Abstract
Background
Exosomes are extracellular vesicles containing a variety of biological molecules including microRNAs (miRNAs). We have recently demonstrated that certain miRNA species are selectively and highly enriched in pancreatic cancer exosomes with miR-1246 being the most abundant. Exosome miRNAs have been shown to mediate intercellular communication in the tumor microenvironment and promote cancer progression. Therefore, understanding how exosomes selectively enrich specific miRNAs to initiate exosome miRNA signaling in cancer cells is critical to advancing cancer exosome biology.
Results
The aim of this study was to identify RNA binding proteins responsible for selective enrichment of exosome miRNAs in cancer cells. A biotin-labeled miR-1246 probe was used to capture RNA binding proteins (RBPs) from PANC-1 cells. Among the RBPs identified through proteomic analysis, SRSF1, EIF3B and TIA1 were highly associated with the miR-1246 probe. RNA immunoprecipitation (RIP) and electrophoretic mobility shift assay (EMSA) confirmed the binding of SRSF1 to miR-1246. Lentivirus shRNA knockdown of SRSF1 in pancreatic cancer cells selectively reduced exosome miRNA enrichment whereas GFP-SRSF1 overexpression enhanced the enrichment as analyzed by next generation small RNA sequencing and qRT-PCR. miRNA sequence motif analysis identified a common motif shared by 36/45 of SRSF1-associated exosome miRNAs. EMSA confirmed that shared motif decoys inhibit the binding of SRSF1 to the miR-1246 sequence.
Conclusions
We conclude that SRSF1 mediates selective exosome miRNA enrichment in pancreatic cancer cells by binding to a commonly shared miRNA sequence motif.
Video Abstract
Graphical abstract
Keywords: SRSF1, Exosome, miRNA, miR-1246, Pancreatic cancer
Background
Exosomes are endosome-derived extracellular vesicles (EVs) [1] that can be transferred from cancer cells to stromal cells in the tumor microenvironment [2, 3]. These membrane vesicles are 40–120 nm in size, and contain proteins, lipids and nucleic acids, including small RNAs such as microRNAs (miRNAs) [1, 4]. Exosome-mediated intercellular communication between cancer cells, endothelial cells [5, 6], fibroblasts [7, 8], or immune cells [9, 10] can facilitate tumor progression. Furthermore, cancer exosomes are released into the circulation and contribute to pre-metastatic niche formation in distant organs [11, 12].
How cancer exosomes interact with stromal cells to promote tumor progression has been extensively investigated. One critical signaling event in the tumor microenvironment is the exosome miRNA-mediated intercellular communication [1, 13–15]. Studies have shown that exosome miRNA signaling promotes tumor progression in various model systems [16, 17]. Notably, it has been reported that miRNAs contained in exosomes are delivered to recipient cells in the tumor microenvironment or distant organs where they can regulate target gene expression and promote tumor angiogenesis and metastasis [13, 14, 18].
In the context of exosome miRNA signaling, we and others have reported that certain miRNA species are selectively enriched in cancer exosomes as compared to exosomes derived from normal epithelial cells [13, 19–21]. Results from several studies have also indicated that selective enrichment of exosome miRNAs is relevant to tumor progression [22]. For example, exosome sorting of miR-193a was found to promote colon cancer progression [23]. Likewise, miR-122, a cancer exosome enriched miRNA [19, 24], was shown to reprogram glucose metabolism in a pre-metastatic niche to facilitate metastasis in a breast cancer model system [25]. Moreover, the exosome enriched miR-1246 [26] was reported to promote tumor invasion in both breast cancer [27] and oral squamous cell carcinoma [28]. It seems clear that selective enrichment of exosome miRNAs drives cancer exosome miRNA signaling in the tumor microenvironment, which in turn reinforces tumor invasiveness and progression. However, how exosome miRNAs are enriched or how exosome miRNA signaling is initiated in cancer cells remains largely unknown. Elucidating the mechanisms of selective exosome miRNA enrichment in cancer cells may help identify new cancer therapeutic opportunities that are urgently needed.
Recent reports have indicated that certain RNA binding proteins (RBPs) are involved in exosome miRNA sorting in eukaryotic cells, and the type of RBPs involved seems to differ among various model systems [23, 29, 30], suggesting that exosome miRNA sorting is a tissue or cell type specific process. Furthermore, there have been no reports on the identification of RBPs that regulate exosome miRNA sorting in pancreatic cancer cells. We have recently characterized the biogenesis of exosome miR-1246 [26], which is the most highly enriched miRNA in pancreatic cancer cell-derived exosomes [21]. The aim of this study was to utilize our established cell model systems to identify RBPs that are involved in exosome miRNA loading in pancreatic cancer cells. Using a labeled miR-1246 probe as “bait”, we fished out several RBPs from pancreatic cancer cells, including serine and arginine rich splicing factor 1 (SRSF1), eukaryotic translation initiation factor 3 subunit B (eIF3B), and T cell-restricted intracellular antigen 1 (TIA1). We found that SRSF1, a recently claimed oncoprotein [31], is predominantly involved in regulating exosome miRNA enrichment in pancreatic cancer model systems.
Methods
Cell culture
The human pancreatic cancer cell lines PANC-1, MIAPaCa-2 and BxPC-3, and breast cancer cell line MDA-MB-231 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured following ATCC’s instructions except that exosome-depleted fetal bovine serum (FBS) and horse serum were applied whenever needed. Exosome-depleted FBS and horse serum were prepared by pelleting the serum exosomes at 200,000×g for 2 h at 4 °C. Cells were routinely incubated in a humidified environment at 37 °C and 5% CO2.
Exosome isolation
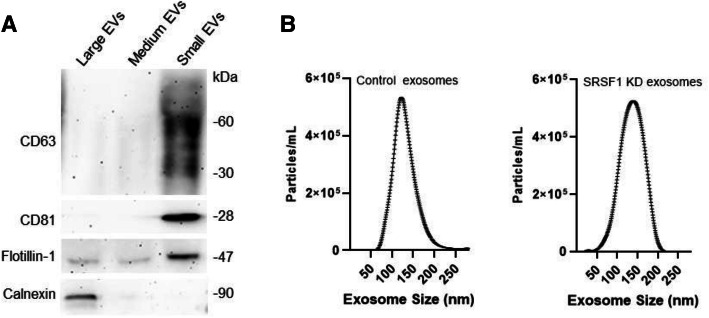
Exosomes were isolated from the culture medium utilizing a combination of centrifugation, ultracentrifugation, and filtration as we recently described [13, 19, 26], with minor modifications. In brief, the culture medium of PANC-1 cells was pre-cleared by 10,000 g centrifugation for 30 min at 4 °C, and the resulting supernatant was filtered through a 0.22 μm PVDF centrifuge filter. The large size EVs were trapped in the filter and recovered in PBS. The filtered supernatant was then applied to a 0.1 μm PVDF centrifuge filter. The medium size EVs were trapped in the second filter and re-suspended in PBS. The small size EVs (exosomes) in the final supernatant were recovered by ultracentrifugation (100,000 g, 70 min at 4 °C). The isolated exosomes were verified by western blot detecting positive and negative exosome marker proteins and nanoparticle analysis (Nanosight NS300 System, Malvern Instruments, UK) measuring both sizes and concentrations of the isolated exosomes (Fig. 1).
Fig. 1.
Verification of the exosomes derived from PANC-1 cells. a Representative western blot analysis of CD63 (non-reducing condition), CD81, flotillin, and calnexin in the EVs isolated from PANC-1 cells. Positive exosome markers are only detected in small EVs (exosomes). b Representative nanoparticle tracking analysis of exosomes (small EVs) derived from control and SRSF1 knockdown PANC-1 cells. Three individual experiments were performed for both a and b
miRNA binding protein pull-down
Pull-down experiment was performed using the Pierce™ Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific). Briefly, 100 pmol of biotin-labeled miR-1246 or poly-A RNA oligonucleotides (Integrated DNA Technologies) were hybridized to 100 μl streptavidin magnetic beads (Prod#1862766, Thermo Fisher Scientific). The miR-1246-biotin-streptavidin beads were incubated with PANC-1 lysate for 60 min at 4 °C. The lysate-bead mixture was washed three times with washing buffer from the above-mentioned kit. To elute bound proteins, 50 μl of elution buffer was applied, and a magnetic separator was applied to separate the beads from the eluted protein, following the manufacturer’s protocol (Pierce™ Magnetic RNA-Protein Pull-Down Kit, Thermo Fisher scientific). Proteins were separated by SDS-PAGE before mass spectrometry (MS) analysis.
Liquid chromatography–mass spectrometry (LC-MS)/mass spectrometry (MS) measurement
The experiment was performed by the Laboratory for Molecular Biology and Cytometry Research Core Facility at OUHSC. Proteins were digested with trypsin according to the FASP [32] protocol. Briefly the eluate was buffer exchanged in 8 M urea, the proteins were reduced with 10 mM dithiothreitol and then alkylated with 10 mM iodoacetamide. The peptides were eluted, dried and resuspended. Liquid chromatography tandem mass spectrometry was performed by coupling a nanaoAcquity UPLC (Waters Corp., Manchester, UK) to a Q-TOF SYNAPT G2S instrument (Waters Corp., Manchester, UK). Each protein digest (about 100 ng of peptide) was delivered to a trap column (300 μm × 50 mm nanoAcquity UPLC NanoEase Column 5 μm BEH C18, Waters Corp, Manchester, UK) at a flow rate of 2 μl/min in 99.9% solvent A (10 mM ammonium formate pH 10, in HPLC grade water). Tandem mass spectra were generated in the trapping region of the ion mobility cell by using a collisional energy ramp from 20 V (low mass, start/end) to 35 V (high mass, start/end). The pusher/ion mobility synchronization for the HDMSe method was performed using MassLynx V4.1 and DriftScope v2.4. LockSpray of Glufibrinopeptide-B (m/z 785.8427) was acquired every 60 s and lock mass correction was applied post acquisition.
Protein identification
Raw MS data were processed by PLGS (ProteinLynx Global Server, Waters Corp., Manchester, UK) for peptide and protein identification. MS/MS spectra were searched against the Uniprot Human database (containing 20,417 reviewed sequences) with the following search parameters: full tryptic specificity, up to two missed cleavage sites; carbamidomethylation of cysteine residues was set as a fixed modification; and N-terminal protein acetylation and methionine oxidation were set as variable modifications.
Small RNA library preparation and next generation sequencing
Total RNA was extracted from cell and exosome pellets using the TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, California). The small RNA libraries were constructed and run on the Illumina MiSeq platform as we recently described [21, 26].
RNA immunoprecipitation assay
PANC-1 cells or MDA-MB-231 cell lysates were prepared using IP buffer (10 mM Tris-HCl, pH 7.4, 50 mM NaCl, 0.5 mM EDTA, 1 mM PMSF, and 1% Triton X-100). The lysate was sonicated for 1 min on ice, and insoluble material was removed by centrifugation. Supernatants were collected, and protein concentrations were measured. The supernatant was pre-cleared by Protein G Dynabeads™(Thermo Fisher Scientific), and then mixed with antibody: SRSF1 (Santa Cruz, sc-33,652), EIF3B (Santa Cruz, sc-137,214), TIA1 (Santa Cruz, sc-166,247), GAPDH (ProMab, 20,035), and IgG (Santa Cruz sc-2025) in a ratio of 1:100 at 4 °C overnight with gentle rotation. To capture the antibody-protein-RNA complexes, 50 μl of protein G magnetic beads were added, and the complexes were rotated for 2 h at 4 °C. The sample was separated by magnetic separation. Trizol reagent (Invitrogen/Life Technologies) was applied to isolated RNA from the complex. The miRNA expression was analyzed by qRT-PCR.
Co-immunoprecipitation (co-IP)
Co-immunoprecipitation (co-IP) using PANC-1 cell lysate and antibody of SRSF1 (Santa Cruz, sc-33,652), EIF3B (Santa Cruz, sc-137,214), TIA1 (Santa Cruz, sc-166,247), GAPDH (ProMab, 20,035), and IgG (Santa Cruz sc-2025) was performed as described previously [33], and the protein complex was detected by western blot.
Western blot analysis
Western blot was performed as we recently described [21, 26]. Primary antibodies raised against SRSF1 (Santa Cruz, sc-33,652), EIF3B (Santa Cruz, sc-137,214), TIA1 (Santa Cruz, sc-166,247), beta-actin (A5441), and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Santa Cruz, sc-47,724) were used for detection. Nuclear and cytoplasmic protein extraction was extracted following ROCKLAND Nuclear & Cytoplasmic Extract Protocol [34], and verified by Histone-H3 (CST, 4499S) and GAPDH (Santa Cruz, sc-47,724) detection. Antibodies used for exosome marker detection include: CD63, CD81 (Santa Cruz Bio Technology Inc., CA, USA), Flotillin-1 and Calnexin (Cell Signaling Technology, Inc., MA, USA).
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was performed as we described [21, 26] with specific primers: Cel-54 (5′-GCGCGCCCGTAATCTTCATAATCC-3′), miR-1246 (5′-GCGCGATGGATTTTTGGAGCAG-3′), miR-320c (5′-GCAAAAGCUGGGUUGAGAGGGU-3′), and miR-320d (5′-GCGAAAAGCUGGGUUGAGAGGA-3′).
SRSF1 shRNA expression plasmid construction
Target specific oligonucleotides were designed using online tool RNAi Codex (Cold Spring Harbor Laboratory), and were synthesized (Integrated DNA Technologies) with the addition of overhangs according to the cutting site of BamH1 and EcoRI. The shRNA expression plasmid was constructed by annealing the oligonucleotides to pSIH-H1 vector following the user manual of pSIH-H1 shRNA system (SBI system Bioscience). The oligonucleotide sequences for shRNA of SRSF1, EIF3B or TIA1 are provided in Supplemental Table 1.
Lentivirus transduction
Lentiviral particles were produced as previously described [35] using the shRNA expression plasmid and the 3rd generation packaging plasmids pMD2.G (Addgene plasmid #12259), pMDL/RREg/p (Addgene plasmid #12251), and pRSV-Rev (Addgene plasmid #12253). The packaging plasmids were co-transfected with the lentiviral expression vector into 293 T cells using the polyethyleneimine (Polysciences Inc.) to produce replication deficient lentivirus. After transfection, the supernatant was pooled and filtered with a 0.45 μm membrane and concentrated by ultracentrifugation to acquire lentivirus. Infection was performed by using lentivirus in the presence of 8 μg/ml polybrene (Sigma-Aldrich). Approximately 48 h post-infection cells were selected by treating with 10 μg/ml puromycin (InvivoGen, San Diego, CA).
GFP-SRSF1 expression
The GFP-SRSF1 expression plasmid was a gift from Dr. Massimo Caputi [36]. DNA transfection was performed using Lipofectamine 3000 (Thermo Fisher Scientific) to PANC-1 cells and the expression of GFP-SRSF1 was verified by western blot.
GST-SRSF1 protein purification
BL21 (Thermo-Fisher Scientific, C600003) competent cells transformed with pGEX6P-SRSF1 DNA (Addgene plasmid # 99020, [37] were cultured at 37 °C for 3.5 h, and after OD600 reached to 0.6–0.8, bacteria were treated with 0.1 mM isopropyl β-D-1-thiogalactopyranoside for 24 h at 16 °C. GST-tagged-SRSF1 was purified with Glutathione Sepharose beads (GE Health Care). The purity of the recombinant proteins was determined by SDS–PAGE with Coomassie blue staining.
Electrophoretic mobility shift assay (EMSA)
IRD-800 labeled miR-1246 (0.1 μM) (Integrated DNA Technologies) was mixed with 4 μl of GST slurry, or GST-SRSF1 in binding buffer (Tris pH 8.0 40 mM, KCL 30 mM, MgCL2 1 mM, NP40 0.001%, DTT 1 mM, glycerol 5%) and incubated at room temperature for 40 min avoiding light. 5X loading buffer (KCL 60 mM, Tris PH 7.6 10 mM, glycerol 10%, xylene cyanol 0.01%, bromophenol blue 0.01%) was then added, and the complex was separated on a 4% native gel (40% polyacrylamide, 1 M Tris pH 7.5, 1 M glycine, 0.5 M EDTA, 10% APS, TEMED) at 120 voltage for 40 min. The signal was detected using the Li-Cor Odyssey 9120 Imaging system (LI-COR. Inc., USA).
Design of decoy motif mimics
The decoy motif mimics were designed by permutation and combination of the identified motif sequences in the length of 24 nucleotides. The secondary structure of the designed sequences was analyzed in RNAfold WebServer (University of Vienna). Sequences without self-complementary were selected (Decoy mimics 1: 5′-UUGGACUAGGACUAGGAU-3′, Decoy mimics 2: 5′-AGGAAGGAAGGAAGGA-3′).
Bioinformatics analysis
The miRNA motif analysis was performed using MEME Suite [38]. The protein profile analysis for the result of mass spectrometry was performed using DAVID Bioinformatics (ABCC at SAIC-Frederick, Inc). The RNA binding protein and miRNA sequence binding analysis was performed using the database of RNA-binding specificities (RBPDB) [39]. SRSF1 expression in cancer tissues was examined using ONCOMINE [40]. The correlation of gene expression with cancer patient survival was extracted from The Human Protein Atlas (SciLifeLab, Sweden) [41].
Statistics
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc. La Jolla, CA, USA). The heatmap was made in RStudio (RStudio, Inc) with the ggplot2 package [42]. Student’s t-test was applied to determine significant differences among control and experimental groups.
Results
Identification of miR-1246 associated proteins
Because RBPs are involved in exosome miRNA sorting, we first sought to identify proteins that bind to miRNAs highly enriched in cancer exosomes. miR-1246, the most highly enriched miRNA in pancreatic cancer exosomes, was biotin-labeled and incubated with a cellular lysate from PANC-1 cells. The biotin-miR-1246 probe was captured with streptavidin-coated magnetic beads. Biotin labeled poly-A mimics were used as control. The miRNA-protein complexes were eluted and the proteins were analyzed by liquid chromatography/mass spectrometry in triplicate (Table 1). There were total of 593 proteins specifically pulled down by the miR-1246 probe. Interestingly, about half of the proteins that associate with miR-1246 are vesicle-associated proteins (Supplement Fig. 1A). Based on the intensity of detection, RNA binding property, and cancer relevance, we ranked the RBPs using “The Database for Annotation, Visualization and Integrated Discovery (DAVID)”. This resulted in ten candidate RBPs that complex with the miR-1246 sequence and are relevant to eukaryotic exosomes (Table 2). Among them, SRSF1 (also called SFRS1) was predicted to bind to the miR-1246 sequence (Supplement Fig. 2) by in silico analysis using the database of RNA-binding specificities (RBPDB) [39].
Table 1.
Over view of the result of mass spectrometry
| Experiments’ condition | Number of proteins detected |
|---|---|
| Poly A + PANC-1 | 575 |
| Poly A + MDA-MB-231 | 609 |
| miR-1246 + PANC-1 | 793 |
| miR-1246 + MDA-MB-231 | 582 |
Table 2.
miR-1246 RNA binding protein candidates obtained from the mass spectrometric analysis
| Protein symbol | Protein full name |
|---|---|
| SRSF1 | Serine/arginine-rich splicing factor 1 |
| PARK7 | Parkinson disease protein 7 |
| EIF3B | Eukaryotic translation initiation factor 3 subunit B |
| THOC4 | THO complex subunit 4 (Aly/REF export factor) |
| ACOC | Cytoplasmic aconitate hydratase |
| DDX5 | Probable ATP-dependent RNA helicase DDX5 |
| TIA1 | T-cell-restricted intracellular antigen-1 |
| IF5A1 | Eukaryotic translation initiation factor 5A-1 |
| EIF2A | Eukaryotic translation initiation factor 2A |
| IMDH2 | Inosine-5′-monophosphate dehydrogenase 2 |
Verification of SRSF1 binding to miR-1246
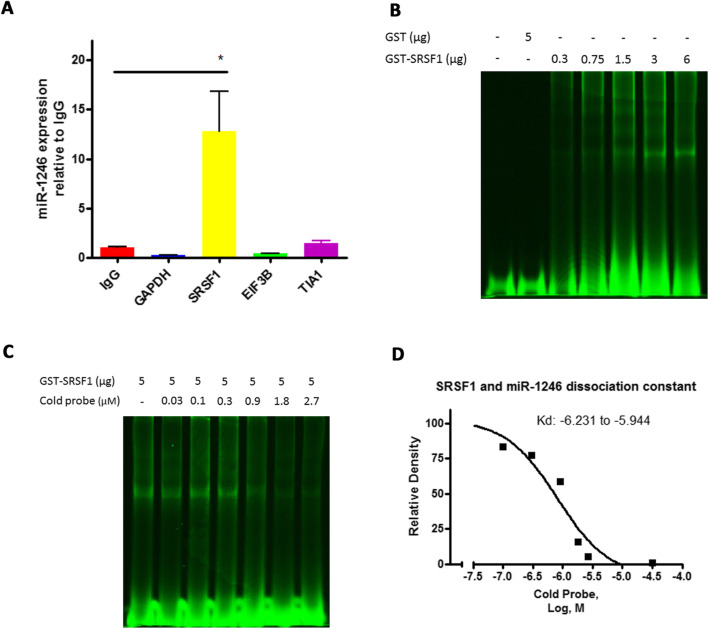
RNA immunoprecipitation (RIP) was performed to verify the association of several identified RBPs with miR-1246, including SRSF1, EIF3B and TIA1. IgG and GAPDH antibody was used as controls for immunoprecipitation. As shown in Fig. 2a, miR-1246 expression is more than 12-fold higher in the SRSF1-precipitants, as compared to that of IgG precipitants, indicating a specific association of SRSF1 with miR-1246. miR-1246 expression was moderately increased in the TIA1-precipitants, and near IgG control levels in the EIF3B precipitants. Co-Immunoprecipitation (Co-IP) experiments were performed to verify the immunoprecipitation procedures (data not shown). To directly determine the binding of SRSF1 to the miR-1246 sequence, glutathione s-transferase (GST) conjugated human SRSF1 protein was expressed in BL21 competent E. coli, captured by glutathione sepharose beads, and eluted by glutathione. The purity of eluted GST-SRSF1 protein was shown by SDS-PAGE and Coomassie blue staining (Supplement Fig. 3).
Fig. 2.
SRSF1 binds to miR-1246. a qRT-PCR detection of miR-1246 in IgG, GAPDH, SRSF1, EIF3B, and TIA1 immunoprecipitants of PANC-1 lysate (n = 3, *p < 0.001, student t-test). b-c EMSA detection of the SRSF1-miR-1246 complex (hot probe: IRD-800 labeled miR-1246 mimics; cold probe: miR-1246 mimics, n = 3). Direct binding of GST-SRSF1 and miR-1246 (b); and concentration-dependent competition between the cold and hot miR-1246 probe for binding to GST-SRSF1 (c). d Semi-quantification of SRSF1 and miR-1246 binding in C and calculated dissociation constant (n = 3)
The binding of GST-SRSF1 to a fluorescent-tagged miR-1246 probe was determined by RNA EMSA. As shown in Fig. 2b, binding of the labeled probe was specific to GST-SRSF1, but not GST, and increased with greater protein input. The specific binding of GST-SRSF1 to the miR-1246 probe was evident as the unlabeled miR-1246 probe effectively competed with the labeled miR-1246 probe in a concentration-dependent manner (Fig. 2c). The detected bands were semi- quantified and the Kd was calculated from the detected signals (Fig. 2d). These data confirmed the direct binding of SRSF1 to the miR-1246 sequence.
Exosome miRNA enrichment by SRSF1 in cancer cells
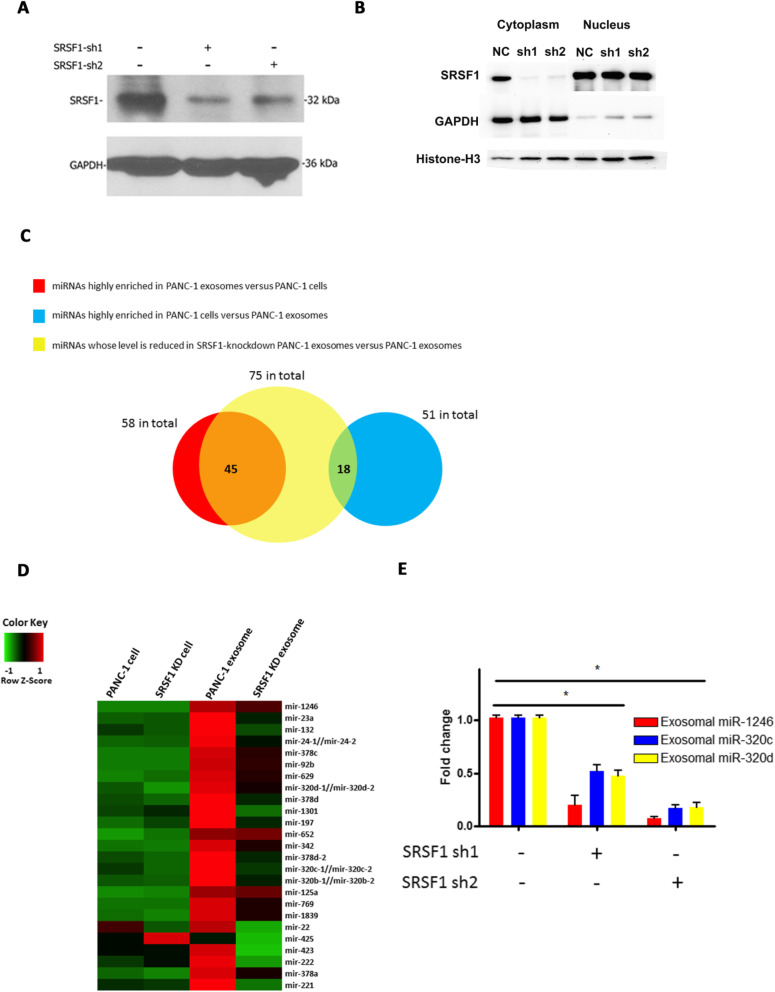
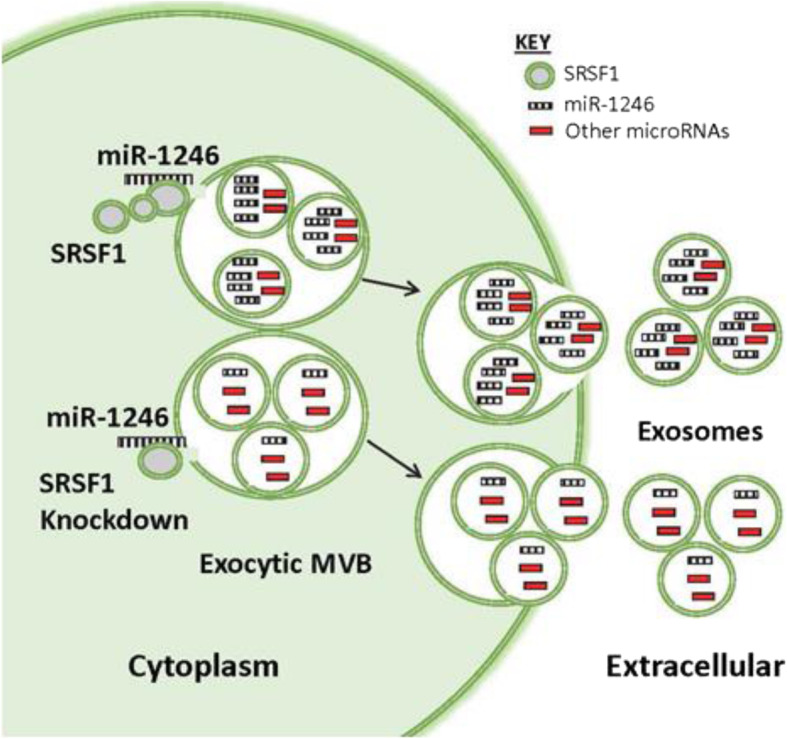
Because SRSF1 is a key splicing factor that is essential to eukaryotic cells [31], a knockout model could not be established. Therefore, to determine whether SRSF1 miRNA binding activity is relevant to exosome miRNA enrichment, we established a lentivirus SRSF1 shRNA construct to knockdown SRSF1 expression in PANC-1 cells (Fig. 3a). Interestingly, though SRSF1 protein was detected both in the nucleus and cytoplasm, the knockdown was more pronounced in the cytoplasm (Fig. 3b). Knockdown of SRSF1 did not significantly alter the concentration and size distribution of the exosomes released by PANC-1 cells (Fig. 1b). Cellular and exosome RNA from control and SRSF1-shRNA cells were isolated and small RNA sequencing was performed. Among the 58 highly enriched PANC-1 exosome miRNAs, expression of 45 miRNAs (77.6%) was significantly down-regulated in exosomes derived from SRSF1-shRNA PANC-1 cells as compared to exosomes derived from control PANC-1 cells (Fig. 3c), strongly indicating the involvement of SRSF1 in exosome miRNA enrichment. A heatmap showing the expression of the top 25 miRNAs enriched in PANC-1 exosomes demonstrates the dramatic drop in expression levels of miRNAs in SRSF1-shRNA PANC-1 exosomes compared to PANC-1 exosomes (Fig. 3d). Notably, miR-1246 was the highest enriched exosome miRNA (data not shown) and its expression in exosomes was significantly reduced by SRSF1 knockdown (Fig. 3d). On the other hand, among 51 of the miRNAs less enriched in exosomes, only 18 (35.3%) were expressed at lower levels in exosomes derived from SRSF1 knockdown cells as compared to exosomes derived from wild type PANC-1 cells (Fig. 3c), suggesting that SRSF1 knockdown mainly affects exosome enriched miRNAs.
Fig. 3.
Cellular and exosome miRNA profiles after SRSF1 knockdown in PANC-1 cells. a Detection of SRSF1 knockdown by shRNAs in PANC-1 cells. b PANC-1 SRSF1 protein levels in nuclear and cytoplasmic fractions (NC: normal control). c Venn Diagram of overlap of miRNAs detected by next generation small RNA sequencing in SRSF1 knockdown and control PANC-1 cells and exosomes. d Heatmap showing the expression of top 25 exosome miRNAs in cells and exosomes after SRSF1 knockdown. e qRT-PCR analysis of miR-1246, miR-320c and miR-320d in exosomes derived from control and SRSF1 knockdown PANC-1 cells (*p < 0.001, Student’s t-test). Shown are representatives of three independent experiments (a-e)
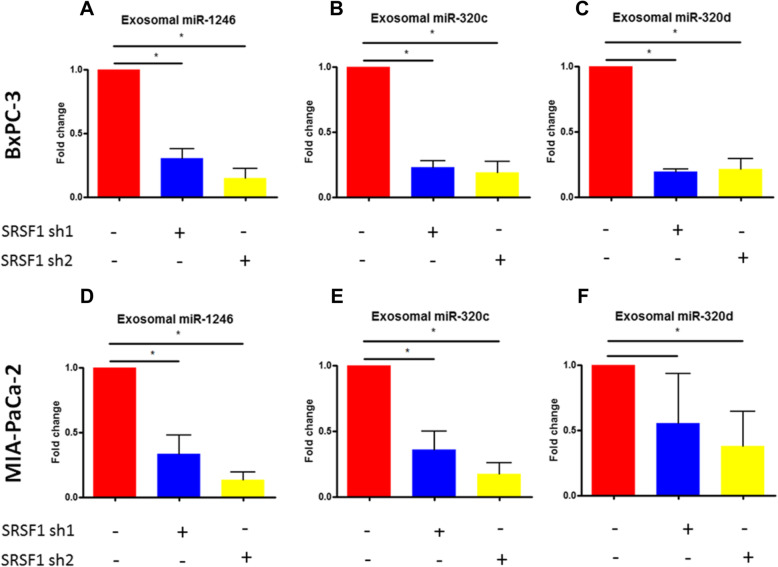
To further confirm the effect of SRSF1 knockdown on exosome miRNA enrichment, the expression levels of several representative miRNAs were quantified by qRT-PCR. SRSF1 knockdown in PANC-1 cells significantly reduced exosome levels of miR-1246, miR-320c and miR-320d, confirming the small RNA sequencing results (Fig. 3e). In contrast, knockdown of EIF3B or TIA1 did not reduce exosome miR-1246 expression, suggesting that these RBPs may not promote exosome miRNA enrichment (Supplement Fig. 4 and 5). Our observations were extended to two additional pancreatic cancer cell lines, MIAPaCa-2 and BxPC-3 (Fig. 4a-f). In addition, expression of let-7c, which is less enriched in exosomes, was unchanged in exosomes after SRSF1 knockdown (data not shown).
Fig. 4.
qRT-PCR analysis of miR-1246, miR-320c miR-320d expression in exosomes derived from SRSF1 knockdown BxPC-3 and MIAPaCa-2 cells. a-c qRT-PCR detection of miR-1246, miR-320c and miR-320d in exosomes derived from SRSF1 knockdown BxPC-3 cells (n = 3, *p < 0.001, Student t-test). d-f qRT-PCR detection of miR-1246, miR-320c and miR-320d in exosomes derived from SRSF1 knockdown MIAPaCa-2 cells (n = 3, *p < 0.001, Student’s t-test)
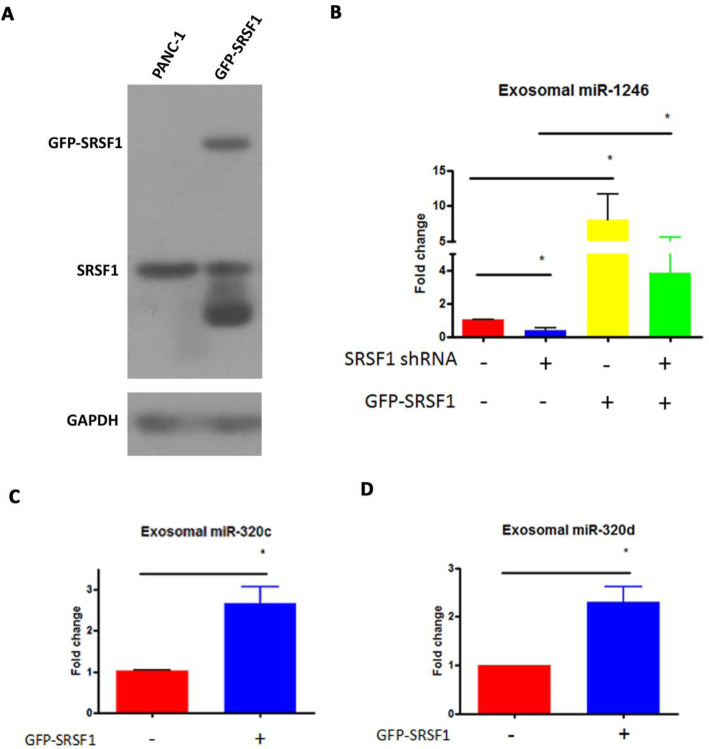
To verify the involvement of SRSF1 in exosome miRNA enrichment in cancer cells, we also exogenously overexpressed SRSF1 in PANC-1 cells. A GFP-SRSF1 expression plasmid was introduced into PANC-1 cells, and SRSF1 over-expression was confirmed by western blot (Fig. 5a). Expression of miR-1246, miR-320c, and miR-320d in the exosomes derived from GFP-SRSF1 PANC-1 cells was analyzed by qRT-PCR (Fig. 5b-c). As shown in Fig. 5b, over-expression of GFP-SRSF1 increased exosome expression of miR-1246 and rescued miR-1246 levels in exosomes derived from SRSF1-shRNA cells. Levels of miR-320c and miR-320d were also increased in exosomes derived from the GFP-SRSF1 cells, further supporting the involvement of SRSF1 in exosome miRNA enrichment (Fig. 5c-d).
Fig. 5.
qRT-PCR analysis of exosome enriched miRNAs derived from SRSF1 overexpression PANC-1 cells. a Confirmation of GFP-SRSF1 overexpression in PANC-1 cells. b qRT-PCR detection of miR-1246 in exosomes derived from wild type and SRSF1 knockdown PANC-1 cells with GFP-SRSF1 overexpression. c qRT-PCR detection of miR-320c in exosomes derived from GFP-SRSF1 overexpression PANC-1 cells. d qRT-PCR detection of miR-320d in exosomes derived from GFP-SRSF1 overexpression PANC-1 cells. *p < 0.001, Student’s t-test, n = 3 for (b-d)
Identification of RNA sequence motifs involved in exosome miRNA enrichment
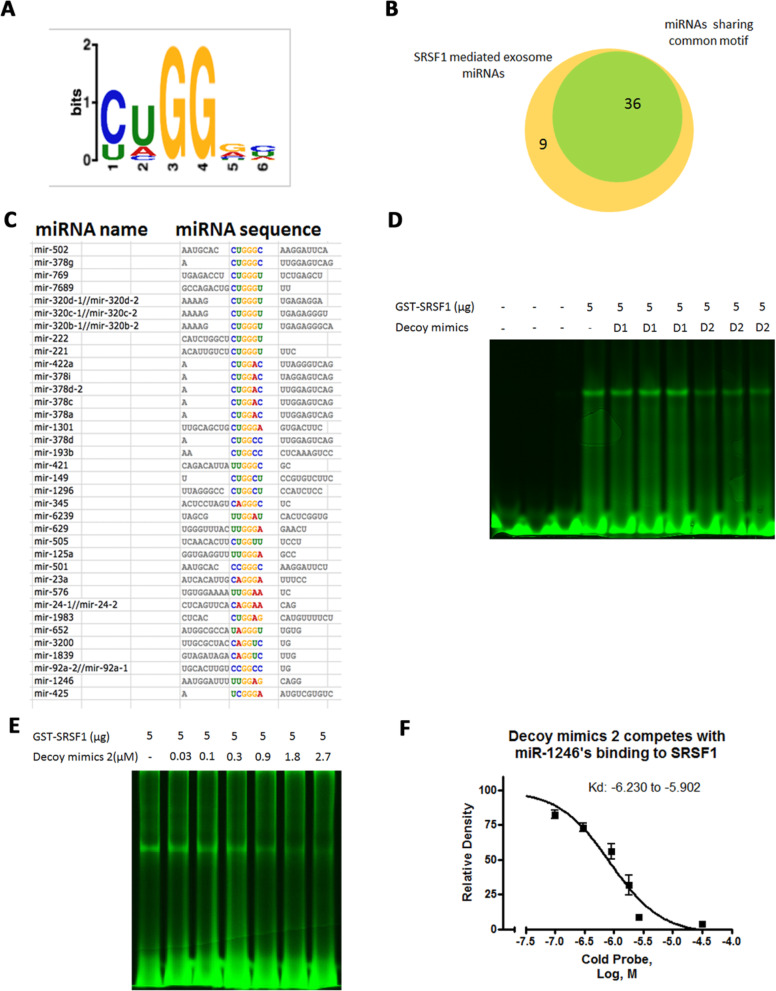
According to the RBPDB, SRSF1 binds specifically to a motif present in the miR-1246 sequence (Supplement Fig. 2). To understand the contribution of specific RNA motifs involved in exosome miRNA enrichment, we applied an unbiased approach to identify the RNA motifs that contribute to exosome miRNA enrichment. For this purpose, we analyzed the RNA sequences of the miRNAs highly enriched in cancer exosomes and regulated by SRSF1, using the bioinformatics tool MEME Suite [38]. A 6-bp length motif was found to be shared in 36 of the 45 exosome enriched miRNAs, including miR-1246 (Fig. 6 A-C). To test whether the binding of SRSF1 to miR-1246 depends on this motif, two decoy mimics were designed according to the shared motif sequences and their secondary structure (determined with the RNAfold WebServer, http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi). The binding of the decoy mimics to SRSF1 protein was determined by RNA EMSA analysis. Addition of decoy motif #1 did not alter the binding of SRSF1 to the miR-1246 probe (Fig. 6d), whereas decoy motif #2 competed with miR-1246 binding to SRSF1 in a concentration-dependent manner (Fig. 6d-f), indicating that SRSF1 directly interacts with this sequence motif.
Fig. 6.
SRSF1-associated exosome miRNA sequence motif analysis. a The motif commonly shared among SRSF1-associated exosome miRNAs. b Venn diagram showing the number of SRSF1-associated exosome miRNAs that share the motif. c List of miRNAs sharing the common motif. d EMSA analysis demonstrating the inhibition of GST-SRSF1 binding to miR-1246 by RNA decoys (D1: decoy 1, 5′-UUGGACUAGGACUAGGAU-3′; D2: decoy 2, 5′-AGGAAGGAAGGAAGGA-3′). e Concentration-dependent inhibition of GST-SRSF1 binding to miR-1246 by D2. f Semi-quantification of the detected bands in Fig. 5e and the calculated dissociation constant
Discussion
The role of exosome miRNA signaling in promoting cancer progression has been intensely investigated and well recognized in recent years [43, 44]. The higher enrichment of certain miRNAs in cancer exosomes [13, 19–21] indicates that exosome miRNA encapsulation is an active cellular process that initiates exosome miRNA signaling in the tumor microenvironment. However, the specific cellular process responsible for selective exosome miRNA enrichment has not been well established in eukaryotic cells. The most significant finding from the present study is that we have identified SRSF1 as a mediator of exosome miRNA enrichment in pancreatic cancer cells. A specific miRNA sequence motif was also identified that may be involved in the exosome miRNA enrichment process. These findings provide new insight into how miRNAs are enriched in cancer cell exosomes to initiate exosome-mediated miRNA signaling.
We recently reported that exosome miR-1246, the most highly enriched miRNA in pancreatic cancer cell-derived exosomes [21], is derived from RNU2–1, a small nuclear RNA important for mRNA splicing [26]. Along this line of our research, we sought to determine how this miRNA is enriched in cancer exosomes using our established model systems. In the present study, we have provided several lines of evidence demonstrating that SRSF1, a vital splicing factor [45] and established oncoprotein [46], is significantly involved in exosome miRNA enrichment in pancreatic cancer cells. The first line of evidence indicating SRSF1 involvement in exosome miRNA enrichment was obtained from the biotin-labeled miR-1246 pull-down experiment, followed by proteomic analysis. Among the RBPs identified, several were selected based on their detection intensity, relevance to extracellular vesicles, and reported connections to human cancer [40], including SRSF1, EIF3B, and TIA1. Of note, SRSF1 was the only RBP among them that was also predicted by the RBPDB to bind to a motif in the miR-1246 sequence. Furthermore, the direct binding of SRSF1 to the miR-1246 sequence was verified by RIP and RNA EMSA analysis, strongly indicating the physical interaction of SRSF1, and not EIF3B or TIA1, with the miR-1246 sequence. The most convincing evidence demonstrating the involvement of SRSF1 in cancer exosome miRNA enrichment was the observation that knockdown of SRSF1 significantly reduces exosome miRNA enrichment for a majority of the selectively enriched exosome miRNAs, without altering the expression levels of less enriched exosome miRNAs. These results were based on small RNA sequencing and confirmed by RT-PCR analysis. The observations were also extended to additional human pancreatic cancer cell lines, including MIAPaCa-2 and BxPC-3.
SRSF1 was initially identified as a splicing factor in eukaryotic cells [45], but SRSF1 was later revealed to shuttle between the nucleus and cytoplasm [47] to regulate RNA metabolism, miRNA procession [48] and other cellular events independent of the mRNA splicing process [31]. Importantly, SRSF1 is over-expressed in different cancer types and is considered a potent oncogene [46, 49]. Moreover, SRSF1 over expression in different types of cancer is associated with worse prognosis (Supplement Fig. 6). While the full spectrum of SRSF1 function remains to be determined, our results reveal that SRSF1 binds to specific miRNAs and is significantly involved in exosome miRNA enrichment in cancer cells. This function is likely independent of the splicing process as the reduced expression of the detected exosome miRNAs after SRSF1 knockdown is greater than their expression change in the cells. Because exosome miRNA signaling contributes to tumor development through intercellular communication in the tumor microenvironment [6, 50, 51], the involvement of SRSF1 in exosome miRNA signaling initiation likely represents a part of its oncogenic action, which may lead to new therapeutic strategies to intervene with exosome miRNA signaling in cancer. Several RBPs have been previously identified as mediators of exosome miRNA sorting in various model systems, including major vault protein in colon cancer cells [23], hnRNPA2B1 [29] in T cells, and YBX1 in HEK293T cells [30]. The identification of SRSF1 involvement in exosome miRNA enrichment in pancreatic cancer cells further supports the notion that the cellular exosome miRNA sorting process in eukaryotic cells may differ among different cell types.
We have also identified a miRNA motif commonly shared by the SRSF1-associated exosome miRNAs using the MEME Suite program (meme-suite.org). This motif was specifically bound by SRSF1 as evidenced by our RNA EMSA analysis. A similar motif, albeit slightly shorter, was identified in our recent report that describes exosome miR-1246 enrichment in pancreatic cancer cells [26]. Our results reinforce the concept that specific miRNA motifs are involved in exosome miRNA sorting [29]. The fact that a decoy motif was able to compete with the miR-1246 probe for binding to SRSF1 indicates a possibility that decoy motifs can be applied to alter exosome miRNA enrichment or exosome miRNA signaling in cancer cells. This assumption merits further investigation.
Conclusions
In summary, we have demonstrated that SRSF1 binds to a specific motif in the miR-1246 sequence and is significantly involved in selective exosome miRNA enrichment in cancer cells. These findings reveal new insights into our understanding of the cellular process that initiates exosome miRNA signaling in cancer cells and may lead to the development of new therapeutic strategies against cancer.
Supplementary information
Additional file 1: Supplementary Table 1. shRNA oligonucleotide sequences. Supplementary Figure 1. Gene ontology analysis of miR-1246 associated proteins. The enriched A. cellular component and B. molecular function categories of the 593 proteins found to associate with miR-1246. Supplementary Figure 2. Results of the RNA-binding specificities (RBPDB) database analysis of proteins predicted to bind to the mature miR-1246 sequence. Supplement Figure 3. SDS-PAGE of purified GST-SRSF1. A total of 5 μl of lysate or elute was loaded per lane. The gel was stained with Coomassie blue dye. Supplementary Figure 4. Exosomal miR-1246 levels in EIF3B knockdown PANC-1 cells. A. EIF3B knockdown verified by western blot. B. Exosomal miR-1246 in EIF3B knockdown PANC-1 cells. Supplementary Figure 5. Exosomal miR-1246 in TIA1 knockdown PANC-1 cells. A. TIA1 knockdown verified by western blot. B. Exosomal miR-1246 in TIA1 knockdown PANC-1 cells. Supplementary Figure 6. Association of SRSF1’s expression level with cancer patient survival. Data were from THE HUMAN PROTEIN ATLAS database: A. SRSF1 expression and survival years in pancreatic cancer patients. B. SRSF1 expression and survival years in liver cancer patients. C. SRSF1 expression and survival years in renal cancer patients.
Acknowledgements
Technical services were provided by the core facility at the Peggy and Charles Stephenson Cancer Center, University of Oklahoma Health Sciences Center. We thank the Laboratory for Molecular Biology and Cytometry Research at OUHSC for the next generation small RNA sequencing and mass spectrometry service (P20GM103447). We appreciate Dr. Virginie Sjoelund’s help in providing assistance with the LC-MS analysis.
Abbreviations
- ATCC
American type culture collection
- EIF3B
Eukaryotic translation initiation factor 3 subunit B
- Co-IP
Co-immunoprecipitation
- EMSA
Electrophoretic mobility shift assay
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- miRNA
MicroRNA
- qRT-PCR
Quantitative reverse transcription PCR
- RBP
RNA binding protein
- RIP
RNA immunoprecipitation
- SRSF1
Serine and arginine rich splicing factor 1
- TIA1
T cell-restricted intracellular antigen 1
Authors’ contributions
YX carried out and/or supervised the experiments and drafted the manuscript. XX, AG and JND assisted with cellular assays and western blots. BNH, BHMM, and MC participated in the design of the experiments and assisted with drafting the manuscript. WQD conceived of the study and participated in its design and coordination, and finalized the manuscript. The authors read and approved the final manuscript.
Author’s information
Not applicable.
Funding
This study was supported in part by grants from the National Institute of General Medical Sciences of the National Institutes of Health (U54GM104938, P20GM103640), the National Cancer Institute (CA235208–01), the Oklahoma Center for the Advancement of Science and Technology (HR14–147), the Presbyterian Health Foundation, and the Peggy and Charles Stephenson Cancer Center.
Availability of data and materials
The data generated or analyzed during this study are included in this manuscript and its supplementary files or are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read this manuscript and approved for the submission.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi-Fan Xu, Email: yian-xu@ouhsc.edu.
Xiaohui Xu, Email: Xiaohui-xu@ouhsc.edu.
Amy Gin, Email: amy-gin@ouhsc.edu.
Jean D. Nshimiyimana, Email: jean-nshimiyimana@ouhsc.edu
Blaine H. M. Mooers, Email: blaine-mooers@ouhsc.edu
Massimo Caputi, Email: mcaputi@health.fau.edu.
Bethany N. Hannafon, Email: Bethany-hannafon@ouhsc.edu
Wei-Qun Ding, Email: weiqun-ding@ouhsc.edu.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12964-020-00615-9.
References
- 1.Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14:14240–14269. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 7.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126:1106–1117. doi: 10.1182/blood-2014-12-618025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 10.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 11.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 13.Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding WQ. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA) Mol Cancer. 2015;14:133. doi: 10.1186/s12943-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 15.Su MJ, Aldawsari H, Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of MicroRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci Rep. 2016;6:30110. doi: 10.1038/srep30110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292. doi: 10.3402/jev.v5.31292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126:1139–1143. doi: 10.1172/JCI87316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirzaei H, Sahebkar A, Jaafari MR, Goodarzi M, Mirzaei HR. Diagnostic and therapeutic potential of exosomes in cancer: the beginning of a new tale? J Cell Physiol. 2017; 232:3251–60. [DOI] [PubMed]
- 19.Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90. doi: 10.1186/s13058-016-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5:e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu YF, Hannafon BN, Zhao YD, Postier RG, Ding WQ. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget. 2017;8:77028–77040. doi: 10.18632/oncotarget.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng Y, Ren Y, Hu X, Mu J, Samykutty A, Zhuang X, et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun. 2017;8:14448. doi: 10.1038/ncomms14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu YF, Hannafon BN, Ding WQ. microRNA regulation of human pancreatic cancer stem cells. Stem Cell Investig. 2017;4:5. doi: 10.21037/sci.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu YF, Hannafon BN, Khatri U, Gin A, Ding WQ. The origin of Exosomal miR-1246 in human cancer cells. RNA Biol. 2019. [DOI] [PMC free article] [PubMed]
- 27.Li XJ, Ren ZJ, Tang JH, Yu Q. Exosomal MicroRNA MiR-1246 promotes cell proliferation, invasion and drug resistance by targeting CCNG2 in breast cancer. Cell Physiol Biochem. 2017;44:1741–1748. doi: 10.1159/000485780. [DOI] [PubMed] [Google Scholar]
- 28.Sakha S, Muramatsu T, Ueda K, Inazawa J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci Rep. 2016;6:38750. doi: 10.1038/srep38750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276. [DOI] [PMC free article] [PubMed]
- 31.Das S, Krainer AR. Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer. Mol Cancer Res. 2014;12:1195–1204. doi: 10.1158/1541-7786.MCR-14-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Yu H, Lou JR, Zheng J, Zhu H, Popescu NI, et al. MicroRNA-19 (miR-19) regulates tissue factor expression in breast cancer cells. J Biol Chem. 2011;286:1429–1435. doi: 10.1074/jbc.M110.146530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 35.Hannafon BN, Gin AL, Xu YF, Bruns M, Calloway CL, Ding WQ. Metastasis-associated protein 1 (MTA1) is transferred by exosomes and contributes to the regulation of hypoxia and estrogen signaling in breast cancer cells. Cell Commun Signal. 2019;17:13. doi: 10.1186/s12964-019-0325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paz S, Lu ML, Takata H, Trautmann L, Caputi M. SRSF1 RNA recognition motifs are strong inhibitors of HIV-1 replication. J Virol. 2015;89:6275–6286. doi: 10.1128/JVI.00693-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang X, Zheng M, Wang P, Mok BW, Liu S, Lau SY, et al. An NS-segment exonic splicing enhancer regulates influenza a virus replication in mammalian cells. Nat Commun. 2017;8:14751. doi: 10.1038/ncomms14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook KB, Kazan H, Zuberi K, Morris Q, Hughes TR. RBPDB: a database of RNA-binding specificities. Nucleic Acids Res. 2011;39:D301–D308. doi: 10.1093/nar/gkq1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 42.Wickham H. ggplot2, elegant graphics for data analysis: 2016;Springer-Verlag New York, ISBN 978-3-319-24277-4.
- 43.Salehi M, Sharifi M. Exosomal miRNAs as novel cancer biomarkers: challenges and opportunities. J Cell Physiol. 2018;233:6370–6380. doi: 10.1002/jcp.26481. [DOI] [PubMed] [Google Scholar]
- 44.Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17:147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krainer AR, Conway GC, Kozak D. Purification and characterization of pre-mRNA splicing factor SF2 from HeLa cells. Genes Dev. 1990;4:1158–1171. doi: 10.1101/gad.4.7.1158. [DOI] [PubMed] [Google Scholar]
- 46.Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caceres JF, Screaton GR, Krainer AR. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 1998;12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H, Sun S, Tu K, Gao Y, Xie B, Krainer AR, et al. A splicing-independent function of SF2/ASF in microRNA processing. Mol Cell. 2010;38:67–77. doi: 10.1016/j.molcel.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anczukow O, Rosenberg AZ, Akerman M, Das S, Zhan L, Karni R, et al. The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat Struct Mol Biol. 2012;19:220–228. doi: 10.1038/nsmb.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moradi-Chaleshtori M, Hashemi SM, Soudi S, Bandehpour M, Mohammadi-Yeganeh S. Tumor-derived exosomal microRNAs and proteins as modulators of macrophage function. J Cell Physiol. 2019;234:7970–7982. doi: 10.1002/jcp.27552. [DOI] [PubMed] [Google Scholar]
- 51.Bell E, Taylor MA. Functional roles for Exosomal MicroRNAs in the tumour microenvironment. Comput Struct Biotechnol J. 2017;15:8–13. doi: 10.1016/j.csbj.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. shRNA oligonucleotide sequences. Supplementary Figure 1. Gene ontology analysis of miR-1246 associated proteins. The enriched A. cellular component and B. molecular function categories of the 593 proteins found to associate with miR-1246. Supplementary Figure 2. Results of the RNA-binding specificities (RBPDB) database analysis of proteins predicted to bind to the mature miR-1246 sequence. Supplement Figure 3. SDS-PAGE of purified GST-SRSF1. A total of 5 μl of lysate or elute was loaded per lane. The gel was stained with Coomassie blue dye. Supplementary Figure 4. Exosomal miR-1246 levels in EIF3B knockdown PANC-1 cells. A. EIF3B knockdown verified by western blot. B. Exosomal miR-1246 in EIF3B knockdown PANC-1 cells. Supplementary Figure 5. Exosomal miR-1246 in TIA1 knockdown PANC-1 cells. A. TIA1 knockdown verified by western blot. B. Exosomal miR-1246 in TIA1 knockdown PANC-1 cells. Supplementary Figure 6. Association of SRSF1’s expression level with cancer patient survival. Data were from THE HUMAN PROTEIN ATLAS database: A. SRSF1 expression and survival years in pancreatic cancer patients. B. SRSF1 expression and survival years in liver cancer patients. C. SRSF1 expression and survival years in renal cancer patients.
Data Availability Statement
The data generated or analyzed during this study are included in this manuscript and its supplementary files or are available from the corresponding author on reasonable request.