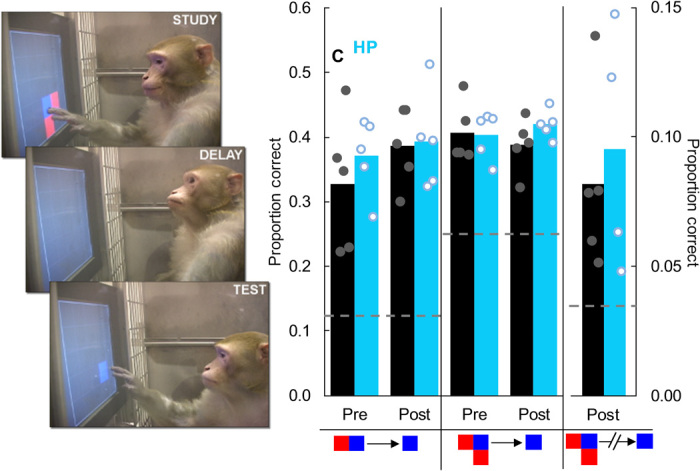
(Left) A monkey reproduces a three-block shape from memory after a retention interval. (Right) Hippocampal damage did not impair reproduction of two-block shapes (t8 = 0.16, P = 0.88), three-block shapes (t8 = 1.52, P = 0.17), or three-block shapes with a distractor task during the retention interval to interfere with working memory (t7 = 0.05, P = 0.96). Dotted lines represent chance in the different conditions. Dots represent individual monkeys and are jittered along the x axis to help visualize individual performance. Photo credit: Benjamin M. Basile, Emory University.
Monkeys with selective hippocampal damage were as accurate as control monkeys in shape recall. This was true when reproducing two-block shapes (Fig. 5) and three-block shapes (Fig. 5) and when reproducing three-block shapes following a cognitive distraction imposed to limit the availability of working memory (Fig. 5). The lack of impairment in shape reproduction across these conditions is evidence against the selective involvement of the hippocampus in recall.
Image recognition
Evidence about whether the hippocampus is necessary for image recognition has been mixed. Some studies of rodents, monkeys, and humans report that that individuals with hippocampal damage show recognition deficits (3, 21, 48), while others report normal recognition (6, 7, 22–24). The inconsistency of findings about hippocampal involvement in recognition may result from the use of different task parameters, such as size of the stimulus set, type of recognition decision required, or use of single-item samples versus lists. It is possible that different parameters differentially encourage recollection or familiarity, either of which can support successful recognition performance. One prominent hypothesis is that the hippocampus supports only the recollective component of recognition and that subjects with hippocampal damage depend on a vague sense of item familiarity to recognize images (2, 49, 50). This shift to recognition based on familiarity might result in preserved overall accuracy but markedly altered criteria for when to report a partially familiar test item as being remembered.
We tested monkeys on a variety of image recognition tests. These include single image recognition using both large and small sets of images, different retention intervals, different decisions (four-alternative forced-choice and yes/no), serial-probe recognition of items studied in lists, tendency to falsely accept recently seen lures, and reaction time analyses that have been proposed to measure the contribution of a vague familiarity signal. Details of these tasks have been published (30–32). Based on previous findings from monkeys, we expected no impairment in overall recognition accuracy (22, 23) or in the shape of the serial position curve in memory for lists (24). Based on the hypothesis that hippocampal damage should cause a shift to a recognition strategy that relies on a vague familiarity signal (2, 49, 50), we predicted that monkeys with hippocampal damage would show a change in the pattern of false alarms made during normal recognition and speeded recognition and to recently seen lures.
As expected, selective hippocampal damage did not affect overall accuracy, regardless of recognition paradigm, size of the image set, or length of the retention interval (fig. S4A). In both monkeys with intact and damaged hippocampi, accuracy was worse with a small set of images than a large set and worse at longer retention intervals than shorter intervals, and the memory decay with increasing retention interval was more pronounced for small sets than large sets (fig. S4A). Similarly, selective hippocampal damage did not impair primacy or change any aspect of the serial position curve when monkeys were required to recognize one item from a previously studied list (fig. S4B), confirming the previous monkey study of memory for lists (24).
To determine whether hippocampal damage caused monkeys to shift strategies toward greater dependence on familiarity, perhaps to compensate for impaired recollection, we evaluated three patterns of false alarms during normal item recognition. First, we assessed the typical U-shaped false alarm pattern in which quick responses are disproportionally false alarms to incorrect lures (32). In monkeys, this pattern is robust across single-sample tests, multi-sample lists, multiple stimulus types, and multiple delay lengths. Selective hippocampal damage did not produce any measurable change in overall accuracy (Fig. 6B) or in the U-shaped pattern of false alarms (Fig. 6A). Second, we assessed the increase in false alarms that typically occurs under a response deadline that increases reliance on the quicker familiarity signal (32). As expected, if monkeys shifted to responding based on vague stimulus familiarity, adding a response deadline selectively increased false alarms without affecting misses (false alarms: t9 = 5.41, P < 0.001; misses: t9 = 0.21, P = 0.836); however, hippocampal damage did not alter this change in false alarms due to a response deadline (Fig. 6C). Third, we assessed false alarms on probe trials on which the to-be rejected lure was seen on the previous trial and thus should be highly familiar. Monkeys made more false alarms to recently seen lures, and this effect was larger after a longer retention interval (Fig. 6D); however, hippocampal damage did not alter monkeys’ rate of false alarms to recently seen lures.
Fig. 6. Selective hippocampal damage did not change the pattern of false alarms during item recognition.
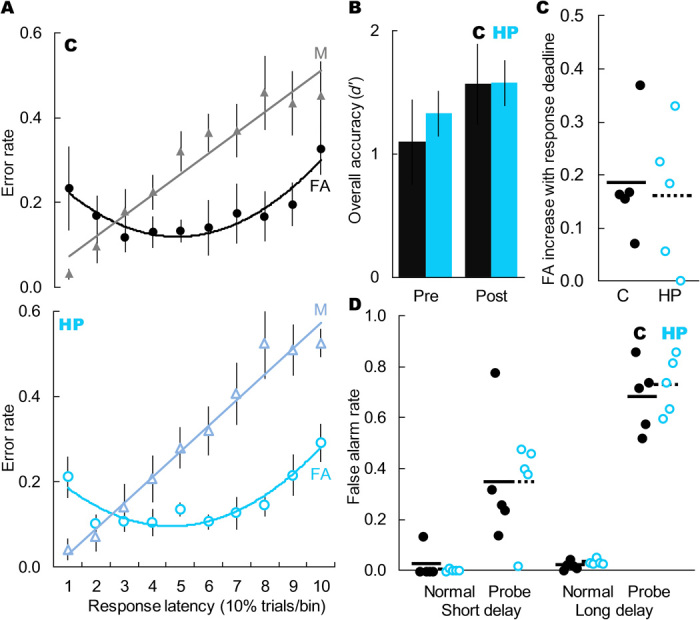
(A) Error rates for control monkeys (solid black shapes) and monkeys with selective hippocampal damage (open blue shapes) as a function of error type (triangles = misses, circles = false alarms) and response latency. Hippocampal damage did not alter the classic pattern of U-shaped false alarms associated with quick but vague familiarity (main effect of response speed: F9,80 = 3.09, P = 0.003; main effect of group: F1,9 = 0.51, P = 0.475; interaction: F9,80 = 0.15, P = 0.999). Dots represent group means (±SEM) within each bin, and each bin contains 10% of trials. (B) Groups did not differ in overall recognition accuracy (pre: t8 = 0.02, P = 0.982; post: t8 = 0.59, P = 0.592). Bars represent group means (±SEM) and chance is 0. (C) Hippocampal damage did not affect the increase in false alarm rate due to addition of a response deadline (t8 = 0.33, P = 0.748). Dots represent individual monkeys, and lines represent group means. (D) False alarm rate as a function of trial type (normal nonmatch trial or probe nonmatch trial with recently seen lure) and retention interval (4 or 20 s). Monkeys showed elevated false alarms to recently seen lures (short delay: t9 = 10.30, P < 0.001; long delay: t9 = 86.33, P < 0.001), and this effect was greater with a longer retention interval (t9 = 7.21, P < 0.001), but it was not affected by selective hippocampal damage (short delay: t8 = 0.18, P = 0.858; long delay: t8 = 0.38, P = 0.716). Lines represent group means, each dot represents one monkey, and dots are jittered along the x axis to allow better visualization of individual performance.
To test whether group differences would emerge at longer memory intervals or with reduced within-group variance, we substituted completely novel stimuli and titrated long delay intervals for each monkey such that all monkeys scored in a restricted accuracy range of 60 to 70% correct. The groups did not differ in the titrated delay that achieved matched accuracy (mean retention interval = 20.8 s for both groups). Novel stimuli and longer retention intervals did not reveal group differences or group interactions in patterns of false alarms [analysis of variance (ANOVA) on U-shaped false alarm curve: main effect of group: F1,9 = 2.07, P = 0.154; interaction: F9,80 = 0.45, P = 0.902; t test between groups on false alarm difference sped-unsped, t8 = 0.41, P = 0.696].
To interfere with working memory, we extended the delay interval to 40 s and interposed a competing cognitive load during the retention interval (perceptual classification as in fig. S6). Limiting the use of working memory, even at the relatively long delay of 40 s, did not reveal group differences or group interactions (ANOVA on U-shaped false alarm curve: main effect of group: F1,7 = 0.10, P = 0.762; interaction: F9,63 = 1.57, P = 0.145; t test between groups on false alarm difference sped-unsped, t7 = 0.75, P = 0.478).
Together, these findings show that hippocampal damage did not negatively affect the ability to recognize items and did not cause monkeys to switch to a more familiarly based strategy for identifying studied images at test. Overall, hippocampal damage did not affect whether or how monkeys recognized items.
Source memory
One hypothesis for the function of the primate hippocampus is that it binds studied items to contextual details about the study episode (51). This is broadly termed source memory. In typical human studies, item memory is operationalized as the ability to discriminate between studied items and unstudied items. In contrast, source memory is operationalized as the ability to discriminate between multiple studied items based on some secondary aspect of the study event, such as the color of the font in which words were presented at study or the judgment the subject was required to make about the item at study. Hippocampal blood oxygen levels track source memory strength in humans (52), and hippocampal lesions in rats impair memory for how a memory was acquired but not whether it was remembered (4).
We assessed the involvement of the hippocampus in source memory by testing monkeys on a nonhuman primate source memory test. Six of our monkeys, three in each group, had previously learned to discriminate between two studied images based on whether they had simply touched or had classified the image during study (29). Monkeys studied two images on each trial, one by touching it and the other by classifying it as a bird, fish, flower, or person. At test, they were cued by the screen background as to whether to select the touched sample or the classified sample from among unstudied distractors (fig. S5A). If the hippocampus is selectively necessary for source memory but not item memory, then monkeys with selective hippocampal damage should still be able to discriminate between studied and unstudied items but should be impaired at discriminating between the two types of studied items.
Monkeys with selective hippocampal damage were unimpaired both at discriminating studied items from unstudied items and at discriminating between studied items based on how they were presented at study (fig. S5B). Because the monkeys could not know until the test phase which sample would be cued, the only way to discriminate between the samples is to remember not only which images were studied but also how they were studied. This provides preliminary evidence against the hypothesis that the primate hippocampus is selectively involved in source memory. However, because only half the monkeys in each group learned this task before surgery, the sample size for this test was not as large as that of other tests in this report and thus these results need additional verification.
Perceptual classification
As a control task to evaluate potential nonmnemonic deficits, we tested monkeys’ ability to perform a perceptual classification task. On each trial, monkeys classified a central image as a bird, fish, flower, or person by touching one of four associated symbols (fig. S6) (33). Monkeys learn this perceptual discrimination well and fully transfer performance to novel stimuli (35). Based on evidence from human memory-impaired patients who show largely normal visual perception (53), we hypothesized that our monkeys with selective hippocampal damage would also show normal perceptual classification performance. As expected, selective hippocampal damage did not affect perceptual classification accuracy (fig. S6).
DISCUSSION
Contrary to dominant theories, we found no evidence that selective hippocampal damage in rhesus monkeys produced disordered relational cognition or impaired visual memory. Across a substantial battery of cognitive tests, monkeys with hippocampal damage were as accurate as intact monkeys and we found no evidence that the two groups of monkeys solved the tasks in different ways.
Our findings are consistent with the proposal that we need to reassess the relative contributions of the hippocampus proper compared to other regions in visual memory and relational cognition. The hippocampus is one part of a broader set of memory-related brain structures including the mammillary bodies, anterior thalamus, retrosplenial cortex, and prefrontal cortex (11, 16). Some of the tests used in this study, as well as similar tests, are known to be sensitive to damage to some of these structures. For example, order judgments are sensitive to fornix transection in monkeys (44), although they were not affected by damage to the hippocampus per se in the current study. Recent evidence from developmental amnesic patients has shifted focus from their hippocampal atrophy to their thalamic atrophy (11). A striking report has also recently found that hippocampal damage in monkeys does not produce any anterograde memory impairment in the object-in-context “scenes” task, a long-used test of episodic memory that is sensitive to fornix transections and mammillary body damage (12). Although the current experiments did not directly evaluate the role of these nonhippocampal areas in these tasks, the current findings should inform research into the functional selectivity of different structures in the Papez circuit.
To our knowledge, this is the largest battery of cognitive tests that include both pre- and postsurgical measures given to a single group of monkeys with hippocampal damage. These include tasks that are previously unreported for monkeys with hippocampal lesions, such as temporal order memory and shape recall, and some that have been tested before with mixed results, such as item recognition. For tasks that had been tested before, we used both standard analyses of accuracy and more fine-grained analyses to evaluate the hypothesis that monkeys with hippocampal damage and normal monkeys solve these tasks via different strategies. Other well-done and informative nonhuman primate studies have also addressed the role of the hippocampus in visual memory, episodic memory, relational memory, and relational cognition. These individual studies often present conflicting results. For example, hippocampal damage in monkeys has been previously reported both to impair (54) and to improve (55) transverse patterning performance, a test of relational cognition. It is often difficult to identify why two individual studies carried out in different laboratories and using different subjects might come to different conclusions. The present study’s striking lack of impairment across multiple tasks, often testing similar cognitive abilities in different ways, with the same subjects should tip the scales in favor of the individual studies showing a lack of impairment.
These results must be interpreted with the caveat that it is logically impossible to prove a negative. Although we tried to be thorough and use multiple tests to approach the hypothesis from converging angles, it remains possible that these tasks would have been hippocampally dependent if tested under some yet-to-be-identified alternate parameters. However, it is also true that negative results mean more in some study designs than others. Others have argued that null results from lesion studies are as informative as positive results when there is a strong a priori hypothesis that a particular brain structure is necessary for a particular task (56). We believe that the current study represents such a case.
Much of the work in humans with hippocampal damage historically de-emphasized the contribution of nonhippocampal damage, even when such damage was documented. The classic case of H.M.’s memory has often been characterized as reflecting his hippocampal damage. However, postmortem analysis revealed substantially spared hippocampi (H.M.’s spared volume: left = 2.02 cm3, right = 1.96 cm3; age-matched intact hippocampus volume: 3.04 cm3), confirmed the substantial damage to other temporal lobe structures, and discovered focal frontal damage (8). It is not known whether H.M.’s spared hippocampal tissue was functional, and the dominant anatomical model suggests that it would be deafferented (10). Similarly, work with developmental amnesic patients initially identified only hippocampal shrinkage (6), but recent reexamination found that these patients also had damage to mammillary bodies and anterior thalamus, and the thalamus damage correlated better with memory impairment than did hippocampal damage (11). Such damage likely confounds evidence in most cases of human brain damage. This uncertainty calls for the type of work we described here.
In contrast to studies with humans, studies with rodents use selective lesions and achieve more controlled results. Extending findings from rodents to humans, while often highly informative, is not always straightforward because there are several major differences between how rodents and primates, including humans, are typically tested. First, some major rodent paradigms use odor stimuli (7, 49). In rodents, olfactory inputs are found along 100% of the entorhinal cortex, which, in turn, projects to the hippocampus, whereas in monkeys that density is closer to 15% and is likely even lower in humans (57). These species-typical olfactory projections to the hippocampus may cause differences in the role of the hippocampus in analogous tasks using different sensory dimension. Theoretically, using species-appropriate modalities “equates” tests across species, but whether modality differences explain species differences in outcomes after hippocampal damage is an open and empirical question. Notably, the literature on visual recognition in rodents, where odors are eliminated as cues to recognition, is mixed, with reports of no impairments following hippocampal damage (58) or significantly less impairment than after perirhinal damage (59). Second, given the undisputed role of the hippocampus in spatial navigation across taxa (17, 18, 60) and the immense importance of spatial information to rodent cognition, it is possible that the very presence of spatial information in many rodent tests that involve walking between locations, even if technically task-irrelevant, results in a spatially tagged memory trace that relies on the hippocampus. Monkeys and humans are rarely given tests that require locomoting through the environment to encounter test stimuli. Third, rodents are sometimes tested for just a few sessions after surgery. Here, we found an impairment on the temporal order task in the first postlesion session only. Thus, some discrepancies between monkeys and rats may be due to the duration of postlesion testing. But amnesia in humans is not transient; if it could be corrected with training or time, it would not be the debilitating condition that it is. We do not argue that there is strong evidence for species differences in hippocampal function or that good evidence from rodent studies should be discounted. Instead, we hope to draw attention to the fact that applying findings from rodents—or monkeys—to humans requires nuance and comes with a host of caveats that are too often left unstated. Because results from different species present different advantages and limitations, a robust theory of hippocampal function must rest on a confluence of evidence from multiple species.
It is unlikely that all the memory tests in this study failed to tap the target memory functions. Many of the tests we used were designed to be directly analogous to those used in human or rodent studies. For example, our temporal order test was designed after one that is affected by hippocampal lesions in rodents (7) and is notably similar to one affected by fornix transection in monkeys (44). Although some of these tasks can be accomplished via multiple cognitive mechanisms, and it is impossible to completely rule out the possibility that monkeys adopted alternative strategies, analyses of response patterns beyond mere accuracy revealed no evidence that monkeys in the surgical and control groups used different cognitive mechanisms. It is possible that our computerized test battery preferentially engaged prefrontal working memory rather than memories traditionally thought to depend on the hippocampus. However, in several tests, we attenuated working memory with a concurrent cognitive load or a long delay interval and monkeys with hippocampal lesions were still unimpaired. It is also unlikely, but possible, that these tasks are normally hippocampally dependent but that all monkeys in both groups shifted to performing them in a hippocampally independent way during testing. Except for the transient effect in the temporal order test, in which the SDE was modestly flattened in the first postoperative session but still significant, we did not find changes that would indicate that strategy had changed over time (e.g., in symbolic distance, false alarm patterns, and susceptibility to intervening tasks). Many of the baseline effects, such as symbolic distance or false alarm curves, were the same as in the previous behavioral reports for which data were collected a year or more before this study (25, 26, 32), suggesting that the monkeys used stable strategies. If selective hippocampal damage caused major relational or visual memory deficits, our test battery should have detected it. One of the key conclusions from the lack of impairments on these varied tests is that we may not yet fully understand the essential operating characteristics of episodic memory that depend on the primate hippocampus.
It is unlikely that our monkeys showed intact performance because they actually had fully functional hippocampi. All monkeys showed substantial hippocampal shrinkage in both hemispheres (Fig. 1), and we conducted additional follow-up surgeries to augment incomplete damage. The damage produced here compared favorably in extent to the damage reported in previous studies of excitotoxic hippocampal lesions with monkeys (fig. S1). The amount of hippocampal damage we obtained overlaps almost entirely with the amount of damage that caused severe spatial memory impairments in a previous study using the same lesion methods and carried out by one of the same authors (17). It is also comparable or superior to the range of damage seen in human patients with memory loss (6) and the amount of damage necessary to produce significant memory impairments in rodents (49). Last, the behavior of these specific monkeys is affected by their lesions in other ways. The lesion group shows blunted habituation to videos, compared to the control group (61), sometimes considered a marker of incidental memory. Also, the lesion group shows reduced response latencies compared to the control group, which is a common hallmark of hippocampal damage (62). Thus, these are effective lesions. If performance on these tests depended on a fully functional hippocampus, these monkeys would have been impaired.
It is possible but unlikely that the spared performance was due to brain plasticity. Neurotoxic lesions in monkeys do cause neuron differentiation in connected structures (63). However, this plasticity is not normally sufficient to counteract the behavioral effects of major brain damage. For example, monkeys with selective brain damage still show marked behavioral impairments, altered morphology, and disrupted functional connectivity years later (64–66). If brain plasticity were so effective in ameliorating cognitive deficits, human cases of brain damage would not result in lifelong memory and relational deficits.
The current evidence does not invalidate the substantial existing literature implicating the hippocampus in some types of memory, notably allocentric spatial memory. However, these findings must now constrain future theorizing and future work. They caution against over-generalizing from human correlational studies or rodent experimental studies. Last, they compel a new generation of nonhuman primate studies. It is possible that we need to reassess the relative contributions of the hippocampus proper compared to other regions in visual memory and relational cognition.
MATERIALS AND METHODS
General methods
Subjects
We tested 10 adult male rhesus monkeys (mean age at surgery: 10.37 years) in their home cages. When possible, monkeys were pair-housed. Pair-housed monkeys were separated during testing by a protected-contact divider that allowed them limited visual, auditory, and tactile access to their partner but not their partner’s computer screen. Monkeys received full food rations after each day’s testing, and water was available ad lib. All monkeys had previous experience with touch screen–based cognitive tasks including all tasks mentioned in Table 1. One monkey, Mi, died midway through these experiments from unrelated causes, and thus, some follow-up tests contain four monkeys in the experimental group, as noted. All testing complied with U.S. law and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Apparatus
We tested subjects 6 days a week using portable testing rigs equipped with a 15-inch color LCD touch screen (3M, St. Paul, MN; and ELO, Milpitas, CA) running at a resolution of 1024 pixels × 768 pixels, stereo speakers, and two automatic food dispensers (Med Associates Inc., St. Albans, VT), which dispensed nutritionally complete food pellets into cups below the screen. Testing equipment was available to the monkeys approximately 7 hours a day.
Surgery
Procedures for creating selective excitotoxic lesions of the hippocampus have been described in detail elsewhere (67). We obtained a T1-weighted MR scan for each individual and plotted a single injection path along the length of the hippocampus in each hemisphere. Injection sites were tailored to each individual hemisphere and comprised eight or nine sites separated by ~2 mm. Before surgery, monkeys were anesthetized using a mixture of dexmedetomidine (0.02 mg/kg, intramuscularly) and low-dose ketamine (5 mg/kg, intramuscularly) and maintained on isoflurane gas (1 to 4% to effect). Once under gas anesthesia, dexmedetomidine was reversed with atipamezole (0.02 mg/kg, intravenously). Blood pressure, respiratory rate, heart rate, temperature, blood oxygen saturation, and exhaled/inhaled CO2 were monitored throughout surgery. Using aseptic procedures, we opened a small incision (~2 to 3 cm) in anatomical layers on the top of the skull and drilled a small hole to visualize the central sinus for purposes of adjusting medial-lateral injection coordinates. We then opened a second incision on the back of the skull, just superior to the occipital ridge, retracted the temporalis muscles, and drilled two small entry holes in line with our two injection paths. We inserted both needles to the most anterior injection sites in the hippocampus, injected 2 μl of N-methyl-d-aspartate (NMDA) (62.5 mg/ml; 0.42 M) at .25 μl/min, waited 3 min for the excitotoxin to diffuse, and retracted the needles to the next site. This process was repeated until the needle tip reached the most posterior site, and then we waited five additional minutes before withdrawing the needle from the brain. For most monkeys, we paused midway through the injection series to allow tachycardia to subside. We then closed all incisions in anatomical layers. Postsurgical seizures were managed with diazepam (0.5 mg/kg) as needed. Pain and infection risk were managed with a combination of flunixin (1 mg/kg), buprenorphine (0.03 mg/kg), and ceftriaxone (25 mg/kg) as directed by veterinary staff.
Lesion assessment
Hippocampal lesions can be accurately predicted in vivo via MRI (68). Six or seven days after surgery, each monkey received a T2-weighted MR scan to visualize the edema that indicates cell death. For monkeys in which this T2 scan indicated that less than half of the hippocampus was affected, we proceeded with a second surgery targeting the remaining tissue. Second surgeries followed a recovery period of at least 2 weeks, before completing a second T1-weighted structural scan to develop a new set of coordinates.
We assessed final damage by comparing the volume of the hippocampus pre- and postsurgery, as described previously (17, 62, 67, 68). Each monkey received a T1-weighted MR scan ~150 days after their final surgery (range = 119 to 231 days). Volume was calculated by tracing the hippocampus in ImageJ on successive coronal MR images and then summing those areas across all images. For tracing, we included all subfields of the hippocampus as well as the subiculum. Each hemisphere was traced four times per tracing session to give a mean volume, each session was repeated three times during different sittings and without reference to the previous tracing sessions, and the final measure used in analyses was the mean of these three sessions to provide an average that corrected for individual hand jitter in an individual tracing. Percent estimated damage was then calculated via the regression function based on data comparing MRI to histology (68) and as used previously (62, 67).
Lesions were largely as intended and included most of the hippocampus bilaterally (Fig. 1 and table S1). The resulting estimated damage compared favorably to previous studies using excitotoxic lesions in monkey hippocampus and to the hippocampal shrinkage seen in human cases of developmental amnesia [fig. S1; tables 1 to 3 of (67)]. As a group, spared tissue occurred bilaterally in the most anterior sections, and unilaterally in the most posterior portion of the left hemisphere. We observed minor amounts of unintended damage in the surrounding tissue, but this was usually unilateral.
General procedures
For prelesion measures, monkeys engaged in all tasks in a set order over the course of 2 to 3 days. If monkeys did not meet a priori accuracy criteria for every test, the task progression was repeated. Once monkeys were performing all tasks accurately, we pseudo-randomly divided them into two groups such that each group had similar overall performance as measured by a composite accuracy score derived from accuracy across all tasks. Five monkeys received selective excitotoxic lesions of the hippocampus, and five were retained as unoperated controls. The lesion group rested for a minimum of 2 weeks after the final surgery, and the control group rested for a yoked period such that each control monkey rested for the same number of days as one of the surgical monkeys.
For postlesion measures, monkeys repeated the same task progression as during the prelesion testing. This task progression was repeated five total times over the course of approximately 18 testing days to capture any potential transient effects of the hippocampal damage. Follow-up tests were then performed as warranted for individual paradigms.
Unless otherwise noted, all tasks shared the following features. Trials were self-initiated by the monkey touching a green start square (150 pixels × 150 pixels) located at the bottom center of the screen. Responses required two consecutive touches to prevent accidental responding. Correct responses were rewarded with a 95% chance of one grain-based food pellet and a positive audio reinforcer (“excellent!”) and 5% chance of one miniature chocolate candy and a positive audio reinforcer (“woo-hoo!”). Incorrect responses produced no reward, a negative audio cue (“doh!”), and an unfilled time-out.
General analyses
Performance was compared across time points (preoperation to postoperation) and between groups (experimental versus control) using ANOVAs or t tests with independent samples or repeated samples as appropriate. For our targeted a priori hypotheses (e.g., postoperative comparisons between groups), we did not correct for multiple comparisons unless noted. For bigger multiway analyses and exploratory analyses, we did correct for multiple comparisons. Specifically, although ANOVAs protect against multiple comparisons due to added levels within a factor, they do not protect against multiple comparisons due to added factors, and the possibility of the type I error for a three-way ANOVA is actually 30% and not 5% (69). To protect against this high type I error rate when using three-way ANOVAs, exploratory main effects and interactions for three-way ANOVAs were evaluated against a Bonferroni corrected α of 0.007 (P = 0.05/seven possible main effects and interactions). To better approximate normality, proportions were arcsine transformed before statistical analysis. All tests were two-tailed with α = 0.05. Unless otherwise stated, analyses were performed on the average of two prelesion sessions and the average of five postlesion sessions, with follow-up tests as appropriate and as stated in individual experiments.
Individual tasks
Transitive inference
On a given trial, two photographic color images (350 pixels × 350 pixels) appeared on the left and right sides of the screen. During training, trials presented one of six overlapping pairs of object discriminations (i.e., A + B-, B + C-, C + D-, D + E-, E + F-, F + G-). Twenty-five trials of each of the six adjacent training pairs were presented semi-randomly intermixed in 150-trial sessions until monkeys performed above 80% correct on all six pairs. Monkeys then moved on to testing, in which one iteration of each of the possible nonadjacent test pairs (i.e., AC, AD, AE, AF, AG, BD, BE, BF, BG, CE, CF, CG, DF, DG, EG) were presented intermixed with training pairs as nondifferentially reinforced probe trials for four 165 trial sessions. The location of the correct image on the screen was semi-randomly counterbalanced.
Monkeys were trained and tested on three unique seven-item TI sets: one that was trained and tested prelesion (prelesion set), one that was trained prelesion and tested postlesion (cross-lesion set), and one that was trained and tested postlesion (postlesion set). This design allowed us to determine the contributions of the hippocampus to performance on both learning new TI sets and tests of nonadjacent pairs. If the hippocampus is involved in learning new TI sets, monkeys should show decreased performance on the postlesion set compared to the prelesion and cross-lesion sets. However, if the hippocampus is involved specifically in the integration of pair information during testing, monkeys should show decreased performance on both the postlesion and cross-lesion sets compared to the prelesion set. Presurgical criterion was completion of training and testing in the prelesion set and 80% accuracy on the six adjacent training pairs in the cross-lesion set.
Temporal order memory
The 10 test types were randomly intermixed in 120-trial sessions such that 12 tests of each of the 10 trial types occurred per session. The study lists consisted of trial-unique images drawn from a large set of 6000 images with randomization without replacement. Monkeys were required to perform at or above 65% correct, averaged across all trial types, before surgeries were performed. Further details of the methods are described elsewhere (26).
Simultaneous chaining
Monkeys were trained to touch five images in a predefined order. On each trial, each image in the list was randomly assigned to one of 12 locations on the touch-screen, ensuring that order of the images rather than image location was the to-be-remembered information. Because the trial ended with negative auditory reinforcement and a time-out if images were selected out of order (e.g., A➔B➔D; B; A➔C), the probability of selecting all five images in the correct order was 0.078%. After each of the five-image lists was executed to at least 70% correct in 50-trial sessions, monkeys received two-image probe tests randomly intermixed with normal five-image lists. Sessions consisted of 120 trials: 40 trials were normal five-image lists and 80 trials were two-image probe tests, 40 trials of which were within-list tests consisting of 10 distinct trial types for each list (listed above by SD), and 40 of which were between-list tests, in which images were either from lists 1 and 2 or from lists 3 and 4 (e.g., A4 versus E3).
Monkeys were required to perform at or above 65% correct on all within-list probe tests and at 10% or above on each five-image list before receiving lesions. Accuracy on five-image lists was averaged across the four lists, and accuracy on between-list probe tests was also averaged trials from lists 1 and 2 and from lists 3 and 4. Accuracy on probe tests was based on the overall ordinal position of images (e.g., A4➔E3). Further details of the methods have been published (27).
Shape recall
After each trial was started, a 5 × 5 grid appeared on the screen with two or three contiguous boxes of the grid filled in to produce a shape. One box was blue, and the remaining boxes were red. The monkey made an observing response by touching the blue box once, which caused the shape to disappear and started the retention interval. After the retention interval, the blue box reappeared in a different location in the grid and the monkey had to reproduce the studied shape by touching appropriate grid locations, relative to the new location of the blue box, to turn them red. Trials were correct if the monkey successfully completed the studied shape in the new location and incorrect if the monkey touched a relative location that would produce an unstudied shape. The intertrial interval was 10s after all trials.
For two-block shapes, sessions were 144 trials, the retention interval was 1 s, and presurgical criterion was ≥20% correct. All eight adjacent boxes were available as response options (chance = 12.5%). For three-block shapes, sessions were 252 trials, retention intervals were 1 s, and presurgical criterion was ≥35% correct. Four of the adjacent boxes were available for reproducing the first two blocks, and two of the adjacent boxes were available for reproducing the remaining block (chance for each block = 50%, chance for the full shape = 25%). For three-block shapes with a cognitive distractor during the retention interval, sessions were 504 trials, retention intervals were 4 s, and there was no presurgical criterion because this was a postsurgical follow-up test. The mid-retention interval distractor task was the perceptual classification task as described below, with the exception that the center stimulus and choice shapes all appeared simultaneously. To ensure that monkeys devoted cognitive resources to the distractor task, trials were aborted if the monkey did not correctly classify the central image. All eight adjacent boxes were available as response options at test (conditional probability of reproducing both boxes = 2/8 * 1/7 = 3.6%).
Item recognition
For the four alternative forced choice matching test (4AFC), after each trial was started, a color photograph (300 pixels × 300 pixels) appeared in the center of the screen. The monkey made an observing response by touching the image twice, which caused the sample to disappear and started the unfilled retention interval. Retention intervals were 4, 8, or 16 s and were pseudorandomly intermixed such that each block of six trials contained each retention interval twice. After the retention interval, four test images appeared in the four corners of the screen, one of which was the studied image and the other three were unstudied distractor images. The correct image appeared equally often in all test locations. A correct response was to touch the studied image, and an incorrect response was to touch any of the unstudied images. The intertrial interval was 10 s following correct trials and 12 s following incorrect trials. Images were drawn from either a set of 400 possible images in the large set condition or 4 possible images in the small set condition. Sessions were 120 trials long, and criterion was ≥70% correct at the shortest retention interval for each stimulus set size.
The yes/no recognition test was similar to the 4AFC test with the exception that the test screen now only presented one test item and a “no-memory” symbol in two of the four screen corners. The test image either matched the studied image or was an unstudied lure. A correct response was to touch the test image if it matched the studied image (a hit) or the no-memory symbol if the test image did not match the studied image (a correct rejection). An incorrect response was to touch the test image if it did not match the studied image (a false alarm) or touch the no-memory symbol if the test image did match the studied image (a miss). Match and nonmatch trials were pseudorandomly intermixed such that each block of four trials contained two of each type. Criterion was d′ ≥ 1.5 for each stimulus set size.
List memory was similar to the yes/no recognition test with the exception that five images were studied instead of one. The five studied images were presented sequentially, each requiring an observing response, and separated by 200 ms. The experienced retention interval varied depending on list position tested and individual monkey response speed and ranged from 1 s with no intervening items to an average of 9.2 s with four intervening items. On matching trials, each studied item was presented equally often as the test image. The image set contained six possible images, five of which were presented on every trial as the samples. Criterion was d′ ≥ 0.5 for each list position.
The analysis of false alarm rates as a function of natural response latency and in response to a response deadline was conducted as reported previously (32). Briefly, to provide the initial correlation of false alarms with response speed, monkeys completed 2000 trials of yes/no matching as described above. The retention interval was 4 s and the stimuli consisted of a small set of two perceptually similar items so as to maximize interference from item familiarity. To investigate the effect of a response deadline, monkeys first learned about the response deadline by completing an initial 1000 trials in which trials aborted if the monkey did not respond at test within 800 ms. Then, critical data came from ten 200-trial sessions that alternated between having the response deadline and having no response deadline.
To test for increased false alarms to recently seen lures, monkeys completed two 600-trial sessions of yes/no recognition, as described above. The stimuli were session-unique. Thus, on normal trials, the to-be-rejected lure had not been seen that week. On unpredictable probe trials, the to-be-rejected lure was the sample from the previous trial and thus should produce elevated false alarm rates to the degree that recognition is controlled by a vague sense of item familiarity. Sessions consisted of 100 normal trials as a warm-up and then 100 probe trials pseudorandomly intermixed with 400 normal trials. Because the effect of lure familiarity should be more pronounced when the studied sample was less well remembered, we repeated this at two different retention intervals: 4 and 20 s.
We substituted two novel, perceptually similar stimuli and titrated retention intervals individually in 200-trial sessions until accuracy was between 60 and 75%. We then repeated the methods for assessing false alarm rates as a function of response time and response deadline.
Last, we increased the retention interval for all monkeys to 40 s and required that they complete a distractor task during the retention interval. The task was the perceptual classification task as described below, with the exception that the center stimulus and choice shapes all appeared simultaneously. Again, we repeated the methods for assessing false alarm rates as a function of response time and response deadline.
Source memory
Trials ran as described previously reported (29). After each trial was started, subjects saw two consecutive color photographs (400 pixels × 300 pixels) as samples. The first was presented centrally and required the monkey to touch it as an observing response. After a 10-s unfilled retention interval, the second sample was presented centrally flanked by four symbols that corresponded to the four possible categories to which the sample could belong: birds, fish, flowers, or people. The monkey was required to categorize this second sample but not required to touch it. After an additional 200-ms delay, four images appeared at test in four different screen locations. Two images were the studied samples and two were unstudied distractors. The background color of the screen cued the monkeys whether to respond to the touched sample or the classified sample and was counterbalanced across monkeys. Three monkeys from each group learned this task before scheduled surgeries.
Perceptual classification
After each trial was started, a color photograph (300 pixels × 400 pixels) appeared in the center of the screen. The monkey made an observing response by touching the image twice, which produced four distinctive colored shapes on white backgrounds (200 pixels × 200 pixels) in the four corners of the screen. Monkeys knew from previous training that each shape corresponded to one of four categories: birds, fish, flowers, or people (33–35). The correct response was to touch the shape associated with the central image, and the incorrect response was to touch any other shape. Presurgical criterion was overall accuracy ≥ 70%. The intertrial interval was 3 s after correct trials and 5 s after incorrect trials. Sessions were 100 trials and stimuli were 100 images, seen once per session and equally split among categories.
Supplementary Material
Acknowledgments
We thank T. A. Dove-VanWormer, E. Brown, R. Diamond, and T. Hassett for help running subjects. Funding: This project was supported, in part, by ORIP/OD P51OD011132, the NSF (grants BCS-0745573, IOS-1146316, and BCS-1632477), the National Institute of Mental Health (NIMH) (grant R01M H082819), the Intramural Research Program of the NIMH (ZIAMH002887), and the Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the NIH (grant P20GM103430). Author contributions: All authors designed the study and wrote the paper. B.M.B., V.L.T., and R.P.G. conducted the study and analyzed the results. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/29/eaaz0484/DC1
REFERENCES AND NOTES
- 1.Templer V. L., Hampton R. R., Episodic memory in nonhuman animals. Curr. Biol. 23, R801–R806 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt K. R., Gardiner J. M., Vargha-Khadem F., Baddeley A. D., Mishkin M., Impairment of recollection but not familiarity in a case of developmental amnesia. Neurocase 15, 60–65 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark R. E., West A. N., Zola S. M., Squire L. R., Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus 11, 176–186 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Crystal J. D., Alford W. T., Zhou W., Hohmann A. G., Source memory in the rat. Curr. Biol. 23, 387–391 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scoville W. B., Milner B., Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargha-Khadem F., Gadian D. G., Watkins K. E., Connelly A., Van Paesschen W., Mishkin M., Differential effects of early hippocampal pathology on episodic and semantic memory. Science 277, 376–380 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Fortin N. J., Agster K. L., Eichenbaum H. B., Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 5, 458–462 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annese J., Schenker-Ahmed N. M., Bartsch H., Maechler P., Sheh C., Thomas N., Kayano J., Ghatan A., Bresler N., Frosch M. P., Klaming R., Corkin S., Postmortem examination of patient H.M.'s brain based on histological sectioning and digital 3D reconstruction. Nat. Commun. 5, 3122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corkin S., Amaral D. G., González R. G., Johnson K. A., Hyman B. T., H. M.'s medial temporal lobe lesion: Findings from magnetic resonance imaging. J. Neurosci. 17, 3964–3979 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustinack J. C., van der Kouwe A. J. W., Salat D. H., Benner T., Stevens A. A., Annese J., Fischl B., Frosch M. P., Corkin S., H.M.'s contributions to neuroscience: A review and autopsy studies. Hippocampus 24, 1267–1286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzieciol A. M., Bachevalier J., Saleem K. S., Gadian D. G., Saunders R., Chong W. K. K., Banks T., Mishkin M., Vargha-Khadem F., Hippocampal and diencephalic pathology in developmental amnesia. Cortex 86, 33–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Froudist-Walsh S., Browning P. G. F., Croxson P. L., Murphy K. L., Shamy J. L., Veuthey T. L., Wilson C. R. E., Baxter M. G., The rhesus monkey hippocampus critically contributes to scene memory retrieval, But not new learning. J. Neurosci. 38, 7800–7808 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaffan D., Amnesia for complex naturalistic scenes and for objects following fornix transection in the rhesus monkey. Eur. J. Neurosci. 4, 381–388 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Parker A., Gaffan D., Mamillary body lesions in monkeys impair object-in-place memory: Functional unity of the fornix-mamillary system. J. Cogn. Neurosci. 9, 512–521 (1997). [DOI] [PubMed] [Google Scholar]
- 15.Aggleton J. P., Understanding amnesia: Is it time to forget HM. Psychologist 26, 612–615 (2013). [Google Scholar]
- 16.Aggleton J. P., Pralus A., Nelson A. J. D., Hornberger M., Thalamic pathology and memory loss in early Alzheimer’s disease: Moving the focus from the medial temporal lobe to Papez circuit. Brain 139, 1877–1890 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hampton R. R., Hampstead B. M., Murray E. A., Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus 14, 808–818 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Lavenex P. B., Amaral D. G., Lavenex P., Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J. Neurosci. 26, 4546–4558 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray E. A., What have ablation studies told us about the neural substrates of stimulus memory? Semin. Neurosci. 8, 13–22 (1996). [Google Scholar]
- 20.Beason-Held L. L., Rosene D. L., Killiany R. J., Moss M. B., Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus 9, 562–574 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Zola S. M., Squire L. R., Teng E., Stefanacci L., Buffalo E. A., Clark R. E., Impaired recognition memory in monkeys after damage limited to the hippocampal region. J. Neurosci. 20, 451–463 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray E. A., Mishkin M., Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J. Neurosci. 18, 6568–6582 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heuer E., Bachevalier J., Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behav. Neurosci. 125, 137–149 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachevalier J., Wright A. A., Katz J. S., Serial position functions following selective hippocampal lesions in monkeys: Effects of delays and interference. Behav. Processes 93, 155–166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazes R. P., Chee N. W., Hampton R. R., Cognitive mechanisms for transitive inference performance in rhesus monkeys: Measuring the influence of associative strength and inferred order. J. Exp. Psychol. Anim. Behav. Process. 38, 331–345 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Templer V. L., Hampton R. R., Cognitive mechanisms of memory for order in rhesus monkeys (Macaca mulatta). Hippocampus 23, 193–201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Templer V. L., Gazes R. P., Hampton R. R., Co-operation of long-term and working memory for ordinal position in rhesus monkeys (Macaca mulatta). Q. J. Exp. Psychol. 72, 2208–2224 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basile B. M., Hampton R. R., Monkeys recall and reproduce simple shapes from memory. Curr. Biol. 21, 774–778 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basile B. M., Hampton R. R., Dissociation of item and source memory in rhesus monkeys. Cognition 166, 398–406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basile B. M., Hampton R. R., Rhesus monkeys (Macaca mulatta) show robust primacy and recency in memory for lists from small, but not large, image sets. Behav. Processes 83, 183–190 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basile B. M., Hampton R. R., Dissociation of active working memory and passive recognition in rhesus monkeys. Cognition 126, 391–396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basile B. M., Hampton R. R., Recognition errors suggest fast familiarity and slow recollection in rhesus monkeys. Learn. Mem. 20, 431–437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basile B. M., Hampton R. R., Monkeys show recognition without priming in a classification task. Behav. Processes 93, 50–61 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamond R. F. L., Stoinski T. S., Mickelberg J. L., Basile B. M., Gazes R. P., Templer V. L., Hampton R. R., Similar stimulus features control visual classification in orangutans and rhesus monkeys. J. Exp. Anal. Behav. 105, 100–110 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazes R. P., Brown E. K., Basile B. M., Hampton R. R., Automated cognitive testing of monkeys in social groups yields results comparable to individual laboratory-based testing. Anim. Cogn. 16, 445–458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazes R. P., Lazareva O. F., Bergene C. N., Hampton R. R., Effects of spatial training on transitive inference performance in humans and rhesus monkeys. J. Exp. Psychol. Anim. Learn. Cogn. 40, 477–489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazareva O. F., Kandray K., Acerbo M. J., Hippocampal lesion and transitive inference: Dissociation of inference-based and reinforcement-based strategies in pigeons. Hippocampus 25, 219–226 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Zalesak M., Heckers S., The role of the hippocampus in transitive inference. Psychiatry Res. 172, 24–30 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith C., Squire L. R., Declarative memory, awareness, and transitive inference. J. Neurosci. 25, 10138–10146 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dusek J. A., Eichenbaum H., The hippocampus and memory for orderly stimulus relations. Proc. Natl. Acad. Sci. U.S.A. 94, 7109–7114 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckmaster C. A., Eichenbaum H., Amaral D. G., Suzuki W. A., Rapp P. R., Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. J. Neurosci. 24, 9811–9825 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kesner R. P., Gilbert P. E., Barua L. A., The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav. Neurosci. 116, 286–290 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Lehn H., Steffenach H.-A., van Strien N. M., Veltman D. J., Witter M. P., Håberg A. K., A specific role of the human hippocampus in recall of temporal sequences. J. Neurosci. 29, 3475–3484 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charles D. P., Gaffan D., Buckley M. J., Impaired recency judgments and intact novelty judgments after fornix transection in monkeys. J. Neurosci. 24, 2037–2044 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Templer V. L., Brown E. K., Hampton R. R., Rhesus monkeys metacognitively monitor memories of the order of events. Sci. Rep. 8, 11541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terrace H. S., Son L. K., Brannon E. M., Serial expertise of rhesus macaques. Psychol. Sci. 14, 66–73 (2003). [DOI] [PubMed] [Google Scholar]
- 47.D'Amato M. R., Columbo M., Representation of serial order in monkeys (Cebus apella). J. Exp. Psychol. Anim. Behav. Process. 14, 131–139 (1988). [PubMed] [Google Scholar]
- 48.Manns J. R., Squire L. R., Impaired recognition memory on the doors and people test after damage limited to the hippocampal region. Hippocampus 9, 495–499 (1999). [DOI] [PubMed] [Google Scholar]
- 49.Fortin N. J., Wright S. P., Eichenbaum H., Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature 431, 188–191 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yonelinas A. P., The nature of recollection and familiarity: A review of 30 years of research. J. Mem. Lang. 46, 441–517 (2002). [Google Scholar]
- 51.Davachi L., Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 16, 693–700 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Yu S. S., Johnson J. D., Rugg M. D., Hippocampal activity during recognition memory co-varies with the accuracy and confidence of source memory judgments. Hippocampus 22, 1429–1437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shrager Y., Gold J. J., Hopkins R. O., Squire L. R., Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J. Neurosci. 26, 2235–2240 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarado M. C., Bachevalier J., Selective neurotoxic damage to the hippocampal formation impairs performance of the transverse patterning and location memory tasks in rhesus macaques. Hippocampus 15, 118–131 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Saksida L. M., Bussey T. J., Buckmaster C. A., Murray E. A., Impairment and facilitation of transverse patterning after lesions of the perirhinal cortex and hippocampus, respectively. Cereb. Cortex 17, 108–115 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Vaidya A. R., Pujara M. S., Petrides M., Murray E. A., Fellows L. K., Lesion studies in contemporary neuroscience. Trends Cogn. Sci. 23, 653–671 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Insausti R., Marcos P., Arroyo-Jiménez M. M., Blaizot X., Martínez-Marcos A., Comparative aspects of the olfactory portion of the entorhinal cortex and its projection to the hippocampus in rodents, nonhuman primates, and the human brain. Brain Res. Bull. 57, 557–560 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Rothblat L. A., Kromer L. F., Object recognition memory in the rat: The role of the hippocampus. Behav. Brain Res. 42, 25–32 (1991). [DOI] [PubMed] [Google Scholar]
- 59.Prusky G. T., Douglas R. M., Nelson L., Shabanpoor A., Sutherland R. J., Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proc. Natl. Acad. Sci. U.S.A. 101, 5064–5068 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherry D. F., Jacobs L. F., Gaulin S. J. C., Spatial memory and adaptive specialization of the hippocampus. Trends Neurosci. 15, 298–303 (1992). [DOI] [PubMed] [Google Scholar]
- 61.Brady R. J., Basile B. M., Hampton R. R., Hippocampal damage attenuates habituation to videos in monkeys. Hippocampus 29, 1121–1126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basile B. M., Hampton R. R., Nonnavigational spatial memory performance is unaffected by hippocampal damage in monkeys. Hippocampus 29, 93–101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chareyron L. J., Amaral D. G., Lavenex P., Selective lesion of the hippocampus increases the differentiation of immature neurons in the monkey amygdala. Proc. Natl. Acad. Sci. U.S.A. 113, 14420–14425 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng Y., Hu X., Bachevalier J., Zhang X., Decreased functional connectivity in dorsolateral prefrontal cortical networks in adult macaques with neonatal hippocampal lesions: Relations to visual working memory deficits. Neurobiol. Learn. Mem. 134, 31–37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.E. Bliss-Moreau, G. Moadab, D. G. Amaral, Lifetime consequences of early amygdala damage in rhesus monkeys, in Living Without an Amygdala, D. G. Amaral, R. Adolphs, Eds. (The Guilford Press, 2016), chap. 6, pp. 149–185. [Google Scholar]
- 66.J. Bachevalier, M. Sanchez, J. Raper, S. B. Z. Stephens, K. Wallen, The effects of neonatal amygdala lesions in rhesus monkeys living in a species-typical social environment, in Living Without an Amygdala, D. G. Amaral, R. Adolphs, Eds. (The Guilford Press, 2016), chap. 7, pp. 186–217. [Google Scholar]
- 67.Hampton R. R., Buckmaster C. A., Anuszkiewicz-Lundgren D., Murray E. A., Method for making selective lesions of the hippocampus in macaque monkeys using NMDA and a longitudinal surgical approach. Hippocampus 14, 9–18 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Málková L. E., Lex C. K., Mishkin M., Saunders R. C., MRI-based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus 11, 361–370 (2001). [DOI] [PubMed] [Google Scholar]
- 69.Cramer A. O. J., van Ravenzwaaij D., Matzke D., Steingroever H., Wetzels R., Grasman R. P. P. P., Waldorp L. J., Wagenmakers E.-J., Hidden multiplicity in exploratory multiway ANOVA: Prevalence and remedies. Psychon. Bull. Rev. 23, 640–647 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlesimo G. A., Marfia G. A., Loasses A., Caltagirone C., Recency effect in anterograde amnesia: Evidence for distinct memory stores underlying enhanced retrieval of terminal items in immediate and delayed recall paradigms. Neuropsychologia 34, 177–184 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/29/eaaz0484/DC1


